Abstract
Sickness induced by gastrointestinal malaise or by microbial pathogens is more than a private experience. Sick individuals share their illness within their social environment by communicating their sickness to others. In turn, recipients of the communication respond with appropriate behavioral adaptations. Avoidance of sick individuals and the events associated with their sickness is advantageous for members of the group. However, these responses can conflict with the need for comfort or social support expressed by sick individuals. There is evidence that the relationship between the sick individual and its social environment involves neurobiological mechanisms that are similar to those that mediate social bonding. Despite their commonality the feelings of love and fear/disgust that are associated with the sociality of sickness have thus far been neglected by mainstream affective neuroscience.
Keywords: Sickness, Behavior, Inflammation, Conditioned taste aversion, Fitness, Parasites, Infection, Love, Fear
1. Introduction
Sickness refers to the state of being sick or ill, accompanied or not by nausea. In the behavioral sciences, sickness is studied mainly in relation to post-ingestive poisonous episodes that lead to conditioned taste aversion and microbial infections that induces sickness behavior. In both cases, the emphasis is mainly on the individual’s state of sickness. The objective generally implied by this approach is to understand how a poisonous substance or an immune stimulus can promote learning about chemosensory cues in the food that have been associated with sickness or give rise to behavioral signs characteristic of sickness such as decreased spontaneous activity, withdrawal from the environment, reduced appetite and altered sleep. Relatively little attention has been paid to either the behavior of the sick individual toward those who surround him or vice versa, the behavior of the individuals who are part of the same social environment toward the sick person.
The question of whether these behavioral responses reflect feelings of love - love for the person who is sick - or fear - fear of death of a loved one or fear of what the cause of the sickness entitles for those who are surrounding them - is usually not a scientific matter.
This obviously contrasts with the popular views of sickness. In several oil paintings and drawings entitled “The Sick Child” (https://www.tate.org.uk/art/artworks/munch-the-sick-child-n05035), Edward Munch represented his older sister on her deathbed, suffering from the pain of tuberculosis and attended by her loving aunt Karen, overwhelmed by the specter of her niece’s impending death. We have witnessed too many examples of such feelings in the context of the COVID-19 pandemic to not accept the idea that love and fear are inseparable parts of the social environment in which sickness occurs.
The objective of the present paper is to determine whether feelings of love and fear can be deduced from the few behavioral observations of the relationship between animals made experimentally sick and their conspecifics. For such feelings to happen, there must be some form of communication between the sick individual and the individuals in its immediate environment resulting in observable alterations in behavior.
2. Sickness as a motivational state
Many people have experiences with the state of being sick. In addition, many people can easily recognize this state in others and most of them have experienced the feelings of sickness repeatedly. There are of course, some rare individuals who are proud to tell others they have never been sick in their life. Still, it has taken time for sickness to be considered as an entity worth being studied scientifically. The first noticeable incursion in the field of sickness can be traced back to John Garcia following his work on radiation-induced sickness during his postdoctoral stay at the U.S. Naval Radiological Defense Lab in San Francisco in the mid-1950s [16]. Garcia demonstrated that conditioned taste aversion, i.e., the ability of rats to associate a new taste with sickness caused by irradiation or whatever poisonous episode occurred after ingestion of the taste solution, does not obey the temporal contiguity laws of classical Pavlovian conditioning in which the conditioned stimulus must precede the unconditioned stimulus by no longer than a few seconds.
Even in the event that the sickness (the unconditioned stimulus) is experienced hours after the new taste (the conditioned stimulus), the sick individual will still associate the new taste it previously sampled with the sickness it experienced. Thus, the individual will avoid any food or drinking solution with the same taste in the future. This observation led to the theoretical formulation of an interoceptive defense system distinct from the exteroceptive system previously studied by behaviorists [15]. The interoceptive defense system is geared to detect gastrointestinal malaise caused by a poisonous agent, and to relate it to the specific new taste or smell that the individual recently encountered. In a typical conditioned taste aversion experiment, animals are presented with a new taste solution such as a saccharin solution. The ingestion of this solution is followed by sickness induced by injection of a sickness-inducing agent, such as lithium chloride. Upon re-exposure to the taste solution, conditioned animals display an aversive response, characterized by a decrease in the consumption of the taste solution compared to the consumption of the same taste solution by animals that have not been submitted to the conditioning process.
The interoceptive defense system differs from the exteroceptive defense system in the inability of exteroceptive signals (e.g., light or sound) to elicit the same type of conditioning. Presenting animals with distinct lights or sounds before the poisonous episode does not induce aversion to these exteroceptive stimuli. Although not crucial for the present discussion, it is useful to mention that this does not mean that interoceptive sensations cannot be paired to exteroceptive environmental stimuli. Experiments carried out mainly in the context of drugs of abuse showed that animals can form associations between: 1) sickness or relief from sickness and 2) distinct environmental cues, resulting in conditioned place aversions or place preferences [45]. These outcomes make evolutionary sense, as it advantageous to remember in which place you encountered the new flavored food that turned out to be either poisonous or safe, in order to maximize the probability of avoiding further encounters with it, or, conversely, in order to maximize the probability of finding it again.
