Abstract
Background
Despite emerging evidence regarding the reversibility of stunting at older ages, most stunting research continues to focus on children below five years of age. We aimed to assess stunting prevalence and examine the sociodemographic distribution of stunting risk among older children and adolescents in a Malaysian population.
Methods
We used cross-sectional data on 6759 children and adolescents aged 6-19 years living in Segamat, Malaysia. We compared prevalence estimates for stunting defined using the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) references, using Cohen’s kappa coefficient. Associations between sociodemographic indices and stunting risk were examined using mixed effects Poisson regression with robust standard errors.
Results
The classification of children and adolescents as stunted or normal height differed considerably between the two references (CDC versus WHO; kappa for agreement: 0.73), but prevalence of stunting was high regardless of reference (crude prevalence: CDC 29.2%; WHO: 19.1%). Stunting risk was approximately 19% higher among underweight versus normal weight children and adolescents (P = 0.030) and 21% lower among overweight children and adolescents (P = 0.001), and decreased strongly with improved household drinking water sources (risk ratio [RR] for water piped into house: 0.35, 95% confidence interval [95% CI]: 0.30, 0.41, P < 0.001). Protective effects were also observed for improved sanitation facilities (RR for flush toilet: 0.41, 95% CI: 0.19, 0.88, P = 0.023). Associations were not materially affected in multiple sensitivity analyses.
Conclusions
Our findings justify a framework for strategies addressing stunting across childhood, and highlight the need for consensus on a single definition of stunting in older children and adolescents to streamline monitoring efforts.
Keywords: South East Asia, health and demographic surveillance, child stunting, child nutrition, water and sanitation
Introduction
Notable progress has been made in the reduction of child undernutrition in recent decades. However, child stunting remains an important global health issue (1). In 2014, 157 million out of 667 million children under the age of five years were estimated to be stunted, over half of whom were in Asia (1). Understood to be a direct result of poor nutrition and chronic infection, stunting is associated with a number of later life adverse outcomes, including cognitive impairment, lower educational achievement, adverse pregnancy outcomes among women, and increased cardiometabolic disease risk (2–6). Given its high burden and far-reaching consequences, comprehensive and well-informed strategies addressing stunting must remain a priority (6–8).
Until recently, stunting was generally understood to occur during the first 1000 days of life, with little potential for recovery in later years (4,6). Most research and programmes to date addressing stunting and associated risk factors have thus focused on children up to five years of age (9–20). However, a growing body of evidence from diverse populations indicates the potential to transition between stunted and non-stunted status up till at least 15 years of age (21–29). Recent research also suggests cognitive gains among children and adolescents who show catch-up growth to normal height at older ages, as well as deficits among older children and adolescents who transition to being stunted (24–28). More data are needed to better characterise linear growth trajectories and stunting throughout childhood, assess influences on the same, and understand subsequent later life outcomes. This includes a clearer assessment of potential differences in stunting classification by growth reference, given the implications this may have in terms of comparing estimates of stunting burden across populations and informing resource planning for strategies addressing stunting (30), and an exploration of potential determinants. Such evidence would facilitate a more comprehensive understanding of the risk of stunting and potential for recovery across childhood, and contribute to a clearer picture of implications for population health and productivity. We sought to address the current gap in understanding of stunting among older children and adolescents by assessing the prevalence and sociodemographic correlates of stunting in 6759 children and adolescents aged six to 19 years in Segamat, Malaysia.
Subjects and methods
Study population and measures of interest
We used data from two rounds of enumeration (2012-2013 and 2013-2014) and a cross-sectional health survey (2013-2014) conducted by the South East Asia Community Observatory health and demographic surveillance system (SEACO HDSS) operating in Segamat, Malaysia (31, 32). Enumerations included all consenting individuals living within the area covered by SEACO, whilst the health survey collected measures from individuals aged six years and above using standardized tools; information was linked across databases (33–37). Ethical approval for all data collections was obtained from the Monash University Human Research Ethics Committee, and informed consent was obtained from all participants prior to data collection.
Analyses included all children and adolescents with information on age, sex, ethnicity and height (N=6759). Children were defined as individuals aged less than 10 years, while adolescents were defined as individuals aged 10-19 years (38–41). Information from the 2012-2013 enumeration was used to match children and adolescents to their parents and determine birth order. Data from both enumerations were used to identify the child’s household indices including number of household members, bedrooms, bathrooms and living areas, type of toilet, whether the toilet was shared with other households, main source of drinking water, and main method of garbage disposal. All other information was obtained from the health survey. This included the child’s age, sex and ethnicity, the child’s and parents’ current height and weight (measured using a Transtek digital weighing scale with height gauge, model GBS-721), parents’ age, and parents’ and household head’s education.
Variable transformation
We used the World Health Organization (WHO) 2007 and Centers for Disease Control and Prevention (CDC) 2000 child growth references to express height-for-age z-score and classify stunting among children and adolescents (41, 42). These references are distinct in terms of the reference populations chosen and methods used to derive height-for-age reference curves across childhood and adolescence, and in terms of their recommended cut-offs to define stunting (41–43). Conversion of absolute height to reference-specific height was done using the Zanthro package in Stata (44). The WHO 2007 reference classifies stunting as height <-2 standard deviations (SD) from the median (41), whilst the CDC 2000 reference classifies stunting as height <5th percentile (42, 45). Body mass index (BMI) was calculated as weight (kg) divided by the square of the height (m), and BMI-for-age status was also expressed relative to the WHO and CDC references (41, 42, 45) using the Zanthro package (44). The WHO 2007 reference classifies underweight, normal BMI, overweight and obesity as BMI-for-age <-2 SD, -2 to 2 SD, >2 to 3 SD and >3 SD respectively (41). The CDC 2000 reference defines underweight, normal BMI, overweight and obesity as <5th percentile, 5th to 84th percentile, 85th to 94th percentile, and ≥ 95th percentile respectively (42, 45). Maternal BMI was calculated as described above, and maternal underweight was defined as BMI less than 18.5 kg/m2. Parental height was assessed as a continuous variable and also categorized into five groups, as has been done previously (160 cm and above, 155-159 cm, 150-154 cm, 145-149 cm and less than 145 cm) (12). The number of bedrooms, bathrooms and living areas in the household was divided by the number of members to obtain the ratio of rooms to people.
Statistical analysis
We first examined crude stunting prevalence obtained with stunting classified using the WHO versus the CDC reference in the full study population (N=6759). Prevalence was calculated overall and across demographic subgroups (sex, age and ethnicity). We used Cohen’s kappa statistic to assess the agreement in classification of stunting between the WHO and CDC references, overall and across demographic subgroups. Kappa <0.6 was defined as poor agreement, 0.6 – <0.8 as moderate agreement, 0.8 – <0.9 as good agreement and ≥0.9 as excellent agreement (46). We examined within-household and within-sub-district clustering of stunting in the full population using both references. We then compared adjusted estimates for stunting prevalence using the WHO and CDC references, overall and across demographic subgroups, generated using mixed effects Poisson regression adjusted for sex, age and ethnicity, and for clustering at the household level.
