Abstract
High dietary sodium intake triggers increased blood pressure. Animal studies show that dietary salt loading results in dermal Na+ accumulation and lymphangiogenesis mediated by vascular endothelial growth factor-C, both attenuating the rise in blood pressure. Our objective was to determine if these mechanisms function in humans. We assessed skin electrolytes, blood pressure and plasma vascular endothelial growth factor-C in 48 healthy participants randomized to placebo (70 mmol sodium/day) and slow sodium (200 mmol/day) for 7 days. Skin Na+ and K+ concentrations were measured in mg/g of wet tissue and expressed as the ratio Na+:K+ to correct for variability in sample hydration. Skin Na+:K+ increased between placebo and slow sodium phases (2.91±0.08 vs. 3.12±0.09; p=0.01). In post hoc analysis there was a suggestion of a sex specific effect, with a significant increase in skin Na+:K+ in men (2.59±0.09 vs. 2.88±0.12, p=0.008), but not women (3.23±0.10 vs. 3.36±0.12, p=0.31). Women showed a significant increase in 24-hour mean blood pressure with salt loading (93±1 vs. 91±1 mmHg, p<0.001) while men did not (96±2 vs. 96±2 mmHg, p=0.91). Skin Na+:K+ correlated with blood pressure, stroke volume and peripheral vascular resistance in men, but not in women. No change was noted in plasma vascular endothelial growth factor-C. These findings suggest that the skin may buffer dietary Na+, reducing the hemodynamic consequences of increased salt and this may be influenced by sex.
Keywords: Blood pressure, salt, sex-specific, skin, sodium, VEGF-C
Introduction
Large population studies suggest that excessive dietary sodium, principally as the chloride salt, is an important trigger for hypertension.1,2 The mechanisms for this relationship are still debated.3,4 In the classical paradigm, increased salt intake leads to increased sodium accumulation in the extracellular space with a corresponding increase in extracellular volume – which can be partly counterbalanced by pressure natriuresis.5 However, more recent studies looking at sodium balance in humans have shown that large amounts of sodium can accumulate without commensurate water retention.6–8 These observations oppose the traditional view that sodium balance functions as a two-compartment model, supporting the existence of non-osmotic sodium retention in a third compartment. In support of this, studies in rat models have shown that the skin is capable of osmotically inactive Na+ storage, via glycosaminoglycans (glycocalyx), serving as an important mechanism for buffering volume and blood pressure changes with salt intake.9–11 High salt intake in rats also stimulates tonicity-responsive enhancer binding protein (TonEBP) secretion by mononuclear phagocyte system (MPS) cells, which mediates vascular endothelial growth factor C (VEGF-C) expression. This results in enhanced interstitial lymphatic drainage and increased expression of endothelial nitric oxide synthase (eNOS), which buffers the hemodynamic response to salt loading.10,11 However, the relevance of these mechanisms in humans is unclear. In humans, the skin is the largest organ in the body, constituting 6% of body weight and receiving 20-30% of the cardiac output (CO) under normal conditions and up to 60% in erythroderma and other pathological conditions, and its role in blood pressure (BP) control is of current interest.12,13 Recent 23Na magnetic resonance imaging (MRI) studies in humans show a direct relationship between skin Na+ and BP, as well as age and sex differences in the capacity to store skin Na+.14,15 Although MRI data were confirmed by direct ashing of human cadaveric samples, they have not yet been confirmed by direct chemical analysis of skin electrolytes in humans.16 Moreover, 23Na MRI has not been used to measure changes in skin Na+ with dietary salt modulation. We hypothesized that the degree of change in skin sodium would relate to the BP change seen with dietary salt loading. We tested this hypothesis in a group of young healthy adults in whom we studied change in skin Na+ with increased dietary salt intake, by direct chemical measurements. We also sought to study the correlation between skin sodium, blood pressure, other hemodynamic variables and plasma VEGF-C.
Methods
Subjects
Participants were healthy individuals aged between 18 - 50 years and recruited by advertisement. Exclusion criteria included hypertension (sustained blood pressure>140/90 mmHg), current use of antihypertensive drugs, diuretics, salt supplements, renal impairment, or pregnancy. All participants gave informed consent prior to study participation. Ethical approval for the study was obtained from a National Research Ethics Committee (REC Reference: 11/H0304/003) and was performed according to Good Clinical Practice and according to the principles of the Declaration of Helsinki.
Study protocol
We conducted a four-week single-center, double-blind, randomized, crossover study as depicted in Figure S1 (online supplement). Volunteers were screened and placed on a 4g low salt diet (equivalent to 70 mmol of sodium/day) at visit 1 to standardize the background sodium intake. Participants were outpatients and given a printed booklet provided by practicing dietitians on how to maintain a low salt diet, with email and telephone advice offered throughout the duration of the study. Baseline salt consumption was assessed with 24-hour urinary sodium excretion (UNaV) up to 3 days after visit 1. Participants with high baseline salt intakes were given further advice on dietary salt restriction. After a 1-week run-in period on a low-sodium diet, participants received slow sodium (200 mmol/day for 7 days) or placebo tablets during weeks 2 and 4, in random order. Study compliance was assessed by 24-hour UNaV within 48 hours of visits 2, 3, and 4, with participants and investigators blinded to the results. Medication compliance was assessed by participants returning used tablet bottles. Each participant was seen at approximately the same time at each study visit in a temperature-controlled room. Participants were required to refrain from caffeine, alcohol, strenuous exercise and the application of moisturizer or fake tan to their lower back for 6 hours prior to study visits. At each visit, weight was measured using the same calibrated scales, with one layer of clothing on no shoes, and seated brachial BP was recorded after a minimum of 5 minutes rest. After a further 10 minutes supine rest, brachial BP, CO, stroke volume (SV), pulse wave analysis and aortic pulse wave velocity were taken. Ambulatory blood pressure monitoring (ABPM) was recorded with 24-hour UNaV collections within 48 hours of visits 2, 3, and 4. At visits 2 and 4, after hemodynamic measurements, a skin biopsy was taken. The skin biopsies were taken from the lower back to minimize any impact of scarring. The first biopsy was done on the right and the second on the left, in a symmetrical position. Skin was analysed by inductively coupled plasma optical emission spectrometry (ICP-OES) to determine Na+ and K+ concentrations in milligrams per gram of wet skin. ICP-OES is a highly sensitive analytical tool capable of simultaneous multi-elemental determinations down to the sub-parts-per-billion level.17 We summarize the technique in the online supplement (Figures S2 and S3), together with the specific methods for haemodynamic and biochemical measurements, and skin biopsies.
