Abstract
XBP1 is a multitasking transcription factor and a key component of the unfolded protein response (UPR). Despite the wealth of knowledge about the role of XBP1 in luminal/ER-positive breast cancer not much is known about the effectors of XBP1 in this context. Here we show that NCOA3 is a transcriptional target of XBP1. We observed increased expression of NCOA3 during conditions of UPR and estrogen (E2) stimulation. Further investigations revealed a role for IRE1-XBP1 axis in the induction of NCOA3 during UPR and estrogen signalling. We identify a novel role for NCOA3 in activation of PERK-ATF4 axis during UPR where knockdown of NCOA3 compromised the optimal activation of PERK-ATF4 pathway. We found that NCOA3 is required for induction of XBP1 during E2-stimulation and uncover a positive feedback regulatory loop that maintains high levels of NCOA3 and XBP1 in breast cancer. Furthermore upregulated NCOA3 was required for XBP1-mediated resistance to anti-hormonal agents. Increased expression of NCOA3 was associated with poor prognosis and higher levels of XBP1-S in breast cancer tissues. Our results uncover a novel steroid hormone independent role for NCOA3 in UPR signalling. Further we identify a positive feedback regulatory loop consisting of XBP1 and NCOA3 that maintains high levels of NCOA3 and XBP1 expression in breast cancer tissues. Taken together our data identify XBP1-NCOA3 axis that regulates cell fate decisions in ER-positive breast cancer cells.
Keywords: unfolded protein response, estrogen signalling, XBP1, NCOA3, breast cancer
INTRODUCTION
Physiological or pathological processes that disturb protein folding in the endoplasmic reticulum activate a set of signalling pathways termed as the Unfolded Protein Response (UPR). This concerted and complex cellular response is mediated by three molecular sensors, PKR-like ER kinase (PERK), activated transcription factor 6 (ATF6), and Inositol-requiring enzyme 1 (IRE1) present in the membrane of endoplasmic reticulum.(1) The luminal domain of PERK, IRE1, and ATF6 interacts with the endoplasmic reticulum chaperone Glucose-regulated protein 78 (GRP78). However, upon accumulation of unfolded proteins, GRP78 dissociates from these molecules, leading to their activation. The most salient feature of UPR is to increase the functional activity of a variety of transcription factors (ATF6, ATF4, XBP1, and CHOP). Once activated, these transcription factors coordinate transcriptional induction of genes encoding for endoplasmic reticulum-resident chaperones, endoplasmic reticulum-associated degradation machinery, amino acid transport and metabolism proteins, phospholipid biosynthesis enzymes, and several others, including many that have no obvious direct relationship to secretory pathway function.(1, 2)
Invasive breast cancer (IBC) is a heterogeneous disease with varied molecular features, behaviour, and response to therapy. Estrogen receptor α (ER) is the primary therapeutic target in breast cancer and is expressed in 70% of cases. Endocrine therapy is the mainstay of treatment for patients with advanced ER-positive breast cancer. One-third of women treated with hormonal therapy for 5 years will have recurrent disease within 15 years, and therefore endocrine-resistant disease may constitute up to one-quarter of all breast cancers.(3) The Cancer Genome Atlas (TCGA) consortium reported that most dominant feature of Luminal/ER-positive breast cancers is increased mRNA and protein levels of ESR1, GATA3, FOXA1, XBP1 and MYB. Most notably ESR1 and XBP1 were highly expressed and infrequently mutated.(4) The expression of XBP1-S mRNA and protein can be upregulated following 17β-estradiol (E2) treatment of ER-positive human breast cancer cell lines.(5, 6) XBP1 physically interacts with ER and potentiates ER-dependent transcriptional activity in a ligand-independent manner.(7) Ectopic expression of XBP1-S in ER-positive breast cancer cells can lead to estrogen-independent growth and reduced sensitivity to anti-estrogens.(8) Downregulation of XBP1 reduces the survival of transformed human cells under hypoxic conditions and impairs their ability to grow as tumour xenografts in SCID mice.(9) Thus accumulating evidence suggests an active role of the IRE1-XBP1 pathway in estrogen signalling.(10) Despite the wealth of knowledge about the role of XBP1-S in luminal/ER-positive breast cancer not much is known about the molecular effectors (transcriptional targets) of XBP1-S in context of estrogen signalling.
Nuclear receptor coactivator 3 (NCOA3/SRC-3/AIB1/ACTR/pCIP/RAC3) is a member of p160 family of coactivators.(11) It is an oncogenic coactivator and interacts with nuclear receptors (NR) to enhance the expression of cognate target genes.(12) By modulating gene expression, NCOA3 regulates diverse physiological functions and has been implicated in the development of breast cancer.(13) Transgenic mice overexpressing NCOA3 shows increased mammary epithelial cell proliferation, development of mammary hyperplasia and tumorigenesis(11). The ablation of NCOA3 in mouse mammary tumour virus (MMTV)/v-Ha-ras mice suppresses mammary gland ductal hyperplasia and mammary gland tumorigenesis.(14) NCOA3 not only functions to promote breast cancer development, it also participates in resistance to anti-hormonal therapy.(15) Increased expression of NCOA3 is strongly correlated with shorter disease-free and overall survival.(16) NCOA3 was found to be overexpressed in >60% of primary breast tumours; however its gene is amplified in only 5%–10% of breast cancers.(17, 18) Nonetheless, how NCOA3 becomes overexpressed in breast cancers is not well understood.
In this study we demonstrate that expression of NCOA3 is regulated by XBP1-S during the conditions of UPR, as well as estrogen stimulation in human breast cancer cells. We show that inhibition of IRE1 activity and knockdown of XBP1 expression both compromised the induction of NCOA3 during UPR and estrogen signalling. Our results describe an important non-nuclear receptor (NR) function of NCOA3 where IRE1-XBP1 dependent upregulation of NCOA3 regulates optimal activation of PERK-ATF4 axis during UPR. We also show that NCOA3 is required for induction of XBP1 and cellular proliferation upon estrogen stimulation. Higher expression of NCOA3 was associated with poor prognosis and mRNA levels of NCOA3 correlated with spliced XBP1 transcript levels in breast cancer tissues. These findings provide novel insights into the biological function of XBP1 in ER-positive breast cancer.
RESULTS
Upregulation of NCOA3 expression during conditions of UPR
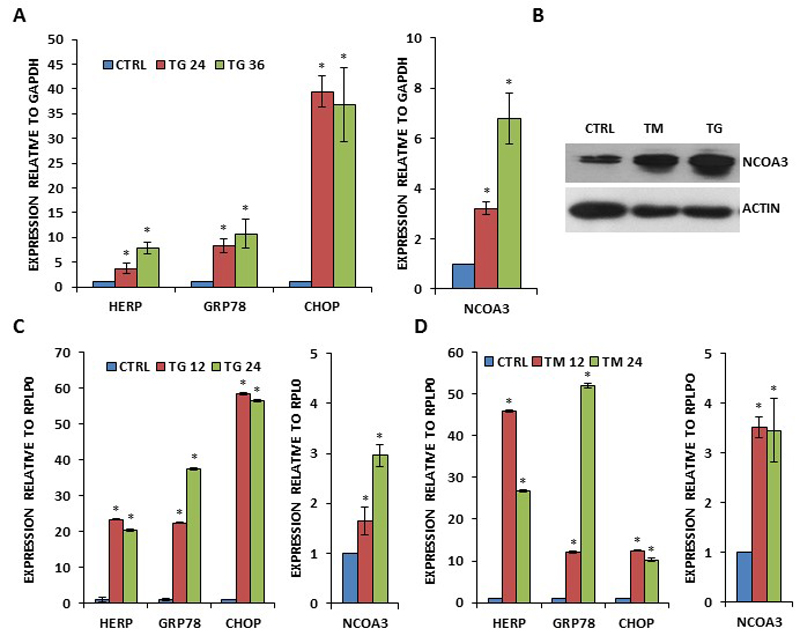
During the analysis of the microarray gene expression dataset (GSE63252) we found that expression of NCOA3 was robustly induced (logFC >2) upon treatment with two different pharmacological inducers of endoplasmic reticulum (EnR) stress. To experimentally assess if UPR upregulates NCOA3 gene expression in breast cancer cells, MCF7 and T47D cells were exposed to different EnR stressors: N-linked glycosylation inhibitor Tunicamycin (TM), and ER Ca-ATPase family (SERCA) inhibitor Thapsigargin (TG)(19). TG increased the expression of HERP, GRP78, CHOP (bonafide UPR-responsive genes) and NCOA3 mRNA levels in a time-dependent manner (Fig. 1A). We observed increase in NCOA3 protein upon treatment with TG and TM of MCF7 cells (Fig. 1B). Next we treated T47D cells with TG and TM. We observed significant increase in the expression of HERP, GRP78, CHOP (bonafide UPR-responsive genes) and NCOA3 mRNA levels in TG and TM-treated T47D cells (Fig. 1C-D). In order to confirm that regulation of NCOA3 during EnR stress was not restricted to ER-positive breast cancer cells, we examined levels of NCOA3 during conditions of EnR stress in MDA-MB231 cells. We observed significant increase in the expression of HERP, GRP78, CHOP (bonafide UPR-responsive genes) and NCOA3 mRNA levels in Bortezomib-treated MDA-MB231 cells (SF 1).
