Abstract
The brain changes of Alzheimer’s disease and other degenerative dementias begin long before cognitive dysfunction develops, and in people with subtle cognitive complaints, clinicians often struggle to predict who will develop dementia. The public increasingly sees benefits to accessing dementia risk evidence (DRE) such as biomarkers, predictive algorithms, and genetic information, particularly as this information moves from research to demonstrated usefulness in guiding diagnosis and clinical management. For example, the knowledge that one has high levels of amyloid in the brain may lead one to seek amyloid reducing medications, plan for disability, or engage in health promoting behaviors to fight cognitive decline. Researchers often hesitate to share DRE data, either because they are insufficiently validated or reliable for use in individuals, or there are concerns about assuring responsible use and ensuring adequate understanding of potential problems when one’s biomarker status is known. Concerns include warning people receiving DRE about situations in which they might be compelled to disclose their risk status potentially leading to discrimination or stigma. The Advisory Group on Risk Evidence Education for Dementia (AGREEDementia) welcomes all concerned with how best to share and use DRE. Supporting understanding in clinicians, stakeholders, and people with or at risk for dementia and clearly delineating risks, benefits, and gaps in knowledge is vital. This brief overview describes elements that made this group effective as a model for other health conditions where there is interest in unfettered collaboration to discuss diagnostic uncertainty and the appropriate use and communication of health-related risk information.
Keywords: Alzheimer’s disease, amyloid, biomarkers, dementia, genetics
THE IMPORTANCE OF CONSULTATION: AGREEDementia
As various forms of dementia risk evidence (DRE), such as biomarkers for brain amyloid, demonstrate clinical utility and transition beyond research use only, the public increasingly wants access to their personal DRE. Participants in dementia research want their test results [1]. When researchers and clinicians hesitate in responding to requests for this personal information, requestors feel frustrated and patronized by barriers designed to protect them from potential harm. A person potentially facing a progressive degenerative disease may feel time pressure to know if they have or will develop dementia. Informed, autonomous decision making around appropriate access to DRE in people with or at risk for dementia is vital and depends on promoting understanding in all, including patients, research participants, and clinicians, by clearly delineating evidence of benefits, risks, and gaps in knowledge. In contrast to open discussion and consultation around best practices, publications are slower and content is often filtered by fear of misstatement and subjective judgments of whether questions are worthy of being published. To address the need for agile, informed, and responsive communication between all users of DRE, the Advisory Group on Risk Evidence Education for Dementia (AGREEDementia.org) was convened initially by Nina Silverberg of the National Institute on Aging (NIA) and originally was comprised largely of members of NIA supported Alzheimer’s Disease Centers. Over time the group evolved into an independent body that sought to be widely inclusive and to promote open dialogue. Such inclusiveness has been demonstrated by the attraction of interested centers from Brazil, Germany, and the United Kingdom. The aims are 1) to evaluate the current evidence on putative predictors of dementia risk, 2) to guide whether, when, and how to disclose DRE, and 3) to consult to support ethical and appropriate use of and communication about biomarkers and other dementia risk-related information. Here we describe elements that support the group’s effectiveness as a guide to future working groups with similar aims.
GROUP COMPOSITION
As of the beginning of 2022 there were 122 members, although the level of activity of individual people has varied. The majority (82%) of people were from academia, broadly including clinical researchers, students, research staff, genetic counselors, and healthcare lawyers. The remaining 18% were people living with or at risk for dementia, care partners, United States government staff (National Institutes of Health, Food and Drug Administration) or were representatives from advocacy groups (e.g., the Lewy Body Dementia Association, the Alzheimer’s Association, the Association for Frontotemporal Degeneration, and Cohen Veterans Bioscience).
A strength of the group is that it draws individuals from vastly different disciplines and perspectives with a universal interest in how DRE is developed and communicated. AGREEDementia has become a forum for early career investigators and those new to Alzheimer’s Disease and Related Disorders work to seek mentorship and networking in addition to specific resources. Participants learn about AGREEDementia through a variety of means. The bulk of members came initially from the US NIA funded Alzheimer’s centers. Members also come from the Alzheimer’s Association International Conference meetings and workgroups. Members tell their students, staff, and colleagues. The Journal of Alzheimer’s Disease also publicizes the organization to its editors and authors. People also find us through our website (AGREEDementia.org). To be maximally sensitive to novel issues, membership is open and inclusive. Informal conversations among group members enable people to quickly and informally ask questions of colleagues with diverse expertise when their own institution or role limits their access to wide, public, consultation. For example, research staff often have few interactions outside of their site, yet one discussant performing data entry identified an implicit privacy risk in their rare genetic cohort and that observation informed national practices. Specifically, the global unique identifier (GUID) enables the US National Institutes of Health (NIH) to link individuals across studies; however, this identifier also poses a risk to privacy as the detail accumulated on individual participants increases. This discussant raised concern that identifying the researcher/creator of a GUID implicitly identifies their participants as having rare genetic mutations, which could increase the risk of identifying those participants. Still more troubling is that the participants have a highly penetrant gene for dementia and a breach of privacy could have devastating consequences on those participants and their families.
