Abstract
IMPORTANCE
Administration of corticosteroids to women at high risk for delivery in the late preterm period (34–36 weeks’ gestation) improves short-term neonatal outcomes. The cost implications of this intervention are not known.
OBJECTIVE
To compare the cost-effectiveness of treatment with antenatal corticosteroids with no treatment for women at risk for late preterm delivery.
DESIGN, SETTING, AND PARTICIPANTS
This secondary analysis of the Antenatal Late Preterm Steroids trial, a multicenter randomized clinical trial of antenatal corticosteroids vs placebo in women at risk for late preterm delivery conducted from October 30,2010, to February 27,2015. took a third-party payer perspective. Maternal costs were based on Medicaid rates and included those of betamethasone, as well as the outpatient visits or inpatient stay required to administer betamethasone. All direct medical costs for newborn care were included. For infants admitted to the neonatal intensive care unit, comprehensive daily costs were stratified by the acuity of respiratory illness. For infants admitted to the regular newborn nursery, nationally representative cost estimates from the literature were used. Effectiveness was measured as the proportion of infants without the primary outcome of the study: a composite of treatment in the first 72 hours of continuous positive airway pressure or high-flow nasal cannula for 2 hours or more, supplemental oxygen with a fraction of inspired oxygen of 30% or more for 4 hours or more, and extracorporeal membrane oxygenation or mechanical ventilation. This secondary analysis was initially started in June 2016 and revision of the analysis began in May 2017.
EXPOSURES
Bethamethasone treatment.
MAIN OUTCOMES AND MEASURES
Incremental cost-effectiveness ratio.
RESULTS
Costs were determined for 1426 mother-infant pairs in the betamethasone group (mean [SD] maternal age, 28.6 [6.3] years; 827 [58.0%] white) and 1395 mother-infant pairs in the placebo group (mean [SD] maternal age, 27.9 [6.2] years; 794 [56.9%] white). Treatment with betamethasone was associated with a total mean (SD) woman-infant-pair cost of $4681 ($5798), which was significantly less than the mean (SD) amount of $5379 ($8422) for women and infants in the placebo group (difference, $698; 95% Cl, $186-$1257; P = .02). The Antenatal Late Preterm Steroids trial determined that betamethasone use is effective: respiratory morbidity deceased by 2.9% (95% Cl, −0.5% to −5.4%). Thus, the cost-effectiveness ratio was −23 986. Inspection of the bootstrap replications confirmed that treatment was the dominant strategy in 5000 samples (98.8%). Sensitivity analyses showed that these results held under most assumptions.
CONCLUSIONS AND RELEVANCE
The findings suggest that antenatal betamethasone treatment is associated with a statistically significant decrease in health care costs and with improved outcomes; thus, this treatment may be an economically desirable strategy.
Preterm delivery remains an important and costly problem in the United States.1 It is estimated that approximately 10% of births in the United States are preterm, and of those, approximately 70% occur between 34 and 36 weeks of gestation (ie, the late preterm period).2 The neonatal morbidities associated with late preterm delivery are well described, with respiratory morbidity among the most common.3–6 Specifically, infants born in the late preterm period are at higher risk of respiratory distress syndrome, transient tachypnea of the newborn, and ventilator and surfactant use compared with their term counterparts.5 Late preterm birth is associated with higher costs than term births because of longer hospitalizations related in part to respiratory morbidity.7–11 It has been suggested that a substantial proportion of prematurity-related costs in the neonatal period and in later childhood are associated with infants born moderately or late preterm because of the magnitude of their numbers compared with the fewer early preterm births, although such estimates must be interpreted cautiously because of potential issues of generalizability.12–14
To address the problem of respiratory morbidity in late preterm neonates, the Maternal-Fetal Medicine Units Network conducted the Antenatal Late Preterm Steroids (ALPS) trial. The study, in which the objective was to evaluate the role of antenatal corticosteroids in women at risk for late preterm birth, demonstrated that administration of betamethasone to women at high risk for late preterm delivery improved the rate of shortterm neonatal respiratory complications.15 Subsequently, administration of late preterm corticosteroids has been incorporated widely into practice in the United States.16,17 However, the cost implications of this practice are not known. Therefore, our objective was to assess whether administration of antenatal betamethasone, compared with standard of care without betamethasone therapy, was a cost-effective strategy.
