Abstract
Background
Screening and treatment for latent tuberculosis infection (LTBI) are key for TB control. In the UK, the National Institute for Health and Care Excellence (NICE) and the British HIV Association (BHIVA) give conflicting guidance on which groups of people with HIV (PWH) should be screened, and previous national analysis demonstrated heterogeneity in how guidance is applied. There is an urgent need for a firmer clinical effectiveness evidence base on which to build screening policy.
Methods
We conducted a systematic, programmatic LTBI-screening intervention for all PWH receiving care in Leicester, UK. We compared yields (percentage IGRA positive) and number of tests required when applying the NICE and BHIVA testing strategies, as well as strategies targeting screening by TB incidence in patients’ countries of birth.
Results
Of 1053 PWH tested, 118 were IGRA-positive (11.2%). Positivity was associated with higher TB incidence in country-of-birth [adjusted odds ratio, 50–149 cases compared with <50 cases/100 000: 11.6; 95% confidence interval (CI) 4.79–28.10)]. There was high testing uptake (1053/1069, 98.5%). Appropriate chemoprophylaxis was commenced in 100 of 117 (85.5%) patients diagnosed with LTBI, of whom 96 of 100 (96.0%) completed treatment. Delivering targeted testing to PWH from countries with TB incidence greater than 150 per 100000 population or any sub-Saharan African country, would have correctly identified 89.8% of all LTBI cases while cutting tests required by 46.1% compared with NICE guidance, performing as well as BHIVA 2018 guidance.
Conclusion
Targeting screening to higher risk PWH increases yield and reduces the number requiring testing. Our proposed ‘PWH-LTBI streamlined guidance’ offers a simplified approach, with the potential to improve national LTBI-screening implementation.
Keywords: HIV, interferon gamma release assay, latent tuberculosis, screening, testing, tuberculosis
1. Introduction
In 2019, tuberculosis (TB) incidence in England rose for the first time in 9 years, from 4615 in 2018 to 4725 (2.4%) [1]. Incidence remains higher in the UK than most other countries in western and northern Europe [2] and its public health importance remains: until the SARS-CoV-2 pandemic, TB was the leading cause of death from an infectious disease among adults worldwide, with an estimated 10 million people falling ill with TB in 2019, a number that has been declining only very slowly in recent years [3].
There is now increasing focus on latent TB infection (LTBI) screening to reduce TB incidence in high-risk populations. Once latently infected, an individual is at highest risk of developing TB disease within the first 2 years, but can remain at risk for their lifetime [4]. As the global community looks to meet ambitious targets for reduction (90% reduction in TB incidence by 2035) and elimination of TB (<1 incident cases/1 000 000 per year) by 2050 [5], reducing the LTBI reservoir will be essential and is one of the WHO’s key performance indicators [6].
The WHO has published guidelines on groups at high risk to target for LTBI screening and treatment: people with HIV (PWH) are prime amongst these. HIV accelerates progression from LTBI to active TB from around a 10% lifetime risk to as high as 15% per year [7]. Although antiretroviral therapy (ART) reduces TB risk, it does not return to that of the HIV-negative population [8,9]. Screening and treatment (chemoprophylaxis) for LTBI reduce the risk of developing active TB, thereby preventing active TB with its attendant morbidity and mortality, transmission as well as additional costs for the health system [10].
Both 2019 European Centre for Disease Control (ECDC) guidance for use in the European Union and European Economic Area [11] and WHO guidelines for low-TB burden countries [12] recommend that all PWH should be targeted for LTBI screening. In the UK, national guidance is conflicting: the updated 2016 National Institute for Health and Care Excellence (NICE) guidance aligns with international recommendations to test all PWH [13]. By contrast, the British HIV Association (BHIVA) updated 2018 guidance recommends offering interferon gamma release assay (IGRA) testing to allPWHfrom high (≥ 150/100 000 population) or medium (40–150/100 000 population) TB incidence countries, and only screening those from low-TB-burden countries (<40/100 000 population) if additional risk factors for TB are present (listed in the guidance [14]).
This contradictory guidance may have contributed to the extreme heterogeneity in how LTBI testing for PWH is applied in the UK. Our national evaluation of practice highlighted that no widespread LTBI programmatic screening has been implemented in the UK in this population, with approximately half of HIV services offering no LTBI testing [15] despite LTBI screening and treatment being highly acceptable to this population [16]. Reasons for this heterogeneity in screening practice remain unclear but it likely represents a lack of confidence in existing guidelines and uncertainty as to which individuals should be offered LTBI screening. With patchy testing coverage, it is unsurprising that there are few previously published data on prospective, programmatic screening in low-TB-burden settings. Those which are available from cohort studies in low-incidence settings, including the UK, have included only a proportion of the active cohort being treated in that centre [17,18], contained estimated data [19], or had small sample sizes [17,20]. This highlights the need for a firmer clinical effectiveness evidence base on which to base national, and potentially international, screening policy.
We aimed to address this evidence gap by implementing a prospective screening programme for LTBI among PWH to understand levels of LTBI-testing uptake, prevalence of LTBI and levels of LTBI chemoprophylaxis uptake and completion for those testing positive, amongst this population. We also explored factors associated with LTBI in PWH, such as ethnicity and TB incidence in country-of-birth, to evaluate the performance of targeted screening strategies including the 2018 BHIVA [14] and NICE [13] guidance and formulate an alternative targeted testing strategy identifying groups of PWH to prioritize for testing, which optimizes testing yield (IGRA positivity rate amongst those tested) and efficiency (minimal IGRA tests required).
Methods
Study design and setting
We implemented a LTBI-screening programme in Leicester, UK, an ethnically diverse city with one of the highest TB incidence rates in the UK (40.5/100 000 general population in 2018 [21]). HIV prevalence is 3.96 of 1000 population aged 15 –59 years, making Leicester one of 84 (out of 317) local authorities in England with ‘high-diagnosed prevalence’ (≥2/1000 population) [22]. Only inconsistent, patchy LTBI screening amongst PWH had been occurring in Leicester since the introduction of IGRA tests.
