Abstract
Given the importance of the cerebellum in controlling movements, it might be expected that its main role in eating would be the control of motor elements such as chewing and swallowing. Whilst such functions are clearly important, there is more to eating than these actions, and more to the cerebellum than motor control. This review will present evidence that the cerebellum contributes to homeostatic, motor, rewarding and affective aspects of food consumption.
Prediction and feedback underlie many elements of eating, as food consumption is influenced by expectation. For example, circadian clocks cause hunger in anticipation of a meal, and food consumption causes feedback signals which induce satiety. Similarly, the sight and smell of food generate an expectation of what that food will taste like, and its actual taste will generate an internal reward value which will be compared to that expectation. Cerebellar learning is widely thought to involve feedforward predictions to compare expected outcomes to sensory feedback. We therefore propose that the overarching role of the cerebellum in eating is to respond to prediction errors arising across the homeostatic, motor, cognitive, and affective domains.
Keywords: Cerebellum, Feeding behaviour, Hunger, Satiation, Reward
Introduction
The cerebellum is the largest sensorimotor structure in the brain and has traditionally been associated with motor control and the coordination of voluntary movements, balance, and posture [1, 2, 3]. The cerebellum is now known to contribute to a wide range of behaviours extending beyond motor control including higher-order functions such as cognitive processing [4, 5, 6], reward signalling [4, 7, 8], and affective processing [9, 10], as well as fundamental functions including visceral control [4, 7, 8] and survival behaviours [11, 12, 13, 14]. Contributions to such an array of functions mean the cerebellum is well placed to act as a hub for processing multi-modal information involved in mediating complex behaviours.
One such complex behaviour is eating—an essential function to provide the energy and nutrition required to live [15, 16, 17]. But we do not just eat to survive, we also eat for pleasure [18, 19, 20]. Hunger and hedonic desire provide motivation to seek food, movement is required to locate and consume food, and then digestive processes break down food in the gut and provide feedback to the brain to regulate the amount eaten [21, 22]. Eating can also be influenced by emotional and pathological states, such as overeating in obesity and undereating in anorexia nervosa [23, 24], emphasising that food consumption has both homeostatic and higher-order elements.
This review will provide a brief introduction to the cerebellar organisation to inform the following discussion of its role in (1) motor aspects of eating, (2) homeostatic and hedonic elements of appetite, (3) reward processing in relation to healthy and disordered eating, and (4) affective processing related to appetite and reward. The cerebellum is implicated in many of the processes involved in food consumption, and its activity and network connectivity are altered in both overeating (obesity) and undereating (anorexia nervosa) disorders which also span the above domains. We therefore propose that the cerebellum serves as a central regulator of information processing across the homeostatic, motor, cognitive and affective domains. More specifically, given that the cerebellum is known to create predictive representations of the environment and that eating behaviours are underpinned by expectation, we will present evidence that the universal role of the cerebellum in eating is to generate behaviourally relevant responses to prediction errors.
Overview of Cerebellar Structure
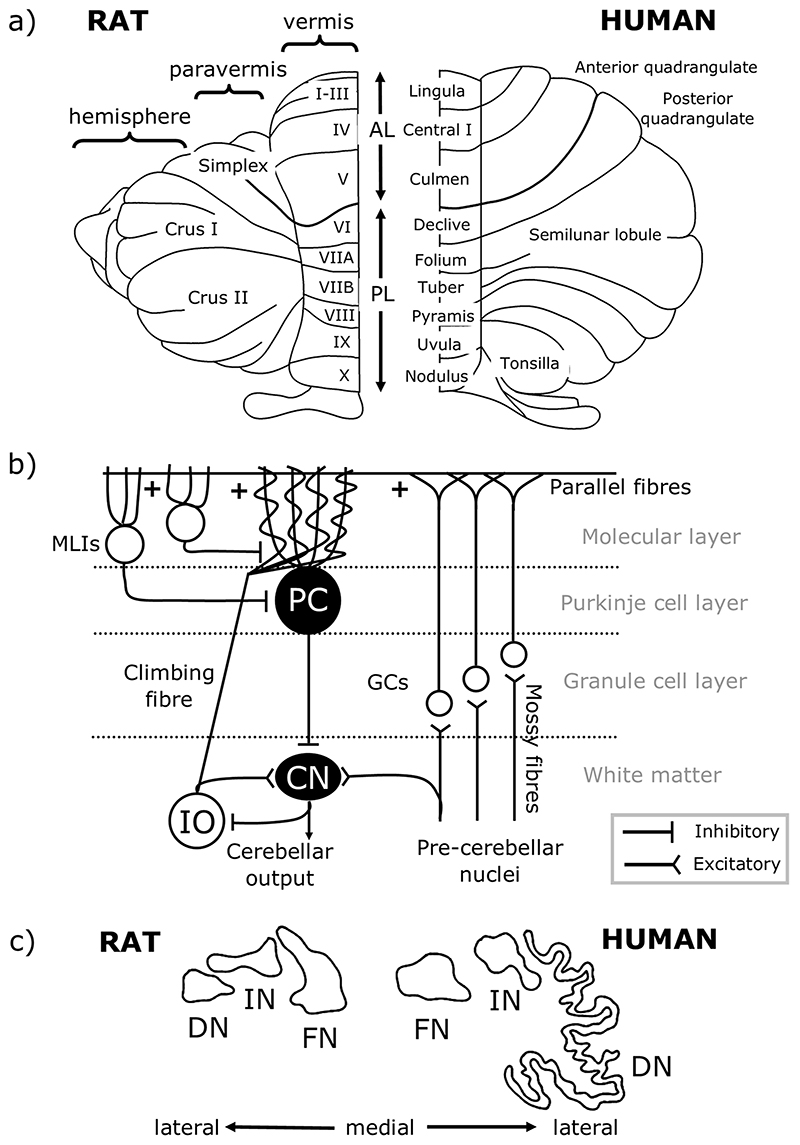
To understand cerebellar function, it is necessary to appreciate its basic anatomical organisation. At a macroscale the cerebellar cortex has three rostro-caudally oriented longitudinal divisions: from medial to lateral on each side of the cerebellar midline these are the vermis, paravermis and hemisphere (Fig. 1a). The cerebellar cortex is intricately folded and has a tri-laminar structure comprised of the granule cell layer, Purkinje cell layer, and molecular layer, the circuitry of which is summarised in Fig. 1b. The cells within these cortical layers process cerebellar inputs and Purkinje cells form the sole output of the cerebellar cortex, projecting to neurons of the cerebellar nuclei (Fig. 1b), which, in turn, provide the final cerebellar output signal. Purkinje cells located within the vermis, paravermis, and hemispheres project mainly to the fastigial (medial), interpositus and dentate (lateral) nuclei respectively (Fig. 1c) [25].
Fig. 1. Cerebellar anatomical organisation.
a Dorsal view of the rat (left) and human (right) cerebellum. There are three main longitudinal compartments of the cerebellar cortex, from medial to lateral the vermis, paravermis and hemisphere. AL, anterior lobe; PL, posterior lobe. b Simplified cerebellar circuitry. Inputs to the cerebellum are from mossy fibres of various pre-cerebellar nuclei and climbing fibres of the inferior olive (IO), both of which are glutamatergic. Mossy fibres synapse onto granule cells (GCs) which form bifurcating axons, known as parallel fibres, targeting Purkinje cell (PC) dendrites, and climbing fibres synapse onto PC dendrites directly. Both mossy fibres and climbing fibres also form collaterals targeting neurons of the cerebellar nuclei (CN). PCs are the sole output neuron of the cerebellar cortex, and these GABAergic neurons target neurons of the CN which form cerebellar output. Several types of interneurons also act within the cerebellar cortex, including molecular layer interneurons (MLIs), not all of which are shown. c Outlines of the rat (left) and human (right) cerebellar nuclei. The vermis, paravermis and hemispheres of the cerebellar cortex project to the fastigial nuclei (FN, also known as medial nuclei), interpositus nuclei (IN) and dentate nuclei (DN, also known as lateral nuclei), respectively. Scaled so that FN is a similar size in both species. Adapted from Altman and Bayer [26]
The medial parts of the cerebellum are the oldest in evolutionary terms, with roles in motor control, proprioception and autonomic functions [27]. In contrast, the hemispheres are more highly developed in higher-order species in line with the expansion of the cerebral cortex [28]. These lateral cerebellar regions are related to goal-directed behaviour, including cognition, due to extensive cerebro-cerebellar connections [29, 30]. The cerebellar nuclei have also expanded disproportionately with evolutionary development; by comparison to fastigial and interpositus, the dentate nucleus is larger and more convoluted in higher species in accordance with an increase in the size of the cerebellar hemispheres from which it receives input (Fig. 1c) [26].
At a finer level of anatomical organisation, the cerebellum contains a series of “modules” consisting of rostrocaudally oriented “zones” of Purkinje cells in the cortex together with the cerebellar nuclear territory that they target, and the inferior olive neurons from which they receive climbing fibre input (for further detail on modules and zones see [31, 32, 33, 34, 35]). These olivo-cerebellar loops are thought to be the basic functional units of the cerebellum. The function of each is thought to be dictated by its input and output connectivity with other regions of the brain [32, 33, 36, 37, 38, 39]. The modular organisation of the cerebellum should therefore be taken into account when considering the contributions of individual cerebellar regions to behaviour, including eating.
An additional important consideration is that the cerebellum has been proposed to act as a ‘prediction machine’ [40, 41], participating in the formation and updating of internal models which allow ongoing behaviours to be modified based on prior experience (Wolpert et al. [42]). As such, the cerebellum is likely to control behaviour by generating predictions about future behavioural outcomes which are updated based on the comparison of actual and expected outcomes [40]. Similar predictive mechanisms may apply across multiple domains via cerebellar connections with a multitude of brain regions, allowing the cerebellum to optimise many types of behaviour, including those involved in eating.
Cerebellar Contributions to Motor Control of Eating
Perhaps the most obvious role of the cerebellum in eating is its contribution to motor behaviour. Aside from its involvement in the movement required to locate food and bring it to the mouth, the cerebellum contributes to the two main motor components of consuming food: mastication (chewing) and swallowing [43]. Cerebellar injuries, stroke, and ataxia are associated with difficulties swallowing (dysphagia) and chewing [44, 45, 46, 47, 48], demonstrating the importance of an intact cerebellum in the physical ability to eat. This section will discuss the role of the cerebellum in both the voluntary (mastication and initiation of swallowing) and involuntary (passive swallowing) motor aspects of food ingestion.
Cerebellum in Mastication
Mastication is voluntary and recruits different groups of facial and neck muscles depending on the difficulty encountered at food breakdown [22]. The cerebellum indirectly innervates facial muscles via the red nucleus [49], a key component of the lateral descending motor system (for review see [50]).
Functional magnetic resonance imaging (fMRI) is commonly used to non-invasively assess brain activity, particularly in humans. Increased regional blood flow is linked to neuronal activation, and the changes in levels of oxygenated and deoxygenated blood can be detected as a blood oxygen level-dependent (BOLD) contrast signal which indicates whether a brain region is showing relatively increased or decreased activity [51]. Such imaging studies in humans have shown that most regions of the posterior cerebellum change their activity in relation to chewing, with activation of the anterior cerebellum and of lobules V, VI, VIII, and IX of the posterior cerebellum during chewing and associated facial movements [52, 53, 54, 55, 56, 57]. Co-activation of the cerebellum (lobule VI), thalamus and supplementary motor areas in the cerebral cortex during chewing and clenching of the jaw indicate that the cerebellum may modulate masticatory activity via cerebello-thalamo-cerebral connections [54, 56, 58, 59].
