Abstract
In mammalian male meiosis, the heterologous X and Y chromosomes remain unsynapsed and, as a result, are subject to meiotic sex chromosome inactivation (MSCI). MSCI is required for the successful completion of spermatogenesis. Following the initiation of MSCI, the X and Y chromosomes undergo various epigenetic modifications and are transformed into a nuclear body termed the XY body. Here, we review the mechanisms underlying the initiation of two essential, sequential processes in meiotic prophase I: MSCI and XY-body formation. The initiation of MSCI is directed by the action of DNA damage response (DDR) pathways; downstream of the DDR, unique epigenetic states are established, leading to the formation of the XY body. Accumulating evidence suggests that MSCI and subsequent XY-body formation may be driven by phase separation, a physical process that governs the formation of membraneless organelles and other biomolecular condensates. Thus, here we gather literature-based evidence to explore a phase separation hypothesis for the initiation of MSCI and the formation of the XY body.
Keywords: Sex chromosomes, Germ cells, Germline, Epigenetics, Liquid–liquid phase separation, Sex body
Introduction
Mounting evidence has revealed that phase separation is a driving mechanism for the formation of biomolecular condensates, including various membraneless organelles [1, 2]. Liquid–liquid phase separation is a physical process that sees the spontaneous separation of a supersaturated liquid mixture into stable, distinct, coexisting liquid phases. In the nucleus, phase separation drives the organization of fundamental structures such as nuclear pore complexes [3], nucleoli [4], heterochromatin [5, 6], and transcription hubs associated with super-enhancers [7]. Additionally, in the germline, phase separation underlies the formation of various germline-specific biomolecular condensates [8, 9]. For example, in a 2009 study of C. elegans, P granules, the sites of germline-specific RNA regulation, were the first membraneless organelles shown to form from phase separation [10]. In meiosis, phase separation also drives the formation of synaptonemal complexes, which are required for the association of homologous chromosomes [11] and, during meiotic recombination, the assembly of meiotic DNA break machinery at sites of DNA double-strand breaks (DSBs) [12]. Thus, various biomolecular condensates interact to control gene expression and other cellular functions in germ cells.
Here, we gather literature-based evidence to propose a phase separation hypothesis for meiotic sex chromosome inactivation (MSCI) and the subsequent formation of the “XY body” (also known as the “sex body”), a distinct membrane-free nuclear body (Fig. 1A). An essential process in mammalian male germ cells, MSCI is a male sex chromosome-specific manifestation of meiotic silencing of unsynapsed chromatin (MSUC), a general mechanism for transcriptional silencing in both male and female meiosis [13–17]. MSUC operates as a surveillance mechanism for chromosome asynapsis after homologous autosomes have completed synapsis in the pachytene stage of meiotic prophase I. In normal male meiosis, MSUC is confined to unsynapsed X and Y chromosomes, where there is pairing and synapsis at only a small region of homologous sequence termed the pseudoautosomal region. Following the initiation of MSCI, at the onset of the mid-pachytene stage, sex chromosomes are sequestered away from recombining and transcriptionally active autosomes, and are transformed into an ellipsoidal nuclear body known as the XY body (MSCI and XY bodies are extensively reviewed in [17–24]; Fig. 1B).
Fig. 1.
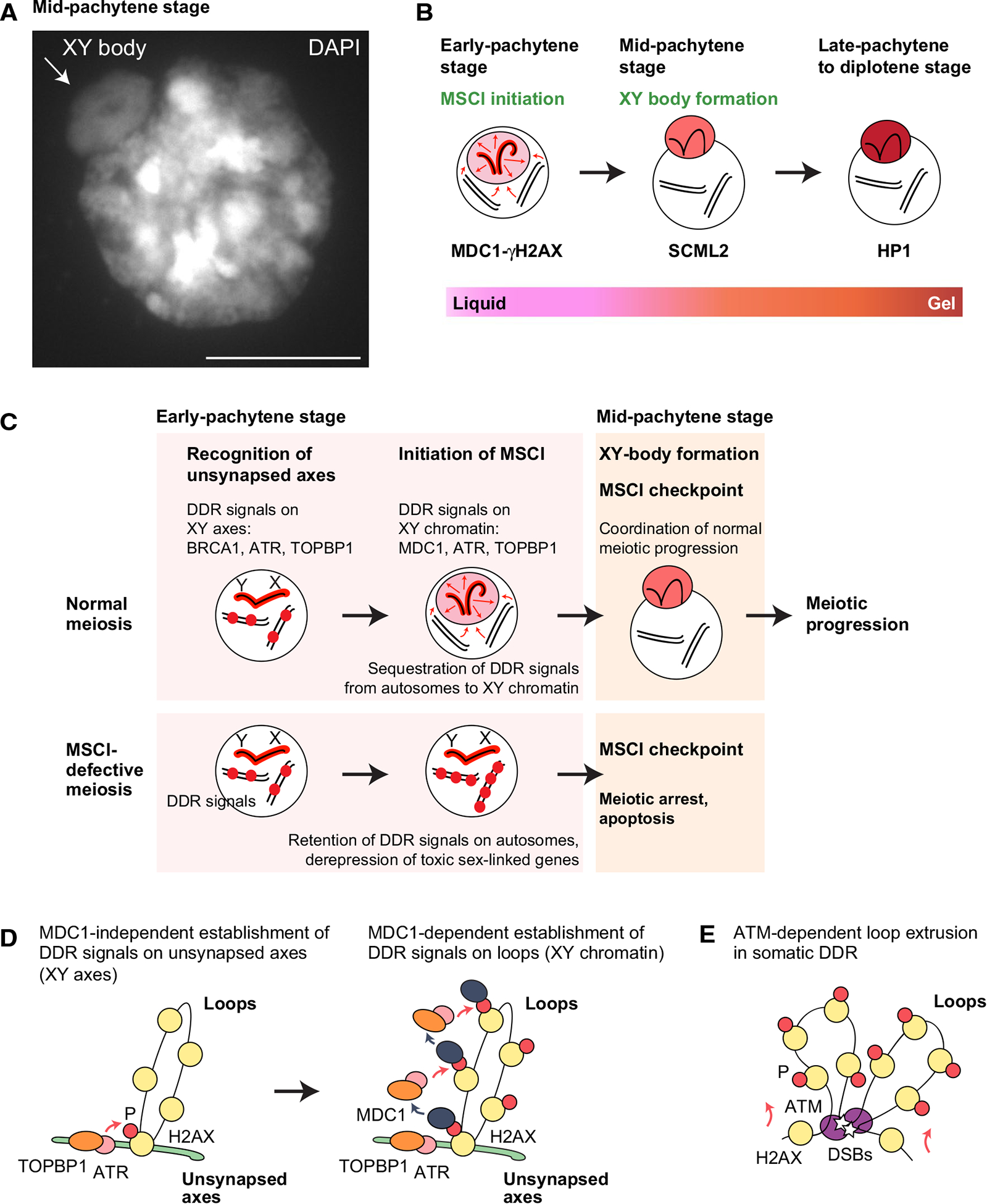
Mechanisms of MSCI initiation. A DAPI counterstaining of a “3D slide,” i.e., a slide prepared such that the gross three-dimensional organization of chromatin is preserved [94]. The dashed circle indicates the XY body. Scale bar: 10 μm. The image is originally from Abe et al. 2020 [30]. B Model of phase separation of the sex chromosomes. Key proteins involved at each step of the process are shown. C Model of the MSCI checkpoint: the physical seclusion of DDR factors from autosomes to the XY body is a critical checkpoint in the progression of meiosis and the development of gametes. At the onset of MSCI, DDR factors (shown as red) are excluded from autosomes and sequestered to the sex chromosomes. The physical seclusion of DDR factors on/at the XY body, which may involve phase separation, is a critical step in the MSCI checkpoint in the mid-pachytene stage of meiotic prophase I. While the MSCI checkpoint ensures meiotic stage progression in normal meiosis, the abolishment of MSCI enables the ectopic retention of DDR signals on/at autosomes; in turn, this triggers complete meiotic arrest and cell death in response to the checkpoint. D Model for the initiation of MSCI. ATR and its activator, TOPBP1, are recruited to unsynapsed axes in an MDC1- and H2AX-Y142-independent manner, resulting in phosphorylation of H2AX (γH2AX) on axes (left). Then, γH2AX recruits MDC1, which facilitates the progressive recruitment of ATR and TOPBP1, resulting in γH2AX and MDC1 spreading throughout loops (right). E Model for ATM-dependent loop extrusion in the somatic DDR. DSBs trigger the recruitment of ATM, which phosphorylates H2AX. As loop extrusion progresses, DNA passes by ATM enzymes at sites of DSBs, facilitating the phosphorylation of histones across large tracts of DNA
Mechanistically, MSUC and MSCI are directed by DNA damage response (DDR) pathways that mediate the phosphorylation of histone variant H2AX at serine 139 (γH2AX) [23–25] (Fig. 1C). In the initial step of this mechanism, unsynapsed chromosome axes (XY axes in MSCI) are recognized by ATR, a serine/threonine-protein kinase, and TOPBP1, an ATR activator [26–28]. ATR phosphorylates H2AX at serine 139, which attracts MDC1, a γH2AX-binding partner [25]. The subsequent spread of γH2AX into a chromosome-wide domain (XY chromatin in MSCI) is directed by MDC1 in what comes to comprise an expansive feedforward mechanism: ATR and TOPBP1 are recruited to proximal nucleosomes, H2AX is again phosphorylated, MDC1 binds γH2AX, more ATR and TOPBP1 are incorporated, and so on [25] (Fig. 1D). It is this mechanism that commences the chromosome-wide silencing of the XY.
Failure to initiate MSCI is linked to the complete arrest and elimination of male germ cells in the pachytene stage of meiotic prophase I [25, 29, 30]. The initiation of MSCI sequesters DDR signaling from autosomes to the sex chromosomes, leading to the timely progression of male germ cells through meiotic prophase I [30] (Fig. 1C). In this way, the XY body acts as a “trap” or “sink” for a diverse array of proteins [31], which facilitate the epigenetic regulation of XY chromosomes in later stages of spermatogenesis [23, 25, 32–35]. Related to this, in MSCI, the 3D chromatin organization of the sex chromosomes is spatially segregated from autosomes, and the X chromosome lacks several prominent features of higher-order chromatin organization [36–40]. Here, in summarizing such biochemical and chromatin features, we propose a hypothesis in which the sex chromosomes make for an energetically favorable environment for extensive epigenetic programming by the DDR. Then, we propose a model for the initiation of MSCI and XY-body formation in which a DDR-mediated liquid state experiences a progressive increase in ordered, less reversible interactions through the recruitment of various proteins, thereby transitioning to a gel-like state (Fig. 1B). Such a phase separation mechanism may explain how the DDR directs the regulation of the sex chromosomes in the male germline.
An overview of meiosis and the meiotic DNA damage response
In spermatogenesis and oogenesis, germ cells undergo extended, distinct periods of development that result in the formation of mature, functional gametes: sperm and eggs, respectively. One period of germ cell development stands out among the others for its length and intricacy: meiosis. In males, germ cells differentiate from a stem cell state and, following a period of premeiotic DNA replication, enter meiosis as primary spermatocytes. Now containing two complements of the diploid genome, primary spermatocytes undergo homologous recombination between maternal and paternal alleles—a biochemical mechanism that is a hallmark of sexual reproduction, ensuring accurate chromosome segregation for euploid gametes and fostering genetic diversity in offspring. The first phase of meiosis, prophase I, is followed by two quick meiotic divisions that result in four haploid round spermatids [41].
In meiotic prophase I, spermatocytes undergo the programmed induction of DNA double-strand breaks (DSBs). These DSBs are formed through the activity of SPO11, a topoisomerase II-like enzyme, and DSB-induction is essential for homologous recombination repair [42]. By resolving DSBs, homologous recombination repair promotes the proper pairing of homologous chromosomes, which in turn facilitates the shuffling of genetic material between maternal and paternal alleles [42–45]. A small portion of these homologous recombination repair events result in genetic crossovers, which ensure the proper segregation of homologs to daughter cells at the first meiotic division [42–46]. DSB repair is tightly linked to chromosome synapsis, and persistent DSBs on the unsynapsed chromosomes are thought to trigger the DDR that underlies MSUC and MSCI [23, 24, 47, 48]. Failure to repair DSBs in a timely manner triggers the developmental arrest and death of spermatocytes [44, 49, 50]. In another form of genetic quality control, improper synapsis also triggers spermatocyte arrest and death [50–52]. Taken together, MSCI is sensitive to DNA damage and altered DSB-processing on the autosomes [51, 53]. Thus, it is posited that certain DDR proteins are available in limited quantities such that autosomal defects draw away proteins needed for MSCI, thereby disrupting the MSCI process and triggering spermatocyte death [53].
