Abstract
Background
Attention-Deficit/Hyperactivity Disorder (ADHD) often comorbid with sleep disturbances can produce profound disruption in daily life and impact negatively on quality of life of both the child and family. However, the temporal relationship between ADHD and sleep impairment is unclear, as are underlying common brain mechanisms.
Methods
This study used data from the Quebec Longitudinal Study of Child Development (n=1,601, 52% female) and the Adolescent Brain Cognitive Development Study (n=3,515, 48% female). Longitudinal relationships between symptoms were examined using cross-lagged panel models. Gray matter volume (GMV) neural correlates were identified using linear regression. The transcriptomic signature of the identified brain-ADHD-sleep relationship was characterized by gene enrichment analysis. Confounding factors, such as stimulant drugs for ADHD and social economic status, were controlled for.
Results
ADHD symptoms contributed to sleep disturbances at one or more subsequent time-points in both cohorts. Lower GMVs were associated with both ADHD symptoms and sleep disturbances in the middle frontal gyrus and inferior frontal gyrus, amygdala, striatum, and insula. ADHD symptoms significantly mediated the link between these structural brain abnormalities and sleep dysregulation, and genes differentially expressed in the implicated brain regions, included those involved in neurotransmission and circadian entrainment.
Conclusions
This study indicates that ADHD symptoms and sleep disturbances have common neural correlates, including structural changes of the ventral attention system and fronto-striatal circuitry. Leveraging data from large datasets, these results offer new mechanistic insights into this clinically important relationship between ADHD and sleep impairment, with potential implications for neurobiological models and future therapeutic directions.
Keywords: ADHD, dyssomnia, parasomnia, neurodevelopmental, development, longitudinal study
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is the most prevalent neurodevelopmental disorder in children, and persists into adulthood in 50-66% of cases (1–3). Sleep disturbances, composed of two main classifications (i.e. dyssomnia and parasomnia) (4), are extremely common, occurring in 25-55% of children with ADHD (5), and are associated with poor outcomes (6–8). Left untreated, such symptoms lead to untoward functional consequences in young people, including worse academic outcomes (9–11). Both ADHD symptoms and sleep problems can produce profound disruption in daily life and impact negatively on quality of life of both the child and family (12–14). ADHD symptoms such as hyperactivity can lead to longer sleep onset latency, more night awakening and lower sleep quality (15), but disrupted sleep can also impair daytime attention, overlapping with the core symptoms of ADHD (16). Thus, these two conditions could mutually exacerbate each other (17). This complex relationship is of particular concern during childhood and adolescence when brain structures and associated functions are undergoing significant developmental changes (18–20). Both conditions have separately been linked to aberrant brain development (21–25). Therefore, greater knowledge of the temporal and neurobiological relationships between these two conditions is of considerable clinical and public health importance (26).
At the behavioral level, studies have reported associations between ADHD symptoms and sleep disturbance (27–30), albeit most of this research has been cross-sectional. Furthermore, these studies often did not control for stimulant medication, nor examine brain substrates. At the neuroanatomical level, a wide range of brain abnormalities have been associated with ADHD (31), including structural abnormalities in both subcortical and cortical regions (32, 33). In studies not considering ADHD symptoms, neuroanatomical correlates of sleep disturbances in patients with insomnia have also been identified in overlapping cortico-striatal circuitry (34–37). These findings often varied across studies, owing to both the heterogeneous nature of these conditions and methodological inconsistencies (e.g. age group recruited, symptom measurements used) (38, 39). Nevertheless, one hypothesis is that delayed development of the cognitive control system (fronto-striatal circuit) coupled with lower daytime arousal, implicating the salience/ventral attention pathway, contribute to these problems (40–44). While it has been hypothesized that common structural abnormalities of the fronto-striatal and salience/ventral attention networks contribute to both conditions, this hypothesis has yet to be tested (45).
At the molecular level, ADHD has been associated with neurotransmitter systems especially dopamine (46–48) and norepinephrine (49–51), which also play an important role in sleep regulation (52–54). For example, the locus coeruleus noradrenergic system, with widely projecting noradrenergic axons from brainstem to the central nervous system (e.g. hippocampus and neocortex), has been implicated in both attention and arousal (55). Dysregulation of this system has been hypothesized to be involved not only sleep disturbances, but also the pathophysiology of ADHD (56). Therefore, investigating the interplay between sleep disturbances and ADHD symptoms may be particularly informative to shed new light on this hypothesis.
To our knowledge, no study to date has identified common brain abnormalities in both ADHD and sleep disturbance, using large-scale datasets. Therefore, the objectives of the present study were: (i) to uncover the temporal relationship between ADHD symptoms and sleep disturbances; (ii) to identify the common neuroanatomical association shared between both symptom types; (iii) to quantify the extent to which the identified neuroanatomical association was mediated through ADHD; and (iv) to examine the gene expressions of which biological processes or functional pathways are associated with the mediation effect. To achieve these goals, we used three datasets: a longitudinal cohort of child development (n=1,601), a neuroimaging cohort with a longitudinal design (n=3,515), and an "all genes, all structures" gene expression survey in human brains (3,702 samples with >62,000 gene probes per profile). We hypothesized that ADHD symptoms would contribute to subsequent sleep disturbances; that reduced gray matter volumes (GMV) of fronto-striatal and salience/ventral attention pathways would be common to both conditions; and that brain gene expression regulating the neurochemical systems above (dopamine, norepinephrine) would be related to mediating effects between brain structure and symptoms. Specifically, we postulated mechanistically that changes in brain structure and gene expression would contribute to ADHD, which in turn would contribute to sleep disturbance.
Methods and Materials
Participants and behavioral measures
We used data from the Quebec Longitudinal Study of Child Development (QLSCD) (57) as the discovery dataset for longitudinal analysis. Participants with at least one observation of ADHD symptoms or sleep disturbances at age 7, 8, 10, 12 and 13 years were included in the present study (n=1,601). ADHD symptoms (total score, hyperactivity-impulsivity score, and inattention score) were measured using the teacher-rated Social Behavior Questionnaire (58). Sleep disturbances were assessed by seven questions, which were answered by the mother (i.e. daytime sleepiness, sleep talking, sleep walking, night terror, nightmare, bruxism, and enuresis). The protocol of QLSCD was approved by the Quebec Institute of Statistics (Quebec City, Quebec, Canada) and the St-Justine Hospital Research Center (Montreal) ethics committees. Written informed consent was obtained from all the participating families at each assessment. This cohort focused on behavioral measures and not brain imaging (Method S1).
The Adolescent Brain Cognitive Development (ABCD) Study is tracking the brain development and health of over 10,000 children aged 9 to 11 years from 21 centers throughout the United States (https://abcdstudy.org). These centers obtained parents’ full written informed consent and all children’s assent, and research procedures and ethical guidelines were followed in accordance with the Institutional Review Boards. We used data from 3,515 subjects for whom both completed behavioral and magnetic resonance imaging (MRI) data were available at baseline, and 3,076 of them had the one-year follow-up data available (Method S2). ADHD symptoms were measured using the parent-reported DSM-Oriented Attention Problem Scale of the Child Behavior Checklist (59). Sleep disturbances rated using the parent Sleep Disturbance Scale for Children (60) were further summarized into two dimensions: dyssomnias (disorders of initiating and maintaining sleep, sleep breathing disorders and disorders of excessive somnolence) and parasomnias (disorders of arousal, sleep-wake transition disorders and sleep hyperhidrosis) (61).