Internal and external cerebellar functional connectivity is also increased during chewing; fMRI has shown enhancements in both inter-hemispheric connectivity within the cerebellum and with other brain regions including sensorimotor cortices, left temporal gyrus and left cingulate cortex [60]. The pattern of cerebral connections suggests the cerebellum is involved in the motor planning element of mastication, in line with theories of cerebellar prediction and anticipatory activity [60, 61]. Furthermore, patients who adjust their chewing movements after structural changes in the dental arch show in fMRI studies an increased involvement of the cerebellum [57], supporting the potential involvement of the cerebellum in chewing pattern generation [52]. This is consistent with the cerebellum containing an internal model related to chewing, which is updated when oral modifications change the most efficient form of chewing. Taken together, imaging studies in humans therefore suggest a role for the cerebellum in the motor activity, planning and updating of internal models related to mastication.
Cerebellum in Swallowing
Swallowing can be divided into three phases: (i) the oral preparatory phase, comprising of the formation of the bolus and voluntary guidance towards the larynx; (ii) the pharyngeal transfer phase, where a series of reflexes induce involuntary closure of the epiglottis and guidance of the bolus towards the oesophagus; and (iii) the oesophageal transport phase, when the bolus is transported towards the stomach by synchronised contraction and relaxation of the circular muscles [21]. Based on anatomical, physiological, imaging, and clinical evidence, the cerebellum has an established role in all phases of swallowing (for a more extensive review, see [48]). For example, fMRI studies in humans have shown cerebellar activation during swallowing, particularly within the left-hand side of the cerebellum (around lobule VI, vermal culmen and pyramis) [62, 63, 64].
The cerebellum has reciprocal connections with cranial nerves involved in mediating pharyngeal and oesophageal phases of swallowing; typically, these nerves have a sensory component with projections to the cerebellum, and a motor component which controls and coordinates pharyngeal and oesophageal muscle activity according to feedback [65]. Somatotopic representations of the facial area are found across the cerebellar cortex [66]. In particular, the tongue is a critical component of the voluntary phase of swallowing [67], and there is a somatotopic representation of the tongue across lobules VII and VIII of the cerebellar cortex in humans [62]. A perioral representation with fractured somatotopic organisation has also been demonstrated in the cerebellar hemisphere granule cell layer in other species, including rodents [68, 69], suggesting conservation of function across species.
The cerebellum also has reciprocal connections with the nucleus of the solitary tract (NTS, Fig. 2), which integrates visceral information from cranial nerves, and amongst other functions, initiates the voluntary phase of swallowing [70]. The NTS has direct projections to the cerebellar cortex, particularly the vermis, and receives inputs from the medial nucleus of the cerebellum [70, 71], which relays output from the vermis, thereby forming a reciprocal NTS-cerebellar circuit. Transcranial magnetic stimulation of the cerebellum in humans interferes with swallowing, indicating that the cerebellum, like the NTS, plays a pivotal role in coordinating voluntary swallowing [72].
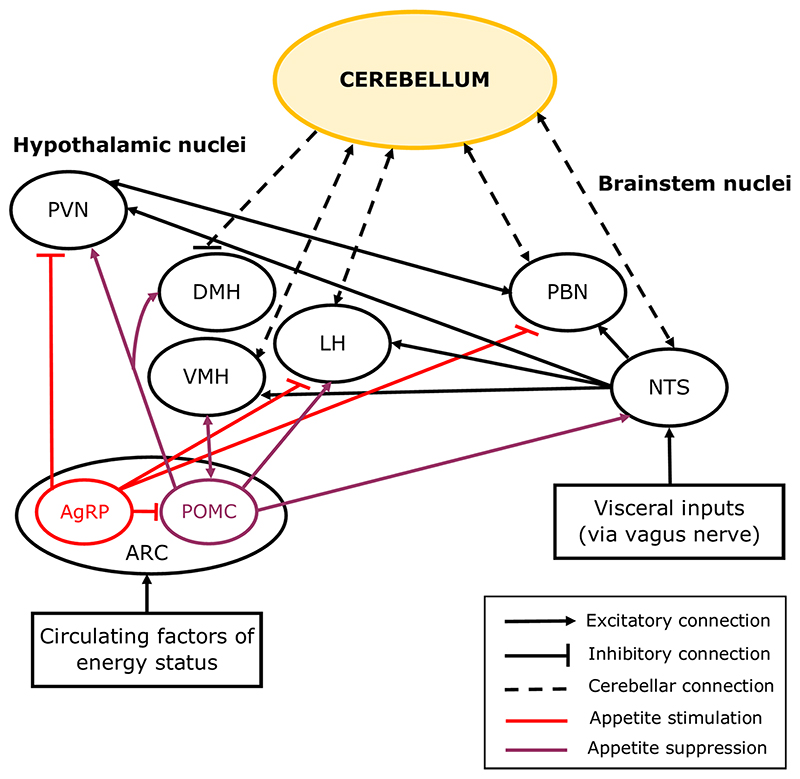
Fig. 2.
Simplified diagram showing cerebellar connections with feeding circuits in the brain. The hypothalamic nuclei are central to a network of brain regions which regulate appetite. Distinct subtypes of neurons in the arcuate (ARC) nucleus of the hypothalamus are involved in the initiation (AgRP neurons) or cessation of food consumption (POMC neurons) via their inputs to the other hypothalamic nuclei including the paraventricular hypothalamic nucleus (PVN), ventromedial hypothalamic nucleus (VMH), lateral hypothalamic nucleus (LH), and dorsomedial hypothalamic nucleus (DMH). Short-term appetite regulation involves the parabrachial nucleus (PBN) and the solitary tract nucleus (NTS) of the brainstem, which respond to feedback from the gut and form connections with the hypothalamus to initiate satiation. The cerebellum has reciprocal connections with the VMH, LH, PBN and NTS, and sends inhibitory projections to the DMH
Physiological studies have also provided direct evidence for a role of the cerebellum in swallowing. In the awake cat and rat, electrical stimulation of the cerebellar vermis and medial nucleus elicits swallowing, gnawing and grooming behaviours [73, 74]. In addition, cerebellectomy in anaesthetised cats reduces motor recruitment in the pharyngeal-oesophageal area [75].
Together, the available evidence from human and animal studies demonstrates that the cerebellum is involved in all of the key stages of food ingestion, from voluntary preparatory and transfer phases of swallowing [63, 64], to the involuntary transport phase [73, 76]. Chewing and swallowing are stereotypical, repetitive behaviours and therefore predictable, making them well suited to being represented by cerebellar internal models. In the future, the advance of cell-specific genetic methods to manipulate cerebellar circuitry in animal models will help reveal the mechanisms and precise networks by which the cerebellum mediates these motor behaviours.
Cerebellar Contributions to Appetite Control
Appetite control is a complex process balancing feedback information from energy stores and reward centres relating to hunger and desire to eat, and feed-forward information from the body’s internal clock in anticipation of mealtimes. Although not widely recognised, the cerebellum contributes to these processes through its connections with well-established brain circuits involved in feeding behaviour.
Cerebellum in Hunger and Satiation
Homeostatic mechanisms associated with eating maintain energy stores by either increasing or decreasing food intake to match energy requirements [15]. Food can also be rewarding, so hedonic mechanisms can initiate or limit food intake and can sometimes overrule homeostatic mechanisms [19]. For example, hunger produces an unpleasant sensation in response to low energy stores that drives appetitive behaviours to replenish the energy stores [15, 17, 77], but the sensation of hunger can also be induced by exposure to food cues and cravings [19]. The hypothalamus is the central regulator of appetite and has reciprocal connections with the cerebellum [15, 16, 19, 78, 79, 80]. Both the cerebellum and hypothalamus interact with other brain structures regulating homeostatic and hedonic appetite (Fig. 2, section The Cerebellum in the Homeostatic and Hedonic Appetite Regulation Circuitry).
Cerebellar Activation in Hunger and Satiation
Cerebellar activity changes with hunger state, reflecting its contributions to the feeding circuitry described above. For example, imaging studies in humans using positron emission tomography (PET) in combination with injection of radioactive water found increased regional blood flow in the cerebellum following a 36-h fast in healthy-weight participants, particularly within the anterior and midline vermis [81]. Cerebellar activation during hunger was significantly decreased when satiation was induced via liquid meal ingestion [81, 82] suggesting that the cerebellum may be responsive to nutrient intake, perhaps by responding to one of the circulating factors released during food consumption (e.g. glucose or cholecystokinin) which provide fast-acting signals regulating appetite [83, 84, 85, 86].
A comparison between healthy-weight and overweight participants, using the same PET technique as above, found that a larger area of the anterior-midline cerebellar vermis had decreased regional blood flow when satiation was induced following a 36-h fast in overweight participants [87, 88]. This suggests that the cerebellum functions differently in healthy-weight and overweight participants and raises the possibility that modified cerebellar processing plays a role in the pathophysiology of overeating disorders.
During food consumption, stomach stretch is communicated to the brain via the vagus nerve, providing feedback from the gut that food ingestion has begun and serving as a satiation signal [89]. Imaging studies in humans have reported increased activation in the cerebellar uvula (vermal lobule IX) in response to stomach stretch [90]. The strength of the BOLD signal in response to stomach stretch increased linearly with body mass index [90] indicating that the responsiveness of the cerebellum to mechanical, and possibly chemical, feedback from the gut increases with body weight.
Whilst the anterior midline cerebellar vermis is activated during hunger, posterior areas of the vermis are responsive to feedback generated by food ingestion [81, 82, 87, 88, 90]. As outlined in the introduction, this may be related to differential connectivity of cerebellar regions, and food anticipatory processes may vary depending on the size of the metabolic store.
The Cerebellum in the Homeostatic and Hedonic Appetite Regulation Circuitry
The hypothalamic nuclei involved in appetite regulation form an intricate network [15, 19, 91]. In brief, the lateral hypothalamic nucleus (LH) is widely considered to be a hub for regulating homeostatic and hedonic feeding [19]. The LH integrates information from the arcuate nucleus of the hypothalamus (ARC, Fig. 2), which contains both hunger-related neurons (expressing Agouti-related protein, AgRP) and satiation-related neurons (expressing pro-opiomelano-cortin, POMC). AgRP neurons project to the paraventricular nucleus of the hypothalamus (PVN), LH and brainstem parabrachial nucleus (PBN) in response to lowering levels of the carbohydrate energy store, and POMC neurons mainly project to the PVN, but also to the dorsal medial hypothalamic nucleus (DMH), LH, ventromedial hypothalamic nucleus (VMH), and NTS, in response to nutrient ingestion (Fig. 2) [15, 17, 92, 93, 94].
Whilst the cerebellum has generally been overlooked as a component of this homeostatic feeding circuit, anatomical evidence from a range of animal species (including rat, cat, and monkey) has demonstrated a direct reciprocal connection between the cerebellum and hypothalamic nuclei including the DMH, VMH and LH (Fig. 2) [83, 95, 96]. Activation of lateral cerebellar nuclei neurons in mice reduced AgRP neuron-mediated food consumption, indicating that the cerebellum can influence homeostatic control of hunger [97]. The cerebellum also forms reciprocal connections with satiety centres in the brainstem; the NTS (see section Cerebellum in Swallowing for roles of this connection in swallowing) [71, 98] and the PBN [99, 100].
Upon food ingestion, feedback from the gut is communicated to the NTS and the brainstem satiation centre in the PBN, which is passed on to hypothalamic nuclei responsible for inducing satiation and meal termination (Fig. 2) [17, 70, 86, 101]. Circulating factors are also released from the gut and accessory organs and are transported across the blood–brain barrier to reach receptors in a number of areas including the brainstem, hypothalamus, amygdala and cerebellum [15, 101, 102, 103, 104].