Statistical Analysis
The primary outcome measure was change in skin sodium concentration between the placebo and slow sodium phases. Due to technical limitations of drying small tissue samples, encountered when validating the ICP-OES, we had to change our measure from absolute dry Na+ to Na+:K+ ratio. As published data available on changes in skin sodium concentration in response to salt loading in humans were lacking, the initial sample size of 48 was based on other salt loading studies of a similar design, with a planned interim analysis to assess effect size and power. Normally distributed data are presented as mean±SEM and non-normal data as median and interquartile range(IQR). Tests for normality were carried out using the Shapiro Wilk’s test. Student’s paired t-tests were applied to paired observations after placebo and slow sodium for normally distributed data and the Wilcoxon signed-rank test for non-normally distributed data. Independent samples t-tests were applied to unpaired observations (normally distributed data) and the Man Whitney test (non-normally distributed data). Correlation coefficients between skin Na+:K+ and putative parameters such as age, sex, body mass index (BMI), body surface area (BSA) and haemodynamic variables were calculated using Pearson’s method (normally distributed variables) and Spearman’s method (non-normally distributed variables). We then performed multiple regression analysis to examine the parameters that independently infuence skin Na+:K+. In analyzing the data it became apparent that that skin Na+:K+ differed by sex and a post-hoc sex specific analysis was therefore undertaken. The presence of carry-over effect was checked using univariate analysis of variance(ANOVA). A probability of <5% was used to reject the null hypothesis. Statistical analysis was performed with SPSS software (version 23.0).
Results
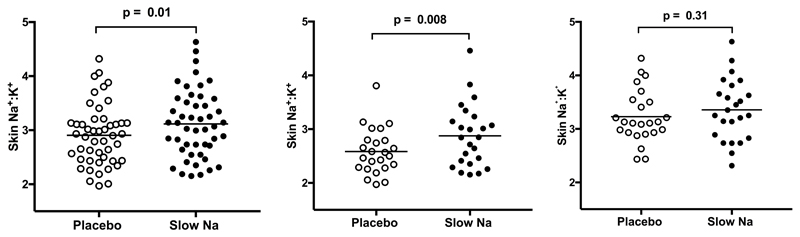
In all, 24 men and 24 women completed the study (mean age of 30±2 years, range 18–49 years). Baseline characteristics are shown in Supplementary Table S1. All women were pre-menopausal, and 10 were using an oral contraceptive pill or contraceptive implant. Our study population had a lower baseline sodium intake compared to the current average intake in England (approximately 130 mmol/day).18 The primary endpoint was the change in skin Na+:K+ between placebo and slow sodium phases. There was an increase in skin Na+:K+ between placebo and slow sodium phases (2.91±0.08 to 3.12±0.09; p=0.01; Figure. 1A). In multiple regression analysis including age, BMI, clinic mean arterial pressure (MAP) and sex, only sex remained independently associated with skin Na+:K+ values (R2 =0.316, P<0.001). This was primarily because in women, skin K+ was lower and the Na+:K+ ratio was consequently higher. Therefore subsequent analyses were sex-specific. Skin Na+, K+ and Na+:K+ values are presented in Table 1 and Figure 1. Men showed a significant increase in skin Na+:K+ of 11.2% (p=0.008) from placebo to slow sodium phases, whilst women showed non-significant increase of 4.0% (p=0.31). However, there was no significant difference between genders on formal testing (repeated measures ANOVA p=0.31). Neither sex showed a significant change in skin K+ with a change in salt intake, in keeping with previous animal studies.19,20 No evidence of carry-over effect was seen for skin Na+:K+ or skin Na+ for either sex using univariate analysis of variance with order of treatment (placebo or slow sodium) as a fixed factor(p=0.68).
Figure 1. Changes in skin Na+:K+ ratios between placebo and slow sodium phases.
A. All 48 participants. B. 24 males. C. 24 females.
The change in skin Na+ :K+ between placebo and slow sodium for males and females respectively was analysed using the student’s paired t test. P value < 0.05 taken to be significant.
Table 1. Differences in skin biochemical responses to placebo vs. slow sodium by sex.
Women have a lower skin K compared to men (p < 0.01 post placebo and slow sodium) and consequently have higher Na+:K+ ratio than men (p < 0.001 post placebo and salt).
| Variable | Men (n=24) | Women (n=24) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Placebo | Slow sodium | P-value | Placebo | Slow sodium | P-value | |
| Skin Na+, mg/g | 2.02±0.06 | 2.27±0.08 | 0.02 | 2.15±0.05 | 2.14±0.03 | 0.84 |
| Skin K+, mg/g | 0.80±0.03 | 0.82±0.04 | 0.59 | 0.68±0.03 | 0.65±0.02 | 0.28 |
| Skin Na+:K+ | 2.59±0.09 | 2.88±0.12 | 0.008 | 3.23±0.10 | 3.36±0.12 | 0.31 |
Hemodynamic responses to placebo vs. slow sodium are shown in Table 2. In men there were no differences in hemodynamics following salt loading, whilst women had higher 24-hour systolic BP (SBP), 24-hour MAP, daytime SBP, nighttime SBP, diastolic BP (DBP) and MAP. Only women had an increase in body weight post slow sodium (63.5±1.6 vs. 64.2±1.6kg; p=0.01). Baseline characteristics and haemodynamic responses to salt loading were similar in women with and without contraceptive use (Supplementary Table S3 and S4). Differences in biochemical responses to placebo vs. slow sodium are shown in Table 3. As expected, slow sodium increased 24-hour UNaV, serum Na+ and Cl-, and suppressed plasma renin and aldosterone. There were no significant changes in plasma VEGF-C levels or sFLT-4 (soluble receptor for VEGF-C) between placebo and slow sodium in either sex or in the whole study population.