Figure 1. Increased expression of NCOA3 by UPR in human breast cancer cells.
(A) MCF7 cells were either untreated (CTRL) or treated with (1.0 μM) TG for indicated time points. The expression level of UPR-responsive genes (GRP78, HERP and CHOP) and NCOA3 was quantified by real-time RT-PCR, normalizing against GAPDH. Error bars represent mean ± S.D. from three independent experiments performed in triplicate. (B) MCF7 cells were either untreated (CTRL) or treated with (1.0 μM) TG and (1.0 μg/ml) TM for 24 hours. Western blotting of total protein was performed using antibodies against NCOA3 and β-actin. (C-D) T47D cells were either untreated (CTRL) or treated with (1.0 μM) TG (C) and (1.0 μg/ml) TM (D) for indicated time points. The expression level of UPR-responsive genes (GRP78, HERP and CHOP) and NCOA3 was quantified by real-time RT-PCR, normalizing against RPLP0. Error bars represent mean ± S.D. from three independent experiments performed in triplicate. *P < 0.05, two-tailed unpaired t-test compared with untreated cells.
Induction of NCOA3 during EnR stress is dependent on IRE1-XBP1 axis
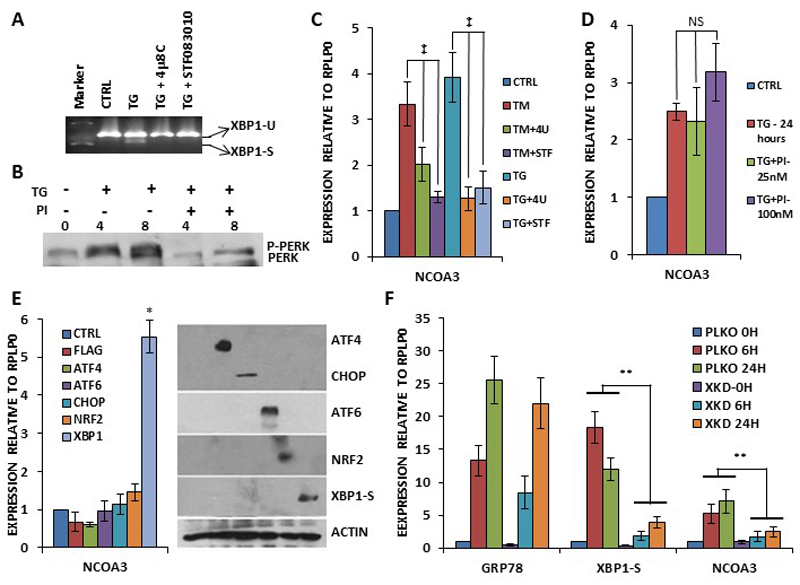
Next we investigated the role of PERK and IRE1 arms of the UPR in the regulation of the NCOA3 expression. MCF7 cells were treated with TG alone or in combination with the GSK PERK inhibitor(20) and (4μ8C and STF083010) IRE1 inhibitors.(21, 22) As shown in Figure 2A, 4μ8C and STF083010 efficiently attenuated the TG induced production of spliced XBP1. Further we observed that GSK PERK inhibitor (PI) abrogated the TG induced auto-phosphorylation of PERK (Fig. 2B). These results confirmed that both PERK and IRE1 inhibitors were blocking their respective targets. We observed that both 4μ8C and STF083010 compromised the TG and TM mediated increase in the expression of NCOA3 (Fig. 2C), whereas GSK PERK inhibitor (PI) had no effect on TG mediated increase in the expression of NCOA3 (Fig. 2D). To determine which mammalian UPR transcriptional activators regulate NCOA3 expression, we transfected MCF7 cells with plasmids encoding indicated gene products (Fig. 2E). These ectopic transcription factors were functional and regulated the expression of their cognate target genes (SF2). We observed that ectopic expression of spliced XBP1 resulted in a significant increase in the NCOA3 transcripts level (Figure 2E). To further confirm the role of XBP1 in the induction of NCOA3, we generated the control (MCF7-PLKO) and XBP1 knockdown sub-clones (MCF7-XKD) of MCF7 cells. For this purpose we tested a panel of XBP1 targeting shRNAs for their knockdown efficiency (SF3). MCF7 cells were transduced with pLKO.1-Puro or XBP1-targeting shRNAs (TRCN0000019805) lentiviruses followed by puromycin selection to obtain control (MCF7-PLKO) and XBP1 knockdown sub-clones (MCF7-XKD) of MCF7 cells. We found that XBP1 knockdown clones were compromised in the induction of spliced XBP1 and NCOA3 upon TG treatment (Fig. 2F). The knockdown of XBP1 did not alter the induction of GRP78 upon TG treatment (Fig. 2F). Collectively, these results suggest that induction of NCOA3 during UPR is dependent on IRE1-XBP1 axis.
Figure 2. Upregulation of NCOA3 during UPR is mediated by IRE1-XBP1 pathway.
(A) MCF7 cells were either untreated (CTRL) or treated with (1.0 μM) TG in absence and presence of IRE1 inhibitors, (10 μM) 4μ8C (4U) and (100 μM) STF083010 (STF) for 24 hours. Cells were harvested and expression of XBP1 (unspliced and spliced) was analysed by RT-PCR followed by gel electrophoresis. (B) MCF7 cells were treated with TG (1.0 μM) in absence and presence of (25 nM) GSK-PERK inhibitor (PI) for indicated time points. Whole cell lysates were subjected to SDS-PAGE followed by immunoblotting using PERK antibody. (C) MCF7 cells were either untreated (CTRL) or treated with TG (1.0 μM) and and (1.0 μg/ml) TM in absence and presence of IRE1 inhibitors as in A, and expression level of NCOA3 was quantified by real-time RT-PCR, normalizing against RPLP0. Error bars represent mean ± S.D. from three independent experiments performed in triplicate. (D) MCF7 cells were either untreated (CTRL) or treated with (1.0 μM) TG in absence and presence of (25 nM) GSK-PERK inhibitor (PI) for 24h hours. The expression level of NCOA3 was quantified by real-time RT-PCR, normalizing against RPLP0. Error bars represent mean ± S.D. from three independent experiments performed in triplicate. (E) MCF7 cells were transfected with (CTRL) pcDNA3-FLAG or plasmids expressing indicated UPR transcription factors (ATF4, ATF6, CHOP, NRF2 and XBP1-S). Cells were harvested 24 hours post transfection normalizing against RPLP0. Error bars represent mean ± S.D. from three independent experiments performed in triplicate. Equivalent amounts of cell lysates were resolved by SDS-PAGE and immunoblotting was performed using antibodies against FLAG, ATF6, spliced XBP1, and β-actin. ATF4, CHOP and NRF2 are FLAG-tagged. β-actin served as a loading control. (F) MCF7-control (PKLO) and MCF7-XBP1 knockdown (XKD) cells were treated with (1.0 μM) TG for indicated time points. The expression levels of GRP78, XBP1-S and NCOA3 was quantified by real-time RT-PCR, normalizing against RPLP0. Error bars represent mean ± S.D. from three independent experiments performed in triplicate. *P < 0.05, two-tailed unpaired t-test compared with untreated cells; ‡, P< 0.05 for one-way ANOVA; ** P < 0.05, two-tailed unpaired t-test comparing respective time points; NS, not significant at P<0.05.
NCOA3 is a transcriptional target of XBP1
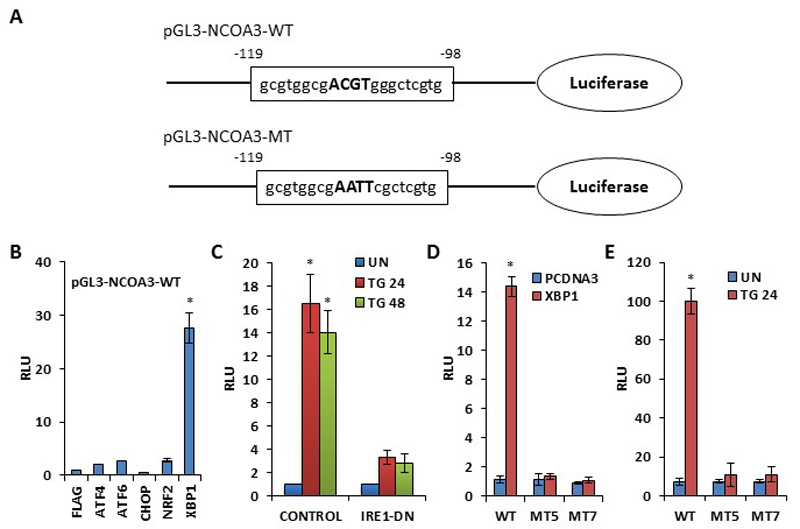
Examination of the nucleotide sequence of human NCOA3 promoter showed a sequence homologous to the consensus XBP1-binding site at nucleotide position -119 to -98 relative to the transcriptional start site (Fig. 3A). A NCOA3 promoter reporter construct (pGL3-NCOA3-WT) containing this region was activated over 25-fold by cotransfection with spliced XBP1 in 293T cells (Fig. 3B). To determine the role of endogenous XBP1 in regulating NCOA3 promoter, 293T cells were transfected with NCOA3 promoter-reporter plasmid along with dominant-negative IRE1 (IRE1∆C) and after 24 h they were treated with TG for 24 or 48 h. IRE1∆C mutant of IRE1has been shown to attenuate EnR stress-induced production of spliced of XBP1.(23) TG treatment resulted in up to 16-fold increase in NCOA3 promoter activity that was abolished by cotransfection of IRE1∆C mutant (Fig. 3C). Mutation of the putative XBP1-binding site in NCOA3 promoter (pGL3-NCOA3-MT) completely abolished its transactivation by ectopic XBP1 (Fig. 3D) and TG treatment (Fig. 3E). These observations suggest that there is only one functional XBP1-responsive site in this region of NCOA3 promoter.
