Abstract
Background:
Pre-existing cardiovascular disease (CVD) is perceived as a risk factor for poor outcomes in patients with COVID-19. We sought to determine whether CVD is associated with in-hospital death and cardiovascular events in critically ill patients with COVID-19.
Methods:
This study used data from a multicenter cohort of adults with laboratory confirmed COVID-19 admitted to intensive care units (ICUs) at 68 centers across the United States from March 1-July 1, 2020. The primary exposure was CVD, defined as pre-existing coronary artery disease, congestive heart failure, or atrial fibrillation/flutter. Myocardial injury on ICU admission defined as a troponin I or T level above the 99th percentile upper reference limit of normal was a secondary exposure. The primary outcome was 28-day in-hospital mortality. Secondary outcomes included cardiovascular events (cardiac arrest, new-onset arrhythmias, new-onset heart failure, myocarditis, pericarditis, or stroke) within 14 days.
Results:
Among 5,133 patients (3,231 male [62.9%]; mean age 61 years [standard deviation 15]), 1,174 (22.9%) had pre-existing CVD. A total of 1,178 (34.6%) died and 920 (17.9%) had a CV event. After adjusting for age, sex, race, body mass index, history of smoking, and comorbidities, pre-existing CVD was associated with a 1.15 (95% CI: 0.98–1.34) higher odds of death. No independent association was observed between pre-existing CVD and CV events. Myocardial injury on ICU admission was associated with higher odds of death (adjusted odds ratio [aOR]=1.93, 95%CI [1.61–2.31]) and CV events (aOR=1.82, 95%CI [1.47–2.24]), regardless of the presence of CVD.
Conclusions:
CVD risk factors, rather than CVD itself, were the major contributors of outcomes in critically ill patients with COVID-19. The occurrence of myocardial injury, regardless of CVD, and its association with outcomes suggests it is likely due to multi-organ injury related to acute inflammation rather than exacerbation of preexisting CVD.
Clinical Trial Registration:
NCT04343898; https://clinicaltrials.gov/ct2/show/NCT04343898
Keywords: cardiovascular disease, COVID-19, risk factors, death, myocardial injury, troponin, inflammation
INTRODUCTION
Coronavirus disease 2019 (COVID-19) was declared a global pandemic as of March 2020 by the World Health Organization. Since March 2020, there have been nearly 90 million cases and over 1 million deaths attributed to COVID-19 in the United States alone.1 The overall case fatality rate of COVID-19 is 1.8% in the United States, with estimates much higher for patients admitted to intensive care units (ICUs) and those with pre-existing conditions, such as cardiovascular disease (CVD).2, 3
COVID-19 is recognized as a hyper-inflammatory syndrome with aberrant immune activation and fulminant cytokine release resulting in multi-organ dysfunction, including adverse effects on the heart.4 The relationship between COVID-19 and CVD is complex. Pre-existing CVD is common in patients hospitalized for COVID-19.5 Additionally, COVID-19 has also been linked to cardiovascular complications, such as myocardial injury or infarction, myocarditis, heart failure, arrhythmias, and stroke, even in patients without a history of CVD.6 Accordingly, patients with pre-existing CVD may be at a higher risk of poor in-hospital outcomes related to COVID-19, including death and cardiovascular complications.7–9
Prior studies examining the relationship between CVD and adverse outcomes in patients with COVID-19 were limited by being single center, having small sample sizes, or relying on billing codes and administrative databases lacking in data granularity, and thus were unable to comprehensively account for potential confounders.10–15 Further, many studies were focused on patient populations outside the United States with highly differing risk factor profiles, or defined patients based on SARS-CoV-2 positivity rather than a clinical diagnosis of COVID-19, which could result in selection bias.12, 16–18
We performed a comprehensive analysis of the relationship between CVD and outcomes in severe COVID-19 through leveraging the Study of the Treatment and Outcomes in Critically Ill Patients with COVID-19 (STOP-COVID); a large multicenter cohort study of critically ill adults hospitalized for COVID-19 across the United States.
METHODS
Data availability
Due to restrictions on patient privacy and data sharing, data from STOP-COVID is not available for purposes of reproducing the results or replicating the procedure. Syntax and output files of statistical analyses can be made available upon reasonable request by contacting the corresponding author.
Study Population & Design
STOP-COVID is a multicenter observational cohort study that enrolled 5,133 adult patients (≥18 years of age) with laboratory-confirmed COVID-19 admitted to ICUs at 68 hospitals across the United States.9, 19–27 Patients were admitted to ICUs between March 1 and July 1, 2020, and were followed until the first of hospital discharge, death, or September 1, 2020. The study was approved by the institutional review boards at each participating site under a waiver of informed consent. Additional details regarding STOP-COVID are reported elsewhere.9, 19–27 A list and map of participating sites are shown in Table S1 and Figure S1 in the Supplemental Material.
Data Collection & Procedures
Medical records were reviewed by study personnel at each participating site and data were entered into REDCap, a secure, HIPAA-compliant web-based application. A standardized electronic case report form was used to ensure consistent data collection across sites. A variety of relevant patient data were collected from medical records, including demographics, coexisting conditions, home medications, physiologic data, and daily data on laboratory values and outcomes during the first 14 days after ICU admission. All data were validated using a series of automated verifications and were manually reviewed to assess for potential errors or incongruent values.9
Exposure and Outcome Definitions
Exposures
The primary exposure was pre-existing CVD defined as a history of coronary artery disease (CAD), congestive heart failure (CHF), atrial fibrillation, or atrial flutter based on diagnoses documented in the medical record prior to or at the time of admission. CHF included patients with or without reduced left ventricular ejection fraction.
We explored myocardial injury as a secondary exposure. Myocardial injury at ICU admission was defined as a troponin I or T level above the 99th percentile upper reference limit of normal (URL) reported at each site (Table S2) and measured within 24-hours of ICU admission. If more than one troponin value was measured in this 24-hour period, the first value was recorded. We also assessed myocardial injury by grouping troponin levels according to multiples of the 99th percentile of the URL as follows: 1–2x, 2–3x, 3–4x, and > 4x the URL. Lastly, we examined the change in troponin defined as the absolute fold change between the maximum and minimum troponin concentrations during hospitalization. All troponin measurements were recorded up to 14 days after ICU admission.
Outcomes
The primary outcome was in-hospital death within 28 days of ICU admission. Patients discharged alive prior to 28 days were considered alive at 28 days, an assumption that we validated in the original STOP-COVID report by contacting a subset of patients by phone after they were discharged.9 The secondary outcome was a composite endpoint of cardiovascular (CV) events (cardiac arrest, new-onset arrhythmias, new-onset heart failure, myocarditis, pericarditis, or stroke) occurring within 14 days following ICU admission. New-onset arrhythmias were further stratified as ventricular fibrillation or sustained ventricular tachycardia, non-sustained ventricular tachycardia, and atrial fibrillation or flutter. Patients with pre-existing atrial fibrillation or flutter (n=187) were excluded from analyses of incident atrial fibrillation or flutter.
Statistical Analysis
Clinical characteristics are reported as means and standard deviation for normally distributed continuous variables, medians and interquartile range (IQR) for non-normally distributed continuous variables, and frequencies and proportions for categorical variables. Group comparisons were made using t-tests, Wilcoxon rank-sum test, or chi-square tests for normal continuous, non-normal continuous, and categorical variables, respectively. To determine whether pre-existing CVD was independently associated with higher levels of thrombo-inflammation markers, we used linear regression models with C-reactive protein and D-dimer levels as the dependent variables, and CVD, age, race/ethnicity, smoking status, diabetes mellitus, hypertension, and chronic kidney disease as independent variables.
