Abstract
The endogenous neuropeptide galanin and its associated receptors GalR1 and GalR2 are highly localized in the brain limbic structures and play an important role in the control of seizures in animal epilepsy models. As such, galanin receptors provide an attractive target for the development of novel anticonvulsant drugs. Our efforts to engineer galanin analogs that can penetrate the blood-brain-barrier and suppress seizures, yielded NAX 5055 (Gal-B2), a systemically-active analog that maintains low nanomolar affinity for galanin receptors and displays a potent anticonvulsant activity. In this report, we show that NAX 5055 is active in three models of epilepsy; i.e., the Frings audiogenic seizure-susceptible mouse, the mouse corneal kindling model of partial epilepsy and the 6 Hz model of pharmacoresistant epilepsy. NAX 5055 was not active in the traditional maximal electroshock and subcutaneous pentylenetetrazol seizure models. Unlike most antiepileptic drugs (AEDs), NAX 5055 showed high potency in the 6 Hz model of epilepsy across all three different stimulation currents; i.e., 22, 32 and 44 mA, suggesting a potential use in the treatment of pharmacoresistant epilepsy. Furthermore, NAX 5055 was found to be biologically active following intravenous, intraperitoneal and subcutaneous administration and efficacy was associated with a linear pharmacokinetic profile. The results of the present investigation suggest that NAX 5055 is a first-in-class neurotherapeutic for the treatment of epilepsy in patients refractory to currently approved AEDs.
Keywords: Neuropeptide, anticonvulsant drug, audiogenic seizures, 6 Hz seizure, corneal kindled mouse
Introduction
It is estimated that there are 50-60 million people with epilepsy worldwide. Epilepsy affects approximately three million people in the United States alone. Each year, 125,000 new cases are diagnosed. Unfortunately, only 70% of patients are effectively treated with currently available AEDs. For a substantial number of patients with epilepsy, the currently available therapies are often not effective in controlling their symptoms. As such, there is a substantial need for more effective therapies.
Neuropeptides are potent modulators of neuronal excitability1, 2. Under ambient conditions, peptides are “silent” and exert little effect on normal neurotransmission. However, under conditions of excessively high neuronal firing; i.e., epileptic seizures, neuropeptides are released and exert a modulatory effect on neurotransmission. Unlike small molecule neurotransmitters, neuropeptides are released distal to the synaptic cleft, and instead of their action being eliminated by reuptake or rapid degradation, it is limited by diffusion and enzymatic degradation. Unlike classical neurotransmitters, the action of neuropeptides is slow, sustained, and can extend to beyond the synaptic region.3 One such neuropeptide is galanin and it has been implicated in such diverse behaviors as sleep, feeding, reproduction, nociception, and cognition. Galanin is found along with many neurotransmitters, including glutamate, GABA, acetylcholine, norepinephrine, serotonin, dopamine and histamine, suggesting it has many roles in the brain4, 5. Galanin, which can be found both centrally and peripherally, is highly expressed along with the galanin receptor 1 (GalR1) in the hippocampus, a plastic structure highly susceptible to seizure activity.
There have been numerous studies linking galanin to seizure activity. Expression levels of galanin and its receptors change in response to, or as a result of, seizures.6 For example, galanin levels and galanin receptor GalR2 protein are decreased following status epilepticus. Since the work of Mazarati and coworkers7, there has been increasing evidence that galanin is a potent anticonvulsant peptide. Previous investigations have demonstrated that galanin and galanin agonists, when delivered directly to the brain, possess anticonvulsant properties8, 9. Moreover, the acute administration of galanin receptor agonists or virus-mediated overexpression of galanin in the hippocampus has been found to inhibit limbic status epilepticus, pentylenetetrazol- and picrotoxin-induced seizures in rats and mice7, 8, 10. Furthermore, several reports have suggested that centrally administered galanin may modify the damage associated with limbic seizures and delay or prevent the development of epilepsy (i.e. disease modifying). Kokaia et al.11 reported delayed kindling in galanin peptide overexpressing mice. esults obtain Furthermore, red from studies with GalR1 knockout mice and rats treated with antisense GalR2 oligonucleotides suggests that galanin exerts its anticonvulsant effect through an action at both GalR1 and GalR212, 13.
Previous attempts to pharmacologically target the galanin system have been plagued by the finding that peptides are generally not metabolically stable and do not readily penetrate the blood-brain-barrier (BBB). Two galanin-based agonists, galmic and galnon, that possess anticonvulsant activity have been reported in the literature14, 15. However, these compounds show little receptor subtype specificity (2-3 fold difference in affinity for GalR1 and GalR2), and have a 10,000-100,000-fold lower affinity for galanin receptors relative to the native peptide. Furthermore, significant off-target activities have been described for both galmic and galnon16.
In the present investigation we describe the anticonvulsant actions and pharmacological profile of the novel BBB permeable galanin analog NAX 5055 (Gal-B2) which is bioavailable, metabolically stable, and retains nanomolar affinity and selectivity for galanin receptors17. Previously published results from our laboratory have described in detail the physicochemical properties of this and other galanin analogs. Here we describe the activity of NAX 5055 in various animal seizure and epilepsy models and discuss its pharmacokinetic profile and systemic bioavailability. In addition, our results demonstrate that the anticonvulsant activity of this modified peptide is specific for the intact structure of NAX 5055.
Materials and Methods
Peptide synthesis
NAX 5055 and two galanin analogs (a truncated and a scrambled peptide) were chemically synthesized using identical methods as described previously17. Briefly, the peptides were assembled on solid support using the Fmoc protocol and an automated peptide synthesizer. The analogs were cleaved from the resin by a treatment with a reagent K and purified using reverse-phase HPLC separations. Chemical identities of the analogs were confirmed by mass spectroscopy analyses.