Structural MRI data
In ABCD, 3D T1-weighted images were collected using 3T scanners at 21 data collecting sites. The detailed MRI acquisition protocol is described elsewhere (62). We obtained minimally preprocessed MRI data using the ABCD Pipeline (https://abcdstudy.org/scientists-protocol.html), and voxel-based morphometry analysis was conducted using the Computational Anatomy Toolbox (CAT12; http://dbm.neuro.uni-jena.de/cat) and Statistical Parameter Mapping software version 12 (SPM12; http://www.fil.ion.ucl.ac.uk/spm). Briefly, images were segmented into gray matter (GM), white matter, and cerebrospinal fluid based on tissue probability maps for age 9 to 11 produced by the TOM8 Toolbox (https://irc.cchmc.org/software/tom.php). Next, images were normalized to the MNI space using the DARTEL and Geodesic Shooting approach. The registered GM images were multiplied with the Jacobian determinants derived from the spatial normalization, and then smoothed with an 8 mm full width at half maximum Gaussian kernel with the resulting voxel size 1.5mm3. Finally, we calculated the mean image of all these smoothed GM images and focused our following analyses within a mask of GM by retaining only those voxels with more than 10% GM tissue.
Transcriptomic data
We used the transcriptomic data from six neurotypical adult brains in the Allen Human Brain Atlas (63) (AHBA; https://human.brain-map.org). Since right hemisphere data were only available for two of the six donors in AHBA, we used samples in the left hemisphere only. We followed the preprocessing pipeline recommended by Arnatkevičiūtė et al. (64), including probe-to-gene re-annotation, intensity-based data filtering, probe selection by mean, separating tissue samples into subcortical and cortical regions based on the Harvard-Oxford atlas (65), and within-donor normalization, finally resulting 15,408 unique genes (Method S3).
Statistical analysis
Cross-lagged panel analysis
In QLSCD, the longitudinal associations between ADHD total score and sleep disturbance were examined using a random-intercepts cross-lagged panel model (RI-CLPM) (66). Compared with the traditional CLPM, RI-CLPM requires at least three data waves, and more closely approximates causal inference by separating the within-person process from stable between-person differences through the inclusion of random intercepts (67). We followed the two analytical steps in (68). First, the standard RI-CLPM was estimated; then we examined the contribution of covariates (i.e. sex, social economic status [SES] and ADHD medication at age 7) to the between-person factors. We also performed RI-CLPMs between sleep and ADHD symptom dimensions (i.e. hyperactivity-impulsivity and inattention). We conducted a false discovery rate (FDR) correction for the 16 between-wave associations examined (i.e. 8 auto-regressive and 8 cross-lagged paths).
To provide more supporting evidence using an independent cohort, we conducted traditional CLPMs for ADHD symptoms and each of the three sleep disturbance scores (i.e. total score, dyssomnia and parasomnia) in ABCD. We controlled for several stable variables (i.e. sex, race and site) and time-variant parameters (i.e. ADHD medication, household income, educational level of parents, BMI and puberty) in these models. Accounting for the family relatedness (i.e. the records of single, sibling, twin and triplet provided in a questionnaire and also the kinship reconstructed from the genetic data; Method S4), the statistical significance, denoted by Pperm, was established by conducting 5,000 times multi-level block permutations (69). We compared the strength between the sleep→ADHD path and the ADHD→sleep path by the Wald test. To test whether the findings were robust across data collection sites, we conducted a meta-analysis of the significant cross-lagged coefficient identified above (Method S5). The model parameters were estimated by the full information maximum likelihood method (70), and the model fit was interpreted using common thresholds of good fit (71).
Whole-brain and voxel-wise analysis (ABCD cohort)
A linear regression model was conducted to investigate the relationship between GMVs and ADHD symptoms at baseline, using age, sex, handedness, race, puberty, BMI, site, household income, parental education, head motion and total intracranial volume (TIV) as covariates of no interest. We conducted a multi-level block permutation-based cluster-level correction (5,000 times) for multiple comparisons in the neuroimaging analysis (69, 72, 73) (Method S4). At voxel level, we used a two-sided test with a significance level of α=0.001, whereas, at cluster level, we used a permutation-based family-wise error (FWE) correction with α=0.05. Similarly, we examined the GMV correlates with the total sleep disturbance score (pFWE<0.05), and also tested such correlations for two dimensions (i.e. dyssomnia and parasomnia, pFWE<0.025). Significant overlapping GMVs of ADHD and sleep were defined on the basis of a cluster having more than 217 voxels falling into the 90% confidence interval of the smoothing kernel) voxels (74).
Mediation analysis
As the directional association between ADHD symptoms and sleep disturbances was determined by the CLPM, we assessed the mediation effect of ADHD on the association between sleep and the overlapping clusters identified above. The analyses were performed using the mediation toolbox developed by Tor Wager (75) (http://wagerlab.colorado.edu/tools) with 10,000 bootstraps.
Furthermore, we conducted a whole-brain and voxel-wise exploratory analysis of this mediation effect with 3,000 bootstraps at each voxel and FDR correction among all voxels. We additionally required a significant GMV-sleep association (p<0.005, two-sided, uncorrected). The unthresholded bootstrap-based t map of the mediation effect was further used in the following analyses.
Transcriptomic analysis
We used partial least square (PLS) regression to relate the mediation effect to the gene expression data in AHBA (76–79). The response variable was an n×1 matrix, which was calculated by the average t value of a spherical region of interests (ROI; r=4mm) centered by the MNI coordinates of each gene expression sampling site (80). The predictor variable was an n (number of tissue samples) × 15,408 (number of genes) matrix. AHBA provided 182 tissue samples in left subcortical regions, and 784 in left cortical regions. Using a permutation test (5,000 times), we selected those PLS components that explained more variance of the mediation effect than could be accounted for by chance (79, 81). The first PLS component (PLS1) was the linear combination of the weighted gene expression scores, maximizing the covariance between the expression profile and the mediation profile in the brain. A Z score was calculated for each weight in a PLS component as the ratio between each weight estimation and the standard error (SE) given by 5,000 bootstraps. Therefore, the genes could be ranked by their normalized contributions to the PLS component. We adapted the codes for PLS provided by (76, 79). Leave-one-out cross-validation (i.e. repeating the analysis by leaving 1 donor out at a time) was used to test the influence of individual donors on the results, and PLS analyses using 6-mm ROIs or a refined brain atlas (78, 82) were also performed and compared to ensure the findings were not dependent on a particular definition of ROI size.
We used GSEA Pre-ranked (version 6.0.12) (83) with default settings to identify sets of genes associated with Gene Ontology (GO) terms of biological processes and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. The two lists of genes (n=15,408) for subcortical and cortical regions were ranked by Z score and passed to GSEA Pre-ranked. From the top positively and negatively correlated genes in each list, we obtained separate sets of enriched gene sets (S+ and S–, respectively). To demonstrate the robustness of the findings, we also applied a more stringent threshold (i.e. top 1% and bottom 1% genes based on Z score) to identify significant enrichments using DAVID 6.8 (84). Gene sets were considered significantly enriched with FDR q-values<0.05.