Table 2. Differences in haemodynamic responses to placebo vs. slow sodium by sex.
| Variable | Males (n=24) | Females (n=24) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Placebo | Slow sodium | P-value | Placebo | Slow sodium | P-value | |
| Body weight, kg | 73.6±2.5 | 73.6±2.5 | 0.93 | 63.5±1.6 | 64.2±1.6 | 0.01 |
| Office measurements | ||||||
| Seated SBP, mmHg | 120±2 | 119±2 | 0.85 | 114±2 | 114±2 | 0.72 |
| Seated DBP, mmHg | 71±2 | 71±2 | 0.98 | 73±2 | 73±1 | 0.75 |
| Seated MAP, mmHg | 87±1 | 87±2 | 0.65 | 87±2 | 88±1 | 0.43 |
| Seated HR, bpm | 72±2 | 68±2 | 0.12 | 74±2 | 72±2 | 0.30 |
| Supine SBP, mmHg | 116±2 | 116±4 | 0.91 | 111±2 | 113±2 | 0.27 |
| Supine DBP, mmHg | 65±2 | 65±2 | 0.72 | 68±2 | 69±1 | 0.55 |
| Supine MAP, mmHg | 83±2 | 82±1 | 0.62 | 83±2 | 84±2 | 0.33 |
| Supine HR, bpm | 60±2 | 58±2 | 0.15 | 64±2 | 63±2 | 0.23 |
| Ambulatory BP, mmHg | ||||||
| 24-hr SBP | 121±1 | 122±2 | 0.57 | 114±1 | 118±1 | < 0.001 |
| 24-hr DBP | 73±2 | 73±2 | 0.46 | 71±1 | 73±1 | 0.05 |
| 24-hr MAP | 96±2 | 96±2 | 0.89 | 91±1 | 93 ± 1 | < 0.001 |
| Day SBP | 124±1 | 125±2 | 0.32 | 118±1 | 121 ± 2 | 0.02 |
| Day DBP | 76±2 | 76±2 | 0.34 | 75±2 | 76 ± 2 | 0.33 |
| Day MAP | 98±2 | 98±2 | 0.93 | 95±1 | 97 ± 2 | 0.05 |
| Night SBP | 114±2 | 115±2 | 0.52 | 105±1 | 110 ± 1 | < 0.001 |
| Night DBP | 65±2 | 66±2 | 0.65 | 63±1 | 65 ± 1 | 0.01 |
| Night MAP | 88±2 | 89±2 | 0.80 | 82±1 | 86 ± 1 | < 0.001 |
| Central haemodynamics | ||||||
| CSBP, mmHg | 99±1 | 99±2 | 0.99 | 95±2 | 98±2 | 0.11 |
| CDBP, mmHg | 67±2 | 66±2 | 0.21 | 69±2 | 70±1 | 0.22 |
| CMAP, mmHg | 82±2 | 80±2 | 0.12 | 83±2 | 85±2 | 0.22 |
| Augmentation index, % | 4.5±2 | 4.5±2 | 0.98 | 13.7±3 | 14.5±3 | 0.58 |
| PWV ms-1 | 5.2(4.7 – 5.6) | 5.3(4.9 – 6.0) | 0.10 | 5.0(4.7 – 5.5) | 5.2(4.8 – 5.4) | 0.93 |
| Cardiac output, litres/min | 6.6±0.3 | 7.0±0.4 | 0.08 | 5.6±0.3 | 5.8±0.3 | 0.10 |
| Stroke volume, ml | 107.9±5.2 | 116.8±6.5 | 0.09 | 86.0±4.0 | 88.9±3.6 | 0.29 |
| PVR, dynes s-1 cm-5 | 1048±51 | 1045±64 | 0.57 | 1251±72 | 1212±66 | 0.46 |
Normally distributed data presented as mean±SEM. Non-normally distributed data are presented as median and IQR.
Table 3. Differences in biochemical responses to placebo vs. slow sodium by sex.
