Abstract
Background:
Solid organ transplant recipients (SOTRs) are at increased risk for severe COVID-19 and exhibit lower antibody responses to SARS-CoV-2 vaccines. This study aimed to determine if prevaccination cytokine levels are associated with antibody response to SARS-CoV-2 vaccination.
Methods:
A cross-sectional study was performed among 58 SOTRs before and after two-dose mRNA vaccine series, 35 additional SOTRs before and after a third vaccine dose, and comparison to 16 healthy controls (HCs). Anti-spike antibody was assessed using the IgG Euroimmun ELISA. Electrochemiluminescence detection-based multiplexed sandwich immunoassays (Meso Scale Diagnostics) were used to quantify plasma cytokine and chemokine concentrations (n=20 analytes) and compare concentrations between SOTRs and HCs, stratified by ultimate antibody response to the vaccine using Wilcoxon-rank-sum test with false discovery rates (FDR) computed to correct for multiple comparisons.
Results:
In the study population, 100% of HCs, 59% of SOTRs after 2 doses and 63% of SOTRs after 3 doses had a detectable antibody response. Multiple baseline cytokines were elevated in SOTRs versus HCs. There was no significant difference in cytokine levels between SOTRs with high vs low-titer antibodies after 2 doses of vaccine. However, as compared to poor antibody responders, SOTRs who went on to develop high-titer antibody response to a third dose of vaccine had significantly higher pre-third dose levels of several innate immune cytokines including IL-17, IL-2Ra, IL-6, IP-10, MIP-1α, and TNF-α (FDR <0.05).
Conclusions:
A specific inflammatory profile may be associated with developing higher antibodies in response to a third dose of SARS-CoV-2 vaccine in SOTRs.
1. INTRODUCTION
Solid organ transplant recipients (SOTRs) are less likely than immunocompetent populations to develop a positive antibody response to the mRNA based SARS-CoV-2 vaccines.1-4 Additionally, SOTRs are more likely to die from COVID-19,5,6 and are at increased risk of breakthrough infections after vaccination.7,8 Due to these factors, a third dose is now recommended for immunocompromised individuals.9 These recommendations were based on several studies demonstrating that at least a portion of SOTRs who do not mount a positive immune response to 2 doses do respond to a third.10-12 A large percentage (40-50%) of SOTRs who do not respond to 2 doses of vaccine do not mount a humoral response even to a third dose of vaccine, and those who do have antibody titers markedly lower than healthy controls (HCs).12,13 Therefore, understanding which SOTRs may respond to a third dose and which are unlikely to do so is critical to developing strategies to protect this vulnerable group.
The mechanisms underlying blunted immune response to mRNA vaccination in SOTRs is unknown. Some clinical factors associated with an increased likelihood of a positive vaccine response in SOTRs include younger age, receipt of the mRNA-1273 (Moderna) vaccine, and absence of anti-metabolite maintenance immunosuppression.14 Given the association between maintenance immunosuppression regimen and antibody response, we hypothesized that a certain inflammatory profile may represent resting immunoreactive potential and be associated with improved vaccine response, as was seen with influenza vaccination.15
2. MATERIALS AND METHODS
2.1. Study Participants
The SOTR participants were enrolled in a national prospective, observational cohort: COVID-19 Antibody Testing of Recipients of Solid Organ Transplants and Patients with Chronic Diseases, which was approved by the Johns Hopkins IRB (00248540), as previously described.1,11 All SOTR participants were recruited virtually and provided detailed transplant history as well as oral informed consent (waiver of written consent granted). All vaccines were also administered independently in the community. For participants in the two-dose cohort, blood samples were obtained 0-4 weeks before and 2 weeks after vaccine doses. The three-dose cohort consisted of participants who did not have a substantial antibody response after the two-dose series. For this cohort, blood samples were obtained (0-22 days or median 1 (IQR 0-5) days) before dose 3 (median of 83 days after dose 2) and 2 weeks after dose 3.
HC participants were enrolled under Johns Hopkins IRB00027183 and all received 2 doses of BNT162b2 (Pfizer). All HCs provided written informed consent, and contributed limited demographic data (e.g., sex, race, decade of age).