Methods
Study Framing
In this secondary analysis of the ALPS randomized clinical trial, which was performed from October 30,2010, to February 27, 2015, we conducted a retrospective economic evaluation of antenatal corticosteroids vs no treatment for women at risk for late preterm delivery using patient-level data for costs reflected by resource use and effectiveness in that trial. To be eligible for the ALPS trial, women had to have a nonanoma-lous singleton gestation and be deemed at high risk for preterm delivery between 34 weeks 6 days to 36 weeks 0 days of gestation. Eligible women could be at risk for medically indicated or spontaneous preterm delivery, and study drug could be administered in an inpatient or outpatient setting, depending on the clinical scenario. Other details of the ALPS trial have been published previously.15 This secondary analysis was initially started in June 2016 and revision of the analysis began in May 2017. The institutional review boards at the participating intitutions provided approval for this study. Written informed consent was obtained from all participants before randomization.
Resource Utilization and Costs
The current cost-effectiveness analysis included participants who received at least 1 dose of the study drug and who were not lost to follow-up. The primary neonatal outcome in the ALPS trial was assessed at 72 hours of life, but resource utilization information was collected through hospital discharge of mother and infant. New-onset respiratory compromise associated with late preterm status after 72 hours after delivery is uncommon; thus, effectiveness was determined based on the difference between groups in the frequency of respiratory morbidity at 72 hours. In contrast, because therapy often persists for longer than this 72-hour duration, we measured costs through hospital discharge. The analysis took the perspective of a third-party payer in which we included direct medical costs and associated overhead accruing to hospitals and medical payers for the care of enrolled patients and their infants.
Maternal costs included those of betamethasone and of the outpatient visits or inpatient stay required to administer betamethasone (Table 1). Betamethasone was administered as two 12-mg intramuscular doses 24 hours apart. If the infant remained undelivered 24 hours after the first dose, the second dose was administered. Enrolled women who received betamethasone during the stay in which they delivered incurred no additional costs for drug administration. Women who received betamethasone before the delivery admission had the location (inpatient or outpatient) of the second dose documented. It was deemed that the first injection was part of routine clinical care during the initial assessment, and an additional cost was assigned for the second dose if the patient was discharged before betamethasone administration. This additional cost was not assigned to the placebo (ie, no treatment) group because these women would not need to return for a visit specifically for antenatal corticosteroid administration. Because there were no differences noted between allocation groups for any other aspects of maternal resource use, other maternal costs were considered to be equal. Prices for maternal interventions were based on Medicaid rates.18,19
Table 1.
Costs of Care
| Item | Cost, $ |
|---|---|
| Maternal costs | |
| Betamethasone, 12 mg per dose | 29.36 |
| Office visita | 12.56 |
| Hospital visita,b | 2133.60 |
| Neonatal costsb | |
| NICU/SCN | |
| Ventilator day 1 | 3600.45 |
| Ventilator days 2–28 | 3024.36 |
| Ventilator day ≥29 | 3036.84 |
| CPAP day 1 | 3215.10 |
| CPAP days 2–28 | 2639.01 |
| CPAPday≥29 | 2651.49 |
| Nonventilator/CPAP day 1 | 1224.58 |
| Nonventilator/CPAP day ≥2 | 988.08 |
| Well-child nursery total stayc | 1443.00 |
Abbreviations: CPAP, continuous positive airway pressure; NICU, neonatal intensive care unit; SCN, special care nursery.
Cost (in 2015 US dollars) assessed for mothers returning to the obstetrician’s office or remaining in the hospital for the second dose but before the admission for delivery.
Includes hospital and physician components.
Cost for a mean of 2 to 4 days.
We estimated newborn costs by assigning each day of an infant’s admission to 1 of the following mutually exclusive respiratory categories: (1) positive pressure ventilation (including conventional mechanical ventilation and high-frequency ventilation); (2) continuous positive airway pressure without endotracheal intubation; (3) supplemental oxygen via nasal cannula or hood; or (4) no respiratory support. This method of allocation was reflective of the ALPS trial’s primary outcome, which was the need for respiratory support and included the following parameters: continuous positive airway pressure or high-flow nasal cannula for at least 2 consecutive hours, an oxygen requirement with a fraction of inspired oxygen of at least 30% for at least 4 continuous hours, extracor-poreal membrane oxygenation, or the need for mechanical ventilation, all within 72 hours of birth.15
We then further estimated costs by applying daily costs for each of these mutually exclusive neonatal intensive care unit (NICU) hospital-day categories as previously calculated and used in several other neonatal randomized clinical trial economic evaluations.20,21 These daily costs were derived from infants’ summed total daily charges for respiratory and non-respiratory care at each illness acuity level. Infants with hypoglycemia that required management in the NICU but not respiratory support were assigned costs of NICU care without respiratory support. The charges were converted to costs by applying the appropriate ratio of costs to charges at the level of the hospital cost center, such as pharmacy, laboratory, and radiology; costs derived in this way are generally considered to be the best available typical estimates of the relevant daily expenditures across multiple hospitals. For infants admitted to the regular newborn nursery, we used nationally representative per-admission cost estimates from the published literature.22 Physician professional fees for the hospitalization, based on information derived from the ALPS case report forms, were based on Centers for Medicare & Medicaid Services19 reimbursement levels for each day of stay and non-bundled procedure.