Animals and Test Substances
Male albino CF1 mice (18-25 g, Charles River, Portage, MI) and male and female Frings audiogenic seizure (AGS)-susceptible (20-30 g, obtained from an in-house colony at the University of Utah) were used as experimental animals. All animals were allowed free access to both food (Prolab RMH 3000) and water except when they were removed from their cages for the experimental procedure. All mice were housed, fed, and handled in a manner consistent with the recommendations in the National Research Council Publication, “Guide for the Care and Use of Laboratory Animals.” No insecticides capable of altering hepatic drug metabolism enzymes were used in the animal facilities. Except for corneal kindling studies, animals were used once. All animals were euthanized in accordance with the Institute of Laboratory Resources policies on the humane care of laboratory animals.
NAX 5055 was administered in 0.9% saline intravenously (i.v.), intraperitoneally (i.p.), subcutaneously (s.c.), or orally (p.o.) in a volume of 0.01 ml/g body weight.
Anticonvulsant Tests
The anticonvulsant activity of NAX 5055 was established by both electrical and chemical tests. The electrical tests used were the maximal electroshock (MES) seizure, the 6 Hz psychomotor seizure, and the corneal kindled mouse18-20. The chemical tests included the s.c. pentylenetetrazol (PTZ) seizure18.
MES and 6 Hz Tests
For the MES and 6 Hz tests, a drop of anesthetic/electrolyte solution (0.5% tetracaine hydrochloride in 0.9% saline) was applied to the eyes of each animal prior to placement of the corneal electrodes. The electrical stimulus in the MES test was 50 mA, 60 Hz delivered for 0.2 sec by an apparatus similar to that originally described by Woodbury and Davenport 21. Elimination of the hindleg tonic extensor component of the seizure was used as the endpoint.
The ability of the test substance to prevent seizures induced by 6 Hz corneal stimulation (6 Hz, 0.2 msec rectangular pulse width, 3 sec duration) was evaluated at convulsive currents (CC) of 22, 32 and 44 mA. Current was delivered with a Grass S48 stimulator. Six Hz seizures are characterized by a minimal clonic phase that is followed by stereotyped automatistic behaviors described originally as being similar to the aura of human patients with partial seizures19, 22. Animals not displaying this behavior were considered protected.
Corneal Kindling
NAX 5055 was evaluated for its ability to block the fully expressed corneal kindled seizure. Individual mice were corneal kindled according to the procedures established by Matagne and Klitgaard 23. Briefly, each mouse received a twice daily corneal stimulation of 3mA for 3 seconds Monday through Friday. Prior to each of the stimulations, a drop of 0.5% tetracaine was applied to the cornea of each mouse. The kindling procedure was continued until each mouse displayed at least five consecutive secondarily generalized seizures; i.e., Stage 4 to 5 seizures according to the Racine scale 24. On the day of the experiment, fully kindled mice were treated i.p. with NAX 5055. One hour after NAX 5055 administration, mice were challenged with the same current stimulus used to kindle them; i.e., 3 mA for 3 sec. Mice not displaying a prototypical seizure were considered protected.
Pentylenetetrazol (PTZ)-induced Seizures
NAX 5055 was evaluated for its ability to block a minimal 3 second episode of a clonic seizure induced by 85 mg/kg PTZ (dissolved in 0.9% saline) administered s.c. into a loose fold on the back of the neck. Animals were observed for at least 30 min for the presence or absence of a seizure.
Audiogenic Seizures
NAX 5055 was tested for its ability to block audiogenic seizures in the Frings AGS-susceptible mouse following i.p. administration. At the previously determined time to peak effect (TPE) individual male and female Frings mice were placed into a plexiglass cylinder (diameter, 15cm; height 18 cm) fitted with an audio transducer (Model AS-ZC; FET Research and Development, Salt Lake City, UT) and exposed to a sound stimulus of 110 decibels (11 KHz) delivered for 20 sec. Sound-induced AGS are characterized by wild running followed by loss of righting reflex with forelimb and hindlimb tonic extension. Mice not displaying hindlimb tonic extension were considered protected.
Minimal Toxicity Tests
Minimal toxicity was identified in mice by the rotorod procedure 25. When a mouse is placed on a 1-inch knurled rod that rotates at a speed of 6 rpm, the animal can maintain its equilibrium for long periods of time. Mice were considered toxic if they fell off this rotating rod three times during a 1-min period. In addition, individual mice were observed for the presence or absence of ataxia, abnormal gait, somnolence, and other signs of acute toxicity. In addition, the effect of NAX 5055 on core body temperature was evaluated at various times after i.p. administration of 4 mg/kg.
Determination of Median Effective (ED50) or Toxic Dose (TD50)
All quantitative in vivo anticonvulsant/toxicity studies were conducted at the time to peak effect (TPE) following i.v., i.p., s.c, and p.o. administration. Groups of at least eight mice were tested with various doses of NAX 5055 until at least two points were established between the limits of 100% protection or maximal toxicity and 0% protection or minimal toxicity. The dose of NAX 5055 required to produce the desired endpoint in 50% of animals (ED50 or TD50) and the 95% confidence intervals were then calculated by a computer program based on the method described by Finney 26.
