Abstract
Sesquiterpenoids, with ca. 5000 structures, are the most diverse class of plant volatiles, with manifold hypothesized functions in defense, stress tolerance, and signaling between and within plants. These hypotheses have often been tested by transforming plants with sesquiterpene synthases (STPS’s) expressed behind the constitutively active 35S promoter, which may have physiological costs, measured as inhibited growth and reduced reproduction, or require augmentation of substrate pools to achieve enhanced emission, complicating the interpretation of data from affected transgenic lines. Here, we expressed Zea mays TPSlO (ZmTPSl0), which produces (E)-α-bergamotene and (E)-β-farnesene, or a point mutant, ZmTPSlOM, producing primarily (E)-β-farnesene, under control of the 35S promoter in the ecological model plant Nicotiana attenuata. Transgenic N. attenuata plants had specifically enhanced emission of target sesquiterpene(s) with no changes detected in their emission of any other volatiles. Treatment with herbivore or jasmonate elicitors induces emission of (E)-α-bergamotene in wild-type (WT) plants, and also tended to increase emission of (E)-α-bergamotene and (E)-β-farnesene in transgenics. However, transgenics did not differ from WT in defense signaling or chemistry, and did not alter defense chemistry in neighboring WT plants. These data are inconsistent with within- or between-plant signaling functions of (E)-β-farnesene and (E)-α-bergamotene in N. attenuata. Ectopic sesquiterpene emission was apparently not costly for transgenics, which were similar to WT plants in their growth and reproduction even when forced to compete for common resources. These transgenics would be well suited for field experiments to investigate indirect, ecological effects of sesquiterpenes for a wild plant in its native habitat.
Introduction
Isoprenoids, derived from the 5-carbon precursors isoprene diphosphate (IDP) and dimethylallyl diphosphate (DMADP), are the largest and most diverse group of plant metabolites (Thulasiram et al., 2007; Vranová et al., 2012). They are produced from two different biosynthetic pathways in the plant cell: the mevalonate (MVA) pathway in the cytosol and peroxisomes, and the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway in the plastid (reviewed in Rodríguez-Concepción, 2006; Tholl and Lee, 2011; Rodríguez-concepción et al., 2013). These pathways are at an intersection of general and specialized metabolism. The plastidial MEP/DOXP pathway produces the precursors of carotenoids and of the chlorophyll side chain and, in addition, precursors of e.g. abscisic acid, gibberellins, cytokinins, volatile monoterpenes and, in some plants, highly volatile isoprene. The largest metabolic sink for products of the MVA pathway is sterol biosynthesis (Rodríguez-Concepción, 2006), but substrate from the MVA pathway also provides precursors of sesquiterpene volatiles, as well as of signaling molecules such as cytokinins and brassinosteroids, and prenyl moieties for protein modification – among other things. The two pathways are furthermore able to exchange intermediates. Thus, the synthesis of any one terpene product may affect metabolic flux towards a large array of general and specialized plant metabolites and signaling molecules (reviewed in Nagegowda, 2010; Vranová et al., 2012).
Of the volatile terpenes – isoprene (C5), monoterpenes (C10), sesquiterpenes (C15), and a few homoterpenes (C11, C16) derived from the degradation of sesqui- and diterpenes – sesquiterpenes and their derivatives are the most diverse, with ca. 5 000 structures (Seigler). Sesquiterpenes are often components of stress-induced plant volatile blends (Duhl et al., 2008; Holopainen and Gershenzon, 2010), and they have a multitude of possible functions in plant physiology and ecological interactions (Vickers et al., 2009; Dicke and Baldwin, 2010; Holopainen and Gershenzon, 2010). For example, several herbivore-induced sesquiterpenes have been shown to contribute to defense in multiple plant species by attracting predators or parasitoids of herbivores feeding on plant roots or shoots, or by deterring ovipositing herbivores in laboratory assays as well as field experiments (Kessler and Baldwin, 2001; Kappers et al., 2005; Rasmann et al., 2005; Schnee et al., 2006; Halitschke et al., 2008; Degenhardt et al., 2009a). Furthermore, there is evidence that herbivory-induced sesquiterpenes function as stress signals between leaves of single plants, as well as between plants (Paschold et al., 2006; Heil and Silva Bueno, 2007; Ton et al., 2007; Heil and Karban, 2010; Zebelo et al., 2012). As has been shown for isoprene and some monoterpenes (reviewed in Vickers et al., 2009), sesquiterpenes could also help to protect plants from oxidative stress resulting from abiotic factors.
The stress-induced emission of sesquiterpenes, as well as their diversity among genotypes and species, is thought to be largely controlled via the polymorphism, subcellular localization, and transcript levels of sesquiterpene synthase enzymes (reviewed in Chen and Tholl, 2011). These enzymes belong to the family of terpene synthases (TPS’s) which includes members that produce both general and specialized metabolites, and are all thought to be derived from an ancestral kaurene synthase of general metabolism (Trapp and Croteau, 2001; Chen and Tholl, 2011). Sesquiterpene synthases use the 15-carbon substrate farnesyl diphosphate (FDP) to produce a multitude of mono- and polycyclic sesquiterpene products and derivatives, as well as linear products (Degenhardt et al., 2009b).
Hypotheses about the physiological and ecological functions of specific sesquiterpenes in plants have frequently been tested by ectopically expressing sesquiterpene synthases under the control of the CaMV 35S promoter, resulting in constitutive emission of the engineered sesquiterpenes (e.g. Degenhardt et al., 2003; Aharoni et al., 2005; Rasmann et al., 2005; Dudareva and Pichersky, 2008; Degenhardt et al., 2009a; Kos et al., 2013). These transgenic plants have helped demonstrate a role for sesquiterpenes in resisting herbivore damage and attracting enemies of herbivores in several plant species. However, constitutive transgenic production of sesquiterpenes can result in negative physiological effects likely related to altering flux into essential terpenoid products, including stunted growth and reduced reproductive yield (Aharoni et al., 2003; Robert et al., 2013), complicating the use of such transgenic plants in ecological studies. In some cases, ectopic expression of terpene synthases has been thought to reveal a so-called silent metabolism due to the appearance of additional products derived from the ectopic terpenes (Nagegowda, 2010). Measures have sometimes been taken to increase available substrate pools in order to achieve enhanced emission, such as altered subcellular localization, or co-expression of FDP SYNTHASE (FDPS) (Kappers et al., 2005; Nagegowda, 2010; Houshyani et al., 2013, Kos et al., 2013). These engineering efforts demonstrate that substrate flux can also regulate sesquiterpene emission (Vranová et al., 2012), and that ectopic engineering of sesquiterpenes can not only relieve, but also produce stress in plants (Holopainen and Gershenzon, 2010; Nagegowda, 2010; Robert et al., 2013).
To learn more about the physiological and ecological roles of volatile sesquiterpenes in Nicotiana attenuata, we enhanced the emission of (E)-α-bergamotene and (E)-β-farnesene in this wild tobacco via ectopic expression of the sesquiterpene synthase Zea mays TPS10 (ZmTPS10) or a point mutant, ZmTPS10M, producing primarily (E)-β-farnesene (Köllner et al., 2009), under the control of the CaMV 35S promoter. The sesquiterpene (E)-α-bergamotene is ubiquitously emitted by natural genotypes of N. attenuata in response to herbivore elicitation (Halitschke et al., 2000; Kessler and Baldwin, 2001), and natural variation in (E)-α-bergamotene emission is related to the variation in magnitude of endogenous jasmonate signaling response to herbivore attack (Schuman et al., 2009). (E)-α-Bergamotene has been shown to reduce herbivore populations on N. attenuata plants in their native habitat by attracting predatory arthropods, and deterring oviposition of the specialist herbivore Manduca sexta (Kessler and Baldwin, 2001; Halitschke et al., 2008). Furthermore, (E)-α-bergamotene regulates a subset of genes in N. attenuata plants exposed to herbivore-induced volatiles from conspecifics (Paschold et al., 2006). (E)-β-Farnesene has occasionally been detected in small amounts in the headspace of N. attenuata plants, and in larger amounts from plants in which the JAZH negative regulator of jasmonate responses was silenced (Oh et al., 2012), or from WT plants which have been treated with strong jasmonate elicitors (Kallenbach et al., 2014). The engineered emission of these two sesquiterpenes, which are released upon caterpillar damage to maize leaves, has been shown to attract a hymenopteran parasitoid of the caterpillar to Arabidopsis thaliana plants constitutively emitting the sesquiterpenes, but only after the parasitoids first had an oviposition experience in the presence of the sesquiterpenes (Schnee et al., 2006).
In the present study, we found that enhanced emission of (E)-α-bergamotene and/or (E)-β-farnesene in N. attenuata TPS10 and TPS10M plants had no measurable effects on plant growth, reproductive yield, or defense physiology, even when TPS10 plants were forced to compete with WT plants for common resources. Moreover, the growth and defense physiology of WT plants was not affected by their neighbors’ (E)-β-farnesene and (E)-α-bergamotene emissions. These data are not consistent with within- or between-plant signaling functions for (E)-α-bergamotene and (E)-β-farnesene in N. attenuata. However given that enhanced levels of emission occurred without any alterations in growth and physiology, the engineered plants are well-suited for use in ecological experiments.
Results
Ectopic expression of TPS10 or TPS10M results in elevated levels of (E)-β-farnesene and (E)-α-bergamotene in the foliar headspace
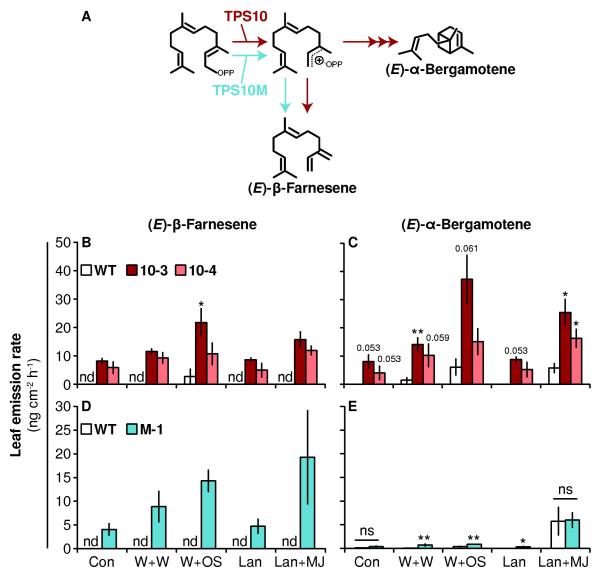
We ectopically expressed Z. mays TPS10 or the point mutation TPS10M under the control of the 35S promoter in multiple transgenic lines of N. attenuata, and selected diploid, homozygous lines with single insertion events for further screening (Supplemental Fig. S1). We then measured the abundance of the target volatiles (E)-β-farnesene and (E)-α-bergamotene in the headspace of unwounded leaves, and leaves treated with wounding and the addition of Manduca sexta oral secretions to wounds (W+OS), which elicits emission of (E)-α-bergamotene in WT plants (Halitschke et al., 2000). Headspace samples from transgenic lines contained (E)-β-farnesene, while samples from WT did not, and samples from transgenics also contained 2- to 25-fold as much (E)-α-bergamotene as those from WT (Supplemental Fig. S1). As expected, a TPS10M line (M-1) emitted more than twice as much (E)-β-farnesene and less than half as much (E)-α-bergamotene in comparison to TPS10 lines (10-1 to 10-5). Interestingly, emission of both (E)-β-farnesene and (E)-α-bergamotene was enhanced by W+OS treatment (Supplemental Fig. S1): differences among transgenic lines in (E)-β-farnesene abundance were not significant in measurements of constitutive emission (Kruskal-Wallis test, χ2 = 7.419, df = 5, Bonferroni corrected P = 0.3821) but became significant after W+OS treatment (χ2 = 14.96, df = 5, corrected P = 0.0211), and the overall effect of W+OS treatment on (E)-β-farnesene abundance was also significant (Wilcoxon rank-sum test, W = 143, corrected P < 0.001). While (E)-α-bergamotene was detected in all headspace samples, its abundance increased after W+OS treatment (W = 317, corrected P = 0.0167); the effect of line on (E)-α-bergamotene abundance was significant both in measurements of constitutive emission (χ2 = 21.16, df = 6, corrected P = 0.0034) and after W+OS treatment (χ2 = 23.81, df = 6, corrected P = 0.0011).