Total costs per patient were the summed products ofma- ternal and neonatal costs incurred. Only direct medical costs were considered, and all costs were expressed in 2015 US dollars, consistent with the completion of enrollment in the clinical trial. When necessary, cost inputs were converted to 2015 US dollars by using the Centers for Medicare & Medicaid Services Personal Consumption Expenditure Health price deflator.23,24 The decision tree is shown in eFigure 1 in the Supplement.
Statistical Analysis
In the first phase of the analysis, we directly compared mean cost between the study groups. Because cost data are typically right skewed, we modeled the logarithm of mean costs using generalized linear modeling with a logarithmic link function and y distribution.25–27 Randomization stratum was included as the only covariate in this equation.
We calculated the incremental cost-effectiveness ratio (ICER),28 defined as the difference in mean total cost per patient in the betamethasone and placebo arms divided by the difference in the effectiveness (defined as the proportion of patients in each study arm without the primary neonatal outcome) between the 2 groups.29 This cost-effectiveness phase of the analysis used raw, nontransformed, and nonmodeled costs.
To assess statistical uncertainty in the joint distribution of costs and effects, we used nonparametric bootstrapping with 5000 random samples, each including 2821 mother-infant pairs drawn with replacement from the 2821 mother-infant pairs from the cost analysis set.30,31 For each sample, we calculated the difference in mean total cost, difference in percent effectiveness, and the ICER. We determined the proportion of the costs and effects pairs that resided in the dominant quadrant of a cost-effect graph and plotted the values against a range of willingness-to-pay thresholds on a cost-effectiveness acceptability curve.32,33
Finally, a deterministic sensitivity analysis was implemented to assess parameter uncertainty of cost values in which we recalculated the cost-effectiveness ratio after varying numerous maternal and neonatal costs across their plausible ranges. Specifically, the cost of betamethasone varied from 125% to 200% of the baseline cost, and all other costs varied from 50% to 200% of their baseline costs. For each cost-varying scenario, the same nonparametric bootstrapping procedure was used. All tests were 2-tailed, and P < .05wascon-sidered to be statistically significant.
Results
Costs were determined for 1426 mother-infant pairs in the betamethasone group (mean [SD] maternal age, 28.6 [6.3] years; 827 [58.0%] white) and 1395 mother-infant pairs in the placebo group (mean [SD] maternal age, 27.9 [6.2] years; 794 [56.9%] white). Of 2831 mother-infant pairs enrolled in the ALPS trial, 4 (0.14%) were lost to follow-up and 6 (0.21%) did not receive the first dose of study medication, leaving 2821 (99.6%) mother-infant pairs for whom costs could be esti-mated for the analysis. Of those, there were 1426 mother- infant pairs (50.5%) in the betamethasone group and 1395 mother-infant pairs (49.5%) in the placebo group. Baseline characteristics were similar between allocation groups (Table 2). Treatment with betamethasone for mother-infant pairs at risk for late preterm birth was associated with a total mean (SD) cost of $4681 ($5798), which was significantly less than the $5379 ($8422) cost for pairs who were randomized to placebo (ie, who did not receive betamethasone) (difference, $698; 95% CI, $186-$1257; P = .02) (Figure). According to the results of the ALPS trial, betamethasone use resulted in a 2.9% reduction (95% CI, −0.5% to −5.4%) in respiratory morbidity. Thus, because the treated group had lower costs and this strategy was more effective, administration of betamethasone to women at risk for late preterm birth was judged to be a dominant strategy, which is defined as one in which costs are lower and effectiveness is higher than a comparator (ICER, −23 986).
Table 2.
