Abstract
Cytotoxic T lymphocytes (CTLs) are effective components of the immune system capable of destroying tumor cells. Generation of CTLs using peptide vaccines is a practical approach to treat cancer. We have previously described a peptide vaccination strategy that generates vast numbers of endogenous tumor-reactive CTLs after 2 sequential immunizations (prime-boost) using poly-ICLC adjuvant, which stimulates endosomal Toll-like receptor 3 (TLR3) and cytoplasmic melanoma differentiation antigen 5 (MDA5). Dendritic cells (DCs) play an important role not only in antigen presentation but are critical in generating costimulatory cytokines that promote CTL expansion. Poly-ICLC was shown to be more effective than poly-IC in generating type-I interferon (IFN-I) in various DC subsets, through its enhanced ability to escape endosomal compartment and stimulate MDA5. In our system IFN-I did not directly function as a T cell costimulatory cytokine, but enhanced CTL expansion through the induction of IL15. With palmitoylated peptide vaccines, CD8a+ DCs were essential for peptide crosspresentation. For vaccine boosts, nonprofessional antigen presenting cells were able to present minimal epitope peptides, but DCs were still required for CTL expansions through the production of IFN-I mediated by poly-ICLC. Overall, these results clarify the roles of DCs, TLR3, MDA5, IFN-I and IL15 in the generation of vast and effective antitumor CTL responses using peptide and poly-IC vaccines.
Keywords: Peptide vaccines, poly-IC, dendritic cells, MDA5, type-I interferon, Interleukin-15
Precis
Poly-ICLC activation of MDA5 on DCs mediates T cell expansion by regulating IFN-I and IL15 production. IFN-I does not directly function as a signal-3 cytokine on T cells but induces production of IL15 by professional and non-professional APCs
Introduction
T cells, in particular cytotoxic T lymphocytes (CTLs) are the most effective component of the immune system capable of destroying tumor cells (1). CTLs recognize antigens on tumor cells as small peptides derived from tumor antigens that associate with cell surface major histocompatibility class I (MHC-I) molecules. Synthetic peptide vaccines are a practical and cost-effective strategy to generate antitumor CTL responses. However, for effective clinical benefits, these vaccines need to induce potent CTL responses. For several years, our laboratory has been developing therapeutic peptide vaccines to elicit strong and lasting anti-tumor CTL responses (2–5). Amongst several adjuvants tested, poly-IC was the most effective in generating vast endogenous CTL responses (2, 3). Poly-IC is a synthetic double stranded RNA mimic that activates antigen-presenting cells (APCs) via endosomal Toll-like receptor 3 (TLR3) and cytoplasmic melanoma differentiation associated protein 5 (MDA5) (6).
The objective of the current studies was to dissect the role of APC subsets during the CTL expansion phase and assess the roles of TLR3 and MDA5 using peptide/poly-IC vaccines. We report that dendritic cells (DCs) are essential for CTL expansion not only for peptide processing and crosspresentation but also for providing signal 3 (S3) cytokines such as IFN-I and IL15. Certain poly-IC formulations have a higher capacity to activate MDA5 and induce IFN-I production. IFN-I supports CTL expansion indirectly through the production of IL15. Collectively, our findings indicate that both the peptide construct and the formulation of poly-IC are crucial for the induction and expansion of CTL responses to peptide vaccination, which correlate with antitumor efficacy.
Materials and Methods
Mice.
C57BL/6 (WT) and CD45.1 mice were from NCI/Charles River. Mice deficient for MHC-I (b2M-KO), MDA5, TLR3, BATF-3 and mice expressing diphtheria toxin receptor (DTR) under CD11c promoter (CD11cDTR) were from Jackson Laboratory. IFNαβ receptor–deficient (IFNαβR-KO) and MDA5/TLR3 double knockout mice were bred in our facility. OT-I transgenic mice (CD45.1) expressing TCR specific for Ova257–264 were bred with IFNαβR-KO mice to generate IFNαβR deficient OT-I mice. Transnuclear TgTR1 mice expressing TCR recognizing the CTL epitope Trp1455–463 were described (7).
Bone marrow chimeras and DC depletion.
CD45.1 mice were irradiated (1000 rads) followed by intravenous (i.v.) injection of 0.5–1 × 107 bone marrow (BM) cells from CD11cDTR mice (8). CD45.1 mice were also reconstituted with 1:1 ratio of BM cells from CD11cDTR and b2M-KO mice. For CD11c+ cell depletions, reconstituted mice were injected with 100 ng diphtheria toxin (DT)/mouse −2, 0 and +2 days of the vaccination time. For plasmacytoid DC (pDC) depletions, mice were injected with 400 μg anti-CD317 (BST2, PDCA-1; BioXcell clone BX444) on days −3 and −1 before poly-ICLC injections. pDC depletion was assessed by flow cytometry using a different anti-CD317 mAb (ThermoFischer, clone eBio927).
Cells.
DC lines CD8aDC (1940 CD8 MuTu, (9) were provided by H. Acha-Orbea, U. Lausanne); D1-DC (10) were provided by O. Finn (U. Pittsburgh) and a clone of DC2.4 (11) was provided by C-F Hung (Johns Hopkins University). B16F10 was obtained from the ATCC and the ovalbumin transfected B16 (B16-Ova) was described (12). Mouse BM-derived DCs were generated as described (10).
Peptides, adjuvants, adoptive CTL transfers (ACT) and vaccination.
Synthetic peptides representing minimal CTL epitopes, Ova257–264 (SIINFEKL), Trp1455–463/9M (TAPDNLGYM) and their di-palmitoylated forms pam2-KMVESIINFEKL (pam-Ova), pam2-KMVFTAPDNLGYM (pam-Trp1) were from A&A Labs. Two different formulations of poly-IC were used: high molecular weight poly-IC (InvivoGen) or poly-ICLC stabilized with poly-L-Lysine and carboxymethyl cellulose (Hiltonol, Oncovir, Inc.). Poly-ICPEI was prepared with in-vivo-JetPEI (Polyplus) at an N/P ratio of 6. For ACT, naive CD8 (CD44-negative) T cells were enriched using negative isolation kits (Miltenyi). One day after ACT, mice received i.v. vaccinations with 100 μg (minimal peptide) or 120 μg (pam-peptide) and 50 μg of poly-IC, poly-ICLC or poly-ICPEI. Where indicated, some mice also received one of the following treatments: 100 μg anti-mouse CD40 mAb during the boost (clone FGK4.5, BioXcell, Inc.; endotoxin, <2EU/mg); 3 doses of IL-2 immune complexes (IL2Cx) on days 1, 3 and 5 after the boost; mouse IFNb (10000 IU every 12 h, days 1–3 post-boost); 100 μg aIL15 mAb (M96, Amgen, days 1, 3 and 5 post-boost) or 300 μg aIL2 mAb (JES6–5H4, days 1, 3 and 5 post-boost). Each dose of IL2Cx was prepared by mixing 2 μg of recombinant mouse IL2 (BioLegend) with 10 μg of anti-mouse IL2 (clone JES6–5H4, BioXcell). CTL responses were measured in peripheral blood or spleen by flow cytometry using PE-labeled tetramers (NIH Tetramer Core Facility).
Cytokine analysis.
Cells were stimulated for 18 h. Elisa kits were used to measure cytokine production: mouse IL15/IL15aR Complex ELISA Ready-SET-Go kit (eBioScience). Mouse IL12/IL23 (p40) ELISA MAX™ Standard (Biolegend). IFN-I assays were done with B16-Blue™ IFN-α/β cells (InvivoGen).
In vivo tumor model.
Mice were inoculated s.c. with B16F10 (3 × 105/mouse) or B16F10-Ova (3 × 105/mouse) and growth was monitored every 2–3 days. Results are presented as the mean tumor size (area in mm2) ± SD.