Subsequent research on conditioned taste aversions has focused mainly on the neuroanatomical bases of this form of conditioning. The objective was to delineate which brain areas are involved in this phenomenon, and how they differ from brain areas mediating associations between exteroceptive stimuli and aversive responses having a fear dimension. At the psychological level, there has been some discussion about whether conditioned taste aversions are really learned or they just represent the additive result of neophobic tendencies (the tendency to sample with precaution new foodstuff combined to the novel experience of sickness) [30]. Whatever the case, the possibility of an interoceptive defense system distinct from the exteroceptive defense system in its modalities of functioning but possessing the same finality, (i.e., maximizing fitness), represents an important step toward attributing to sickness some motivational value. In this context, motivation refers to a central state that organizes perception (e.g., attention to possible predictors of danger, perception of the relation between the feeling of sickness and the previous ingestion of new food) and action (subsequent avoidance of the “poisonous” food).
Although research on conditioned taste aversion remained quite active during Garcia’s life time it became gradually eclipsed in psychology by the new surge of research on cognition, contributing to a decrease of interest in interoception. It took time for sickness to resurge to the surface, thanks to progress in immunology, and, with it, the discovery of interleukins or cytokines. Cytokines are molecules that are produced by immune cells in response to pathogen-associated molecular patterns. They allow communication between the different subtypes of immune cells and their target cells [11]. Studies on the evolutionary adaptive value of fever were the driving force in this resurgence [25]. Fever is a regulated increase in body temperature during the course of an infection. During a fever, there is an increase in the set point at which body temperature is normally regulated. The feverish individual maintains a higher body temperature by increasing heat production and decreasing heat losses. In contrast, during hyperthermia due to intense exercise or prolonged exposure to excessive heat, the hyperthermic individual increases heat loss to get rid of the excessive heat its organism produces or is exposed to.
Fever is a very expensive process. An increase of 1 °C in body temperature requires a 13% increase in basal metabolism. This means that all activities necessitating energy need to be minimized in favor of activities promoting conservation of energy. In other words, fever cannot develop without the concomitant behavioral adaptations that define sickness behavior [18] (Fig. 1). Not surprisingly, sickness behavior was found to be induced by the same cytokines as the ones that are responsible for fever, i.e., proinflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor (TNF), and IL-6. Administration of these cytokines or the pathogen-associated molecular patterns that cause their production and release from innate immune cells was found to be able to induce sickness behavior in healthy animals via mechanisms different from those mediating fever.
Fig. 1.
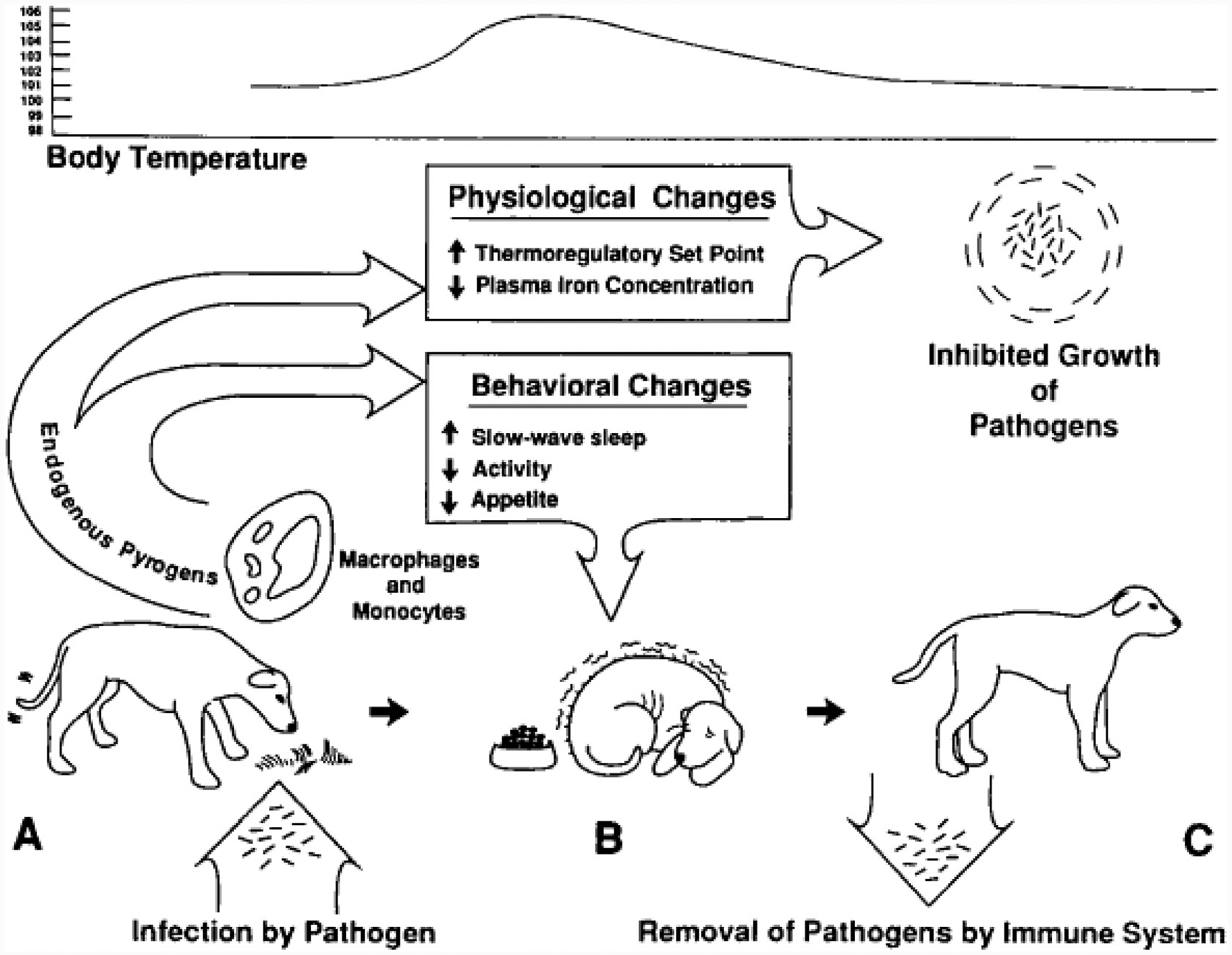