Figure 3. NCOA3 is a transcriptional target of XBP1.
(A) Schematic representation of wild type (pGL3-NCOA3-WT) and XBP1-binding site mutant (pGL3-NCOA3-MT) human NCOA3 promoter-reporter constructs. The nucleotide sequence of human NCOA3 promoter from position -119 to -98 relative to the transcription start site is shown. (B) 293T cells were transfected with pGL3-NCOA3-WT along with control (FLAG) or expression plasmid for indicated UPR transcription factors. Luciferase activity was measured 24 h after transfection and normalized luciferase activity (Firefly/Renilla) relative to control is shown. Error bars represent mean±S.D. from three independent experiments performed in duplicate. (C) 293T cells were transfected with pGL3-NCOA3-WT along pcDNA3 (control) and pIRE1-ΔC (IRE1-DN). After 24 h of transfection, cells were either untreated (UN) or treated with (1.0 μM) TG for indicated time points. Normalized luciferase activity (Firefly/Renilla) relative to untreated control is shown. Error bars represent mean±S.D. from three independent experiments performed in duplicate. (D) 293T cells were transfected with pGL3-NCOA3-WT or mutant pGL3-NCOA3-MT (MT5 and MT7) along with control (PCDNA3) or spliced XBP1 (XBP1) expression plasmid. Luciferase activity was measured 24 hours post transfection and normalized luciferase activity (Firefly/Renilla) relative to control is shown. Error bars represent mean±S.D. from three independent experiments performed in duplicate. (E) 293T cells were transfected with pGL3-NCOA3-WT or mutant pGL3-NCOA3-MT (MT5 and MT7). 24 hours post transfection, cells were either untreated (UN) or treated with (1.0 μM) TG for indicated time points. Normalized luciferase activity (Firefly/Renilla) relative to untreated control is shown. Error bars represent mean±S.D. from three independent experiments performed in duplicate. *P < 0.05, two-tailed unpaired t-test compared with untreated cells.
NCOA3 modulates optimal activation of PERK-eIF2α-ATF4 axis during UPR
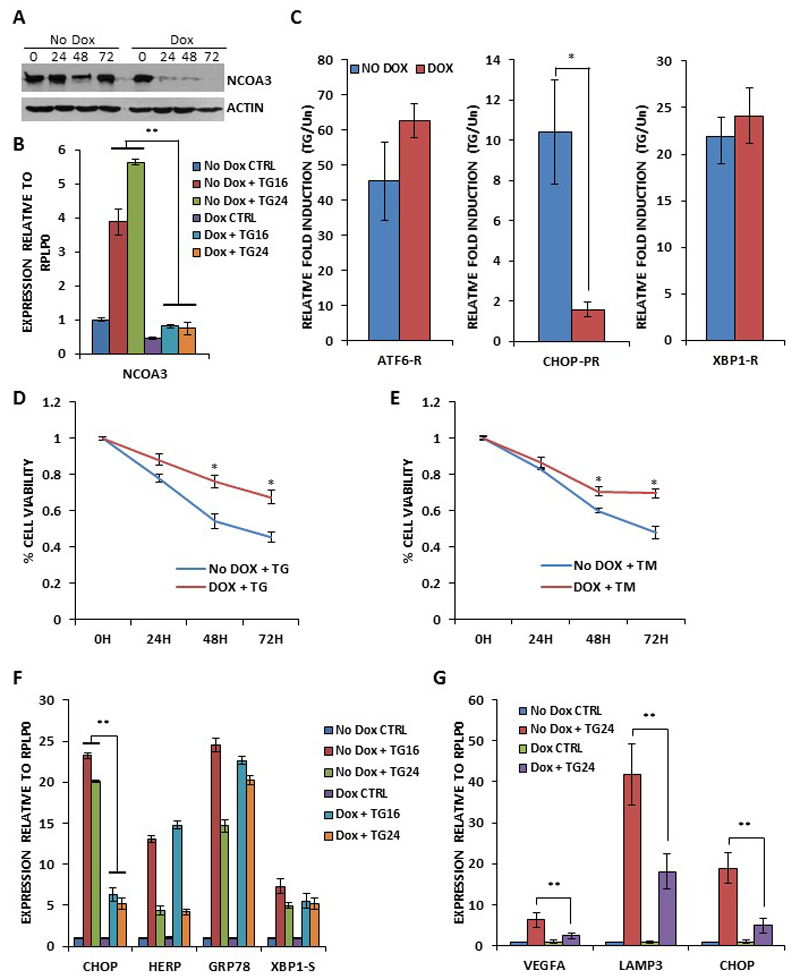
Next we generated the clones of MCF7 cells expressing NCOA3 shRNA. For this purpose MCF7 cells were transduced with tetracycline-inducible lentivirus engineered to produce RFP and NCOA3 targeting shRNA upon addition of doxycycline and co-expression of the tetracycline regulatory protein, rtTA3. We observed significant knockdown of NCOA3 protein after the addition of (500 ng/ml) doxycycline to MCF7-NCOA3-shRNA clone (Fig. 4A). For subsequent experiments MCF7-NCOA3-shRNA cells were pre-treated with doxycycline (500 ng/ml) for 48 hours to knockdown the expression of NCOA3. We observed that TG-induced increase in the expression of NCOA3 was attenuated by doxycycline in MCF7-NCOA3-shRNA clone (Fig. 4B). Next, we tested whether NCOA3 modulated the activation of three branches of the UPR. For this purpose we used synthetic luciferase reporter constructs having ATF6- or XBP1-binding sites and CHOP-promoter reporter. The induction of the XBP1-binding site reporter and ATF6-binding site reporter in response to thapsigargin was not affected by knockdown of NCOA3 (Fig. 4C). In contrast, the response of the CHOP-promoter reporter to thapsigargin was significantly decreased in absence of NCOA3 (Fig. 4C). PERK activation promotes both adaptive, as well as apoptotic, responses depending on the severity of the stress and context. In agreement with the proapoptotic role of PERK pathway we observed that knockdown of NCOA3 provided resistance to EnR stress-mediated cell death (Fig. 4D-E). Next we examined levels of UPR target genes to determine if NCOA3 modulated their expression. Levels of CHOP, HERP, GRP78 and XBP1-S were examined by quantitative RT-PCR. No difference in UPR target genes expression was observed, with the notable exception of CHOP whose induction were consistently decreased in presence of doxycycline (Fig. 4F). PERK-eIF2α-ATF4 branch of UPR has been shown to upregulate VEGFA and LAMP3 to induce angiogenesis and cell migration respectively.(24, 25) Indeed we observed that PERK inhibitor compromised the induction of VEGFA and LAMP3 during conditions of EnR stress in MCF7 cells (SF4). In agreement with its effect on induction of CHOP gene expression during UPR, knockdown of NCOA3 attenuated the TG-mediated induction of VEGFA and LAMP3 in MCF7 cells (Fig. 4G). These results suggest that NCOA3 does not affect the ATF6 or IRE1-XBP1 axis but is required for the optimal activation of PERK-eIF2α-ATF4 pathway in response to EnR stress.
Figure 4. NCOA3 is required for ER stress-induced activation of PERK-ATF4-CHOP axis.
