Abstract
Background:
Postural orthostatic tachycardia syndrome (POTS) is a disorder of orthostatic intolerance that primarily affects females of child-bearing age. While the underlying pathophysiology of POTS is not fully understood, it has been suggested that autoimmunity may play a role. The aim of this study was to compare concentrations of autoantibodies to cardiovascular G-protein coupled receptors (GPCR) between POTS patients and healthy controls.
Methods:
Sera were collected from 116 POTS patients (91% female; age 29y) and 81 healthy controls (84% female; age 27y) from Calgary, Canada and Malmö, Sweden. Samples were evaluated for autoantibodies to 11 receptors (adrenergic, muscarinic, angiotensin-II, and endothelin) using a commercially available enzyme-linked immunosorbent assay (ELISA).
Results:
Autoantibody concentrations against all of the receptors tested were not significantly different between controls and POTS patients. The majority of POTS patients (98.3%) and all controls (100%) had alpha-1 adrenergic receptor (α1-AR) autoantibody concentrations above the seropositive threshold provided by the manufacturer (7 units/mL). The proportion of POTS patients versus healthy controls who fell above the diagnostic thresholds were not different for any tested autoantibodies. Similarly, receiver-operating characteristic curves showed a poor ability to discriminate between POTS patients and controls.
Conclusion:
POTS patients and healthy controls do not differ in their ELISA-derived autoantibody concentrations to cardiovascular GPCRs. These findings suggest that these tests are not useful for establishing the role of autoimmunity in POTS.
Keywords: Postural orthostatic tachycardia syndrome, autoimmunity, enzyme-linked immunosorbent assay, G-protein coupled receptors
Introduction
Postural orthostatic tachycardia syndrome (POTS) is a disorder of orthostatic intolerance that primarily affects females of child-bearing age 1. It is characterized by orthostatic tachycardia (≥ 30bpm) within 10 minutes of standing in the absence of orthostatic hypotension (≥20/10mmHg) and symptoms that are worse when upright and improve with recumbence 2,3. The cause and underlying pathophysiology of this condition are not yet fully understood. Several pathophysiological mechanisms have been described in POTS patients, often with multiple mechanisms co-existing within the same patient 3. Included among these potential mechanisms are partial autonomic denervation 4, hypovolemia 5, and deconditioning 6. One area of particular interest has been the role of autoimmunity in the pathophysiology of POTS 7. This hypothesis is supported by the abnormally high rates of autoimmune disorders in POTS patients 8. A number of studies have explored this area by studying the prevalence and activity of autoantibodies against cardiovascular G-protein couple receptors (GPCR) in POTS patients 7,9–15. Some studies have found increased concentrations of GPCR autoantibodies, as well as a variety of others, in POTS patients 10,11. Despite this extensive research, there is still no consensus as to what role autoantibodies against GPCRs and other receptors play in the pathophysiology of POTS. There are important differences between different autoantibody assay methods: some assays detect the presence of autoantibody binding while others measure biological effects of specific antibodies. Additionally, autoantibodies have been found in healthy controls as well as in patients 15. Importantly, most studies of GPCR autoantibodies in POTS have had small samples sizes and have lacked appropriate internal controls 16. Using a relatively large multicenter cohort, we sought to test the null hypothesis that GPCR-autoantibody concentrations are not different between POTS patients and healthy controls using an established, commercially-available enzyme-linked immunosorbent assay (ELISA).
Methods:
Participants:
The data that support this study’s findings can be made available from the corresponding author upon reasonable request. POTS diagnosis was based on the current consensus criteria 2: an orthostatic increase in HR of ≥30 bpm within 10 minutes of standing and in the absence of hypotension, the reproduction of orthostatic intolerance symptoms during the test, and a duration of characteristic symptoms > 6 months. POTS patient and healthy control data came from both Calgary, Canada and Malmö, Sweden. POTS patients (ntotal=116) from both Calgary (n=52) and Malmö (n=64) had a physician-confirmed POTS diagnosis. None of the healthy controls (ntotal=81) from either Calgary (n=16) nor Malmö (n=65) had a known history of autonomic dysfunction, active autoimmune disease or any other chronic inflammatory condition.
In Calgary, patient and healthy control data and samples came from participants enrolled in the Pathophysiological Role of Adrenergic Antibodies in POTS study. POTS patients and controls were recruited for this study as of February 2016. Participants were included if they were between 18 and 60 years old and provided their written informed consent. POTS patients and controls were both excluded if they had conflicting health conditions (e.g. were smokers, had significant cardiovascular, pulmonary, hepatic, or hematological disease). This study was approved by the Calgary Conjoint Health Research Ethics Board and was registered with http://www.clinicaltrials.gov (NCT02673996).
In Malmö, both POTS patient and healthy control data and samples were from the POTS sub-study of the Syncope Study of Unselected Population in Malmö (SYSTEMA). Details of the SYSTEMA – POTS cohort are described elsewhere 17. The SYSTEMA study protocol consisted of cardiovascular autonomic testing including head-up tilt (HUT) testing with continuous hemodynamic monitoring. Data from 64 POTS patients with a heart rate increase of ≥30 bpm during HUT and chronic symptoms for ≥6 months from the SYSTEMA cohort were selected between October 2017 and January 2020. Sixty-five controls were recruited through personal invitation, e.g. healthy medical students, Skåne University Hospital staff and younger participants of parallel population-based epidemiological programs in Malmö, Sweden. Controls had no history of syncope, orthostatic intolerance, POTS or endocrine disease. All cardiovascular pharmacological agents such as beta-blockers, ivabradine, midodrine and droxidopa were discontinued 72 hours prior to examination. All participants in the SYSTEMA study and sub-study provided informed consent prior to their involvement. These studies were approved by the regional ethical review board in Lund (DNR 08/82 and 17/295) and all procedures were performed in accordance with the Helsinki Declaration.