Pre-Existing CVD, Myocardial Injury, and Outcomes
We used multivariable logistic regression models to investigate the relationship between exposures and outcomes (death and CV events at 28 and 14 days, respectively). The following exposures were examined in separate models: (1) CVD vs. no CVD; (2) myocardial injury vs. no myocardial injury; (3) myocardial injury categorized as troponin level on ICU admission 1–2x, 2–3x, 3–4x, and >4x the URL vs. no myocardial injury; and (4) absolute fold change in troponin levels >URL categorized as <1.29-fold change, 1.3–9.3-fold change%, and >9.3-fold change (tertiles), with troponin levels in the normal range as the reference.
We created stepwise models with each outcome as the dependent variable. Model 0 was unadjusted; model 1 included the following demographic covariates: age group (18–39, 40–49, 50–59, 60–69, 70–79, ≥80 years), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, other), and sex; model 2 included model 1 covariates in addition to the following cardiac risk factors: body-mass index (BMI; < 25, 25–29.9, 30–34.9, 35–39.9, ≥ 40 kg/m2), smoking status (current, former, never), diabetes mellitus, hypertension, and chronic kidney disease (CKD); model 3 included model 2 covariates in addition to the modified Sequential Organ Failure Assessment (SOFA) score, a measure of illness severity calculated on ICU admission.22, 28 Models 2 and 3 included adjustment for pre-existing CVD when myocardial injury is the exposure. We repeated the modeling separately for patients with CAD vs. CHF to investigate their independent association with outcomes. In sensitivity analyses, we assessed whether treatment with remdesivir or corticosteroids impacted the association between pre-existing CVD and outcomes. To explore the possibility of competing events, we examined associations between pre-existing CVD,myocardial injury and the composite outcome of in-hospital death and cardiovascular events within 14 days. We further accounted for hospital-level differences by conducting a generalized linear mixed-effects model with a random effect for institution. Given that troponin is often only measured in high-risk patients or those with presenting symptoms, we conducted an analysis in which patients with missing troponin levels were assumed to have normal troponin. Lastly, we examined the interaction term myocardial injury*CVD to assess whether the association between myocardial injury and outcomes differed according to CVD status.
We computed the relative importance of clinical characteristics in their association with the outcomes based on the Gini index using a random forest approach.29 To assess the contribution of CVD to outcomes, we computed the area under the curves (AUC) for models with clinical characteristics (model 2) with and without CVD and compared them using the Delong test.
For multivariable models, we used complete case analysis. A two-sided P-value < 0.05 was used to determine statistical significance. All analyses were performed using R Version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Characteristics of the Study Cohort
Of the 5,133 patients included in STOP-COVID, 1,174 (22.9%) had pre-existing CVD with a mean (SD) age of 61 (15) years. Of those with CVD, 492 (41.9%) had CAD without CHF, and 521 (44.4%) had CHF. Compared to those without CVD, patients with CVD were older (mean age 69 vs. 58) and more likely to have a smoking history (54.4% vs. 39.9%) and co-morbid conditions (hypertension [85% vs. 55%], diabetes mellitus [56% vs. 38%], CKD [20% vs. 9%]) (Table 1). Patients with CVD were also more likely to have higher serum creatinine on arrival to the ICU compared to those without CVD. Pre-existing CVD was not independently associated with higher C-reactive protein or D-dimer levels on ICU admission after adjusting for clinical characteristics (Table S3). Among those with CVD, patients with CHF were more likely to be women, non-Hispanic Black, have a history of atrial fibrillation or flutter and CKD, and be prescribed a mineralocorticoid receptor antagonist compared to those with CAD alone (Table 1).
Table 1.
Demographics and clinical characteristics of STOP-COVID cohort
| Overall cohort | Patients with cardiovascular disease | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| No cardiovascular disease (n=3,959) |
Cardiovascular disease (n=1,174) |
P-value | Coronary artery disease (n=492) |
Congestive heart failure (n=521) |
P-value | |
|
| ||||||
| Demographics | ||||||
| Age, years, mean (SD) | 59 (15) | 69 (12) | <0.001 | 69 (10) | 68 (13) | 0.009 |
| Male, n (%) | 2476 (62.5) | 755 (64.3) | 0.29 | 354 (72.0) | 291 (55.9) | <0.001 |
| Race/Ethnicity, n (%) | <0.001 | <0.001 | ||||
| Hispanic/Latino | 1030 (26.0) | 166 (14.1) | 78 (15.9) | 61 (11.7) | ||
| Non-Hispanic Black | 1034 (26.1) | 369 (31.4) | 129 (26.2) | 210 (40.3) | ||
| Non-Hispanic White | 940 (23.7) | 397 (33.8) | 169 (34.3) | 159 (30.5) | ||
| Other | 955 (24.1) | 242 (20.6) | 116 (23.6) | 91 (17.5) | ||
| Cardiac risk factors | ||||||
| BMI, kg/m2, mean (SD)* | 34 (22) | 33 (22) | 0.40 | 31 (16) | 34 (22) | 0.05 |
| Smoking Status, n (%) | <0.001 | 0.57 | ||||
| Non-Smoker | 2379 (60.1) | 535 (45.6) | 215 (43.7) | 239 (45.9) | ||
| Former Smoker | 799 (20.2) | 448 (38.2) | 203 (41.3) | 195 (37.4) | ||
| Current Smoker | 189 (4.8) | 73 (6.2) | 28 (5.7) | 37 (7.1) | ||
| Coexisting conditions, n (%) | ||||||
| Hypertension | 2159 (54.5) | 995 (84.8) | <0.001 | 414 (84.1) | 453 (86.9) | 0.24 |
| Diabetes mellitus | 1511 (38.2) | 654 (55.7) | <0.001 | 291 (59.1) | 298 (57.2) | 0.57 |
| Atrial fibrillation or flutter | 0 (0.0) | 392 (33.4) | <0.001 | 62 (12.6) | 169 (32.4) | <0.001 |
| Chronic kidney disease | 356 (9.0) | 316 (26.9) | <0.001 | 111 (22.6) | 179 (34.4) | <0.001 |