We then examined associations between stunting and a range of sociodemographic indices in a subset of the population with complete information on all measures of interest, including individual (age, sex, ethnicity, birth order and BMI-for-age status), maternal (height and current underweight), and household measures (bedrooms, bathrooms and living areas per household member, type of toilet, sharing of toilet with other households, main source of drinking water and main method of garbage disposal) (N=3791). Initially, we assessed differences in measures of interest between children and adolescents included in regressions versus those who were excluded. Following this, we examined sociodemographic measures of interest across categories of stunting among included children and adolescents. Differences in distributions of measures by inclusion status or by stunting status were assessed using Pearson’s chi squared tests for categorical variables (Fisher’s exact tests for cell counts less than five) and Student’s t-test for continuous variables. We then examined univariable associations between each measure and stunting (using Poisson regression). The linearity of continuous measures was assessed by comparing models with continuous versus categorised measures using likelihood ratio tests.
Following this, we used mixed effects Poisson regression models with robust standard errors to assess the relation between sociodemographic indices and stunting expressed using the CDC reference. Regression models were built with progressive adjustment for groups of variables, starting with individual measures, and then additionally including maternal and then household measures. Fully-adjusted models included all individual, maternal and household variables described above, and were additionally adjusted for clustering at the household level. To confirm trends and obtain more concise estimates of risk, we additionally examined models (i) including maternal height as a continuous variable, and (ii) including variables with collapsed categories for maternal height, type of toilet, and main source of drinking water.
Effect modification of stunting risk by sex was explored by comparing minimally-adjusted mixed effects Poisson regression models (age, ethnicity, sex, measure of interest) with those including an additional term for interaction (sex x measure of interest) using likelihood ratio tests. Furthermore, we performed a number of sensitivity analyses to assess the robustness of associations observed. Crude and fully adjusted associations between maternal age and stunting were assessed, and the effect of its inclusion on other associations was examined. We similarly examined education of the mother, father and head of household, and paternal height. We assessed the specificity of associations by checking the effect of inclusion of the following orthogonal variables in regression models: maternal heart rate, maternal diastolic blood pressure, availability of internet from the provider Streamyx in the household, and the ratio of motorcycles in the household to the number of household members. Analysis of complete records was undertaken for these sensitivity analyses.
Additionally, we examined the relation between stunting risk and the co-occurrence of associated sociodemographic indices identified from the preceding regression models. These indices were binarised to facilitate clearer interpretation, and included Malay ethnicity, child underweight, maternal height less than 145 cm, living in a household with an unprotected water source, living in a household with a bucket, hanging or no latrine, and living in a household with a shared toilet. Mixed effects Poisson regression with robust standard errors was undertaken with stunting as the outcome and the number of associated indices as the primary exposure. The final model was adjusted for all other sociodemographic indices of interest, and for clustering at the household level.
Finally, we checked associations observed using mixed effects logistic regression models with robust standard errors, and with mixed effects linear regression models; final models were built identically to Poisson regression models above. We also ran all analyses using the WHO reference to express height-for-age and classify stunting, to assess comparability in the direction and effect size of estimates of association.
All analyses were performed using Stata 14 (Statacorp, Texas).
Results
Analyses covered a total of 6759 children and adolescents (49.3% boys). Children aged 6-9 years comprised 26.2% of the population, and approximately 37% each were aged 10-14 and 15-19 years. The majority of children and adolescents were of Malay ethnicity, followed by Chinese and then Indian ethnicity (67.3%, 19.9% and 9.8% respectively). There was no difference in age or ethnicity distributions by sex (Table 1).
Table 1. Demographic characteristics of study population.
| Overall | Boys | Girls | P | ||||
|---|---|---|---|---|---|---|---|
| N (%) | 6759 | 3335 | (49.3) | 3424 | (50.7) | ||
| Age (years) | |||||||
| 6-9 | 1772 | (26.2) | 899 | (27.0) | 873 | (25.5) | |
| 10-14 | 2519 | (37.3) | 1257 | (37.7) | 1262 | (36.9) | |
| 15-19 | 2468 | (36.5) | 1179 | (35.4) | 1289 | (37.6) | 0.127 |
| Ethnicity, n (%) | |||||||
| Malay | 4548 | (67.3) | 2246 | (67.3) | 2302 | (67.2) | |
| Indian | 662 | (9.8) | 332 | (10.0) | 330 | (9.6) | |
| Chinese | 1344 | (19.9) | 658 | (19.7) | 686 | (20.0) | |
| Bumiputera/Orang | |||||||
| Asli | 122 | (1.8) | 61 | (1.8) | 61 | (1.8) | |
| Other | 83 | (1.2) | 38 | (1.1) | 45 | (1.3) | 0.952 |
Differences in distributions across categories between girls and boys were compared using Pearson's Chi squared test.
We first assessed the agreement between the CDC and WHO references in stunting classification and subsequent estimation of prevalence. Stunting prevalence was notably higher when using the CDC reference compared with the WHO reference, with only moderate agreement between the two (crude prevalence using WHO reference: 19.1%, using CDC reference: 29.2%, kappa for agreement: 0.73) (Table 2, Supplementary Table S1). Differences in prevalence estimates, and demographic patterns in prevalence, were generally consistent between references (Table 2, Supplementary Table S1). Stunting was strongly clustered within households, regardless of the reference used (Supplementary Table S2).
Table 2. Prevalence of stunting amongst children in Segamat, Malaysia according to the World Health Organization 2007 versus Centers for Disease Control 2000 reference.
| WHO | CDC | Agreement1 | |||
|---|---|---|---|---|---|
| Overall | 16.5 | (15.1, 17.9) | 29.1 | (27.8, 30.4) | 0.73 |
| Sex, n (%) | |||||
| Male | 19.9 | (17.7, 22.2) | 28.8 | (27.0, 30.6) | 0.77 |
| Female | 17.9 | (15.7, 20.0) | 29.4 | (27.5, 31.2) | 0.69 |
| Age, years, n (%) | |||||
| 6-9 | 12.9 | (11.3, 14.6) | 20.1 | (18.0, 22.2) | 0.75 |
| 10-14 | 18.4 | (15.8, 21.0) | 26.9 | (24.9, 28.9) | 0.77 |
| 15-19 | 23.7 | (21.8, 25.7) | 37.8 | (35.4, 40.3) | 0.68 |
| Ethnicity, n (%) | |||||
| Malay | 18.1 | (16.4, 19.7) | 31.8 | (30.2, 33.5) | 0.72 |
| Indian | 13.1 | (10.3, 15.9) | 24.1 | (20.3, 27.8) | 0.73 |
| Chinese | 12.8 | (10.7, 14.9) | 22.3 | (19.7, 24.8) | 0.75 |
| Bumiputera/Orang Asli | 19.2 | (11.4, 27.0) | 34.4 | (24.0, 44.8) | 0.70 |
| Other | 13.1 | (5.5, 20.7) | 18.7 | (9.3, 28.2) | 0.91 |
WHO, World Health Organization; CDC, Centers for Disease Control.
Agreement in classification of stunting between references was calculated using Cohen's kappa coefficient.
Estimates are for adjusted prevalence, based on mixed effects Poisson regression models including sex, age and ethnicity and adjusted for clustering at the household level.
