Abstract
Introduction:
The older population is growing and with this growth there is a parallel rise in the operations performed on this vulnerable group. The perioperative pain management strategy for older adults is unique and requires a team-based approach for provision of high-quality surgical care.
Methods:
Literature search was performed using PubMed in addition to review of relevant protocols and guidelines from geriatric, surgical, and anesthesia societies. Systematic reviews and meta-analyses, randomized trials, observational studies, and society guidelines were summarized in this review.
Management:
The optimal approach to a pain management strategy for older adults undergoing surgery involves addressing all phases of perioperative care. For example, preoperative assessment of a patient’s cognitive function and presence of chronic pain may impact the pain management plan. Consideration should be also given to intraoperative strategies to improve pain control and minimize both the dose and side effects from opioids (e.g. regional anesthetic techniques). Postoperative pain control (e.g. under or over treatment of pain) may impact the development of elderly-specific complications such as postoperative delirium and functional decline. Finally, pain management does not stop after the older adult patient leaves the hospital. Both discharge planning and post-operative clinic follow-up provide important opportunities for collaboration and intervention.
Conclusions:
An opioid-sparing pain management strategy for older adults can be accomplished with a comprehensive and collaborative interdisciplinary strategy addressing all phases of perioperative care.
Mini-abstract:
This narrative review summarizes the unique perioperative pain management issues for older adults undergoing surgery. The focus of this comprehensive review is an opioid-sparing multimodality approach to ensure high quality and safe care for older adults throughout all phases of the perioperative period.
Introduction:
The population is aging and the number of older adults undergoing surgery is increasing.1 In addition to worse traditional surgical outcomes (i.e. mortality, morbidity), older adults (age 65 years and older) are prone to geriatric-specific complications such postoperative delirium and functional decline.2 Aging affects every organ system and these physiologic changes need to be recognized and incorporated into geriatric-specific perioperative care.3
Pain management after surgery is becoming an increasingly important topic. This is particularly important in the older adult due to increased opioid sensitivity and risk of postoperative delirium.4 There is also growing attention to the use of opioids after surgery including recent research on excessive opioid prescriptions for common surgery procedures.4,5 The current momentum surrounding opioid overuse and abuse also highlights the need to minimize opioids in older adults undergoing surgery. While there are practice guidelines for pharmacologic management of persistent pain in older persons,6 there are no comprehensive reviews on perioperative pain management issues for older adults. The overarching goal of this narrative review is to highlight the unique perioperative pain management needs for older adults undergoing surgery with an emphasis on opioid-sparing and multimodal pain management techniques. An additional goal is to identify common pain management strategies for older adults that are relevant to and can be used across a range of surgical disciplines and operations.
Materials and Methods:
Key areas for perioperative pain management in older adults undergoing surgery were identified through a review of the literature across the following phases of care: preoperative, immediate preoperative, intraoperative, postoperative, and peri-/post-discharge. Example search terms included: “pain management”, “opioid-sparing”, “multimodal”, “geriatric”, “older adult”, and “elderly.” It should be noted that an opioid-sparing multimodality pain management protocol is one of the thirty standards required for implementation of the American College of Surgeons Geriatric Surgery Verification Program which was the impetus behind this review to help identify the potential key components of this protocol for older adults undergoing surgery.7 We performed a non-systematic literature review of the PubMed database in addition to a gray literature review of relevant protocols and guidelines from geriatric, surgical, and anesthesia societies.8
Phases of Care:
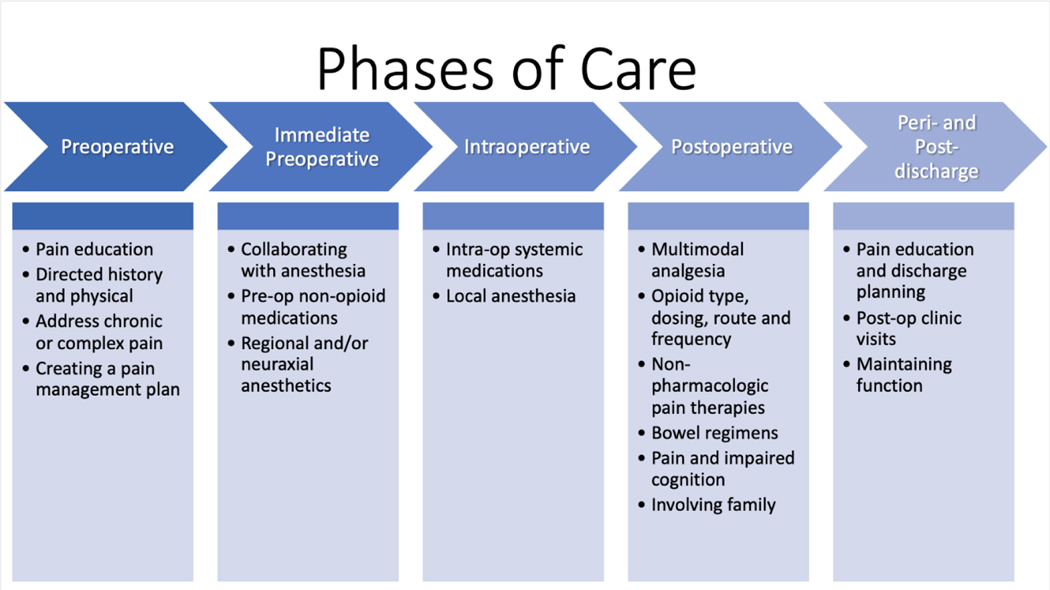
Pain management can be addressed across all phases of surgical care. Figure 1 provides a framework to approach perioperative pain management in the older adult. This systematic approach to management of pain by phase of surgical care will assist providers in caring for this unique population. The preoperative phase emphasizes the patients’ medical history and forms the basis of pain expectations through patient education. The immediate preoperative, intraoperative, and postoperative phases highlight the collaboration between provider teams, namely anesthesia and surgery, and focus on the multi-modality approaches available to address diverse pain issues in this vulnerable population. The peri- and post-discharge phase again focuses on expectations with patient education as well as continued care with close follow-up to address any issues that may arise.
Figure 1:
Pain management strategy overview for phase of perioperative care
Preoperative Phase (subheading):
Arguably the most important part of an optimal pain management strategy, the preoperative phase of caresets the stage for successful pain management after surgery, particularly in the geriatric population.
Several meta-analyses have suggested that preoperative education can decrease postoperative pain and anxiety.9,10 The provider should explain the importance of pain control in the context of participating in postoperative recovery activities such as incentive spirometry and mobilization.11,12 The older adult should understand that they will have pain after surgery and that no combination of medications can make them completely pain free. Stronger pain medications (i.e. opioids) should be reserved for pain that limits critical function or activities after surgery (i.e. ambulation) as there are potential downstream side effects of opioid use in older adults such as postoperative delirium.
Discussing the anticipated pain assessment tools with the older adult prior to surgery is a key step that can be easily forgotten. Pain assessment, and thus pain treatment, is also more effective when the older adult understands the pain assessment tool. There are several assessment tools that are useful in measuring pain intensity in older adults, but some may be more useful depending on the patient’s sensory and cognitive abilities. For example, the Faces Pain Scale—Revised may be useful for more visually inclined patients as well as those with mild to moderate cognitive impairment, whereas the Numeric Rating Scale should be used in individuals who can understand a numerical scale and self-report their pain intensity.13 The Verbal Descriptor Scale (Pain Thermometer) can be used in patients with moderate to severe cognitive impairment, which is a common geriatric-specific comorbidity in older adults.14
For non-pharmacologic treatments, the provider should explain evidence-based options that are available postoperatively such as distraction strategies, guided imagery, or mindful breathing.15 Once the older adult has chosen preferred modalities, the provider should ensure that the older adult has the physical and mental abilities to utilize the chosen non-pharmacologic pain management. Physical and mental fatigue may interfere with the learning process, so for these modalities to be maximally effective, it is important for the older adult to learn them preoperatively when not yet afflicted by pain.15,16
Older adults and their caregivers should also be educated on the various pharmacologic treatment options. A simple explanation of how the drug works (e.g. non-steroidal anti-inflammatory drugs (NSAIDs)) should be discussed (Table 1). Misconceptions about pain treatments are common among older adults and can interfere with willingness to take certain analgesics, particularly fears regarding addiction and tolerance affecting patients’ willingness to take opioids.16–18 Infographics and visuals about pain management with easy to understand language can also be useful in the preoperative setting. A recent single-institution study by Angelo et al. showed that a patient-friendly infographic in the preoperative area listing the common “do’s and don’ts” of pain medications was a useful adjunct to help decrease opioid consumption postoperatively.19 Similar visual reminders can help both patients and caregivers in the outpatient clinic or post-hospital discharge setting.