| Variable | Males (n=24) | Females (n=24) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Placebo | Slow sodium | P-value | Placebo | Slow sodium | P-value | |
| Serum measurements | ||||||
| Na+, mmol/l | 140±0.4 | 141±0.3 | 0.001 | 139±0.3 | 140±0.4 | < 0.001 |
| Cl-, mmol/l | 103±0.4 | 106±0.5 | < 0.001 | 103±0.4 | 105±0.6 | < 0.001 |
| K+, mmol/l | 4.4±0.06 | 4.4±0.07 | 0.97 | 4.3±0.07 | 4.3±0.09 | 0.69 |
| eGFR, ml/min/1.72m2 | 114.7 (103.9 – 127.5) | 117.1(107.3 – 136.7) | 0.24 | 99.2 (84.1 – 119.2) | 105.9 (94.2-127.8) | < 0.001 |
| Plasma measurements | ||||||
| Renin, mU/l | 24(15–30) | 11(4–13) | < 0.001 | 17 (9 – 21) | 3 (2 – 7) | < 0.001 |
| Aldosterone, pmol/l | 221(143–318) | 80(69–111) | < 0.001 | 240 (137 – 435) | 69 (69 – 75) | < 0.001 |
| VEGF-C, pg/ml | 540 (352 – 881) | 625 (460 – 1066) | 0.20 | 830 (467 – 1404) | 755 (412 – 1130) | 0.39 |
| SFLT-4, pg/ml | 8.9±1.1 | 9.1±1.0 | 0.79 | 10.3±1.0 | 10.6±0.9 | 0.57 |
| Urinary measurements | ||||||
| Volume, ml/24hr | 1540±174 | 1678±203 | 0.25 | 1935±166 | 2299±186 | 0.004 |
| Na+, mmol/24hr | 86.3±10.3 | 221.9±16.5 | < 0.001 | 60.0±6.8 | 227.1±19.9 | < 0.001 |
| Cl-, mmol/24hr | 103.7±11.6 | 228.1±14.4 | < 0.001 | 83.8±8.2 | 241.3±21.5 | < 0.001 |
| K+, mmol/24hr | 63.3(51.2 – 93.5) | 55.1(39.9 – 73.8) | 0.15 | 56.9(40.5 – 76.7) | 58.7(41.6 – 77.9) | 0.49 |
| Creatinine clearance, ml/min | 111.1±9.1 | 125.3±9.6 | 0.14 | 108.1±8.0 | 122.4±7.6 | 0.06 |
| Fractional ExcretionNa, % | 0.44±0.06 | 0.93±0.08 | < 0.001 | 0.28±0.03 | 0.94±0.09 | < 0.001 |
Normally distributed data presented as mean±SEM. Non-normally distributed data are presented as median and IQR
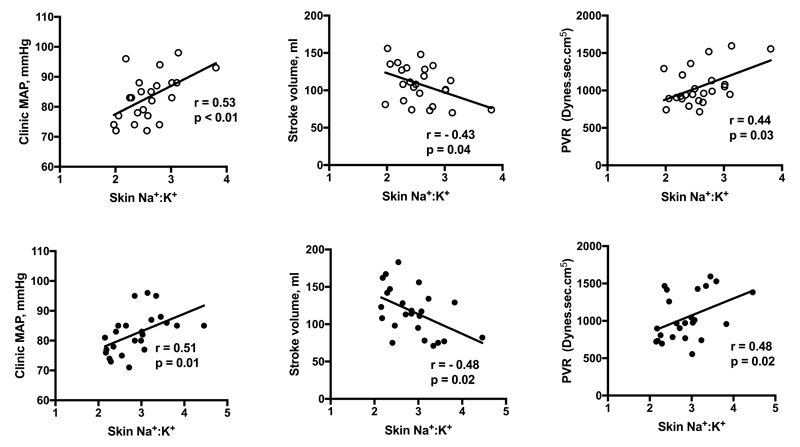
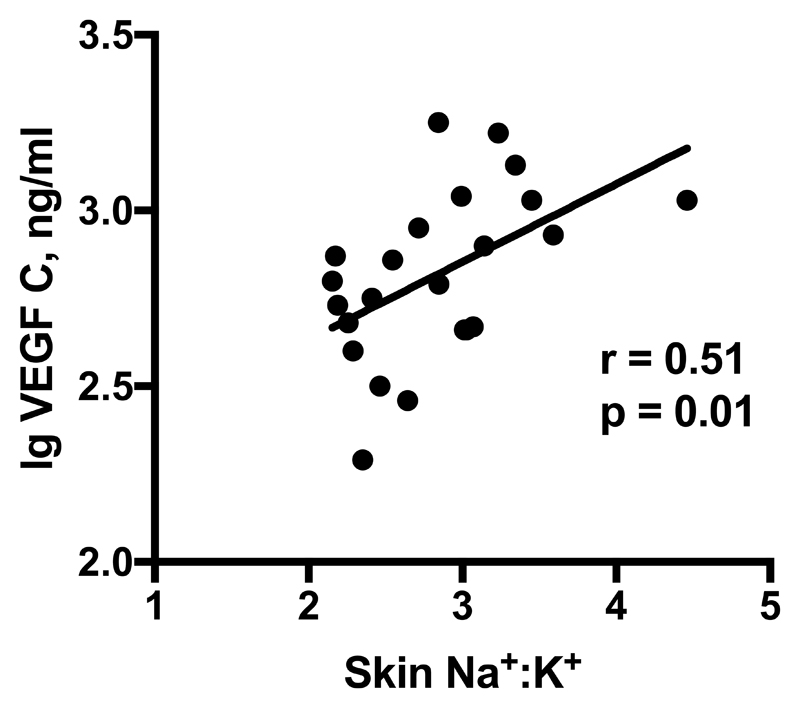
Univariate analyses revealed significant correlations between skin Na+:K+ and hemodynamic variables in men only, as shown in Figure 2. Skin Na+:K+ correlated positively with supine MAP post placebo (r=0.53, p<0.01) and post slow sodium (r=0.51, p=0.01). We observed that skin Na+:K+ was negatively correlated with SV and positively correlated with PVR post placebo and slow sodium. Plasma VEGF-C, after logarithmic transformation, showed a positive correlation with skin Na+:K+ post slow sodium (r=0.51, p=0.01) but not placebo (r=0.06, p=0.79) (fig. 3). All correlations were independent of age and BMI on multiple regression analysis. Correlations between haemodynamic parameters and skin Na+:K+ in women and urine Na+:K+ are shown in Supplementary Figure S6 and Supplementary Table S5 respectively.
Figure 2. Correlation between skin Na+:K+ and haemodynamic variables in 24 male participants.
A. Supine brachial MAP post placebo. B. Stroke volume post placebo. C. PVR post placebo. D. Supine brachial MAP post slow sodium. E. Stroke volume post slow sodium. F. PVR post slow sodium.
Fig 3. Correlation between skin Na+:K+ & VEGF-C post slow sodium in 24 male participants.
Discussion
The main findings of this study are that skin sodium increases with dietary salt loading and this may be influenced by sex. The dietary salt load we used (200 mmol Na+ for 7 days) is within the normal daily intakes of many individuals in urban societies, and thus would be clinically relevant.2 The primary endpoint was significant, with skin Na+:K+ increasing between the placebo and slow sodium phases for the whole study population, but in our study this appeared to be mainly driven by men.
Our measurements show that women have lower skin K+ and consequently a higher Na+:K+ ratio (Table 1). The epidermis has a significant K+ content and this difference could be explained by the epidermis being thinner in women.21,22 Therefore changes in Na+:K+ ratio with salt loading can be compared, but it is not possible to directly compare values for skin Na+:K+ between men and women. The use of contraceptives appeared to influence skin K+ values(supplementary table S2). The reasons for this are unclear, although estrogen administration increases epidermal thickness.23 Importantly, women have the same response in skin Na+:K+ with salt loading regardless of contraceptive use (supplementary Table S2 and Figure S5).