We further explored the relation between stunting and a range of sociodemographic indices in a subset of the population with complete information on all measures (N=3791). Children and adolescents included in this subset were generally comparable to those who were excluded in terms of measures of interest (Supplementary Table S3). Similar to the full study population, 28.5% of children and adolescents were stunted (CDC reference) (Table 3). Compared with children and adolescents of normal height, stunted children and adolescents were marginally older, and were less commonly of Chinese or Indian ethnicity, overweight or obese, of birth order four or above, and with mothers of height 150 cm or above (P < 0.001 for all). Stunted children and adolescents were also less likely to be living in a household with a flush toilet or a toilet not shared with other households (P = 0.009 and P = 0.025 respectively), or a household where the main source of drinking water was piped into the yard or house (P < 0.001) (Table 3).
Table 3. Sociodemographic characteristics of study population across categories of stunting.
| Overall | Normal height | Stunted1 | P | ||||
|---|---|---|---|---|---|---|---|
| N (%) | 3791 | 2712 | (71.5) | 1079 | (28.5) | ||
| Sex, n (%) | |||||||
| Male | 1870 | (49.3) | 1372 | (50.6) | 498 | (46.2) | |
| Female | 1921 | (50.7) | 1340 | (49.4) | 581 | (53.9) | 0.014 |
| Age, years, mean (SD) | 12.6 | (3.9) | 12.2 | (3.8) | 13.6 | (3.7) | <0.001 |
| Ethnicity, n (%) | |||||||
| Malay | 2586 | (68.2) | 1792 | (66.1) | 794 | (73.6) | |
| Indian | 405 | (10.7) | 316 | (11.7) | 89 | (8.3) | |
| Chinese | 694 | (18.3) | 530 | (19.5) | 164 | (15.2) | |
| Bumiputera/Orang Asli | 65 | (1.7) | 43 | (1.6) | 22 | (2.0) | |
| Other | 41 | (1.1) | 31 | (1.1) | 10 | (0.9) | <0.001 |
| BMI-for-age status1, n (%) | |||||||
| Underweight | 232 | (6.1) | 255 | (9.4) | 134 | (12.4) | |
| Normal | 2460 | (64.9) | 1712 | (63.1) | 736 | (68.2) | |
| Overweight or obese | 1099 | (29.0) | 745 | (27.5) | 209 | (19.4) | <0.001 |
| Birth order, n (%) | |||||||
| 1 | 1604 | (42.3) | 1101 | (40.6) | 503 | (46.6) | |
| 2 | 1150 | (30.3) | 821 | (30.3) | 329 | (30.5) | |
| 3 | 635 | (16.8) | 481 | (17.7) | 154 | (14.3) | |
| 4 + | 402 | (10.6) | 309 | (11.4) | 93 | (8.6) | <0.001 |
| Maternal height (cm) category, n (%) | |||||||
| 160 + | 613 | (16.2) | 462 | (17.0) | 151 | (14.0) | |
| 155 - 159 | 886 | (23.4) | 663 | (24.5) | 223 | (20.7) | |
| 150 - 154 | 1204 | (31.8) | 893 | (32.9) | 311 | (28.8) | |
| 145-149 | 758 | (20.0) | 491 | (18.1) | 267 | (24.8) | |
| < 145 | 330 | (8.7) | 203 | (7.5) | 127 | (11.8) | <0.001 |
| Maternal current underweight, n (%) | 92 | (2.4) | 69 | (2.5) | 23 | (2.1) | 0.456 |
| Rooms per household member, mean (SD) | |||||||
| Bedrooms | 0.6 | (0.3) | 0.6 | (0.3) | 0.6 | (0.3) | 0.582 |
| Bathrooms | 0.4 | (0.2) | 0.4 | (0.2) | 0.4 | (0.2) | 0.785 |
| Living areas2 | 0.3 | (0.2) | 0.3 | (0.2) | 0.3 | (0.2) | 0.607 |
| Type of toilet, n (%) | |||||||
| None, bucket or hanging latrine | 10 | (0.3) | 4 | (0.2) | 6 | (0.6) | |
| Bore hole toilet3 | 110 | (2.9) | 67 | (2.5) | 43 | (4.0) | |
| Pour flush toilet | 1017 | (26.8) | 706 | (26.0) | 311 | (28.8) | |
| Flush toilet with septic tank | 1548 | (40.8) | 1140 | (42.0) | 408 | (37.8) | |
| Flush toilet connected with sewerage system | 1106 | (29.2) | 795 | (29.3) | 311 | (28.8) | 0.009 |
| Toilet shared with other household, n (%) | 17 | (0.5) | 8 | (0.3) | 9 | (0.8) | 0.025 |
| Main source of drinking water, n (%) | |||||||
| Unprotected source4 | 3 | (0.1) | 1 | (0.0) | 2 | (0.2) | |
| Public standpipe or other protected source5 | 96 | (2.5) | 57 | (2.1) | 39 | (3.6) | |
| Piped into yard | 133 | (3.5) | 106 | (3.9) | 27 | (2.5) | |
| Piped into house | 3559 | (93.9) | 2548 | (94.0) | 1011 | (93.7) | <0.001 |
| Main method of garbage disposal, n (%) | |||||||
| Buried, burned or thrown | 1042 | (27.5) | 746 | (27.5) | 296 | (27.4) | |
| Collected and thrown for recycling | 250 | (6.6) | 177 | (6.5) | 73 | (6.8) | |
| Collected irregularly by local authority | 133 | (3.5) | 89 | (3.3) | 44 | (4.1) | |
| Collected regularly by local authority | 2366 | (62.4) | 1700 | (62.7) | 666 | (61.7) | 0.665 |
Descriptive analyses presented for a subset of children with complete information on all sociodemographic variables of interest above (N=3791), whose data were used for regression analyses (Table 4, Figures 1–3).
BMI, body mass index.
Stunting and BMI-for-age were classified using the Centers for Disease Control 2000 reference.
Living areas include dining rooms but not kitchens
Bore hole toilet both with or without cover
Unprotected sources: unprotected dug well, or water taken directly from pond or stream
Protected sources: protected dug well or spring, or water from bottles or tanker truck.
Differences in distributions across categories between normal height and stunted were compared using Pearson’s Chi squared test, or Fisher’s exact test for variables with cell frequencies < 5.
We examined associations between sociodemographic indices and child stunting (CDC reference) using mixed effects Poisson regression. In fully adjusted analyses, stunting risk increased by 8% per year increase in age (fully adjusted risk ratio [RR]: 1.08, 95% confidence interval [95% CI]: 1.06, 1.09, P < 0.001). Children and adolescents of Indian or Chinese ethnicity had an approximately 30% decreased risk of stunting compared with those of Malay ethnicity (RR for Indian ethnicity: 0.66, 95% CI: 0.52, 0.83, P < 0.001; for Chinese ethnicity: 0.68, 95% CI: 0.55, 0.83, P < 0.001) (Table 4, Figure 1, Supplementary Table S4). Overweight children and adolescents also had a reduced risk of stunting compared with those of normal BMI, whilst underweight was positively associated with stunting risk (RR for overweight: 0.79, 95% CI: 0.69, 0.90, P = 0.001; for underweight: 1.19, 95% CI: 1.02, 1.39, P = 0.030). Children and adolescents with mothers of height less than 145 cm had a 53% increased risk of stunting compared with those whose mothers were 160 cm or taller (RR: 1.53, 95% CI: 1.21, 1.92, P < 0.001). We found no evidence of associations between sex, birth order or current maternal underweight and child stunting risk (Table 4).