Blood was collected in acid citrate dextrose (ACD) or heparin tubes and plasma was isolated by centrifugation and stored at −80°C until cytokines were measured.
2.2. SARS-CoV-2 Antibody Detection
Plasma specimens were analyzed using the Euroimmun Anti-SARS-CoV-2 ELISA (Mountain Lakes, NJ), which measures anti-SARS-CoV-2 IgG specific to spike (S1 subunit). All ELISA kits were purchased from the manufacturer and assays were performed according to the manufacturers’ instructions. The Euroimmun results are reported as an arbitrary unit ratio (AU), which is the optical density [OD] of the sample divided by calibrator provided.16,17 Plasma IgG in World Health Organization binding antibody units (BAU) was measured using the chemiluminescent Meso Scale Diagnostics (MSD, Rockville, MD) V-PLEX COVID-19 Respiratory Panel 3 Kit according to the manufacture’s protocol at a dilution of 1:5000. BAU were calculated by multiplying AU by the manufacturer’s verified conversion factor.
2.3. Pseudoneutralization/ACE2 Inhibition Measurement:
Plasma from study participants was thawed and ACE2 blocking/pseudoneutralization was measured using the MSD ACE2 V-PLEX SARS-CoV-2 kit according to the manufacturer’s instructions at a dilution of 1:100.
2.4. Cytokine Measurement
Twenty cytokines and chemokines were measured in plasma from HCs and SOTRs before receiving the first dose of a two-dose SARS-CoV-2 vaccine series, and immediately before a third dose of vaccine among SOTRs. The choice of cytokines and chemokines was based on previous studies on SARS-CoV-2 infection and studies of cytokines in SOTRs before receiving influenza vaccines.15,18,19
Plasma was thawed and cytokines (interferon (IFN)-α2a, IFN-γ, IFN-λ, interleukin(IL)-10, IL-15, IL-16, IL-17A, IL-18, IL-1RA, IL-21, IL-22, IL-2Ra, IL-6, IL-7, IL-8, interferon-γ-inducible protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), tumor necrosis factor (TNF)-α, vascular endothelial growth factor (VEGF)) were measured using a custom multiplex kit from MSD according to the manufacture’s protocol and data were acquired on a MESO QuickPlex SQ 120.
2.5. Statistical Analysis
Each sample was tested twice, and the mean value of 2 runs was used in analysis. The actual read of the values were kept for samples below the lower limit of detection. Below fitted curve values were set to 0. The SOTR dose 2 and dose 3 cohorts were subdivided based on antibody response after the second dose (for the dose 2 cohort) or after the third dose (for the dose 3 cohort) into low-titer (< 3.5 AU) and high-titer (≥ 3.5 AU) based on the classification of low-titer versus high-titer convalescent plasma set by the FDA.20 Cytokines and chemokines prevaccine were then compared among HCs, low-titer SOTRs, and high-titer SOTRs to determine if 1 or more cytokines or chemokines were associated with development of a high-titer response to the two-dose or three-dose series. Wilcoxon-Rank-Sum test was used to compare median cytokine values between groups. Multiple comparisons were adjusted using Benjamini-Hochberg procedure with a false discovery rate (FDR) cutoff of 0.05. Sensitivity analyses were also performed to assess differences between type of vaccine received (mRNA-1273 vs. BNT162b), use of antimetabolite immunosuppression, and seroconversion prior to third dose as described below.
3. RESULTS
3.1. Cohort Characteristics
A total of 93 SOTRs participants were included in the study along with 16 HCs. The SOTR cohort (50% <60 years of age) was older than the HCs (100% <60 years of age) with more females (54% vs 31%) (Table 1). The SOTR cohort was subdivided into those who received a two-dose vaccine regimen (SOTR dose 2) and those who received a third dose (SOTR dose 3). Both SOTR groups were similar with regard to age, sex, race, type of transplanted organ, baseline use of immunosuppressants, and type of vaccine received. 59% of the SOTR dose 2 cohort seroconverted after the two-dose vaccine series. Among the SOTR dose 3 cohort, 6 (17%) had already seroconverted after 2 doses and after 3 doses a total of 22 (63%) were then seropositive (Table 1).