From 22 February 2014 onwards, we prospectively screened all remaining active HIV patients in Leicester for LTBI followed by treatment, irrespective of ethnicity, country-of-birth, age, sex or comorbidities, to assess acceptability and uptake of LTBI screening and treatment among PWH, IGRA positivity rate, LTBI treatment completion rate and correlates with IGRA positivity.
Study population and participants
We included all PWH who had sought care for HIV at University Hospitals Leicester (UHL) NHS Trust (which is the sole provider for HIV and TB care in Leicester city and Leicestershire) up until 30 June 2017. We excluded those who had had active TB or LTBI treated previously and those who had died, moved away from Leicester, been lost to follow-up or who had been screened for LTBI previously. Results from IGRA screening of the cohort, together with chemoprophylaxis uptake and completion data, were included until 30 June 2021 for the purposes of this analysis.
Ethics
No ethics approval was required as this was considered to be implementation of clinical care in line with national recommendations. Approval was given by the UHL Trust TB Board, the UHL HIV Department and the UHL Microbiology Department.
Screening and management
In our prospective screening study, we used Quanti-FERON-TB Gold In-Tube (QFN-GIT), with gradual switching to QuantiFERON-TB Gold Plus (QFT-Plus) between May 2016 and January 2017. Results were positive, negative or indeterminate, dependent on manufacturers’ criteria. Indeterminate results were included in the denominator.
The majority of PWH with CD4+ cell counts at least 200 cells/μl received a single IGRA test. Those with CD4+ cell counts less than 200 cells/μl generally received two tests (T-SPOT. TB plus a QuantiFERON-TB test), in most cases performed on the same day (where this was not possible, dual results were included if tests were taken within 14 days). PWH taking two tests were classed IGRA positive if either test was positive.
The most recent previous CD4+ cell count to an IGRA test was used as the CD4+ cell count classification at the time of the test. Individuals who had CD4+ cell counts performed more than a year prior to the planned time of IGRA testing had testing withheld until a more recent CD4+ cell count became available.
Individuals with positive IGRA tests were recalled for chest radiography and further clinical assessment to exclude active TB [13]. We defined LTBI as PWH with a positive IGRA and normal chest radiography in the absence of any clinical features that would suggest active disease [23]. PWH diagnosed with LTBI were offered chemoprophylaxis, in most cases, 6 months of isoniazid, in accordance with UK guidelines [13], although individual clinicians made the final decision dependent on clinician and patient preference. Where active TB was diagnosed [23], treatment again followed UK guidelines [13].
Data acquisition
Date of birth and sex at birth were recorded, together with NHS number wherever available, to verify records. Ethnicity and country-of-birth were ascertained from electronic hospital, HIV records or paper hospital records, and ethnicity was coded according to the national NHS data dictionary [24]. Countries of birth were further classified into regions according to the World Bank Analytical Grouping [25]. We took TB incidence in country-of-birth (<50, 50–149, 150–249, 250–349 and ≥350 per 100 000 population) from WHO’s Global Health Observatory and used figures available in March 2019 [26].
Statistical analysis
Continuous data were summarized with median and interquartile range (IQR) and categorical responses as proportions/percentages. Comparisons were made using Pearson’s chi-square test (or Fisher’s exact test, if appropriate). We assessed univariate associations of IGRA positivity with age at IGRA test, CD4+ cell count at IGRA test, year of HIV diagnosis, sex, ethnicity, UK birth status, region of birth, TB incidence level in country-of-birth and type of IGRA performed using logistic regression, reported as crude odds ratios (OR) and 95% confidence intervals (95% CIs). Region of birth and black ethnicity categories were collapsed because of small numbers for some regions/groups. Multivariable models were adjusted for age, sex and year of HIV diagnosis (selected a priori). On the basis of univariate associations, models were further adjusted for CD4+ cell count at IGRA test. However, as ethnicity, UK birth status and region of birth were closely linked to the TB incidence in country-of-birth, only TB incidence in country-of-birth was included in the multivariable logistic regression.
To evaluate the performance of NICE and BHIVA guidelines alongside other targeted testing strategies using different thresholds of TB incidence in country-of-birth, for each screening scenario we calculated number of PWH needing to be screened, LTBI yield and the proportion of all those IGRA-positives correctly identified. TB incidence thresholds evaluated were: more than 350 of 100 000, more than 250 of 100 000, more than 150 of 100 000, more than 50 of 100 000 and no threshold, that is, screening all PWH, the strategy recommended by NICE 2016 guidelines [13], ECDC guidelines for the EU/EEA [11] and WHO guidelines for low-TB-incidence countries [12]. We compared these strategies to 2018 BHIVA guidance, which recommends targeted IGRA testing for those born in low-TB-incidence countries (<40/100 000) for those with TB risk factors including recent exposure to a known TB case, injecting drug use and diabetes mellitus [14]. We, therefore, prospectively collected risk factor data for patients testing IGRA-positive and from a low incidence (<40/100 000) country from 2018 onwards, and retrospectively extracted data from medical notes for those testing IGRA-positive pre-2018. As BHIVA guidance changed part-way through our study, we did not prospectively collect data on risk factors for patients who tested IGRA-negative from low-incidence countries.
Finally, we compared these guidelines to our proposed ‘PWH-LTBI streamlined guidance’: targeting testing to PWH with country-of-birth TB incidence more than 150 cases per 100 000 population or any sub-Saharan African country. This alternative guidance was formulated to maximize yield while minimizing testing required, while streamlining guidance to be as simple and user-friendly for physicians as possible.
All data were analysed using Stata v15.1 (StataCorp, College Station, Texas, USA). All statistical tests were considered significant when P value 0.05 or less.
