Abstract
Complement activation is an important mediator of kidney injury in glomerulonephritis. Complement factor H (FH) and FH-related protein 5 (FHR-5) influence complement activation in C3 glomerulopathy (C3G) and IgA nephropathy (IgAN) by differentially regulating glomerular complement. FH is a negative regulator of complement C3 activation. Conversely, FHR-5 in vitro promotes C3 activation either directly or by competing with FH for binding to complement C3b. The FH-C3b interaction is enhanced by surface glycosaminoglycans (GAGs) and the FH-GAG interaction is well-characterised. In contrast, the contributions of carbohydrates to the interaction of FHR-5 and C3b is unknown. Using plate-based and microarray technologies we demonstrate that FHR-5 interacts with sulfated GAGs and that this interaction is influenced by the pattern and degree of GAG sulfation. The FHR-5-GAG interaction that we identified has functional relevance as we could show that the ability of FHR-5 to prevent binding of FH to surface C3b is enhanced by surface kidney heparan sulfate. Our findings are important in understanding the molecular basis of the binding of FHR-5 to glomerular complement and the role of FHR-5 in complement-mediated glomerular disease.
Introduction
The complement system is a complex group of proteins that contributes to host defence through effector pathways that result in surface opsonisation, inflammatory cell recruitment and membrane damage. A critical aspect of the system is the ability to regulate activation so that complement activation occurs appropriately, for example on the surface of a pathogen or damaged or dying cells, and not on healthy host tissue [reviewed in ref (1)]. Complement activation can occur following the binding of antibodies to antigens (the classical pathway); following binding of pattern recognition receptors in the lectin pathway (for example the interaction between mannose-binding lectin and mannose-terminating glycans) and also spontaneously on surfaces through the activation of the alternative pathway (AP). Due to the ability of the AP to activate spontaneously and to contribute to amplification of surface complement generated through the lectin or classical pathways (AP amplification loop), regulation of this pathway is particularly important(2). Regulation of the AP is mediated by factor I (FI) and factor H (FH) [reviewed in (3)]). FI is an enzyme that, in the presence of a cofactor, cleaves the central complement activation protein, C3b, preventing further activation. FH is an essential fluid-phase cofactor for the FI-mediated cleavage of C3b. FH also has a key role in determining whether or not surface C3b is inactivated by FI or can continue to amplify through the AP. FH consists of 20 domains, each of approximately 60 amino acids termed short consensus repeat domains (SCR). FI cofactor activity is located within the first four SCR domains (SCR1-4, also termed complement regulatory domains). The C3b-binding domains are located within SCR1-4 and SCR19-20 (also termed complement recognition domains).
The interaction between FH and surface C3b depends not only on direct interaction with C3b through the recognition domains but critically on low affinity interactions with glycan markers (‘host polyanions’) namely, sulfated glycosaminoglycans (GAGs) and sialic acid-terminating glycans (4–9). The key role of sialic acid in influencing the ability of FH to regulate surface AP activation was first revealed in vitro: removal of sialic acid from sheep erythrocytes resulted in complement-mediated haemolysis due to loss of the protective interaction of FH with sialic acid on the erythrocytes surface (10). The FH-sialic acid recognition site is within SCR20 and there is an interaction with α2-3- (rather than α2-6- or α2-8-linked) N-acetylneuraminic acid [(Neu5Ac) (4)] and with N-glycolylneuraminic acid [(Neu5Gc) (9)]. SCRs 6-8 and 19-20 contain GAG-binding sites. Binding to Bruchs membrane in the eye is particularly dependent on interactions between SCR6-8 and heparan sulfate, and to a lesser extent dermatan sulfate (7). In contrast, interaction within the kidney appears to be more dependent on SCR19-20 GAG interactions (7). The FH SCR19-20 domains are also important for interactions with endothelial cells (11). Mutations within this region (predominantly the SCR19-20 domains), result in impaired binding of FH to C3b, heparin and endothelial cells, and are associated with susceptibility to atypical hemolytic uremic syndrome (aHUS), a thrombotic disorder of the kidney triggered by an inability to down-regulate complement activation along the renal endothelium (12).
The regulation of C3b on surfaces by FH is also influenced by factor H-related (FHR) proteins (reviewed in ref (13)). There are five FHR proteins (FHR-1 to FHR-5); these, like FH, are composed of SCR domains of varying number and sequence similarity to those in FH. Importantly none of the FHR protein family contain complement regulatory domains comparable to those within FH SCR1-4 whereas FHR-1, -2 and -5 contain carboxyl terminal domains with sequence similarity to SCR19-20 domains of FH [(reviewed in (14)]. Consequently, these proteins can compete with FH for binding to surface C3b (15, 16). But, unlike FH, they are unable to negatively regulate C3b amplification. In fact FHR-1, FHR-4 (17) and FHR-5 (18) are each able to promote C3b activation in vitro. The biological relevance of the FHR proteins within the kidney is exemplified by the finding that FHR proteins influence surface complement activation even in the presence of intact FH proteins (19). The prototypical example of this was the observation that a heterozygous mutation in FHR-5 resulted in familial C3 glomerulopathy (20). FHR-5 is able to bind to C3b and its cleavage products iC3b and C3d (15). It can also bind to laminins in basement membranes and to malondialdehyde-acetaldehyde epitopes on cell surfaces (21). In FHR-5 nephropathy the mutated FHR-5 protein is thought to promote C3 activation along surfaces within the kidney, by interfering with the ability of FH to negatively regulate glomerular complement by out-competing FH for binding to glomerular C3 (FH de-regulation)(15). FHR-5 also influences the development of kidney injury in IgA nephropathy(22–24), a renal condition associated with abnormal accumulation of IgA and complement within the renal glomeruli. As FHR-5 is associated with both rare (C3 glomerulopathy) and common (IgA nephropathy) kidney diseases we wanted to elucidate the role of GAG ligands in the interaction between FHR-5 and renal complement. Using plate-based assays and GAG microarrays we demonstrate here the interactions of FHR-5 with GAG ligands and show that this interaction influences the binding of FH to surface C3b.
Methods
Proteins and Antibodies
Recombinant FHR-5 (rFHR-5) was transiently expressed in human embryonic kidney 293T cells as previously described (15). rFHR-5 was purified by affinity chromatography using the 2C6 antibody (mouse monoclonal anti-FHR1/2/5 antibody; Dr Claire Harris, Newcastle University, UK) cross-linked to an N-hydroxysuccinimide-activated Hitrap column (GE Healthcare, 17071601). C3b and FH were sourced commercially (Comptech, C3b-A114; FH-A137). The homogeneity of the C3b was shown by SDS-polyacrylamide gel electrophoresis [(PAGE) (Supplemental Figure 1]). Desialylated FH (ΔSIA-FH) was prepared by incubating 250μg FH with 0.3 units of agarose-conjugated Clostridium perfringens neuraminidase (Sigma, N5254) for 13 hours at 37°C in 100mM NaOAc, 2mM CaCl2 Buffer, pH 5.0. The antibodies used in the present study include rabbit anti-full-length human FHR-5 (Abnova, H00081494-D01); mouse human anti-FHR-5 (Abnova, H00081494-B01) monoclonal mouse anti-human FH (Pierce, OX24, MA1-70057); and polyclonal goat anti-serum to human FH (Quidel, A312), which was biotinylated using biotinamidohexanoic acid N-hydroxysuccinimide (NHS) ester (ref) following protein G affinity purification. The molarity provided for FHR-5 is calculated for the dimer which is its native form in circulation.
