Abstract
Cellular cryopreservation is a platform technology which underpins cell biology, biochemistry, biomaterials, diagnostics, and the cold chain for emerging cell-based therapies. This technique relies on effective methods for banking and shipping to avoid the need for continuous cell culture. The most common method to achieve cryopreservation is to use large volumes of organic solvent cryoprotective agents which can promote either a vitreous (ice free) phase or dehydrate and protect the cells. These methods are very successful but are not perfect: not all cell types can be cryopreserved and recovered, and the cells do not always retain their phenotype and function post-thaw. This Perspective will introduce polyampholytes as emerging macromolecular cryoprotective agents and demonstrate they have the potential to impact a range of fields from cell-based therapies to basic cell biology and may be able to improve, or replace, current solvent-based cryoprotective agents. Polyampholytes have been shown to be remarkable (mammalian cell) cryopreservation enhancers, but their mechanism of action is unclear, which may include membrane protection, solvent replacement, or a yet unknown protective mechanism, but it seems the modulation of ice growth (recrystallization) may only play a minor role in their function, unlike other macromolecular cryoprotectants. This Perspective will discuss their synthesis and summarize the state-of-the-art, including hypotheses of how they function, to introduce this exciting area of biomacromolecular science.
Introduction
For the cryopreservation of mammalian cells, the standard protocol is freezing in a solution containing 5–10 wt % of the cryoprotective agent (CPA) dimethyl sulfoxide (DMSO), which was introduced in 19591 and remains the gold standard. DMSO is able to enter cells and, at least partly, reduce injury by moderating the increase in solute concentration during freezing.2–4 While freezing in DMSO works for most cell lines (and indeed is a key underpinning technology), there remains many where it is not satisfactory. For example, DMSO causes differentiation of human leukemic cell lines,5 as well as certain cells such as leukocytes and RAW 264.7 (murine macrophage) cells, which are highly sensitive to DMSO, with concentrations even below 1% significantly affecting ROS production.6 In addition to these specific instances, there is a concern with using DMSO for all cell lines due to its cytotoxicity at high concentrations and/or at room temperature.7,8 Emerging cell-based therapies such as CAR-T (chimeric antigen receptor T-cell) are now approved for some cancers including acute lymphoblastic leukemia. One potential side effect of these therapies is from the DMSO transfusion, which in the case of Tisagenlecleucel is 7.5 wt %, leading to nausea/vomiting.9 Removal of DMSO prior to bone marrow transfusion complicates the process but has been shown to reduce side effects.10 DMSO can also interfere with cellular processes such as altering the epigenetic profile and hypermethylation of cardiac and hepatic cells,11 disrupting morphology and reducing the viability of primary neuronal cells,12 inducing protein aggregation,13 damaging mitochondrial integrity and promoting apoptosis in astrocytes,14 and inducing significant alterations in gene expression, protein content, and functionality of differentiated hepatic cells.15
It is, at this point, important to point out that DMSO cryopreservation is a highly successful technology which is essential to modern bioscience, and our aim is not to say otherwise. But, there exists major opportunities to improve upon it; this could be by increased cell recovery (function and yield) or by reducing the concentration required, while ensuring the new additives also have minimal side effects. Alternatives such as trehalose have been widely explored, but trehalose is non-cell-permeating and hence requires either to be used alongside a cell-penetrating cryoprotectant (such as DMSO)16 or loading into the cell using poration methods.17,18
Currently, cells are typically frozen as suspensions in cryovials and stored in liquid nitrogen, but a large amount of biomedical research is conducted on adherent cells as monolayers. However, preserving cells as attached monolayers “ready for assays” is challenging. Inclusion of DMSO alone does not work well for cell monolayers,19 typically resulting in only around 20–35% recovery.20,21 Adherent attached human embryonic stem cells yield extremely low survival rates of <5% which has been shown to be due to apoptosis rather than necrosis from freeze–thaw injury.19,20 The ability to cryopreserve cell monolayers would facilitate drug development by providing phenotypically identical cells for assays as well as provide insights into the cryopreservation of more complex biological material such as spheroids or tissues. Compared to cryopreservation of suspended cells, protocols for adherent cell monolayers are significantly lacking. To date, there have been few studies on the slow-freezing (nonvitrified) cryopreservation of monolayered cells.21–33 3-D cell cultures such as spheroids or organoids present even more complexity, such as the significant impact of spheroid size on recovery (large <40% recovery vs small <70%) along with required equilibration times,34 and hence result in lower cell yields post-thaw. This is clearly observed with human embryonic kidney cells, where 3-D cryopreservation led to recovery of only 36%,30 and improved methods are obviously required.
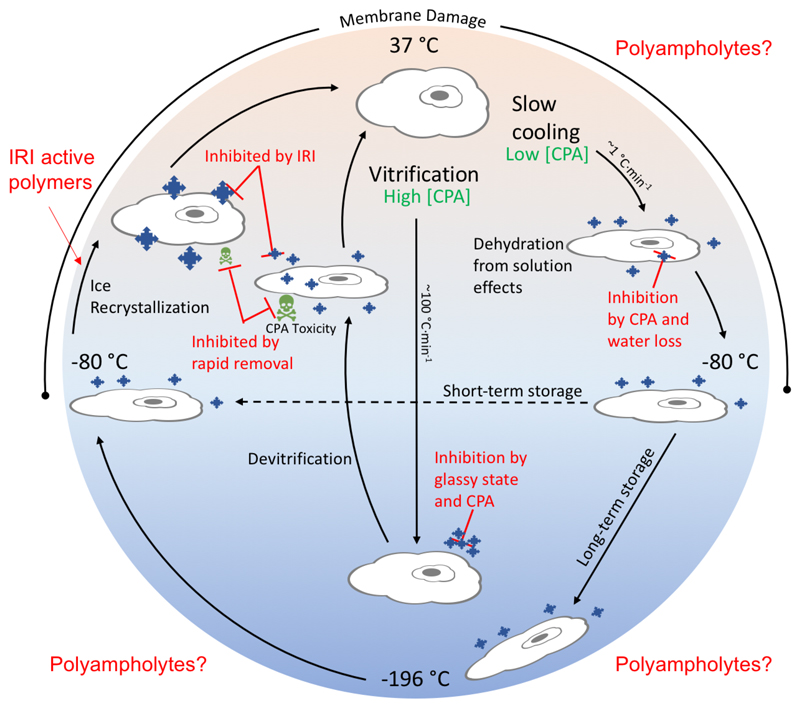
In order to improve cryopreservation outcomes and introduce new disruptive technology, one must first understand the mechanisms by which cryopreserved tissues are damaged. Regardless of the format the cells are frozen in, there are two main conventional methods for freezing: slow cooling and vitrification (Figure 1). Vitrification uses a high concentration of solutes and ultrarapid lowering of temperature generating a glassy solid while avoiding ice nucleation.35 The high CPA concentration results in dehydration of the cells and the potential for ice crystal growth exists upon thawing.36 Additionally, the high concentration of CPA must be quickly removed to prevent toxicity, which can involve challenging and complex processes. Due to the high rate of freezing and high CPA concentration required to reach a glassy state, vitrification is not wholly practical for everyday lab use. Slow cooling involves freezing at a controlled rate of 1 °C·min−1 in the presence of a CPA which promotes dehydration to avoid intracellular ice formation.37 Cells can be stored short-term at −80 °C or moved to liquid nitrogen for long-term storage at −196 °C. Upon thawing, as for cells that have been vitrified, ice recrystallization can damage the cells through the growth of ice crystals at temperatures close to the freezing point of the cryoprotectant solution. This crystal growth can cause both mechanical and osmotic damage to cells, and being able to control this process by using ice recrystallization inhibitors has been shown to result in enhanced post-thaw recovery.38 In either process, membrane damage can occur at any point where there is molecular movement (>−80 °C). Cellular membranes function universally as barriers between the environment as well as individual cellular components, play a major role in molecule transport and bioenergetics, and are of critical importance in cell signaling processes. The bilayer typically exists in what is commonly termed a liquid-crystalline phase (a balance between flexibility and order) and at sufficiently low temperatures the regions of the bilayer enter what is termed a gel phase, however, due to the heterogeneity of the bilayer, liquid- and gel-phase regions may coexist in the membrane throughout the cooling process, which results in phase separation.39 Phase separated membranes have been shown to have an increased permeability at the interface of the two regions40 and upon reheating, phase separated membranes can form nonbilayer lipid structures, due to lipid aggregation,39 which results in membrane integrity damage.41 There are clearly many opportunities to tackle these challenges using both organic and macromolecular chemistry.
Figure 1.
Schematic showing conventional cryopreservation process and sites of damage. Pathways for vitrification and slow cooling processes are indicated. Point where ice recrystallization inhibiting polymers (outside of context of this Perspective) can impact is shown, and the range of processes where membrane damage (a possible mechanism of action of polyampholytes) is also shown.