ATM- and ATR-mediated DNA damage response signaling
Meiotic prophase I corresponds to the G2 phase of the cell cycle, and the DDR pathways that function in the G2/M checkpoint in somatic cells have major functions in meiotic prophase I [54]. These pathways are driven by the ATM and ATR phosphatidylinositol 3-kinase-related protein kinases (PIKKs). ATM responds to DSBs, and ATR responds to stalled replication forks and the resected single strands of DNA that appear following DSB formation [55, 56]. In meiotic prophase I, in response to the programmed DSBs that initiate meiotic recombination, ATM-driven DDR signals cascade and propagate throughout the nucleus to facilitate homologous recombination repair [50, 57]. Accordingly, in the initial stage of meiotic prophase I, the leptotene stage, ATM-mediated accumulation of γH2AX is observed nucleus-wide [58]. ATM is typically activated and functions before ATR, both in the somatic DDR and in meiosis. As a result, ATR is subsequently required for the completion of meiotic recombination [59, 60] and MSCI [27, 61, 62]. In the next stage of meiotic prophase I, the zygotene stage, ATR-dependent γH2AX formation takes place on unsynapsed regions of autosomes and sex chromosomes; upon completion of synapsis at the onset of the subsequent pachytene stage, ATR-dependent γH2AX is confined to the unsynapsed sex chromosomes [27].
In meiotic prophase I, meiotic chromosome assembly is mediated by loop extrusion [36, 37, 63], a biochemical mechanism in which DNA is threaded through the ring-shaped, multi-subunit protein cohesin, thereby forming loops of DNA [64]. A recent study using somatic cell lines revealed a loop extrusion mechanism as the basis for ATM-dependent spreading of the γH2AX domain in response to DSBs [65]. In somatic cells, γH2AX is confined within preexisting topologically associating domains (TADs), where chromatin interacts with chromatin inside the domain at a higher frequency than that which is outside the domain [65]. Formation of γH2AX-confined TADs is mediated by rapid loop extrusion from sites of DSBs, where ATM is located (Fig. 1E). Because ATM-dependent DSB repair takes place via similar mechanisms in somatic cells and leptotene spermatocytes, it is possible—perhaps even likely—that loop extrusion underlies the initial spread of γH2AX in early meiotic prophase I. In this mechanism, the γH2AX-binding protein, MDC1, is likely recruited downstream of ATM-dependent γH2AX domain formation and, indeed, MDC1 is not required for the first wave of γH2AX domain formation in leptotene nuclei [25]. By contrast, on meiotic sex chromosomes, MDC1 may spread ATR to preexisting meiotic chromatin loops to generate a γH2AX “mega domain” that comprises the whole of the X and Y chromosomes (Fig. 1D). As discussed later in “DDR pathways and phase separation”, we propose that this ATR-dependent establishment of a γH2AX mega domain drives phase separation of the sex chromosomes during male meiosis.
Our understanding of how DDR signaling controls XY-body formation is based in part on loss-of-function mouse models for H2ax and Mdc1 [25, 29, 30]. In these mutants, not a single spermatocyte has been observed to have escaped and/or to have survived defective MSCI. Thus, this surveillance mechanism in male germ cells is highly specific and potent. The mechanism that underlies the completely penetrant death of MSCI-defective spermatocytes is a mystery. One study proposed a model in which the ectopic expression of toxic Y-linked genes, such as Zfy1 and Zfy2, induces cell death when MSCI is abrogated [66]. However, given that both ZFY1 and ZFY2 function in normal spermatogenesis [67–69], derepression of sex-linked genes is unlikely to be the sole mechanism inducing cell death (as discussed in [30]). Further insight comes from a recent study that developed a mouse model with a point mutation in which tyrosine 142 (Y142) of H2AX is converted to alanine (H2ax-Y142A); with this tyrosine converted to an alanine, phosphorylation of amino acid 142 is no longer possible [30]. Like serine 139, H2AX’s tyrosine 142 is an important amino acid residue for the DDR; in H2ax-Y142A meiosis, the establishment of DDR signals on the XY chromatin is completely impaired, as is the case in Mdc1-deficient meiosis [25]. Indeed, in H2ax-Y142A and Mdc1-deficient meiosis, ATR-mediated DDR signaling is retained on autosomes [30]. The study thereby shows that the initiation of MSCI sequesters DDR factors from autosomes to the sex chromosomes at the onset of the pachytene stage, and that the subsequent formation of an isolated XY nuclear compartment, the XY body, sequesters DDR factors to permit meiotic progression from the mid-pachytene stage onward (Fig. 1C). These findings suggest that MSCI functions as a checkpoint that coordinates and orders events during the pachytene stage of meiotic prophase I, and is directly regulated by the same DDR pathway that functions in the G2 DNA damage checkpoint in somatic cells [30]. Of note, pachytene arrest and cell death were still observed in Atr mutant mice [27] as well as triple mutant mice deficient for all three DDR-related PIKKs: ATM, ATR, and PRKDC (also known as DNA-PKcs) [59]. Therefore, while meiotic progression is regulated by ATM and ATR, pachytene arrest and cell death per se are independent of ATM, ATR, and PRKDC, although it is possible that the sequestration of other checkpoint proteins (or a network of DDR proteins) by MSCI is required for normal meiotic progression.
The establishment of DNA damage response signals along unsynapsed axes
During the initiation of MSCI, the DDR mediates two genetically separable steps: The first is the establishment of DDR signals along the XY axes, and the second is the MDC1-dependent amplification of γH2AX through the XY chromatin [25] (Fig. 1D). ATR, TOPBP1, and the ATR-interacting protein ATRIP localize on the unsynapsed axes of the sex chromosomes [26, 70, 71], and ATR and TOPBP1 are essential for MSCI [27, 28]. Another DDR factor, BRCA1, the protein product of breast cancer susceptibility gene 1, is also a marker of unsynapsed sex-chromosome axes [72] and is required for MSCI [73]. Starting from sites of persistent DSBs on the XY axes, BRCA1 establishes and amplifies DDR signals (e.g., ATR-TOPBP1; Fig. 2) [48]. Along with BRCA1, ATR and TOPBP1 are also required for the amplification of DDR signals, along the XY axes [27, 28]. Thus, BRCA1, ATR, and TOPBP1 likely work together along the XY axes.
Fig. 2.
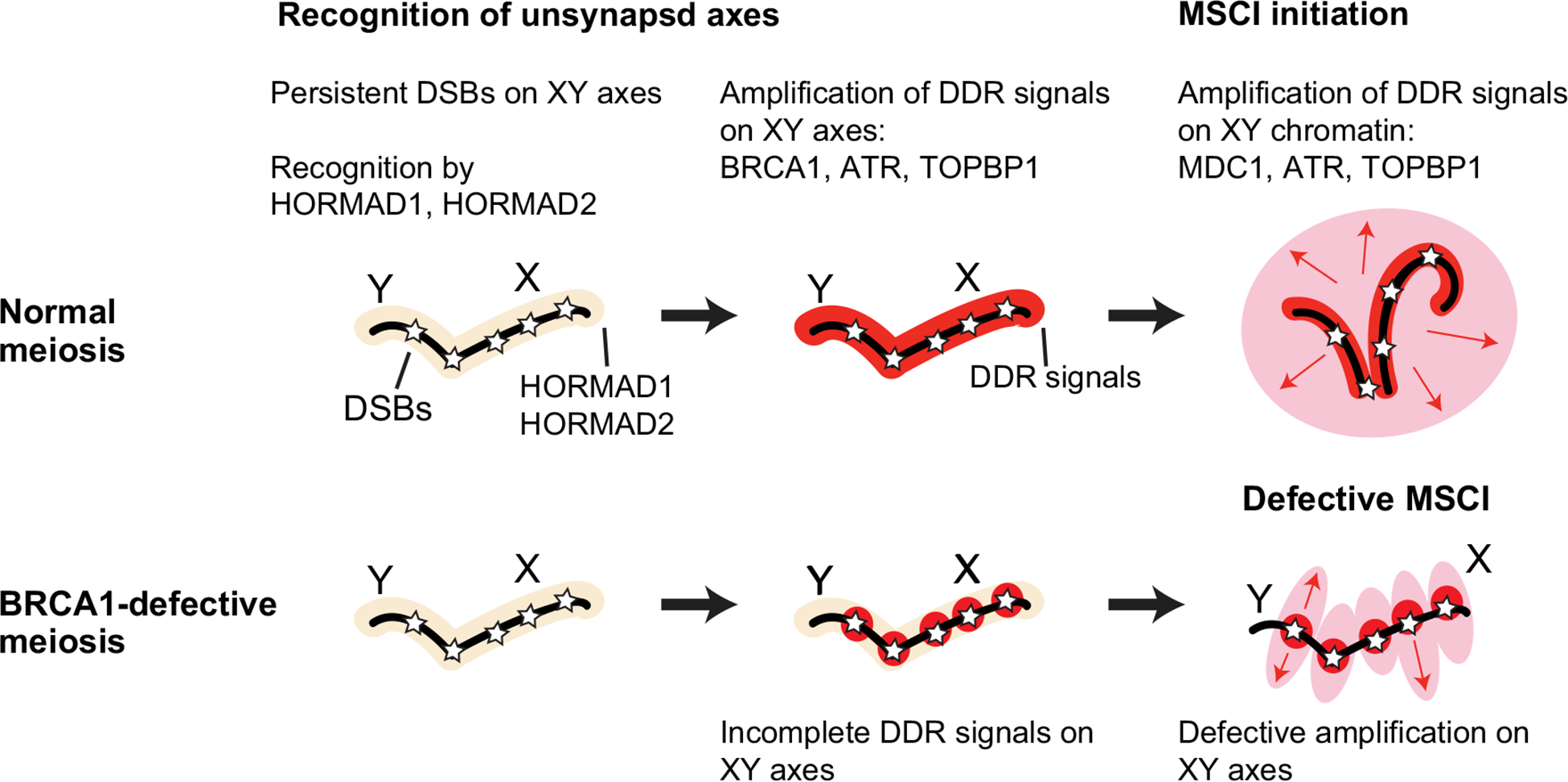
Depiction of the role of BRCA1 in meiosis. Model for BRCA1-dependent amplification of DDR signals along the XY axes followed by the initiation of MSCI
During MSCI, the accumulation of BRCA1 on unsynapsed XY axes precedes γH2AX domain formation; this is evident from studies of H2ax and Mdc1 loss-of-function spermatocytes, because even though γH2AX domain formation does not take place, BRCA1 accumulates normally on unsynapsed XY axes [25, 29, 30]. Since BRCA1 also accumulates at stalled DNA replication forks in somatic cells [74], the DDR signaling associated with pachytene-stage XY asynapsis resembles the replication stress response [23]. Interestingly, the order of events in MSCI differs from that of another DDR pathway in somatic cells, the “DSB response,” where BRCA1 accumulates downstream of γH2AX [75]. This difference raises an interesting possibility: The order of accumulation of BRCA1 and γH2AX may be distinct between ATM- and ATR-dependent DDR pathways. If this is indeed the case, then it is likely to have consequences for loop extrusion, which presumably involves ATM, and the ultimate size of γH2AX foci or domains that assemble in response to DNA damage.
Related to this, the BRCA1-A complex, one of the major BRCA1-containing complexes, is detected along unsynapsed XY axes but not throughout the XY chromatin domain [76]. Comprised of BRCA1, RAP80, CCDC98/Abraxas, and BRCC45, the BRCA1-A complex regulates G2 checkpoint arrest in response to DNA damage in somatic cells [75]. Thus, the BRCA1-A complex could have a checkpoint function in meiotic cells that parallels its function in somatic cells. CTIP, a protein that functions in DNA end resection, is also detected on unsynapsed XY axes [76]. Although their functions in MSCI are largely unknown, the presence of BRCA1-A and CTIP suggests the existence of DNA damage and/or ongoing repair along the XY axes [77]. Indeed, their presence could be the basis for the sustained DDR that mediates signaling on the XY axes and through XY chromatin loops, thereby culminating in MSCI.
How does DDR signaling recognize unsynapsed chromatin? Although the mechanism is largely unknown, several meiosis-specific proteins are implicated. SYCP3, a component of the synaptonemal complex that forms between homologous chromosomes in meiotic prophase I, is required for the loading of BRCA1 along unsynapsed axes in female meiosis [78]. Furthermore, SMC1β, a meiosis-specific component of the cohesin complex, is required for chromosome synapsis and, consequently, for MSCI [79]. Also, the meiosis-specific HORMA domain proteins, HORMAD1 and HORMAD2, accumulate on unsynapsed axes independent of BRCA1 [48] and ATR [27]; in analyses of meiosis using loss-of-function of Hormad1 and Hormad2 models, DDR signaling along unsynapsed axes was attenuated [80–84]. Thus, in response to chromosome asynapsis and persistent DSBs, meiosis-specific proteins elicit ATR-mediated DDR signaling along the unsynapsed axes, and although phosphorylation of HORMAD proteins is regulated by ATR [27, 85], HORMAD proteins appear to be upstream of DDR factors (Fig. 2).