Glycosaminoglycans
GAG polysaccharides sourced from Sigma-Aldrich include heparin (sodium salt, from porcine intestinal mucosa, H3393), heparin-biotin sodium salt (B9806), heparan sulfate (sodium salt, from bovine kidney, H7640), dermatan sulfate (sodium salt, from porcine intestinal mucosa, C3788), chondroitin sulfate A (sodium salt, from bovine trachea, C8529); chondroitin sulfate C (sodium salt, from shark cartilage, C4384), hyaluronic acid (sodium salt, from bovine vitreous humor, H7630). Heparan sulfate (sodium salt, from porcine intestinal mucosa, GAG-HS01), and 2-O-desulfated (DSH001/2), 6-O-desulfated (DSH002/6) and N-desulfated/re-acetylated porcine intestinal mucosa heparin (DSH004/NAc) were from Iduron (Alderley Edge, England). GAG oligosaccharides were prepared by partial depolymerisation of the polysaccharides using GAG specific lyases or nitrous acid and size-fractionated by gel filtration chromatography as previously described (25–27). The enzymatic digestions were monitored by absorption of UV 232 nm and terminated at 50% digestion. The chain lengths (the number of monosaccharide units or the degree-of-polymerization, DP) of the major oligosaccharide components were determined by negative ion electrospray ionization mass spectrometry (MS) after conversion into their ammonium salts for the fractions with lower DPs (e.g. DP2 to DP6) (27) or deduced by gel filtration profiles of those with higher DPs (Supplemental Figure 1).
Plate-based GAG binding assays
Heparin-binding plates (BD Biosciences, 354676) were coated overnight with 50 μl GAG solution [(40 μg/ml in 10 mM phosphate buffer, pH 7.4, 150 mM NaCl (PBS)]. This coating level was selected based on initial binding studies (not shown) which indicated that at 40 μg/ml, there was efficient coating of the positively charged (allyl amine) surface not only with the highly sulfated GAG, heparin, but also the non-sulfated hyaluronan and the low sulfated chondroitin sulfate A. The coating with hyaluronan was detected using the carbohydrate-binding module of Streptococcus pneumoniae hyaluronate lyase (28) and with chondroitin sulfate A using monoclonal antibody CS-56 (29). Wells were washed twice with 100 μl wash buffer (10 mM HEPES, 150 mM NaCl, pH 7.3) to remove unbound GAGs and blocked with Carbo-Free blocking reagent (Vector Laboratories, SP-5040) for 2 h at room temperature. After washing the wells 4 times with 100 μl of wash buffer, serial dilutions of 50 μl of FH or rFHR-5 were added and incubated for 2 h. Surface-bound FH was detected using biotinylated goat anti-human FH (1 μg/ml; in-house preparation from goat antiserum to FH, Quidel A312) and streptavidin-HRP conjugate (1:200 dilution; R&D, DY998). Surface-bound rFHR-5 was detected using mouse anti-FHR-5 (1 μg/ml), HRP-conjugated rabbit anti-mouse IgG (1:1000 dilution; DAKO/Agilent, P0260). The same chromogenic substrate 3,3’,5,5’-Tetramethylbenzidine (BD Biosciences, 555214) was used throughout. As protein and antibody diluent 5% w/v bovine serum albumin [(BSA) (Sigma, A7030)] was used in acetate buffer (50 mM NaOAC, 100mM NaCl, pH 7.3). The colorimetric reaction was terminated by adding 20 μl of 2N sulfuric acid. The optical density was measured at 450 nm using a spectrophotometer (ThermoScientific, Multiskan Ascent). After each incubation with protein/antibody the wells were washed 4 times with 100 μl wash buffer. Non-specific binding signals of FH or rFHR-5 to the positively charged polymer-surface (wells not coated with GAG) as well as antibody alone signals (GAG coated wells incubated with detection antibodies, in absence of FH or rFHR-5) were subtracted from the raw signals.
GAG oligosaccharide microarray
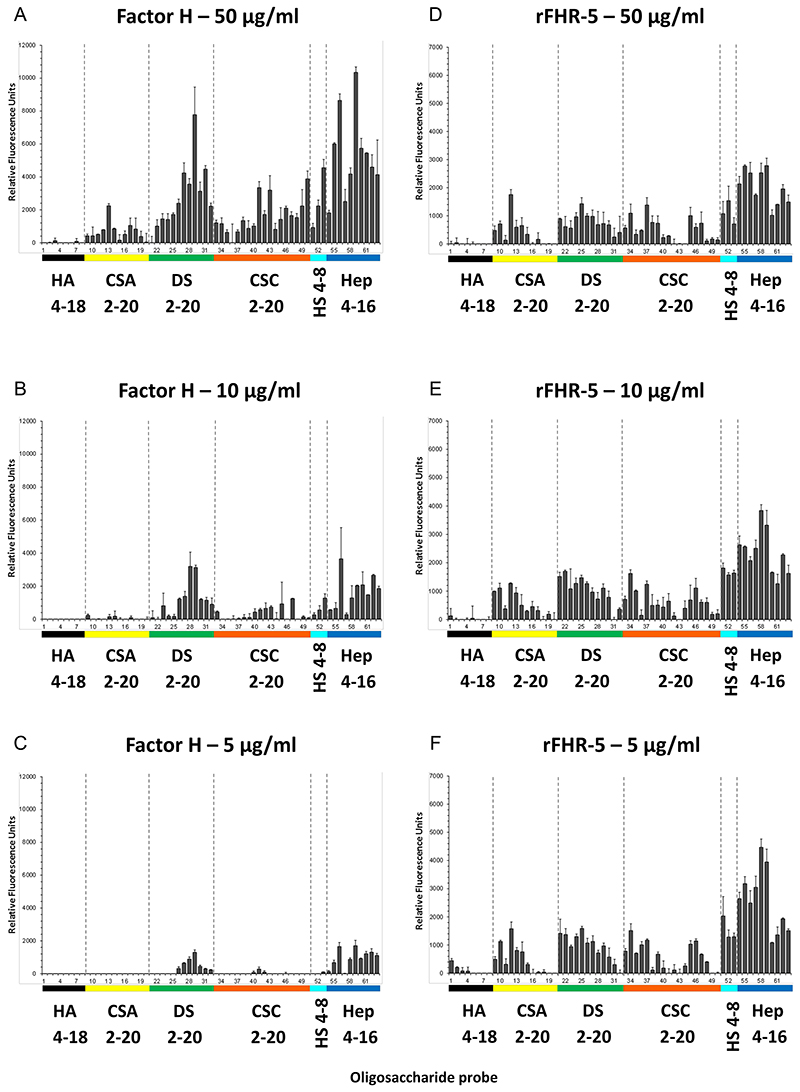
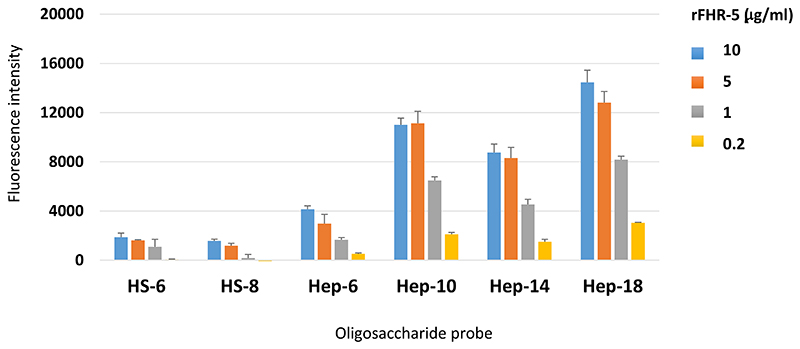
Microarray analyses were carried out using the neoglycolipid (NGL)-based microarray system(30). Details of the glycan probe library, the generation of the microarrays, imaging and data analysis are in Supplementary Glycan Microarray Document (Supplemental Table 1) in accordance with guidelines for reporting glycan microarray-based data: MIRAGE [(Minimum Information Required for A Glycomics Experiment) (31)]. Microarray analyses of FH and rFHR-5 were performed at ambient temperature. In brief, the arrayed slides were blocked for 60 min with 3% BSA (w/v) in PBS. The proteins were diluted in a 1% BSA-PBS (w/v) solution at 50, 10 and 5 μg/ml (Figure 2) and overlaid onto the pre-blocked microarray surface for 90 min. Binding of FH was detected using goat anti-human FH serum (1:200, dilution Quidel) and rFHR-5 was detected using polyclonal mouse anti-FHR-5 (5 μg/ml, Abnova) followed by biotin-conjugated rabbit anti-goat IgG (1:200 dilution; Sigma, B7014) and biotin-conjugated goat anti-mouse IgG (1:200 