Summary
Background
Basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC), together known as keratinocyte cancers (KCs), are the commonest cancer in white ethnic populations. Recent improvements to registry data collection in England has allowed more accurate analysis of the epidemiology of BCC and cSCC and for the first time we are able to provide an accurate (representative) tumour burden for KC in the U.K.
Objectives
To estimate the incidence of BCC and cSCC in the U.K.
Methods
A cohort of patients with KCs between 2013 and 2015 were identified using linkage to diagnostic codes derived from pathology reports collected into the national cancer registry. Data from England’s cancer registry were combined with data from Scotland, Northern Ireland and Wales. European age-standardized incidence rates (EASRs) of the first BCC and cSCC per patient per annum (PPPA) were calculated.
Results
In the U.K, the EASR of the first BCC and cSCC PPPA in 2013–15 were 285 and 77 per 100 000 person years, respectively (211 120 KCs total in 2015). The mean annual percentage increase was 5% between 2013 and 2015 for both BCC and cSCC. By counting the first KC PPPA, we include an additional 51% KCs compared with the previous reporting technique which counts only the first BCC and cSCC in a patient’s lifetime, yet it represents a probable underestimation of 5–11% of the true tumour count.
Conclusions
Based on an improved methodology, a more representative incidence of KC is presented, which is essential to healthcare planning and will lead to improved understanding of the epidemiology of KC.
Keratinocyte cancers (KCs), the collective term for basal cell carcinomas (BCCs) and cutaneous squamous cell carcinomas (cSCCs), are the most common cancers in the U.K. and the most common cancers in white ethnic populations worldwide. However, epidemiological data in the U.K. have historically been of relatively poor quality.1–5 Due to high volume and multiplicity of KCs, they have frequently been excluded from national cancer registries and statistics. Previous studies have reported that incidence rates of KCs are increasing worldwide and evidence from local audits suggest that the rate of increase in the U.K. may be higher than in European counterparts.6–13 Increasing tumour incidence is presumed to be a result of an ageing population, increased ultraviolet radiation (UVR) exposure with easier access to travel abroad and a higher proportion of fairer skin types in the U.K. compared with other countries,6,14 but little is known about the epidemiology of KCs in the U.K.
The healthcare workload burden and cost of KCs is substantial within dermatology departments in the U.K., where these cancers are often seen urgently within 2 weeks of referral. In 2008, skin cancer management costs were estimated to be between £106 and £112 million and approximately £8891-226 per KC in England.15 A decade later, these estimates are likely to be substantially higher, with advances in treatment approaches and increase in patient volume. Furthermore, despite a relatively low mortality, morbidity through cosmetic disfigurement and functional morbidity is considerable given that KCs are frequently multiple and most commonly located on the face.
Despite the high volume and associated costs of KCs, limited research progress has been reported. This discrepancy was highlighted by a U.K. Translational Research Network in Dermatology (UKTREND) e-DELPHI exercise that assessed the research needs of clinicians in the U.K. and identified research into KCs as a priority area.16
In 1999, the United Kingdom and Ireland Association of Cancer Registries (UKIACR) ruled that due to complexities in registering multiple pathology reports accurately, only the first BCC or cSCC per patient is reported, despite all pathology reports now being routinely collected since 2013 in England, Scotland and Northern Ireland.17 This rule is also used in many cancer registries worldwide.6 The impact of this registration approach was that previous figures were clearly significant underestimations of national incidence in the U.K. Metachronous and synchronous BCCs and cSCCs are common.18 After a diagnosis of BCC, the 3-year risk of a subsequent BCC is estimated to be 44%, with 10% developing a further BCC within 6 months.10,19–21 Likewise, after an initial cSCC, the 5-year risk of a further cSCC is estimated to be 37%.21 Previous studies have shown that when counting all BCCs as opposed to the first registered BCC, an additional 30–50% of tumours are counted.10,22–24 Changes in cancer registration processes in England, including the introduction of national automated registration have enabled the development of a more comprehensive BCC and cSCC dataset for the first time.
The objective of this study was to validate improved data collection methods and report the incidence and survival for BCC and cSCC from the cancer registry data at a national level for the first time.
Materials and methods
Study design, setting and participants
Data were provided by the National Cancer Registration and Analysis Service (NCRAS), England. It is mandatory for all National Health Service pathology laboratories, and recommended to all private pathology laboratories in England, to provide all cancer pathology reports to NCRAS and NCRAS data quality teams to ensure that pathology laboratories are compliant. These pathology reports are combined with information from the Patient Administration System (PAS) and Cancer Outcomes and Services Dataset (COSD) to form a cancer registration record. In England, this process has been nationalized and mostly automated since 2013; a majority of tumours are registered based on the pathology report laboratory codes and text information provided. The minority that do not meet the automated processor’s selection criteria are manually registered.
BCCs and cSCCs were identified using the ICD-10 (International Classification of Diseases) site codes and ICD-02 morphology and behaviour codes (Table S1, see Supporting Information). The date of receipt of the pathology sample was used as the date of diagnosis, to identify the first BCC and cSCC per patient per annum (PPPA).
