Abstract
A subset of patients started on a selective serotonin reuptake inhibitor (SSRI) initially experience increased anxiety, which can lead to early discontinuation before therapeutic effects are manifest. The neural basis of this early SSRI effect is not known. Presynaptic dorsal raphe neuron (DRN) 5-HT1A receptors are known to play a critical role in affect processing. Thus we investigated the effect of acute citalopram on emotional processing and the relationship between DRN 5-HT1A receptor availability and amygdala reactivity. Thirteen (mean age 48±9 years) healthy male subjects received either a saline or citalopram infusion intravenously (10 mg over 30 minutes) on separate occasions in a single-blind, random order, cross-over design. On each occasion, participants underwent a block design face-emotion processing task during fMRI known to activate the amygdala. Ten subjects also completed a positron emission tomography (PET) scan to quantify DRN 5-HT1A availability using [11C]CUMI-101.Citalopram infusion when compared to saline resulted in a significantly increased bilateral amygdala responses to fearful vs. neutral faces (Left p=0.025; Right p=0.038 FWE-corrected). DRN [11C]CUMI-101availability significantly positively correlated with the effect of citalopram on the left amygdala response to fearful faces (Z=2.51, p=0.027) and right amygdala response to happy faces (Z=2.33, p=0.032). Our findings indicate that the initial effect of SSRI treatment is to alter processing of aversive stimuli, and that this is linked to DRN 5-HT1A receptors in line with evidence that 5-HT1A receptors have a role in mediating emotional processing.
Keywords: positron emission tomography (PET), serotonin (5-HT), Citalopram, SSRI, emotion, amygdala, neuroimaging, functional magnetic resonance imaging, 5-HT1A receptor, [11C]-CUMI-101, face processing, dorsal raphe nucleus
Introduction
Selective serotonin reuptake inhibitors (SSRI) are the most commonly prescribed medications for anxiety and depressive disorders worldwide (Olfson & Marcus, 2009). However, despite being used to treat anxiety disorders, a subgroup of patients experience an initial increase in anxiety after initiation of SSRI treatment (Gollan et al., 2012; Sinclair et al., 2009). Whilst this generally ameliorates over a few weeks, it can be clinically problematic as these patients with high anxiety are less likely to reach remission (Gollan et al., 2012). The neural basis of this early effect of SSRIs on anxiety and subsequent heterogeneity in treatment response is not known.
Serotonin, or 5-hydroxytryptamine (5-HT), is thought to be critical for affect regulation in the brain (Dayan & Huys, 2008), and SSRIs are thought to act primarily by altering 5-HT function. The administration of single doses of citalopram, a commonly used SSRI, in healthy human subjects is associated with enhanced startle responses and fear recognition (Browning, Reid, Cowen, Goodwin, & Harmer, 2007; Burghardt, Sullivan, McEwen, Gorman, & LeDoux, 2004; Grillon, Levenson, & Pine, 2007), and altered serotonin release (Selvaraj et al., 2012b). Functional magnetic resonance imaging (fMRI) studies have revealed that depressed patients have exaggerated amygdala reactivity as measured using blood-oxygen-level-dependent (BOLD) responses when presented with emotions of negative valence (fearful or sad faces) and 8 weeks of SSRI treatment attenuates this to ‘normalise’ the amygdala responses (Sheline et al., 2001). Bigos et al (2008) using a double-blind balanced crossover study design, found that citalopram 20 mg infusion compared to saline in healthy male participants (N=8) caused concentration-dependent increases in human amygdala reactivity to aversive facial stimuli (Bigos et al., 2008). However, in contrast, Del-Ben et al used a covert (aversive) face emotion recognition task and found attenuated amygdala response to fear after a 7.5 mg citalopram infusion compared to saline in male volunteers (N=12) (Del-Ben et al., 2005).
5-HT1A receptors are a key regulator of brain 5-HT activity through inhibitory autoreceptors located presynaptically on 5-HT dorsal raphe neurons (DRN), as well as on postsynaptic neurons in projection sites (Barnes & Sharp, 1999). Activation of the DRN 5-HT1A receptors causes hyperpolarization and reduces 5-HT neuronal firing which results in decreased 5-HT release from the 5-HT nerve terminals in the synapses. Acute SSRI administration increases 5-HT by blocking 5-HTT which then activates raphe 5-HT1A autoreceptor and thus reducing neuronal firing. Raphe 5-HT1A activation causes internalization which immediately returns to baseline level (Riad, Watkins, Doucet, Hamon, & Descarries, 2001) and this phenomenon is not observed in postsynaptic 5-HT1A receptors (Riad et al., 2001). 5-HT1A receptors have been consistently shown to modulate anxiety related behaviour in animal models. Specifically, 5-HT1A receptor knock-out mice exhibit increased fear-related behaviour (Ramboz et al., 1998; Richardson-Jones et al., 2011) and an altered fear response (Gross, Santarelli, Brunner, Zhuang, & Hen, 2000). A common functional variation (C(-1019)G) in the human 5-HT1A gene (HTR1A) is associated with increased 5-HT1A autoreceptor expression and decreased threat-related amygdala reactivity (Fakra et al., 2009). Finally, psychotropic drugs such as buspirone and vilazodone with 5-HT1A receptor binding properties have been found to be clinically useful for anxiety symptoms (Akimova, Lanzenberger, & Kasper, 2009; Gommoll et al., 2015; Sramek, Hong, Hamid, Nape, & Cutler, 1999).
An inverse relationship between 5-HT1A receptor binding in the dorsal raphe and amygdala reactivity has been reported in healthy human subjects (Fisher et al., 2006). In a combined fMRI and positron emission tomography (PET) imaging study using the 5-HT1A receptor tracer [11C]-CUMI-101, we similarly found DRN 5-HT1A receptor binding to be inversely related to amygdala BOLD responses to fear vs neutral faces (Selvaraj et al., 2014). The above findings suggest DRN 5-HT1A receptors may play a critical role in regulating amygdala reactivity during aversive emotion processing.
Citalopram is one of the most selective SSRI compared to fluoxetine, paroxetine sertraline or fluvoxamine and has high affinity to serotonin transporter (5-HTT) without any significant affinity for other serotonergic (5-HT1A, 5-HT1B, or 5-HT2A/C), adrenergic, cholinergic or other neurotransmitters and (Hyttel, 1994). A single administration of citalopram 1 mg/kg in rodents increases 5-HT levels in raphe but not in frontal cortex. A 10 mg/kg increases 5-HT release to 400% in raphe but only 170% in frontal cortex (Invernizzi, Belli, & Samanin, 1992). This dose-dependent and differential regional effect of SSRI on 5-HT release is consistent with 5-HT1A mediated negative feedback mechanism (Chaput, de Montigny, & Blier, 1986; Gartside, Umbers, Hajos, & Sharp, 1995; Riad et al., 2001). Thus, SSRI induced 5-HT release in 5-HT neuronal projection regions could be a balance of SSRIs ability to block 5-HTT at local neuronal terminals and to decrease DRN neuronal firing (Fuller, 1994; Gartside et al., 1995; Hjorth & Auerbach, 1996; Richardson-Jones et al., 2011). Interestingly, mice selectively engineered to express lower 5-HT1A autoreceptor levels compared to those with higher DRN 5-HT1A autoreceptor levels had increased raphe firing rate, greater 5-HT release in fronto-limbic regions and produced robust response to SSRI in reducing the aversive behavior (Richardson-Jones et al., 2010). In addition to 5-HT1A autoreceptor mediated negative feedback, post synaptic 5-HT1A heteroreceptor and 5-HT1B mediate the inhibitory actions and 5-HT2A mediates the excitatory actions of 5-HT on target neurons in prefrontal and limbic cortices along with other 5-HT receptors such as 5-HT3, 5-HT4, and 5-HT7 and also regulate 5-HT neuronal firing and release through postsynaptic feedback (Sharp, Boothman, Raley, & Quérée, 2007).