Baseline Characteristics by Treatment Assignmenta
| Characteristic | Betamethasone (n = 1426) |
Placebo (n = 1395) |
|---|---|---|
| Gestational age at trial entry | ||
| ≤34 wk 6 d | 368 (25.8) | 398 (28.5) |
| 35 wk 0dto35 wk 6 d | 571 (40.0) | 527 (37.8) |
| ≥36 wk 0 d | 487 (34.2) | 470 (33.7) |
| Indication for trial entry | ||
| Preterm labor | 398 (27.9) | 391 (28.0) |
| Preterm PROM | 315 (22.1) | 302 (21.7) |
| Medically indicated delivery | 713 (50.0) | 702 (50.3) |
| Maternal age, mean (SD), y | 28.6 (6.25) | 27.9(6.15) |
| Smoking during current pregnancy | 204(14.3) | 182 (13.1) |
| Gestational diabetes | 153 (10.7) | 153 (11.0) |
| Nulliparous | 455 (31.9) | 447 (32.0) |
| Racial/ethnic group | ||
| Black | 375 (26.3) | 381 (27.3) |
| White | 827 (58.0) | 794(56.9) |
| Asian | 56(3.9) | 39(2.8) |
| Other, unknown, or >1 race | 168(11.8) | 181 (13.0) |
| Hispanic | 405 (28.5) | 447 (32.2) |
Abbreviation: PROM, premature ruptureofmembranes.
Data are presented as number(percentage) ofmothers or infants unless otherwise indicated.
Figure. Total Costs by Treatment Allocation.
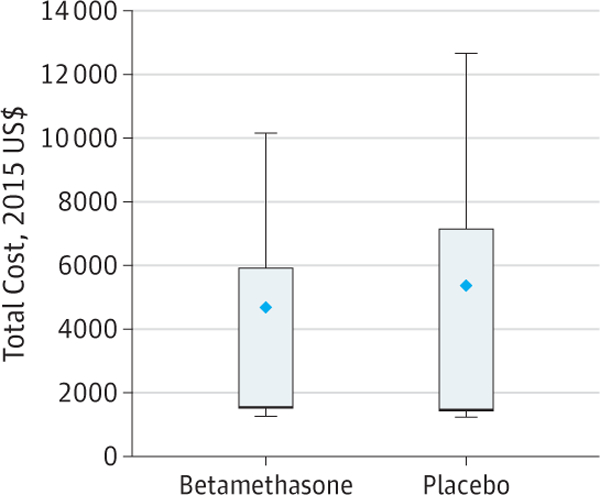
Mean costs were $4681 (interquartile range, $1502-$5913) for betamethasone and $5379 (interquartile range, $1443-$7153) for placebo. Diamonds indicate means; error bars, extreme data points within 1 times the interquartile range.
Dominance of the betamethasone strategy was maintained in 4940 of 5000 bootstrap estimates (98.8%) (eFigure 2 in the Supplement). Bivariable sensitivity analyses demonstrated that results were similarly robust when the costs of betamethasone and aggregate physician and institution costs were varied along plausible ranges (eTable and eFigure 3 in the Supplement). Effectiveness was 0.884% in the betamethasone group and 0.855% in the placebo group (change in incremental effectiveness, 0.029%). Dominance of treatment was also maintained in deterministic analyses when the efficacy of betamethasone was set to its extremes, at the lower and upper limits of the 95% CI in the parent trial; this confirmed the results seen in the bootstrapped sample (ICER with efficacy of 0.005, −139 600; ICER with efficacy of 0.05, −13 960).
Discussion
In this analysis, we found that health care costs were decreased in women at risk for late preterm birth who received betamethasone compared with no treatment. In the ALPS trial, betamethasone treatment was shown to improve neonatal respiratory outcomes (the effectiveness measure used in the present analysis); these combined findings suggest that its administration is a dominant strategy. On the basis of a 6.9% late preterm birth rate in 2015 and approximately $700 cost savings for each late preterm birth,34 assuming only 50% are eligible, this intervention has a potential cost saving in the United States of approximately $100 million dollars annually from the benefit in the immediate neonatal outcome alone.