Results
Cohort description
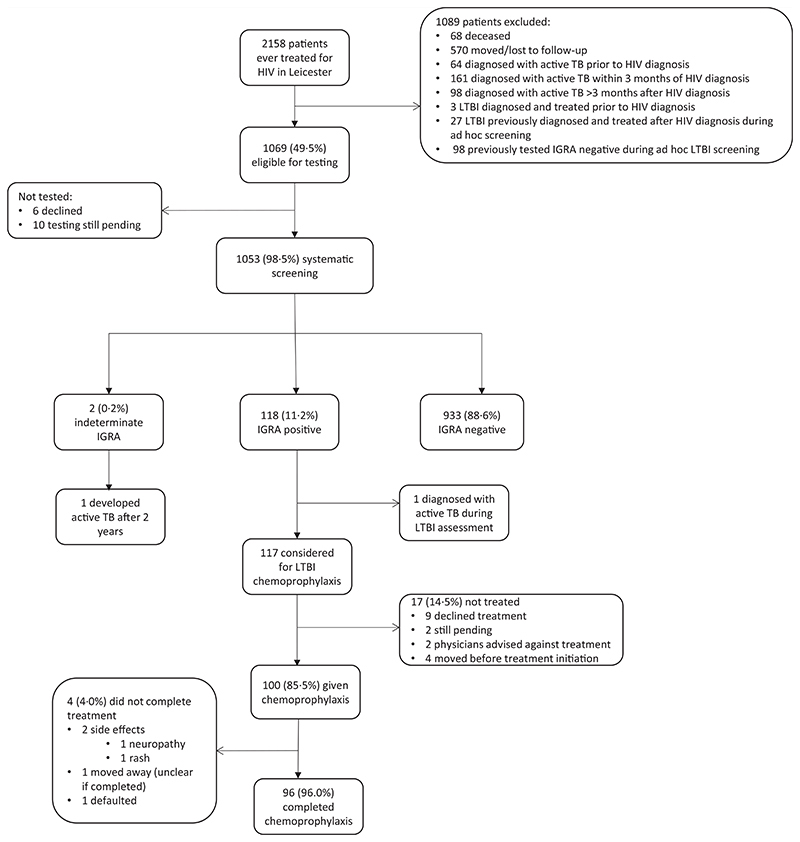
Recruitment into the study is outlined in Fig. 1. Three hundred and twenty-three patients had already had active TB and were excluded from screening, as were 30 patients who had already been diagnosed with and treated for LTBI. Ten of 1069 patients eligible for screening (0.9%) remained untested at the end of follow-up and six (0.6%) patients declined screening, leaving 1053 who underwent LTBI screening.
Fig. 1. Latent tuberculosis infection screening and treatment cascade of care.
IGRA, interferon gamma release assay; LTBI, latent TB infection.
Table 1 shows the demographic and HIV and LTBI testing-related characteristics of the screened population (n = 1053). Median age at IGRA testing was 42 years (IQR 36–49) and median CD4+ cell count was 530 cells/μl (IQR 380–700). Only 48 (4.6%) had a CD4+ cell count less than 200 cells/μl. The dominant ethnic groups were black African (498, 47.3%) and white (388, 36.8%). Sub-Saharan Africa and Europe and Central Asia were the most common regions of birth (51.1 and 40%, respectively).
Table 1. Description of total cohort and those testing interferon gamma release assay-positive.
| Variable | Total screened cohort [n (%)] | Proportion IGRA-positive [x/n (%)] | ||
|---|---|---|---|---|
| Total | 1053 | (100) | 118/1053 | (11.2) |
| Median age at IGRA test, years (IQR) | 42 | (36–49) | 42 | (38–48) |
| Year of HIV diagnosis | ||||
| 1985–1989 | 8 | (0.8) | 2/8 | (25.0) |
| 1990–1999 | 68 | (6.5) | 3/68 | (4.4) |
| 2000–2009 | 604 | (57.4) | 74/604 | (12.3) |
| 2010–2017a | 373 | (35.4) | 39/373 | (10.5) |
| Year of IGRA test | ||||
| 2014 | 393 | (37.3) | 43/393 | (10.9) |
| 2015 | 358 | (34.0) | 47/358 | (13.1) |
| 2016 | 147 | (14.0) | 18/147 | (12.2) |
| 2017 | 125 | (11.9) | 8/125 | (6.4) |
| 2018 | 24 | (2.3) | 2/24 | (8.3) |
| 2019 | 3 | (0.3) | 0/3 | (0.0) |
| 220 | 1 | (0.1) | 0/1 | (0.0) |
| 2021b | 2 | (0.2) | 0/2 | (0.0) |
| Sex | ||||
| Male | 597 | (56.7) | 53/597 | (8.9) |
| Female | 456 | (43.3) | 65/456 | (14.3) |
| CD4+ cell count at IGRA testing (cells/μl) | ||||
| Median (IQR) | 530 | (380–700) | 545 | (440–720) |
| Range | 10–2260 | 90–1350 | ||
| ≤200 | 48 | (4.6) | 2/48 | (4.2) |
| 201–350 | 181 | (17.2) | 12/181 | (6.6) |
| 351–500 | 250 | (23.7) | 36/250 | (14.4) |
| >500 | 574 | (54.5) | 68/574 | (11.8) |
| Ethnicity | ||||
| Black African | 498 | (47.3) | 90/498 | (18.1) |
| South Asian | 94 | (8.9) | 17/94 | (18.1) |
| White | 388 | (36.8) | 9/388 | (2.3) |
| Mixed | 16 | (1.5) | 0/16 | (0.0) |
| Black Caribbean | 14 | (1.3) | 0/14 | (0.0) |
| Black other | 10 | (0.9) | 0/10 | (0.0) |
| Other | 32 | (3.0) | 2/32 | (6.3) |
| Unknown | 1 | (0.1) | 0/1 | (0.0) |
| UK birth status | ||||
| UK born | 361 | (34.3) | 10/361 | (2.8) |
| Non-UK born | 692 | (65.7) | 108/692 | (15.6) |
| Region of birth | ||||
| Sub-Saharan Africa | 538 | (51.1) | 96/538 | (17.8) |
| South Asia | 50 | (4.7) | 8/50 | (16) |
| Europe and Central Asia | 421 | (40.0) | 12/421 | (2.9) |
| East Asia and Pacific | 22 | (2.1) | 2/22 | (9.1) |
| Latin America and Caribbean | 13 | (1.2) | 0/13 | (0.0) |
| Middle East and North Africa | 5 | (0.5) | 0/5 | (0.0) |
| North America | 4 | (0.4) | 0/4 | (0.0) |
| TB incidence in country-of-birth | ||||
| <50/100 000 population | 439 | (41.7) | 12/439 | (2.7) |
| 50–149/100 000 population | 58 | (5.5) | 12/58 | (20.7) |
| 150–249/100 000 population | 427 | (40.6) | 74/427 | (17.3) |
| 250–349/100 000 population | 63 | (6.0) | 11/63 | (17.5 |
| ≥350/100 000 population | 66 | (6.3) | 9/66 | (13.6) |
| Type of IGRA performed | ||||
| QuantiFERON-TB testc only | 1013 | (96.2) | 115/1013 | (11.4) |
| QuantiFERON-TB testsc and T- SPOT.TB | 25 | (2.4) | 2/25 | (8) |
| T-SPOT.TB only | 15 | (1.4) | 1/15 | (6.7) |
IGRA, interferon gamma release assay; IQR, interquartile range.