Results
Demographics
In QLSCD, 1,601 participants (829 [52%] female) with behavioral measurements that were longitudinally collected at the ages of 7, 8, 10, 12, and 13 years old were entered into the following analyses (Table 1). In ABCD, 3,515 participants (1,664 [48%] female, aged 10±0.61 years) who had complete both MRI data and behavioral measurements at baseline were used in the current study, of whom 3,076 had complete behavioral assessments at a 1-year follow-up (aged 11.03±0.63 years; Table 2).
Table 1. Characteristics of the study population in QLSCD.
| Wave 1 | Wave 2 | Wave 3 | Wave 4 | Wave 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | |
| Male, No. (%) | 772 (48.2%) | 1,601 | ||||||||
| Age (year) | 7.15 (0.25) | 1,468 | 8.15 (0.26) | 1,421 | 10.15 (0.26) | 1,295 | 12.14 (0.25) | 1,353 | 13.14 (0.26) | 1,252 |
| ADHD Medication, No. (%) | 56 (3.8%) | 1,468 | ||||||||
| SES | -0.01 (1.00) | 1,467 | ||||||||
| ADHD total score | 2.64 (2.56) | 1,303 | 2.55 (2.43) | 1,267 | 2.31 (2.37) | 986 | 2.13 (2.21) | 1,004 | 2.52 (2.56) | 992 |
| H-I score | 2.05 (2.52) | 1,302 | 1.90 (2.40) | 1,266 | 1.62 (2.33) | 986 | 1.39 (2.09) | 999 | 1.48 (2.39) | 937 |
| IN score | 3.79 (3.39) | 1,303 | 3.77 (3.32) | 1,281 | 3.63 (3.27) | 987 | 3.57 (3.32) | 1,004 | 3.76 (3.25) | 1,023 |
| Sleep disturbance | 0.23 (0.25) | 1,601 | 0.26 (0.25) | 1,601 | 0.32 (0.24) | 1,062 | 0.29 (0.22) | 1,188 | 0.28 (0.22) | 832 |
Abbreviation: SES, socioeconomic status. H-I, hyperactivity-impulsivity. IN, inattention.
Table 2. Characteristics of the study population in ABCD.
| Baseline (n=3,515) | Follow-up (n=3,076) | |
|---|---|---|
| Age (year) | 10.00 (0.61) | 11.03 (0.63) |
| Male, No. (%) | 1,851 (52.7%) | 1,610 (52.3%) |
| Puberty | 1.56 (0.46) | 1.79 (0.59) |
| BMI | 18.59 (4.00) | 19.45 (4.52) |
| Parental education | 17.01 (2.47) | 17.16 (2.33) |
| Household income | 7.52 (2.19) | 7.76 (2.06) |
| Race, No. (%) | ||
| White | 2,554 (72.7%) | 2,307 (75%) |
| Black/African American | 327 (9.3%) | 244 (7.9%) |
| Asian | 182 (5.2%) | 155 (5%) |
| Other | 452 (12.9%) | 370 (12%) |
| Family relationship a | ||
| Single | 2,680 (76.2%) | 2,346 (76.3%) |
| Sibling | 196 (5.6%) | 166 (5.4%) |
| Twin | 630 (17.9%) | 558 (18.1%) |
| Triplet | 9 (0.3%) | 6 (0.2%) |
| ADHD symptom | 2.54 (2.91) | 2.36 (2.86) |
| ADHD medication, No. (%) | ||
| No medication | 3,204 (91.2%) | 2,820 (91.7%) |
| Stimulant only | 233 (6.6%) | 193 (6.3%) |
| Non-stimulant only | 31 (0.9%) | 27 (0.9%) |
| Stimulant + non-stimulant | 46 (1.3%) | 36 (1.2%) |
| Sleep disturbance | ||
| Total score | 36.24 (7.87) | 36.41 (7.75) |
| Dyssomnia | 22.19 (5.49) | 22.66 (5.67) |
| Parasomnia | 14.05 (3.58) | 13.75 (3.32) |
Descriptive statistics is mean (SD) unless noted otherwise.
Abbreviation: BMI, body mass index
provided by a questionnaire (“acspsw02”) from ABCD dataset.
ADHD symptoms contributed to sleep disturbance in school-aged children
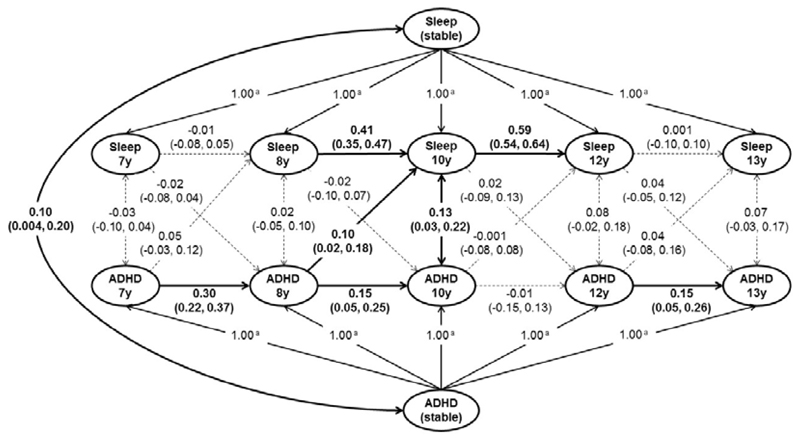
To test the directionality, if any, between ADHD symptoms and sleep disturbances, we conducted a longitudinal analysis using the QLSCD cohort. We found a between-person and time-invariant association between ADHD total score and sleep disturbance (β=0.10, 95%CI [0.004, 0.20]). In the within-person and dynamic component of the model, we found that higher ADHD total score at age 8 was associated with the worse sleep disturbance at age 10 (noted as ADHD8y→sleep10y; β=0.10, 95%CI [0.02, 0.18], FDR q<0.05; Table S1 and Figure 1). Considering the covariates (i.e. sex, SES and ADHD medication), ADHD8y→sleep10y remained significant (β=0.11, 95%CI [0.03, 0.19], FDR q=0.02; Figure S1). Both additional analyses using the participants without ADHD medication only (n=1,095; β=0.09, 95%CI [0.004, 0.18]) and using the participants who had data at both ages 8 and 10 years only (n=1,263; β=0.09, 95%CI [0.01,0.18]; Result S1) confirmed the significance of ADHD8y→sleep10y. Similar results held for ADHD subscales (i.e. hyperactivity-impulsivity symptom and inattention symptom; Figures S2-3).
Figure 1. Cross-lagged analysis between ADHD and sleep disturbance in QLSCD.
Random-intercepts cross-lagged panel model of ADHD total score and sleep disturbance from ages 7 to 13 years in QLSCD (n=1,601). Standardized estimates (95%CIs) are presented. Solid lines represent statistically significant (p<0.05), whereas dashed lines present non-significant (p>0.05). Model fit: root mean square error of approximation (RMSEA)=0.04; comparative fit index (CFI)=0.98; Tucker-Lewis index (TLI)=0.96; standardized root mean square residual (SRMR)=0.04.
a Pathways constrained to 1.00 to isolate between-person factor.