ELISA Autoantibody Assay:
In both Calgary and Malmö, patient and control blood samples were collected during dedicated study visits following overnight fasting. A trained nurse performed an antecubital venipuncture in a designated room after 10 minutes rest in a supine position. Serum was separated by centrifugation, divided into aliquots, and stores at −80°C. The serum aliquots were thereafter collected in an automatized way from the freezer, blinded, and shipped on dry ice to CellTrend GmbH (14943 Luckenwalde, Germany) for evaluation9. According to the manufacturer, at this stage the appropriate human G-protein coupled receptor was pre-coated onto a microtiter pate. During the first incubation, the anti-GPCR antibodies of the studied sample were immobilized on the plate. The auto-antibodies were detected with a peroxidase labeled anti-human IgG antibody. In the following enzymatic substrate reaction, the intensity of the colour correlated with the concentration and/or avidity of respective anti-GPCR receptor antibodies. Serum samples were evaluated for autoantibody concentrations to several cardiovascular GPCRs: angiotensin II receptor type 1 (AT1R), endothelin receptor A (ETAR), alpha-1 adrenergic receptors (α1-AR), alpha-2 adrenergic receptors (α2-AR), beta-1 adrenergic receptors (β1-AR), beta-2 adrenergic receptors (β2-AR), and muscarinic receptor 1 to 5 (M1R, M2R, M3R, M4R, M5R). These concentrations were determined using CellTrend’s commercially available enzyme-linked immunosorbent assay (ELISA).
Statistical Analysis
Continuous results for demographic information and autoantibody concentrations are reported as median [interquartile range (IQR)]. Statistical analyses were conducted through a Mann-Whitney U test. Categorical demographic information was compared using a Pearson chi-square test. Analyses of “positive” versus “negative” serotypes on an individual basis were done according to the threshold concentrations in units/mL (U/mL) that were provided by the manufacturer (CellTrend), except for autoantibodies to M1R, M2R, or M5R, where thresholds were not provided. As an alternative to the manufacturer-provided thresholds, we determined our own thresholds for each receptor as 2 standard deviations (SD) above the mean autoantibody concentration of the control sample. Participants who had an autoantibody concentration above the threshold value for a given receptor autoantibody were considered seropositive for that autoantibody. The proportions of seropositive participants are reported as percentages. Statistical analysis for these categorical data were conducted using a Fisher’s exact test.
Receiver operating characteristic (ROC) curves were generated to create a graphical representation of the diagnostic ability of a given autoantibody concentration to discriminate between POTS patients and healthy controls. The area under the curve (AUC; Harrel’s C statistic) is a reliable indication of the validity of a given diagnostic test, where an AUC of 0.5 suggests that the ability of a test to discriminate between those with or without the disease is left to chance 18. In general, an AUC above 0.7 has good discrimination ability, whereas an AUC between 0.9 and 1.00 is able to discriminate between healthy and diseased with excellent accuracy 19. ROC data are reported as AUC (95% confidence interval).
In an effort to generate ROC curves that encompassed several GPCR-autoantibodies, we combined data from GPCR-autoantibodies that were most promising when evaluated separately. For instance, ROC curves for autoantibodies to AT1R, ETAR, and a1-AR each had a greater AUC compared to the other receptors tested. The results of these tests were combined and then divided by results of autoantibodies to receptors which were higher in the control population, such as M3R.
Tests results were considered statistically significant if a 2-sided p value was ≤ 0.05. Statistical analyses were performed using IBM SPSS Statistics version 26 (IBM Corp., Armonk, N.Y., USA). Figures were made in GraphPad Prism version 7 (GraphPad Software, La Jolla, CA, USA).
Results:
Study population characteristics