GROUP FORMAT
One critical aspect that adds value to the group is the hybrid structure of a meeting that everyone attends (hereafter referred to as the All Hands meeting), and smaller working subgroups that enable discussion and development of work products. In the first half of the All Hands meeting, members hear announcements and identify key concerns of general interest, as well as updates from each of the working groups. Having a time when all members hear these updates enables them to collaborate with other subgroups if there is a potential synergy. In the second half of the All Hands meeting, a speaker presents relevant topics. These presentations ensure that the members have a shared foundational knowledge, thus making advice and guidance more informed. Where possible, lecturers from other fields that addressed similar problems are recruited, for example pharmacogenetics of depression [2] or genetics of movement disorders [3]. Most workgroup meetings are open to all members; however, there have been some exceptions when workgroups requested privacy to develop ideas or voice concerns. For example, a group dedicated to enhancing cultural sensitivity of research staff and outreach workers requested privacy from their supervisors. In sum, with these few exceptions, the group is structured so that all members can attend all monthly groups including 1) All Hands where members can address the entire group and 2) their home working group where they are responsible for a project. All Hands meetings and minutes update members on all workgroup activities so that members can also attend or join any other additional workgroups as much or as little as they wish.
Having both an internal workspace and an externally facing website has enhanced operations. Initially, potential new members felt overwhelmed and confused by the multiple meetings and we (typically AR) needed to meet with each new potential member individually to explain that there were two types of meetings involved in participation: the All Hands meeting and a workgroup of their choosing. Integration of new members improved with a public website that explains the group structure (All Hands, Workgroups) and the aims of the different workgroups they can choose to join. Informal collaboration has been facilitated with a separate, online, private workspace for group members. This space enables sharing of articles, contact information, personal skills and knowledge, and drafts, without fear that work products under development will be misused.
RATIONALE UNDERLYING THE STRUCTURE OF THE SUBGROUPS
Working groups enable more free and in-depth discussion and the structure of the six working subgroups powerfully supports the mission of AGREEDementia. Figure 1 lists the working groups and broad general missions. To facilitate group interactions, the schedule for all meetings are listed in the All Hands agenda. This information enables members to attend meetings of a subgroup based on something they heard at the All Hands call.
Fig. 1.
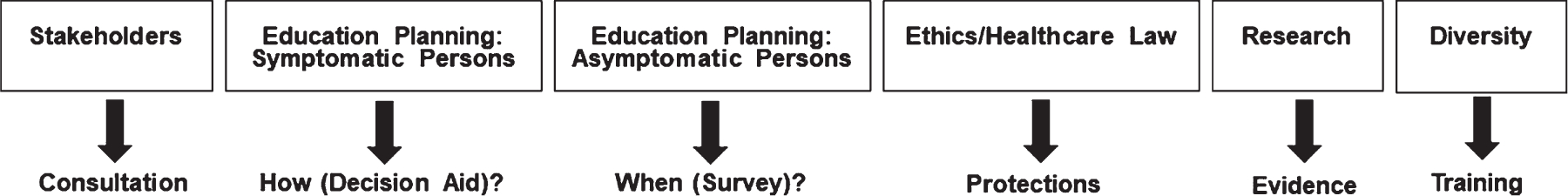
Subgroup structure and missions.
Stakeholders
This group consists of people living with dementia, their care partners, research participants, individuals with elevated risk for dementia (based on genetic or Alzheimer’s disease (AD) biomarkers), and members from advocacy organizations for people with dementia and care partners. One research participant and co-chair of the group has authored a book on the impact of learning APOE ε4 carrier status [4]. The group was formed with the primary purpose of providing feedback on AGREEdementia priorities, focus, and on the deliverables generated by the larger group and subgroups. Members of the Stakeholder group also participate in standing meetings and discussions of subgroups of AGREEDementia, ensuring ongoing integration of their feedback into the work and priority setting of the groups. Multiple rounds of stakeholder input was provided for both the survey on disclosure used for US NIA supported Alzheimer’s Disease Research Centers [5] and the Amyloid imaging decision aid developed for individuals with mild cognitive impairment (MCI) or AD dementia [6]. Following advances in blood-based biomarkers, the stakeholders have urged decision aids to be developed for these new emerging tests, which are now under development. The stakeholders also have piloted a program for engaging general audiences in scientific conferences, which included small group discussions to accommodate interactions with researchers and led to better understanding of scientific advances [7]. The Stakeholder group is developing a Bill of Rights for Disclosure of Research Results to Participants, which advocates preference of participants to be considered in research study design and practice of disclosing clinically meaningful results. A glossary of relevant scientific terms rephrased for non-specialists is provided to improve understanding has also been provided on the AGREEDementia website (https://www.AGREEDementia.org/Education).