Like fever sickness behavior promotes recovery in organisms exposed to an infection by microbial pathogens. Sickness behavior develops in response to production of endogenous pyrogens (called cytokines nowadays) by innate immune cells in response to microbial pathogens. By acting directly or indirectly on the brain, these molecules induce fever and a number of behavioral adaptations aimed to minimize energy expenditure and increase heat production (e.g., shivering) in addition to the biochemical changes induced by cytokines (e.g., sequestration of zinc and iron). This response is adaptive and promotes survival of the host by helping fighting microbial pathogens (reproduced from Ref. [18].
Inflammation-induced sickness behavior involves far more than a simple decrease in all activities that tax energy metabolism, such as moving around, foraging or courting a sexual partner. Sickness behavior encourages the sick individual to engage in those activities that conserve energy, such as hunching to decrease body surface, anorexia and apathy. Sickness is a pre-programmed motivated response to microbial pathogens. In terms of perception and action, the suffering individual switches its attention to its sick body while remaining attentive to any external danger that can compromise its survival due to its impaired ability to respond to it.
As with any motivated behavior, the ultimate expression of sickness behavior depends not just on the intensity of its driving force, inflammation in this case, but on the interaction between sickness and other motivational priorities [29]. The form of sickness behavior that emerges depends on the relative strength of the competing incentive stimuli. As a typical example, the predominant motivation in lactating dams is normally maternal behavior. In female mice kept with their litters, maternal behavior manifests itself by the arching posture of the dam to facilitate access of its teats to the pups when they need sucking, and by pup retrieval when the pups are removed from the nest and dispersed in the cage. Another maternal behavior that is observable is nest building. It occurs when the nest is removed and replaced by cotton wool. If dams are made sick by administration of the cytokine inducer lipopolysaccharide, they remain lethargic in their cage and do not respond to the solicitations of their pups [3] (Fig. 2). However, if the pups are removed from the nest and dispersed in the cage, their sick mother will overcome its apathetic state and take them one by one in its mouth to bring them back to the nest. If the nest is removed and replaced by cotton wool, the sick dam will not engage in nest building unless the environmental temperature drops. This simple experiment clearly demonstrates that sickness behavior competes with maternal behavior and its expression depends on the relative strengths of the incentive stimuli for each motivational state.
Fig. 2.
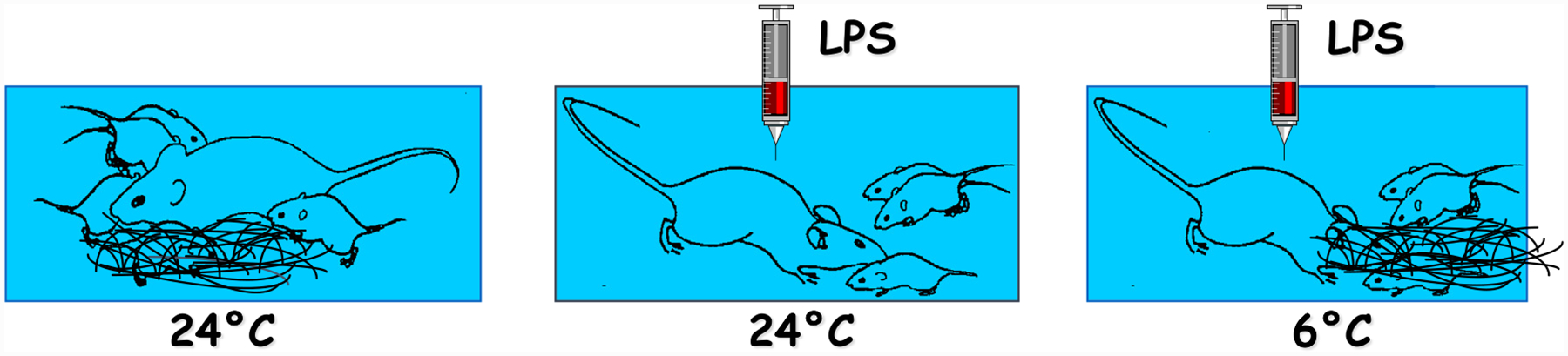
Competition between sickness and maternal motivational states. In the experiments illustrated in this figure the sickness motivational state was maintained constant thanks to a fixed dose of lipopolysaccharide (LPS) sufficient to cause apathy and disinterest toward its pups in a lactating dam while the strength of the incentive stimuli for maternal behavior was gradually increased. The left figure represents the normal behavior of a dam with its litter. The figure in the middle shows what happens when the nest is removed and replaced by cotton wool and the pups are dispersed in the cage. The sick dam emerges from its lethargy and engages in pup retrieval but not in nest building when tested at 24 °C. The figure in the right shows what happens when the ambient temperature decreased from 24 to 6 °C. In this case the sick dam engages in both pup retrieval and nest building [3].