Data were also provided by Information Services Division Scotland, the Northern Ireland cancer registry and the Welsh Cancer Intelligence & Surveillance Unit (WCISU). Welsh data collection is acknowledged by the registry to be incomplete (personal communication with WCISU), awaiting changes to be enforced from 2016 data onwards. Welsh data were therefore excluded from the analyses, except for overall tumour count.
Validation
To confirm the accuracy of the data and the use of the first KC PPPA technique, a randomized selection of 500 patients with BCC and 500 patients with cSCC in 2013 in England were reviewed by a single dermatologist (Z.C.V.). Tumours diagnosed in the previous calendar year, recurrences of previous tumours and incorrectly coded tumours were excluded, e.g. Bowen disease. Additional tumours collected but not counted with the first KC PPPA technique (i.e. more than one tumour per annum) were analysed to provide an estimate of how the first KC PPPA compares with manually registering all tumours per patient and also to assess the validity of the registration process in England. Furthermore, in Scotland, because all cSCCs are registered except in specific cases such as patients with genetic predisposition to multiple cSCCs, Scottish data was also used to assess the first KC PPPA technique vs. registering all tumours manually.
Variables
Patient demographics such as age (taken as a continuous variable taken from day of diagnosis), sex and self-reported ethnicity were analysed from NCRAS, which derives information from PAS and COSD. Deprivation quintiles were calculated using the patient's Lower Super Output Area at diagnosis linked to the Index of Multiple Deprivation 2015.25
Statistical analysis
The NCRAS data were extracted using Oracle SQL developer© version 4·1·5·21 (Oracle; Redwood City, CA, U.S.A.). Microsoft© Excel version 2010 (Microsoft; Los Angeles, CA, U.S.A.) and Stata© version 14 (Stata Corporation; College Station, TX, U.S.A.) were used for statistical analyses. Randomization as listed above was performed using a random number generator from Excel© 2010.
European age-adjusted incidence rates (EASR) are weighted based on European standard populations in 2013 in 5-year age bands. The EASR of first cSCC and BCC PPPA were calculated across sex and nations (England, Scotland and Northern Ireland). Welsh data were excluded from EASR and age-specific rates due to lack of completeness. The estimated annual percentage change (EAPC) was calculated as the mean change in EASR per year.
Statistics for absolute number of deaths attributed to nonmelanoma skin cancer was as reported by the Office of National Statistics.26
The Pohar Perme estimator was used to calculate age-standardized net survival with the ‘stns' command in Stata 14. This analysis was age-standardized using weights from the International Cancer Survival Standard,27 i.e. survival was standardized based on expected survival of age- and sex-specific groups. Life tables were obtained from the Cancer Survival Group at the London School of Hygiene and Tropical Medicine. Vital status of patients was determined until 31 December 2016.28
Results
Validation
Overall, an additional 51% KC tumours were identified using first KC PPPA technique compared with identifying only the first incident tumour of all time. Analysing the first BCC PPPA in England 2013-15 (n = 410 716) compared with first registered (n = 268 565) resulted in 53% further BCC tumours recorded over 3 years. Likewise, when counting first cSCC PPPA (n = 104 529) compared with first registered (n = 76 977) an additional 36% cSCC tumours were recorded.
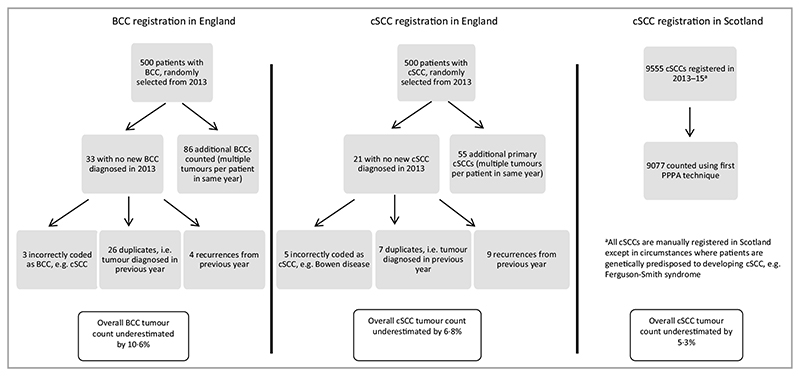
To assess the first KC PPPA technique and use of an automated processor in England, a randomly selected cohort of 500 patients with BCC and 500 with cSCC from the English cancer registry 2013 were selected and the number of tumours per patient was counted. Counting the first KC PPPA resulted in 10·6% fewer BCC tumours and 6·8% fewer cSCC tumours (Fig. 1).
Fig. 1.
Analysis of first KC per patient per annum (PPPA) technique for counting keratinocyte cancers from registry data. BCC, basal cell carcinoma; cSCC, cutaneous squamous cell carcinoma.
In Scotland, all cSCC tumours are registered manually except in cases of genetic predisposition; therefore, we compared the registered cSCC tumour count with the first KC PPPA technique in Scotland. This found an underestimation by 5·3% of all cSCCs by using the first KC PPPA (Fig. 1).