In contrast to traditional cryopreservation strategies, Nature has evolved a remarkable series of ice binding (also known as antifreeze) proteins and biomacromolecules, enabling extremophiles to survive at subzero temperatures. Antifreeze proteins from polar fish are the most studied class, and provide a noncolligative freezing point depression of their internal fluids, preventing damage caused by freezing.42–44 Cryoprotectant macromolecules have also been found in insects and plants (including proteins45,46 and polysaccharides47). In particular their ice recrystallization inhibition (IRI) activity has generated interest, due to the detrimental role of ice recrystallization in cell cryopreservation. A challenge in their application, however, is that their efficient ice binding causes dynamic ice shaping of the crystals, leading to spicular (needle like) growth which can pierce cells and disrupt cell membranes, limiting their effectiveness.48 There has therefore been significant interest in macromolecular cryoprotectants (see reviews49–51) which show IRI activity and have shown some benefit in the cryopreservation of cells.52–55 Similarly small molecule IRI’s from Ben and co-workers have shown potential to modulate this mode of damage.56–59 However, it is clear that IRI alone does not modulate all damage to cells, and that other mechanism of cell death, and hence other materials, are required to transform the biologic and biotechnological cold chain for cells.
In 2009, Matsumura and Hyun reported the remarkable cryopreservative activity of carboxylated poly(ε-lysine)—a polyampholyte.60 Addition of 10 wt % of this polymer enabled the recovery of viable cells, but its protective effect does not seem to be down to the ice recrystallization inhibition alone. Its core structure (balance of cationic/anionic groups) also does not seem to link to known biological macromolecular cryoprotectants, but the need for a polymeric backbone is clear. It is now emerging that many polymers based on the ampholyte structure show cryoprotective benefits without any significant impact on ice recrystallization,61–65 but the mechanism of action, core structural motifs and translational potential is still to be explored.
In this Perspective, we will discuss the new but rapidly evolving field of polyampholytes for cryopreservation. This will include a short commentary on their synthesis and properties followed by a critical evaluation of their application and potency in cell cryopreservation and thoughts on how the field will develop.
Synthesis of Polyampholytes
Polyampholytes, or mixed charge polymers, were first synthesized by Alfrey in 1950, as a “synthetic alternative to proteins using copolymers of 2-vinylpyridine and methacrylic acid which demonstrated similar solubility and electrophoretic mobility to proteins.66 The definition of a polyampholyte can overlap with that of polyzwitterions and polybetaines depending on the nature of the functional group.67 In the context of this article, polyampholytes will be defined as polymers which contain mixed cationic/anionic groups but not those where the charge is on the same repeat unit, where we will refer to it as polybetaines. This Perspective will only consider polyampholytes in the context of cryopreservation, but it is important to highlight the many other application areas of polyzwitterions in general, such as in drag reducers or anti-fouling coatings.68–70
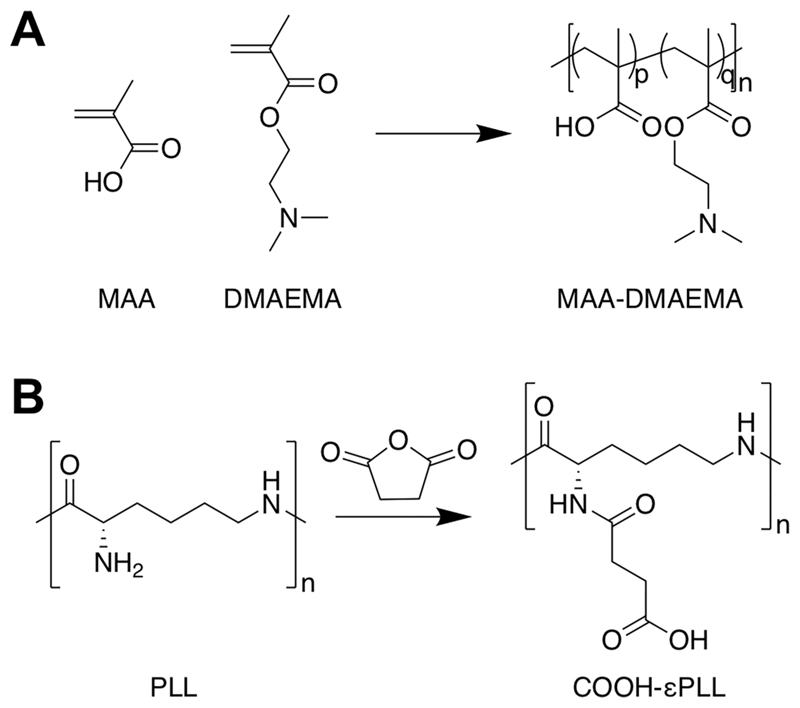
There are a variety of synthetic strategies which can be used to access polyampholytes, either by the direct copolymerization of the appropriate monomers (Figure 2A)71,72 (with or without protecting groups) or by postpolymerization modification (Figure 2B).60 A number of polyampholytes with more complex architectures have been synthesized by exploiting controlled radical polymerization methods, for example triblock copolymers of methacrylic acid (MAA) and 2-(dimethylamino)ethyl methacrylate (DMAEMA) and either methyl methacrylate or 2-phenylethyl methacrylate using group transfer polymerization,73 or polyampholytes based on substituted styrenes using nitroxide mediated polymerization.74
Figure 2.
Common synthetic strategies used to access polyampholytes. (A) Copolymerization of methacrylic acid (MAA) and 2-(dimethylamino)ethyl methacrylate (DMAEMA). (B) Postpolymerization modification of poly(ε-lysine) using succinic anhydride.
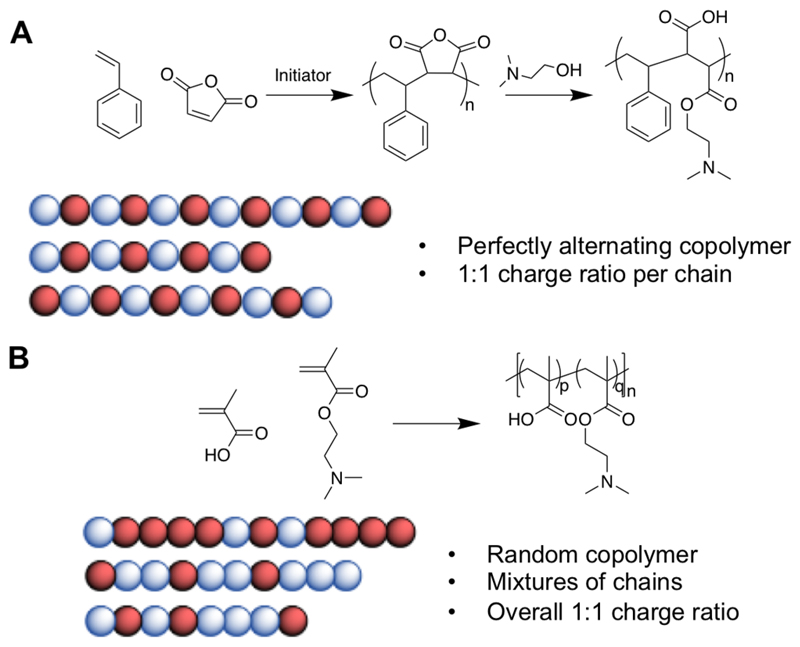
More recently, the ring-opening polymerization of an N-maleamic acid functionalized homocysteine thiolactone monomer has been exploited by Du Prez and co-workers to synthesize purely alternating polyampholytes with no compositional drift.75 Polyampholytes made by most copolymerization methods are limited by their inherent compositional variability, with few individual chains having monomer compositions matching the feed ratio. A powerful strategy to overcome this is to use the unique reactivity of maleic anhydride (MAH). Maleic anhydride cannot homopolymerize as the reaction between the growing MAH polymer radical and the monomer unit is strongly disfavored when compared to cross propagation. Therefore, with appropriate choice of comonomer, perfectly alternating materials can be obtained (Figure 3A). The anhydride ring in the polymer backbone can be ring opened using appropriate nucleophiles to introduce the cationic functionality (e.g., a tertiary amine) and simultaneously a carboxylic acid. This method is particularly useful as it ensures a 1:1 ratio of the charged groups, whereas other copolymerization strategies lead to some compositional variation (Figure 3B). Advances in sequence controlled polymers will no doubt offer opportunities in the future to access precision polyampholytes.76
Figure 3.
(A) Synthetic scheme for the copolymerization of maleic anhydride with styrene to produce a perfectly alternating copolymer, followed by ring opening of the anhydride to produce a polyampholyte. (B) Synthetic scheme for the synthesis of a poly(DMAEMA-MAA) ampholyte, and a schematic showing how 50:50 monomer incorporation across all chains may not be evenly distributed within a single chain.