The concept of sickness as a motivational state is important because it raises the question of its adaptive value not only for the sick individual but also for the social group in which the individual belongs. When the driving force of sickness is a live, pathogenic microorganism, the question arises: which entity is going to win? The pathogen, whose evolutionary fitness is dependent on proliferation and dissemination, or the infected host, whose evolutionary fitness is dependent on survival. Ecoimmunologists study the ways strategies of the host and the pathogen interact and determine both the fate of the host and the dynamics of disease transmission in the population [12,19,20,44]. Their work provides detailed accounts of the various ways the behavior of the host contributes to propagation of the infection, and the elaborate mechanisms that can allow apparently unfavorable elements of sickness behavior such as anorexia to ultimately benefit the host by limiting pathogenicity and propagation of the microbial pathogen [28].
3. Sick individuals are recognized from non-sick individuals
Animals that live in groups benefit considerably from the possibility of using social signals to thrive, especially when it comes to threats that are not easily detectable. Threats do not emanate only from predators. Threats can also be present in ingested foodstuffs, in the form of poisons or pathogenic micro-organisms. As pointed out by Garcia it makes sense for eclectic gastronomes or members of an opportunistic species to restrict their initial consumption of a novel food or drinking solution to very small quantities, without regards to its palatability. In doing so, there is an opportunity to ensure the novel food or drink is safe and to communicate this information to other members of the group. A priori, this should be no more difficult to take place than the social transmission of food preferences. There is ample evidence that in rodents a naïve observer having the possibility to interact with a demonstrator consuming a given food will develop robust preference for the same food even in the absence of direct access to the food [31,32]. Information about safety of the consumed food is based on visual and olfactory cues emanating from the food and on chemosensory signals present in the demonstrator’s breath, urine or feces. Keep the observer and replace the demonstrator consuming palatable food by a demonstrator consuming poisonous food and a priori that is all there is to it!
However, much to the chagrin of researchers it has proven very difficult, if not impossible, to demonstrate any evidence of social transmission of conditioned taste aversions in rodents. Does this mean that animals are not apt at recognizing sick conspecifics and making use of this knowledge to inform their own behavior? Apparently not, as adult birds and even day-old chicks are able to learn to avoid novel food based on demonstrators that become sick after ingesting this novel food [33]. In addition, there is evidence that rats can recognize sick from non-sick conspecifics as demonstrated by the poisoned partner effect. In a typical experiment, pairs of rats are allowed to consume water in the presence of a distinct odor. One of them is then injected with lithium chloride to make it sick and returned later to the cage in which the drinking took place with its non-poisoned partner. In a subsequent test, in which all animals are allowed individually to drink water in presence of the odor, the non-poisoned partner drinks less than control animals not exposed to a sick partner [7].
Of note, the poisoned partner effect does not require an association between the tasting experience and the perception of sickness in the partner. Exposure of rats to a poisoned conspecific independently of any association process is sufficient to decrease their later consumption of a novel saccharin solution, which can be explained by sensitization rather than by associative learning [21]. However, this does not change the conclusion that healthy animals have the ability to perceive the sickness state of a conspecific. Although a recent study shows that sickness induced by lithium chloride injections at doses that elicit gastro-intestinal malaise is associated with a distinct emotion-driven facial expression in mice [13], there is no indication yet that this information is used by healthy conspecifics to recognize this form of emotional expression.
The potential of inflammation-induced sickness to serve as a social cue has been investigated in rodents made sick by injection of inflammatory stimuli. Male rats placed in front of two estrous females, one injected with IL-1 and the other with saline, performed less sexual behavior and spent less time with the sick female than with the control female [4]. However, the reverse was not true as an estrous female placed with two males, one injected with IL-1 and the other one with saline, did not discriminate between the two unless the dosage of IL-1 was increased to the point of negatively impacting the male courtship [4]. Once more, this does not mean that females cannot recognize healthy from non-healthy males. Actually, female mice can discriminate the urine odors of males sub-clinically infected with influenza virus from the odors of other uninfected mice [36]. Based on a detailed analysis of the behavior of healthy mice towards conspecifics made sick by injection of lipopolysaccharide, Aubert and colleagues proposed that mice are able to discriminate the state of sickness of other mice and switch their attitude from a controlled exposure strategy to a pathogen avoidance strategy; the latter is characterized by social distancing and changes in the modalities of social exploration (increased proportion of muzzle sniffing and decreased proportion of ano-genital sniffing) [38]. Comparison of adults and prepubertal rats revealed that the recognition of sickness is based on sexually dependent olfactory signals [1]. Social distancing was only observed in response to smell of adult rats from both sexes injected with lipopolysaccharide. The smell of prepubertal rats was not aversive unless they were injected with testosterone or estradiol. These olfactory stimuli are sensed by the vomeronasal organ rather than by the main olfactory tract.