Statistics.
Experiments were repeated 2–3 times to ensure reproducibility. Statistical significance to assess numbers of antigen specific CD8 T cells (tetramer analyses), surface markers expression and cytokines production were performed using Student’s-t-tests or one-way ANOVA as appropriate. Results are presented as mean ± SD. (*<0.05, **p<0.01, ***p<0.001, ****p<0.0001, and ns: not significant). Statistical analyses were performed using GraphPad Prism (v6).
Results
DCs are crucial for T cell expansion induced by peptide vaccination
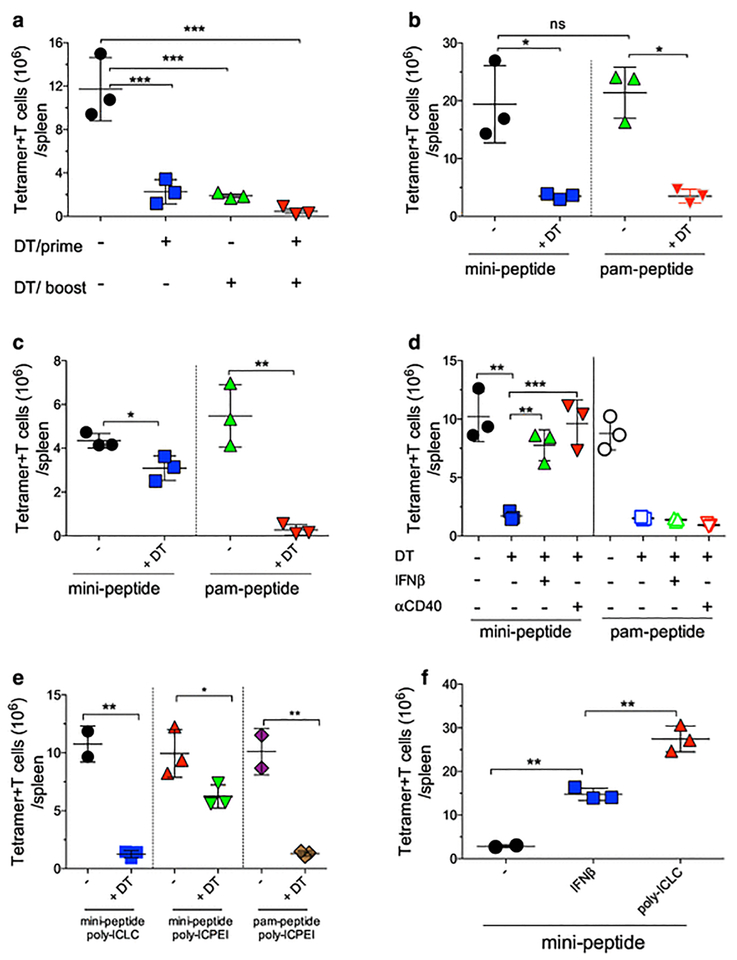
Systemic (i.v.) vaccination using modified palmitoylated peptides (pam-peptides) administeredin combination with a stabilized formulation of poly-IC (poly-ICLC) generates vast endogenous CTL responses in mice after 2 sequential immunizations (prime-boost, 7–12 days apart) (2–4, 13). Most of the T cell expansion occurs after the boost, and while priming required the use of pam-peptide, the boost can be performed using either pam-peptide or the minimal peptide (mini-peptide) epitope (2, 14). This suggests that priming requires professional antigen-presenting cells (pAPC) capable of antigen crosspresentation such as DCs (15–17), whereas the boost can be mediated by either DCs (with pam-peptide) or non-professional APCs (npAPC) because the mini-peptide epitope may be presented by any MHC-I expressing cell. The role of DCs during the priming and the boosting phases was examined using CD11c-DTR (18) BM chimeras. Naïve CTLs from OT-I mice were adoptively transferred into CD11c-DTR BM chimeric mice to assess the effects of depleting DCs prior to the vaccine prime, boost or both prime and boost. DC depletion before the prime or before the boost using a pam-peptide vaccine significantly reduced CTL expansion (Fig. 1a; Supplemental Fig. 1a). Furthermore, CTL expansion was absent when DCs were depleted prior to both the prime and boost. Thus, DCs are required for both prime and boost phases with peptides requiring antigen processing and crosspresentation. Next, we examined whether DCs were required during the expansion phase when a mini-peptide was used in the boost. Mice received a pam-peptide prime and DCs were depleted during the boost using pam-peptide or mini-peptide. Although mini-peptide does not require antigen processing, CTL expansion in the boost also depended on DCs (Fig. 1b; Supplemental Fig. 1b), suggesting that these pAPCs need to present the peptide to previously activated CTLs to induce expansion. However, the possibility exists that npAPCs can present peptide to CTLs and that the DCs when activated by poly-ICLC provide the necessary costimulatory cytokines for expansion (i.e., signal-3 cytokines). This possibility was supported by the observation that DC depletion decreased the production of IFN-I, IL15/IL15Ra complexes and IL12 after poly-ICLC injection Supplemental Fig. 2). To evaluate whether npAPCs can present antigen and generate CTL expansion in circumstances where DCs cannot present antigen but can provide costimulation, mixed chimeras using BM cells from CD11c-DTR and MHC-I deficient mice were used. DT injection depleted MHC-I+ DCs but not the MHC-I deficient DCs Supplemental Fig. 3a). Production of IFN-I by poly-ICLC was slightly reduced by DT and not affected for IL15/IL15Ra or IL12 (Supplemental Fig. 3b–d). Depletion of the MHC-I+ DCs prior to mini-peptide boost slightly reduced CTL expansion, but abolished expansion by pam-peptide (Fig. 1c; Supplemental Fig. 1c). Thus, npAPCs (CD11c-negative) can present mini-peptide to previously primed CTLs, but DCs are necessary to provide costimulation, possibly in the form of signal-3 cytokines in order to promote CTL expansion.
Fig. 1. Role of DCs for T cell expansion induced by peptide vaccination.
a, CD11cDTR BM chimeric mice received 105 naïve OT-I cells followed a day later by prime-boost vaccinations with pam-Ova (pam2-KMVESIINFEKL) and poly-ICLC administered 14 days apart. DCs were depleted with diphtheria toxin (± DT) injections prior to the prime, boost or both as indicated. Numbers of OT-I cells in the spleens were evaluated 7 days after the boost. b, CD11cDTR BM chimeric mice received 105 naïve OT-I cells, were primed with pam-Ova + poly-ICLC and were boosted with pam-Ova or mini-Ova (SIINFEKL) + poly-ICLC. Numbers of OT-I cells in the spleens were determined 7 days after the boost. c Mixed BM chimeric (CD11cDTR and b2M-KO) mice were treated and evaluated as described in b. d, CD11cDTR BM chimeric mice were treated and evaluated as described in b with the addition of twice daily injections of IFNb or one dose of 100 μg aCD40 mAb. e, CD11cDTR BM chimeric mice were treated and evaluated as described in b with boosters using mini-Ova with poly-ICIC or poly-ICPEI or using pam-Ova with poly-ICPEI. f, WT mice received 105 naïve OT-I cells followed by a pam-Ova + poly-ICLC prime and 14 days later were boosted with mini-Ova alone, mini-Ova + 2 daily injections of IFNb or mini-Ova+ poly-ICLC. Results are presented as individual mice (each symbol) with the mean ± SD for each group.
An outcome of DC stimulation by poly-ICLC is the production of IFN-I, promoting CTL expansion directly, or indirectly by the production of IL15 (19–22). Partial depletion of DCs in the mixed BM chimeras resulting in some decrease of IFN-I production by poly-ICLC (Supplemental Fig. 3b) could explain the slight reduction of CTL expansion by the mini-peptide boost (Fig. 1c). Indeed, the addition of IFN-I or anti-CD40 costimulatory antibody (aCD40), which generates signal-3 cytokines (23, 24) with the mini-peptide boost (but not with pam-peptide boost) enhanced CTL expansion in the absence of DCs (Fig. 1d; Supplemental Fig. 1d).