Analysing the automated processing technique in England, only 0·8% (8 of 1000) tumours were incorrectly coded, e.g. Bowen disease coded as an invasive cSCC.
U.K. incidence of keratinocyte cancers
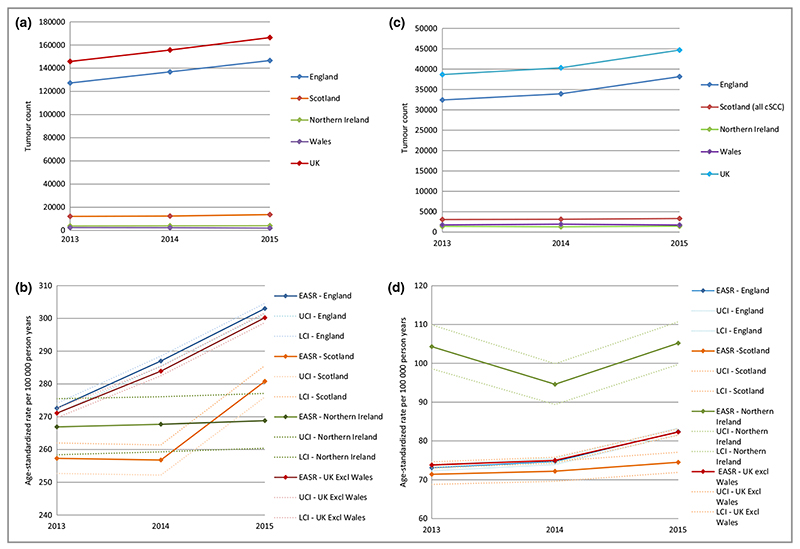
The absolute first BCC PPPA count increased in the 3-year period from 145 817 to 166 448 in 2015 (Fig. 2a). The EASR of first BCC PPPA in the U.K. from 2013 to 2015 was 352 per 100 000 person years (PY) in males and 219 per 100 000 PY in females. More than 85% of BCCs in the U.K. occurred in England and the EASR was highest in England compared with other U.K. nations (Fig. 2b). For the U.K., the EAPC was 5% for BCC, but much lower in Northern Ireland (0·3%).
Fig. 2.
(a-d) National tumour count and incidence rate of BCC and cSCC using the first tumour per patient per annum technique except for cSCC data for Scotland where all cSCCs are registered; Welsh data are excluded from incidence rates due to incomplete data collection. (a) National BCC tumour count 2013-15. (b) National EASR of BCCs 2013-15. (c) National cSCC tumour count 2013-15. (d) National EASR of cSCCs 2013-15. EASR, European age-standardized rate; BCC, basal cell carcinoma; cSCC, cutaneous squamous cell carcinoma; LCI, lower 95% confidence interval; UCI, upper 95% confidence interval.
The absolute count of first cSCC PPPA increased from 38 664 in 2013 to 44 672 in 2015 in the U.K. (Fig. 2c). The EASR of first cSCC PPPA from 2013 to 2015 was 111 in men and 42 per 100 000 PY in women with the highest rates seen in Northern Ireland (Fig. 2d). The EAPC of cSCC was around 6% in the 3-year period.
Geographic incidence differences in the U.K
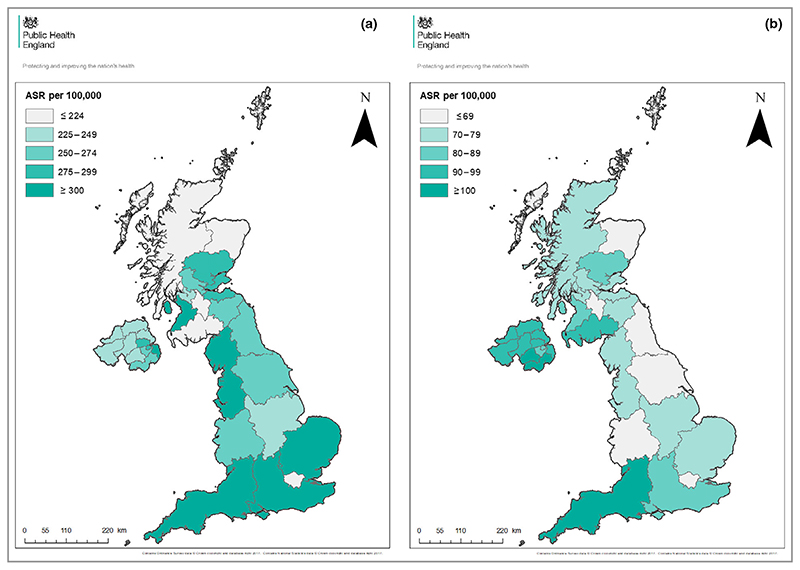
The incidence rates of first KC PPPA differ across the U.K. and are highest in southern and coastal regions (Fig. 3). The highest BCC rates were observed in Southwest England (EASR 362 per 100 000 PY) and the lowest EASR was in Dumfries and Galloway (39 per 100 000 PY). For cSCC, the highest rates were observed in Southwest England and the lowest in Shetland (EASR 107 and 345 per 100 000 PY, respectively). Several regions such as London and Northern Scotland have notably lower EASR as expected.