Pharmacokinetics
To determine the plasma levels of NAX 5055, trunk blood was collected at appropriate time points into heparin coated tubes and centrifuged for 3 min at 5,000 rpm. Plasma samples were then collected, immediately frozen on dry ice and stored in a -80 °C freezer. Prior to the LC/MS analysis, 50 μL of plasma samples were transferred to 96-well plates, diluted with 50 μL of 5% formic acid in water, mixed by vortexing for 1 min, followed by the addition of 600 μL acetonitrile. Diluted samples were mixed by vortexing for 1 min, centrifuged at 3,000 rpm for 3 min, and the resulting supernatant was transferred to a clean 96-well plate. Samples were dried under nitrogen in the Turbovap, and then reconstituted with 300 μL of 0.1% formic acid in 20% acetonitrile in aqueous solution. To produce the standard curve, the plasma samples from untreated mice were spiked with known amounts of NAX 5055. Samples were separated by reversed-phase HPLC (Shimadzu SCL-10A controller with LC-10AD pump), using a C18 column (Varian Metasil, AQ C18, 50 × 2 mm), a linear gradient of acetonitrile in 0.2% formic acid and a flow rate of 0.5 mL/min. The separations were monitored by electrospray ionization mass spectrometry (API 5000) in the positive ion ionization mode. The standard curve was linear within the range of 12.5 ng/mL to 20,000 ng/mL.
Results
Anticonvulsant profile
The results obtained with NAX 5055 in a battery of well-established anticonvulsant tests are summarized in Table 1 and Figures 2 and 3. The anticonvulsant activity of NAX 5055 was initially defined in the Frings audiogenic seizure (AGS)-susceptible mouse model of reflex epilepsy following i.p. administration of 4 mg/kg. At various times after i.p. administration each mouse was placed into a cylindrical test chamber fitted with an audio transducer and challenged with a high-intensity sound stimulus. The results obtained from this study demonstrate that NAX 5055 displays a time-dependent anticonvulsant effect with a time to peak effect of 1 h. The ED50 for protection against sound-induced seizures was determined to be 3.2 mg/kg, i.p. (Table 1).
Table 1. Anticonvulsant profile of NAX 5055.
| Seizure Model | ED50 (mg/kg i.p.) | PIa |
|---|---|---|
| Corneal Kindling | 0.65 | ∼30 |
| Audiogenic Seizures | 3.2 | ∼7 |
| Maximal Electroshock | Inactive at 20 mg/kg | <1 |
| s.c. Pentylenetetrazol | 25% at 20 mg/kg | <1 |
PI, protective index; i.e., TD50 (21 mg/kg)/ED50
s.c., subcutaneous injection
Figure 2.
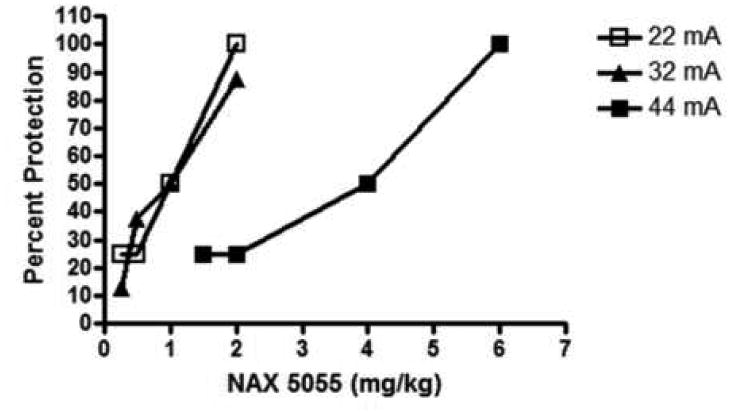
NAX 5055 maintains potent anticonvulsant activity in the mouse 6 Hz seizure model with increasing stimulation intensities (22 mA, 32 mA and 44 mA). Bolus injections of NAX 5055 were administered intraperitoneally and dose-response data were generated at the 1 h time point (time-to-peak effect). Based on these results, ED50 values were calculated as 0.7 mg/kg for 22 mA, 0.8 mA for 32 mA and 2.9 mg/kg for 44 mA stimulations.
Figure 3.
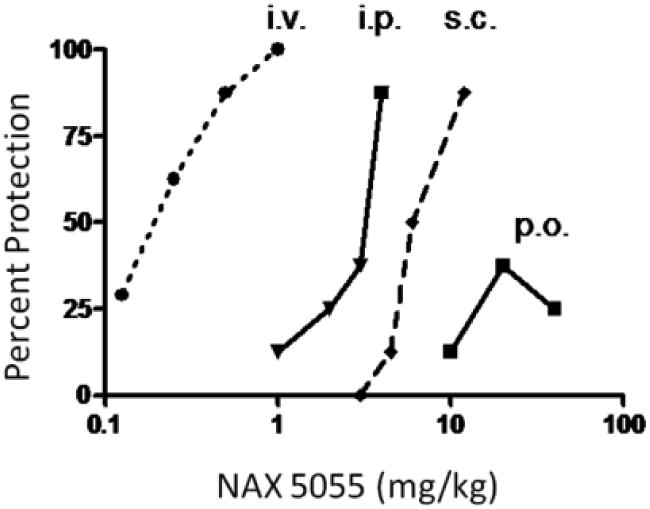
Bioavailability of NAX 5055 following several routes of systemic administration. NAX 5055 was administered intravenously (i.v.), intraperitoneally (i.p.), subcutaneously (s.c.) and orally (p.o.) in CF-1 mice. Dose-response data were generated at the following time-points for each route of administration 1 h (i.v., i.p., s.c.) and 2 hr (p.o.).
Following i.p. administration, NAX 5055 was found to be highly potent against acute psychomotor seizures induced by 6 Hz corneal stimulation regardless of the stimulus intensity employed (Figure 2). For example, ED50s determined at the i.p. TPE against 6 Hz seizures induced at 22, 32, and 44 mA were 0.7, 0.8, and 2.9 mg/kg, respectively.
NAX 5055 was also extremely potent against the fully expressed secondarily generalized seizure in the mouse corneal kindling model of partial seizures (ED50: 0.63 mg/kg; Table 1). In this test, NAX 5055 reduced the seizure severity from an average Racine score of 5 to < 1 at the highest dose evaluated; i.e., 6 mg/kg, i.p.