In somatic cells, ATR is activated by both the single-strand DNA-binding protein RPA, which binds ATRIP to recruit ATR, and the RAD9A‐RAD1‐HUS1 (9A‐1‐1) checkpoint clamp, which is loaded at recessed 5’ ends associated with DSBs [86, 87]. Interestingly, RPA foci are detected on unsynapsed axes in meiosis, and 9A-1–1 accumulates along unsynapsed axes too. However, in analyses of spermatocytes in which Rpa1, Rad9a, and Hus1 were conditionally deleted, MSCI appeared to take place [88–90]. Although the mechanism to activate ATR signaling in MSCI remains a longstanding question [86, 87], it is possible that persistent DSBs may provide single-strand DNA structures that result from DSB resectioning, thereby triggering the DDR [47].
Taken together, these studies establish that DDR protein networks induce MSCI through two steps of signal amplification: the first on unsynapsed axes and the second on the whole of the XY chromatin (Figs. 2 and 3).
Fig. 3.
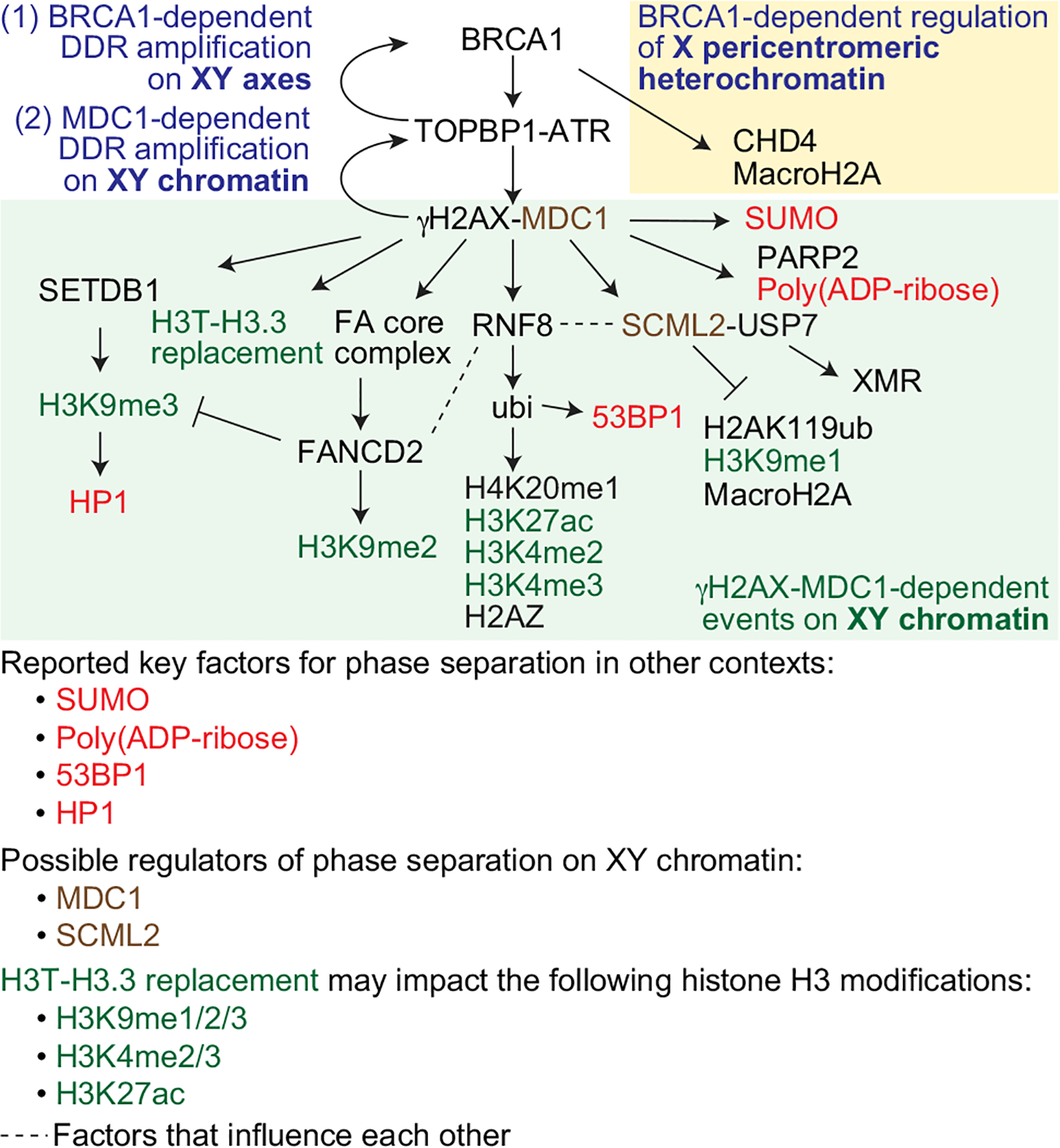
Model for molecular mechanisms in MSCI, including roles for DDR factors, other chromatin-associated proteins, and histone modifications. MSCI is initiated by BRCA1-dependent DDR amplification on the XY axes (1). BRCA1 has an additional function in the establishment of X pericentromeric heterochromatin (yellow box). MDC1-dependent DDR amplification subsequently takes place on XY chromatin (2; green box). Here, we focus on DDR signaling events, recruitment of chromatin factors such as HP1, and histone modifications that occur on XY chromatin. Since the formation of the XY body may involve phase separation, it should be noted that SUMO, Poly(ADP-ribose), 53BP1, and HP1 (shown in red), all of which are related to MSCI, and which are regulated downstream of MDC1, are reported to be key factors for phase separation in other contexts. MDC1 and SCML2 (shown in brown) are possible regulators of phase separation on XY chromatin, as discussed in this review. H3T-H3.3 replacement may impact the following histone H3 modifications: H3K9me1/2/3, H3K4me2/3, and H3K27ac (shown in green). Dashed lines signify factors that influence each other. A key of color codes for various factors is shown at the bottom
DDR pathways and phase separation
Recent studies of 3D chromatin conformation in pachytene spermatocytes revealed striking X-chromosome structural features associated with MSCI [36–40]: X appears to lack TADs and A/B compartments, i.e., alternating states of chromatin—termed A and B—in which each state preferentially interacts with other loci of the same state. Thus, the 3D chromatin conformation of the X appears to be random in the population of pachytene spermatocytes. This raises at least a few questions. Does the DDR pathway that drives MSCI promote this apparent lack of TADs and genomic A/B compartments? If so, then how? And what could be the purpose of such an organizational scheme?
The answers may lie in phase separation, a physical phenomenon in which stable, distinct liquid phases form from the surrounding liquid environment [1, 2]. It is through liquid–liquid phase separation mechanisms that many membraneless organelles self-organize and behave as liquid droplets in the cyto- and nucleoplasm [91, 92]. Heterochromatin, for example, has been proposed to form through phase separation [5, 6], and phase separation establishes and maintains distinct forms of chromatin [93]. Therefore, it could be that an MDC1-driven, chromosome-wide DDR initiates a phase separation mechanism that culminates in XY chromatin—which is largely heterochromatic—coalescing into a self-associating, droplet-like domain that lacks several obvious features of higher-order chromatin organization such as TADs and A/B compartments. While not formally demonstrated, the possibility of phase separation of the sex chromosomes is supported by the fact that this structure does not appear to be separated from the nucleus by a membrane (Fig. 1A) [30] and by the exclusion of particular proteins such as RNA polymerase II [94].
As a basis for nuclear compartmentalization in the absence of membranes, phase separation has emerged as a key feature of biochemical processes such as transcriptional regulation and the DDR. Molecular evidence has established links between DDR signaling and phase separation. For example, poly(ADP-ribosyl)ation (PARylation) seeds phase separation and the assembly of various intrinsically disordered proteins—proteins that, on their own, lack an ordered 3D structure—at DNA break sites in somatic cells [95, 96]. Intriguingly, in MSCI, although the function of PARylation is yet to be determined, PARylation and poly(ADP-ribose) polymerase 2 (PARP2) are detected on the sex chromosomes [97] (Fig. 3). Additionally, the DDR scaffold protein 53BP1 was shown to be a major player in phase separation at DNA lesions in somatic cells [98]. In MSCI, 53BP1 is an XY marker [99] recruited to the sex chromosomes downstream of RNF8, an MDC1-interacting protein [32] (Fig. 3). Despite its localization to the sex chromosomes, MSCI occurs without obvious incident in 53bp1-knockout mice, and 53bp1-knockout mice are fertile [32]. Thus, in MSCI, 53BP1 could be a non-essential factor for DDR-mediated phase separation. In contrast, SUMOylation, a type of post-translational modification involving small ubiquitin-related modifier (SUMO) proteins, is the primary mechanism driving the formation of phase-separated promyelocytic leukemia nuclear bodies [100]. Interestingly, SUMOylation is among the earliest XY modifications at the onset of MSCI [101, 102], and SUMOylation in MSCI is MDC1-dependent [25] (Fig. 3). However, the sex-chromosome substrates subject to SUMOylation are unknown. Based on a growing number of observations reported in the literature, phase separation facilitates distinct environments for DDR-related processes, possibly including MSCI.
In the pachytene stage, DSBs prefigure the localization of ATR signaling on the XY axes. Studies of Spo11-deficient spermatocytes support this idea: In Spo11-deficient spermatocytes, in which chromosomes undergo aberrant synapsis in the absence of programmed DSBs [49, 58, 103], a small number of DSBs nevertheless arise and persist [103]. These breaks colocalize with transcriptionally inactive XY body-like domains, termed “pseudo-sex bodies” because they localize outside of the XY chromosomes but are similarly enriched with γH2AX, ATR, and other DDR factors. This indicates that DSBs trigger the localization of the ATR-driven DDR signaling that gives rise to ectopic MSUC [48, 49, 58, 103]. Importantly, these ectopic γH2AX domains appear to coalesce into a single large domain, and the same is true for ectopic MSUC that recognize large asynaptic segments of autosomes [104, 105]. Thus, the γH2AX domains that form from MSUC appear to demonstrate self-assembly, a key characteristic of liquid–liquid phase separation [106].
At the onset of MSCI, the MDC1-dependent amplification of γH2AX on XY chromatin may be the major driver of phase separation. Indeed, MDC1 is enriched with intrinsically disordered regions (IDRs; Fig. 4A), regions of proteins that do not fold into fixed structures. IDRs are essential features of many proteins implicated in phase separation [107]. Furthermore, because conformational flexibility and electrostatic interactions typically underlie phase separation events [95, 108], it is possible that phase separation of the entire sex chromosomes is driven by large-scale changes in post-translational modifications such as phosphorylation of the highly basic histone H2AX. In MSCI, XY chromosomes lack functional TADs despite the presence of CTCF [39], a ubiquitous transcription factor that demarcates TADs in somatic cells by binding to insulators and domain boundaries [109]. Thus, it is possible that the spread of DDR factors is not constrained by the boundary-inducing effects of CTCF and may thereby promotes phase separation of XY chromosomes.
Fig. 4.