The majority of both POTS patients (91%) and healthy controls (84%) were female (p=0.2). The median age for POTS patients 29.0 [23.0–37.0] years was not significantly different from that of healthy controls 27.0 [23.5–38.5] years (p=0.9). When analyzed by centre, the findings were concordant. In Calgary, the majority of POTS patients (96%) and healthy controls (100%) were female (p=0.4). In Malmö, POTS patients (86%) and healthy controls (80%) were also mostly female (p=0.4). The median age of POTS patients did not differ between Calgary (29.5 [24.3–36.8] years) and Malmö (26.5 [23.0–37.0] years; p=0.4), but the median age of healthy controls was lower in Calgary (24.5 [22.0–27.8] years) than in Malmö (29.0 [24.0–40.5] years; p=0.041).
Antibody Concentrations
There were no significant differences between POTS patients and healthy controls in median autoantibody concentration against any of the receptors evaluated (Table 1 and Figure 1). The same result was observed when data from each centre was evaluated separately (Table 1).
Table 1.
Median autoantibody concentrations split by center
| AutoAb Against: | Overall | Calgary | Malmö | POTS Calgary vs. Malmö | Control Calgary vs. Malmö | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| POTS (n=116) | Control (n=81) | p-value | POTS (n=52) | Control (n=16) | p-value | POTS (n=64) | Control (n=65) | p-value | p-value | p-value | |
| AT1R | 12.8 (8.9–22.8) | 10.9 (7.5–18.9) | 0.065 | 15.0 (11.6–41.9) | 13.9 (10.2–37.4) | 0.761 | 10.4 (7.9–17.1) | 10.4 (6.8–16.9) | 0.486 | 0.001 | 0.017 |
| ETAR | 9.9 (7.5–15.8) | 8.8 (5.8–13.4) | 0.081 | 13.3 (8.6–21.5) | 11.4 (8.0–20.0) | 0.745 | 9.1 (6.5–14.2) | 8.6 (5.3–13.1) | 0.486 | 0.002 | 0.044 |
| α1-AR | 16.0 (12.6–26.4) | 14.0 (11.3–24.6) | 0.195 | 19.7 (13.2–29.0) | 16.0 (12.3–29.8) | 0.573 | 15.2 (10.6–23.6) | 13.4 (10.5–24.5) | 0.797 | 0.013 | 0.182 |
| α2-AR | 11.7 (9.3–15.3) | 11.6 (9.4–15.8) | 0.868 | 10.5 (8.7–15.2) | 11.2 (9.3–17.3) | 0.553 | 12.5 (10.0–15.6) | 11.8 (9.4–15.8) | 0.423 | 0.037 | 0.704 |
| β1-AR | 5.3 (4.3–7.7) | 5.6 (4.0–7.8) | 0.863 | 6.1 (4.3–10.5) | 6.9 (5.2–32.9) | 0.318 | 5.3 (3.9–6.4) | 5.3 (4.0–7.5) | 0.812 | 0.015 | 0.017 |
| β2-AR | 5.2 (3.8–7.50 | 5.1 (3.6–8.0) | 0.822 | 5.5 (4.1–10.4) | 7.8 (4.9–10.7) | 0.236 | 4.8 (3.6–7.2) | 4.9 (3.3–7.5) | 0.949 | 0.042 | 0.011 |
| M1R | 2.0 (1.5–2.8) | 2.0 (1.5–3.1) | 0.831 | 2.3 (1.8–3.3) | 3.1 (1.6–3.3) | 0.548 | 1.9 (1.4–2.4) | 1.9 (1.5–2.6) | 0.543 | 0.002 | 0.063 |
| M2R | 3.0 (2.3–4.4) | 2.6 (2.0–4.2) | 0.226 | 4.2 (3.1–5.6) | 4.7 (3.4–9.5) | 0.294 | 2.6 (1.6–3.3) | 2.3 (1.9–3.5) | 0.895 | <0.001 | <0.001 |
| M3R | 7.7 (5.8–9.6) | 8.1 (6.7–10.9) | 0.053 | 6.2 (5.1–8.2) | 7.5 (5.5–9.1) | 0.256 | 8.2 (6.8–10.2) | 8.5 (7.0–11.1) | 0.801 | <0.001 | 0.122 |
| M4R | 7.8 (6.2–10.4) | 7.5 (6.5–10.1) | 0.890 | 7.0 (5.7–11.3) | 8.5 (6.0–11.6) | 0.378 | 8.3 (6.7–9.3) | 7.5 (6.6–9.8) | 0.262 | 0.176 | 0.413 |
| M5R | 6.0 (4.7–7.7) | 5.7 (4.9–7.0) | 0.861 | 6.1 (4.7–8.3) | 7.1 (5.3–9.2) | 0.393 | 5.9 (4.8–7.1) | 5.5 (4.9–6.8) | 0.772 | 0.242 | 0.042 |
AT1R: angiotensin II receptor type 1; α1-AR: alpha-1 adrenergic receptors; α2-AR: alpha-2 adrenergic receptors; β1-AR: beta-1 adrenergic receptors; β2-AR: beta-2 adrenergic receptors; ETAR: endothelin receptor A; M1R-M5R: muscarinic receptor 1 to 5; POTS: Postural Orthostatic Tachycardia Syndrome.
Figure 1. Autoantibody concentrations to 11 cardiovascular GPCRs in POTS patients versus healthy controls.
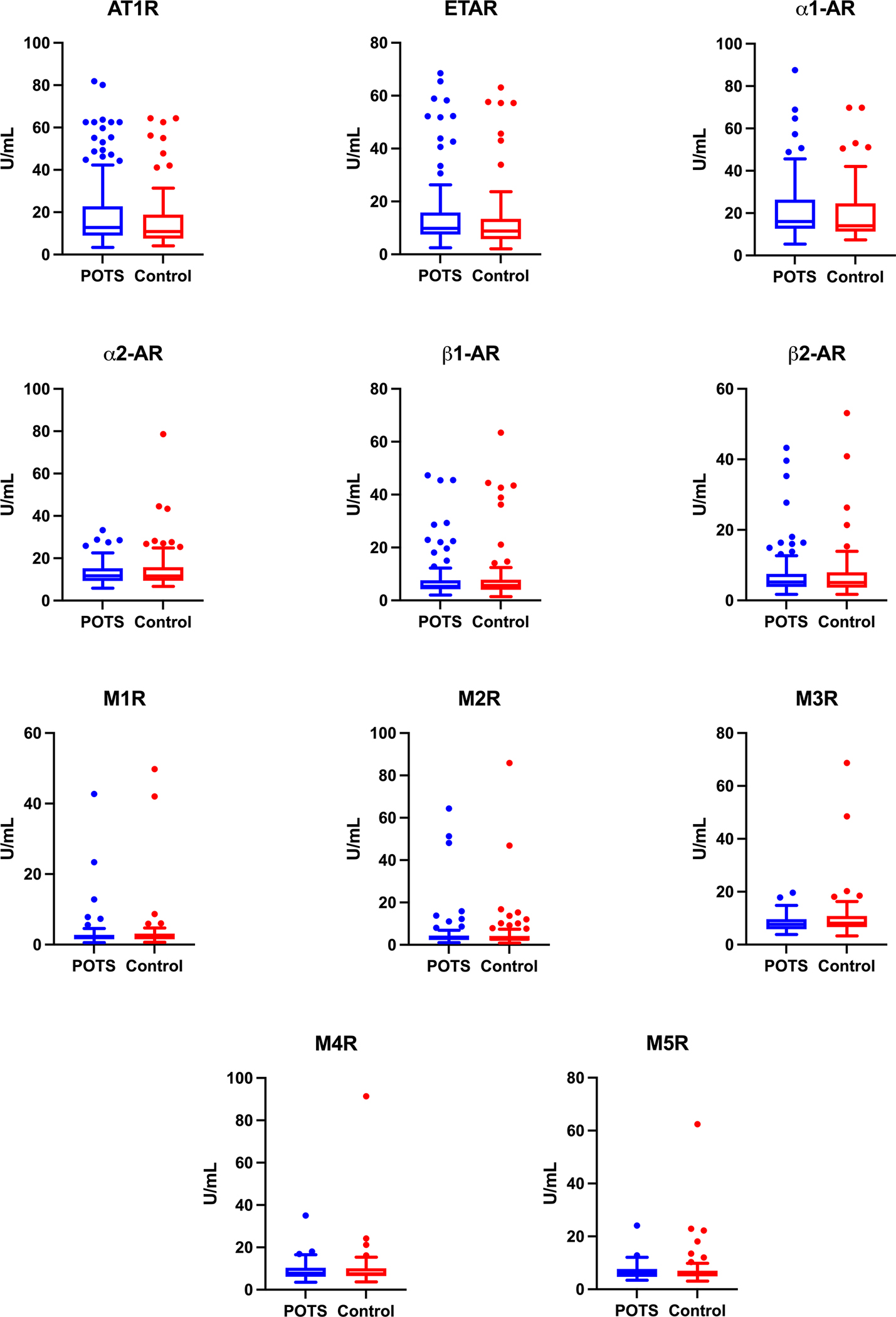
Autoantibody concentrations in (units/mL) to angiotensin II receptor type 1 (AT1R), endothelin receptor A (ETAR), alpha-1 adrenergic receptors (α1-AR), alpha-2 adrenergic receptors (α2-AR), beta-1 adrenergic receptors (β1-AR), beta-2 adrenergic receptors (β2-AR), and muscarinic receptor 1 to 5 (M1R, M2R, M3R, M4R, M5R). Data are presented as box and whiskers plots where the box represents the interquartile range (IQR) and the line represents the median. The whisker (error bar) length is 1.5x the IQR. The individual points are outliers above or below the whiskers. GPCRs: G-protein coupled receptors; POTS: Postural Orthostatic Tachycardia Syndrome.