Polyampholytes as Macromolecular Cryoprotectants
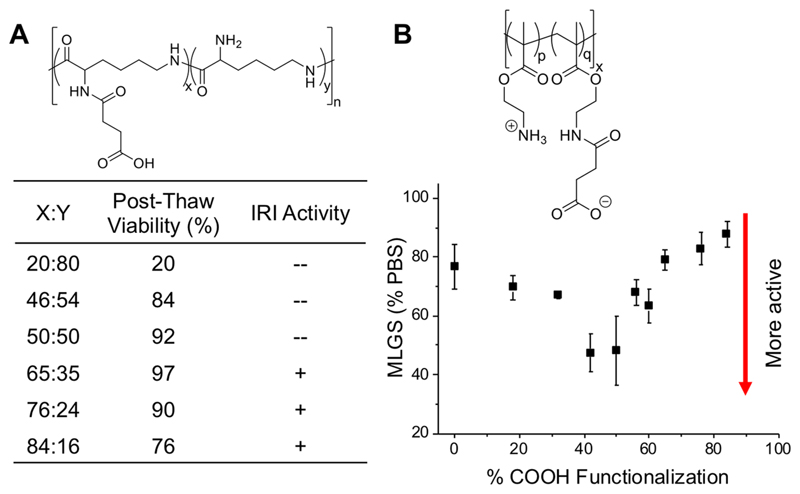
In the context of macromolecular cryopreservation, there have been several reports on using polymers which have IRI activity to enhance post-thaw cell recovery, which is outside the context of this Perspective.49,50,58,77–79 However, evidence is emerging that IRI activity alone often only gives incremental benefits, and has so far not enabled to dramatically remove organic solvents from mammalian cell cryopreservation. In short, ice recrystallization is part of the cryopreservation problem but not the only one, with issues such as membrane damage and apoptosis not addressed. In contrast polyampholytes, which have only modest/weak IRI and seem to function by an alternative mechanism, are attracting attention. Carboxylated poly(ε-lysine) was the first polyampholyte cryoprotectant, and was observed to enhance the viability of cryopreserved L929 cells to as high as 95% [note, viability is not the same as total cell recovery] using a slow freezing (1 °C· min−1) process (Figure 4). The ratio of anionic and cationic groups was found to be crucial, with a ratio close to 1:1 essential for the best cryopreservation results, demonstrating the ampholyte structure is essential (Figure 4A).60
Figure 4.
(A) IRI (ice recrystallization inhibition) activity and viability of a carboxylated poly(ε-lysine) with varying ratios of carboxylation.60 (B) IRI activity represented as mean largest grain size for varying ratios of anionic to cationic monomers in a vinyl-based polymer.80
To investigate if polyampholytes have IRI activity (as a potential mechanism of action for cryopreservation), Mitchell et al. used reversible addition–fragmentation chain transfer (RAFT) polymerization to produce poly(aminoethyl methacrylate) (PAEMA), followed by reaction with succinic anhydride to give a polyampholyte. The ratio of positive to negative charges was systematically varied, and the IRI activity evaluated. It was observed that IRI activity was highest when a 1:1 ratio of charges were used, with activity being lost as one group starts to dominate (Figure 4B).80 It must be noted, however, that the overall activity was rather low compared to other IRI active materials.81 Interestingly, zwitterionic polymers (where both charges are on each repeat unit) were found to have no activity, indicating the correct location, chemical nature and distribution of the charges is a key structural requirement.80
In order to investigate the importance of regioregularity on the IRI activity of polyampholytes, Stubbs et al. synthesized a selection of alternating copolymers using RAFT/MADIX polymerization of maleic anhydride to generate polymer materials using comonomers of varying hydrophobicity, followed by the subsequent ring-opening of the anhydride to install the charged groups. It was shown that the appropriate balance of hydrophobicity in both the comonomer and pendant amine are required in order to maximize IRI activity. Polymers functionalized with dimethylaminoethanol displayed more IRI activity than those functionalized with either NH2 or NiPr2 groups, suggesting a balance of hydrophobicity/philicity is required. These copolymers were then also compared to structurally similar materials synthesized through random copolymerization of acrylic acid (AA) and dimethylaminoethyl acrylate (DMEA) demonstrating the importance of this regioregular distribution of charges.82
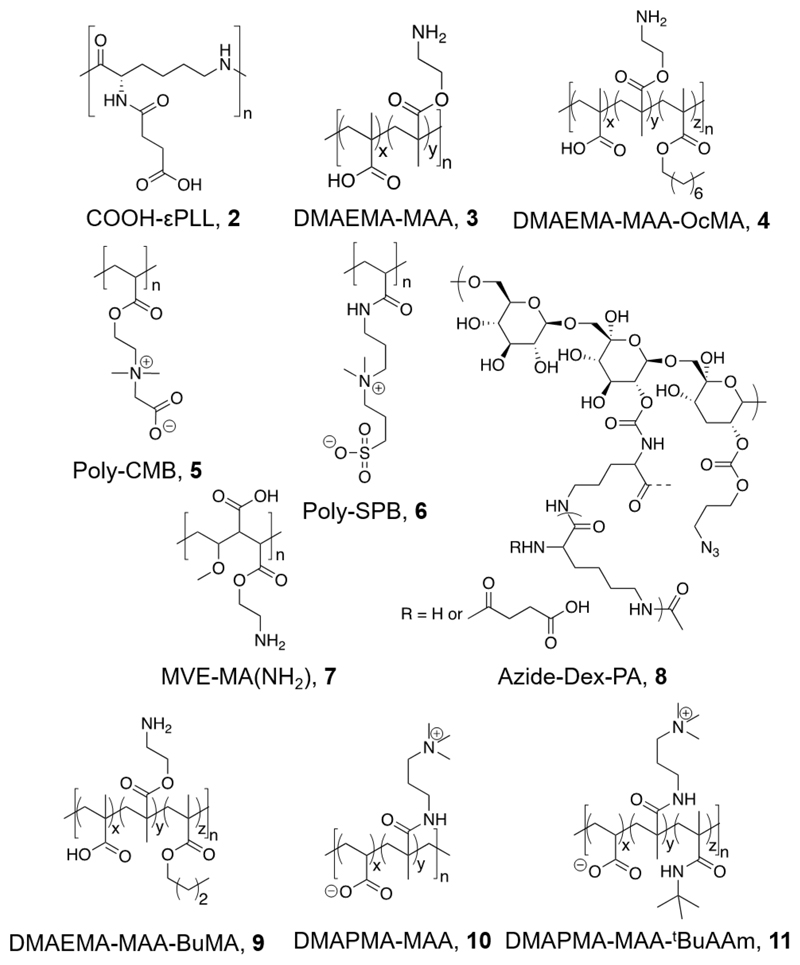
Copolymers obtained from 2-(dimethylamino)ethyl methacrylate and methacrylic acid (at 1:1 ratio) showed some increase in cryoprotective and IRI activity when hydrophobicity was increased (noting that the concentrations used for IRI testing were very high and hence activity in these cases was very weak).62 The effect of this hydrophobicity increase to cryoprotectant activity was then investigated further, with hydrophobically modified p(DMAEMA-MAA) compared to poly carboxymethyl betaine (poly(CMB)) and poly sulfobetaine (poly(SPB)), and their hydrophobically modified counterparts. Hydrophobic modification of p(DMAEMA-MAA) enhanced activity from ~60% to 90% recovery. Interestingly, poly(CMB), which has a remarkably similar structure to poly(DMAEMA-MAA), was shown to demonstrate no cryoprotective properties, and that hydrophobic modification had no effect on its activity. Poly(SPB), however, showed no change in activity after hydrophobic modification, suggesting that this is not a guaranteed method by which to enhance cryoprotectant activity.63 Table 1 summarizes some polyampholytes, their IRI activity, and post-thaw cell viability, highlighting the lack of trend between the two. [Note; IRI activity is defined by the mean largest grain size here–the values can be between 0 and 100%, with 100% representing no activity (the same as a negative control).]
Table 1. Summary of IRI Activity and Cell Recovery Using a Panel of Polyampholytes and Polyzwitterions61.
| Structure(a) | Cell viability post-thaw(b) (%) | MLGS (%PBS)(c) | Structure(a) | Cell viability post-thaw(b) (%) | MLGS (%PBS)(c) |
|---|---|---|---|---|---|
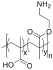 |
62 | 48 | 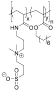 |
42 | 22 |
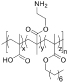 |
96 | 2 | 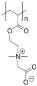 |
3 | 63 |
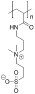 |
48 | 39 | 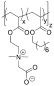 |
2 | 27 |
Structure of the polyampholyte tested.
Post-thaw viability of L929 cells cryopreserved using 100 mg·mL−1 polyampholyte, and cooling to −80 °C without controling the cooling rate.
IRI activity given as MLGS (mean largest grain size) and compared to a phosphate-buffered saline control. Polymers were tested at 100 mg·mL−1.
It is crucial to highlight here that all the reported polyampholytes only have very weak IRI (or other properties associated with ice growth modulation), requiring concentrations 10–50-fold higher than PVA, and 100s of times higher than antifreeze proteins. It is therefore important to highlight that it is not clear if IRI or another entirely separate mechanism is responsible for their observed cryoprotective properties and it may be possible that the weak IRI is coincidental and not crucial. See previous reviews for details of IRI and macromolecules.49,50,81
Other investigations into fully synthetic ampholytes have demonstrated that a copolymer of dimethylaminopropyl methacrylamide and acrylic acid are efficient cryoprotectants for the DMSO-free cryopreservation of 3T3 cells. It was observed that with the inclusion of 9% hydrophobic N-tbutyl acrylamide, 90% recovery (relative to 10% DMSO) was observed immediately post-thaw; however, long-term (up to 72 h) viability was poorer. This highlights a key issue in that many additives can enable cell recovery, but retaining viable cells after >24 h of culture is challenging, but also essential for all downstream applications. The addition of 2% DMSO helped to reduce this loss, with results comparable to 10% DMSO alone.83 This highlights a crucial point for assessing cryopreservation success, since the process of freezing and thawing cells has been shown to induce stress and the upregulation of apoptosis proteins in many cell types,19,20,84 leading to subsequent cell death occurring up to 24 h post-thaw.85 Analyzing cell recovery immediately post-thaw often overlooks this process and can lead to false-positives of cryopreservation success.86 Therefore, it is crucial in this field to enable sufficient time (normally at least 24 h) post-thaw to be confident of a cryopreservation result.