(A) pTRIPZshNCOA3-MCF7 cells were either untreated (No Dox) or treated (Dox) with (500 ng/ml) of doxycycline for indicated time points. Upper panel, Equivalent amounts of cell lysates were resolved by SDS-PAGE and immunoblotting was performed using antibodies against NCOA3 and β-actin. (B) pTRIPZshNCOA3-MCF7 cells were either untreated (CTRL) or treated with (1.0 μM) TG for indicated time points in absence and presence of doxycycline. The expression level of NCOA3 was quantified by real-time RT-PCR, normalizing against RPLP0. Error bars represent mean ± S.D. from three independent experiments performed in triplicate. (C) pTRIPZshNCOA3-MCF7 cells were transfected with the indicated UPR pathway reporter genes (ATF6-R, CHOP-PR, XBP1-R). Transfected cells were treated with TG (1.0 μM) in absence and presence of doxycycline for 24 hours. Normalized luciferase activity (Firefly/Renilla) relative to untreated control is shown. Error bars represent mean±S.D. from three independent experiments performed in duplicate. (D-E) pTRIPZshNCOA3-MCF7 cells were untreated (CTRL) or treated with (1.0 μM) TG and (1.0 μg/ml) TM in absence and presence of (500 ng/ml) of doxycycline for indicated time points. Line graphs show the absorbance in cells at the indicated time points after the treatment. Error bars represent mean ± S.D. from three independent experiments performed in triplicate. (F-G) pTRIPZshNCOA3-MCF7 cells were either untreated (CTRL) or treated with (1.0 μM) TG for indicated time points in absence and presence of doxycycline. The expression level of CHOP, HERP, GRP78, XBP1-S, VEGFA and LAMP3 was quantified by real-time RT-PCR, normalizing against RPLP0. Error bars represent mean ± S.D. from three independent experiments performed in triplicate. *P < 0.05, two-tailed unpaired t-test comparing TG induced samples; ** P < 0.05, two-tailed unpaired t-test comparing respective time points.
Estrogen upregulates NCOA3 expression in IRE1-XBP1 dependent manner
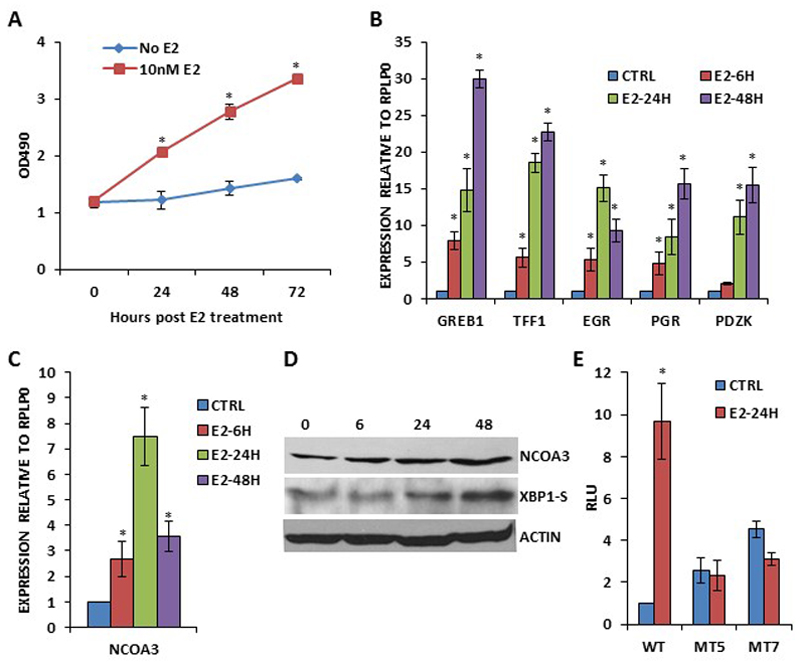
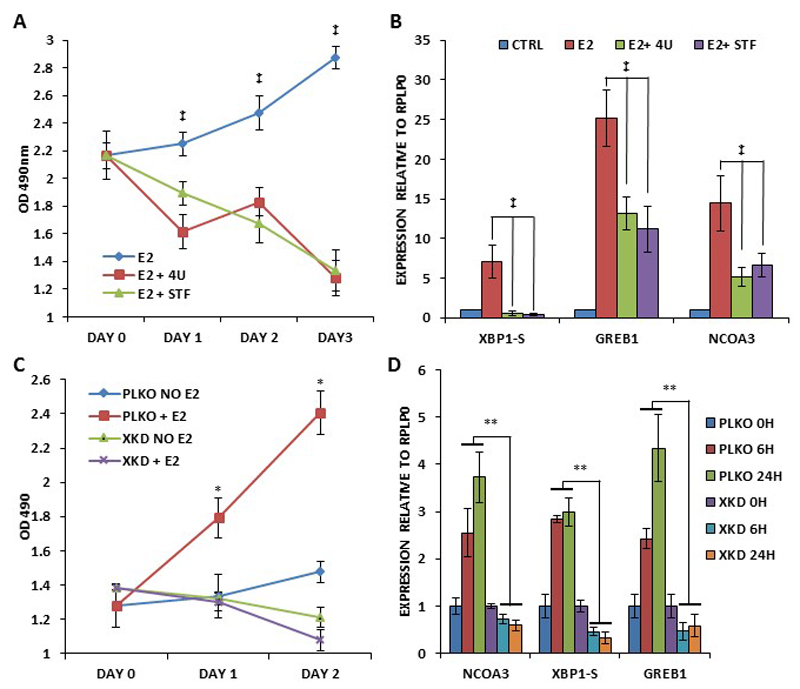
Next we determined whether XBP1 regulates NCOA3 expression upon E2 stimulation. We first optimized the conditions for the E2-dependent growth and induction of bonafide E2-target genes in MCF7 cells. After synchronization for 72 h, MCF7 cells were treated with (10 nM) E2 in 1% DCC-FBS supplemented medium. We observed a time-dependent growth of MCF7 cells (Fig. 5A) and induction of (GREB1, TFF1, EGR, PGR and PDZK) bonafide E2-responsive genes (Fig. 5B) under these conditions. We observed significant increase in NCOA3 mRNA and protein levels starting 6 h after the onset of E2-treatment and lasting for 48 h (Fig. 5C-D). Similar increase in the expression of NCOA3 following E2 stimulation was observed in T47D cells (SF5). Next we investigated the role of IRE1-XBP1 axis in the regulation of the NCOA3 expression upon E2 signalling. We found that wild type NCOA3 promoter reporter construct (pGL3-NCOA3-WT) was upregulated 8-12 fold by E2-treatment (Fig. 5E). This upregulation was completely abrogated in XBP1-binding site mutant NCOA3 promoter reporter construct (Fig. 5E). Next we determined the effect of IRE1 inhibitors (4μ8C and STF083010) on E2-mediated induction of NCOA3 expression. We found that 4μ8C and STF083010 efficiently attenuated the E2-stimulated growth and increase in the expression of XBP1-S, GREB1 and NCOA3 (Fig. 6A-B). These results suggest that induction of NCOA3 during E2-stimulation is dependent on RNase activity of IRE1. Next we used the control (MCF7-PLKO) and XBP1 knockdown sub-clones (MCF7-XKD) of MCF7 cells to determine the role of XBP1 in the regulation of the NCOA3 expression upon E2 signalling. We found that knockdown of XBP1 compromised E2-stimulated growth as well as expression of XBP1-S, GREB1 and NCOA3 (Fig. 6C-D). Collectively, these results suggest that induction of NCOA3 during E2 stimulation is dependent on IRE1-XBP1 axis.
Figure 5. Induction of NCOA3 expression during estrogen signalling.
(A) MCF7 cells were synchronized before estrogen treatment as described in methods section. After synchronization, cells were either untreated (No E2) or treated with (10nM E2) estrogen in 1% DCC-FBS supplemented medium. Line graphs show the absorbance in cells at the indicated time points after E2 treatment. Error bars represent mean ± S.D. from three independent experiments performed in triplicate. (B-C) MCF7 cells were treated as in A and induction of E2-responsive genes (GREB1, TFF1, EGR, PGR and PDZK) and NCOA3 was quantified by real-time RT-PCR, normalizing against RPLP0. Error bars represent mean ± S.D. from three independent experiments performed in triplicate. (D) MCF7 cells were treated as in A, and western blotting of total protein was performed using antibodies against NCOA3, XBP1-S and β-actin. (E) MCF7 cells were transfected with pGL3-NCOA3-WT or mutant pGL3-NCOA3-MT (MT5 and MT7). Transfected cells were left either untreated (CTRL) or treated (E2-24H) with (10 nM) estrogen. Normalized luciferase activity (Firefly/Renilla) relative to untreated control is shown. Error bars represent mean±S.D. from three independent experiments performed in duplicate. *P < 0.05, two-tailed unpaired t-test compared with untreated cells.
Figure 6. IRE1-XBP1 signalling is required for E2-mediated growth and expression of NCOA3.
(A) MCF7 cells were treated with (10 nM) estrogen in absence and presence of IRE-1 inhibitors, (10 μM) 4μ8C (4U) and (100 μM) STF083010 (STF) for indicated time points. Line graphs show the absorbance in cells at the indicated time points after the treatment with IRE1 inhibitors. (B) MCF7 cells were treated as in A, for 24 hours. Expression of XBP1-S, GREB1 and NCOA3 was quantified by real-time RT-PCR, normalizing against RPLP0. Error bars represent mean ± S.D. from three independent experiments performed in triplicate. (C) MCF7-control (PKLO) and MCF7-XBP1 knockdown (XKD) cells were synchronized as described in methods section. After synchronization, cells were treated with (10 nM) E2 in 1% DCC-FBS supplemented medium. Line graphs show the absorbance in cells at the indicated time points after the E2 treatment. Error bars represent mean ± S.D. from three independent experiments performed in triplicate. (D) MCF7-control (PKLO) and MCF7-XBP1 knockdown (XKD) cells were treated as in C, for indicated time points. Expression of spliced XBP1 (XBP1-S), total XBP1 (XBP1-F), GREB1 and NCOA3 was quantified by real-time RT-PCR, normalizing against RPLP0. Error bars represent mean ± S.D. from three independent experiments performed in triplicate. *P < 0.05, two-tailed unpaired t-test compared with untreated cells; ‡, P< 0.05 for one-way ANOVA; ** P < 0.05, two-tailed unpaired t-test comparing respective time points.