Fig. 3.
(a) Regional BCC EASRs in the U.K. 2013–15 using the first KC PPPA technique. Welsh data are excluded due to incomplete data collection. (b) Regional cSCC EASR in the U.K. 2013–15 using the first KC PPPA technique except in Scotland where all cSCC are registered. Welsh data are excluded due to incomplete data collection. EASR, European age-standardized incidence rates; BCC, basal cell carcinoma; cSCC, cutaneous squamous cell carcinoma; PPPA, per patient per annum.
Demographics of patients with keratinocyte cancers in England
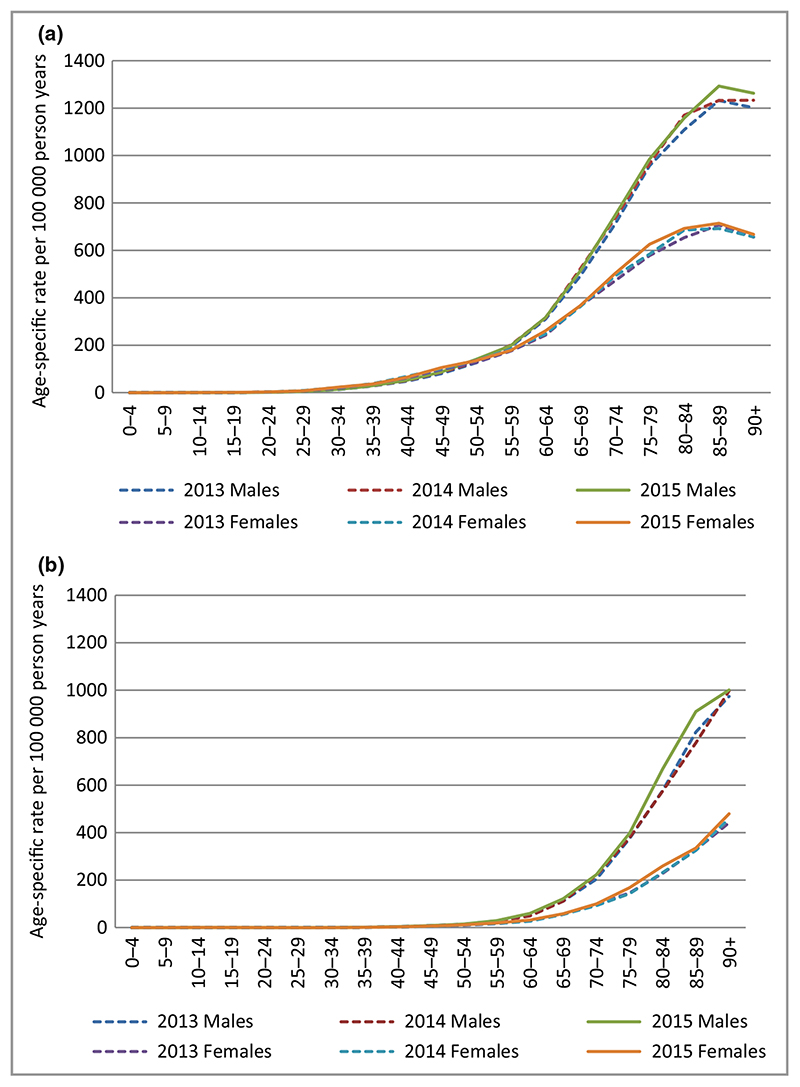
Patients with first all-time BCC and cSCC were more commonly males, with a male : female ratio 1·2 : 1 for BCC and 1·7 : 1 for cSCC (Table 1). The median age at time of incident tumour was approximately 71 years [interquartile range (IQR) = 62–80] for BCC and 79 years (IQR 71–85) for cSCC. The age-specific rates for both BCC and cSCC clearly increase with age and are much higher and steeper among men than women (Fig. 4). For both BCC and cSCC, fewer than 1% of individuals self-reported as nonwhite, with a substantial proportion of missing data (up to 34%). The distribution of the deprivation quintiles is comparable for both tumour types between men and women.
Table 1. Patient demographics of first all-time registered BCC and cSCC, England 2013–15.
