Abstract
The synthesis of acute-phase protein serum amyloid A (SAA) is largely regulated by inflammation-associated cytokines and a high concentration of circulating SAA may represent an ideal marker for acute and chronic inflammatory diseases. However, SAA is also synthesized in extrahepatic tissues, e.g. human carcinoma metastases and cancer cell lines. An increasing body of in vitro data supports the concept of involvement of SAA in carcinogenesis and neoplastic diseases. Accumulating evidence suggests that SAA might be included in a group of biomarkers to detect a pattern of physiological events that reflect the growth of malignancy and host response. This review is meant to provide a broad overview of the many ways that SAA could contribute to tumour development, and accelerate tumour progression and metastasis, and to gain a better understanding of this acute-phase reactant as a possible link between chronic inflammation and neoplasia.
Keywords: SAA, inflammation, renal cell carcinoma, extracellular matrix, rheumatoid arthritis, FPRL-1, MMP, proteomics
Introduction
Inflammation is generally regarded as an organism’s protective mechanism against trauma, irritation, injury or infection. Whereas acute inflammation has mainly beneficial effects, clinical and epidemiological studies have suggested a strong association between chronic infection, chronic inflammation and cancer. Recurrent or persistent chronic inflammation may induce, promote, or influence susceptibility to carcinogenesis by causing DNA damage, inciting tissue reparative proliferation, and/or by creating an environment that is enriched with tumour-promoting cytokines and growth factors. Clinically and pathologically classifiable inflammatory diseases are established precursors of cancers occurring in gastrointestinal, respiratory, anogenital, and lymphoid organs and tissues [1]. It has been clear for some time now that the tumour microenvironment, largely orchestrated by inflammatory cells and a network of signalling molecules, are indispensable participants in the neoplastic process, fostering proliferation, survival and migration [2, 3]. Important components in this linkage are cytokines produced by activated innate immune cells that stimulate tumour growth. Subsequently, soluble mediators synthesized by cancer cells may also recruit and activate inflammatory cells that, in turn, may stimulate cancer progression [4, 5]. Basically, a number of pro-inflammatory gene products have been described as mediators of tumour development. Recent evidence suggested a possible involvement of serum amyloid A (SAA) in carcinogenesis. SAA is a generic term for a family of acute-phase proteins coded for by different genes with a high allelic variation and a high degree of homology between species [6, 7]. In humans, the bona fide acute-phase SAA genes SAA1 and SAA2 share approx. 95% overall sequence identity in their promoter regions, exons and introns [8]. The different alleles of the SAA1 and SAA2 loci encode for non-glycosylated SAA proteins (104 amino acids, 11.7 kDa). The dominant isotype, SAA1, consists of at least five allelic variants, while less have been reported for SAA2 [9].
Hepatic synthesis of acute-phase proteins is largely regulated by inflammation-associated cytokine-peptide hormone signals produced by endothelial cells, lymphocytes and, in particular, activated monocytes and macrophages. Indeed, the acute-phase response is an orchestrated response to tissue injury, infection, and inflammation, and the primary function of acute-phase proteins is the restoration of homeostasis. Depending on the extent of inflammation, major acute-phase proteins may reach levels up to 1000-fold greater than those in the non-inflammatory state. As compared to the currently widely used C-reactive protein, SAA is frequently a more sensitive marker of inflammation, particularly in some conditions [10], while it has the advantage of also being involved in the acute-phase response in species other than humans, such as mice. As concentrations of acute-phase reactants might correlate with the amount of damaged tissue, measurements of SAA are of value in the assessment of activity and response to therapy during several inflammatory diseases [10]. Normally, SAA levels peak on the third day after the onset of the acute event and, approximately four days later, levels of SAA – the major apolipoprotein of high-density lipoprotein (HDL) during inflammation – return to baseline [10]. Thus the ”natural” role of SAA seems to be one of maintaining homeostasis. In chronic inflammation, however, which is a driving force in tumour development, SAA levels increase substantially and, together with other pro-inflammatory molecules, contribute to the vicious positive feedback cycle, unable to be balanced by anti-inflammatory mediators. Indeed, serum levels of SAA are increased in a number of chronic inflammatory and neoplastic diseases that may predispose to amyloidosis, a clinical disorder caused by extracellular deposition of insoluble abnormal fibrils, derived from aggregation of misfolded, normally soluble protein. Sustained SAA levels may give rise to secondary amyloidosis, now called reactive systemic amyloid A (AA) amyloidosis, where the 76 amino acid N-terminal portion of intact SAA is deposited as AA fibrils [11, 12]. AA amyloidosis always involves the spleen but typically presents with proteinuria and/or hepatosplenomegaly while cardiac involvement is very rare [13]. Basically, amyloid deposits contain abundant heparan sulfate and dermatan sulfate proteoglycans and glycosaminglycan chains, some of which are tightly bound to the fibrils [13].
Malignant transformation can be closely associated with chronic infection and inflammation, while biosynthesis and secretion of pro-inflammatory cytokines represent the primary cause for this close connection. Key molecular links between inflammation and tumour promotion/progression involve various signal transduction pathways, which are activated by many pro-inflammatory cytokines. A number of previous and recent studies proposed a direct correlation between SAA concentrations and tumour grading [14, 15]. This led to the assumption that SAA might be considered a marker to monitor tumour progression and that SAA might act as a biomarker candidate for specific cancer types [16, 17]. Finally, as elevated SAA levels accompany neoplastic processes, and poor prognosis correlates with the level of SAA, it is most interesting to clarify whether SAA plays an active role in the pathophysiology of cancer.
Serum levels of SAA in cancer
SAA is found at low levels in sera of healthy donors [18]. Although fluctuations with time for various individuals may occur as a reflection of subclinical infection, inflammation or even malnutrition, no age-related increase in plasma SAA concentrations was observed in healthy subjects [19]. Thus, the high SAA levels measured in octogenarians [20] were apparently due to occult inflammatory and neoplastic diseases [21]. In 277 patients with a broad spectrum of neoplastic diseases, including ten classes of solid tumours (stomach, colon, pancreas, prostate, breast, ovary, testis, lung, endocrine, and sarcoma) and three classes of haematological malignancies (Hodgkin’s disease, non Hodgkin’s disease, and leukaemia), SAA levels in these patients varied substantially but were generally elevated [22]. SAA levels were significantly higher in patients with quantitatively advanced diseases. The difference between metastatic and limited disease was consistent within all the individual tumour categories and histologic groups, i.e., adenocarcinoma, squamous carcinoma, sarcoma, lymphoma, and acute leukaemia [22]. SAA levels were increased in patients with proven carcinoma of the lung (squamous cell carcinoma, adenocarcinoma, oat cell carcinoma, and anaplastic large cell carcinoma) [23–25], prostate cancer [26], and colorectal carcinoma [27] suggesting that SAA could act at least as a non-specific tumour marker and independent prognostic factor as obtained by univariate and multivariate analysis. Most importantly, SAA levels were also high in renal cell carcinoma (RCC) [28], a disease found to be associated with reactive systemic AA amyloidosis, consisting of β-sheets of SAA-derived AA protein deposited in tissues and organs. Other authors have suggested SAA as an indicator of distant metastases but not as an early tumour marker in patients with RCC [29].
Rosenthal and Sullivan [30] even proposed SAA as a biochemical marker that discriminates between disseminated and localized or regional disease. Biran and coworkers [14] further addressed a direct correlation between SAA concentrations and cancer activity, stage (early to metastasis) and prognosis. Initial SAA values had prognostic significance: a value below 10 µg/ml correlated with survival advantage, whereas a higher initial value indicated a greater likelihood of poor outcome. Gastric cancer patients with SAA levels above 97 µg/ml had a nearly four-fold increase in risk of death [31]. In 233 patients with a number of different tumours (lung, bladder, stomach, and colorectal) a higher percent of raised SAA levels was found in patients with more advanced disease [32]; patients with breast carcinoma (stages I–III) had a poor acute-phase response. These findings are in line with others [33] where elevated SAA levels were seen in stage IV patients with the highest levels in ulcerating tumours; these observations suggest that, in the absence of metastatic disease, breast cancer is a poor stimulus for the production of acute-phase proteins. High levels of SAA in cancer patients (progressive metastatic renal cell cancer, malignant melanoma, stage III/IV breast cancer or non small cell lung cancer) were drastically increased when patients received human recombinant interleukin 6 (rIL-6) as an anti-tumour immunotherapy in phase I and phase II studies [34–36]. SAA levels increased dose-dependently during the first week of rIL-6 administration, with peak values occurring at day 3 [34–36]. However, SAA levels decreased slightly during the following weeks of immunotherapy [34–36]. In contrast to rIL-6 immunotherapy, treatment of ovarian cancer patients (stage III/IV) with rIL-3 only slightly increased SAA levels [37]. In patients with colorectal liver metastasis, partial hepatectomy is associated with increased serum SAA levels [38, 39] probably a consequence of alterations in the liver proteome. Serum SAA levels increased postoperatively in laparoscopy-assisted distal gastrectomy as well as in open distal gastrectomy, but temporal increase was lower in the laparoscopy-assisted distal gastrectomy group [40]. Mean plasma SAA concentrations were also high in gastric cancer patients and SAA concentrations were associated with tumour stage and location [41]. Most importantly, SAA turned out to be useful in predicting survival of patients with gastric cancer, but also as a valuable tool for postoperative follow-up [31]. SAA levels were high in sera from patients with multiple myeloma compared to controls [42]. Whether SAA can be used as a specific clinical marker in cancer patients undergoing febrile neutropenia is still a matter of debate [43–45].
SAA was found to be up-regulated around 30-fold using microarray gene expression profiling and analysis in RCC [46]. Antibody microarray analysis of samples from lung cancer patients showed a two-fold increase of SAA above control samples. A distinct serum protein profile involving abundant acute-phase proteins was observed and could be useful as a utility for detecting lung cancers [47]. Microarray analysis from prostate cancer tissue samples revealed that 466 network and 423 functions-pathways eligible genes were upregulated; SAA was found as a member of the network around the IL-1β pathway [48].
SAA is synthesized in cancer tissues of non-hepatic origin
Although hepatoma cells retain the ability to produce SAA in response to cytokines, they express less SAA in the transformed state in comparison to primary hepatocytes [49–51]. Nevertheless, human hepatoblastoma HepG2 cell lines [52] and various hepatocellular carcinoma cell lines, e.g. Hep3B [52, 53], HuH-7 [52, 54, 55], and NPLC/PRF/5 [56], served as in vitro models to mimic time- and cytokine concentration-dependent hepatic expression of SAA at the RNA and protein levels [57, 58]. Using quantitative RT-PCR and immunohistochemistry, SAA has been identified as a specific marker for hepatocellular adenoma subtype classification [59]. As SAA1/2 transcripts are also expressed in epithelial cells, fibroblasts, endothelial cells, and monocytes/macrophages [52, 60, 61], the question has arisen as to whether tumour cell lines of non-hepatic-origin are also prone to express SAA at the RNA and protein levels?
Using serial analysis of gene expression in normal and tumour tissues/cell lines (colon and pancreas), SAA was among 183 genes elevated in human pancreatic cancer but not colon cancer; the ratio of SAA in pancreatic tumour to normal colon and pancreatic cell line to normal colon was 11 and 25, respectively [62]. Serial analysis of gene expression further revealed high SAA expression in a primary colon tumour cell line (SW480) compared to its low abundance in an isogenic lymph-node metastasis cell line (SW620) and other metastatic colon cancer cell lines (LoVo and Colo201) isolated from the same patient [63]. In a model of p53-induced apoptosis, SAA was among 14 genes markedly increased in the colorectal cancer cell line DLD-1 (containing an inactive endogenous p53 gene), that has been infected with a replication-defective adenovirus encoding p53 [64]. Gene expression in macroscopically normal colonic mucosa revealed a higher expression of SAA in individuals with a family history of sporadic colon cancer [65]. Using suppression subtractive hybridization, SAA was found to be one out of nine highly expressed genes in the cancerous region of human renal biopsies [66]. Gene expression analyses identified a protein signature for tumour aggressiveness in clear cell RCC; SAA was one out of six candidate biomarkers upregulated in aggressive primary and metastatic clear cell RCC compared with non-aggressive primaries [67]. Validation by quantitative reverse transcription PCR revealed SAA among three candidate biomarkers with most significant upregulation [67]. SAA transcripts and/or SAA protein were found in different cell lines of tumour origin, e.g. adrenal cortex carcinoma (SW13), ileoceal carcinoma (HCT-8), and oral epidermal carcinoma (KP) [52, 68]. SAA expression was also observed in colonic adenocarcinoma cells (CaCo-2), colonic carcinoma cell lines (colo-205 and T-84), and in neoplastic human colonic mucosa [69]; the local and differential expression of SAA in human colon cancer tissues suggests its role in colonic tumourigenesis and may have both prognostic and therapeutic applications. SAA is also highly expressed in the colorectal carcinoma cell line SW620 [70].
SAA1/2 transcripts are expressed in highly proliferating trophoblast-like choriocarcinoma cell lines (JAR and Jeg-3) and non-malignant first trimester trophoblasts, supporting the notion that SAA is a product of local inflammation probably contributing to cholesterol homeostasis during embryonic development [71]. Also, osteoblast-like human osteosarcoma cell lines (SAOS-2 and MG-63) express SAA1/2 transcripts upon cytokine-mediated stimulation; SAOS-2 cells express both SAA transcripts even under non-stimulating conditions [72]. Most importantly, SAA activating factor-1 (SAF-1), a transcriptional regulator of SAA, is also expressed in osteosarcoma cells, prior to and post cytokine stimulation [72]. In addition, SAA1/2 transcripts are present in non-differentiated human embryonic stem cells, but to a higher extent in culture-expanded human embryonic stem cells differentiated to osteogenic lineage [72]. The expression of SAA in normal and cancerous extrahepatic tissues has been confirmed by immunohisto- and immunocytochemistry [69, 71–73] and the predominant localization of SAA is the epithelium [73].
