Abstract
Introduction:
Neonates are less responsive to vaccines than adults, making it harder to protect newborns against infection. Neonatal differences in antigen presenting cell, B and T cell function, all likely contribute. A key question is whether novel adjuvants might be able to make neonatal vaccines more effective.
Areas Covered:
This review addresses the issues of how to improve neonatal vaccines, which we have defined as vaccines given in the first 4 weeks of life in a human infant or the first week of life in a mouse. A search was performed using keywords including ‘neonatal immunity’, ‘neonatal immunisation’, ‘vaccine’ and ‘adjuvant’ of PubMed articles published between 1960 and 2018.
Expert Opinion:
Sugar-like structures have recently been shown to prime the infant adaptive immune system to respond to vaccines, being potentially more effective than traditional adjuvants. Sugar-based compounds with beneficial adjuvant effects in neonatal vaccine models include delta inulin (Advax), curdlan, and trehalose 6,6’-dibehenate. Such compounds make interesting neonatal adjuvant candidates, either used alone or in combination with traditional innate immune adjuvants.
Keywords: Neonate, immunity, vaccine, adjuvant, Advax, delta inulin, carbohydrate, influenza
1. Pediatric respiratory virus disease burden
According to the World Health Organization (WHO), 2·8 – 3.3 million deaths annually occur in the first four weeks of neonatal life [1]. Fifty-two percent of the 6.3 million children who died under 5 years of age in 2013, died of infectious causes, with viruses, bacteria and parasites all contributing to infant morbidity and mortality [2]. Influenza and respiratory syncytial virus (RSV) are common causes of acute lower respiratory infections (ALRI) in children. Influenza having an annual global attack rate of 20–30% in children (versus 5– 10% in adults) [3]. Children aged <5 years have greater rates of hospitalisation and complications, particularly in the presence of co-existing illnesses [4–8]. In a cohort of 209 US infants monitored weekly from birth to 1 year of age, 33% developed influenza infection [8]. In a retrospective cohort study of children hospitalized with community-acquired influenza during 4 consecutive influenza seasons (July 2000 through June 2004), 25% were <6 months old and 77% were children <5 years old [6]. In 2008 alone, an estimated 28,000 to 111,500 children died worldwide as a result of influenza-associated ALRI [9]. Annual global burden of RSV-associated ALRI is estimated at 33 million cases resulting in > 3 million hospitalizations and ~60,000 deaths in those under 5 years of age, with RSV in children under the 1 year of age accounting for 9 times more deaths than influenza [10–16]. Around 80% of children have developed RSV-specific antibodies by 18 months of age and by 3 years virtually all children have been exposed [17].
2. The neonatal immune system
The newborn immune system has traditionally been viewed as immature [19,20] with Medawar and colleagues proposing the immaturity of the newborn immune system functioned as a barrier to autoimmunity [21]. Although mature αβ T and B cells can be detected in the fetal liver from 8 weeks of gestation [22], the newborn immune system exhibits a reduced response to active immunization with neonates responding to immunization with qualitatively and quantitatively lower memory immune responses[23]. It has more recently been suggested that reduced neonatal immunity is not a defective state of immaturity, but rather reflects necessary adaptation of the immune system to the demands placed on it in early life[22]. Regardless, the problem remains that vaccination during the neonatal period results in lower antibody and T cell responses compared to adults, resulting in reduced ability to use vaccines to prevent neonatal morbidity and mortality from infections.
3. Potential role of adjuvants in enhancing neonatal vaccine responses
Successful neonatal vaccines will require development of strategies to overcome these reduced adaptive immune responses in infancy. Traditional inflammatory adjuvants, improve adult vaccine responses, but are less successful in neonates [24,25]. Adjuvants act in a variety of ways to increase recruitment of immune cells to the site of injection, enhance migration of activated APCs to the draining lymph node, increase uptake of the Ag by APCs, increase APC antigen presentation and co-stimulation, all resulting in enhanced adaptive immune responses (reviewed in [26–28]). In adults, this manifests in a higher frequency of CD4+ T helper cells, and higher antibody (Ab) titers. A key question is whether such benefits of adjuvants can be utilised to create more effective neonatal vaccines. This review explores the problems of neonatal immunization and identifies potential adjuvant solutions, focussing on new findings in respect of unique benefits of sugar-based adjuvant compounds.
4. Factors underlying reduced neonatal vaccine responses
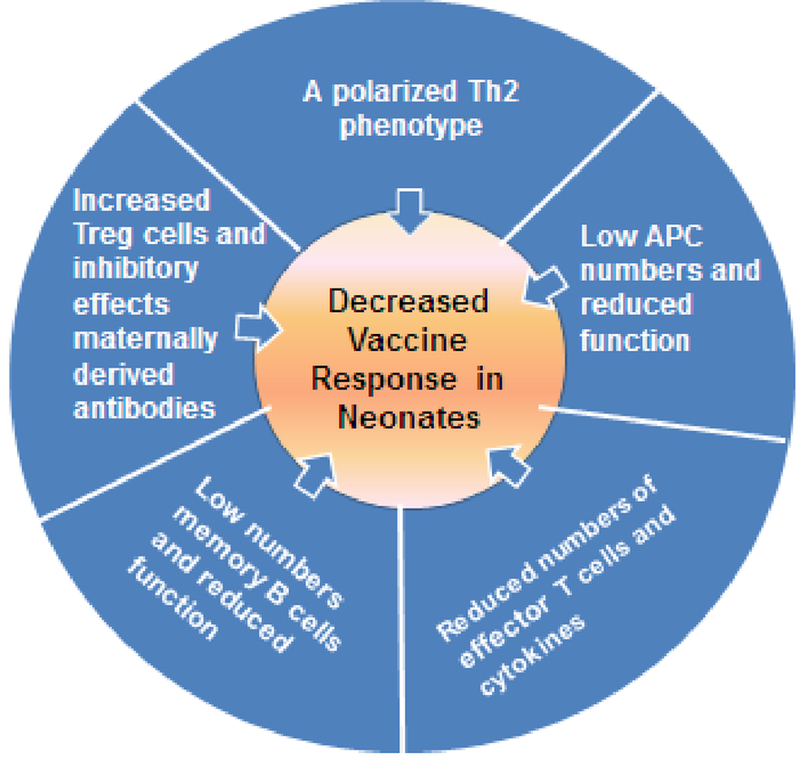
Low and/or delayed responses to many vaccines are seen in neonatal animal models [19,39,40] reflecting what is seen in human newborns. Neonates have reduced vaccine responses because of differences in both innate and adaptive immune pathways when are compared to adults [23,41,42]. Furthermore, passively acquired maternal Abs which help protect neonates against early-life infections, may also contribute to inhibition of neonatal vaccine responses by antigen interference [43–45]. The features of the neonatal immune system are illustrated in Figure 1 [20,23,44]. The immune system in early-life is skewed towards immune tolerance because of the need to avoid autoimmunity and inflammatory immunopathology. This is reflected in increased activity of suppressive T regulatory cells [46,47], reduced antigen presenting cell (APC) function, a higher threshold for B cell activation, reduced production of immune effectors such as inflammatory cytokines, and a bias to a T helper type 2 (Th2) phenotype [48].
Figure 1. Challenges to neonatal immunization related to characteristics of the newborn immune system.
Differences in the neonatal immune system has an effect on increased susceptibility to infection and decreased vaccine efficacy. The recognition of infectious agents is reduced in early life and it is therefore easier for a pathogen to invade the host. Neonates are also less experienced so have no immune memory against infection. (2) Decreased vaccine efficacy. In a similar fashion to infection, reduced recognition of vaccine antigens as foreign means that induction of protective memory responses to vaccines are reduced. There is also an effect of maternally derived antibody that may mask key epitopes of the vaccine. Therefore, a successful vaccine formulation should be able to circumvent or overcome the functional peculiarities of the neonatal period. Abbreviations: APC = Antigen presenting cells. (Refs: [20,23,44])
APCs, including dendritic cells (DC), macrophages and monocytes, express a range of receptors/molecules that respond to pathogen-associated molecular patterns (PAMPS) and damage-associated molecular patterns (DAMPs). There are four major classes of such receptors which includes the toll-like receptors (TLRs), Nod-like receptors (NLRs), retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) and C-type lectin receptors (CLRs). Activation of these receptors results in innate immune stimulation and increased pro-inflammatory cytokine production and upregulation of co-stimulatory molecules (CD40, CD80, CD86, MHC-II) facilitating increased Ag presentation to memory T and B cells. In neonates, APCs express low basal levels of co-stimulatory molecules and MHC-II, and have a decreased ability to produce cytokines, particularly interleukin-12 (IL-12), in response to TLR stimulation [23,49,50]. The downstream effect is reduced activation and expansion of memory T and B cells. While inflammatory adjuvants based on PAMPs and DAMPs enhance APC function and are effective in adults [28], these are on the whole poorly effective if at all in neonates. This suggests that the development of effective neonatal adjuvants will require a fresh approach and cannot simply rely on repurposing adjuvants shown to work in adults.