dilution; Sigma, B7264). For final signal detection, the arrays were incubated with Alexa 647-conjugated streptavidin (Molecular Probes, 1 μg/ml) for 30 min. The sequences of the sixty three GAG NGL probes included in the focused GAG array set (in house designation ‘GAG Oligosaccharide Array Set 4’) are in Supplemental Table 2. Binding signals were glycan dose-related. The results at 5 fmol NGL probe per spot are presented as histogram charts (Figure 2) and Supplemental Table 3, which include the fluorescence intensities and error bars (half of the difference of signal intensities of duplicate spots of each glycan probe). Additional analyses of rFHR-5 binding were carried out at a lower concentration range (10, 5, 1 and 0.2 μg/ml) (Figure 3) using a more recent GAG array (in house designation ‘GAG Oligosaccharide Array Set 11’) that included two HS and four heparin oligosaccharide probes.
Figure 2. Binding of FH and rFHR-5 to GAG oligosaccharide microarrays.
FH (A-C) or rFHR-5 (D-F) were applied to GAG oligosaccharide microarrays at concentrations of 50, 10 and 5 μg protein per ml. Hep, heparin; other abbreviations are defined in legend to Figure 1. Results with increasing oligosaccharide chain lengths are shown from left to right. Sequences of the GAG NGL probes (numbered 1 to 63 and listed in Table 1) are in Supplemental Table 2. Binding signals (fluorescence intensities) represent means of duplicates at 5 fmol per spot of each oligosaccharide probe. Error bars represent half of the difference between two values. Binding data shown are representative of at least two independent experiments.
Figure 3. Protein concentration-dependent binding of rFHR-5 to HS and heparin oligosaccharide microarrays.
FHR-5 was overlaid at 10, 5, 1 and 0.2 μg protein per ml onto microarrays that contain HS and heparin NGL probes with selected saccharide chain lengths as indicated. Binding signals (fluorescence intensities) represent mean of quadruplicate spots at 5 fmol/spot and error bars represent standard deviation. Binding data shown are representative of at least two independent experiments.
Inhibition assays of heparin binding by fluid-phase GAGs
Heparin-binding plates were coated overnight with 100 μl of 20 μg/ml heparin in PBS solution. Wells were washed twice with 100 μl wash buffer to remove unbound heparin and blocked with 100 μl Carbo-free blocking reagent (1:10 dilution) for 2 hours at room temperature. rFHR-5 was diluted in 5% BSA binding buffer (5% BSA w/v, 50 mM NaOAC, 100 mM NaCl, pH 7.3) to 1.56 μg/ml for incubation with GAG polysaccharides and diluted to 0.5 μg/ml for incubation with heparin oligosaccharides(32). rFHR-5 was pre-incubated with GAG polysaccharides (160 μg/ml) or heparin oligosaccharides (160 μg/ml) for 1 h at 4°C. For assessment of the influence of ionic strength on the rFHR-5/FH heparin interaction in the fluid-phase, rFHR-5 (1.56 μg/ml) and FH (500 ng/ml) solutions were pre-incubated for 1 h with 160 μg/ml heparin solutions which were prepared in buffers (5% BSA w/v, 20 mM Tris) with different molarities of NaCl (20, 40, 50, 60, 80, 100, 150 mM). Following this, 50 μl of the protein-GAG mixtures were transferred onto the heparin-coated plates. After 2 h incubation at room temperature, the wells were washed 4 times with 100 μl wash buffer. Detection of the surface bound FH and rFHR-5 was performed as described above, using anti-human FH or anti-FHR-5 antibodies followed by secondary antibodies and colour development. The signals were corrected against antibody blank values and non-specific protein binding to the plate surface as described above. The optical density was displayed as percentage of rFHR-5/FH binding using as 100% the optical density values of FHR-5 or FH in the absence of soluble heparin.
Analyses of FH binding to surface bound C3b with or without HS in the presence of rFHR-5
Heparin-binding plates were coated overnight at 4°C with 25 μg/ml human C3b alone or in combination with either 40 μg/ml kidney HS and 40 μg/ml hyaluronic acid, respectively. Following a wash step to remove any unbound C3b or GAG, the plates were blocked with 100 μl of 1x Carbo-Free for 2 h at room temperature. Increasing concentrations of rFHR-5 (7, 14, 28, 55, 111 and 221 nM; conrresponding to a concentration range of 0.938 to 30 μg/ml of dimeric rFHR-5) were added to fixed concentration of FH 16 nM (2.5 μg/ml) in 5 % BSA binding buffer (5% BSA w/v, 100 mM NaCl, 50 mM NaOAC, pH 7.3) and 50 μl thereof incubated in the wells for 2 h at room temperature. Surface-bound FH was detected using a monoclonal mouse IgG anti-FH (2.5 μg/ml, Invitrogen/ThermoFischer, MA1-70057) followed by HRP-conjugated polyclonal rabbit IgG anti-mouse IgG (1:1000 dilution, DAKO/Agilent, P0260). FH binding in the absence of rFHR-5 was denoted 100%.