SAA and its role as a candidate tumour-specific surrogate biomarker
Recent developments in cancer biotherapy introduced protein profiling as a high-throughput platform that allows the simultaneous analysis of multiple low-molecular weight proteins from small quantities of material. Among different techniques, mass spectrometry (MS) may serve as a diagnostic and a cancer biomarker discovery tool [74] and matrix-assisted laser desorption and ionisation time-of-flight (MALDI-TOF) and surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF) MS can profile proteins in the low-molecular-weight range [75].
SAA was identified as a biomarker in serum that distinguished prostate cancer patients with and without bone lesions (bone metastases) using SELDI-TOF-MS and two-dimensional gel electrophoresis, ingel trypsin digestion and tandem MS; SAA identification was confirmed by ELISA and immunodepletion experiments [76]. In the same report, prostate specific antigen serum concentrations were reported to be useful for initial diagnosis; however, when used alone prostate specific antigen can be associated with a high degree of false positives and cannot predict the presence of metastases. Li and coworkers [77] suggested the 11.7 kDa SAA protein as one of the plasma proteins in the proteomic signature able to differentiate between pediatric osteosarcoma and benign osteochondroma.
Using the SELDI-TOF-MS technique, the 11.7 kDa SAA protein has been identified in plasma as a human ovarian cancer marker [78] and as an important component of the proteome diagnostic profiling in ovarian cancer [17]. Performing serum proteomic patterns for human ovarian cancer, Helleman and coworkers [79] discovered eight potential biomarkers, among them SAA, for monitoring disease progression. In both studies [78, 79], SELDI-TOF-MS data for the 11.7 kDa SAA protein and the SAA immunoassay were combined with cancer antigen 125 data. SELDI-TOF-MS analyses confirmed haptoglobin and SAA as differentiating proteins in serum from RCC patients (n = 25) compared to their normal counterparts (n = 26) [80]. Of particular emphasis is the detection of a cluster of multiple SAA peaks; in addition to the 11.7 kDa peak, a 11.5 kDa (SAA lacking the N-terminal arginine) and a 11.4 kDa peak (SAA lacking the N-terminal arginine-serine residues) have been identified [80]. This is of importance as the corresponding des-arginine forms (11.5 kDa) have been used previously to identify SAA1, SAA2 or other isoelectric focusing SAA variants [10, 81]. Using the SELDI-TOF-MS technique, the 11.7 kDa and 11.5 kDa peaks of SAA have further been identified in serum of RCC patients after rIL-2 treatment [82]; this observation parallels high SAA levels measured in cancer patients following rIL-6 therapy [34, 35]; however, it is indicative that SAA may not necessarily act as a candidate biomarker in RCC [80].
Engwegen and coworkers [83] further confirmed the expression of SAA and the corresponding peak cluster in two sets of RCC; the first set (40 RCC patients and 32 healthy controls) consisted of mainly pre-surgery samples from patients with disease stage I-IV, while the second set (26 RCC patients and 27 healthy controls) were mostly sera from patients with stage-IV disease, drawn after nephrectomy. The SAA peak cluster (11.7, 11.5, and 11.4 kDa) was validated as a robust RCC biomarker candidate [83]. This peak cluster is also present in sera from patients undergoing laparoscopic colon resection to remove a colon carcinoma [84] and during plasma protein profiling for diagnosis of pancreatic cancer [85] when SELDI-TOF-MS or MALDI-TOF-MS was used. Applying the PVDF-aided MALDI-TOF-MS technique, the same SAA peak cluster was observed with high SAA levels in a group of gastric cancer patients [41]. When performing a serum proteome profile that discriminates lung cancer patients from matched controls, the same SAA peak cluster (indicative for N-terminal fragmentation) was found by MALDI-based analysis [86]. Using SELDI-TOF-MS techniques, SAA (11.7 kDa) and transthyretin were identified in a group of proteins specific for RCC, although the value of SAA in monitoring and therapy response needs evaluation in further studies [87–89]. Furthermore, the SELDI-TOF-MS technology was useful to identify SAA as a candidate serum biomarker that strongly correlates with prognosis in neuroblastoma, the most common extra-cranial solid tumour in children [90]. Serum protein profiling using the MALDI-TOF-MS technology revealed SAA among four proteins up-regulated in patients with stage IV neuroblastoma [91].
A comprehensive, longitudinal follow-up study using serum proteomic profiling suggested SAA as a useful marker to monitor relapse of nasopharyngeal cancer with a gradual elevation of SAA occurring on metastasis of nasopharyngeal cancer to bone, lung or liver and a dramatic decrease in the protein to background level with chemotherapy [92, 93]. Two-dimensional gel electrophoresis and MALDI-TOF-MS analysis revealed SAA as a potential nasopharyngeal carcinoma metastasis-specific serum biomarker [94]; ELISA and immunohistochemistry confirmed identification of SAA. In lung squamous cell carcinoma patients, SAA was one out of ten proteins with high abundance level in sera [95].
SAA and its role in tumour pathogenesis
SAA proteins are highly conserved in vertebrates with respect to their sequence and inductive capacity [6–8]. Their function in inflammation seems to be one of a protective nature and is over-ridingly necessary in the acute-phase response and probably also as an immune-effector molecule [7]. However, in chronic diseases the role of SAA becomes one with adverse effects. There is a strong relationship between inflammation and cancer progression and it will be further interesting to determine whether cancer tissue-derived cytokines stimulate SAA synthesis in liver or epithelial cells.
Regulation of SAA
Expression of SAA is primarily regulated at the transcriptional level; cytokines, e.g. IL-1, IL-6, and tumour-necrosis factor α (TNFα), or glucocorticoids, alone or in combination, act by binding to their respective receptors. As a consequence, induction of a series of transcription factors (CCAAT/enhancer binding proteins, NF-κB, and SAF-1), either by activation of resident pools of inactive factors in the cytoplasma and/or by increased factor biosynthesis, occurs; alternatively, post-transcriptional regulation of human SAA genes has been reported [17, 96]. rSAA may activate transcription factors mentioned above [97, 98]. SAA (purified from plasma, rSAA or the rSAA-HDL complex [99]) exerts cytokine-like properties by promoting secretion of IL-1β, IL-1 receptor antagonist, IL-8, IL-10, IL-12, IL-23, and TNFα from granulocytes [97, 100], lymphocytes [101], and monocytes/macrophages [98, 99, 102].
The influence of elevated SAA on cancer development
Besides known utilities of SAA as an inflammatory and clinical marker [10], its role of modulating properties of HDL during the inflammation [103], and its association with amyloidosis [104], accumulating evidence supports a role of SAA in human malignancies. A potential role of SAA in tumour pathogenesis may be deduced from its likely role of acting as an extracellular matrix (ECM) adhesion protein. The ECM is a highly organized network of proteins, glycoproteins, hyaluronan and proteoglycans, which form a molecular scaffold that provides mechanical strength, elasticity, and binding sites for cells and also controls the diffusion and migration of cells. Alterations in ECM composition and its degradation have a major influence on tumour initiation and development. SAA contains binding sites for ECM-components, laminin [105] and heparin/heparan sulfate [106] and has YIGSR-like and RGD-like adhesion epitopes (residues 29–42), that correspond to laminin and fibronectin cell-binding domains, respectively. Sequence-specific antibodies raised against various synthetic peptides of SAA were used as an immunological tool to identify surface-located epitopes present on lipid-free and lipid(HDL)-associated SAA [107, 108]. A hypothetical structural model for SAA further suggested that the proposed binding sites for laminin, fibronectin, and calcium are segregated to one face of the molecule and that the heparin/heparan binding site is located in the putatively disordered region of the protein [106, 109]. Via these adhesion motifs, SAA may interact with ECM [110, 111], thereby changing the affinity of different cell types to ECM. This implies the role of SAA in various pathologic conditions, including cancer. Either synthetic peptides (corresponding to SAA residues 29–42), rSAA (corresponding to human SAA1) or human AA protein inhibited the binding of human T-lymphocytes and mouse M-4 melanoma cells to surfaces coated with laminin or fibronectin [110]. The inhibitory effects of SAA and SAA-peptides on cell adhesion to glycoproteins of the ECM might have a role in metastasis, by inhibiting the adhesion of tumour cells to the ECM.
SAA interacts specifically with the vessel wall and ECM-linked glycoprotein moieties (including laminin, but not fibronectin or collagen), attaching temporarily to those matrix substrates and thereby providing proadhesive stimuli for resting CD4+ T cells [112]. SAA-ECM complex (achieved via binding of SAA domain in the 2–82 region to laminin) is also shown to enhance secretion of TNF-α by human T lymphocytes in a dose-dependent manner [112]. Similarly, SAA influences murine mast cell adhesion to ECM or laminin [113]. rSAA binds to mast cells, and when preincubated with ECM, SAA and its amyloidogenic AA fragment (also containing an RGD-like adhesion epitope), cause the binding of inactive mast cells to ECM or laminin, at least partly through an integrin recognition site [113, 114]. The ECM is known to affect intracellular signalling mostly mediated by interactions between the matrix within the pericellular microenvironment and integrins on the cell surface.
Furthermore, SAA may modulate platelet adhesion and influences adhesion of tumour cells to platelets. Immobilized SAA supports adhesion of washed platelets, which may further be induced by Mn2+ and thrombin. SAA-derived peptide 29–42 (containing an incomplete RGD motif) inhibits platelet adhesion to fibronectin, and the adhesion of human platelets to SAA occurs in an RGD-and αIIββ3-dependent manner [111, 115]. The tetrapeptide RGDS inhibits adhesion of tumour cells to platelets in the same manner [116]. Thus, SAA produced locally at the sites of tumour invasion may modulate adhesion of tumour cells to platelets.
Michaeli and coworkers [117] recently reported that SAA enhances plasminogen activation, a process that points towards a potential role of SAA in colon cancer progression. Indeed, plasminogen activation, i.e. conversion of plasminogen to the active serine protease plasmin, is a feature of many physiologic and pathologic processes involving ECM degradation and tissue remodelling, including inflammation and tumour metastasis. Although the main effects of inflammation on tumour development are exerted at the promotion and progression stages, it is also accepted that chronic inflammation might enhance tumour initiation. Perhaps the most compelling clinical evidence for a causative link between chronic inflammation and cancer development comes from epidemiological studies reporting that usage of anti-inflammatory drugs such as selective cyclooxygenase-2 inhibitors significantly reduces cancer risk. This indicates that cyclooxygenase-2 could act as a mediator per se (at least in colon cancer) [118] or in concert with other key molecules involved in prostaglandin biosynthesis, and might thus be effective anticancer targets. One of the crucial molecules that mediate these effects is the prostaglandin E2 receptor EP2 subtype; however, SAA has been reported to induce expression of cyclooxygenase and to promote formation of cyclooxygenase metabolites, e.g. prostaglandin E2, in neutrophils and preactivated monocytes [119, 120]. A second pathway that, however, favours induction of apoptosis and downregulation of cyclooxygenase-2 occurs via activation of peroxisome-proliferating activated receptor-γ. Clinical trials have further shown that specific peroxisome-proliferating activated receptor-γ ligands reduce serum concentrations of SAA and matrix metalloproteinases (MMPs), in particular MMP-9 [121].
Indeed, one of the main causes of ECM alterations during cancer development is its degradation by MMPs. MMPs, a family of zinc-dependent endopeptidases, are not only capable of targeting the ECM, but also influence growth factors, cytokines and cell surface-associated adhesion and signalling receptors. MMPs are important factors in tissue remodelling during various physiological responses including embryogenesis, wound healing and bone tissue formation. Because of their role in ECM degradation and angiogenesis, and the ability to initiate epithelial-mesenchymal transition and genomic instability, aberrant expression of MMPs contributes to pathogenesis in various diseases, including cancer [122]. An increased expression of MMPs in human malignant tissue has often been correlated with poor prognosis. Elevated expression of many MMPs, including MMP-1, -2, -3, -7, -9, -13, -14, in both primary tumours and/or metastases, are positively associated with tumour progression, i.e. poor tumour differentiation, invasive stage of cancer, poor prognosis, metastasis and shorter survival time [123].
Several reports suggest a link between the expression of SAA and MMPs. Different members of the MMP family (MMP-1, MMP-2, MMP-3 and MMP-9) have been identified in tissue sections of amyloidotic patients [124], and both, SAA (purified from human plasma) and AA protein (isolated from spleenic tissue), are prone to be cleaved by MMP-2 and MMP-3 in vitro [125]. These findings not only imply a role of MMPs in amyloidosis, but also point towards a potential negative feedback mechanism of MMPs in the regulation by SAA.
Since cancer is often seen as a consequence of chronic inflammation, SAA might influence tumour invasion through the ECM by stimulating the production of MMPs. An increasing number of studies report the induction of different MMPs by rSAA. Recently, Lee and coworkers [126] showed that rSAA selectively stimulated the production of MMP-9 in THP-1 cells and human monocytes. SAA-induced upregulation of MMP-9 was mediated via the formyl peptide receptor like-1 (FPRL-1) and was achieved at the transcriptional level via NF-κB.
In synovial fibroblasts from patients with rheumatoid arthritis, SAA stimulated the production of MMP-2 and MMP-3 in a dose-dependent manner, whereas pre-treatment of cells with cycloheximide or immunodepletion of SAA-containing media by anti-SAA-specific antibodies prevented SAA-mediated MMP-2 and MMP-3 secretion [127]. When stimulated with rSAA, fibroblast-like synoviocytes from patients with inflammatory arthritis had increased production of MMP-1 and MMP-3 by a mean of 2.5-fold and 9-fold, respectively, 24 h upon stimulation [128]. The fact that IL-1β-induced MMP-1 and MMP-3 production was observed after 12 h, and that MMP levels were over three-fold greater than SAA-induced levels suggested the possibility that SAA-induced MMP production might be mediated through IL-1 signalling pathways. Furthermore, rSAA was shown to induce expression of intercellular adhesion molecule 1 and vascular cell adhesion molecule 1, as well as the expression of MMP-1 on human microvascular endothelial cells and synovial fibroblasts from patients with rheumatoid arthritis, a mechanism that is, at least partly, regulated through the NF-κB/IκB signalling pathway. In consequence, the binding of peripheral blood monocytes to human microvascular endothelial cells and synovial fibroblasts from patients with rheumatoid arthritis increased, and angiogenesis through endothelial cell migration and tubule formation was induced [129].