Because late preterm birth comprises a large proportion of all preterm births, our findings have the potential for a large influence on public health. In evaluating the cost influence of late preterm birth, McLaurin et al9 conducted a retrospective cohort study using the MedStat MarketScan Commercial Claims and Encounters database, which can link patient-level health care use with expenditures. They found that the mean hospital stay was longer for a late preterm infant compared with an infant born at term (8.8 vs 2.2 days). This longer length of stay was associated with a higher mean cost for the late preterm infant compared with its term counterpart ($26 054 vs $2087). Furthermore, the authors found that the increased costs continued through the first year of life in part because of higher rates of additional hospitalization. Jacob et al7 found late preterm birth was associated with higher health care costs compared with term birth and that these increased costs persisted into the first year of life. Before their introduction, Bastek et al35 performed a decision and economic analysis to understand the potential cost implications of antenatal corticosteroid treatments for late preterm infants. They found that a full course of corticosteroids at 34, 35, or 36 weeks was associated with reduced cost and morbidity of late preterm birth. Their findings are consistent with the findings of the ALPS trial and this economic analysis. However, a recent cost-effectiveness analysis based on aggregate data from the ALPS trial used trial secondary outcomes of transient tachypnea of the newborn and respiratory distress syndrome as the measure of effectiveness.36 The authors did not assess the primary outcome. Because the parent trial found no difference in respiratory distress syndrome, any model of costs and effectiveness of respiratory distress syndrome based on the parent trial would be unlikely to show economic desirability. Furthermore, a quality-adjusted life year assessment at 7 days of life is of uncertain significance.
Strengths and Limitations
A strength of our study was the original randomized trial design, limiting bias by treatment assignment. There was little loss to follow-up, and we had well-defined and detailed respiratory outcomes for the first 72 hours of life. Our study approach also has limitations. Because costs were not assigned prospectively, we used a top-down cost estimation approach based on hospital length of stay and illness acuity. It is possible that this approach would have missed subtle differences in measured line-item resource utilization, but this would again unlikely be of significant magnitude given the stratification by the most important indicator of neonatal costs. Furthermore, costs based on specific diagnoses or resource utilization should be reflected in the analysis given their implications for NICU and physician costs. This study used a third-party payer approach compared with a societal approach. The latter would have required prospective assessment of cost, including cost to the family and lost patient productivity time, on which we did not have data. The effectiveness measure in our study was chosen to match that of a clinical trial. It was not possible to convert this to a nonsurrogate outcome, such as quality-adjusted life years orto include caregiverutility without a modeling process that would entail extensive assumptions. Use of betamethasone that was inconsistent withthe protocol in the trial (eg, being given to women likely to deliver at term, those ineligible by study criteria, or other real-world permutations not evaluated in the clinical trial) may not yield similar cost-effectiveness results. Finally, in this third-party payer perspective, costs were limited to those related to direct medical care. Costs accruing to other parties, notably families, were not collected but are known to be significant for newborn hospital stays, which may be of policy relevance. However, because these costs are related to the length of stay, it is unlikely that the central conclusion (ie, dominance of the treatment) would be altered. Also, the use of Medicaid reimbursement rates represents a conservative approach to the estimation of costs, albeit one that continues to be frequently used in economic evaluations in the perinatal arena, given that it is the most common payer for such care. Recognizing the limitation of any specific source of price points, we used a broad range for the cost estimates in deterministic sensitivity analysis. The conclusions remained constant throughout this range.
Conclusions
We found a decrease in health care costs associated with administration of late preterm antenatal betamethasone to women who were at risk for delivery. This treatment may be an economically desirable strategy.
Supplementary Material
Key Points.
Question
Is administration of antenatal corticosteroids to women at risk for late preterm delivery a cost-effective strategy?
Findings
In this secondary analysis of a randomized clinical trial, treatment with betamethasone was associated with a total mean woman-infant-pair cost that was significantly less than that for women and infants in the placebo group.
Meaning
The findings suggest that antenatal betamethasone treatment is associated with a statistically significant decrease in health care costs and with improved outcomes; thus, the treatment may be an economically desirable strategy.
Acknowledgments
Funding/Support: This study was supported by grants HL098554and HL098354 from the NHLBI, grants HD21410, HD27915, HD27917, HD27869, HD34116, HD34208, HD40485, HD40500, HD40512, HD40544, HD40545, HD40560, HD53097, HD53118, HD68268, HD68258, HD68282, and HD36801 from the NICHD, and by grant UL1TR000040 from the National Center for Advancing Translational Sciences, NIH.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval ofthe manuscript; and decision to submit the manuscript for publication.
Footnotes
Group Information: The Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units network members and additional network members are listed at the end of this article.