Table 1:
Common pain medications with mechanisms of action (MOA) for pain relief
| Medication | MOA |
|---|---|
| Acetaminophen | Nonspecific central cyclooxygenase inhibitor |
|
Non-steroidal anti-inflammatory drugs (NSAIDs) - Non-selective COX inhibitors (e.g. ibuprofen, naproxen, ketorolac) - Selective COX2 inhibitors (e.g. celecoxib) |
Inhibit cyclooxygenase enzymes, thereby reducing the synthesis of prostaglandins which are involved in inflammation |
|
GABA analogues (e.g. gabapentin and pregabalin) |
Inhibits calcium influx, which reduces the excitability of dorsal horn neurons after tissue damage |
|
Opioids (e.g. morphine, hydrocodone, oxycodone, hydromorphone) |
Mimic natural opioids and interact with mu, delta or kappa opioid receptors |
Every older adult should receive a directed pain history and physical examination during the preoperative period.20,21 The pain history should focus on previous experiences with painful conditions, pain strategies that have worked in the past, preferences for pain control and history of geriatric events (e.g. delirium, fall).22 In addition, a history of abuse of other substances that may affect pain management (e.g. benzodiazepines, alcohol) is an important component of the preoperative conversation.20 Although no studies have proven the efficacy of a preoperative pain history and physical exam, multiple anesthesia and pain societies agree that this information is essential in personalizing and optimizing perioperative pain management. This is particularly true in the aging adult who may have geriatric-specific comorbidities such as cognitive impairment or history of falls that influence the pain management strategy after surgery.20,21 In addition, assessment and documentation of the older adult’s cognitive function with a screening test such as the Mini-Cog can help identify patients with cognitive impairment or dementia who are at increased risk of postoperative delirium. Instruments like this can provide an important baseline reference of cognitive ability and guide choice of pain assessment tools as well as both pharmacologic (e.g. minimization of opioids) and non-pharmacologic treatment options.23,24
In older adults with complex pain management needs (i.e. chronic opioid use, opioid tolerance or addiction), a pain specialist should be consulted for preoperative planning and postoperative management. When a designated pain specialist is unavailable or resources are limited, surgeons can collaborate with others that may have pain management experience such as anesthesiologists, physical medicine and rehabilitation physicians, palliative care physicians or healthcare providers with geriatrics expertise (e.g. geriatrician or hospitalist/internist/advance practice provider with geriatric training). An issue for this vulnerable population is balancing adequate pain treatment with risk of worsening addiction, as poorly treated pain can be a trigger for relapse as well as postoperative delirium which is commonly seen in the geriatric population.20,25 These patients may also be taking medications aimed at treating an opioid use disorder (e.g., methadone, buprenorphine, naltrexone), further complicating their perioperative pain management.26
A preoperative pain management plan should be developed and individualized for each older adult given the unique comorbidities, previous experiences with pain, and patient preferences. In addition, it is also important to develop the plan in collaboration with the older adult’s family member/caregiver.20 An example of a pain management plan template is available from the American College of Surgeons Safe and Effective Pain Control After Surgery.18 The pain management plan should be centered around specific functional targets rather than a defined level of pain.20,27 Functional goals in the postoperative period should be both provider-driven (e.g. early mobility or incentive spirometry) and patient-driven (e.g. sleep comfortably). Importantly, these goals may impact other downstream outcomes in older adults such as postoperative delirium and functional decline.
Immediate preoperative phase (subheading):
The immediate preoperative phase is a crucial time period for collaboration with the anesthesiologists as well as implementing adjuncts for a successful pain management strategy. The surgeon should work with the anesthesiologist to review and incorporate the preoperative pain history and pain management plan to help close the loop between the preoperative and intraoperative phases of care.
Non-opioid adjuncts like acetaminophen, NSAIDs and GABA (γ-aminobutyric acid) analogues should be considered in the preoperative period for older adults. Table 2 depicts timing, example dosages for older adults, and side effects of common preoperative medications. The pathophysiology of preemptive analgesia is based on the idea of prevention of central sensitization, or the activation of central neurons by afferent signals that occur during surgery, leading to hypersensitivity postoperatively.28 A meta-analysis of 11 randomized controlled trials (RCTs) showed that a single dose of preoperative acetaminophen reduced early pain as well as postoperative opioid consumption.29 Acetaminophen is generally well tolerated, even in the older adult population.30 The available evidence supports a strong recommendation for preoperative acetaminophen use, delivered either orally or intravenously. However, caution is advised when using acetaminophen near maximal dosages, especially in the older adult demographic, as it can cause hepatotoxicity.
Table 2:
Perioperative medications, timing, dosages and potential side effects in older adults
| Phase of Care | Medication | Timing | Example Dosing42 | Max dose | Side effects | Special Considerations |
|---|---|---|---|---|---|---|
| Pre-operative | Acetaminophen | Within 30–90min of surgery | 500 to 1000mg i.v. or p.o. × 1 dose | 3000mg daily | Hepatotoxicity | Reduce max dose (2000mg) if malnourished (<50kg) or hepatic insufficiency42 |
| NSAIDs (e.g. Celecoxib) | 200mg p.o. celecoxib × 1 dose | Celecoxib: 400mg daily | GI bleeding, renal dysfunction | Non-selective potentially inappropriate: risk of GI bleed, PUD and AKI34 | ||
| GABA analogues (e.g. gabapentin, pregabalin) | 600mg p.o. Gabapentin OR 150mg p.o. of pregabalin × 1 dose | Gabapentin: 2400mg/day Pregabalin: 300mg/day | Respiratory depression, sedation, dizziness | Potential drug-drug interaction with opioids34 | ||
| Intra-operative Peri-induction | Neuraxial/Local | At time of induction or incision | Variable dose and route | Variable | Cardiotoxicity | Route specific to surgery |
| Dexamethasone | 0.11–0.2mg/kg | 8 to 25mg per day | Blood glucose alterations | Anesthesia to dose | ||
| Magnesium | 30–50mg/kg bolus followed by infusion | 30 to 40gm per day | Hypotension | Anesthesia to dose | ||
| Post-operative | Acetaminophen | Initiate <24hrs of surgery completion | 500 to 1000mg p.o. or i.v. q6hrs | 3000mg daily | Hepatotoxicity | Reduce max dose (2000mg) if malnourished (<50kg) or hepatic insufficiency |
| NSAIDs | 200mg mg BID Celecoxib OR 15mg i.v. q6hrs Ketorolac |
-Celecoxib: 400mg daily -Ketorolac: 60mg/day |
GI bleeding, renal dysfunction | Non-selective potentially inappropriate: risk of GI bleed, PUD and AKI | ||
| GABA analogues | 600mg Gabapentin OR 150mg pregabalin q12hrs | See above | Respiratory depression, Sedation, dizziness | Potential drug-drug interaction with opioids | ||
| Topical patches | 5% lidocaine patch (Lidoderm) 12hr/day | N/a | Local skin reaction | |||
| Opioids | - Reduce by 25–50% (e.g. 2.5mg oxycodone q6hrs) - PCA when i.v. required (e.g. morphine 1mg q10min or hydromorphone 0.1mg q10min) |
Variable | Respiratory depression, sedation, tolerance and addiction risks | Use equianalgesic table when converting67 |
Abbreviations: NSAIDs (non-steroidal anti-inflammatory drugs), GABA (gamma-Aminobutyric acid), GI (gastrointestinal), PUD (peptic ulcer disease), AKI (acute kidney injury), PCA (patient-controlled analgesia)
Other preoperative premedication regimens include NSAIDs and GABA analogues (gabapentinoids). Clinical Practice Guidelines from the American Pain Society (APS) recommend the consideration of a preoperative dose of oral celecoxib, given that a number of RCTs have shown an associated reduction in postoperative opioid requirements and lower postoperative pain scores.20,31,32 A meta-analysis including thirteen RCTs examining a single perioperative dose of ketorolac showed improvements in early pain at rest and a reduction in opioid consumption.33 However, it should be noted that non-cyclooxygenase-selective NSAIDs (e.g. ibuprofen, naproxen, ketorolac) were included on the 2019 Beers criteria of potentially inappropriate medications for older adults due to increased risk of gastrointestinal bleeding/peptic ulcer disease and acute kidney injury.34 However, the specific recommendation is to avoid chronic use of non-selective NSAIDs, unless alternatives are not effective and patient can take a gastroprotective agent (i.e. proton pump inhibitor). Particularly high-risk groups for chronic NSAID use include older adults >75 years, those taking steroids or anticoagulants, those with a history of gastric or duodenal ulcers, and patients with chronic kidney disease stage 4 or higher (moderate quality of evidence, strong recommendation based on 2019 AGS Beers Criteria).34 However, it should be noted that thoughtful use of short-term NSAIDS may be appropriate in select older adults depending on comorbidities and desire to minimize opioids.