Most importantly, both SAA and MMPs may be activated by SAF-1, a transcription factor similar to human MAZ zinc finger protein. SAF-1 is known for cytokine-mediated induction of SAA genes [130], and recently has been found responsible for the upregulation of MMPs [131–133]. SAF-1 was found to interact with a novel promoter element in the human MMP-1 gene, and overexpression of SAF-1, in human and canine chondrocytes of osteoarthritic cartilage, led to an increase in the activity of the MMP-1 promoter. This effect could be inhibited by an interference of endogenous SAF-1 activity by antisense SAF-1 messenger RNA [131]. Mouse MMP-14 promoter has also been shown to contain the DNA-binding site for SAF-1. When THP-1 cells were transfected with an SAF-1 expression plasmid, the transcription from MMP-14 promoter and the level of endogenous MMP-14 protein increased. The fact that MMP-14 promoter was markedly reduced, but not completely inhibited with an antisense SAF-1 oligonucleotide, identified SAF-1 as one of the mediators of MMP-14 promoter activation [132]. Similarly, SAF-1 binding elements were identified in the MMP-9 promoter of rabbit synoviocyte cells and human chondrocytes [134]. The high SAF-1 expression in differentiated over non-differentiated osteoblast-like human mesenchymal stem cells suggested that differentiated cells contain nuclear factor(s) that synergize SAF-1 activity [72]. SAF-1 is also constitutively expressed in human MG-63 osteosarcoma cells, while the presence of cytokines led to a decrease in expression of SAF-1 in these cells [72].
Receptors for SAA
FPRL-1/ALX
Local expression of SAA at the RNA and protein levels is tightly coupled with induced transcription of MMPs [135] and expression of FPRL-1 or genes in disease [128]. Indeed, SAA has been reported to be a specific ligand for FPRL-1 [136, 137]. FPRL-1, found to be identical with the lipoxin A4 (LXA4) receptor (termed ALX) [138] has also been shown to mediate SAA-induced IL-6, IL-8 and MMP-3 protein release and to up-regulate NF-κB and AP-1 DNA binding activity in fibroblast-like synoviocytes [139]. Most importantly, Kd-values for LXA4 (0.1–1.0 nM [140]) and SAA (20–40 nM, [136]) are markedly different.
ALX undergoes post-translational glycosylation as predicted by putative N-glycosylation sites and shown by the apparent molecular mass of the expressed protein of ~75 kDa (SDS-PAGE) vs. the expected size of ~40 kDa (deduced from the primary structure containing 351 amino acids) [141]. Treatment of human fibroblast-like synoviocytes with glycosidase PNGaseF attenuated SAA-dependent NF-κB activation, presumably by causing reduced ALX N-glycosylation, while sparing LXA4-dependent inhibition of the IL-1β response (Sodin-Semrl et al., unpublished). These findings are compatible with results from an earlier study indicating that LXA4 binding to its receptor was insensitive to glycosidase treatment [140] but targets the seventh transmembrane domain of ALX [142]. These observations thus suggest that receptor interactions of SAA and LXA4 involve different domains of ALX, with SAA (but not LXA4) requiring the glycosylated extracellular loops of the receptor.
SAA may contribute to inflammation by rescuing neutrophils from apoptosis. SAA suppression of neutrophil apoptosis is mediated via phosphorylation of the mitogen-activated protein kinase (MAPK) p42/44 and Akt signalling, and it can be reversed by aspirin-triggered LXA4 [143]. Culturing neutrophils with 15-epi-LXA4 alone, however, failed to produce changes in the development of apoptosis [143]. This is in contrast to LXA4-stimulated apoptosis in rat fibroblasts as reported earlier [144] and implies organism-as well as cell-specificity. Both LXA4 and 15-epi-LXA4 can facilitate phagocytosis of apoptotic neutrophils by macrophages, specifying a role in the clearance mechanisms [145, 146]. SAA may further rescue human neutrophils from constitutive apoptosis by preventing mitochondrial dysfunction and subsequent activation of caspase-3 [147]. However, SAA may also inhibit apoptosis in human neutrophils via an FPRL-1/ALX-independent pathway [148]. Contrary to human neutrophils, overexpression of SAA1/2 isoforms in mouse mammary epithelial cells accelerated their apoptosis by increasing caspase activity [149] indicating a different mechanism by which SAA influences epithelial cell death and tissue remodelling. The binding of SAA to FPRL-1 may stimulate upregulation of MMPs in human monocytic THP-1 cells [126]. An association between overexpressed SAA and FPRL-1 with the production of MMPs in inflamed synovial tissue has been determined [128] and SAA has been shown to induce synovial hyperplasia that can lead to the development of pannus. The rheumatoid pannus has some properties that are similar to those exhibited by localized tumour: synovial fibroblasts from rheumatoid arthritic patients can proliferate abnormally, resist apoptosis, invade the local environment [150, 151] and may have somatic mutations in the p53 tumour suppressor gene and point mutations in the oncogene H-ras [152, 153]. Lee and coworkers [154] showed that rSAA acts to protect rheumatoid synoviocytes against apoptosis via FPRL-1. This mechanism appears to occur via phosphorylation of p42/44 and Akt signalling pathway, similarly to the mechanism occurring in neutrophils [143].
FPRL-1/ALX, a member of the chemoattractant subfamily of G protein-coupled receptors, is involved in regulating leukocyte migration in inflammation. Chemoattractant properties of SAA on monocytes, neutrophils, and T-cells have been demonstrated [155, 156] and several physiological processes that are necessary for tumour development, such as increased cell survival, tissue remodelling, angiogenesis and suppression of anti-tumour adaptive immune responses, are regulated by leukocytic infiltrates in neoplastic environments. Recently, it has been reported that SAA can mediate the production of reactive oxygen species in primary human neutrophils independently of FPRL-1/ALX [157].
SR-BI and ABCA1
Numerous studies carried out on tumour cell lines, experimental tumours, and human tumours have shown an abnormal cholesterol metabolism that is reflected by an increase in intracellular cholesteryl esters [158, 159] apparently via receptor-mediated uptake of cholesteryl esters from circulating plasma lipoproteins, primarily HDL [159–161]. SR-BI, a class B type I scavenger receptor, is considered the prime receptor for native HDL. SAA as apoA-I, the major apolipoprotein of HDL under physiological, i.e. non-inflammatory, conditions, is a high affinity ligand for SR-BI and is efficiently internalized by transfected cells in an SR-BI-dependent manner. As a consequence, markedly enhanced levels of phosphorylation of the MAPK p42/44, p38, and JNK [162] have been reported. SAA, when present on HDL, did not affect interaction of the lipoprotein particle with SR-BI, although it did not reduce SR-BI-mediated selective lipid uptake from HDL [163, 164]. The selective uptake of cholesteryl esters by monocytes from acute-phase HDL (containing between 22% and 42% SAA in the total apolipoprotein content) is higher compared to native HDL [165]; most importantly, expression of SR-BI is upregulated in human monocytes and macrophages during inflammatory conditions and high SR-BI expression has been demonstrated in lipid-laden macrophages in human atherosclerotic lesions [166] where SAA is also present [60].
A major physiological function of native HDL is to promote cellular cholesterol efflux. However, HDL experimentally remodelled with purified human SAA showed a decreased cellular cholesterol efflux from human monocytic THP-1 cells [167]. A decrease in cellular cholesterol efflux by acute-phase HDL, compared with native HDL, from cells of hepatic origin might be compatible with the role of SR-BI mediating bidirectional cholesterol flux [168]. Indeed, SAA has been reported to promote cellular cholesterol efflux via SR-BI [169]; however, the lipidation status of SAA, an amphipathic apolipoprotein similar as apoA-I, seems to be a critical factor governing its cholesterol acceptor properties [164]. SAA and apoA-I mediate cellular cholesterol efflux via SR-BI and/or the human ATP binding cassette protein ABCA1 [164, 170–172]. In principal, ABCA1 and SR-BI may act in concert; ABCA1 is involved in the lipidation process (cholesterol and phospholipids) of the corresponding apolipoprotein (apoA-I and/or SAA), while SR-BI may promote cholesterol flux to the lipidated HDL-like particles. Recent in vivo experiments performed in abca1-/- mice have confirmed that SAA and apoA-I generate HDL largely in hepatocytes only in the presence of ABCA1; both apolipoproteins will be secreted in a lipid-free form and may then interact with cellular ABCA1 [172]. Most importantly, human SAA binds cholesterol [173] apparently via its amphipathic helical content in the N-terminus [109, 174]. However, the role(s) of SAA during acute inflammation to either enhance cholesterol removal from sites of tissue destruction and/or to direct HDL during the acute-phase to deliver phospholipids and cholesterol/cholesteryl esters to cells involved in tissue repair at sites of inflammation has/have not been completely resolved yet [175, 176].
RAGE
Recently, the receptor for advanced glycation end products (RAGE), has been identified to bind SAA [177]. This multiligand receptor of the immunoglobulin superfamily also binds, in addition to nonenzymatically glycated adducts, the ß-sheet fibrils characteristic of amyloid, pro-inflammatory cytokine-like mediators of the S100/calgranulin family, and amphoterin, a nuclear protein sometimes found in the ECM [178]. Activation of cell-surface expressed RAGE by extracellular ligands results in a specific signalling cascade ultimately leading to the activation of NF-κB and MAPK. Increased expression of RAGE is associated with a number of pathological conditions (e.g. rheumatoid arthritis, atherosclerosis and some tumours) with high SAA concentrations. RAGE has been reported to bind human rSAA1 and to promote expression of monocyte tissue factor via activation of NF-κB through the p42/44 and p38 MAPK pathway [177]. An elevated SAA level is considered a marker of disease activity in patients with rheumatoid arthritis, and peripheral blood monocytes from these patients were more reactive to SAA than normals; this suggests a new link between inflammation and thrombosis via the SAA-RAGE axis. SAA also binds to sRAGE [177], the soluble and extracellular form of RAGE. Thus, sRAGE may be a promising therapeutic target to prevent vascular damage as a consequence of chronic inflammatory conditions by acting as a decoy for circulating RAGE ligands [178]. Indeed, preincubation of SAA with sRAGE prevented SAA-induced IκBα degradation, suggesting that the SAA-RAGE-NF-κB axis is operative in rheumatoid synovial fibroblasts [179]. Yan and coworkers [180] further reported that 125I-labelled-RAGE specifically binds to murine SAA1 (forming amyloidogenic fibrils) and SAA1-derived AA fibrils (isolated from mouse spleenic tissues) but not to murine SAA2 [180]. Most importantly, incubation of murine microglial BV2 cells with SAA1 fibrils resulted in nuclear translocation of NF-κB [180].
Other receptors
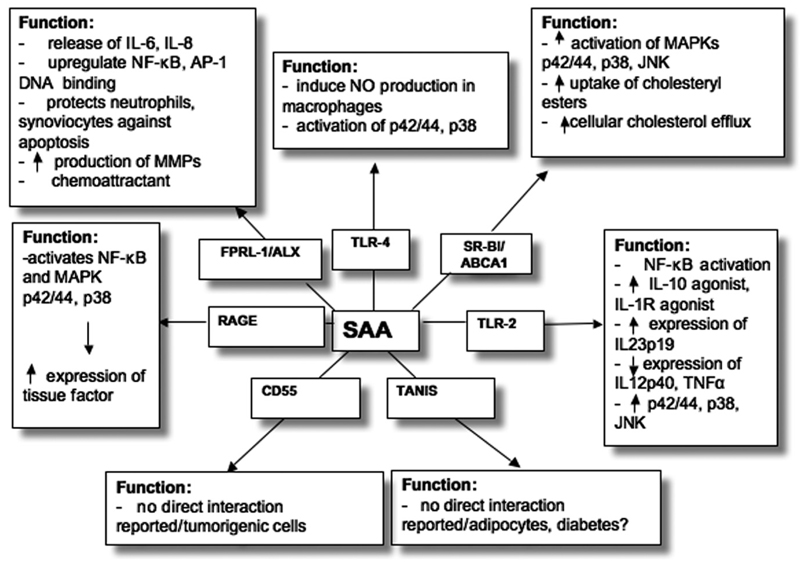
In addition to FPRL-1/ALX, SR-BI/ABCA1, and RAGE (see Fig. 1 for overview) other receptors have been identified to bind/interact with SAA. In line with observations that the glycosylphosphatidylinositol-anchored protein CD55 (also termed decay accelerating factor) acts as binding protein for native HDL [181], a binding protein that comigrates with CD55 was identified for SAA-enriched (acute-phase) HDL on macrophages [165]. Although CD55 is abundantly expressed in malignant tumours [182] where high SAA concentrations may occur, no direct interaction of SAA with CD55 in relation to tumour cell growth or the malignant potential of cancer cells has been reported.
Figure 1.
Candidate SAA receptors with selected biological activities; for details see text.
Another SAA binding protein/receptor, identified by yeast two-hybrid screening, is TANIS [183]. TANIS, a membrane selenoprotein predicted to have a single transmembrane region close to its N-terminus, was shown to be regulated by glucose and is differentially expressed in type-2 diabetes.