Table 4. Relative risk of stunting1 associated with sociodemographic indices.
| Risk ratio (95% confidence interval) | P | ||
|---|---|---|---|
| Female sex (versus male) | 1.10 | (1.00, 1.22) | 0.057 |
| Age | 1.08 | (1.06, 1.09) | <0.001 |
| Ethnicity | |||
| Malay | 1.00 | ||
| Indian | 0.66 | (0.52, 0.83) | <0.001 |
| Chinese | 0.68 | (0.55, 0.83) | <0.001 |
| Bumiputera/Orang Asli | 0.96 | (0.65, 1.40) | 0.818 |
| Other | 0.91 | (0.42, 1.93) | 0.796 |
| BMI-for-age1 status | |||
| Underweight | 1.19 | (1.02, 1.39) | 0.030 |
| Normal | 1.00 | ||
| Overweight or obese | 0.79 | (0.69, 0.90) | 0.001 |
| Birth order | |||
| 1 | 1.00 | ||
| 2 | 1.03 | (0.92, 1.14) | 0.628 |
| 3 | 0.99 | (0.84, 1.17) | 0.936 |
| 4 + | 1.05 | (0.83, 1.31) | 0.704 |
| Maternal height (cm) category | |||
| 160 + | 1.00 | ||
| 155 - 159 | 1.03 | (0.83, 1.28) | 0.765 |
| 150 - 154 | 1.06 | (0.87, 1.29) | 0.586 |
| 145-149 | 1.44 | (1.18, 1.76) | <0.001 |
| < 145 | 1.53 | (1.21, 1.92) | <0.001 |
| Maternal current underweight (versus BMI ≥ 18.5 kg/m2) | 0.86 | (0.58, 1.27) | 0.440 |
| Rooms per household member | |||
| Bedrooms | 1.04 | (0.79, 1.36) | 0.787 |
| Bathrooms | 0.67 | (0.46, 0.98) | 0.036 |
| Living areas2 | 1.16 | (0.73, 1.85) | 0.519 |
| Type of toilet | |||
| None, bucket or hanging latrine | 1.00 | ||
| Bore hole toilet3 | 0.57 | (0.25, 1.30) | 0.182 |
| Pour flush toilet | 0.41 | (0.18, 0.89) | 0.025 |
| Flush toilet with septic tank | 0.38 | (0.18, 0.84) | 0.017 |
| Flush toilet connected with sewerage system | 0.46 | (0.21, 1.02) | 0.055 |
| Toilet shared with other household (versus not shared) | 1.72 | (1.07, 2.74) | 0.025 |
| Main source of drinking water | |||
| Unprotected source4 | 1.00 | ||
| Public standpipe or other protected source5 | 0.52 | (0.38, 0.73) | <0.001 |
| Piped into yard | 0.25 | (0.15, 0.39) | <0.001 |
| Piped into house | 0.35 | (0.30, 0.41) | <0.001 |
| Main method of garbage disposal | |||
| Buried, burned or thrown | 1.00 | ||
| Collected and thrown for recycling | 1.06 | (0.84, 1.34) | 0.603 |
| Collected irregularly by local authority | 1.39 | (1.02, 1.89) | 0.036 |
| Collected regularly by local authority | 1.22 | (1.05, 1.41) | 0.011 |
BMI: body mass index.
Stunting and BMI-for-age were classified using the Centers for Disease Control 2000 reference.
Living areas include dining rooms but not kitchens
Bore hole toilet both with or without cover
Unprotected sources: unprotected dug well, or water taken directly from pond or stream
Protected sources: protected dug well or spring, or water from bottles or tanker truck.
Estimates based on mixed-effects Poisson regression models adjusted for all other variables above, and for clustering at the household level.
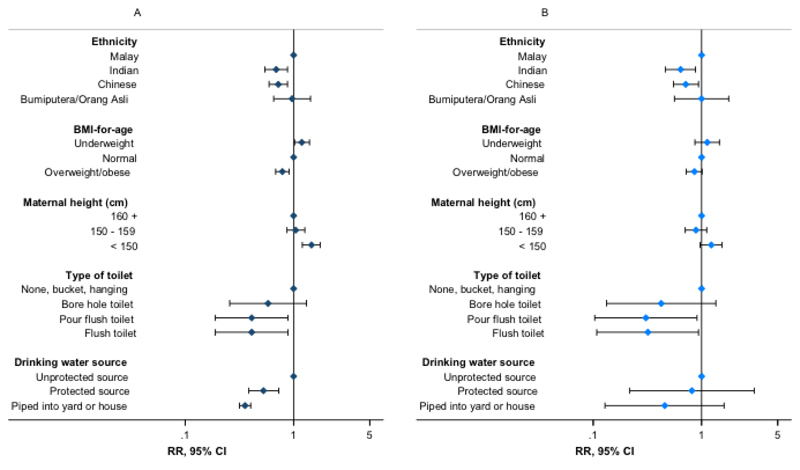
Figure 1.
Association of stunting with selected individual, parental and household water and sanitation indices – stunting expressed using the (A) Centers for Disease Control and Prevention 2000 reference and (B) World Health Organization 2007 reference. Estimates presented from Poisson regression models described in Supplementary Tables S5 and S10. RR: risk ratio; 95% CI: 95% confidence interval (error bars). Flush toilet includes that connected with septic tank or sewerage system; unprotected drinking water sources: unprotected dug well, or water taken directly from pond or stream; protected sources: public standpipe, protected dug well or spring, or water from bottles or tanker truck.
Additionally, children and adolescents whose main source of drinking water was pumped into the house or yard had an approximately 70% reduced risk of stunting versus those with access to unprotected sources, with smaller inverse associations for other protected water sources (RR for water piped into yard: 0.25, 95% CI: 0.15, 0.39, P < 0.001; for other protected sources: 0.52, 95% CI: 0.38, 0.73, P < 0.001). Furthermore, children and adolescents with a flush toilet in the household had an approximately 60% decreased risk of stunting versus those with a bucket, hanging or no latrine (RR for flush toilet with septic tank: 0.38, 95% CI: 0.18, 0.84, P = 0.017) (Table 4, Figure 1, Supplementary Table S4). Consistent trends were observed for related sanitation indices (RR for shared household toilets versus not shared: 1.72, 95% CI: 1.07, 2.74, P = 0.025; RR per unit increase in bathrooms per household member: 0.67, 95% CI: 0.46, 0.98, P = 0.036 respectively) (Table 4). We found no evidence of effect modification of associations between measures of interest and stunting risk by sex (Supplementary Table S6).