GABA analogues (e.g. gabapentin, pregabalin) are another adjunct frequently used in the preoperative period. Several meta-analyses of RCTs have suggested beneficial effects of preoperative GABA analogues on postoperative pain.35,36 However, GABA analogues are associated with side effects that may disproportionately affect older adults (e.g. respiratory depression, sedation, dizziness). Furthermore, the combination of opioids and GABA analogues have been added to the 2019 AGS Beers Criteria as a potentially inappropriate medication combination for older adults, due to a drug-drug interaction increasing the risk of opioid overdose and sedation-related adverse events. However, the recommendation includes an exception when transitioning from opioid therapy to gabapentin or pregabalin or when using gabapentinoids to reduce opioid dosages (moderate quality of evidence, strong recommendation based on 2019 AGS Beers Criteria).34 Given the recommendation from the 2019 AGS Beers Criteria regarding GABA analogues, thoughtful use of GABA analogues can be considered as part of enhanced recovery after surgery (ERAS) pathways tailored specifically to older adults.
Regional or neuraxial anesthetic techniques can be safely used in older adults and should be considered if appropriate for the operation.20,21,37 Examples of specific operations for which regional or neuraxial anesthetic techniques are commonly used include the following: thoracotomy (e.g. paravertebral block), colectomy (e.g. epidural or transversus abdominis plane blocks), and extremity operations (e.g. site-specific regional blocks for orthopedic or vascular surgery).20 Several meta-analyses and systematic reviews across a variety of surgical procedures suggest that regional and neuraxial techniques are associated with decreased pain scores and decreased need for analgesic medication postoperatively.38–41 Regional anesthesia is associated with earlier ambulation, earlier return of bowel function and improved mental status.42 Additionally, regional techniques have minimal effect on hemodynamics and do not cause urinary retention, a common complication following any surgical procedure in the older adult population. Neuraxial techniques may allow for reduced opioid dosing and thus can benefit the older adult in terms of cognitive function, improved mobility, and prevention of pulmonary complications.17 Increased attention to use of regional or neuraxial techniques can be an important component of an opioid sparing multimodality pain management protocol in older adults with the goal of minimizing downstream outcomes like postoperative delirium and functional decline.
Intraoperative phase (subheading):
There are limited but important interventions that can occur during the intraoperative phase of care in the geriatric population which necessitate collaboration and communication with the anesthesia team. Local anesthetic should be considered for intraoperative administration via at least one method (i.e. regional/neuraxial or subcutaneous). Multiple systematic reviews of RCTs have found improved pain scores and greater pain relief when neuraxial opioid is combined with local anesthetic, as compared to neuraxial opioid alone.43,44 APS Guidelines recommend that providers consider surgical site-specific local anesthetic infiltration for select surgical procedures. Although there are no RCTs showing the efficacy of subcutaneous local anesthetic, there is a low risk of harm when using appropriate doses and should be considered for a wide variety of surgical procedures in older adults.45 One example of local anesthetic infiltration is continuous local anesthetic infusion catheters (i.e. ON-Q pumps) which provide non-opioid pain relief delivered directly to a surgical site for up to five days. These pumps have been shown to be useful in a variety commonly performed surgeries in the geriatric population, for example orthopedic procedures and open abdominal operations such as laparotomy for colorectal resections.46,47 While there is no data specific to use of local anesthetic in older adults, it is a low risk intervention that may reduce opioid use and improve mobility, therefore minimizing the risk of postoperative delirium and functional decline.
Several drugs, including dexamethasone and magnesium, delivered either before induction of anesthesia, at induction or intraoperatively have shown to be important adjuncts in preventive analgesia, a central tenet for optimal pain management after surgery in older adults. Dexamethasone, which is frequently given to decrease postoperative nausea and vomiting, has also been shown to have analgesic and opioid sparing effects.48 Magnesium, which serves as an antagonist to N-methyl-d-aspartate glutamate receptors and can therefore alter pain response, has been used for many decades in an attempt to reduce postoperative pain.49,50 Meta-analyses of randomized controlled trials examining both of these medications in a variety of procedures have shown promising results with low rates of adverse effects.48,51,52 The dosages of these medications are weight based, and should be administered by the anesthesiologist (Table 2).
Intravenous ketamine is another medication that could be considered for intraoperative administration (weak recommendation, moderate quality of evidence per the 2019 AGS Beers Criteria).20 A meta-analysis of RCTs in adults of all ages showed that intraoperative ketamine may offer protection against postoperative cognitive dysfunction, which is particularly relevant to the older adult population.53 However, a recent randomized trial in older adults showed that while ketamine did not have increased rates of delirium, it also did not affect postoperative opioid requirements or pain and can cause negative experiences like hallucinations or nightmares.54 In summary, local anesthetic, dexamethasone, magnesium and ketamine are intraoperative medications that can be consider for use in older adults undergoing surgery with the goal of optimizing pain control while minimizing opioid use.