DCs express a diverse array of scavenger receptors, which are probably involved in the capture and receptor-mediated phagocytosis of poly-ICLC, resulting in the production of signal-3 cytokines. Polyethylenimine (PEI) can be used as transfection reagent in vivo (25), allowing the delivery of nucleic acids to cells independently of receptor-mediated internalization. Thus, we examined whether PEI formulated poly-IC (poly-ICPEI) could bypass the requirement of DCs for T cell costimulatory cytokine production. In DC-depleted animals, IFN-I and IL15/IL15Ra production decreased only by ~30% with poly-ICPEI compared to ~70% reduction using poly-ICLC (Supplemental Fig. 4). Furthermore, vaccine boosts in DC-depleted mice using poly-ICPEI allowed CTL expansion using mini-peptide but not pam-peptide (Fig. 1e; Supplemental Fig. 1e). Moreover, simple IFN-I administration circumvented the requirement of poly-ICLC for CTL expansion with mini-peptide boosts, but the magnitude of the response was somewhat lower as compared to poly-ICLC (Fig. 1f; Supplemental Fig. 1f).
Role of DC subsets in responses to poly-ICLC and pam-peptide crosspresentation
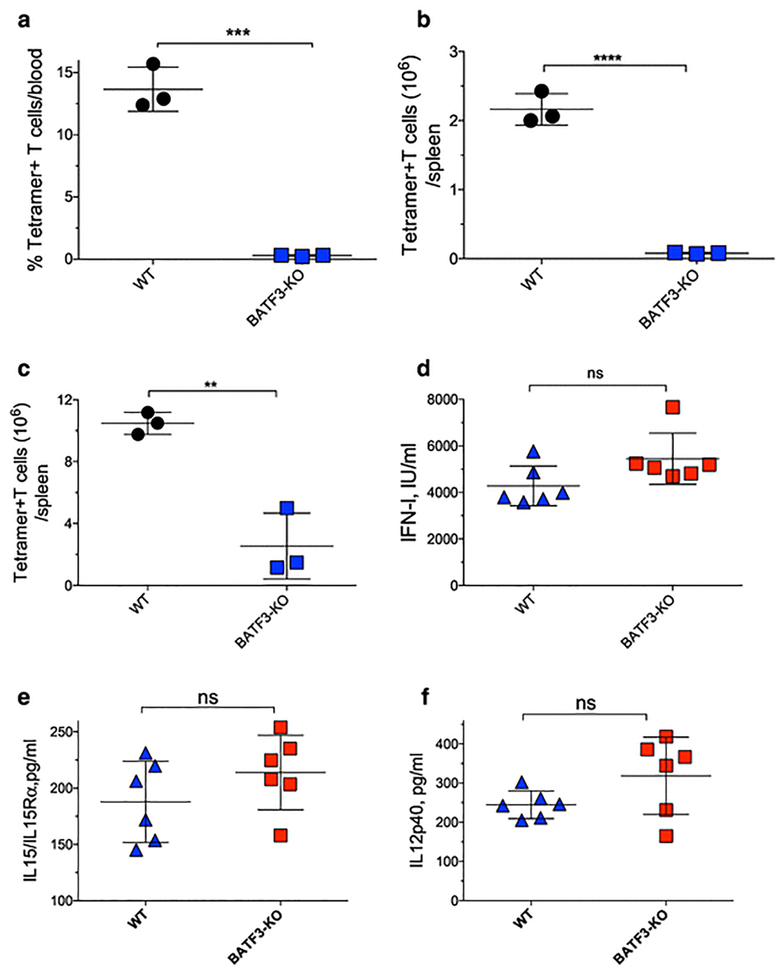
Our results so far indicate that IFN-I is important for the CTL expansion phase, and that DCs are the primary source of IFN-I after poly-ICLC injection. Plasmacytoid DCs (pDCs) are the highest IFN-I producers in response to viral infection (26, 27). However, pDC depletion only slightly reduced the in vivo production of IFN-I by poly-ICLC with no effects in IL12 or IL15/IL15Ra (Supplemental Fig. 5a–d), indicating that other DC subsets are primarily responsible for the production of these cytokines. CD8a+ DCs are critical for antigen crosspriming (16) and to test their role in peptide vaccine priming, we used BATF3-KO mice (28). Lack of CD8a DCs prevented CTL priming using pam-peptide in either endogenous or ACT CTL responses (Fig. 2a–c). However, BATF3-KO mice produced similar levels of costimulatory cytokines after poly-ICLC injection as compared to wild type (WT) mice (Fig. 2d–f). These results indicate that signal-3 cytokines can be readily produced by other DC subsets besides pDCs and CD8a+ DCs, and that the lack of CTL responses in CD8a DC-deficient mice is due to the inability of other DC subsets to crosspresent the pam-peptide and not due to a lack of costimulatory cytokines.
Fig. 2. Role of DC subsets in responses to poly-ICLC and pam-peptide crosspresentation.
a–b, WT or BATF3-KO mice were vaccinated with pam-Trp1 (pam2-KMVFTAPDNLGYM) + poly-ICLC and the Trp1-specific CTL percentages in blood (a) and total numbers in spleens (b) were evaluated. c, WT or BATF3-KO mice received 105 naïve OT-I cells followed by one dose of pam-Ova + poly-ICLC and 7 days later the absolute numbers of OT-I cells were evaluated in spleens. d–f, WT or BATF3-KO mice were injected with poly-ICLC (50 μg, i.v.) and 18 h later the serum levels of IFN-I, IL15/IL15Ra complexes, and IL12p40 were measured. Results are presented as individual mice (each symbol) with the mean ± SD for each group.
Differences in poly-IC formulations affect CTL responses
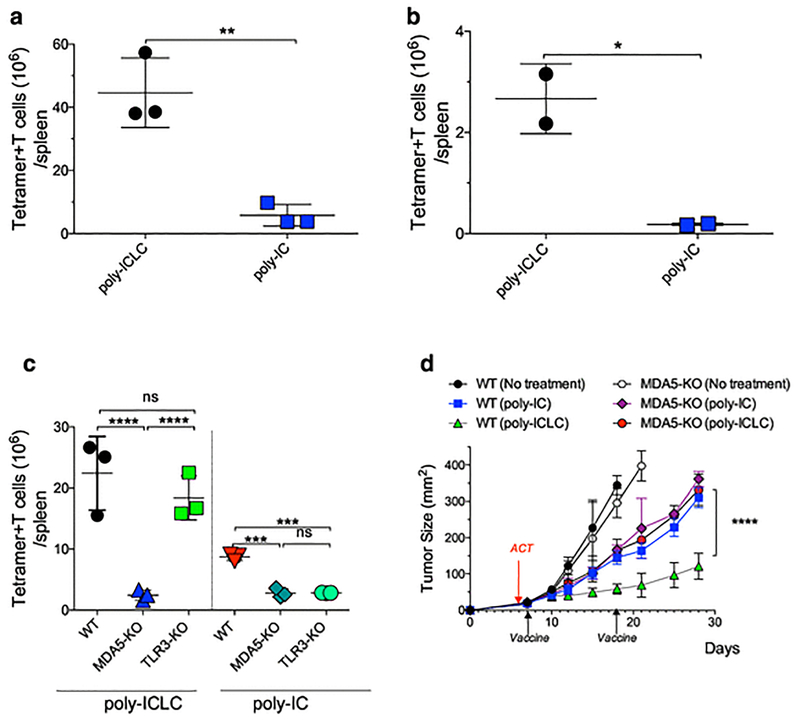
Most of the work performed in our laboratory has used poly-ICLC, a poly-IC formulation using poly-lysine and carboxyl-methylcellulose to prevent RNAse degradation, a problem when used in humans but not in mice (29–31). When comparing side-by-side the adjuvant efficacy of poly-IC with poly-ICLC (made with the same poly-IC lot) using a pam-peptide vaccine, poly-ICLC was substantially more efficient for CTL expansion as compared to poly-IC either with ACT (Fig. 3a; Supplemental Fig. 6a) or with endogenous CTL responses (Fig. 3b; Supplemental Fig. 6b). Allegedly, poly-IC and poly-ICLC can function as immune adjuvants by stimulating 2 different pattern recognition receptors, TLR3 and MDA5 (6, 32). To test the requirement of TLR3 and MDA5, naïve OT-I CTLs were transferred into WT, TLR3 deficient (TLR3-KO) or MDA5 deficient (MDA5-KO) mice and CTL responses were evaluated after prime-boost vaccination with pam-peptide using poly-ICLC or poly-IC. CTL expansion in MDA5-KO mice was impaired with poly-ICLC and poly-IC (Fig. 3c; Supplemental Fig. 6c). Responses in TLR3-KO were not significantly reduced with poly-ICLC but were decreased with poly-IC and the magnitude of the response using poly-ICLC was superior as compared to poly-IC in WT and TLR3-KO mice. Consistent with the effects in CTL expansion, poly-ICLC exerted superior antitumor effects compared to poly-IC when used as vaccine adjuvants in WT mice, and these differences were absent in MDA5-KO mice (Fig. 3d). Thus, the adjuvant activity of poly-IC requires both TLR3 and MDA5 but the enhanced adjuvant activity of poly-ICLC relies mostly in MDA5.