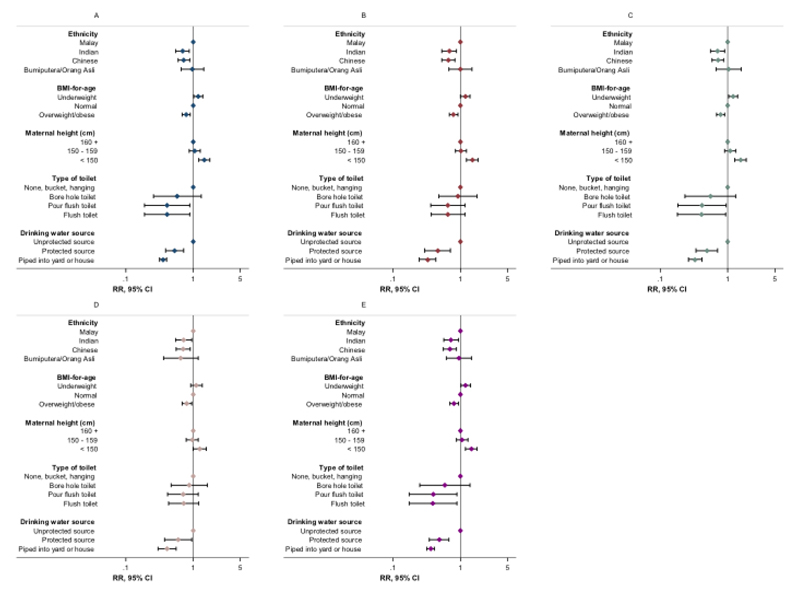
We confirmed the robustness and specificity of observed associations through multiple additional checks and sensitivity analyses. Similar magnitudes and directions of association were observed when analyses were repeated with height-for-age and stunting expressed according to the WHO reference (Figure 1, Supplementary Table S7). The additional inclusion of maternal age, paternal height, or parental or household head’s education to fully adjusted models did not materially change observed associations (Figure 2, Supplementary Tables S8-S11), with no evidence of association between parental or head of household’s education and stunting risk (Supplementary Table S9). Furthermore, we observed no association between stunting and theoretically uncorrelated maternal and household variables in fully adjusted regression models, with no effect of their inclusion on other associations (Figure 2, Supplementary Table S12). Trends in associations when using linear or logistic regression were consistent with those observed for Poisson regression models (Supplementary Table S5, data not shown for logistic regression).
Figure 2.
Summary of effect of sensitivity analyses on associations between key indices and stunting risk. (A) Original regression model (Table S5; N=3791); (B) model with maternal age included (N=3337); (C) model with maternal education included (N=3625); (D) model with paternal height included (N=2723); (E) model with orthogonal variables included (N=3434). RR: risk ratio; 95% CI: 95% confidence interval (error bars); BMI: body mass index. Stunting and BMI-for-age were defined using the Centers for Disease Control and Prevention 2000 reference. Flush toilet includes that connected with septic tank or sewerage system; unprotected drinking water sources: unprotected dug well, or water taken directly from pond or stream; protected sources: public standpipe, protected dug well or spring, or water from bottles or tanker truck. Orthogonal variables: maternal heart rate, maternal diastolic blood pressure, household Streamyx internet, and motorcycles per household member.
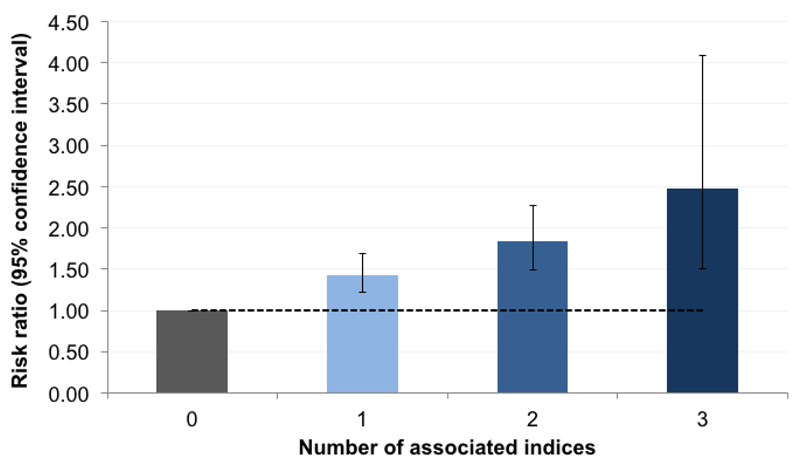
We further examined the relation between stunting and the co-occurrence of associated sociodemographic indices identified in the previous regressions. Indices included Malay ethnicity, child underweight, maternal height less than 145 cm, unprotected drinking water source, none or hanging or bucket latrine in the household, and shared household toilet. Thirty four (0.9%) children and adolescents had a maximum of three of any of these measures (Supplementary Table S13). The risk of stunting increased consistently with the number of co-occurring indices, with a greater than twofold increased risk among children and adolescents with three indices versus none (RR: 2.48, 95% CI: 1.51, 4.09, P < 0.001) (Figure 3, Supplementary Table S14).
Figure 3.
Relative risk of stunting associated with the number of co-occurring risk factors. Stunting was classified using the Centers for Disease Control and Prevention 2000 reference. Dashed line represents null association (relative risk of 1); error bars denote 95% confidence interval. Risk factors included: Malay ethnicity, child underweight, maternal height less than 145 cm, living in a household with unprotected drinking water source (unprotected dug well, or water taken directly from pond or stream), living in household with no bucket or hanging latrine, and living in a household with a shared toilet. Estimates are based on Poisson regression models adjusted for the child's age, sex and body mass index-for-age status, birth order, maternal current underweight, rooms per household member, household's main method of garbage disposal, and for clustering at the household level.
Discussion
In this large population of Malaysian children and adolescents, we observed a high burden of stunting, with notable discrepancies in prevalence estimated using two international height references. Stunting was independently associated with indices related to nutritional deficits and poor household environment; associations were robust to a series of sensitivity analyses and to the height reference used. The risk of stunting increased with the number of co-occurring indices. Our results provide strong support for a framework for strategies addressing stunting across childhood, and highlight the need for consensus on a single definition of stunting among older children and adolescents to streamline monitoring efforts.
Evidence on the burden and determinants of stunting among children and adolescents aged over five years is limited (22, 47–52). This includes research on the comparability of height references in the classification of stunting and subsequent estimation of prevalence. We identified only three other studies comparing the WHO and CDC references among older children and adolescents, based on smaller populations of narrower age ranges than in this study (47–49). All reported discrepancies in stunting classification by reference, although smaller than those observed here (47–49). The large differences in prevalence estimates by reference in our study population have important implications for decisions relating to targeting of interventions and health resource planning. Furthermore, our results indicate a fundamental gap in the current understanding of normal and compromised linear growth at older ages, including whether this is distinct to population. Further longitudinal research is required in this area, along with a more immediate consensus on the universal use of one reference to harmonise monitoring of stunting in this older age group across populations.
The associations between stunting and environmental and nutritional indices observed in our study are consistent with previous work, which has mainly been undertaken in younger children (9–20). Evidence from older children and adolescents is scarce, generally based on pre-adolescents and on smaller populations than examined here, with limited exploration of environmental or nutritional measures (22, 50–52). Among younger children, poor sanitation and drinking water facilities are understood to contribute to stunting through increased risk of acute and chronic enteric infections, leading to malabsorption of nutrients essential to growth (6, 53). Measures such as underweight or wasting are established indicators of undernutrition resulting from insufficient nutrient intake or absorption, and are thought to at least partly share biological pathways leading to stunting (6, 54). Our results suggest that poor environment and nutrition are also important determinants of linear growth and stunting in later childhood. Importantly, our study indicates that these indices contribute to stunting risk independently from the potential genetic, intrauterine and shared environmental influences of both parents’ height (55–62). Targeting these modifiable factors throughout childhood may thus be a useful approach to improving linear growth in this population, with potential for further subsequent intergenerational benefits. On the other hand, the implications of the increased risk of stunting observed among Malay children and adolescents are unclear. There has been debate regarding whether ethnicity-specific differences observed in child growth beyond five years of age may be a result of modifiable environmental exposures or genetic differences in growth potential (41). While we accounted for potential differences in household environment and BMI in this study, we were unable to account for other ethnicity-specific factors such as diet that may additionally explain such differences in risk. Our observations point to an important area of inquiry that may be best addressed through additional studies, including qualitative or genetic research.