Postoperative phase (subheading):
Pain control in the older adult requires adequate treatment of pain while simultaneously avoiding over-treatment with opioids. Uncontrolled pain is more common in older adults, and is also a precipitating factor for postoperative delirium.55 However, the risk of harmful side effects of opioids in older adults (e.g. triggering postoperative delirium) must be balanced with appropriate pain control to facilitate mobility and minimize postoperative functional decline after surgery.17,42
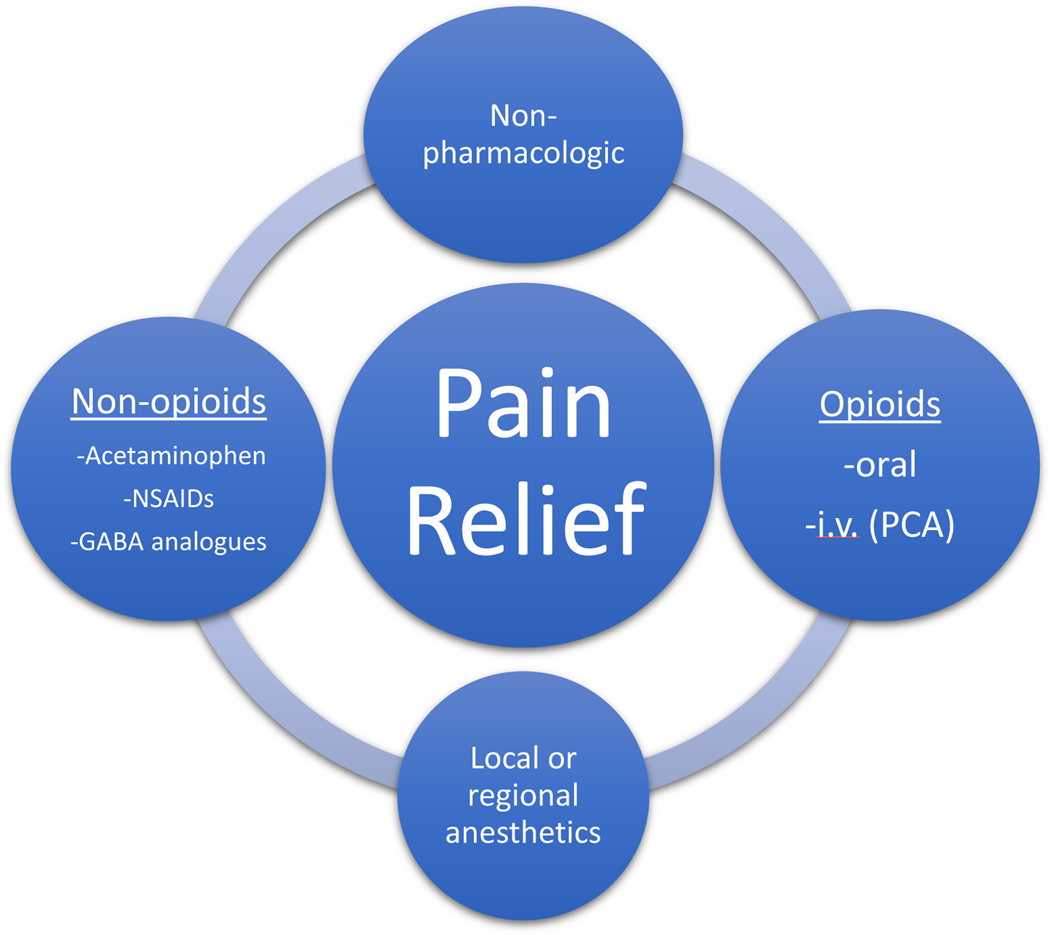
The cornerstone of the postoperative phase of care in the older adult population involves a multimodal pain management strategy, employing medications with different mechanisms as well as non-pharmacologic pain relief therapies (Figure 2) with the goal of minimizing opioid use.21,51 RCTs with numerous combinations of drugs acting at different receptors, medications administered via different techniques, and non-pharmacologic modalities have shown that these strategies result in a reduction in postoperative pain, a reduction in the consumption of any one analgesic drug, and in many cases a reduction in the consumption of opioid analgesics.20,56,57 Older adults have increased sensitivity to opioid and nonopioid medications, so this population may have the most to benefit from multimodal pain regimens.42 However, the choice of a specific multimodal pain regimen should be individualized to both the older adult and type of surgery. Particular attention must be paid to drug-drug interactions and potential medication side effects when designing a multimodal pain plan for older adults as this population is at increased risk for side effects from polypharmacy and postoperative delirium.
Figure 2:
Postoperative multimodal pain strategy for successful pain relief in the older adult
There are multiple medications that can be scheduled around-the-clock to help decrease the need for opioids in the older adult population. Acetaminophen is an excellent drug to use in older adults, as it does not cause any gastrointestinal disturbance and has low risk for toxicity except in those with severely impaired liver function.42 As discussed in the immediate preoperative section, the use of NSAIDs in the older adult population is controversial. The optimal duration for administration of NSAIDs to avoid adverse side effects in older adults has not been directly studied so it is recommended that they be used with caution and for the shortest duration possible to minimize the risk of side effects.58 Similar to regional strategies addressed in the preoperative phases, nerve blocks (either single-shot or continuous) can be safely employed postoperatively in the older adult population for certain operations including knee replacement therapy59 or in the setting of hip fractures in the elderly.60 In addition, patient-controlled epidural analgesia (PCEA) is an effective method to consider for a variety of procedures including laparotomy for general surgery or gynecologic operations. The exact PCEA regimen should be tailored to the older adult to avoid adverse events such as hypotension or inadequate pain control which are commonly seen in this cohort of older adult patients.61 Topical anesthetic agents (e.g. lidocaine patch, vapocoolant anesthetic sprays) can also be considered for postoperative administration although these have variable data to support routine use.6,62,63
The evidence-based non-pharmacologic pain management therapies learned and practiced preoperatively (e.g. guided imagery, mindful breathing) should be implemented as early as possible in the postoperative setting in the older adult population. Overall, studies of these modalities have shown positive effects on postoperative pain, anxiety, and analgesic use, with no significant harm.20 Other therapies that do not require preoperative learning can be introduced by nursing staff (e.g. superficial massage, repositioning, superficial heat or cold, and vibration). Furthermore, certain interventions (e.g. music) have been shown in older adults to reduce discomfort and increase family involvement in care.17
When treating older adults with opioids, careful dose titration is essential. The ASA guidelines include a short section on geriatric patients where it is acknowledged that vigilant dose titration is necessary due to altered physiology, comorbidities, and the concurrent use of other medications.21 Many are familiar with the adage “start low and go slow,” and it is generally well-accepted by the geriatrics community that a dose reduction of 25 – 50% of the normal adult dose of opioids is a reasonable starting point (Table 2).17,42 For example, a more appropriate starting dose of oxycodone in an older adult would be 2.5 mg rather than 5 mg. In addition, when increasing the dose of opioids, an increase of 25% is reasonable until there is a 50% reduction in the patient’s pain rating or the patient reports satisfactory pain relief.64
When opioids are used in the older adult population, the oral route should be used over the intravenous (IV) route whenever possible.20,65 IV opioids have the highest risk of adverse events such as sedation, respiratory depression, cognitive impairment and postoperative delirium.42 However, there are many instances where IV opioids are necessary, such as in the immediate postoperative period or if patients must be nil per os (NPO) after abdominal surgery. When IV opioids are necessary, patient-controlled analgesia (PCA) is the gold standard in terms of successful analgesia as well as patient satisfaction (see Table 2 for example starting doses).42 However, this modality should not be used in older adults with severe dementia or delirium who are unable to understand how to push the button as this may result in inadequate analgesia.17 Intramuscular (IM) administration of medications should not be used in the older adults due to increased pain and unreliable absorption.20,21,66 Older adults have muscle wasting and decreased fatty tissue that leads to slower IM absorption and potentially delayed or prolonged effects, which can subsequently lead to accumulated toxicity with repeated injections.17 Lastly, if changing to a new opioid agent or different route of administration in the older adult patient, the new dose should be calculated with an equianalgesic table67 and the dose should be lowered by 25–50% of oral morphine equivalents.64
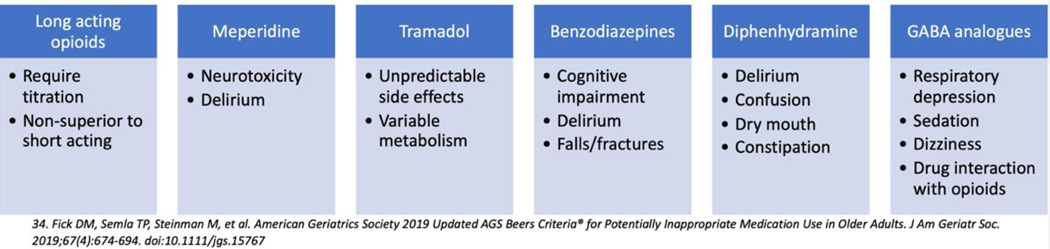
While opioids can be a critical part of the postoperative pain management plan, there are certain opioids that should be avoided in older adults (Figure 3). For example, long acting opioids (e.g. MS Contin, OxyContin, fentanyl patch) should be avoided for opioid naïve older adults as there is no evidence that they provide better pain control than short acting opioids.20,68 Meperidine is another potentially inappropriate medication for older adults as it has a higher risk of neurotoxicity, including postoperative delirium, compared to other opioids (moderate quality of evidence, strong recommendation based on 2019 AGS Beers Criteria).34 Lastly tramadol should be used with caution due to unpredictable effects69 and concern for increased risk of delirium.42,70 Finally, tramadol was recently included in the 2019 AGS Beers Criteria34 and has also been referred to as “tramadon’t” in the geriatrics community due to the variable metabolism and unfavorable side effect profile in the older adult.71
Figure 3:
Medications to be avoided in older adult based on 2019 Beers Criteria34
Older adults are more sensitive to opioids and their adverse effects, including constipation.42,72 Analgesic plans for all older adults postoperatively should include a prophylactic pharmacologic bowel regimen.37 An optimal prophylactic pharmacologic bowel regimen should include more than just a stool softener such as a bulking agent (e.g. fiber), stimulant laxative (e.g. Bisacodyl, Senna) or osmotic laxative (e.g. polyethylene glycol).73