From the lines having a single insertion, we selected two each of TPS10 and TPS10M for further experiments (10-3 = A-09-389, homozygote 4 or 6; 10-4 = A-09-391, homozygote 1; M-1 = A-09-596, homozygote 4; M-2 = A-09-334, homozygote 4). Because line M-2 was generated later than the other three lines, many experiments were conducted only with line M-1 of TPS10M, but line M-2 was included in experiments to demonstrate transgene transcript abundance and to test for off-target effects.
We analyzed the abundance of (E)-α-bergamotene and (E)-β-farnesene in the headspace of leaves on WT plants compared to lines 10-3 and 10-4, or compared to M-1, after no treatment, or after treatment with herbivory or jasmonate elicitors known to induce (E)-α-bergamotene emission from WT plants (Halitschke et al., 2000; Schuman et al., 2009; Fig. 1). Again, (E)-β-farnesene was detected in all headspace samples from transgenic plants but in only a few samples from W+OS-treated WT plants; (E)-α-bergamotene was more abundant in headspace samples from 10-3 and 10-4 than from WT regardless of treatment, and was enhanced in the headspace of M-1 compared to WT after W+W, W+OS, or Lan treatment (Fig. 1, Tables S1, S2).
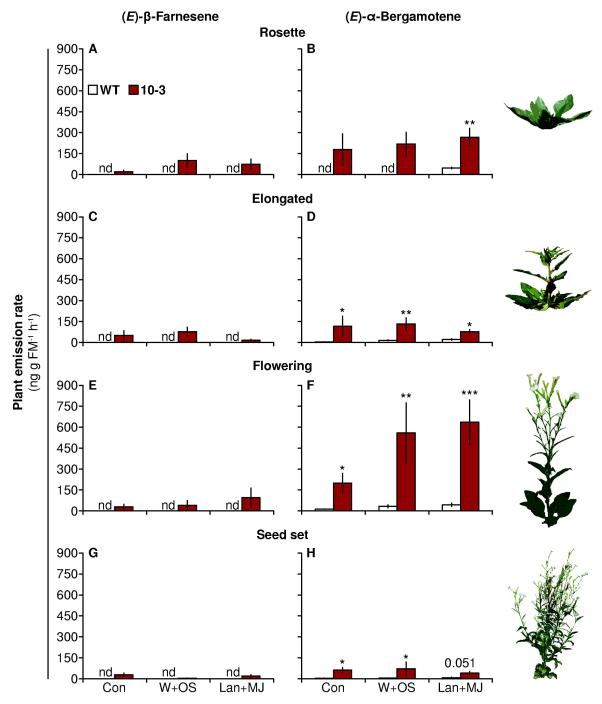
Figure 1. Ectopic expression of TPS10 and the point mutant TPS10M results in greater headspace abundance of (E)-β-farnesene and/or (E)-α-bergamotene versus WT.
(A) Simplified mechanism of TPS10 and TPS10M (modified from Köllner et al., 2009). TPS10 (B, C) and TPS10M lines (D, E) consistently emit more (E)-β-farnesene (left panels) and/or (E)-α-bergamotene (right panels) than WT plants from leaves treated with several herbivory-related elicitors (mean ± SEM, n = 4). Note different scales: data in (B, C) and (D, E) are from two separate experiments conducted within the same week; all plants were in the same growth stage (elongated). The headspace of the second fully expanded leaf (+2) on plants was collected without treatment (Con) or for 24-32 h after treatment with wounding and water (W+W; W+OS control), wounding and Manduca sexta oral secretions (W+OS), lanolin (Lan; Lan+MJ control), or lanolin containing 150 μg methyl jasmonate (Lan+MJ). In the constitutive and lanolin treatments, there was very low but detectable (E)-α-bergamotene emission from WT (<0.1 ng); nd, not detected. *P < 0.05, ** P < 0.01 versus WT within each treatment after Holm-Bonferroni corrections for multiple testing if required (WT tested against lines 10-3 and 10-4) following Wilcoxon rank-sum tests or Welch’s t-tests; marginally significant corrected P-values (<0.1) are written above bars; ns, not significant. Non-target volatiles from these plants did not differ among transgenic lines and WT (PCAs in Supplemental Figs. S2 and S3).
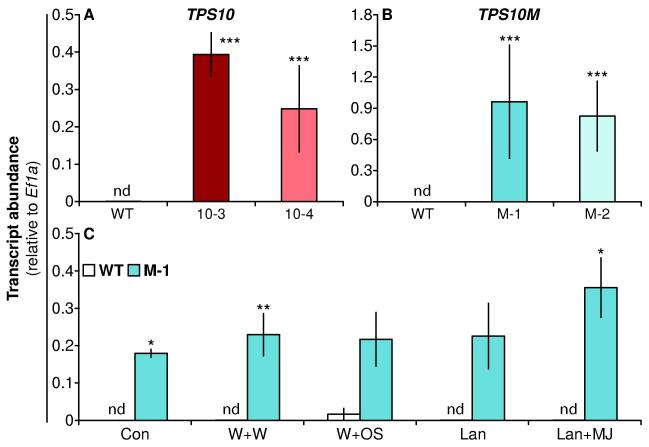
We also assayed the expression of the TPS10 and TPS10M transgenes in lines 10-3, 10-4, M-1, and M-2 (Fig. 2), both constitutively and after the various treatments used to elicit (E)-β-farnesene and (E)-α-bergamotene emission (shown for line M-1). There was a very low signal detected in WT plants from non-specific amplification (judged from melting curves), but abundance of the specifically amplified TPS10 and TPS10M transcripts was more than 100-fold the nonspecific abundance in WT (Welch’s t-tests followed by Holm-Bonferroni corrections for testing WT twice: 10-3 v. WT, t = 20.75, df = 3.129, P = 0.0004; 10-4 v. WT, t = 10.79, df = 4.644, P = 0.0002; M-1 v. WT, t = 5.596, df = 3.563, P = 0.0070; M-2 v. WT, t = 13.99, df = 5.866, P < 0.0001). Transgene transcript abundance was generally not affected by damage, herbivore, or jasmonate treatments (Welch’s t-tests, M-1 v. WT: Con, t = 8.787, df = 2.018, P = 0.0124; W+W, t = 7.790, df = 3.458, P = 0.0026; W+OS, t = 1.126, df = 4.885, P = 0.3126; Lan, t = 1.765, df = 3.038, P = 0.1745; Lan+MJ, t = 4.439, df = 3, P = 0.0213; treatment effect in M-1, Kruskal-Wallis test followed by a Holm-Bonferroni correction for testing both line and treatment: χ2 = 3.414, df = 4, P = 0.4910).
Figure 2. Transcript levels of TPS10 and TPS10M are high in transgenic lines and stable across several treatments (mean ± SEM, n = 4).
Transcripts were quantified in the first fully expanded leaf (+1) in two lines of TPS10 (A), in two lines of TPS10M (B), or in line M-1 1 h after treatment with herbivory-related elicitors (C), which did not change TPS10M transcript abundance (P = 0.491 in a Kruskal-Wallis test across treatments). Similarly, no effect of treatment was observed on transcripts in the other lines in separate experiments (not shown). There was a very low signal due to nonspecific amplification in WT negative controls (nd, < 0.001 units) which increased to a maximum of ca. 0.03 units after W+OS treatment. *P < 0.05, **P < 0.01, ***P < 0.001 after Holm-Bonferroni correction of Welch’s t-tests versus WT (WT tested twice in A and B, M-1 tested twice in C).
Neither (E)-β-farnesene nor (E)-α-bergamotene were detected in the floral headspace of transgenics, which was dominated by benzyl acetone as in WT plants.
Emission of non-target plant volatiles is similar to WT in TPS10 and TPS10M lines
We determined the abundance of non-target volatiles in the same experiment for which (E)-β-farnesene and (E)-α-bergamotene measurements are shown in Fig. 1. To this end we sampled the headspace of leaves both for 3 h immediately after treatment, when the rapidly emitted green leaf volatiles (GLVs) are most abundant (Allmann et al., 2010), and for 24-32 h after treatment, when terpenoids are most abundant (Halitschke et al., 2000). All volatiles other than (E)-β-farnesene and (E)-α-bergamotene detected in the headspace were analyzed by PCAs for each time point, demonstrating near-complete overlap of the profile of non-target plant volatiles in the headspace of lines 10-3, 10-4, and M-1 with WT regardless of treatment (Supplemental Figs. S2 and S3).
Ectopic expression of TPS10 and TPS10M does not alter plants’ jasmonate-mediated defense response
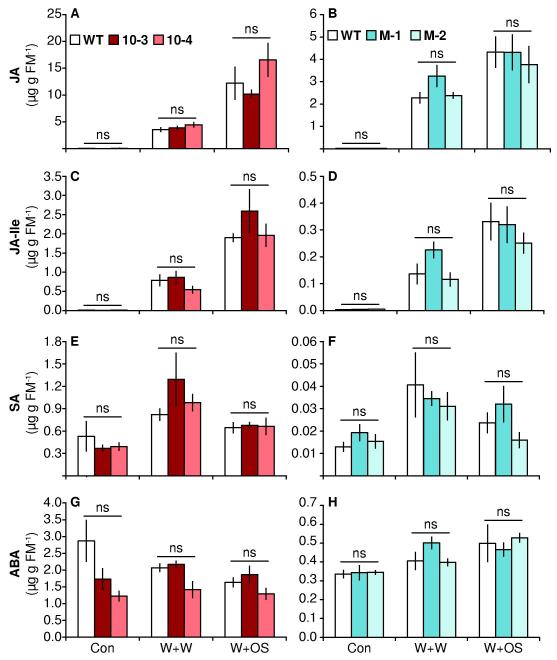
There is evidence from N. attenuata and other plant species that sesquiterpenes elicited by herbivory, such as (E)-α-bergamotene, may be involved in the priming or elicitation of defense against herbivores (Baldwin et al., 2006; Paschold et al., 2006; Heil and Silva Bueno, 2007; Ton et al., 2007; Asai et al., 2009; Zebelo et al., 2012). We therefore hypothesized that lines producing elevated (E)-α-bergamotene and/or (E)-β-farnesene might respond to elicitation with elevated levels of defense. To determine whether TPS10 and TPS10M plants showed an elevated response to treatment at the signaling level, we measured jasmonic acid (JA) and its active conjugate jasmonoyl isoleucine (JA-Ile), which largely regulate accumulation of defense metabolites after wounding or herbivory (Wang et al., 2008); as well as the antagonistically acting salicylic acid (SA), and the terpenoid abscisic acid (ABA) which elicits responses to herbivory in synergy with JA (Gilardoni et al., 2011; Robert-Seilaniantz et al., 2011; Dinh et al., 2013; Vos et al., 2013). These phytohormones were measured both in untreated leaves and 1 h after treatment at the peak time of JA and JA-Ile (Gilardoni et al., 2011). Lines 10-3, 10-4, M-1, and M-2 all were similar to WT in their concentrations of JA, its active conjugate JA-Ile, SA, and ABA, regardless of treatment (Fig. 3, P > 0.18 in Wilcoxon rank sum tests or Welch’s t-tests between WT and individual lines after Holm-Bonferroni corrections for testing WT twice).
Figure 3. Expression of TPS10 and TPS10M does not alter levels of defense-related phytohormones constitutively, or after elicitation (mean ± SEM, n = 4).