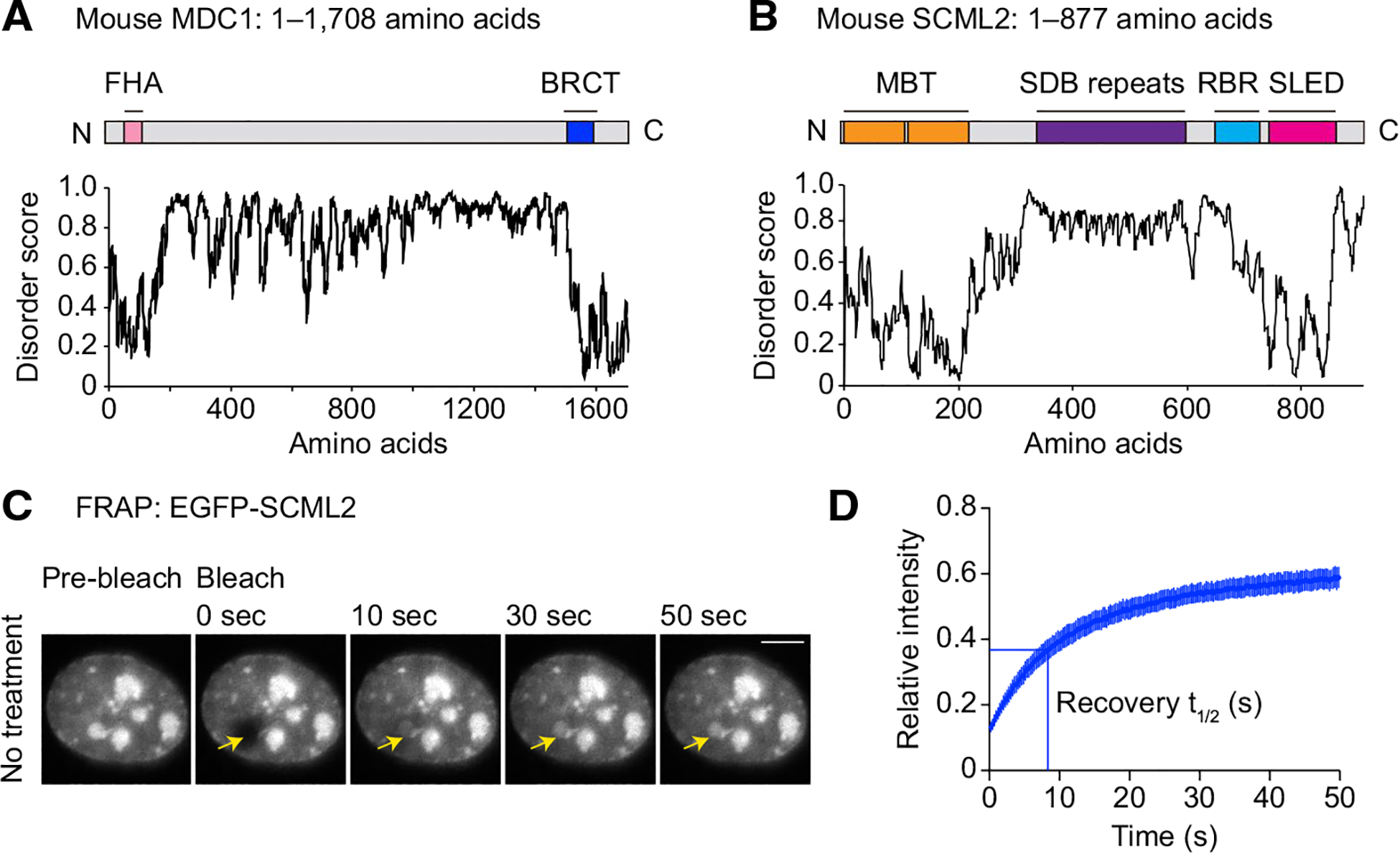
A phase separation hypothesis for the initiation of MSCI and for XY-body formation. A, B Predictions of protein disorder in MDC1 (A) and SCML2 (B; IUPRED score, Dosztányi et al., 2018 [161]). C Representative live images of FRAP assays in mK4 cells expressing EGFP-SCML2. Bars: 5 μm. The images are originally from Maezawa et al., 2018 [140]. Arrows show the site of photobleaching. D Relative intensity from FRAP assays of full-length SCML2. Recovery represents 50% of the plateaued intensity: t1/2 (s). Error bars for FRAP curves and recovery t1/2 represent 95% confidence intervals of the mean. The data are originally from Maezawa et al. 2018 [140]
Apart from X-chromosome regulation in male meiosis, it has been proposed that somatic X chromosome inactivation in females occurs via a phase separation mechanism [110, 111]. However, the mechanism of female X chromosome inactivation depends on Xist, an X-linked long non-coding RNA. The inactive female X is folded into two separated “mega domains,” an organization scheme which is distinct from that of autosomes and the active X chromosome [112–115]. In contrast, the inactive male X in male meiosis lacks two separated mega domains. It is intriguing to consider the possibility that the DDR pathway may phase-separate the male meiotic sex chromosomes, giving rise to a nuclear domain with what is an apparently near-patternless chromatin organization scheme (the structural features of meiotic sex chromosomes have been discussed in detail elsewhere [116]).
What could be the function of XY phase separation in male meiosis? Phase-separated sex chromosomes may serve as a microenvironment for the functional activity of DDR factors, chromatin remodelers, and other factors associated with MSCI while also excluding non-essential factors. In the case of the sex chromosomes, phase separation could (1) lead to the concentration of specific factors necessary for MSCI, (2) generate specific signals within the XY chromatin by dampening those signals elsewhere, and/or (3) exclude unwanted signals or transcription factors from the XY chromatin. Importantly, phase separation-mediated MSCI could also function as a means to suppress the illegitimate recombination of unsynapsed chromosomes [19] and as a surveillance mechanism warding against other meiotic abnormalities (for example, a checkpoint to recognize ectopic asynapsis).
XY-body formation and its molecular regulators
Following the DDR-directed initiation of MSCI in the early-pachytene stage, the sex chromosomes form a distinct nuclear compartment, the XY body, in the mid-pachytene stage (Fig. 1A, B). To some extent, the XY body can be considered a heterochromatic structure, bearing epigenetic marks typically associated with transcriptional inactivation and chromatin compaction. The XY body is maintained into postmeiotic spermatids in the form of another heterochromatic nuclear body: postmeiotic sex chromatin (PMSC) [94, 104, 117]. In placental mammals and marsupials, MSCI, XY-body formation, and PMSC formation are evolutionarily conserved processes [118–121]. DDR signaling is an evolutionarily conserved mechanism to drive MSCI, and so is the deposition of H3K9me3, a histone post-translational modification typically associated with heterochromatin [118, 120].
But to describe the XY body as solely heterochromatic elides another essential fact: the XY body accumulates active post-translational modifications throughout the entire XY chromatin [32], including H4K20 monomethylation (H4K20me1) and H3K4 dimethylation (H3K4me2). This post-translational information supports “escape gene activation” in postmeiotic spermatids [32, 122]. In escape gene activation, a select set of sperm-related genes escape transcriptional repression. Such active modifications are established downstream of RNF8, an E3 ubiquitin ligase that interacts with MDC1 and mediates poly- and monoubiquitination of XY chromatin [32, 122] (Fig. 3). Downstream of RNF8-mediated ubiquitination, the histone post-translational modifications H4K20me1, H3K4me2, and H3K27 acetylation (H3K27ac), a marker of active enhancers, are deposited. Thus, RNF8-dependent open chromatin and active enhancers are enriched on the X chromosome in pachytene spermatocytes [34, 123–125]. After meiotic prophase I, the histone variant H2AZ is also incorporated into XY chromosomes [117] in an RNF8-dependent mechanism [32]. Of note, RNF8-targeted escape genes are marked with H3K9me3 in pachytene spermatocytes, presumably suppressing the genes until they are subsequently activated [126]. Together, RNF8-mediated processes function in mechanisms associated with the transcriptional activation of sex chromosome-linked genes in the postmeiotic spermatid phase of spermatogenesis. Thus, the XY body is subject to forms of epigenetic and post-translational information that are associated with both active and inactive chromatin.
Beyond those associated with DDR signaling, a variety of proteins have been identified on the sex chromosomes in meiosis (Fig. 3). This includes the histone methyltransferase SETDB1, which mediates the deposition of H3K9me3 and is recruited to the sex chromosomes downstream of the DDR [127]. This also includes several proteins known to influence the gene silencing associated with MSCI, among which are Senataxin (SETX), a DNA repair protein [128]; AGO4, an Argonaute family member that functions in microRNA regulation [129]; SCML2, a germline-specific Polycomb protein [35, 130]; and MAPS, a male germline-specific protein [131].
There is a dynamic temporal aspect to the protein accumulation and epigenetic programming that occurs on the XY body. At the onset of MSCI, XY chromatin is enriched with SETDB1-mediated H3K9me3 [127, 132]. However, H3K9me3 is lost in the mid pachytene stage only to return when spermatocytes progress into the subsequent diplotene stage [132]. Presumably, this is due to the replacement of histones H3.1 and H3.2 with histone H3.3 [132]. Following histone eviction and replacement, H3K9me3 is deposited along with various proteins and other heterochromatic histone post-translational modifications. Concomitant with this, the testis-specific histone variant H3T—a predominant histone H3 isoform in differentiating spermatogenic cells—is excluded from the XY body [133]. Thus, independent of DNA replication, extensive histone eviction and replacement takes place, and H3.3 becomes an important substrate for H3 modifications during and after its incorporation into XY chromatin (Fig. 3).
Notably, in conjunction with SETDB1-mediated establishment of H3K9me3, a group of DDR/DNA repair proteins associated with Fanconi anemia (FA) function on the sex chromosomes to regulate H3K9 methylation. FA is a genetic disease associated with bone marrow failure, increased cancer susceptibility, and severe germline defects [134], and patients are said to have FA if they are deficient for the function of any one of > 20 FA genes. On the sex chromosomes, FA proteins function in a network to regulate the deposition of epigenetic marks such as repressive H3K9me2/3 and active H3K4me2 [33, 135]. FA proteins such as FANCB [135], FANCM, BRCA1 (FANCS) [73], BRCA2 (FANCD1) [72], FANCD2, PALB2 (FANCN), SLX4 (FANCP/BTBD12) [136], and FANCI [137] localize on the sex chromosomes in meiosis, where FA proteins function in a network to positively regulate H3K4me2 and H3K9me2 while counteracting H3K9me3 [33, 137] (Fig. 3).
In addition to these DDR-mediated processes, the Polycomb protein SCML2 regulates key epigenetic and post-translational modifications associated with the XY body. SCML2 works with USP7, another XY-body component, to suppress the ubiquitination of histone H2A at lysine 119, which is mediated by Polycomb repressive complex 1 (PRC1) [35, 130] and influences RNF8-mediated ubiquitination on the sex chromosomes (Fig. 3). SCML2 is also required for the localization of XMR, a classic marker of the XY body [138] (although the identity of this antigen is unknown so far [139]). Furthermore, SCML2 suppresses the deposition of H3K9me1 on, and the incorporation of histone variant macroH2A1 into, XY chromatin. Interestingly, in male meiosis, BRCA1 establishes various forms of protein signaling on X-pericentromeric heterochromatin, including the accumulation of macroH2A1 and the chromatin-remodeling protein CHD4 [48] (Fig. 3). Thus, the action of SCML2 on XY chromatin is mutually exclusive with that of BRCA1 on X-pericentromeric heterochromatin in male meiosis.
A closeup view of the relationship between XY-body formation and phase separation
A notable feature of the formation of the XY body is the gradual establishment of specific epigenetic states following DDR-directed initiation of MSCI (Fig. 1B and 3). In the early-to-mid pachytene stage transition, SCML2 is recruited to the XY chromatin [35, 130]. Unlike the DDR factors discussed above, SCML2 is not an early marker of DDR-mediated MSUC, as SCML2 is not detected on the pseudo-XY body in Spo11 mutants [130]. SCML2 contains a domain comprised of 10 repeats of 28-amino-acid units enriched with basic amino acids, termed the SCML2 DNA binding (SDB) repeats [140]; the domain is made up of IDRs (Fig. 4B). Fittingly, when SCML2 was ectopically expressed in somatic cells, SCML2 self-assembled into several nuclear bodies [140] (Fig. 4C), a phenomenon associated with phase separation [106]. A fluorescence recovery after photobleaching (FRAP) assay revealed rapid recovery of SCML2 ectopically expressed in somatic cells—another phenomenon associated with phase separation. After photobleaching, it took 8.6 s to recover 50% of the plateaued intensity (t1/2) [140] (Fig. 4C, D), a timescale that appears to be comparable to 53BP1 nuclear bodies, which are DDR-mediated phase separation condensates [141].
It is increasingly apparent that the formation and regulation of the XY body may parallel heterochromatin formation and regulation in somatic cells. Importantly, heterochromatin has been reported to form in somatic cells through a phase separation mechanism [5, 6]. Proteins in the heterochromatin protein 1 (HP1) family such as HP1β and HP1γ—both of which bind H3K9me3—associate with the XY body [142, 143]. Notably, HP1 accumulation is a relatively late event in the sequence of steps that comprise XY chromatin remodeling in meiosis; this is presumably due to the histone H3 replacement that takes place within XY chromatin in the mid-pachytene stage [132] (Fig. 1B and 3). A newly incorporated histone H3 variant, H3.3, is the likely target of H3K9me3 deposition, which in turn recruits HP1 proteins. This raises the possibility that H3K9me3-binding HP1 proteins, which accumulate later in the pachytene-diplotene transition on the sex chromosomes, further modulate the status of phase separation following the initiation of DDR-mediated phase separation in MSCI.