Accurate assessment of pain in the older patient with impaired cognition can be difficult, and unfortunately, clinicians often under-treat pain in this patient population.72 The cognitively impaired older adult may have trouble verbally communicating pain levels, so behavioral indicators can act as an indirect measure by which the clinician can judge the presence and intensity of pain.73 Examples include nonverbal cues/behaviors such as restlessness and agitation, vocalizations such as groaning or moaning, facial expressions such as brow lowering with jaw drop or mouth open, and a change in usual behavior such as new onset of confusion or aggression.73 Appropriate pain assessment tools should be utilized in order to guide interventions. Examples of appropriate pain assessment tools in this population include the Checklist of Nonverbal Pain Indicators (CNPI), Pain Assessment in Advanced Dementia (PAINAD), and the Critical Care Pain Observation Tool (CPOT).74–76 Frequent assessments at regular intervals after surgery help determine adequacy of pain relief, monitor progress toward functional goals, and recognize early signs of postoperative delirium which is of utmost importance in the older adult patient. Postoperative delirium is associated with increased rates of postoperative mortality, morbidity and need for discharge to skilled nursing facility as well as increased healthcare costs77
Post-operative delirium is the most common complication in older adults and rates can vary by type of operation. Delirium deserves special attention as this complication disproportionately affects older adults and profoundly affects their recovery after surgery.78,79 Importantly, up to 40% of cases of delirium are preventable.80 Prophylactic strategies to prevent delirium include non-pharmacologic strategies such as sensory enhancement, for example, to ensure that glasses, hearing aids, or listening amplifiers are available in addition to early mobility. Additionally, frequent reorientation and appropriate sleep hygiene such as preserving the sleep/wake cycle and maximizing cues that orient the older patient to time, place, and person are crucial in this population post-operatively.
Once a delirium episode has been identified, further workup is needed to identify a potential underlying cause for the delirium such as infection, electrolyte abnormalities, environmental contributors, and medications. Pharmacotherapy for management of delirium may be initiated if non-pharmacologic methods or management of precipitating conditions are not effective. The AGS recommends against the routine use of benzodiazepines as a first-line agent for the treatment of postoperative delirium, even when complicated by agitation or threats of harm to self/others in the elderly. In the severely agitated elderly patient experiencing delirium, the recommended treatment is use of an antipsychotic at the lowest effective dose for the shortest possible duration after all non-pharmacologic interventions have been exhausted.81 Practitioners should exhibit caution in prescribing antipsychotics for delirium and reserve pharmacotherapy for the treatment of older adults who are agitated or threatening substantial harm to self or others. Recent data shows that low-dose haloperidol (< 3.0 mg/day) is equally efficacious as the atypical antipsychotics olanzapine and risperidone in the treatment of delirium.82 High-dose haloperidol (> 4.5 mg/day) is associated with extrapyramidal adverse effects (parkinsonism) and should be avoided in the older age patient population.83
Peri-and Post-Discharge Phase (subheading):
The transition home after surgery can be challenging for patients, and this is especially true for older adults and their caregivers. With older adults, it is important to involve family members/caregivers in discharge education, which includes medication education, as older adults may need more support during the transition from hospital to home. Topics specific to pain management that should be addressed include expectations for pain, tapering of opioid medications and how to dispose of unused opioids. In addition, recognition of problems related to pain management including constipation, functional decline and postoperative delirium should be reviewed. Easy to follow instructions and patient infographics for pain after surgery can be used to reinforce the importance of these topics.19 Equally important, the older adult should be counseled on mobility exercises (e.g. daily walking program) to perform after discharge to prevent functional decline and must be informed that these may also be associated with some pain and discomfort.84 At the same time, it is important that the older adult treats his or her pain adequately so as not to impair function. A post discharge pain management plan including a list of instructions for the older adult and family member/caregiver should be reviewed with the older adult and caregiver prior to discharge.18
Conclusions:
The pain management needs and strategy are unique for older adults undergoing surgery. While pain management often needs to be tailored to specific operations, the current narrative review aimed to highlight important themes for older adults that can be applied across a myriad of surgical disciplines. A multi-disciplinary approach spanning all phases of perioperative care is necessary to provide a safe and effective pain management plan for older adults undergoing surgery. In addition, it should be noted that opioid-sparing, multimodality pain management is one of the hospital standards for the recently launched American College of Surgeons (ACS) Geriatric Surgery Verification Program.7
The overarching goal of improving pain management in older adults is to optimize pain control while minimizing the side effects from opioids which can contribute to other downstream outcomes in this vulnerable population such as postoperative delirium and functional decline. A summary of the pain management issues unique to older adults are outlined by phase of care below.
Preoperative Phase:
This phase is hallmarked by a thorough history focusing on prior issues with pain and geriatric-specific issues unique to the older adults such as prior falls or delirium. Additionally, there is an emphasis on education of both the older adult and their family/caregiver about expectations for pain after surgery and the importance of meeting functional goals such as ambulation.
Immediate Preoperative Phase:
This phase is highlighted by a structured coordination between the operative and anesthesiology teams. Preoperative consideration of non-opioid medications such as acetaminophen, NSAIDs, and GABA analogues should be considered based on the older adult’s other medical problems. In addition, an emphasis on incorporation of regional or neuraxial anesthetic techniques are important as a way to minimize opioids and improve pain control.
Intraoperative Phase:
This phase focuses on optimizing patient’s pain control prior to awakening from anesthesia as well as minimizing narcotics. Strong consideration should be given to judicious use of local anesthetics as well as continuation of neuraxial/regional anesthesia. Medications such as dexamethasone, magnesium, and ketamine may be used as adjuncts for an opioid-sparing multimodal pain plan.
Postoperative Phase:
This phase proves to be a delicate balance between adequate pain control using a multimodality approach and avoidance of side effects seen with common opioid pain medications. Minimization of opioids in older adults is a combination of use of non-pharmacologic methods for pain control, non-opioid medications, and use of smaller doses of opioids in this vulnerable population. The relationship between pain and postoperative delirium is reviewed including a brief overview of delirium prevention, workup, and treatment.
Peri-and Post-discharge Phase:
This phase again emphasizes pain expectations and communication between the provider, patient, and importantly, the patient’s family/caretakers regarding management of pain after discharge from the hospital. Th goal is to return patients to their prior level of function while minimizing complications related to pain management (e.g. constipation, postoperative delirium, functional decline).
Acknowledgments
Funding: GEMSSTAR - Grants for Early Medical/Surgical Specialists’ Transition to Aging Research to Dr. Russell - 1R03AG056350-01 funded by National Institute on Aging. Dr. Sarkisian was supported by National Institute on Aging [grant number 1K24AG047899-01] and UCLA Clinical and Translational Science Institute (NIH/NCATS Grant # UL1TR001881).