| First BCC, n = 268 565 | First cSCC n = 76 977 | |||
|---|---|---|---|---|
| Patient demographics of first registered tumour | Male, n = 144 680 | Female, n = 123 885 | Male, n = 48 254 | Female, n = 28 723 |
| Age (years) | ||||
| Median (IQR) | 72 (63–79) | 71 (60–80) | 78 (71–84) | 80 (71–87) |
| 0—29 | 464 (0·3) | 641 (0·5) | 30 (0·1) | 26 (0·1) |
| 30–39 | 2229 (1·5) | 3066 (2·5) | 123 (0·3) | 98 (0·3) |
| 40–49 | 7685 (5·3) | 9680 (7·8) | 655 (1·4) | 489 (1·7) |
| 50–59 | 16 660 (1 1·5) | 16 148 (13·0) | 2117 (4·4) | 1491 (5·2) |
| 60—69 | 35 487 (24·5) | 27 893 (22·5) | 7352 (15·2) | 3930 (13·7) |
| 70—79 | 46 551 (32·2) | 33 990 (27·4) | 16 158 (33·5) | 7735 (26·9) |
| 80—89 | 30 631 (21·2) | 25 913 (20·9) | 17 822 (36·9) | 10 385 (36·2) |
| 90+ | 4973 (3·4) | 6554 (5·3) | 3997 (8·3) | 4569 (15·9) |
| Ethnicity | ||||
| White | 97 418 (67·3) | 80 690 (65·1) | 43 283 (89·7) | 25 226 (87·8) |
| Mixed | 63 (0·0) | 69 (0·1) | 29 (0·1) | 22 (0·1) |
| Indian/other Asian background | 108 (0·1) | 114 (0·1) | 73 (0·2) | 50 (0·2) |
| Afro-Caribbean/other black background | 57 (0·0) | 61 (0·0) | 42 (0·1) | 38 (0·1) |
| Chinese | 22 (0·0) | 23 (0·0) | 7 (0·0) | 8 (0·0) |
| Other ethnic group | 402 (0·3) | 371 (0·3) | 169 (0·4) | 96 (0·3) |
| Unknown | 46 610 (32·2) | 42 557 (34·4) | 4651 (9·6) | 3283 (11·4) |
| Deprivation quintiles | ||||
| 1 (least deprived) | 40 366 (27·9) | 32 572 (26·3) | 12 792 (26·5) | 7096 (24·7) |
| 2 | 37 363 (25·8) | 31 004 (25·0) | 12 451 (25·8) | 7064 (24·6) |
| 3 | 30 101 (20·8) | 26 108 (21·1) | 10 200 (21·1) | 6295 (21·9) |
| 4 | 21 915 (15·1) | 20 045 (16·2) | 7642 (15·8) | 4825 (16·8) |
| 5 (most deprived) | 14 935 (10·3) | 14 156 (11·4) | 5169 (10·7) | 3443 (12·0) |
| Site of first registered BCC or cSCC | ||||
| Lip (cutaneous) | 1491 (1·0) | 3219 (2·6) | 815 (1·7) | 679 (2·4) |
| Eyelid incl. canthus | 6353 (4·4) | 8019 (6·5) | 584 (1·2) | 483 (1·7) |
| Ear | 9439 (6·5) | 1568 (1·3) | 7601 (15·8) | 363 (1·3) |
| Face | 55 213 (38·2) | 50 691 (40·9) | 13 126 (27·2) | 9308 (32·4) |
| Scalp/neck | 11 315 (7·8) | 8265 (6·7) | 11 566 (24·0) | 1669 (5·8) |
| Trunk incl. perianal | 22 630 (15·6) | 14 484 (11·7) | 3080 (6·4) | 2201 (7·7) |
| Upper limb incl. shoulder | 9870 (6·8) | 7479 (6·0) | 6947 (14·4) | 5416 (18·9) |
| Lower limb incl. hip | 5637 (3·9) | 10 951 (8·8) | 2367 (4·9) | 7205 (25·1) |
| Skin NOS | 22 732 (15·7) | 19 209 (15·5) | 2168 (4·5) | 1399 (4·9) |
Data are n (%) unless otherwise specified. BCC, basal cell carcinoma; CI, confidence interval; cSCC, cutaneous squamous cell carcinoma; IQR, interquartile range; NOS, not otherwise specified
Fig. 4.
(a–b) Age-specific rates of basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC) in males and females 2013–15, England. Using data for first tumour all time. (a) Age-specific rates of BCC. (b) Age-specific rates of cSCC.
Body site of keratinocyte cancers in England
The majority of KCs are located in the head and neck region, especially on the face (between 27% and 40·9%; Table 1). Compared with cSCCs, BCCs tend to be more common on the trunk and are less frequent on the upper extremities. In men, 15·8% of cSCCs were located on the ear whereas the ear was affected in only 1·3% of women. A quarter of cSCCs in women were located on the lower extremities, which is fivefold more than in men. In approximately 15% of BCCs and 5% of cSCCs, body site was not reported (Table 1).
Keratinocyte cancer-specific mortality in England
The absolute number of deaths with an underlying cause of nonmelanoma skin cancers (which includes other skin cancers such as Merkel cell carcinomas) in England were 489 in 2013, 626 in 2014 and 624 in 2015.
Three-year net survival was 101·9% [95% confidence interval (CI) 101·8–102·0] for BCC and 96·2% (95% CI 95·9–96·6) for cSCC overall. For men, 3-year net survival was 101·9% (95% CI 101·8–102·1) for BCC and 95·7% (95% CI 95·3–96·2) for cSCC. For women, 3-year net survival was 101·9% (95% CI 101·7–102·0) for BCC and 97·1% (96% CI 96·6–97·6) for cSCC.