Citalopram is the only SSRI available in intravenous form and is relatively well tolerated by volunteers in clinical studies (Attenburrow, Mitter, Whale, Terao, & Cowen, 2001). Intravenous citalopram 10 mg has been successfully used as a probe to study brain serotonin function in clinical studies (Attenburrow et al., 2001; Bhagwagar, Cowen, Goodwin, & Harmer, 2004). In addition, we have used intravenous citalopram in PET imaging studies to characterize the specificity of serotonin transporter radioligand [11C]DASB occupancy (Hinz et al., 2008) and to study serotonin displacement (Selvaraj et al., 2012b).
In the present study, we aimed to investigate the effect of acute citalopram infusion on the neural processing of aversive emotional stimuli and to determine its relationship with DRN 5-HT1A receptors as measured with [11C]-CUMI-101 in healthy human subjects. We hypothesized that intravenous citalopram would increase amygdala reactivity to fear vs neutral faces. It is not known how the DRN 5-HT1A is related to the effect of acute citalopram on emotion processing. Based on our work and other studies (Richardson-Jones et al., 2010; Richardson-Jones et al., 2011; Selvaraj et al., 2014; Selvaraj et al., 2012b), we hypothesized that subjects with higher DRN 5-HT1A receptor availability would show a greater increase in amygdala response to emotional facial expressions following intravenous citalopram infusion.
Materials and Methods
A total of 13 healthy male participants took part in the citalopram and saline infusion fMRI study. All participants had undergone Structured Clinical Interview for DSM IV Disorders (SCID)(Spitzer, Williams, Gibbon, & First, 2004) screening interview administered by study investigators to ascertain past and current psychiatric and medical history. Inclusion criteria were male and female subjects, aged 35–65 years, in good physical health and capable of giving informed consent. Exclusion criteria were contraindication to PET scanning (pregnancy or breast feeding was an absolute contraindication), current or past history of major psychiatric disorder, present or recent (previous 3 months) use of psychotropic medication, current significant illicit substance/alcohol misuse or current significant other co-morbidity and no MRI contraindications. Electrocardiogram (ECG) was carried out before the infusion to rule out any prolonged corrected QT (cQT) interval. All the subjects had urine drug screen on all scan days to check for illegal drug use. All subjects also completed validated subjective scales to assess mood and anxiety and also a visual analogue scale to quantify side effects, if any. The subjects were paid a small honorarium for taking part in the study. The study was approved by the local research ethics committee. The PET and fMRI scans were carried out at the MRC London Institute of Medical Sciences, Hammersmith Hospital, London, UK.
Research Design
Thirteen subjects first took part in a PET scan experiment in which healthy subjects received either a placebo (saline) or citalopram infusion before a [11C]CUMI PET scan to index 5-HT1A receptor availability (Supplemental Figure 1). The results of this experiment are described in our previous publication (Selvaraj et al., 2012b). Subjects then went on to participate in the new fMRI experiment reported here. Of the 13 subjects who took part in the PET experiment, 3 dropped out, leaving 10 subjects who completed the fMRI component as well and we recruited an additional 3 new subjects who only participated in the fMRI component. We used the data from the 5-HT1A [11C]CUMI PET placebo (saline) scan as an index of baseline DRN 5-HT1A availability (Supplemental Figure 1).
In this new fMRI experiment, all participants received either saline or an intravenous infusion of 10 mg citalopram over 30 minutes in a single-blinded (participants), random order cross-over design on alternate days. About 15-30 minutes after the end of infusion, the subjects underwent the fMRI emotion processing task (Selvaraj et al., 2012a). Blood samples were collected for citalopram levels at (t=0) and after the infusion (t=45 mins). Mood was assessed before and after each scans using visual analogue scales (VAS) to ascertain subjective affective responses on emotions (including anxiety, sadness, happiness, anger, and irritability) across sessions. VAS scale was divided into a ten point scale for each emotion. There was a gap of at least one week between the two scans (mean and standard deviation (SD) was 30 (42.9) days).
Measurement of neural response to emotional stimuli
fMRI Data Acquisition
MRI was performed on 3T scanner (3T Intera Philips Medical Systems (Best, The Netherlands) to acquire T2*-weighted transverse echoplanar images (EPI).A total of 132 whole-brain EPI volumes were collected with 44 slices acquired in an even-odd interleave in a descending direction (TR = 3 s; TE = 30 ms; slice thickness = 3.25 mm; 2.19 mm x 2.19 mm in-plane resolution; phase encoding direction = anterior → posterior; field of view = 280 mm2; matrix size 128x128). Real-time reconstruction, z-shimming correction and a slice tilt of –30° to the anterior commissure–posterior commissure line were used to minimize orbitofrontal and temporal signal dropout as a result of magnetic field inhomogeneities due to air tissue susceptibility differences in these regions (Weiskopf, Hutton, Josephs, & Deichmann, 2006; Weiskopf, Hutton, Josephs, Turner, & Deichmann, 2007). A whole-brain 3D-MPRAGE scan was acquired (TR=9.6 ms, TE=4.5 ms, flip angle=8°, slice thickness = 1.2 mm, 0.94 mm × 0.94 mm in plane resolution, 150 slices) after the EPI scans.
fMRI task
A well-characterized incidental facial emotional processing task was employed as described in our previous studies (O'Nions, Dolan, & Roiser, 2011; Selvaraj et al., 2014). Subjects were shown a series of faces on a projector screen and asked to respond by classifying if each face was male or female. Emotional faces representing a single emotion (i.e., happy, fearful, or neutral) were presented in 16 second blocks of eight faces, with a total of 12 blocks (4 per emotion). Subjects were instructed to fixate on a cross during a 16 second rest period between stimulus blocks.
Measurement of 5-HT1A receptor availability
PET scan acquisition
All PET scans were performed on the GE Discovery RX PET/CT scanner with a PET axial field of view of 15.7 cm and 47 reconstructed transaxial image planes. [11C]CUMI-101 is a selective 5-HT1A radioligand with high signal to noise ratio in the brain. CUMI-101 has higher affinity (Ki= 0.15 nM) and better selectivity for 5-HT1A receptor than 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) (Kumar et al., 2013; Kumar et al., 2007). It was initially developed as 5-HT1A partial agonist ligand with specific binding to high affinity 5-HT1A receptors and thus to be more sensitive to study 5-HT release than older antagonist radiotracers such as [11C]WAY-100635 (Milak et al., 2011). However, the exact nature of [11C]CUMI-101 intrinsic activity as 5-HT1A receptor agonist or antagonist is not clear (Hendry, Christie, Rabiner, Laruelle, & Watson, 2011; Kumar et al., 2013; Shrestha et al., 2014). [11C]CUMI-101 was administered via injection into an antecubital vein as a smooth bolus over 30 s. The dynamic PET scan was acquired over 90 min in (simultaneous) frame and list mode (Selvaraj et al., 2012a).