Individuals were included up and including to 30 June 2017.
30 June 2021 was used as the cut-off for following up patients for IGRA testing.
QuantiFERON-TB GIT or QuantiFERON-TB Plus.
Interferon gamma release assay-testing outcomes
IGRA results were available for all participants. Overall, 118 (11.2%) PWH had a positive IGRA result (Fig. 1 and Table 1), and two had indeterminate results (0.2%, further information in Table S1, Supplemental Digital Content, http://links.lww.com/QAD/C621).
All PWH with a positive IGRA test were diagnosed with LTBI apart from one individual who was found to have active TB disease during clinical/radiological assessment of a positive T-SPOT. TB test, performed at a CD4+ cell count of 340 cells/μl but with a detectable HIV viral load of 182 copies/ml.
Of the 117 PWH diagnosed with LTBI, 100 (85.5%) commenced LTBI chemoprophylaxis (Fig. 1). Nine of 117 (7.7%) declined; treatment was not advised by the treating physician in two of 117 (1.7%) cases; and four of 117 (3.4%) moved away before chemoprophylaxis could be given. Treatment is pending in two of 117 (1.7%) cases. Reasons behind declination were not well documented in patient notes. Ninety-eight of 100 (98%) of those initiating chemoprophylaxis had isoniazid monotherapy; the remaining two (2%) had combined rifampicin/ isoniazid.
Of the 100 patients commencing chemoprophylaxis, 96 (96%) completed treatment to the satisfaction of the treating physician. One individual moved away and it was unclear whether chemoprophylaxis had been completed, and one defaulted from treatment. Only two of 100 (2%) had to stop treatment prematurely because of adverse drug effects.
Factors associated with interferon gamma release assay-positivity and latent tuberculosis infection
Non-UK born individuals were significantly more likely than UK-born individuals to be IGRA-positive (15.6 versus 2.8%, P < 0.0001). The majority of those testing positive were from sub-Saharan Africa (96/118, 81.4%), with the IGRA positivity rate for this group being 17.8%. Black African and South Asian patients had the highest IGRA positivity rates (both 18.1%). Patients from a country where TB incidence was more than 50 per 100 000 population had higher positivity rates: 17.3% (106/614) compared with 2.7% (12/439) for patients from low-TB-incidence countries (<50/100,000). Of the 12 from low-TB-incidence countries, only four (33.3%) had risk factors (Table S2, Supplemental Digital Content, http://links.lww.com/QAD/C621).
In univariable analysis, being born abroad, specifically in sub-Saharan Africa and the South Asia and East Asia and Pacific regions, and being of black African or South Asian ethnicities, were associated with positive IGRA (Table 2). TB incidence in country-of-birth was significant in both univariable and multivariable analysis with increasing likelihood of having a positive IGRA amongst individuals born in countries with TB incidence more than 50 per 100 000 population.
Table 2. Univariate and multivariable logistic regression for having a positive interferon gamma release assay test at latent tuberculosis infection screening.