If indeed the XY body forms through phase separation, then what is its material state? Our understanding of the DDR-mediated initiation of MSCI lays the groundwork for a predictive framework for domain formation. Because many different proteins, including SCML2, accumulate in the XY body in a time-dependent manner, one could frame a testable hypothesis that the XY body begins in a liquid-like state, predominated by non-covalent interactions between proteins that are uniform and shorter-range in occurrence; then, over time and with the addition of various proteins, the XY body comes to acquire a gel-like state, comprised of non-uniform, longer-range non-covalent interactions. Various biomolecular condensates continue to change material states across time, such as from liquid to gel to solid states, with the mobility of components gradually decreasing, forming interaction regimes that are less and less reversible [1, 96]. The Balbiani body in oocytes is reported to undergo just such a transition [144]. Thus, it is conceivable that the gradual establishment of various proteins on the XY body change it from a liquid state to a gel state (Fig. 1B). We hypothesize that the XY body originates with DDR signaling, transitions to a network of unknown numbers of various proteins, among which is SCML2, then changes to a stabler network that includes HP1 proteins. To test this model, researchers will need to evaluate the XY body in living cells as was done for the initial characterization of P-granule phase separation [10] and for the formation of meiotic chromosome synapses via phase separation [11]. Caveats and technical considerations associated with such approaches and associated assays have been considered in detail elsewhere [145, 146].
Our phase separation hypothesis has the potential to guide the study of MSCI through a number of instructive and testable predictions, opening researchers to the application of assays that test for XY-body formation, maintenance, and maturation. Since IDRs, noncovalent inter-protein interactions, and noncovalent intra-protein interactions are critical for phase separation in other contexts, it is important to perform experiments that test the roles of various protein domains and/or disrupt hydrophobic interactions (relevant experiments and approaches are discussed in [147]). However, care should be taken to avoid over-interpretation of resulting data, as it is increasingly clear that results from such experiments can and do sometimes support models beyond that of phase separation [148, 149].
Because the XY body persists and changes through time, it will be important to understand what factors are essential and nonessential for the maintenance and/or maturation of the XY body downstream of initiation, either dependent on or independent of phase separation. In what sequences are XY-body components deposited? And what are their structural properties, alone and in combinations with each other? How does the XY body respond to changes in interaction strengths? And what about non-protein macromolecules that potentially make up the XY body? The phase separation model allows for predictions that, for example, RNA species exert specific influences on condensation. These questions, among many others, will fuel MSCI and XY-body research for years to come.
Looking forward
In a previous study, we presented the idea that MSCI is an apt model system to dissect the roles of different DDR factors in epigenetic programming [33]; i.e., in cell biology, the MSCI model allows for the formulation of hypotheses with the potential to unlock our understanding of how proteins, epigenetic information, and/or post-translational information are regulated and interrelated in time and space. We propose to extend this MSCI model to incorporate the phenomenon of phase separation. When considering experimental design, this is useful: Given the origins of the XY body and its persistent and dynamic nature, experiments that focus on the XY body—and/or the greater nuclear system that gives rise to the XY body—have the potential to yield a better understanding of how cells regulate phase separation in a spatiotemporal manner. With such a perspective, various lines of inquiry and testable hypotheses are made available to researchers interested in MSCI and/or phase separation. Also, it is important to note that, regardless of whether or not empirical tests confirm the XY body as a biomolecular condensate that arises from phase separation, the initial perspective of a phase-separated XY body establishes a useful framing device for experimental design. Put another way, if the hypothetical phase-separated XY body is disconfirmed, the experimental design originating from the initial perspective is likely adaptable to other hypothetical mechanisms that facilitate the sequestration and concentration of a vast sub-proteome on the meiotic sex chromosomes.
But a phase-separation mechanism should not be discounted yet. Consistent with previous reports regarding other biomolecular condensates in the germline [8, 9], compartmentalization of the XY body could serve to promote the fidelity of spermatogenesis. Of note, in pachytene spermatocytes, the XY body is adjacent to a nucleolus [150], itself a phase-separated compartment [151]. The relationship between the XY body and the XY-neighboring nucleolus is a longstanding mystery. And that is far from the only mystery concerning the XY body: for example, a recent study revealed that the dosage of sex-linked proteins in MSCI is compensated by the high translation of sex-linked proteins in pachytene spermatocytes [152], consistent with the enrichment of translational machinery on the XY body [153]. Thus, in a certain sense, the “silent” XY body “continues to speak.” And since the epigenetic and post-translational states of the XY body are largely maintained into the PMSC of postmeiotic spermatids, MSCI may prepare heritable epigenetic states [154]. Ultimately, MSCI is considered a driving force for genomic evolution [120, 155–158], and since MSCI defects are tightly associated with the hybrid sterility of heterogametic males [159, 160], such a mechanism could serve to influence speciation. Taken together, a mechanistic exploration of MSCI and the XY body addresses a role for phase separation in nuclear biology, with strong potential to reveal insights into sex-chromosome regulation and function—including, in a grander sense, sex-chromosome function in evolutionary adaptation. A phase-separation hypothesis for MSCI lights the path to a greater understanding of meiosis, epigenetics, and evolution.
Acknowledgements
We thank Huanyu Qiao, Hironori Abe, Yu-Han Yeh, and Aidan M. Fenix for critical readings of the manuscript, and we thank Huanyu Qiao for related discussions.
Funding
This work was supported by an Albert J. Ryan Fellowship to KGA; Grant-in-Aid for Research Activity Start-up (19K21196), the Takeda Science Foundation (2019), and the Uehara Memorial Foundation Research Incentive Grant (2018) to SM; NIH Grant GM134731 to PRA; and NIH Grants GM098605 and GM141085 to SHN.
Footnotes
Conflict of interest The authors declare no competing interests.
Availability of data and materials
All data relevant to this review is included in the text, references, and figures.
References
- 1.Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, Schymkowitz J, Shorter J, Wolozin B, Van Den Bosch L, Tompa P, Fuxreiter M (2018) Protein phase separation: a new phase in cell biology. Trends Cell Biol 28(6):420–435. 10.1016/j.tcb.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao YG, Zhang H (2020) Phase separation in membrane biology: the interplay between membrane-bound organelles and membraneless condensates. Dev Cell 55(1):30–44. 10.1016/j.devcel.2020.06.033 [DOI] [PubMed] [Google Scholar]
- 3.Schmidt HB, Görlich D (2016) Transport selectivity of nuclear pores, phase separation, and membraneless organelles. Trends Biochem Sci 41(1):46–61. 10.1016/j.tibs.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 4.Brangwynne CP, Mitchison TJ, Hyman AA (2011) Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc Natl Acad Sci USA 108(11):4334–4339. 10.1073/pnas.1017150108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strom AR, Emelyanov AV, Mir M, Fyodorov DV, Darzacq X, Karpen GH (2017) Phase separation drives heterochromatin domain formation. Nature 547(7662):241–245. 10.1038/nature22989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson AG, Elnatan D, Keenen MM, Trnka MJ, Johnston JB, Burlingame AL, Agard DA, Redding S, Narlikar GJ (2017) Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature 547(7662):236–240. 10.1038/nature22822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabari BR, Dall’Agnese A, Boija A, Klein IA, Coffey EL, Shrinivas K, Abraham BJ, Hannett NM, Zamudio AV, Manteiga JC, Li CH, Guo YE, Day DS, Schuijers J, Vasile E, Malik S, Hnisz D, Lee TI, Cisse II, Roeder RG, Sharp PA, Chakraborty AK, Young RA (2018) Coactivator condensation at super-enhancers links phase separation and gene control. Science. 361(6400):aar3958. 10.1126/science.aar3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodson AE, Kennedy S (2020) Phase separation in germ cells and development. Dev Cell 55(1):4–17. 10.1016/j.devcel.2020.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.So C, Cheng S, Schuh M (2021) Phase separation during germline development. Trends Cell Biol. 10.1016/j.tcb.2020.12.004 [DOI] [PubMed] [Google Scholar]
- 10.Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Jülicher F, Hyman AA (2009) Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324(5935):1729–1732. 10.1126/science.1172046 [DOI] [PubMed] [Google Scholar]
- 11.Rog O, Köhler S, Dernburg AF (2017) The synaptonemal complex has liquid crystalline properties and spatially regulates meiotic recombination factors. Elife 6:21455. 10.7554/eLife.21455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claeys Bouuaert C, Pu S, Wang J, Oger C, Daccache D, Xie W, Patel DJ, Keeney S (2021) DNA-driven condensation assembles the meiotic DNA break machinery. Nature. 10.1038/s41586-021-03374-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner JM, Mahadevaiah SK, Fernandez-Capetillo O, Nussenzweig A, Xu X, Deng CX, Burgoyne PS (2005) Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet 37(1):41–47. 10.1038/ng1484 [DOI] [PubMed] [Google Scholar]
- 14.Baarends WM, Wassenaar E, van der Laan R, Hoogerbrugge J, Sleddens-Linkels E, Hoeijmakers JH, de Boer P, Grootegoed JA (2005) Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol Cell Biol 25(3):1041–1053. 10.1128/MCB.25.3.1041-1053.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schimenti J (2005) Synapsis or silence. Nat Genet 37(1):11–13. 10.1038/ng0105-11 [DOI] [PubMed] [Google Scholar]
- 16.Burgoyne PS, Mahadevaiah SK, Turner JM (2009) The consequences of asynapsis for mammalian meiosis. Nat Rev Genet 10(3):207–216. 10.1038/nrg2505 [DOI] [PubMed] [Google Scholar]
- 17.Turner JM (2007) Meiotic sex chromosome inactivation. Development 134(10):1823–1831. 10.1242/dev.000018 [DOI] [PubMed] [Google Scholar]
- 18.Solari AJ (1974) The behavior of the XY pair in mammals. Int Rev Cytol 38:273–317. 10.1016/s0074-7696(08)60928-6 [DOI] [PubMed] [Google Scholar]
- 19.McKee BD, Handel MA (1993) Sex chromosomes, recombination, and chromatin conformation. Chromosoma 102(2):71–80 [DOI] [PubMed] [Google Scholar]
- 20.Hoyer-Fender S (2003) Molecular aspects of XY body formation. Cytogenet Genome Res 103(3–4):245–255. 10.1159/000076810 [DOI] [PubMed] [Google Scholar]
- 21.Handel MA (2004) The XY body: a specialized meiotic chromatin domain. Exp Cell Res 296(1):57–63. 10.1016/j.yexcr.2004.03.008 [DOI] [PubMed] [Google Scholar]
- 22.Yan W, McCarrey JR (2009) Sex chromosome inactivation in the male. Epigenetics 4(7):452–456. 10.4161/epi.4.7.9923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichijima Y, Sin HS, Namekawa SH (2012) Sex chromosome inactivation in germ cells: emerging roles of DNA damage response pathways. Cell Mol Life Sci 69(15):2559–2572. 10.1007/s00018-012-0941-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner JM (2015) Meiotic silencing in mammals. Annu Rev Genet 49:395–412. 10.1146/annurev-genet-112414-055145 [DOI] [PubMed] [Google Scholar]