NCOA3 is required for E2-mediated upregulation of XBP1
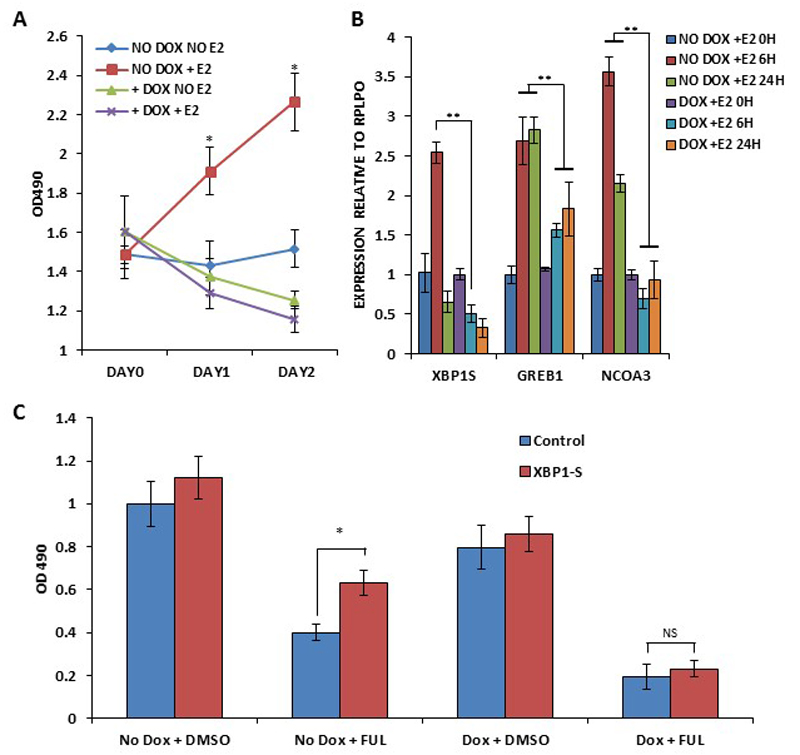
Next we determined the role of upregulated NCOA3 in estrogen signalling and XBP1 mediated anti-estrogen resistance. After synchronization for 72 h, MCF7-NCOA3-shRNA cells were treated with (10 nM) E2 in absence and presence of doxycycline (500 ng/ml). We observed that knockdown of NCOA3 expression attenuated the E2-stimulated growth as well as expression of XBP1-S, GREB1 and NCOA3 (Fig. 7A-B). To evaluate the role of NCOA3 in XBP1-mediated resistance to anti-estrogens MCF7-NCOA3-shRNA cells were transfected with XBP1-S expressing plasmid. Fulvestrant, a selective estrogen receptor down-regulator (SERD) is a pure competitive antagonist of estrogen receptor alpha(26). We found that ectopic XBP1-S provided the resistance to fulvestrant and knockdown of NCOA3 abrogated the resistance provided by XBP1-S (Fig. 7C). In addition, NCOA3 knockdown cells showed increased sensitivity to fulvestrant, further underscoring a role for NCOA3 in anti-estrogen resistance.
Figure 7. NCOA3 regulates induction of XBP1 upon estrogen signalling and is required for XBP1-mediated antiestrogen resistance.
(A) pTRIPZshNCOA3-MCF7 cells were treated with (10 nM) estrogen in absence and presence of (500 ng/ml) of doxycycline for indicated time points. Line graphs show the absorbance in cells at the indicated time points after the E2 treatment. Error bars represent mean ± S.D. from three independent experiments performed in triplicate. (B) pTRIPZshNCOA3-MCF7 cells were treated with (10 nM) estrogen in absence and presence of (500 ng/ml) of doxycycline for indicated time points. The expression level of spliced XBP1 (XBP1-S, GREB1 and NCOA3 was quantified by real-time RT-PCR, normalizing against RPLP0. Error bars represent mean ± S.D. from three independent experiments performed in triplicate. (C) pTRIPZshNCOA3-MCF7 cells transfected with control or XBP1-S plasmid and were treated with (1 μM) fulvestrant in absence and presence of (500 ng/ml) of doxycycline for 48 hours. MTS assay was performed to assess the changes in cell density. *P < 0.05, two-tailed unpaired t-test compared with untreated cells; ** P < 0.05, two-tailed unpaired t-test comparing respective time points.
Higher levels of NCOA3 mRNA in breast tumours are associated with a poor prognosis
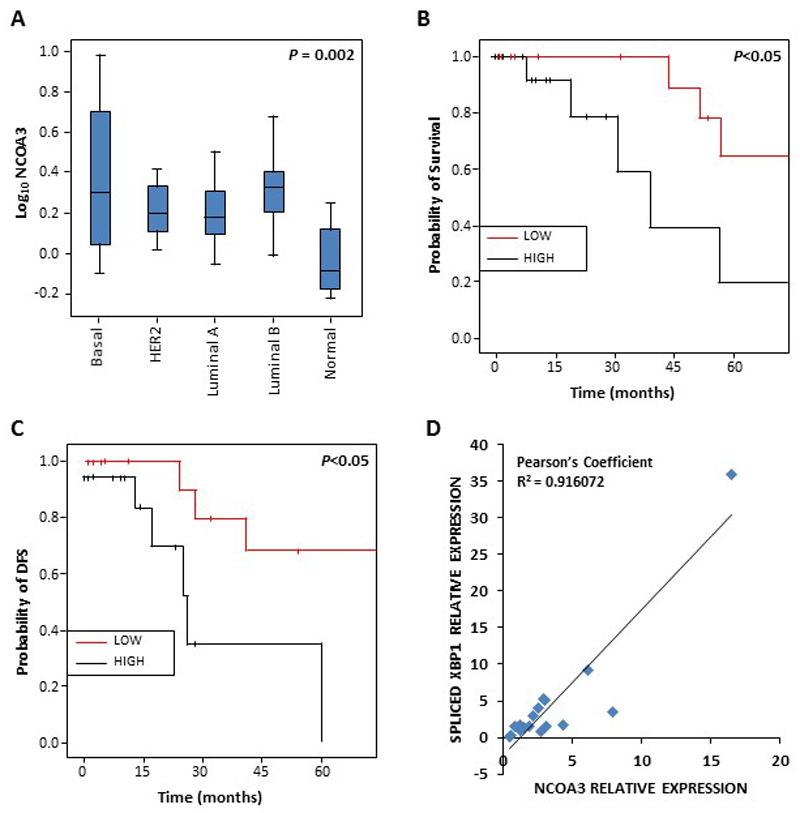
Breast tumour specimens (n = 60) were retrieved from patients undergoing primary curative resection at University Hospital Galway, Ireland. Matched tumour-associated normal breast tissue was also obtained from a subset (n = 10) of these patients where possible. Clinical and pathological data related to the samples are presented in SF6. The total RNA from breast tumour (n = 60) and tumour-associated normal breast tissue (n = 10) specimens was used to quantify levels of NCOA3 gene expression. NCOA3 levels were observed to be dysregulated in different sub-types of breast cancer samples that in normal breast tissue (Fig. 8A). An increase in tumour stage (log-rank; p=0.009) and NCOA3 expression was associated with shorter overall survival (log-rank; p=0.043). Prognostic significance of these parameters remained after multivariate analysis. No other statistically significant associations between high NCOA3 expression and clinico-pathological variables (age at diagnosis, macroscopic tumour size and lymph node status), or biomarker expression were found. Associations between NCOA3 expression and outcome were examined by dichotomising the NCOA3 expression as low and high at the median. We observed that in the patient samples, higher NCOA3 expression was associated with reduced overall survival (Fig. 8B) and disease free survival (Fig. 8C) as compared with low NCOA3 expression. Furthermore, higher XBP1 mRNA in breast tumour samples correlated with a higher NCOA3 mRNA level (Fig. 8D). Overall, these results suggest that higher XBP1 is associated with increased NCOA3 gene expression in human breast cancer tumours and poor outcome in human patients.
Figure 8. NCOA3 expression is higher in XBP1 expressing breast cancer tumours.