Autoantibody concentrations against all but 2 of the GPCRs tested, M4R (p=0.176) and M5R (p=0.242), were different between POTS patients in Calgary and in Malmö. The autoantibody concentrations that were different between centres were significantly higher in Calgary except those to α2-AR (p=0.037) and M3R (p<0.001), which were higher in Malmö (Table 1). Likewise, healthy controls in Calgary had significantly higher autoantibody concentrations against 6 of the 11 GPCR’s tested compared to the healthy controls in Malmö (Table 1).
Categorical Seropositivity Using Manufacturer Thresholds
The assay manufacturer provided threshold (upper limit of normal) autoantibody concentrations for 8 of the 11 GPCR’s tested. Based upon these thresholds, a large number of both POTS patients and healthy controls were considered seropositive for several autoantibodies (Table 2). The majority of POTS patients (98%) and all healthy controls (100%) were seropositive for anti-α1-AR (p=0.5). While the seropositivity rate for the remaining autoantibodies was lower, there were no significant differences in the proportion of POTS versus healthy controls who were seropositive for any of the autoantibodies tested.
Table 2.
Percent seropositive based on manufacturer-provided diagnostic thresholds
| AutoAb Against: | Overall | Calgary | Malmö | POTS Calgary vs. Malmö | Control Calgary vs. Malmö | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| POTS (n=116) | Control (n=81) | p-value | POTS (n=52) | Control (n=16) | p-value | POTS (n=64) | Control (n=65) | p-value | p-value | p-value | |
| AT1R | 35.3% | 27.2% | 0.277 | 46.2% | 37.5% | 0.579 | 26.6% | 24.6% | 0.842 | 0.033 | 0.351 |
| ETAR | 20.7% | 18.5% | 0.856 | 32.7% | 25.0% | 0.759 | 10.9% | 16.9% | 0.447 | 0.005 | 0.481 |
| α1-AR | 98.3% | 100.0% | 0.513 | 100.0% | 100.0% | - | 96.9% | 100.0% | 0.244 | 0.501 | - |
| α2-AR | 26.7% | 27.2% | 1 | 26.9% | 25.0% | 1 | 26.6% | 27.7% | 1 | 1 | 1 |
| β1-AR | 9.5% | 8.6% | 1 | 19.2% | 25.0% | 0.726 | 1.6% | 4.6% | 0.619 | 0.002 | 0.026 |
| β2-AR | 7.8% | 6.2% | 0.782 | 11.5% | 18.8% | 0.430 | 4.7% | 3.1% | 0.680 | 0.295 | 0.050 |
| M1R | - | - | - | - | - | - | - | - | - | - | - |
| M2R | - | - | - | - | - | - | - | - | - | - | - |
| M3R | 20.7% | 28.4% | 0.237 | 13.5% | 18.9% | 0.689 | 26.6% | 30.8% | 0.698 | 0.108 | 0.537 |
| M4R | 24.1% | 18.5% | 0.385 | 30.8% | 31.3% | 1 | 18.8% | 15.4% | 0.646 | 0.190 | 0.161 |
| M5R | - | - | - | - | - | - | - | ||||
Abbreviations as in Table 1.
When comparing POTS patients to healthy controls, both centres had a similar proportion of POTS patients and healthy controls who were seropositive for each of the autoantibodies tested (Table 2).
Between centres, POTS patients in Calgary were more likely to be seropositive for anti-AT1R (p=0.033), anti-ETAR (p=0.005), and anti-β1-AR (p=0.002) than POTS patients in Malmö. Similarly, healthy controls in Calgary were more likely to be seropositive for anti-β1-AR (p=0.026) and anti-β2-AR (p=0.050) (Table 2).
Categorical Seropositivity Using the “Mean+2SD” Threshold
Using a threshold based on the control sample’s mean + 2SD, there were no significant differences in the proportion of POTS patients versus healthy controls who were seropositive for antibodies against any of the GPCRs tested. Using this criterion, very few participants in either the POTS group or the healthy control group were considered positive for autoantibodies to any of the receptors. The receptors with the greatest seropositive rates from both POTS patients and healthy controls were AT1R (12.9% of POTS patients vs. 7.4% of controls; p=0.2) and ETAR (8.6% of POTS patients vs. 7.4% of controls; p=0.8; Table 3). These findings were unchanged when the two sites were analyzed individually.
Table 3.
Percent seropositive based off mean+2SD threshold
| Autoantibody Against | POTS (n=116) | Control (n=81) | p-value |
|---|---|---|---|
| AT1R | 12.9% | 7.4% | 0.249 |
| ETAR | 8.6% | 7.4% | 0.798 |
| α1-adr | 5.2% | 6.2% | 0.763 |
| α2-adr | 0.0% | 3.7% | 0.068 |
| β1-adr | 2.6% | 7.4% | 0.165 |
| β2-adr | 3.4% | 3.7% | 1.000 |
| M1R | 1.7% | 2.5% | 1.000 |
| M2R | 2.6% | 2.5% | 1.000 |
| M3R | 0.0% | 2.5% | 0.168 |
| M4R | 0.9% | 1.2% | 1.000 |
| M5R | 0.9% | 3.7% | 0.308 |
Abbreviations as in Table 1.
Receiver Operating Characteristic Curves
The ROC curves for each of the GPCR-autoantibodies tested are shown in Figure 2. None of the autoantibody tests had significant ability to discriminate between POTS patients and healthy controls. Most provided a C-statistic below 0.51, and no autoantibody had a C-statistic >0.6. These findings held true for the individual centres.
Figure 2. Receiver operating characteristic (ROC) curves for autoantibodies to 11 cardiovascular GPCRs, split by group (POTS patients versus healthy controls).