Recent findings revealed that SAA could act as an endogenous agonist for the Toll-like receptor 4 (TLR4) [184]. SAA stimulated macrophage production of nitric oxide radical in a TLR4-dependent manner that requires phosphorylation of p42/44 and p38 MAPK but not the myeloid differentiation factor 88 pathway [184]. Macrophages from C3H/HeJ and C57BL/10ScCr mice lacking this functional receptor complex (associated with innate immunity) did not respond to SAA stimulation. In contrast to these observations, Cheng and coworkers [185] reported that TLR2, but not TLR4, is involved in NF-κB activation, a process that promotes phosphorylation of all three major MAPK, p42/44, p38, and JNK, respectively. A neutralizing antibody against TLR2 significantly reduced SAA-stimulated NF-κB activation in TLR2 overexpressing HeLa cells. SAA-induced expression of the two anti-inflammatory cytokines IL-10 and IL-1 receptor antagonist was completely abrogated in bone marrow-derived macrophages from tlr2-/- mice. However, expression of IL-12p40 (a common subunit shared by immunomodulatory cytokines IL-12 and IL-23) and TNFα was partially reduced [185]. The expression of IL-23p19 (a unique subunit of IL-23, a member of the IL-12 family) by SAA and TLR2 contribute further support that SAA may act as an endogenous ligand for IL-23 expression [98].
SAA levels in tumour bearing mice
The link between cancer and inflammation in an organ or tissue has firmly been established on the basis that cancer tends to occur at sites of chronic inflammation and that local inflammatory processes can accelerate the growth of the pre-existing tumours not only in humans but also in animals. Serum SAA concentrations in CD1 mice transplanted with S-180 mouse sarcoma cells or two other tumours (MN MCA/1 and mFS6 sarcomas) were markedly increased from day three following transplantation and reached a plateau at about day six [186]. Surgical removal of the tumour decreased SAA levels similar to those in control mice within three days of surgery, while SAA levels in recipient mice injected with serum from tumour-bearing mice were massively increased [186]. Subcutaneous injection of cells of the mastocytoma line P815 in syngenic DBA/2 mice revealed elevated SAA plasma concentrations starting at day five and decreasing to normal values around day 21 [187]. Injection of human pancreatic cancer cell line L3.9pl into the pancreas of an orthotopic nude mouse model identified significant positive correlation between tumour weight and expression of the 11.7 kDa SAA protein in serum, clearly identified by protein profiling using SELDI-MS, N-terminal sequencing, and Western blot analysis [188]; ELISA experiments revealed a significant positive correlation between tumour weight and serum SAA levels [188]. In another tumour model, BALB/c mice inoculated with Line-1 (a weakly immunogenic tumour cell line derived from a spontaneously arising lung alveolar carcinoma of the BALB/c mouse) expressed elevated levels of SAA, but not IL-1β or TNF-α, in their sera in comparison to naïve mice [189]. While the SAA levels within each group displayed considerable variability, the correlation between primary tumour size and SAA levels was significant. As an SAA level of > 0.05 ng/ml was predictive for the presence of metastatic lung tumour, SAA has been considered to be more useful as a diagnostic (tumour vs. no tumour) than a quantitative marker for metastatic disease in this model [189]. Using a surgical spontaneous metastasis model as a clinically relevant tool for the evaluation of anti-cancer therapy, comparative time-dependent analysis of potential inflammation biomarkers in RcsX lymphoma-bearing SJL mice revealed SAA as a representative within a group of highly up-regulated genes [190]; protein digests were analyzed by LC-tandem MS technique and resulting data were processed by ProQuant and Spectrum software packages [191]. Identification of tumour-associated plasma proteins using proteomic analysis (two-dimensional gel electrophoresis and MS techniques) revealed overexpression of haptoglobin and SAA in plasma only in nude mice subcutaneously inoculated with SC-M1 cells (human adenocarcinoma), while inoculation with HONE-1 (human nasopharyngeal carcinoma), CC-M1 (human colon adenocarcinoma), OECM1 (human oral cancer), and GBM 8401 (human glioblastoma multiforme) was accompanied by expression of haptoglobin only [192]. These authors [192] further suggested that acute-phase proteins may be used as non-specific tumour-associated serum markers, but SAA may serve as a potential marker for detecting stomach cancer. SAA is also expressed in As4.1 cells isolated from a kidney tumour from transgenic mice [193]. Intraperitoneal injection of C6 (rat astrocytoma) cell supernatant into C57BL/10 ScCR mice induced production of SAA [194]. To acquire further insight into the pre-clinical relevance of biomarkers in identifying neuroblastoma, nude mice were inoculated with a human neuroblastoma cell line (SK-N-DZ); SAA was among five proteins overexpressed in neuroblastoma tumour-bearing mice [91].
In a murine model of fibrosarcoma, tumour growth was paralleled by a massive increase in SAA concentrations reaching levels of 5 mg/ml serum 35 days after tumour growth [195]. Intraperitoneal injection of a single dose of dexamethasone (0.75 mg/kg) reduced parameters of systemic inflammation to almost baseline levels. Furthermore, partial hepatectomy in C57BL6 mice revealed SAA as one out of 12 proteins – identified by two-dimensional gel electrophoresis, MS, and mass fingerprinting – to be upregulated at least two-fold, indicating that liver regeneration followed by partial hepatectomy affects various signalling and metabolic pathways [196].
Future perspectives
There is a critical need for cancer biomarkers in order to make clinical decisions about patient treatment early and aid in shortening the development time for vaccines. It has become very clear that tumour markers with absolute specificity are not currently available. This lack of biomarkers is also a result of problems associated with their validation and development. A key difficulty is that tumour markers are also expressed in normal/healthy tissue or in other disease states at elevated concentrations. However, in addition to those used for diagnosis, there is a need for prognostic biomarkers and biomarkers that can stratify patients into potential chemoradiotherapy responders and non-responders [197]. During treatment, signatures or profiles of multiple markers are generally necessary to distinguish between development and progression of various types of cancers. Clusters of biomarkers, when used in combination, can achieve a diagnostic specificity and sensitivity greater than single markers [92]. We will increasingly rely on bioinformatics tools that allow for the diagnosis, prognosis and monitoring of cancer using clusters of cancer associated biomarkers with well-defined specificities [198] (author’s response). A line of thought is that elevated SAA levels are cancer epiphenomena, unlikely to be of clinical use for more effective diagnoses and monitoring of cancer [74, 198, 199]. Elevated serum SAA might be a primary product of tumour lesions, but could, additionally or alternatively, be the product of hepatocytes. The aim of this review reaches beyond the purpose of promoting SAA as a surrogate biomarker for cancer, and it does not promote a certain analytical technique as a suitable diagnostic and discovery tool for its analysis [74]. However, the elevation of SAA during cancer relapse is so high that its role in disease monitoring in cancer patients is not only warranted but also critical, especially when monitoring disease outcome and survival prediction. A number of studies has underscored that SAA is involved in tumourigenesis, apparently due to its capacity to interact with the ECM. Alternatively, a potential role of SAA is its protection from infection [200] and its capacity to bind to a surprisingly large number of Gram-negative bacteria [201]. Although a number of key molecular players linking cancer to inflammation have been identified and chronic inflammation is a common and important factor in the pathogenesis of neoplasia [1, 2], the whole story between inflammation and cancer is still far from being completely understood. For instance, the question regarding the intriguing feedback loop between cytokines and NF-κB is, which activation is the initial event [3]? Future studies may reveal the underlying mechanisms for how SAA may promote tumour development and accelerate tumour progression and metastasis [3]. Animal models for inflammation-derived cancers in combination with molecular approaches, such as specific saa-/- animals or mice overexpressing SAA [202] even in specific tissues, will be helpful. Clinical studies and basic research may further help to clarify whether SAA may act as an individual biomarker for the detection, the monitoring of disease activity, and the staging of specific types of cancer.
Acknowledgements
The authors thank Dr. A.S. Whitehead (Philadelphia, PA, USA) and Dr. W. Sattler (Graz, Austria) for helpful comments and suggestions. This work was supported by grants from the Austrian Science Fund (FWF, P14186-B05 and P19074-B05) to E.M.
Footnotes
Note added in proof. Using two-dimensional mass fingerprinting and SELDI-TOF analysis, He and coworkers [203] reported that the 11.6 kDa SAA protein might be considered as one out of three specific biomarkers for hepatitis B virus hepatocellular carcinoma. The authors would like to recognize Dr. Urieli-Shoval in being instrumental by bringing to the foreground the novel field of SAA and tumorigenesis in her published articles.
References
- 1.Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006;56:69–83. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 4.Bhardwaj N. Harnessing the immune system to treat cancer. J Clin Invest. 2007;117:1130–1136. doi: 10.1172/JCI32136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malle E, Steinmetz A, Raynes JG. Serum amyloid A (SAA): an acute phase protein and apolipoprotein. Atherosclerosis. 1993;102:131–146. doi: 10.1016/0021-9150(93)90155-n. [DOI] [PubMed] [Google Scholar]
- 7.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 8.Uhlar CM, Burgess CJ, Sharp PM, Whitehead AS. Evolution of the serum amyloid A (SAA) protein superfamily. Genomics. 1994;19:228–235. doi: 10.1006/geno.1994.1052. [DOI] [PubMed] [Google Scholar]
- 9.Sipe J. Revised nomenclature for serum amyloid A (SAA). Nomenclature Committee of the International Society of Amyloidosis. Part 2. Amyloid. 1999;6:67–70. doi: 10.3109/13506129908993291. [DOI] [PubMed] [Google Scholar]
- 10.Malle E, De Beer FC. Human serum amyloid A (SAA) protein: a prominent acute-phase reactant for clinical practice. Eur J Clin Invest. 1996;26:427–435. doi: 10.1046/j.1365-2362.1996.159291.x. [DOI] [PubMed] [Google Scholar]
- 11.Husby G, Marhaug G, Sletten K. Amyloid A in systemic amyloidosis associated with cancer. Cancer Res. 1982;42:1600–1603. [PubMed] [Google Scholar]
- 12.Pras M, Franklin EC, Shibolet S, Frangione B. Amyloidosis associated with renal cell carcinoma of the AA type. Am J Med. 1982;73:426–428. doi: 10.1016/0002-9343(82)90747-1. [DOI] [PubMed] [Google Scholar]
- 13.Pepys MB. Amyloidosis. Annu Rev Med. 2006;57:223–241. doi: 10.1146/annurev.med.57.121304.131243. [DOI] [PubMed] [Google Scholar]
- 14.Biran H, Friedman N, Neumann L, Pras M, Shainkin-Kestenbaum R. Serum amyloid A (SAA) variations in patients with cancer: correlation with disease activity, stage, primary site, and prognosis. J Clin Pathol. 1986;39:794–797. doi: 10.1136/jcp.39.7.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu DH, Wang XM, Zhang LJ, Dai SW, Liu LY, Liu JF, Wu SS, Yang SY, Fu S, Xiao XY, He DC. Serum amyloid A protein: a potential biomarker correlated with clinical stage of lung cancer. Biomed Environ Sci. 2007;20:33–40. [PubMed] [Google Scholar]
- 16.Howard BA, Wang MZ, Campa MJ, Corro C, Fritzgerald MC, Patz EF., Jr Identification and validation of a potential lung cancer serum biomarker detected by matrix-assistend laser desoption/ionization-time of flight spectra analysis. Proteomics. 2003:1720–1724. doi: 10.1002/pmic.200300514. [DOI] [PubMed] [Google Scholar]