| Variable | Observation (%) |
Unadjusted OR (univariate analysis) |
P value | Adjusted OR (multivariable analysis) |
P value | |
|---|---|---|---|---|---|---|
| Age at IGRA test (years) | 1.01 (0.992–1.028) | 0.26 | 1.022 (1.0–1.05) | 0.05 | ||
| Year of HIV diagnosis | 0.99 (0.96–1.02) | 0.53 | 1.03 (0.99–1.07) | 0.23 | ||
| Sex | Male | 53/118 (44.9) | 1 | 1 | ||
| Female | 65/118 (55.1) | 1.71 (1.16–2.51) | 0.01 | 0.95 (0.62-1.45) | 0.79 | |
| CD4+ cell count at IGRA test (cells/μl) |
<200 | 2/118 (1.7) | 1 | 1 | ||
| 200–349 | 12/118 (10.2) | 1.63 (0.36–7.56) | 0.53 | 1.67 (0.35–7.97) | 0.52 | |
| 350–499 | 36/118 (30.5) | 3.87 (0.90–16.65) | 0.008 | 4.39 (0.99–19.53) | 0.05 | |
| ≥500 | 68/118 (57.6) | 3.09 (0.73–13.02) | 0.12 | 3.92 (0.89–17.12) | 0.07 | |
| Ethnicity | Blacka | 90/118 (76.3) | 1 | – | – | |
| South Asian | 17/118 (14.4) | 1.06 (0.60–1.88) | 0.84 | – | – | |
| White | 9/118 (7.6) | 0.11 (0.06–0.23) | <0.0001 | – | – | |
| Mixed/other | 2/118 (1.7) | 0.20 (0.05–0.86) | 0.03 | – | – | |
| UK birth status | Non-UK born | 108/118 (91.5) | 1 | – | – | |
| UK born | 10/118 (8.5) | 0.15 (0.08–0.30) | <0.0001 | – | – | |
| World Bank region of birth | Europe and Central Asia, | 12/118 (10.2) | 1 | - | - | |
| North America and | ||||||
| Latin America and | ||||||
| Caribbean and Middle | ||||||
| East and North Africab | ||||||
| South Asia and East Asia and Pacificc | 10/118 (8.5) | 5.79 (2.40–13.97) | <0.0001 | – | – | |
| Sub-Saharan Africa | 96/118 (81.4) | 7.80 (4.22–14.42) | <0.0001 | – | – | |
| TB incidence in country-of-birth [26]′ |
Less than 50 of 100 000 population | 12/118 (10.2) | 1 | 1 | ||
| 50–149/100 000 population | 12/118 (10.2) | 9.28 (3.94–21.85) | <0.0001 | 11.6 (4.79–28.10) | <0.0001 | |
| 150–249/100 000 population | 74/118 (62.7) | 7.46 (3.99–13.95) | <0.0001 | 8.26 (4.27–15.98) | <0.0001 | |
| 250–349/100 000 population | 11/118 (9.3) | 7.53 (3.16–17.92) | <0.0001 | 8.13 (3.33–19.86) | <0.0001 | |
| ≥350/100000 population | 9/118 (7.6) | 5.62 (2.27–13.92) | <0.0001 | 6.16 (2.42–15.67) | <0.0001 | |
IGRA, interferon gamma release assay; OR, odds ratio.
All were black African; none were black Caribbean or black other.
All were from Europe and Central Asia; none were from Latin America and Caribbean, North America or the Middle East and North Africa.
Eight of 10 were from South Asia; 2/11 were from East Asia and Pacific region.
Yields by testing threshold
Table 3 outlines the outcome of PWH IGRA test screening in Leicester stratified by TB incidence in country-of-birth, as well as outcomes for other screening strategies including BHIVA 2018 [14] and NICE [13] guidance and our proposed alternative ‘PWH-LTBI streamlined guidance’ for targeted testing. As the incidence at which screening is instigated increases, fewer PWH are eligible to be screened and, consequently, the number of identified LTBI cases also decreases. The yield (IGRA positivity rate amongst those tested) does not correspondingly increase once above the 40 per 100 000 population on BHIVA 2018 incidence threshold because we did not observe a linear increase in IGRA positivity for PWH from countries with TB incidence in country-of-birth more than 40 per 100 000 population (Table 1).
Table 3. Yield and percentage of interferon gamma release assay-positive results obtained by implementing latent tuberculosis infection screening at different tuberculosis incidence thresholds.
| Threshold: TB incidence in country-of-birth |
Number screened (%) |
Number IGRA positive |
Yield (% IGRA positive of those tested) |
Percent of all IGRA positives correctly identified |
|
|---|---|---|---|---|---|
| >350/100 000 | 66 | (6.3) | 9 | 13.6% | 7.6% |
| >250/100 000 | 129 | (12.3) | 20 | 15.5% | 16.9% |
| >150/100 000 | 556 | (52.8) | 94 | 16.9% | 79.7% |
| >150/100 000 plus all sub-Saharan African countries: the proposed ‘PWH-LTBI streamlined guidance’ |
568 | (53.9) | 106 | 18.7% | 89.8% |
| >50/100 000 | 614 | (58.3) | 106 | 17.3% | 89.8% |
| ≥40/100 000 plus risk factors: BHIVA 2018 guidelinesa [14] |
622b | (59.1) | 110 | 17.7% | 93.2% |
| Screen all PWH:* 2016 NICE | 1053 | (100) | 118 | 11.2% | 100% |
| guidelines [13], ECDC guidelines | |||||
| for the EU/EEA [11], WHO | |||||
| guidelines for low tuberculosis | |||||
| burden countries [12] | |||||
BHIVA, British HIV Association; ECDC, European Centre for Disease Prevention and Control; EEA, European Economic Area; EU, European Union; IGRA, interferon gamma release assay; NICE, National Institute for Health and Care Excellence. All included guidelines mention dual use of IGRA/Mantoux testing in some, or all PWH. We have assumed in this table that IGRA is as effective at diagnosing LTBI as Mantoux.
Recommends screening all those from high (≥ 150/100 000 population) or medium (40-150/100 000 population) TB incidence countries;only screening those from low-TB-burden countries (<40/100 000 population) if additional risk factors for TB are present: CD4+ cell count less than 200 cells/μl; recent exposure to a known TB case; diabetes mellitus;stage 4/5 chronic kidney disease; receipt of chemotherapy for malignancy; immunosuppression following transplantation; biological disease modifiers for inflammatory conditions; prolonged duration of high-dose corticosteroids (prednisolone 20 mg o.d., or equivalent, for ≥2 months); travel to or periods of time spent in medium-incidence or high-incidence countries; history of working in medical settings in countries with medium or high TB incidence; injecting drug use (detailed in Table 6.1 of guidance [14]).
This figure is an underestimate (includes all patients from countries where TB at least 40/100 000 population; plus four IGRA-positive patients from countries where TB incidence less than 40/100 000 for whom BHIVA cited additional risk factors were evident, but does not include patients with negative IGRA results from countries where TB incidence less than 40 of 100 000 because BHIVA-cited risk factors were not collected prospectively.