In an effort to determine the relative bioavailability of NAX 5055, it was administered by i.v., i.p., s.c., and p.o. routes in the 32 mA 6 Hz seizure model. The rank order of potency was i.v. > i.p.> s.c. > p.o. (Figure 3). When compared to the i.p. route, NAX 5055 was approximately 7 times more potent following i.v. administration and approximately 8-fold less potent following s.c. administration (ED50's: 0.25, 1.8, and 7.8 mg/kg, respectively). NAX 5055 was not fully efficacious following p.o. administration. As shown in Figure 3, a maximum of 37.5% protection was observed at a dose of 20 mg/kg, p.o. Moreover, increasing the dose to 40 mg/kg, p.o. resulted in a slightly lower level of protection (25%).
In contrast to the modified galanin-based analog NAX 5055, the unmodified native peptide GAL(1-16) was inactive at a dose of 4 mg/kg, i.p. when tested at a current intensity of 32 mA (Table 2). Previously we have demonstrated that the native peptide fragment was active when it was administered directly to the brain via intracerebroventricular administration17. Two NAX 5055 analogs, scrambled and truncated, were also inactive in this model when tested across time at a dose of 4 mg/kg, i.p. (Table 2). These two analogs had critical residues for the galanin receptors binding either exchanged (NAX 5055 scrambled) or removed (NAX 5055 truncated). Collectively, these results suggest that anticonvulsant activity of NAX 5055 is dependent on penetration of the BBB and selectivity for the target GalR1 receptor.
Table 2.
Anticonvulsant activity of NAX 5055 and control analogs, “scrambled” and “truncated” in the 6 Hz (32 mA) model of epilepsy in CF-1 mice.
| Analog | Structure | Dose 4 mg/kg, i.p. | ||||
|---|---|---|---|---|---|---|
| 15′ | 30′ | 60′ | 120′ | 240′ | ||
| Gal(1-16)a | GWTLNSAGYLLGPHAV-NH2 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| NAX 5055a | (Sar)WTLNSAGYLLGPKK(Lys-P)K | 3/4 | 4/4 | 4/4 | 4/4 | 0/4 |
| NAX 5055 scrambled | (Sar)YTLLSAGWLLGPKK(Lys-P)K | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| NAX 5055 truncated | Ac-YLLGPKK(Lys-P)K | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
Values shown represent a number of mice protected from seizures in groups of four mice per time point. Sar is sarcosine, Lys-P is a lipoamino acid, Lysine-palmitoyl. Note that Trp2, Asn5 and Tyr9 are critical residues for binding to the galanin receptors. Any modifications or replacement of these amino acid residues results in a loss of the affinity to the galanin receptors.42, 43
values reported previously from Bulaj et al, 2008.17
Pharmacokinetics
To assess the pharmacokinetic profile of NAX 5055, plasma levels of the galanin analog were determined in mice at different time points following a bolus i.p. administration. As illustrated in Figure 4A, detectable plasma levels of NAX 5055 were observed after 15 min post drug administration (2 mg/kg) and peaked at 1 hour. Based on a time course of disappearance of NAX 5055, the half-life was estimated to be 120 minutes. As shown in Figure 4B, the time-dependent protection against seizures in the 32 mA 6 Hz model was correlated with NAX 5055 plasma levels. In addition, NAX 5055 displayed linear pharmacokinetics between 0.1 mg/kg to 4 mg/kg. Full protection of animals from seizures was obtained with plasma levels of 4,500 ng/mL.
Figure 4.
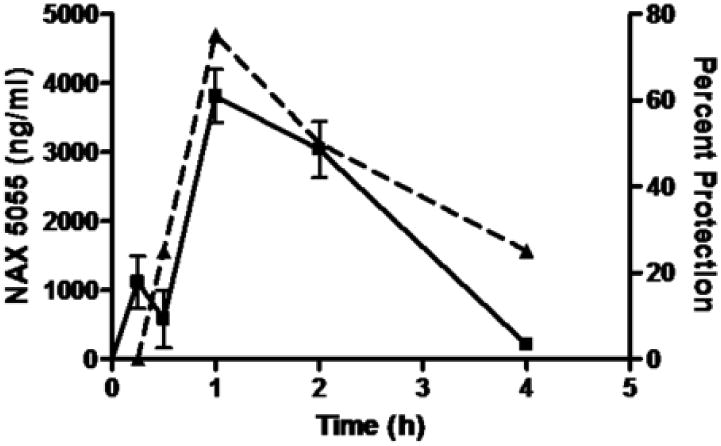
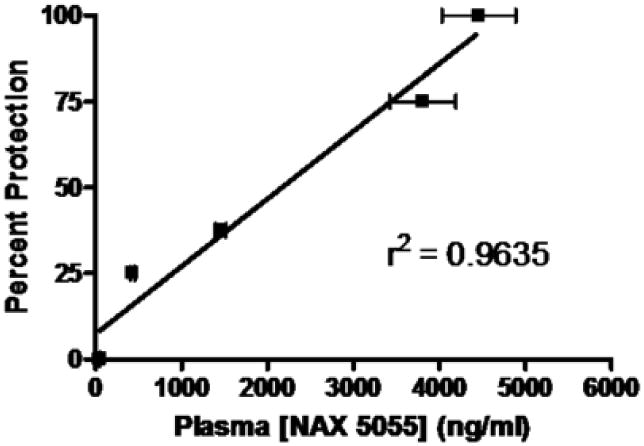
Pharmacokinetic analysis of NAX 5055 in CF-1 mice. A) time-dependent changes of NAX 5055 (solid line) following a bolus intraperitoneal administration of 2 mg/kg dose. Dash line represents a time-response of NAX 5055 at the same dose in protecting mice from seizures in the 6 Hz (32 mA) seizure model. B) a linear correlation between plasma levels of NAX 5055 and percent of seizure protection at doses 0.1, 0.25, 0.5, 2 and 4 mg/kg. Plasma levels were determined at 1 h after the administration of NAX 5055.