Discussion
We report the largest and most complete dataset for national incidence of KCs ever published, with over 210 000 tumours reported in 2015 in the U.K. With significant increases in tumour count between 2013 and 2015, KCs represent an overwhelming burden on the workload of healthcare resources. KCs occur mainly in older people, and because of steadily ageing populations, the pressure on the health service is likely to further increase. Of note, skin cancers were four times more common than any other cancer in the U.K. in 2015.26 This study provides much needed information, as highlighted in recent epidemiological reviews of KC.3,7–9,29
Due to the frequent multiplicity of KCs, the UKIACR method of counting only one tumour per patient has resulted in substantial underestimation of national tumour counts. We validate a technique to assess the first BCC and cSCC PPPA, which identified 51% additional tumours compared with the standard first registered BCC and cSCC UKIACR method. While less accurate than manually registering all KCs, this method allows improved incidence reports with minimal additional workload and is easily achievable with access to pathology or cancer registry data. Our validation study would imply that when counting all tumours, i.e. multiple tumours per patient per year, the true tumour count is likely to be 5–11% higher than those provided by the first KC PPPA technique.
There are strengths and limitations to our study. The combined U.K. registries form the largest population-based KC registry in the world, an essential tool for assessing the epidemiology of these tumours and the main strength of our study. However, inherent to large national pathology-based cancer registries there are several potential sources of under reporting such as use of topical and destructive treatments without histological confirmation, miscoding or the impact of long waiting lists. Also, there are regional differences in KC registration within the U.K. that may have affected comparisons between regions. For example, all cSCCs are manually registered in Scotland; therefore, rates will be comparatively higher than first KC PPPA counts; despite this, rates remain lower than in England and Northern Ireland. Due to the introduction of new automated report-processing technology in 2013, the data collection prior to 2013 may be less complete. Therefore, the increase in first registered tumours in subsequent years may be an overestimation, but this does not apply to first KC PPPA reporting. Because the KC registry is based on routinely collected data and linked to other national data sources, the granularity of the data is not always sufficient, e.g. missing data in self-reported ethnicity, tumour localization and lack of UVR exposure data.
Primary cSCCs affecting perianal sites have a different pathogenesis, with human papillomavirus infection thought to be an important cause rather than UVR.30 However, ICD-10 coding of perianal tumours classifies these to be coded as ‘truncal’ tumours and therefore this precludes accurate identification of these tumours.
Regarding interpretation, the distribution of recorded anatomical sites for primary KCs differed between men and women, which is in line with other studies.4,20 This may relate to varying UVR exposure as a result of male-pattern baldness and cultural preferences, i.e. shorter hair for men and women wearing dresses/skirts. This may explain why men are more likely to develop KCs on the ear and scalp, and for women the lower limb is preferentially affected. However, the commonest site in both sexes for KC was the face.
The regional variations identified in KC incidence may reflect reducing incidence with higher latitude/lower UVR exposure. In addition, in urban areas ethnicity may be more diverse, and behaviour may differ in terms of outdoor work and activities (Fig. 3). It is unclear why Northern Ireland has a higher cSCC incidence compared with other nations; this could relate to a higher number of outdoor occupations, recent public health campaigns, variation in clinical practice or data collection.31,32 Compared with elsewhere in the world, incidence rates of KC in the U.K. are lower than those published in Australia, but higher than elsewhere in Europe and the U.S.A., with varying rates presumably due to skin type/genetic predisposition and UVR exposure.6,10,18,24
Similar to previous studies, we show that BCC and cSCC are both significantly associated with lower deprivation quintiles.33 This is likely to be the result of the expense of foreign travel and increased leisure time being more affordable in these quintiles, equating to higher UVR exposure in the generations affected as well as increased awareness of skin cancer and access to health care.
According to national statistics, 86% of the population in England and Wales are of ‘white’ ethnicity; however, of those with known ethnicity, 99·3% of patients with primary cSCC are of white ethnicity, which may be mainly due to these skin types having reduced protection from UVR.34
Three year net survival of KC is over 95% for both types and the overall prognosis of KC is very good with only a minority of patients with cSCC developing advanced disease. Despite low mortality rates, KCs represent an overwhelming tumour burden in the population.35 We confirm an interesting observation that patients who develop their first BCC have a 3-year net survival of over 100%.36 This can be in part the result of the association of BCC with lower deprivation quintiles, fitness for biopsy excluding older more frail patients from our cohort and general adherence to healthier outdoor lifestyles. Figure 4 shows that at the age of 85 years a peak in incidence rate occurs, suggesting that patients are excluded where the risk outweighs the benefit for BCC treatment, i.e. frailer, older patients with more comorbidities.
In conclusion, a new technique taking advantage of the modernization of national cancer registration and data collection in the U.K. has resulted in significantly improved reporting of KC incidence. We have validated this registration and data collection approach here and now demonstrate more accurately the huge and steadily increasing trend in KC incidence in the U.K. The scale of the disease burden posed by KC inevitably has implications for healthcare resources. The more accurate incidence data reported here, and now available prospectively through improved KC registration, will undoubtedly facilitate improved service planning and provision in the future. In addition, it will lead to improved understanding of the natural history and prognosis of KC. Finally, it provides further evidence for the importance of future skin cancer prevention initiatives as part of strategies to reduce the morbidity and future healthcare resource implications of KC.
Supplementary Material
What’s already known about this topic?
Keratinocyte cancers (KCs) are the most common cancers affecting white ethnic populations.