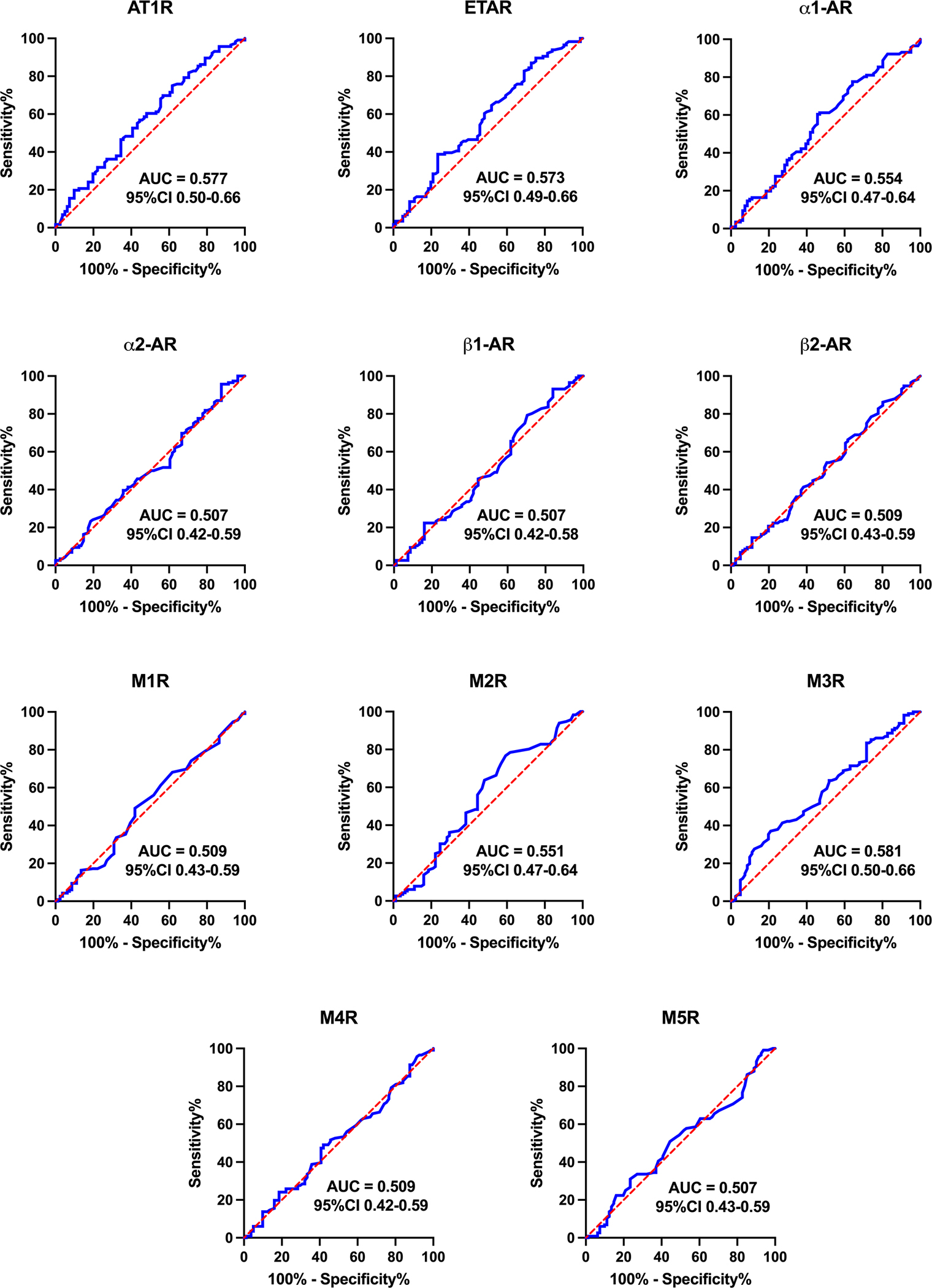
Data are presented as area under the curve (AUC) and 95% confidence interval (CI). A greater AUC indicates greater ability for the concentration of that autoantibody to discriminate whether a person has POTS. If 0.5 is contained within the 95% CI, then there is no significant difference between POTS patients and healthy controls. Abbreviations as in Figure 1.
The most promising combinations of GPCR-autoantibody improved the ROC C-statistic to between 0.60–0.65 and are shown in Figure 3.
Figure 3. Receiver operating characteristic (ROC) curves for combinations of autoantibody concentrations to GPCRs.