Previous human data on skin electrolytes are sparse. Oral salt loading experiments conducted over 80 years ago, in a small number of humans, showed that 10g NaCl administered daily over one week increased the Na+ content of human skin by 15–20%, suggesting the skin was a repository for NaCl.24 Direct chemical measurements in human skin, performed over 70 years ago, yielded values of between 1.00-2.14 mg/g for Na+ and 0.64-0.91 mg/g for K+ per wet weight of skin.25–27 Our values for Na+ and K+ fell within this range, but are difficult to compare with skin Na+ measured using MRI, which are expressed as mmol/l of water.14,16 Elemental measurements per wet weight of tissue may have potential inaccuracies introduced by changes in sample hydration during sample processing, which is why previous animal studies expressed skin Na+ and K+ concentrations per unit dry weight following dry ashing.11,19,20 Our samples had an average wet weight of ~15 mg compared with rat skin samples (~60g) and, unfortunately, were not suitable for dry ashing. Because our freeze-drying technique could not remove moisture uniformly in small samples, we corrected for sample hydration by expressing skin Na+ as a ratio Na+:K+ because of our ability to measure K+ reliably with ICP-OES and evidence from animal work that skin K+ remains stable with extremes of salt intake.11,19,20 Importantly, skin K+ did not change with salt loading. Furthermore, the Na+ content of injected local anesthetic did not appear to contaminate our skin samples. (Online supplementary methods and supplementary Figure S4.)
In our post hoc analyses skin Na+:K+ differed between men and women following placebo and slow sodium and it appeared that skin Na+:K+ rose with salt loading in men but not in women. Although this sex difference failed to achieve significance with formal ANOVA comparison, no doubt due to the small sample size, it is potentially interesting. Women had a significant increase in day and night ambulatory BP while men did not. This was associated with significant weight gain in women, in keeping with previous data demonstrating greater sensitivity to dietary sodium in women.28–30 Baseline sex differences in haemodynamic and biochemical variables were also in keeping with previous studies in healthy young adults.31–35 Augmentation index is higher in women and this is thought to be due to women having shorter stature and higher PVR, when corrected for body surface area. 33,36 Our study was not powered to show a sex difference in PVR or PWV. There could be several explanations for the sex differences observed with salt loading.
The reason for observed sex difference in salt sensitivity is unclear and the importance of addressing this issue has been highlighted recently.3,4 In our study there was a modest, but statistically significant difference in 24-hour UNaV, where women were lower than men post placebo, suggesting that women were either more adherent to a low salt diet or simply ate less. This difference was statistically significant but modest. There are known limitations of using 24-hour UNaV to estimate Na+ intake, with recent evidence showing ±25 mmol deviations in urinary Na+ from recorded Na+ intake.37 An alternative explanation is that BP in men did not change with a short-term changes in salt intake because they could buffer the additional dietary Na+ with their skin via mechanisms described in animal studies, while in women, this ability was attenuated.
In animals the skin buffers dietary salt, and a lower capacity to store Na+ in the skin is associated with a greater BP rise during acute salt loading.38 Recent evidence in humans suggests that the ability to store Na+ in the interstitium serves as a protective factor against the pressor effect of salt.30 Our results are consistent with these data, albeit with a sex difference. We propose 2 reasons why the sex differences may have been observed. Firstly, men have a thicker skin at all ages and may have higher levels of dermal glycosaminoglycans.39,40 This could imply that the skin is a more effective buffer for dietary Na+ in men. Indeed animal data demonstrate that with a high salt intake, male rats have a higher capacity for osmotically inactive skin Na+ storage compared to fertile female rats.38 Secondly, comparison of skin Na+ measurements not corrected for differences in sample hydration, reveals a trend for women to have a higher skin Na+ post-placebo than men. This suggests that men had greater passage of Na+ through the skin than women, rather than greater storage of skin Na+, and this passage of Na+ was protective in short-term salt loading. As shown in animal studies, the passage of skin Na+ into the skin would have resulted in efflux of Na+ via VEGF-C mediated lymphangiogenesis, which relates to NO production by VEGF-C.10,11. This is consistent with the known adaptation of salt-resistant subjects to a salt-load, which is vasodilation concomitant to the increase in cardiac output.30,41–43
We found significant correlations between skin Na+:K+ and various haemodynamic parameters in men, which were present regardless of salt intake. This suggests a physiological role for skin Na+ or Na+:K+ ratio in regulating normal haemodynamics. The negative correlation between skin Na+:K+ and stroke volume could reflect increased osmotically inactive Na+ binding to glycosaminoglycans, allowing Na+ without commensurate water accumulation, and therefore a less pronounced rise in circulatory volume.44 The positive correlation between BP and Na+:K+ support recent 23Na MRI data, which showed a positive correlation between BP and skin Na+. 14,15 The mechanisms for these observations remain to be explained, but could potentially involve interactions between hypoxia-inducible transcription factors (HIF), nitric oxide (NO) and skin Na+. The skin is a rich source of NO and the expression of NO is altered by HIF, which is in turn modulated by salt intake. 13,45,46 This interaction could potentially modulate PVR and the resulting BP. Alternatively, dermal capillary rarefaction, which refers to a reduction in capillary blood flow and increase in PVR, is associated with increased dietary salt and hypertension in humans.47 These mechanisms could potentially explain the positive correlation between skin Na+ and BP as well as PVR although, clearly, further studies are required. A distinction should be made between the role of skin Na+ in influencing BP in response to short and long term high-salt intake. In short-term salt loading, as in our study, dermal Na+ storage may buffer the BP response to salt. However, in the long-term (months), the ability to maintain normal BP in the face of high salt intake is dependent on the ability to decrease PVR.48 The positive correlation seen between skin Na+:K+ and PVR suggests that skin Na+ accumulation may, in the longer-term, lead to higher BP by increasing PVR. This is supported by cross-sectional 23Na MRI data showing that skin Na+ storage higher in hypertensives.14. In women skin Na+:K+ was positively correlated with PVR post salt, but this was not independent of age. There was a trend for skin Na+:K+ to be lower in women on contraceptives. Therefore, the Na+:K+ ratio may have been less reliable for assessing correlations in women in our study.