Results
rFHR-5 and FH bind to sulfated GAG polysaccharides and the interaction is influenced by the degree and the pattern of sulfation
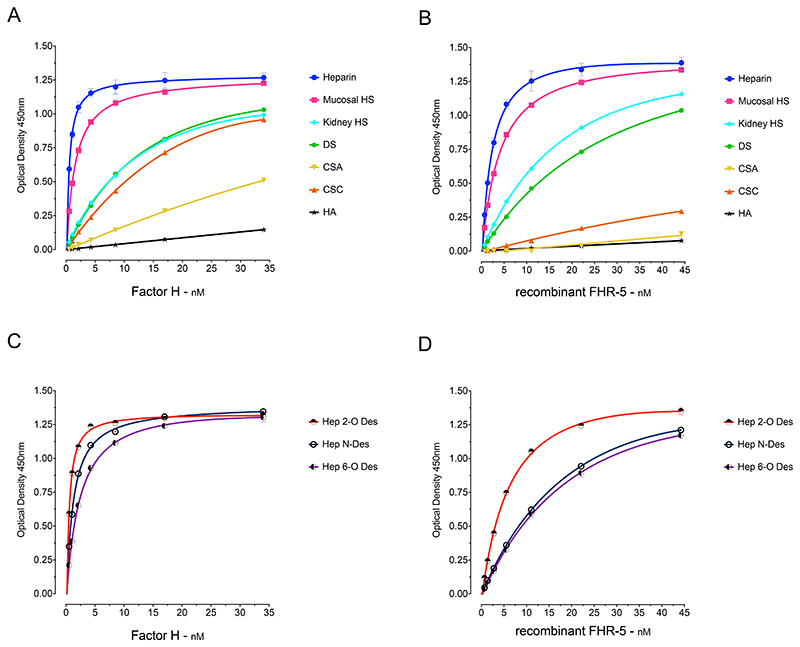
To examine the GAG-binding properties of FH and FHR-5 we first analysed the binding of serum-derived FH and recombinant FHR-5 to GAG polysaccharides immobilized in ‘heparin-binding plates’ (Figure 1). The polysaccharides included heparin, heparan sulfate (HS) derived from porcine intestinal mucosa and bovine kidney, dermatan sulfate (DS), chondroitin sulfate A (CSA) and C (CSC), and hyaluronic acid (HA). The two proteins were added to the wells at different concentrations and the binding signals analysed. The strongest binding by both proteins was to the most highly sulfated GAG, heparin, and no interaction was evident with the non-sulfated polysaccharide HA (Figure 1). The binding to porcine intestinal mucosal HS was slightly less intense than to heparin. Lower binding intensities were observed between FH and bovine kidney HS, DS and CSC, followed by CSA (Figure 1A). A broadly similar binding profile was seen for rFHR-5, including relatively strong binding to heparin, HS and DS. Both proteins interacted less strongly with HS from kidney compared to HS from porcine intestinal mucosa. Although FH bound relatively strongly to CSC, rFHR-5 showed only weak binding to CSC and little binding to CSA (Figure 1B). The results correlate with the degree-of-sulfation which progressively decreases from heparin (27) to intestinal mucosal (33) and kidney HS (34).
Figure 1. Binding of Factor H (FH) and recombinant Factor H FHR-5 (rFHR-5) to surface-immobilised GAGs.
(A) FH and (B) rFHR-5 showed dose-dependent binding correlating with the degree of GAG sulfation and lack of binding to the non-sulfated GAG, hyaluronic acid. (C) FH and (D) rFHR-5 binding to surface-immobilized, selectively desulfated GAGs showing reduction of binding after desulfation. The data are representative of more than ten independently performed experiments using the GAGs and representative of two independently performed experiments using the selectively de-sulphated GAGs. Data points represent mean of two replicates with standard deviation. HS, heparan sulfate; DS, dermatan sulfate; CSA, chondroitin sulfate A; CSC, chondroitin sulfate C; HA, hyaluronic acid. Hep 2-O-deS, heparin desulfated at the 2-O position of the iduronic acid; Hep 6-O-deS, heparin desulfated at the 6-O position of the iduronic acid; Hep N-deS-reAc, heparin desulfated at the N-position of the glucosamine followed by N-reacetylation.
The majority of heparin disaccharide units contain three sulfate groups (with 2-O-sulfate at iduronic acid and 6-O-sulfate and 2-N-sulfate at glucosamine residues). To characterise the influence of pattern of sulfation on FH and rFHR-5 binding, we compared the interactions of the proteins with heparin and selectively modified forms of heparin (Figure 1C and 1D). We used heparins that were chemically 2-O-desulpahted, 6-O-desulfated, or N-desulfated and re-N-acetylated. Both FH (Figure 1C) and rFHR-5 (Figure 1D) showed a reduction in binding strength to 6-O-desulfated heparin. rFHR-5 showed reduction in binding to N-desulfated heparin to a similar extent as 6-O-desulfated, whereas N-desulfation had less impact on FH binding. The rFHR-5 showed reduced binding to 2-O-desulfated heparin in comparison to its binding to heparin, although to a lesser extent compared to the 6-O-desulfated or N-desulfated heparin. Binding of FH to 2-O-desulfated and heparin was almost identical. Clearly the pattern of sulfation has an effect on the binding intensities of the two proteins In summary, the 6-O-sulfation has a similar contribution to the binding strengths of the two proteins whereas the influences of N-sulfation and 2-O-sulfation are greater for rFHR-5 than for FH.
rFHR-5 and FH share similar GAG oligosaccharide-binding profiles but of differing strength
The GAG binding properties of FH and FHR-5 were further investigated using a GAG oligosaccharide microarray which contains NGLs of 63 size-defined oligosaccharide fractions derived from HA, CSA, DS, CSC, HS and heparin (Figure 2). The GAG NGL arrays were overlaid with FH and rFHR-5 each at three concentrations (50, 10 and 5 μg/ml). The two protein preparations bound exclusively to the sulfated GAG oligosaccharide probes and not to the non-sulfated HA probes. Both proteins showed preferential binding to oligosaccharide probes of heparin followed by DS derived probes, in accord with observations with plate assays (Figure 1). The binding signals with FH were dependent on protein concentrations at the levels tested (Figure 2A-C) but not with rFHR-5 (Figure 2D-F), indicating a higher avidity of binding for the latter. This was confirmed using an array encompassing 6 and 8 mers of HS and 6, 10, 14 and 18 mers of heparin where rFHR-5 showed decreased binding when overlaid at concentrations lower than 5 μg/ml (Figure 3).