| Home medications, n (%) | ||||||
| ACE-I/ARB | 1140 (28.8) | 556 (47.4) | <0.001 | 244 (49.6) | 256 (49.1) | 0.93 |
| MRA | 47 (1.2) | 72 (6.1) | <0.001 | 12 (2.4) | 55 (10.6) | 0.002 |
| Beta-blocker | 642 (16.2) | 721 (61.4) | <0.001 | 309 (62.8) | 318 (61.0) | 0.61 |
| Statin | 1167 (29.5) | 787 (67.0) | <0.001 | 373 (75.8) | 330 (63.3) | 0.001 |
| Aspirin | 589 (14.9) | 580 (49.4) | <0.001 | 302 (61.4) | 241 (46.3) | <0.001 |
| Anticoagulation | 170 (4.3) | 352 (30.0) | <0.001 | 91 (18.5) | 174 (33.4) | <0.001 |
| Laboratory findings on ICU admission, median (IQR) | ||||||
| Creatinine, mg/dL† | 1.0 (0.8, 1.5) | 1.4 (1, 2.6) | <0.001 | 1.3 (1, 2.3) | 1.7 (1.1, 3.1) | <0.001 |
| Hemoglobin, g/dL‡ | 12.7 (11.2, 14.1) | 11.9 (10.1, 13.6) | <0.001 | 12.4 (10.8, 13.8) | 11.2 (9.5, 13.1) | <0.001 |
| Lactate, mmol/L§ | 1.6 (1.1, 2.3) | 1.6 (1.1, 2.6) | 0.10 | 1.6 (1.1, 2.6) | 1.6 (1.1, 2.70) | 0.81 |
| C-reactive protein, mg/|| | 151 (80, 233) | 133 (75, 218) | 0.007 | 138 (82, 219) | 124 (70, 202) | 0.09 |
| D-dimer, mg/L# | 1287 (647, 3690) | 1190 (653, 2555) | 0.08 | 1208 (689, 2820) | 1177 (551, 2220) | 0.11 |
| White blood cell count, /uL** | 8.7 (6.2, 12) | 7.7 (5.6, 11.1) | <0.001 | 7.8 (5.5, 11.3) | 7.6 (5.5, 10.9) | 0.34 |
| Lymphocyte count, /uL†† | 10 (6, 15.3) | 10 (6.1, 15) | 0.95 | 9.4 (5.4, 15) | 10.9 (7.0, 15.4) | 0.048 |
| Severity of illness on ICU admission, mean (SD) | ||||||
| mSOFA score | 12.8 (3.2) | 13.4 (3.4) | <0.001 | 13.5 (3.2) | 13.4 (3.6) | 0.41 |
| In-hospital treatment, n (%) | ||||||
| Corticosteroids | 1491 (37.7) | 460 (39.2) | 0.35 | 206 (41.9) | 196 (37.6) | 0.19 |
| Remdesivir | 66 (1.7) | 17 (1.4) | 0.60 | 7 (1.4) | 5 (1.0) | 0.70 |
| In-hospital outcomes, n (%) | ||||||
| Death within 28 days | 1245 (31.4) | 533 (45.4) | <0.001 | 232 (47.2) | 227 (43.6) | 0.17 |
| Cardiovascular events within 14 days | 674 (17.0) | 246 (21.0) | 0.002 | - | - | - |
| Ventricular fibrillation or sustained VT | 65 (1.6) | 32 (2.7) | 0.023 | 12 (2.4) | 15 (2.9) | 0.81 |
| Non-sustained VT | 43 (1.1) | 38 (3.2) | <0.001 | 12 (2.4) | 19 (3.6) | 0.35 |
| Atrial fibrillation or flutter | 404 (10.2) | 126 (19.2)‡‡ | <0.001 | 77 (17.9)|||| | 49 (13.9)## | <0.001 |
| New-onset heart failure | 123 (3.1) | 33 (5.3)§§ | <0.001 | 28 (5.8)*** | - | - |
| Myocarditis or pericarditis | 127 (3.2) | 49 (4.2) | 0.08 | 27 (5.5) | 19 (3.6) | 0.043 |
| Stroke | 37 (0.9) | 15 (1.3) | 0.39 | 6 (1.2) | 7 (1.3) | 0.99 |
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; ICU, intensive care unit; IQR = interquartile range; MRA, mineralocorticoid receptor; mSOFA, modified Sequential Organ Failure Assessment score; SD, standard deviation; VT, ventricular tachycardia
Missing data for 200 patients (3.9%)
Missing data for 286 patients (5.6%)
Missing data for 328 patients (6.4%)
Missing data for 1961 patients (38.6%)
Missing data for 1890 patients (37.0%)
Missing data for 2263 patients (44.3%)
Missing data for 332 patients (6.5%)
Missing data for 1037 patients (20.3%)
Out of 656 patients without atrial fibrillation or flutter at admission
Out of 624 patients without congestive heart failure at admission
Out of 430 patients without atrial fibrillation or flutter at admission
Out of 352 patients without atrial fibrillation or flutter at admission
Out of 468 patients without congestive heart failure at admission
Incidence of Primary and Secondary Outcomes Stratified by Pre-Existing CVD
A total of 1,778 (34.6%) patients died within 28 days of ICU admission, and 920 (17.9%) experienced a cardiovascular event within 14 days following ICU admission. Compared to patients without CVD, those with CVD had a higher incidence of 28-day mortality (45.4% vs. 31.4%; P<0.001) and CV events (21.0% vs. 17.0%; P=0.002) (Table 1). Among patients with CVD, the incidence of death was similar in patients with CAD compared to those with CHF (47.2% and 43.6%).
Associations Between CVD and Death and Cardiovascular Events
Pre-existing CVD was associated with higher odds of 28-day mortality in both unadjusted models (model 0) and models adjusted for age, sex, and race (model 1) (adjusted odds ratio [aOR]=1.28 [95%CI: 1.11–1.48]). The association was attenuated after adjusting for comorbidities (model 2) and modified SOFA score (model 3) (aOR=1.15 [95%CI: 0.98–1.34]) (Figure 1). Similarly, whereas pre-existing CVD had been associated with higher odds of CV events in unadjusted analyses, it was no longer significant in multivariable models (aOR=0.95 [95%CI: 0.79–1.14]) (Figure 1 and Table S4). Trends were similar when examining the association between CAD and CHF individually with outcomes (Figure 1 and Table S4). These associations did not differ according to treatment with corticosteroids or remdesivir (Table S5).Findings were consistent when examining the association between pre-existing CVD and a composite of in-hospital death and CV events within 14 days (Figure S2).
Figure 1. Associations between cardiovascular disease, coronary artery disease, and congestive heart failure with 28-day mortality and cardiovascular events.
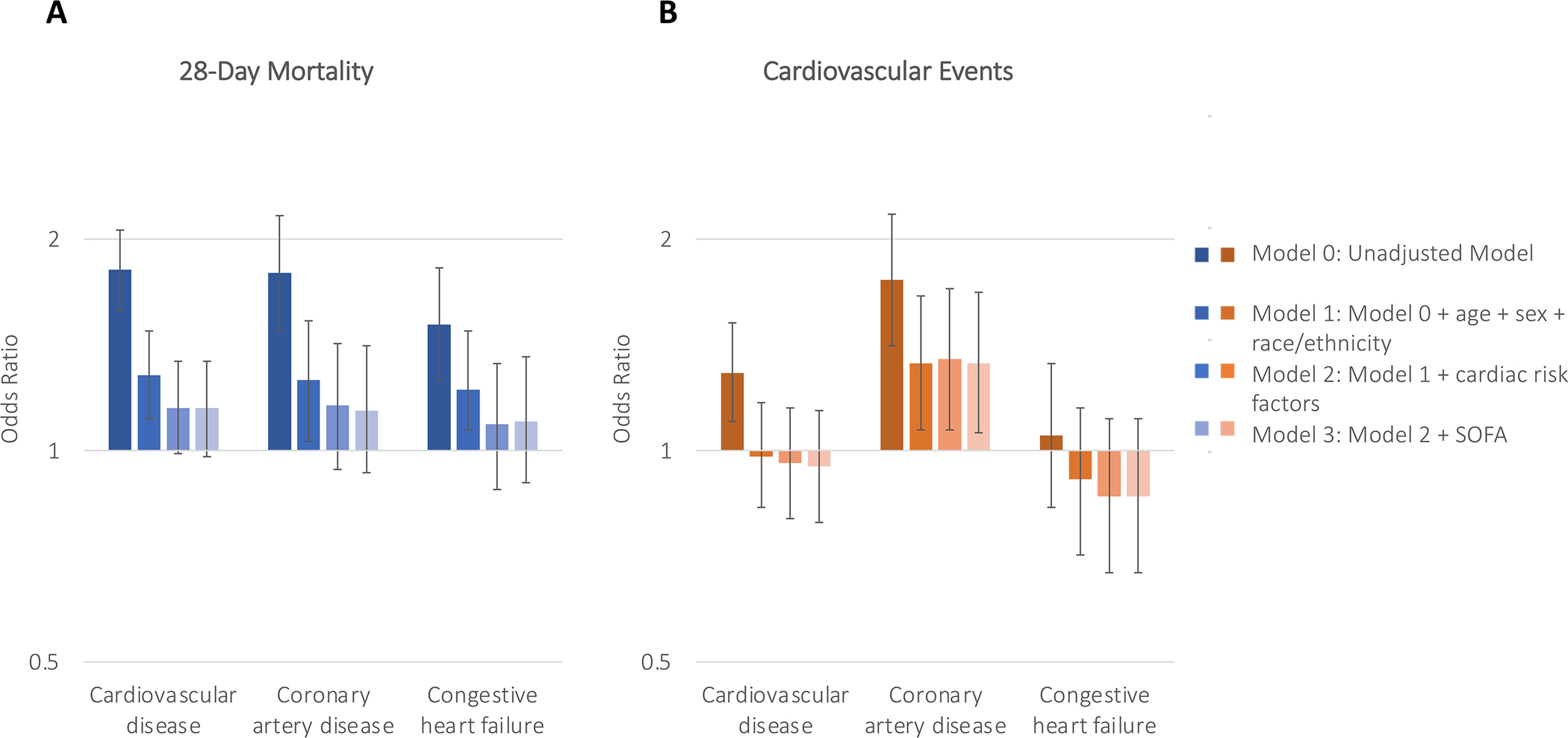
Bar graphs depicting the odds ratio and 95% confidence intervals for 28-day mortality (Panel A) and cardiovascular events (Panel B) using four different models. Model 0 was unadjusted. Model 1 was adjusted for age, race/ethnicity, and sex. Model 2 incorporated model 1 in addition to body mass index, smoking status, and history of pre-existing diabetes mellitus, hypertension, and chronic kidney disease. Model 3 included the modified Sequential Organ Failure Assessment score. Based on model 3, neither cardiovascular disease, coronary artery disease, nor congestive heart failure were associated with 28-day mortality or cardiovascular events.