We did not observe significant changes in plasma VEGF-C or sFLT4 between placebo and slow sodium phases. A previous study looking at plasma VEGF-C in healthy adults noted no change on dietary salt loading. 49 As far as we are aware, this was the first study to examine plasma sFLT4. We did not measure skin VEGF-C levels and therefore cannot draw any conclusions on skin VEGF-C response. However, plasma VEGF-C in men correlated positively with skin Na+:K+ post sodium, suggesting that in the salt-loaded state, skin Na+ induced VEGF-C production, as seen previously in animal studies.10,11
This study has several limitations. The study size was small but we used a state-of-the art technique to measure skin Na+, ICP-OES, which is more sensitive than 23Na MRI. Our participants were non-resident and the control of Na+ intake was challenging, especially in men, most likely due to high salt levels in processed foods, which even highly motivated participants would have struggled to avoid over the 4-week study period. 50 We could not normalize dietary Na+ intakes for body weight. Participants also did not have their K+ and calorie intakes strictly controlled. Women were not salt loaded on the same day of the menstrual cycle. Our skin biopsies were small and we had no means of ascertaining if they were representative of the whole skin, or if skin Na+ varies with time. We did not quantify glycosaminoglycans in our skin samples or show how much of this Na+ was osmotically inactive. We also could not measure Cl- with ICP-OES. A further limitation was that we performed post–hoc analysis by sex and this was due to unexpected sex differences in skin K+ and the consequent limitations of using the Na+:K+. The sex differences in BP and skin Na+ are interesting but warrant further investigation and confirmation in larger studies. Our study was not primarily designed to determine salt sensitivity: it examined skin Na+ in response to changes in dietary Na+. Our target Na+ intake during the placebo phase was 70 mmol, which is high compared to other studies examining salt sensitivity. 28,29 The use of ambulatory BP monitoring made the detection of significant BP changes in women possible. Similar studies should also be conducted in older people, other ethnicities and hypertensives. We conducted a short-term study, as salt loading over a longer period may not have been ethical and chronic skin electrolyte changes in response to changes in salt intake may be different. Despite these limitations, we were able to provide a unique insight into the influence of interstitial Na+ on the haemodynamic response to dietary salt and will inform further studies on skin Na+.
Perspectives
Worldwide, hypertension has a significant prevalence and associated morbidity. An elevated BP is the single most important cause of cardiovascular disease, being responsible for 62% of strokes and 49% of coronary heart disease.51 Excessive salt intake is believed to play a crucial role in this epidemic.52 Our understanding of how salt affects BP and how we handle Na+ is lacking and the traditional “nephrocentric” view of sodium and fluid balance provides an incomplete explanation for this phenomenon. Our study provides novel data on how skin Na+ is altered by dietary salt, influences systemic haemodynamics and may influence the regulation of salt sensitivity in a sex-specific manner.
Supplementary Material
Novelty and significance.
What is new?
Skin Na+ increases with dietary salt loading in humans.
The skin may buffer dietary Na+, reducing the hemodynamic consequences of increased salt. This may be influenced by sex.
Skin Na+ may influence blood pressure, stroke volume and PVR.
What is relevant?
Excessive salt intake is believed to play a crucial role in the development of hypertension, but the exact mechanisms have not been fully elucidated.
Sex differences have been observed in the haemodynamic response to dietary salt, with some studies showing women have a greater BP response to dietary salt modulation. This has still not been explained.
Summary.
Skin Na+ is influenced by dietary salt and influences systemic hemodynamics. It may influence the regulation of salt sensitivity in a sex specific manner.
Acknowledgements
VEGF-C AND SFLT-4 assays were performed by the NIHR Cambridge Biomedical Research Centre, Core Biochemical Assay Laboratory.
Dr Paul Norris and Dr Maysoon Elkhawed contributed to the pilot study included in the online supplement.
Funding
VS was funded by the British Heart Foundation (grant number FS/12/33/29561) and NIHR.
IBW is funded by the British Heart Foundation.
IBW, CMM and KMP receive Cambridge NIHR BRC CCRN support.
The Human Research Tissue Bank is supported by the NIHR Cambridge Biomedical Research Centre.
We thank the Medical Research Council (grant number MC_US_A090_0008/Unit Programme number U1059) for their support.