Data in left and right panels are from two separate experiments with elongated plants (legends in A and B). Jasmonic acid (JA, A-B), jasmonoyl isoleucine (JA-Ile, C-D), salicylic acid (SA, E-F), and abscisic acid (ABA, G-H) were quantified in the first fully expanded leaf (+1) after no treatment (Con) or 1 h after treatment with W+W or W+OS, which is within the peak of elicited jasmonates in Nicotiana attenuata. A very small amount of JA and JA-Ile was detected in constitutive measurements (<0.1 μg for JA, <0.01 μg for JA-Ile). There were no significant differences (corrected P>0.05) in Welch’s t-tests or Wilcoxon rank-sum tests between lines and WT within each treatment; the Holm-Bonferroni method was used to correct for multiple testing (WT tested twice within each treatment).
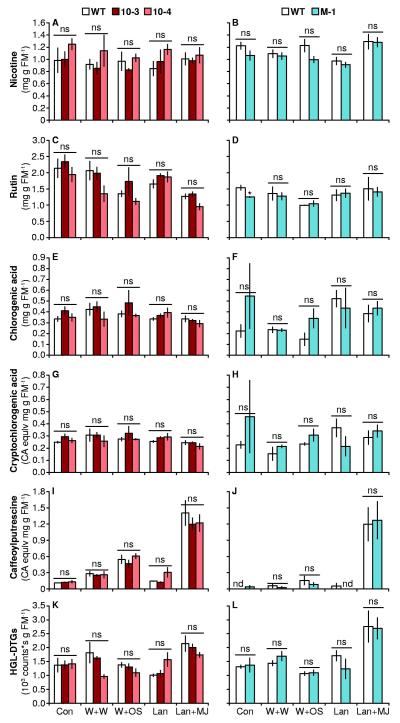
We furthermore measured the levels of several defense metabolites: nicotine, several phenolic compounds, and hydroxygeranyllinalool diterpene glycosides (HGL-DTGs) (Steppuhn et al., 2004; Heiling et al., 2010; Kaur et al., 2010), in leaves from WT and lines 10-3, 10-4, and M-1 at peak accumulation 72 h after elicitation or in simultaneously collected untreated leaves (constitutive, Con; Fig. 4, Tables S3, S4). There were few significant differences among lines (P > 0.08 in Wilcoxon rank sum tests or Welch’s t-tests between WT and individual lines, after Holm-Bonferroni corrections for testing WT twice against 10-3 and 10-4), except for constitutive samples of M-1 which had slightly reduced rutin levels compared to WT (< 20% reduction, t = −4.100, df = 2.268, P = 0.0440). In addition, there were only a few significant differences in individual HGL-DTGs (Welch’s t-tests followed by Bonferroni corrections for testing WT v. 10-3 and 10-4, and testing individual as well as total HGL-DTGs; 10-4 v. WT, W+W treatment: Nicotianoside V, t = −4.330, df = 5.408, P = 0.0190, Nicotianoside VII, t = −5.514, df = 4.557, P = 0.0110; Lan treatment: Nicotianoside I; t = 5.131, df = 5.528, P = 0.0080; all other comparisons, P > 0.1).
Figure 4. Expression of TPS10 and TPS10M does not alter levels of defense metabolites constitutively, or after elicitation (mean ± SEM, n = 4).
Data in left and right panels are from two separate experiments with elongated plants (legends above A and B). Samples were taken from the 0 leaf (source-sink transition) 3 d after treatment, to permit elicited metabolite synthesis and accumulation. Nicotine (A-B), rutin (C-D), chlorogenic acid (E-F), chryptochlorogenic acid (G-H), caffeoylputrescine (I-J), and total hydroxygeranyllinalool diterpene glycosides (HGL-DTGs, K-L) were quantified after no treatment (Con) or after treatment with W+W, W+OS, Lan, or Lan+MJ. Cryptochlorogenic acid and caffeoylputrescine were quantified as chlorogenic acid equivalents (CA equiv); nd, not detected. Peak areas of individual HGL-DTGs are shown in Tables S1 and S2. *P < 0.05 in a Welch’s t-test between line M-1 and WT in the Con treatment (rutin, D). There were no other significant differences (ns, not significant: P>0.05) after Holm-Bonferroni correction of Welch’s t-tests or Wilcoxon rank-sum tests between lines and WT within each treatment (WT tested twice versus lines 10-3 and 10-4, individual and total HGL-DTGS tested).
TPS10-expressing plants maintain elevated (E)-β-farnesene and (E)-α-bergamotene emission throughout growth and development
In order to investigate the effects of (E)-β-farnesene and (E)-α-bergamotene on plants’ ecological interactions, and to assay potential functions – which are defined by their contribution to plant fitness – it is essential to use plants which maintain emission through several developmental stages. We enclosed the entire aboveground portions of WT and 10-3 plants in a push-pull trapping set-up and measured the headspace 24-32 h after no treatment (Con), W+OS, or Lan+MJ treatment, at four stages starting with establishment of the rosette stage and ending with maturation of seed capsules (Fig. 5). At every stage, the headspace around 10-3 plants contained (E)-β-farnesene, which was not detected in the WT headspace, as well as 2-fold to more than 10-fold the amount of (E)-α-bergamotene measured in the WT headspace (Welch’s t-tests or Wilcoxon rank-sum tests between WT and 10-3, followed by Bonferroni corrections for also testing emission by treatment: Rosette, Lan+MJ, t = 4.558, df = 5.974, P = 0.0078; Elongated, Con, t = 3.240, df = 6.344, P = 0.0327, W+OS, t = 4.220, df = 6.999, P = 0.0079, Lan+MJ, t = 3.329, df = 6.967, P = 0.0254; Flowering, Con, W = 25, n = 5, P = 0.0159, W+OS, t = 4.380, df = 7.617, P = 0.0053, Lan+MJ, t = 6.300, df = 7.729, P = 0.0005; Seed set, Con, W = 25, n = 5, P = 0.0239, W+OS, t = 3.198, df = 7.787, P = 0.0262, Lan+MJ, t = 2.752, df = 7.842, P = 0.0510). Treatment did not significantly alter the emission of (E)-β-farnesene or (E)-α-bergamotene in line 10-3 at any growth stage (Kruskal-Wallis tests followed by Bonferroni corrections for testing (E)-α-bergamotene emission twice, P > 0.2). After normalization to g fresh mass, the headspace of flowering plants contained the most (E)-α-bergamotene: about twice as much as rosette-stage and elongated plants, and about 10 times as much as plants setting seed; (E)-β-farnesene abundance did not vary strongly with plant growth stage (Fig. 5).
Figure 5. TPS10 expression results in elevated (E)-β-farnesene and (E)-α-bergamotene emission throughout plant growth (mean ± SEM, n = 5).
The legend in (A) applies to all panels. Volatiles were collected from whole shoots of rosette-stage (A, B), elongated (C, D), early flowering (E, F) or late flowering plants (G, H), either left untreated (Con), or treated with W+OS or Lan+MJ. In these measurements, W+OS and Lan+MJ elicited larger amounts of (E)-α-bergamotene emission from WT but not from 10-3. * P < 0.05, ** P < 0.01 after Bonferroni corrections following Welch’s t-tests or Wilcoxon rank-sum tests between lines within a treatment (tests were additionally conducted among treatments); P = 0.051 for WT vs. 10-3 after Lan+MJ treatment in plants setting seed; nd, not detected. Plant growth stage icons are modified from photos by D. Kessler.
TPS10 plants do not alter the defense responses of their neighbors
Although TPS10 and TPS10M plants did not alter their own defense responses to herbivory-related elicitation, we hypothesized that they might modify the defense responses of neighboring plants since priming and defense elicitation effects have frequently been shown in plants exposed to externally supplied volatiles, either as pure compounds or from the headspace of an elicited plant or plant part. Thus we tested whether WT plants growing in pots together with either of the two independently transformed TPS10 lines differed in their levels of defense metabolites and herbivore-induced volatiles. In order to assay both primed and directly elevated defenses, we sampled TPS10 plants and their neighbors both before elicitation, and after multiple applications of W+OS to the second fully-expanded leaf (position +2) for both plants in the pot. The same procedure was conducted with plants of WT and both TPS10 lines grown individually in pots of the same size, as a control for competition effects.
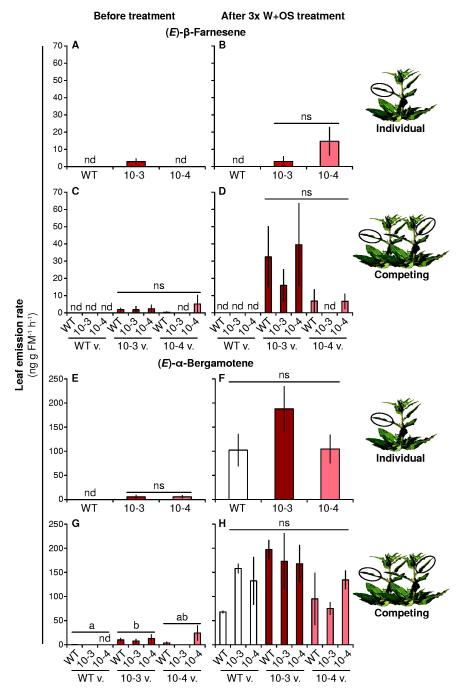
As expected, prior to elicitation, (E)-β-farnesene and (E)-α-bergamotene could be quantified in the headspace around TPS10 plants, but inconsistently and in very low amounts in WT plants (< 10% the amounts detected in the TPS10 headspace), even for WT plants with TPS10 neighbors (thus indicating little or no headspace contamination by neighbors). However, multiple rounds of elicitation resulted in greatly elevated emission of (E)-α-bergamotene in all plants, with WT becoming similar to both TPS10 lines; in contrast, (E)-β-farnesene emission was less affected by treatment, and remained undetected in the WT headspace (Fig. 5). Emission of both engineered volatiles was more robust in line 10-3 than 10-4. There was no significant effect of competition or neighbor identity on the abundance of either (E)-β-farnesene or (E)-α-bergamotene (P > 0.3 in Wilcoxon rank-sum tests or Kruskal-Wallis tests after Bonferroni corrections for between 2 and 5 tests of each data set). There was furthermore no difference among lines in the abundance of other volatiles either before or after elicitation, regardless of whether plants were grown alone or in competition; likewise, there was no effect of neighbor identity on the emission of non-target volatiles (PCAs Supplemental Fig. S4).
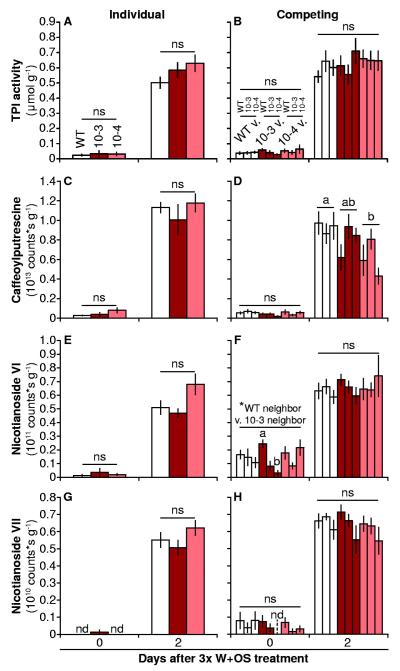
We also measured TPI activity, caffeoylputrescine, two abundant hydroxygeranyllinalool diterpene glycosides (HGL-DTGs), and nicotine in the same plants before, and 48 h after the first of the multiple W+OS elicitations. Most of these metabolites reach peak levels 72-96 h after elicitation (Steppuhn et al., 2004; Wu et al., 2006; Heiling et al., 2010); while priming effects were detected in N. attenuata plants within 3 d of exposure to sagebrush volatiles and 48 h after the onset of M. sexta feeding (Kessler et al., 2006). Thus we chose the time point 48 h after the first of multiple W+OS elicitations in order to most sensitively detect the results of priming effects on increasing metabolite levels (Fig. 7). Treatment strongly elevated the levels of all defensive metabolites measured except for nicotine, which is suppressed by M. sexta OS (von Dahl et al., 2007). However, lines did not differ, except that competing TPS10 plants had slightly lower levels of caffeoylputrescine after elicitation (ANOVA minimal model, significant effect of line: F2,47 = 4.947, corrected P = 0.0224, Tukey HSD WT v. 10-4: corrected P = 0.0163; P-values were adjusted using the Bonferroni correction for additionally testing plants grown individually v. in competition for each line, 2 to 3 tests total).