Discussion
The goal of the present study was to extend the pharmacological profile of NAX 5055 in epilepsy models. We previously showed that NAX 5055 was active in the 32 mA 6 Hz model of refractory epilepsy17. The present study demonstrated that NAX 5055 retained its potency in the 6 Hz test across all stimulation intensities (22 mA to 44 mA). The ED50 value at each stimulation intensity was well below the dose producing motor impairment in mice. As discussed below, these results are in contrast to those for several other marketed AEDs which show a significant loss of potency or become inactive in the 6 Hz test at higher stimulation intensities. In addition to the 6 Hz model, NAX 5055 was found to be a potent blocker of secondarily generalized seizures in the corneal kindled mouse. NAX 5055 was also effective in controlling sound-induced motor seizures in Frings audiogenic mice. However, NAX 5055 was not active in the mouse maximal electroshock test and was only minimally active in the s.c. PTZ at high dose. We previously showed that NAX 5055 binds the GalR1 and GalR2 receptors17 and the antiepileptic effects for NAX 5055 observed in this study do not appear to be mediated by the chemical modifications to the analog. Neither the scrambled nor truncated peptides showed antiepileptic activity when evaluate in the 6 Hz test.
These study results revealed that NAX 5055 was active following several routes of systemic administration (i.v., i.p., s.c. and p.o.) and possesses predictable pharmacokinetic properties. It was most potent with i.v. administration and only weakly active following oral administration. One hour following i.p. administration there was a linear correlation between antiepileptic activity and measured plasma concentration. The time-course of activity in the 6 Hz test also tracked closely with measured plasma levels of the compound. Behavioral activity was apparent at 15 minutes with measurable plasma levels and declined after 2 hours, which is near the half-time for measured plasma levels.
Galanin and its receptors have been validated as antiepileptic therapeutic targets using genetic and pharmacological tools 27-29. Galanin immunoreactivity in the hippocampus is highest in the dentate gyrus, which receives excitatory innervations from the entorhinal cortex. The entorhinal cortex-dentate gyrus fiber tract is also known as the perforant pathway, which can be stimulated to evoke seizure activity30. The Gal-R1 and R2 subtypes are both expressed in the hippocampus and are believed to contribute to different components of seizure generation and epileptogenesis31. There is obviously great therapeutic potential in the targeting of these two receptors. Compounds active at the galanin receptors which also penetrate the BBB have been previously synthesized. Galnon and galmic are non-peptide small molecules and both can be administered systemically to inhibit seizures in animal models of PTZ- and perforant path-stimulated seizures32, 33. However, these compounds have been of limited value due to low binding affinity and the lack of receptor subtype discrimination. Our efforts have instead been directed towards making a galanin peptide analog that not only can penetrate the BBB and remain metabolically stable, but also retain nanomolar affinity. We have shown that by using a combination of lipidization and cationization on the galanin backbone, these goals can be achieved17. Furthermore, we have been able to engineer GalR1 or GalR2 selectivity into these galanin analogs. In this paper we demonstrate the functionality of one compound, NAX 5055, in halting the seizure activity in particular forms of evoked seizures.
As summarized above, NAX 5055 is not active in the MES and scPTZ tests. In this regard, the anticonvulsant profile of NAX 5055 is very much like that of the SV2a modulator levetiracetam which is also inactive in these two acute seizure models34, 35. It is also important to note that NAX 5055, unlike the two previously described small molecule galanin agonists galnon and galmic, is not effective against PTZ-induced seizures. This apparent discrepancy in anticonvulsant profile is likely due to the low μM affinity of galnon and galmic for galanin receptors and their reported off-target actions. For example, at 10 μM, both galnon and galmic interact with a variety of non-galanin receptors including 5-HT-1A and 1B receptors, D2 dopamine receptors, ghrelin and melanocortin receptors, NPY receptors (galnon only) or μ-opioid receptors (galmic only). Activities at one or more of these non-galanergic targets likely contribute to their efficacy in the PTZ test. Thus, the primary limitation of these first generation galanin agonists is that they are both non-selective, low affinity molecules that can interact with other pharmacological targets16. In this respect, high affinity and receptor selectivity of NAX 5055 for galanin receptors provides two distinct advantages over currently available galanin agonists for dissecting the role of galanin receptors in epilepsy and other CNS disorders.
In the present investigation, NAX 5055 was uniquely active in the 6 Hz psychomotor seizure test. Interestingly, the pharmacological profile of the 6 Hz model is somewhat dependent on the intensity of the stimulation (Table 3). For example, at a convulsive current (CC) of 22 mA is sufficient to induce a prototypical seizure in 97% of the population tested (i.e., the CC97), the 6 Hz seizure test is relatively nondiscriminating; that is, the large majority of AEDs evaluated (phenytoin (PHT), lamotrigine (LTG), ethosuximide (ETS), levetiracetam (LEV), and valproic acid (VPA)) are effective in blocking the acute seizure. As the current intensity is increased to a level that is 1.5 times the CC97 (32 mA), several of the AEDs lose their ability to protect against a 6 Hz seizure. At a current equivalent to two times the CC97 (i.e., 44 mA), only VPA and LEV retained their ability to block 6 Hz seizures; albeit, the potency of both drugs at two times the CC97 was markedly reduced. Given the pharmacological profile of this model, it has evolved as a unique model for differentiating the potential anticonvulsant compounds that might be useful for the treatment of refractory partial epilepsy. In this regard, the results obtained in this study suggest that NAX 5055 may provide some advantages over other available AEDs. Among the various AEDs that have been evaluated in the 6 Hz model, NAX 5055 is extremely potent following i.p. administration (Table 3). Moreover, it retained excellent efficacy at all three stimulus currents evaluated. Unlike the sodium channel blockers PHT, carbamazepine (CBZ), and LTG, the SV2A modulator LEV and the broad-spectrum AED VPA, the potency of NAX 5055 was largely retained as the stimulus intensity was increased from 22 to 44 mA. It is also of interest to note that among the various AEDs evaluated in this model, NAX 5055 was the most potent at all three stimulus intensities.