Using the ABCD cohort, we found more supporting evidence that ADHD symptoms at age 10 were also associated with sleep disturbances at a 1-year follow-up (sleep total score: β=0.09, 95%CI [0.06, 0.13], Pperm<0.001; Figure S4A), and the meta-analysis across all data collection sites showed that this association was also robust (meta-β=0.09, 95%CI [0.04, 0.13]). In contrast, although the path coefficient of the opposite direction was significant in this large cohort, its effect size was weaker (p<0.005 by Wald test) and not robust (Figure S5A-B). Again, both additional analyses using the participants without ADHD medication only (n=2,902; β=0.04, 95%CI [0.01, 0.08]) and using the participants who were diagnosed as ADHD at baseline (n=281; β=0.10, 95% CI [0.01, 0.20]; Result S2) confirmed the significance of ADHD→sleep. Similar results held for sleep subscales (i.e. dyssomnia and parasomnia; Figure S4-5).
Shared neural correlates between ADHD symptoms and sleep disturbances
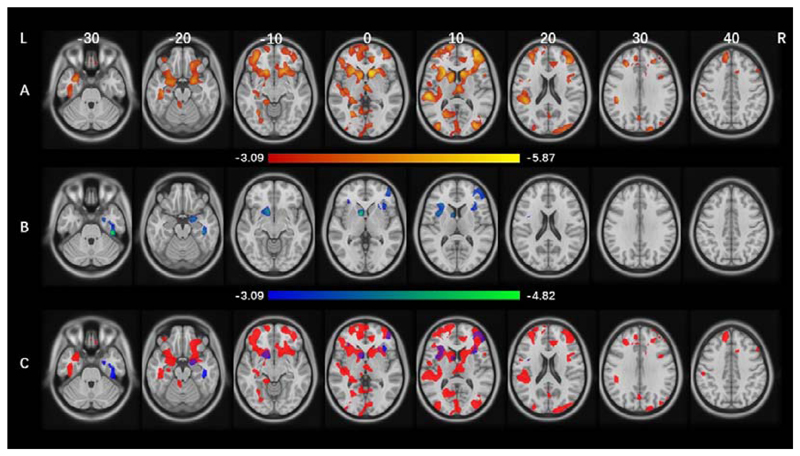
To test the hypothesis whether ADHD symptoms and sleep disturbances share common neural correlates, we conducted a neuroimaging analysis using the ABCD cohort at baseline. We found that ADHD symptoms were associated with lower GMV in two brain clusters (Figure 2A, Tables S2-3, Figures S6-7A), while only the dyssomnia subscale was associated with lower GMV in three brain clusters (Figure 2B, Tables S3-4, Figure S7B). Among these clusters, we found three overlapping areas, including in the bilateral insula, left caudate and putamen (2,762 voxels), in the right middle frontal gyrus and inferior frontal gyrus (2,296 voxels), and in the right parahippocampus, hippocampus and amygdala (419 voxels) (Figure 2C, Figure S7C).
Figure 2. Significant brain clusters associated with ADHD symptoms and dyssomnia in ABCD at baseline.
Multiple comparison correction includes voxel-level p<0.001 and cluster-level pFWE<0.05 estimated by a multi-level block permutation accounting for family relatedness. The color bar represents t-value. Age, sex, handedness, race, puberty, BMI, site, household income, parental education, head motion and TIV were controlled for in all analyses. A. Brain regions significantly associated with ADHD symptoms. B. Brain regions significantly associated with dyssomnia. C. Brain regions significantly associated with ADHD or dyssomnia. Red areas are associated with ADHD, blue areas are associated with dyssomnia, and purple areas are the overlapping regions.
Mediation analysis: The identified neural correlates contributed to both ADHD symptoms and dyssomnia
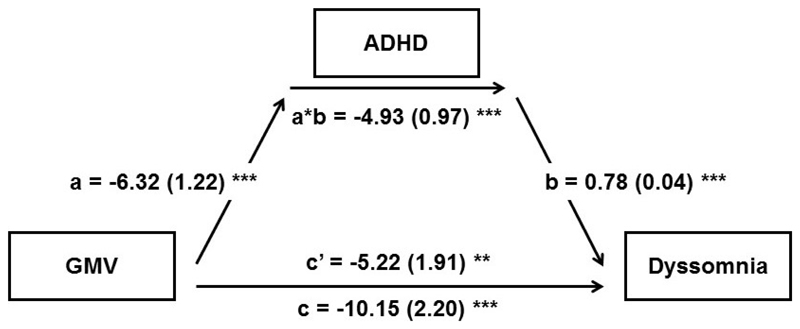
Having identified that ADHD contributed to subsequent sleep problems in the longitudinal datasets, we were then interested in examining whether ADHD statistically contributed to anatomical brain changes linked with sleep problems cross-sectionally. Thus, we conducted a mediation analysis to quantify the brain→ADHD→sleep relationship. We found that 48.6% (95%CI [31.7%, 75.9%]) of the association between lower average GMV of the three overlapping areas identified above and higher dyssomnia was mediated by ADHD symptoms (Figure 3; the mediation effect of each area was between 45.2% and 49.7%, Figure S8). Controlling for ADHD medication, this mediation effect remained significant (44.2%, 95%CI [27.3%,77.2%]; path a*b: -3.92, 95%CI [-5.64, -2.35]). The whole-brain exploratory analysis of the mediation effect identified a significant cluster that covered the overlapping areas identified above (Figure S9). We also confirmed that ADHD symptoms at baseline significantly mediated the association between the baseline GMV and the follow-up dyssomnia (Figure S10).
Figure 3. Associations of the average overlapping gray matter volume, ADHD symptoms and dyssomnia.
Mediation model using the average overlapping GMV as the predictor, ADHD as the mediator and dyssomnia as the dependent variable. Age, sex, handedness, race, puberty, BMI, site, household income, parental education, head motion and TIV were used as covariates of no interest. Path a measures the association between the predictor and the mediator; path b represents the effect of the mediator on the dependent variable while controlling for the predictor; path c measures the total relationship between the predictor and the dependent variable; path c’ measures the direct effect; the mediation effect is the product of path a and path b (a*b).
*p<0.05, **p<0.01, ***p<0.001.
Relationship between the mediation effect and brain gene-expression profiles
Given that the strength of the brain→ADHD→sleep relationship had a heterogeneous spatial distribution among different brain areas, we further conducted a transcriptomic analysis to identify its association with the gene expressions in brain tissues. In the model for the subcortical regions, only the PLS1 was significant (i.e. explained 30% of the variance of the mediation effect, p<0.001 by permutation; additional details and validations in Results S3-6). After the FDR correction, the S– gene sets in subcortical regions were enriched in 104 relevant biological processes (Table S7) and 26 relevant KEGG pathways (Tables S8). Enriched biological processes included “chemical synaptic transmission”, while enriched KEGG pathways included “circadian entrainment” and “dopaminergic synapse”. These findings were also enriched in the top 1% gene set (Table S6). More enrichment findings of the S+ gene sets and the cortical regions were listed in Tables S9-14.
Discussion
This study demonstrated that the cross-lagged association of ADHD at baseline with sleep disturbance at follow-up was stronger than the cross-lagged association in the opposite direction. Neuroimaging analysis revealed that smaller volumes mainly in the cognitive control system and the salience/ventral attention system, constituted a common neurobiological substrate linking both ADHD and sleep disturbance. Among the subcortical structures, we identified a number of genes with higher expression levels in those brain areas where a greater proportion of the brain-sleep association was mediated by ADHD. These genes included those playing key roles in dopamine signaling and in the circadian cycle. The findings are in keeping with our hypothesis that changes in brain structure and gene expression contribute to ADHD, which in turn lead to sleep disturbance.