Fig. 3. Differences in poly-IC formulations affect CTL responses.
a, WT mice received 105 naïve OT-I cells followed by prime-boost vaccination (administered 14 days apart) with pam-Ova with poly-ICLC or poly-IC and the OT-I cells numbers in spleens were measured 7 days after the boost. b, Similar experiment as in a, except that endogenous CTL responses to the Trp1 epitope were evaluated. c, WT, TLR3-KO or MDA5-KO mice were vaccinated and evaluated as described in a. d, WT and MDA5-KO mice were inoculated s.c. with 3×105 B16F10 melanoma cells followed by ACT of 105 naïve TgTR1 CTLs on day 6 after tumor inoculation. Mice were vaccinated on days 7 and 18 with pam-Trp1 using either poly-ICLC or poly-IC. Tumor growth was monitored over time measuring two opposing diameters. Results are presented for individual mice (each symbol) with the mean ± SD for each group (a–c) or mean tumor size for 5 mice/group with SD (d).
Poly-ICLC stimulates MDA5 more effectively than poly-IC via the proton sponge effect
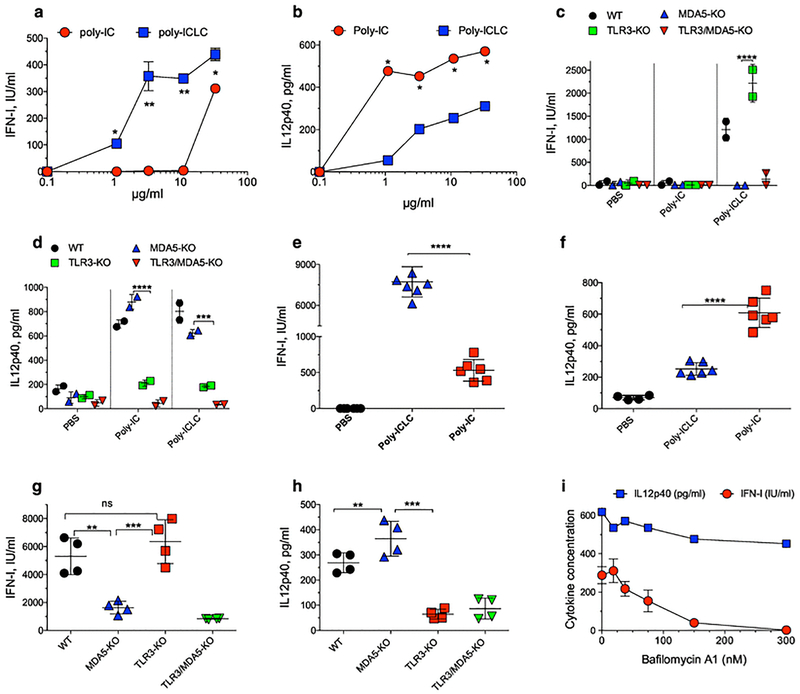
While TLR3 activation takes place in the endosomal compartment after poly-IC internalization, poly-IC must reach the cytoplasm to stimulate MDA5. In addition, whereas TLR3 activation results mostly in IL12 production (which may be more important for the prime), MDA5 stimulation has been linked to high IFN-I production (6, 32, 33). Thus, we compared the ability of poly-IC and poly-ICLC to induce IFN-I and IL12 production by DCs in vitro. While poly-ICLC stimulated higher IFN-I levels than poly-IC in CD8aDCs, poly-IC generated higher amounts of IL12 (Fig. 4a–b). The ability of poly-ICLC to generate superior IFN-I responses than poly-IC was also observed with other DC lines (Supplemental Fig. 7a–b). Poly-ICLC and poly-IC were compared for their capacity to produce these cytokines using BM-derived DCs from WT, MDA5-KO, TLR3-KO, and MDA5/TLR3 double-KO mice. Again, higher IFN-I levels were observed with poly-ICLC as compared with poly-IC and these depended on MDA5 but not on TLR3 (Fig. 4c). Conversely, both poly-ICLC and poly-IC were effective in generating IL12, which required TLR3 but not MDA5 (Fig. 4d). The requirements of MDA5 and TLR3 for IFN-I and IL12 production in vivo by poly-ICLC and poly-IC were evaluated. WT mice injected with poly-ICLC produced more IFN-I and less IL12 as compared to those injected with poly-IC (Fig. 4e–f). The in vivo production of IFN-I by poly-ICLC was decreased in MDA5-KO and TLR3/MDA5-KO mice but not in TLR3-KO mice, while IL12 production was independent of MDA5 and required TLR3 (Fig. 4g–h).
Fig. 4. Poly-ICLC stimulates MDA5 more effectively than poly-IC through the proton sponge effect.
a–b, The CD8aDC cell line was stimulated with various doses of poly-IC or poly-ICLC and 18 h later the levels of IFN-I and IL12p40 were measured in the supernatants. c–d, BM DCs from WT, TLR3-KO, MDA5-KO or TLR3/MDA5 KO mice were stimulated with 10 μg poly-IC or poly-ICLC and 18 h later the levels of IFN-I and IL12p40 were measured in the supernatants. e–f, WT mice were injected (i.v.) with 50 μg poly-ICLC or poly-IC and 18 h later the amounts of IFN-I and IL12p40 were measured in serum. g–h, WT, TLR3-KO, MDA5-KO or TLR3/MDA5-KO were injected (i.v.) with 50 μg poly-ICLC and 18 h later the amounts of IFN-I and IL12p40 were measured in serum. i, The CD8aDC cell line was incubated with bafilomycin A1 6 h before stimulation with 50 μg/ml poly-ICLC and the production of IFN-I and IL12p40 was measured. Results are presented for individual replicate samples/mice (each symbol) with the mean ± SD for each group.