The binding of rFHR-5 to surface bound heparin is inhibited by fluid-phase pre-incubation with sulfated GAGs
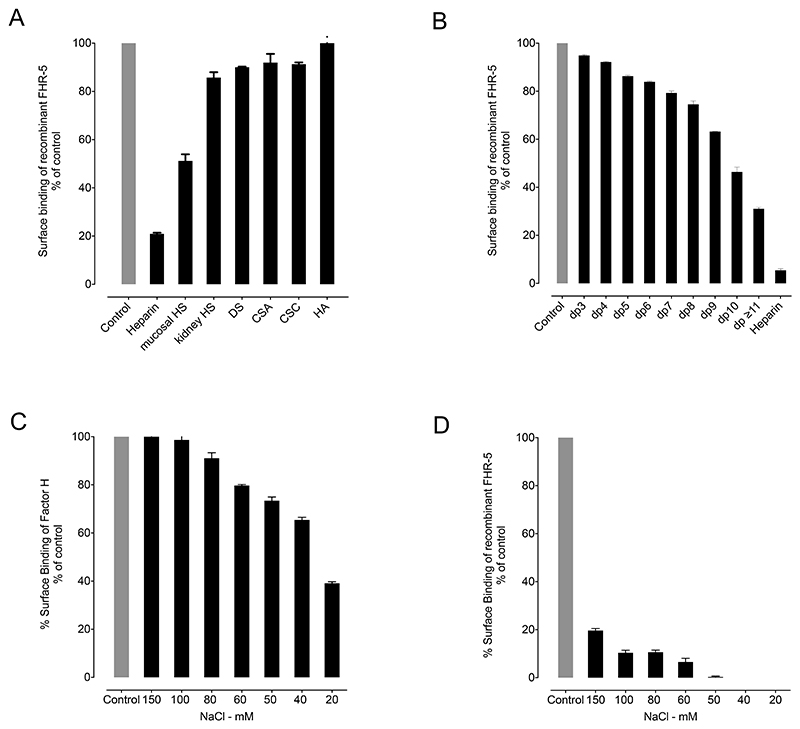
To investigate further the ability of FH and rFHR-5 to interact with GAG polysaccharides in the fluid-phase, we incubated the proteins with different GAG polysaccharides and then examined how this affected the interaction of the two proteins with surface bound heparin. The binding of rFHR-5 to immobilized heparin was inhibited by pre-incubation with heparin polysaccharide (Figure 4A). No inhibition was observed following pre-incubation with hyaluronic acid. The degree of inhibition seen following pre-incubation with other GAG polysaccharides in the fluid-phase correlated with the degree of GAG sulfation: The greatest inhibition was with intestinal mucosal HS. To determine the minimal GAG oligosaccharide chain length required for the interaction of rFHR-5 and soluble GAG polysaccharides, we pre-incubated rFHR-5 with heparin oligosaccharides of defined chain lengths (2 to 20mers). The degree of inhibition of rFHR-5 to surface heparin increased as the chain length of the soluble heparin increased (Figure 4B). In contrast to rFHR-5, fluid-phase pre-incubation of FH with heparin polysaccharide at physiological ionic strength did not affect the strength of FH binding to surface heparin. Only when the ionic strength was reduced to below 100mM NaCl were we able to detect inhibition of binding to surface heparin (Figure 4C and D).
Figure 4. The binding of recombinant FHR-5 (rFHR-5) to surface heparin is inhibited by fluid-phase pre-incubation with sulfated GAGs.
(A) Binding of rFHR-5 (1.56μg/ml) to surface heparin following pre-incubation with GAG polysaccharides (160μg/ml). (B) Binding of rFHR-5 (0.5μg/ml) to surface heparin following pre-incubation with heparin oligosaccharides (160μg/ml). Control represents surface binding of rFHR-5 in the absence of fluid-phase GAGs. DP, degree of polymerization; HS, heparan sulfate; DS, dermatan sulfate; CSA, chondroitin sulfate A; CSC, chondroitin sulfate C; HA, hyaluronic acid. The effect of ionic strength on the fluid-phase inhibition by heparin (160μg/ml) of the surface binding of (C) FH (0.5μg/ml) and (D) rFHR-5 (1.56μg/ml) to immobilized heparin. The data are representative of two independently performed experiments. Data points represent mean of two replicates with standard deviation.
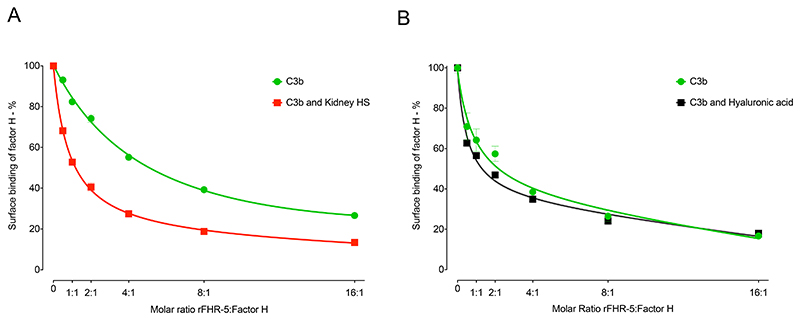
Surface bound sulfated GAG influences the relative binding strengths of FH and rFHR-5 to C3b
FHR-5 can act as a competitive antagonist of FH, a process termed FH de-regulation (15). To explore the contribution of the protein interactions with polysaccharides to this process, we examined the ability of rFHR-5 to inhibit the interaction of FH with surfaces coated with either C3b alone, with C3b and kidney HS, or with C3b and hyaluronic acid. As previously shown (15), there was a dose-dependent inhibition of the binding of FH to C3b as the concentration of rFHR-5 was increased (Figure 5). This dose-dependent inhibition of FH surface binding was comparable when surfaces coated with C3b alone and with both C3b and hyaluronic acid (Figure 5A). However, inhibition of FH surface binding by rFHR-5 was enhanced on surfaces coated with C3b and kidney HS compared to surfaces coated with C3b alone (Figure 5B). At a 1:1 molar ratio of rFHR-5 and FH (Figure 5B) surface FH binding to C3b alone was reduced to approximately 80% but fell to approximately 50% when surfaces were coated with C3b and kidney HS. These data indicate that the immobilized sulfated polysaccharide can alter binding of FH and FHR-5 to surface C3b.
Figure 5. Influence of surface-coated heparan sulfate (HS) on the relative binding of FH and rFHR-5 to surface C3b.
(A) FH binding to either surface C3b (green) or surface C3b together with kidney HS (red) in the presence of increasing concentrations of rFHR-5. The data are representative of two independently performed experiments. Data points represent mean of two replicates with standard deviation. (B) FH binding to either surface C3b (green) or surface C3b together with hyaluronic acid (black) in the presence of increasing concentrations of rFHR-5. The data are from one experiment. Data points represent mean of two replicates with standard deviation.