Abbreviations:
- CPOT
Critical Care Pain Observation Tool
- CNPI
Checklist of Nonverbal Pain Indicators
- GABA
γ-aminobutyric acid
- IM
intramuscular
- IV
intravenous
- NPO
nil per os
- NSAIDS
non-steroidal anti-inflammatory drugs
- PAINAD
Pain Assessment in Advanced Dementia
- PCA
patient-controlled analgesia
- PFEC
patient and family engaged care
- RCT
randomized controlled trials
Societies:
- ACS
American College of Surgeons
- AGS
American Geriatrics Society
- APS
American Pain Society
- ASRA
Society of Regional Anesthesia and Pain Medicine
- ASA
American Society of Anesthesiologists
Footnotes
1. Adam D. Shellito, MD: ashellito@dhs.lacounty.gov, Dr. Shellito participated in research design, writing of the paper and in data analysis
2. Jill Q. Dworsky, MD, MS: JDworsky@mednet.ucla.edu, Dr. Dworsky participated in research design, writing of the paper, performance of the research and in data analysis
3. Patrick J. Kirkland, MD: pkirkland@dhs.lacounty.gov; Dr. Kirkland participated in writing of the paper and in data analysis
4. Ronnie A. Rosenthal, MD: ronnie.rosenthal@yale.edu; Dr. Rosenthal participated in research design, writing of the paper, and in data analysis
5.Catherine A. Sarkisian, MD, MSPH: CSarkisian@mednet.ucla.edu; Dr. Sarkisian participated in research design, writing of the paper, and in data analysis
6. Clifford Y. Ko, MD, MS, MSHS: cko@facs.org; Dr. Ko participated in research design, writing of the paper, and in data analysis
7. Marcia M. Russell, MD: MRussell@mednet.ucla.edu; Dr. Russell participated in research design, writing of the paper, performance of the research and in data analysis
Footnotes
Disclosures: The authors declare no conflicts of interest
References
- 1.Werner CA. The Older Population: 2010 (U. S. Census Bureau Report No. C2010BR-09). 2011;(November):1–19. www.census.gov/population. Accessed July 10, 2019.
- 2.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a Predictor of Surgical Outcomes in Older Patients. J Am Coll Surg. 2010;210(6):901–908. doi: 10.1016/j.jamcollsurg.2010.01.028 [DOI] [PubMed] [Google Scholar]
- 3.Aalami OO, Fang TD, Song HM, Nacamuli RP. Physiological features of aging persons. Arch Surg. 2003;138(10):1068–1076. doi: 10.1001/archsurg.138.10.1068 [DOI] [PubMed] [Google Scholar]
- 4.Hill MV, McMahon ML, Stucke RS, Barth RJ. Wide Variation and Excessive Dosage of Opioid Prescriptions for Common General Surgical Procedures. Ann Surg. 2017;265(4):709–714. doi: 10.1097/SLA.0000000000001993 [DOI] [PubMed] [Google Scholar]
- 5.Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and Risk Factors for Chronic Opioid Use Among Opioid-Naive Patients in the Postoperative Period. JAMA Intern Med. 2016;176(9):1286. doi: 10.1001/jamainternmed.2016.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ickowicz E. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x [DOI] [PubMed] [Google Scholar]
- 7.Geriatric Surgery Verification Program. https://www.facs.org/quality-programs/geriatric-surgery. Accessed January 28, 2020.
- 8.Paez A. Gray literature: An important resource in systematic reviews. J Evid Based Med. 2017;10(3):233–240. doi: 10.1111/jebm.12266 [DOI] [PubMed] [Google Scholar]
- 9.Szeverenyi C, Kekecs Z, Johnson A, Elkins G, Csernatony Z, Varga K. The Use of Adjunct Psychosocial Interventions Can Decrease Postoperative Pain and Improve the Quality of Clinical Care in Orthopedic Surgery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Pain. 2018;19(11):1231–1252. doi: 10.1016/j.jpain.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 10.Ramesh C, Nayak BS, Pai VB, et al. Effect of Preoperative Education on Postoperative Outcomes Among Patients Undergoing Cardiac Surgery: A Systematic Review and Meta-Analysis. J perianesthesia Nurs Off J Am Soc PeriAnesthesia Nurses. 2017;32(6):518–529.e2. doi: 10.1016/j.jopan.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 11.McGory ML, Kao KK, Shekelle PG, et al. Developing quality indicators for elderly surgical patients. Ann Surg. 2009;250(2):338–347. doi: 10.1097/SLA.0b013e3181ae575a [DOI] [PubMed] [Google Scholar]
- 12.Lee TG, Kang SB, Kim DW, Hong S, Heo SC, Park KJ. Comparison of early mobilization and diet rehabilitation program with conventional care after laparoscopic colon surgery: A prospective randomized controlled trial. Dis Colon Rectum. 2011;54(1):21–28. doi: 10.1007/DCR.0b013e3181fcdb3e [DOI] [PubMed] [Google Scholar]
- 13.Ware LJ, Epps CD, Herr K, Packard A. Evaluation of the Revised Faces Pain Scale, Verbal Descriptor Scale, Numeric Rating Scale, and Iowa Pain Thermometer in Older Minority Adults. Pain Manag Nurs. 2006;7(3):117–125. doi: 10.1016/j.pmn.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 14.University of Iowa, Pain Management Resources | Geriatric Pain. https://geriatricpain.org/education-clinicians/clinical-practice-guidelines/university-iowa-pain-management-resources. Accessed November 16, 2019.
- 15.Tick H, Nielsen A, Pelletier KR, et al. Evidence-Based Nonpharmacologic Strategies for Comprehensive Pain Care: The Consortium Pain Task Force White Paper. Explore. 2018;14(3):177–211. doi: 10.1016/j.explore.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 16.Patient & Community Education | Michigan OPEN. https://michigan-open.org/patient-community-education/. Accessed November 16, 2019.
- 17.Ardery G, Herr KA, Titler MG, Sorofman BA, Schmitt MB. Assessing and managing acute pain in older adults: a research base to guide practice. Medsurg Nurs. 2003;12(1). [PubMed] [Google Scholar]
- 18.Safe Pain Control: Opioid Abuse and Surgery. https://www.facs.org/safepaincontrol. Accessed November 15, 2019.