Table 1.
Demographic and Clinical Characteristics of the Cohort
| SOTR dose 2, n = 58 |
SOTR dose 3, n =35 |
P values‡ | HC, n = 16 | |
|---|---|---|---|---|
| Age | ||||
| 20-39 | 15 (26) | 3 (9) | 0.108 | 7 (44) |
| 40-59 | 15 (26) | 13 (37) | 9 (56) | |
| 60-79 | 28 (48) | 19 (54) | 0 (0) | |
| Sex | ||||
| Female | 31 (53) | 19 (54) | 1.000 | 5 (31) |
| Male | 27 (47) | 16 (46) | 11 (69) | |
| Race | ||||
| White | 53 (91) | 33 (94) | 0.417 | 11 (69) |
| Asian | 3 (5) | 0 (0) | 3 (19) | |
| African American | 1 (2) | 0 (0) | 2 (13) | |
| Multiple race | 1 (2) | 1 (3) | 0 (0) | |
| Other | 0 (0) | 1 (3) | 0 (0) | |
| Type of vaccine received | ||||
| First two doses | ||||
| BNT162b2 | 33 (57) | 17 (49) | 0.665 | - |
| mRNA-1273 | 25 (43) | 17 (49) | - | |
| Missing | 0 (0) | 1 (3) | - | |
| Third dose | ||||
| BNT162b2 | - | 8 (23) | - | - |
| mRNA-1273 | - | 14 (40) | - | |
| Ad26.COV2.S | - | 13 (37) | - | |
| Graft transplanted | ||||
| Kidney | 37 (67) | 19 (54) | 0.815 | - |
| Heart | 5 (9) | 3 (9) | - | |
| Liver | 12 (21) | 9 (26) | - | |
| Lung | 2 (3) | 1 (3) | - | |
| Pancreas | 0 (0) | 1 (3) | - | |
| Multi* | 2 (0) | 1 (3) | - | |
| Anti-rejection medication† | ||||
| Prednisone | 38 (66) | 17 (49) | 0.130 | - |
| Calcineurin Inhibitors | 52 (90) | 28 (80) | 0.226 | - |
| mTOR inhibitors | 8 (14) | 3 (9) | 0.526 | - |
| anti-metabolites | 40 (69) | 23 (66) | 0.820 | - |
| Treated for rejection in the past 6 months | ||||
| Not treated | 52 (90) | 32 (91) | 1.000 | |
| Was treated | 3 (5) | 1 (3) | ||
| Missing | 3 (5) | 2 (6) | ||
| post vaccine SARS-Cov-2 IgG | ||||
| Negative | 24 (41) | 13 (37) | 0.827 | 0 (0) |
| Positive | 34 (59) | 22 (63) †† | 16 (100) | |
| Low titer | 32 (55) | 19 (54) | 1.000 | 0 (0) |
| High titer | 26 (45) | 16 (46) | 16 (100) |
Note: One participant was included in both SOTR dose 2 cohort and SOTR dose 3 cohort.
In the SOTR dose 2 cohort one participant received both kidney and heart transplants, and one participant received both kidney and liver transplants; In the SOTR dose 3 cohort, one participant received both kidney and pancreas transplants, and one participant received both lung and other specified transplant.
Anti-rejection medication use was not mutually exclusive.
Six participants in the dose 3 cohort were seropositive prior to receiving a third dose.
P value comparing SOTR dose 2 and SOTR dose 3 cohort were computed using two-tailed Fisher’s exact test.