- 17.Vlasova MA, Moshkovskii SA. Molecular interactions of acute phase serum amyloid A: possible involvement in carcinogenesis. Biochemistry (Mosc) 2006;71:1051–1059. doi: 10.1134/s0006297906100014. [DOI] [PubMed] [Google Scholar]
- 18.d’Eril GM, Anesi A, Maggiore M, Leoni V. Biological variation of serum amyloid A in healthy subjects. Clin Chem. 2001;47:1498–1499. [PubMed] [Google Scholar]
- 19.Hijmans W, Sipe JD. Levels of the serum amyloid A protein (SAA) in normal persons of different age groups. Clin Exp Immunol. 1979;35:96–100. [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenthal CJ, Franklin EC. Variation with age and disease of an amyloid A protein-related serum component. J Clin Invest. 1975;55:746–753. doi: 10.1172/JCI107985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benson MD, Cohen AS. Serum amyloid A protein in amyloidosis, rheumatic, and enoplastic diseases. Arthritis Rheum. 1979;22:36–42. doi: 10.1002/art.1780220106. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein PS, Skinner M, Sipe JD, Lokich JJ, Zamcheck N, Cohen AS. Acute-phase proteins or tumour markers: the role of SAA, SAP, CRP and CEA as indicators of metastasis in a broad spectrum of neoplastic diseases. Scand J Immunol. 1984;19:193–198. doi: 10.1111/j.1365-3083.1984.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 23.Nel AE, Strachan AF, Welke HE, de Beer FC. Acute phase response in bronchiectasis and bronchus carcinoma. Respiration. 1984;45:406–410. doi: 10.1159/000194647. [DOI] [PubMed] [Google Scholar]
- 24.Benson MD, Eyanson S, Fineberg NS. Serum amyloid A in carcinoma of the lung. Cancer. 1986;57:1783–1787. doi: 10.1002/1097-0142(19860501)57:9<1783::aid-cncr2820570912>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 25.Khan N, Cromer CJ, Campa M, Patz EF., Jr Clinical utility of serum amyloid A and macrophage migration inhibitory factor as serum biomarkers for the detection of nonsmall cell lung carcinoma. Cancer. 2004;101:379–384. doi: 10.1002/cncr.20377. [DOI] [PubMed] [Google Scholar]
- 26.Kaneti J, Winikoff Y, Zimlichman S, Shainkin-Kestenbaum R. Importance of serum amyloid A (SAA) level in monitoring disease activity and response to therapy in patients with prostate cancer. Urol Res. 1984;12:239–241. doi: 10.1007/BF00256147. [DOI] [PubMed] [Google Scholar]
- 27.Glojnaric I, Casl MT, Simic D, Lukac J. Serum amyloid A protein (SAA) in colorectal carcinoma. Clin Chem Lab Med. 2001;39:129–133. doi: 10.1515/CCLM.2001.022. [DOI] [PubMed] [Google Scholar]
- 28.Kimura M, Tomita Y, Imai T, Saito T, Katagiri A, Ohara-Mikami Y, Matsudo T, Takahashi K. Significance of serum amyloid A on the prognosis in patients with renal cell carcinoma. Cancer. 2001;92:2072–2075. doi: 10.1002/1097-0142(20011015)92:8<2072::aid-cncr1547>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 29.Ramankulov A, Lein M, Johannsen M, Schrader M, Miller K, Loening SA, Jung K. Serum amyloid A as indicator of distant metastases but not as early tumor marker in patients with renal cell carcinoma. Cancer Lett. 2008 doi: 10.1016/j.canlet.2008.04.022. (in press) [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal CJ, Sullivan LM. Serum amyloid A to monitor cancer dissemination. Ann Intern Med. 1979;91:383–390. doi: 10.7326/0003-4819-91-3-383. [DOI] [PubMed] [Google Scholar]
- 31.Chan DC, Chen CJ, Chu HC, Chang WK, Yu JC, Chen YJ, Wen LL, Huang SC, Ku CH, Liu YC, Chen JH. Evaluation of serum amyloid A as a biomarker for gastric cancer. Ann Surg Oncol. 2007;14:84–93. doi: 10.1245/s10434-006-9091-z. [DOI] [PubMed] [Google Scholar]
- 32.Raynes JG, Cooper EH. Comparison of serum amyloid A protein and C-reactive protein concentrations in cancer and non-malignant disease. J Clin Pathol. 1983;36:798–803. doi: 10.1136/jcp.36.7.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Hanlon DM, Lynch J, Cormican M, Given HF. The acute phase response in breast carcinoma. Anticancer Res. 2002;22:1289–1293. [PubMed] [Google Scholar]
- 34.Nieken J, Mulder NH, Buter J, Vellenga E, Limburg PC, Piers DA, de Vries EG. Recombinant human interleukin-6 induces a rapid and reversible anemia in cancer patients. Blood. 1995;86:900–905. [PubMed] [Google Scholar]
- 35.van Gameren MM, Willemse PH, Mulder NH, Limburg PC, Groen HJ, Vellenga E, de Vries EG. Effects of recombinant human interleukin-6 in cancer patients: a phase I-II study. Blood. 1994;84:1434–1441. [PubMed] [Google Scholar]
- 36.de Jong KP, van Gameren MM, Bijzet J, Limburg PC, Sluiter WJ, Slooff MJ, de Vries EG. Recombinant human interleukin-6 induces hepatocyte growth factor production in cancer patients. Scand J Gastroenterol. 2001;36:636–640. doi: 10.1080/003655201750163132. [DOI] [PubMed] [Google Scholar]
- 37.Biesma B, Willemse PH, Mulder NH, Sleijfer DT, Gietema JA, Mull R, Limburg PC, Bouma J, Vellenga E, de Vries EG. Effects of interleukin-3 after chemotherapy for advanced ovarian cancer. Blood. 1992;80:1141–1148. [PubMed] [Google Scholar]
- 38.de Jong KP, von Geusau BA, Rottier CA, Bijzet J, Limburg PC, de Vries EG, Fidler V, Slooff MJ. Serum response of hepatocyte growth factor, insulin-like growth factor-I, interleukin-6, and acute phase proteins in patients with colorectal liver metastases treated with partial hepatectomy or cryosurgery. J Hepatol. 2001;34:422–427. doi: 10.1016/s0168-8278(00)00030-1. [DOI] [PubMed] [Google Scholar]
- 39.de Jong KP, Hoedemakers RM, Fidler V, Bijzet J, Limburg PC, Peeters PM, de Vries EG, Slooff MJ. Portal and systemic serum growth factor and acute-phase response after laparotomy or partial hepatectomy in patients with colorectal liver metastases: a prognostic role for C-reactive protein and hepatocyte growth factor. Scand J Gastroenterol. 2004;39:1141–1148. doi: 10.1080/00365520410009609. [DOI] [PubMed] [Google Scholar]
- 40.Jung IK, Kim MC, Kim KH, Kwak JY, Jung GJ, Kim HH. Cellular and peritoneal immune response after radical laparoscopy-assisted and open gastrectomy for gastric cancer. J Surg Oncol. 2008;98:54–59. doi: 10.1002/jso.21075. [DOI] [PubMed] [Google Scholar]
- 41.Chen SH, Liao HK, Chang CY, Juo CG, Chen JH, Chan SI, Chen YJ. Targeted protein quantitation and profiling using PVDF affinity probe and MALDI-TOF MS. Proteomics. 2007;7:3038–3050. doi: 10.1002/pmic.200700393. [DOI] [PubMed] [Google Scholar]
- 42.Biro L, Domjan G, Falus A, Jakab L, Cseh K, Kalabay L, Tarkovacs G, Tresch J, Malle E, Kramer J, Prohaszka Z, et al. Cytokine regulation of the acute-phase protein levels in multiple myeloma. Eur J Clin Invest. 1998;28:679–686. doi: 10.1046/j.1365-2362.1998.00333.x. [DOI] [PubMed] [Google Scholar]
- 43.Persson L, Engervall P, Magnuson A, Vikerfors T, Söderquist B, Hansson LO, Tidefelt U. Use of inflammatory markers for early detection of bacteraemia in patients with febrile neutropenia. Scand J Infect Dis. 2004;36:365–371. doi: 10.1080/00365540410020217. [DOI] [PubMed] [Google Scholar]
- 44.Uys A, Rapoport BL, Fickl H, Meyer PW, Anderson R. Prediction of outcome in cancer patients with febrile neutropenia: comparison of the Multinational Association of Supportive Care in Cancer risk-index score with procalcitonin, C-reactive protein, serum amyloid A, and interleukins-1beta, -6, -8 and -10. Eur J Cancer Care (Engl.) 2007;16:475–483. doi: 10.1111/j.1365-2354.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 45.Persson L, Soderquist B, Engervall P, Vikerfors T, Hansson LO, Tidefelt U. Assessment of systemic inflammation markers to differentiate a stable from a deteriorating clinical course in patients with febrile neutropenia. Eur J Haematol. 2005;74:297–303. doi: 10.1111/j.1600-0609.2004.00387.x. [DOI] [PubMed] [Google Scholar]
- 46.Liou LS, Shi T, Duan ZH, Sadhukhan P, Der SD, Novick AA, Hissong J, Skacel M, Almasan A, DiDonato JA. Microarray gene expression profiling and analysis in renal cell carcinoma. BMC Urol. 2004;4:9. doi: 10.1186/1471-2490-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao WM, Kuick R, Orchekowski RP, Misek DE, Qiu J, Greenberg AK, Rom WN, Brenner DE, Omenn GS, Haab BB, Hanash SM. Distinctive serum protein profiles involving abundant proteins in lung cancer patients based upon antibody microarray analysis. BMC Cancer. 2005;5:110. doi: 10.1186/1471-2407-5-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savli H, Szendr A, Romics I, Nagy B. Gene network and canonical pathway analysis in prostate cancer: a microarray study. Exp Mol Med. 2008;40:176–185. doi: 10.3858/emm.2008.40.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moshage HJ, Roelofs HM, van Pelt JF, Hazenberg BP, van Leeuwen MA, Limburg PC, Aarden LA, Yap SH. The effect of interleukin-1, interleukin-6 and its interrelationship on the synthesis of serum amyloid A and C-reactive protein in primary cultures of adult human hepatocytes. Biochem Biophys Res Commun. 1988;155:112–117. doi: 10.1016/s0006-291x(88)81056-8. [DOI] [PubMed] [Google Scholar]
- 50.Yap SH, Moshage HJ, Hazenberg BP, Roelofs HM, Bijzet J, Limburg PC, Aarden LA, van Rijswijk MH. Tumor necrosis factor (TNF) inhibits interleukin (IL)-1 and/or IL-6 stimulated synthesis of C-reactive protein (CRP) and serum amyloid A (SAA) in primary cultures of human hepatocytes. Biochim Biophys Acta. 1991;1091:405–408. doi: 10.1016/0167-4889(91)90207-e. [DOI] [PubMed] [Google Scholar]
- 51.Gabay C, Genin B, Mentha G, Iynedjian PB, Roux-Lombard P, Guerne PA. IL-1 receptor antagonist (IL-1Ra) does not inhibit the production of C-reactive protein or serum amyloid A protein by human primary hepatocytes.Differential regulation in normal and tumour cells. Clin Exp Immunol. 1995;100:306–313. doi: 10.1111/j.1365-2249.1995.tb03669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steel DM, Donoghue FC, O’Neill RM, Uhlar CM, Whitehead AS. Expression and regulation of constitutive and acute phase serum amyloid A mRNAs in hepatic and non-hepatic cell lines. Scand J Immunol. 1996;44:493–500. doi: 10.1046/j.1365-3083.1996.d01-341.x. [DOI] [PubMed] [Google Scholar]
- 53.Ganapathi MK, May LT, Schultz D, Brabenec A, Weinstein J, Sehgal PB, Kushner I. Role of interleukin-6 in regulating synthesis of C-reactive protein and serum amyloid A in human hepatoma cell lines. Biochem Biophys Res Commun. 1988;157:271–277. doi: 10.1016/s0006-291x(88)80043-3. [DOI] [PubMed] [Google Scholar]
- 54.Raynes JG, Eagling S, McAdam KP. Acute-phase protein synthesis in human hepatoma cells: differential regulation of serum amyloid A (SAA) and haptoglobin by interleukin-1 and interleukin-6. Clin Exp Immunol. 1991;83:488–491. doi: 10.1111/j.1365-2249.1991.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malle E, Leonhard B, Knipping G, Sattler W. Effects of cytokines, butyrate and dexamethasone on serum amyloid A and apolipoprotein A-I synthesis in human HUH-7 hepatoma cells. Scand J Immunol. 1999;50:183–187. doi: 10.1046/j.1365-3083.1999.00574.x. [DOI] [PubMed] [Google Scholar]
- 56.Steel DM, Whitehead AS. Heterogeneous modulation of acute-phase-reactant mRNA levels by interleukin-1 beta and interleukin-6 in the human hepatoma cell line PLC/PRF/5. Biochem J. 1991;277:477–482. doi: 10.1042/bj2770477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thorn CF, Lu ZY, Whitehead AS. Tissue-specific regulation of the human acute-phase serum amyloid A genes, SAA1 and SAA2, by glucocorticoids in hepatic and epithelial cells. Eur J Immunol. 2003;33:2630–2639. doi: 10.1002/eji.200323985. [DOI] [PubMed] [Google Scholar]
- 58.Thorn CF, Lu ZY, Whitehead AS. Regulation of the human acute phase serum amyloid A genes by tumour necrosis factor-alpha, interleukin-6 and glucocorticoids in hepatic and epithelial cell lines. Scand J Immunol. 2004;59:152–158. doi: 10.1111/j.0300-9475.2004.01369.x. [DOI] [PubMed] [Google Scholar]
- 59.Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, Rullier A, Cubel G, Couchy G, Imbeaud S, Balabaud C, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–748. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]