Graphical analysis of reversible radioligand binding together with the metabolite corrected plasma input function was used to quantify the binding potential BPND in ROIs (Selvaraj et al., 2012b). The specific binding was quantified as BPND (37) where: BPND = (VT target region - VT reference region) / VT reference region VT is the volume of distribution (mL/cm3) defined as the ratio of the tracer concentration in the region to the metabolite corrected plasma concentration at equilibrium (Innis et al., 2007).
fMRI Analysis
The fMRI preprocessing and analysis were carried out in FSL using FEAT (Smith et al., 2004) and mirrored the analyses reported in our previous study (Selvaraj et al., 2014). Functional MRI data for individual runs were high pass filtered at 0.0078 Hz and motion corrected using a 6 degree of freedom (DOF) rigid body transformation (MCFLIRT). Lastly, data were smoothed with an 8 mm FWHM Gaussian kernel prior to a two stage standard space transformation. fMRI data and individual high resolution T1 images were registered to a 2 mm MNI standard template using a 12 DOF linear transformation (FLIRT) followed by nonlinear warping of T1 images to standard space (FNIRT). Both linear and nonlinear transformations were concatenated and applied to first-level, native space statistical images before higher level analyses.
Task regressors for happy, fearful, and neutral face blocks were modeled using a double-gamma function convolved with a 16 second square wave. Motion parameter estimates were included in the model to account for residual motion artifacts. All regressors were temporally filtered to match fMRI data preprocessing parameters (Hallquist, Hwang, & Luna, 2013). Time series data were pre-whitened (FILM) prior to modeling. As per Selvaraj et al. (Selvaraj et al., 2014), three contrasts were calculated at this level to compare: (1) faces vs baseline; (2) fearful vs neutral faces; and (3) happy vs neutral faces. At the second level of analysis, each first-level contrast was submitted to a fixed effect analysis which computed a contrast estimate for each subject comparing citalopram vs placebo. Second level contrast estimates were submitted for a final mixed effect analysis using FSL’s FLAME2 tool which employs Bayesian estimation of mean contrast estimates to determine the group level effect of citalopram on face (average within contrasts 1, 2, and 3) and valence processing (contrast 2 vs. contrast 3).
To constrain the number of simultaneous tests, we defined two spherical regions-of-interest (ROIs), in the left and right amygdala, by setting a 6 mm radius around the MNI coordinates [x=±21 y=-6 z= -15] adapted from our previous study (O'Nions et al., 2011; Selvaraj et al., 2014) using the same paradigm. This ensured an unbiased ROI definition. Voxelwise corrections for family-wise error inflation were applied using Gaussian random field (GRF)-theory-based height thresholding of Z-statistical maps at p < 0.05 (corrected). The values reported in the text represent the mean lower-level contrast estimates for citalopram and placebo extracted from voxels showing a significant citalopram vs. placebo difference, and thus represents a potential selection bias (Kriegeskorte, Simmons, Bellgowan, & Baker, 2009). Therefore, mean condition and contrast estimates across the independently-defined spherical amygdala ROIs are presented in Table 1.
Table 1. Condition and contrast parameter estimates to face stimuli during placebo (PBO) and citalopram (CITA) infusions in the amygdala.
| LEFT AMYGDALA – M
(SD) |
RIGHT AMYGDALA – M
(SD) |
|||
|---|---|---|---|---|
| Condition | PBO | CITA | PBO | CITA |
| Neutral Faces | 17.98 (39.60) | 13.89 (32.69) | 15.73 (27.56) | 20.08 (38.40) |
| Fearful Faces | 20.26 (35.23) | 30.92 (30.59) | 15.06 (41.77) | 22.23 (32.38) |
| Happy Faces | 7.57 (37.08) | 30.53 (53.00) | 11.79 (35.13) | 19.07 (22.35) |
| Contrast (Level 1) | ||||
| All vs Baseline | 50.61 (85.23) | 77.72 (82.18) | 46.79 (98.94) | 66.56 (76.02) |
| Fearful vs Neutral | 1.46 (25.78) | 16.97 (37.33) | -1.88 (23.92) | 3.11 (36.42) |
| Happy vs Neutral | -12.14 (54.84) | 16.79 (60.70) | -5.40 (30.81) | -1.54 (29.46) |
PET data analysis
Subjects’ structural MRIs were segmented (into gray/white matter/cerebrospinal fluid) using the segmentation tool in SPM (www.fil.ion.ucl.ac.uk/spm) and were re-sliced (1 x 1 x 1 mm3) and co-registered to the corresponding subject’s denoised, head movement-corrected and summed PET image using SPM5. Amygdala, post-synaptic cortical regions and cerebellum were defined using a probabilistic brain atlas template (Hammers et al., 2003). The atlas was spatially normalized to the coregistered individual MRI scans with deformation parameters obtained from the normalization to the standard MNI T1 template in SPM. The normalized brain atlas was resliced to the individual’s PET space and fused with the individual grey matter map to obtain a grey matter template for the amygdala and post-synaptic cortical regions. These were then used to sample the dynamic PET to obtain the regional time-activity courses. The presynaptic DRN was manually defined as a fixed-size region (215 mm3) in the midbrain area on the summed PET images of each individual (Bose et al., 2011a; Selvaraj et al., 2014; Selvaraj et al., 2012b). Finally, cerebellar grey matter was used as the reference region (Selvaraj et al., 2012b). See Selvaraj et al for further details of the [11C]CUMI PET analysis (Selvaraj et al., 2012b).
Multi-modal PET-MR analysis
The third level analysis described in the fMRI analysis above was repeated for the 10 subjects who had also completed the PET imaging protocol. To estimate the relationship between fMRI changes associated with citalopram and DRN 5-HT1A receptor availability, the [11C]CUMI-101 binding potential (BND) values from the DRN were included as a continuous predictor variable in the analysis. Extracted data represents lower level contrast estimates summarizing mean parameter estimate differences as in the fMRI-only analysis. Correlation coefficients were computed between DRN [11C]CUMI-101 BPND and the citalopram vs placebo contrast estimates for the independent amygdala ROI (rAMYG) to display general strength and direction of association. Extracted Z-statistics represent the mean of significant Z-scores with corresponding GRF-theory based p-values (Beckmann, Jenkinson, & Smith, 2003; Jenkinson, Bannister, Brady, & Smith, 2002; Woolrich, Ripley, Brady, & Smith, 2001). Owing to the possible presence of an outlier (i.e., DRN [11C]CUMI-101 BND > 2.4 SD of mean; q.v., Fig 2 B&C), these analyses were repeated with FEAT’s outlier deweighting tool. Statistical results were identical. Furthermore, our review of the specific outlier case found that [11C]CUMI-101 availability in other brain regions (e.g., amygdala) and the first-level fMRI results were comparable to other participants’ PET/first-level results. We deemed the case to exhibit a real physiological effect and the final reported statistics reflect its inclusion.