The strategy we identified as optimizing yield and efficiency of testing (the ‘PWH-LTBI streamlined guidance’) involves testing all PWH with country-of-birth TB incidence greater than 150 per 100 000 plus all sub-Saharan African countries. Application of NICE [13] and international [11,12] guidance, that is, screening all PWH in our cohort, identifies 100% of IGRA-positive cases with yield 11.2%. Applying BHIVA 2018 [14] guidance or our proposed ‘PWH-LTBI streamlined guidance’ both reduce the number of patients eligible for screening (to 622, 59.1% and 568, 53.9%, respectively). These screening strategies produce yields of 17.7 and 18.7%. Both yields are significantly higher than NICE [13] guidelines (proposed guidance v NICE, P < 0.0001; BHIVA guidance v NICE, P = 0.0002). BHIVA 2018 [14] guidance misses marginally fewer infections than in our proposed strategy (percentage IGRA positives correctly identified 93.2% versus 89.8%). There was no statistically significant difference in any of the outcomes shown in Table 3 between BHIVA 2018 [14] and the ‘PWH-LTBI streamlined guidance’ (P = 0.66).
Discussion
Our study describes a large prospective, systematic LTBI-screening programme implemented among PWH in a low-TB-incidence country and is the first to report chemoprophylaxis treatment uptake and completion rates. Overall, 11.1% (117/1053) of screened patients had LTBI, confirming that there is significant potential to reduce incident TB rates amongst PWH in the UK. TB incidence in this Leicester cohort is extremely high: of the 2158 patients ever treated for HIV in Leicester, 325 (15%) have had active TB, with 100 of these (31%) having incident TB occurring more than 3 months after HIV diagnosis [15,27]. Therefore, it is imperative that the burden of LTBI amongst PWH is addressed to prevent incidence of active infection. Our study showed high acceptance of LTBI testing among PWH, with high chemoprophylaxis uptake and completion for IGRA-positive patients. It is, therefore, feasible to achieve high levels of retention at each stage of the cascade of care.
Our assessment of the outcomes of IGRA screening at different incidence thresholds and using different testing guidelines showed that an alternative to current NICE [13] and BHIVA [14] guidelines, the ‘PWH-LTBI streamlined guidance’, performed statistically significantly as well as BHIVA guidelines in reducing number of IGRA tests performed and increasing yield of LTBI identified. Additionally, it offered a simpler, more streamlined approach to testing than BHIVA guidance, without the need to consult a complex set of TB risk factors to determine test eligibility that may constitute a barrier to effective implementation. 89.8% of IGRA positive cases could have been identified by restricting screening to those from countries with TB more than 150 per 100 000 population or any sub-Saharan African country. This strategy led to a significantly higher yield (LTBI positivity rate) in those tested than if all patients were screened, as is currently proposed in the ECDC, WHO and 2016 NICE guidelines [11–13].
Extremely few patients declined IGRA testing (0.6%), although a higher proportion of these IGRA-positive individuals declined chemoprophylaxis (7.7%). Over 85% of IGRA-positive individuals started chemoprophylaxis, comparing favourably with rates of 17–87% from elsewhere in the UK and other low-TB-incidence countries [18,20,28]. Ninety-six percent successfully completed treatment, and adverse drug effects from chemoprophylaxis led to cessation of therapy in only two of 100 (2%) cases, supporting previous evidence showing that chemoprophylaxis regimens, and particularly isoniazid monotherapy regimens, are well tolerated in PWH [10,29]. Although there was high retention at each stage of the cascade of care, small drop-outs at each stage still led to 14.5% of IGRA-positive cases not being treated. Further research to identify barriers and facilitators to improve uptake are required in order to avert reactivation to active TB cases as far as possible.
We were fortunate to have all data available on the country of birth for patients in our cohort, which made analysis straightforward. Encouraging the recording of country-of-birth without stigma or discrimination is helpful in health systems, so that any targeted testing based on country-of-birth can be implemented effectively UK-wide.
Our work has several limitations. Most notable of these is generating testing eligibility estimates according to the 2018 BHIVA guidance testing criteria, which recommends offering IGRA testing to PWH from low-TB-burden countries (<40/100 000 population) only if additional risk factors for TB are present (see details in Table 3 footnotes) [14]. TB risk factor data were not collected prospectively for IGRA tests performed pre-2018 (date of BHIVA guidance [14] publication). Information on risk factors was retrieved from medical records only for IGRA-positive cases from low-TB-burden countries. Therefore, our estimate of IGRA eligibility under BHIVA guidance [14] is likely to be an underestimate. This would make our proposed ‘PWH-LTBI streamlined guidance’ even more efficient than BHIVA 2018 guidance [14] in reducing IGRA tests required.
Secondly, we included indeterminate results in the denominator, which will lead to an under-estimation of the overall IGRA positivity rate. As there were only two cases of indeterminate results, however, this effect will be marginal. A further limitation was that we used country-specific TB incidence data available at a single time-point in our analysis, rather than using incidence estimates corresponding to year of entry to the UK for non-UK-born PWH. TB incidence may have changed in individual countries over time. However, date of UK entry was incomplete in our dataset and may not be routinely available, and an accessible, risk-based testing approach requires a simplified approach.
LTBI prevalence was moderately high at 11.1% for the whole cohort compared with 7–10% in other settings [18–20]. IGRA positivity for PWH from low-TB-incidence countries was comparable: 3.1% among PWH born in countries with TB incidence less than 30 per 100 000 in London [20] compared with 2.7% for those from less than 50 per 100 000 in our study. Key to the performance of screening criteria dependent on TB incidence in country-of-birth is LTBI prevalence amongst those from countries below the determined threshold. It is reassuring to observe a similar prevalence from a contrasting UK region, but more evidence on IGRA positivity rates by TB incidence in country-of-birth for other PWH populations in the UK would be useful to validate our proposed testing strategy and determine the generalizability of the results.