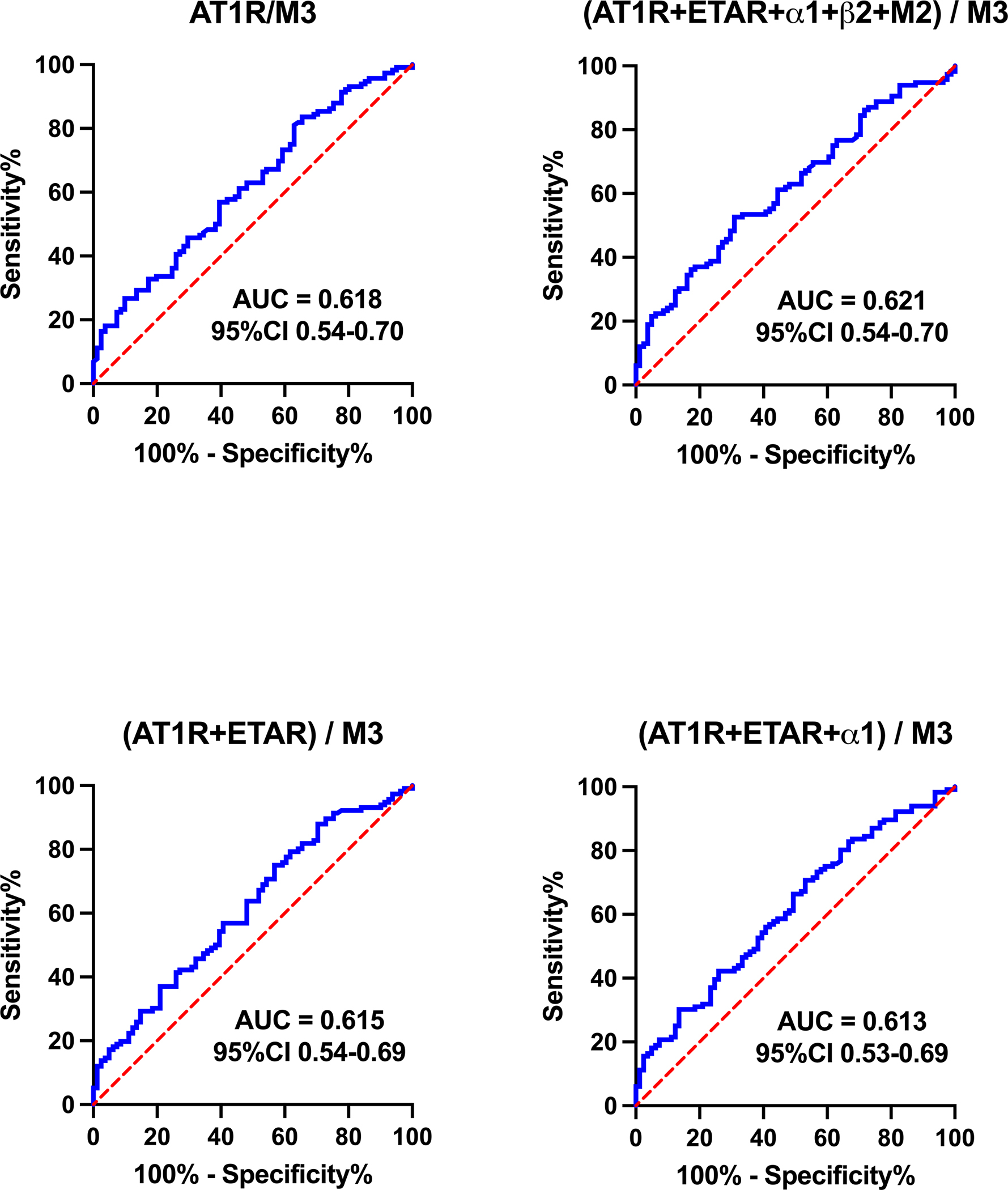
Data are presented as area under the curve (AUC) and 95% confidence interval (CI). A greater AUC indicates greater ability for the concentration of that autoantibody to discriminate whether a person has POTS. Abbreviations as in Figure 1.
Discussion:
The results of our study indicate that the commercially-available ELISA assays offered by the manufacturer used in this study have no diagnostic value when evaluating GPCR-autoantibody levels in patients with POTS, and should not be used as a clinical test. Based on this assay, there are no significant differences in GPCR-autoantibody concentrations, categorical seropositivity rates, and ROC curves between POTS patients and healthy controls. Previous research focused on POTS patients only, without a control group, providing misleading results supporting the validity of these tests in patients POTS10. However, our study includes healthy controls, allowing for direct comparison between POTS patients and the control group. With the addition of this control group, we have demonstrated that there are no differences in GPCR-autoantibody concentrations between POTS patients and healthy controls based on the results of this assay.
Importantly, these findings do not negate the potential role of immune dysregulation in the pathophysiology of POTS. However, this role cannot be shown using the ELISA-based tests of GCPR-autoantibodies in serum presented here, and these tests should not be used in the clinical diagnosis of POTS.
Antibody concentrations:
Autoantibody concentrations to AT1R, ETAR, α1-AR, α2-AR, β1-AR, β2-AR, and M1R to M5R, were not different between POTS patients and healthy controls. This was consistent whether analyzed in total or separately in two geographically distinct populations. These results are in line with other studies which have found that autoantibodies exist in healthy populations at the same concentrations that they do in patients with autoimmune disease20. As such, all future studies of autoantibody markers in POTS must include an adequate number of relevant matched controls.
Categorical Seropositivity:
There were no significant differences in the proportion of participants in each group who were seropositive based on the manufacturer-provided antibody concentration thresholds. Gunning et al. have previously reported that 89% of POTS patients demonstrated seropositivity to α1-AR, similar to our finding of 98% 10. However, 100% of healthy controls in our study were also positive, highlighting again the importance of a control group. These data questions the idea that the presence of autoantibodies above a certain concentration, as measured by ELISA, can be used to diagnose POTS. These results were also consistent across centres, highlighting the fact that results were not skewed by extraneous, centre-dependent variables.
There were no differences between POTS patients and healthy controls for the percentage of participants who were seropositive when using the “mean + 2SD” threshold. In contrast to the manufacturer thresholds, few participants from either population were seropositive for any of the autoantibodies tested using this more rigorous threshold. Regardless of the threshold value used, there were no differences between the POTS patients and healthy controls.
Discriminating Value of GPCR Autoantibody Seropositivity
ROC curves allowed us to examine the use of GPCR-autoantibody concentrations in the diagnosis of POTS. The largest C-statistic for any of the GPCR-autoantibodies individually was 0.581 (with 0.5 being totally uninformative), suggesting that none of the GPCR-autoantibodies tested are accurately able to discriminate between POTS patients and healthy controls. Even when we added several GPCR-autoantibody concentrations together, the best area under the curve we found was 0.621. This diagnostic yield is not high enough to be of clinical significance for the diagnosis of POTS. Our results highlight the fact that GPCR-autoantibody concentrations derived from the commercially available ELISA-based assays that are currently available cannot be used to discriminate POTS patients from healthy patients, and for therapy monitoring.
Calgary vs. Sweden:
Autoantibody concentrations and seropositivity were the same in POTS patients compared to controls in both the Calgary and Malmö cohorts. These data increase our confidence that these findings are likely representative of the broader patient population. Interestingly, when we compared autoantibody concentrations between POTS patients in Calgary and Malmö, we found that autoantibodies against several GPCRs were significantly higher in the Calgary POTS population. Likewise, healthy controls in Calgary tended to have higher median autoantibody concentrations compared to healthy controls in Malmö. These differences in GPCR-autoantibody concentrations between centres parallel previous findings of geographic differences in autoantibody profiles and highlights the need for studies to have locally recruited healthy controls for comparison 21.