Figure 7. TPS10 plants and WT accumulate similar levels of defense metabolites after multiple elicitations, when grown alone or in competition with WT or TPS10 neighbors (mean ± SEM, n = 6).
Labels in A apply to left panels, while labels in B apply to right panels. Leaves were harvested from the same plants before (+1), and 48 h after the first of three elicitations with W+OS over 18 h across 2 d (+2), so that differences in metabolite accumulation due either to direct induction or priming of the induced response could be amplified and detected. Levels of several defense metabolites elicited by herbivory, including trypsin protease inhibitors (TPIs, A-B), caffeoylputrescine (C-D), and two malonylated hydroxygeranyllinalool diterpene glycosides (HGL-DTGs), nicotianoside VI (E-F) and VII (G-H), showed few or no differences among lines or in response to different neighbors. The Holm-Bonferroni method was used to correct for 2-3 tests of each data set (effect of line within individuals or competing pairs, effect of neighbor within competing pairs, effect of competition, and post-hoc pairwise tests); each ANOVA and its accompanying post-hoc tests were counted as single tests for P-value correction. a,bDifferent letters indicate significant differences (P < 0.05) after Holm-Bonferroni correction of Tukey’s HSD tests of genotype for caffeoylputrescine at 2 d; or of neighbor identity (WT neighbor or 10-3 neighbor) and of the interaction between line and neighbor identity (10-3 v. WT and 10-3 v. 10-4) for Nicotianoside VI at 0 d; ns, not significant.
Although constitutive levels of the HGL-DTG Nicotianoside VI were low overall, there was significant variance in competing 10-3 plants prior to treatment (ANOVA minimal model: neighbor, F2,40 = 4.697, corrected P = 0.0294; line, F2,40 = 0.570, corrected P = 1.000; neighbor:line interaction, F4,40 = 3.260, corrected P = 0.0418; Tukey HSD WT v. 10-3: corrected P = 0.0374; Tukey HSD 10-3 with WT v. 10-3 with 10-4: corrected P = 0.0351). However, there were no other significant effects of neighbor identity: during model reduction, neighbor and neighbor:line interactions were the most insignificant factors.
Individuals differed significantly from competing pairs in some measures of caffeoylputrescine (WT before treatment, W = 13, n = 5-6, corrected P = 0.0386; 10-4 after treatment, t = 4.643, df = 10.07, corrected P = 0.0018), nicotianoside VI (WT before treatment, W = 12.5, n = 5-6, corrected P = 0.0392, 10-4 before treatment, W = 11, n = 5, corrected P = 0.0297; 10-3 after treatment, t = −4.213, df = 10.86, corrected P = 0.0030), and nicotine accumulation (10-4 before treatment, t = −2.689, df = 14.99, corrected P = 0.0337). There were no other significant effects of line, neighbor, or growth in competition on metabolite levels either before or following multiple W+OS treatments.
Ectopic expression of TPS10 does not reduce growth or reproduction, nor do TPS10 plants affect the growth and reproduction of neighbors
Costs of producing defense compounds are best quantified in plants forced to compete for common resources (van Dam and Baldwin, 2001; Schwachtje et al., 2008). We therefore measured vegetative growth, and counted the production of flowers and seed capsules for WT and both lines of TPS10 grown separately and in competition. These measurements were conducted with the same plants used for the chemical measurements described above (4 replicates were used for headspace analysis and 6 for analysis of defense compounds from a total of 10 replicates for which growth and reproduction were monitored). Rosette diameter was measured from the time that rosettes were established until they ceased expanding at 40 d post-germination, and stem length was measured from the time that plants began to elongate at 25 d post-germination until the end of the experiment (Fig. 8). Multiple W+OS treatments for defense metabolite measurements were conducted from 36 to 37 d post-germination; thus, growth measurements represent relative plant sizes both before and after elicitation.
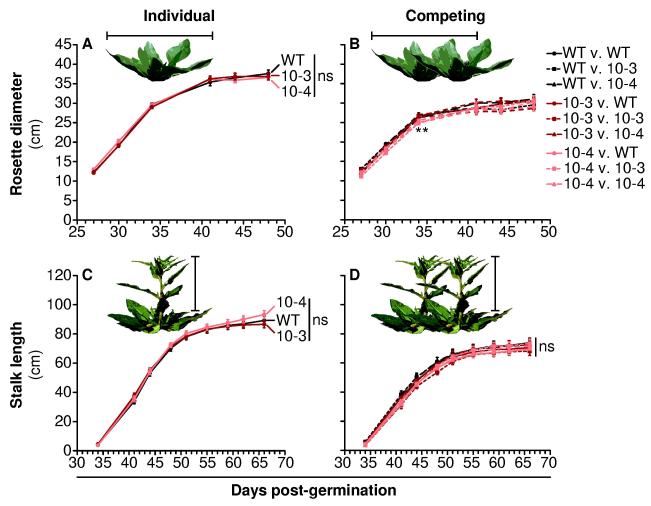
Figure 8. TPS10 plants grow similarly to WT plants both alone and in competition, and do not affect their neighbors’ growth differently than WT competitors (mean ± SEM, n = 10).
Rosette diameter (A-B) and stalk length (C-D) of plants grown individually (left panels) were greater than for plants grown in competition (right panels) for all lines (see statistical analyses in Table S5), but differed little between either of two independent lines of TPS10 and WT. Within competing pairs, neither rosette diameter nor stalk length was affected by neighbor identity (Table S5). Line 10-4 had smaller rosettes than WT or TPS10 line 10-3 on day 34 post-germination, when grown in competition: **corrected P-value < 0.01 in a Wilcoxon rank-sum tests following a significant result (corrected P = 0.0024) in a Kruskal-Wallis test among lines; ns, not significant. The Holm-Bonferroni method was used to correct for 2-4 tests of each data set (effect of line within individuals or competing pairs, effect of neighbor within competing pairs, effect of competition, and post-hoc pairwise tests).
Throughout their growth, TPS10 plants were similar in size to WT, with the exception that competing plants of line 10-4 had slightly smaller rosettes on day 34; this difference disappeared within one week, at which point rosette growth had nearly ceased (Fig. 8). There was a strong effect of competition on both rosette diameter and stalk length, with competing plants being smaller than individuals, but there was no effect of line (except for the transient difference observed in line 10-4) or neighbor identity on either growth measure (Table S5).
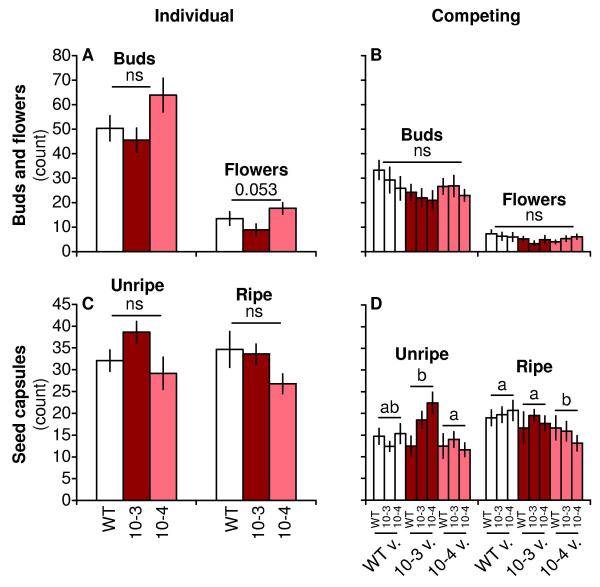
Reproduction was monitored by counting buds, flowers, and ripe and unripe seed capsules 72-73 d after germination when all plants were ripening seed. TPS10 plants were similar to WT in their production of buds, flowers, and ripe and unripe seed capsules, and neighbors of TPS10 plants were similar to neighbors of WT plants (Fig. 9). Overall, plants in competition produced fewer buds, flowers, unripe and ripe seed capsules than did individuals (statistical analysis in Table S6), and there were a few small differences among lines in seed capsule numbers when plants competed, but the only significant difference from WT was that line 10-4 produced ca. 25% fewer ripe seed capsules by the end of the experiment. There were no other significant effects of line or neighbor (Table S6).
Figure 9. TPS10 plants produce similar numbers of buds, flowers and seed capsules as WT when grown alone or in competition, and do not affect their neighbors’ reproduction differently than WT competitors (mean ± SEM, n = 10).
Plants of all lines produced more buds, flowers (A-B), and ripe and unripe seed capsules (C-D) when grown individually (left panels) versus in competition (right panels), but reproductive measures differed little between either of two independent lines of TPS10 and WT; within competing pairs, none of these parameters was affected by neighbor identity (see statistical analyses in Table S6). a,bDifferent letters indicate significant differences (corrected P < 0.05) in Tukey’s HSD tests between lines, conducted following a significant effect of line (corrected P < 0.05) in an ANOVA minimal model; ns, not significant. The Holm-Bonferroni method was used to correct for 2-4 tests of each data set (effect of line within individuals or competing pairs, effect of neighbor within competing pairs, effect of competition, and post-hoc pairwise tests).
Discussion
We specifically enhanced both constitutive and inducible emission of two sesquiterpene volatiles, (E)-β-farnesene and (E)-α-bergamotene, by ectopically expressing Zea mays TPS10 and the point mutant TPS10M (Köllner et al., 2009) under the control of the CaMV 35S promoter in Nicotiana attenuata (Figs. 1, 2, S1; Tables S1, S2). We profiled the herbivory- and jasmonate-inducible defense responses of the transformed plants at the level of phytohormone signaling and metabolite accumulation, and showed that transformants were similar to WT plants constitutively, and in response to several herbivory-related elicitors (Figs. 3, 4, S2, S3; Tables S2, S3). Our results are inconsistent with either volatile serving as a within-plant stress signal. To determine whether effects of ectopic emission could be assayed throughout plant growth, we measured the emission of (E)-β-farnesene and (E)-α-bergamotene from rosette stage through seed set and saw that TPS10 plants consistently had higher levels of emission (Fig. 5).
We then forced TPS10 plants to compete with other plants for resources and measured growth, reproduction, and defense responses in transgenic plants and their neighbors. There was no effect of transformants’ (E)-β-farnesene and (E)-α-bergamotene emission on the defense chemistry of their neighbors, even after multiple elicitation events; the sole exception was a small change in constitutive levels of a single HGL-DTG for neighbors of only one of two independently transformed lines (Figs. 6, 7, S4). This is inconsistent with a role for either sesquiterpene in eliciting or priming defense in neighboring N. attenuata plants. Furthermore, by eliciting plants multiple times with wounding and OS from the specialist herbivore Manduca sexta, we were able to produce (E)-α-bergamotene, but not (E)-β-farnesene, emission from WT plants which was similar in magnitude to the emission from TPS10 plants (Fig. 6). Finally, we observed little to no cost of ectopic TPS10 expression in terms of growth and reproduction of transformants versus their WT competitors (Figs. 8, 9; Tables S5, S6). We discuss the significance of these results in the following sections.
Figure 6. Emission of (E)-α-bergamotene, but not (E)-β-farnesene, is as great from WT plants as from TPS10 lines after multiple elicitations, but neither volatile is affected by growth of plants in close competition with WT or TPS10 (mean ± SEM, n = 4).
The headspace around +2 leaves on plants, grown either alone or in competing pairs, was sampled for 6 h before treatment (left panels), or 0-6 h after the last of three elicitations with W+OS over 18 h across 2 d (right panels). Multiple elicitations were conducted so that differences in emission due either to direct induction or priming of the induced response could be amplified and detected. (E)-β-Farnesene (A-D) and (E)-α-bergamotene (E-H) emission rates are shown here, while PCA analyses of non-target volatiles measured in the same samples are shown in Supplemental Fig. S4. a,bDifferent letters indicate significant differences (P < 0.05) in Wilcoxon rank-sum tests or Welch’s t-tests between lines following significant Kruskal-Wallis tests, after Bonferroni corrections for multiple testing; nd, not detected; ns, not significant (P > 0.05).