- 60.Meek RL, Urieli-Shoval S, Benditt EP. Expression of apolipoprotein serum amyloid A mRNA in human atherosclerotic lesions and cultured vascular cells: implications for serum amyloid A function. Proc Natl Acad Sci U S A. 1994;91:3186–3190. doi: 10.1073/pnas.91.8.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Urieli-Shoval S, Meek RL, Hanson RH, Eriksen N, Benditt EP. Human serum amyloid A genes are expressed in monocyte/macrophage cell lines. Am J Pathol. 1994;45:650–660. [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 63.Parle-McDermott A, McWilliam P, Tighe O, Dunican D, Croke DT. Serial analysis of gene expression identifies putative metastasis-associated transcripts in colon tumour cell lines. Br J Cancer. 2000;83:725–728. doi: 10.1054/bjoc.2000.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 65.Hao CY, Moore DH, Wong P, Bennington JL, Lee NM, Chen LC. Alteration of gene expression in macroscopically normal colonic mucosa from individuals with a family history of sporadic colon cancer. Clin Cancer Res. 2005;11:1400–1407. doi: 10.1158/1078-0432.CCR-04-1942. [DOI] [PubMed] [Google Scholar]
- 66.Nishie A, Masuda K, Otsubo M, Migita T, Tsuneyoshi M, Kohno K, Shuin T, Naito S, Ono M, Kuwano M. High expression of the Cap43 gene in infiltrating macrophages of human renal cell carcinomas. Clin Cancer Res. 2001;7:2145–2151. [PubMed] [Google Scholar]
- 67.Kosari F, Parker AS, Kube DM, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Vasmatzis G. Clear cell renal cell carcinoma: gene expression analyses identify a potential signature for tumor aggressiveness. Clin Cancer Res. 2005;11:5128–5139. doi: 10.1158/1078-0432.CCR-05-0073. [DOI] [PubMed] [Google Scholar]
- 68.Vreugdenhil AC, Dentener MA, Snoek AM, Greve JW, Buurman WA. Lipopolysaccharide binding protein and serum amyloid A secretion by human intestinal epithelial cells during the acute phase response. J Immunol. 1999;163:2792–2798. [PubMed] [Google Scholar]
- 69.Gutfeld O, Prus D, Ackerman Z, Dishon S, Linke RP, Levin M, Urieli-Shoval S. Expression of serum amyloid A, in normal, dysplastic, and neoplastic human colonic mucosa: implication for a role in colonic tumorigenesis. J Histochem Cytochem. 2006;54:63–73. doi: 10.1369/jhc.5A6645.2005. [DOI] [PubMed] [Google Scholar]
- 70.Liang L, Qu L, Ding Y. Protein and mRNA characterization in human colorectal carcinoma cell lines with different metastatic potentials. Cancer Invest. 2007;25:427–434. doi: 10.1080/07357900701512258. [DOI] [PubMed] [Google Scholar]
- 71.Kovacevic A, Hammer A, Sundl M, Pfister B, Hrzenjak A, Ray A, Ray BK, Sattler W, Malle E. Expression of serum amyloid A transcripts in human trophoblast and fetal-derived trophoblast-like choriocarcinoma cells. FEBS Lett. 2006;580:161–167. doi: 10.1016/j.febslet.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 72.Kovacevic A, Hammer A, Stadelmeyer E, Windischhofer W, Sundl M, Ray A, Schweighofer N, Friedl G, Windhager R, Sattler W, Malle E. Expression of serum amyloid A transcripts in human bone tissues, differentiated osteoblast-like stem cells and human osteosarcoma cell lines. J Cell Biochem. 2008;103:994–1004. doi: 10.1002/jcb.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Urieli-Shoval S, Cohen P, Eisenberg S, Matzner Y. Widespread expression of serum amyloid A in histologically normal human tissues. Predominant localization to the epithelium. J Histochem Cytochem. 1998;46:1377–1384. doi: 10.1177/002215549804601206. [DOI] [PubMed] [Google Scholar]
- 74.Diamandis EP. Mass spectrometry as a diagnostic and a cancer biomarker discovery tool: opportunities and potential limitations. Mol Cell Proteomics. 2004;3:367–378. doi: 10.1074/mcp.R400007-MCP200. [DOI] [PubMed] [Google Scholar]
- 75.Petricoin EF, Ardekani AM, Hitt BA, Levine PJ, Fusaro VA, Steinberg SM, Mills GB, Simone C, Fishman DA, Kohn EC, Liotta LA. Use of proteomic patterns in serum to identify ovarian cancer. Lancet. 2002;359:572–577. doi: 10.1016/S0140-6736(02)07746-2. [DOI] [PubMed] [Google Scholar]
- 76.Le L, Chi K, Tyldesley S, Flibotte S, Diamond DL, Kuzyk MA, Sadar MD. Identification of serum amyloid A as a biomarker to distinguish prostate cancer patients with bone lesions. Clin Chem. 2005;51:695–707. doi: 10.1373/clinchem.2004.041087. [DOI] [PubMed] [Google Scholar]
- 77.Li Y, Dang TA, Shen J, Perlaky L, Hicks J, Murray J, Meyer W, Chintagumpala M, Lau CC, Man TK. Identification of a plasma proteomic signature to distinguish pediatric osteosarcoma from benign osteochondroma. Proteomics. 2006;6:3426–3435. doi: 10.1002/pmic.200500472. [DOI] [PubMed] [Google Scholar]
- 78.Moshkovskii SA, Serebryakova MV, Kuteykin-Teplyakov KB, Tikhonova OV, Goufman EI, Zgoda VG, Taranets IN, Makarov OV, Archakov AI. Ovarian cancer marker of 11.7 kDa detected by proteomics is a serum amyloid A1. Proteomics. 2005;5:3790–3797. doi: 10.1002/pmic.200401205. [DOI] [PubMed] [Google Scholar]
- 79.Helleman J, van der Vlies D, Jansen MP, Luider TM, van der Burg ME, Stoter G, Berns EM. Serum proteomic patterns for ovarian cancer monitoring. Int J Gynecol Cancer. 2008 doi: 10.1111/j.1525-1438.2007.01139.x. (in press) [DOI] [PubMed] [Google Scholar]
- 80.Tolson J, Bogumil R, Brunst E, Beck H, Elsner R, Humeny A, Kratzin H, Deeg M, Kuczyk M, Mueller GA, Mueller CA, et al. Serum protein profiling by SELDI mass spectrometry: detection of multiple variants of serum amyloid alpha in renal cancer patients. Lab Invest. 2004;84:1220–1221. doi: 10.1038/labinvest.3700097. [DOI] [PubMed] [Google Scholar]
- 81.Kluve-Beckerman B, Malle E, Vitt H, Pfeiffer C, Benson M, Steinmetz A. Characterization of an isoelectric focusing variant of SAA1 (ASP-72) in a family of Turkish origin. Biochem Biophys Res Commun. 1991;181:1097–1102. doi: 10.1016/0006-291x(91)92051-k. [DOI] [PubMed] [Google Scholar]
- 82.Rossi L, Martin BM, Hortin GL, White RL, Foster M, Moharram R, Stroncek D, Wang E, Marincola FM, Panelli MC. Inflammatory protein profile during systemic high dose interleukin-2 administration. Proteomics. 2006;6:709–720. doi: 10.1002/pmic.200500004. [DOI] [PubMed] [Google Scholar]
- 83.Engwegen JY, Mehra N, Haanen JB, Bonfrer JM, Schellens JH, Voest EE, Beijnen JH. Validation of SELDI-TOF MS serum protein profiles for renal cell carcinoma in new populations. Lab Invest. 2007;87:161–172. doi: 10.1038/labinvest.3700503. [DOI] [PubMed] [Google Scholar]
- 84.Roelofsen H, Alvarez-Llamas G, Dijkstra M, Breitling R, Havenga K, Bijzet J, Zandbergen W, de Vries MP, Ploeg RJ, Vonk RJ. Analyses of intricate kinetics of the serum proteome during and after colon surgery by protein expression time series. Proteomics. 2007;7:3219–3228. doi: 10.1002/pmic.200601047. [DOI] [PubMed] [Google Scholar]
- 85.Koomen JM, Shih LN, Coombes KR, Li D, Xiao LC, Fidler IJ, Abbruzzese JL, Kobayashi R. Plasma protein profiling for diagnosis of pancreatic cancer reveals the presence of host response proteins. Clin Cancer Res. 2005;11:1110–1118. [PubMed] [Google Scholar]
- 86.Yildiz PB, Shyr Y, Rahman JS, Wardwell NR, Zimmerman LJ, Shakhtour B, Gray WH, Chen S, Li M, Roder H, Liebler DC, et al. Diagnostic accuracy of MALDI mass spectrometric analysis of unfractionated serum in lung cancer. J Thorac Oncol. 2007;2:893–901. doi: 10.1097/JTO.0b013e31814b8be7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Junker K, von Eggeling F, Muller J, Steiner T, Schubert J. [Identification of biomarkers and therapeutic targets for renal cell cancer using Protein Chip Technology. Urologe A. 2006;45:305–315. doi: 10.1007/s00120-006-1001-2. [DOI] [PubMed] [Google Scholar]
- 88.Junker K, Gneist J, Melle C, Driesch D, Schubert J, Claussen U, Von Eggeling F. Identification of protein pattern in kidney cancer using ProteinChip arrays and bioinformatics. Int J Mol Med. 2005;15:285–290. [PubMed] [Google Scholar]
- 89.Junker K, Sanjmyatav J, Mueller J, Steiner T, Wunderlich H, Chyhrai A, Pilchowski R, Reichelt O, Schubert J. Molecular tumour profiling for detection of biomarkers in renal cell tumours. European Urology Supplements. 2007:611–615. [Google Scholar]
- 90.Combaret V, Bergeron C, Brejon S, Iacono I, Perol D, Negrier S, Puisieux A. Protein chip array profiling analysis of sera from neuroblastoma patients. Cancer Lett. 2005;228:91–96. doi: 10.1016/j.canlet.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 91.Sandoval JA, Turner KE, Hoelz DJ, Rescorla FJ, Hickey RJ, Malkas LH. Serum protein profiling to identify high-risk neuroblastoma: preclinical relevance of blood-based biomarkers. J Surg Res. 2007:268–274. doi: 10.1016/j.jss.2007.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cho WC, Yip TT, Yip C, Yip V, Thulasiraman V, Ngan RK, Yip TT, Lau WH, Au JS, Law SC, Cheng WW, et al. Identification of serum amyloid A protein as a potentially useful biomarker to monitor relapse of nasopharyngeal cancer by serum proteomic profiling. Clin Cancer Res. 2004;10:43–52. doi: 10.1158/1078-0432.ccr-0413-3. [DOI] [PubMed] [Google Scholar]
- 93.Cho WC, Yip TT, Ngan RK, Yip TT, Podust VN, Yip C, Yiu HH, Yip V, Cheng WW, Ma VW, Law SC. ProteinChip array profiling for identification of disease- and chemotherapy-associated biomarkers of nasopharyngeal carcinoma. Clin Chem. 2007;53:241–250. doi: 10.1373/clinchem.2005.065805. [DOI] [PubMed] [Google Scholar]
- 94.Liao Q, Zhao L, Chen X, Deng Y, Ding Y. Serum proteome analysis for profiling protein markers associated with carcinogenesis and lymph node metastasis in nasopharyngeal carcinoma. Clin Exp Metastasis. 2008;25:465–476. doi: 10.1007/s10585-008-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dowling P, O’Driscoll L, Meleady P, Henry M, Roy S, Ballot J, Moriarty M, Crown J, Clynes M. 2-D difference gel electrophoresis of the lung squamous cell carcinoma versus normal sera demonstrates consistent alterations in the levels of ten specific proteins. Electrophoresis. 2007;28:4302–4310. doi: 10.1002/elps.200700246. [DOI] [PubMed] [Google Scholar]
- 96.Jensen LE, Whitehead AS. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem J. 1998;334:489–503. doi: 10.1042/bj3340489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He R, Sang H, Ye RD. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood. 2003;101:1572–1581. doi: 10.1182/blood-2002-05-1431. [DOI] [PubMed] [Google Scholar]
- 98.He R, Shepard LW, Chen J, Pan ZK, Ye RD. Serum amyloid A is an endogenous ligand that differentially induces IL-12 and IL-23. J Immunol. 2006;177:4072–4079. doi: 10.4049/jimmunol.177.6.4072. [DOI] [PubMed] [Google Scholar]
- 99.Patel H, Fellowes R, Coade S, Woo P. Human serum amyloid A has cytokine-like properties. Scand J Immunol. 1998;48:410–418. doi: 10.1046/j.1365-3083.1998.00394.x. [DOI] [PubMed] [Google Scholar]
- 100.Furlaneto CJ, Campa A. A novel function of serum amyloid A: a potent stimulus for the release of tumor necrosis factor-alpha, interleukin-1beta, and interleukin-8 by human blood neutrophil. Biochem Biophys Res Commun. 2000;268:405–408. doi: 10.1006/bbrc.2000.2143. [DOI] [PubMed] [Google Scholar]
- 101.Preciado-Patt L, Cahalon L, Hershkovitz R, Lider O, Pras M, Fridkin M. Serum amyloid A complexed with extracellular matrix induces the secretion of tumor necrosis factor-α by human T-lymphocytes. Letters in Peptide Science. 1998;5:349–355. [Google Scholar]
- 102.Lee HY, Kim MK, Park KS, Shin EH, Jo SH, Kim SD, Jo EJ, Lee YN, Lee C, Baek SH, Bae YS. Serum amyloid A induces contrary immune responses via formyl peptide receptor-like 1 in human monocytes. Mol Pharmacol. 2006;70:241–248. doi: 10.1124/mol.105.022103. [DOI] [PubMed] [Google Scholar]
- 103.van der Westhuyzen DR, de Beer FC, Webb NR. HDL cholesterol transport during inflammation. Curr Opin Lipidol. 2007;18:147–151. doi: 10.1097/MOL.0b013e328051b4fe. [DOI] [PubMed] [Google Scholar]
- 104.Husebekk A, Skogen B, Husby G, Marhaug G. Transformation of amyloid precursor SAA to protein AA and incorporation in amyloid fibrils in vivo. Scand J Immunol. 1985;21:283–287. doi: 10.1111/j.1365-3083.1985.tb01431.x. [DOI] [PubMed] [Google Scholar]
- 105.Ancsin JB, Kisilevsky R. Laminin interactions with the apoproteins of acute-phase HDL: preliminary mapping of the laminin binding site on serum amyloid A. Amyloid: Int J Exp Clin Invest. 1999;6:37–47. doi: 10.3109/13506129908993286. [DOI] [PubMed] [Google Scholar]
- 106.Ancsin JB, Kisilevsky R. The heparin/heparan sulfate-binding site on apo-serum amyloid A. Implications for the therapeutic intervention of amyloidosis. J Biol Chem. 1999;274:7172–7181. doi: 10.1074/jbc.274.11.7172. [DOI] [PubMed] [Google Scholar]
- 107.Malle E, Munscher G, Muller T, Vermeer H, Ibovnik A. Quantification and mapping of antigenic determinants of serum amyloid A (SAA) protein utilizing sequence-specific immunoglobulins and Eu3+ as a specific probe for time-resolved fluorometric immunoassay. J Immunol Methods. 1995;182:131–144. doi: 10.1016/0022-1759(95)00035-9. [DOI] [PubMed] [Google Scholar]