Based on a random forest approach, we identified the most important variables associated with 28-day mortality as age, BMI, race/ethnicity, history of smoking, hypertension, diabetes mellitus, male sex, and pre-existing CKD, CHF, atrial fibrillation or flutter, and CAD in descending order of importance. Age, BMI, race, and history of smoking also had the highest importance scores for CV events (Figure S3). The AUC for clinical characteristics in their association with 28-day mortality and CV events was 0.69 (95% CI: 0.67–0.70) and 0.63 (95% CI: 0.62–0.65) respectively. The addition of CVD to the model had minimal impact on the AUC of mortality (ΔAUC=0.001, P=0.27) and CV events (ΔAUC=0.000, P=0.76).
Prevalence of Myocardial Injury at ICU Admission and Measures of Illness Severity
A total of 2,741 patients had at least one troponin measured within 24 hours of ICU admission. Compared to patients without troponin measured at ICU admission, patients who had troponin measured were older, had higher BMIs, and were more likely to have a history of smoking, hypertension, and chronic kidney disease as well as a higher cumulative incidence of 28-day mortality (35.8% vs 33.4%) and CV events (20.4% vs 15.1%) (Table S6). Of those with troponin levels, 1,263 (46.1%) had troponin values >URL, consistent with myocardial injury. Among patients with myocardial injury, 334 (26.4%), 211 (16.7%), 114 (9.0%), and 604 (47.8%) had troponin values 1–2x, 2–3x, 3–4x, and > 4x the URL, respectively. A total of 2,533 patients had at least two troponin measurements during hospitalization, with n=901 having levels below the URL, and n=1632 with at least 1 measure >URL.
Patients with myocardial injury were more likely to be mechanically ventilated and had higher SOFA scores, c-reactive protein, and creatinine levels on ICU admission compared to those without myocardial injury (Figure 2). Patients with CVD had a significantly higher prevalence of myocardial injury on ICU admission (66.6% vs. 39.2%; P<0.001). After adjusting for demographics and clinical characteristics, patients with pre-existing CVD had 1.67-fold higher odds (95% CI: 1.33–2.11]) of experiencing myocardial injury compared to patients without CVD (Table S7).
Figure 2. Bar graphs comparing measures of COVID-19 illness severity by myocardial injury on admission for mechanical ventilation, modified SOFA score, creatinine, and c-reactive protein.
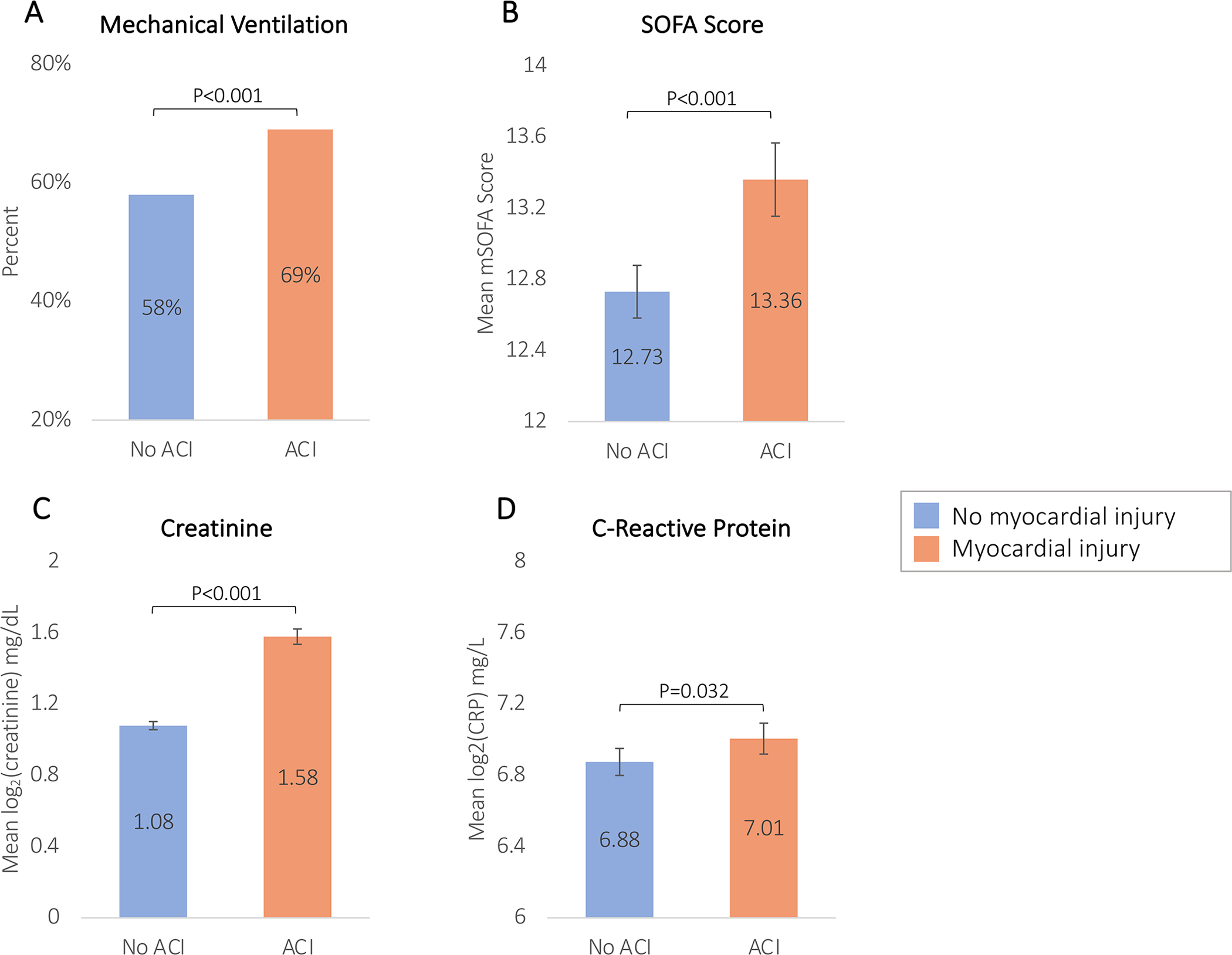
Panel A displays the proportion of patients on mechanical ventilation at ICU admission. Panels B, C, and D compare the means of modified SOFA scores, creatinine, and c-reactive protein between patients with and without myocardial injury at ICU admission. Creatinine and c-reactive protein are log2 transformed. Abbreviations: ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment.
Myocardial Injury and Death and Cardiovascular Events
Patients with myocardial injury at ICU admission compared to those without had a higher incidence of 28-day mortality (47.2% vs. 26.0%; P<0.001) and CV events (26.8% vs. 14.9%; P<0.001) (Table S8). New onset atrial fibrillation or flutter, new onset CHF, and myocarditis or pericarditis were each more common in patients with myocardial injury (Table S8).