Constitutive expression of TPS10 or TPS10M enhances emission of (E)-β-farnesene and/or (E)-α-bergamotene, even after elicitation
Transcription of the transformed terpene synthases was constitutive (Fig. 2), as expected given that the transgenes were under control of a 35S promoter. Yet emission of (E)-α-bergamotene and (E)-β-farnesene were altered by induction treatment (Figs. 1, S1) and growth stage (Fig. 5). TPS10 plants roughly doubled emission of both target volatiles when treated with methyl jasmonate (Fig. 1B & C); in TPS10M, methyl jasmonate treatment tripled (E)-β-farnesene emission (Fig. 1D). Likewise, (E)-α-bergamotene varied over a 4-fold range between elongated and flowering growth stages (Fig. 5). The independence of emission rate from transcript level suggests that production of volatiles is also regulated by substrate supply or post-transcriptional processes.
The lack of close transcriptional control over emission contrasts with studies of terpene emission from other plant species, including poplar, hops, citrus, strawberry, and Clarkia breweri, in which emission rate and TPS transcript level were tightly correlated (Nagegowda, 2010). However, studies of Picea abies and Arabidopsis thaliana showed that levels of substrate-supplying enzymes, including DXS, DXR, GDPS, and GGDPS, can also be upregulated during times of increased isoprenoid emission (Nagegowda, 2010). Hence, in wild-type plants, production of terpene synthases and their substrates may be coordinated across ontogeny and environmental conditions by well-regulated genetic networks (Vranová et al., 2012). Constitutive expression of an ectopic TPS as in this study may disturb this network due to the occurrence of high terpene synthase levels accompanied by low substrate supply. Meeting this increased substrate demand could negatively affect primary metabolic processes, such as sterol biosynthesis, that depend on supply of the sesquiterpene precursor FDP (Vranová et al., 2012). However, the normal growth, development, and emission of non-target volatiles from of our transgenic lines indicate that they were able to compensate for this increased demand, perhaps by upregulating FDP production. The increased sesquiterpene production from both WT and transgenic plants following herbivore-related elicitation may be at least partially attributable to increased FDP supply (Figs. 1, S1).
Production of (E)-β-farnesene and (E)-α-bergamotene does not induce or prime defense responses in transgenic plants or their neighbors
Previous studies have implicated plant volatiles in within- and between-plant signaling. In a variety of species, exposure to the volatiles of herbivore-damaged tissues results in induction of defense against herbivores, increased transcription of defense-related genes, and priming – increased responsiveness to subsequent herbivore challenge (Heil and Karban, 2010). Terpenes specifically have been shown to alter transcriptional profiles in A. thaliana (Godard et al., 2008). Likewise, volatile-mediated induction of extrafloral nectar production by Phaseolus lunatus is compromised by destruction of several terpenoid components under high-ozone conditions (Blande et al., 2010). About 60% of genes regulated in neighboring N. attenuata plants after 48 h exposure to the headspace of W+OS-elicited neighbors may be regulated by induced terpenes including (E)-α-bergamotene (Paschold et al., 2006), although no direct or priming effects were observed in neighbors as a result of these changes in gene expression.
To test whether (E)-β-farnesene and (E)-α-bergamotene induced defenses in emitters themselves or in their neighbors, we measured constitutive levels of secondary metabolites in transgenic plants and neighboring WT plants. If exposure to these sesquiterpenes induced defenses directly, we would have expected increased levels of secondary metabolites and phytohormones in both the transgenics, which were exposed to their own volatile emissions, and in neighboring WT growing close to the emitters. In contrast, our experiments showed that in uninduced plants, emitters did not differ from WT in levels of phytohormones (Fig. 3), defense metabolites (Fig. 4), or non-target volatiles (Supplemental Figs. S2 and S3). When transgenic plants were grown in competition with WT, neither emitters nor their neighbors differed from WT in levels of defense metabolites (Fig. 7) or non-target volatiles (Supplemental Fig. S4) compared to WT grown with other WT.
If exposure to (E)-β-farnesene and (E)-α-bergamotene had primed defenses rather than inducing them directly, we would have expected greater responsiveness to elicitation in volatile-producing or -exposed plants, i.e., in transgenics themselves and in WT plants competing with transgenic neighbors. On the contrary, responses to induction were similar between WT and transgenics in terms of phytohormones (Fig. 3) and defense metabolites (Fig. 4), as well as non-target volatiles (Supplemental Figs. S2 and S3). Likewise, when WT was grown with transgenic neighbors, responses to induction were similar in magnitude to WT grown with other WT, as measured by volatile emission (Fig. 6) and defense metabolite levels (Fig. 7). Although N. attenuata has been shown to respond to both conspecific (Paschold et al., 2006) and heterospecific plant volatiles (Kessler et al., 2006), (E)-β-farnesene and (E)-α-bergamotene appear not to alter defense responses in this species.
There is little or no physiological cost of the enhanced emission of (E)-β-farnesene and (E)-α-bergamotene
Our experiments did not demonstrate any consistent physiological costs of either transformation or emission of (E)-β-farnesene and (E)-α-bergamotene. Production of secondary metabolites is believed to be costly, which may explain why many compounds are synthesized under specific environmental conditions rather than constitutively (Karban and Baldwin, 1997). Physiological costs result from diversion of resources away from growth and reproduction. If production of sesquiterpenes imposed a high physiological cost, we would have expected impaired vegetative growth or reproductive output from transformed plants. For example, transformation of maize with a constitutively expressed sesquiterpene synthase resulted in reduced seed germination, growth, and yield under field conditions (Robert et al., 2013).
However, our transgenic plants grew and developed normally: when plants were grown alone, neither rosette diameter nor stalk diameter differed (Fig. 8), and reproductive output was also similar to that of WT plants (Fig. 9 A & C). Because physiological costs may be more readily apparent under competition (Van Dam and Baldwin, 1998), we also assessed growth and reproduction with WT and transgenic plants grown together in the same pot. Even under direct competition, transgenic plants had no consistent disadvantages in rosette growth, stalk elongation, or flower or seed production when compared to WT (Fig. 9 B & D).
The seemingly negligible physiological costs of (E)-β-farnesene and (E)-α-bergamotene emission, combined with their demonstrated benefits, raise the question of why these volatiles are not produced constitutively in nature. One possible explanation may be that physiological costs only become apparent under adverse environmental conditions. Our characterization of the transgenic plants was conducted on amply watered and fertilized plants grown in a climate-controlled glasshouse environment, and under these conditions, growth was likely limited by rooting volume and not by other factors. Results might differ under field conditions that present stresses such as water scarcity, nutrient limitation, and variable temperature, as well as leaf area removal from herbivores or possible attack by pathogens.
Another possibility is that sesquiterpene production carries ecological costs that can only be observed in the plant’s natural habitat (Karban and Baldwin, 1997). One ecological cost of emission could be the attraction of specialists. For example, maize plants that constitutively emitted (E)-β-caryophyllene and α-humulene attracted more larvae of the specialist herbivore Diabrotica virgifera (Robert et al., 2013). A second possible ecological cost results from “crying wolf”–plants which emit large amounts of herbivore-induced volatiles independently of prey abundance may attract naive natural enemies of herbivores, but experienced natural enemies may learn that those volatiles are unreliable indicators of prey presence (Shiojiri et al., 2010). Thus it is interesting to what extent the inducibility of sesquiterpene emission is frequency-dependent in wild populations, and the transgenic plants described here could be used to set up otherwise isogenic experimental populations to test hypotheses about frequency-dependent costs and benefits of constitutively enhanced sesquiterpene emission.
A third ecological cost could include repellence of beneficial insects, such as pollinators. Herbivore-induced changes in volatile emission have been shown to decrease pollinator services to Solanum peruvianum (Kessler et al., 2011), and pollinating moths of the herbivore M. sexta have been shown to avoid oviposition on – and perhaps also pollination of – N. attenuata plants supplemented with (E)-α-bergamotene (Kessler and Baldwin, 2001). Suggestive evidence that pollinators are specifically repelled by sesquiterpenes comes from a comparison of A. thaliana with its sister species, A. lyrata. In the self-pollinating A. thaliana, an (E)-β-caryophyllene synthase is expressed constitutively in floral tissue, whereas in the outcrossing A. lyrata, the homologous sesquiterpene synthase is expressed only after herbivory (Abel et al., 2009). This phylogenetic comparison suggests that (E)-β-caryophyllene, and possibly other herbivore-induced sesquiterpenes, could decrease floral attractiveness to pollinators as well as herbivores. Finally, a fourth possible ecological cost is the deterrence of beneficial microbes. Sesquiterpenes have demonstrated antimicrobial properties both in vitro and in planta, which can protect plants from pathogens (Huang et al., 2010). However, these antimicrobial properties might be undesirable if they reduce colonization by mutualist or commensalist flora. In Tropaeolum majus and Carica papaya, for example, jasmonate treatment impaired colonization of roots with mycorrhizae (Ludwig-Müller et al., 2002), and monoterpenes and a sesquiterpene from Pinus sylvestris inhibited mycorrhizal growth in vitro (Melin and Krupa, 1971). Since the transgenic lines described here seem to bear few or no physiological costs, they may be useful tools with which to test hypotheses about ecological costs of sesquiterpene production under field conditions.
Conclusions
In conclusion, we propose that in N. attenuata, (E)-α-bergamotene and (E)-3-farnesene have ecological rather than physiological functions in plants’ response to herbivory, and furthermore that these ecological effects are mediated by the response of arthropods, such as herbivores and their predators, to these volatiles. The transgenic lines described here are excellent candidates for experiments to test the ecological consequences of constitutively enhanced emission of both volatiles, especially because the ectopic expression seems to have little or no direct effect on plant defense, growth, or reproduction, permitting the straightforward interpretation of ecological data.
Materials and Methods
Plants and growth conditions
N. attenuata Torr. Ex. Wats. (Solanaceae) wild-type (WT) plants were from a 31st-generation inbred line described by Krügel et al. (2002). The full-length Zea mays TERPENE SYNTHASE 10 (TPS10) B73 allele (Schnee et al., 2006; GenBank Accession Number AY928078), or the gene for the point mutant TPS10-B73 L356F (TPS10M) (Köllner et al., 2009) were transferred out of plasmids kindly provided by E. Pichersky and T. Küllner into the pSOL9 plasmid, after first confirming correct gene sequences by Sanger sequencing of BigDye reactions (www.lifetechnologies.com) using vector primers and primers designed mid-sequence to obtain full sequence coverage. Vector construction and the pSOL9 plasmid have been described previously (Gase et al., 2011). The A. tumefaciens (strain LBA 404)-mediated transformation procedure described by Krügel et al. (2002) was used to generate multiple stably transformed N. attenuata lines expressing TPS10 and TPS10M under control of the CaMV 35S promoter in the pSOL9 plasmid.
Homozygosity of T2 plants was determined by screening for resistance to hygromycin (Gase et al., 2011) provided by the HYGROMYCIN PHOSPHOTRANSFERASE II [HPTII] gene from pCAMBIA-1301 in the pSOL9 vector. High-quality genomic DNA was extracted using a modified cetyltrimethylammonium bromide (CTAB) method (Bubner et al., 2004) from non-senescent leaf tissue collected from transformed plants, flash-frozen and ground as described below. Southern blotting was performed to identify lines with single transgene insertions using a probe for HPTII as previously described (Gase et al., 2011) after digestion of genomic DNA with Xbai or EcoRI (Supplemental Fig. S1). A PCR was conducted with genomic DNA template to confirm full T-DNA insertions in these lines as previously described (Gase et al., 2011). Flow cytometric analysis (described by Bubner et al., 2006) confirmed that all lines were diploid. Two independently transformed diploid, homozygous lines of each construct with single transgene insertions and having the predicted pattern of (E)-β-farnesene and (E)-α-bergamotene emission (Supplemental Fig. S1) were chosen for further characterization.