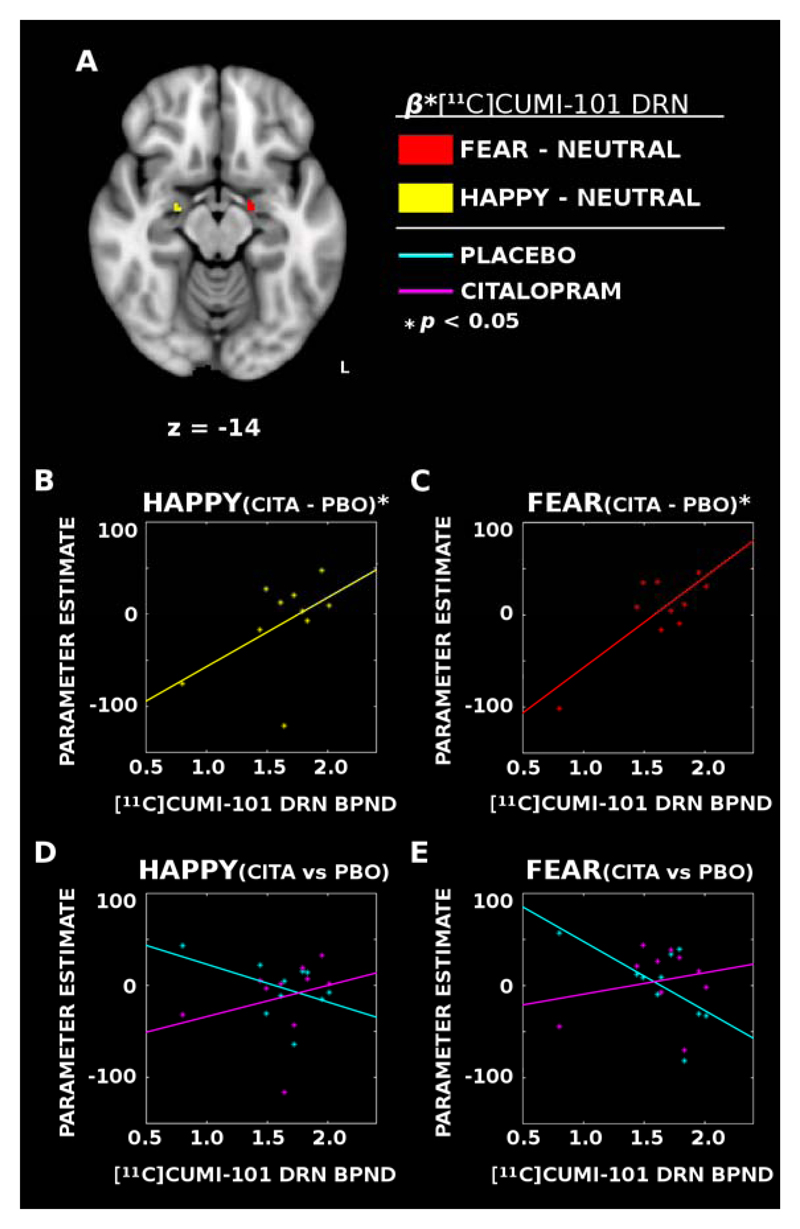
Figure 2. Dorsal raphe nucleus (DRN) 5-HT1A availability was positively associated with the degree of modulation induced by citalopram infusion in the amygdala.
(A) PET [11C]CUMI-101 binding estimates demonstrated a functionally lateralized positive association with activity in the left amygdala between the response to fearful faces and DRN 5-HT1A availability (RED) and the right amygdala showing an association with happy faces (YELLOW). (B & C) Citalopram (CITA) minus placebo (PBO) differences show that individuals with greater DRN 5-HT1A availability have reduced citalopram-induced modulation of the amygdala to happy (YELLOW) and fearful (RED) faces in the right and left hemispheres, respectively. (D & E) Same as B & C, but with citalopram and placebo data presented separately. Whereas individuals with less DRN 5-HT1A availability show heightened amygdala responses during placebo infusions (CYAN), the association largely disappears with citalopram (MAGENTA) suggesting individual differences in the response of the amygdala to emotional content is associated with differences in DRN 5-HT1A availability.
Results
Thirteen male subjects (mean (SD) age=48±9 years) completed the emotion processing task on both days. Study participants generally tolerated intravenous citalopram well with either no or minimal self-limiting adverse effects, which included mild nausea, hot flush, lightheadedness and tiredness. One subject reported mild nausea after saline (placebo). None of the subjects stopped the scan procedures or dropped out of study during the study day. There were no significant differences in behavioral measures between sessions on subjective VAS affective state measures (paired t tests, all Ps>0.1) especially no significant change in anxiety measures. The mean serum citalopram concentration at 45 minutes after the start of infusion is 757.45 µg/L (SD 802.75). There was no significant correlation was observed between serum citalopram concentration and citalopram effect on amygdala reactivity to fearful faces (p>0.1).
Effect of citalopram on amygdala reactivity to aversive faces
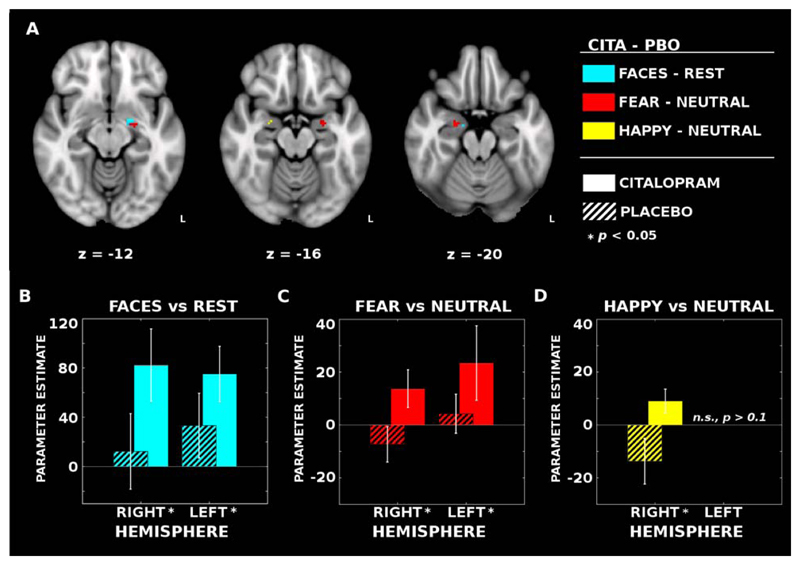
For the all faces vs baseline contrast, citalopram infusions resulted in a significantly increased BOLD response bilaterally in the amygdala with a larger cluster in the left hemisphere: right amygdala (k = 2, peak MNI coordinates: [x=16, y=-10, z=-18]; MPBO = 12.31, SDPBO = 110.48; MCITA = 82.32, SDCITA = 106.10), Z=2.46, p=0.039 (FWE-corrected); left amygdala (k = 11, peak MNI coordinates: [x=-20, y=-4, z=-12]; MPBO = 33.30, SDPBO = 94.71; MCITA = 75.06, SDCITA = 81.33), Z=2.33, p=0.046 (FWE-corrected) (Fig 1 A,B). See Table 1 for individual condition and contrast parameter estimates across the spherical amygdala ROIs.
Figure 1. Functional imaging reveals bilateral patterns of increased face-dependent activation with citalopram infusion.