Matsumura has also developed dextran-based polyampholyte cryoprotectants, which form in situ hydrogels via a biocompatible copper-free click reaction. These hydrogels were then used for the DMSO-free cryopreservation of mammalian cells.87 Given the successes observed using polyampholytes to aid in suspension freezing, Matsumura also investigated the applicability of these materials for the cryopreservation of cellular monolayers.25 Monolayers are significantly more difficult to freeze88 while also acting as a more realistic substitute for organs and tissues.23 It was observed that DMSO can be omitted during slow vitrification by using an alternative cryoprotectant solution of 6.5 M ethylene glycol, 10 wt % COOH-εPLL and 0.5 M sucrose. Freezing rates as low as 4.9 °C·min−1 were used, with good recovery of the cells immediately after thawing and after 1 day of cell culture. COOH-εPLL has since been shown to be effective for the cryopreservation of a wide variety of other cell types, for example chondrocyte cell sheets,89 mouse oocytes,90 pig91 and mouse92 embryos and long-term studies on human mesenchymal stem cells.93
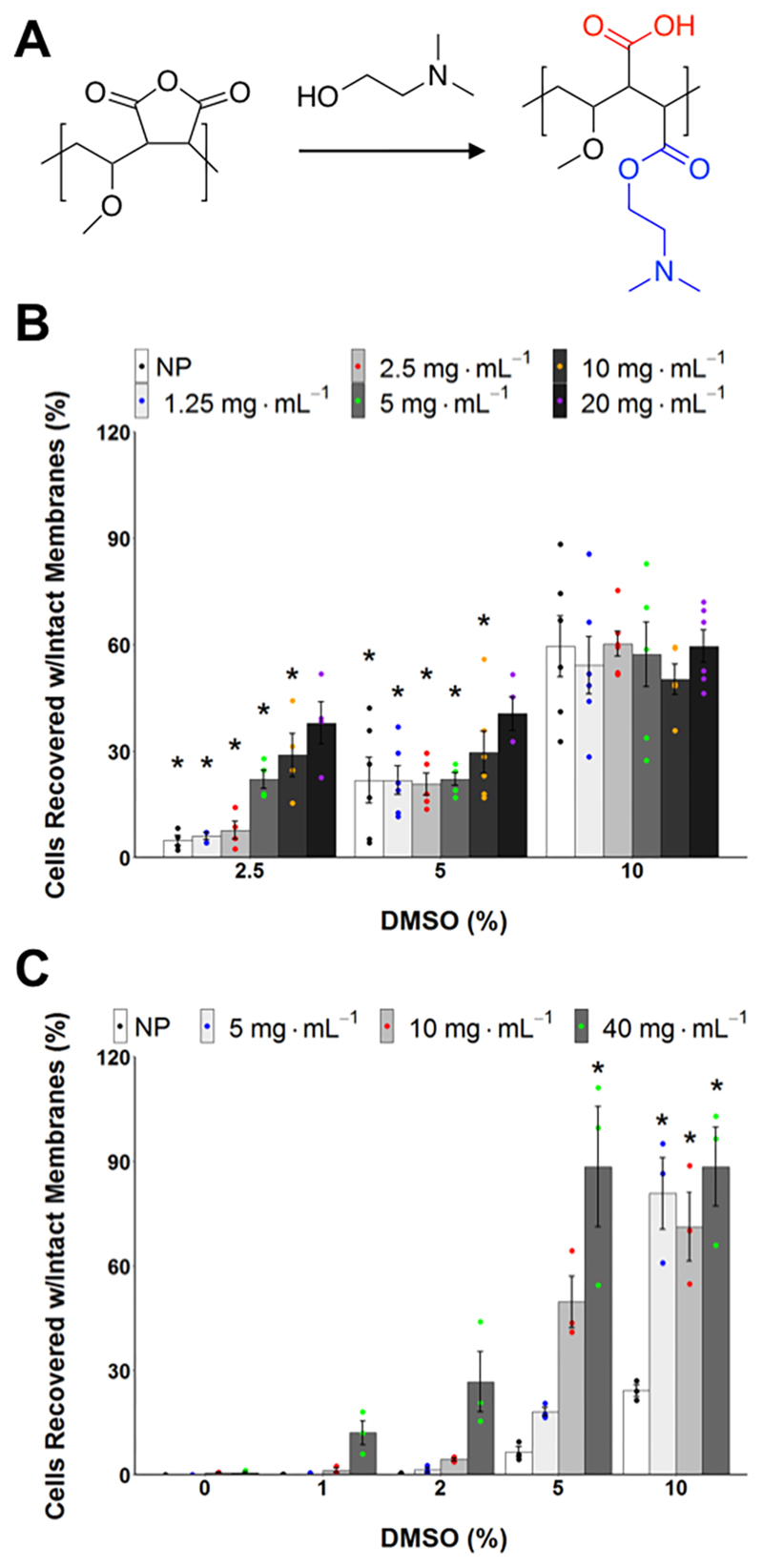
However, these polymers are based on relatively expensive precursors (such as poly(ε-lysine)); for larger scale cryopreservation, grams to kilograms are required. To address this issue, Mitchell et al. reported using the commercially available ampholyte precursor poly(methyl vinyl ether-alt-maleic anhydride), (poly(MVE-alt-MA)). This material was functionalized with a Boc protected aminoethanol, followed by deprotection to generate a polyampholyte with exact 1:1 charge ratios, and then applied to the cryopreservation of blood cells via a slow thawing mechanism that gave excellent recovery when paired with hydroxyethyl starch as a cocryoprotectant.65 Given the activity of these materials, the potential of maleic anhydride copolymers as ampholyte precursors was determined to be of interest. Poly(MVE-alt-MA) is produced using a standard free radical polymerization, which while efficient and low cost, leads to little control over the molecular weight, dispersity, and monomer incorporation of the polymer. Following on from this, Gibson and co-workers have developed a polymer based on poly(MVE-alt-MA) which is available commercially on a multikilogram basis, is used as a bioadhesive and available to good manufacturing practice (GMP) quality, which is important for biomedical applications.94 The poly(MVE-alt-MA) copolymer is easily ring opened with dimethylaminoethanol to give an ampholytic structure (Figure 5A). The choice of dimethylamine (rather than, e.g., primary amine) as the positive charge was essential to maximize performance versus solubility, as found using previous polyampholytes.82 This polymer was shown to enable suspension cryopreservation of A549 cells using just 2.5 wt % DMSO (in place of the normal 10 wt %) (Figure 5). Additionally, it was found to improve the cryopreservation of A549 monolayers from around 20 to almost 90%, with a similar fold increase seen with other cell lines. This showed that polyampholytes may be translatable from the laboratory to a real-world scenario by using a commercially valid scaffold.
Figure 5.
(A) Synthesis and application of a polyampholyte derived from the commodity polymer poly(methyl vinyl ether-alt-maleic anhydride). (B) Suspension cryopreservation of A549 cells using the polyampholyte showing total cells recovered. (C) Monolayer cryopreservation of A549 cells using the polyampholyte showing total cells recovered. Results show total cell recovery as a function of both polyampholyte and DMSO concentration. Reproduced with permission from Bailey et al.24 Copyright 2019 American Chemical Society.
Table 2/Figure 6 collates the currently (to the best of our knowledge) reported cells and cryopreservation conditions where polyampholytes have been used. It is crucial to note there is no standardized test for cryopreservation, so many features such as cell density, freezing/thawing rates and the addition or absence of serum can all affect the outcomes. Also, each cell type has a different growth rate and different media requirements (which may also affect cryopreservation outcomes), so it is not possible to compare between them. What is crucial is to note, in Table 2, which cases are vitrification (ice free, using larger volumes of solvents) and which are slow freezing (ice is allowed to form), as these are technically different processes and the polyampholytes appear to give benefits in both cases. Furthermore, only cell viability is reported, not cell recovery, as this is the most commonly used output, enabling comparison. We would highlight, however, that obtaining viable cells is important, but so is the total cell yield; for example, obtaining cells with viability >90%, but only recovering 1% of what went in the vial is clinically and biochemically not a useful process, but this number is less commonly reported.