To our knowledge, this is the first study using RI-CLPM (random-intercepts cross-lagged panel model) to address the longitudinal associations between ADHD with sleep disturbances. RI-CLPM offers potential advantages over other statistical approaches, such as providing closer approximation of causal inference (67). Although cross-sectional associations between ADHD and sleep disturbances have been reported, little is known about the longitudinal relationships. Most longitudinal studies examined unidirectional associations only, such as early sleep patterns predicting later ADHD symptoms (85, 86); or childhood ADHD symptoms being associated with adulthood sleep quality (87). In the QLSCD cohort, we found the strength of the ADHD→sleep relationship peaked between ages 8 and 10 years. However, this finding did not necessarily suggest an age-restricted relationship. For example, the ADHD symptoms at age 10 might indirectly influence the sleep disturbances at age 12 via elevating the sleep disturbances at age 10 (Figure 1).
We found that ADHD symptoms and dyssomnia were associated with common reductions of GM in the right frontal gyrus, bilateral insula, left striatum, right amygdala and hippocampus. Structural abnormalities of these regions have previously been reported separately in ADHD (88–90) and dyssomnia (35, 36, 91–93). Our findings are in keeping with prior data, but crucially extend beyond it, to identify common underpinnings of these two related pathologies, by leveraging a large dataset (94). These neural regions play a cardinal role in high-level cognitive functions (fronto-striatal circuitry) (95) and in the salience/ventral attention system (96).
Cognitive domains contingent on the fronto-striatal circuitry are often impaired in ADHD (97) and in people with sleep disturbances (98). The striatum is connected to prefrontal cortex (99, 100), and is particularly implicated in ADHD (101), as well as being important for sleep-wake regulation (102), and arousal (35). Disturbances in the maturation of such fronto-striatal circuitry may contribute to cognitive problems often found in ADHD, such as difficulties in self-regulation (103), cognitive control (104), and reward processing (105). Notably, attention and arousal closely interact with each other (106), and their interaction has been hypothesized to mainly located at the salience/ventral attention pathway. Our findings are in keeping with disruption of these pathways being associated with ADHD and sleep disturbances, particularly with regards to the right inferior frontal cortex, right temporoparietal junction, right middle frontal gyrus, anterior insular cortex (106–108). Such ADHD-related brain abnormalities have been posited to reflect delayed development of fronto-striatal circuitry underlying cognitive control (109, 110).
Notably, our findings provide novel insights into neurobiological mechanisms contributing to the relationship between ADHD symptoms and sleep disturbances. Specifically, we demonstrated that lower GMV in key brain regions was associated with ADHD and dyssomnia; and that 40% of this neuroanatomical association was mediated by ADHD’s impact on sleep. Furthermore, our enrichment analysis of this mediation effect highlighted several overlapping pathways (Figure S15), including the circadian entrainment pathway and neural signaling pathways (e.g. the chemical synaptic transmission, dopaminergic synapse, glutamatergic synapse). Particularly, the neuroanatomical associations of sleep disturbances were mediated to a greater extent by ADHD symptoms in those subcortical regions with a higher gene expression levels of these pathways (Figure S16). Therefore, if a given ADHD treatment targets one of these pathways, it might reduce the ADHD-component in sleep disturbances (111). However, approximately half of the brain-sleep association was not mediated by ADHD symptoms, suggesting that not all GMV reductions common to ADHD and dyssomnia stem from ADHD itself. This suggests additional sleep management strategies are needed from a treatment perspective, even though treating ADHD itself may lead to sleep improvements, provided such treatments do not have their own deleterious effects (112).
There are several limitations in our study. Using two large longitudinal cohorts, we observed a significant temporal relationship between ADHD symptoms and subsequent sleep disturbances in school-aged children. This relationship was greatest between ages 8 and 10 years in QLSCD and between ages 10 and 11 years in ABCD. There are several possible reasons for this cohort difference, including: 1) As these two cohorts were collected 10 years apart (i.e. the QLSCD children were born in 1997-98, while the ABCD children were born in 2007-08), the same chronological age may not reflect the same pubertal stage in these two cohorts; 2) Given the significant development during adolescence, it is also possible that a 1-year follow-up after 10 years old can be different from a 2-year follow-up after the same age. It is possible that future longitudinal studies could directly investigate these points.
Conclusion
Analysis of two large longitudinal cohorts combined with the largest neuroimaging cohort of school-aged children, to date, revealed a strong ADHD-driven effect on subsequent sleep disturbance; and also identified common neuroanatomical correlates of both ADHD symptoms and sleep disturbances. We found that ADHD substantially mediated common neuroanatomical changes linked with both problems, highlighting the need to develop precision treatment approaches that integrate multimodal approaches, in order to mitigate both ADHD symptoms and sleep disturbances.
Supplementary Material
Acknowledgments and Disclosures
Dr Luo was supported by the National Natural Science Foundation of China (grant 81873909 and 81930095), Natural Science Foundation of Shanghai (grant 17ZR1444400). During the preparation of the manuscript, Dr Luo was a Visiting Fellow at Clare Hall, Cambridge, UK. Dr Chamberlain’s role in this project was funded by a Clinical Fellowship from the Wellcome Trust (reference 110049/Z/15/Z). Dr Zhao was supported by the National Natural Science Foundation of China (grant 61932008, 61772368) and National Key Research and Development Program of China (2018YFC0910500). Dr Morgan holds a Henslow Fellowship at Lucy Cavendish College, University of Cambridge, funded by the Cambridge Philosophical Society. Dr Feng was supported by the 111 project (grant B18015), the Key Project of Shanghai Science &Technology (grant 16JC1420402), Shanghai Municipal Science and Technology Major Project (grant 2018SHZDZX01), Zhangjiang Lab, National Key Research and Development Program of China (grant 2018YFC1312900) and National Natural Science Foundation of China (grant 91630314). This research was also supported by the NIHR Cambridge Biomedical Research Centre. In particular, we wish to thank Dr Simon R White for statistical advice and support. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
We thank the children and families whose ongoing participation made this study possible. We also acknowledge the considerable contribution of the coordinators of the Quebec Longitudinal Study of Child Development and the Quebec Institute of Statistics and the tireless work of all the interviewers who assessed the mothers and children during this study. The Quebec Longitudinal Study of Child Development was made possible thanks to the funding provided by the Fondation Lucie et André Chagnon, the Institut de la Statistique du Québec, the Ministère de l’Éducation et de l’Enseignement supérieur, the Ministère de la Famille, the Institut de recherche Robert-Sauvé en santé et en sécurité du travail, the Centre hospitalier universitaire Sainte-Justine, and the Ministère de la Santé et des Services sociaux du Québec. Source: Data compiled from the final master file ‘E1-E20’ from the Quebec Longitudinal Study of Child Development (1998-2017), ©Gouvernement du Québec, Institut de la statistique du Québec. Part of data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9-10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/nih-collaborators. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from National Institute of Mental Health data (DOI: 10.15154/1460410). The codes used in this paper are available at the following website: https://github.com/qluo2018/FamilyPermutationABCD.