(A) Intravenous citalopram (CITA) significantly increased activation bilaterally for the all faces vs. rest (BLUE) and fearful vs neutral face (RED) contrasts when compared to placebo (PBO). Trend-level activation increases were also observed in the right amygdala for the happy vs neutral face contrast (YELLOW). (B; CYAN) Mean parameter estimates (± standard error of the mean; SEM) extracted across the voxels with significant differences in the second level contrasts (i.e., CITA – PBO). Within each hemisphere, the blood oxygen level dependent (BOLD) signal elicited by faces increased after CITA infusions compared to PBO (striped bars indicate PBO estimates). (C; RED) A similar pattern of activity was observed in the left hemisphere for the fearful vs neutral face contrasts as well; however, (D; YELLOW) the right hemisphere effects for fearful vs neutral and happy vs neutral faces are less clear owing to a task-related deactivation in the PBO condition.
To test the hypothesis that intravenous citalopram would specifically increase the response to fearful faces, we repeated the citalopram contrast analysis for the fearful vs neutral faces contrast estimates and again identified increased bilateral amygdala activation associated with citalopram. Statistical differences were identified in both the right amygdala (k = 14, peak MNI coordinates: [x=24, y=-6, z=-20]; MPBO = -7.31, SDPBO = 24.15, MCITA = 13.74, SDCITA = 25.65), Z=2.59, p=0.025 (FWE-corrected), and the left amygdala (k = 18, peak MNI coordinates: [x=-22, y=-6, z=-18]; MPBO = 4.25, SDPBO = 26.64, MCITA = 23.43, SDCITA = 50.89), Z=2.29, p=0.038 (FWE-corrected) (Fig 1 A,C). There were no significant differences for the happy vs. neutral faces contrast in the left amygdala after citalopram; though a significant increase was observed in the right amygdala (k = 3, peak MNI coordinates: [x=24, y=-4, z=-16]; MPBO = -13.70, SDPBO = 31.01, MCITA = 9.07, SDCITA = 16.27), Z=2.12, p=0.042 (FWE-corrected) (Fig 1 A,D). Despite qualitative hemispheric differences between the fearful and happy face response, a direct comparison only demonstrated a trend level effect of greater fearful face response modulation by citalopram compared to the happy face response in the left amygdala (k = 2, peak MNI coordinates: [x=-20, y=-10, z=-12]; MFEARFUL = 3.50, SDFEARFUL = 24.73, MHAPPY = -5.24, SDHAPPY = 18.42), Z=2.02, p=0.062.
There was no significant correlation was observed between serum citalopram concentration and citalopram effect on amygdala reactivity to fearful faces (p>0.1).
Relationship between citalopram induced changes in Amygdala reactivity and DRN 5-HT1A receptor
Of the total of 13 subjects, ten completed the [11C]CUMI-101 PET imaging protocol as well. 5-HT1A receptor BPND values from the DRN (Mean = 1.63, SD = 0.34) were included as a continuous predictor in the citalopram contrast model, and analyses were repeated within this subset to identify associations within the amygdala. There was a significant positive association between DRN 5-HT1A and the all faces vs baseline citalopram contrast in the right amygdala, (k = 1, peak MNI coordinates: [x=16, y=-8, z=-12]; MPBO = 36.57, SDPBO = 67.94, MCITA = 33.07, SDCITA = 56.66), rAMYG(10) = 0.05, Z=2.59, p=0.032, (FWE-corrected).
A significant positive association was identified within the left amygdala for the fearful faces vs neutral faces contrast. The citalopram vs placebo difference in the response to fearful faces vs neutral faces was larger in participants with greater DRN [11C]CUMI-101 binding (k = 11, peak MNI coordinates: [x=-16, y=-10, z=-14]; MPBO = 0.74, SDPBO = 38.78, MCITA = 5.29, SDCITA = 35.19), rAMYG(10) = 0.38, Z = 2.51, p = 0.027 (FWE-corrected) (Fig 2 A,C&E). There was a smaller positive association between DRN [11C]CUMI-101 binding and the citalopram vs placebo difference in response to fearful vs neutral faces in the right amygdala (k = 1, peak MNI coordinates: [x=24, y=-10, z=-14]; MPBO = 4.51, SDPBO = 27.91, MCITA = -8.51, SDCITA = 48.77), rAMYG(10) = 0.06, Z = 2.16, p = 0.042, FWE-corrected.
For the happy vs neutral face contrasts, DRN values demonstrated positive associations in the right amygdala (k = 6, peak MNI coordinates: [x=24, y=-10, z=-14]; MPBO = -2.85, SDPBO = 28.63, MCITA = -12.60, SDCITA = 40.37), rAMYG(10) = 0.21, Z = 2.33, p = 0.032, (FWE-corrected) (Fig 2 A,B&D), and again, a smaller effect in the left amygdala (k = 1, peak MNI coordinates: [x=-16, y=-10, z=-14]; MPBO = -13.53, SDPBO = 60.41, MCITA = -7.38, SDCITA = 32.36), rAMYG(10) = 0.34, Z = 2.29, p = 0.033, (FWE-corrected). DRN [11C]CUMI-101 binding associations did not differ significantly between fearful and happy faces. Collectively, this pattern of results suggests greater [11C]CUMI-101 binding potential in the DRN is positively associated with the degree of increase in BOLD signaling to emotional faces induced by citalopram infusion. However, the specificity of the amygdala response to particular valences is still unclear.
Discussion
In this multimodal brain imaging study, we report the effect of acute intravenous citalopram on amygdala reactivity and its relationship with DRN 5-HT1A receptor availability (as indexed by [11C]CUMI-101 PET) in healthy human male subjects. The main findings of this study are that: 1) acutely citalopram increased the BOLD response bilaterally in the amygdala to fearful faces and in the right amygdala to happy faces with a trend-level left amygdala selectivity to fearful vs. happy faces; and 2) DRN 5-HT1A receptor availability is positively associated with the degree of increase in amygdala BOLD response to emotional faces (both fearful and happy vs neutral) induced by citalopram infusion. The current findings, when combined with other findings (Fisher et al., 2006; Richardson-Jones et al., 2010; Richardson-Jones et al., 2011; Selvaraj et al., 2014) support the critical role of presynaptic DRN 5-HT1A receptors in regulating emotion processing.
Intravenous citalopram increased amygdala BOLD response bilaterally for fearful vs. neutral faces. This finding is consistent with our a priori hypothesis regarding amygdala reactivity to SSRI and in agreement with Bigos et al (Bigos et al., 2008) but not Del-Ben et al (Del-Ben et al., 2005). The differences could be due to differences in the nature of emotion task paradigms of using covert or explicit emotion faces task. Curiously, voxels demonstrating significant increases in BOLD response were not positively active for the placebo in the fearful and happy faces vs. neutral contrasts (Fig 1C&D). One possibility for this result is that our analytical approach highlighted those voxels which were found to be maximally different between the two sessions rather than which voxels are maximally activated by the task per se. However, parameter estimates extracted from the condition regressors presented in Table 1 demonstrate consistent positive activation across all conditions, suggesting that the amygdala was sensitive to faces, but not uniquely sensitive to emotional faces during the placebo visit. Thus, only during the citalopram infusion were amygdala responses sensitive to affect. Another possibility is that the voxels responsive to emotional faces during the placebo visit were already active at ceiling and the voxels identified in the present analysis represent an increase in the spatial extent of activation (i.e. similar peak, but wider spread). Future investigations may consider varying the valence of affective stimuli to better address this question of state-based reactivity versus activation span.