LH neurons also integrate information from the ventral tegmental area (VTA) and nucleus accumbens (NAc), which are involved in processing food and reward-related cues [105, 106, 107, 108]. More specifically, the dopaminergic systems in the VTA, NAc and the striatum are involved in driving rewarding behaviours and attributing a rewardvalue to food to prolong feeding [15, 19, 109]. Recently the cerebellum has also been found to signal various elements of reward as further described in the section Cerebellar Reward Processing and Contributions to Over-Eating. In addition, a subclass of glutamatergic neurons in the mouse lateral cerebellar nuclei have been shown to reduce food intake by increasing the release of dopamine from the VTA [97]. When dopamine is released during consumption, the ingested food is given a reward value indicating that the hedonic need for consumption has been met [19, 97]. Therefore, various lines of evidence point to the cerebellum being involved in the modulation of central mechanisms linked to hedonic satiation as well as homeostatic regulation of feeding behaviour.
Cerebellum and Leptin-Mediated Appetite Regulation
Short-term feeding regulation limits food intake per meal, whilst long-term regulation dictates daily food intake [84, 86]. One circulating hormone that limits food intake in the short-term is cholecystokinin, which is secreted in the duodenum and induces satiety [83, 85, 110]. Circulating hormones regulating long-term appetite are insulin, which is secreted by the pancreas and governs glucose metabolism [111], and ghrelin, an appetitive stimulant secreted by the stomach [109, 112]. An additional hormone, leptin, is continuously secreted by adipose tissue [113, 114] and is an important regulator of appetite [113, 115, 116, 117], contributing to the process of meal termination [118, 119, 120].
Leptin is of particular interest to the role of the cerebellum in appetite regulation. High levels of leptin and leptin receptor expression have been reported in the rodent and human cerebellum [113], particularly in the cerebellar cortical granule cell layer but also in Purkinje cells and the lateral nucleus [121, 122]. High levels of cerebellar leptin have been associated with various physiological processes, for example during embryological development, the effect of leptin in the cerebellum is to promote survival, growth, and development of Purkinje cells [123]. Leptin has also been shown to facilitate NMDA receptor-mediated calcium influx in cultured cerebellar granule cells [124].
Low circulating levels of leptin are a hallmark of obesity [119, 125, 126] and deficiencies in leptin production, transport and signalling are known causes of increased weight [127, 128, 129, 130]. Leptin replacement therapies are therefore often used as a treatment for obesity [131]. Individuals with hereditary leptin deficiency have cerebellar activation within lobule VI and Crus I in response to food cues which decrease as patients undergo leptin-replacement therapy [132]. This suggests that leptin replacement counteracts abnormal levels of cerebellar activation in response to food cues associated with obesity [133]. Cerebellar activation patterns in relation to varying weights and volumes of adipose tissue might therefore contribute to changes in leptin signalling in overweight patients [87, 88].
It is well established that fluctuations in circulating leptin are detected by neurons in the hypothalamus which can increase or decrease appetitive behaviours accordingly [86, 134]. Given that the cerebellum is responsive to leptin replacement therapies used in obesity, this raises the interesting possibility that leptin also regulates appetite through its effects on the cerebellum. Further studies investigating the effects of leptin receptor activation on cerebellar neuronal activity would shed light on the mechanisms by which this may occur.
Cerebellum in Mealtime Anticipation
The suprachiasmatic nucleus of the hypothalamus (SCN) is the circadian master clock [135] and has the primary responsibility for controlling and synchronising neural activity in relation to daylight cycles [136]. The basis of SCN function is differential expression of a number of genes that peak in expression at different times of the day, collectively termed Clock genes [137]. Clock gene expression can peak around anticipated mealtimes, thereby inducing hunger in a predictive, feed-forward manner [138, 139]. Secondary clocks also exist around the body; these are areas that express a number of Clock genes, and gene expression in these areas peaks with an approximate 6-h phase delay to the master SCN clock [138, 139].
Clock genes of particular interest for this review are period genes, Per1 and Per2 [137]. These are expressed in the granule cell layer and Purkinje cell layer of the cerebellar cortex and are involved in mediating food anticipatory activity around expected mealtimes [140, 141]. In food-restricted animals, expression of Per 1 and Per 2 in the cerebellum is phase-shifted to peak around expected mealtime, several hours earlier than the peak Per 1 and Per 2 expression when animals have free access to food and therefore no set mealtime [142, 143]. Accordingly, depletion of Purkinje cells or disruption of the cerebellar circuitry can reduce food anticipatory activity [140, 141, 142]. Peaks in the cerebellar expression of Clock genes are delayed by several hours relative to the SCN, and it is likely this is controlled (perhaps indirectly) by the SCN [144].
In summary, the cerebellum has been shown to be involved in various mechanisms of appetite regulation, including key hormones and circadian rhythm genes, and cerebellar processing may differ between healthy and disordered eating. One outstanding question concerns how Clock genes, and their regulation, differ in those with disordered eating, as this could provide evidence for underlying genetic causes in such disorders. Similarly, there may also be genetic causes of differences in leptin receptor expression and responsivity in obesity.
Cerebellar Reward Processing and Contributions to Over-Eating
Appetitive behaviours are governed by pleasurable sensations and desire of food, so it is important to consider the rewarding nature of eating [145]. The cerebellum is connected with reward centres in the brain including the VTA, striatum and neocortex [105, 146, 147, 148, 149] and outputs from the cerebellar lateral nucleus have been found to mediate hedonic aspects of satiation by increasing dopaminergic release in the VTA [97]. The cerebellum is likely therefore to be involved in assigning food with a reward value. This section will outline recent animal studies providing evidence that reward information is indeed processed by the cerebellum and the implications of abnormal cerebellar responses to food cues in over-eating disorders.
Reward-Related Signalling in the Cerebellum
An increasing body of evidence suggests that different elements of reward-related information are encoded within the cerebellum. For example, in mice trained to perform a voluntary movement for reward, a population of cerebellar granule cells in lobules VIa, VIb and lobulus simplex were shown to respond to reward delivery or reward omission, whilst others encoded reward anticipation [150]. These response profiles developed over the learning period, suggesting that the cerebellum learns to predict reward delivery and adapt its responses based on experience. Granule cells receive synaptic input from mossy fibres (Fig. 1b), which are therefore likely to carry this information.
The other main input to the cerebellum is from climbing fibres (Fig. 1b), which also carry multiple types of information related to reward. Climbing fibre inputs to the cerebellar flocculus have been shown to encode reward size; in monkeys cued to the size of a reward in an eye movement task, climbing fibre activity increased in response to a large but not a small reward cue [151]. In the lateral cerebellum of mice, climbing fibres have also been shown to signal reward prediction (lobule simplex, Crus I and II) during learning, and can also signal reward delivery and omission [152].
In agreement with classical theories of cerebellar-dependent motor learning [153], reward omission information conveyed by climbing fibres may serve as an error signal which occurs when the outcome is unexpected. In support of this idea, climbing fibre responses to predictable rewards were suppressed during learning of a visuomotor integration task [154], and the phenomenon can also be generalised to the cerebellar mossy fibre-granule cell-parallel fibre system because reward-related error signals in PC simple spike responses diminish as monkeys learn a reward-association task [155]. Reward-based learning in the cerebellum would seem therefore to be driven by similar mechanisms as errorbased motor learning, where the cerebellum learns to predict the expectation of a reward and an error signal occurs when an expected reward does not materialise.
Cerebellum in Pathophysiological Cue Processing
In addition to evidence from animal models that the cerebellum processes rewarded-related cue information, imaging studies in humans suggest that this function is conserved across species. The cerebellum can learn to selectively respond to rewarding cues, including highly palatable food [156], and altered cue processing may underlie several pathophysiological states including over- and under-eating disorders (the latter are explored further in the section Cerebellar Contributions to Affective Aspects of Eating).
Activation of the anterior cerebellum (hemispheric lobule VI) and VTA is enhanced in overweight participants compared to healthy weight participants when exposed to cues for highly palatable foods [157]. Overweight individuals also find palatable foods more appealing when full compared to full healthy-weight individuals [133]. This is correlated with over-responsiveness in the cerebellum, as obese children show increased cerebellar activation in response to exposure to highly palatable foods once satiated, as compared to healthy weight children [158]. Participants with Prader-Willi syndrome have obesity characterised by dysfunctional reward processing circuits, including potential overactivation of subcortical reward circuitry and under activation of cortical inhibitory regions after eating [159]. Prader-Willi participants also show increased activation in regions of the cerebellum, likely corresponding to the cerebellar nuclei, in response to food cues post-meal compared to controls [97].
Changes in perception of hunger and satiety may be regulated by cerebellar connections with other reward centres in the brain. For example, neuromodulation of prefronto-cerebellar connections using transcranial direct current stimulation (tDCS) increased the desire to eat upon exposure to visual food cues [160], suggesting that disrupting this circuit could impair normal regulation of food intake. Decreased functional connectivity between the LH and the cerebellar cortex has also been reported in overweight compared to healthy-weight participants [161]. As the LH is involved in processing both physiological and pleasurable eating, this decreased connectivity could indicate that the cerebellar-LH connection contributes to food being perceived as less rewarding in obese participants, which may lead to overeating to obtain a similar reward value. Altered cerebellar-VTA connections are also associated with overeating in mice [97].
In summary, animal studies have demonstrated that reward information is processed in the cerebellum and that the cerebellum interacts with other reward centres [152, 154, 162]. Imaging studies have suggested that altered reward processing within the cerebellum could be a key contributor to the pathophysiological events of compulsive eating experienced by obese and overweight individuals. Understanding how the cerebellum learns pathological reward-related responses and how it could ‘re-learn’ healthier associations could provide insight to approaches aimed at preventing or treating overeating and obesity.
Cerebellar Contributions to Affective Aspects of Eating
Appetite is influenced by affective state, and the cerebellum is a key node for affective processing through its connections with brain regions including the limbic system and prefrontal cortex [8, 9, 10, 12, 163, 164, 165, 166, 167, 168]. This section will discuss how cerebellar involvement in affective processing may be linked to appetite in both negative emotional states (stress and anxiety) and survival (fear and innate survival).
The influence of Negative Emotions on Eating
Stress and anxiety strongly influence appetite; whilst anxiety is predominantly an appetite suppressant, stress can either suppress or stimulate appetite depending on the palatability of available food and whether the stressor is acute or chronic [23]. Stress has been linked with changes in weight and corresponding neural activity. Alterations in functional connectivity between the cerebellum and regions such as the LH and the hippocampus have been linked with a higher risk of weight gain and overeating in stressed participants [169, 170, 171, 172]. Stress can also interfere with dopaminergic signalling in the VTA [169, 172], which may change the way in which the cerebellum controls VTA dopamine release in relation to hedonic satiation [97].
The cerebellum has been linked to emotional processing disorders in which anxiety is commonly reported, including schizophrenia, autism and depression [173, 174, 175, 176, 177]. As well as affecting appetite in healthy weight individuals, anxiety is also strongly related to eating disorders including anorexia nervosa. Imaging studies have shown an altered cerebellar network connectivity in anorexia nervosa patients compared to healthy controls, in particular increased activity within the vermis [178, 179, 180]. The disorder is associated with altered food-cue processing and decreased appetite upon presentation of food cues [172, 181, 182]. This contrasts with the cerebellar over-responsiveness to food cues described in obese participants [97, 157].
The cerebellum is therefore involved in processing negative emotions as well as the regulation of appetite (see Cerebellar Contributions to Appetite Control) which has been shown to vary in several affective disorders [23, 172, 183]. The cerebellum may have an impaired predictive ability in affective conditions which could alter the perceived reward value of foods and impact the anticipation of appropriate times to eat. This could lead to fluctuations in the volume of food consumed at individual meals and increase or decrease number of meals over a longer term, leading to changes in weight.