Next, we explored the mechanism by which exogenously administered poly-ICLC can reach the cytoplasm of DCs to activate MDA5. A likely possibility is that the poly-lysine and carboxymethylcellulose components of the poly-ICLC facilitate a proton sponge effect allowing the escape of poly-IC from endosomes into the cytosol. Pretreatment of DCs with bafilomycin A1 (H+ ATPase inhibitor that decreases endosomal swelling and rupturing) (34) diminished poly-ICLC induced IFN-I but not IL12 production (Fig. 4i). On the other hand, bafilomycin A1 did not inhibit IFN-I production by DCs induced by poly-IC (Supplemental Fig. 7c), suggesting that the production of this cytokine by poly-IC is not facilitated via the proton sponge effect. Since bafilomycin A1 may have other effects besides H+ ATPase inhibition, we measured endosomal escape by flow cytometry. These results showed that poly-ICLC, but not poly-IC enhanced endosomal escape of acridine orange into the cytoplasm as measured by red fluorescence (35) (Supplemental Fig. 7d). Collectively, these results indicate that poly-ICLC is more potent inducer of IFN-I, than poly-IC due to its increased ability to escape from the endosomal compartment into the cytoplasm most likely via the proton sponge effect in order to stimulate MDA5.
Role of IFN-I in the T cell expansion mediated by peptide and poly-ICLC
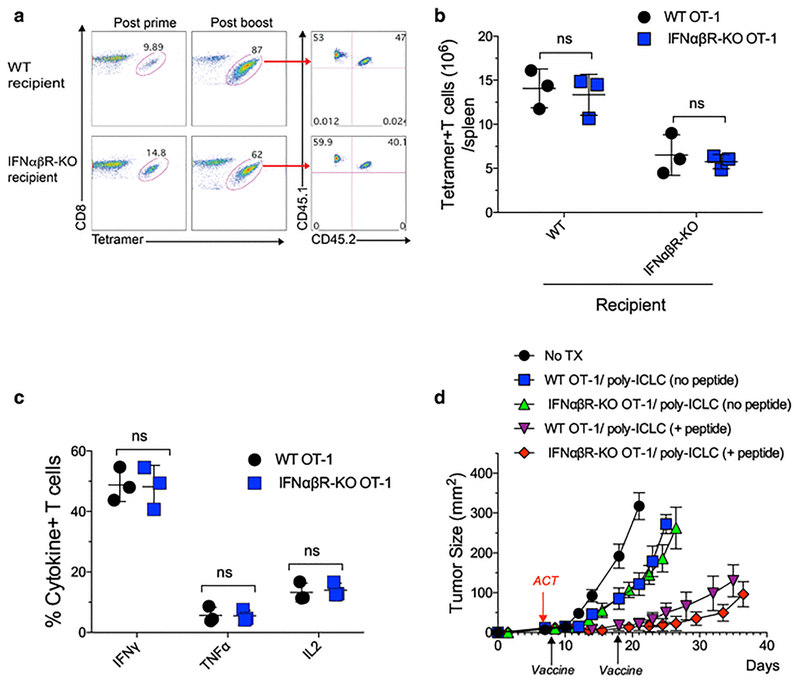
IFN-I has been shown to support in vivo T cell expansion either directly, or indirectly after viral infections (36–38). To test the mechanism by which IFN-I enhanced T cell expansion with peptide vaccination and poly-ICLC, equal numbers of naïve OT-I (50,000 CD45.1+/CD45.2 negative T cells) and IFNabR deficient OT-I (50,000 CD45.1+/CD45.2+ T cells) were transferred into WT or IFNabR-KO host mice. Both populations of OT-I cells expanded equally well after pam-peptide vaccination in the WT or IFNabR-KO hosts (Fig. 5a–b). Nevertheless, the total numbers of OT-I cells in spleens were higher in WT mice compared to IFNabR-KO mice (Fig. 5b) suggesting that IFN-I signals in other cells besides the CTLs within the host participate in the expansion of the transferred CTLs. Similar results were obtained with adoptive transfer of much lower numbers of naïve OT-I and IFNabR deficient OT-I (1,000 each), implying that these observations can be extended to endogenous T cell responses, where the numbers of T cell precursors are low (Supplemental Fig. 8).
Fig. 5. Role of IFN-I in the T cell expansion mediated by peptide and poly-ICLC.
a, WT and IFNabR-KO mice received equal numbers (5 × 104) of IFNabR-KO OT-I cells (CD45.1+/CD45.2+) and WT OT-I cells (CD45.1+/CD45.2-) followed by pam-Ova + poly-ICLC vaccination. The percentages of OT-I cells were evaluated in blood 7 days after prime and boost and the percentages of IFNabR-KO OT-I and WT OT-I cells after the boost are shown. b, Total numbers of the OT-I populations in spleens from the experiment shown in a. c, Cytokine production in OT-I cells from a stimulated with 10 μg/ml mini-Ova peptide evaluated by intracellular cytokine staining. d, WT mice were inoculated s.c. with 3 × 105 B16F10-Ova cells and after 6 days received ACT of 105 IFNabR-KO OT-I or WT OT-I cells, followed one day later by pam-Ova + poly-ICLC prime and a boost on day 18. Results are presented for individual replicate samples (b–c) or mean tumor size for 5 mice/group with SD (d).
Both the IFNabR deficient and IFNabR+ OT-I cells exhibited comparable effector function as they produced similar levels of IFNg, TNFa and IL2 after in vitro antigen stimulation (Fig. 5c). Moreover, IFNabR deficient and IFNabR+ OT-I cells were equally effective in controlling the growth of B16F10-Ova tumors after pam-peptide/poly-ICLC vaccination (Fig. 5d). The antitumor effects of adoptively transferred cells were significantly lower with poly-ICLC alone (no peptide). Thus, the absence of direct IFN-I signals on the CTLs does not impair their ability to expand to vaccination, perform effector function and control tumor growth.
IFN-I signaling leads to IL15 production enabling CTL expansion
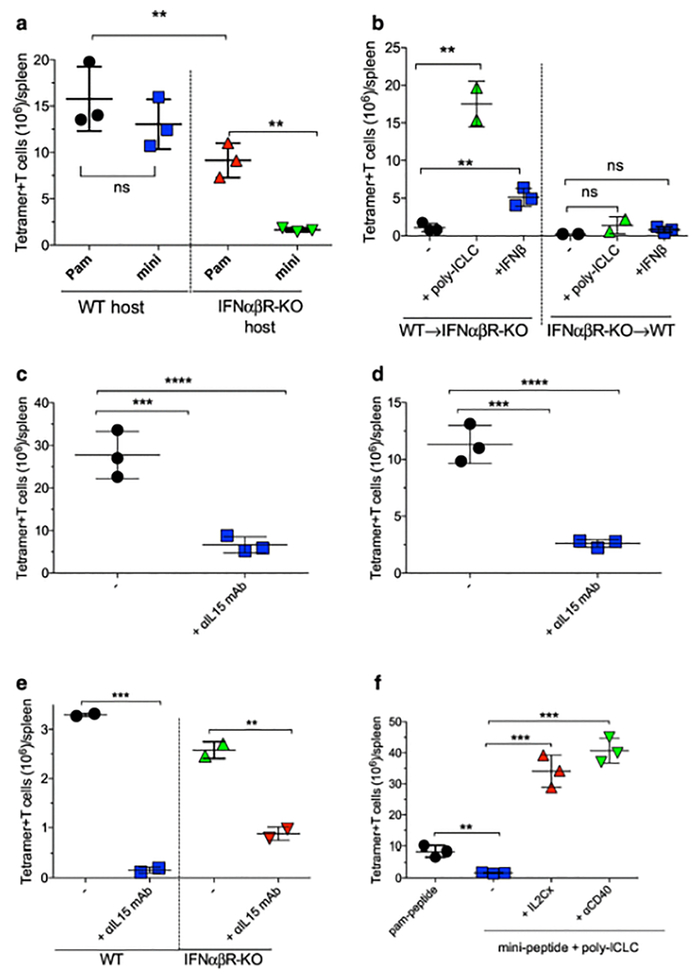
CTL expansion is reduced in IFNabR-KO hosts as compared to WT mice (Fig. 6a). However, the deficiency of CTL expansion when host lacks IFN-I signaling was more severe with the mini-peptide boost. To test whether the IFN-I signals that lead to CTL expansion occur in hematopoietic and/or stromal cells, BM chimeras of IFNabR-KO into WT mice or WT into IFNabR-KO hosts were used. OT-I cells expanded efficiently to mini-peptide/poly-ICLC boosts in IFNabR-KO mice reconstituted with WT BM cells (Fig. 6b). On the other hand, the CTLs failed to expand when IFN-I signaling was limited to stromal cells in WT mice reconstituted with IFNabR-KO BM. Administration of IFN-I as adjuvant instead of poly-ICLC promoted some degree of expansion in the mice reconstituted with WT BM but not in those reconstituted with IFNabR-KO BM. These data suggest that IFN-I signaling on hematopoietic cells is crucial to support CTL expansion.