- 25.Ichijima Y, Ichijima M, Lou Z, Nussenzweig A, Camerini-Otero RD, Chen J, Andreassen PR, Namekawa SH (2011) MDC1 directs chromosome-wide silencing of the sex chromosomes in male germ cells. Genes Dev 25(9):959–971. 10.1101/gad.2030811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perera D, Perez-Hidalgo L, Moens PB, Reini K, Lakin N, Syvaoja JE, San-Segundo PA, Freire R (2004) TopBP1 and ATR colocalization at meiotic chromosomes: role of TopBP1/Cut5 in the meiotic recombination checkpoint. Mol Biol Cell 15(4):1568–1579. 10.1091/mbc.e03-06-0444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Royo H, Prosser H, Ruzankina Y, Mahadevaiah SK, Cloutier JM, Baumann M, Fukuda T, Hoog C, Toth A, de Rooij DG, Bradley A, Brown EJ, Turner JM (2013) ATR acts stage specifically to regulate multiple aspects of mammalian meiotic silencing. Genes Dev 27(13):1484–1494. 10.1101/gad.219477.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ElInati E, Russell HR, Ojarikre OA, Sangrithi M, Hirota T, de Rooij DG, McKinnon PJ, Turner JMA (2017) DNA damage response protein TOPBP1 regulates X chromosome silencing in the mammalian germ line. Proc Natl Acad Sci U S A 114(47):12536–12541. 10.1073/pnas.1712530114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez-Capetillo O, Mahadevaiah SK, Celeste A, Romanienko PJ, Camerini-Otero RD, Bonner WM, Manova K, Burgoyne P, Nussenzweig A (2003) H2AX is required for chromatin remodeling and inactivation of sex chromosomes in male mouse meiosis. Dev Cell 4(4):497–508 [DOI] [PubMed] [Google Scholar]
- 30.Abe H, Alavattam KG, Hu YC, Pang Q, Andreassen PR, Hegde RS, Namekawa SH (2020) the initiation of meiotic sex chromosome inactivation sequesters DNA damage signaling from autosomes in mouse spermatogenesis. Curr Biol 30(3):408–420. e405. 10.1016/j.cub.2019.11.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Handel MA (2020) The XY body: an attractive chromatin domain. Biol Reprod 102(5):985–987. 10.1093/biolre/ioaa021 [DOI] [PubMed] [Google Scholar]
- 32.Sin HS, Barski A, Zhang F, Kartashov AV, Nussenzweig A, Chen J, Andreassen PR, Namekawa SH (2012) RNF8 regulates active epigenetic modifications and escape gene activation from inactive sex chromosomes in post-meiotic spermatids. Genes Dev 26(24):2737–2748. 10.1101/gad.202713.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alavattam KG, Kato Y, Sin HS, Maezawa S, Kowalski IJ, Zhang F, Pang Q, Andreassen PR, Namekawa SH (2016) Elucidation of the Fanconi anemia protein network in meiosis and its function in the regulation of histone modifications. Cell Rep 17(4):1141–1157. 10.1016/j.celrep.2016.09.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams SR, Maezawa S, Alavattam KG, Abe H, Sakashita A, Shroder M, Broering TJ, Sroga Rios J, Thomas MA, Lin X, Price CM, Barski A, Andreassen PR, Namekawa SH (2018) RNF8 and SCML2 cooperate to regulate ubiquitination and H3K27 acetylation for escape gene activation on the sex chromosomes. PLoS Genet 14(2):e1007233. 10.1371/journal.pgen.1007233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasegawa K, Sin HS, Maezawa S, Broering TJ, Kartashov AV, Alavattam KG, Ichijima Y, Zhang F, Bacon WC, Greis KD, Andreassen PR, Barski A, Namekawa SH (2015) SCML2 establishes the male germline epigenome through regulation of histone H2A ubiquitination. Dev Cell 32(5):574–588. 10.1016/j.devcel.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Wang H, Zhang Y, Du Z, Si W, Fan S, Qin D, Wang M, Duan Y, Li L, Jiao Y, Li Y, Wang Q, Shi Q, Wu X, Xie W (2019) Reprogramming of meiotic chromatin architecture during spermatogenesis. Mol Cell. 73(3):547–561. 10.1016/j.molcel.2018.11.019 [DOI] [PubMed] [Google Scholar]
- 37.Patel L, Kang R, Rosenberg SC, Qiu Y, Raviram R, Chee S, Hu R, Ren B, Cole F, Corbett KD (2019) Dynamic reorganization of the genome shapes the recombination landscape in meiotic prophase. Nat Struct Mol Biol 26(3):164–174. 10.1038/s41594-019-0187-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alavattam KG, Maezawa S, Sakashita A, Khoury H, Barski A, Kaplan N, Namekawa SH (2019) Attenuated chromatin compartmentalization in meiosis and its maturation in sperm development. Nat Struct Mol Biol 26(3):175–184. 10.1038/s41594-019-0189-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vara C, Paytuví-Gallart A, Cuartero Y, Le Dily F, Garcia F, Salvà-Castro J, Gómez HL, Julià E, Moutinho C, Aiese Cigliano R, Sanseverino W, Fornas O, Pendás AM, Heyn H, Waters PD, Marti-Renom MA, Ruiz-Herrera A (2019) Three-dimensional genomic structure and cohesin occupancy correlate with transcriptional activity during spermatogenesis. Cell Rep 28(2):352–367.e359. 10.1016/j.celrep.2019.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y, Qiao H (2021) A hypothesis: linking phase separation to meiotic sex chromosome inactivation and sex-body formation. Front Cell Dev Biol 9:674203. 10.3389/fcell.2021.674203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolcun-Filas E, Handel MA (2018) Meiosis: the chromosomal foundation of reproduction. Biol Reprod 99(1):112–126. 10.1093/biolre/ioy021 [DOI] [PubMed] [Google Scholar]
- 42.Lam I, Keeney S (2014) Mechanism and regulation of meiotic recombination initiation. Cold Spring Harb Perspect Biol 7(1):a016634. 10.1101/cshperspect.a016634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gray S, Cohen PE (2016) Control of meiotic crossovers: from double-strand break formation to designation. Annu Rev Genet 50:175–210. 10.1146/annurev-genet-120215-035111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Handel MA, Schimenti JC (2010) Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet 11(2):124–136. 10.1038/nrg2723 [DOI] [PubMed] [Google Scholar]
- 45.Hunter N (2015) Meiotic recombination: the essence of heredity. Cold Spring Harb Perspect Biol. 7(12):a016618. 10.1101/cshperspect.a016618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cole F, Kauppi L, Lange J, Roig I, Wang R, Keeney S, Jasin M (2012) Homeostatic control of recombination is implemented progressively in mouse meiosis. Nat Cell Biol 14(4):424–430. 10.1038/ncb2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inagaki A, Schoenmakers S, Baarends WM (2010) DNA double strand break repair, chromosome synapsis and transcriptional silencing in meiosis. Epigenetics 5(4):255–266. 10.4161/epi.5.4.11518 [DOI] [PubMed] [Google Scholar]
- 48.Broering TJ, Alavattam KG, Sadreyev RI, Ichijima Y, Kato Y, Hasegawa K, Camerini-Otero RD, Lee JT, Andreassen PR, Namekawa SH (2014) BRCA1 establishes DNA damage signaling and pericentric heterochromatin of the X chromosome in male meiosis. J Cell Biol 205(5):663–675. 10.1083/jcb.201311050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barchi M, Mahadevaiah S, Di Giacomo M, Baudat F, de Rooij DG, Burgoyne PS, Jasin M, Keeney S (2005) Surveillance of different recombination defects in mouse spermatocytes yields distinct responses despite elimination at an identical developmental stage. Mol Cell Biol 25(16):7203–7215. 10.1128/MCB.25.16.7203-7215.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subramanian VV, Hochwagen A (2014) The meiotic checkpoint network: step-by-step through meiotic prophase. Cold Spring Harb Perspect Biol 6(10):a016675. 10.1101/cshperspect.a016675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Homolka D, Ivanek R, Capkova J, Jansa P, Forejt J (2007) Chromosomal rearrangement interferes with meiotic X chromosome inactivation. Genome Res 17(10):1431–1437. 10.1101/gr.6520107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Odorisio T, Rodriguez TA, Evans EP, Clarke AR, Burgoyne PS (1998) The meiotic checkpoint monitoring synapsis eliminates spermatocytes via p53-independent apoptosis. Nat Genet 18(3):257–261. 10.1038/ng0398-257 [DOI] [PubMed] [Google Scholar]
- 53.Mahadevaiah SK, Bourc’his D, de Rooij DG, Bestor TH, Turner JM, Burgoyne PS (2008) Extensive meiotic asynapsis in mice antagonises meiotic silencing of unsynapsed chromatin and consequently disrupts meiotic sex chromosome inactivation. J Cell Biol 182(2):263–276. 10.1083/jcb.200710195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burgoyne PS, Mahadevaiah SK, Turner JM (2007) The management of DNA double-strand breaks in mitotic G2, and in mammalian meiosis viewed from a mitotic G2 perspective. BioEssays 29(10):974–986. 10.1002/bies.20639 [DOI] [PubMed] [Google Scholar]
- 55.Ciccia A, Elledge SJ (2010) The DNA damage response: making it safe to play with knives. Mol Cell 40(2):179–204. 10.1016/j.molcel.2010.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lanz MC, Dibitetto D, Smolka MB (2019) DNA damage kinase signaling: checkpoint and repair at 30 years. Embo j 38(18):e101801. 10.15252/embj.2019101801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keeney S, Lange J, Mohibullah N (2014) Self-organization of meiotic recombination initiation: general principles and molecular pathways. Annu Rev Genet 48:187–214. 10.1146/annurev-genet-120213-092304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bellani MA, Romanienko PJ, Cairatti DA, Camerini-Otero RD (2005) SPO11 is required for sex-body formation, and Spo11 heterozygosity rescues the prophase arrest of Atm−/− spermatocytes. J Cell Sci 118(Pt 15):3233–3245. 10.1242/jcs.02466 [DOI] [PubMed] [Google Scholar]
- 59.Widger A, Mahadevaiah SK, Lange J, ElInati E, Zohren J, Hirota T, Pacheco S, Maldonado-Linares A, Stanzione M, Ojarikre O, Maciulyte V, de Rooij DG, Tóth A, Roig I, Keeney S, Turner JMA (2018) ATR is a multifunctional regulator of male mouse meiosis. Nat Commun 9(1):2621. 10.1038/s41467-018-04850-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pacheco S, Maldonado-Linares A, Marcet-Ortega M, Rojas C, Martínez-Marchal A, Fuentes-Lazaro J, Lange J, Jasin M, Keeney S, Fernández-Capetillo O, Garcia-Caldés M, Roig I (2018) ATR is required to complete meiotic recombination in mice. Nat Commun 9(1):2622. 10.1038/s41467-018-04851-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fedoriw AM, Menon D, Kim Y, Mu W, Magnuson T (2015) Key mediators of somatic ATR signaling localize to unpaired chromosomes in spermatocytes. Development 142(17):2972–2980. 10.1242/dev.126078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Menolfi D, Jiang W, Lee BJ, Moiseeva T, Shao Z, Estes V, Frattini MG, Bakkenist CJ, Zha S (2018) Kinase-dead ATR differs from ATR loss by limiting the dynamic exchange of ATR and RPA. Nat Commun 9(1):5351. 10.1038/s41467-018-07798-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schalbetter SA, Fudenberg G, Baxter J, Pollard KS, Neale MJ (2019) Principles of meiotic chromosome assembly revealed in S. cerevisiae. Nat Commun. 10(1):4795. 10.1038/s41467-019-12629-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishiguro KI (2019) The cohesin complex in mammalian meiosis. Genes Cells 24(1):6–30. 10.1111/gtc.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arnould C, Rocher V, Finoux AL, Clouaire T, Li K, Zhou F, Caron P, Mangeot PE, Ricci EP, Mourad R, Haber JE, Noordermeer D, Legube G (2021) Loop extrusion as a mechanism for formation of DNA damage repair foci. Nature. 10.1038/s41586-021-03193-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Royo H, Polikiewicz G, Mahadevaiah SK, Prosser H, Mitchell M, Bradley A, de Rooij DG, Burgoyne PS, Turner JM (2010) Evidence that meiotic sex chromosome inactivation is essential for male fertility. Curr Biol 20(23):2117–2123. 10.1016/j.cub.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 67.Vernet N, Mahadevaiah SK, Yamauchi Y, Decarpentrie F, Mitchell MJ, Ward MA, Burgoyne PS (2014) Mouse Y-linked Zfy1 and Zfy2 are expressed during the male-specific interphase between meiosis I and meiosis II and promote the 2nd meiotic division. PLoS Genet 10(6):e1004444. 10.1371/journal.pgen.1004444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vernet N, Mahadevaiah SK, de Rooij DG, Burgoyne PS, Ellis PJI (2016) Zfy genes are required for efficient meiotic sex chromosome inactivation (MSCI) in spermatocytes. Hum Mol Genet 25(24):5300–5310. 10.1093/hmg/ddw344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakasuji T, Ogonuki N, Chiba T, Kato T, Shiozawa K, Yamatoya K, Tanaka H, Kondo T, Miyado K, Miyasaka N, Kubota T, Ogura A, Asahara H (2017) Complementary critical functions of Zfy1 and Zfy2 in mouse spermatogenesis and reproduction. PLoS Genet 13(1):e1006578. 10.1371/journal.pgen.1006578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reini K, Uitto L, Perera D, Moens PB, Freire R, Syvaoja JE (2004) TopBP1 localises to centrosomes in mitosis and to chromosome cores in meiosis. Chromosoma 112(7):323–330. 10.1007/s00412-004-0277-5 [DOI] [PubMed] [Google Scholar]