Dr Chamberlain consults for Promentis, and Ieso Digital Health. Dr Chamberlain receives a stipend from Elsevier for editorial journal work (Neuroscience & Biobehavioral Reviews; and Comprehensive Psychiatry). Dr. Sahakian consults for Cambridge Cognition, Greenfield BioVentures and Cassava Sciences. The other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Polanczyk G, De Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 2.Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol. 2002;111:279–289. [PubMed] [Google Scholar]
- 3.Lara C, Fayyad J, De Graaf R, Kessler RC, Aguilar-Gaxiola S, Angermeyer M, et al. Childhood predictors of adult attention-deficit/hyperactivity disorder: results from the World Health Organization World Mental Health Survey Initiative. Biol Psychiatry. 2009;65:46–54. doi: 10.1016/j.biopsych.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petit D, Touchette E, Tremblay RE, Boivin M, Montplaisir J. Dyssomnias and parasomnias in early childhood. Pediatrics. 2007;119:1016–1025. doi: 10.1542/peds.2006-2132. [DOI] [PubMed] [Google Scholar]
- 5.Lunsford-Avery JR, Krystal AD, Kollins SH. Sleep disturbances in adolescents with ADHD: A systematic review and framework for future research. Clin Psychol Rev. 2016;50:159–174. doi: 10.1016/j.cpr.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Z, Lichtenstein P, D'Onofrio BM, Sjolander A, Larsson H. Serious transport accidents in adults with attention-deficit/hyperactivity disorder and the effect of medication: a population-based study. JAMA Psychiatry. 2014;71:319–325. doi: 10.1001/jamapsychiatry.2013.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mick E, Biederman J, Jetton J, Faraone SV. Sleep Disturbances Associated with Attention Deficit Hyperactivity Disorder: The Impact of Psychiatric Comorbidity and Pharmacotherapy. J Child Adolesc Psychopharmacol. 2000;10:223–231. doi: 10.1089/10445460050167331. [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb DJ, Ellenbogen JM, Bianchi MT, Czeisler CA. Sleep deficiency and motor vehicle crash risk in the general population: a prospective cohort study. BMC Med. 2018;16:44. doi: 10.1186/s12916-018-1025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loe IM, Feldman HM. Academic and educational outcomes of children with ADHD. J Pediatr Psychol. 2007;32:643–654. doi: 10.1093/jpepsy/jsl054. [DOI] [PubMed] [Google Scholar]
- 10.Efron D, Sciberras E, Anderson V, Hazell P, Ukoumunne OC, Jongeling B, et al. Functional status in children with ADHD at age 6–8: a controlled community study. Pediatrics. 2014;134:992–1000. doi: 10.1542/peds.2014-1027. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 12.Danckaerts M, Sonuga-Barke EJ, Banaschewski T, Buitelaar J, Dopfner M, Hollis C, et al. The quality of life of children with attention deficit/hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry. 2010;19:83–105. doi: 10.1007/s00787-009-0046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin J, Hiscock H, Hardy P, Davey B, Wake M. Adverse associations of infant and child sleep problems and parent health: an Australian population study. Pediatrics. 2007;119:947–955. doi: 10.1542/peds.2006-2569. [DOI] [PubMed] [Google Scholar]
- 14.Dey M, Castro RP, Haug S, Schaub MP. Quality of life of parents of mentally-ill children: a systematic review and meta-analysis. Epidemiology and psychiatric sciences. 2019;28:563–577. doi: 10.1017/S2045796018000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker SP, Langberg JM, Eadeh HM, Isaacson PA, Bourchtein E. Sleep and daytime sleepiness in adolescents with and without ADHD: differences across ratings, daily diary, and actigraphy. Journal of Child Psychology and Psychiatry. 2019 doi: 10.1111/jcpp.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawyer AC, Clark CR, Keage HA, Moores KA, Clarke S, Kohn MR, et al. Cognitive and electroencephalographic disturbances in children with attention-deficit/hyperactivity disorder and sleep problems: New insights. Psychiatry Res. 2009;170:183–191. doi: 10.1016/j.psychres.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Hvolby A. Associations of sleep disturbance with ADHD: implications for treatment. Atten Defic Hyperact Disord. 2015;7:1–18. doi: 10.1007/s12402-014-0151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowley SJ, Wolfson AR, Tarokh L, Carskadon MA. An update on adolescent sleep: New evidence informing the perfect storm model. J Adolesc. 2018;67:55–65. doi: 10.1016/j.adolescence.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colrain IM, Baker FC. Changes in sleep as a function of adolescent development. Neuropsychol Rev. 2011;21:5–21. doi: 10.1007/s11065-010-9155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- 21.Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D. Development of Cortical Surface Area and Gyrification in Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2012;72:191–197. doi: 10.1016/j.biopsych.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jan JE, Reiter RJ, Bax MC, Ribary U, Freeman RD, Wasdell MB. Long-term sleep disturbances in children: a cause of neuronal loss. Eur J Paediatr Neurol. 2010;14:380–390. doi: 10.1016/j.ejpn.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Feinberg I, Campbell IG. Longitudinal sleep EEG trajectories indicate complex patterns of adolescent brain maturation. Am J Physiol Regul Integr Comp Physiol. 2013;304:R296–303. doi: 10.1152/ajpregu.00422.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Telzer EH, Goldenberg D, Fuligni AJ, Lieberman MD, Galvan A. Sleep variability in adolescence is associated with altered brain development. Dev Cogn Neurosci. 2015;14:16–22. doi: 10.1016/j.dcn.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon SYR, Jain U, Shapiro C. Sleep in attention-deficit/hyperactivity disorder in children and adults: past, present, and future. Sleep Med Rev. 2012;16:371–388. doi: 10.1016/j.smrv.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Owens JA, Maxim R, Nobile C, McGuinn M, Msall M. Parental and self-report of sleep in children with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2000;154:549–555. doi: 10.1001/archpedi.154.6.549. [DOI] [PubMed] [Google Scholar]
- 28.Cortese S, Faraone SV, Konofal E, Lecendreux M. Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. J Am Acad Child Adolesc Psychiatry. 2009;48:894–908. doi: 10.1097/CHI.0b013e3181ac09c9. [DOI] [PubMed] [Google Scholar]
- 29.Paavonen EJ, Raikkonen K, Lahti J, Komsi N, Heinonen K, Pesonen AK, et al. Short sleep duration and behavioral symptoms of attention-deficit/hyperactivity disorder in healthy 7- to 8-year-old children. Pediatrics. 2009;123:857–864. doi: 10.1542/peds.2008-2164. [DOI] [PubMed] [Google Scholar]
- 30.Didriksen M, Thorner LW, Erikstrup C, Pedersen OB, Paarup HM, Petersen M, et al. Self-reported restless legs syndrome and involuntary leg movements during sleep are associated with symptoms of attention deficit hyperactivity disorder. Sleep Med. 2019;57:115–121. doi: 10.1016/j.sleep.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 31.Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015;1 doi: 10.1038/nrdp.2015.20. 15020. [DOI] [PubMed] [Google Scholar]