Fig. 6. IFN-I signaling leads to IL15 production enabling CTL expansion.
a, WT or IFNabR-KO mice received 105 naïve IFNabR-KO OT-I cells followed a day later with a pam-Ova + poly-ICLC vaccine prime. Fourteen days later mice received pam-Ova + poly-ICLC or mini-Ova + poly-ICLC boosts, and the total numbers of OT-I cells in spleens were evaluated 7 days later. b, WT BM into IFNabR-KO or IFNabR-KO BM into WT chimeric mice received 105 naïve IFNabR-KO OT-I cells followed by a pam-Ova + poly-ICLC prime followed 14 days later by mini-Ova boost administered alone (−) or in combination with poly-ICLC or IFNb. Numbers of OT-I cells in spleens were evaluated 7 days after the boost. c, WT mice received 105 naïve OT-I cells followed by 2 doses of pam-Ova + poly-ICLC vaccine 14 days apart. Some mice received aIL15 mAb on days 14, 16 and 18 and the numbers of OT-I cells in spleens were evaluated 7 days after the boost. d, Similar experiment as in c, except that IFNabR-KO OT-I cells were transferred into IFNabR-KO mice. e, WT or IFNabR-KO mice were vaccinated with 2 doses of pam-Trp1 + poly-ICLC 14 days apart with or without aIL15 mAb treatment on days 14, 16 and 18 and the endogenous responses to the Trp1 epitope were evaluated 7 days post-boost. f, IFNabR-KO mice received 105 naïve IFNabR-KO OT-I cells followed by pam-Ova + poly-ICLC vaccine prime followed 14 days later with pam-Ova + poly-ICLC or mini-Ova + poly-ICLC boosts alone or in combination with 100 μg of aCD40 mAb or IL2Cx (on days 14, 16 and 18). Numbers of OT-I cells were evaluated in spleens 7 days after the boost. Results are presented as individual mice (each symbol) with the mean ± SD for each group.
Collectively, these results indicate that in our vaccination system IFN-I does not have a direct effect on CTLs to promote their expansion and that IFN-I mediates CTL expansion through other signal-3 cytokines produced by BM-derived cell populations. Although DCs are an excellent source of these IFN-I stimulated signal-3 cytokines, efficient CTL boosting can be induced in DC-depleted mice with mini-peptide using IFN-I or poly-ICPEI (Fig. 1d–e) indicating that CD11c-negative cells are also capable of producing signal-3 cytokines as a consequence of IFN-I signaling.
To study the downstream events of IFN-I signaling on hematopoietic cells, which lead to CTL expansion, we assessed the role of IFN-I in the poly-ICLC-induced production of IL15, known to be a quintessential T cell growth factor (19–22). Poly-ICLC injections induced the production of soluble IL15/IL15Ra complexes in an IFN-I dependent manner (Supplemental Fig. 9a). Furthermore, administration of IFN-I into CD11c cell-depleted mice stimulated the production of IL15/IL15Ra (Supplemental Fig. 9b). The role of IL15 in CTL expansion during the peptide vaccine boost was validated with a neutralizing anti-IL15 mAb. Blocking IL15 inhibited the expansion of adoptively transferred OT-I cells both in WT and IFNabR-KO mice (Fig. 6c–d; Supplemental Fig. 10a–b). Anti-IL15 mAb also inhibited an endogenous Trp1-specific CD8 T cell response (Fig. 6e). On the other hand, blocking IL2 using a neutralizing anti-IL2 mAb during the vaccine boost did not significantly reduce CTL expansion (Supplemental Fig. 10c). Blocking IL15 during the priming phase of CTL responses had a minimal effect on initial T cell expansion (data not shown), indicating that naïve CTLs require TCR stimulation (i.e., priming) in order to express the receptor for IL15 (CD122).
It has been reported that some IL2/aIL2 antibody complexes (IL2Cx) resemble IL15 signaling by delivering IL2 to IL2Rb (CD122) and preventing binding to IL2Ra (CD25) (39) and that aCD40 mAb injections stimulate the production of IL15/IL15Ra complexes (2, 40, 41). The lack of CTL expansion with mini-peptide boost observed in IFNabR-KO mice was restored with the use of IL2Cx or aCD40 mAb (Fig. 6f; Supplemental Fig. 10d). CTL expansion mediated by aCD40 mAb also depended on IL15, since it was abrogated by anti-L15 mAb (data not shown). The above results indicate that the IFN-I pathway plays an important role in the expansion of CTLs indirectly, by promoting the production of IL15 mostly by BM-derived cells. However, these finding do not explain why CTLs can expand to pam-peptide boosts in the absence of IFN-I signaling (Fig. 6a) but still requires IL15 (Fig. 6d–e).
Upregulation of CD86 expression in npAPCs is sufficient to support T cell expansion in the absence of IFN-I signaling
IFN-I not only increases the production of IL15/IL15Ra but also upregulates CTL costimulatory receptors on different cell subsets (19–22). Poly-ICLC enhanced CD86 surface expression in DCs and significant increased levels were still observed in the absence of IFN-I signaling (Supplemental Fig. 11a). This observation could explain why pam-peptide and poly-ICLC are able to boost CTL responses in the absence of IFN-I signaling. Poly-ICLC was also able to increase the expression of CD86 in CD11c negative cells, but this required IFN-I signaling. On the other hand, poly-ICPEI enhanced CD86 expression on both CD11c+ and CD11c-negative cells in an IFN-I-independent manner (Supplemental Fig. 11b), suggesting that the use of poly-ICPEI (instead of poly-ICLC) would be able to function in a mini-peptide boost in the absence of IFN-I signaling. Indeed, effective CTL expansions induced with a mini-peptide boost were obtained in IFNabR-KO hosts using poly-ICPEI but not with poly-ICLC (Supplemental Fig. 11c). In addition, aCD40 mAb injection also enhanced CD86 expression in all CD45+ spleen cell subsets and independently of both MDA5 and IFN-I (Supplemental Fig. 12), which would help explain the ability of this costimulatory antibody to restore CTL expansion in boosts with mini-peptide in IFNabR-KO mice (Fig. 6f). Collectively, these results demonstrate that enhancing the expression of costimulatory molecules such as CD86 on the surface of npAPCs can mediate T cell expansion by mini-peptide boosts in the absence of IFN-I signaling.