Table 2. Cryoprotective Outcomes Using Polyampholytes.
| Structurea | [Polymer] (wt %) | Cell type | Cell viabilityb | Freezing ratec | Other CPAsd | Ref |
|---|---|---|---|---|---|---|
| 2 | 7 | L929 | ∼95% | Slow freezing 1 °C·min−1 | None | 60 |
| 3 | 15 | L929 | ∼90% | Slow freezing NCe | None | 62 |
| 4 | 10 | L929 | ∼95% | Slow freezing NCe | None | 63 |
| 5 | 15 | <10% | ||||
| 6 | 15 | ∼70% | ||||
| 7 | 2 | RBC | ∼65% | Vitrification direct into LN2 | 350 mg·mL−1 HES, 30 mg·mL−1 mannitol and 6.5 mg·mL−1 NaCl | 65 |
| 8 | 12 | L929 | ∼90% | Slow freezing NCe | None | 87 |
| 2 | 10 | MSC Monolayer | ∼80% | Slow freezing 4.9 °C·min−1 | 6.5 M EGf, 0.5 M sucrose | 25 |
| 3 | 10 | L929 | ∼60% | Slow freezing NCe | None | 61 |
| 9 | 10 | ∼70% | ||||
| 4 | 10 | 96% | ||||
| 10 | 10 | 3T3 | 70% | Slow freezing 1 °C·min−1 | 2% DMSO | 83 |
| 11 | 10 | 90% | ||||
| 2 | 20 | PN Pig embryo | Higher development rate | Vitrification direct into LN2 | 30% EGf, 0.5 M sucrose | 91 |
| 2 | 10 | Chondrocyte sheet | All sheets recovered | Vitrification direct into LN2 | 20% DMSO, 20% EGf, 0.5 M sucrose | 89 |
| 2 | 10 | Mouse oocyte | 95% Survival after fertilization | Vitrification direct into LN2 | 20% EGf, 0.5 M sucrose | 90 |
| 2 | 7.5 | Human mesenchymal stem cells | 90% Viability after 24 months | Slow freezing NCe | None | 93 |
| 1 | 2 | A549 Suspension | 50% | Slow freezing 1 °C·min−1 | 5% DMSO | 24 |
| 1 | 4 | A549 Monolayer | 90% | Slow freezing 1 °C·min−1 | 5% DMSO |
Representative structure reproduced from reference.
Viability of the cells as reported.
If included in original ref.
Any other materials added to the cryopreservation solution.
Not controlled, final storage temperature −80 °C.
EG is ethylene glycol.
Figure 6.
Chemical structures of polyampholytes referred to in Table 2.
Mechanism of Cryoprotection
In an attempt to understand the mechanism by which polyampholytes provide efficient protection, Matsumura investigated the effect of three different cryoprotective polymers on the integrity of the cell membrane. Samples which gave greater cellular recovery post-thaw were shown to cause a greater depression in the gel–liquid phase-transition temperature of model phospholipid membranes. Leakage experiments also suggested that the polymers were interacting with the membranes, with improved cryoprotective activity corresponding to less post-thaw leakage from a cryopreserved liposome.61 This membrane interaction has also been exploited by Matsumura for the introduction of lysozyme proteins into cells through a freeze–thaw mechanism, which enables a 4-fold increase in uptake when compared to nonfrozen cells.95 It was then demonstrated that these ampholyte-based nanocarrier complexes allowed more protein internalization when compared to 10% DMSO, which is known to enhance membrane permeability.96 Taken together, these results suggest that while also providing superior cell viability post-thaw, polyampholytes interact with the cell membrane during the freezing process to allow greater payload uptake when incorporated into a protein–nanocarrier complex.
Poly(zwitterions) have been investigated for gene and drug delivery, to mitigate the toxicity of fully cationic polymers. While beyond the scope of this Perspective, it appears these delivery mechanisms are also reliant on the ability of polyampholytes to engage with cell membranes. Interestingly, bacteria cell membranes have a higher density of anionic lipids compared to mammalian cell membranes. A study by Hasan et al. has shown that polyampholytes fail to offer any cryoprotective effect to a range of bacteria, despite their near-universal role in enhancing mammalian cryopreservation, providing further evidence for the role of membrane interactions (while remembering there are other significant differences between prokaryotes and eukaryotic cells).97
Future Perspective
This Perspective has gathered compelling examples that polyampholytes are potent macromolecular cryopreservatives which enhance the cryopreservation of a vast range of cell types. The cryoprotective capability of these appears to be remarkably tolerant of the actual structure of the polyampholytes, based on available evidence, with a range of backbones and functionalities possible. However, this diversity of structures does mean there are major knowledge gaps in exactly how these polymers function, and to what extent than can be employed within both basic biomedical research and also clinical applications, where formulations using DMSO as the major cryoprotective agent are successfully used and integrated into supply chains. There is also the issue of cost, compared to DMSO alone, and the role of other cryoprotectants such as trehalose to look for synergistic benefits. Furthermore, there is the question of whether routine addition of these polymers can improve workflows or enable the cryopreservation of emerging, more challenging, cell systems particularly in 2 and 3 dimensions, where current methods are not suitable. In order to advance this field, a detailed molecular level understanding of their function is essential as well as consideration of how they can be incorporated into both routine (e.g., lab based) and therapeutic cryopreservation protocols. This will include issues such as ensuring shelf stability, solubility (due to the high concentrations required) as well as biocompatibility. We also postulate it is key to highlight that a polymer which controls ice growth may give a benefit to cryopreservation; however, a material which benefits cryopreservation may not necessarily impact ice growth. We therefore anticipate significant interest in these materials in the coming years, especially to obtain quantitative structure–activity relationships to help guide them to real-world applications.
Acknowledgements
M.I.G. holds an ERC starter grant 638661) and PoC Grant (789182).
Footnotes
ORCID
Matthew I. Gibson: 0000-0002-8297-1278
Notes
The authors declare the following competing financial interest(s): M.I.G., T.L.B. and C.S. are named inventors on a patent application using materials related to those mentioned in this Perspective.
References
- (1).Lovelock JE, Bishop MW. Prevention of Freezing Damage to Living Cells by Dimethyl Sulfoxide. Nature. 1959;183(4672):1394–1395. doi: 10.1038/1831394a0. [DOI] [PubMed] [Google Scholar]
- (2).Stéphenne X, Najimi M, Sokal EM. Hepatocyte Cryopreservation: Is It Time to Change the Strategy? World J Gastroenterol. 2010;16(1):1–14. doi: 10.3748/wjg.v16.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Mazur P. Cryobiology: The Freezing of Biological Systems. Science (Washington, DC, U. S.) 1970;168(3934):939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- (4).Mazur P, Farrant J, Leibo SP, Chu EH. Survival of Hamster Tissue Culture Cells after Freezing and Thawing. Interactions between Protective Solutes and Cooling and Warming Rates. Cryobiology. 1969;6(1):1–9. doi: 10.1016/s0011-2240(69)80002-7. [DOI] [PubMed] [Google Scholar]
- (5).Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC. Terminal Differentiation of Human Promyelocytic Leukemia Cells Induced by Dimethyl Sulfoxide and Other Polar Compounds. Proc Natl Acad Sci U S A. 1978;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Timm M, Saaby L, Moesby L, Hansen EW. Considerations Regarding Use of Solvents in in Vitro Cell Based Assays. Cytotechnology. 2013;65(5):887–894. doi: 10.1007/s10616-012-9530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Hengstler JG, Utesch D, Steinberg P, Platt KL, Diener B, Ringel M, Swales N, Fischer T, Biefang K, Gerl M, Böttger T, et al. Drug Metabolism Reviews. Taylor & Francis; 2000. Cryopreserved Primary Hepatocytes as a Constantly Available in Vitro Model for the Evaluation of Human and Animal Drug Metabolism and Enzyme Induction; pp. 81–118. [DOI] [PubMed] [Google Scholar]