Footnotes
Conflicts of Interest Disclosures
None
References
- 1.Intersalt Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24-hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. Br Med J. 1988;297:319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mente A, O'Donnell MJ, Rangarajan S, et al. Association of Urinary Sodium and Potassium Excretion with Blood Pressure. N Engl J Med. 2014;371:601–611. doi: 10.1056/NEJMoa1311989. [DOI] [PubMed] [Google Scholar]
- 3.Elijovich F, Weinberger MH, Anderson CAM, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL. Salt Sensitivity of Blood Pressure: A Scientific Statement From the American Heart Association. Hypertension. 2016;68:e7–e46. doi: 10.1161/HYP.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 4.Oh YS, Appel LJ, Galis ZS, et al. National Heart, Lung, and Blood Institute Working Group Report on Salt in Human Health and Sickness: Building on the Current Scientific Evidence. Hypertension. 2016;68:281–288. doi: 10.1161/HYPERTENSIONAHA.116.07415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall JE. Guyton and Hall Textbook of Medical Physiology. 12ed. Elsevier Health Sciences; 2010. [Google Scholar]
- 6.Heer M, Baisch F, Kropp J, Gerzer R, Drummer C. High dietary sodium chloride consumption may not induce body fluid retention in humans. Am J Physiol Renal Physiol. 2000;278:F585–595. doi: 10.1152/ajprenal.2000.278.4.F585. [DOI] [PubMed] [Google Scholar]
- 7.Titze J, Maillet A, Lang R, Gunga HC, Johannes B, Gauquelin-Koch G, Kihm E, Larina I, Gharib C, Kirsch KA. Long-term sodium balance in humans in a terrestrial space station simulation study. American Journal of Kidney Diseases. 2002;40:508–516. doi: 10.1053/ajkd.2002.34908. [DOI] [PubMed] [Google Scholar]
- 8.Rakova N, Jüttner K, Dahlmann A, et al. Long-Term Space Flight Simulation Reveals Infradian Rhythmicity in Human Na+ Balance. Cell Metabolism. 2013;17:125–131. doi: 10.1016/j.cmet.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Ivanova LN, Archibasova VK, Shterestal IS. Sodium-depositing function of the skin in white rats. Fiziol Zh SSSR Im I M Sechenova. 1978;64:358–363. [PubMed] [Google Scholar]
- 10.Machnik A, Neuhofer W, Jantsch J, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C–dependent buffering mechanism. Nature Medicine. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 11.Machnik A, Dahlmann A, Kopp C, et al. Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhancer binding protein/vascular endothelial growth factor C expression and induces salt-sensitive hypertension in rats. Hypertension. 2010;55:755–761. doi: 10.1161/HYPERTENSIONAHA.109.143339. [DOI] [PubMed] [Google Scholar]
- 12.Tobin DJ. Biochemistry of human skin—our brain on the outside. Chem Soc Rev. 2006;35:52–67. doi: 10.1039/b505793k. [DOI] [PubMed] [Google Scholar]
- 13.Johnson RS, Titze J, Weller R. Cutaneous control of blood pressure. Current Opinion in Nephrology and Hypertension. 2016;25:11–15. doi: 10.1097/MNH.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Muller DN, Schmieder RE, Cavallaro A, Eckardt KU, Uder M, Luft FC, et al. 23Na Magnetic Resonance Imaging-Determined Tissue Sodium in Healthy Subjects and Hypertensive Patients. Hypertension. 2013;61:635–640. doi: 10.1161/HYPERTENSIONAHA.111.00566. [DOI] [PubMed] [Google Scholar]
- 15.Schneider MP, Raff U, Kopp C, et al. Skin Sodium Concentration Correlates with Left Ventricular Hypertrophy in CKD. Journal of the American Society of Nephrology. 2017;28:1867–1876. doi: 10.1681/ASN.2016060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopp C, Linz P, Wachsmuth L, et al. 23Na Magnetic Resonance Imaging of Tissue Sodium. Hypertension. 2012;59:167–172. doi: 10.1161/HYPERTENSIONAHA.111.183517. [DOI] [PubMed] [Google Scholar]
- 17.Hou X, Jones BT. Inductively Coupled Plasma-Optical Emission Spectrometry. Encyclopedia of Analytical Chemistry. 2000:9468–9485. [Google Scholar]
- 18.Bates B, Cox L, Maplethorpe N, Mazumder A, Nicholson S, Page P, Prentice A, Rooney K, Ziaudden, Swan G. National diet and nutrition survey—assessment of dietary sodium in adults (aged 19–64 years)in England. 2014. https://www.gov.uk/government/statistics/national-diet-and-nutrition-survey-assessment-of-dietary-sodium-in-adults-in-england-2014. [Google Scholar]
- 19.Titze J, Baur K, Schafflhuber M, Dietsch P, Lang R, Schwind KH, Luft FC, Kai-Uwe E, Hilgers KF. Internal sodium balance in DOCA-salt rats: a body composition study. AJP: Renal Physiology. 2005;289:F793–F802. doi: 10.1152/ajprenal.00096.2005. [DOI] [PubMed] [Google Scholar]
- 20.Titze J, Luft FC, Bauer K, Dietsch P, Lang R, Veelken R, Wagner H, Kai-Uwe E, Hilgers KF. Extrarenal Na+ Balance, Volume, and Blood Pressure Homeostasis in Intact and Ovariectomized Deoxycorticosterone-Acetate Salt Rats. Hypertension. 2006;47:1101–1107. doi: 10.1161/01.HYP.0000221039.17735.1a. [DOI] [PubMed] [Google Scholar]
- 21.Hodgson C. The sodium and potassium content of the epidermis in eczema, psoriasis and lichen simplex. British Journal of Dermatology. 1960;72:409–415. doi: 10.1111/j.1365-2133.1960.tb13827.x. [DOI] [PubMed] [Google Scholar]
- 22.Sandby-Møller J, Poulsen T, Wulf HC. Epidermal thickness at different body sites: relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta dermato-venereologica. 2003;83:410–413. doi: 10.1080/00015550310015419. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson S, Thornton J. Effect of estrogens on skin aging and the potential role of SERMs. Clin Interv Aging. 2007;2:283–297. doi: 10.2147/cia.s798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urbach E, LeWinn EB. Skin Diseases, Nutrition and Metabolism. Vol. 1. Grune and Stratton Publishers; 1946. pp. 23–24. [Google Scholar]
- 25.Brown H. The mineral content of human skin. Journal of Biological Chemistry. 1927;75:789–794. [Google Scholar]
- 26.Urbach E. Contributions to a physiological and pathological chemical of the skin II Note Water, common salt, residual nitrogen and fat content of the skin normally and under pathological relations. Archiv Fur Dermatologie Und Syphilis. 1928;156:73–101. [Google Scholar]