Cerebellum and Survival
Cerebellar activation has been associated with survival functions [171, 184, 185], for example cerebellar activation, particularly within the vermis and anterior paravermis, has been shown in response to hypercapnia (hunger for air) and thirst [186, 187, 188, 189, 190, 191]. It may therefore be hypothesised that the cerebellum drives appetitive functions in response to an innate need to replenish energy stores. The cerebellar vermis is a common link between affective disorders, survival networks, homeostatic functions, and hunger [81, 192, 193, 194], indicating that it could be a key node which regulates affective influences on appetite. It remains to be determined if separate vermal regions are related to each of these functions, or whether there are overlapping roles of individual regions.
Associative fear learning, like reward-based learning and classical cerebellar motor learning (e.g., eyeblink conditioning), can be driven by prediction errors, and negative prediction errors drive the extinction of conditioned fear responses [40, 195, 196, 197]. A universal mechanism of cerebellar learning, based on prediction, may therefore apply across multiple types of behaviour, including eating. In this case, the cerebellum may regulate appetite by integrating metabolic (leptin and other circulating factors), sensory (food cues), and proprioceptive (hedonic satiation) factors in a predictive error-correction manner.
Cerebellum and Prediction
The traditional error-based model of cerebellar learning uses feed forward predictions (internal models) about the sensory outcomes of movement and compares these against movement-related sensory feedback to supervise error correction, thereby improving performance accuracy (for more details see [198]. An extension of this theory is to consider the cerebellum as a ‘prediction machine’ [1, 2, 40, 41]. This extends cerebellar involvement in specific forms of motor learning, for example eyeblink conditioning, to more complex neocortical prediction paradigms involving interactions between multiple brain regions [199, 200, 201, 202]. For example, preparatory changes in firing rate have been reported in both the medial [203] and lateral cerebellar nuclei in mice [204], and inhibiting these regions has been shown to disrupt preparatory activity in the anterolateral motor cortex thereby impairing motor planning [203]. This suggests that the predictive capabilities of the cerebellum influence neocortical processing and could underpin its involvement in cognitive processing.
Prediction and feedback underlie many elements of eating described in this review, as food consumption is influenced by expectation. As well as short- and long-term regulation of appetite which works in a feedback fashion, appetite can also be regulated in a feed forward manner by circadian clocks, for example becoming hungry in anticipation of a meal [15]. The sight and smell of food will generate an internal expectation of what that food will taste like, and sensory information upon eating that food will provide feedback to match or contradict that expectation. As outlined in the section Cerebellar Reward Processing and Contributions to Over-Eating, evidence is accumulating that the cerebellum encodes information about reward prediction and is likely to do so for other properties of reward, such as preferred taste. This internal prediction may be relayed to the hypothalamus to influence satiety-inducing neural circuits depending upon expectation and outcome.
This predictive model may explain disordered eating (both under- and over-eating), where often the feedback signals are maladaptive. For example, overweight individuals are more responsive to food cues and have altered satiation signalling. This lack of accurate feedback could prevent the learning of healthy eating behaviours, and affective or rewarding elements may override basic homeostatic regulation, in line with evidence that prediction errors are also present in the emotional domain [195]. We therefore propose that the overarching role of the cerebellum in eating is to generate a behaviourally relevant response to prediction errors which arise from a variety of domains including homeostatic, motor, reward, and affective signals.
The ability of the cerebellum to contribute to such a variety of functions related to eating stems from its extensive interconnectivity with other brain regions, as explored in this review. Altered connectivity within this brain-wide network may contribute to disordered eating. It follows that manipulating this network to restore altered connectivity may provide a therapeutic approach for disorders associated with eating. The feasibility of such an approach is now possible with the development of non-invasive neuromodulatory techniques including tDCS, transcranial alternating current stimulation and transcranial magnetic stimulation [205, 206]. Applying these techniques to the cerebellum has been shown to alter cerebellar connectivity with cognitive regions of the cerebral cortex [207], improve performance in motor tasks likely via altering excitability in motor cortical regions [208], and improve cognitive performance in patients with bipolar disorder [209]. As outlined in the section Cerebellum in Pathophysiological Cue Processing, increasing prefrontal cortical activity and decreasing cerebellar activity using tDCS can increase the propensity to eat [160], showing that such approaches can directly influence eating behaviour. Therefore, non-invasive stimulation of the cerebellum would seem to be a promising approach to explore in future efforts to treat pathological eating processes.
Summary
This review has outlined cerebellar contributions to the motor, homeostatic, rewarding, and affective aspects of eating. The regions of the cerebellar cortex involved in each of these domains are summarised in Fig. 3a (and corresponding Table 1). The distribution of cerebellar contributions suggests that the medial parts of the cerebellum, which are phylogenetically the oldest, largely contribute to the homeostatic, motor and survival aspects of eating. In contrast, the phylogenetically newer regions of the cerebellum, the hemispheres, are related to higher-order aspects of eating, including reward processing and associative learning (both appetitive and affective). Cerebellar contributions to such a wide range of functions can be achieved through its wide-spread connections with other brain regions involved in each function, as summarised in Fig.3b.
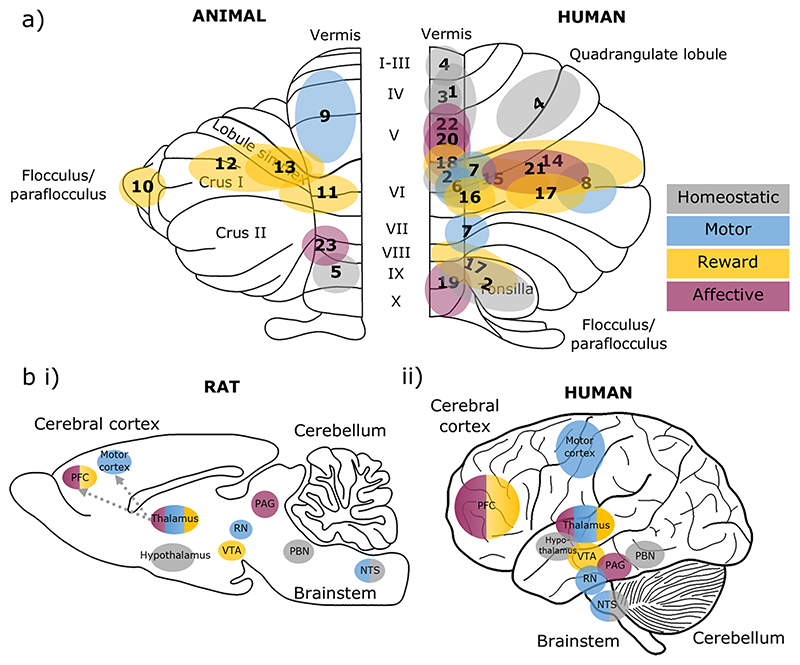
Fig. 3. Cerebellar and brain-wide networks involved in eating behaviours.
a Cerebellar regions shown to be involved in homeostatic (grey), motor (blue), reward (yellow) and affective (purple) aspects of eating. Animal studies are depicted on an outline of the rat cerebellum on the left, and human studies are shown on the right. The numbers correspond to the studies detailed in Table 1. b The cerebellum has connections with brain regions contributing to homeostatic (grey), motor (blue), reward (yellow) and affective (purple) domains of eating behaviours, depicted in a (i) rat and (ii) human brain outline. Connections may be direct or indirect; the latter is the case for cerebello-thalamo-cortical pathways. We propose that the cerebellum has a unifying role via prediction signals which contributes to each of these components. Note that this diagram is not comprehensive, but represents key structures discussed in this review. PFC, prefrontal cortex; VTA, ventral tegmental area; RN, red nucleus; PAG, periaqueductal grey; PBN, parabrachial nucleus; NTS. nucleus tractus solitaries
Table 1.
Summary of cerebellar regions involved in different aspects of eating behaviours. Colours correspond to homeostatic (grey), motor (blue), reward (yellow) and affective (purple) functions. These areas are depicted on a cerebellar outline in Fig. 3a
| Function | Cerebellar Area | Study | Species | Fig. 3 |
|---|---|---|---|---|
| Hunger | Vermis (anterior midline) | Tataranni et al. (1999) | Human | 1 |
| Stomach stretch | Vermal lobule IX, declive, tonsil, uvula | Tomasi et al. (2009) | Human | 2 |
| Air hunger | Midline regions of central lobule | Parsons et al. (2001) | Human | 3 |
| Thirst | Vermis; Quadrangulate, Lingula | Parsons et al. (2000) | Human | 4 |
| Blood pressure and heart rate | Posterior Vermis – Lobule IX | Bradley et al. (1987) | Rabbit | 5 |
| Chewing, clenching | Vermal Lobule VI | Onozuka et al. (2002), Lin (2018b) | Human | 6 |
| Sensory inputs from the tongue | Lobules VI, VIIb/VIIIa | Boillat et al. (2020) | Human | 7 |
| Swallowing | Left posterior lobe | Suzuki et al. (2003) | Human | 8 |
| Oral behaviours (eating, grooming, gnawing) | Rostro-ventral anterior lobe vermis, fastigial nucleus, superior cerebellar peduncle | Ball et al. (1974), Berntson et al. (1973) | Rat, cat | 9 |
| Reward size encoding | Flocculus | Larry et al. (2019) | Monkey | 10 |
| Reward expectancy and omission | Lobules VI and Simplex | Wagner et al. (2017) | Mouse | 11 |
| Reward prediction learning | Lobule Simplex, Crus I and II | Heffley and Hull (2019) | Mouse | 12 |
| Prediction of reward | Lobules V, VI and Simplex | Kostadinov et al. (2019) | Mouse | 13 |
| Food and drug cue | Crus I, II and Lobules V, VI | Tomasi et al. (2015) | Human | 14 |
| Food cue reactivity + responsiveness to leptin replacement therapies | Lobule VI and Crus I | Berman et al. (2013) | Human | 15 |
| Food cue reactivity + overactivity in obesity | Lobule VI | Carnell et al. (2014) | Human | 16 |
| Decreased grey matter volume in obesity | Pyramis, Tonsil and Semilunar Lobule | Weise et al. (2017) | Human | 17 |
| Dietary disinhibition | Lobules V, VI | English et al. (2019) | Human | 18 |
| Stress | Vermis; Culmen, Declive | Yang et al. (2019) | Human | 19 |
| Altered connectivity in eating disorders | Lobule IX, X | Amianto et al. (2013) | Human | 20 |
| Fear conditioning; prediction error | Crus I and Lobule VI | Utz et al. (2015), Ernst et al. (2019) | Human | 21 |
| Fear extinction | Anterior Vermis (Lobules III-V) | Utz et al. (2015) | Human | 22 |
| Innate freezing to predatory odour | Lateral vermal lobule VIII | Koutsikou et al. (2014) | Rat | 23 |
Evidence for a role of the cerebellum in feeding behaviour exists at a molecular (e.g., via leptin signalling), anatomical (neuronal connections with regions including the hypothalamus, PBN and NTS), and functional level (imaging studies showing activation during hunger and in the presence of pleasant taste sensations). The cerebellum is involved in processing reward and associated cues which may induce cravings and the false sensations of hunger. In obesity, this could contribute to overeating by both inhibiting the sensation of satiety and being hyperreactive to food cue processing. On the other hand, underactive food cue processing impacts appetite and, together with the involvement of the cerebellum in affective processing, could contribute to the symptomatology of eating disorders. We propose that the cerebellum may control each of these elements in its role as a prediction machine. Future experiments could investigate this by examining predictive behaviours, such as mealtime or reward anticipation, and abnormal eating patterns (under- or over-eating) following targeted cerebellar inactivation in animal models and observing changes in eating habits of cerebellar patients.
A number of outstanding questions remain, including:
Is the same cerebellar computation carried out when predicting the different types of behaviour involved in eating (motor, homeostatic, reward, affective), or is it region-specific? Could it depend on the time course of individual predictions, given that these behaviours happen over different time scales? For example, motor control of swallowing versus circadian control of appetite.