Rodents are not the only species that make use of olfactory signals to identify sickness. Olfactory stimuli are also effective to transmit information concerning the sickness status of conspecifics in other species such as bullfrog tadpoles (Rana catesbeiana) infected with the pathogen Candida humicola [24]. Moreover, there is plenty of evidence to suggest that the ability to discriminate sick from healthy individuals based on olfaction also applies to sickness caused by external or internal parasite infections [22], which is in accordance with the concept that chemical stimuli are an important source of information about the threats and dangers present in the social world.
Humans are not as good as microsmatic animals in using olfaction to discriminate between individuals. In an experiment carried out in donors before and during a respiratory illness, armpit odors collected by nursing pads sewn in a t-shirt worn during 2 nights in each condition could not be used by raters to discriminate between sick and non-sick body odor donors [40]. This does not mean that body odors of sick individuals cannot be used to recognize the presence of a disease because a few centuries ago, physicians smelled body fluids to recognize diseases such as typhoid (“baked bread”) and yellow fever (“butcher shop smell”) [35].
In a separate experiment, volunteers injected with endotoxin wore t-shirts, as, in the previous experiment, and their body odors were collected over 4 h after the injection [34]. Compared to body odors from saline-treated individuals, the body odors of sick individuals were evaluated as more intense and less pleasant even if the scoring differences were small. The same results were obtained when urine was used as a source of odor [17]. In this last case, experimental inflammation resulted in an alteration of the volatile composition of urine and an increased odor aversiveness. Obviously, an acute sickness episode does not have the same qualities as a chronic infection, which could explain the differences between body odors from individuals with a respiratory illness and those from individuals made sick by injection of endotoxin. In addition, breath could have been a better vehicle than sweat for vehiculating disease-related odors.
Whatever the case, humans rarely use odors to recognize sick individuals. They are more likely to do it on the basis of visual cues including general body appearance, slowing of movement, and facial features. Facial photographs of volunteers injected with endotoxin were rated as more aversive than facial photographs of volunteers injected with saline, and the averseness rating was facilitated by simultaneous presentation of the body odor from these persons [37]. Facial cues associated with sickness were unsurprisingly pallor of skin and lips, swelling of the face, dropping corners of the mouth, hanging eyelids, red eyes, and tired appearance [5]. Whether such characteristics are associated with alterations in modalities of vocal communication has not yet been tested.
As previously mentioned, the evolutionary advantage of being able to recognize sickness in individuals of the same social group is that it allows group members to physically isolate those individuals who are at risk of contaminating the rest of the group. The advent of medicine has modified this situation by delegating to specialized personnel the burden of taking care of sick persons (i.e., healthcare personnel) helped by families when the risk of contagion is minimum. Still, there is always the risk of social prejudice toward diseased individuals, especially when they have other features such as race, non-heteronormative sexualities, existing chronic conditions or disabilities, or cultural differences that distinguish them from the general population. The reader is referred to more specialized articles for further discussion of the important aspect of what has been called the behavioral immune system and its intersection with the psychology of human sociality [41].
4. Sick individuals display altered social behavior
Based on the close association between sickness and fever, sick individuals might search for closer contact with conspecifics that have the potential to be comforting and to bring a needed source of heat. Although there has been no systematic study of the expression of sickness over the lifespan, it can be speculated that attraction of sick individuals toward social partners should be at its peak during the early and late periods of their life, when they are weak and unable to care for themselves. However, the attraction toward healthy conspecifics is likely to be counteracted by the necessity of minimizing the risk of transmissible infection, demonstrated by the social withdrawal attitude of sick individuals. We have already seen that sick individuals are perceived as aversive by conspecifics. Reciprocally, rodents made sick by injection of lipopolysaccharide or cytokines display social withdrawal. Whether this reflects true altruistic behavior can be disputed. In most cases, social withdrawal is measured in situations in which sick individuals are exposed to unfamiliar conspecifics. For an individual whose physical abilities are impaired by sickness it certainly makes sense to be careful about strangers.
This should not be the case in familiar social surroundings. In an experiment designed to test the social behavior of male rats made sick by administration of lipopolysaccharide, the rats that were tested for their social behavior in response to lipopolysaccharide had been housed with cage mates of the same sex since after weaning [48]. When placed in a semi-natural environment in which they were able to distance themselves, lipopolysaccharide-injected rats still spent 10% of their time in close contact with their familiar cage mates. However, they spent the rest of the time as far as possible from them. These findings were interpreted in terms of social ambivalence, sick rats still keeping the motivation to engage in social contact but showing at the same time, an increased avoidance of social environments [48].