Follicular DCs (FDCs) play an essential role in B cell responses as they capture/retain Ag in the form of immune complexes for extended periods and thereby draw together Ag-specific B and T cells [51–53]. FDCs develop slowly after birth, resulting in delayed germinal center (GC) formation and ability to produce high affinity Ab [54]. Abs currently define the correlate of protection for the majority of successful vaccines against infectious agents [56]. Even if vaccines induce Ab responses in neonates, the induced Ab are quantitatively lower and qualitatively inferior compared with older individuals [20]. Lower neonatal Ab responses reflect limitations of both GC and extrafollicular plasma cell responses. Low APC Ag expression combined with low expression of costimulatory molecules causes reduced B cell receptor (BCR) signalling and hence less Ab avidity maturation and isotype switching [23,57,58–61]. In neonates there is slow maturation of marginal zone neonatal B cells, a subset of PAMP-responsive B cells that mount rapid T-independent extra follicular B cell responses [40]. Extrinsic factors that influence the magnitude, persistence, or quality of neonatal vaccine responses also include maternally-derived Abs [44,62] and an increased frequency of CD4+ Foxp3+ regulatory T cells (Treg) [46,63]. Maternal Abs reduce neonatal vaccine responses through masking neutralizing Ab epitopes, binding to the Fcγ-receptor 2B, disposing of antigen and neutralizing attenuated vaccine vectors [43,44]. Tregs are present at higher levels at birth and administration of BCG or HBV vaccines to mice together with anti-CD25 Ab treatment to deplete Treg resulted in higher vaccine responses [65][66]. In human infants, malaria infection was associated with increased malaria-specific Tregs [68] and this correlated with decreased responses to BCG vaccination persisting to 1 year of age [69]. Hence adjuvants that reduced Tregs could be beneficial for neonatal vaccines. A problem is that traditional PAMP-based adjuvants such as TLR agonists typically stimulate Tregs and anti-inflammatory cytokines such as IL-10 in neonates thereby hindering rather than helping neonatal vaccine responses [64].
CD4+ and CD8+ αβ T lymphocytes are the main adaptive immune cells responsible for B-cell help and cytotoxic killing of infected cells, respectively. During responses to vaccination or acute infection, Ag-specific CD4+ T cells proliferate and differentiate into different Th subsets: e.g. Th1, Th2, Th17, T follicular helper (TFH) cells, which are characterized by different cytokine profiles that mediate their functions. Diminished T cell responses predispose neonates to infections by intracellular pathogens. Impaired T-cell function in early life is attributed to a low T cell precursor frequency plus reduced quantity and quality of APCs [23,70]. The dominant anti-inflammatory cytokine milieu preferentially supports development of Th2 rather than Th1 responses. Moreover, as shown in mice the expression of the IL4Rα/IL13Rα heteromeric receptors by neonatal Th1 cells renders them susceptible to apoptosis upon IL-4 binding [71]. Nevertheless, the reduced Th1 capacity in neonates is not absolute because they can develop Th1 responses to some infections and vaccines e.g. BCG [72,73], pertussis [74] and cytomegalovirus infections [75]. Memory T-cell subtypes are critical to vaccination, as they affect the type and quality of B-cell responses. In mice, Th1 cytokines induce class switching to IgG2 and IgA [80], whereas Th2-type cytokines primarily induce IgM, IgG1, IgA and IgE antibodies [79]. TFH cells are essential for the generation of high-affinity memory B cells through the GC reaction producing IL-21 plus IFN-γ or IL-4, inducing IgG2a/c and IgG1 Ab production, respectively [83–86]. Also, TFH cells play an important role in promoting IgA production, and homing of IgA-committed B cells to mucosal sites. TFH cells in tonsils and Peyer’s patches produce IL-21 that synergizes with transforming growth factor β1 (TGF-β1) to skew isotype switching toward IgA [87,88]. IL-21 also downregulates CXCR5, the chemokine receptor that promotes follicular localization of B cells and upregulates CCR10, the chemokine receptor that facilitates the migration of IgA class-switched B cells to local mucosal effector sites [89,90]. Limited neonatal GC B cell and plasma cell responses have been correlated with delayed maturation of FDCs and reduced expansion of TFH cells [91,92]. As TFH cells likely limit humoral responses to vaccines in early life [92], adjuvants that support neonatal TFH cell differentiation could be beneficial in improving neonatal vaccine responses
5. Lessons from successful neonatal vaccines
Given the difficulties of neonatal vaccination, much could be learned from the few vaccines that have proved successful in the neonatal period [42,94–96]. BCG and HBV vaccines are routinely administered to neonates as part of the Expanded Program of Immunization (EPI) schedule. Gaining insight into how these vaccines induce neonatal protection may help in development of strategies to induce protective neonatal responses to other vaccines. BCG is a live attenuated Mycobacterium bovis vaccine administered as a single dose within the first few days of life in developing countries to prevent tuberculous meningitis and disseminated TB in children. BCG contains inherent adjuvant activity, expressing factors that stimulate PAMP receptors including TLR-2, TLR-4 and TLR-9, NOD2 and MINCLE [97,98]. The effectiveness of BCG has been linked to its ability to induce a strong neonatal Th1 immune response due to activation of all these PAMP receptors [72,73,99]. This indicates that the right immune signals can induce favourable adaptive immune responses in early life. Nevertheless, poor BCG efficacy in regions closer to the equator has been associated with prior Treg induction by environmental mycobacteria [103], highlighting the challenges even for a successful neonatal vaccine like BCG.
HBV vaccine formulated with aluminium salt adjuvants (hydroxide or phosphate), is the other immunization administered at birth to reduce the risk of mother-to-infant transmission of HBV [99,105–107]. Children routinely receive three doses of HBV vaccine as part of childhood immunization schedules with the first dose administered within 24 hours of birth and the second and third dose administered at 1 − 2 months and 6 − 18th mouths of age, respectively. This strategy is highly effective in preventing acute and chronic HBV infection in infants born to mothers who are HBV positive [37]. Notably, the dynamics of preventing infection at the time of birth by a birth dose of HBV vaccine is different to the use of birth doses of vaccine to protect against prospective infections such as influenza or RSV, where short duration low level immunity induced by a birth dose may not be sufficient to protect for more than a few weeks after vaccine administration, if at all.
WHO also recommends an oral polio vaccine (OPV) dose at birth followed by a series of three further OPV doses in polio-endemic countries [108]. More than half of newborns that received a monovalent-OPV had seroconverted 30 days after priming [110], showing this strategy to be effective. However, OPV is a live infectious vaccine that also contains RNA thereby ensuring its ability to activate both DAMPS and PAMPS, effectively acting as inbuilt adjuvants [109], and this likely explains its effectiveness in neonates.
As opposed to the few vaccines mentioned above, most vaccines are only administered after the neonatal phase has passed. Starting at 2 months of age, vaccines administered to infants include rotavirus vaccine (2 and 4 moths), diphtheria, pertussis, tetanus (DTaP) (2, 4, 6, and 15–18 months), Hib conjugate (2, 4, 6, 12–15 months), pneumococcal conjugate (PCV; 2, 4, 6, 12–15 months), IPV (2, 4, 6–18 months), seasonal influenza (6 months and older), measles, mumps, and rubella (MMR) (12 months), varicella (12 months), and hepatitis A (12–18 months) (reviewed in [99,117]). The major reason for this delay is two-fold; first, there is an age-dependent increase in seroconversion rates if vaccination is delayed and also this delay avoids much of the potential problem of maternal antibody interference [40] [20,40,43].
Many attempts to immunize children at birth with other vaccines have been unsuccessful. For example, immunization at birth with pertussis vaccines resulted in a reduced response to pertussis boosters at up to 5 months of age, which was independent of maternal Abs [113]. Conversely, if immunization was delayed until 3 weeks of age the serological response to pertussis was significantly improved [114,115]. A study of rotavirus vaccine similarly found the response was less robust, the earlier in life the vaccine was given [116]. Questions are also raised of whether neonatal vaccination might induce persistent immune hypo-responsiveness, due to immune paralysis, tolerance or Ag interference [99,118,119]. For example, DTaP vaccine given at birth was associated with significantly reduced Ab responses to DTaP boosters through to 7 months of age [120].
In summary, two of the 3 vaccines given at birth are live organisms (BCG, rotavirus) that express multiple inbuilt PAMPs and in the act of infection also induce endogenous DAMPs, thereby explaining their effectiveness. The third example is a recombinant antigen (HBsAg) where the role of the birth dose of this vaccine is just to protect against immediate infection transmission at the time of birth, with subsequent immunity provided by additional immunisations starting at 2 months. The key question is whether specific adjuvants or adjuvant combinations could be used to replicate the effects of the live BCG and rotavirus vaccines and thereby improve neonatal vaccine responses.
6. Neonatal vaccines against influenza and RSV
ALRI are the most common cause of infant mortality worldwide, with influenza, RSV, parainfluenza and human metapneumovirus being the most common causes of ALRI in children [9,123,124] [125,126]. Vaccination is the primary strategy for prevention of influenza [127,128]. Currently licensed influenza vaccines include trivalent or quadrivalent inactivated vaccines (TIV/QIV) and intranasal live attenuated influenza vaccine (LAIV) [129]. Even though both TIV/QIV and LAIV are effective in preventing influenza and its associated complications in children 6 months and older, neither of them are indicated for neonates due to poor responses to inactivated vaccines and increased side effects from LAIV vaccines [130,131]. A single dose of TIV failed to induce seroconversion against H1N1 strains in infants less than 3–5 months of age and seroprotection was only seen in ~29% of infants even after a second dose, with a correlation between the age of first immunisation and the rate of seroconversion [132]. This leaves neonates highly vulnerable to infection in the absence of an effective neonatal influenza vaccine.