Table 3. Anticonvuslant profile of NAX 5055 compared to other antiepileptic drugs in the 6 Hz model.
| ED50, mg/kg, i.p. (95% confidence interval) | |||
|---|---|---|---|
| AED | Stimulation Intensity | ||
| 22 mA | 32 mA | 44 mA | |
| NAX 5055 | 0.7 (0.4 – 1.2) PIa = 30 |
0.8 (0.4 – 1.6) PI = 26.3 |
2.9 (1.9 – 4.3) PI = 7.2 |
| Valproic Acidb | 41.5 (16.1 – 68.8) PI = 11.6 |
126 (94.5 – 152) PI = 3.8 |
310 (258 – 335) PI = 1.6 |
| Leviteracitamb | 4.6 (1.1 – 8.7) PI >217 |
19.4 (9.9 – 36.0) PI >51 |
1089 (787 – 2650) PI ≥ 1 |
| Ethosuximideb | 86.9 (37.8 – 156) PI =3.7 |
167 (114 – 223) PI = 1.9 |
>600 PI < 0.6 |
| Phenytoinb | 9.4 (4.7 – 14.9) PI = 4.6 |
>60 PI < 0.7 |
>60 PI < 0.7 |
| Lamotrigineb | 4.4 (2.2 – 6.6) PI = 6.8 |
>60 PI < 0.5 |
>60 PI < 0.5 |
| Lacosamidec | -- | 9.9 (7.7 – 12.8) PI = 2.7 |
-- |
NAX 5055 was also shown to be highly efficacious and potent in a rodent model believed to mimic temporal lobe epilepsy, the most common type in adult humans36, 37. The pharmacology of the corneal kindled mouse model is very consistent with that observed in more traditional electrical kindling models such as the hippocampal kindled rat (Rowley et al., manuscript in preparation). For example, LTG, CBZ, and VPA are all active in this model at doses of 9.9, 33, and 121 mg/kg, i.p. PHT is only active at doses that produce marked motor impairment; i.e., >50 mg/kg, i.p. NAX 5055 decreased the seizure severity in a dose-dependent manner at doses well below those that produced behavioral toxicity (ED50; 0.65 mg/kg, i.p.). Thus, NAX 5055 is at least one order of magnitude more potent in this model that several of the established AEDs. It is also important to note that activity of NAX 5055 in the corneal kindled mouse is an activity that it shares with the SV2A modulator LEV20. This finding further supports the potential therapeutic utility of galanin-based therapeutics for the symptomatic treatment of partial epilepsy.
Since galanin plays a role in pleiotropic neurological functions, including seizure control, epileptogenesis, antinociception, neuroprotection and neurotrophic activities, NAX 5055 represents a drug prototype that may have broad clinical applications. Firstly, the potent antiepileptic activity of NAX 5055 in the pharmacoresistant model of epilepsy makes this class of analogs drug candidates for treatment of refractory seizures. As a potential first-in-class therapeutic, NAX 5055 or its analogs will have to be carefully evaluated for their long-term toxicity, safety and tolerance before entering the clinical development stage. Antiepileptogenic and neuroprotective actions of galanin may also be retained in NAX 5055, but more studies are needed to evaluate these beneficial aspects of galanin-based antiepileptic therapy. Secondly, GalR1 and GalR2 selective agonists also display anxiolytic activity, thus applications of NAX 5055 may extend to controlling anxiety and modulating mood disorders38. Thirdly, the potential of NAX 5055 as an analgesic is also apparent: numerous studies have demonstrated a role of galanin in neuropathic pain39-41. Our results indeed suggest that NAX 5055 is an effective analgesic in various animal pain models (Adkins-Scholl et al, manuscript in preparation). Given the broad spectrum bioavailability of NAX 5055, various routes of administration (ranging from subcutaneous depo-formulations to a rapid intranasal delivery) may facilitate multiple clinical applications. Current efforts of our research group are focused on selecting optimal indications for NAX 5055 analogs to enter a clinical development stage.
Figure 1.

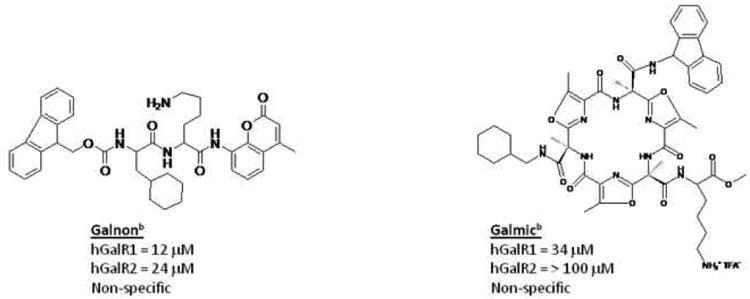
Structures of selected their affinities to the hGalR1 and hGalR2 receptors. A) the peptides NAX 5055 (Gal B2) and galanin. B) two small molecule galanin agonists, galnon and galmic.
aPreviously reported Ki values in Bulaj et al. 2008.17
bPreviously reported Kd values in Lundstrom et al. 2005.15
Acknowledgments
This work was supported in part by the Epilepsy Therapy Grants Program from the Epilepsy Research Foundation, the University of Utah Startup Funds, the University of Utah Research Foundation, and NIH grant R21 NS059669 (GB and HSW). We thank the Anticonvulsant Screening Program (ASP) Project Officer, James Stables, and his group at the NIH/NINDS for their support of the galanin project. We also thank Dan McDougle for his help with purification of the galanin analogs.