- (8).Fahy GM. Cryoprotectant Toxicity Neutralization. Cryobiology. 2010;60(3 Suppl):S45–S53. doi: 10.1016/j.cryobiol.2009.05.005. [DOI] [PubMed] [Google Scholar]
- (9).Yáñez L, Sánchez-Escamilla M, Perales M-A. CAR T Cell Toxicity: Current Management and Future Directions. Hema Sphere. 2019;3(2):e186. doi: 10.1097/HS9.0000000000000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Syme R, Bewick M, Stewart D, Porter K, Chadderton T, Glück S. The Role of Depletion of Dimethyl Sulfoxide before Autografting: On Hematologic Recovery, Side Effects, and Toxicity. Biol Blood Marrow Transplant. 2004;10(2):135–141. doi: 10.1016/j.bbmt.2003.09.016. [DOI] [PubMed] [Google Scholar]
- (11).Verheijen M, Lienhard M, Schrooders Y, Clayton O, Nudischer R, Boerno S, Timmermann B, Selevsek N, Schlapbach R, Gmuender H, Gotta S, et al. DMSO Induces Drastic Changes in Human Cellular Processes and Epigenetic Landscape in Vitro. Sci Rep. 2019;9(1):4641–4653. doi: 10.1038/s41598-019-40660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Zhang C, Deng Y, Dai H, Zhou W, Tian J, Bing G, Zhao L. Effects of Dimethyl Sulfoxide on the Morphology and Viability of Primary Cultured Neurons and Astrocytes. Brain Res Bull. 2017;128:34–39. doi: 10.1016/j.brainresbull.2016.11.004. [DOI] [PubMed] [Google Scholar]
- (13).Giugliarelli A, Urbanelli L, Ricci M, Paolantoni M, Emiliani C, Saccardi R, Mazzanti B, Lombardini L, Morresi A, Sassi P. Evidence of DMSO-Induced Protein Aggregation in Cells. J Phys Chem A. 2016;120(27):5065–5070. doi: 10.1021/acs.jpca.6b00178. [DOI] [PubMed] [Google Scholar]
- (14).Yuan C, Gao J, Guo J, Bai L, Marshall C, Cai Z, Wang L, Xiao M. Dimethyl Sulfoxide Damages Mitochondrial Integrity and Membrane Potential in Cultured Astrocytes. PLoS One. 2014;9(9):e107447. doi: 10.1371/journal.pone.0107447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Pal R, Mamidi MK, Das AK, Bhonde R. Diverse Effects of Dimethyl Sulfoxide (DMSO) on the Differentiation Potential of Human Embryonic Stem Cells. Arch Toxicol. 2012;86(4):651–661. doi: 10.1007/s00204-011-0782-2. [DOI] [PubMed] [Google Scholar]
- (16).Katenz E, Vondran FWR, Schwartlander R, Pless G, Gong X, Cheng X, Neuhaus P, Sauer IM. Cryopreservation of Primary Human Hepatocytes: The Benefit of Trehalose as an Additional Cryoprotective Agent. Liver Transplant. 2007;13(1):38–45. doi: 10.1002/lt.20921. [DOI] [PubMed] [Google Scholar]
- (17).Sharp DMC, Picken A, Morris TJ, Hewitt CJ, Coopman K, Slater NKH. Amphipathic Polymer-Mediated Uptake of Trehalose for Dimethyl Sulfoxide-Free Human Cell Cryopreservation. Cryobiology. 2013;67(3):305–311. doi: 10.1016/j.cryobiol.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Dovgan B, Barlič A, Knežević M, Miklavčič D. Cryopreservation of Human Adipose-Derived Stem Cells in Combination with Trehalose and Reversible Electroporation. J Membr Biol. 2017;250(1):1–9. doi: 10.1007/s00232-016-9916-z. [DOI] [PubMed] [Google Scholar]
- (19).Xu X, Cowley S, Flaim CJ, James W, Seymour L, Cui Z. The Roles of Apoptotic Pathways in the Low Recovery Rate after Cryopreservation of Dissociated Human Embryonic Stem Cells. Biotechnol Prog. 2010;26(3):827–837. doi: 10.1002/btpr.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Heng BC, Ye CP, Liu H, Toh WS, Rufaihah AJ, Yang Z, Bay BH, Ge Z, Ouyang HW, Lee EH, Cao T. Loss of Viability during Freeze-Thaw of Intact and Adherent Human Embryonic Stem Cells with Conventional Slow-Cooling Protocols Is Predominantly Due to Apoptosis Rather than Cellular Necrosis. J Biomed Sci. 2006;13(3):433–445. doi: 10.1007/s11373-005-9051-9. [DOI] [PubMed] [Google Scholar]
- (21).Bailey TL, Wang M, Solocinski J, Nathan BP, Chakraborty N, Menze MA. Protective Effects of Osmolytes in Cryopreserving Adherent Neuroblastoma (Neuro-2a) Cells. Cryobiology. 2015;71(3):472–480. doi: 10.1016/j.cryobiol.2015.08.015. [DOI] [PubMed] [Google Scholar]
- (22).Stokich B, Osgood Q, Grimm D, Moorthy S, Chakraborty N, Menze MA. Cryopreservation of Hepatocyte (HepG2) Cell Monolayers: Impact of Trehalose. Cryobiology. 2014;69(2):281–290. doi: 10.1016/j.cryobiol.2014.08.001. [DOI] [PubMed] [Google Scholar]
- (23).Pasch J, Schiefer A, Heschel I, Rau G. Cryopreservation of Keratinocytes in a Monolayer. Cryobiology. 1999;39(2):158–168. doi: 10.1006/cryo.1999.2197. [DOI] [PubMed] [Google Scholar]
- (24).Bailey TL, Stubbs C, Murray K, Tomas RMF, Otten L, Gibson MI. A Synthetically Scalable Poly(Ampholyte) Which Dramatically Enhances Cellular Cryopreservation. Biomacromolecules. 2019;20:3104–3114. doi: 10.1021/acs.biomac.9b00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Matsumura K, Kawamoto K, Takeuchi M, Yoshimura S, Tanaka D, Hyon S-HH. Cryopreservation of a Two-Dimensional Monolayer Using a Slow Vitrification Method with Polyampholyte to Inhibit Ice Crystal Formation. ACS Biomater Sci Eng. 2016;2(6):1023–1029. doi: 10.1021/acsbiomaterials.6b00150. [DOI] [PubMed] [Google Scholar]
- (26).Stevenson DJ, Morgan C, Goldie E, Connel G, Grant MH. Cryopreservation of Viable Hepatocyte Monolayers in Cryoprotectant Media with High Serum Content: Metabolism of Testosterone and Kaempherol Post-Cryopreservation. Cryobiology. 2004;49(2):97–113. doi: 10.1016/j.cryobiol.2004.05.006. [DOI] [PubMed] [Google Scholar]
- (27).Pless-Petig G, Knoop S, Rauen U. Serum- and Albumin-Free Cryopreservation of Endothelial Monolayers with a New Solution. Organogenesis. 2018;14(2):107–121. doi: 10.1080/15476278.2018.1501136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Graham B, Bailey TL, Healey JRJ, Marcellini M, Deville S, Gibson MI. Polyproline Is a Minimal Antifreeze Protein Mimetic and Enhances the Cryopreservation of Cell Monolayers. Angew Chem, Int Ed. 2017;56:15941–15944. doi: 10.1002/anie.201706703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Horacek JM, Jebavy L, Jakl M, Zak P, Mericka P, Maly J. Cardiovascular Changes Associated with Infusion of Hematopoietic Cell Grafts in Oncohematological Patients – Impact of Cryopreservation with Dimethylsulfoxide. Exp Oncol. 2009;31(2):121–122. [PubMed] [Google Scholar]
- (30).Hara J, Tottori J, Anders M, Dadhwal S, Asuri P, Mobed-Miremadi M. Trehalose Effectiveness as a Cryoprotectant in 2D and 3D Cell Cultures of Human Embryonic Kidney Cells. Artif Cells, Nanomed, Biotechnol. 2017;45(3):609–616. doi: 10.3109/21691401.2016.1167698. [DOI] [PubMed] [Google Scholar]
- (31).Miyamoto Y, Enosawa S, Takeuchi T, Takezawa T. Cryopreservation in Situ of Cell Monolayers on Collagen Vitrigel Membrane Culture Substrata: Ready-to-Use Preparation of Primary Hepatocytes and ES Cells. Cell Transplant. 2009;18(5–6):619–626. doi: 10.1177/096368970901805-618. [DOI] [PubMed] [Google Scholar]
- (32).Eskandari N, Marquez-Curtis LA, McGann LE, Elliott JAW. Cryopreservation of Human Umbilical Vein and Porcine Corneal Endothelial Cell Monolayers. Cryobiology. 2018;85:63–72. doi: 10.1016/j.cryobiol.2018.10.001. [DOI] [PubMed] [Google Scholar]
- (33).Pasch J, Schiefer A, Heschel I, Dimoudis N, Rau G. Variation of the HES Concentration for the Cryopreservation of Keratinocytes in Suspensions and in Monolayers. Cryobiology. 2000;41(2):89–96. doi: 10.1006/cryo.2000.2270. [DOI] [PubMed] [Google Scholar]
- (34).Von Mach MA, Schlosser J, Weiland M, Feilen PJ, Ringel M, Hengstler JG, Weilemann LS, Beyer J, Kann P, Schneider S. Size of Pancreatic Islets of Langerhans: A Key Parameter for Viability after Cryopreservation. Acta Diabetol. 2003;40(3):123–129. doi: 10.1007/s00592-003-0100-4. [DOI] [PubMed] [Google Scholar]