Could genetic predispositions to eating-related disorders directly affect gene expression patterns in the cerebellum?
Can we truly separate the motor, homeostatic, cognitive, and affective aspects of feeding behaviours? Are all feeding networks likely to be involved in multiple domains to some extent?
Could non-invasive stimulation techniques be useful therapeutic approaches for treatment of disordered eating?
Acknowledgements
This work was supported by the Biotechnology and Biological Sciences Research Council-funded South West Biosciences Doctoral Training Partnership [BB/M009122/1] and the Biotechnology and Biological Sciences Research Council [BB/R017336/1].
Funding
Biotechnology and Biological Sciences Research Council, BB/R017336/1, Richard Apps, BB/M009122/1
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ito M. Neurophysiological aspects of the cerebellar motor control system. Int J Neurol. 1970;7(2):162–76. [PubMed] [Google Scholar]
- 2.Ito M. Neural design of the cerebellar motor control system. Brain Res. 1972;40(1):81–4. doi: 10.1016/0006-8993(72)90110-2. [DOI] [PubMed] [Google Scholar]
- 3.Ito M. Adaptive control of reflexes by the cerebellum. Prog Brain Res. 1976;44:435–44. doi: 10.1016/s0079-6123(08)60750-5. [DOI] [PubMed] [Google Scholar]
- 4.Schmahmann JD. The cerebellum and cognition. Neurosci Lett. 2019;688:62–75. doi: 10.1016/j.neulet.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 6.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–44. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medina JF. Teaching the cerebellum about reward. Nat Neurosci. 2019;22(6):846–8. doi: 10.1038/s41593-019-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strick PL, Dum RP, Fiez JA. Cerebellum and Nonmotor Function. Annu Rev Neurosci. 2009;32:413–34. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 9.Adamaszek M, D’Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, Leggio M, Mariёn P, Molinari M, Moulton E, Orsi L, et al. Consensus Paper: Cerebellum and Emotion. Cerebellum. 2017;16(2):552–76. doi: 10.1007/s12311-016-0815-8. [DOI] [PubMed] [Google Scholar]
- 10.Apps R, Strata P. Neuronal circuits for fear and anxiety — the missing link. Nat Rev Neurosci. 2015;16(10):642. doi: 10.1038/nrn4028. [DOI] [PubMed] [Google Scholar]
- 11.Apps R, Hawkes R, Aoki S, Bengtsson F, Brown AM, Chen G, Ebner TJ, Isope P, Jörntell H, Lackey EP, Lawrenson C, et al. Cerebellar Modules and Their Role as Operational Cerebellar Processing Units: A Consensus paper [corrected] Cerebellum. 2018;17(5):654–82. doi: 10.1007/s12311-018-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koutsikou S, Apps R, Lumb BM. Top down control of spinal sensorimotor circuits essential for survival. J Physiol. 2017;595(13):4151–8. doi: 10.1113/jp273360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koutsikou S, Crook JJ, Earl EV, Leith JL, Watson TC, Lumb BM, Apps R. Neural substrates underlying fear-evoked freezing: the periaqueductal grey-cerebellar link. J Physiol-Lond. 2014;592(10):2197–213. doi: 10.1113/jphysiol.2013.268714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odeh F, Ackerley R, Bjaalie JG, Apps R. Pontine Maps Linking Somatosensory and Cerebellar Cortices Are in Register with Climbing Fiber Somatotopy. J Neurosci. 2005;25(24):5680–90. doi: 10.1523/jneurosci.0558-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andermann ML, Lowell BB. Toward a Wiring Diagram Understanding of Appetite Control. Neuron. 2017;95(4):757–78. doi: 10.1016/j.neuron.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz GJ. The-pole of gastrointestinal vagal afferents in the control of food intake: Current prospects. Nutrition. 2000;16(10):866–73. doi: 10.1016/s0899-9007(00)00464-0. [DOI] [PubMed] [Google Scholar]
- 17.Sternson SM, Eiselt AK. Three Pillars for the Neural Control of Appetite. In Julius D Ann Rev Physiol Palo Alto. 2017;79(1):401–23. doi: 10.1146/annurev-physiol-021115-104948. [DOI] [PubMed] [Google Scholar]
- 18.Morales I, Berridge KC. “Liking” and “wanting” in eating and food reward: Brain mechanisms and clinical implications. Physiol Behav. 2020;227:113152. doi: 10.1016/j.physbeh.2020.113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi MA, Stuber GD. Overlapping Brain Circuits for Homeostatic and Hedonic Feeding. Cell Metab. 2018;27(1):42–56. doi: 10.1016/j.cmet.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15(1):37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasegbon A, Hamdy S. The anatomy and physiology of normal and abnormal swallowing in oropharyngeal dysphagia. Neurogastroenterol Motil. 2017;29(11):e13100. doi: 10.1111/nmo.13100. [DOI] [PubMed] [Google Scholar]
- 22.van der Bilt A, Engelen L, Pereira LJ, van der Glas HW, Abbink JH. Oral physiology and mastication. Physiol Behav. 2006;89(1):22–7. doi: 10.1016/j.physbeh.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Macht M. How emotions affect eating: a five-way model. Appetite. 2008;50(1):1–11. doi: 10.1016/j.appet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 24.van Strien T. Causes of Emotional Eating and Matched Treatment of Obesity. Curr Diab Rep. 2018;18(6):35. doi: 10.1007/s11892-018-1000-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voogd J, Glickstein M. The anatomy of the cerebellum. Trends Neurosci. 1998;21(9):370–5. doi: 10.1016/s0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]
- 26.Altman J, Bayer SA. Development of the cerebellar system: in relation to its evolution, structure, and functions. CRC Press; Boca Raton: 1997. [Google Scholar]
- 27.Glickstein M, Sultan F, Voogd J. Functional localization in the cerebellum. Cortex. 2011;47(1):59–80. doi: 10.1016/j.cortex.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Herculano-Houzel S. Coordinated scaling of cortical and cerebellar numbers of neurons. Front Neuroanat. 2010;4:8. doi: 10.3389/fnana.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barton RA, Venditti C. Rapid Evolution of the Cerebellum in Humans and Other Great Apes. Curr Biol. 2014;24(20):2440–4. doi: 10.1016/j.cub.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 30.Smaers JB, Turner AH, Gómez-Robles A, Sherwood CC. A cerebellar substrate for cognition evolved multiple times independently in mammals. eLife. 2018;7 doi: 10.7554/eLife.35696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apps R. Columnar organisation of the inferior olive projection to the posterior lobe of the rat cerebellum. J Comp Neurol. 1990;302(2):236–54. doi: 10.1002/cne.903020205. [DOI] [PubMed] [Google Scholar]
- 32.Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci. 2005;6(4):297–311. doi: 10.1038/nrn1646. [DOI] [PubMed] [Google Scholar]
- 33.Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. 2009;10(9):670–81. doi: 10.1038/nrn2698. [DOI] [PubMed] [Google Scholar]
- 34.Garwicz M, Jorntell H, Ekerot CF. Cutaneous receptive fields and topography of mossy fibres and climbing fibres projecting to cat cerebellar C3 zone. J Physiol. 1998;512(Pt 1):277–93. doi: 10.1111/j.1469-7793.1998.277bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oscarsson O. Functional units of the cerebellum - sagittal zones and microzones. Trend Neurosci. 1979;2:143–5. doi: 10.1016/0166-2236(79)90057-2. [DOI] [Google Scholar]
- 36.Cerminara NL, Lang EJ, Sillitoe RV, Apps R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat Rev Neurosci. 2015;16(2):79–93. doi: 10.1038/nrn3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruigrok TJ. Cerebellar nuclei: the olivary connection. Prog Brain Res. 1997;114:167–92. doi: 10.1016/s0079-6123(08)63364-6. [DOI] [PubMed] [Google Scholar]
- 38.Ruigrok TJ, Voogd J. Organization of projections from the inferior olive to the cerebellar nuclei in the rat. J Comp Neurol. 2000;426(2):209–28. doi: 10.1002/1096-9861(20001016)426:2<209::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 39.Ruigrok TJH. Ins and Outs of Cerebellar Modules. Cerebellum. 2011;10(3):464–74. doi: 10.1007/s12311-010-0164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hull C. Prediction signals in the cerebellum: beyond supervised motor learning. Elife. 2020;9 doi: 10.7554/eLife.54073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramnani N, Toni I, Josephs O, Ashburner J, Passingham RE. Learning- and expectation-related changes in the human brain during motor learning. J Neurophysiol. 2000;84(6):3026–35. doi: 10.1152/jn.2000.84.6.3026. [DOI] [PubMed] [Google Scholar]
- 42.Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2(9):338–47. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- 43.Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19(4):691–707. doi: 10.1016/j.pmr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dehaghani SE, Yadegari F, Asgari A, Chitsaz A, Karami M. Brain regions involved in swallowing: Evidence from stroke patients in a cross-sectional study. J Res Med Sci. 2016;21:45. doi: 10.4103/1735-1995.183997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreira B, Palinkas M, Gonçalves L, da Silva G, Arnoni V, Regalo I, Vasconcelos P, Junior WM, Hallak J, Regalo S, Siéssere S. Spinocerebellar ataxia: Functional analysis of the stomatognathic system. Med Oral Patol Oral Cir Bucal. 2019;24(2):e165–71. doi: 10.4317/medoral.22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia After Stroke. Stroke. 2005;36(12):2756–63. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- 47.Rönnefarth M, Hanisch N, Brandt AU, Mähler A, Endres M, Paul F, Doss S. Dysphagia Affecting Quality of Life in Cerebellar Ataxia-a Large Survey. Cerebellum. 2020;19(3):437–45. doi: 10.1007/s12311-020-01122-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasegbon A, Hamdy S. The Role of the Cerebellum in Swallowing. Dysphagia. 2021 doi: 10.1007/s00455-021-10271-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pong M, Horn KM, Gibson AR. Pathways for control of face and neck musculature by the basal ganglia and cerebellum. Brain Res Rev. 2008;58(2):249–64. doi: 10.1016/j.brainresrev.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Basile GA, Quartu M, Bertino S, Serra MP, Boi M, Bramanti A, Anastasi GP, Milardi D, Cacciola A. Red nucleus structure and function: from anatomy to clinical neurosciences. Brain Struct Funct. 2021;226(1):69–91. doi: 10.1007/s00429-020-02171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hillman EM. Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci. 2014;37:161–81. doi: 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang H, Liu H, Liu G, Jin Z, Wang L, Ma J, Li H. Analysis of brain activity involved in chewing-side preference during chewing: an fMRI study. J Oral Rehabil. 2015;42(1):27–33. doi: 10.1111/joor.12224. [DOI] [PubMed] [Google Scholar]
- 53.Lin C-s. Revisiting the link between cognitive decline and masticatory dysfunction. BMC Geriatr. 2018;18(1):5. doi: 10.1186/s12877-017-0693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin CS. Meta-analysis of brain mechanisms of chewing and clenching movements. J Oral Rehabil. 2018;45(8):627–39. doi: 10.1111/joor.12657. [DOI] [PubMed] [Google Scholar]
- 55.Lotze M, Domin M, Kordass B. Symmetry of fMRI activation in the primary sensorimotor cortex during unilateral chewing. Clin Oral Investig. 2017;21(4):967–73. doi: 10.1007/s00784-016-1858-4. [DOI] [PubMed] [Google Scholar]