No vaccine is licensed anywhere in the world for the prevention of RSV disease. The initial RSV vaccine tested in 1960s was a formalin-inactivated whole-virus vaccine formulated with alum adjuvant [135]. Although infants immunized with a 3-dose series administered between 2 and 7 months of age developed RSV-specific serum Ab responses, these were non-neutralizing, failed to protect against RSV infection and ultimately exacerbated infection outcomes (vaccine-enhanced disease) [136][137]. Major obstacles remain to successful development of a neonatal RSV vaccine. Although a high serum neutralizing Ab titer is able to prevent ALRI due to RSV in adults, it is more difficult to induce high Ab levels in neonates. Even if an adjuvant could induce high Ab responses in neonates, there is still the issue of vaccine-enhanced disease. Data from rodent models suggest that vaccine-enhanced disease is associated with priming of an excessive Th2 response, a factor exacerbated by the Th2 adjuvant, alum [144,145]. Although alternative adjuvants, most notably TLR agonists, have been suggested to overcome the problem of RSV vaccine-enhanced disease in animal models, even TLR agonists have a Th2 bias in young infants and ability to use this strategy to prevent vaccine-enhanced disease has yet to be confirmed in human infants.
7. Adjuvants for neonatal vaccines
Novel adjuvants may assist the development of effective neonatal vaccines with the current major focus being on TLR agonists. TLR2 and TLR5 ligands primarily enhance Ab production whereas TLR3, 7, 8 and 9 ligands promote a Th1 polarized response (reviewed in [28,151]). Monophosphoryl Lipid A (MPLA), a TLR4 ligand when combined with alum or QS21 enhances Ab response. However, while these TLR adjuvants have been demonstrated to be effective in older children and adults, there is still no guarantee that they will work in human neonates. Neonates exhibit reduced responsiveness of TLR and inflammasome pathways [64,155,156]. In particular, they have a defect in pro-caspase 1 cleavage that contributes to the low responses of human neonatal cells to adjuvants targeting the IL-1β/inflammasome pathway [155]. Endosomal TLRs such as TLR-3 or −7 may have better preserved activity in neonates [157–159] but in our experience are still not able to fully compensate for the reduced responses to influenza vaccine in neonatal mice (manuscript in preparation).
As depicted in Figure 2, addition of an adjuvant to neonatal vaccine formulations might help accelerate maturation of the neonatal immune system, thereby enhancing Ag presentation, increasing T and B cell co-stimulatory signals and overriding reduced neonatal vaccine responses. Adjuvants could potentially be used to enhance neonatal TFH T cell responses, increase the frequency and quality of GC and memory B cell responses and thereby enhance high-affinity broadly neutralizing Ab production in addition to enhancing cellular (Th1) immunity [163–165]. Unfortunately, there is a paucity of available data on the utility of adjuvants to enhance immune responses to vaccines in human neonates. While alum adjuvanted HBV vaccine is effective in infants and adults, it was not effective in preterm neonates [169–172].
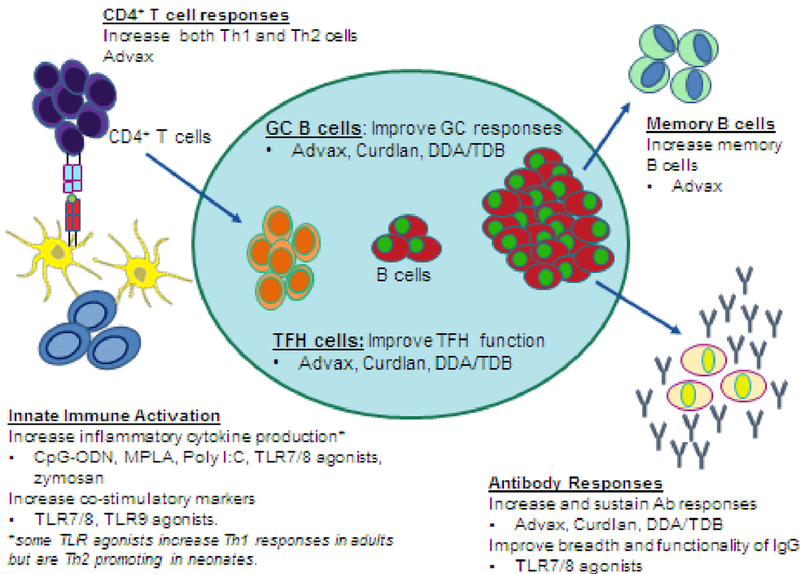
Figure 2. Overcoming functional differences in neonatal immune system through use of vaccine adjuvants.
Adjuvants enhance immunogenicity of neonatal vaccines through innate activation and via enhancement of multiple aspects of adaptive immunity. Adjuvants can activate APC, such as monocytes and dendritic cells and increase cytokine production and co-stimulatory marker expression, which improves the priming of naïve CD4+ T cells. Following activation and antigen presentation, CD4+ T cells can differentiate into T follicular helper cells, which are necessary in the germinal center reaction to assist B cells in generating effective antibodies. Improvement in memory and plasma B cells enhances antigen detection and neutralization through increased production of high-affinity antibodies. Abbreviations/key to understanding: TLR=Toll-like receptor; CpG-ODN = CpG-oligodeoxynucleotides; MPLA = Monophosphoryl lipid A; DDA/TDB = dimethyldioctadecyl ammonium (DDA) and trehalose 6,6′-dibehenate (TDB); 3M-052-SE = a TLR7/8 agonist containing imidazoquinoline/Oil-in-water squalene emulsion (SE); Advax = delta inulin adjuvant.
Most neonatal data on novel adjuvants still comes from neonatal animal models, with uncertainty as to how well this might reflect the situation in human neonates. The squalene oil emulsion adjuvant, MF59, failed to induce protective influenza antibody responses when used to immunize 7 day old mice vaccine, even when a second booster dose was administered [93]. By contrast, a lipidated TLR7/8 agonist was reported to enhance B cell responses to a polysaccharide pneumococcal vaccine when administered to rhesus macaques on the first day of life [164]. Overall, there is a paucity of data to demonstrate that traditional adjuvants used in adults, are effective in neonates.
8. Benefits of sugar-based adjuvants in neonatal vaccines
As noted previously, BCG is one of the few vaccines that is effective in newborns. BCG expresses glycolipids, glycopeptides and other sugar-based structures that are potent activators of the innate immune system. The success of BCG raises the question of whether sugar-based immune activators may be able to preferentially activate the neonatal immune system. Trehalose 6,6’-dibehenate (TDB) is a synthetic analog of mycobacterially-expressed trehalose-6,6-dimycolate (TDM) that targets the PAMP receptor, MINCLE (macrophage inducible Ca2+-dependent lectin receptor). TDB was shown to activate human newborn DCs and enhanced Th1 polarizing cytokine production by DCs when given in combination with a TLR7/8-ligand [166]. TDB enhanced protection against influenza in immunized neonatal mice through the induction of Tfh cells and high-affinity plasma cells [165]. CAF01, a cationic adjuvant formulation consisting of TDB in dimethyl dioctadecyl ammonium (DDA) delivery vehicle successfully induced Th1/Th17 responses in murine neonates [167]. Curdlan, a water-insoluble linear beta-1,3-glucan produced by bacteria was also been shown to enhance Th1 responses to a subunit TB vaccine [165] and an influenza vaccine in neonatal mice [168]. Zymosan, a glucose-based polysaccharide expressed by yeast that activates multiple PAMP receptors including TLR2 and the glucan receptor, induced comparable cytokine levels in cord blood and adult blood monocytes and DCs [178]), raising the possibility it may also be an effective neonatal adjuvant.
The above data supports the hypothesis that sugar-based compounds may uniquely activate the neonatal adaptive immune system in a way that supports the induction of more adult-like memory responses. Although the exact mechanism for this favourable action is not known, TDB is known to activate MINCLE and Curdlan activates Dectin-1. MINCLE and Dectin-1 are both C-type lectin receptors. TDB and Curdlan have been shown in neonatal mice to enhance protection against influenza through the induction of TFH, GCs, and bone marrow high-affinity plasma cells [165]. This suggests that activation of C type lectin receptors by sugar-based compounds may specifically bypass blocks to other innate immune pathways in neonates and thereby boost neonatal vaccine responses.
9. Delta inulin (Advax) as a neonatal vaccine adjuvant
Delta inulin, a plant-based polysaccharide, is the basis of Advax adjuvant. Inulin is a fructan comprised of a linear chain of fructose units connected by β-(2–1) glycosidic bonds and capped at the reducing end by an α-D-(1–2)-glucopyranoside ring [182,183]. The inulin must be crystallized into microparticles known as delta inulin to become adjuvant active, with soluble inulin having no measurable immunological activity. Advax alone or in combination with TLR agonists has proved effective in enhancing humoral and cellular immunity in adult animals against a broad range of pathogens including influenza, Japanese encephalitis virus, West Nile virus, tuberculosis, anthrax, African horse sickness, HIV, listeria and HBV, amongst others (reviewed in [183]). Advax adjuvant similarly afforded complete protection to murine pups vaccinated at seven days of age with a single-dose of inactivated influenza vaccine whereas the influenza vaccine alone provided no protection [163]. The Advax-formulated vaccine induced significantly higher levels of serum influenza-specific Ab of both Th1 and Th2 isotypes plus a 3–4 fold increase in the frequency of influenza-specific IgM and IgG secreting cells in the spleen and bone marrow [163]. Pups immunized with Advax-formulated vaccine also had significantly higher influenza-specific T cell production of IFN-γ, IL-2, IL-4, and IL-10 together with a 3–10-fold higher frequency of influenza-specific T cells on ELISPOT secreting IFN-γ IL-2, IL-4 and IL-17 [163]. No adverse effects were observed in the Advax-immunized pups even although they received the same 1 mg dose of Advax used in adult mice. The ability of Advax adjuvant to enhance neonatal influenza vaccine protection so effectively raises many questions, including the nature of the mechanisms whereby it achieves this effect. Notably, in exactly the same neonatal influenza model we have shown that the TLR9 agonist, CpG2006, had no adjuvant activity and provided no protection to the 7 day old immunised pups (manuscript in preparation). Neonatal protection with Advax-adjuvanted influenza vaccine was dependent on functional B cells as protection was not seen in immunized μMT neonates that were B cell deficient [163].