- (35).Meryman HT. Cryopreservation of Living Cells: Principles and Practice. Transfusion. 2007;47(5):935–945. doi: 10.1111/j.1537-2995.2007.01212.x. [DOI] [PubMed] [Google Scholar]
- (36).Asghar W, El Assal R, Shafiee H, Anchan RM, Demirci U. Preserving Human Cells for Regenerative, Reproductive, and Transfusion Medicine. Biotechnol J. 2014 Jul;:9895–903. doi: 10.1002/biot.201300074. NIH Public Access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).De Santis L, Coticchio G. Theoretical and Experimental Basis of Slow Freezing. Reprod BioMed Online. 2011;22(2):125–132. doi: 10.1016/j.rbmo.2010.10.012. [DOI] [PubMed] [Google Scholar]
- (38).Chao H, Davies PL, Carpenter JF. Effects of Antifreeze Proteins on Red Blood Cell Survival during Cryopreservation. J Exp Biol. 1996;199(Pt 9):2071–2076. doi: 10.1242/jeb.199.9.2071. [DOI] [PubMed] [Google Scholar]
- (39).Hazel JR. Thermal Adaptation in Biological Membranes: Is Homeoviscous Adaptation the Explanation? Annu Rev Physiol. 1995;57(1):19–42. doi: 10.1146/annurev.ph.57.030195.000315. [DOI] [PubMed] [Google Scholar]
- (40).Cordeiro RM. Molecular Structure and Permeability at the Interface between Phase-Separated Membrane Domains. J Phys Chem B. 2018;122(27):6954–6965. doi: 10.1021/acs.jpcb.8b03406. [DOI] [PubMed] [Google Scholar]
- (41).Quinn PJ. A Lipid-Phase Separation Model of Low-Temperature Damage to Biological Membranes. Cryobiology. 1985;22(2):128–146. doi: 10.1016/0011-2240(85)90167-1. [DOI] [PubMed] [Google Scholar]
- (42).Harding MM, Anderberg PI, Haymet ADJ. Antifreeze” Glycoproteins from Polar Fish. Eur J Biochem. 2003 Apr;270:1381–1392. doi: 10.1046/j.1432-1033.2003.03488.x. [DOI] [PubMed] [Google Scholar]
- (43).DeVries AL, Wohlschlag DE. Freezing Resistance in Some Antarctic Fishes. Science (Washington, DC, U. S.) 1969;163(3871):1073–1075. doi: 10.1126/science.163.3871.1073. [DOI] [PubMed] [Google Scholar]
- (44).Devries AL. Glycoproteins as Biological Antifreeze Agents in Antarctic Fishes. Science (Washington, DC, U. S.) 1971;172(3988):1152–1155. doi: 10.1126/science.172.3988.1152. [DOI] [PubMed] [Google Scholar]
- (45).Graether SP, Kuiper MJ, Gagné SM, Walker VK, Jia Z, Sykes BD, Davies PL. β-Helix Structure and Ice-Binding Properties of a Hyperactive Antifreeze Protein from an Insect. Nature. 2000;406(6793):325–328. doi: 10.1038/35018610. [DOI] [PubMed] [Google Scholar]
- (46).Duman JG. Antifreeze and Ice Nucleator Proteins in Terrestrial Arthropods. Annu Rev Physiol. 2001;63(1):327–357. doi: 10.1146/annurev.physiol.63.1.327. [DOI] [PubMed] [Google Scholar]
- (47).Dreischmeier K, Budke C, Wiehemeier L, Kottke T, Koop T. Boreal Pollen Contain Ice-Nucleating as Well as Ice-Binding ‘Antifreeze’ Polysaccharides. Sci Rep. 2017;7:41890. doi: 10.1038/srep41890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Matsumoto S, Matsusita M, Morita T, Kamachi H, Tsukiyama S, Furukawa Y, Koshida S, Tachibana Y, Nishimura SI, Todo S. Effects of Synthetic Antifreeze Glycoprotein Analogue on Islet Cell Survival and Function during Cryopreservation. Cryobiology. 2006;52(1):90–98. doi: 10.1016/j.cryobiol.2005.10.010. [DOI] [PubMed] [Google Scholar]
- (49).Voets IK. From Ice-Binding Proteins to Bio-Inspired Antifreeze Materials. Soft Matter. 2017;13(28):4808–4823. doi: 10.1039/c6sm02867e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Biggs CI, Bailey TL, Graham B, Stubbs C, Fayter A, Gibson MI. Polymer Mimics of Biomacromolecular Antifreezes. Nat Commun. 2017;8(1):1546. doi: 10.1038/s41467-017-01421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Gibson MI. Slowing the Growth of Ice with Synthetic Macromolecules: Beyond Antifreeze(Glyco) Proteins. Polym Chem. 2010;1(8):1141–1152. [Google Scholar]
- (52).Deller RC, Pessin JE, Vatish M, Mitchell DA, Gibson MI. Enhanced Non-Vitreous Cryopreservation of Immortalized and Primary Cells by Ice-Growth Inhibiting Polymers. Biomater Sci. 2016;4(7):1079–1084. doi: 10.1039/c6bm00129g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Mitchell DE, Fayter AER, Deller RC, Hasan M, Gutierrez-Marcos J, Gibson MI. Ice-Recrystallization Inhibiting Polymers Protect Proteins against Freeze-Stress and Enable Glycerol-Free Cryostorage. Mater Horiz. 2019;6:364–368. doi: 10.1039/c8mh00727f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Deller RC, Vatish M, Mitchell DA, Gibson MI. Synthetic Polymers Enable Non-Vitreous Cellular Cryopreservation by Reducing Ice Crystal Growth during Thawing. Nat Commun. 2014;5:3244. doi: 10.1038/ncomms4244. [DOI] [PubMed] [Google Scholar]
- (55).Geng H, Liu X, Shi G, Bai G, Ma J, Chen J, Wu Z, Song Y, Fang H, Wang J. Graphene Oxide Restricts Growth and Recrystallization of Ice Crystals. Angew Chem Int Ed. 2017;56(4):997–1001. doi: 10.1002/anie.201609230. [DOI] [PubMed] [Google Scholar]
- (56).Balcerzak AK, Capicciotti CJ, Briard JG, Ben RN. Designing Ice Recrystallization Inhibitors: From Antifreeze (Glyco)- Proteins to Small Molecules. RSC Adv. 2014;4(80):42682–42696. [Google Scholar]
- (57).Capicciotti CJ, Leclere M, Perras FA, Bryce DL, Paulin H, Harden J, Liu Y, Ben RN. Potent Inhibition of Ice Recrystallization by Low Molecular Weight Carbohydrate-Based Surfactants and Hydrogelators. Chem Sci. 2012;3(5):1408–1416. [Google Scholar]
- (58).Wu LK, Tokarew JM, Chaytor JL, Von Moos E, Li Y, Palii C, Ben RN, Allan DS. Carbohydrate-Mediated Inhibition of Ice Recrystallization in Cryopreserved Human Umbilical Cord Blood. Carbohydr Res. 2011;346(1):86–93. doi: 10.1016/j.carres.2010.10.016. [DOI] [PubMed] [Google Scholar]
- (59).Capicciotti CJ, Poisson JS, Boddy CN, Ben RN. Modulation of Antifreeze Activity and the Effect upon Post-Thaw HepG2 Cell Viability after Cryopreservation. Cryobiology. 2015;70(2):79–89. doi: 10.1016/j.cryobiol.2015.01.002. [DOI] [PubMed] [Google Scholar]
- (60).Matsumura K, Hyon SH. Polyampholytes as Low Toxic Efficient Cryoprotective Agents with Antifreeze Protein Properties. Biomaterials. 2009;30(27):4842–4849. doi: 10.1016/j.biomaterials.2009.05.025. [DOI] [PubMed] [Google Scholar]
- (61).Rajan R, Hayashi F, Nagashima T, Matsumura K. Toward a Molecular Understanding of the Mechanism of Cryopreservation by Polyampholytes: Cell Membrane Interactions and Hydrophobicity. Biomacromolecules. 2016;17(5):1882–1893. doi: 10.1021/acs.biomac.6b00343. [DOI] [PubMed] [Google Scholar]
- (62).Rajan R, Jain M, Matsumura K. Cryoprotective Properties of Completely Synthetic Polyampholytes via Reversible Addition-Fragmentation Chain Transfer (RAFT) Polymerization and the Effects of Hydrophobicity. J Biomater Sci Polym Ed. 2013;24(15):1767–1780. doi: 10.1080/09205063.2013.801703. [DOI] [PubMed] [Google Scholar]
- (63).Rajan R, Matsumura K. Preparation of Novel Synthetic Cryoprotectants. Cryobiol Cryotechnol. 2014;60(2):99–103. [Google Scholar]
- (64).Vorontsov DA, Sazaki G, Hyon SH, Matsumura K, Furukawa Y. Antifreeze Effect of Carboxylated ε-Poly-l-Lysine on the Growth Kinetics of Ice Crystals. J Phys Chem B. 2014;118(34):10240–10249. doi: 10.1021/jp507697q. [DOI] [PubMed] [Google Scholar]
- (65).Mitchell DE, Cameron NR, Gibson MI. Rational, yet Simple, Design and Synthesis of an Antifreeze-Protein Inspired Polymer for Cellular Cryopreservation. Chem Commun. 2015;51(65):12977–12980. doi: 10.1039/c5cc04647e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Alfrey T, Morawetz H, Fitzgerald EB, Fuoss RM. Synthetic Electrical Analog of Proteins. J Am Chem Soc. 1950;72(4):1864. [Google Scholar]
- (67).Hess M, Jones RG, Kahovec J, Kitayama T, Kratochvíl P, Kubisa P, Mormann W, Stepto RFT, Tabak D, Vohlídal J, Wilks ES. Terminology of Polymers Containing Ionizable or Ionic Groups and of Polymers Containing Ions (IUPAC Recommendations 2006) Pure Appl Chem. 2006;78(11):2067–2074. [Google Scholar]