Discussion
The effectiveness of peptide vaccines will depend on their ability to produce large numbers of antigen-specific CTLs. Previous work in our laboratory (2, 13, 42, 43) and results presented herein indicate that most of the CTL expansion induced by our peptide vaccines using poly-ICLC takes place in the boost. Poly-ICLC is a unique adjuvant for CTL responses due to its ability to stimulate TLR3 and MDA5, and while TLR3 is important for the prime, MDA5 is critical for the boost (2). Vaccine priming is more effective when the peptide is presented as an amphiphilic construct that leads to the formation of nanostructures that resemble viral particles (13). These amphiphilic peptide constructs such as pam-peptides, require the antigen crosspresentation mediated by CD8a+ DCs. On the other hand, the peptide boost can be mediated by either a pam-peptide, which again must be crosspresented by CD8a+ DCs or by a mini-peptide that can be presented by npAPCs since it does not require antigen processing. Costimulation on the form of signal-3 cytokines or costimulatory ligands such as CD86 plays a critical role in antigen-mediated CTL expansion. Our results show that different MDA5-mediated costimulatory requirements exist depending on the APC presenting the peptide during the boost. When mini-peptide is presented by npAPCs, it is necessary that poly-ICLC stimulates (via MDA5) the production of IFN-I by DCs. However, IFN-I does not act directly as a signal-3 cytokine on T cells but induces the production of IL15, which is responsible for the massive CTL expansion obtained with our vaccines. Our results show that IFN-I induced IL15 can be produced not only by DCs but by other not yet identified BM-derived cells. In boosts using pam-peptide (which require crosspresentation by CD8a+ DCs) CTL expansion also required MDA5 stimulation and was mediated via IL15 but could take place in the absence of IFN-I signals. Effective CTL stimulation leading to expansion under these circumstances is likely due to the ability of poly-ICLC to enhance CD86 expression in DCs in an IFN-I independent way.
The large molecular mass of poly-IC makes it difficult for non-phagocytic cells to internalize it in order to stimulate TLR3 and MDA5. Using DC depletions, we showed that DCs are the main cell type capable of producing IFN-I, IL12 and IL15/IL15Ra complexes as a consequence of poly-ICLC stimulation. Nonetheless, low levels of IFN-I were still detected after DC depletion suggesting that either depletion was incomplete or that other cells may contribute to IFN-I production by poly-ICLC. It has been reported that stromal cells are capable of producing IFN-I in an MDA5-dependent manner in mice after poly-IC injections (33, 44). However, the mechanisms by which non-phagocytic stromal cells can engulf poly-IC and stimulate MDA5 requires further investigation. Vascular endothelial cells can be stimulated in vitro with poly-ICLC (but not poly-IC) to produce IFN-I (H. Sultan, unpublished) suggesting that scavenger receptor mediated endocytosis and the proton sponge effect may participate in this process. Poly-ICPEI (formulated with a transfection reagent) was able to generate sufficient IFN-I allowing effective CTL expansion by mini-peptide boost in the absence of DCs.
The mechanism by which exogenously administered poly-ICLC reaches the cytoplasm to activate MDA5 was not well understood. Previous reports showed that poly-IC could induce endosomal destabilization and allow escape of poly-IC into the cytoplasm (35), which could explain the ability of poly-IC to produce some IFN-I. However, our results clearly show that poly-ICLC is substantially more efficient than poly-IC in stimulating IFN-I production. Cationic polymers such as poly-lysine (which is a component of poly-ICLC) can increase the endosomal pH by consuming free protons in the endosome followed by swelling, rupture and release of the endosome contents into the cytosol (proton sponge effect) (45–47). Carboxymethyl cellulose (another component of poly-ICLC) may play a role in endosomal rupture by increasing the water retention into the endosome.
In summary, the present study demonstrates that combination of peptide-based cancer vaccines with poly-IC formulations highly stimulatory for MDA5 (poly-ICLC, poly-ICPEI), or the administration of signal-3 cytokines such as IFNb, IL15 or IL2Cx can result in huge expansion of CTLs responses and hence improved antitumor effects.
Supplementary Material
Funding
This work was supported by National Cancer Institute grant R01CA157303, and by start-up funds from Augusta University, Georgia Cancer Center and the Georgia Research Alliance (GRA).
Abbreviations
- ACT
Adoptive cell transfer
- APC
Antigen-presenting cell
- BM
Bone marrow
- CTL
Cytotoxic T lymphocyte
- DC
Dendritic cell
- DT
Diphtheria toxin
- DTR
Diphtheria toxin receptor
- IFN-I
Type-I interferon
- IFNαβR
Type-I interferon receptor
- IFNβ
Interferon beta
- IL2Cx
IL-2 anti-IL2 mAb immune complex
- KO
Knockout
- mAb
Monoclonal antibody
- MDA5
Melanoma differentiation associated protein 5
- MHC-I
MHC class I
- MHC-II
MHC class II
- npAPC
Non-professional antigen presenting cell
- Ova
Ovalbumin
- pam
Palmitoylated
- pAPC
Professional antigen-presenting cell
- pDC
Plasmacytoid DC
- PEI
Polyethylenimine
- poly-IC
Polyinosinic-polycytidylic acid
- poly-ICLC
poly-IC stabilized with poly-lysine and carboxymethyl cellulose
- poly-ICPEI
poly-IC stabilized with PEI
- S3
Signal 3
- TCR
T cell receptor for antigen
- TLR3
Toll-like receptor 3
- Trp1
Tyrosinase-related protein 1
- WT
Wild type
Footnotes
Conflict of interest
A. Salazar is President and CEO of Oncovir, Inc. and is developing poly-ICLC (Hiltonol ™) for the clinic. Esteban Celis has filed patent applications based on the use of synthetic peptides and poly-IC combinatorial vaccines. The rights of the patent applications have been transferred to the Moffitt Cancer Center (Tampa, FL). Other authors declare no conflict of interest.
Statement on the welfare of animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in the experiments involving animals were in accordance with the ethical standards of the Augusta University Institutional Animal Care and Use Committee where all the studies were conducted (Protocol No. 2013–0598, approved on 11/21/2016).
Human subjects
This article does not contain any studies with humans done by any of the authors.