Our proposed ‘PWH-LTBI streamlined guidance’ performed statistically significantly as well as BHIVA 2018 guidance in terms of yield, number screened and proportion of latent infections identified. The next step is to undertake a full cost-effectiveness analysis of this and other LTBI testing strategies for PWH, both for the Leicester cohort and more generally across the UK. This would bring together the costs of the intervention, not only in terms of IGRA tests and chemoprophylaxis but also costs saved by averting cases of active TB and associated health benefits of reducing active TB morbidity and mortality, under a single framework, to inform formation of the next round of UK guidance. A previous cost-effectiveness analysis of LTBI screening among PWH based in London found that a targeted approach to screening was more cost-effective than universal testing but at the expense of missing some cases [30,31]. We now have the empirical data to inform new health economic analyses with realistic assumptions regarding IGRA positivity rates by risk group, chemoprophylaxis uptake and treatment.
This large, prospective screening cohort showed that PWH from high-TB-burden countries are at the highest risk of having LTBI but also that programmatic LTBI screening is achievable and can lead to impressive outcomes in terms of chemoprophylaxis completion. We now recommend that a full cost-effectiveness analysis is undertaken in order to produce the most user-friendly, evidence-based guidelines for screening in the UK and other low-TB-incidence settings, to enable consistent implementation.
Supplementary Material
Acknowledgements
Footnotes
Author contributions: H.A.W. and M.P. conceived the study and wrote the study protocol. H.A.W., H.P. and C. B. undertook data collection and prepared the dataset, with data verification undertaken by H.A.W. and M.P.. Analysis was conducted by H.A.W. and H.O. H.A.W. and R.F.B. drafted the manuscript, with M.P. and R.F.B. providing ongoing advice and consultation on the analysis plan and manuscript preparation. All authors contributed to manuscript writing and approved the final, submitted version.
Conflicts of interest
M.P. received grants and personal fees from Gilead Sciences and personal fees from QIAGEN, outside the submitted work. All other authors have no conflicts of interest to declare.
Data sharing
The patient cohort was extracted under Caldicott Guardian approval for a specific purpose and as part of our undertaking with them, we are not to further routinely share the data presented here. The data are held in an institutional repository and interested parties, with appropriate approvals, can apply for data access through the Corresponding author. Reasonable requests will be assessed on a case-by-case basis in discussion with the Caldicott Guardian.
R.F.B. is supported by a Wellcome Trust Institutional Strategic Support Fund Fellowship (204801/Z/16/Z). M.P. is supported by a NIHR Development and Skills Enhancement Award and also acknowledges support/funding from the NIHR Leicester BRC and UKRI. The funding source played no role in study design; in the collection, analysis and interpretation of data; in the writing of the report or in the decision to submit the paper for publication.
References
- 1.PHE. Public Health England. Tuberculosis in England 2020 report (presenting data to end of 2019) 2020. Nov, [Accessed 21 June 2021]. Available at: https://assets.publishing.service.gov.uk/government/up-loads/system/uploads/attachment_data/file/943356/TB_Annual_Report_2020.pdf.
- 2.WHO Ea. European Centre for Disease Prevention and Control and World Health Organization Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2021 (2019 data) [Accessed 21 June 2021]. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/tuberculosis-surveillance-monitoring-Europe-2021.pdf.
- 3.WHO. Global Tuberculosis Report 2020. [Accessed 21 June 2021]. ISBN 978-92-4-001313-1. Available at: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf.
- 4.Lillebaek T, Dirksen A, Baess I, Strunge B, Thomsen VO, Andersen AB. Molecular evidence of endogenous reactivation of Mycobacterium tuberculosis after 33 years of latent infection. J Infect Dis. 2002;185:401–404. doi: 10.1086/338342. [DOI] [PubMed] [Google Scholar]
- 5.WHO. World Health Organization. Framework towards tuberculosis elimination in low-incidence countries. The Organization; Geneva: [Accessed 21 June 2021]. Available at: http://www.who.int/tb/publications/elimination_framework/en. [PubMed] [Google Scholar]
- 6.WHO. World Health Organization. The End TB Strategy. Geneva: [Accessed 26 August 2021]. Available at: https://www.who.int/tb/strategy/End_-TB_Strategy.pdf. [Google Scholar]
- 7.Diedrich CR, Flynn JL. HIV-1/mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect Immun. 2011;79:1407–1417. doi: 10.1128/IAI.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawn SD, Harries AD, Williams BG, Chaisson RE, Losina E, De Cock KM, et al. Antiretroviral therapy and the control of HIV-associated tuberculosis. Will ART do it? Int J Tuberc Lung Dis. 2011;15:571–581. doi: 10.5588/ijtld.10.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta RK, Rice B, Brown AE, Thomas HL, Zenner D, Anderson L, et al. Does antiretroviral therapy reduce HIV-associated tuberculosis incidence to background rates? A national observational cohort study from England, Wales, and Northern Ireland. Lancet HIV. 2015;2:e243–e251. doi: 10.1016/S2352-3018(15)00063-6. [DOI] [PubMed] [Google Scholar]
- 10.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;1:CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosales-Klintz S, Bruchfeld J, Haas W, Heldal E, Houben R, van Kessel F, et al. Guidance for programmatic management of latent tuberculosis infection in the European Union/European Economic Area. Eur Respir J. 2019;53:1802077. doi: 10.1183/13993003.02077-2018. [DOI] [PubMed] [Google Scholar]
- 12.Getahun H, Matteelli A, Abubakar I, Aziz MA, Baddeley A, Barreira D, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46:1563–1576. doi: 10.1183/13993003.01245-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NICE. National Institute for Health and Care Excellence. Tuberculosis NICE Guideline NG33 London. [Accessed 14 May 2021]. Published: 13 January 2016 Last updated: 12 September 2019. Available at: https://www.nice.org.uk/guidance/ng33.