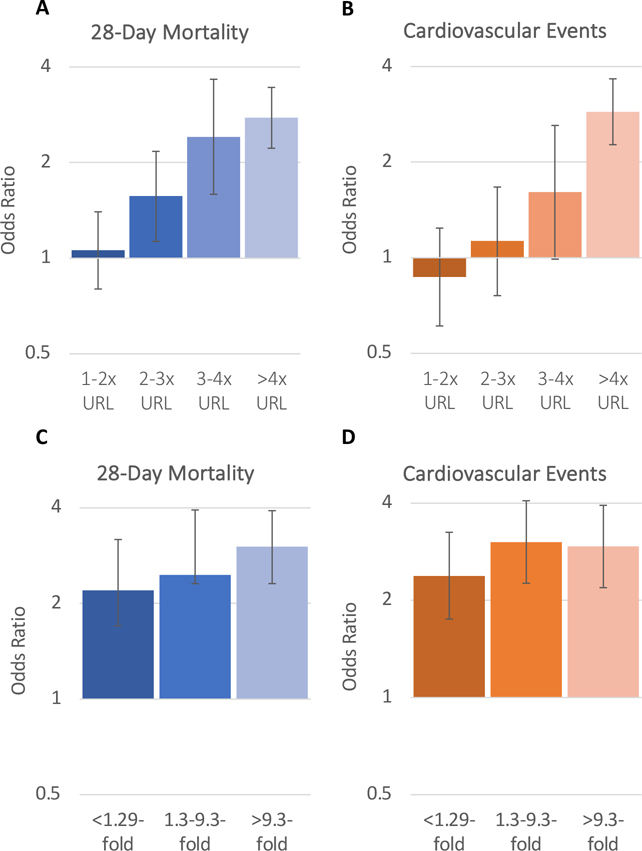
The presence of myocardial injury on admission was associated with higher odds of both 28-day mortality and CV events. In fully adjusted models, the odds of 28-day mortality and cardiovascular events was 1.93-fold (95% CI: 1.61–2.31) and 1.88-fold (95% CI: 1.53–2.32) higher, respectively, compared to those without myocardial injury on admission (Figure S4). Troponin elevation and a greater absolute change in troponin during hospitalization were monotonically associated with higher odds of 28-day mortality and CV events (Figure 3). In fully adjusted models, patients in the highest troponin elevation category (>4x URL) had a 2.77-fold (95% CI: 2.22–3.45) and 3.00-fold (95% CI: 2.35–3.81) higher odds of death and CV events compared to those without myocardial injury (Figure 3, Table S9). Likewise, patients with an absolute fold change in troponin of greater than 9.3 had a 3.02-fold (95% CI: 2.31, 3.92) and 2.94-fold (95% CI: 2.19, 3.94) higher odds of death and CV events compared to patients who did not have elevated troponin during hospitalization (Figure 3). These associations were unchanged after accounting for institution or assuming normal troponin levels in patients with missing troponin (Table S10 & Table S11). Patients with myocardial injury were also at a higher odds of new onset atrial fibrillation or flutter, new-onset heart failure, and myocarditis or pericarditis (Table S4). Consistent findings were observed when examining a composite outcome of death and cardiovascular events within 14 days (Figure S2). In sensitivity analyses, the associations between myocardial injury and death were similar in those with vs. without pre-existing CVD (P interaction=0.31), CAD (P interaction=0.67), or CHF (P interaction=0.10). The association between myocardial injury and CV events was also similar in those with vs. without pre-existing CVD (P interaction=0.11), CAD (P interaction=0.43) or CHF (P interaction=0.06) (Table S12).
Figure 3. Associations between troponin elevation on ICU admission and troponin fold change during hospitalization with 28-day mortality (A,C) and cardiovascular events (B,D).
Bar graphs depicting the odds ratios and 95% confidence intervals for 28-day mortality (Panel A) and cardiovascular events (Panel B) based on acute cardiac injury on ICU admission categorized as troponin elevation 1–2x, 2–3x, 3–4x, and > 4x the URL vs. no acute cardiac injury (reference) based on model 3. Panels C and D depict odds ratios and 95% confidence intervals based on the absolute fold change in troponin during hospitalization categorized as an absolute fold change of <1.29, 1.3–9.3%, and >9.3% compared to patients with no elevated troponin measurements during hospitalization (reference) for 28-day mortality (Panel C) and cardiovascular events (Panel D) based on model 3. Abbreviations: ICU, intensive care unit; URL, upper reference limit of normal
DISCUSSION
In this multicenter cohort study of over 5,000 critically ill adult patients hospitalized for COVID-19 in the United States, nearly one fourth of patients had pre-existing CVD. Patients with CVD had a close to 30% higher age and sex adjusted 28-day mortality compared to those without CVD. The association was heavily attenuated when accounting for comorbidities, suggesting that CV risk factors rather than CVD (defined here by the presence of CAD, HF or atrial fibrillation) are the main contributors to in-hospital outcomes in patients with severe COVID-19. Indeed, age, BMI, smoking, hypertension, and diabetes mellitus were the most important contributors to mortality. Myocardial injury at ICU admission was common, occurring in nearly half of the patients with available troponin levels, and associated with measures of illness severity. We found a monotonic association between myocardial injury with odds of death and CV events, which was not dependent on the presence of CVD. Overall, our findings support the characterization of severe COVID-19 as a pulmonary disease with multi-organ injury related to systemic inflammation. The occurrence of myocardial injury independently of the presence of CVD and its association with outcomes suggests it is a marker of the severity of the acute illness from COVID-19 rather than exacerbation of pre-existing disease.
CVD is an unsurprisingly common comorbidity among critically ill patients with COVID-19 given its relation to age and chronic inflammation. The reported prevalence of CVD in patients with COVID-19 varied widely (2.5 to 40%).5, 10–12, 14, 15, 30–33 The large variability in estimates could reflect differences in sample sizes across studies (with many having fewer than 200 patients), geographic location, definitions of CVD, and COVID-19 severity. One large cohort study of 5,700 critically and non-critically ill patients hospitalized with COVID-19 in New York City reported that 11% of patients had a history of CAD and 6.9% had CHF.5 These estimates were similar to those in our study despite the difference in severity of illness between cohorts, and suggests that CVD itself may not be a direct contributor to the severity of COVID-19 disease.