3.2. Prevaccine Cytokines in the Two-Dose Cohort (HC versus SOTR dose 2)
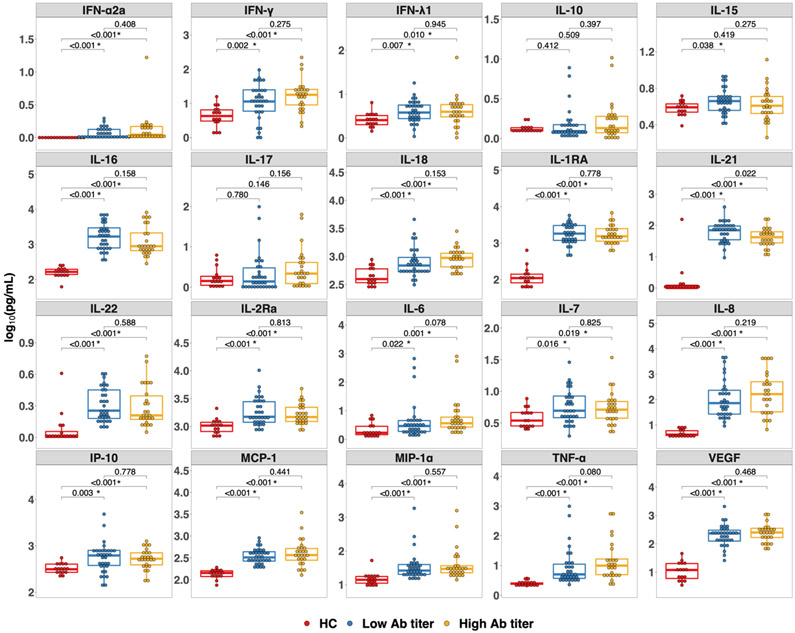
Overall, the median level of baseline cytokines and chemokines in the HCs were lower than both SOTR subgroups. After correcting for multiple comparisons IFN-α2a, IFN-γ, IFN-λ1, IL-16, IL-18, IL-1RA, IL-21, IL-22, IL-2Ra, IL-6, IL-7, IL-8, IP-10, MCP-1, MIP-1α, TNF-α, and VEGF were all significantly lower in the HCs compared to both the low-titer SOTR group and the high-titer SOTR group (FDR <0.05) (Figure 1). IL-15 was also significantly lower in HCs compared to the low-titer group, but not the high-titer group. When comparing the low-titer SOTR group to the high-titer SOTR group several cytokines and chemokines were higher in the high-titer group including IFN-γ, IL-17, IL-18, IL-6, IL-8, and TNF-α, but none of these differences reached statistical significance after correcting for multiple comparisons (Figure 1). When sub-dividing the SOTR dose 2 cohort into those who had any positive antibody response after receiving 2 doses of an mRNA vaccine (positive) and those with an undetectable response (negative), the same trends were observed; however, no statistically significant differences were detected after correcting for multiple comparisons (Figure S1).
Figure 1.
Cytokines in solid organ transplant recipients (SOTRs) and healthy controls prior to first dose of a two-dose messenger RNA-based vaccine series. Each cytokine or chemokine measured is indicated in the grey header above each panel. Differences among the healthy controls (red, n = 16), SOTRs who developed low-titer responses (blue, n = 32), and SOTRs who developed high-titer responses (yellow, n = 26) after vaccination were determined by a two-tailed Wilcoxon-Rank-Sum test. Multiple comparison was controlled using the Benjamini-Hochberg procedure with false discovery rate of 0.05. Significant P-values after adjusting for multiple comparison are marked with *. The boxplots represent the interquartile range. The median is represented by a horizontal line in the box. The lower and upper whiskers represent 1.5x the interquartile range beyond the quartiles. Each dot represents an individual sample. Low-titer (ratio < 3.5) and High-titer (ratio ≥ 3.5) were based on the classification of low-titer versus high-titer convalescent plasma set by the FDA.20 Ab, antibody; HC, healthy control; IFN, interferon; IL, interleukin; IP-10, Interferon-γ-inducible protein 10; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
3.3. Pre-Third Dose Cytokines in the SOTR Dose 3 Cohort
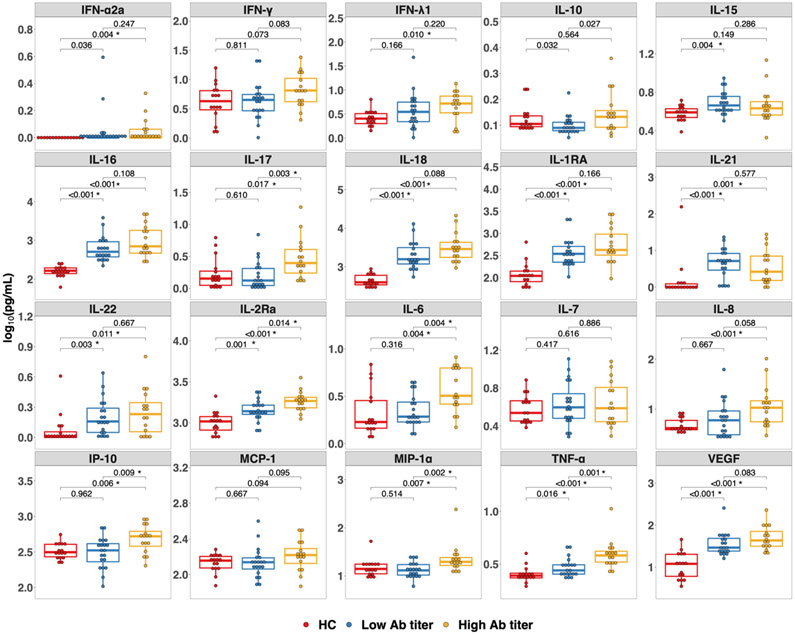