- 56.Onozuka M, Fujita M, Watanabe K, Hirano Y, Niwa M, Nishiyama K, Saito S. Mapping brain region activity during chewing: a functional magnetic resonance imaging study. J Dent Res. 2002;81(11):743–6. doi: 10.1177/0810743. [DOI] [PubMed] [Google Scholar]
- 57.Shoi K, Fueki K, Usui N, Taira M, Wakabayashi N. Influence of posterior dental arch length on brain activity during chewing in patients with mandibular distal extension removable partial dentures. J Oral Rehabil. 2014;41(7):486–95. doi: 10.1111/joor.12169. [DOI] [PubMed] [Google Scholar]
- 58.Hintzen A, Pelzer EA, Tittgemeyer M. Thalamic interactions of cerebellum and basal ganglia. Brain Struct Funct. 2018;223(2):569–87. doi: 10.1007/s00429-017-1584-y. [DOI] [PubMed] [Google Scholar]
- 59.Sakai ST. In: Handbook of the Cerebellum and Cerebellar Disorders. M M, S J, R F, G DL, K N, editors. Springer; Dordrecht: 2013. Cerebellar Thalamic and Thalamocortical Projections; pp. 529–547. [DOI] [Google Scholar]
- 60.Quintero A, Ichesco E, Schutt R, Myers C, Peltier S, Gerstner GE. Functional Connectivity of Human Chewing: An fcMRI Study. J Dent Res. 2013;92(3):272–8. doi: 10.1177/0022034512472681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manto M, Bower JM, Conforto AB, Delgado-García JM, da Guarda SN, Gerwig M, Habas C, Hagura N, Ivry RB, Mariёn P, Molinari M, et al. Consensus paper: roles of the cerebellum in motor control-the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2012;11(2):457–87. doi: 10.1007/s12311-011-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boillat Y, Bazin PL, van der Zwaag W. Whole-body somatotopic maps in the cerebellum revealed with 7T fMRI. Neuroimage. 2020;211:116624. doi: 10.1016/j.neuroimage.2020.116624. [DOI] [PubMed] [Google Scholar]
- 63.Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum Brain Mapp. 2009;30(10):3209–26. doi: 10.1002/hbm.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki M, Asada Y, Ito J, Hayashi K, Inoue H, Kitano H. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia. 2003;18(2):71–7. doi: 10.1007/s00455-002-0088-x. [DOI] [PubMed] [Google Scholar]
- 65.Costa MMB. Neural control of swallowing. Arq Gastroenterol. 2018;55(Suppl 1):61–75. doi: 10.1590/s0004-2803.201800000-45. [DOI] [PubMed] [Google Scholar]
- 66.Grodd W, Hülsmann E, Lotze M, Wildgruber D, Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp. 2001;13(2):55–73. doi: 10.1002/hbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Logemann JA. Critical Factors in the Oral Control Needed for Chewing and Swallowing. J Texture Stud. 2014;45(3):173–9. doi: 10.1111/jtxs.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hartmann MJ, Bower JM. Tactile responses in the granule cell layer of cerebellar folium crus IIa of freely behaving rats. J Neurosci. 2001;21(10):3549–63. doi: 10.1523/jneurosci.21-10-03549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shambes GM, Gibson JM, Welker W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain Behav Evol. 1978;15(2):94–140. doi: 10.1159/000123774. [DOI] [PubMed] [Google Scholar]
- 70.Ross CA, Ruggiero DA, Reis DJ. Afferent projections to cardiovascular portions of the nucleus of the tractus solitarius in the rat. Brain Res. 1981;223(2):402–8. doi: 10.1016/0006-8993(81)91155-0. [DOI] [PubMed] [Google Scholar]
- 71.Somana R, Walberg F. Cerebellar afferents from the nucleus of the solitary tract. Neurosci Lett. 1979;11(1):41–7. doi: 10.1016/0304-3940(79)90053-3. [DOI] [PubMed] [Google Scholar]
- 72.Sasegbon A, Watanabe M, Simons A, Michou E, Vasant DH, Magara J, Bath PM, Rothwell J, Inoue M, Hamdy S. Cerebellar repetitive transcranial magnetic stimulation restores pharyngeal brain activity and swallowing behaviour after disruption by a cortical virtual lesion. J Physiol. 2019;597(9):2533–46. doi: 10.1113/jp277545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ball GG, Micco DJ, Jr, Berntson GG. Cerebellar stimulation in the rat: complex stimulation-bound oral behaviors and self-stimulation. Physiol Behav. 1974;13(1):123–7. doi: 10.1016/0031-9384(74)90313-8. [DOI] [PubMed] [Google Scholar]
- 74.Berntson GG, Potolicchio SJ, Jr, Miller NE. Evidence for higher functions of the cerebellum: eating and grooming elicited by cerebellar stimulation in cats. Proc Natl Acad Sci USA. 1973;70(9):2497–9. doi: 10.1073/pnas.70.9.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reed MD, English M, English C, Huff A, Poliacek I, Musselwhite MN, Howland DR, Bolser DC, Pitts T. The Role of the Cerebellum in Control of Swallow: Evidence of Inspiratory Activity During Swallow. Lung. 2019;197(2):235–40. doi: 10.1007/s00408-018-00192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martner J. Cerebellar influences on autonomic mechanisms. An experimental study in the cat with special reference to the fastigial nucleus. Acta Physiol Scand Suppl. 1975;425:1–42. [PubMed] [Google Scholar]
- 77.MacLean PS, Blundell JE, Mennella JA, Batterham RL. Biological control of appetite: A daunting complexity. Obesity (Silver Spring) 2017;25(Suppl 1):S8–s16. doi: 10.1002/oby.21771. (Suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dietrichs E. Divergent axon collaterals to cerebellum and amygdala from neurons in the parabrachial nucleus, the nucleus locus coeruleus and some adjacent nuclei - a fluorescent double labeling study using rhodamine labeled latex microspheres and fast blue as retrograde tracers. Anat Embryol. 1985;172(3):375–82. doi: 10.1007/bf00318986. [DOI] [PubMed] [Google Scholar]
- 79.Dietrichs E, Haines DE. Interconnections between hypothalamus and cerebellum. Anat Embryol. 1989;179(3):207–20. doi: 10.1007/bf00326585. [DOI] [PubMed] [Google Scholar]
- 80.Dietrichs E, Haines DE, Røste GK, Røste LS. Hypothalam-ocerebellar and cerebellohypothalamic projections-circuits for regulating nonsomatic cerebellar activity? Histol Histopathol. 1994;9(3):603–14. [PubMed] [Google Scholar]
- 81.Tataranni PA, Gautier JF, Chen KW, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA. 1999;96(8):4569–74. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gautier JF, Chen KW, Uecker A, Bandy D, Frost J, Salbe AD, Pratley RE, Lawson M, Ravussin E, Reiman EM, Tataranni PA. Regions of the human brain affected during a liquid-meal taste perception in the fasting state: a positron emission tomography study. Am J Clin Nutr. 1999;70(5):806–10. doi: 10.1093/ajcn/70.5.806. [DOI] [PubMed] [Google Scholar]
- 83.Desai AJ, Dong M, Harikumar KG, Miller LJ. Cholecystokinin-induced satiety, a key gut servomechanism that is affected by the membrane microenvironment of this receptor. Int J Obes Suppl. 2016;6(Suppl 1):S22–s27. doi: 10.1038/ijosup.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Domingos AI, Vaynshteyn J, Voss HU, Ren XY, Gradinaru V, Zang F, Deisseroth K, de Araujo IE, Friedman J. Leptin regulates the reward value of nutrient. Nat Neurosci. 2011;14(12):1562–U1592. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peters JH, Simasko SM, Ritter RC. Modulation of vagal afferent excitation and reduction of food intake by leptin and cholecystokinin. Physiol Behav. 2006;89(4):477–85. doi: 10.1016/j.physbeh.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 86.Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 87.Gautier JF, Chen KW, Salbe AD, Bandy D, Pratley RE, Heiman M, Ravussin E, Reiman EM, Tataranni PA. Differential brain responses to satiation in obese and lean men. Diabetes. 2000;49(5):838–46. doi: 10.2337/diabetes.49.5.838. [DOI] [PubMed] [Google Scholar]
- 88.Gautier JF, Del Parigi A, Chen KW, Salbe AD, Bandy D, Pratley RE, Ravussin E, Reiman EM, Tataranni PA. Effect of satiation on brain activity in obese and lean women. Obes Res. 2001;9(11):676–84. doi: 10.1038/oby.2001.92. [DOI] [PubMed] [Google Scholar]
- 89.Maljaars PW, Peters HP, Mela DJ, Masclee AA. Ileal brake: a sensible food target for appetite control. Rev Physiol Behav. 2008;95(3):271–81. doi: 10.1016/j.physbeh.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 90.Tomasi D, Wang GJ, Wang RL, Backus W, Geliebter A, Telang F, Jayne MC, Wong C, Fowler JS, Volkow ND. Association of Body Mass and Brain Activation during Gastric Distention: Implications for Obesity. PLoS ONE. 2009;4(8):11. doi: 10.1371/journal.pone.0006847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sternson SM. Hunger: The carrot and the stick. Mol Metab. 2016;5(1):1–2. doi: 10.1016/j.molmet.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14(3):351–5. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488(7410):172. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Betley JN, Cao ZFH, Ritola KD, Sternson SM. Parallel, Redundant Circuit Organization for Homeostatic Control of Feeding Behavior. Cell. 2013;155(6):1337–50. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, Elmquist JK, et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci. 2015;18(6):863–U299. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garfield AS, Shah BP, Burgess CR, Li MM, Li C, Steger JS, Madara JC, Campbell JN, Kroeger D, Scammell TE, Tannous BA, et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat Neurosci. 2016;19(12):1628–35. doi: 10.1038/nn.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Low AYT, Goldstein N, Gaunt JR, Huang K-P, Zainolabidin N, Yip AKK, Carty JRE, Choi JY, Miller AM, Ho HST, Lenherr C, et al. Ch’ngBruceMartinHalkoB radyHolsenAlhadeffChenBetley THASLEMAROLMALAIJN. Reverse-translational identification of a cerebellar satiation network. Nature. 2021;600(7888):269–73. doi: 10.1038/s41586-021-04143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Norgren R. Projections from the nucleus of the solitary tract in the rat. Neuroscience. 1978;3(2):207–18. doi: 10.1016/0306-4522(78)90102-1. [DOI] [PubMed] [Google Scholar]
- 99.Hashimoto M, Yamanaka A, Kato S, Tanifuji M, Kobayashi K, Yaginuma H. Anatomical Evidence for a Direct Projection from Purkinje Cells in the Mouse Cerebellar Vermis to Medial Parabrachial Nucleus. Front Neural Circuits. 2018;12:6. doi: 10.3389/fncir.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Supple WF, Kapp BS. Anatomical and physiological relationships between the anterior cerebellar vermis and the pontine parabrachial nucleus in the rabbit. Brain Res Bull. 1994;33(5):561–74. doi: 10.1016/0361-9230(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 101.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell. 2016;166(1):209–21. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cano V, Merino B, Ezquerra L, Somoza B, Ruiz-Gayo M. A cholecystokinin-1 receptor agonist (CCK-8) mediates increased permeability of brain barriers to leptin. Br J Pharmacol. 2008;154(5):1009–15. doi: 10.1038/bjp.2008.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23(4):775–86. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 104.May AA, Liu M, Woods SC, Begg DP. CCK increases the transport of insulin into the brain. Physiol Behav. 2016;165:392–7. doi: 10.1016/j.physbeh.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The Inhibitory Circuit Architecture of the Lateral Hypothalamus Orchestrates Feeding. Science. 2013;341(6153):1517–21. doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496(7444):224–8. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jennings JH, Ung RL, Resendez SL, Stamatakis AM, Taylor JG, Huang J, Veleta K, Kantak PA, Aita M, Shilling-Scrivo K, Ramakrishnan C, et al. Visualizing Hypothalamic Network Dynamics for Appetitive and Consummatory Behaviors. Cell. 2015;160(3):516–27. doi: 10.1016/j.cell.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nieh EH, Matthews GA, Allsop SA, Presbrey KN, Leppla CA, Wichmann R, Neve R, Wildes CP, Tye KM. Decoding Neural Circuits that Control Compulsive Sucrose Seeking (vol 160, pg 528, 2015) Cell. 2015;161(6):1468–71. doi: 10.1016/j.cell.2015.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Al Massadi O, Nogueiras R, Dieguez C, Girault JA. Ghrelin and food reward. Neuropharmacology. 2019;148:131–8. doi: 10.1016/j.neuropharm.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 110.Miller LJ, Harikumar KG, Wootten D, Sexton PM. Roles of Cholecystokinin in the Nutritional Continuum. Physiology and Potential Therapeutics. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.684656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qiu J, Zhang C, Borgquist A, Nestor CC, Smith AW, Bosch MA, Ku S, Wagner EJ, Rønnekleiv OK, Kelly MJ. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab. 2014;19(4):682–93. doi: 10.1016/j.cmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pradhan G, Samson SL, Sun Y. Ghrelin: much more than a hunger hormone. Curr Opin Clin Nutr Metab Care. 2013;16(6):619–24. doi: 10.1097/MCO.0b013e328365b9be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harvey J. Leptin - A multifaceted hormone in the central nervous system. Mol Neurobiol. 2003;28(3):245–58. doi: 10.1385/mn:28:3:245. [DOI] [PubMed] [Google Scholar]