Previous Research on Autoimmune Involvement in POTS
ELISA:
Previous work asserted that elevated autoantibody levels to various GPCR’s in POTS patients could be used to help diagnose POTS10. Unfortunately, these studies failed to consider whether high GPCR autoantibody levels were unique to POTS patients, or if comparably high levels were present in healthy controls. Our main finding is that we cannot use the current commercially available ELISA methods of determining the presence of autoantibodies to GPCRs as a diagnostic criterion for POTS, since these metrics are similar between individuals who do and do not have POTS. These data also demonstrate the importance of having control data available to ensure that a given variable is truly different in a diseased group.
Functional Assays:
Functional assays evaluate the ability of autoantibodies to activate GPCR receptors, rather than just evaluating for the presence of the autoantibodies. Previous studies have found that cardiovascular GPCR activity, to both α1-AR and β1-AR9, is elevated when exposed to sera from POTS patients compared to controls22. This has raised the question of whether the mere presence of autoantibodies in POTS can be used as a surrogate measure of altered autoantibody activity. The results of the current study suggest that exclusively measuring autoantibody presence through an ELISA may not be a good surrogate. Yu et al.13 found that POTS patients displayed significantly higher autoantibody activity to the angiotensin-II type I receptor, even when seropositivity was the same between POTS patients and controls. Alternative methods to commercial ELISA are needed to evaluate the role of GPCR autoantibodies in POTS.
Our findings do not reject a role for autoantibodies in the pathophysiology of POTS, and several prior studies have shown that serum- or IgG-dependent GPCR activity may be altered in the POTS patient population 9,13,14,22. It is important that POTS research explores the mechanisms which underlie altered autoantibody activity in POTS, what the downstream effects of this altered activity are, and how this contributes to the pathogenesis of this syndrome.
Limitations
This study looked only at a single proprietary ELISA assay method, and not functional autoantibody assays. The latter may be more relevant to the role of autoimmunity in POTS, and should be the focus of future studies. Our approach was reasonable given that it is currently in clinical use by POTS patients. Another limitation is that the present study did not control of the role of disease ‘flares’ in the detection of autoantibodies. Autoimmune conditions can go through active and inactive states. Thus, differences between the POTS patients and controls may be absent during quiescent periods. That being said, despite the fact that disease flares may play a role, it remains that based on the manufacturer threshold concentrations that were used to deem a given participant ‘positive’ or ‘negative’ for a certain autoantibody, even some control patients were ‘positive’ in unflared states. As such, the conclusion that the commercially-available ELISA method is unable to differentiate between POTS patients and controls based on these thresholds holds true.
Conclusions
Our results support the hypothesis that GPCR-autoantibody concentrations, as detected by standard ELISA, are not different between POTS patients and healthy controls. Future studies are needed to further characterize the role of autoimmunity in POTS using alternative assays and methodology.
Clinical Perspective.
What is new?
Commercially available autoantibody concentrations to G-protein coupled receptors (GCPR) are not increased or altered in POTS patients relative to healthy controls as assessed using ELISA.
While this study suggests that GCPR autoantibody concentrations alone cannot explain the pathophysiology of POTS, autoantibody activity and signals not picked up by ELISA should be explored as these results may provide more insights into POTS.
Clinical complications
Commercially available autoantibody concentrations alone cannot be used to differentiate between POTS patients and healthy controls.
The mere presence of GPCR autoantibodies is not diagnostic of POTS.
Acknowledgements:
The authors deeply thank all participants who agreed to participate in these studies.
Sources of Funding:
This work was supported by the Canadian Institutes of Health Research (CIHR; Ottawa, ON, Canada) grant MOP142426, Dysautonomia International Grant-in-Aid (2019), the Vanderbilt Institute for Clinical and Translational Research (NIH UL1-TR000445), the Swedish Heart and Lung Foundation (HLF: 20190383) and the Crafoord Foundation (CF: 20190006).
Non-standard Abbreviations and Acronyms:
- POTS
postural orthostatic tachycardia syndrome
- GPCR
G-protein coupled receptor
- ELISA
enzyme-linked immunosorbent assay
- SYSTEMA
Syncope Study of Unselected Population in Malmö
- HUT
head-up tilt
- AT1R
angiotensin II receptor type 1
- ETAR
endothelin receptor A
- α1-AR
alpha-1 adrenergic receptors
- α2-AR
alpha-2 adrenergic receptors
- β1-AR
beta-1 adrenergic receptors
- β2-AR
beta-2 adrenergic receptors
- M1R-M5R
muscarinic receptor 1 to 5
- ROC
receiver operating characteristic
- AUC
area under the curve
Footnotes
Disclosures: S.V. reports grants from Dysautonomia International and NIH; contracts from Genentech, Alterity, and BioHaven; licensing contract to Quest Diagnostics; consulting fees from Alterity, Genentech, ArgenX, and Sage Therapeutics; honoraria from ACLI, AANEM, AAN, Texas Neurological Society, and is an unpaid Board member for the American Autonomic Society. V.H. reports payment for a lecture at The Swedish Society of Cardiology, financial support for attending congresses at the Crafoord Foundation. A.F. reports funding for the present manuscript from Dysautonomia International, Hearth and Lung Foundation, and the Crafoord Foundation; consulting fees from Medtronic Inc; payment from Medtronic Inc and Biotronik for presentations, and participation on a Board for Medtronic Inc. S.R.R. reports receiving funding for the present manuscript from the Canadian Institutes of Health Research; grants from Dysautonomia International, the Canadian Institutes of Health Research, and the Cardiac Arrhythmia Network of Canada; consulting feess from Lundbeck LLC and Theravance Biopharma USA; payment for development of teaching materials by Medscape LCC, Spire Learning, and the Academy for Continued Healthcare Learning; payment for expert testimony by Faris Law; participation on a Data Safety Monitoring Board for Arena Pharmaceuticals; past President and member of the Board for the American Autonomic Society, and on the Board of Directors for the Canadian Cardiovascular Society Academy. All other authors report no conflicts of interest.
Data Availability Statement:
Data will be available to investigators with reasonable requests.