The exact mechanism(s) by which Advax enhances the immunogenicity of influenza vaccine in animal models and human clinical trials has yet to be determined. Delta inulin does not activate classical inflammatory innate immune receptors such as the TLRs, but instead works through another receptor or non-receptor mechanism to modulate the function of DCs [182,184] inducing chemotaxis and enhancing expansion of memory T and B cells. In murine TB studies, addition to Advax to a TLR9 agonist, CpG oligonucleotide (Advax-CpG), improved both the immunogenicity and protective efficacy of a novel TB vaccine candidate, CysVac2 [192]. Immunization with CysVac2 with Advax-CpG resulted in heightened release of the chemoattractants, CXCL1, CCL3, and TNF-α, and rapid influx of monocytes and neutrophils to the site of vaccination, with pronounced early priming of CysVac2-specific CD4+ T cells.
Human subjects immunized with Advax-adjuvanted seasonal influenza vaccine had increased serum neutralising antibody titers with an increased frequency of plasmablasts seen in their peripheral blood days 7 post vaccination [185]. These plasmablasts when sorted and sequenced exhibited a 2–3 fold higher rate of non-silent mutations in the B cell receptor (BCR) heavy chain complementarity-determining region (CDR3). This was associated with higher plasmablast expression of activation-induced cytidine deaminase (AICDA), the major enzyme controlling BCR affinity maturation, providing a mechanism to explain the enhanced neutralising antibody responses seen in subjects receiving Advax adjuvant.
While studies are ongoing in animal models to determine how Advax adjuvant enhances humoral immune responses, preliminary results suggest this is dependent on enhanced activation of CD4+ T cells. We thereby hypothesize that Advax increases the frequency of TFH cells which then act to enhance antigen-specific B cell activation, BCR affinity maturation, IgG switching and production of high avidity Ab. Whether this action of Advax on enhanced activation of CD4+ T cells might be mediated by interaction with one or more C type lectin receptors expressed on APC is not known. However, Advax’s non-inflammatory effect on human PBMC suggests it is not acting through one of the pro-inflammatory types of C type lectin receptors such as MINCLE, Dectin-1 or-2 or DC-SIGN.
10. Use of combination adjuvants in neonatal vaccines
Compared with the effects in adult blood, LPS (TLR4), Pam3CSK4 (TLR2), flagellin (TLR5) or Poly I:C (TLR3) stimulated weaker inflammatory cytokine production in cord blood monocytes. A Haemophilus influenza B vaccine, adjuvanted with the outer membrane protein (OMP, Porin B (PorB) from Neisseria meningitidis), a TLR2 agonist, induced minimal Ab in neonates and was only protective when the first dose was given after 2 months of age. This suggests that TLR signals alone may be relatively inefficient in stimulating neonatal vaccine responses. Furthermore, TLR-7/8 and TLR-9 adjuvants, which induce Th1 responses in adults, paradoxically induce Th2 responses in neonates. Notably, Advax can be combined with traditional innate immune activators, such as TLR and NOD2 agonists, to further enhance its adjuvant activity. Notably when co-formulated with CpG oligonucleotide, Advax not only enhanced the immunogenicity of a SARS coronavirus vaccine but also prevented vaccine-associated eosinophilic lung pathology, a problem exacerbated by formulation of SARS vaccine with an alum adjuvant [191]. Hence, it will be interesting to see how the co-formulation of Advax and CpG oligonucleotide performs in a neonatal setting.
11. Conclusion
There is a major need for vaccines that can be used to protect young infants. Emerging data suggests that particular immune pathways, including the C type lectin receptors, that respond to sugar structures may be particularly effective at activating the infant immune system. This may help explain the beneficial effects on neonatal vaccine responses of sugar-based compounds including delta inulin (Advax), curdlan, and TDB. Hence, additional research into these alternative immune pathways induced by sugar-based compounds might help elucidate additional compounds able to enhance neonatal vaccine responses. This is a promising area of investigation for development of neonatal vaccines against pathogens that cause significant infant morbidity and mortality including influenza and RSV.
7. Expert Opinion
The millions of deaths in neonates and infants from infectious disease annually, emphasize the need to develop more effective strategies for early-life immunization. The success of BCG and rotavirus vaccines given at birth is proof-of-concept that effective and safe neonatal immunization is feasible [34,193]. A framework to allow discovery of neonatal age-specific adjuvants is needed. Comparing responses of various immune-stimulators in cord and adult blood may not translate to complex adjuvant effects in vivo [176,194–196]. Ultimately, answers will only come from human neonatal studies of vaccines containing novel adjuvants. To assist progress, there is a need to define and validate specific neonatal biomarkers of vaccine and adjuvant safety and efficacy. The beneficial effects in neonatal animal models of sugar-based adjuvants such as delta inulin, curdlan, and TDB on neonatal vaccine responses highlights the ability to identify compounds able to enhance neonatal vaccine responses. To understand the specific benefits of these compounds, more research is needed into the differences in immune signalling pathways between newborns and adults. Systems biological analysis of the host response to vaccination has provided new insights into the adult immune response to vaccination, [197,198]. Systems biology approaches employ high-throughput molecular and cellular measurements called ‘OMICs’ analysis [199]. For example, transcriptomic analyses showed early gene signatures following vaccination were predictive of subsequent measures of immunogenicity for seasonal influenza [200]. These high-dimensional methodologies could be applied to characterization of neonatal vaccine induced molecular signatures that correspond to protection [201–203]. The importance of formulation to vaccine effectiveness is often overlooked, and hence there is also a need for research into development of new and improved formulations and stable delivery systems to improve neonatal vaccine immunogenicity. New knowledge will help in the rational design of neonatal vaccines and adjuvants, reduce empiricism and lessen the risk of failed vaccine candidates, thereby expediting the development of effective neonatal vaccines.
Article Highlights.
Influenza and RSV infections cause high morbidity in neonates, requiring better vaccines
Neonatal responses to non-living vaccines are low and unprotective
Live BCG vaccines that are effective in neonates express immune activators including trehalose-6,6-dimycolate
Multidose Hepatitis B and polio vaccine regimens have shown benefit when the first dose is given to neonates
Sugar-based adjuvants including delta inulin (Advax), curdlan and trehalose 6,6’-dibehenate enhance neonatal vaccine responses
Such sugar-based adjuvants may help solve the need for neonatal influenza and RSV vaccines
Funding
This work was supported in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272200800039C and Collaborative Research Contact No. U01AI061142 (to NP), and Clinical and translational Science Institute award TL1 TR001858 (to Katherine ME). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Declaration of interest
I Sakala is an employee of Vaxine Pty Ltd, which developed the Advax adjuvant, and is an academic affiliate of the Flinders University. N Petrovsky is the founder and Research Director of Vaxine Pty Ltd, and is an academic affiliate of the Flinders University. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.WHO, PMNCH. Newborn death and illness. Partnership for Maternal, Newborn & Child Health, News and Media Centre Fact sheets. http://www.who.int/pmnch/media/press_materials/fs/fs_newborndealth_illness/en/, (2011). [Google Scholar]
- 2.Liu L, Oza S, Hogan D et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet, 385(9966), 430–440 (2015). [DOI] [PubMed] [Google Scholar]
- 3.WHO. Vaccines against influenza WHO position paper - November 2012. Releve epidemiologique hebdomadaire / Section d’hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations, 87(47), 461–476 (2012). [Google Scholar]
- 4.Poehling KA, Edwards KM, Weinberg GA et al. The underrecognized burden of influenza in young children. The New England journal of medicine, 355(1), 31–40 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Poehling KA, Edwards KM, Griffin MR et al. The Burden of Influenza in Young Children, 2004–2009. Pediatrics, 131(2), 207–216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffin SE, Zaoutis TE, Rosenquist ABW et al. Incidence, Complications, and Risk Factors for Prolonged Stay in Children Hospitalized With Community-Acquired Influenza. Pediatrics, 119(4), 740–748 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Ampofo K, Gesteland PH, Bender J et al. Epidemiology, Complications, and Cost of Hospitalization in Children With Laboratory-Confirmed Influenza Infection. Pediatrics, 118(6), 2409–2417 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Glezen WP, Taber LH, Frank AL, Gruber WC, Piedra PA. Influenza virus infections in infants. The Pediatric infectious disease journal, 16(11), 1065–1068 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Nair H, Brooks WA, Katz M et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet, 378(9807), 1917–1930 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Dudas RA, Karron RA. Respiratory Syncytial Virus Vaccines. Clinical Microbiology Reviews, 11(3), 430–439 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the united states. Jama, 289(2), 179–186 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Welliver RC. Respiratory syncytial virus and other respiratory viruses. The Pediatric infectious disease journal, 22(2 Suppl), S6–10; discussion S10–12 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Shi T, McAllister DA, O’Brien KL et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet, 390(10098), 946–958 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. WHO strategy to pilot global respiratory syncytial virus surveillance based on the Global Influenza Surveillance and Response System (GISRS). (2017). [Google Scholar]