Studies reporting on the link between pre-existing CVD and COVID-19 related outcomes are conflicting.34–39 Critical illness related to COVID-19 is thought to exacerbate pre-existing CVD by altering hemodynamics and the hypercoagulable milieu.40 However, we did not find pre-existing CVD to be a major contributor to in-hospital mortality or CV events in patients with COVID-19 such as myopericarditis and arrhythmias independently of risk factors. Findings were similar when stratified between patients with CAD vs. CHF, in whom we would have expected outcomes would be worse. Circulating markers of acute inflammation were also not independently associated with pre-existing CVD. Conversely, hypertension and diabetes mellitus were much stronger predictors of mortality in COVID-19. However, findings should not be construed as implying patients with CVD are not at high risk as most have a high burden of risk factors for COVID-19 such as diabetes mellitus, hypertension, obesity, and smoking.41
Myocardial injury on ICU admission was common in this cohort, with estimates higher than those reported in prior studies of hospitalized patients with COVID-19, likely due to the current study being comprised of ICU patients only.17, 30, 42, 43 The magnitude of myocardial injury correlates with COVID-19 disease severity and has been consistently associated with adverse outcomes across studies.7, 12, 18, 42–46 In addition, a recent study found that hospitalized patients with COVID-19 who develop a ST-segment elevation myocardial infarction had higher rates of in-hospital mortality.47 We found that a greater change in troponin values during hospitalization was associated with worse outcomes. The association between myocardial injury and death and CV events did not differ between patients with and without pre-existing CVD, supporting the notion that myocardial injury and CV events in COVID-19 are related to injury from mechanisms pertaining to the acute illness, such as endothelial dysfunction and a hypercoagulable state, rather than an exacerbation of pre-existing CVD.18
Strengths and Limitations
STOP-COVID is the one of the largest and most comprehensive multicenter cohort studies of critically ill patients with COVID-19, which provided considerable statistical power and the ability to perform detailed multivariable adjustment in our analyses. Through its focus on critically ill patients, STOP-COVID allows us to identify the clinical relevance of pre-existing CVD and myocardial injury in the COVID-19 population at highest risk of death and CV events. There are several limitations to this analysis. The focus on ICU patients limits generalizability to the non-ICU COVID-19 population. Our definition of CVD was limited to the presence of CAD, CHF, or atrial fibrillation or flutter, and does not capture the full breadth of CVD. Pre-existing CVD and CV events were also determined based on documentation in the medical records rather than objective measures such as cardiac imaging or cardiac markers. Due to its observational nature, troponin levels were not measured systematically, lending a risk of selection bias. While we adjusted for demographics and clinical characteristics associated with whether a patient had troponin measured and severe outcomes, we acknowledge this will not fully account for the risk of bias. Due to different troponin assays across sites, we modeled the change in troponin during hospitalization as a relative fold-change. However, given patients with at least two troponin measurements were included in this analysis, survival bias is possible. We additionally adjusted for hospital-level characteristics, including institution, which did not change our findings. Findings regarding the association between troponin and outcomes are consistent with previous reports. Data on left ventricular ejection fraction were not available, precluding performing subgroup analyses differentiated CHF with and without left ventricular dysfunction. Additionally, because CV events were collected for only the first 14 days following ICU admission, their incidence is likely an underestimate. Based on prior data9, we assumed patients discharged alive prior to 28 days were alive at 28 days, however it is possible that a subset of patients may have died after discharge and were unaccounted for in this analysis. Lastly, these data were collected prior to the implementation of the COVID-19 vaccine, thus it is unknown how the current trajectory of the COVID-19 pandemic would influence these findings.
Conclusion
In summary, critically ill patients with COVID-19 and pre-existing CVD had higher mortality than those without CVD. However, CVD risk factors rather than CVD itself appear to be the most important contributors to outcomes. Myocardial injury was common and strongly associated with death and CV events regardless of underlying CVD status, reflecting the severity of the hyper-inflammatory phase of COVID-19. Patients with CVD should be construed as a high-risk patient group due to their burden of shared risk factors with severe COVID-19 outcomes such as hypertension, diabetes mellitus, obesity, and smoking. Studies on subpopulations with more severe underlying CVD, such as those with advanced heart failure or high-risk CAD, are warranted to further refine risk profiles in patients with COVID-19.
Supplementary Material
What is known?
COVID-19 is a hyper-inflammatory syndrome resulting in multi-organ dysfunction, including the heart.
Patients with pre-existing cardiovascular disease are perceived to be at higher risk of poor outcomes in COVID-19 based on small, single center studies.
What this study adds?
This study includes data from one the largest and most comprehensive multicenter cohort studies of critically ill patients hospitalized for COVID-19.
Cardiovascular risk factors, rather than pre-existing cardiovascular disease, were the main contributors to in-hospital in patients with severe COVID-19.
Myocardial injury was strongly associated with death and cardiovascular events regardless of a history of cardiovascular disease and likely reflected the severity of the acute illness rather than exacerbation of pre-existing disease.
Sources of Funding:
Dr. Vasbinder is supported by a National Heart, Lung, Blood Institute funded post-doctoral fellowship (T32HL007853). Dr. Leaf is supported by R01HL144566. Dr. Hayek is supported by 1R01HL153384 and the Frankel Cardiovascular Center COVID-19: Impact Research Ignitor (U-M G024231).
Non-standard Abbreviations and Acronyms
- AUC
area under curve
- BMI
body mass index
- CAD
coronary artery disease
- CV
cardiovascular
- CHF
congestive heart failure
- CKD
chronic kidney disease
- COVID-19
coronavirus disease 2019
- CVD
cardiovascular disease
- ICU
intensive care unit
- IQR
interquartile range
- SOFA
Sequential Organ Failure Assessment
- STOP-COVID
Study of the Treatment and Outcomes in Critically Ill Patients with COVID-19
- URL
upper reference limit of normal
Appendix
STOP-COVID INVESTIGATORS
Baylor College of Medicine: Carl P. Walther, Samaya J. Anumudu
Baylor University Medical Center: Justin Arunthamakun, Kathleen F. Kopecky, Gregory P. Milligan, Peter A. McCullough, Thuy-Duyen Nguyen
Beth Israel Deaconess Medical Center: Shahzad Shaefi, Megan L. Krajewski, Sidharth Shankar, Ameeka Pannu, Juan D. Valencia