Discussion
We have explored the interaction between FHR-5 and GAG ligands using both a plate-based assay and a carbohydrate microarray. Using the plate-based assay the binding profiles of FH and rFHR-5 were broadly similar and correlated with the degree of sulfation of the GAGs examined, with strongest binding to heparin and no binding to hyaluronic acid. However, there were some differences in that, whilst FH bound strongly to CSC the rFHR-5 did not. The significance of this is unclear. The predominant GAG in human glomerular and tubular basement membranes is heparan sulfate, constituting at least 75% of total GAGs present (35). However approx. 20% is CS (35) and differences in the interaction of FH and FHR-5 with renal basement membrane CS may be relevant during complement activation on these surfaces. Based on our data using selectively desulfated heparins, we deduce that the pattern, as well as the degree of GAG sulfation, influences the interaction between rFHR-5 and heparin. This has previously been shown for the FH interactions (7). Using FH, the SCR19-20 domains and immobilized heparins, the interaction progressively diminished following N-desulfation-N-re-acetylation; 2-O-desulfation; 6-O-desulfation; and 2,6-O-desulfation (7). Using a similar approach, we corroborated reduction in FH binding to heparin after desulfation, and showed that this also occurs for rFHR-5. In our studies the contributions of N-sulfation and 2-O-sulfation to heparin binding were greater for rFHR-5 than for FH.
To further characterize the GAG interactions of FHR-5 we studied the interaction of rFHR-5 with 63 NGLs derived from HA, CSA, DS, CSC, HS and heparin (Supplemental Table 2). The NGLs were derived from size-defined GAG chains and we demonstrated that rFHR-5 bound only to sulfated GAG oligosaccharide probes. Interestingly whilst both FH and FHR-5 showed greater binding to heparin and DS probes, a protein dose-dependent interaction was evident only at a lower protein concentration range for FHR-5 than FH. This indicates that the interaction between FHR-5 and these NGLs is stronger and this may derive from the fact that FHR-5 exists as an obligate ‘head-to-tail’ dimer mediated by the first two amino-terminal SCR domains (15). FHR-1, FHR-2 and FHR-5 share high sequence similarity within these first two domains and all circulate as homo-dimers. Whilst heterodimerization is possible, studies have shown that the only heterodimer to be identified physiologically is the FHR-1-FHR-2 heterodimer (36). So, our use of rFHR-5 homodimers corresponds to the FHR-5 that is circulates in vivo.
We were able to inhibit the binding of rFHR-5 to immobilized heparin by pre-incubation of rFHR-5 with heparin. This inhibition was dependent on sulfation as (i) no inhibition was seen if rFHR-5 was pre-incubated with hyaluronic acid; (ii) the degree of inhibition with other GAGs correlated with the degree of sulfation; and (111) inhibition increased as the chain length of the heparin oligosaccharide increased. These data suggest that FHR-5 may interact with GAGs in the fluid-phase in vivo. Interestingly, pre-incubation of FH with heparin reduced the subsequent binding to surface heparin only when the ionic strength was below physiological sodium levels. The inhibition studies with FH were not pursued further
FHR-5 has been shown to be present in association with complement C3 within glomeruli in a variety of glomerular conditions (37). And mutations in FHR-5 are associated with C3 glomerulopathy (19, 20). Using surface plasmon resonance it has been shown that FHR-5 binds to the C3 activation fragments: C3b, iC3b and C3dg (15). FH binds to C3b and the association between abnormal FHR-5 proteins and C3 glomerulopathy has been proposed to be due to an ability of FHR-5 to out-compete FH for interaction with C3b. This has been demonstrated in vitro using haemolytic assays (15). However, the contribution of GAG ligands to the binding of FHR-5 to surface C3b had not been explored. Our data show that surface-bound sulfated GAG (kidney heparan sulfate) influences the interactions of rFHR-5 and FH with immobilized C3b. Whereas the binding of FH to surface C3b could be reduced by rFHR-5 independently of the presence of surface GAG, this reduction was enhanced when surface GAG was present. These data indicate that GAGs in the kidney can influence to the interaction of FHR-5 with renal C3b.
The biological roles of FHR-5 and of the other FHR proteins are still poorly understood. FHR-5 is present within glomeruli in C3 glomerulopathy (38), IgA nephropathy (23), and other glomerular conditions associated with complement activation (37). Understanding the molecular basis of this interaction will be key to designing approaches to prevent it. The FHR-5-heparin interaction was located within FHR-5 SCR5-7 (21, 39). The subunit domains of the FHR-5 protein that mediate other GAG interactions remain to be defined. The influence of the FHR-5-GAG interaction on other binding partners of FHR-5 also remains unclear; these ligands include properdin (40) and laminins (21) and pentraxin-3 (18). Specifically, FHR-5 binds to laminin-521 and laminin-211 and this may be relevant to FHR-5 binding within the kidney as these laminins are components of the glomerular basement membrane and mesangial matrix, respectively.
In sum, we have studied the interactions of FHR-5 with GAG ligands using both plate-based assays and a microarray platform and shown that the interaction of rFHR-5 with kidney heparan sulfate reduces the ability of FH to interact with surface C3b. Understanding the molecular basis of the FHR-5-GAG interactions will be important in elucidating the relationship between glomerular complement activation and FHR-5.
Supplementary Material
Key Points.
The pattern and degree of sulfation influenced FHR5-GAG interactions.
Surface heparan sulphate enhanced the ability of FHR5 to limit FH binding to C3b.
Table 1. List of GAG NGLs in the GAG oligosaccharide microarrays.
| Position | Probe Name | Position | Probe Name | Position | Probe Name |
|---|---|---|---|---|---|
| 1 | HA-S4 | 22 | DS-5 | 43 | CSC-18 |
| 2 | HA-S6 | 23 | DS-2 | 44 | CSC-20 |
| 3 | HA-S8 | 24 | DS-4 | 45 | CSC-S4 |
| 4 | HA-S10 | 25 | DS-6 | 46 | CSC-S6 |
| 5 | HA-S12 | 26 | DS-8 | 47 | CSC-S8 |
| 6 | HA-S14 | 27 | DS-10 | 48 | CSC-S10 |
| 7 | HA-S16 | 28 | DS-12 | 49 | CSC-S12 |
| 8 | HA-S18 | 29 | DS-14 | 50 | CSC-S14 |
| 9 | CSA-3 | 30 | DS-16 | 51 | HS-S4-AO |
| 10 | CSA-5 | 31 | DS-18 | 52 | HS-S6-AO |
| 11 | CSA-2 | 32 | DS-20 | 53 | HS-S8-AO |
| 12 | CSA-4 | 33 | CSC-3 | 54 | Hep-S4-AO |
| 13 | CSA-6 | 34 | CSC-5 | 55 | Hep-S6-AO |
| 14 | CSA-8 | 35 | CSC-2 | 56 | Hep-S8-AO |
| 15 | CSA-10 | 36 | CSC-4 | 57 | Hep-4-AO |
| 16 | CSA-12 | 37 | CSC-6 | 58 | Hep-6-AO |
| 17 | CSA-14 | 38 | CSC-8 | 59 | Hep-8-AO |
| 18 | CSA-16 | 39 | CSC-10 | 60 | Hep-10-AO |
| 19 | CSA-18 | 40 | CSC-12 | 61 | Hep-12-AO |
| 20 | CSA-20 | 41 | CSC-14 | 62 | Hep-14-AO |
| 21 | DS-3 | 42 | CSC-16 | 63 | Hep-16-AO |
Acknowledgments
MCP is a Wellcome Trust Senior Fellow in Clinical Science (212252/Z/18/Z). We acknowledge support by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare National Health Service Trust and Imperial College London, and from the NIHR Clinical Research Network. The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health research, or the Department of Health. We thank past and present members of the Glycosciences Laboratory, in particular Lisete Silva, for their collaboration in the establishment and refinements of the neoglycolipid-based microarray system. We are grateful to Barbara Mulloy and the MRC Biomedical NMR Facility directed by Tomas Frenkiel (Francis Crick Institute UK) for conducting NMR analysis of GAG oligosaccharides included in the GAG microarray studies.