- 15.Liu L, Oza S, Hogan D et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet, 388(10063), 3027–3035 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nair H, Simoes EA, Rudan I et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet, 381(9875), 1380–1390 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simoes EAF. Respiratory syncytial virus infection. The Lancet, 354(9181), 847–852 (1999). [DOI] [PubMed] [Google Scholar]
- 18.Levy O, Netea MG. Innate immune memory: implications for development of pediatric immunomodulatory agents and adjuvanted vaccines. Pediatric research, 75(1–2), 184–188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morein B, Abusugra I, Blomqvist G. Immunity in neonates. Veterinary immunology and immunopathology, 87(3–4), 207–213 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Siegrist CA. Neonatal and early life vaccinology. Vaccine, 19(25–26), 3331–3346 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature, 172(4379), 603–606 (1953). [DOI] [PubMed] [Google Scholar]
- 22.Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, Levy O. Protecting the Newborn and Young Infant from Infectious Diseases: Lessons from Immune Ontogeny. Immunity, 46(3), 350–363 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Basha S, Surendran N, Pichichero M. Immune responses in neonates. Expert review of clinical immunology, 10(9), 1171–1184 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riese P, Schulze K, Ebensen T, Prochnow B, Guzman CA. Vaccine adjuvants: key tools for innovative vaccine design. Current topics in medicinal chemistry, 13(20), 2562–2580 (2013). [DOI] [PubMed] [Google Scholar]
- 25.O’Hagan DT, Fox CB. New generation adjuvants--from empiricism to rational design. Vaccine, 33 Suppl 2, B14–20 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Savelkoul HF, Ferro VA, Strioga MM, Schijns VE. Choice and Design of Adjuvants for Parenteral and Mucosal Vaccines. Vaccines, 3(1), 148–171 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson-Welder JH, Torres MP, Kipper MJ, Mallapragada SK, Wannemuehler MJ, Narasimhan B. Vaccine adjuvants: current challenges and future approaches. Journal of pharmaceutical sciences, 98(4), 1278–1316 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity, 33(4), 492–503 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. International journal of epidemiology, 22(6), 1154–1158 (1993). [DOI] [PubMed] [Google Scholar]
- 30.Wolstenholme A, Duffy C, Smith C. National Immunisation Research — Qualitative (2017) research reports: https://beta.health.gov.au/resources/publications/national-immunisation-research-qualitative-2016-and-quantitative-2017. Australian Government, Department of Health, Canberra ACT., (2017). [Google Scholar]
- 31.al-Kassimi FA, al-Hajjaj MS, al-Orainey IO, Bamgboye EA. Does the protective effect of neonatal BCG correlate with vaccine-induced tuberculin reaction? American journal of respiratory and critical care medicine, 152(5 Pt 1), 1575–1578 (1995). [DOI] [PubMed] [Google Scholar]
- 32.Lanckriet C, Levy-Bruhl D, Bingono E, Siopathis RM, Guerin N. Efficacy of BCG vaccination of the newborn: evaluation by a follow-up study of contacts in Bangui. International journal of epidemiology, 24(5), 1042–1049 (1995). [DOI] [PubMed] [Google Scholar]
- 33.Colditz GA, Berkey CS, Mosteller F et al. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics, 96(1 Pt 1), 29–35 (1995). [PubMed] [Google Scholar]
- 34.Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nature reviews. Microbiology, 3(8), 656–662 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Soares AP, Scriba TJ, Joseph S et al. Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. Journal of immunology, 180(5), 3569–3577 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tena-Coki NG, Scriba TJ, Peteni N et al. CD4 and CD8 T-cell responses to mycobacterial antigens in African children. American journal of respiratory and critical care medicine, 182(1), 120–129 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenberg DP. Pediatric experience with recombinant hepatitis B vaccines and relevant safety and immunogenicity studies. The Pediatric infectious disease journal, 12(5), 438–445 (1993). [DOI] [PubMed] [Google Scholar]
- 38.Halsey N, Galazka A. The efficacy of DPT and oral poliomyelitis immunization schedules initiated from birth to 12 weeks of age. Bulletin of the World Health Organization, 63(6), 1151–1169 (1985). [PMC free article] [PubMed] [Google Scholar]
- 39.Morein B, Blomqvist G, Hu K. Immune responsiveness in the neonatal period. Journal of comparative pathology, 137 Suppl 1, S27–31 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nature reviews. Immunology, 9(3), 185–194 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Levy O Innate immunity of the newborn: basic mechanisms and clinical correlates. Nature reviews. Immunology, 7(5), 379–390 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nature reviews. Immunology, 4(7), 553–564 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Niewiesk S Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Frontiers in immunology, 5, 446 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siegrist CA. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine, 21(24), 3406–3412 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Siegrist CA. The challenges of vaccine responses in early life: selected examples. Journal of comparative pathology, 137 Suppl 1, S4–9 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Michaelsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. Journal of immunology, 176(10), 5741–5748 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Fernandez MA, Puttur FK, Wang YM, Howden W, Alexander SI, Jones CA. T regulatory cells contribute to the attenuated primary CD8+ and CD4+ T cell responses to herpes simplex virus type 2 in neonatal mice. Journal of immunology, 180(3), 1556–1564 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nature immunology, 16, 343 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun CM, Deriaud E, Leclerc C, Lo-Man R. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity, 22(4), 467–477 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum Immunol, 68(10), 813–822 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Seminars in immunology, 20(1), 14–25 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacLennan IC. Germinal centers. Annual review of immunology, 12, 117–139 (1994). [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Cho B, Suzuki K et al. Follicular dendritic cells help establish follicle identity and promote B cell retention in germinal centers. The Journal of experimental medicine, 208(12), 2497–2510 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pihlgren M, Tougne C, Bozzotti P et al. Unresponsiveness to lymphoid-mediated signals at the neonatal follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T-dependent antigens. Journal of immunology, 170(6), 2824–2832 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Bjarnarson SP, Adarna BC, Benonisson H, Del Giudice G, Jonsdottir I. The adjuvant LT-K63 can restore delayed maturation of follicular dendritic cells and poor persistence of both protein- and polysaccharide-specific antibody-secreting cells in neonatal mice. Journal of immunology, 189(3), 1265–1273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plotkin SA. Complex correlates of protection after vaccination. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 56(10), 1458–1465 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Zemlin M, Hoersch G, Zemlin C et al. The postnatal maturation of the immunoglobulin heavy chain IgG repertoire in human preterm neonates is slower than in term neonates. Journal of immunology, 178(2), 1180–1188 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunological reviews, 229(1), 152–172 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawabe T, Naka T, Yoshida K et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity, 1(3), 167–178 (1994). [DOI] [PubMed] [Google Scholar]
- 60.van Essen D, Kikutani H, Gray D. CD40 ligand-transduced co-stimulation of T cells in the development of helper function. Nature, 378(6557), 620–623 (1995). [DOI] [PubMed] [Google Scholar]
- 61.Clark EA, Ledbetter JA. How B and T cells talk to each other. Nature, 367(6462), 425–428 (1994). [DOI] [PubMed] [Google Scholar]
- 62.Glezen WP. Effect of maternal antibodies on the infant immune response. Vaccine, 21(24), 3389–3392 (2003). [DOI] [PubMed] [Google Scholar]
- 63.Burt TD. Fetal regulatory T cells and peripheral immune tolerance in utero: implications for development and disease. Am J Reprod Immunol, 69(4), 346–358 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity, 37(5), 771–783 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moore AC, Gallimore A, Draper SJ, Watkins KR, Gilbert SC, Hill AVS. Anti-CD25 Antibody Enhancement of Vaccine-Induced Immunogenicity: Increased Durable Cellular Immunity with Reduced Immunodominance. The Journal of Immunology, 175(11), 7264–7273 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Ho P, Wei X, Seah GT. Regulatory T cells induced by Mycobacterium chelonae sensitization influence murine responses to bacille Calmette-Guérin. Journal of Leukocyte Biology, 88(6), 1073–1080 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Burl S, Adetifa UJ, Cox M et al. Delaying Bacillus Calmette-Guérin Vaccination from Birth to 4 1/2 Months of Age Reduces Postvaccination Th1 and IL-17 Responses but Leads to Comparable Mycobacterial Responses at 9 Months of Age. The Journal of Immunology, 185(4), 2620–2628 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Flanagan KL, Halliday A, Burl S et al. The effect of placental malaria infection on cord blood and maternal immunoregulatory responses at birth. European journal of immunology, 40(4), 1062–1072 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Walther B, Miles DJ, Waight P et al. Placental malaria is associated with attenuated CD4 T-cell responses to tuberculin PPD 12 months after BCG vaccination. BMC Infectious Diseases, 12(1), 6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.CDC. Flu-related hospitalizations and deaths in the United States from April 2009 – January 30, 2010. https://www.cdc.gov/h1n1flu/hosp_deaths_ahdra.htm Accessed on 7 February 2019, (2010). [Google Scholar]