Boston Medical Center: Sushrut S. Waikar, Zoe A. Kibbelaar
Cook County Health: Ambarish M. Athavale, Peter Hart, Shristi Upadhyay, Ishaan Vohra
Cooper University Health Care: Adam Green, Jean-Sebastien Rachoin, Christa A. Schorr, Lisa Shea
Duke University Medical Center: Daniel L. Edmonston, Christopher L. Mosher
Hackensack Meridian Health Mountainside Medical Center: Alexandre M. Shehata, Zaza Cohen, Valerie Allusson, Gabriela Bambrick-Santoyo, Noor ul aain Bhatti, Bijal Mehta, Aquino Williams
Hackensack Meridian Health Hackensack University Medical Center: Samantha K. Brenner, Patricia Walters, Ronaldo C. Go, Keith M. Rose
Harvard T.H. Chan School of Public Health: Miguel A. Hernán
Harvard University: Rebecca Lisk, Amy M. Zhou, Ethan C. Kim
Icahn School of Medicine at Mount Sinai: Lili Chan, Kusum S. Mathews, Steven G. Coca, Deena R. Altman, Aparna Saha, Howard Soh, Huei Hsun Wen, Sonali Bose, Emily A. Leven, Jing G. Wang, Gohar Mosoyan, Girish N. Nadkarni, Pattharawin Pattharanitima, Emily J. Gallagher
Indiana University School of Medicine/Indiana University Health: Allon N. Friedman, John Guirguis, Rajat Kapoor, Christopher Meshberger, Katherine J. Kelly
Johns Hopkins Hospital: Chirag R. Parikh, Brian T. Garibaldi, Celia P. Corona-Villalobos, Yumeng Wen, Steven Menez, Rubab F. Malik, Elena Cervantes, Samir Gautam
Kings County Hospital Center: Mary C. Mallappallil, Jie Ouyang, Sabu John, Ernie Yap, Yohannes Melaku, Ibrahim Mohamed, Siddartha Bajracharya, Isha Puri, Mariah Thaxton, Jyotsna Bhattacharya, John Wagner, Leon Boudourakis
Loma Linda University: H. Bryant Nguyen, Afshin Ahoubim
Mass General Brigham: Meghan E. Sise, Erik T. Newman, Samah Abu Omar, Kapil K. Pokharel, Shreyak Sharma, Harkarandeep Singh, Simon Correa, Tanveer Shaukat, Omer Kamal, Wei Wang, Heather Yang, Jeffery O. Boateng, Meghan Lee, Ian A. Strohbehn, Jiahua Li, Ariel L. Mueller
Mayo Clinic, Arizona: Leslie F. Thomas, Dheeraj Reddy Sirganagari
Mayo Clinic, Florida: Pramod K. Guru
Mayo Clinic, Rochester: Kianoush Kashani, Shahrzad Tehranian
Medical College of Wisconsin: Yan Zhou, Paul A. Bergl, Jesus Rodriguez, Jatan A. Shah, Mrigank S. Gupta
MedStar Georgetown University Hospital: Princy N. Kumar, Deepa G. Lazarous, Seble G. Kassaye
Montefiore Medical Center/Albert Einstein College of Medicine: Michal L. Melamed, Tanya S. Johns. Ryan Mocerino, Kalyan Prudhvi, Denzel Zhu, Rebecca V. Levy, Yorg Azzi, Molly Fisher, Milagros Yunes, Kaltrina Sedaliu, Ladan Golestaneh, Maureen Brogan, Neelja Kumar, Michael Chang, Jyotsana Thakkar
New York-Presbyterian Queens Hospital: Ritesh Raichoudhury, Akshay Athreya, Mohamed Farag
New York-Presbyterian/Weill Cornell Medical Center: Edward J. Schenck, Soo Jung Cho, Maria Plataki, Sergio L. Alvarez-Mulett, Luis G. Gomez-Escobar, Di Pan, Stefi Lee, Jamuna Krishnan, William Whalen
New York University Langone Hospital: David Charytan, Ashley Macina, Sobaata Chaudhry, Benjamin Wu, Frank Modersitzki
Northwestern Memorial Hospital: Anand Srivastava, Alexander S. Leidner, Carlos Martinez, Jacqueline M. Kruser, Richard G. Wunderink, Alexander J. Hodakowski
Ochsner Medical Center: Juan Carlos Q. Velez, Eboni G. Price-Haywood, Luis A. Matute-Trochez, Anna E. Hasty, Muner MB. Mohamed
Oregon Health and Science University Hospital: Rupali S. Avasare, David Zonies
ProMedica Health System: Roberta Redfern, Nicholas S. Cairl, Gabriel Naimy, Abeer Abu-Saif, Danyell Hall, Laura Bickley
Renown Health: Chris Rowan, Farah Madhani-Lovely
Rush University Medical Center: Vasil Peev, Jochen Reiser, John J. Byun, Andrew Vissing, Esha M. Kapania, Zoe Post, Nilam P. Patel, Joy-Marie Hermes
Rutgers/New Jersey Medical School: Anne K. Sutherland, Amee Patrawalla, Diana G. Finkel, Barbara A. Danek, Sowminya Arikapudi, Jeffrey M. Paer, Peter Cangialosi, Mark Liotta
Rutgers/Robert Wood Johnson Medical School: Jared Radbel, Jag Sunderram, Sonika Puri, Jayanth S. Vatson, Matthew T. Scharf, Ayesha Ahmed, Ilya Berim,
Stanford Healthcare: Stanford University School of Medicine – Shuchi Anand, Joseph E. Levitt, Pablo Garcia
Temple University Hospital: Suzanne M. Boyle, Rui Song, Ali Arif
Thomas Jefferson Health: Jingjing Zhang, Sang Hoon Woo, Xiaoying Deng, Goni Katz-Greenberg, Katharine Senter
Tulane Medical Center: Moh’d A. Sharshir, Vadym V. Rusnak
United Health Services Hospitals: Muhammad Imran Ali, Terri Peters, Kathy Hughes
University of Colorado Anschutz Medical Campus: Anip Bansal, Amber S. Podoll, Michel Chonchol, Sunita Sharma, Ellen L. Burnham
University Hospitals Cleveland Medical Center: Arash Rashidi, Rana Hejal
University of Alabama-Birmingham Hospital: Eric Judd, Laura Latta, Ashita Tolwani
University of California-Davis Medical Center: Timothy E. Albertson, Jason Y. Adams
University of California-Los Angeles Medical Center: Steven Y. Chang, Rebecca M. Beutler; Santa Monica-UCLA Medical Center – Carl E. Schulze
University of California-San Diego Medical Center: Etienne Macedo, Harin Rhee
University of California-San Francisco Medical Center: Kathleen D. Liu, Vasantha K. Jotwani
University of Chicago Medical Center: Jay L. Koyner
University of Florida Health-Gainesville: Chintan V. Shah
University of Florida-Health-Jacksonville: Vishal Jaikaransingh
University of Illinois Hospital and Health Sciences System: Stephanie M. Toth-Manikowski, Min J. Joo, James P. Lash
University of Kentucky Medical Center: Javier A. Neyra, Nourhan Chaaban
University Medical Center of Southern Nevada: Alfredo Iardino, Elizabeth H. Au, Jill H. Sharma
University of Miami Health System: Marie Anne Sosa, Sabrina Taldone, Gabriel Contreras, David De La Zerda, Alessia Fornoni, Hayley B. Gershengorn
University of Michigan: Rafey Feroze, Abbas Bitar, Jeff Leya, John P. Donnelly, Andrew J. Admon
University of North Carolina School of Medicine: Jennifer E. Flythe, Matthew J. Tugman, Emily H. Chang
University of Oklahoma Health Sciences Center: Brent R. Brown
University of Pennsylvania Health System: Amanda K. Leonberg-Yoo, Ryan C. Spiardi, Todd A. Miano, Meaghan S. Roche, Charles R. Vasquez
University of Pittsburgh Medical Center: Amar D. Bansal, Natalie C. Ernecoff, Sanjana Kapoor, Siddharth Verma, Huiwen Chen
University of Tennessee Health Science Center and Memphis VA Medical Center/Methodist University Hospital – Csaba P. Kovesdy, Miklos Z. Molnar, Ambreen Azhar
University of Texas Southwestern Medical Center and Parkland Health and Hospital System: S. Susan Hedayati, Mridula V. Nadamuni, Shani Shastri, Duwayne L. Willett
University of Vermont Larner College of Medicine: Samuel A.P. Short
University of Virginia Health System: Amanda D. Renaghan, Kyle B. Enfield
University of Washington Medical Center: Pavan K. Bhatraju, A. Bilal Malik
Vanderbilt University Medical Center: Matthew W. Semler
Washington University in St. Louis/Barnes Jewish Hospital: Anitha Vijayan, Christina Mariyam Joy, Tingting Li, Seth Goldberg, Patricia F. Kao
Wellforce Health System: Greg L. Schumaker, Nitender Goyal, Anthony J. Faugno, Greg L. Schumaker, Caroline M. Hsu, Asma Tariq, Leah Meyer, Ravi K. Kshirsagar, Daniel E. Weiner
Westchester Medical Center: Marta Christov, Jennifer Griffiths, Sanjeev Gupta, Aromma Kapoor
Yale School of Medicine: Perry Wilson, Tanima Arora, Ugochukwu Ugwuowo
Footnotes
Disclosures: The authors have no conflicts of interests to disclose.