- 108.Malle E, Herz R, Artl A, Ibovnik A, Andreae F, Sattler W. Mapping of antigenic determinants of purified, lipid-free human serum amyloid A proteins. Scand J Immunol. 1998;48:557–561. doi: 10.1046/j.1365-3083.1998.00439.x. [DOI] [PubMed] [Google Scholar]
- 109.Stevens FJ. Hypothetical structure of human serum amyloid A protein. Amyloid. 2004;11:71–80. doi: 10.1080/13506120412331272296. [DOI] [PubMed] [Google Scholar]
- 110.Preciado-Patt L, Levartowsky D, Prass M, Hershkoviz R, Lider O, Fridkin M. Inhibition of cell adhesion to glycoproteins of the extracellular matrix by peptides corresponding to serum amyloid A. Toward understanding the physiological role of an enigmatic protein. Eur J Biochem. 1994;223:35–42. doi: 10.1111/j.1432-1033.1994.tb18963.x. [DOI] [PubMed] [Google Scholar]
- 111.Urieli-Shoval S, Shubinsky G, Linke RP, Fridkin M, Tabi I, Matzner Y. Adhesion of human platelets to serum amyloid A. Blood. 2002;99:1224–1229. doi: 10.1182/blood.v99.4.1224. [DOI] [PubMed] [Google Scholar]
- 112.Preciado-Patt L, Hershkoviz R, Fridkin M, Lider O. Serum amyloid A binds specific extracellular matrix glycoproteins and induces the adhesion of resting CD4+ T cells. J Immunol. 1996;156:1189–1195. [PubMed] [Google Scholar]
- 113.Hershkoviz R, Preciado-Patt L, Lider O, Fridkin M, Mekori YA. Mast cell adhesion to extracellular matrix: local effects of acute phase reactants. Int Arch Allergy Immunol. 1997;113:295–296. doi: 10.1159/000237579. [DOI] [PubMed] [Google Scholar]
- 114.Hershkoviz R, Preciado-Patt L, Lider O, Fridkin M, Dastych J, Metcalfe DD, Mekori YA. Extracellular matrix-anchored serum amyloid A preferentially induces mast cell adhesion. Am J Physiol. 1997;273:C179–187. doi: 10.1152/ajpcell.1997.273.1.C179. [DOI] [PubMed] [Google Scholar]
- 115.Zimlichman S, Danon A, Nathan I, Mozes G, Shainkin-Kestenbaum R. Serum amyloid A, an acute phase protein, inhibits platelet activation. J Lab Clin Med. 1990;116:180–186. [PubMed] [Google Scholar]
- 116.Nierodzik ML, Klepfish A, Karpatkin S. Role of platelets, thrombin, integrin IIb-IIIa, fibronectin and von Willebrand factor on tumor adhesion in vitro and metastasis in vivo. Thromb Haemost. 1995;74:282–290. [PubMed] [Google Scholar]
- 117.Michaeli A, Finci-Yeheskel Z, Dishon S, Linke RP, Levin M, Urieli-Shoval S. Serum amyloid A enhances plasminogen activation: implication for a role in colon cancer. Biochem Biophys Res Commun. 2008;368:368–373. doi: 10.1016/j.bbrc.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 118.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 119.Malle E, Bollmann A, Steinmetz A, Gemsa D, Leis HJ, Sattler W. Serum amyloid A (SAA) protein enhances formation of cyclooxygenase metabolites of activated human monocytes. FEBS Lett. 1997;419:215–219. doi: 10.1016/s0014-5793(97)01459-2. [DOI] [PubMed] [Google Scholar]
- 120.Lee HY, Jo SH, Lee C, Baek SH, Bae YS. Differential production of leukotriene B4 or prostaglandin E2 by WKYMVm or serum amyloid A via formyl peptide receptor-like 1. Biochem Pharmacol. 2006;72:860–868. doi: 10.1016/j.bcp.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 121.Marx N, Froehlich J, Siam L, Ittner J, Wierse G, Schmidt A, Scharnagl H, Hombach V, Koenig W. Antidiabetic PPAR gamma-activator rosiglitazone reduces MMP-9 serum levels in type 2 diabetic patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2003;23:283–288. doi: 10.1161/01.atv.0000054195.35121.5e. [DOI] [PubMed] [Google Scholar]
- 122.Folgueras AR, Pendas AM, Sanchez LM, Lopez-Otin C. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int J Dev Biol. 2004;48:411–424. doi: 10.1387/ijdb.041811af. [DOI] [PubMed] [Google Scholar]
- 123.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 124.Muller D, Roessner A, Rocken C. Distribution pattern of matrix metalloproteinases 1, 2, 3, and 9, tissue inhibitors of matrix metalloproteinases 1 and 2, and alpha 2-macroglobulin in cases of generalized AA- and AL amyloidosis. Virchows Arch. 2000;437:521–527. doi: 10.1007/s004280000271. [DOI] [PubMed] [Google Scholar]
- 125.Stix B, Kahne T, Sletten K, Raynes J, Roessner A, Rocken C. Proteolysis of AA amyloid fibril proteins by matrix metalloproteinases-1, -2, and -3. Am J Pathol. 2001;159:561–570. doi: 10.1016/S0002-9440(10)61727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee HY, Kim MK, Park KS, Bae YH, Yun J, Park JI, Kwak JY, Bae YS. Serum amyloid A stimulates matrix-metalloproteinase-9 upregulation via formyl peptide receptor like-1-mediated signaling in human monocytic cells. Biochem Biophys Res Commun. 2005;330:989–998. doi: 10.1016/j.bbrc.2005.03.069. [DOI] [PubMed] [Google Scholar]
- 127.Migita K, Kawabe Y, Tominaga M, Origuchi T, Aoyagi T, Eguchi K. Serum amyloid A protein induces production of matrix metalloproteinases by human synovial fibroblasts. Lab Invest. 1998;78:535–539. [PubMed] [Google Scholar]
- 128.O’Hara R, Murphy EP, Whitehead AS, FitzGerald O, Bresnihan B. Local expression of the serum amyloid A and formyl peptide receptor-like 1 genes in synovial tissue is associated with matrix metalloproteinase production in patients with inflammatory arthritis. Arthritis Rheum. 2004;50:1788–1799. doi: 10.1002/art.20301. [DOI] [PubMed] [Google Scholar]
- 129.Mullan RH, Bresnihan B, Golden-Mason L, Markham T, O’Hara R, FitzGerald O, Veale DJ, Fearon U. Acute-phase serum amyloid A stimulation of angiogenesis, leukocyte recruitment, and matrix degradation in rheumatoid arthritis through an NF-kappaB-dependent signal transduction pathway. Arthritis Rheum. 2006;54:105–114. doi: 10.1002/art.21518. [DOI] [PubMed] [Google Scholar]
- 130.Ray A, Ray BK. Isolation and functional characterization of cDNA of serum amyloid A-activating factor that binds to the serum amyloid A promoter. Mol Cell Biol. 1998;18:7327–7335. doi: 10.1128/mcb.18.12.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ray A, Kuroki K, Cook JL, Bal BS, Kenter K, Aust G, Ray BK. Induction of matrix metalloproteinase 1 gene expression is regulated by inflammation-responsive transcription factor SAF-1 in osteoarthritis. Arthritis Rheum. 2003;48:134–145. doi: 10.1002/art.10706. [DOI] [PubMed] [Google Scholar]
- 132.Ray BK, Shakya A, Turk JR, Apte SS, Ray A. Induction of the MMP-14 gene in macrophages of the atherosclerotic plaque: role of SAF-1 in the induction process. Circ Res. 2004;95:1082–1090. doi: 10.1161/01.RES.0000150046.48115.80. [DOI] [PubMed] [Google Scholar]
- 133.Ray A, Shakya A, Ray BK. Inflammation-responsive transcription factors SAF-1 and c-Jun/c-Fos promote canine MMP-1 gene expression. Biochim Biophys Acta. 2005;1732:53–61. doi: 10.1016/j.bbaexp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 134.Ray BK, Shakya A, Ray A. Vascular endothelial growth factor expression in arthritic joint is regulated by SAF-1 transcription factor. J Immunol. 2007;178:1774–1782. doi: 10.4049/jimmunol.178.3.1774. [DOI] [PubMed] [Google Scholar]
- 135.Vallon R, Freuler F, Desta-Tsedu N, Robeva A, Dawson J, Wenner P, Engelhardt P, Boes L, Schnyder J, Tschopp C, Urfer R, et al. Serum amyloid A (apoSAA) expression is up-regulated in rheumatoid arthritis and induces transcription of matrix metalloproteinases. J Immunol. 2001;166:2801–2807. doi: 10.4049/jimmunol.166.4.2801. [DOI] [PubMed] [Google Scholar]
- 136.Su SB, Gong W, Gao JL, Shen W, Murphy PM, Oppenheim JJ, Wang JM. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J Exp Med. 1999;189:395–402. doi: 10.1084/jem.189.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Badolato R, Johnston JA, Wang JM, McVicar D, Xu LL, Oppenheim JJ, Kelvin DJ. Serum amyloid A includes calcium mobilization and chemotaxis of human monocytes by activating a pertussis toxin-sensitive signaling pathway. J Immunol. 1995;155:4004–4010. [PubMed] [Google Scholar]
- 138.Brink C, Dahlen SE, Drazen J, Evans JF, Hay DW, Nicosia S, Serhan CN, Shimizu T, Yokomizo T. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol Rev. 2003;55:195–227. doi: 10.1124/pr.55.1.8. [DOI] [PubMed] [Google Scholar]
- 139.Sodin-Semrl S, Spagnolo A, Mikus R, Barbaro B, Varga J, Fiore S. Opposing regulation of interleukin-8 and NF-kappaB responses by lipoxin A4 and serum amyloid A via the common lipoxin A receptor. Int J Immunopathol Pharmacol. 2004;17:145–156. doi: 10.1177/039463200401700206. [DOI] [PubMed] [Google Scholar]
- 140.Fiore S, Romano M, Reardon EM, Serhan CN. Induction of functional lipoxin A4 receptors in HL-60 cells. Blood. 1993;81:3395–3403. [PubMed] [Google Scholar]
- 141.Kang Y, Taddeo B, Varai G, Varga J, Fiore S. Mutations of serine 236–237 and tyrosine 302 residues in the human lipoxin A4 receptor intracellular domains result in sustained signaling. Biochemistry. 2000;39:13551–13557. doi: 10.1021/bi001196i. [DOI] [PubMed] [Google Scholar]
- 142.Chiang N, Fierro IM, Gronert K, Serhan CN. Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J Exp Med. 2000;191:1197–1208. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.El Kebir D, Jozsef L, Khreiss T, Pan W, Petasis NA, Serhan CN, Filep JG. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: a novel mechanism for resolution of inflammation. J Immunol. 2007;179:616–622. doi: 10.4049/jimmunol.179.1.616. [DOI] [PubMed] [Google Scholar]
- 144.Wu SH, Lu C, Dong L, Zhou GP, He ZG, Chen ZQ. High dose of lipoxin A4 induces apoptosis in rat renal interstitial fibroblasts. Prostaglandins Leukot Essent Fatty Acids. 2005;73:127–137. doi: 10.1016/j.plefa.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 145.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 146.Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, Petasis NA, Erwig L, Rees AJ, Savill J, Brady HR, et al. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–2507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- 147.El Kebir D, Jozsef L, Filep JG. Opposing regulation of neutrophil apoptosis through the formyl peptide receptor-like 1/lipoxin A4 receptor: implications for resolution of inflammation. J Leukoc Biol. 2008 doi: 10.1189/jlb.1107765. (in press) [DOI] [PubMed] [Google Scholar]
- 148.Christenson K, Bjorkman L, Tangemo C, Bylund J. Serum amyloid A inhibits apoptosis of human neutrophils via a P2X7-sensitive pathway independent of formyl peptide receptor-like 1. J Leukoc Biol. 2008;83:139–148. doi: 10.1189/jlb.0507276. [DOI] [PubMed] [Google Scholar]
- 149.Kho Y, Kim S, Yoon BS, Moon JH, Kim B, Kwak S, Woo J, Oh S, Hong K, Kim H, You S, et al. Induction of serum amyloid A genes is associated with growth and apoptosis of HC11 mammary epithelial cells. Biosci Biotechnol Biochem. 2008;72:70–81. doi: 10.1271/bbb.70374. [DOI] [PubMed] [Google Scholar]
- 150.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 151.Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996;39:1781–1790. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- 152.Firestein GS, Echeverri F, Yeo M, Zvaifler NJ, Green DR. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc Natl Acad Sci U S A. 1997;94:10895–10900. doi: 10.1073/pnas.94.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Roivainen A, Jalava J, Pirila L, Yli-Jama T, Tiusanen H, Toivanen P. H-ras oncogene point mutations in arthritic synovium. Arthritis Rheum. 1997;40:1636–1643. doi: 10.1002/art.1780400913. [DOI] [PubMed] [Google Scholar]
- 154.Lee MS, Yoo SA, Cho CS, Suh PG, Kim WU, Ryu SH. Serum amyloid A binding to formyl peptide receptor-like 1 induces synovial hyperplasia and angiogenesis. J Immunol. 2006;177:5585–5594. doi: 10.4049/jimmunol.177.8.5585. [DOI] [PubMed] [Google Scholar]
- 155.Xu L, Badolato R, Murphy WJ, Longo DL, Anver M, Hale S, Oppenheim JJ, Wang JM. A novel biologic function of serum amyloid A. Induction of T lymphocyte migration and adhesion. J Immunol. 1995;155:1184–1190. [PubMed] [Google Scholar]
- 156.Badolato R, Wang JM, Murphy WJ, Lloyd AR, Michiel DF, Bausserman LL, Kelvin DJ, Oppenheim JJ. Serum amyloid A is a chemoattractant: induction of migration, adhesion, and tissue infiltration of monocytes and polymorphonuclear leukocytes. J Exp Med. 1994;180:203–209. doi: 10.1084/jem.180.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Björkman L, Karlsson J, Karlsson A, Rabiet M, Boulay F, Fu H, Bylung J, Dahlgren C. Serum amyloid A mediates human neutrophil production or reactive oxygen species through a receptor independent of formyl peptide receptor like-1. J Leukoc Biol. 2008;83:245–253. doi: 10.1189/jlb.0607-408. [DOI] [PubMed] [Google Scholar]