Certain observations in our study stand somewhat in contrast to the totality of previous evidence on determinants of stunting risk. Measures of overcrowding and family size (17, 51, 52), maternal age and underweight (12, 15, 18, 56), and parental education (6, 10, 11, 14–16, 19) or related measures of socioeconomic status (63, 64), have previously been implicated in poor linear growth, but were not associated with stunting here. As has been observed with risk factors in other studies, it may be that these measures are not important determinants of stunting in our study population (9, 10, 15, 50). Importantly, we observed increased stunting risk with a low bathroom to household member ratio, and with sharing of toilets between households, suggesting that overcrowding may be relevant in more extreme contexts in this population. As such, these results reiterate the importance of population-specific research to inform suitable strategies addressing stunting.
Valuable insights for the design and targeting of interventions may be gained by examining the co-occurrence of risk factors and their collective effect on stunting risk. Yet, to our knowledge, this has not been explored in earlier studies. In our study population, out of six measures associated with stunting, a small group of children and adolescents had the maximum of three co-occurring indices, and stunting risk increased generally consistently with the number of co-occurring indices. Our population size limited our ability to assess in greater detail the risk of stunting associated with specific combinations of indices, which could be more informative for the purposes of intervention targeting. Nonetheless, we highlight here a potentially useful approach to identify sub-populations at greater risk of stunting through examining the extent and patterns of distribution of co-occurring risk factors.
Child undernutrition is being increasingly recognised as a public health issue of concern in Malaysia, with recent estimates suggesting a notable and rising burden of stunting among children under five years of age (65). Both governmental and non-governmental organisations have acknowledged the growing need to implement targeted strategies to address undernutrition among children in Malaysia (65–67). Importantly, these would have to be undertaken in coordination with interventions addressing the simultaneously increasing burden of overweight and obesity among children (66, 68–72). Given the high overweight and obesity prevalence observed among children and adolescents in our study population, our results provide further support for the need for a continued research and programmatic focus on the double burden of malnutrition throughout childhood in Malaysia.
To our knowledge, ours is among the largest of the few studies to date that have examined the prevalence and determinants of stunting in children and adolescents older than five years of age (22, 47–52). Our study provides strong evidence for associations between environmental and nutritional indices and stunting risk: observed associations were specific, robust to adjustment for multiple covariates, and independent of the height reference used to express stunting. For household sanitation, we assessed three measures capturing distinct aspects of the availability and quality of facilities, all of which were independently and consistently related to stunting risk. As this is a cross-sectional analysis, we are unable to make definitive statements regarding causality, and to more clearly distinguish between the relative contribution to stunting risk of current versus historical nutritional and environmental exposures, which may be correlated (50). Furthermore, we were unable to consider more detailed measures capturing dimensions relating to nutrition, such as diet or food security. However, in light of the previous research suggesting the potential for transition between stunted and non-stunted status at older ages (21–29), we believe our study provides key information regarding potential factors that may inform such transitions. Importantly, our study indicates that these are no different from factors influencing linear growth at younger ages, suggesting that broadening the current conceptual view of stunting to one that extends across childhood may ultimately allow for greater public health gains.
To conclude, we report independent associations between stunting and nutritional and household environmental indices in this population of older children and adolescents from Malaysia, with notable increases in risk associated with the co-occurrence of indices. Importantly, we observed a high burden of stunting in this population, with large differences in stunting classification by height reference. In the context of the increasing prevalence of overweight and obesity in this and similar populations (71, 73), our data on stunting point to the complex impact of malnutrition. Our study supports a framework for stunting across childhood, and highlights the need for consensus on a single height reference to monitor stunting at older ages. It provides a basis for further longitudinal research on the exact definition, determinants and consequences of linear growth and stunting throughout childhood in diverse populations. Such work will be fundamental to the design of strategies that may more comprehensively and effectively address childhood stunting worldwide.
Supplementary Material
Financial support
SEACO is funded by the office of the Vice Provost Research, Monash University Australia; the office of the Deputy Dean Research, Faculty of Medicine, Nursing and Health Sciences, Monash University Australia; the Monash University Malaysia Campus and the Jeffrey Cheah School of Medicine and Health Sciences. SEACO is an associate member of the INDEPTH Network.
This work was supported by the Wellcome Trust (grant number 098051). MS is supported by the National Institute for Health Research Cambridge Biomedical Research Centre (UK). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. UP is supported by the Dr Herchel Smith Fellowship.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- 1.IFPRI. Global Nutrition Report 2016: From Promise to Impact, Ending Malnutrition by 2030. Washington, DC: Institute of Food Policy Research; 2016. [Google Scholar]
- 2.Adair LS, Fall CH, Osmond C, Stein AD, Martorell R, Ramirez-Zea M, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382(9891):525–34. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller AC, Murray MB, Thomson DR, Arbour MC. How consistent are associations between stunting and child development? Evidence from a meta-analysis of associations between stunting and multidimensional child development in fifteen low- and middle-income countries. Public Health Nutr. 2016;19(8):1339–47. doi: 10.1017/S136898001500227X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onis M, Branca F. Childhood stunting: a global perspective. Matern Child Nutr. 2016;12(S1):12–26. doi: 10.1111/mcn.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewey KG, Begum K. Long-term consequences of stunting in early life. Matern Child Nutr. 2011;7(s3):5–18. doi: 10.1111/j.1740-8709.2011.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–51. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 7.Shekar M, Dayton Eberwein J, Kakietek J. The costs of stunting in South Asia and the benefits of public investments in nutrition. Matern Child Nutr. 2016;12(Suppl 1):186–95. doi: 10.1111/mcn.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoddinott J, Alderman H, Behrman JR, Haddad L, Horton S. The economic rationale for investing in stunting reduction. Matern Child Nutr. 2013;9(S2):69–82. doi: 10.1111/mcn.