Funding was from a studentship from the Imperial College London Wellcome Trust Institutional Strategic Support Fund (FGQ); the Wellcome Trust (grants 212252/Z/18/Z, 108430/Z/15/Z and 218304/Z/19/Z); the China Scholarship Council and the March of Dimes (Arlington, Virginia, U.S.A.) Prematurity Research Center grant (22-FY18-821). The MRC Biomedical NMR Facility is jointly funded by Cancer Research UK, Medical Research Council and the Wellcome Trust (FC001029).
References
- 1.Jozsi M. Factor H Family Proteins in Complement Evasion of Microorganisms. Front Immunol. 2017;8:571. doi: 10.3389/fimmu.2017.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pickering MC, Cook HT. Translational mini-review series on complement factor H: renal diseases associated with complement factor H: novel insights from humans and animals. Clin Exp Immunol. 2008;151:210–230. doi: 10.1111/j.1365-2249.2007.03574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt CQ, Lambris JD, Ricklin D. Protection of host cells by complement regulators. Immunol Rev. 2016;274:152–171. doi: 10.1111/imr.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaum BS, Hannan JP, Herbert AP, Kavanagh D, Uhrin D, Stehle T. Structural basis for sialic acid-mediated self-recognition by complement factor H. Nat Chem Biol. 2015;11:77–82. doi: 10.1038/nchembio.1696. [DOI] [PubMed] [Google Scholar]
- 5.Clark SJ, Higman VA, Mulloy B, Perkins SJ, Lea SM, Sim RB, Day AJ. His-384 allotypic variant of factor H associated with age-related macular degeneration has different heparin binding properties from the non-disease-associated form. J Biol Chem. 2006;281:24713–24720. doi: 10.1074/jbc.M605083200. [DOI] [PubMed] [Google Scholar]
- 6.Clark SJ, Perveen R, Hakobyan S, Morgan BP, Sim RB, Bishop PN, Day AJ. Impaired binding of the age-related macular degeneration-associated complement factor H 402H allotype to Bruch’s membrane in human retina. J Biol Chem. 2010;285:30192–30202. doi: 10.1074/jbc.M110.103986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark SJ, Ridge LA, Herbert AP, Hakobyan S, Mulloy B, Lennon R, Wurzner R, Morgan BP, Uhrin D, Bishop PN, Day AJ. Tissue-specific host recognition by complement factor H is mediated by differential activities of its glycosaminoglycan-binding regions. J Immunol. 2013;190:2049–2057. doi: 10.4049/jimmunol.1201751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannakis E, Jokiranta TS, Male DA, Ranganathan S, Ormsby RJ, Fischetti VA, Mold C, Gordon DL. A common site within factor H SCR 7 responsible for binding heparin, C-reactive protein and streptococcal M protein. Eur J Immunol. 2003;33:962–969. doi: 10.1002/eji.200323541. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt CQ, Hipgrave Ederveen AL, Harder MJ, Wuhrer M, Stehle T, Blaum BS. Biophysical analysis of sialic acid recognition by the complement regulator Factor H. Glycobiology. 2018;28:765–773. doi: 10.1093/glycob/cwy061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fearon DT. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978;75:1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jokiranta TS, Cheng ZZ, Seeberger H, Jozsi M, Heinen S, Noris M, Remuzzi G, Ormsby R, Gordon DL, Meri S, Hellwage J, et al. Binding of complement factor H to endothelial cells is mediated by the carboxy-terminal glycosaminoglycan binding site. Am J Pathol. 2005;167:1173–1181. doi: 10.1016/S0002-9440(10)61205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manuelian T, Hellwage J, Meri S, Caprioli J, Noris M, Heinen S, Jozsi M, Neumann HP, Remuzzi G, Zipfel PF. Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J Clin Invest. 2003;111:1181–1190. doi: 10.1172/JCI16651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jozsi M, Tortajada A, Uzonyi B, Goicoechea de Jorge E, Rodriguez de Cordoba S. Factor H-related proteins determine complement-activating surfaces. Trends Immunol. 2015;36:374–384. doi: 10.1016/j.it.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Medjeral-Thomas N, Pickering MC. The complement factor H-related proteins. Immunol Rev. 2016;274:191–201. doi: 10.1111/imr.12477. [DOI] [PubMed] [Google Scholar]
- 15.Goicoechea de Jorge E, Caesar JJ, Malik TH, Patel M, Colledge M, Johnson S, Hakobyan S, Morgan BP, Harris CL, Pickering MC, Lea SM. Dimerization of complement factor H-related proteins modulates complement activation in vivo. Proc Natl Acad Sci U S A. 2013;110:4685–4690. doi: 10.1073/pnas.1219260110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tortajada A, Yebenes H, Abarrategui-Garrido C, Anter J, Garcia-Fernandez JM, Martinez-Barricarte R, Alba-Dominguez M, Malik TH, Bedoya R, Cabrera Perez R, Lopez Trascasa M, et al. C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation. J Clin Invest. 2013;123:2434–2446. doi: 10.1172/JCI68280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hebecker M, Jozsi M. Factor H-related protein 4 activates complement by serving as a platform for the assembly of alternative pathway C3 convertase via its interaction with C3b protein. J Biol Chem. 2012;287:19528–19536. doi: 10.1074/jbc.M112.364471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Csincsi AI, Kopp A, Zoldi M, Banlaki Z, Uzonyi B, Hebecker M, Caesar JJ, Pickering MC, Daigo K, Hamakubo T, Lea SM, et al. Factor H-related protein 5 interacts with pentraxin 3 and the extracellular matrix and modulates complement activation. J Immunol. 2015;194:4963–4973. doi: 10.4049/jimmunol.1403121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbour TD, Ruseva MM, Pickering MC. Update on C3 glomerulopathy. Nephrol Dial Transplant. 2016;31:717–725. doi: 10.1093/ndt/gfu317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gale DP, de Jorge EG, Cook HT, Martinez-Barricarte R, Hadjisavvas A, McLean AG, Pusey CD, Pierides A, Kyriacou K, Athanasiou Y, Voskarides K, et al. Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet. 2010;376:794–801. doi: 10.1016/S0140-6736(10)60670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudnick RB, Chen Q, Stea ED, Hartmann A, Papac-Milicevic N, Person F, Wiesener M, Binder CJ, Wiech T, Skerka C, Zipfel PF. FHR5 Binds to Laminins, Uses Separate C3b and Surface-Binding Sites, and Activates Complement on Malondialdehyde-Acetaldehyde Surfaces. J Immunol. 2018;200:2280–2290. doi: 10.4049/jimmunol.1701641. [DOI] [PubMed] [Google Scholar]