Our data provide evidence that variations in DRN 5-HT1A receptor availability are related to the SSRI effect on emotion processing. However, the mechanistic pathway of this relationship cannot be determined from the correlations we report. Our finding is however consistent with preclinical research on the role of presynaptic DRN 5-HT1A receptors. Preclinical studies show that stimulation of 5-HT1A receptors decreases the firing rate of 5-HT neurones (Sprouse & Aghajanian, 1987) and 5-HT release (F. Bosker, Vrinten, Klompmakers, & Westenberg, 1997; F. J. Bosker et al., 2001). Mice selectively expressing high DRN 5-HT1A auto-receptors compared to low DRN 5-HT1A auto-receptors have decreased 5-HT cell firing and therefore reduced 5-HT tone in the projection sites such as the amygdala (Richardson-Jones et al., 2011). Acute SSRI induced increases in extracellular raphe 5-HT also activates 5-HT1A autoreceptors, thereby decreases 5-HT firing and release in projection sites (Auerbach, Lundberg, & Hjorth, 1995; Fuller, 1994; Gartside et al., 1995; Haddjeri, Lavoie, & Blier, 2004; Romero & Artigas, 1997). Thus high DRN 5-HT1A may be associated with low 5-HT in amygdala. Based on our findings, we speculate that individuals with high DRN 5-HT1A autoreceptors are more sensitive to the autoreceptor activation and thus show greater amygdala response when given acute dose of SSRI. However, acute citalopram treatment also increases 5-HT levels in the amygdala in rodent models (F. J. Bosker et al., 2001) and elevated 5-HT in amygdala increases fear learning and acquisition (Bocchio, McHugh, Bannerman, Sharp, & Capogna, 2016; Deakin & Graeff, 1991). Furthermore, the net effect of synaptic 5-HT at projection sites may be influenced by regional variations in 5-HT transporters (Bose et al., 2011b). Finally, the modulatory effect of 5-HT on anxiety response depends upon a balance of excitatory (5-HT2A) and inhibitory (5-HT1A and 5-HT1B) 5-HT signaling on cortical pyramidal and interneurons in the prefrontal and amygdala circuitry (Albert, Vahid-Ansari, & Luckhart, 2014; Fisher et al., 2011). Therefore multiple mechanisms may be involved in SSRI induced amygdala response.
When taken together, these findings indicate that combined knowledge of 5-HTT and 5-HT1A autoreceptor density may have predictive value in understanding antidepressant response. Based on our preliminary findings in healthy volunteers, we speculate that patients with high DRN 5-HT1A receptor availability would be predicted to have more severe anxiety responses to SSRIs. Further research in patients with major depression might help clarify the contribution of this mechanism to the increase in anxiety levels reported by some patients after initiation of antidepressant treatment.
This study has a number of limitations; first, we only studied male participants. Future studies should assess whether gender has an effect on the relationship between DRN 5-HT1A receptor and amygdala reactivity as some studies have reported associations between DRN 5-HT1A auto-receptor availability and sex (Parsey et al., 2002). Second, although N=13 is comparable to the size of similar studies (Bigos et al., 2008; Del-Ben et al., 2005), the sample size for the combined PET/fMRI experiment does not permit investigation of additional potential moderators (e.g. 5-HTT genetic polymorphisms) on the influence of the 5-HT1A receptors on amygdala reactivity. This will have to be addressed in future larger studies. Furthermore, analysis of smaller sample sizes, as reported here, are known to provide inflated estimates of effect sizes (Button et al., 2013). As such, we have presented means and standard deviations from voxels showing significant differences and from our a priori amygdala ROI as a whole. A substantially larger sample (e.g., N > 78 for the fearful vs. neutral face citalopram contrasts; cf., fmripower.org; (Mumford & Nichols, 2008) will be required to establish well-powered effect size estimates. Therefore, we put forward our own results with caution to be interpreted as indicating the presence of an association rather than a specific magnitude of effect. Third, correlation between DRN 5-HT1A receptor availability and amygdala BOLD response to emotional faces after citalopram does not prove causality. Further experimental research studies will be needed to study the direct role of 5-DRN HT1A in emotion processing in humans. Finally, the average interval between PET and fMRI data acquisition was 125 (SD 7.7) weeks (2.4 years). Although the PET and fMRI scans were performed at different time points, several lines of evidence indicate that the binding potential measures in this study would be stable over the time scale of the experiment. A previous 5-HT1A [11C]CUMI-101 study reported high test-retest reliability for raphe measurements, with an intraclass correlation coefficient of 0.8 (Milak et al., 2010), indicating that it can be reliably measured. A selective 5-HT1A receptor antagonist ligand (18F-MPPF) study that collected test-retest scans with a mean delay 27 weeks between scans in healthy volunteers, reported high reliability of dorsal raphe binding potential measurements (ICC 0.78) (Costes et al., 2007). This result suggests 5-HT1A PET measures from the dorsal raphe are reliable over time, albeit using a different tracer (18F-MPPF). In addition, PET studies report no significant decline of 5-HT1A availability (as indexed by [11C]WAY-100635) in pre or postsynaptic regions over time with age (age range of 24 to 53) years in a large cohort (N=61) (Rabiner et al., 2002), suggesting that ageing does not significantly affect 5-HT1A availability. Thus, brain 5-HT1A receptors availability in vivo using PET provides stable measure of 5-HT1A binding and the time interval between acquisitions of the PET/MRI scan data is less likely to have influenced the results. Nevertheless we cannot exclude variation over time, although this would, if anything, be expected to increase noise and weaken the results.”
Conclusion
An acute intravenous administration of citalopram increased amygdala reactivity to aversive emotion, and this was positively associated with DRN 5-HT1A receptor availability. Our findings indicate that the initial effect of SSRI treatment is to alter processing of aversive stimuli, and that this is linked to DRN 5-HT1A receptors in line with evidence that 5-HT1A receptors have a role in mediating emotional processing.
Supplementary Material
Acknowledgements
The authors thank the staff at Hammersmith Imanet (Andrew Blyth, Hope McDevitt, Andreanna Williams, Safiye Osman and Noora Ali) and Robert Steiner MRI unit at Hammersmith Hospital for the technical expertise they provided.
Funding and Disclosure
This study was supported by an Academy of Medical Sciences, UK clinical lecturer starter grant to Sudhakar Selvaraj (grant number: AMS-SGCL6). This study was funded by a Medical Research Council (UK) grant to Oliver Howes (grant number: MC-A656-5QD30). Maudsley Charity (no. 666), Brain and Behavior Research Foundation, and Wellcome Trust (no. 094849/Z/10/Z) grants to Oliver Howes and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. Paul Faulkner was supported by an MRC studentship. Danilo Arnone was supported by the Academy of Medical Sciences, UK (grant number: AMS SGCL8) and has received travel grants from Jansenn-Cilag and Servier. Philip Cowen has been a member of advisory boards of Servier and Lundbeck and has been a paid lecturer for Servier and, Lundbeck. Jonathan Roiser has been a member of a media advisory board for Lundbeck and consults for Cambridge Cognition Ltd. Intravenous citalopram was kindly provided by Lundbeck, UK. Dr Howes has received investigator-initiated research funding from and/or participated in advisory/ speaker meetings organised by Astra-Zeneca, Autifony, BMS, Eli Lilly, Heptares, Janssen, Lundbeck, Lyden-Delta, Otsuka, Servier, Sunovion, Rand and Roche. Chris Walker and Bo Cao have nothing to disclose.