- (68).Mumick PS, Welch PM, Salazar LC, McCormick CL. Water-Soluble Copolymers. 56. Structure and Solvation Effects of Polyampholytes in Drag Reduction. Macromolecules. 1994;27(2):323–331. [Google Scholar]
- (69).Li G, Xue H, Gao C, Zhang F, Jiang S. Nonfouling Polyampholytes from an Ion-Pair Comonomer with Biomimetic Adhesive Groups. Macromolecules. 2010;43(1):14–16. doi: 10.1021/ma902029s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Blackman LD, Gunatillake PA, Cass P, Locock KES. An Introduction to Zwitterionic Polymer Behavior and Applications in Solution and at Surfaces. Chem Soc Rev. 2019;48(3):757–770. doi: 10.1039/c8cs00508g. [DOI] [PubMed] [Google Scholar]
- (71).Alfrey T, Morawetz H. Amphoteric Polyelectrolytes. I. 2-Vinylpyridine-Methacrylic Acid Copolymers. J Am Chem Soc. 1952;74(2):436–438. [Google Scholar]
- (72).Ehrlich G, Doty P. Macroions. III. The Solution Behavior of a Polymeric Ampholyte 1. J Am Chem Soc. 1954;76(14):3764–3777. [Google Scholar]
- (73).Patrickios CS, Hertler WR, Abbott NL, Hatton TA. Diblock, ABC Triblock, and Random Methacrylic Polyampholytes: Synthesis by Group Transfer Polymerization and Solution Behavior. Macromolecules. 1994;27(4):930–937. [Google Scholar]
- (74).Gabaston LI, Furlong SA, Jackson RA, Armes SP. Direct Synthesis of Novel Acidic and Zwitterionic Block Copolymers via TEMPO-Mediated Living Free-Radical Polymerization. Polymer. 1999;40(16):4505–4514. [Google Scholar]
- (75).Du Prez F, Kaya NU, Badi N, Frank D, Resetco C. Precisely Alternating Functionalized Polyampholytes Prepared in a Single Pot from Sustainable Thiolactone Building Blocks. ACS Macro Lett. 2017;6(3):277–280. doi: 10.1021/acsmacrolett.7b00079. [DOI] [PubMed] [Google Scholar]
- (76).Lutz J-F. An Introduction to Sequence-Controlled Polymers. ACS Symp Ser. 2014;1170:1. [Google Scholar]
- (77).Balcerzak AK, Febbraro M, Ben RN. The Importance of Hydrophobic Moieties in Ice Recrystallization Inhibitors. RSC Adv. 2013;3(10):3232–3236. [Google Scholar]
- (78).Briard JG, Jahan S, Chandran P, Allan D, Pineault N, Ben RN. Small-Molecule Ice Recrystallization Inhibitors Improve the Post-Thaw Function of Hematopoietic Stem and Progenitor Cells. ACS Omega. 2016;1(5):1010–1018. doi: 10.1021/acsomega.6b00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Briard JG, Poisson JS, Turner TR, Capicciotti CJ, Acker JP, Ben RN. Small Molecule Ice Recrystallization Inhibitors Mitigate Red Blood Cell Lysis during Freezing, Transient Warming and Thawing. Sci Rep. 2016;6:23619. doi: 10.1038/srep23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Mitchell DE, Lilliman M, Spain SG, Gibson MI. Quantitative Study on the Antifreeze Protein Mimetic Ice Growth Inhibition Properties of Poly(Ampholytes) Derived from Vinyl-Based Polymers. Biomater Sci. 2014;2(12):1787–1795. doi: 10.1039/c4bm00153b. [DOI] [PubMed] [Google Scholar]
- (81).Biggs CI, Stubbs C, Graham B, Fayter AER, Hasan M, Gibson MI. Mimicking the Ice Recrystallization Activity of Biological Antifreezes. When Is a New Polymer “Active”? Macromol Biosci. 2019:1900082. doi: 10.1002/mabi.201900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Stubbs C, Lipecki J, Gibson MI. Regioregular Alternating Polyampholytes Have Enhanced Biomimetic Ice Recrystallization Activity Compared to Random Copolymers and the Role of Side Chain versus Main Chain Hydrophobicity. Biomacromolecules. 2017;18(1):295–302. doi: 10.1021/acs.biomac.6b01691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Zhao J, Johnson MA, Fisher R, Burke NAD, Stöver HDH. Synthetic Polyampholytes as Macromolecular Cryoprotective Agents. Langmuir. 2019;35(5):1807–1817. doi: 10.1021/acs.langmuir.8b01602. [DOI] [PubMed] [Google Scholar]
- (84).Baust JM, Buskirk RVAN, Baust JG. Cell Viability Improves Following Inhibition of Cryopreservation-Induced Apoptosis. Vitr Cell Dev Biol - Anim. 2000;36(April):262–270. doi: 10.1290/1071-2690(2000)036<0262:cvifio>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- (85).Suzuki K, Kostin S, Person V, Elsässer A, Schaper J. Time Course of the Apoptotic Cascade and Effects of Caspase Inhibitors in Adult Rat Ventricular Cardiomyocytes. J Mol Cell Cardiol. 2001;33(5):983–994. doi: 10.1006/jmcc.2001.1364. [DOI] [PubMed] [Google Scholar]
- (86).Baust JM, Vogel MJ, Van Buskirk R, Baust JGA. Molecular Basis of Cryopreservation Failure and Its Modulation to Improve Cell Survival. Cell Transplant. 2001;10(7):561–571. [PubMed] [Google Scholar]
- (87).Jain M, Rajan R, Hyon S-H, Matsumura K. Hydrogelation of Dextran-Based Polyampholytes with Cryoprotective Properties via Click Chemistry. Biomater Sci. 2014;2(3):308–317. doi: 10.1039/c3bm60261c. [DOI] [PubMed] [Google Scholar]
- (88).Armitage WJ, Juss BK. The Influence of Cooling Rate on Survival of Frozen Cells Differs in Monolayers and in Suspensions. Cryo-Letters. 1996;17:213–218. [Google Scholar]
- (89).Maehara M, Sato M, Watanabe M, Matsunari H, Kokubo M, Kanai T, Sato M, Matsumura K, Hyon SH, Yokoyama M, Mochida J, et al. Development of a Novel Vitrification Method for Chondrocyte Sheets. BMC Biotechnol. 2013;13(1):58. doi: 10.1186/1472-6750-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Watanabe H, Kohaya N, Kamoshita M, Fujiwara K, Matsumura K, Hyon SH, Ito J, Kashiwazaki N. Efficient Production of Live Offspring from Mouse Oocytes Vitrified with a Novel Cryoprotective Agent, Carboxylated ε-Poly-L-Lysine. PLoS One. 2013;8(12):e83613. doi: 10.1371/journal.pone.0083613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Kamoshita M, Kato T, Fujiwara K, Namiki T, Matsumura K, Hyon SH, Ito J, Kashiwazaki N. Successful Vitrification of Pronuclear-Stage Pig Embryos with a Novel Cryoprotective Agent, Carboxylated ϵ-Poly-L-Lysine. PLoS One. 2017;12(4):e0176711. doi: 10.1371/journal.pone.0176711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Shibao Y, Fujiwara K, Kawasaki Y, Matsumura K, Hyon S-H, Ito J, Kashiwazaki N. The Effect of a Novel Cryoprotective Agent, Carboxylated ε-Poly-l-Lysine, on the Developmental Ability of Re-Vitrified Mouse Embryos at the Pronuclear Stage. Cryobiology. 2014;68(2):200–204. doi: 10.1016/j.cryobiol.2014.01.008. [DOI] [PubMed] [Google Scholar]
- (93).Matsumura K, Hayashi F, Nagashima T, Hyon SH. Long-Term Cryopreservation of Human Mesenchymal Stem Cells Using Carboxylated Poly-l-Lysine without the Addition of Proteins or Dimethyl Sulfoxide. J Biomater Sci, Polym Ed. 2013;24(12):1484–1497. doi: 10.1080/09205063.2013.771318. [DOI] [PubMed] [Google Scholar]
- (94).Iglesias T, Dusinska M, El Yamani N, Irache JM, Azqueta A, López de Cerain A. In Vitro Evaluation of the Genotoxicity of Poly(Anhydride) Nanoparticles Designed for Oral Drug Delivery. Int J Pharm. 2017;523(1):418–426. doi: 10.1016/j.ijpharm.2017.03.016. [DOI] [PubMed] [Google Scholar]
- (95).Ahmed S, Fujita S, Matsumura K. Enhanced Protein Internalization and Efficient Endosomal Escape Using Polyampholyte-Modified Liposomes and Freeze Concentration. Nanoscale. 2016;8(35):15888–15901. doi: 10.1039/c6nr03940e. [DOI] [PubMed] [Google Scholar]
- (96).Ahmed S, Miyawaki O, Matsumura K. Enhanced Adsorption of a Protein-Nanocarrier Complex onto Cell Membranes through a High Freeze Concentration by a Polyampholyte Cryoprotectant. Langmuir. 2018;34(6):2352–2362. doi: 10.1021/acs.langmuir.7b03622. [DOI] [PubMed] [Google Scholar]
- (97).Hasan M, Fayter AER, Gibson MI. Ice Recrystallization Inhibiting Polymers Enable Glycerol-Free Cryopreservation of Microorganisms. Biomacromolecules. 2018;19(8):3371–3376. doi: 10.1021/acs.biomac.8b00660. [DOI] [PMC free article] [PubMed] [Google Scholar]