Conflict of Interest Disclosures:
Dr Gyamfi-Bannerman reported receivinggrants from the National Heart, Lung, and Blood Institute (NHLBI)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD); the National Institutes of Health (NIH); and the SocietyforMaternalFetalMedicineand AMAG pharmaceuticals during the conduct of the study. Mr Sandoval reported receiving grants from the NIH during the conduct of the study. Dr Grobman reportedreceivinggrantsfromtheNICHDduring the conduct of the study. Dr Chien reported receiving grants NICHD during the conduct of the study. Dr Peaceman reported receiving grants from NICHD during the conduct of the study. Dr Swamy reported receiving grants from Duke University during the conduct of the study. Dr Tolosa reported receiving grants from NICHD during the conduct of the study. No other disclosures were reported.
Group Members: In addition to the authors, other members of the Eunice Kennedy Shriver National Institute ofChild Health and Human Development Maternal-Fetal Medicine Units Network are as follows: Columbia University, New York, New York: S. Bousleiman, MSN, R. Wapner, MD, M. DiVito, MSN, M. Talucci, MSN, L. Plante, MD (Drexel University), C. Tocci, BSN (Drexel University), M. Hoffman, MD (Christiana Care Health Systems), S. Lynch (Christiana Care Health Systems), BSN, A. Ranzin, MD (St. Peter’s University Hospital), M. Lake (St. Peter’s University Hospital), J. Smulian, MD (Lehigh Valley Health Network), D. Skupski, MD (New York Hospital Queens); The University of Texas Health Science Center at Houston-Children’s Memorial Hermann Hospital, Houston: F. Ortiz, BSN, B. Sibai, MD, M. Hutchinson, P. Givens, and L. Garcia (LBJ General Hospital); University of Alabama at Birmingham, Birmingham: S. Harris, BSN, J. Biggio, MD, A. Todd, MSN, L. Merin, RN, G. Adams, BSN, M. Tew, BSN, J. Grant, MSN; University of Texas Medical Branch, Galveston: A. Salazar, WHNP, L. McCoy, RN, B. Aguillon, RN, M. Wilson, RN, J. Sikes, RN, G. Hankins, MD, G. Olson, MD, H. Harirah, MD; Brown University, Providence, Rhode Island: D. Allard, RNC, L. Beati, BSN, B. Wallin, BSN, J. Rousseau, BSN, B. Hughes, MD; The Ohio State University, Columbus: F. Johnson, M. Prasad, MD, D. McKenna, MD, R. Ozug, MSN,T. Dible, MS, K. Snow, BSN, K. Fennig, MS, S. Webster, MSN, M. Donohue, MSN; University of Utah Health Sciences Center, Salt Lake City: K. Hill, A. Sowles, S. Timothy, P. Reed (deceased; Intermountain Healthcare), M. Varner, MD; University of North Carolina at Chapel Hill, Chapel Hill: K. Clark, BSN, S. Timlin, MSN, R. Bass, RN, K. Dorman, MS, S. Brody, MD (WakeMed Health & Hospitals), J. Warren (Mission Health System); MetroHealth Medical Center, Case Western Reserve University, Cleveland, Ohio: M. Duchon, MD, W. Dalton, RNC, C. Milluzzi, MSN, L. Wolfe, CNP, K. Kushner, RN, B. Mercer,MD; Northwestern University, Chicago, Illinois: G. Mallett, MS, L. Stein, RN, M. Dinsmoor, MD (NorthShore University HealthSystem), K. Paychek, BSN, (NorthShore University HealthSystem); University of Colorado School ofMedicine, Anschutz Medical Campus, Aurora: K. Hale, BSN, M. Hoffman, MD, J.C. Carey, MD, H. Galan, MD, K. Heyborne, MD, T. Metz, MD, A. Rosenberg, MD; Duke University, Durham, North Carolina: T. Bishop, MSN, A. Murtha, MD, R. Heine, MD, C. Grotegut, MD, L. Brancazio, MD; Stanford University, Stanford, California: K. Kushniruk, PhD, Y. El-Sayed, MD, D. Lyell, MD, A. Sit, MD, C. Willson, BSN, A. Monk, BSN, E. Kogut, BSN, R. Knapp, MPH; University of Texas Southwestern Medical Center, Dallas: L. Moseley, BSN, J. Price, RN, M. Santillan, MSN, J. Gerald, BSN A. Sias, K. Gonzales; University of Pittsburgh, Pittsburgh, Pennsylvania: H. Simhan, MD, H. Birkland, RN, P. Cotroneo, RN; Oregon Health & Science University, Portland: L. Pereira, MD, C. McEvoy, MD, M. Rincon, MD, J. Snyder, RN; Wayne State University, Detroit, Michigan: N. Hauff, PhD; The George Washington University Biostatistics Center, Washington, DC: E. Thom, PhD, K. Jablonski, PhD, V. Momirova, MS, G. Heinrich, MS, T. Billingsley, T. Spangle; National Heart, Lung, and Blood Institute, Bethesda, Maryland: C. Blaisdell, MD; Eunice Kennedy Shriver National Institute ofChild Health and Human Development, Bethesda, Maryland: C. Spong, MD, S. Tolivaisa.