Cytokines and chemokines were also measured in the SOTR dose 3 cohort prior to receiving a third dose of SARS-CoV-2 vaccine. This cohort was also subdivided based on low-titer or high-titer antibody response after the third dose. When comparing these groups with the HCs there were again differences between the HCs and the SOTR cohorts. Specifically, IL-15, IL-16, IL-18, IL-1RA, IL-21, IL-22, IL-2Ra, TNF-α, VEGF were significantly lower in HCs compared to the low-titer group and IFN-α2a, IFN-λ1, IL-16, IL-17, IL-18, IL-1RA, IL-21, IL-22, IL-2Ra, IL-6, IL-8, IP-10, MIP-1α, TNF-α, VEGF were significantly lower in HCs compared to the high-titer group (FDR < 0.05) (Figure 2). When comparing the low-titer to high-titer SOTR groups, IL-17, IL-2Ra, IL-6, IP-10, MIP-1α, and TNF-α were all significantly higher in the high-titer group even after adjusting for multiple comparisons (FDR <0.05) (Figure 2). When comparing cytokines and chemokines in SOTRs with no detectable antibody response after a third dose to those with any positive response, these same cytokines and chemokines levels were higher in the positive subgroup, but the differences did not reach statistical significance after adjustment for multiple comparisons (Figure S2).
Figure 2.
Cytokines in solid organ transplant recipients (SOTRs) prior to a third dose of SARS-CoV-2 vaccine and healthy controls prior to first dose of a two-dose mRNA-based vaccine series. Each cytokine or chemokine measured is indicated in the grey header above each panel. Differences among the healthy controls (red, n = 16), SOTRs who developed low-titer responses (blue, n = 19), and SOTRs who developed high-titer responses (yellow, n = 16) after a third dose of SARS-CoV-2 vaccine were determined by a two-tailed Wilcoxon-Rank-Sum test. Multiple comparison was controlled using the Benjamini-Hochberg procedure with false discovery rate of 0.05. Significant P-values after adjusting for multiple comparison are marked with *. Low-titer (ratio < 3.5) and High-titer (ratio ≥ 3.5) were based on the classification of low-titer versus high-titer convalescent plasma set by the FDA.20 Ab, antibody; HC, healthy control; IFN, interferon; IL, interleukin; IP-10, Interferon-γ-inducible protein 10; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
In order to provide another measure of the immune response between the low-titer and high-titer group, BAU and pseudoneutralization were measured in the dose 3 cohort using a quantitative ELISA and ACE2-spike inhibition assay, respectively. Those who developed high-titer responses as measured by the Euroimmun assay had significantly higher BAU and pseudoneutralization compared to the low-titer group (Figure S3). The median BAU values (IQR) were 15 (1-49) for the low-titer group and 644 (250-1648) for the high titer group.