References:
- 1.Shaw BH, Stiles LE, Bourne K, Green EA, Shibao CA, Okamoto LE, Garland EM, Gamboa A, Diedrich A, Raj V, Sheldon RS, Biaggioni I, Robertson D, Raj SR. The face of postural tachycardia syndrome – insights from a large cross-sectional online community-based survey. J Intern Med. 2019;286:438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheldon RS, Grubb BP, Olshansky B, Shen WK, Calkins H, Brignole M, Raj SR, Krahn AD, Morillo CA, Stewart JM, Sutton R, Sandroni P, Friday KJ, Hachul DT, Cohen MI, Lau DH, Mayuga KA, Moak JP, Sandhu RK, Kanjwal K. 2015 Heart Rhythm Society Expert Consensus Statement on the Diagnosis and Treatment of Postural Tachycardia Syndrome, Inappropriate Sinus Tachycardia, and Vasovagal Syncope. Hear Rhythm. 2015;12:e41–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raj SR, Guzman JC, Harvey P, Richer L, Schondorf R, Seifer C, Thibodeau-Jarry N, Sheldon RS. Canadian Cardiovascular Society Position Statement on Postural Orthostatic Tachycardia Syndrome (POTS) and Related Disorders of Chronic Orthostatic Intolerance. Can J Cardiol. 2020;36:357–372. [DOI] [PubMed] [Google Scholar]
- 4.Benarroch EE. Postural tachycardia syndrome: A heterogeneous and multifactorial disorder. Mayo Clin Proc. 2012;87:1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, Robertson D. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111:1574–1582. [DOI] [PubMed] [Google Scholar]
- 6.Fu Q, Vangundy TB, Galbreath MM, Shibata S, Jain M, Hastings JL, Bhella PS, Levine BD. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2010;55:2858–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vernino S, Stiles LE. Autoimmunity in postural orthostatic tachycardia syndrome: Current understanding. Auton Neurosci Basic Clin. 2018;215:78–82. [DOI] [PubMed] [Google Scholar]
- 8.Blitshteyn S Autoimmune markers and autoimmune disorders in patients with postural tachycardia syndrome (POTS). Lupus. 2015;24:1364–1369. [DOI] [PubMed] [Google Scholar]
- 9.Fedorowski A, Li H, Yu X, Koelsch KA, Harris VM, Liles C, Murphy TA, Quadri SMS, Scofield RH, Sutton R, Melander O, Kem DC. Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace. 2017;19:1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunning WT, Kvale H, Kramer PM, Karabin BL, Grubb BP. Postural Orthostatic Tachycardia Syndrome Is Associated With Elevated G-Protein Coupled Receptor Autoantibodies. J Am Heart Assoc. 2019;8:e013602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Yu X, Liles C, Khan M, Vanderlinde-Wood M, Galloway A, Zillner C, Benbrook A, Reim S, Collier D, Hill MA, Raj SR, Okamoto LE, Cunningham MW, Aston CE, Kem DC. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. 2014;3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruzieh M, Batizy L, Dasa O, Oostra C, Grubb B. The role of autoantibodies in the syndromes of orthostatic intolerance: a systematic review. Scand Cardiovasc J. 2017;51:243–247. [DOI] [PubMed] [Google Scholar]
- 13.Yu X, Li H, Murphy TA, Nuss Z, Liles J, Liles C, Aston CE, Raj SR, Fedorowski A, Kem DC. Angiotensin II type 1 receptor autoantibodies in postural tachycardia syndrome. J Am Heart Assoc. 2018;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kharraziha I, Axelsson J, Ricci F, Di Martino G, Persson M, Sutton R, Fedorowski A, Hamrefors V. Serum activity against g protein–coupled receptors and severity of orthostatic symptoms in postural orthostatic tachycardia syndrome. J Am Heart Assoc. 2020;9. doi: 10.1161/JAHA.120.015989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryarly M, Raj SR, Phillips L, Hynan LS, Okamoto LE, Arnold AC, Paranjape SY, Vernino M, Black BK, Vernino S. Ganglionic Acetylcholine Receptor Antibodies in Postural Tachycardia Syndrome. Neurol Clin Pract. 2021;: 10.1212/CPJ.0000000000001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miglis MG, Muppidi S. Is postural tachycardia syndrome an autoimmune disorder? And other updates on recent autonomic research. Clin Auton Res. 2020;30:3–5. [DOI] [PubMed] [Google Scholar]
- 17.Johansson M, Ricci F, Schulte J, Persson M, Melander O, Sutton R, Hamrefors V, Fedorowski A. Circulating levels of growth hormone in postural orthostatic tachycardia syndrome. Sci Rep. 2021;11:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habibzadeh F, Habibzadeh P, Yadollahie M. On determining the most appropriate test cut-off value: The case of tests with continuous results. Biochem Medica. 2016;26:297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter JV, Pan J, Rai SN, Galandiuk S. ROC-ing along: Evaluation and interpretation of receiver operating characteristic curves. Surg (United States). 2016;159:1638–1645. [DOI] [PubMed] [Google Scholar]
- 20.Cabral-Marques O, Marques A, Giil LM, De Vito R, Rademacher J, Günther J, Lange T, Humrich JY, Klapa S, Schinke S, Schimke LF, Marschner G, Pitann S, Adler S, Dechend R, Müller DN, Braicu I, Sehouli J, Schulze-Forster K, Trippel T, Scheibenbogen C, Staff A, Mertens PR, Löbel M, Mastroianni J, Plattfaut C, Gieseler F, Dragun D, Engelhardt BE, Fernandez-Cabezudo MJ, Ochs HD, al-Ramadi BK, Lamprecht P, Mueller A, Heidecke H, Riemekasten G. GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis. Nat Commun. 2018;9. doi: 10.1038/s41467-018-07598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapira Y, Katz BSP, Gilburd B, Barzilai O, Ram M, Blank M, Lindeberg S, Frostegård J, Anaya JM, Bizzaro N, Jara LJ, Damoiseaux J, Shoenfeld Y, Levin NA. Geographical differences in autoantibodies and anti-infectious agents antibodies among healthy adults. Clin Rev Allergy Immunol. 2012;42:154–163. [DOI] [PubMed] [Google Scholar]
- 22.Badiudeen T, Forsythe EA, Bennett G, Li H, Yu X, Beel M, Nuss Z, Blick KE, Okamoto LE, Arnold AC, Paranjape SY, Black BK, Maxey C, Kem DC, Raj SR. A functional cell-based bioassay for assessing adrenergic autoantibody activity in postural tachycardia syndrome. J Transl Autoimmun. 2019;2:100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available to investigators with reasonable requests.