- 19.Angelo JL, Wu J, Sirody J, Deugarte DA. Reduction in prescribed opioids after general surgery procedures at a public hospital. Am Surg. 2019;85(10):1198–1203. [PubMed] [Google Scholar]
- 20.Chou R, Gordon DB, De Leon-Casasola OA, et al. Management of postoperative pain: A clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of anesthesiologists’ committee on regional anesthesia, executive commi. J Pain. 2016;17(2):131–157. doi: 10.1016/j.jpain.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 21.Practice guidelines for acute pain management in the perioperative setting: An updated report by the american society of anesthesiologists task force on acute pain management. Anesthesiology. 2012;116(2):248–273. doi: 10.1097/ALN.0b013e31823c1030 [DOI] [PubMed] [Google Scholar]
- 22.Tan H, Saliba D, Kwan L, et al. Burden of Geriatric Events Among Older Adults Undergoing Major Cancer Surgery. J Clin Oncol. 2016;34(11). doi: 10.1200/JCO.2015.63.4592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Simplifying detection of cognitive impairment: Comparison of the Mini-Cog and Mini-Mental State examination in a multiethnic sample. J Am Geriatr Soc. 2005;53(5):871–874. doi: 10.1111/j.1532-5415.2005.53269.x [DOI] [PubMed] [Google Scholar]
- 24.Riley McCarten J, Anderson P, Kuskowski MA, McPherson SE, Borson S, Dysken MW. Finding dementia in primary care: The results of a clinical demonstration project. J Am Geriatr Soc. 2012;60(2):210–217. doi: 10.1111/j.1532-5415.2011.03841.x [DOI] [PubMed] [Google Scholar]
- 25.Samuel M, Inouye SK, Robinson T, et al. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63(1):142–150. doi: 10.1111/jgs.13281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison TK, Kornfeld H, Aggarwal AK, Lembke A. Perioperative Considerations for the Patient with Opioid Use Disorder on Buprenorphine, Methadone, or Naltrexone Maintenance Therapy. Anesthesiol Clin. 2018;36(3):345–359. doi: 10.1016/j.anclin.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 27.Brinson ZS, Tang VL, Finlayson E. Postoperative Functional Outcomes in Older Adults. Curr Surg Reports. 2016;4(6). doi: 10.1007/s40137-016-0140-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woolf CJ, Chong MS. Preemptive analgesia - Treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77(2):362–379. doi: 10.1213/00000539-199377020-00026 [DOI] [PubMed] [Google Scholar]
- 29.De Oliveira GS, Castro-Alves LJ, McCarthy RJ. Single-dose systemic acetaminophen to prevent postoperative pain: A meta-analysis of randomized controlled trials. Clin J Pain. 2015;31(1):86–93. doi: 10.1097/AJP.0000000000000081 [DOI] [PubMed] [Google Scholar]
- 30.Shamoon M, Hochberg MC. The role of acetaminophen in the management of patients with osteoarthritis. Am J Med. 2001;110(3 SUPPL. 1):46–49. doi: 10.1016/s0002-9343(01)00618-0 [DOI] [PubMed] [Google Scholar]
- 31.Ekman EF, Wahba M, Ancona F. Analgesic Efficacy of Perioperative Celecoxib in Ambulatory Arthroscopic Knee Surgery: A Double-Blind, Placebo-Controlled Study. Arthrosc - J Arthrosc Relat Surg. 2006;22(6):635–642. doi: 10.1016/j.arthro.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 32.Huang YM, Wang CM, Wang CT, Lin WP, Horng LC, Jiang CC. Perioperative celecoxib administration for pain management after total knee arthroplasty - A randomized, controlled study. BMC Musculoskelet Disord. 2008;9. doi: 10.1186/1471-2474-9-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Oliveira GS, Agarwal D, Benzon HT. Perioperative single dose ketorolac to prevent postoperative pain: A meta-analysis of randomized trials. Anesth Analg. 2012;114(2):424–433. doi: 10.1213/ANE.0b013e3182334d68 [DOI] [PubMed] [Google Scholar]
- 34.Fick DM, Semla TP, Steinman M, et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674–694. doi: 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 35.Arumugam S, Lau CSM, Chamberlain RS. Use of preoperative gabapentin significantly reduces postoperative opioid consumption: A meta-analysis. J Pain Res. 2016;9:631–640. doi: 10.2147/JPR.S112626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han C, Kuang MJ, Ma JX, Ma XL. The efficacy of preoperative gabapentin in spinal surgery: A Meta-analysis of randomized controlled trials. Pain Physician. 2017;20(7):649–661. [PubMed] [Google Scholar]
- 37.Mohanty S, Rosenthal RA, Russell MM, Neuman MD, Ko CY, Esnaola NF. Optimal Perioperative Management of the Geriatric Patient: A Best Practices Guideline from the American College of Surgeons NSQIP and the American Geriatrics Society. J Am Coll Surg. 2016;222(5):930–947. doi: 10.1016/j.jamcollsurg.2015.12.026 [DOI] [PubMed] [Google Scholar]
- 38.Liu SS, Strodtbeck WM, Richman JM, Wu CL. A comparison of regional versus general anesthesia for ambulatory anesthesia: A meta-analysis of randomized controlled trials. Anesth Analg. 2005;101(6):1634–1642. doi: 10.1213/01.ANE.0000180829.70036.4F [DOI] [PubMed] [Google Scholar]
- 39.Yeung JH, Gates S, Naidu BV., Wilson MJ, Gao Smith F. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2016;2016(2). doi: 10.1002/14651858.CD009121.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Oliveira GS, Castro-Alves LJ, Nader A, Kendall MC, McCarthy RJ. Transversus abdominis plane block to ameliorate postoperative pain outcomes after laparoscopic surgery: A meta-analysis of randomized controlled trials. Anesth Analg. 2014;118(2):454–463. doi: 10.1213/ANE.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 41.Barreveld A, Witte J, Chahal H, Durieux ME, Strichartz G. Preventive analgesia by local anesthetics: The reduction of postoperative pain by peripheral nerve blocks and intravenous drugs. Anesth Analg. 2013;116(5):1141–1161. doi: 10.1213/ANE.0b013e318277a270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKeown JL. Pain Management Issues for the Geriatric Surgical Patient. Anesthesiol Clin. 2015;33(3):563–576. doi: 10.1016/j.anclin.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 43.Joshi GP, Bonnet F, Shah R, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg. 2008;107(3):1026–1040. doi: 10.1213/01.ane.0000333274.63501.ff [DOI] [PubMed] [Google Scholar]
- 44.Nishimori M, Ballantyne JC, Low JH. Epidural pain relief versus systemic opioid-based pain relief for abdominal aortic surgery. In: Nishimori M, ed. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2006. doi: 10.1002/14651858.CD005059.pub2 [DOI] [PubMed] [Google Scholar]
- 45.Ferrada P, Callcut R, Zielinski MD, et al. Loop ileostomy versus total colectomy as surgical treatment for Clostridium difficile-associated disease: An Eastern Association for the Surgery of Trauma multicenter trial. J Trauma Acute Care Surg. 2017;83(1):36–40. doi: 10.1097/TA.0000000000001498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raines S, Hedlund C, Franzon M, Lillieborg S, Kelleher G, Ahlén K. Ropivacaine for Continuous Wound Infusion for Postoperative Pain Management: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur Surg Res. 2014;53(1–4):43–60. doi: 10.1159/000363233 [DOI] [PubMed] [Google Scholar]
- 47.Liang SS, Ying AJ, Affan ET, et al. Continuous local anaesthetic wound infusion for postoperative pain after midline laparotomy for colorectal resection in adults. Cochrane Database Syst Rev. 2019;2019(10). doi: 10.1002/14651858.CD012310.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Oliveira GS, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: A meta-analysis of randomized controlled trials. Anesthesiology. 2011;115(3):575–588. doi: 10.1097/ALN.0b013e31822a24c2 [DOI] [PubMed] [Google Scholar]
- 49.McCarthy RJ, Kroin JS, Tuman KJ, Penn RD, Ivankovich AD. Antinociceptive potentiation and attenuation of tolerance by intrathecal co-infusion of magnesium sulfate and morphine in rats. Anesth Analg. 1998;86(4):830–836. doi: 10.1097/00000539-199804000-00028 [DOI] [PubMed] [Google Scholar]
- 50.ANSTETT M. [ACTION OF MAGNESIUM FLAVONE CHELATES ON PAINFUL POSTOPERATIVE PULMONARY CICATRICES]. Lyon Med. 1963;210:589–592. http://www.ncbi.nlm.nih.gov/pubmed/14096802. Accessed February 16, 2020. [PubMed] [Google Scholar]