3.4. Subgroup Analysis in the SOTR Dose 3 Cohort
In an effort to understand whether other factors influenced these findings, subgroups were explored. To determine if type of vaccine received impacted cytokine levels prior to a third dose, cytokines from those SOTRs who received BNT162b were compared to those who received mRNA-1273. No cytokines or chemokines were significantly different between these 2 groups (Figure S4). The use of antimetabolite immunosuppression has been associated with decreased response to the SARS-CoV-2 vaccines,14 and could influence cytokine levels. Therefore, cytokines were compared between the subgroup that was on antimetabolite immunosuppression and the subgroup that was not; there were no significant differences (Figure S5). As described, 6 patients from the dose 3 cohort already had seroconverted prior to a third dose. Cytokines from these patients were compared to those who had not seroconverted prior to a third dose, but no significant differences were observed (Figure S6). Finally, given that the third dose cohort was composed of SOTRs who had minimal response to 2 doses, we compared the cytokines in this group prior to a third dose to the cytokines in the two-dose cohort prior to any vaccination. IFN-γ, IL-16, IL-1RA, IL-21, IL-8, IP-10, MCP-1, MIP-1α, TNF-α, and VEGF were all significantly lower in the dose 3 cohort while IL-18 was higher in the dose 3 cohort (Figure S7).
4. DISCUSSION
Despite widespread recognition of poor humoral immune responses to SARS-CoV-2 vaccination in SOTRs, mechanisms underlying these suboptimal responses and prediction of response to additional vaccine doses remain obscure. Data in this series suggest that a specific pattern of immune activation, as measured by plasma cytokines and chemokines, may distinguish those SOTRs who are likely to develop higher antibody titers to a third dose of SARS-CoV-2 vaccine. The majority of cytokines identified as significantly different are innate immune cytokines, consistent with the role of the innate immune system in initiating and modulating the adaptive, including the antibody, response to pathogens. IL-6, TNF-α, MIP-1α, and IP-10 are all produced by innate immune cells such as macrophages and natural killer cells in response to pathogen recognition.21-23 IL-17 and IL-2Ra can be produced by innate immune cells, but are also associated with ongoing T cell activation.24,25 Several of these cytokines, particularly TNF-α and IL-6, have previously been shown to be important in developing immune response against viral vaccines.15,26,27 Thus, these data possibly reflect a role for the innate immune system in the response to SARS-CoV-2 vaccines in SOTRs. Given that these cytokines were elevated in the high-titer group, this may signify a state of less immunosuppression and greater ability to respond to additional vaccine antigen exposure. This is further supported by the fact that the dose 2 cohort had mostly higher cytokine and chemokine levels than the dose 3 cohort which consisted of participants with low or no response to the initial two-dose series. However, despite this trend in SOTRs, HCs had overall very distinct cytokine and chemokine profiles. This likely reflects major alterations in baseline immune state following organ transplantation and subsequent use of immunosuppressive medications. Furthermore, this suggests that strategies to enhance the response of SOTRs to SARS-CoV-2 vaccines should be evaluated independently of strategies employed for other populations.
While this study demonstrated similar trends in innate cytokines in the high-titer group within the two-dose cohort, these differences did not reach statistical significance. This could be explained by the relatively small sample size but could also be due to greater heterogeneity of immune state within the two-dose cohort. An important consideration in interpreting these data is that the three-dose cohort comprised SOTRs who had a poor response to 2 doses, and therefore, were uniformly composed of those with weak or no response. As a result, it may be easier to distinguish differences in response among this more immunological homogenous group with more uniformly low baseline SARS-CoV-2 response.