(A) Significant differences in relative expression of NCOA3 transcripts in breast tumour subtypes (n=60) and tumour-associated normal (n=10) breast tissue. Box-plot shows the expression of NCOA3 in breast cancer subtypes [Luminal A (n=18); Luminal B (n=19); Basal-like (n=15); HER2 overexpressing (n=80)] and tumour associated normal breast tissue (ANOVA p<0.002). (B-C) Kaplan–Meier survival analysis and log-rank test was used to assess the statistical significance of survival difference. Kaplan-Meier curves for Overall Survival (B) and Disease Free Survival (C) in IBC categorised according to NCOA3 expression. (D) Correlation between the expression of spliced XBP1 and NCOA3 in the breast cancer samples. The expression level of spliced XBP1 and NCOA3 was quantified by real-time RT-PCR, normalizing against RPLP0. The square of the Pearson correlation coefficient is 0.91.
DISCUSSION
Spliced XBP1 (XBP1-S), a member of the activated transcription factor (ATF) family of transcription factors is a key component of the UPR. XBP1-S plays crucial role in development of highly secretory cells, such as exocrine pancreas, Paneth cells and antibody-producing plasma cells.(27) Several gene expression profiling studies have revealed that XBP1-S induces the expression of a core group of genes involved in constitutive maintenance of endoplasmic reticulum function in almost all cell types.(28) In addition, there is a unique subset of XBP1-regulated genes that vary in the context of specific stimuli and cell types, such as Wolfram syndrome 1 (WFS1) in neuronal cells(29), basic helix-loop-helix family, member a15 (BHLHA15) in myoblasts.(27) Recently Chen et al have shown that XBP1 plays an important role in progression of triple negative breast cancer (TNBC) by regulating the HIF1α transcriptional program.(30) In this report we show that XBP1-S plays an important role in increased expression of NCOA3 during conditions of UPR and estrogen stimulation (Fig. 9). NCOA3 has been shown to play an important role in the tumourigenesis and progression of hormone-dependent as well as hormone-independent cancers.(11, 13) The expression of NCOA3 is elevated in human cancers in the absence of gene amplification and relatively little is known about mechanisms of NCOA3 overexpression.(18) The stressful conditions in the tumour microenvironment including low oxygen supply, nutrient deprivation and pH changes activate a range of cellular stress-response pathways.(31) Cellular adaptation to stress in tumour microenvironment occurs through multiple mechanisms, including activation of the UPR.(1) Our results showing the increased expression of NCOA3 during conditions of UPR (Fig. 1-3) provides a mechanism for overexpression of NCOA3 in human cancers.
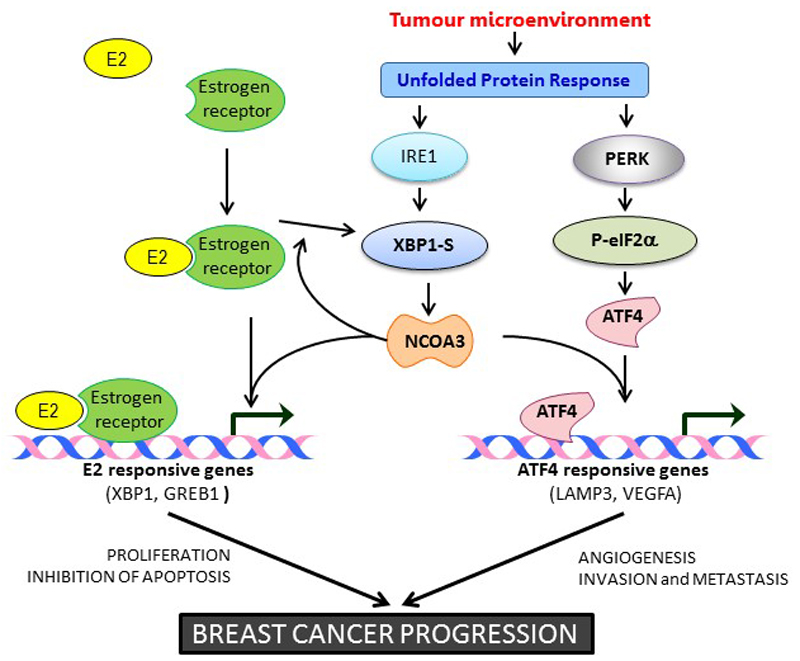
Figure 9. Graphical abstract.
Stressful conditions of the tumour microenvironment activate an adaptive mechanism called the unfolded protein response (UPR). X-box binding protein-1 (XBP1) is a critical transcriptional activator that is induced by the UPR. In estrogen receptor-positive (ER+) breast cancer cells XBP1 is rapidly induced in response to E2-stimulation. In luminal breast cancers estrogen signalling and UPR induce the expression of XBP1s. Here we show that XBP1s upregulates the transcriptional activation of NCOA3 during estrogen signalling and UPR. NCOA3 is required for the induction of estrogen-responsive and PERK-ATF4-responsive genes. Our results suggest that XBP1s regulates growth and proliferation of ER-positive breast cancer cells, in part, by transcriptional activation of NCOA3.
Nuclear receptor coactivators (NCOAs) are associated with diverse array of human diseases such as systemic metabolite homoeostasis, inflammation, energy regulation and several types of human cancer.(32) An important question is how the expression/activity of NCOAs is regulated by diverse metabolic disruptions and stress responses. Chronic EnR stress and defects in UPR signalling are emerging as key contributors to a growing list of human diseases, including immune disorders, cardiovascular diseases, diabetes, neurodegeneration, and cancer. (31, 33) In light of our results showing IRE1-XBP1 dependent upregulation of NCOA3 during UPR and ample overlap of human diseases where a role for NCOAs and UPR has been implicated we posit that UPR-mediated induction of NCOA3 may play a role in coordination of NCOA3 activity in accordance with the metabolic demand.
Our results uncover a novel non-NR role for NCOA3 in the UPR signalling, where NCOA3 plays an important role in optimal activation of PERK-eIF2α-ATF4 pathway, but has no significant effect on IRE1-XBP1 or ATF6 branches of UPR (Fig. 4). As PERK signalling mediates both adaptive, as well as apoptotic, responses depending on the intensity and duration of the stress, it may promote, as well as suppress, malignant transformation depending on the context.(34, 35) Indeed we observed that knockdown of NCOA3 abrogated PERK signalling and provided resistance to EnR stress-mediated cell death (Fig. 4). Loss of NCOA3 has been shown to accelerate polyoma middle-T antigen-induced mammary tumourigenesis and malignant B-cell lymphomas in mice.(36, 37) However further investigation is required to evaluate whether loss of NCOA3 contributes to cancer progression by inhibiting EnR stress-induced apoptosis in tumour microenvironment. PERK-ATF4 arm directly upregulates vascular endothelial growth factor A (VEGFA) and Lysosomal-Associated Membrane Protein 3 (LAMP3) thereby regulating tumour vascularity and invasion.(25, 38) In line, tumours derived from K-Ras-transformed embryonic fibroblasts derived from PERK knockout mice show severely compromised tumour vascularization(39) and PERK-deficient mice have reduced growth of β cell insulinoma tumours as a result of compromised tumour vascularization.(40) Indeed, the PERK inhibitor and knockdown of NCOA3 both attenuated the UPR-mediated increase in the expression of VEGF and LAMP3 (Fig. 4 and SF3). Further epithelial to mesenchymal transition activates PERK-eIF2α signalling, which is required for invasion and metastasis of primary tumour.(41) Taken together our results suggest a role for NCOA3 in PERK-dependent effects on malignant transformation.
Our results show that XBP1-S regulates the expression of NCOA3 upon estrogen stimulation via the XBP1-binding sites in the promoter of NCOA3 (Fig. 5-6). Further we show that NCOA3 is required for induction of XBP1-S upon E2-stimulation (Fig. 7) but not during the conditions of EnR stress (Fig. 4). During conditions of E2-stimulation, estrogen receptor is recruited to the enhancer region of XBP1 gene leading to induction of XBP1 mRNA which is then spliced by IRE1 to produce XBP1-S.(5) However ATF6 induces the expression of XBP1 mRNA during UPR which is then spliced by IRE1 to produce XBP1-S.(42) In agreement with these observations we found that loss of NCOA3 had no effect on transcriptional activity of ATF6 (Fig. 4C) but compromised the induction of E2-responsive genes (Fig. 7B). We further show that NCOA3 is required for E2-dependent proliferation and induction of XBP1 thereby generating a feed forward autoregulatory loop (Fig. 7). These results suggest that in cells with hyperactive XBP1-S, such as the case encountered in stressful conditions of tumour microenvironment, there is a positive feedback regulatory loop consisting of XBP1-S and NCOA3 to maintain high levels of NCOA3 and XBP1-S in ER-positive breast cancer. Indeed we observed very good correlation between the transcript levels of NCOA3 and XBP1-S in breast cancer patient samples (Fig. 8). Overexpression of XBP1-S can confer estrogen-independent growth and resistance to anti-hormonal therapy.(8) Further XBP1-S is overexpressed in anti-estrogen resistant breast cancer cells and increased expression of XBP1-S is associated with poor clinical outcome.(43) Our results show that NCOA3 is required for XBP1-S mediated to anti-estrogens (Fig. 7). XBP1 has been shown to regulate NF-κB activity which plays an important role in XBP1 mediated effects on anti-estrogen responsiveness.(44) NCOA3 interacts with I kappa B kinase (IKK) and is phosphorylated by the IKK complex.(45) Further phosphorylated NCOA3 potentiates NF-κB mediated gene expression.(45) Thus activation of NCOA3 and NF-κB by XBP1 can act in a concerted manner to regulate anti-estrogen responsiveness. Taken together our findings reveal a key function for the IRE1-XBP1-NCOA3 axis in luminal/ER-positive breast cancer and indicate that targeting this pathway may offer alternative treatment strategies for anti-estrogen resistant breast cancer.