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- Akimova E, Lanzenberger R, Kasper S. The serotonin-1A receptor in anxiety disorders. Biol Psychiatry. 2009;66(7):627–635. doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Albert PR, Vahid-Ansari F, Luckhart C. Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci. 2014;8:199. doi: 10.3389/fnbeh.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attenburrow MJ, Mitter PR, Whale R, Terao T, Cowen PJ. Low-dose citalopram as a 5-HT neuroendocrine probe. Psychopharmacology. 2001;155(3):323–326. doi: 10.1007/s002130100729. [DOI] [PubMed] [Google Scholar]
- Auerbach SB, Lundberg JF, Hjorth S. Differential inhibition of serotonin release by 5-HT and NA reuptake blockers after systemic administration. Neuropharmacology. 1995;34(1):89–96. doi: 10.1016/0028-3908(94)00137-h. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38(8):1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Cowen PJ, Goodwin GM, Harmer CJ. Normalization of enhanced fear recognition by acute SSRI treatment in subjects with a previous history of depression. Am J Psychiatry. 2004;161(1):166–168. doi: 10.1176/appi.ajp.161.1.166. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology. 2008;33(13):3221–3225. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio M, McHugh SB, Bannerman DM, Sharp T, Capogna M. Serotonin, Amygdala and Fear: Assembling the Puzzle. Front Neural Circuits. 2016;10:24. doi: 10.3389/fncir.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose SK, Mehta MA, Selvaraj S, Howes OD, Hinz R, Rabiner EA, et al. Murthy V. Presynaptic 5-HT1A is Related to 5-HTT Receptor Density in the Human Brain. Neuropsychopharmacology. 2011a;36(11):2258–2265. doi: 10.1038/npp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose SK, Mehta MA, Selvaraj S, Howes OD, Hinz R, Rabiner EA, et al. Murthy V. Presynaptic 5-HT1A is related to 5-HTT receptor density in the human brain. Neuropsychopharmacology. 2011b;36(11):2258–2265. doi: 10.1038/npp.2011.113. doi:npp2011113 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosker F, Vrinten D, Klompmakers A, Westenberg H. The effects of a 5-HT1A receptor agonist and antagonist on the 5-hydroxytryptamine release in the central nucleus of the amygdala: a microdialysis study with flesinoxan and WAY 100635. Naunyn Schmiedebergs Arch Pharmacol. 1997;355(3):347–353. doi: 10.1007/pl00004953. [DOI] [PubMed] [Google Scholar]
- Bosker FJ, Cremers TI, Jongsma ME, Westerink BH, Wikström HV, den Boer JA. Acute and chronic effects of citalopram on postsynaptic 5-hydroxytryptamine(1A) receptor-mediated feedback: a microdialysis study in the amygdala. J Neurochem. 2001;76(6):1645–1653. doi: 10.1046/j.1471-4159.2001.00194.x. [DOI] [PubMed] [Google Scholar]
- Browning M, Reid C, Cowen PJ, Goodwin GM, Harmer CJ. A single dose of citalopram increases fear recognition in healthy subjects. J Psychopharmacol. 2007;21(7):684–690. doi: 10.1177/0269881106074062. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55(12):1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafò MR. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5):365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: electrophysiological studies in the rat brain. Naunyn-Schmiedeberg's archives of pharmacology. 1986;333(4):342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- Costes N, Zimmer L, Reilhac A, Lavenne F, Ryvlin P, Le Bars D. Test-retest reproducibility of 18F-MPPF PET in healthy humans: a reliability study. J Nucl Med. 2007;48(8):1279–1288. doi: 10.2967/jnumed.107.041905. [DOI] [PubMed] [Google Scholar]
- Dayan P, Huys QJ. Serotonin, inhibition, and negative mood. PLoS computational biology. 2008;4(2):e4. doi: 10.1371/journal.pcbi.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin JF, Graeff FG. 5-HT and mechanisms of defence. Journal of psychopharmacology. 1991;5(4):305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- Del-Ben CM, Deakin JF, McKie S, Delvai NA, Williams SR, Elliott R, et al. Anderson IM. The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an FMRI study. Neuropsychopharmacology. 2005;30(9):1724–1734. doi: 10.1038/sj.npp.1300728. [DOI] [PubMed] [Google Scholar]
- Fakra E, Hyde LW, Gorka A, Fisher PM, Munoz KE, Kimak M, et al. Hariri AR. Effects of HTR1A C(-1019)G on amygdala reactivity and trait anxiety. Archives of general psychiatry. 2009;66(1):33–40. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Ziolko SK, Price JC, Moses-Kolko EL, Berga SL, Hariri AR. Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nat Neurosci. 2006;9(11):1362–1363. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Price JC, Meltzer CC, Moses-Kolko EL, Becker C, Berga SL, Hariri AR. Medial prefrontal cortex serotonin 1A and 2A receptor binding interacts to predict threat-related amygdala reactivity. Biology of mood & anxiety disorders. 2011;1(1):2. doi: 10.1186/2045-5380-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RW. Uptake inhibitors increase extracellular serotonin concentration measured by brain microdialysis. Life sciences. 1994;55(3):163–167. doi: 10.1016/0024-3205(94)00876-0. [DOI] [PubMed] [Google Scholar]
- Gartside SE, Umbers V, Hajos M, Sharp T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. British journal of pharmacology. 1995;115(6):1064–1070. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan JK, Fava M, Kurian B, Wisniewski SR, Rush AJ, Daly E, et al. Trivedi MH. What are the clinical implications of new onset or worsening anxiety during the first two weeks of SSRI treatment for depression? Depress Anxiety. 2012;29(2):94–101. doi: 10.1002/da.20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gommoll C, Forero G, Mathews M, Nunez R, Tang X, Durgam S, Sambunaris A. Vilazodone in patients with generalized anxiety disorder: a double-blind, randomized, placebo-controlled, flexible-dose study. Int Clin Psychopharmacol. 2015;30(6):297–306. doi: 10.1097/YIC.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Levenson J, Pine DS. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacology. 2007;32(1):225–231. doi: 10.1038/sj.npp.1301204. [DOI] [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Altered fear circuits in 5-HT(1A) receptor KO mice. Biol Psychiatry. 2000;48(12):1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Lavoie N, Blier P. Electrophysiological evidence for the tonic activation of 5-HT(1A) autoreceptors in the rat dorsal raphe nucleus. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29(10):1800–1806. doi: 10.1038/sj.npp.1300489. [DOI] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, et al. Duncan JS. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Human brain mapping. 2003;19(4):224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry N, Christie I, Rabiner EA, Laruelle M, Watson J. In vitro assessment of the agonist properties of the novel 5-HT1A receptor ligand, CUMI-101 (MMP), in rat brain tissue. Nuclear medicine and biology. 2011;38(2):273–277. doi: 10.1016/j.nucmedbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Hinz R, Selvaraj S, Murthy NV, Bhagwagar Z, Taylor M, Cowen PJ, Grasby PM. Effects of citalopram infusion on the serotonin transporter binding of [11C]DASB in healthy controls. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28(8):1478–1490. doi: 10.1038/jcbfm.2008.41. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Auerbach SB. 5-HT1A autoreceptors and the mode of action of selective serotonin reuptake inhibitors (SSRI) Behavioural brain research. 1996;73(1–2):281–283. doi: 10.1016/0166-4328(96)00113-1. [DOI] [PubMed] [Google Scholar]