Though these results support that cytokine profiles may identify the SOTRs who are likely to benefit from a third dose of SARS-CoV-2 vaccine, this study does have several limitations. This was a single cohort, single-center, observational study. The samples were not collected at precisely the same time relative to vaccination, different transplanted organ recipients were allowed to participate, and sample size was relatively small. This heterogeneity limits the general applicability of the results. Although a broad cytokine and chemokine panel was employed, this analysis was limited to a subset of these immune signaling molecules and there may be other unmeasured cytokines and chemokines that contribute to vaccine response. While some significant differences were detected, the sample sizes were relatively small, and a larger study might allow identification of additional inflammatory markers associated with robust vaccine responses. Furthermore, comprehensive medical histories or clinical laboratory data were not available for these participants. Therefore, it was not possible to determine whether prior episodes of rejection, infections, immunosuppressant drug levels, or absolute lymphocyte count (ALC) may influence the findings. Moreover, the majority of the SOTRs in this observational study were kidney and/or liver transplant recipients reflecting the demographics of organ transplantation in the United States. Therefore, it is unclear whether these findings would hold for lung or heart transplant recipients who were underrepresented in this study. The HCs were younger than the SOTR group, and therefore some of the observed differences in resting cytokine levels may be related to age rather than SOTR status. Additionally, cellular responses to the vaccine were not measured in this study, though they are thought to be reduced compared to healthy controls3. We also are unable to comment on whether these antibody responses are truly reflective of protection from COVID-19. While the ACE2 inhibition assay reported here has excellent correlation with live-virus neutralization, it is not a perfect correlation, therefore it is not clear if these cytokine signatures are associated with true neutralization and/or protection from infection.28-30
While it is impractical to measure twenty cytokines on an individual prior to vaccination, several of the cytokines associated with a more robust third dose response (such as IL-6 and TNF-α) are available for clinical use. Furthermore, in a more controlled study these cytokines could be correlated with laboratory tests that are routinely available (such as ALC and C-reactive protein) which could be used to guide recommendations about additional vaccine doses or other strategies to increase protection from COVID-19.
Despite these limitations, these novel findings highlight that specific cytokine profiles may be associated with greater antibody responses in some SOTRs. Should this prove to be true in a larger study, this could be used to target strategies for enhancing vaccine responses in specific SOTRs. These results indicate that there might be identifiable and measurable characteristics in SOTRs that could be used to stratify this population into those who would most benefit from additional doses of currently available SARS-CoV-2 vaccines and those who may require alternative protection strategies (i.e. novel vaccine platforms, immunosuppression reduction prior to vaccination, or passive immunoprophylaxis with antibody infusions). Additional investigations with a larger cohort will be required to determine if the data presented here can be extrapolated to the greater transplant population.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to all participants who enrolled in this study and donated plasma.
FUNDING:
This work was supported by the Ben-Dov family, the Johns Hopkins COVID-19 Vaccine-related Research Fund, the National Cancer Institute (U54CA260491), and K23DK115908 (J.M.G.-W.) from the National Institute of Diabetes and Digestive and Kidney Diseases, and grants K24AI144954 (D.L.S.), K08AI156021 (A.H.K.), U01AI138897 (C.M.D./D.L.S.), K23AI157893 (W.A.W.), and R01AI120938S1 (A.A.R.T.) from the National Institute of Allergy and Infectious Disease (NIAID). Additional funding was provided by the Division of Intramural Research, NIAID (S.S.).
ABBREVIATIONS:
- ALC
Absolute Lymphocyte Count
- AU
Arbitrary Unit
- BAU
Binding Antibody Units
- CDC
Centers for Disease Control and Prevention
- EUA
Emergency Use Authorization
- FDR
False discovery rate
- FDA
Food and Drug Administration
- HC
Healthy control
- IFN
Interferon
- IL
Interleukin
- IP-10
Interferon-γ-inducible protein 10, also known as CXCL10
- IQR
Interquartile range
- MCP-1
Monocyte Chemoattractant Protein-1
- MIP-1α
Macrophage Inflammatory Protein 1α
- MSD
Meso Scale Diagnostics
- SOTR
Solid Organ Transplant Recipient
- TNF-α
Tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
Footnotes
DISCLOSURE:
D.L.S. has the following financial disclosures: consulting and speaking honoraria from Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, Thermo Fisher Scientific. C.M.D. receives grants from AbbVie and GlaxoSmithKline and receives an honorarium for a grant review committee for Gilead Sciences. None of the other authors have any relevant competing interests.