Disclaimer: The comments and views expressed in this article are those ofthe authors and do not necessarily represent the views of the National Institutes of Health.
REFERENCES
- 1.Russell RB, Green NS, Steiner CA, et al. Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics. 2007;120(1): e1–e9. doi: 10.1542/peds.2006-2386 [DOI] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: final data for 2015. Natl Vital Stat Rep. 2017;66(1):1. [PubMed] [Google Scholar]
- 3.McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111(1):35–41.doi: 10.1097/01.AOG.0000297311.33046.73 [DOI] [PubMed] [Google Scholar]
- 4.Yoder BA, Gordon MC, Barth WH Jr. Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111(4):814–822. doi: 10.1097/AOG.0b013e31816499f4 [DOI] [PubMed] [Google Scholar]
- 5.Hibbard JU, Wilkins I, Sun L, et al. ; Consortium on Safe Labor. Respiratory morbidity in late preterm births. JAMA. 2010;304(4):419–425. doi: 10.1001/jama.2010.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gyamfi-Bannerman C The scope of the problem: the epidemiology of late preterm and early-term birth. Semin Perinatol. 2011;35(5):246–248. doi: 10.1053/j.semperi.2011.05.013 [DOI] [PubMed] [Google Scholar]
- 7.Jacob J, Lehne M, Mischker A, Klinger N, Zickermann C, Walker J Cost effects of preterm birth: a comparison of health care costs associated with early preterm, late preterm, and full-term birth in the first 3 years after birth. EurJ Health Econ. 2017;18(8):1041–1046. doi: 10.1007/s10198-016-0850-x [DOI] [PubMed] [Google Scholar]
- 8.Bérard A, Le Tiec M, DeVera MA. Study of the costs and morbidities of late-preterm birth. Arch Dis Child Fetal Neonatal Ed. 2012;97(5):F329–F334. doi: 10.1136/fetalneonatal-2011-300969 [DOI] [PubMed] [Google Scholar]
- 9.McLaurin KK, Hall CB, Jackson EA, Owens OV, Mahadevia PJ. Persistence of morbidity and cost differences between late-preterm and term infants during the first year of life. Pediatrics. 2009;123(2): 653–659. doi: 10.1542/peds.2008-1439 [DOI] [PubMed] [Google Scholar]
- 10.Khan KA, Petrou S, Dritsaki M, et al. Economic costs associated with moderate and late preterm birth: a prospective population-based study. BJOG. 2015;122(11):1495–1505. doi: 10.1111/1471-0528.13515 [DOI] [PubMed] [Google Scholar]
- 11.Aly H, Hoffman H, El-Dib M, Said L, Mohamed M. Factor affecting length of stay in late preterm infants: an US national database study. J Matern Fetal Neonatal Med. 2015;28(5):598–604. doi: 10.3109/14767058.2014.927428 [DOI] [PubMed] [Google Scholar]
- 12.Zupancic JA, Dukhovny D. Resource distribution in neonatology: beyond the Pareto principle. Arch Dis Child Fetal Neonatal Ed. 2015;100 (6):F472–F473.doi: 10.1136/archdischild-2014-308136 [DOI] [PubMed] [Google Scholar]
- 13.Petrou S, Khan K. Economic costs associated with moderate and late preterm birth: primary and secondary evidence. Semin Fetal Neonatal Med. 2012;17(3):170–178. doi: 10.1016/j.siny.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 14.Phibbs CS, Schmitt SK, Cooper M, et al. Birth hospitalization costs and days of care for mothers and neonates in California, 2009–2011. J Pediatr. 2019;204:118–125.e14.doi: 10.1016/j.jpeds.2018.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al. ; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016; 374(14):1311–1320. doi: 10.1056/NEJMoa1516783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation ofthe use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215(2):B13–B15. doi: 10.1016/j.ajog.2016.03.013 [DOI] [PubMed] [Google Scholar]
- 17.Committee on Obstetric Practice. Committee Opinion No. 713: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2017;130(2): e102–e109. doi: 10.1097/AOG.0000000000002237 [DOI] [PubMed] [Google Scholar]
- 18.Nicholson WK, Frick KD, Powe NR. Economic burden of hospitalizations for preterm labor in the United States. Obstet Gynecol. 2000;96(1):95–101. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Medicare & Medicaid Services Physician Fee Schedule Look-Up Tool. 2015. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PFSlookup/index.html . Accessed March 1, 2018.