METHODS
Cell culture and treatments – MCF7, T47D and MDA-MB231 cells were purchased from ECACC. HEK 293T cells were from Cells were from Indiana University National Gene Vector Biorepository. Cells maintained in Dulbecco’s modified medium (DMEM) supplemented with 10% FCS, 100 U/ml penicillin and 100 mg/ml streptomycin at 37 °C with 5% CO2. To induce EnR stress, cells were treated with tunicamycin (TM) or thapsigargin (TG) at the indicated concentrations for the indicated time. Thapsigargin (Cat # 1138), tunicamycin (Cat # 3516), ICI 182,780 (Cat # 1047) were from Tocris Bioscience. Estradiol (Cat# 1006315) was from Cayman chemical, USA. To inhibit IRE1 endoribonuclease activity, cells were treated with IRE1 inhibitor (4μ8C) (Cat # 412512 Millipore Ireland B.V) and STF083010 (Cat# 412510 Millipore Ireland B.V). To inhibit PERK activity cells were treated with GSK2606414 (Cat # 516535 Millipore Ireland B.V).
Estrogen stimulated growth – Parental MCF7 cells or sub clones (MCF-PLKO, MCF7-XKD and pTRIPZshNCOA3-MCF7) were synchronized before estrogen treatment by incubation for 72 h in the phenol red free medium supplemented with 1% dextran-coated charcoal-stripped FBS (DCC-FBS). After synchronization, cells were treated with 10 nM E2 in 1% DCC-FBS supplemented medium. Cultures were further incubated at 37°C after which cells were assayed with MTS at time intervals 1-5 days as indicated. Measurements were made in accordance with the manufacturer’s instructions (Promega Corp., Madison, WI).
Plasmid constructs - The expression vector for pBMN-I-GFP, pBMN-hATF6(373)-I-GFP encoding aa 1–373 of human ATF6α and pBMN-hXBP1(S)-I-GFP encoding full-length human XBP1 generated by UPR-mediated splicing were kind gift from Dr Joseph Brewer, University of South Alabama, USA. The expression vector for FLAG-tagged ATF4 (pRK-ATF4) was a gift from Yihong Ye (Addgene plasmid # 26114); FLAG-tagged NRF2 (NC16 pCDNA3.1-FLAG-NRF2) was a gift from Randall Moon (Addgene plasmid # 36971). The expression vector for FLAG-tagged CHOP (pcDNA3-FLAG-CHOP) was kind gift from Dr Wolfgang Dubiel, Humbolt University, Germany. The pGL3-ACTR-1.6kb construct was a kind gift from Dr. Hongwu Chen, University of California at Davis, USA and contains a genomic DNA fragment (1.6 kb, HindIII-NcoI) containing the first exon of NCOA3 into vector pGL3-basic. The ATF6, PERK and IRE1-XBP1 pathway reporters has been described previously.(46)
Generation of stable cell lines - The control and XBP1-targeting shRNA plasmid (TRCN0000019805) was from Sigma. The tetracycline inducible pTRIPZ NCOA3 shRNA plasmid (V2THS_261936) with targeting sequence 5’-GTCAGATAAGCAGGAGGTA-3’ was from Thermo Scientific, St Leon-Rot Germany. Lentivirus was generated by transfecting lentiviral plasmids along with packaging plasmids in 293T cells using jetPEI transfection reagent (Polyplus transfection, VWR International Ltd, Dublin, Ireland) according to manufacturer’s instructions. MCF7 cells were then transduced with the shRNA lentivirus and selection for shRNA-positive cells was performed with 2 μg/ml puromycin for 7 days.
RNA extraction, RT-PCR and real time RT-PCR - Total RNA was isolated using Trizol (Life Technologies) according to the manufacturer's instructions. Reverse transcription (RT) was carried out with 2 μg RNA and random primerss (Promega) using ImProm-II™ Reverse Transcription System (Promega). Real-time PCR method to determine the induction of UPR target genes has been described previously.(47)
Luciferase reporter Assays – The wild type NCOA3 human promoter reporter construct (pGL3-ACTR-1.6kb) was used to generate XBP1-binding site mutant construct. Point mutations in pGL3-ACTR-1.6kb were performed using QuikChange site-directed mutagenesis method. The following forward primers were used to produce point mutations in XBP1-binding site of pGL3-NCOA3-WT construct- (NCOA3-MUT-Forward) 5’-CGGAGGGCGTGGCGAATTCGGCTCGTGCGGCCG-3’, and (NCOA3-MUT-Reverse) 5’-CGGCCGCTCGAGCCGAATTCGCCACGCCCTCCG-3’. The generated mutants were verified by restriction enzyme digestion because the mutations introduced an EcoRI site in the pGL3-NCOA3-MT plasmid and confirmed by sequencing. In promoter assays, 293T cells were grown in 6-well plate and transfected with (1.0 μg) pGL3-NCOA3-WT or pGL3-NCOA3-MT reporter constructs in combination with (100 ng) Renilla luciferase vector as internal control. Twenty four hours post-transfection cells were treated with thapsigargin or tunicamycin for 24 h. Firefly luciferase and Renilla luciferase activities were measured 48 h after transfection using Lucetta™ Luminometer (Lonza) and then normalized for Renilla luciferase activity.
Western blotting – Western blotting procedures has been described previously.(48) The primary antibodies used were ATF6 (Abcam, Cat# ab122897), spliced XBP1 (Biolegend, Cat# 619502), PERK (Cell signalling, Cat# C33E10), GRP78 (Pierce antibodies, Cat# PA1-014A), phospho-eIF2α (Cell signalling, Cat# 9721), total eIF2α (Cell signalling, Cat# 9722) and or β-Actin (Sigma, Cat# A-5060) overnight at 4° C. The membrane was washed 3 times with PBS-0.05% Tween and further incubated in appropriate horseradish peroxidase-conjugated secondary antibody (Pierce) for 90 min. Signals were detected using Western Lightening Plus ECL (Perkin Elmer).
Patients and tumour samples - Breast tumour specimens (n = 60) were retrieved from patients undergoing primary curative resection at University Hospital Galway, Ireland. Matched tumour-associated normal breast tissue was also obtained from a subset (n = 10) of these patients where possible. Following excision, tissue samples were immediately snap-frozen in liquid nitrogen and stored at -80°C until RNA extraction. Prior written and informed consent was obtained from each patient and the study was approved by the ethics review board of University Hospital Galway. Clinical and pathological data related to the samples are presented in SF6.
RNA extraction and NCOA3 expression analysis - Tissue samples (50-100mg) were homogenised using a hand-held homogeniser (Polytron PT1600E, Kinematica AG, Littau-Luzern, Switzerland) in 1-2 mL of Trizol. RNA was extracted and levels of NCOA3 gene expression were quantified by RQ-PCR using TaqMan assays. Relationships between gene expression levels and clinic-pathological parameters, intrinsic subtype and clinical outcomes were analysed using Pearson s correlation coefficient, student t-test, ANOVA, Kaplan-Meier survival curves and Cox proportional hazards model with SPSS software. P<0.05 was considered significant.
Statistical Analysis- The data is expressed as mean ± SD for three independent experiments. Differences between the treatment groups were assessed using Two-tailed paired student’s t-tests. The values with a p<0.05 were considered statistically significant.
Supplementary Material
ACKNOWLWDGEMENTS
This publication has emanated from research conducted with the financial support of Health Research Board (grant number HRA_HSR/2010/24) and Breast Cancer campaign (grant number 2011PR26).
Footnotes
AUTHOR CONTRIBUTION
The author(s) have made the following declarations about their contributions: Conceived and designed the experiments: AG, GC and SG. Performed the experiments: AG, MH, NM and SG. Analysed the data: AG, MH, and SG. Contributed reagents/materials/analysis tools: NM and MK. The text and figures were prepared by AG, NM and SG. All authors reviewed the manuscript.
COMPETING INTERESTS
Authors declare no competing financial interests.