Germination and seedling growth conditions were as described by Krügel et al. (2002). Petri dishes with 20-30 germinated seeds each were kept in a growth chamber Percival Scientific CU-36L, (www.percival-scientific.com) at 26°C/16 h of 105.4 μmol s−1 m−2 light (measured using an LI-COR LI-250A Light Meter und Sensor LI-COR Quantum Q 35195 www.licor.com), 24°C/8 h dark for 10 d and then transferred to TEKU JP 3050 104 pots (www.poeppelmann.com) with plug soil (www.klasmann-deilmann.com). From then on, plants were cultivated in the glasshouse with a day/night cycle of 16 h/8 h under daylight supplemented with 1000-1300 μmol m−2 s−1 PAR supplied by Na-vapor high-intensity discharge bulbs (Master Sun-T PIA Agro 400 W or Master Sun-T PIA Plus 600 W sodium lights, www.philips.com). Air temperatures in the glasshouse were 23-35°C during the day and 19-23°C at night; relative humidity was 40%-55%. Twenty days after germination, individual small rosette plants were transplanted to 1 L plastic pots, or, for the competition experiment, two size-matched plants were paired in a 2 L pot as described by Schwachtje et al. (2008). Pots contained Fruhsdorfer Nullerde (www.hawita-gruppe.de) with 0.5 g/L PG mix (www.yara.co.uk), 0.9 g/L Superphosphat (www.triferto.org), 0.35 g/L MgSO4*7 H2O (www.sigmaaldrich.com), and 0.055 g/L Micro Max (www.everris.com). Water and fertilizer were supplied daily using an automatic flood irrigation system: at 21 days post-germination (dpg) 0.0075 g/L Bor-Folicin (www.jostgroup.com) were added to the 400 L watering tank and at 27 dpg 0.05 g L−1 of Peters Allrounder 20:20:20 (www.scottsprofessional.com) and 0.005 g L−1 Folicin-Bor were added to the tank; at 34 dpg, 0.1 g L−1 Peters Allrounder 20:20:20 and 0.0025 g L−1 Folicin-Bor were added; from 41 dpg, conductivity was maintained by weekly addition of 0.0625-0.075 g L−1 Peters Allrounder 20:20:20.
Treatment with herbivory and jasmonate elicitors
The leaf undergoing the source/sink transition (position 0) and the first two fully expanded leaves (positions +1 and +2) were used to assay defense metabolites, transcript and phytohormone abundance, and volatile emission, which are known to respond strongly to local elicitation in leaves at these positions (Halitschke et al., 2000; van Dam et al., 2001; Wang et al., 2008). Single elicitations with wounding and Manduca sexta oral secretions (W+OS) were performed in order to reproducibly mimic herbivore elicitation, by making 3 rows of holes on either side of the leaf midvein along the adaxial surface using a pattern wheel, applying 20 μL of M. sexta OS (from 4th-5th instar larvae fed on WT N. attenuata plants) diluted 1:5 with distilled water, and rubbing diluted OS into holes with a gloved finger as described (Halitschke et al., 2001; Schittko et al., 2001; Schuman et al., 2012). The same treatment using distilled water instead of M. sexta OS served as a wounding control (W+W). Multiple W+OS treatment of plants grown in competition was used as a standardized method to provide multiple herbivore elicitation events: leaves were wounded by creating two roles of holes from the adaxial surface of the +2 leaf, one on each side of the leaf midvein, and adding 5 μL of 1:5 diluted M. sexta OS to each row; this was done 3 times over 18 h across 2 d, from the edge of the leaf toward the midvein. Methyl jasmonate, which is demethylated by plants to generate jasmonic acid (Wu et al., 2008), was applied to treated leaves by dissolving 150 μg racemic methyl jasmonate in 20 μL lanolin (www.sigmaaldrich.com) and gently applying the lanolin paste to the base of the adaxial leaf surface by the petiole (Lan+MJ); application of lanolin paste in the same manner served as the Lan control (Preston and Baldwin, 1999; Kessler and Baldwin, 2001; Heiling et al., 2010). Plant treatments were always begun in the morning.
Headspace analyses
For constitutive measurements (Con) during the pre-screening of transformed lines (Supplemental Fig. S1), headspaces were collected for 8 h during the day from the +2 leaf on rosette-stage plants. The following morning, the neighboring +1 leaf was treated with W+OS, and the headspace was collected from 24-32 h to measure induced sesquiterpenes (Halitschke et al., 2000). For the volatile collections in Figs. 1, S2 and S3, +2 leaves of plants were treated as described above and the headspaces were immediately collected for 3 h, and then again for 24-32 h after treatment. For whole-plant volatile collections (Fig. 5), treatments were applied to the +2 leaf in the rosette and elongating stage, or on the first true stem leaf (S1) in the early and late flowering stages. For plants in the competition experiment (Fig. 6), the headspace was collected from +2 leaves for 6 h during the day, after which leaves were treated three times with W+OS as described above and then the headspace was immediately collected again for 6 h during the following day.
Headspace collection of single leaves was conducted by enclosing leaves on plants in two 50 mL PET cups (www2.huhtamaki.com) lined on the edges with foam to protect leaves and with an activated charcoal filter attached to one side for incoming air, and secured with miniature claw-style hair clips. Headspace VOCs were collected on 20 mg of Porapak Q (Tholl et al., 2006, www.sigmaaldrich.com) in self-packed filters (bodies and materials from www.ars-fla.com) by drawing ambient air through these clip cages at 300 mL min−1 using a manifold with screw-close valves set to provide equal outflow, via pushing air at 2 to 3 bar through a Venturi aspirator as described previously by Oh et al. (2012) and based on procedures from Halitschke et al. (2000) and Schuman et al. (2009). Background VOCs present in ambient air were collected using empty trapping containers and background signals were later subtracted if necessary from raw intensities of plant samples prior to further processing. To sample from whole shoots (Fig. 5), additional pressurized air was filtered through activated charcoal and pushed into custom-cut clean PET bags of equal volumes for each growth stage (rosette: push 500 mL min−1; all other stages: push 1 L min−1), and 400 mL min−1 were pulled through the Porapak Q filter. The ratio of air in to air out was factored into the final quantification of these headspace samples: signals from rosette-stage plants were multiplied by 5/4, and from other stages by 5/2, to account for loss due to overpressure.
Porapak Q filters were either immediately spiked with 320 ng tetralin as an internal standard and eluted with 250 μL of dichloromethane into a 1.5 mL GC vial containing a 250 μL glass insert, or stored at −20°C until tetralin addition and elution. Samples were analyzed on one of two different GC-MS instruments from Varian with columns from Phenomenex (30 m×0.25 mm i.d., 0.25 μm film thickness; www.phenomenex.com). One μL of each sample was injected in splitless mode, and then the injectors were returned to a 1:70 split ratio from 2 min after injection through the end of each run. He carrier gas was used with a column flow of 1 mL min−1. Samples collected from 0-3 h after treatment were analyzed by a CP-3800 GC Saturn 2000 ion trap MS with a polar ZB-wax column and a CP-8200 autoinjector; the GC and MS were programmed as previously described for this instrument (Schuman et al., 2012), and compounds were separated by a temperature ramp of 5°C min−1 between 40°C and 185°C. All other headspace samples were analyzed on a CP-3800 GC coupled to a Saturn 4000 ion-trap mass spectrometer with a nonpolar ZB5 column and a CP-8400 autoinjector; The GC and MS were programmed as previously described for this instrument (Oh et al., 2012), and compounds were separated by a temperature ramp of 5°C min−1 between 40°C and 180°C.
Individual volatile compound peaks were quantified using the combined peak area of two specific and abundant ion traces per compound using MS Work Station Data Analysis software (Varian) and normalized by the 104+132 ion trace peak area from tetralin in each sample. The identification of compounds was conducted by comparing GC retention times and mass spectra to those of standards and mass spectra databases: Wiley version 6 (www.wiley.com) and NIST (National Institute of Standards and Technology, www.nist.gov) spectral libraries. Values in ng for (E)-α-bergamotene and (E)-β-farnesene in Fig. 1 were determined using standard curves with a co-injected tetralin standard curve to determine response factors versus tetralin. The standard curve for (E)-α-bergamotene used another bicyclic sesquiterpene olefin, (E)-β-caryophyllene, since no pure standard of (E)-α-bergamotene was available. These response factors were used to calculate ng values for (E)-β-farnesene and (E)-α-bergamotene in all other data sets analyzed on the same instrument (Supplemental Figs. S1, 5, and 6).
The area of trapped leaves was quantified for comparison by scanning and calculating areas in pixels using SigmaScan (www.systat.com), then converting pixels to cm2 using a size standard which was scanned with leaves. Volatiles were expressed either in ng tetralin per cm2 leaf area, or in absolute ng per cm2 leaf area.
Collection and processing of plant tissue samples
Treated whole leaves were cut at the petiole, wrapped securely in aluminum foil, and flash-frozen in liquid nitrogen within less than 1 min after removal from the plant. Frozen tissue samples were stored at −80°C until further processing. Leaf tissue was ground over liquid N2 by crushing and transfer to 2 mL microcentrifuge tubes followed by grinding with small plastic pestles or a Genogrinder 2000 (www.spexsampleprep.com) to a fine, homogeneous powder, which was weighed out over liquid N2 for extraction and analysis of hormones, transcripts, or metabolites, or stored at −80°C.
Determination of transcript abundance
Transcript abundance was determined in +1 leaves harvested 1 h after treatment as described above. RNA was extracted from ca. 100 mg ground leaf tissue using TRI reagent © (www.sigmaaldrich.com) according to the manufacturer’s instructions. RNA quality was checked on a 1% agarose gel (agarose from www.sigmaaldrich.com), and concentration was measured at 260 nm using a NanoDrop ND-1000 spectrophotometer (www.nanodrop.com). Synthesis of cDNA from 0.5 μg of total RNA per sample and SYBR® Green qPCR analyses were conducted as in Wu et al. (2007) using reagents from www.thermoscientificbio.com/fermentas, a Mastercycler (www.eppendorf.com) and an Mx3005P qPCR system (www.stratagene.com) and qPCR Core Kit for SYBR® Green I (www.eurogentec.com). We designed a primer pair to amplify a 158 bp product from TPS10 and TPS10M: NaTPS10_FWD1 (TTGTTGGGATGGGTGACA) and NaTPS10_RVS1 (TTGGACCGTGGACAC A), previously used by Fragoso et al. (2011). Transcript abundance was normalized to the abundance of N. attenuata ELONGATION FACTOR 1a (NaEF1a) transcripts in each sample (Kaur et al., 2012) using primers Nt_EF1a_FWD2 (CCACACTTCCCACATTGCTGTCA) and Nt_EF1a_RVS2 (CGCATGTCCCTCACAGCAAAAC). Samples of RNA used to make cDNA were pooled to the same dilution as in cDNA samples and run alongside cDNA in all qPCRs to control for gDNA contamination; no contamination was detected.
Quantification of phytohormones
Phytohormone accumulation was determined in +1 leaves harvested 1 h after treatment as described above. Phytohormones were extracted from ca. 100 mg ground leaf tissue using ethyl acetate spiked with internal standards and quantified by liquid chromatography-electrospray ionization-triple quadrupole tandem mass spectrometry (LC-ESI-MS/MS, Varian) as described by Oh et al. (2012), with the modification that 100 ng rather than 200 ng of [2H2]JA, and 20 ng rather than 40 ng of JA-[13C6]Ile, [2H4]SA, and [2H6]ABA were used as internal standards. Individual hormones were quantified in ng by comparison to the corresponding internal standard peak area and normalized per g leaf tissue fresh mass (FM).
Quantification of defense metabolites
Frozen, ground tissue (100 mg) from 0 leaves collected 3 d after treatment (Fig. 4, Tables S3, S4) as described above was extracted and analyzed as described by Oh et al. (2012) for a combined analysis of nicotine and phenolic metabolites by high performance liquid chromatography-UV diode array detector (HPLC-UV-DAD, Keinänen et al., 2001), and individual DTGs by LC-ESI-MS/MS (Heiling et al., 2010). Nicotine, chlorogenic acid, and rutin were quantified in μg using external standard curves, and the chlorogenic acid standard curve was used to quantify cryptochlorogenic acid and caffeoylputrescine as chlorogenic acid (CA) equivalents; for HGL-DTGs, peak areas were normalized to the peak area of the internal standard glycyrrhizinic acid. Values (μg, CA equivalents or normalized peak areas) were normalized to g leaf tissue fresh mass (FM).