- 20.Zupancic JA, Hibbs AM, Palermo L, et al. ; NO CLDTrial Group. Economicevaluation of inhaled nitric oxide in preterm infants undergoing mechanical ventilation. Pediatrics. 2009;124(5): 1325–1332. doi: 10.1542/peds.2008-3214 [DOI] [PubMed] [Google Scholar]
- 21.Mowitz ME, Zupancic JA, Millar D, et al. Prospective economic evaluation alongside the non-invasive ventilation trial. J Perinatol. 2017;37(1): 61–66. doi: 10.1038/jp.2016.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barradas DT, Wasserman MP, Daniel-Robinson L, et al. Hospital utilization and costs among preterm infants by payer: nationwide inpatient sample, 2009. Matern Child Health J. 2016;20(4): 808–818. doi: 10.1007/s10995-015-1911-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agency for Healthcare Research and Quality Medical Expenditure Panel Survey. https://meps.ahrq.gov/about_meps/Price_Index.shtml . Accessed November 1, 2018.
- 24.Dunn A, Grosse SD, Zuvekas SH. Adjusting health expenditures for inflation: a review of measures for health services research in the United States. HealthServRes. 2018;53(1):175–196. doi: 10.1111/1x475-6773.12612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blough DK, Madden CW, Hornbrook MC. Modeling risk using generalized linear models. J Health Econ. 1999;18(2):153–171. doi: 10.1016/S0167-6296(98)00032-0 [DOI] [PubMed] [Google Scholar]
- 26.Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005;24(3):465–488. doi: 10.1016/j.jhealeco.2004.09.011 [DOI] [PubMed] [Google Scholar]
- 27.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? JHealth Econ. 2001;20(4):461–494. doi: 10.1016/S0167-6296(01)00086-8 [DOI] [PubMed] [Google Scholar]
- 28.Ramsey SD, Willke RJ, Glick H et al. Cost-effectiveness analysis alongside clinical trials II: an ISPOR Good Research Practices Task Force report. ValueHealth. 2015;18(2):161–172. doi: 10.1016/j.jval.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 29.Drummond MF, Sculpher MJ, Claxton K, Stoddard GL, Torrance GW. Methods for the Economic Evaluation ofHealth Care Programmes. 4th ed New York, NY: Oxford University Press; 2015. [Google Scholar]
- 30.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York, NY: Chapman and Hall/CRC; 1993. doi: 10.1007/978-1-4899-4541-9 [DOI] [Google Scholar]
- 31.Polsky D, Glick HA, Willke R, Schulman K. Confidence intervals for cost-effectiveness ratios: a comparison of four methods. Health Econ. 1997;6 (3):243–252. doi: [DOI] [PubMed] [Google Scholar]
- 32.Fenwick E, Claxton K, Sculpher M.Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ. 2001;10(8):779–787. doi: 10.1002/hec.635 [DOI] [PubMed] [Google Scholar]
- 33.Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves: facts, fallacies and frequently asked questions. Health Econ. 2004;13(5):405–415. doi: 10.1002/hec.903 [DOI] [PubMed] [Google Scholar]
- 34.March of Dimes. Peristats. https://www.marchofdimes.org/Peristats/ViewSubtopic.aspx?reg=99&top=3&stop=240&lev=1&slev=1&obj=1 . Accessed February 7,2018.
- 35.Bastek JA, Langmuir H, Kondapalli LA, Paré E, Adamczak JE, Srinivas SK. Antenatal corticosteroids for late-preterm infants: a decision-analytic and economic analysis. ISRN ObstetGynecol. 2012;2012: 491595. doi: 10.5402/2012/491595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenbloom JI, Lewkowitz AK, Sondgeroth KE, et al. Antenatal corticosteroid administration in late-preterm gestations: a cost-effectiveness analysis [published online November15,2018]. J Matern Fetal Neonatal Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glick HA, Doshi JA, Sonnad SS, Polsky D, eds. Economic Evaluation in Clinical Trials. Oxford, England: Oxford University Press; 2014,. doi: 10.1093/med/9780199685028.001.0001 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