- 71.Refolio E, Cavero S, Marcon E, Freire R, San-Segundo PA (2011) The Ddc2/ATRIP checkpoint protein monitors meiotic recombination intermediates. J Cell Sci 124(Pt 14):2488–2500. 10.1242/jcs.081711 [DOI] [PubMed] [Google Scholar]
- 72.Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J, Ashley T, Livingston DM (1997) Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell 88(2):265–275. 10.1016/s0092-8674(00)81847-4 [DOI] [PubMed] [Google Scholar]
- 73.Turner JM, Aprelikova O, Xu X, Wang R, Kim S, Chandramouli GV, Barrett JC, Burgoyne PS, Deng CX (2004) BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr Biol 14(23):2135–2142. 10.1016/j.cub.2004.11.032 [DOI] [PubMed] [Google Scholar]
- 74.Scully R, Chen J, Ochs RL, Keegan K, Hoekstra M, Feunteun J, Livingston DM (1997) Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell 90(3):425–435. 10.1016/s0092-8674(00)80503-6 [DOI] [PubMed] [Google Scholar]
- 75.Huen MS, Sy SM, Chen J (2010) BRCA1 and its toolbox for the maintenance of genome integrity. Nat Rev Mol Cell Biol 11(2):138–148. 10.1038/nrm2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu LY, Xiong Y, Kuang H, Korakavi G, Yu X (2013) Regulation of the DNA damage response on male meiotic sex chromosomes. Nat Commun 4:2105. 10.1038/ncomms3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu LY, Yu X (2015) Double-strand break repair on sex chromosomes: challenges during male meiotic prophase. Cell Cycle 14(4):516–525. 10.1080/15384101.2014.998070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kouznetsova A, Wang H, Bellani M, Camerini-Otero RD, Jessberger R, Höög C (2009) BRCA1-mediated chromatin silencing is limited to oocytes with a small number of asynapsed chromosomes. J Cell Sci 122(Pt 14):2446–2452. 10.1242/jcs.049353 [DOI] [PubMed] [Google Scholar]
- 79.Biswas U, Wetzker C, Lange J, Christodoulou EG, Seifert M, Beyer A, Jessberger R (2013) Meiotic cohesin SMC1β provides prophase I centromeric cohesion and is required for multiple synapsis-associated functions. PLoS Genet 9(12):e1003985. 10.1371/journal.pgen.1003985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shin YH, Choi Y, Erdin SU, Yatsenko SA, Kloc M, Yang F, Wang PJ, Meistrich ML, Rajkovic A (2010) Hormad1 mutation disrupts synaptonemal complex formation, recombination, and chromosome segregation in mammalian meiosis. PLoS Genet 6(11):e1001190. 10.1371/journal.pgen.1001190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Daniel K, Lange J, Hached K, Fu J, Anastassiadis K, Roig I, Cooke HJ, Stewart AF, Wassmann K, Jasin M, Keeney S, Tóth A (2011) Meiotic homologue alignment and its quality surveillance are controlled by mouse HORMAD1. Nat Cell Biol 13(5):599–610. 10.1038/ncb2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kogo H, Tsutsumi M, Ohye T, Inagaki H, Abe T, Kurahashi H (2012) HORMAD1-dependent checkpoint/surveillance mechanism eliminates asynaptic oocytes. Genes Cells 17(6):439–454. 10.1111/j.1365-2443.2012.01600.x [DOI] [PubMed] [Google Scholar]
- 83.Wojtasz L, Cloutier JM, Baumann M, Daniel K, Varga J, Fu J, Anastassiadis K, Stewart AF, Reményi A, Turner JM, Tóth A (2012) Meiotic DNA double-strand breaks and chromosome asynapsis in mice are monitored by distinct HORMAD2-independent and -dependent mechanisms. Genes Dev 26(9):958–973. 10.1101/gad.187559.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kogo H, Tsutsumi M, Inagaki H, Ohye T, Kiyonari H, Kurahashi H (2012) HORMAD2 is essential for synapsis surveillance during meiotic prophase via the recruitment of ATR activity. Genes Cells 17(11):897–912. 10.1111/gtc.12005 [DOI] [PubMed] [Google Scholar]
- 85.Fukuda T, Pratto F, Schimenti JC, Turner JM, Camerini-Otero RD, Höög C (2012) Phosphorylation of chromosome core components may serve as axis marks for the status of chromosomal events during mammalian meiosis. PLoS Genet 8(2):e1002485. 10.1371/journal.pgen.1002485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lyndaker AM, Vasileva A, Wolgemuth DJ, Weiss RS, Lieberman HB (2013) Clamping down on mammalian meiosis. Cell Cycle 12(19):3135–3145. 10.4161/cc.26061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pereira C, Smolka MB, Weiss RS, Brieño-Enríquez MA (2020) ATR signaling in mammalian meiosis: from upstream scaffolds to downstream signaling. Environ Mol Mutagen 61(7):752–766. 10.1002/em.22401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi B, Xue J, Yin H, Guo R, Luo M, Ye L, Shi Q, Huang X, Liu M, Sha J, Wang PJ (2019) Dual functions for the ssDNA-binding protein RPA in meiotic recombination. PLoS Genet 15(2):e1007952. 10.1371/journal.pgen.1007952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vasileva A, Hopkins KM, Wang X, Weisbach MM, Friedman RA, Wolgemuth DJ, Lieberman HB (2013) The DNA damage checkpoint protein RAD9A is essential for male meiosis in the mouse. J Cell Sci 126(Pt 17):3927–3938. 10.1242/jcs.126763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lyndaker AM, Lim PX, Mleczko JM, Diggins CE, Holloway JK, Holmes RJ, Kan R, Schlafer DH, Freire R, Cohen PE, Weiss RS (2013) Conditional inactivation of the DNA damage response gene Hus1 in mouse testis reveals separable roles for components of the RAD9-RAD1-HUS1 complex in meiotic chromosome maintenance. PLoS Genet 9(2):e1003320. 10.1371/journal.pgen.1003320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Banani SF, Lee HO, Hyman AA, Rosen MK (2017) Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18(5):285–298. 10.1038/nrm.2017.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bergeron-Sandoval LP, Safaee N, Michnick SW (2016) Mechanisms and consequences of macromolecular phase separation. Cell 165(5):1067–1079. 10.1016/j.cell.2016.05.026 [DOI] [PubMed] [Google Scholar]
- 93.Gibson BA, Doolittle LK, Schneider MWG, Jensen LE, Gamarra N, Henry L, Gerlich DW, Redding S, Rosen MK (2019) Organization of chromatin by intrinsic and regulated phase separation. Cell 179(2):470–484.e421. 10.1016/j.cell.2019.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Namekawa SH, Park PJ, Zhang LF, Shima JE, McCarrey JR, Griswold MD, Lee JT (2006) Postmeiotic sex chromatin in the male germline of mice. Curr Biol 16(7):660–667. 10.1016/j.cub.2006.01.066 [DOI] [PubMed] [Google Scholar]
- 95.Altmeyer M, Neelsen KJ, Teloni F, Pozdnyakova I, Pellegrino S, Grofte M, Rask MD, Streicher W, Jungmichel S, Nielsen ML, Lukas J (2015) Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose). Nat Commun 6:8088. 10.1038/ncomms9088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, Pozniakovski A, Poser I, Maghelli N, Royer LA, Weigert M, Myers EW, Grill S, Drechsel D, Hyman AA, Alberti S (2015) A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162(5):1066–1077. 10.1016/j.cell.2015.07.047 [DOI] [PubMed] [Google Scholar]
- 97.Dantzer F, Mark M, Quenet D, Scherthan H, Huber A, Liebe B, Monaco L, Chicheportiche A, Sassone-Corsi P, de Murcia G, Ménissier-de Murcia J (2006) Poly(ADP-ribose) polymerase-2 contributes to the fidelity of male meiosis I and spermiogenesis. Proc Natl Acad Sci U S A 103(40):14854–14859. 10.1073/pnas.0604252103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kilic S, Lezaja A, Gatti M, Bianco E, Michelena J, Imhof R, Altmeyer M (2019) Phase separation of 53BP1 determines liquid-like behavior of DNA repair compartments. Embo j 38(16):e101379. 10.15252/embj.2018101379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahmed EA, van der Vaart A, Barten A, Kal HB, Chen J, Lou Z, Minter-Dykhouse K, Bartkova J, Bartek J, de Boer P, de Rooij DG (2007) Differences in DNA double strand breaks repair in male germ cell types: lessons learned from a differential expression of Mdc1 and 53BP1. DNA Repair (Amst) 6(9):1243–1254. 10.1016/j.dnarep.2007.02.011 [DOI] [PubMed] [Google Scholar]
- 100.Banani SF, Rice AM, Peeples WB, Lin Y, Jain S, Parker R, Rosen MK (2016) Compositional control of phase-separated cellular bodies. Cell 166(3):651–663. 10.1016/j.cell.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rogers RS, Inselman A, Handel MA, Matunis MJ (2004) SUMO modified proteins localize to the XY body of pachytene spermatocytes. Chromosoma 113(5):233–243. 10.1007/s00412-004-0311-7 [DOI] [PubMed] [Google Scholar]
- 102.Vigodner M (2009) Sumoylation precedes accumulation of phosphorylated H2AX on sex chromosomes during their meiotic inactivation. Chromosome Res 17(1):37–45. 10.1007/s10577-008-9006-x [DOI] [PubMed] [Google Scholar]
- 103.Carofiglio F, Inagaki A, de Vries S, Wassenaar E, Schoenmakers S, Vermeulen C, van Cappellen WA, Sleddens-Linkels E, Grootegoed JA, Te Riele HP, de Massy B, Baarends WM (2013) SPO11-independent DNA repair foci and their role in meiotic silencing. PLoS Genet 9(6):e1003538. 10.1371/journal.pgen.1003538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Turner JM, Mahadevaiah SK, Ellis PJ, Mitchell MJ, Burgoyne PS (2006) Pachytene asynapsis drives meiotic sex chromosome inactivation and leads to substantial postmeiotic repression in spermatids. Dev Cell 10(4):521–529. 10.1016/j.devcel.2006.02.009 [DOI] [PubMed] [Google Scholar]
- 105.Manterola M, Page J, Vasco C, Berríos S, Parra MT, Viera A, Rufas JS, Zuccotti M, Garagna S, Fernández-Donoso R (2009) A high incidence of meiotic silencing of unsynapsed chromatin is not associated with substantial pachytene loss in heterozygous male mice carrying multiple simple robertsonian translocations. PLoS Genet 5(8):e1000625. 10.1371/journal.pgen.1000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hyman AA, Weber CA, Jülicher F (2014) Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol 30:39–58. 10.1146/annure-v-cellbio-100913-013325 [DOI] [PubMed] [Google Scholar]
- 107.Martin EW, Holehouse AS (2020) Intrinsically disordered protein regions and phase separation: sequence determinants of assembly or lack thereof. Emerg Top Life Sci 4(3):307–329. 10.1042/etls20190164 [DOI] [PubMed] [Google Scholar]
- 108.Mitrea DM, Kriwacki RW (2016) Phase separation in biology; functional organization of a higher order. Cell Commun Signal 14:1. 10.1186/s12964-015-0125-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rowley MJ, Corces VG (2018) Organizational principles of 3D genome architecture. Nat Rev Genet 19(12):789–800. 10.1038/s41576-018-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang CY, Jegu T, Chu HP, Oh HJ, Lee JT (2018) SMCHD1 merges chromosome compartments and assists formation of super-structures on the inactive X. Cell. 174(2):406–421. 10.1016/j.cell.2018.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cerase A, Armaos A, Neumayer C, Avner P, Guttman M, Tartaglia GG (2019) Phase separation drives X-chromosome inactivation: a hypothesis. Nat Struct Mol Biol 26(5):331–334. 10.1038/s41594-019-0223-0 [DOI] [PubMed] [Google Scholar]
- 112.Deng X, Ma W, Ramani V, Hill A, Yang F, Ay F, Berletch JB, Blau CA, Shendure J, Duan Z, Noble WS, Disteche CM (2015) Bipartite structure of the inactive mouse X chromosome. Genome Biol 16:152. 10.1186/s13059-015-0728-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Giorgetti L, Lajoie BR, Carter AC, Attia M, Zhan Y, Xu J, Chen CJ, Kaplan N, Chang HY, Heard E, Dekker J (2016) Structural organization of the inactive X chromosome in the mouse. Nature 535(7613):575–579. 10.1038/nature18589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Minajigi A, Froberg J, Wei C, Sunwoo H, Kesner B, Colognori D, Lessing D, Payer B, Boukhali M, Haas W, Lee JT (2015) Chromosomes A comprehensive Xist interactome reveals cohesin repulsion and an RNA-directed chromosome conformation. Science. 349(6245):aab2276. 10.1126/science.aab2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, Sanborn AL, Machol I, Omer AD, Lander ES, Aiden EL (2014) A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159(7):1665–1680. 10.1016/j.cell.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alavattam KG (2019) Epigenetic regulation of the sex chromosomes and 3D chromatin organization in male germ cells. Dissertation, University of Cincinnati College of Medicine [Google Scholar]