The incidence of basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC) is increasing worldwide including the U.K., most commonly in elderly male Caucasian patients.
These cancers are traditionally substantially underreported and frequently excluded from national cancer statistics.
What does this study add?
Using improved data collection methods in England and validated tumour-reporting techniques, we report the most accurate BCC and cSCC incidence data for the U.K. ever published.
Identifying the first BCC and cSCC per patient per annum, the incidence of BCC and cSCC in the U.K. (excluding Wales) was 285 and 77 per 100 000 person years, respectively, between 2013 and 2015, with more than 210 000 KCs in the U.K. in 2015.
Acknowledgments
U.K. data were provided by the National Cancer Registration and Analysis Service England funded by Public Health England. Scottish data were obtained from Information Services Division Scotland funded by NHS Scotland, while the Northern Ireland Cancer Registry was funded by the Public Health Agency. This work uses data provided by patients and collected by health services as part of their care and support. S.M.L. is supported by a Wellcome Senior Clinical fellowship in Science (205039/Z/16/Z). Z.C.V. is supported through a British Association of Dermatologists and Genetic Medicine (BADGEM) fellowship. Thanks to Jennifer Lai at Public Health England for providing graphics.
Funding sources
No external funding.
Footnotes
Conflicts of interest
None decIared.
References
- 1.Trakatelli M, Ulrich C, del Marmol V, et al. Epidemiology of nonmelanoma skin cancer (NMSC) in Europe: accurate and comparable data are needed for effective public health monitoring and interventions. Br J Dermatol. 2007;156(3):1–7. doi: 10.1111/j.1365-2133.2007.07861.x. [DOI] [PubMed] [Google Scholar]
- 2.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(61):1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 3.Lansbury L, Leonardi-Bee J, Perkins W, et al. Interventions for nonmetastatic squamous cell carcinoma of the skin. Cochrane Database Syst Rev. 2010;4 doi: 10.1002/14651858.CD007869.pub2. CD007869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046–53. doi: 10.1016/j.ejca.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Holterhues C, Vries Ed, Louwman MW, et al. Incidence and trends of cutaneous malignancies in the Netherlands, 1989-2005. J Invest Dermatol. 2010;130:1807–12. doi: 10.1038/jid.2010.58. [DOI] [PubMed] [Google Scholar]
- 6.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166:1069–80. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 7.Nijsten T, Leigh IM. Keratinocyte skin cancers in the spotlight. Br J Dermatol. 2017;177:334–5. doi: 10.1111/bjd.15711. [DOI] [PubMed] [Google Scholar]
- 8.Verkouteren JAC, Ramdas KHR, Wakkee M, Nijsten T. Epidemiology of basal cell carcinoma: scholarly review. Br J Dermatol. 2017;177:359–72. doi: 10.1111/bjd.15321. [DOI] [PubMed] [Google Scholar]
- 9.Green AC, Olsen CM. Cutaneous squamous cell carcinoma: an epidemiological review. Br J Dermatol. 2017;177:373–81. doi: 10.1111/bjd.15324. [DOI] [PubMed] [Google Scholar]
- 10.de Vries E, Micallef R, Brewster DH, et al. EPIDERM Group Population-based estimates of the occurrence of multiple vs first primary basal cell carcinomas in 4 European regions. Arch Dermatol. 2012;148:347–54. doi: 10.1001/archdermatol.2011.2244. [DOI] [PubMed] [Google Scholar]
- 11.Reinau D, Surber C, Jick SS, Meier CR. Epidemiology of basal cell carcinoma in the United Kingdom: incidence, lifestyle factors, and comorbidities. Br J Cancer. 2014;111:203–6. doi: 10.1038/bjc.2014.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carsin AE, Sharp L, Comber H. Geographical, urban/rural and socioeconomic variations in nonmelanoma skin cancer incidence: a population-based study in Ireland. Br J Dermatol. 2011;164:822–9. doi: 10.1111/j.1365-2133.2011.10238.x. [DOI] [PubMed] [Google Scholar]
- 13.Goon PKC, Greenberg DC, Igali L, Levell NJ. Predicted cases of U.K. skin squamous cell carcinoma and basal cell carcinoma in 2020 and 2025: horizon planning for National Health Service dermatology and dermatopathology. Br J Dermatol. 2017;176:1351–3. doi: 10.1111/bjd.15110. [DOI] [PubMed] [Google Scholar]
- 14.Levell NJ, Igali L, Wright KA, Greenberg DC. Basal cell carcinoma epidemiology in the UK: the elephant in the room. Clin Exp Dermatol. 2013;38:367–9. doi: 10.1111/ced.12016. [DOI] [PubMed] [Google Scholar]
- 15.Vallejo-Torres L, Morris S, Kinge JM, et al. Measuring current and future cost of skin cancer in England. J Public Health (Oxf) 2014;36:140–8. doi: 10.1093/pubmed/fdt032. [DOI] [PubMed] [Google Scholar]