- 22.Medjeral-Thomas NR, Lomax-Browne HJ, Beckwith H, Willicombe M, McLean AG, Brookes P, Pusey CD, Falchi M, Cook HT, Pickering MC. Circulating complement factor H-related proteins 1 and 5 correlate with disease activity in IgA nephropathy. Kidney Int. 2017;92:942–952. doi: 10.1016/j.kint.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medjeral-Thomas NR, Troldborg A, Constantinou N, Lomax-Browne HJ, Hansen AG, Willicombe M, Pusey CD, Cook HT, Thiel S, Pickering MC. Progressive IgA Nephropathy Is Associated With Low Circulating Mannan-Binding Lectin-Associated Serine Protease-3 (MASP-3) and Increased Glomerular Factor H-Related Protein-5 (FHR5) Deposition. Kidney Int Rep. 2018;3:426–438. doi: 10.1016/j.ekir.2017.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu L, Guo WY, Shi SF, Liu LJ, Lv JC, Medjeral-Thomas NR, Lomax-Browne HJ, Pickering MC, Zhang H. Circulating complement factor H-related protein 5 levels contribute to development and progression of IgA nephropathy. Kidney Int. 2018;94:150–158. doi: 10.1016/j.kint.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 25.Chai W, Beeson JG, Kogelberg H, Brown GV, Lawson AM. Inhibition of adhesion of Plasmodium falciparum-infected erythrocytes by structurally defined hyaluronic acid dodecasaccharides. Infect Immun. 2001;69:420–425. doi: 10.1128/IAI.69.1.420-425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chai W, Beeson JG, Lawson AM. The structural motif in chondroitin sulfate for adhesion of Plasmodium falciparum-infected erythrocytes comprises disaccharide units of 4-O-sulfated and non-sulfated N-acetylgalactosamine linked to glucuronic acid. J Biol Chem. 2002;277:22438–22446. doi: 10.1074/jbc.M111401200. [DOI] [PubMed] [Google Scholar]
- 27.Chai W, Luo J, Lim CK, Lawson AM. Characterization of heparin oligosaccharide mixtures as ammonium salts using electrospray mass spectrometry. Anal Chem. 1998;70:2060–2066. doi: 10.1021/ac9712761. [DOI] [PubMed] [Google Scholar]
- 28.Suits MDL, Pluvinage B, Law A, Liu Y, Palma AS, Chai W, Feizi T, Boraston AB. Conformational analysis of the Streptococcus pneumoniae hyaluronate lyase and characterization of its hyaluronan-specific carbohydrate-binding module. J Biol Chem. 2014;289:27264–27277. doi: 10.1074/jbc.M114.578435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avnur Z, Geiger B. Immunocytochemical localization of native chondroitin-sulfate in tissues and cultured cells using specific monoclonal antibody. Cell. 1984;38:811–822. doi: 10.1016/0092-8674(84)90276-9. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Childs RA, Palma AS, Campanero-Rhodes MA, Stoll MS, Chai W, Feizi T. Neoglycolipid-based oligosaccharide microarray system: preparation of NGLs and their noncovalent immobilization on nitrocellulose-coated glass slides for microarray analyses. Methods Mol Biol. 2012;808:117–136. doi: 10.1007/978-1-61779-373-8_8. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, McBride R, Stoll M, Palma AS, Silva L, Agravat S, Aoki-Kinoshita KF, Campbell MP, Costello CE, Dell A, Haslam SM, et al. The minimum information required for a glycomics experiment (MIRAGE) project: improving the standards for reporting glycan microarray-based data. Glycobiology. 2016 doi: 10.1093/glycob/cww118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marson A, Robinson DE, Brookes PN, Mulloy B, Wiles M, Clark SJ, Fielder HL, Collinson LJ, Cain SA, Kielty CM, McArthur S, et al. Development of a microtiter plate-based glycosaminoglycan array for the investigation of glycosaminoglycan-protein interactions. Glycobiology. 2009;19:1537–1546. doi: 10.1093/glycob/cwp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leteux C, Chai W, Nagai K, Herbert CG, Lawson AM, Feizi T. 10E4 antigen of Scrapie lesions contains an unusual nonsulfated heparan motif. J Biol Chem. 2001;276:12539–12545. doi: 10.1074/jbc.M010291200. [DOI] [PubMed] [Google Scholar]
- 34.Sugahara K, Tohno-oka R, Yamada S, Khoo KH, Morris HR, Dell A. Structural studies on the oligosaccharides isolated from bovine kidney heparan sulphate and characterization of bacterial heparitinases used as substrates. Glycobiology. 1994;4:535–544. doi: 10.1093/glycob/4.4.535. [DOI] [PubMed] [Google Scholar]
- 35.Van den Heuvel LP, Van den Born J, Jalanko H, Schroder CH, Veerkamp JH, Assmann KJ, Berden JH, Holmberg C, Rapola J, Monnens LA. The glycosaminoglycan content of renal basement membranes in the congenital nephrotic syndrome of the Finnish type. Pediatr Nephrol. 1992;6:10–15. doi: 10.1007/BF00856820. [DOI] [PubMed] [Google Scholar]
- 36.van Beek AE, Pouw RB, Brouwer MC, van Mierlo G, Geissler J, Ooijevaar-de Heer P, de Boer M, van Leeuwen K, Rispens T, Wouters D, Kuijpers TW. Factor H-Related (FHR)-1 and FHR-2 Form Homo- and Heterodimers, while FHR-5 Circulates Only As Homodimer in Human Plasma. Front Immunol. 2017;8:1328. doi: 10.3389/fimmu.2017.01328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy B, Georgiou T, Machet D, Hill P, McRae J. Factor H-related protein-5: a novel component of human glomerular immune deposits. Am J Kidney Dis. 2002;39:24–27. doi: 10.1053/ajkd.2002.29873. [DOI] [PubMed] [Google Scholar]
- 38.Medjeral-Thomas NR, Moffitt H, Lomax-Browne HJ, Constantinou N, Cairns T, Cook HT, Pickering MC. Glomerular Complement Factor H-related protein 5 (FHR5) is highly prevalent in C3 glomerulopathy and associated with renal impairment. Kidney Int Rep. 2019 doi: 10.1016/j.ekir.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McRae JL, Duthy TG, Griggs KM, Ormsby RJ, Cowan PJ, Cromer BA, McKinstry WJ, Parker MW, Murphy BF, Gordon DL. Human factor H-related protein 5 has cofactor activity, inhibits C3 convertase activity, binds heparin and C-reactive protein, and associates with lipoprotein. J Immunol. 2005;174:6250–6256. doi: 10.4049/jimmunol.174.10.6250. [DOI] [PubMed] [Google Scholar]
- 40.Chen Q, Manzke M, Hartmann A, Buttner M, Amann K, Pauly D, Wiesener M, Skerka C, Zipfel PF. Complement Factor H-Related 5-Hybrid Proteins Anchor Properdin and Activate Complement at Self-Surfaces. J Am Soc Nephrol. 2016;27:1413–1425. doi: 10.1681/ASN.2015020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.