Remaining engaged in social contact is important, as group housing is associated with decreased inflammatory responses compared to single housing. When injected with lipopolysaccharide male rats that had been kept in same sex groups of 4 displayed less intense sickness behavior than male rats that had been isolated after weaning [47]. This was associated with a decreased inflammatory response in group housed rats. However, the social environment had the reverse effect in females, as group-housed females were sicker than single housed females [47].
Social dynamics of infection have been studied more recently in natural populations. The results confirm active avoidance of social contacts by sick individuals. Lipopolysaccharide-challenged female vampire bats still received the same amount of social grooming as their healthy conspecifics. However, the infected females engaged less often in allog-rooming [42] and produce fewer contact calls than healthy bats when isolated, thereby decreasing their probability of being met by conspecifics [43]. This decrease in vocalization emitted by sick individuals is observed in several species, especially in birds [14].
Active social isolation by sick individuals has important implications for disease transmission dynamics. In a study conducted in social networks of wild mice living in a barn, lipopolysaccharide-injected mice showed reduced movement compared to controls [27]. They came into contact with fewer conspecifics not because they were avoided by healthy mice but because they actively visited less communal nests. Meanwhile social networks of non-injected mice remained stable. Although 40% of all lipopolysaccharide-injected mice showed this pattern of self-isolation, modeling of disease transmission dynamics showed that this pattern of behavior was sufficient to significantly decrease the potential spread of an infection.
5. Sickness behavior is modulated by social neuropeptides
In view of the close interaction between sickness behavior and sociality it can be speculated that neurobiological processes involved in modulation of social behavior also play a role in the expression of sickness; and, vice versa, that sickness behavior impacts these neurobiological processes. Oxytocin and vasopressin are probably the neuropeptides that have been the most studied in the context of sociality. There is evidence that the involvement of these neuropeptides in social recognition and in bonding extends to socio-affective states induced by exposure to pathogens [23]. Inflammation is well known to induce the release of vasopressin, which, at the periphery, is responsible for decreased micturition during fever. Endogenous brain vasopressin has antipyretic effects and negatively regulates cytokine-induced sickness behavior in a sex-dependent manner, with males more affected than females [6,10]. Similar effects are seen in social contexts. Presentation of odor signals from conspecifics made sick by injection of lipopolysaccharide was found to increase expression of vaopressin receptor V1A and V1B in the medial amygdala of rats whereas presentation of odor signals from healthy conspecifics increased expression of the oxytocin receptor [2].
Conversely, infusion of an oxytocin receptor antagonist into the medial amygdala blocked approach behavior toward the healthy odor while administration of a selective antagonist of vasopressin receptor V1A V1A inhibited avoidance response to the sick odor. Exogenous oxytocin reduced signs of sickness behavior and modified alterations in heart rate variability induced by lipopolysaccharide in rats [39]. Although the anti-inflammatory properties of oxytocin have been extensively studied [8,9], what is probably more interesting is the observation that administration of this neuropeptide can mimic the beneficial effects of group housing on sickness behavior. A recent study demonstrated that sickness induced by a chemotherapeutic cocktail combining doxorubicin and cyclophosphamide was more intense in socially isolated mice than in group housed mice and that centrally administered oxytocin remedied the detrimental effects of social isolation via modulation of IL-6 [46].
6. Conclusion and perspectives
There is clear evidence that sickness induced by gastro-intestinal malaise and by microbial pathogens induces coordinated changes in not only the behavior of individuals who experience it but also the attitude of others toward the sick individual. These changes contribute to inclusive fitness. How love, fear, and disgust intervene in these changes cannot be addressed without asking the question of the relationship between behavior and affect. It has been argued that the complex states of the human brain that underlie feelings and emotions cannot be studied in animals. To paraphrase LeDoux, the survival circuits that mediate social bonding and social avoidance can be seen as hard-wired and orthogonal to the subjective feeling states of love and fear/disgust that presumably involve the higher-order circuits involved in self-awareness [26]. As a consequence, preprogrammed responses modulate but do not determine feelings or emotions.
Nonetheless, it could be argued that if in animals such as rodents, physical contact with conspecifics has a protective role on the deleterious consequences of infection in certain conditions, the same effect could potentially occur in humans. The protective effect of physical contact could be inspired by not only gentle touch from loved ones, but also by the perception of the love of others, even in the absence of physical contact because of the contagious nature of the disease. Of course, it still remains to be determined whether the act of sharing love ultimately benefits the fight against the pathological process, or whether it serves only to improve wellbeing. This debate lies at the essence of what medical care should be about when it cannot save lives and cure diseases.
Financial support
Funded by the National Institute of Health (R01 CA193522 and R01 NS073939). Additional support comes from a National Institute of Health Cancer Center Support (CORE) grant (P30 CA016672) to the University of Texas MD Anderson Cancer Center.