References
- 1.Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer. 2014;14(9):581–97. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- 2.Read DE, Gupta A, Cawley K, Gupta S. Regulation of ER Stress Responses by microRNAs. In: Agostinis P, Afshin S, editors. Endoplasmic Reticulum Stress in Health and Disease. Springer Netherlands: 2012. pp. 143–61. [Google Scholar]
- 3.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9(9):631–43. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 4.Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122(1):33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Sengupta S, Sharma CG, Jordan VC. Estrogen regulation of X-box binding protein-1 and its role in estrogen induced growth of breast and endometrial cancer cells. Horm Mol Biol Clin Investig. 2010;2(2):235–43. doi: 10.1515/HMBCI.2010.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding L, Yan J, Zhu J, Zhong H, Lu Q, Wang Z, et al. Ligand-independent activation of estrogen receptor alpha by XBP-1. Nucleic Acids Res. 2003;31(18):5266–74. doi: 10.1093/nar/gkg731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez BP, Riggins RB, Shajahan AN, Klimach U, Wang A, Crawford AC, et al. Human X-box binding protein-1 confers both estrogen independence and antiestrogen resistance in breast cancer cell lines. FASEB J. 2007;21(14):4013–27. doi: 10.1096/fj.06-7990com. [DOI] [PubMed] [Google Scholar]
- 9.Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H, et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004;64(17):5943–7. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- 10.Clarke R, Cook KL. Unfolding the Role of Stress Response Signaling in Endocrine Resistant Breast Cancers. Frontiers in oncology. 2015;5:140. doi: 10.3389/fonc.2015.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lydon JP, O'Malley BW. Minireview: steroid receptor coactivator-3: a multifarious coregulator in mammary gland metastasis. Endocrinology. 2011;152(1):19–25. doi: 10.1210/en.2010-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres-Arzayus MI, Font de Mora J, Yuan J, Vazquez F, Bronson R, Rue M, et al. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer cell. 2004;6(3):263–74. doi: 10.1016/j.ccr.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Wu RC, O'Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9(9):615–30. doi: 10.1038/nrc2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O'Malley BW. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci U S A. 2000;97(12):6379–84. doi: 10.1073/pnas.120166297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson AB, O'Malley BW. Steroid receptor coactivators 1, 2, and 3: critical regulators of nuclear receptor activity and steroid receptor modulator (SRM)-based cancer therapy. Molecular and cellular endocrinology. 2012;348(2):430–9. doi: 10.1016/j.mce.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao C, Yasui K, Lee CJ, Kurioka H, Hosokawa Y, Oka T, et al. Elevated expression levels of NCOA3, TOP1, and TFAP2C in breast tumors as predictors of poor prognosis. Cancer. 2003;98(1):18–23. doi: 10.1002/cncr.11482. [DOI] [PubMed] [Google Scholar]
- 17.Murphy LC, Simon SL, Parkes A, Leygue E, Dotzlaw H, Snell L, et al. Altered expression of estrogen receptor coregulators during human breast tumorigenesis. Cancer Res. 2000;60(22):6266–71. [PubMed] [Google Scholar]
- 18.Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277(5328):965–8. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 19.Cawley K, Deegan S, Samali A, Gupta S. Assays for detecting the unfolded protein response. Methods in enzymology. 2011;490:31–51. doi: 10.1016/B978-0-12-385114-7.00002-7. [DOI] [PubMed] [Google Scholar]
- 20.Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) Journal of medicinal chemistry. 2012;55(16):7193–207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- 21.Cross BC, Bond PJ, Sadowski PG, Jha BK, Zak J, Goodman JM, et al. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc Natl Acad Sci U S A. 2012;109(15):E869–78. doi: 10.1073/pnas.1115623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117(4):1311–4. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samali A, Fitzgerald U, Deegan S, Gupta S. Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. Int J Cell Biol. 2010;2010:830307. doi: 10.1155/2010/830307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagelkerke A, Bussink J, Mujcic H, Wouters BG, Lehmann S, Sweep FC, et al. Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast cancer research: BCR. 2013;15(1):R2. doi: 10.1186/bcr3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mujcic H, Nagelkerke A, Rouschop KM, Chung S, Chaudary N, Span PN, et al. Hypoxic activation of the PERK/eIF2alpha arm of the unfolded protein response promotes metastasis through induction of LAMP3. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(22):6126–37. doi: 10.1158/1078-0432.CCR-13-0526. [DOI] [PubMed] [Google Scholar]
- 26.Wijayaratne AL, McDonnell DP. The human estrogen receptor-alpha is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. The Journal of biological chemistry. 2001;276(38):35684–92. doi: 10.1074/jbc.M101097200. [DOI] [PubMed] [Google Scholar]
- 27.Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, et al. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell. 2007;27(1):53–66. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Hetz C, Martinon F, Rodriguez D, Glimcher LH. The Unfolded Protein Response: Integrating Stress Signals through the Stress Sensor Ire1 Alpha. Physiol Rev. 2011;91(4):1219–43. doi: 10.1152/physrev.00001.2011. [DOI] [PubMed] [Google Scholar]
- 29.Kakiuchi C, Ishiwata M, Hayashi A, Kato T. XBP1 induces WFS1 through an endoplasmic reticulum stress response element-like motif in SH-SY5Y cells. J Neurochem. 2006;97(2):545–55. doi: 10.1111/j.1471-4159.2006.03772.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Iliopoulos D, Zhang Q, Tang Q, Greenblatt MB, Hatziapostolou M, et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature. 2014;508(7494):103–7. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 32.Reineke EL, Benham A, Soibam B, Stashi E, Taegtmeyer H, Entman ML, et al. Steroid receptor coactivator-2 is a dual regulator of cardiac transcription factor function. The Journal of biological chemistry. 2014;289(25):17721–31. doi: 10.1074/jbc.M113.539908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 34.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5(5):897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 35.Gupta S, Read DE, Deepti A, Cawley K, Gupta A, Oommen D, et al. Perk-dependent repression of miR-106b-25 cluster is required for ER stress-induced apoptosis. Cell death & disease. 2012;3:e333. doi: 10.1038/cddis.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Kuang SQ, Liao L, Zhou S, Xu J. Haploid inactivation of the amplified-in-breast cancer 3 coactivator reduces the inhibitory effect of peroxisome proliferator-activated receptor gamma and retinoid X receptor on cell proliferation and accelerates polyoma middle-T antigen-induced mammary tumorigenesis in mice. Cancer Res. 2004;64(19):7169–77. doi: 10.1158/0008-5472.CAN-04-1176. [DOI] [PubMed] [Google Scholar]
- 37.Coste A, Antal MC, Chan S, Kastner P, Mark M, O'Malley BW, et al. Absence of the steroid receptor coactivator-3 induces B-cell lymphoma. EMBO J. 2006;25(11):2453–64. doi: 10.1038/sj.emboj.7601106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Binet F, Sapieha P. ER Stress and Angiogenesis. Cell metabolism. 2015 doi: 10.1016/j.cmet.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Blais JD, Addison CL, Edge R, Falls T, Zhao H, Wary K, et al. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Molecular and cellular biology. 2006;26(24):9517–32. doi: 10.1128/MCB.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta S, McGrath B, Cavener DR. PERK regulates the proliferation and development of insulin-secreting beta-cell tumors in the endocrine pancreas of mice. PloS one. 2009;4(11):e8008. doi: 10.1371/journal.pone.0008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng YX, Sokol ES, Del Vecchio CA, Sanduja S, Claessen JH, Proia TA, et al. Epithelial-to-mesenchymal transition activates PERK-eIF2alpha and sensitizes cells to endoplasmic reticulum stress. Cancer discovery. 2014;4(6):702–15. doi: 10.1158/2159-8290.CD-13-0945. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107(7):881–91. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 43.Davies MP, Barraclough DL, Stewart C, Joyce KA, Eccles RM, Barraclough R, et al. Expression and splicing of the unfolded protein response gene XBP-1 are significantly associated with clinical outcome of endocrine-treated breast cancer. International journal of cancer Journal international du cancer. 2008;123(1):85–8. doi: 10.1002/ijc.23479. [DOI] [PubMed] [Google Scholar]
- 44.Hu R, Warri A, Jin L, Zwart A, Riggins RB, Fang HB, et al. NF-kappaB signaling is required for XBP1 (unspliced and spliced)-mediated effects on antiestrogen responsiveness and cell fate decisions in breast cancer. Molecular and cellular biology. 2015;35(2):379–90. doi: 10.1128/MCB.00847-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu RC, Qin J, Hashimoto Y, Wong J, Xu J, Tsai SY, et al. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) Coactivator activity by I kappa B kinase. Molecular and cellular biology. 2002;22(10):3549–61. doi: 10.1128/MCB.22.10.3549-3561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta A, Hossain MM, Read DE, Hetz C, Samali A, Gupta S. PERK regulated miR-424(322)-503 cluster fine-tunes activation of IRE1 and ATF6 during Unfolded Protein Response. Sci Rep. 2015;5:18304. doi: 10.1038/srep18304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta A, Read DE, Gupta S. Assays for induction of the unfolded protein response and selective activation of the three major pathways. Methods in molecular biology. 2015;1292:19–38. doi: 10.1007/978-1-4939-2522-3_2. [DOI] [PubMed] [Google Scholar]
- 48.Cawley K, Logue SE, Gorman AM, Zeng Q, Patterson J, Gupta S, et al. Disruption of microRNA biogenesis confers resistance to ER stress-induced cell death upstream of the mitochondrion. PLoS One. 2013;8(8):e73870. doi: 10.1371/journal.pone.0073870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.