Footnotes
Conflict of Interest Disclosure. GB and HSW are scientific co-founders of NeuroAdjuvants, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baraban SC, Tallent MK. Interneuron Diversity series: Interneuronal neuropeptides--endogenous regulators of neuronal excitability. Trends in neurosciences. 2004;27:135–142. doi: 10.1016/j.tins.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Hokfelt T, Broberger C, Xu ZQ, Sergeyev V, Ubink R, Diez M. Neuropeptides--an overview. Neuropharmacology. 2000;39:1337–1356. doi: 10.1016/s0028-3908(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 3.Waxham N. Neuropeptides and nitric oxide. In: Byrne JH, editor. Neuroscience Online. 2007. [Google Scholar]
- 4.Gundlach AL, Jungnickel RF. Galanin and GALP systems in brain - molecular pharmacology, anatomy, and putative roles in physiology and pathology. In: Kastin AJ, editor. Handbook of biologically active peptides. Amsterdam: Elsevier; 2006. pp. 753–761. [Google Scholar]
- 5.Hawes JJ, Picciotto MR. Characterization of GalR1, GalR2, and GalR3 immunoreactivity in catecholaminergic nuclei of the mouse brain. The Journal of comparative neurology. 2004;479:410–423. doi: 10.1002/cne.20329. [DOI] [PubMed] [Google Scholar]
- 6.Gorter JA, van Vliet EA, Aronica E, et al. Potential new antiepileptogenic targets indicated by microarray analysis in a rat model for temporal lobe epilepsy. J Neurosci. 2006;26:11083–11110. doi: 10.1523/JNEUROSCI.2766-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazarati AM, Halaszi E, Telegdy G. Anticonvulsive effects of galanin administered into the central nervous system upon the picrotoxin-kindled seizure syndrome in rats. Brain research. 1992;589:164–166. doi: 10.1016/0006-8993(92)91179-i. [DOI] [PubMed] [Google Scholar]
- 8.Gu XL, Sun YG, Yu LC. Involvement of galanin in nociceptive regulation in the arcuate nucleus of hypothalamus in rats with mononeuropathy. Behavioural brain research. 2007;179:331–335. doi: 10.1016/j.bbr.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 9.Kanter-Schlifke I, Toft Sorensen A, Ledri M, Kuteeva E, Hokfelt T, Kokaia M. Galanin gene transfer curtails generalized seizures in kindled rats without altering hippocampal synaptic plasticity. Neuroscience. 2007 doi: 10.1016/j.neuroscience.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 10.Wynick D, Bacon A. Targeted disruption of galanin: new insights from knock-out studies. Neuropeptides. 2002;36:132–144. doi: 10.1054/npep.2002.0888. [DOI] [PubMed] [Google Scholar]
- 11.Kokaia M, Holmberg K, Nanobashvili A, et al. Suppressed kindling epileptogenesis in mice with ectopic overexpression of galanin. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14006–14011. doi: 10.1073/pnas.231496298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu HX, Brumovsky P, Schmidt R, et al. Receptor subtype-specific pronociceptive and analgesic actions of galanin in the spinal cord: selective actions via GalR1 and GalR2 receptors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9960–9964. doi: 10.1073/pnas.161293598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahoney SA, Hosking R, Farrant S, et al. The second galanin receptor GalR2 plays a key role in neurite outgrowth from adult sensory neurons. J Neurosci. 2003;23:416–421. doi: 10.1523/JNEUROSCI.23-02-00416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branchek TA, Smith KE, Gerald C, Walker MW. Galanin receptor subtypes. Trends in pharmacological sciences. 2000;21:109–117. doi: 10.1016/s0165-6147(00)01446-2. [DOI] [PubMed] [Google Scholar]
- 15.Lundstrom L, Elmquist A, Bartfai T, Langel U. Galanin and its receptors in neurological disorders. Neuromolecular medicine. 2005;7:157–180. doi: 10.1385/NMM:7:1-2:157. [DOI] [PubMed] [Google Scholar]
- 16.Lu X, Lundstrom L, Langel U, Bartfai T. Galanin receptor ligands. Neuropeptides. 2005;39:143–146. doi: 10.1016/j.npep.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Bulaj G, Green BR, Lee HK, et al. Design, synthesis and characterization of high-affinity, systemically-active galanin analogs with potent anticonvulsant activities. Journal of medicinal chemistry. 2008 doi: 10.1021/jm801088x. [DOI] [PubMed] [Google Scholar]
- 18.White HS, Watson WP, Hansen SL, et al. First demonstration of a functional role for central nervous system betaine/{gamma}-aminobutyric acid transporter (mGAT2) based on synergistic anticonvulsant action among inhibitors of mGAT1 and mGAT2. The Journal of pharmacology and experimental therapeutics. 2005;312:866–874. doi: 10.1124/jpet.104.068825. [DOI] [PubMed] [Google Scholar]
- 19.Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy research. 2001;47:217–227. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- 20.Matagne A, Klitgaard H. Validation of corneally kindled mice: a sensitive screening model for partial epilepsy in man. Epilepsy research. 1998;31:59–71. doi: 10.1016/s0920-1211(98)00016-3. [DOI] [PubMed] [Google Scholar]
- 21.Woodbury LA, Davenport VD. Design and use of a new electroshock seizure apparatus, and analysis of factors altering seizure threshold and pattern. Arch Int Pharmacodyn Ther. 1952;92:97–104. [PubMed] [Google Scholar]