- 71.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends in immunology, 30(12), 585–591 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marchant A, Goetghebuer T, Ota MO et al. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. Journal of immunology, 163(4), 2249–2255 (1999). [PubMed] [Google Scholar]
- 73.Ravn P, Boesen H, Pedersen BK, Andersen P. Human T cell responses induced by vaccination with Mycobacterium bovis bacillus Calmette-Guerin. Journal of immunology, 158(4), 1949–1955 (1997). [PubMed] [Google Scholar]
- 74.Mascart F, Verscheure V, Malfroot A et al. Bordetella pertussis Infection in 2-Month-Old Infants Promotes Type 1 T Cell Responses. The Journal of Immunology, 170(3), 1504–1509 (2003). [DOI] [PubMed] [Google Scholar]
- 75.Huygens A, Lecomte S, Tackoen M et al. Functional Exhaustion Limits CD4+ and CD8+ T-Cell Responses to Congenital Cytomegalovirus Infection. The Journal of infectious diseases, 212(3), 484–494 (2015). [DOI] [PubMed] [Google Scholar]
- 76.Finkelman FD, Holmes J, Katona IM et al. Lymphokine Control of In Vivo Immunoglobulin Isotype Selection. Annual review of immunology, 8(1), 303–333 (1990). [DOI] [PubMed] [Google Scholar]
- 77.Sangster MY, Mo XY, Sealy R, Coleclough C. Matching antibody class with pathogen type and portal of entry: cognate mechanisms regulate local isotype expression patterns in lymph nodes draining the respiratory tract of mice inoculated with respiratory viruses, according to virus replication competence and site of inoculation. The Journal of Immunology, 159(4), 1893–1902 (1997). [PubMed] [Google Scholar]
- 78.Marshall D, Sealy R, Sangster M, Coleclough C. TH Cells Primed During Influenza Virus Infection Provide Help for Qualitatively Distinct Antibody Responses to Subsequent Immunization. The Journal of Immunology, 163(9), 4673–4682 (1999). [PubMed] [Google Scholar]
- 79.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual review of immunology, 7, 145–173 (1989). [DOI] [PubMed] [Google Scholar]
- 80.Snapper C, Paul W. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science, 236(4804), 944–947 (1987). [DOI] [PubMed] [Google Scholar]
- 81.Breitfeld D, Ohl L, Kremmer E et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. The Journal of experimental medicine, 192(11), 1545–1552 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crotty S Follicular helper CD4 T cells (TFH). Annual review of immunology, 29, 621–663 (2011). [DOI] [PubMed] [Google Scholar]
- 83.Harada Y, Tanaka S, Motomura Y et al. The 3’ enhancer CNS2 is a critical regulator of interleukin-4-mediated humoral immunity in follicular helper T cells. Immunity, 36(2), 188–200 (2012). [DOI] [PubMed] [Google Scholar]
- 84.Luthje K, Kallies A, Shimohakamada Y et al. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nature immunology, 13(5), 491–498 (2012). [DOI] [PubMed] [Google Scholar]
- 85.Vijayanand P, Seumois G, Simpson LJ et al. Interleukin-4 production by follicular helper T cells requires the conserved Il4 enhancer hypersensitivity site V. Immunity, 36(2), 175–187 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yusuf I, Kageyama R, Monticelli L et al. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). Journal of immunology, 185(1), 190–202 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dullaers M, Li D, Xue Y et al. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity, 30(1), 120–129 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsuji M, Komatsu N, Kawamoto S et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science, 323(5920), 1488–1492 (2009). [DOI] [PubMed] [Google Scholar]
- 89.Hieshima K, Kawasaki Y, Hanamoto H et al. CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. Journal of immunology, 173(6), 3668–3675 (2004). [DOI] [PubMed] [Google Scholar]
- 90.Kunkel EJ, Kim CH, Lazarus NH et al. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. The Journal of clinical investigation, 111(7), 1001–1010 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Debock I, Jaworski K, Chadlaoui H et al. Neonatal follicular Th cell responses are impaired and modulated by IL-4. Journal of immunology, 191(3), 1231–1239 (2013). [DOI] [PubMed] [Google Scholar]
- 92.Mastelic B, Kamath AT, Fontannaz P et al. Environmental and T cell-intrinsic factors limit the expansion of neonatal follicular T helper cells but may be circumvented by specific adjuvants. Journal of immunology, 189(12), 5764–5772 (2012). [DOI] [PubMed] [Google Scholar]
- 93.Mastelic Gavillet B, Eberhardt CS, Auderset F et al. MF59 Mediates Its B Cell Adjuvanticity by Promoting T Follicular Helper Cells and Thus Germinal Center Responses in Adult and Early Life. Journal of immunology, 194(10), 4836–4845 (2015). [DOI] [PubMed] [Google Scholar]
- 94.Sarzotti M, Robbins DS, Hoffman PM. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science, 271(5256), 1726–1728 (1996). [DOI] [PubMed] [Google Scholar]
- 95.Fadel SA, Ozaki DA, Sarzotti M. Enhanced type 1 immunity after secondary viral challenge in mice primed as neonates. Journal of immunology, 169(6), 3293–3300 (2002). [DOI] [PubMed] [Google Scholar]
- 96.Hunt DW, Huppertz HI, Jiang HJ, Petty RE. Studies of human cord blood dendritic cells: evidence for functional immaturity. Blood, 84(12), 4333–4343 (1994). [PubMed] [Google Scholar]
- 97.Heldwein KA, Liang MD, Andresen TK et al. TLR2 and TLR4 serve distinct roles in the host immune response against Mycobacterium bovis BCG. Journal of Leukocyte Biology, 74(2), 277–286 (2003). [DOI] [PubMed] [Google Scholar]
- 98.Pompei L, Jang S, Zamlynny B et al. Disparity in IL-12 release in dendritic cells and macrophages in response to Mycobacterium tuberculosis is due to use of distinct TLRs. Journal of immunology, 178(8), 5192–5199 (2007). [DOI] [PubMed] [Google Scholar]
- 99.Demirjian A, Levy O. Safety and efficacy of neonatal vaccination. European journal of immunology, 39(1), 36–46 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang X, Lepelley A, Azria E et al. Neonatal plasmacytoid dendritic cells (pDCs) display subset variation but can elicit potent anti-viral innate responses. PloS one, 8(1), e52003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X, Casartelli N, Lemoine S et al. Plasmacytoid dendritic cells engagement by influenza vaccine as a surrogate strategy for driving T-helper type 1 responses in human neonatal settings. The Journal of infectious diseases, 210(3), 424–434 (2014). [DOI] [PubMed] [Google Scholar]
- 102.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. Journal of immunology, 136(7), 2348–2357 (1986). [PubMed] [Google Scholar]
- 103.Price DN, Kusewitt DF, Lino CA, McBride AA, Muttil P. Oral Tolerance to Environmental Mycobacteria Interferes with Intradermal, but Not Pulmonary, Immunization against Tuberculosis. PLoS pathogens, 12(5), e1005614 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jaron B, Maranghi E, Leclerc C, Majlessi L. Effect of attenuation of Treg during BCG immunization on anti-mycobacterial Th1 responses and protection against Mycobacterium tuberculosis. PloS one, 3(7), e2833 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wong VC, Ip HM, Reesink HW et al. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin. Double-blind randomised placebo-controlled study. Lancet, 1(8383), 921–926 (1984). [DOI] [PubMed] [Google Scholar]
- 106.Xu Z-Y, Liu C-B, Francis DP et al. Prevention of Perinatal Acquisition of Hepatitis B Virus Carriage Using Vaccine: Preliminary Report of a Randomized, Double-Blind Placebo-Controlled and Comparative Trial. Pediatrics, 76(5), 713–718 (1985). [PubMed] [Google Scholar]
- 107.Gerlich WH. Prophylactic vaccination against hepatitis B: achievements, challenges and perspectives. Medical Microbiology and Immunology, 204(1), 39–55 (2015). [DOI] [PubMed] [Google Scholar]
- 108.WHO. In International travel and health > Vaccines > Poliomyelitis (Polio). https://www.who.int/ith/vaccines/polio/en/ Accessed 4th February 2019. (2019). [Google Scholar]
- 109.Philbin VJ, Levy O. Immunostimulatory activity of Toll-like receptor 8 agonists towards human leucocytes: basic mechanisms and translational opportunities. Biochemical Society Transactions, 35(6), 1485–1491 (2007). [DOI] [PubMed] [Google Scholar]
- 110.el-Sayed N, el-Gamal Y, Abbassy AA et al. Monovalent type 1 oral poliovirus vaccine in newborns. The New England journal of medicine, 359(16), 1655–1665 (2008). [DOI] [PubMed] [Google Scholar]
- 111.Scott JA, Ojal J, Ashton L, Muhoro A, Burbidge P, Goldblatt D. Pneumococcal conjugate vaccine given shortly after birth stimulates effective antibody concentrations and primes immunological memory for sustained infant protection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 53(7), 663–670 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clarke E, Kampmann B, Goldblatt D. Maternal and neonatal pneumococcal vaccination - where are we now? Expert review of vaccines, 15(10), 1305–1317 (2016). [DOI] [PubMed] [Google Scholar]
- 113.Provenzano RW, Wetterlow LH, Sullivan CL. Immunization and antibody response in the newborn infant. I. Pertussis inoculation within twenty-four hours of birth. The New England journal of medicine, 273(18), 959–965 (1965). [DOI] [PubMed] [Google Scholar]