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguayo VM, Badgaiyan N, Paintal K. Determinants of child stunting in the Royal Kingdom of Bhutan: an in-depth analysis of nationally representative data. Matern Child Nutr. 2015;11(3):333–45. doi: 10.1111/mcn.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguayo VM, Nair R, Badgaiyan N, Krishna V. Determinants of stunting and poor linear growth in children under 2 years of age in India: an in-depth analysis of Maharashtra's comprehensive nutrition survey. Matern Child Nutr. 2016;12(Suppl 1):121–40. doi: 10.1111/mcn.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akombi BJ, Agho KE, Hall JJ, Merom D, Astell-Burt T, Renzaho AM. Stunting and severe stunting among children under-5 years in Nigeria: A multilevel analysis. BMC Pediatr. 2017;17(1):15. doi: 10.1186/s12887-016-0770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danaei G, Andrews KG, Sudfeld CR, Fink G, McCoy DC, Peet E, et al. Risk Factors for Childhood Stunting in 137 Developing Countries: A Comparative Risk Assessment Analysis at Global, Regional, and Country Levels. PLoS Med. 2016;13(11):e1002164. doi: 10.1371/journal.pmed.1002164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darteh EK, Acquah E, Kumi-Kyereme A. Correlates of stunting among children in Ghana. BMC Pub Health. 2014;14:504. doi: 10.1186/1471-2458-14-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fregonese F, Siekmans K, Kouanda S, Druetz T, Ly A, Diabate S, et al. Impact of contaminated household environment on stunting in children aged 12-59 months in Burkina Faso. J Epidemiol Community Health. 2017;71(4):356–63. doi: 10.1136/jech-2016-207423. [DOI] [PubMed] [Google Scholar]
- 15.Rachmi CN, Agho KE, Li M, Baur LA. Stunting, Underweight and Overweight in Children Aged 2.0–4.9 Years in Indonesia: Prevalence Trends and Associated Risk Factors. PloS one. 2016;11(5):e0154756. doi: 10.1371/journal.pone.0154756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rah JH, Cronin AA, Badgaiyan B, Aguayo VM, Coates S, Ahmed S. Household sanitation and personal hygiene practices are associated with child stunting in rural India: a cross-sectional analysis of surveys. BMJ open. 2015;5(2) doi: 10.1136/bmjopen-2014-005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rakotomanana H, Gates GE, Hildebrand D, Stoecker BJ. Determinants of stunting in children under 5 years in Madagascar. Matern Child Nutr. 2016 doi: 10.1111/mcn.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saxton J, Rath S, Nair N, Gope R, Mahapatra R, Tripathy P, et al. Handwashing, sanitation and family planning practices are the strongest underlying determinants of child stunting in rural indigenous communities of Jharkhand and Odisha, Eastern India: a cross-sectional study. Matern Child Nutr. 2016;12(4):869–84. doi: 10.1111/mcn.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torlesse H, Cronin AA, Sebayang SK, Nandy R. Determinants of stunting in Indonesian children: evidence from a cross-sectional survey indicate a prominent role for the water, sanitation and hygiene sector in stunting reduction. BMC Pub Health. 2016;16(1):669. doi: 10.1186/s12889-016-3339-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varela-Silva MI, Azcorra H, Dickinson F, Bogin B, Frisancho AR. Influence of maternal stature, pregnancy age, and infant birth weight on growth during childhood in Yucatan, Mexico: a test of the intergenerational effects hypothesis. Am J Hum Biol. 2009;21(5):657–63. doi: 10.1002/ajhb.20883. [DOI] [PubMed] [Google Scholar]
- 21.Adair LS. Filipino children exhibit catch-up growth from age 2 to 12 years. J Nutr. 1999;129(6):1140–8. doi: 10.1093/jn/129.6.1140. [DOI] [PubMed] [Google Scholar]
- 22.Svefors P, Rahman A, Ekstrom EC, Khan AI, Lindstrom E, Persson LA, et al. Stunted at 10 Years. Linear Growth Trajectories and Stunting from Birth to Pre-Adolescence in a Rural Bangladeshi Cohort. PLoS One. 2016;11(3):e0149700. doi: 10.1371/journal.pone.0149700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teivaanmaki T, Cheung YB, Kortekangas E, Maleta K, Ashorn P. Transition between stunted and nonstunted status: both occur from birth to 15 years of age in Malawi children. Acta Paediatr. 2015;104(12):1278–85. doi: 10.1111/apa.13060. [DOI] [PubMed] [Google Scholar]
- 24.Mendez MA, Adair LS. Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. J Nutr. 1999;129(8):1555–62. doi: 10.1093/jn/129.8.1555. [DOI] [PubMed] [Google Scholar]
- 25.Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359(9306):564–71. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- 26.Crookston BT, Penny ME, Alder SC, Dickerson TT, Merrill RM, Stanford JB, et al. Children who recover from early stunting and children who are not stunted demonstrate similar levels of cognition. J Nutr. 2010;140(11):1996–2001. doi: 10.3945/jn.109.118927. [DOI] [PubMed] [Google Scholar]
- 27.Fink G, Rockers PC. Childhood growth, schooling, and cognitive development: further evidence from the Young Lives study. Am J Clin Nutr. 2014;100(1):182–8. doi: 10.3945/ajcn.113.080960. [DOI] [PubMed] [Google Scholar]
- 28.Lundeen EA, Behrman JR, Crookston BT, Dearden KA, Engle P, Georgiadis A, et al. Growth faltering and recovery in children aged 1-8 years in four low- and middle-income countries: Young Lives. Public Health Nutr. 2014;17(9):2131–7. doi: 10.1017/S1368980013003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prentice AM, Ward KA, Goldberg GR, Jarjou LM, Moore SE, Fulford AJ, et al. Critical windows for nutritional interventions against stunting. Am J Clin Nutr. 2013;97(5):911–8. doi: 10.3945/ajcn.112.052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Partap U, Young EH, Allotey P, Sandhu MS, Reidpath DD. The Use of Different International References to Assess Child Anthropometric Status in a Malaysian Population. J Pediatr. 2017;190:63–8.e1. doi: 10.1016/j.jpeds.2017.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allotey P, Reidpath DD, Devarajan N, Rajagobal K, Yasin S, Arunachalam D, et al. Cohorts and community: a case study of community engagement in the establishment of a health and demographic surveillance site in Malaysia. Global Health Action. 2014;7 doi: 10.3402/gha.v7.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Partap U, Young E, Allotey P, Soyiri I, Jahan N, Komahan K, et al. HDSS Profile: The South East Asia Community Observatory Health and Demographic Surveillance System (SEACO HDSS) Int J Epidemiol. 2017 doi: 10.1093/ije/dyx113. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO. STEPwise approach to surveillance (STEPS) Geneva: World Health Organization; 2016. [Available from: http://www.who.int/chp/steps/en/ [Google Scholar]
- 34.WHO. Global Physical Activity Surveillance. Geneva: World Health Organization; 2016. [Available from: http://www.who.int/chp/steps/GPAQ/en/ [Google Scholar]
- 35.WHO. WHO Study on global AGEing and adult health (SAGE) Geneva: World Health Organization; 2016. [Available from: http://www.who.int/healthinfo/sage/en/ [Google Scholar]
- 36.WHO. The World Health Organization Quality of Life (WHOQOL) Geneva: World Health Organization; 2016. [Available from: http://www.who.int/mental_health/publications/whoqol/en/ [Google Scholar]
- 37.PFA. Depression Anxiety Stress Scales. Sydney: University of New South Wales and Psychology Foundation of Australia; 2014. [Available from: http://www2.psy.unsw.edu.au/dass/ [Google Scholar]
- 38.WHO. Adolescent Development. Geneva: World Health Organization; 2015. [Available from: http://www.who.int/maternal_child_adolescent/topics/adolescence/dev/en/ [Google Scholar]