Frozen, ground tissue (100 mg) from +1 leaves harvested before, or +2 leaves collected from the same plants 48 h after multiple W+OS treatments (Fig. 7) was used to extract and profile metabolites by liquid chromatography-electrospray ionization-micro time of flight-mass spectrometry (LC-ESI-microToF-MS) in positive mode as described by Gaquerel et al. (2010) with specific modifications described by Kim et al. (2011) and a 2 μL injection volume. This permitted quantification of nicotine, phenolics, and individual HGL-DTGs in the same run. Metabolites having a robust signal-to-noise ratio (> 10) were selected and peaks were integrated using Data Analysis v 4.0 and Quant Analysis (www.bruker.com). Peak areas were normalized to g leaf tissue fresh mass (FM). TPI activity levels were determined relative to mg protein in 100 mg of frozen, ground tissue from the same leaf samples (Fig. 7) using the extraction, Bradford assay, and radial diffusion assay described by Van Dam et al. (2001) based on the protocol from Jongsma et al. (1993) and using materials from Sigma-Aldrich (www.sigmaaldrich.com).
Measurements of plant growth and reproduction
Plant size (maximum rosette diameter and stem length) was monitored for plants grown in competition and singly grown controls: rosette diameter was measured as the maximum diameter found by gently laying a ruler over the rosette, and stem length was measured from the base of the stem to the tip of the apical inflorescence by placing a ruler beside the stem. Reproductive output was quantified by counting the total number of buds (> 2 mm long), flowers (corolla visible and turgid), unripe seed capsules (green, visible past calyx), and ripe seed capsules (browning, dry).
Statistical analyses
Summary statistics were calculated in Excel (www.microsoft.com). Further statistical tests were performed in R version 3.0.2 using RStudio version 0.98.501 (R core team 2012). We checked treatment groups graphically (quantile-quantile plots, residual v. fitted plots) and statistically (Shapiro-Wilk test, Bartlett test) for normality and homoscedasticity. Multiple groups were compared by ANOVA when requirements of normality and homoscedasticity were met for raw or natural log transformed data, using the stepwise model simplification approach in R to determine the minimal adequate model in which either all factors contributed significantly or else only the least insignificant factor remained (Crawley, 2013), followed by Tukey’s HSD post-hoc tests. Binary comparisons were made using Welch’s t-tests when at least the requirement of normality was met. Non-parametric data were analyzed using Kruskal-Wallis tests when comparing > 2 groups, or Wilcoxon rank-sum tests for binary comparisons.
Bonferroni P-value corrections, employing the Holm modification when applicable, were calculated in Excel (www.microsoft.com) for families of tests on the same data. Both corrections control the familywise error rate and are simple to calculate, but the Holm-Bonferroni method is more powerful (Holm, 1979): briefly, all P-values in a family of tests conducted on the same data set and subsets are listed from smallest to largest. The smallest P-value is multiplied by the total number of tests (n) conducted on that data. If the resulting corrected P-value is < 0.05, then the next-smallest P-value is multiplied by n-1. If the resulting corrected P-value is < 0.05, then the third smallest P-value is multiplied by n-2, and so on. At the first correction resulting in a P-value ≥ 0.05, that P-value and all larger P-values are considered non-significant. The corrected P-values reported are the products of Bonferroni or Holm-Bonferroni corrections as noted, e.g. (P-value)*(n tests) or (second smallest P-value)*(n-1).
Supplementary Material
Acknowledgments
We gratefully acknowledge R. Nagel for assistance during the cloning of TPS10 and TPS10M sequences provided by E. Pichersky and T. Köllner into the pSOL9 plasmid, W. Kröber, S. Kutschbach, and A. Wissgott for plant transformation with A. tumefaciens and generation of T0 plants, the gardening staff of the Max Planck Institute for Chemical Ecology for caring for plants in the glasshouse, and D. Veit for constructing and providing equipment for volatile collection. We thank several capable student interns: K. Barthel, J. Koenig, R. Ludwig, T. Mentrup, B. Naumann, N. Peterson, A. Rauch, I. Schmidt, and D. Schweizer, each of whom assisted the authors with at least one glasshouse experiment requiring careful hands and precise timing, or in preparing samples for analysis.
Financial Sources: Max Planck Society (all), International Max Planck Research School (MCS), European Research Council advanced grant ClockworkGreen (No. 293926) to ITB (ITB, MCS), NSF GRFP (Graduate Research Fellowship Program) Grant No. S121000000211 (ECPY)
Literature Cited
- Abel C, Clauss M, Schaub A, Gershenzon J, Tholl D. Floral and insect-induced volatile formation in Arabidopsis lyrata ssp. petraea, a perennial, outcrossing relative of A. thaliana. Planta. 2009;230:1–11. doi: 10.1007/s00425-009-0921-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, Giri AP, Deuerlein S, Griepink F, Kogel W De, Verstappen FWA, Verhoeven HA, Jongsma MA, Schwab W, Bouwmeester HJ. Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell. 2003;15:2866–2884. doi: 10.1105/tpc.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, Jongsma M a, Bouwmeester HJ. Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci. 2005;10:594–602. doi: 10.1016/j.tplants.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Allmann S, Halitschke R, Schuurink RC, Baldwin IT. Oxylipin channelling in Nicotiana attenuata: lipoxygenase 2 supplies substrates for green leaf volatile production. Plant Cell Environ. 2010;33:2028–2040. doi: 10.1111/j.1365-3040.2010.02203.x. [DOI] [PubMed] [Google Scholar]
- Asai N, Nishioka T, Takabayashi J, Furuichi T. Plant volatiles regulate the activities of Ca2+-permeable channels and promote cytoplasmic calcium transients in Arabidopsis leaf cells. Plant Signal Behav. 2009;4:294–300. doi: 10.4161/psb.4.4.8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT, Halitschke R, Paschold A, Von Dahl CC, Preston CA. Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science. 2006;311:812–815. doi: 10.1126/science.1118446. [DOI] [PubMed] [Google Scholar]
- Blande JD, Holopainen JK, Li T. Air pollution impedes plant-to-plant communication by volatiles. Ecol Lett. 2010;13:1172–1181. doi: 10.1111/j.1461-0248.2010.01510.x. [DOI] [PubMed] [Google Scholar]
- Bubner B, Gase K, Baldwin IT. Two-fold differences are the detection limit for determining transgene copy numbers in plants by real-time PCR. BMC Biotechnol. 2004;4:14. doi: 10.1186/1472-6750-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubner B, Gase K, Berger B, Link D, Baldwin IT. Occurrence of tetraploidy in Nicotiana attenuata plants after Agrobacterium-mediated transformation is genotype specific but independent of polysomaty of explant tissue. Plant Cell Rep. 2006;25:668–675. doi: 10.1007/s00299-005-0111-4. [DOI] [PubMed] [Google Scholar]
- Chen F, Tholl D. The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011;66:212–229. doi: 10.1111/j.1365-313X.2011.04520.x. [DOI] [PubMed] [Google Scholar]
- Crawley MJ. The R Book. Ed 2 John Wiley & Sons; Chichester, West Sussex, UK: 2013. p. 1076. [Google Scholar]
- Von Dahl CC, Winz RA, Halitschke R, Kuhnemann F, Gase K, Baldwin IT. Tuning the herbivore-induced ethylene burst: the role of transcript accumulation and ethylene perception in Nicotiana attenuata. Plant J. 2007;51:293–307. doi: 10.1111/j.1365-313X.2007.03142.x. [DOI] [PubMed] [Google Scholar]
- Van Dam NM, Baldwin I. Costs of jasmonate-induced responses in plants competing for limited resources. Ecol Lett. 1998;1:30–33. [Google Scholar]
- Van Dam NM, Baldwin IT. Competition mediates costs of jasmonate-induced defences , nitrogen acquisition and transgenerational plasticity in Nicotiana attenuata. Funct Ecol. 2001;15:406–415. [Google Scholar]
- Van Dam NM, Horn M, Mares M, Baldwin IT. Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. J Chem Ecol. 2001;27:547–568. doi: 10.1023/a:1010341022761. [DOI] [PubMed] [Google Scholar]
- Degenhardt J, Gershenzon J, Baldwin IT, Kessler A. Attracting friends to feast on foes: engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr Opin Biotechnol. 2003;14:169–176. doi: 10.1016/s0958-1669(03)00025-9. [DOI] [PubMed] [Google Scholar]
- Degenhardt J, Hiltpold I, Köllner TG, Frey M, Gierl A, Gershenzon J, Hibbard BE, Ellersieck MR, Turlings TCJ. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc Natl Acad Sci U S A. 2009a;106:13213–13218. doi: 10.1073/pnas.0906365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt J, Köllner TG, Gershenzon J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry. 2009b;70:1621–1637. doi: 10.1016/j.phytochem.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: beyond the “cry for help.”. Trends Plant Sci. 2010;15:167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Dinh ST, Baldwin IT, Gális I. The HERBIVORE ELICITOR-REGULATED1 (HER1) gene enhances abscisic acid levels and defenses against herbivores in Nicotiana attenuata plants. Plant Physiol. 2013;162:2106–2124. doi: 10.1104/pp.113.221150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Pichersky E. Metabolic engineering of plant volatiles. Curr Opin Biotechnol. 2008;19:181–189. doi: 10.1016/j.copbio.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Duhl TR, Helmig D, Guenther A. Sesquiterpene emissions from vegetation: a review. Biogeosciences. 2008;5:761–777. [Google Scholar]
- Fragoso V, Goddard H, Baldwin IT, Kim S-G. A simple and efficient micrografting method for stably transformed Nicotiana attenuata plants to examine shoot-root signaling. Plant Methods. 2011;7:34. doi: 10.1186/1746-4811-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaquerel E, Heiling S, Schoettner M, Zurek G, Baldwin IT. Development and validation of a liquid chromatography-electrospray ionization-time-of-flight mass spectrometry method for induced changes in Nicotiana attenuata leaves during simulated herbivory. J Agric Food Chem. 2010;58:9418–9427. doi: 10.1021/jf1017737. [DOI] [PubMed] [Google Scholar]
- Gase K, Weinhold A, Bozorov T, Schuck S, Baldwin IT. Efficient screening of transgenic plant lines for ecological research. Mol Ecol Resour. 2011;11:890–902. doi: 10.1111/j.1755-0998.2011.03017.x. [DOI] [PubMed] [Google Scholar]
- Gilardoni PA, Hettenhausen C, Baldwin IT, Bonaventure G. Nicotiana attenuata LECTIN RECEPTOR KINASE1 suppresses the insect-mediated inhibition of induced defense responses during Manduca sexta herbivory. Plant Cell. 2011;23:3512–3532. doi: 10.1105/tpc.111.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godard K-A, White R, Bohlmann J. Monoterpene-induced molecular responses in Arabidopsis thaliana. Phytochemistry. 2008;69:1838–1849. doi: 10.1016/j.phytochem.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Halitschke R, Keßler A, Kahl J, Lorenz A, Baldwin IT. Ecophysiological comparison of direct and indirect defenses in Nicotiana attenuata. Oecologia. 2000;124:408–417. doi: 10.1007/s004420000389. [DOI] [PubMed] [Google Scholar]
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 2001;125:711–717. doi: 10.1104/pp.125.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halitschke R, Stenberg JA, Kessler D, Kessler A, Baldwin IT. Shared signals–“alarm calls” from plants increase apparency to herbivores and their enemies in nature. Ecol Lett. 2008;11:24–34. doi: 10.1111/j.1461-0248.2007.01123.x. [DOI] [PubMed] [Google Scholar]