- 114.Locht C Pertussis: acellular, whole-cell, new vaccines, what to choose? Expert review of vaccines, 15(6), 671–673 (2016). [DOI] [PubMed] [Google Scholar]
- 115.Locht C, Mielcarek N. New pertussis vaccination approaches: en route to protect newborns? FEMS immunology and medical microbiology, 66(2), 121–133 (2012). [DOI] [PubMed] [Google Scholar]
- 116.Saleh E, Eichner B, Clark DW et al. Open-Label Pilot Study to Compare the Safety and Immunogenicity of Pentavalent Rotavirus Vaccine (RV5) Administered on an Early Alternative Dosing Schedule with Those of RV5 Administered on the Recommended Standard Schedule. Journal of the Pediatric Infectious Diseases Society, 7(1), 82–85 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Whittaker E, Goldblatt D, McIntyre P, Levy O. Neonatal Immunization: Rationale, Current State, and Future Prospects. Frontiers in immunology, 9(532) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Siegrist CA. Blame vaccine interference, not neonatal immunization, for suboptimal responses after neonatal diphtheria, tetanus, and acellular pertussis immunization. The Journal of pediatrics, 153(3), 305–307 (2008). [DOI] [PubMed] [Google Scholar]
- 119.Wood N, Siegrist CA. Neonatal immunization: where do we stand? Current opinion in infectious diseases, 24(3), 190–195 (2011). [DOI] [PubMed] [Google Scholar]
- 120.Halasa NB, O’Shea A, Shi JR, LaFleur BJ, Edwards KM. Poor immune responses to a birth dose of diphtheria, tetanus, and acellular pertussis vaccine. The Journal of pediatrics, 153(3), 327–332 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saso A, Kampmann B. Vaccine responses in newborns. Seminars in immunopathology, 39(6), 627–642 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Knuf M, Schmitt HJ, Wolter J et al. Neonatal vaccination with an acellular pertussis vaccine accelerates the acquisition of pertussis antibodies in infants. The Journal of pediatrics, 152(5), 655–660, 660.e651 (2008). [DOI] [PubMed] [Google Scholar]
- 123.Nair H, Nokes DJ, Gessner BD et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet, 375(9725), 1545–1555 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Review on Antimicrobial Resistance. Tackling drug-resistant infections globally: final report and recommendations. May 2016. Available at https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf. (2016). [Google Scholar]
- 125.Meissner HC, Rennels MB. Unpredictable patterns of viral respiratory disease in children. Pediatrics, 113(6), 1814–1816 (2004). [DOI] [PubMed] [Google Scholar]
- 126.Brodzinski H, Ruddy RM. Review of new and newly discovered respiratory tract viruses in children. Pediatr Emerg Care, 25(5), 352–360; quiz 361–353 (2009). [DOI] [PubMed] [Google Scholar]
- 127.Nichol KL, Treanor JJ. Vaccines for seasonal and pandemic influenza. The Journal of infectious diseases, 194 Suppl 2, S111–118 (2006). [DOI] [PubMed] [Google Scholar]
- 128.Fiore AE, Bridges CB, Cox NJ. Seasonal influenza vaccines. Current topics in microbiology and immunology, 333, 43–82 (2009). [DOI] [PubMed] [Google Scholar]
- 129.Fiore AE, Uyeki TM, Broder K et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control, 59(RR-8), 1–62 (2010). [PubMed] [Google Scholar]
- 130.Vesikari T Emerging data on the safety and efficacy of influenza vaccines in children. The Pediatric infectious disease journal, 27(11 Suppl), S159–161 (2008). [DOI] [PubMed] [Google Scholar]
- 131.Vesikari T, Karvonen A, Smith HM et al. Safety and tolerability of cold-adapted influenza vaccine, trivalent, in infants younger than 6 months of age. Pediatrics, 121(3), e568–573 (2008). [DOI] [PubMed] [Google Scholar]
- 132.Groothuis JR, Levin MJ, Rabalais GP, Meiklejohn G, Lauer BA. Immunization of high-risk infants younger than 18 months of age with split-product influenza vaccine. Pediatrics, 87(6), 823–828 (1991). [PubMed] [Google Scholar]
- 133.Chamberlain AT, Seib K, Ault KA et al. Improving influenza and Tdap vaccination during pregnancy: A cluster-randomized trial of a multi-component antenatal vaccine promotion package in late influenza season. Vaccine, 33(30), 3571–3579 (2015). [DOI] [PubMed] [Google Scholar]
- 134.Chamberlain AT, Seib K, Ault KA et al. Factors Associated with Intention to Receive Influenza and Tetanus, Diphtheria, and Acellular Pertussis (Tdap) Vaccines during Pregnancy: A Focus on Vaccine Hesitancy and Perceptions of Disease Severity and Vaccine Safety. PLoS currents, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol, 89(4), 449–463 (1969). [DOI] [PubMed] [Google Scholar]
- 136.Murphy BR, Prince GA, Walsh EE et al. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J Clin Microbiol, 24(2), 197–202 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim HW, Canchola JG, Brandt CD et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol, 89(4), 422–434 (1969). [DOI] [PubMed] [Google Scholar]
- 138.PATH. Respiratory syncytial virus (RSV) Vaccine and mAb Snapshot. Available at PATH Vaccine Development Global Program https://path.org/resources/rsv-vaccine-and-mab-snapshot/ Accessed on 14th May 2019. (2019). [Google Scholar]
- 139.Schmidt AC. Progress in respiratory virus vaccine development. Semin Respir Crit Care Med, 32(4), 527–540 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guvenel AK, Chiu C, Openshaw PJ. Current concepts and progress in RSV vaccine development. Expert review of vaccines, 13(3), 333–344 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jorquera PA, Oakley KE, Tripp RA. Advances in and the potential of vaccines for respiratory syncytial virus. Expert Rev Respir Med, 7(4), 411–427 (2013). [DOI] [PubMed] [Google Scholar]
- 142.Lambert L, Sagfors AM, Openshaw PJ, Culley FJ. Immunity to RSV in Early-Life. Frontiers in immunology, 5, 466 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Taylor G Animal models of respiratory syncytial virus infection. Vaccine, 35(3), 469–480 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Graham BS, Henderson GS, Tang YW, Lu X, Neuzil KM, Colley DG. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. Journal of immunology, 151(4), 2032–2040 (1993). [PubMed] [Google Scholar]
- 145.Knudson CJ, Hartwig SM, Meyerholz DK, Varga SM. RSV vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets. PLoS pathogens, 11(3), e1004757 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC 3rd, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. Journal of virology, 68(8), 5321–5325 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Connors M, Kulkarni AB, Firestone CY et al. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. Journal of virology, 66(12), 7444–7451 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Polack FP, Teng MN, Collins PL et al. A role for immune complexes in enhanced respiratory syncytial virus disease. The Journal of experimental medicine, 196(6), 859–865 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Delgado MF, Coviello S, Monsalvo AC et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nature medicine, 15(1), 34–41 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rappuoli R, Medaglini D. ADITEC: Joining Forces for Next-Generation Vaccines. Science translational medicine, 4(128), 128cm124–128cm124 (2012). [DOI] [PubMed] [Google Scholar]
- 151.Netea MG, Van der Meer JW, Sutmuller RP, Adema GJ, Kullberg BJ. From the Th1/Th2 paradigm towards a Toll-like receptor/T-helper bias. Antimicrobial agents and chemotherapy, 49(10), 3991–3996 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Miller RL, Meng TC, Tomai MA. The antiviral activity of Toll-like receptor 7 and 7/8 agonists. Drug news & perspectives, 21(2), 69–87 (2008). [DOI] [PubMed] [Google Scholar]
- 153.Vasilakos JP, Tomai MA. The use of Toll-like receptor 7/8 agonists as vaccine adjuvants. Expert review of vaccines, 12(7), 809–819 (2013). [DOI] [PubMed] [Google Scholar]
- 154.Bodera P, Stankiewicz W, Kocik J. Synthetic immunostimulatory oligonucleotides in experimental and clinical practice. Pharmacological reports : PR, 64(5), 1003–1010 (2012). [DOI] [PubMed] [Google Scholar]
- 155.Sharma AA, Jen R, Kan B et al. Impaired NLRP3 inflammasome activity during fetal development regulates IL-1β production in human monocytes. European journal of immunology, 45(1), 238–249 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Corbett NP, Blimkie D, Ho KC et al. Ontogeny of Toll-Like Receptor Mediated Cytokine Responses of Human Blood Mononuclear Cells. PloS one, 5(11), e15041 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Morris MC, Surendran N. Neonatal Vaccination: Challenges and Intervention Strategies. Neonatology, 109(3), 161–169 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ganapathi L, Van Haren S, Dowling DJ et al. The Imidazoquinoline Toll-Like Receptor-7/8 Agonist Hybrid-2 Potently Induces Cytokine Production by Human Newborn and Adult Leukocytes. PloS one, 10(8), e0134640 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim D, Niewiesk S. Synergistic induction of interferon α through TLR-3 and TLR-9 agonists stimulates immune responses against measles virus in neonatal cotton rats. Vaccine, 32(2), 265–270 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kamath AT, Rochat AF, Valenti MP et al. Adult-like anti-mycobacterial T cell and in vivo dendritic cell responses following neonatal immunization with Ag85B-ESAT-6 in the IC31 adjuvant. PloS one, 3(11), e3683 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kamath AT, Valenti MP, Rochat A-F et al. Protective anti-mycobacterial T cell responses through exquisite in vivo activation of vaccine-targeted dendritic cells. European journal of immunology, 38(5), 1247–1256 (2008). [DOI] [PubMed] [Google Scholar]