- 32.Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren L, et al. Subcortical brain volume differences of participants with ADHD across the lifespan: an ENIGMA collaboration. The lancet Psychiatry. 2017;4:310–319. doi: 10.1016/S2215-0366(17)30049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoogman M, Muetzel R, Guimaraes JP, Shumskaya E, Mennes M, Zwiers MP, et al. Brain imaging of the cortex in ADHD: a coordinated analysis of large-scale clinical and population-based samples. Am J Psychiatry. 2019 doi: 10.1176/appi.ajp.2019.18091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Someren EJ. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67:182–185. doi: 10.1016/j.biopsych.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Stoffers D, Altena E, van der Werf YD, Sanz-Arigita EJ, Voorn TA, Astill RG, et al. The caudate: a key node in the neuronal network imbalance of insomnia? Brain. 2013;137:610–620. doi: 10.1093/brain/awt329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riemann D, Voderholzer U, Spiegelhalder K, Hornyak M, Buysse DJ, Nissen C, et al. Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep. 2007;30:955–958. doi: 10.1093/sleep/30.8.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen MC, Chang C, Glover GH, Gotlib IH. Increased insula coactivation with salience networks in insomnia. Biol Psychol. 2014;97:1–8. doi: 10.1016/j.biopsycho.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samea F, Soluki S, Nejati V, Zarei M, Cortese S, Eickhoff SB, et al. Brain alterations in children/adolescents with ADHD revisited: A neuroimaging meta-analysis of 96 structural and functional studies. Neurosci Biobehav Rev. 2019;100:1–8. doi: 10.1016/j.neubiorev.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiegelhalder K, Regen W, Baglioni C, Nissen C, Riemann D, Kyle SD. Neuroimaging Insights into Insomnia. Curr Neurol Neurosci Rep. 2015;15:9. doi: 10.1007/s11910-015-0527-3. [DOI] [PubMed] [Google Scholar]
- 40.Hiscock H, Sciberras E. Sleep and ADHD: An Evidence-based Guide to Assessment and Treatment. London: Academic Press; 2019. [Google Scholar]
- 41.Owens J, Gruber R, Brown T, Corkum P, Cortese S, O'Brien L, et al. Future research directions in sleep and ADHD: report of a consensus working group. J Atten Disord. 2013;17:550–564. doi: 10.1177/1087054712457992. [DOI] [PubMed] [Google Scholar]
- 42.Baird AL, Coogan AN, Siddiqui A, Donev RM, Thome J. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol Psychiatry. 2012;17:988–995. doi: 10.1038/mp.2011.149. [DOI] [PubMed] [Google Scholar]
- 43.Hegerl U, Hensch T. The vigilance regulation model of affective disorders and ADHD. Neurosci Biobehav Rev. 2014;44:45–57. doi: 10.1016/j.neubiorev.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Bioulac S, Micoulaud-Franchi J-A, Philip P. Excessive daytime sleepiness in patients with ADHD—diagnostic and management strategies. Current psychiatry reports. 2015;17:69. doi: 10.1007/s11920-015-0608-7. [DOI] [PubMed] [Google Scholar]
- 45.Owens JA. The ADHD and sleep conundrum: a review. J Dev Behav Pediatr. 2005;6:312–322. doi: 10.1097/00004703-200508000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, Wang G-J, Kollins SH, Wigal TL, Newcorn JH, Telang F, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volkow ND, Wang G-J, Newcorn J, Fowler JS, Telang F, Solanto MV, et al. Brain dopamine transporter levels in treatment and drug naive adults with ADHD. Neuroimage. 2007;34:1182–1190. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Volkow ND, Wang G-J, Newcorn JH, Kollins SH, Wigal TL, Telang F, et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry. 2011;16:1147–1154. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arnsten AF, Lombroso PJ. Genetics of childhood disorders: XVIII. ADHD, Part 2: Norepinephrine has a critical modulatory influence on prefrontal cortical function. J Am Acad Child Adolesc Psychiatry. 2000;39:1201–1203. doi: 10.1097/00004583-200009000-00022. [DOI] [PubMed] [Google Scholar]
- 50.Yang L, Wang Y-F, Li J, Faraone SV. Association of norepinephrine transporter gene with methylphenidate response. J Am Acad Child Adolesc Psychiatry. 2004;43:1154–1158. doi: 10.1097/01.chi.0000131134.63368.46. [DOI] [PubMed] [Google Scholar]
- 51.Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:e145–157. doi: 10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 52.Reyes B, Carvalho A, Vakharia K, Van Bockstaele E. Amygdalar peptidergic circuits regulating noradrenergic locus coeruleus neurons: linking limbic and arousal centers. Exp Neurol. 2011;230:96–105. doi: 10.1016/j.expneurol.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berridge CW, Arnsten AF. Psychostimulants and motivated behavior: arousal and cognition. Neurosci Biobehav Rev. 2013;37:1976–1984. doi: 10.1016/j.neubiorev.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Monti JM, Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev. 2007;11:113–133. doi: 10.1016/j.smrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 56.Biederman J, Spencer T. Attention-deficit/hyperactivity disorder (adhd) as a noradrenergic disorder. Biol Psychiatry. 1999;46:1234–1242. doi: 10.1016/s0006-3223(99)00192-4. [DOI] [PubMed] [Google Scholar]
- 57.Jetté M, Des Groseilliers L. Survey Description and Methodology” in Longitudinal Study of Child Development in Québec (ÉLDEQ 1998-2002) Québec: Institut de la statistique du Québec; 2000. Description of the Statistical Methodology of ÉLDEQ 1998-2002 (5-Month-Old Infants) pp. 33–36. [Google Scholar]
- 58.Tremblay RE, Loeber R, Gagnon C, Charlebois P, Larivée S, LeBlanc M. Disruptive boys with stable and unstable high fighting behavior patterns during junior elementary school. J Abnorm Child Psychol. 1991;19:285–300. doi: 10.1007/BF00911232. [DOI] [PubMed] [Google Scholar]
- 59.Aebi M, Winkler Metzke C, Steinhausen H-C. Accuracy of the DSM-oriented attention problem scale of the child behavior checklist in diagnosing attention-deficit hyperactivity disorder. Journal of Attention Disorders. 2010;13:454–463. doi: 10.1177/1087054708325739. [DOI] [PubMed] [Google Scholar]
- 60.Bruni O, Ottaviano S, Guidetti V, Romoli M, Innocenzi M, Cortesi F, et al. The Sleep Disturbance Scale for Children (SDSC) Construct ion and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. 1996;5:251–261. doi: 10.1111/j.1365-2869.1996.00251.x. [DOI] [PubMed] [Google Scholar]
- 61.Spruyt K, Cluydts R, Verleye GB. Pediatric sleep disorders: Exploratory modulation of their relationships. Sleep. 2004;27:495–501. doi: 10.1093/sleep/27.3.495. [DOI] [PubMed] [Google Scholar]
- 62.Casey B, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, et al. The Adolescent Brain Cognitive Development (ABCD) study: imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arnatkevic Iute A, Fulcher BD, Fornito A. A practical guide to linking brain-wide gene expression and neuroimaging data. Neuroimage. 2019;189:353–367. doi: 10.1016/j.neuroimage.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 65.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 66.Hamaker EL, Kuiper RM, Grasman RPPP. A Critique of the Cross-Lagged Panel Model. Psychol Methods. 2015;20:102–116. doi: 10.1037/a0038889. [DOI] [PubMed] [Google Scholar]
- 67.Berry D, Willoughby MT. On the practical interpretability of cross-lagged panel models: Rethinking a developmental workhorse. Child Dev. 2017;88:1186–1206. doi: 10.1111/cdev.12660. [DOI] [PubMed] [Google Scholar]
- 68.Madigan S, Browne D, Racine N, Mori C, Tough S. Association between screen time and children’s performance on a developmental screening test. JAMA pediatrics. 2019;173:244–250. doi: 10.1001/jamapediatrics.2018.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winkler AM, Webster MA, Vidaurre D, Nichols TE, Smith SM. Multi-level block permutation. Neuroimage. 2015;123:253–268. doi: 10.1016/j.neuroimage.2015.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muthén B, Kaplan D, Hollis M. On structural equation modeling with data that are not missing completely at random. Psychometrika. 1987;52:431–462. [Google Scholar]
- 71.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural equation modeling: a multidisciplinary journal. 1999;6:1–55. [Google Scholar]