- 114.Münzberg H, Morrison CD. Structure, production and signaling of leptin. Metabolism. 2015;64(1):13–23. doi: 10.1016/j.metabol.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am. 2008;37(4):811–23. doi: 10.1016/j.ecl.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barrios-Correa AA, Estrada JA, Contreras I. Leptin Signaling in the Control of Metabolism and Appetite: Lessons from Animal Models. J Mol Neurosci. 2018;66(3):390–402. doi: 10.1007/s12031-018-1185-0. [DOI] [PubMed] [Google Scholar]
- 117.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8(1):21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 118.Aotani D, Ebihara K, Sawamoto N, Kusakabe T, Aizawa-Abe M, Kataoka S, Sakai T, Iogawa H, Ebihara C, Fujikura J, Hosoda K, et al. Functional Magnetic Resonance Imaging Analysis of Food-Related Brain Activity in Patients with Lipodystrophy Undergoing Leptin Replacement Therapy. J Clin Endocrinol Metab. 2012;97(10):3663–71. doi: 10.1210/jc.2012-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 120.Zhang YY, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homolog (vol 372, pg 425, 1994) Nature. 1995;374(6521):479. doi: 10.1038/374479a0. [DOI] [PubMed] [Google Scholar]
- 121.Burguera B, Couce ME, Long J, Lamsam J, Laakso K, Jensen MD, Parisi JE, Lloyd RV. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain. Neuroendocrinology. 2000;71(3):187–95. doi: 10.1159/000054536. [DOI] [PubMed] [Google Scholar]
- 122.Savioz A, Charnay Y, Huguenin C, Graviou C, Greggio B, Bouras C. Expression of leptin receptor mRNA (long form splice variant) in the human cerebellum. NeuroReport. 1997;8(14):3123–6. doi: 10.1097/00001756-199709290-00023. [DOI] [PubMed] [Google Scholar]
- 123.Oldreive CE, Harvey J, Dohertya GH. Neurotrophic effects of leptin on cerebellar Purkinje but not granule neurons in vitro. Neurosci Lett. 2008;438(1):17–21. doi: 10.1016/j.neulet.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 124.Irving AJ, Wallace L, Durakoglugil D, Harvey J. Leptin enhances NR2B-mediated N-methyl-D-aspartate responses via a mitogen-activated protein kinase-dependent process in cerebellar granule cells. Neuroscience. 2006;138(4):1137–48. doi: 10.1016/j.neuroscience.2005.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41(12):317–8. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 126.Jeet Singh H. The unfolding tale of leptin. Malays J Med Sci. 2001;8(2):1–6. [PMC free article] [PubMed] [Google Scholar]
- 127.Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392(6674):398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 128.Crujeiras AB, Carreira MC, Cabia B, Andrade S, Amil M, Casanueva FF. Leptin resistance in obesity: An epigenetic landscape. Life Sci. 2015;140:57–63. doi: 10.1016/j.lfs.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 129.de Luis DA, Perez Castrillón JL, Dueñas A. Leptin and obesity. Minerva Med. 2009;100(3):229–36. [PubMed] [Google Scholar]
- 130.Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21(11):643–51. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Paz-Filho G, Mastronardi CA, Licinio J. Leptin treatment: facts and expectations. Metabolism. 2015;64(1):146–56. doi: 10.1016/j.metabol.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 132.Berman SM, Paz G, Wong ML, Kohno M, Licinio J, London ED. Effects of Leptin Deficiency and Replacement on Cerebellar Response to Food-Related Cues. Cerebellum. 2013;12(1):59–67. doi: 10.1007/s12311-012-0360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Burger KS, Cornier MA, Ingebrigtsen J, Johnson SL. Assessing food appeal and desire to eat: the effects of portion size & energy density. Int J Behav Nutr Phys Act. 2011;8:9. doi: 10.1186/1479-5868-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Faber CL, Deem JD, Phan BA, Doan TP, Ogimoto K, Mirzadeh Z, Schwartz MW, Morton GJ. Leptin receptor neurons in the dorsomedial hypothalamus regulate diurnal patterns of feeding, locomotion, and metabolism. ELife. 2021;10:e63671. doi: 10.7554/eLife.63671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cheng AH, Cheng H-YM. Genesis of the Master Circadian Pacemaker in Mice. Frontiers in Neuroscience. 2021;15 doi: 10.3389/fnins.2021.659974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Evans J, Silver R. Neuroscience in the 21st Century: From Basic to Clinical. 2nd Edition. Springer; 233 Spring Street, New York, Ny 10013, United States: 2016. The Suprachiasmatic Nucleus and the Circadian Timekeeping System of the Body. [DOI] [Google Scholar]
- 137.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 138.Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF, Jr, Reppert SM. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron. 1997;19(6):1261–9. doi: 10.1016/s0896-6273(00)80417-1. [DOI] [PubMed] [Google Scholar]
- 139.Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20(6):1103–10. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]
- 140.Challet E, Mendoza J, Dardente H, Pévet P. Neurogenetics of food anticipation. Eur J Neurosci. 2009;30(9):1676–87. doi: 10.1111/j.1460-9568.2009.06962.x. [DOI] [PubMed] [Google Scholar]
- 141.Mendoza J, Albrecht U, Challet E. Behavioural food anticipation in clock genes deficient mice: confirming old phenotypes, describing new phenotypes. Genes Brain Behav. 2010;9(5):467–77. doi: 10.1111/j.1601-183X.2010.00576.x. [DOI] [PubMed] [Google Scholar]
- 142.Mendoza J, Pevet P, Felder-Schmittbuhl MP, Bailly Y, Challet E. The Cerebellum Harbors a Circadian Oscillator Involved in Food Anticipation. J Neurosci. 2010;30(5):1894–904. doi: 10.1523/jneurosci.5855-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mordel J, Karnas D, Pévet P, Isope P, Challet E, Meissl H. The output signal of Purkinje cells of the cerebellum and circadian rhythmicity. PLoS ONE. 2013;8(3):e58457. doi: 10.1371/journal.pone.0058457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rath MF, Rohde K, Møller M. Circadian Oscillations of Molecular Clock Components in the Cerebellar Cortex of the Rat. Chronobiol Int. 2012;29(10):1289–99. doi: 10.3109/07420528.2012.728660. [DOI] [PubMed] [Google Scholar]
- 145.Born JM, Lemmens SGT, Martens MJI, Formisano E, Goebel R, Westerterp-Plantenga MS. Differences between liking and wanting signals in the human brain and relations with cognitive dietary restraint and body mass index. Am J Clin Nutr. 2011;94(2):392–403. doi: 10.3945/ajcn.111.012161. [DOI] [PubMed] [Google Scholar]
- 146.Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci. 2018;19(6):338–50. doi: 10.1038/s41583-018-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Carta F, Chen CH, Schott AL, Dorizan S, Khodakhah K. Cerebellar modulation of the reward circuitry and social behavior. Science. 2019;363(6424):248. doi: 10.1126/science.aav0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27(1-2):99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- 149.Tyree SM, de Lecea L. Lateral Hypothalamic Control of the Ventral Tegmental Area: Reward Evaluation and the Driving of Motivated Behavior. Front Syst Neurosci. 2017;11:50. doi: 10.3389/fnsys.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo LQ. Cerebellar granule cells encode the expectation of reward. Nature. 2017;544(7648):96. doi: 10.1038/nature21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Larry N, Yarkoni M, Lixenberg A, Joshua M. Cerebellar climbing fibers encode expected reward size. eLife. 2019;8:16. doi: 10.7554/eLife.46870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Heffley W, Hull C. Classical conditioning drives learned reward prediction signals in climbing fibers across the lateral cerebellum. eLife. 2019;8:21. doi: 10.7554/eLife.46764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Marr D. A theory of cerebellar cortex. J Physiol. 1969;202(2):437–70. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kostadinov D, Beau M, Blanco-Pozo M, Häusser M. Predictive and reactive reward signals conveyed by climbing fiber inputs to cerebellar Purkinje cells. Nat Neurosci. 2019;22(6):950–62. doi: 10.1038/s41593-019-0381-8. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sendhilnathan N, Semework M, Goldberg ME, Ipata AE. Neural Correlates of Reinforcement Learning in Mid-lateral Cerebellum. Neuron. 2020;106(1):188–198.:e185. doi: 10.1016/j.neuron.2019.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zou Z, Wang H, d’Oleire Uquillas F, Wang X, Ding J, Chen H. Definition of Substance and Non-substance Addiction. Adv Exp Med Biol. 2017;1010:21–41. doi: 10.1007/978-981-10-5562-1_2. [DOI] [PubMed] [Google Scholar]