- 158.Tosi MR, Tugnoli V. Cholesteryl esters in malignancy. Clin Chim Acta. 2005;359:27–45. doi: 10.1016/j.cccn.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 159.Pussinen PJ, Karten B, Wintersperger A, Reicher H, McLean M, Malle E, Sattler W. The human breast carcinoma cell line HBL-100 acquires exogenous cholesterol from high-density lipoprotein via CLA-1 (CD-36 and LIMPII analogous 1)-mediated selective cholesteryl ester uptake. Biochem J. 2000;349:559–566. doi: 10.1042/0264-6021:3490559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wadsack C, Hirschmugl B, Hammer A, Levak-Frank S, Kozarsky KF, Sattler W, Malle E. Scavenger receptor class B, type I on non-malignant and malignant human epithelial cells mediates cholesteryl ester-uptake from high density lipoproteins. Int J Biochem Cell Biol. 2003;35:441–454. doi: 10.1016/s1357-2725(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 161.Wadsack C, Hrzenjak A, Hammer A, Hirschmugl B, Levak-Frank S, Desoye G, Sattler W, Malle E. Trophoblast-like human choriocarcinoma cells serve as a suitable in vitro model for selective cholesteryl ester uptake from high density lipoproteins. Eur J Biochem. 2003;270:451–462. doi: 10.1046/j.1432-1033.2003.03394.x. [DOI] [PubMed] [Google Scholar]
- 162.Baranova IN, Vishnyakova TG, Bocharov AV, Kurlander R, Chen Z, Kimelman ML, Remaley AT, Csako G, Thomas F, Eggerman TL, Patterson AP. Serum amyloid A binding to CLA-1 (CD36 and LIMPII Analogous-1) mediates serum amyloid A protein-induced activation of ERK1/2 and p38 mitogen-activated protein kinases. J Biol Chem. 2005;280:8031–8040. doi: 10.1074/jbc.M405009200. [DOI] [PubMed] [Google Scholar]
- 163.Cai L, de Beer MC, de Beer FC, van der Westhuyzen DR. Serum amyloid A is a ligand for scavenger receptor class B, type I and inhibits high density lipoprotein binding and selective lipid uptake. J Biol Chem. 2005;280:2954–2961. doi: 10.1074/jbc.M411555200. [DOI] [PubMed] [Google Scholar]
- 164.Marsche G, Frank S, Raynes JG, Kozarsky KF, Sattler W, Malle E. The lipidation status of acute-phase protein serum amyloid A determines cholesterol mobilization via scavenger receptor class B type I. Biochem J. 2007;402:117–124. doi: 10.1042/BJ20061406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Artl A, Marsche G, Lestavel S, Sattler W, Malle E. Role of serum amyloid A during metabolism of acute-phase HDL by macrophages. Arterioscler Thromb Vasc Biol. 2000;20:763–772. doi: 10.1161/01.atv.20.3.763. [DOI] [PubMed] [Google Scholar]
- 166.Hirano K, Yamashita S, Nakagawa Y, Ohya T, Matsuura F, Tsukamoto K, Okamoto Y, Matsuyama A, Matsumoto K, Miyagawa J, Matsuzawa Y. Expression of human scavenger receptor class B type I in cultured human monocyte-derived macrophages and atherosclerotic lesions. Circ Res. 1999;85:108–116. doi: 10.1161/01.res.85.1.108. [DOI] [PubMed] [Google Scholar]
- 167.Banka CL, Yuan T, de Beer MC, Kindy M, Curtiss LK, de Beer FC. Serum amyloid A (SAA): influence on HDL-mediated cellular cholesterol efflux. J Lipid Res. 1995;36:1058–1065. [PubMed] [Google Scholar]
- 168.Artl A, Marsche G, Pussinen P, Knipping G, Sattler W, Malle E. Impaired capacity of acute-phase high density lipoprotein particles to deliver cholesteryl ester to the human HUH-7 hepatoma cell line. Int J Biochem Cell Biol. 2002;34:370–381. doi: 10.1016/s1357-2725(01)00132-7. [DOI] [PubMed] [Google Scholar]
- 169.van der Westhuyzen DR, Cai L, de Beer MC, de Beer FC. Serum amyloid A promotes cholesterol efflux mediated by scavenger receptor B-I. J Biol Chem. 2005;280:35890–35895. doi: 10.1074/jbc.M505685200. [DOI] [PubMed] [Google Scholar]
- 170.Abe-Dohmae S, Kato KH, Kumon Y, Hu W, Ishigami H, Iwamoto N, Okazaki M, Wu CA, Tsujita M, Ueda K, Yokoyama S. Serum amyloid A generates high density lipoprotein with cellular lipid in an ABCA1- or ABCA7-dependent manner. J Lipid Res. 2006;47:1542–1550. doi: 10.1194/jlr.M600145-JLR200. [DOI] [PubMed] [Google Scholar]
- 171.Stonik JA, Remaley AT, Demosky SJ, Neufeld EB, Bocharov A, Brewer HB. Serum amyloid A promotes ABCA1-dependent and ABCA1-independent lipid efflux from cells. Biochem Biophys Res Commun. 2004;321:936–941. doi: 10.1016/j.bbrc.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 172.Hu W, Abe-Dohmae S, Tsujita M, Iwamoto N, Ogikubo O, Otsuka T, Kumon Y, Yokoyama S. Biogenesis of HDL by SAA is dependent on ABCA1 in the liver in vivo. J Lipid Res. 2008;49:386–393. doi: 10.1194/jlr.M700402-JLR200. [DOI] [PubMed] [Google Scholar]
- 173.Liang JS, Sipe JD. Recombinant human serum amyloid A (apoSAAp) binds cholesterol and modulates cholesterol flux. J Lipid Res. 1995;36:37–46. [PubMed] [Google Scholar]
- 174.Liang JS, Schreiber BM, Salmona M, Phillip G, Gonnerman WA, de Beer FC, Sipe JD. Amino terminal region of acute phase, but not constitutive, serum amyloid A (apoSAA) specifically binds and transports cholesterol into aortic smooth muscle and HepG2 cells. J Lipid Res. 1996;37:2109–2116. [PubMed] [Google Scholar]
- 175.Kisilevsky R, Lindhorst E, Ancsin JB, Young D, Bagshaw W. Acute phase serum amyloid A (SAA) and cholesterol transport during acute inflammation: A hypothesis. Amyloid: Int J Exp Clin Invest. 1996;3:252–260. [Google Scholar]
- 176.Gonnerman WA, Lim M, Sipe JD, Hayes KC, Cathcart ES. The acute phase response in Syrian hamsters elevates apolipoprotein serum amyloid A (apo-SAA) and disrupts lipoprotein metabolism. Amyloid: Int J Exp Clin Invest. 1996;3:261–269. [Google Scholar]
- 177.Cai H, Song C, Endoh I, Goyette J, Jessup W, Freedman SB, McNeil HP, Geczy CL. Serum amyloid A induces monocyte tissue factor. J Immunol. 2007;178:1852–1860. doi: 10.4049/jimmunol.178.3.1852. [DOI] [PubMed] [Google Scholar]
- 178.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Okamoto H, Katagiri Y, Kiire A, Momohara S, Kamatani N. Serum amyloid A activates nuclear factor-kappaB in rheumatoid synovial fibroblasts through binding to receptor of advanced glycation end-products. J Rheumatol. 2008;35:752–756. [PubMed] [Google Scholar]
- 180.Yan SD, Zhu H, Zhu A, Golabek A, Du H, Roher A, Yu J, Soto C, Schmidt AM, Stern D, Kindy M. Receptor-dependent cell stress and amyloid accumulation in systemic amyloidosis. Nat Med. 2000;6:643–651. doi: 10.1038/76216. [DOI] [PubMed] [Google Scholar]
- 181.Nion S, Briand O, Lestavel S, Torpier G, Nazih F, Delbart C, Fruchart JC, Clavey V. High-density-lipoprotein subfraction 3 interaction with glycosylphosphatidylinositol-anchored proteins. Biochem J. 1997;328:415–423. doi: 10.1042/bj3280415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mikesch JH, Schier K, Roetger A, Simon R, Buerger H, Brandt B. The expression and action of decay-accelerating factor (CD55) in human malignancies and cancer therapy. Cell Oncol. 2006;28:223–232. doi: 10.1155/2006/814816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Walder K, Kantham L, McMillan JS, Trevaskis J, Kerr L, De Silva A, Sunderland T, Godde N, Gao Y, Bishara N, Windmill K, et al. Tanis: a link between type-2 diabetes and inflammation? Diabetes. 2002;51:1859–1866. doi: 10.2337/diabetes.51.6.1859. [DOI] [PubMed] [Google Scholar]
- 184.Sandri S, Rodriguez D, Gomes E, Monteiro HP, Russo M, Campa A. Is serum amyloid A an endogenous TLR4 agonist? J Leukoc Biol. 2008;83:1174–1180. doi: 10.1189/jlb.0407203. [DOI] [PubMed] [Google Scholar]
- 185.Cheng N, He R, Tian J, Ye PP, Ye RD. Cutting Edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J Immunol. 2008;181:22–26. doi: 10.4049/jimmunol.181.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ghezzi P, Bertini R, Bianchi M, Erroi A, Mengozzi M, Delgado R, Giavazzi R, Bartle L, Sipe JD. Serum amyloid A induction in tumor-bearing mice: evidence for a tumor-derived mediator. Int J Immunopathol Pharmacol. 1993;6:169–186. [Google Scholar]
- 187.Kamate C, Baloul S, Grootenboer S, Pessis E, Chevrot A, Tulliez M, Marchiol C, Viguier M, Fradelizi D. Inflammation and cancer, the mastocytoma P815 tumor model revisited: triggering of macrophage activation in vivo with pro-tumorigenic consequences. Int J Cancer. 2002;100:571–579. doi: 10.1002/ijc.10519. [DOI] [PubMed] [Google Scholar]
- 188.Yokoi K, Shih LC, Kobayashi R, Koomen J, Hawke D, Li D, Hamilton SR, Abbruzzese JL, Coombes KR, Fidler IJ. Serum amyloid A as a tumor marker in sera of nude mice with orthotopic human pancreatic cancer and in plasma of patients with pancreatic cancer. Int J Oncol. 2005;27:1361–1369. [PubMed] [Google Scholar]
- 189.McLean M, Wallace HL, Sharma A, Hill HC, Sabel MS, Egilmez NK. A BALB/c murine lung alveolar carcinoma used to establish a surgical spontaneous metastasis model. Clin Exp Metastasis. 2004;21:363–369. doi: 10.1023/b:clin.0000046176.33867.c5. [DOI] [PubMed] [Google Scholar]
- 190.Kristiansson MH, Bhat VB, Babu IR, Wishnok JS, Tannenbaum SR. Comparative time-dependent analysis of potential inflammation biomarkers in lymphoma-bearing SJL mice. J Proteome Res. 2007;6:1735–1744. doi: 10.1021/pr060497x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bhat VB, Choi MH, Wishnok JS, Tannenbaum SR. Comparative plasma proteome analysis of lymphoma-bearing SJL mice. J Proteome Res. 2005;4:1814–1825. doi: 10.1021/pr0501463. [DOI] [PubMed] [Google Scholar]
- 192.Juan HF, Chen JH, Hsu WT, Huang SC, Chen ST, Yi-Chung Lin J, Chang YW, Chiang CY, Wen LL, Chan DC, Liu YC, et al. Identification of tumor-associated plasma biomarkers using proteomic techniques: from mouse to human. Proteomics. 2004;4:2766–2775. doi: 10.1002/pmic.200400785. [DOI] [PubMed] [Google Scholar]
- 193.Thompson HA, Burson JM, Lang JA, Gross KW, Sigmund CD. Tissue-specific expression of novel messenger ribonucleic acids cloned from a renin-expressing kidney tumor cell line (As4.1) Endocrinology. 1995;136:3037–3045. doi: 10.1210/endo.136.7.7789330. [DOI] [PubMed] [Google Scholar]
- 194.Fontana A, McAdam KP, Kristensen F, Weber E. Biological and biochemical characterization of an interleukin 1-like factor from rat C6 glioma cells. Eur J Immunol. 1983;13:685–689. doi: 10.1002/eji.1830130814. [DOI] [PubMed] [Google Scholar]
- 195.Chiarella P, Vulcano M, Bruzzo J, Vermeulen M, Vanzulli S, Maglioco A, Camerano G, Palacios V, Fernandez G, Brando RF, Isturiz MA, et al. Anti-inflammatory pretreatment enables an efficient dendritic cell-based immunotherapy against established tumors. Cancer Immunol Immunother. 2008;57:701–718. doi: 10.1007/s00262-007-0410-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Strey CW, Winters MS, Markiewski MM, Lambris JD. Partial hepatectomy induced liver proteome changes in mice. Proteomics. 2005;5:318–325. doi: 10.1002/pmic.200400913. [DOI] [PubMed] [Google Scholar]
- 197.Hayashida Y, Honda K, Osaka Y, Hara T, Umaki T, Tsuchida A, Aoki T, Hirohashi S, Yamada T. Possible prediction of chemoradiosensitivity of esophageal cancer by serum protein profiling. Clin Cancer Res. 2005;11:8042–8047. doi: 10.1158/1078-0432.CCR-05-0656. [DOI] [PubMed] [Google Scholar]
- 198.Diamandis EP. Identification of serum amyloid A protein as a potentially useful biomarker for nasopharyngeal carcinoma. Clin Cancer Res. 2004;10:5293. doi: 10.1158/1078-0432.CCR-04-0377. author reply 5293-5294. [DOI] [PubMed] [Google Scholar]
- 199.Stroncek DF, Burns C, Martin BM, Rossi L, Marincola FM, Panelli MC. Advancing cancer biotherapy with proteomics. J Immunother. 2005;28:183–192. doi: 10.1097/01.cji.0000162781.78384.95. [DOI] [PubMed] [Google Scholar]
- 200.Lavie M, Voisset C, Vu-Dac N, Zurawski V, Duverlie G, Wychowski C, Dubuisson J. Serum amyloid A has antiviral activity against hepatitis C virus by inhibiting virus entry in a cell culture system. Hepatology. 2006;44:1626–1634. doi: 10.1002/hep.21406. [DOI] [PubMed] [Google Scholar]
- 201.Hari-Dass R, Shah C, Meyer DJ, Raynes JG. Serum amyloid A protein binds to outer membrane protein A of Gram-negative bacteria. J Biol Chem. 2005;280:18562–18567. doi: 10.1074/jbc.M500490200. [DOI] [PubMed] [Google Scholar]
- 202.Zhang N, Ahsan MH, Purchio AF, West DB. Serum amyloid A-luciferase transgenic mice: response to sepsis, acute arthritis, and contact hypersensitivity and the effects of proteasome inhibition. J Immunol. 2005;174:8125–8134. doi: 10.4049/jimmunol.174.12.8125. [DOI] [PubMed] [Google Scholar]
- 203.He QY, Zhu R, Lei T, Ng MY, Luk JM, Sham P, Lau GK, Chiu JF. Toward the proteomic identification of biomarkers for the prediction of HBV related hepatocellular carcinoma. J Cell Biochem. 2008;103:740–752. doi: 10.1002/jcb.21443. [DOI] [PubMed] [Google Scholar]