References
- [1].Arakawa H, Arakawa K, Deak T, Acute illness induces the release of aversive odor cues from adult, but not prepubertal, male rats and suppresses social investigation by conspecifics, Behav. Neurosci 123 (2009) 964–978. [DOI] [PubMed] [Google Scholar]
- [2].Arakawa H, Arakawa K, Deak T, Oxytocin and vasopressin in the medial amygdala differentially modulate approach and avoidance behavior toward illness-related social odor, Neuroscience 171 (2010) 1141–1151. [DOI] [PubMed] [Google Scholar]
- [3].Aubert A, Goodall G, Dantzer R, Gheusi G, Differential effects of lipopolysaccharide on pup retrieving and nest building in lactating mice, Brain Behav. Immun 11 (1997) 107–118. [DOI] [PubMed] [Google Scholar]
- [4].Avitsur R, Cohen E, Yirmiya R, Effects of interleukin-1 on sexual attractivity in a model of sickness behavior, Physiol. Behav 63 (1997) 25–30. [DOI] [PubMed] [Google Scholar]
- [5].Axelsson J, Sundelin T, Olsson MJ, Sorjonen K, Axelsson C, Lasselin J, Lekander M, Identification of acutely sick people and facial cues of sickness, Proc. Biol. Sci 285 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bluthe RM, Dantzer R, Chronic intracerebral infusions of vasopressin and vasopressin antagonist modulate behavioral effects of interleukin-1 in rat, Brain Res. Bull 29 (1992) 897–900. [DOI] [PubMed] [Google Scholar]
- [7].Bond NW, Transferred odor aversions in adult rats, Behav. Neural. Biol 35 (1982) 417–421. [DOI] [PubMed] [Google Scholar]
- [8].Buemann B, Marazziti D, Uvnas-Moberg K, Can intravenous oxytocin infusion counteract hyperinflammation in COVID-19 infected patients? World J. Biol. Psychiatr (2020) 1–12. [DOI] [PubMed] [Google Scholar]
- [9].Carter CS, Kenkel WM, MacLean EL, Wilson SR, Perkeybile AM, Yee JR, Ferris CF, Nazarloo HP, Porges SW, Davis JM, Connelly JJ, Kingsbury MA, Is oxytocin “nature’s medicine”? Pharmacol. Rev 72 (2020) 829–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dantzer R, Bluthe RM, Kelley KW, Androgen-dependent vasopressinergic neurotransmission attenuates interleukin-1-induced sickness behavior, Brain Res 557 (1991) 115–120. [DOI] [PubMed] [Google Scholar]
- [11].Dantzer R, Kelley KW, Twenty years of research on cytokine-induced sickness behavior, Brain Behav. Immun 21 (2007) 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Demas GE, Carlton ED, Ecoimmunology for psychoneuroimmunologists: considering context in neuroendocrine-immune-behavior interactions, Brain Behav. Immun 44 (2015) 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dolensek N, Gehrlach DA, Klein AS, Gogolla N, Facial expressions of emotion states and their neuronal correlates in mice, Science 368 (2020) 89–94. [DOI] [PubMed] [Google Scholar]
- [14].Dreiss A, Navarro C, De Lope F, Moller A, Effects of an immune challenge on multiple components of song display in barn swallows Hirundo rustica; implications for sexual selection, Ethology 114 (2008) 955–964. [Google Scholar]
- [15].Garcia J, Hankins WG, Rusiniak KW, Behavioral regulation of the milieu interne in man and rat, Science 185 (1974) 824–831. [DOI] [PubMed] [Google Scholar]
- [16].Garcia J, Kimeldorf DJ, Koelling RA, Conditioned aversion to saccharin resulting from exposure to gamma radiation, Science 122 (1955) 157–158. [PubMed] [Google Scholar]
- [17].Gordon AR, Kimball BA, Sorjonen K, Karshikoff B, Axelsson J, Lekander M, Lundstrom JN, Olsson MJ, Detection of inflammation via volatile cues in human urine, Chem. Senses 43 (2018) 711–719. [DOI] [PubMed] [Google Scholar]
- [18].Hart BL, Biological basis of the behavior of sick animals, Neurosci. Biobehav. Rev 12 (1988) 123–137. [DOI] [PubMed] [Google Scholar]
- [19].Hawley DM, Etienne RS, Ezenwa VO, Jolles AE, Does animal behavior underlie covariation between hosts’ exposure to infectious agents and susceptibility to infection? Implications for disease dynamics, Integr. Comp. Biol 51 (2011) 528–539. [DOI] [PubMed] [Google Scholar]
- [20].Hennessy MB, Deak T, Schiml PA, Sociality and sickness: have cytokines evolved to serve social functions beyond times of pathogen exposure? Brain Behav. Immun 37 (2014) 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hishimura Y, Re-examination of the poisoned-partner effect with the two-bottle testing method, Behav. Process 50 (2000) 95–99. [DOI] [PubMed] [Google Scholar]
- [22].Kavaliers M, Choleris E, Pfaff DW, Recognition and avoidance of the odors of parasitized conspecifics and predators: differential genomic correlates, Neurosci. Biobehav. Rev 29 (2005) 1347–1359. [DOI] [PubMed] [Google Scholar]