- Heil M, Karban R. Explaining evolution of plant communication by airborne signals. Trends Ecol Evol. 2010;25:137–144. doi: 10.1016/j.tree.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Heil M, Silva Bueno JC. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci U S A. 2007;104:5467–5472. doi: 10.1073/pnas.0610266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiling S, Schuman MC, Schoettner M, Mukerjee P, Berger B, Schneider B, Jassbi AR, Baldwin IT. Jasmonate and ppHsystemin regulate key malonylation steps in the biosynthesis of 17-hydroxygeranyllinalool diterpene glycosides, an abundant and effective direct defense against herbivores in Nicotiana attenuata. Plant Cell. 2010;22:273–292. doi: 10.1105/tpc.109.071449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Holopainen JK, Gershenzon J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010;15:176–184. doi: 10.1016/j.tplants.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Houshyani B, Assareh M, Busquets A, Ferrer A, Bouwmeester HJ, Kappers IF. Three-step pathway engineering results in more incidence rate and higher emission of nerolidol and improved attraction of Diadegma semiclausum. Metab Eng. 2013;15:88–97. doi: 10.1016/j.ymben.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Huang M, Abel C, Sohrabi R, Petri J, Haupt I, Cosimano J, Gershenzon J, Tholl D. Variation of herbivore-induced volatile terpenes among Arabidopsis ecotypes depends on allelic differences and subcellular targeting of two terpene synthases, TPS02 and TPS03. Plant Physiol. 2010;153:1293–1310. doi: 10.1104/pp.110.154864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma MA, Bakker PL, Stiekema WJ. Quantitative determination of serine proteinase inhibitor activity using a radial diffusion assay. Anal Biochem. 1993;212:79–84. doi: 10.1006/abio.1993.1294. [DOI] [PubMed] [Google Scholar]
- Kallenbach M, Oh Y, Eilers EJ, Veit D, Baldwin IT, Schuman MC. A robust, simple, high-throughput technique for time-resolved plant volatile analysis in field experiments. Plant J. 2014;78:1060–1072. doi: 10.1111/tpj.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappers IF, Aharoni A, Van Herpen TWJM, Luckerhoff LLP, Dicke M, Bouwmeester HJ. Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science. 2005;309:2070–2072. doi: 10.1126/science.1116232. [DOI] [PubMed] [Google Scholar]
- Karban R, Baldwin IT. Induced responses to herbivory. University of Chicago Press; Chicago, IL, USA: 1997. p. 319. [Google Scholar]
- Kaur H, Heinzel N, Schöttner M, Baldwin IT, Gális I. R2R3-NaMYB8 regulates the accumulation of phenylpropanoid-polyamine conjugates, which are essential for local and systemic defense against insect herbivores in Nicotiana attenuata. Plant Physiol. 2010;152:1731–1747. doi: 10.1104/pp.109.151738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Shaker K, Heinzel N, Ralph J, Gális I, Baldwin IT. Environmental stresses of field growth allow cinnamyl alcohol dehydrogenase-deficient Nicotiana attenuata plants to compensate for their structural deficiencies. Plant Physiol. 2012;159:1545–1570. doi: 10.1104/pp.112.196717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinänen M, Oldham NJ, Baldwin IT. Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata. J Agric Food Chem. 2001;49:3553–3558. doi: 10.1021/jf010200+. [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Diezel C, Baldwin IT. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia. 2006;148:280–292. doi: 10.1007/s00442-006-0365-8. [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Poveda K. Herbivory-mediated pollinator limitation: negative impacts of induced volatiles on plant-pollinator interactions. Ecology. 2011;92:1769–1780. doi: 10.1890/10-1945.1. [DOI] [PubMed] [Google Scholar]
- Kim S-G, Yon F, Gaquerel E, Gulati J, Baldwin IT. Tissue specific diurnal rhythms of metabolites and their regulation during herbivore attack in a native tobacco, Nicotiana attenuata. PLoS One. 2011;6:e26214. doi: 10.1371/journal.pone.0026214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köllner TG, Gershenzon J, Degenhardt J. Molecular and biochemical evolution of maize terpene synthase 10 , an enzyme of indirect defense. Phytochemistry. 2009;70:1139–1145. doi: 10.1016/j.phytochem.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Kos M, Houshyani B, Overeem A-J, Bouwmeester HJ, Weldegergis BT, van Loon JJ, Dicke M, Vet LE. Genetic engineering of plant volatile terpenoids: effects on a herbivore, a predator and a parasitoid. Pest Manag Sci. 2013;69:302–311. doi: 10.1002/ps.3391. [DOI] [PubMed] [Google Scholar]
- Krügel T, Lim M, Gase K, Halitschke R, Baldwin IT. Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology. 2002;12:177–183. [Google Scholar]
- Ludwig-Muller J, Bennett RN, Garcia-Garrido JM, Piché Y, Vierheilig H. Reduced arbuscular mycorrhizal root colonization in Tropaeolum majus and Carica papaya after jasmonic acid application can not be attributed to increased glucosinolate levels. J Plant Physiol. 2002;159:517–523. [Google Scholar]
- Melin E, Krupa S. Studies on ectomycorrhizae of pine II. Growth inhibition of mycorrhizal fungi by volatile organic constituents of Pinus silvestris (Scots Pine) roots. Physiol Plant. 1971;25:337–340. [Google Scholar]
- Nagegowda DA. Plant volatile terpenoid metabolism: biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010;584:2965–2973. doi: 10.1016/j.febslet.2010.05.045. [DOI] [PubMed] [Google Scholar]
- Oh Y, Baldwin IT, Gális I. NaJAZh regulates a subset of defense responses against herbivores and spontaneous leaf necrosis in Nicotiana attenuata plants. Plant Physiol. 2012;159:769–788. doi: 10.1104/pp.112.193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschold A, Halitschke R, Baldwin IT. Using “mute” plants to translate volatile signals. Plant J. 2006;45:275–291. doi: 10.1111/j.1365-313X.2005.02623.x. [DOI] [PubMed] [Google Scholar]
- Preston CA, Baldwin IT. Positive and negative signals regulate germination in the post-fire annual, Nicotiana attenuata. Ecology. 1999;80:481–494. [Google Scholar]
- Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TCJ. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature. 2005;434:732–737. doi: 10.1038/nature03451. [DOI] [PubMed] [Google Scholar]
- Robert CAM, Erb M, Hiltpold I, Hibbard BE, Gaillard MDP, Bilat J, Degenhardt J, Cambet-Petit-Jean X, Turlings TCJ, Zwahlen C. Genetically engineered maize plants reveal distinct costs and benefits of constitutive volatile emissions in the field. Plant Biotechnol J. 2013;11:628–639. doi: 10.1111/pbi.12053. [DOI] [PubMed] [Google Scholar]
- Robert-Seilaniantz A, Grant M, Jones JDG. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu Rev Phytopathol. 2011;49:317–343. doi: 10.1146/annurev-phyto-073009-114447. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Conceptión M. Early steps in isoprenoid biosynthesis: Multilevel regulation of the supply of common precursors in plant cells. Phytochem Rev. 2006;5:1–15. [Google Scholar]
- Rodríguez-Conceptión M, Campos N, Ferrer A, Boronat A. Isoprenoid synthesis in plants and microorganisms. In: Bach TJ, Rohmer M, editors. Isoprenoid Synthesis in Plants and Microorganisms: New Concepts and Experimental Approaches. Springer Science + Business Media; New York, NY, USA: 2013. pp. 439–456. [Google Scholar]
- Schittko U, Hermsmeier D, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. II. Accumulation of plant mRNAs in response to insect-derived cues. Plant Physiol. 2001;125:701–710. doi: 10.1104/pp.125.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnee C, Köllner TG, Held M, Turlings TCJ, Gershenzon J, Degenhardt J. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA. 2006;103:1129–1134. doi: 10.1073/pnas.0508027103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman MC, Barthel K, Baldwin IT. Herbivory-induced volatiles function as defenses increasing fitness of the native plant Nicotiana attenuata in nature. Elife. 2012;1:e00007. doi: 10.7554/eLife.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman MC, Heinzel N, Gaquerel E, Svatos A, Baldwin IT. Polymorphism in jasmonate signaling partially accounts for the variety of volatiles produced by Nicotiana attenuata plants in a native population. New Phytol. 2009;183:1134–1148. doi: 10.1111/j.1469-8137.2009.02894.x. [DOI] [PubMed] [Google Scholar]
- Schwachtje J, Kutschbach S, Baldwin IT. Reverse genetics in ecological research. PLoS ONE. 2008;3:e1543. doi: 10.1371/journal.pone.0001543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigler D. [Accessed 17th July, 2014];Integrative Biology 425: Sesquiterpenes. http://www.life.illinois.edu/ib/425/
- Shiojiri K, Ozawa R, Kugimiya S, Uefune M, van Wijk M, Sabelis MW, Takabayashi J. Herbivore-specific, density-dependent induction of plant volatiles: Honest or “cry wolf” signals? PLoS ONE. 2010;5:e12161. doi: 10.1371/journal.pone.0012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. Nicotine’s defensive function in nature. PLoS Biol. 2004;2:e217. doi: 10.1371/journal.pbio.0020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC. R: A Language and Environment for Statistical Computing. 2012 [Google Scholar]
- Tholl D, Boland W, Hansel A, Loreto F, Rose USR, Schnitzler J-P. Practical approaches to plant volatile analysis. Plant J. 2006;45:540–560. doi: 10.1111/j.1365-313X.2005.02612.x. [DOI] [PubMed] [Google Scholar]
- Tholl D, Lee S. Terpene specialized metabolism in Arabadopsis thaliana. Arabidopsis Book. 2011;9:e0143. doi: 10.1199/tab.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulasiram HV, Erickson HK, Poulter CD. Chimeras of two isoprenoid synthases catalyze all four coupling reactions in isoprenoid biosynthesis. Science. 2007;316:73–76. doi: 10.1126/science.1137786. [DOI] [PubMed] [Google Scholar]
- Ton J, D’Alessandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TCJ. Priming by airborne signals boosts direct and indirect resistance in maize. Plant J. 2007;49:16–26. doi: 10.1111/j.1365-313X.2006.02935.x. [DOI] [PubMed] [Google Scholar]
- Trapp SC, Croteau RB. Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics. 2001;158:811–832. doi: 10.1093/genetics/158.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers CE, Gershenzon J, Lerdau MT, Loreto F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol. 2009;5:283–291. doi: 10.1038/nchembio.158. [DOI] [PubMed] [Google Scholar]
- Vos IA, Verhage A, Schuurink RC, Watt LG, Pieterse CMJ, Van Wees SCM. Onset of herbivore-induced resistance in systemic tissue primed for jasmonate-dependent defenses is activated by abscisic acid. Front Plant Sci. 2013;4:539. doi: 10.3389/fpls.2013.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranová E, Coman D, Gruissem W. Structure and dynamics of the isoprenoid pathway network. Mol Plant. 2012;5:318–333. doi: 10.1093/mp/sss015. [DOI] [PubMed] [Google Scholar]
- Wang L, Allmann S, Wu J, Baldwin IT. Comparisons of LIPOXYGENASE3- and JASMONATE-RESISTANT4/6-silenced plants reveal that jasmonic acid and jasmonic acid-amino acid conjugates play different roles in herbivore resistance. Plant Physiol. 2008;146:904–915. doi: 10.1104/pp.107.109264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Hettenhausen C, Baldwin IT. Evolution of proteinase inhibitor defenses in North American allopolyploid species of Nicotiana. Planta. 2006;224:750–760. doi: 10.1007/s00425-006-0256-6. [DOI] [PubMed] [Google Scholar]
- Wu J, Hettenhausen C, Meldau S, Baldwin IT. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell. 2007;19:1096–1122. doi: 10.1105/tpc.106.049353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wang L, Baldwin IT. Methyl jasmonate-elicited herbivore resistance: does MeJA function as a signal without being hydrolyzed to JA? Planta. 2008;227:1161–1168. doi: 10.1007/s00425-008-0690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebelo SA, Matsui K, Ozawa R, Maffei ME. Plasma membrane potential depolarization and cytosolic calcium flux are early events involved in tomato (Solanum lycopersicon) plant-to-plant communication. Plant Sci. 2012;196:93–100. doi: 10.1016/j.plantsci.2012.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.