- 27.Eisele CW, Eichelberger L. Water, Electrolyte and Nitrogen Content of Human Skin. Proceedings of the Society for Experimental Biology and Medicine. 1945;58:97–100. [Google Scholar]
- 28.Overlack A, Ruppert M, Kolloch R, Gobel B, Kraft K. Divergent hemodynamic and hormonal responses to varying salt intake in normotensive subjects. Journal of the American Heart Association. 1993;22:331–338. doi: 10.1161/01.hyp.22.3.331. [DOI] [PubMed] [Google Scholar]
- 29.He J, Gu D, Chen J, et al. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. Journal of Hypertension. 2009;27:48–54. doi: 10.1097/hjh.0b013e328316bb87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laffer CL, Scott RC, Titze JM, Luft FC, Elijovich F. Hemodynamics and Salt-and-Water Balance Link Sodium Storage and Vascular Dysfunction in Salt-Sensitive Subjects. Hypertension. 2016;68:195–203. doi: 10.1161/HYPERTENSIONAHA.116.07289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogawa T, Spina RJ, Martin WH, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- 32.Wiinberg N, Høegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielson PE, Svedsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. American Journal of Hypertension. 1995;8:978–986. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 33.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT) J Am Coll Cardiol. 2005;46:1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 34.Berg UB. Differences in decline in GFR with age between males and females. Reference data on clearances of inulin and PAH in potential kidney donors. Nephrol Dial Transplant. 2006;21:2577–2582. doi: 10.1093/ndt/gfl227. [DOI] [PubMed] [Google Scholar]
- 35.James GD, Sealey JE, Müller F, Alderman M, Madhavan S, Laragh JH. Renin relationship to sex, race and age in a normotensive population. J Hypertens Suppl. 1986;4:S387–S389. [PubMed] [Google Scholar]
- 36.Middlemiss JE, Miles KL, McDonnell BJ, Yasmin, Maki-Petaja KM, Cockcroft JR, Wilkinson IB, McEniery CM. Mechanisms underlying elevated SBP differ with adiposity in young adults. Journal of Hypertension. 2016;34:290–297. doi: 10.1097/HJH.0000000000000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lerchl K, Rakova N, Dahlmann A, et al. Agreement Between 24-Hour Salt Ingestion and Sodium Excretion in a Controlled Environment. Hypertension. 2015;66:850–857. doi: 10.1161/HYPERTENSIONAHA.115.05851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Titze J, Lang R, Ilies C, et al. Osmotically inactive skin Na+ storage in rats. Am J Physiol Renal Physiol. 2003;285:F1108–F1117. doi: 10.1152/ajprenal.00200.2003. [DOI] [PubMed] [Google Scholar]
- 39.Giacomoni PU, Mammone T, Teri M. Gender-linked differences in human skin. J Dermatol Sci. 2009;55:144–149. doi: 10.1016/j.jdermsci.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Oh J-H, Kim YK, Jung J-Y, Shin J-E, Chung JH. Changes in glycosaminoglycans and related proteoglycans in intrinsically aged human skin in vivo. Experimental Dermatology. 2011;20:454–456. doi: 10.1111/j.1600-0625.2011.01258.x. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan JM, Prewitt RL, Ratts TE, Josephs JA, Connor MJ. Hemodynamic characteristics of sodium-sensitive human subjects. Hypertension. 1987;9:398–406. doi: 10.1161/01.hyp.9.4.398. [DOI] [PubMed] [Google Scholar]
- 42.Schmidlin O, Forman A, Sebastian A, Morris R Curtis., J Sodium-Selective Salt Sensitivity: Its Occurrence in Blacks. Hypertension. 2007;50:1085–1092. doi: 10.1161/HYPERTENSIONAHA.107.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidlin O, Forman A, Leone A, Sebastian A, Morris RC. Salt sensitivity in blacks: evidence that the initial pressor effect of NaCl involves inhibition of vasodilatation by asymmetrical dimethylarginine. Hypertension. 2011;58:380–385. doi: 10.1161/HYPERTENSIONAHA.111.170175. [DOI] [PubMed] [Google Scholar]
- 44.Titze J. Water-Free Sodium Accumulation. Seminars in Dialysis. 2009;22:253–255. doi: 10.1111/j.1525-139X.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Zhu Q, Xia M, Li PL, Hinton SJ, Li N. Hypoxia-Inducible Factor Prolyl-Hydroxylase 2 Senses High-Salt intake to Increase Hypoxia Inducible Factor-1 alpha Levels in the Renal Medulla. Hypertension. 2010;55:1129–1136. doi: 10.1161/HYPERTENSIONAHA.109.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cowburn AS, Takeda N, Boutin AT, Kim JW, Sterling JC, Nakasaki M, Southwood M, Goldrath AW, Jamora C, Nizet V, Chilvers ER, et al. HIF isoforms in the skin differentially regulate systemic arterial pressure. Proc Natl Acad Sci USA. 2013;110:17570–17575. doi: 10.1073/pnas.1306942110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He FJ, Marciniak M, Markandu ND, Antonios TF, MacGregor GA. Effect of Modest Salt Reduction on Skin Capillary Rarefaction in White, Black, and Asian Individuals With Mild Hypertension. Hypertension. 2010;56:253–259. doi: 10.1161/HYPERTENSIONAHA.110.155747. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan JM, Ratts TE. Hemodynamic mechanisms of adaptation to chronic high sodium intake in normal humans. Hypertension. 1983;5:814–820. doi: 10.1161/01.hyp.5.6.814. [DOI] [PubMed] [Google Scholar]
- 49.Slagman MCJ, Kwakernaak AJ, Yazdani S, Laverman GD, van de Born J, Titze J, Navis G. Vascular endothelial growth factor C levels are modulated by dietary salt intake in proteinuric chronic kidney disease patients and in healthy subjects. Nephrology Dialysis Transplantation. 2012;27:978–982. doi: 10.1093/ndt/gfr402. [DOI] [PubMed] [Google Scholar]
- 50.He FJ, MacGregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. Journal of Human Hypertension. 2009;23:363–384. doi: 10.1038/jhh.2008.144. [DOI] [PubMed] [Google Scholar]
- 51.Mathers C, Stevens G, Mascarenhas M. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: World Health Organisation; 2009. http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf. [Google Scholar]
- 52.He FJ, MacGregor GA. Reducing Population Salt Intake Worldwide: From Evidence to Implementation. Progress in Cardiovascular Diseases. 2010;52:363–382. doi: 10.1016/j.pcad.2009.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.