- 162.Olafsdottir TA, Lingnau K, Nagy E, Jonsdottir I. Novel Protein-Based Pneumococcal Vaccines Administered with the Th1-Promoting Adjuvant IC31 Induce Protective Immunity against Pneumococcal Disease in Neonatal Mice. Infection and Immunity, 80(1), 461–468 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Honda-Okubo Y, Ong CH, Petrovsky N. Advax delta inulin adjuvant overcomes immune immaturity in neonatal mice thereby allowing single-dose influenza vaccine protection. Vaccine, 33(38), 4892–4900 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dowling DJ, van Haren SD, Scheid A et al. TLR7/8 adjuvant overcomes newborn hyporesponsiveness to pneumococcal conjugate vaccine at birth. JCI insight, 2(6), e91020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vono M, Eberhardt CS, Mohr E et al. Overcoming the Neonatal Limitations of Inducing Germinal Centers through Liposome-Based Adjuvants Including C-Type Lectin Agonists Trehalose Dibehenate or Curdlan. Frontiers in immunology, 9(381) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.van Haren SD, Dowling DJ, Foppen W et al. Age-Specific Adjuvant Synergy: Dual TLR7/8 and Mincle Activation of Human Newborn Dendritic Cells Enables Th1 Polarization. The Journal of Immunology, 197(11), 4413–4424 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kamath AT, Mastelic B, Christensen D et al. Synchronization of Dendritic Cell Activation and Antigen Exposure Is Required for the Induction of Th1/Th17 Responses. The Journal of Immunology, 188(10), 4828–4837 (2012). [DOI] [PubMed] [Google Scholar]
- 168.Lemoine S, Jaron B, Tabka S et al. Dectin-1 activation unlocks IL12A expression and reveals the TH1 potency of neonatal dendritic cells. Journal of Allergy and Clinical Immunology, 136(5), 1355–1368.e1315 (2015). [DOI] [PubMed] [Google Scholar]
- 169.Blondheim O, Bader D, Abend M et al. Immunogenicity of hepatitis B vaccine in preterm infants. Archives of disease in childhood. Fetal and neonatal edition, 79(3), F206–208 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Golebiowska M, Kardas-Sobantka D, Chlebna-Sokol D, Sabanty W. Hepatitis B vaccination in preterm infants. European journal of pediatrics, 158(4), 293–297 (1999). [DOI] [PubMed] [Google Scholar]
- 171.Saari TN, American Academy of Pediatrics Committee on Infectious D. Immunization of preterm and low birth weight infants. American Academy of Pediatrics Committee on Infectious Diseases. Pediatrics, 112(1 Pt 1), 193–198 (2003). [DOI] [PubMed] [Google Scholar]
- 172.Freitas da Motta MS, Mussi-Pinhata MM, Jorge SM, Tachibana Yoshida CF, Sandoval de Souza CB. Immunogenicity of hepatitis B vaccine in preterm and full term infants vaccinated within the first week of life. Vaccine, 20(11–12), 1557–1562 (2002). [DOI] [PubMed] [Google Scholar]
- 173.Holbrook BC, D’Agostino RB Jr., Tyler Aycock S et al. Adjuvanting an inactivated influenza vaccine with conjugated R848 improves the level of antibody present at 6months in a nonhuman primate neonate model. Vaccine, 35(45), 6137–6142 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Holbrook BC, D’Agostino RB Jr., Parks GD, Alexander-Miller MA. Adjuvanting an inactivated influenza vaccine with flagellin improves the function and quantity of the long-term antibody response in a nonhuman primate neonate model. Vaccine, 34(39), 4712–4717 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Phillips B, Van Rompay KKA, Rodriguez-Nieves J et al. Adjuvant-Dependent Enhancement of HIV Env-Specific Antibody Responses in Infant Rhesus Macaques. Journal of virology, 92(20) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Surendran N, Simmons A, Pichichero ME. TLR agonist combinations that stimulate Th type I polarizing responses from human neonates. Innate Immun, 24(4), 240–251 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schuller S, Wisgrill L, Sadeghi K et al. The TLR-specific adjuvants R-848 and CpG-B endorse the immunological reaction of neonatal antigen-presenting cells. Pediatr Res, 80(2), 311–318 (2016). [DOI] [PubMed] [Google Scholar]
- 178.Nohmi K, Tokuhara D, Tachibana D et al. Zymosan Induces Immune Responses Comparable with Those of Adults in Monocytes, Dendritic Cells, and Monocyte-Derived Dendritic Cells from Cord Blood. The Journal of pediatrics, 167(1), 155–162.e151–152 (2015). [DOI] [PubMed] [Google Scholar]
- 179.Chen Q, Ross AC. alpha-Galactosylceramide stimulates splenic lymphocyte proliferation in vitro and increases antibody production in vivo in late neonatal-age mice. Clinical and experimental immunology, 179(2), 188–196 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schlecht G, Garcia S, Escriou N, Freitas AA, Leclerc C, Dadaglio G. Murine plasmacytoid dendritic cells induce effector/memory CD8+ T-cell responses in vivo after viral stimulation. Blood, 104(6), 1808–1815 (2004). [DOI] [PubMed] [Google Scholar]
- 181.Gold MC, Donnelly E, Cook MS, Leclair CM, Lewinsohn DA. Purified neonatal plasmacytoid dendritic cells overcome intrinsic maturation defect with TLR agonist stimulation. Pediatr Res, 60(1), 34–37 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cooper PD, Petrovsky N. Delta inulin: a novel, immunologically active, stable packing structure comprising beta-D-[2 -> 1] poly(fructo-furanosyl) alpha-D-glucose polymers. Glycobiology, 21(5), 595–606 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Petrovsky N, Cooper PD. Advax, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine, 33(44), 5920–5926 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hayashi M, Aoshi T, Haseda Y et al. Advax, a Delta Inulin Microparticle, Potentiates In-built Adjuvant Property of Co-administered Vaccines. EBioMedicine, 15, 127–136 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li L, Honda-Okubo Y, Li C, Sajkov D, Petrovsky N. Delta Inulin Adjuvant Enhances Plasmablast Generation, Expression of Activation-Induced Cytidine Deaminase and B-Cell Affinity Maturation in Human Subjects Receiving Seasonal Influenza Vaccine. PloS one, 10(7), e0132003 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim KH, Lee YT, Hwang HS et al. Alum Adjuvant Enhances Protection against Respiratory Syncytial Virus but Exacerbates Pulmonary Inflammation by Modulating Multiple Innate and Adaptive Immune Cells. PloS one, 10(10), e0139916 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schneider-Ohrum K, Cayatte C, Bennett AS et al. Immunization with Low Doses of Recombinant Postfusion or Prefusion Respiratory Syncytial Virus F Primes for Vaccine-Enhanced Disease in the Cotton Rat Model Independently of the Presence of a Th1-Biasing (GLA-SE) or Th2-Biasing (Alum) Adjuvant. Journal of virology, 91(8) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Acosta PL, Caballero MT, Polack FP. Brief History and Characterization of Enhanced Respiratory Syncytial Virus Disease. Clinical and Vaccine Immunology, 23(3), 189–195 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gordon D, Kelley P, Heinzel S, Cooper P, Petrovsky N. Immunogenicity and safety of Advax, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: a randomized controlled Phase 1 study. Vaccine, 32(48), 6469–6477 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gordon DL, Sajkov D, Woodman RJ et al. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax polysaccharide adjuvant. Vaccine, 30(36), 5407–5416 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Honda-Okubo Y, Barnard D, Ong CH, Peng B-H, Tseng C-TK, Petrovsky N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. Journal of virology, 89(6), 2995–3007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Counoupas C, Pinto R, Nagalingam G, Britton WJ, Petrovsky N, Triccas JA. Delta inulin-based adjuvants promote the generation of polyfunctional CD4(+) T cell responses and protection against Mycobacterium tuberculosis infection. Sci Rep, 7(1), 8582 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lavanchy D Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. Journal of viral hepatitis, 11(2), 97–107 (2004). [DOI] [PubMed] [Google Scholar]
- 194.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood, 108(4), 1284–1290 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Philbin VJ, Dowling DJ, Gallington LC et al. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1–dependent pathways. Journal of Allergy and Clinical Immunology, 130(1), 195–204.e199 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dowling DJ, Tan Z, Prokopowicz ZM et al. The ultra-potent and selective TLR8 agonist VTX-294 activates human newborn and adult leukocytes. PloS one, 8(3), e58164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pulendran B Systems vaccinology: probing humanity’s diverse immune systems with vaccines. Proceedings of the National Academy of Sciences of the United States of America, 111(34), 12300–12306 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hagan T, Nakaya HI, Subramaniam S, Pulendran B. Systems vaccinology: Enabling rational vaccine design with systems biological approaches. Vaccine, 33(40), 5294–5301 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang W, Li F, Nie L. Integrating multiple ‘omics’ analysis for microbial biology: application and methodologies. Microbiology (Reading, England), 156(Pt 2), 287–301 (2010). [DOI] [PubMed] [Google Scholar]
- 200.Nakaya HI, Wrammert J, Lee EK et al. Systems biology of vaccination for seasonal influenza in humans. Nature immunology, 12(8), 786–795 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Amenyogbe N, Levy O, Kollmann TR. Systems vaccinology: a promise for the young and the poor. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 370(1671), 20140340 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Blohmke CJ, O’Connor D, Pollard AJ. The use of systems biology and immunological big data to guide vaccine development. Genome Medicine, 7(1), 114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Whittaker E, Goldblatt D, McIntyre P, Levy O. Neonatal Immunization: Rationale, Current State, and Future Prospects. Frontiers in immunology, 9, 532–532 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lee AH, Shannon CP, Amenyogbe N et al. Dynamic molecular changes during the first week of human life follow a robust developmental trajectory. Nature communications, 10(1), 1092 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]