- 51.Rosero EB, Joshi GP. Preemptive, preventive, multimodal analgesia: What do they really mean? Plast Reconstr Surg. 2014;134(4):85S–93S. doi: 10.1097/PRS.0000000000000671 [DOI] [PubMed] [Google Scholar]
- 52.De Oliveira GS, Castro-Alves LJ, Khan JH, McCarthy RJ. Perioperative systemic magnesium to minimize postoperative pain meta-analysis of randomized controlled trials. Anesthesiology. 2013;119(1):178–190. doi: 10.1097/ALN.0b013e318297630d [DOI] [PubMed] [Google Scholar]
- 53.Hovaguimian F, Tschopp C, Beck-Schimmer B, Puhan M. Intraoperative ketamine administration to prevent delirium or postoperative cognitive dysfunction: A systematic review and meta-analysis. Acta Anaesthesiol Scand. 2018;62(9):1182–1193. doi: 10.1111/aas.13168 [DOI] [PubMed] [Google Scholar]
- 54.Avidan MS, Maybrier HR, Ben Abdallah A, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet. 2017;390(10091):267–275. doi: 10.1016/S0140-6736(17)31467-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.The American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society Abstracted Clinical Practice Guideline for Postoperative Delirium in Older Adults. J Am Geriatr Soc. 2015;63(1):142–150. doi: 10.1111/jgs.13281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elia N, Lysakowski C, Tramèr MR. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005;103(6):1296–1304. doi: 10.1097/00000542-200512000-00025 [DOI] [PubMed] [Google Scholar]
- 57.Maund E, McDaid C, Rice S, Wright K, Jenkins B, Woolacott N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: A systematic review. Br J Anaesth. 2011;106(3):292–297. doi: 10.1093/bja/aeq406 [DOI] [PubMed] [Google Scholar]
- 58.Henry D, Lim LLY, Garcia Rodriguez LA, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: Results of a collaborative meta-analysis. Br Med J. 1996;312(7046):1563–1566. doi: 10.1136/bmj.312.7046.1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan EY, Fransen M, Parker DA, Assam PN, Chua N. Femoral nerve blocks for acute postoperative pain after knee replacement surgery. Cochrane Database Syst Rev. 2014;2014(5). doi: 10.1002/14651858.CD009941.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amin NH, West JA, Farmer T, Basmajian HG. Nerve Blocks in the Geriatric Patient With Hip Fracture: A Review of the Current Literature and Relevant Neuroanatomy. Geriatr Orthop Surg Rehabil. 2017;8(4):268–275. doi: 10.1177/2151458517734046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koh JC, Song Y, Kim SY, Park S, Ko SH, Han DW. Postoperative pain and patient-controlled epidural analgesia-related adverse effects in young and elderly patients: A retrospective analysis of 2,435 patients. J Pain Res. 2017;10:897–904. doi: 10.2147/JPR.S133235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai Y, Miller T, Tan M, Law LSC, Gan TJ. Lidocaine patch for acute pain management: A meta-analysis of prospective controlled trials. Curr Med Res Opin. 2015;31(3):575–581. doi: 10.1185/03007995.2014.973484 [DOI] [PubMed] [Google Scholar]
- 63.Cornelius R, Herr KA, Gordon DB, Kretzer K, Butcher HK. Evidence-Based Practice Guideline : Acute Pain Management in Older Adults. J Gerontol Nurs. 2017;43(2):18–27. doi: 10.3928/00989134-20170111-08 [DOI] [PubMed] [Google Scholar]
- 64.Naples JG, Gellad WF, Hanlon JT. The Role of Opioid Analgesics in Geriatric Pain Management. Clin Geriatr Med. 2016;32(4):725–735. doi: 10.1016/j.cger.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruetzler K, Blome CJ, Nabecker S, et al. A randomised trial of oral versus intravenous opioids for treatment of pain after cardiac surgery. J Anesth. 2014;28(4):580–586. doi: 10.1007/s00540-013-1770-x [DOI] [PubMed] [Google Scholar]
- 66.Tramŕr DMR, Williams JE, Carroll D, Wiffen PJ, Moore RA, McQuay HJ. Comparing analgesic efficacy of non-steroidal anti-inflammatory drugs given by different routes in acute and chronic pain: A qualitative systematic review. Acta Anaesthesiol Scand. 1998;42(1):71–79. doi: 10.1111/j.1399-6576.1998.tb05083.x [DOI] [PubMed] [Google Scholar]
- 67.Equianalgesic Dosing of Opioids for Pain Management Approximate Equianalgesic 24 Hr Dose (Assumes Around-the-Clock Dosing) g Usual Starting Dose (Opioid-Naïve Adults) (Doses NOT Equianalgesic).; 2012. www.pharmacistsletter.com~www.prescribersletter.com~www.pharmacytechniciansletter.com. Accessed November 16, 2019.
- 68.New Safety Measures Announced for Extended-release and Long-acting Opioids. https://www.fda.gov/drugs/information-drug-class/new-safety-measures-announced-extended-release-and-long-acting-opioids. Published 2014. Accessed November 16, 2019.
- 69.Nelson LS, Juurlink DN. Tramadol and hypoglycemia one more thing toworry about. JAMA Intern Med. 2015;175(2):194–195. doi: 10.1001/jamainternmed.2014.5260 [DOI] [PubMed] [Google Scholar]
- 70.Swart LM, van der Zanden V, Spies PE, de Rooij SE, van Munster BC. The Comparative Risk of Delirium with Different Opioids: A Systematic Review. Drugs and Aging. 2017;34(6):437–443. doi: 10.1007/s40266-017-0455-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tramadon’t: a podcast with David Juurlink about the dangers of Tramadol. https://www.geripal.org/2018/06/Tramadont-dangers-of-tramadol.html. Accessed November 16, 2019.
- 72.Herr K. Pain Control: Pain Assessment in Cognitively Impaired Older Adults. Am J Nurs. 102:65–66+68. doi: 10.2307/3523062 [DOI] [PubMed] [Google Scholar]
- 73.Rakel B, Herr K. Assessment and treatment of postoperative pain in older adults. J Perianesthesia Nurs. 2004;19(3):194–208. doi: 10.1016/j.jopan.2004.03.005 [DOI] [PubMed] [Google Scholar]
- 74.Feldt KS. The Checklist of Nonverbal Pain Indicators (CNPI). Pain Manag Nurs. 2000;1(1):13–21. doi: 10.1053/jpmn.2000.5831 [DOI] [PubMed] [Google Scholar]
- 75.Warden V, Hurley AC, Volicer L. Development and Psychometric Evaluation of the Pain Assessment in Advanced Dementia (PAINAD) Scale. doi: 10.1097/01.JAM.0000043422.31640.F7 [DOI] [PubMed] [Google Scholar]
- 76.Paulson CM, Monroe T, Mion LC. Pain assessment in hospitalized older adults with dementia and delirium. J Gerontol Nurs. 2014;40(6):10–15. doi: 10.3928/00989134-20140428-02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gou RY, Hshieh TT, Marcantonio ER, et al. One-Year Medicare Costs Associated With Delirium in Older Patients Undergoing Major Elective Surgery. JAMA Surg. February 2021. doi: 10.1001/jamasurg.2020.7260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen CCH, Li HC, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: A cluster randomized clinical trial. JAMA Surg. 2017;152(9):827–834. doi: 10.1001/jamasurg.2017.1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clinical Practice Guideline for Postoperative Delirium in Older Adults American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults Clinical Practice Guideline for Postoperative Delirium in Older Adults; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: A systematic literature review. Age Ageing. 2006;35(4):350–364. doi: 10.1093/ageing/afl005 [DOI] [PubMed] [Google Scholar]
- 81.Postoperative delirium in older adults: Best practice statement from the American geriatrics society. J Am Coll Surg. 2015;220(2):136–148.e1. doi: 10.1016/j.jamcollsurg.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 82.Gilchrist NA, Asoh I, Greenberg B. Atypical antipsychotics for the treatment of ICU delirium. J Intensive Care Med. 2012;27(6):354–361. doi: 10.1177/0885066611403110 [DOI] [PubMed] [Google Scholar]
- 83.Campbell N, Boustani MA, Ayub A, et al. Pharmacological management of delirium in hospitalized adults - A systematic evidence review. J Gen Intern Med. 2009;24(7):848–853. doi: 10.1007/s11606-009-0996-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Falvey JR, Mangione KK, Stevens-Lapsley JE. Rethinking Hospital-Associated Deconditioning: Proposed Paradigm Shift. Phys Ther. 2015;95(9):1307–1315. doi: 10.2522/ptj.20140511 [DOI] [PMC free article] [PubMed] [Google Scholar]