- Hyttel J. Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs) Int Clin Psychopharmacol. 1994;9(Suppl 1):19–26. doi: 10.1097/00004850-199403001-00004. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Belli S, Samanin R. Citalopram's ability to increase the extracellular concentrations of serotonin in the dorsal raphe prevents the drug's effect in the frontal cortex. Brain research. 1992;584(1–2):322–324. doi: 10.1016/0006-8993(92)90914-u. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Somerville LH, Alexander AL, Oakes TR, Davidson RJ, Kalin NH, Whalen PJ. Stability of amygdala BOLD response to fearful faces over multiple scan sessions. Neuroimage. 2005;25(4):1112–1123. doi: 10.1016/j.neuroimage.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12(5):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar JS, Parsey RV, Kassir SA, Majo VJ, Milak MS, Prabhakaran J, et al. Arango V. Autoradiographic evaluation of [3H]CUMI-101, a novel, selective 5-HT1AR ligand in human and baboon brain. Brain Res. 2013;1507:11–18. doi: 10.1016/j.brainres.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar JS, Prabhakaran J, Majo VJ, Milak MS, Hsiung SC, Tamir H, et al. Parsey RV. Synthesis and in vivo evaluation of a novel 5-HT1A receptor agonist radioligand [O-methyl-11C]2-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-methyl-1,2,4-triazine -3,5(2H,4H)dione in nonhuman primates. European journal of nuclear medicine and molecular imaging. 2007;34(7):1050–1060. doi: 10.1007/s00259-006-0324-y. [DOI] [PubMed] [Google Scholar]
- Milak MS, DeLorenzo C, Zanderigo F, Prabhakaran J, Kumar JS, Majo VJ, et al. Parsey RV. In vivo quantification of human serotonin 1A receptor using 11C-CUMI-101, an agonist PET radiotracer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51(12):1892–1900. doi: 10.2967/jnumed.110.076257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milak MS, Severance AJ, Prabhakaran J, Kumar JS, Majo VJ, Ogden RT, et al. Parsey RV. In vivo serotonin-sensitive binding of [11C]CUMI-101: a serotonin 1A receptor agonist positron emission tomography radiotracer. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31(1):243–249. doi: 10.1038/jcbfm.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JA, Nichols TE. Power calculation for group fMRI studies accounting for arbitrary design and temporal autocorrelation. Neuroimage. 2008;39(1):261–268. doi: 10.1016/j.neuroimage.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Nions EJ, Dolan RJ, Roiser JP. Serotonin transporter genotype modulates subgenual response to fearful faces using an incidental task. Journal of cognitive neuroscience. 2011;23(11):3681–3693. doi: 10.1162/jocn_a_00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848–856. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, Mann JJ. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain research. 2002;954(2):173–182. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Messa C, Sargent PA, Husted-Kjaer K, Montgomery A, Lawrence AD, et al. Grasby PM. A database of [(11)C]WAY-100635 binding to 5-HT(1A) receptors in normal male volunteers: normative data and relationship to methodological, demographic, physiological, and behavioral variables. Neuroimage. 2002;15(3):620–632. doi: 10.1006/nimg.2001.0984. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, et al. Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(24):14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad M, Watkins KC, Doucet E, Hamon M, Descarries L. Agonist-induced internalization of serotonin-1a receptors in the dorsal raphe nucleus (autoreceptors) but not hippocampus (heteroreceptors) J Neurosci. 2001;21(21):8378–8386. doi: 10.1523/JNEUROSCI.21-21-08378.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, et al. Leonardo ED. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65(1):40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Nguyen TH, Kung HF, Gardier AM, Dranovsky A, et al. Leonardo ED. Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(16):6008–6018. doi: 10.1523/JNEUROSCI.5836-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero L, Artigas F. Preferential potentiation of the effects of serotonin uptake inhibitors by 5-HT1A receptor antagonists in the dorsal raphe pathway: role of somatodendritic autoreceptors. Journal of neurochemistry. 1997;68(6):2593–2603. doi: 10.1046/j.1471-4159.1997.68062593.x. [DOI] [PubMed] [Google Scholar]
- Sauder CL, Hajcak G, Angstadt M, Phan KL. Test-retest reliability of amygdala response to emotional faces. Psychophysiology. 2013;50(11):1147–1156. doi: 10.1111/psyp.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Mouchlianitis E, Faulkner P, Turkheimer F, Cowen PJ, Roiser JP, Howes O. Presynaptic Serotoninergic Regulation of Emotional Processing: A Multimodal Brain Imaging Study. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Turkheimer F, Rosso L, Faulkner P, Mouchlianitis E, Roiser JP, et al. Howes O. Measuring endogenous changes in serotonergic neurotransmission in humans: a [11C]CUMI-101 PET challenge study. Mol Psychiatry. 2012a;17(12):1254–1260. doi: 10.1038/mp.2012.78. [DOI] [PubMed] [Google Scholar]
- Selvaraj S, Turkheimer F, Rosso L, Faulkner P, Mouchlianitis E, Roiser JP, et al. Howes O. Measuring endogenous changes in serotonergic neurotransmission in humans: a [(11)C]CUMI-101 PET challenge study. Molecular psychiatry. 2012b doi: 10.1038/mp.2012.78. [DOI] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Quérée P. Important messages in the 'post': recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28(12):629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological psychiatry. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Shrestha SS, Liow JS, Lu S, Jenko K, Gladding RL, Svenningsson P, et al. Innis RB. (11)C-CUMI-101, a PET radioligand, behaves as a serotonin 1A receptor antagonist and also binds to α(1) adrenoceptors in brain. J Nucl Med. 2014;55(1):141–146. doi: 10.2967/jnumed.113.125831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair LI, Christmas DM, Hood SD, Potokar JP, Robertson A, Isaac A, et al. Davies SJ. Antidepressant-induced jitteriness/anxiety syndrome: systematic review. Br J Psychiatry. 2009;194(6):483–490. doi: 10.1192/bjp.bp.107.048371. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. Structured Clinical Interview for the DSM-IV (SCID-I/P) Washington: American Psychiatric Press; 2004. [Google Scholar]
- Sprouse JS, Aghajanian GK. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1(1):3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- Sramek JJ, Hong WW, Hamid S, Nape B, Cutler NR. Meta-analysis of the safety and tolerability of two dose regimens of buspirone in patients with persistent anxiety. Depress Anxiety. 1999;9(3):131–134. [PubMed] [Google Scholar]
- Weiskopf N, Hutton C, Josephs O, Deichmann R. Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: a whole-brain analysis at 3 T and 1.5 T. Neuroimage. 2006;33(2):493–504. doi: 10.1016/j.neuroimage.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Hutton C, Josephs O, Turner R, Deichmann R. Optimized EPI for fMRI studies of the orbitofrontal cortex: compensation of susceptibility-induced gradients in the readout direction. MAGMA. 2007;20(1):39–49. doi: 10.1007/s10334-006-0067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.