- 72.Smith SM, Nichols TE, Vidaurre D, Winkler AM, Behrens TE, Glasser MF, et al. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci. 2015;18:1565–1567. doi: 10.1038/nn.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marquand AF, Haak KV, Beckmann CF. Functional corticostriatal connection topographies predict goal directed behaviour in humans. Nature human behaviour. 2017;1 doi: 10.1038/s41562-017-0146. 0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luo Q, Chen Q, Wang W, Desrivieres S, Quinlan EB, Jia T, et al. Association of a Schizophrenia-Risk Nonsynonymous Variant With Putamen Volume in Adolescents: A Voxelwise and Genome-Wide Association Study. JAMA Psychiatry. 2019;76:435–445. doi: 10.1001/jamapsychiatry.2018.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whitaker KJ, Vertes PE, Romero-Garcia R, Vasa F, Moutoussis M, Prabhu G, et al. Adolescence is associated with genomically patterned consolidation of the hubs of the human brain connectome. Proc Natl Acad Sci U S A. 2016;113:9105–9110. doi: 10.1073/pnas.1601745113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Romero-Garcia R, Warrier V, Bullmore ET, Baron-Cohen S, Bethlehem RAI. Synaptic and transcriptionally downregulated genes are associated with cortical thickness differences in autism. Mol Psychiatry. 2019;24:1053–1064. doi: 10.1038/s41380-018-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morgan SE, Seidlitz J, Whitaker KJ, Romero-Garcia R, Clifton NE, Scarpazza C, et al. Cortical patterning of abnormal morphometric similarity in psychosis is associated with brain expression of schizophrenia-related genes. Proc Natl Acad Sci U S A. 2019;116:9604–9609. doi: 10.1073/pnas.1820754116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vertes PE, Rittman T, Whitaker KJ, Romero-Garcia R, Vasa F, Kitzbichler MG, et al. Gene transcription profiles associated with inter-modular hubs and connection distance in human functional magnetic resonance imaging networks. Philos T R Soc B. 2016;371 doi: 10.1098/rstb.2015.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Albaugh MD, Orr C, Chaarani B, Althoff RR, Allgaier N, D’Alberto N, et al. Inattention and reaction time variability are linked to ventromedial prefrontal volume in adolescents. Biol Psychiatry. 2017;82:660–668. doi: 10.1016/j.biopsych.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whitaker KJ, Vertes PE, Romero-Garcia R, Vasa F, Moutoussis M, Prabhu G, et al. Adolescence is associated with genomically patterned consolidation of the hubs of the human brain connectome. Proc Natl Acad Sci U S A. 2016;113:9105–9110. doi: 10.1073/pnas.1601745113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Romero-Garcia R, Atienza M, Clemmensen LH, Cantero JL. Effects of network resolution on topological properties of human neocortex. Neuroimage. 2012;59:3522–3532. doi: 10.1016/j.neuroimage.2011.10.086. [DOI] [PubMed] [Google Scholar]
- 83.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 85.Touchette É, Petit D, Séguin JR, Boivin M, Tremblay RE, Montplaisir JY. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep. 2007;30:1213–1219. doi: 10.1093/sleep/30.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gregory AM, Eley TC, O’connor TG, Plomin R. Etiologies of associations between childhood sleep and behavioral problems in a large twin sample. J Am Acad Child Adolesc Psychiatry. 2004;43:744–751. doi: 10.1097/01.chi/0000122798.47863.a5. [DOI] [PubMed] [Google Scholar]
- 87.Gregory AM, Agnew-Blais JC, Matthews T, Moffitt TE, Arseneault L. ADHD and sleep quality: longitudinal analyses from childhood to early adulthood in a twin cohort. J Clin Child Adolesc Psychol. 2017;46:284–294. doi: 10.1080/15374416.2016.1183499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Norman LJ, Carlisi C, Lukito S, Hart H, Mataix-Cols D, Radua J, et al. Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: a comparative meta-analysis. JAMA psychiatry. 2016;73:815–825. doi: 10.1001/jamapsychiatry.2016.0700. [DOI] [PubMed] [Google Scholar]
- 89.Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal–striatal model. Trends Cogn Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Proal E, Reiss PT, Klein RG, Mannuzza S, Gotimer K, Ramos-Olazagasti MA, et al. Brain gray matter deficits at 33-year follow-up in adults with attention-deficit/hyperactivity disorder established in childhood. Arch Gen Psychiatry. 2011;68:1122–1134. doi: 10.1001/archgenpsychiatry.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Joo EY, Noh HJ, Kim J-S, Koo DL, Kim D, Hwang KJ, et al. Brain gray matter deficits in patients with chronic primary insomnia. Sleep. 2013;36:999–1007. doi: 10.5665/sleep.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Altena E, Van Der Werf YD, Sanz-Arigita EJ, Voorn TA, Rombouts SA, Kuijer JP, et al. Prefrontal hypoactivation and recovery in insomnia. Sleep. 2008;31:1271–1276. [PMC free article] [PubMed] [Google Scholar]
- 93.Guadagni V, Burles F, Ferrara M, Iaria G. Sleep quality and its association with the insular cortex in emotional empathy. Eur J Neurosci. 2018;48:2288–2300. doi: 10.1111/ejn.14124. [DOI] [PubMed] [Google Scholar]
- 94.Cortese S. Sleep and ADHD: what we know and what we do not know. Sleep Med. 2015;16:5–6. doi: 10.1016/j.sleep.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 95.Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 96.Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rubia K. Cognitive neuroscience of attention deficit hyperactivity disorder (ADHD) and its clinical translation. Front Hum Neurosci. 2018;12 doi: 10.3389/fnhum.2018.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 99.Cohen MX, Schoene-Bake J-C, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci. 2009;12:32–34. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]
- 100.Draganski B, Kherif F, Klöppel S, Cook PA, Alexander DC, Parker GJ, et al. : Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry. 2011;168:1154–1163. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- 102.Lazarus M, Huang Z-L, Lu J, Urade Y, Chen J-F. How do the basal ganglia regulate sleep–wake behavior? Trends Neurosci. 2012;35:723–732. doi: 10.1016/j.tins.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 103.Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. Am J Psychiatry. 2009;166:664–674. doi: 10.1176/appi.ajp.2009.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Durston S, van Belle J, de Zeeuw P. Differentiating frontostriatal and fronto-cerebellar circuits in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:1178–1184. doi: 10.1016/j.biopsych.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 105.Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA psychiatry. 2015;72:584–592. doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- 106.Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55:343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 107.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 108.Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- 109.Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;72:191–197. doi: 10.1016/j.biopsych.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51:356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 111.Furrer M, Jaramillo V, Volk C, Ringli M, Aellen R, Wehrle FM, et al. Sleep EEG slow-wave activity in medicated and unmedicated children and adolescents with attention-deficit/hyperactivity disorder. Translational psychiatry. 2019;9:324. doi: 10.1038/s41398-019-0659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sciberras E, Mulraney M, Mensah F, Oberklaid F, Efron D, Hiscock H. Sustained impact of a sleep intervention and moderators of treatment outcome for children with ADHD: a randomised controlled trial. Psychol Med. 2019:1–10. doi: 10.1017/S0033291718004063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.