REFERENCES
- 1.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall VG, Ferreira VH, Ierullo M, et al. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21:3980–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS-CoV2 BNT162b2 (Tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131:e150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rincon-Arevalo H, Choi M, Stefanski AL, et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol 2021;6(60):eabj1031. [DOI] [PubMed] [Google Scholar]
- 5.Kates OS, Haydel BM, Florman SS, et al. ; UW COVID-19 SOT Study Team. COVID-19 in solid organ transplant: a multicenter cohort study. Clin Infect Dis. 2020. IN PRESS. doi: 10.1093/cid/ciaa1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garibaldi BT, Fiksel J, Muschelli J, et al. Patient trajectories among persons hospitalized for COVID-19. Ann Intern Med. 2021;174(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aslam S, Adler E, Mekeel K, et al. Clinical effectiveness of COVID-19 vaccination in solid organ transplant recipients. Transpl Infect Dis. 2021;23:e13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin CX, Moore LW, Anjan S, et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation. 2021;105:e265–e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. COVID-19 vaccines for moderately to severely immunocompromised people. 2021. Available at https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html. Accessed August 18, 2021.
- 10.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174:1330–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karaba AH, Zhu X, Liang T, et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. medRxiv. 2021. PREPRINT. doi: 10.1101/2021.08.11.21261914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández-Ruiz M, Humar A, Baluch A, et al. Baseline serum interleukin-6 to interleukin-2 ratio is associated with the response to seasonal trivalent influenza vaccine in solid organ transplant recipients. Vaccine. 2015;33(51):7176–7182. [DOI] [PubMed] [Google Scholar]
- 16.Patel EU, Bloch EM, Clarke W, et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J Clin Microbiol. 2021;59(2):e02257–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein SL, Pekosz A, Park HS, et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest. 2020;130(11):6141–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonny TS, Patel EU, Zhu X, et al. Cytokine and chemokine levels in coronavirus disease 2019 convalescent plasma. Open Forum Infect Dis. 2020;8:ofaa574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karaba AH, Zhou W, Hsieh LL, et al. Differential cytokine signatures of SARS-CoV-2 and influenza infection highlight key differences in pathobiology. Clin Infect Dis. 2021. IN PRESS. doi: 10.1093/cid/ciab376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Food & Drug Administration. Emergency use authorization. 2021. Available at https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization. Accessed August 19, 2021.
- 21.Duque GA, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002;13(6):455–481. [DOI] [PubMed] [Google Scholar]
- 23.Lei J, Yin X, Shang H, et al. IP-10 is highly involved in HIV infection. Cytokine. 2019;115:97–103. [DOI] [PubMed] [Google Scholar]
- 24.McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity. 2019;50(4):892–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damoiseaux J. The IL-2 – IL-2 receptor pathway in health and disease: the role of the soluble IL-2 receptor. Clin Immunol. 2020;218:108515. [DOI] [PubMed] [Google Scholar]
- 26.Su B, Wang J, Wang X, et al. The effects of IL-6 and TNF-α as molecular adjuvants on immune responses to FMDV and maturation of dendritic cells by DNA vaccination. Vaccine. 2008;26(40):5111–5122. [DOI] [PubMed] [Google Scholar]
- 27.Ovsyannikova IG, Reid KC, Jacobson RM, et al. Cytokine production patterns and antibody response to measles vaccine. Vaccine. 2003;21(25-26):3946–3953. [DOI] [PubMed] [Google Scholar]
- 28.Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. New Engl J Med. 2020;383(16):1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corbett KS, Nason MC, Flach B, et al. Immune correlates of protection by mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. Science. 2021;373(6561):eabj0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Embregts CWE, Verstrepen B, Langermans JAM, et al. Evaluation of a multi-species SARS-CoV-2 surrogate virus neutralization test. One Health. 2021;13:100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.