References
- 1.Rosenberg SA, Dudley ME (2009) Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 21: 233–40. doi: 10.1016/j.coi.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho HI, Barrios K, Lee YR, Linowski AK, Celis E (2013) BiVax: a peptide/poly-IC subunit vaccine that mimics an acute infection elicits vast and effective anti-tumor CD8 T-cell responses. Cancer Immunol Immunother. 62: 787–99. doi: 10.1007/s00262-012-1382-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho HI, Celis E (2009) Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer Res. 69: 9012–9. doi: 10.1158/0008-5472.can-09-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrios K, Celis E (2012) TriVax-HPV: an improved peptide-based therapeutic vaccination strategy against human papillomavirus-induced cancers. Cancer Immunol Immunother. 61: 1307–17. doi: 10.1007/s00262-012-1259-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Celis E (2015) STING activator c-di-GMP enhances the anti-tumor effects of peptide vaccines in melanoma-bearing mice. Cancer Immunol Immunother. 64: 1057–66. doi: 10.1007/s00262-015-1713-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato H, Takeuchi O, Sato S et al. (2006) Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 441: 101–5. doi: 10.1038/nature04734 [DOI] [PubMed] [Google Scholar]
- 7.Dougan SK, Dougan M, Kim J, Turner JA, Ogata S, Cho HI, Jaenisch R, Celis E, Ploegh HL (2013) Transnuclear TRP1-specific CD8 T cells with high or low affinity TCRs show equivalent antitumor activity. Cancer Immunol Res. 1: 99–111. doi: 10.1158/2326-6066.cir-13-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duran-Struuck R, Dysko RC (2009) Principles of bone marrow transplantation (BMT): providing optimal veterinary and husbandry care to irradiated mice in BMT studies. J Am Assoc Lab Anim Sci. 48: 11–22. [PMC free article] [PubMed] [Google Scholar]
- 9.Fuertes Marraco SA, Grosjean F, Duval A et al. (2012) Novel murine dendritic cell lines: a powerful auxiliary tool for dendritic cell research. Front Immunol. 3: 331. doi: 10.3389/fimmu.2012.00331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann VS, Davoust J, Ricciardi-Castagnoli P (1997) Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 185: 317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen Z, Reznikoff G, Dranoff G, Rock KL (1997) Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 158: 2723–30. [PubMed] [Google Scholar]
- 12.Assudani D, Cho HI, DeVito N, Bradley N, Celis E (2008) In vivo expansion, persistence, and function of peptide vaccine-induced CD8 T cells occur independently of CD4 T cells. Cancer Research. 68: 9892–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sultan H, Fesenkova VI, Addis D, Fan AE, Kumai T, Wu J, Salazar AM, Celis E (2017) Designing therapeutic cancer vaccines by mimicking viral infections. Cancer Immunol Immunother. 66: 203–13. doi: 10.1007/s00262-016-1834-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sultan H, Kumai T, Fesenkova VI, Fan AE, Wu J, Cho HI, Kobayashi H, Harabuchi Y, Celis E (2018) Sustained persistence of IL2 signaling enhances the antitumor effect of peptide vaccines through T-cell expansion and preventing PD-1 inhibition. Cancer Immunol Res. doi: 10.1158/2326-6066.CIR-17-0549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature. 392: 245–52. doi: 10.1038/32588 [DOI] [PubMed] [Google Scholar]
- 16.den Haan JM, Lehar SM, Bevan MJ (2000) CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 192: 1685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heath WR, Belz GT, Behrens GM et al. (2004) Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 199: 9–26. doi: 10.1111/j.0105-2896.2004.00142.x [DOI] [PubMed] [Google Scholar]
- 18.Jung S, Unutmaz D, Wong P et al. (2002) In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 17: 211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunzmann V, Kretzschmar E, Herrmann T, Wilhelm M (2004) Polyinosinic-polycytidylic acid-mediated stimulation of human gammadelta T cells via CD11c dendritic cell-derived type I interferons. Immunology. 112: 369–77. doi: 10.1111/j.1365-2567.2004.01908.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baranek T, Manh TP, Alexandre Y et al. (2012) Differential responses of immune cells to type I interferon contribute to host resistance to viral infection. Cell Host Microbe. 12: 571–84. doi: 10.1016/j.chom.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 21.Berard M, Brandt K, Bulfone-Paus S, Tough DF (2003) IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 170: 5018–26. [DOI] [PubMed] [Google Scholar]
- 22.Mattei F, Schiavoni G, Belardelli F, Tough DF (2001) IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol. 167: 1179–87. [DOI] [PubMed] [Google Scholar]
- 23.Yao X, Wu J, Lin M et al. (2016) Increased CD40 Expression Enhances Early STING-Mediated Type I Interferon Response and Host Survival in a Rodent Malaria Model. PLoS Pathog. 12: e1005930. doi: 10.1371/journal.ppat.1005930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buhtoiarov IN, Lum H, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL (2005) CD40 ligation activates murine macrophages via an IFN-gamma-dependent mechanism resulting in tumor cell destruction in vitro. J Immunol. 174: 6013–22. [DOI] [PubMed] [Google Scholar]
- 25.Ellermeier J, Wei J, Duewell P et al. (2013) Therapeutic efficacy of bifunctional siRNA combining TGF-beta1 silencing with RIG-I activation in pancreatic cancer. Cancer Res. 73: 1709–20. doi: 10.1158/0008-5472.can-11-3850 [DOI] [PubMed] [Google Scholar]
- 26.McKenna K, Beignon AS, Bhardwaj N (2005) Plasmacytoid dendritic cells: linking innate and adaptive immunity. J Virol. 79: 17–27. doi: 10.1128/jvi.79.1.17-27.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perussia B, Fanning V, Trinchieri G (1985) A leukocyte subset bearing HLA-DR antigens is responsible for in vitro alpha interferon production in response to viruses. Nat Immun Cell Growth Regul. 4: 120–37. [PubMed] [Google Scholar]
- 28.Hildner K, Edelson BT, Purtha WE et al. (2008) Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 322: 1097–100. doi: 10.1126/science.1164206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sammons ML, Stephen EL, Levy HB, Baron S, Hilmas DE (1977) Interferon induction in cynomolgus and rhesus monkey after repeated doses of a modified polyriboinosinic-polyribocytidylic acid complex. Antimicrob Agents Chemother. 11: 80–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lampson GP, Field AK, Tytell AA, Hilleman MR (1981) Poly I:C/poly-L-lysine: potent inducer of interferons in primates. J Interferon Res. 1: 539–49. [DOI] [PubMed] [Google Scholar]
- 31.Talmadge JE, Adams J, Phillips H et al. (1985) Immunomodulatory effects in mice of polyinosinic-polycytidylic acid complexed with poly-L-lysine and carboxymethylcellulose. Cancer Res. 45: 1058–65. [PubMed] [Google Scholar]
- 32.Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M (2006) Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A. 103: 8459–64. doi: 10.1073/pnas.0603082103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCartney S, Vermi W, Gilfillan S, Cella M, Murphy TL, Schreiber RD, Murphy KM, Colonna M (2009) Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J Exp Med. 206: 2967–76. doi: 10.1084/jem.20091181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y (1998) Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 23: 33–42. [DOI] [PubMed] [Google Scholar]
- 35.Zou J, Kawai T, Tsuchida T, Kozaki T, Tanaka H, Shin KS, Kumar H, Akira S (2013) Poly IC triggers a cathepsin D-and IPS-1-dependent pathway to enhance cytokine production and mediate dendritic cell necroptosis. Immunity. 38: 717–28. doi: 10.1016/j.immuni.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 36.Aichele P, Unsoeld H, Koschella M, Schweier O, Kalinke U, Vucikuja S (2006) CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J Immunol. 176: 4525–9. [DOI] [PubMed] [Google Scholar]
- 37.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K (2005) Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 202: 637–50. doi: 10.1084/jem.20050821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF (2009) Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 182: 2786–94. doi: 10.4049/jimmunol.0803484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J (2006) Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 311: 1924–7. doi: 10.1126/science.1122927 [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Sun S, Hwang I, Tough DF, Sprent J (1998) Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 8: 591–9. [DOI] [PubMed] [Google Scholar]
- 41.Zhang M, Yao Z, Dubois S, Ju W, Muller JR, Waldmann TA (2009) Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proc Natl Acad Sci U S A. 106: 7513–8. doi: 10.1073/pnas.0902637106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sultan H, Trillo-Tinoco J, Rodriguez P, Celis E (2017) Effective antitumor peptide vaccines can induce severe autoimmune pathology. Oncotarget. 8: 70317–31. doi: 10.18632/oncotarget.19688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumai T, Lee S, Cho HI, Sultan H, Kobayashi H, Harabuchi Y, Celis E (2017) Optimization of Peptide Vaccines to Induce Robust Antitumor CD4 T-cell Responses. Cancer Immunol Res. 5: 72–83. doi: 10.1158/2326-6066.cir-16-0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Cella M, Gilfillan S, Colonna M (2010) Cutting edge: polyinosinic:polycytidylic acid boosts the generation of memory CD8 T cells through melanoma differentiation-associated protein 5 expressed in stromal cells. J Immunol. 184: 2751–5. doi: 10.4049/jimmunol.0903201 [DOI] [PubMed] [Google Scholar]
- 45.Pack DW, Hoffman AS, Pun S, Stayton PS (2005) Design and development of polymers for gene delivery. Nat Rev Drug Discov. 4: 581–93. doi: 10.1038/nrd1775 [DOI] [PubMed] [Google Scholar]
- 46.Sonawane ND, Szoka FC Jr., Verkman AS (2003) Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 278: 44826–31. doi: 10.1074/jbc.M308643200 [DOI] [PubMed] [Google Scholar]
- 47.Wagner E, Zatloukal K, Cotten M, Kirlappos H, Mechtler K, Curiel DT, Birnstiel ML (1992) Coupling of adenovirus to transferrin-polylysine/DNA complexes greatly enhances receptor-mediated gene delivery and expression of transfected genes. Proc Natl Acad Sci U S A. 89: 6099–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.