References
- 1.John Hopkins University Coronavirus Resource Center. COVID-19 United states cases by county by the Center for Systems Science and Engineering (CSSE) at Johns Hopskins Univeristy. Available at https://coronavirus.jhu.edu/us-map. Accessed July 14, 2022. [Google Scholar]
- 2.Macedo A, Gonçalves N, Febra C. Covid-19 fatality rates in hospitalized patients: Systematic review and meta-analysis. Ann Epidemiol. 2021;57:14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (covid-19) outbreak in china: Summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239–1242 [DOI] [PubMed] [Google Scholar]
- 4.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (covid-19): A review. JAMA. 2020;324:782–793 [DOI] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, and the Northwell C-RC. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with covid-19 in the new york city area. JAMA. 2020;323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in covid-19. Am J Emerg Med. 2020;38:1504–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bansal M Cardiovascular disease and covid-19. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14:247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakinala RC, Shah CD, Rakholiya JH, Martin M, Kaur N, Singh H, Okafor TL, Nwodika C, Raval P, Yousuf S, et al. Covid-19 outcomes amongst patients with pre-existing cardiovascular disease and hypertension. Cureus. 2021;13:e13420–e13420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, Brenner SK, Leonberg-Yoo A, Schenck EJ, Radbel J, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the us. JAMA Internal Medicine. 2020;180:1436–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and outcomes of 21 critically ill patients with covid-19 in washington state. JAMA. 2020;323:1612–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in wuhan, china: A descriptive study. Lancet. 2020;395:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (covid-19). JAMA Cardiology. 2020;5:811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu K, Fang Y-Y, Deng Y, Liu W, Wang M-F, Ma J-P, Xiao W, Wang Y-N, Zhong M-H, Li C-H, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in hubei province. Chinese Medical Journal. 2020;133:1025–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in wuhan, china. JAMA. 2020;323:1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with covid-19 in wuhan, china: A retrospective cohort study. The Lancet. 2020;395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badawi A, Vasileva D. Comparative profile for covid-19 cases from china and north america: Clinical symptoms, comorbidities and disease biomarkers. World J Clin Cases. 2021;9:118–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Q, Xu L, Dai Y, Ling Y, Mao J, Qian J, Zhu W, Di W, Ge JA-O. Cardiovascular manifestations in severe and critical patients with covid-19. Clinical Cardiology. 2020;43:796–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, et al. Association of cardiac injury with mortality in hospitalized patients with covid-19 in wuhan, china. JAMA Cardiology. 2020;5:802–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Samkari H, Gupta S, Leaf RK, Wang W, Rosovsky RP, Brenner SK, Hayek SS, Berlin H, Kapoor R, Shaefi S, et al. Thrombosis, bleeding, and the observational effect of early therapeutic anticoagulation on survival in critically ill patients with covid-19. Ann Intern Med. 2021;174:622–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flythe JE, Assimon MM, Tugman MJ, Chang EH, Gupta S, Shah J, Sosa MA, Renaghan AD, Melamed ML, Wilson FP, et al. Characteristics and outcomes of individuals with pre-existing kidney disease and covid-19 admitted to intensive care units in the united states. Am J Kidney Dis. 2021;77:190–203.e191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, Brenner SK, Leonberg-Yoo A, Schenck EJ, Radbel J, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with covid-19. JAMA Internal Medicine. 2021;181:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayek SS, Brenner SK, Azam TU, Shadid HR, Anderson E, Berlin H, Pan M, Meloche C, Feroz R, O’Hayer P, et al. In-hospital cardiac arrest in critically ill patients with covid-19: Multicenter cohort study. BMJ. 2020;371:m3513–m3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathews KS, Soh H, Shaefi S, Wang W, Bose S, Coca S, Gupta S, Hayek SS, Srivastava A, Brenner SK, et al. Prone positioning and survival in mechanically ventilated patients with coronavirus disease 2019-related respiratory failure. Crit Care Med. 2021;49:1026–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molnar MZ, Bhalla A, Azhar A, Tsujita M, Talwar M, Balaraman V, Sodhi A, Kadaria D, Eason JD, Hayek SS, et al. Outcomes of critically ill solid organ transplant patients with covid-19 in the united states. Am J Transplant. 2020;20:3061–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaefi S, Brenner SK, Gupta S, O’Gara BP, Krajewski ML, Charytan DM, Chaudhry S, Mirza SH, Peev V, Anderson M, et al. Extracorporeal membrane oxygenation in patients with severe respiratory failure from covid-19. Intensive care medicine. 2021;47:208–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Short SAP, Gupta S, Brenner SK, Hayek SS, Srivastava A, Shaefi S, Singh H, Wu B, Bagchi A, Al-Samkari H, et al. D-dimer and death in critically ill patients with coronavirus disease 2019. Crit Care Med. 2021;49:e500–e511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasquez CR, Gupta S, Miano TA, Roche M, Hsu J, Yang W, Holena DN, Reilly JP, Schrauben SJ, Leaf DE, et al. Identification of distinct clinical subphenotypes in critically ill patients with covid-19. Chest. 2021:S0012–3692(0021)00874–00876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The sofa (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Medicine. 1996;22:707–710 [DOI] [PubMed] [Google Scholar]
- 29.Strobl C, Boulesteix A-L, Zeileis A, Hothorn T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinformatics. 2007;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, china. The Lancet. 2020;395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X Covid-19 and the cardiovascular system. Nature Reviews Cardiology. 2020;17:259–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR, Nahid M, Ringel JB, et al. Clinical characteristics of covid-19 in new york city. New England Journal of Medicine. 2020;382:2372–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x, Liu L, Shan H, Lei C-l, Hui DSC, et al. Clinical characteristics of coronavirus disease 2019 in china. New England Journal of Medicine. 2020;382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Gallagher K, Shek A, Bean DM, Bendayan R, Papachristidis A, Teo JTH, Dobson RJB, Shah AM, Zakeri R. Pre-existing cardiovascular disease rather than cardiovascular risk factors drives mortality in covid-19. BMC Cardiovascular Disorders. 2021;21:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rastad H, Karim H, Ejtahed H-S, Tajbakhsh R, Noorisepehr M, Babaei M, Azimzadeh M, Soleimani A, Inanloo SH, Shafiabadi Hassani N, et al. Risk and predictors of in-hospital mortality from covid-19 in patients with diabetes and cardiovascular disease. Diabetology & Metabolic Syndrome. 2020;12:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Guo T, Dong D, Zhang X, Chen X, Feng Y, Wei B, Zhang W, Zhao M, Wan J. Defining heart disease risk for death in covid-19 infection. QJM. 2020;113:876–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in covid-19. New England Journal of Medicine. 2020;382:e102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Pranata R, Huang I, Lim MA, Wahjoepramono EJ, July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of covid-19-systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis. 2020;29:104949–104949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh AK, Gillies CL, Singh R, Singh A, Chudasama Y, Coles B, Seidu S, Zaccardi F, Davies MJ, Khunti K. Prevalence of co-morbidities and their association with mortality in patients with covid-19: A systematic review and meta-analysis. Diabetes, Obesity and Metabolism. 2020;22:1915–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung MK, Zidar DA, Bristow MR, Cameron SJ, Chan T, Harding CV, Kwon DH, Singh T, Tilton JC, Tsai EJ, et al. Covid-19 and cardiovascular disease. Circulation Research. 2021;128:1214–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, Brenner SK, Leonberg-Yoo A, Schenck EJ, Radbel J, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the us. JAMA Intern Med. 2020;180:1436–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Pan X, Li Y, An N, Xing Y, Yang F, Tian L, Sun J, Gao Y, Shang H, et al. Cardiac injury associated with severe disease or icu admission and death in hospitalized patients with covid-19: A meta-analysis and systematic review. Critical Care. 2020;24:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smilowitz NR, Jethani N, Chen J, Aphinyanaphongs Y, Zhang R, Dogra S, Alviar CL, Keller N, Razzouk L, Quinones-Camacho A, et al. Myocardial injury in adults hospitalized with covid-19. Circulation. 2020;142:2393–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayek SS, Brenner SK, Azam TU, Shadid HR, Anderson E, Berlin H, Pan M, Meloche C, Feroz R, O’Hayer P, et al. In-hospital cardiac arrest in critically ill patients with covid-19: Multicenter cohort study. BMJ. 2020;371:m3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. European Heart Journal. 2020;41:2070–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mengozzi A, Georgiopoulos G, Falcone M, Tiseo G, Pugliese NR, Dimopoulos MA, Ghiadoni L, Barbieri G, Forfori F, Carrozzi L, et al. The relationship between cardiac injury, inflammation and coagulation in predicting covid-19 outcome. Scientific Reports. 2021;11:6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saad M, Kennedy KF, Imran H, Louis DW, Shippey E, Poppas A, Wood KE, Abbott JD, Aronow HD. Association between covid-19 diagnosis and in-hospital mortality in patients hospitalized with st-segment elevation myocardial infarction. JAMA. 2021;326:1940–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to restrictions on patient privacy and data sharing, data from STOP-COVID is not available for purposes of reproducing the results or replicating the procedure. Syntax and output files of statistical analyses can be made available upon reasonable request by contacting the corresponding author.