- 157.Carnell S, Benson L, Pantazatos SP, Hirsch J, Geliebter A. Amodal brain activation and functional connectivity in response to high-energy-density food cues in obesity. Obes (Silver Spring) 2014;22(11):2370–8. doi: 10.1002/oby.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.English LK, Masterson TD, Fearnbach SN, Tanofsky-Kraff M, Fisher J, Wilson SJ, Rolls BJ, Keller KL. Increased brain and behavioural susceptibility to portion size in children with loss of control eating. Pediatr Obes. 2019;14(2):14. doi: 10.1111/ijpo.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Holsen LM, Savage CR, Martin LE, Bruce AS, Lepping RJ, Ko E, Brooks WM, Butler MG, Zarcone JR, Goldstein JM. Importance of reward and prefrontal circuitry in hunger and satiety: Prader-Willi syndrome vs simple obesity. Int J Obes. 2012;36(5):638–47. doi: 10.1038/ijo.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Marron EM, Viejo-Sobera R, Cuatrecasas G, Redolar-Ripoll D, Lorda PG, Datta A, Bikson M, Magerowski G, Alonso-Alonso M. Prefronto-cerebellar neuromodulation affects appetite in obesity. Int J Obes. 2019;43(10):2119–24. doi: 10.1038/s41366-018-0278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Contreras-Rodriguez O, Vilar-Lopez R, Andrews ZB, Navas JF, Soriano-Mas C, Verdejo-Garcia A. Altered cross-talk between the hypothalamus and non-homeostatic regions linked to obesity and difficulty to lose weight. Sci Rep. 2017;7:9. doi: 10.1038/s41598-017-09874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Heffley W, Song EY, Xu Z, Taylor BN, Hughes MA, McKinney A, Joshua M, Hull C. Coordinated cerebellar climbing fiber activity signals learned sensorimotor predictions. Nat Neurosci. 2018;21(10):1431–41. doi: 10.1038/s41593-018-0228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Koutsikou S, Watson TC, Crook JJ, Leith JL, Lawrenson CL, Apps R, Lumb BM. The Periaqueductal Gray Orchestrates Sensory and Motor Circuits at Multiple Levels of the Neuraxis. J Neurosci. 2015;35(42):14132–47. doi: 10.1523/jneurosci.0261-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lawrenson C, Paci E, Pickford J, Drake RAR, Lumb BM, Apps R. Cerebellar modulation of memory encoding in the periaqueductal grey and fear behaviour. Elife. 2022;11 doi: 10.7554/eLife.76278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Middleton FA, Strick PL. Cerebellar Projections to the Prefrontal Cortex of the Primate. J Neurosci. 2001;21(2):700–12. doi: 10.1523/jneurosci.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sacchetti B, Scelfo B, Strata P. Cerebellum and emotional behavior. Neuroscience. 2009;162(3):756–62. doi: 10.1016/j.neuroscience.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 167.Watson TC, Cerminara NL, Lumb BM, Apps R. Neural Correlates of Fear in the Periaqueductal Gray. J Neurosci. 2016;36(50):12707–19. doi: 10.1523/jneurosci.1100-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Watson TC, Koutsikou S, Cerminara NL, Flavell CR, Crook JJ, Lumb BM, Apps R. The olivo-cerebellar system and its relationship to survival circuits. Front Neural Circuits. 2013;7:72. doi: 10.3389/fncir.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Michels N. Biological underpinnings from psychosocial stress towards appetite and obesity during youth: research implications towards metagenomics, epigenomics and metabolomics. Nutr Res Rev. 2019;32(2):282–93. doi: 10.1017/s0954422419000143. [DOI] [PubMed] [Google Scholar]
- 170.Nakamura C, Ishii A, Matsuo T, Ishida R, Yamaguchi T, Takada K, Uji M, Yoshikawa T. Neural effects of acute stress on appetite: A magnetoencephalography study. PLoS ONE. 2020;15(1):e0228039. doi: 10.1371/journal.pone.0228039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Roelofs K. Freeze for action: neurobiological mechanisms in animal and human freezing. Philos Trans R Soc Lond B Biol Sci. 2017;372(1718):20160206. doi: 10.1098/rstb.2016.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yau YH, Potenza MN. Stress and eating behaviors. Minerva Endocrinol. 2013;38(3):255–67. [PMC free article] [PubMed] [Google Scholar]
- 173.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trend Neurosci. 2008;31(3):137–45. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 174.Becker EB, Stoodley CJ. Autism spectrum disorder and the cerebellum. Int Rev Neurobiol. 2013;113:1–34. doi: 10.1016/b978-0-12-418700-9.00001-0. [DOI] [PubMed] [Google Scholar]
- 175.Gallimore AR, Kim T, Tanaka-Yamamoto K, De Schutter E. Switching On Depression and Potentiation in the Cerebellum. Cell Rep. 2018;22(3):722–33. doi: 10.1016/j.celrep.2017.12.084. [DOI] [PubMed] [Google Scholar]
- 176.Mothersill O, Knee-Zaska C, Donohoe G. Emotion and Theory of Mind in Schizophrenia-Investigating the Role of the Cerebellum. Cerebellum. 2016;15(3):357–68. doi: 10.1007/s12311-015-0696-2. [DOI] [PubMed] [Google Scholar]
- 177.Schutter DJ, van Honk J. The cerebellum on the rise in human emotion. Cerebellum. 2005;4(4):290–4. doi: 10.1080/14734220500348584. [DOI] [PubMed] [Google Scholar]
- 178.Gomes-de-Souza L, Costa-Ferreira W, Mendonça MM, Xavier CH, Crestani CC. Lateral hypothalamus involvement in control of stress response by bed nucleus of the stria terminalis endocannabinoid neurotransmission in male rats. Sci Rep. 2021;11(1):16133. doi: 10.1038/s41598-021-95401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Martín-Pérez C, Contreras-Rodríguez O, Vilar-López R, Verdejo-García A. Hypothalamic Networks in Adolescents With Excess Weight: Stress-Related Connectivity and Associations With Emotional Eating. J Am Acad Child Adolesc Psychiatry. 2019;58(2):211–220.:e215. doi: 10.1016/j.jaac.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 180.Yang X, Casement M, Yokum S, Stice E. Negative affect amplifies the relation between appetitive-food-related neural responses and weight gain over three-year follow-up among adolescents. Neuroimage Clin. 2019;24:102067. doi: 10.1016/j.nicl.2019.102067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Groesz LM, McCoy S, Carl J, Saslow L, Stewart J, Adler N, Laraia B, Epel E. What is eating you? Stress and the drive to eat. Appetite. 2012;58(2):717–21. doi: 10.1016/j.appet.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosom Med. 2003;65(3):450–60. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- 183.Moreno-Rius J. The cerebellum in fear and anxiety-related disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;85:23–32. doi: 10.1016/j.pnpbp.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 184.Gilbey MP. Sympathetic rhythms and nervous integration. Clin Exp Pharmacol Physiol. 2007;34(4):356–61. doi: 10.1111/j.1440-1681.2007.04587.x. [DOI] [PubMed] [Google Scholar]
- 185.Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61(3):137–59. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- 186.Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RSJ, Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol. 2002;88(3):1500–11. doi: 10.1152/jn.2002.88.3.1500. [DOI] [PubMed] [Google Scholar]
- 187.Parsons LM, Denton D, Egan G, McKinley M, Shade R, Lancaster J, Fox PT. Neuroimaging evidence implicating cerebellum in Support of sensory/cognitive processes associated with thirst. Proc Natl Acad Sci USA. 2000;97(5):2332–6. doi: 10.1073/pnas.040555497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Parsons LM, Egan G, Liotti M, Brannan S, Denton D, Shade R, Robillard R, Madden L, Abplanalp B, Fox PT. Neuroimaging evidence implicating cerebellum in the experience of hypercapnia and hunger for air. Proc Natl Acad Sci USA. 2001;98(4):2041–6. doi: 10.1073/pnas.98.4.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Saker P, Farrell MJ, Egan GF, McKinley MJ, Denton DA. Influence of anterior midcingulate cortex on drinking behavior during thirst and following satiation. Proc Natl Acad Sci USA. 2018;115(4):786–91. doi: 10.1073/pnas.1717646115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Stroh MA, Winter MK, McCarson KE, Thyfault JP, Zhu H. NCB5OR Deficiency in the Cerebellum and Midbrain Leads to Dehydration and Alterations in Thirst Response, Fasted Feeding Behavior, and Voluntary Exercise in Mice. Cerebellum. 2018;17(2):152–64. doi: 10.1007/s12311-017-0880-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhu JN, Yung WH, Chow BKC, Chan YS, Wang JJ. The cerebellar-hypothalamic circuits: Potential pathways underlying cerebellar involvement in somatic-visceral integration. Brain Res Rev. 2006;52(1):93–106. doi: 10.1016/j.brainresrev.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 192.Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: Implications for affective disorders. Biol Psychiatry. 2000;48(1):30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- 193.Sacchetti B, Scelfo B, Strata P. The cerebellum: synaptic changes and fear conditioning. Neuroscientist. 2005;11(3):217–27. doi: 10.1177/1073858405276428. [DOI] [PubMed] [Google Scholar]
- 194.Utz A, Thürling M, Ernst TM, Hermann A, Stark R, Wolf OT, Timmann D, Merz CJ. Cerebellar vermis contributes to the extinction of conditioned fear. Neurosci Lett. 2015;604:173–7. doi: 10.1016/j.neulet.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 195.Ernst TM, Brol AE, Gratz M, Ritter C, Bingel U, Schlamann M, Maderwald S, Quick HH, Merz CJ, Timmann D. The cerebellum is involved in processing of predictions and prediction errors in a fear conditioning paradigm. ELife. 2019;8 doi: 10.7554/eLife.46831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.McNally GP, Johansen JP, Blair HT. Placing prediction into the fear circuit. Trend Neurosci. 2011;34(6):283–92. doi: 10.1016/j.tins.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Popa LS, Ebner TJ. Cerebellum, Predictions and Errors. Frontiers in Cellular Neuroscience. 2019;12 doi: 10.3389/fncel.2018.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Popa LS, Ebner TJ. Cerebellum, Predictions and Errors. Front Cell Neurosci. 2018;12:524. doi: 10.3389/fncel.2018.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bracha V. Role of the cerebellum in eyeblink conditioning. Prog Brain Res. 2004;143:331–9. doi: 10.1016/s0079-6123(03)43032-x. [DOI] [PubMed] [Google Scholar]
- 200.Gerwig M, Kolb FP, Timmann D. The involvement of the human cerebellum in eyeblink conditioning. Cerebellum. 2007;6(1):38–57. doi: 10.1080/14734220701225904. [DOI] [PubMed] [Google Scholar]
- 201.Li DB, Yao J, Sun L, Wu B, Li X, Liu SL, Hou JM, Liu HL, Sui JF, Wu GY. Reevaluating the ability of cerebellum in associative motor learning. Sci Rep. 2019;9(1):6029. doi: 10.1038/s41598-019-42413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.De Zeeuw CI, Ten Brinke MM. Motor Learning and the Cerebellum. Cold Spring Harb Perspect Biol. 2015;7(9):a021683. doi: 10.1101/cshperspect.a021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gao Z, Davis C, Thomas AM, Economo MN, Abrego AM, Svoboda K, De Zeeuw CI, Li N. A cortico-cerebellar loop for motor planning. Nature. 2018;563(7729):113–6. doi: 10.1038/s41586-018-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chabrol FP, Blot A, Mrsic-Flogel TD. Cerebellar Contribution to Preparatory Activity in Motor Neocortex. Neuron. 2019;103(3):506–519.:e504. doi: 10.1016/j.neuron.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Grimaldi G, Argyropoulos GP, Boehringer A, Celnik P, Edwards MJ, Ferrucci R, Galea JM, Groiss SJ, Hiraoka K, Kassavetis P, Lesage E, et al. Non-invasive cerebellar stimulation-a consensus paper. Cerebellum. 2014;13(1):121–38. doi: 10.1007/s12311-013-0514-7. [DOI] [PubMed] [Google Scholar]
- 206.van Dun K, Manto M. Non-invasive Cerebellar Stimulation: Moving Towards Clinical Applications for Cerebellar and Extra-Cerebellar Disorders. The Cerebellum. 2018;17(3):259–63. doi: 10.1007/s12311-017-0908-z. [DOI] [PubMed] [Google Scholar]
- 207.Rastogi A, Cash R, Dunlop K, Vesia M, Kucyi A, Ghahremani A, Downar J, Chen J, Chen R. Modulation of cognitive cerebello-cerebral functional connectivity by lateral cerebellar continuous theta burst stimulation. Neuroimage. 2017;158:48–57. doi: 10.1016/j.neuroimage.2017.06.048. [DOI] [PubMed] [Google Scholar]
- 208.Naro A, Bramanti A, Leo A, Manuli A, Sciarrone F, Russo M, Bramanti P, Calabrò RS. Effects of cerebellar transcranial alternating current stimulation on motor cortex excitability and motor function. Brain Struct Funct. 2017;222(6):2891–906. doi: 10.1007/s00429-016-1355-1. [DOI] [PubMed] [Google Scholar]
- 209.Bersani FS, Minichino A, Bernabei L, Spagnoli F, Corrado A, Vergnani L, Mannarelli D, Pauletti C, Fattapposta F, Biondi M, Delle Chiaie R. Prefronto-cerebellar tDCS enhances neurocognition in euthymic bipolar patients. Findings from a placebo-controlled neuropsychological and psychophysiological investigation. J Affect Disord. 2017;209:262–9. doi: 10.1016/j.jad.2016.11.037. [DOI] [PubMed] [Google Scholar]