- [23].Kavaliers M, Ossenkopp KP, Choleris E, Pathogens, odors, and disgust in rodents, Neurosci. Biobehav. Rev 119 (2020) 281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kiesecker JM, Skelly DK, Beard KH, Preisser E, Behavioral reduction of infection risk, Proc. Natl. Acad. Sci. U. S. A 96 (1999) 9165–9168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kluger MJ, Fever: role of pyrogens and cryogens, Physiol. Rev 71 (1991) 93–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].LeDoux JE, Brown R, A higher-order theory of emotional consciousness, Proc. Natl. Acad. Sci. U. S. A 114 (2017) E2016–E2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lopes PC, Block P, Konig B, Infection-induced behavioural changes reduce connectivity and the potential for disease spread in wild mice contact networks, Sci. Rep 6 (2016) 31790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McMillan LE, Miller DW, Adamo SA, Eating when ill is risky: immune defense impairs food detoxification in the caterpillar Manduca sexta, J. Exp. Biol 221 (2018). [DOI] [PubMed] [Google Scholar]
- [29].Miller NE, Experiments on motivation. Studies combining psychological, physiological, and pharmacological techniques, Science 126 (1957) 1271–1278. [DOI] [PubMed] [Google Scholar]
- [30].Mitchell D, Kirschbaum EH, Perry RL, Effects of neophobia and habituation on the poison-induced avoidance of exteroceptive stimuli in the rat, J. Exp. Psychol. Anim. Behav. Process 1 (1975) 47–55. [PubMed] [Google Scholar]
- [31].Morozov A, Behavioral modulation by social experiences in rodent models, Curr Protoc Neurosci 84 (2018) e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nielsen M, Subiaul F, Galef B, Zentall T, Whiten A, Social learning in humans and nonhuman animals: theoretical and empirical dissections, J. Comp. Psychol 126 (2012) 109–113. [DOI] [PubMed] [Google Scholar]
- [33].Noble J, Todd PM, Tuci E, Explaining social learning of food preferences without aversions: an evolutionary simulation model of Norway rats, Proc. Biol. Sci 268 (2001) 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Olsson MJ, Lundstrom JN, Kimball BA, Gordon AR, Karshikoff B, Hosseini N, Sorjonen K, Olgart Hoglund C, Solares C, Soop A, Axelsson J, Lekander M, The scent of disease: human body odor contains an early chemosensory cue of sickness, Psychol. Sci 25 (2014) 817–823. [DOI] [PubMed] [Google Scholar]
- [35].Penn D, Potts WK, Chemical signals and parasite-mediated sexual selection, Trends Ecol. Evol 13 (1998) 391–396. [DOI] [PubMed] [Google Scholar]
- [36].Penn D, Schneider G, White K, Slev P, Potts W, Influenza infection neutralizes the attractiveness of male odours to female mice (Mus musculus), Ethology 104 (1998) 685–694. [Google Scholar]
- [37].Regenbogen C, Axelsson J, Lasselin J, Porada DK, Sundelin T, Peter MG, Lekander M, Lundstrom JN, Olsson MJ, Behavioral and neural correlates to multisensory detection of sick humans, Proc. Natl. Acad. Sci. U. S. A 114 (2017) 6400–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Renault J, Gheusi G, Aubert A, Changes in social exploration of a lipopolysaccharides-treated conspecific in mice: role of environmental cues, Brain Behav. Immun 22 (2008) 1201–1207. [DOI] [PubMed] [Google Scholar]
- [39].Reyes-Lagos JJ, Hadamitzky M, Pena-Castillo MA, Echeverria JC, Bosche K, Luckemann L, Schedlowski M, Pacheco-Lopez G, Exogenous oxytocin reduces signs of sickness behavior and modifies heart rate fluctuations of endotoxemic rats, Physiol. Behav 165 (2016) 223–230. [DOI] [PubMed] [Google Scholar]
- [40].Sarolidou G, Tognetti A, Lasselin J, Regenbogen C, Lundstrom JN, Kimball BA, Garke M, Lekander M, Axelsson J, Olsson MJ, Olfactory communication of sickness cues in respiratory infection, Front. Psychol 11 (2020) 1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schaller M, The behavioural immune system and the psychology of human sociality, Philos. Trans. R. Soc. Lond. B Biol. Sci 366 (2011) 3418–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Stockmaier S, Bolnick D, Page R, Carter G, An immune challenge reduces social grooming in vampire bats, Anim. Behav 140 (2018) 141–149. [Google Scholar]
- [43].Stockmaier S, Bolnick DI, Page RA, Josic D, Carter GG, Immune-challenged vampire bats produce fewer contact calls, Biol. Lett 16 (2020), 20200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sylvia KE, Demas GE, A return to wisdom: using sickness behaviors to integrate ecological and translational research, Integr. Comp. Biol 57 (2017) 1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tzschentke TM, Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues, Prog. Neurobiol 56 (1998) 613–672. [DOI] [PubMed] [Google Scholar]
- [46].Walker WH 2nd, Melendez-Fernandez OH, Pascoe JL, Zhang N, DeVries AC, Social enrichment attenuates chemotherapy induced pro-inflammatory cytokine production and affective behavior via oxytocin signaling, Brain Behav. Immun 89 (2020) 451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yee JR, Prendergast BJ, Sex-specific social regulation of inflammatory responses and sickness behaviors, Brain Behav. Immun 24 (2010) 942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yee JR, Prendergast BJ, Endotoxin elicits ambivalent social behaviors, Psychoneuroendocrinology 37 (2012) 1101–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]