- 16.Healy E, Brown SJ, Langan SM, et al. Identification of translational dermatology research priorities in the U. K.: results of an electronic Delphi exercise. Br J Dermatol. 2015;173:1191–8. doi: 10.1111/bjd.14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.UKIACR, Coding and Classification Group (CCG) Training document: created 2 March 1999; updated June 2005. London: Public Health England; The practise on coding and classification policy and practice agreed by the CCG. 1999 and 2005. Last accessed 19 June 2018 Available at: https://www.mylearningspace.me.uk/moodle/pluginfile.php/8354/mod_resource/content/2/The-Practise.pdf (last accessed 19 June 2018) [Google Scholar]
- 18.Bielsa I, Soria X, Esteve M, Ferrándiz C, Skin Cancer Study Group of Barcelonès Nord Population-based incidence of basal cell carcinoma in a Spanish Mediterranean area. Br J Dermatol. 2009;161:1341–6. doi: 10.1111/j.1365-2133.2009.09468.x. [DOI] [PubMed] [Google Scholar]
- 19.Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136:1524–30. doi: 10.1001/archderm.136.12.1524. [DOI] [PubMed] [Google Scholar]
- 20.Flohil SC, de Vries E, Neumann HA, et al. Incidence, prevalence and future trends of primary basal cell carcinoma in the Netherlands. Acta Derm Venereol. 2011;91:24–30. doi: 10.2340/00015555-1009. [DOI] [PubMed] [Google Scholar]
- 21.Flohil SC, van der Leest RJ, Arends LR, et al. Risk of subsequent cutaneous malignancy in patients with prior keratinocyte carcinoma: a systematic review and meta-analysis. Eur J Cancer. 2013;49:2365–75. doi: 10.1016/j.ejca.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Lucke TW, Hole DJ, Mackie RM. An audit of the completeness of non-melanoma skin cancer registration in Greater Glasgow. Br J Dermatol. 1997;137:761–3. [PubMed] [Google Scholar]
- 23.Poirier V. The Role of the South West Public Health Observatory as the Lead Cancer Registry for Skin Cancer (poster presentation). Presented at the British Association Dermatologist’s Non-Melanoma Skin Cancer Conference; London: 2013. [Google Scholar]
- 24.Pandeya N, Olsen CM, Whiteman DC. The incidence and multiplicity rates of keratinocyte cancers in Australia. Med J Aust. 2017;207:339–43. doi: 10.5694/mja17.00284. [DOI] [PubMed] [Google Scholar]
- 25.Department for Communities and Local Government. Index of Multiple Deprivation 2015. London: Ministry of Housing, Communities & Local Government; 2015. Available at: https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015 (last accessed 27 April 2019) [Google Scholar]
- 26.Office of National Statistics. Cancer Registration Statistics, England: 2015. London: ONS; 2017. May 23, Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancerregistrationstatisticscancerregistrationstatisticsengland 2017; (last accessed 27 April 2019) [Google Scholar]
- 27.Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40:2307–16. doi: 10.1016/j.ejca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 28.London School of Hygiene and Tropical Medicine. Life tables. Available at: http://csg.lshtm.ac.uk/tools-analysis/uk-life-tables/ (last accessed 27 April 2019)
- 29.Leigh IM. Progress in skin cancer: the U.K. experience. Br J Dermatol. 2014;171:443–5. doi: 10.1111/bjd.13258. [DOI] [PubMed] [Google Scholar]
- 30.Dawson H, Serra S. Tumours and inflammatory lesions of the anal canal and perianal skin revisited: an update and practical approach. J Clin Pathol. 2015;68:971–81. doi: 10.1136/jclinpath-2015-203056. [DOI] [PubMed] [Google Scholar]
- 31.Public Health Agency. Skin Cancer Prevention. Belfast: PHA; n.d. Available at: http://www.publichealth.hscni.net/directorate-public-health/health-and-social-wellbeing-improvement/skin-cancer-prevention (last accessed 27 April 2019) [Google Scholar]
- 32.Public Health Agency. Care in the Sun: Outdoor Work, Sports and Leisure. Belfast: Cancer Focus Northern Ireland; n.d. Available at: https://careinthesun.org/sun-protection/outdoor-work-sports-and-leisure/ (last accessed 27 April 2019) [Google Scholar]
- 33.Doherty VR, Brewster DH, Jensen S, Gorman D. Trends in skin cancer incidence by socioeconomic position in Scotland, 1978-2004. Br J Cancer. 2010;102:1661–4. doi: 10.1038/sj.bjc.6605678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Office of National Statistics. Ethnicity and National Identity in England and Wales: 2011. London: ONS; 2012. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/articles/ethnicityandnationalidentityinenglandandwales/2012-12-11 (last accessed 27 April 2019) [Google Scholar]
- 35.Venables ZC, Autier P, Nijsten T, et al. Nationwide incidence of metastatic cutaneous squamous cell carcinoma in England. JAMA Dermatol. 2019;155:298–306. doi: 10.1001/jamadermatol.2018.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisemann N, Jansen L, Castro FA, et al. GEKID Cancer Survival Working Group Survival with nonmelanoma skin cancer in Germany. Br J Dermatol. 2016;174:778–85. doi: 10.1111/bjd.14352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.