- 22.Brown WC, Schiffman DO, Swinyard EA, Goodman LS. Comparative assay of antiepileptic drugs by “psychomotor” seizure test and minimal electroshock threshold test. The Journal of pharmacology and experimental therapeutics. 1953;107:273–283. [PubMed] [Google Scholar]
- 23.Matagne A, Klitgaard H. Validation of corneally kindled mice: a sensitive screening model for partial epilepsy in man. Epilepsy Res Suppl. 1998;31:59–71. doi: 10.1016/s0920-1211(98)00016-3. [DOI] [PubMed] [Google Scholar]
- 24.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 25.Dunham MS, Miya TA. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Amer Pharm Ass Sci Ed. 1957;46:208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- 26.Finney DJ. Probit Analysis. 3rd. London: Cambridge University Press; 1971. [Google Scholar]
- 27.Lang R, Gundlach AL, Kofler B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacology & therapeutics. 2007;115:177–207. doi: 10.1016/j.pharmthera.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Lerner JT, Sankar R, Mazarati AM. Galanin and epilepsy. Cell Mol Life Sci. 2008;65:1864–1871. doi: 10.1007/s00018-008-8161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitsukawa K, Lu X, Bartfai T. Galanin, galanin receptors and drug targets. Cell Mol Life Sci. 2008;65:1796–1805. doi: 10.1007/s00018-008-8153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinemann U, Schmitz D, Eder C, Gloveli T. Properties of entorhinal cortex projection cells to the hippocampal formation. Annals of the New York Academy of Sciences. 2000;911:112–126. doi: 10.1111/j.1749-6632.2000.tb06722.x. [DOI] [PubMed] [Google Scholar]
- 31.Mazarati A, Lundstrom L, Sollenberg U, Shin D, Langel U, Sankar R. Regulation of kindling epileptogenesis by hippocampal galanin type 1 and type 2 receptors: The effects of subtype-selective agonists and the role of G-protein-mediated signaling. The Journal of pharmacology and experimental therapeutics. 2006;318:700–708. doi: 10.1124/jpet.106.104703. [DOI] [PubMed] [Google Scholar]
- 32.Bartfai T, Lu X, Badie-Mahdavi H, et al. Galmic, a nonpeptide galanin receptor agonist, affects behaviors in seizure, pain, and forced-swim tests. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10470–10475. doi: 10.1073/pnas.0403802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saar K, Mazarati AM, Mahlapuu R, et al. Anticonvulsant activity of a nonpeptide galanin receptor agonist. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7136–7141. doi: 10.1073/pnas.102163499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klitgaard H, Matagne A, Gobert J, Wulfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. European journal of pharmacology. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- 35.Loscher W, Honack D. Profile of ucb L059, a novel anticonvulsant drug, in models of partial and generalized epilepsy in mice and rats. European journal of pharmacology. 1993;232:147–158. doi: 10.1016/0014-2999(93)90768-d. [DOI] [PubMed] [Google Scholar]
- 36.Loscher W. Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy research. 2002;50:105–123. doi: 10.1016/s0920-1211(02)00073-6. [DOI] [PubMed] [Google Scholar]
- 37.Stables JP, Bertram EH, White HS, et al. Models for epilepsy and epileptogenesis: report from the NIH workshop, Bethesda, Maryland. Epilepsia. 2002;43:1410–1420. doi: 10.1046/j.1528-1157.2002.06702.x. [DOI] [PubMed] [Google Scholar]
- 38.Wrenn CC, Holmes A. The role of galanin in modulating stress-related neural pathways. Drug news & perspectives. 2006;19:461–467. doi: 10.1358/dnp.2006.19.8.1043963. [DOI] [PubMed] [Google Scholar]
- 39.Hygge-Blakeman K, Brumovsky P, Hao JX, et al. Galanin over-expression decreases the development of neuropathic pain-like behaviors in mice after partial sciatic nerve injury. Brain research. 2004;1025:152–158. doi: 10.1016/j.brainres.2004.07.078. [DOI] [PubMed] [Google Scholar]
- 40.Liu H, Hokfelt T. Effect of intrathecal galanin and its putative antagonist M35 on pain behavior in a neuropathic pain model. Brain research. 2000;886:67–72. doi: 10.1016/s0006-8993(00)02791-8. [DOI] [PubMed] [Google Scholar]
- 41.Wiesenfeld-Hallin Z, Xu XJ, Langel U, Bedecs K, Hokfelt T, Bartfai T. Galanin-mediated control of pain: enhanced role after nerve injury. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:3334–3337. doi: 10.1073/pnas.89.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisone G, Berthold M, Bedecs K, et al. N-terminal galanin-(1-16) fragment is an agonist at the hippocampal galanin receptor. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:9588–9591. doi: 10.1073/pnas.86.23.9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Land T, Langel U, Low M, Berthold M, Unden A, Bartfai T. Linear and cyclic N-terminal galanin fragments and analogs as ligands at the hypothalamic galanin receptor. International journal of peptide and protein research. 1991;38:267–272. doi: 10.1111/j.1399-3011.1991.tb01438.x. [DOI] [PubMed] [Google Scholar]
- 44.Stohr T, Kupferberg HJ, Stables JP, et al. Lacosamide, a novel anti-convulsant drug, shows efficacy with a wide safety margin in rodent models for epilepsy. Epilepsy research. 2007;74:147–154. doi: 10.1016/j.eplepsyres.2007.03.004. [DOI] [PubMed] [Google Scholar]