- 39.WHO. Adolescents: health risks and solutions. World Health Organization; 2017. [Available from: http://www.who.int/mediacentre/factsheets/fs345/en/ [Google Scholar]
- 40.Patton GC, Sawyer SM, Santelli JS, Ross DA, Afifi R, Allen NB, et al. Our future: a Lancet commission on adolescent health and wellbeing. Lancet. 2016;387(10036):2423–78. doi: 10.1016/S0140-6736(16)00579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–7. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1–190. [PubMed] [Google Scholar]
- 43.Borghi E, De Onis M, Garza C, Van den Broeck J, Frongillo E, Grummer-Strawn L, et al. Construction of the World Health Organization child growth standards: selection of methods for attained growth curves. Stat Med. 2006;25(2):247–65. doi: 10.1002/sim.2227. [DOI] [PubMed] [Google Scholar]
- 44.Vidmar SI, Cole TJ, Pan H. Standardizing anthropometric measures in children and adolescents with functions for egen: Update. Stata Journal. 2013;13(2):366–78. [Google Scholar]
- 45.CDC. Overview of the CDC growth charts. Atlanta: Centers for Disease Control; undated. [Google Scholar]
- 46.McHugh ML. Interrater reliability: the kappa statistic. Biochemia Medica. 2012;22(3):276–82. [PMC free article] [PubMed] [Google Scholar]
- 47.Fetuga MB, Ogunlesi TA, Adekanmbi AF, Alabi AD. Growth pattern of schoolchildren in Sagamu, Nigeria using the CDC standards and 2007 WHO standards. Indian Pediatr. 2011;48(7):523–8. doi: 10.1007/s13312-011-0094-x. [DOI] [PubMed] [Google Scholar]
- 48.Khasnutdinova SL, Grjibovski AM. Prevalence of stunting, underweight, overweight and obesity in adolescents in Velsk district, north-west Russia: a cross-sectional study using both international and Russian growth references. Public health. 2010;124(7):392–7. doi: 10.1016/j.puhe.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 49.Mushtaq MU, Gull S, Mushtaq K, Abdullah HM, Khurshid U, Shahid U, et al. Height, weight and BMI percentiles and nutritional status relative to the international growth references among Pakistani school-aged children. BMC Pediatr. 2012;12:31. doi: 10.1186/1471-2431-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dearden KA, Schott W, Crookston BT, Humphries DL, Penny ME, Behrman JR. Children with access to improved sanitation but not improved water are at lower risk of stunting compared to children without access: a cohort study in Ethiopia, India, Peru, and Vietnam. BMC Pub Health. 2017;17(1):110. doi: 10.1186/s12889-017-4033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mushtaq MU, Gull S, Khurshid U, Shahid U, Shad MA, Siddiqui AM. Prevalence and socio-demographic correlates of stunting and thinness among Pakistani primary school children. BMC Pub Health. 2011;11:790. doi: 10.1186/1471-2458-11-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Senbanjo IO, Oshikoya KA, Odusanya OO, Njokanma OF. Prevalence of and risk factors for stunting among school children and adolescents in Abeokuta, southwest Nigeria. J Health Popul Nutr. 2011;29(4):364–70. doi: 10.3329/jhpn.v29i4.8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cumming O, Cairncross S. Can water, sanitation and hygiene help eliminate stunting? Current evidence and policy implications. Matern Child Nutr. 2016;12(Suppl 1):91–105. doi: 10.1111/mcn.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khara T, Dolan C. The relationship between wasting and stunting, policy, programming and research implications. Washington DC: USAID Technical Brief Paper; 2014. [Google Scholar]
- 55.Ozaltin E, Hill K, Subramanian SV. Association of maternal stature with offspring mortality, underweight, and stunting in low- to middle-income countries. JAMA. 2010;303(15):1507–16. doi: 10.1001/jama.2010.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–60. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 57.Stein AD, Barros FC, Bhargava SK, Hao W, Horta BL, Lee N, et al. Birth status, child growth, and adult outcomes in low- and middle-income countries. J Pediatr. 2013;163(6):1740–6.e4. doi: 10.1016/j.jpeds.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corvalan C, Gregory CO, Ramirez-Zea M, Martorell R, Stein AD. Size at birth, infant, early and later childhood growth and adult body composition: a prospective study in a stunted population. Int J Epidemiol. 2007;36(3):550–7. doi: 10.1093/ije/dym010. [DOI] [PubMed] [Google Scholar]
- 59.Christian P, Lee SE, Donahue Angel M, Adair LS, Arifeen SE, Ashorn P, et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int J Epidemiol. 2013;42(5):1340–55. doi: 10.1093/ije/dyt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Addo OY, Stein AD, Fall CH, Gigante DP, Guntupalli AM, Horta BL, et al. Maternal height and child growth patterns. J Pediatr. 2013;163(2):549–54. doi: 10.1016/j.jpeds.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh A, Upadhyay AK, Kumar K. Birth Size, Stunting and Recovery from Stunting in Andhra Pradesh, India: Evidence from the Young Lives Study. Maternal and child health journal. 2016 doi: 10.1007/s10995-016-2132-8. [DOI] [PubMed] [Google Scholar]
- 62.Garza C, Borghi E, Onyango AW, de Onis M. Parental height and child growth from birth to 2 years in the WHO Multicentre Growth Reference Study. Matern Child Nutr. 2013;9(Suppl 2):58–68. doi: 10.1111/mcn.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang N, Becares L, Chandola T. Patterns and Determinants of Double-Burden of Malnutrition among Rural Children: Evidence from China. PLoS One. 2016;11(7):e0158119. doi: 10.1371/journal.pone.0158119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kien VD, Lee HY, Nam YS, Oh J, Giang KB, Van Minh H. Trends in socioeconomic inequalities in child malnutrition in Vietnam: findings from the Multiple Indicator Cluster Surveys, 2000-2011. Glob Health Action. 2016;9 doi: 10.3402/gha.v9.29263. 29263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.UNICEF. Children Without: a study of urban poverty and deprivation in low-cost flats in Kuala Lumpur. Putrajaya: UNICEF; 2018. [Google Scholar]
- 66.IPH. National Health and Morbidity Survey 2015 (NHMS 2015). Vol II: Non-Communicable Diseases, Risk Factors & Other Health Problems. Putrajaya: Institute for Public Health, National Institutes of Health, Ministry of Health; 2015. [Google Scholar]
- 67.IPH. National Health and Morbidity Survey 2016 (NHMS 2016). Vol II: Maternal and Child Health Findings. Putrajaya: Institute for Public Health, National Institutes of Health, Ministry of Health; 2016. [Google Scholar]
- 68.Cheah WL, Wan Muda WA, Mohd Hussin ZA, Thon CC. Factors associated with undernutrition among children in a rural district of Kelantan, Malaysia. Asia Pac J Public Health. 2012;24(2):330–42. doi: 10.1177/1010539510380737. [DOI] [PubMed] [Google Scholar]
- 69.Sumarni Mohd G, Muhammad Amir K, Ibrahim Md S, Mohd Rodi I, Izzuna Mudla MG, Nurziyana I. Obesity among schoolchildren in Kuala Selangor: a cross-sectional study. Trop Biomed. 2006;23(2):148–54. [PubMed] [Google Scholar]
- 70.Naidu BM, Mahmud SZ, Ambak R, Sallehuddin SM, Mutalip HA, Saari R, et al. Overweight among primary school-age children in Malaysia. Asia Pac J Clin Nutr. 2013;22(3):408–15. doi: 10.6133/apjcn.2013.22.3.18. [DOI] [PubMed] [Google Scholar]
- 71.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MOH. Clinical Practice Guidelines on Management of Obesity. Putrajaya: Ministry of Health Malaysia; 2004. [Google Scholar]
- 73.Pell C, Allotey P, Evans N, Hardon A, Imelda JD, Soyiri I, et al. Coming of age, becoming obese: a cross-sectional analysis of obesity among adolescents and young adults in Malaysia. BMC Pub Health. 2016;16(1):1082. doi: 10.1186/s12889-016-3746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.