- 117.Greaves IK, Rangasamy D, Devoy M, Marshall Graves JA, Tremethick DJ (2006) The X and Y chromosomes assemble into H2A Z-containing [corrected] facultative heterochromatin [corrected] following meiosis. Mol Cell Biol. 26(14):5394–5405. 10.1128/mcb.00519-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Namekawa SH, VandeBerg JL, McCarrey JR, Lee JT (2007) Sex chromosome silencing in the marsupial male germ line. Proc Natl Acad Sci U S A 104(23):9730–9735. 10.1073/pnas.0700323104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hornecker JL, Samollow PB, Robinson ES, Vandeberg JL, McCarrey JR (2007) Meiotic sex chromosome inactivation in the marsupial Monodelphis domestica. Genesis 45(11):696–708. 10.1002/dvg.20345 [DOI] [PubMed] [Google Scholar]
- 120.Sin HS, Ichijima Y, Koh E, Namiki M, Namekawa SH (2012) Human postmeiotic sex chromatin and its impact on sex chromosome evolution. Genome Res 22(5):827–836. 10.1101/gr.135046.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Vries M, Vosters S, Merkx G, D’Hauwers K, Wansink DG, Ramos L, de Boer P (2012) Human male meiotic sex chromosome inactivation. PLoS One 7(2):e31485. 10.1371/journal.pone.0031485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sin HS, Namekawa SH (2013) The great escape: active genes on inactive sex chromosomes and their evolutionary implications. Epigenetics 8(9):887–892. 10.4161/epi.25672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maezawa S, Yukawa M, Alavattam KG, Barski A, Namekawa SH (2018) Dynamic reorganization of open chromatin underlies diverse transcriptomes during spermatogenesis. Nucleic Acids Res 46(2):593–608. 10.1093/nar/gkx1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sakashita A, Maezawa S, Takahashi K, Alavattam KG, Yukawa M, Hu YC, Kojima S, Parrish NF, Barski A, Pavlicev M, Namekawa SH (2020) Endogenous retroviruses drive species-specific germline transcriptomes in mammals. Nat Struct Mol Biol 27(10):967–977. 10.1038/s41594-020-0487-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maezawa S, Sakashita A, Yukawa M, Chen X, Takahashi K, Alavattam KG, Nakata I, Weirauch MT, Barski A, Namekawa SH (2020) Super-enhancer switching drives a burst in gene expression at the mitosis-to-meiosis transition. Nat Struct Mol Biol 27(10):978–988. 10.1038/s41594-020-0488-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ernst C, Eling N, Martinez-Jimenez CP, Marioni JC, Odom DT (2019) Staged developmental mapping and X chromosome transcriptional dynamics during mouse spermatogenesis. Nat Commun 10(1):1251. 10.1038/s41467-019-09182-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hirota T, Blakeley P, Sangrithi MN, Mahadevaiah SK, Encheva V, Snijders AP, ElInati E, Ojarikre OA, de Rooij DG, Niakan KK, Turner JMA (2018) SETDB1 links the meiotic DNA damage response to sex chromosome silencing in mice. Dev Cell 47(5):645–659.e646. 10.1016/j.devcel.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Becherel OJ, Yeo AJ, Stellati A, Heng EY, Luff J, Suraweera AM, Woods R, Fleming J, Carrie D, McKinney K, Xu X, Deng C, Lavin MF (2013) Senataxin plays an essential role with DNA damage response proteins in meiotic recombination and gene silencing. PLoS Genet 9(4):e1003435. 10.1371/journal.pgen.1003435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Modzelewski AJ, Holmes RJ, Hilz S, Grimson A, Cohen PE (2012) AGO4 regulates entry into meiosis and influences silencing of sex chromosomes in the male mouse germline. Dev Cell 23(2):251–264. 10.1016/j.devcel.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Luo M, Zhou J, Leu NA, Abreu CM, Wang J, Anguera MC, de Rooij DG, Jasin M, Wang PJ (2015) Polycomb protein SCML2 associates with USP7 and counteracts histone H2A ubiquitination in the XY chromatin during male meiosis. PLoS Genet 11(1):e1004954. 10.1371/journal.pgen.1004954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li M, Zheng J, Li G, Lin Z, Li D, Liu D, Feng H, Cao D, Ng EHY, Li RHW, Han C, Yeung WSB, Chow LT, Wang H, Liu K (2021) The male germline-specific protein MAPS is indispensable for pachynema progression and fertility. Proc Natl Acad Sci USA. 118(8):2025421118. 10.1073/pnas.2025421118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van der Heijden GW, Derijck AA, Posfai E, Giele M, Pelczar P, Ramos L, Wansink DG, van der Vlag J, Peters AH, de Boer P (2007) Chromosome-wide nucleosome replacement and H3.3 incorporation during mammalian meiotic sex chromosome inactivation. Nat Genet 39(2):251–258. 10.1038/ng1949 [DOI] [PubMed] [Google Scholar]
- 133.Ueda J, Harada A, Urahama T, Machida S, Maehara K, Hada M, Makino Y, Nogami J, Horikoshi N, Osakabe A, Taguchi H, Tanaka H, Tachiwana H, Yao T, Yamada M, Iwamoto T, Isotani A, Ikawa M, Tachibana T, Okada Y, Kimura H, Ohkawa Y, Kurumizaka H, Yamagata K (2017) Testis-specific histone variant H3t gene is essential for entry into spermatogenesis. Cell Rep 18(3):593–600. 10.1016/j.celrep.2016.12.065 [DOI] [PubMed] [Google Scholar]
- 134.Tsui V, Crismani W (2019) The fanconi anemia pathway and fertility. Trends Genet 35(3):199–214. 10.1016/j.tig.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 135.Kato Y, Alavattam KG, Sin HS, Meetei AR, Pang Q, Andreassen PR, Namekawa SH (2015) FANCB is essential in the male germline and regulates H3K9 methylation on the sex chromosomes during meiosis. Hum Mol Genet 24(18):5234–5249. 10.1093/hmg/ddv244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Holloway JK, Mohan S, Balmus G, Sun X, Modzelewski A, Borst PL, Freire R, Weiss RS, Cohen PE (2011) Mammalian BTBD12 (SLX4) protects against genomic instability during mammalian spermatogenesis. PLoS Genet 7(6):e1002094. 10.1371/journal.pgen.1002094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu L, Xu W, Li D, Yu X, Gao F, Qin Y, Yang Y, Zhao S (2021) FANCI plays an essential role in spermatogenesis and regulates meiotic histone methylation. Cell Death Dis 12(8):780. 10.1038/s41419-021-04034-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Escalier D, Garchon HJ (2000) XMR is associated with the asynapsed segments of sex chromosomes in the XY body of mouse primary spermatocytes. Chromosoma 109(4):259–265. 10.1007/s004120000075 [DOI] [PubMed] [Google Scholar]
- 139.Reynard LN, Turner JM, Cocquet J, Mahadevaiah SK, Touré A, Höög C, Burgoyne PS (2007) Expression analysis of the mouse multi-copy X-linked gene Xlr-related, meiosis-regulated (Xmr), reveals that Xmr encodes a spermatid-expressed cytoplasmic protein. SLX/XMR Biol Reprod 77(2):329–335. 10.1095/biolreprod.107.061101 [DOI] [PubMed] [Google Scholar]
- 140.Maezawa S, Alavattam KG, Tatara M, Nagai R, Barski A, Namekawa SH (2019) A rapidly evolved domain, the SCML2 DNA-binding repeats, contributes to chromatin binding of mouse SCML2†. Biol Reprod 100(2):409–419. 10.1093/biolre/ioy181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kilic S, Lezaja A, Gatti M, Bianco E, Michelena J, Imhof R, Altmeyer M (2019) Phase separation of 53BP1 determines liquid-like behavior of DNA repair compartments. EMBO J. 38:e101379. 10.15252/embj.2018101379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Motzkus D, Singh PB, Hoyer-Fender S (1999) M31, a murine homolog of Drosophila HP1, is concentrated in the XY body during spermatogenesis. Cytogenet Cell Genet 86(1):83–88. 10.1159/000015418 [DOI] [PubMed] [Google Scholar]
- 143.Metzler-Guillemain C, Luciani J, Depetris D, Guichaoua MR, Mattei MG (2003) HP1beta and HP1gamma, but not HP1alpha, decorate the entire XY body during human male meiosis. Chromosome Res 11(1):73–81. 10.1023/a:1022014217196 [DOI] [PubMed] [Google Scholar]
- 144.Woodruff JB, Hyman AA, Boke E (2018) Organization and function of non-dynamic biomolecular condensates. Trends Biochem Sci 43(2):81–94. 10.1016/j.tibs.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 145.McSwiggen DT, Mir M, Darzacq X, Tjian R (2019) Evaluating phase separation in live cells: diagnosis, caveats, and functional consequences. Genes Dev 33(23–24):1619–1634. 10.1101/gad.331520.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alberti S, Gladfelter A, Mittag T (2019) Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 176(3):419–434. 10.1016/j.cell.2018.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bracha D, Walls MT, Brangwynne CP (2019) Probing and engineering liquid-phase organelles. Nat Biotechnol 37(12):1435–1445. 10.1038/s41587-019-0341-6 [DOI] [PubMed] [Google Scholar]
- 148.McSwiggen DT, Hansen AS, Teves SS, Marie-Nelly H, Hao Y, Heckert AB, Umemoto KK, Dugast-Darzacq C, Tjian R, Darzacq X (2019) Evidence for DNA-mediated nuclear compartmentalization distinct from phase separation. Elife 8:47098. 10.7554/eLife.47098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chong S, Dugast-Darzacq C, Liu Z, Dong P, Dailey GM, Cattoglio C, Heckert A, Banala S, Lavis L, Darzacq X, Tjian R (2018) Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science 361(6400):aar2555. 10.1126/science.aar2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tres LL (2005) XY chromosomal bivalent: nucleolar attraction. Mol Reprod Dev 72(1):1–6. 10.1002/mrd.20334 [DOI] [PubMed] [Google Scholar]
- 151.Frottin F, Schueder F, Tiwary S, Gupta R, Körner R, Schlichthaerle T, Cox J, Jungmann R, Hartl FU, Hipp MS (2019) The nucleolus functions as a phase-separated protein quality control compartment. Science 365(6451):342–347. 10.1126/science.aaw9157 [DOI] [PubMed] [Google Scholar]
- 152.Wang ZY, Leushkin E, Liechti A, Ovchinnikova S, Mößinger K, Brüning T, Rummel C, Grützner F, Cardoso-Moreira M, Janich P, Gatfield D, Diagouraga B, de Massy B, Gill ME, Peters A, Anders S, Kaessmann H (2020) Transcriptome and translatome co-evolution in mammals. Nature 588(7839):642–647. 10.1038/s41586-020-2899-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hu J, Sun F, Handel MA (2018) Nuclear localization of EIF4G3 suggests a role for the XY body in translational regulation during spermatogenesis in mice. Biol Reprod 98(1):102–114. 10.1093/biolre/iox150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huynh KD, Lee JT (2003) Inheritance of a pre-inactivated paternal X chromosome in early mouse embryos. Nature 426(6968):857–862. 10.1038/nature02222 [DOI] [PubMed] [Google Scholar]
- 155.Wu CI, Xu EY (2003) Sexual antagonism and X inactivation–the SAXI hypothesis. Trends Genet 19(5):243–247. 10.1016/s0168-9525(03)00058-1 [DOI] [PubMed] [Google Scholar]
- 156.Potrzebowski L, Vinckenbosch N, Marques AC, Chalmel F, Jégou B, Kaessmann H (2008) Chromosomal gene movements reflect the recent origin and biology of therian sex chromosomes. PLoS Biol 6(4):e80. 10.1371/journal.pbio.0060080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang YE, Vibranovski MD, Landback P, Marais GA, Long M (2010) Chromosomal redistribution of male-biased genes in mammalian evolution with two bursts of gene gain on the X chromosome. PLoS Biol 8(10):e1000494. 10.1371/journal.pbio.1000494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hu YC, Namekawa SH (2015) Functional significance of the sex chromosomes during spermatogenesis. Reproduction 149(6):R265–277. 10.1530/rep-14-0613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bhattacharyya T, Gregorova S, Mihola O, Anger M, Sebestova J, Denny P, Simecek P, Forejt J (2013) Mechanistic basis of infertility of mouse intersubspecific hybrids. Proc Natl Acad Sci U S A 110(6):E468–477. 10.1073/pnas.1219126110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Campbell P, Good JM, Nachman MW (2013) Meiotic sex chromosome inactivation is disrupted in sterile hybrid male house mice. Genetics 193(3):819–828. 10.1534/genetics.112.148635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dosztányi Z (2018) Prediction of protein disorder based on IUPred. Protein Sci 27(1):331–340. 10.1002/pro.3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to this review is included in the text, references, and figures.