- 14.BHIVA. British HIV Association. British HIV Association guidelines for the management of tuberculosis in adults living with HIV 2018 (interim update 2019) [Accessed 14th May 2021]. Available at: https://www.bhiva.org/TB-guidelines. [DOI] [PubMed]
- 15.White HA, Miller RF, Pozniak AL, Lipman MC, Stephenson I, Wiselka MJ, et al. Latent tuberculosis infection screening and treatment in HIV: insights from evaluation of UK practice. Thorax. 2017;72:180–182. doi: 10.1136/thoraxjnl-2016-209063. [DOI] [PubMed] [Google Scholar]
- 16.White HA, Okhai H, Sahota A, Maltby J, Stephenson I, Patel H, et al. Latent tuberculosis screening and treatment in HIV: highly acceptable in a prospective cohort study. ERJ Open Res. 2022;8:00442–2021. doi: 10.1183/23120541.00442-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheallaigh CN, Fitzgerald I, Grace J, Singh GJ, El-Eraki N, Gibbons N, et al. Interferon gamma release assays for the diagnosis of latent TB infection in HIV-infected individuals in a low TB burden country. PLoS One. 2013;8:e53330. doi: 10.1371/journal.pone.0053330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gow N, Briggs S, Nisbet M. Screening for latent tuberculous infection in people living with HIV infection in Auckland, New Zealand. Int J Tuberc Lung Dis. 2017;21:1008–1012. doi: 10.5588/jtld.17.0103. [DOI] [PubMed] [Google Scholar]
- 19.Ramos JM, Robledano C, Masia M, Belda S, Padilla S, Rodriguez JC, et al. Contribution of interferon gamma release assays testing to the diagnosis of latent tuberculosis infection in HIV-infected patients: a comparison of QuantiFERON-TB Gold In Tube, T-SPOT.TB and tuberculin skin test. BMC Infect Dis. 2012;12:169. doi: 10.1186/1471-2334-12-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kall MM, Coyne KM, Garrett NJ, Boyd AE, Ashcroft AT, Reeves I, et al. Latent and subclinical tuberculosis in HIV infected patients: a cross-sectional study. BMC Infect Dis. 2012;12:107. doi: 10.1186/1471-2334-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.PHE. Public Health England. Tuberculosis in the East Midlands: Annual review Data from 2000 to 2018. [Accessed 21 June 2021]. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/833893/TB_2018_East_Midlands.pdf.
- 22.O’Halloran C, Sun S, Nash S, Brown A, Croxford S, Connor N, et al. Public Health England HIV in the United Kingdom: Towards Zero HIV transmissions by 2030 2019 report: appendices Data to end of December 2018 December 2019,Public Health England, London. [Accessed 21 June 2021]. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/965766/HIV_in_the_UK_2019_towards_zero_-HIV_transmissions_by_2030_appendix.pdf.
- 23.Lalvani A, Pareek M. A 100 year update on diagnosis of tuberculosis infection. Br Med Bull. 2010;93:69–84. doi: 10.1093/bmb/ldp039. [DOI] [PubMed] [Google Scholar]
- 24.NHS Data Model and Dictionary Services. Ethnic category code. 2019. [cited 27th May 2019]. [Internet] updated 04/25]. Available at: http://www.datadictionary.nhs.uk/data_dictionary/attributes/e/end/ethnic_category_code_de.asp?shownav=1 [Accessed January 2014]
- 25.Bank TW. How does the World Bank classify countries? 2018. [cited 27th May 2019]. [Internet] Available at: https://data-helpdesk.worldbank.org/knowledgebase/articles/378834-how-does-the-world-bank-classify-countries2018 [Accessed January 2014]
- 26.WHO. World Health Organization. The Global Health Observatory. Incidence of tuberculosis (per 100 000 population per year). Data accessed January 2019. [Accessed 13 September 2021]. Available at: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/cases-and-deaths.
- 27.White HA, Okhai H, Kirwan P, Rafeeq SH, Dillon H, Hefford P, et al. Tuberculosis incidence in country of origin is a key determinant of the risk of active tuberculosis in people living with HIV: data from a 30-year observational cohort study. HIV Med. 2021;23:650–660. doi: 10.1111/hiv.13222. [DOI] [PubMed] [Google Scholar]
- 28.Sultan B, Benn P, Mahungu T, Young M, Mercey D, Morris-Jones S, et al. Comparison of two interferon-gamma release assays (QuantiFERON-TB Gold In-Tube and T-SPOT.TB) in testing for latent tuberculosis infection among HIV-infected adults. Int J STD AIDS. 2013;24:775–779. doi: 10.1177/0956462413486459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zenner D, Beer N, Harris RJ, Lipman MC, Stagg HR, van der Werf MJ. Treatment of latent tuberculosis infection: an updated network meta-analysis. Ann Intern Med. 2017;167:248–255. doi: 10.7326/M17-0609. [DOI] [PubMed] [Google Scholar]
- 30.van Halsema CL, Okhai H, Hill T, Sabin CA, Study UKCHC Incidence of and risk factors for tuberculosis among people with HIV on antiretroviral therapy in the United Kingdom. AIDS. 2020;34:1813–1821. doi: 10.1097/QAD.0000000000002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capocci S, Smith C, Morris S, Bhagani S, Cropley I, Abubakar I, et al. Decreasing cost effectiveness of testing for latent TB in HIV in a low TB incidence area. Eur Respir J. 2015;46:165–174. doi: 10.1183/09031936.00067114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The patient cohort was extracted under Caldicott Guardian approval for a specific purpose and as part of our undertaking with them, we are not to further routinely share the data presented here. The data are held in an institutional repository and interested parties, with appropriate approvals, can apply for data access through the Corresponding author. Reasonable requests will be assessed on a case-by-case basis in discussion with the Caldicott Guardian.
R.F.B. is supported by a Wellcome Trust Institutional Strategic Support Fund Fellowship (204801/Z/16/Z). M.P. is supported by a NIHR Development and Skills Enhancement Award and also acknowledges support/funding from the NIHR Leicester BRC and UKRI. The funding source played no role in study design; in the collection, analysis and interpretation of data; in the writing of the report or in the decision to submit the paper for publication.