Education planning: People with symptoms
One critical distinction impacting how DRE is used is whether or not the stakeholders have symptoms of dementia. Hence, these workgroups are separated. In general, for people living with symptoms of dementia, when DRE is communicated it is integrated with differential diagnosis (e.g., biomarkers may be used to determine the etiology of cognitive impairment) and care management. There may also be evidence of disability, and diagnosis may guide treatment and/or planning for future decline. Furthermore, in people with symptoms, DRE is typically more predictive of progression than in asymptomatic individuals. The focus of this subgroup is thus more often to provide guidance about how to communicate DRE when the DRE is used appropriately, and hence this group has studied [8] and created decision tools to assist people in deciding whether or not to learn their personal DRE, such as amyloid PET scan results. This group discusses in this forum the appropriate uses of a blood amyloid measure [9]. Additional relevant tools are/will be housed on NIA’s Alzheimer’s & Dementia Outreach, Recruitment & Engagement Resources (ADORE) [10].
Education planning: People who are asymptomatic
In contrast, for people who are asymptomatic, an indicator of an elevated risk of dementia may be less predictive than for symptomatic individuals. Hence, sharing DRE is rarely part of routine clinical care, and much of the discussion focused on communication is in research settings. In this setting, discussions around the benefits and risks of sharing DRE should include warnings around the risk of stigma and discrimination since there are circumstances when the stakeholder may be compelled to disclose their status [11]. The focus of this group is more typically whether to communicate DRE, how to communicate the uncertainty around risk, and how to explain the disadvantage of knowing this information. Empirical approaches to studying opinions are helpful, for example there are Delphi approaches of experts that can have gradations of the strength of consensus, e.g., [12]. This group produced a survey of the US NIA-funded Alzheimer’s Disease Research Centers in communicating risk information [5]. The group also produced a scenario-driven discussion of communication of APOE ε4 associated risk for primary care practitioners to address the growing number of individuals who know their APOE genotype from research studies, cardiology risk assessments, and direct to consumer genetic testing [13]. Clinical trials for people without symptoms in which they would learn their DRE (e.g., A4 Study, NCT02008357) typically explain the implications of being biomarker positive or at genetic risk. For example, being amyloid positive and asymptomatic does not mean there is a certain progression to dementia. With the advent of approval of aducanumab (and subsequent other) therapy by the US Food and Drug Administration to reduce amyloid, discussions regarding the availability of treatments need to change in clinical trials of this population to warn about off label use in the setting of unclear risk of progression [14].
Research evidence
Ultimately the strength of evidence for the clinical utility of DRE, such as for the prediction of dementia, is important for guiding what is shared, and hence there is a dedicated subgroup to evaluate the studies and evidence. This Research/Analytic subgroup focuses on types of DRE that are used in research, and potentially may be included in clinical settings at some point in the future (genetics, neuroimaging, cerebrospinal fluid biomarkers, and plasma biomarkers). Early discussions focused on difficulties with the precision and interpretation of APOE results, with APOE representing the susceptibility gene with the best current predictive value for AD dementia because of large sample sizes, low measurement error, and multiple studies to provide replication. Many concerns with sharing APOE ε4 carrier status are common to other DRE and include limitations in generalizing evidence collected in research studies to different settings. Specifically, many studies of the predictive value of these biomarkers have been conducted in small, largely white, highly educated, urban samples, and participants with a family history of AD and/or memory concerns are often overrepresented. For most biomarkers, the sample sizes are too small to provide accurate individual risk estimates, or to reasonably consider more than one biomarker at a time in analysis. Examples of other important studies that are highly relevant to the other workgroups include the impact of positive amyloid PET findings on clinical diagnosis [15, 16]; studies validating biomarkers against postmortem brains [17], and analyses of discrepant findings between different biomarkers.
Ethics and healthcare law
The landscape of ethical concerns in communicating dementia diagnosis are well summarized elsewhere [18]; however, here are some issues identified. With respect to group dynamics, supporting stakeholder autonomy and choice including respect for wishes to know or not know DRE is important in addressing disagreements around best practices, particularly in balancing individual differences in personal decisions surrounding beneficence and non-maleficence. Specifically, supporting knowledge of risks, protections, and benefits of knowing and using DRE and how they can be balanced has been an important theme. Several members in AGREEDementia have studied the risk of psychological distress [19] and stigma [20] in learning of increased risk for dementia. Building education around the risks/benefits and supporting choice in direct to consumer (DTC) testing for APOE genotype (https://genetestornot.org/) has served as a wonderful model for supporting autonomy in a situation where the benefit of DRE discovery is questionable [21, 22]. Zallen often finds stakeholders seek direct to consumer testing to avoid data entering the medical record, raising questions about its role in healthcare. Other risks that were addressed included privacy concerns and the need for software protections to avoid people from being uniquely identified based on their genetics or biomarkers such as by using their magnetic resonance image (MRI) [23]. Examples of benefits of using DRE involve choosing therapies more effectively with pharmacogenetics [24, 25], and well-designed, trial-supporting patient registries that enable selection of cohorts based on biomarker status [26], European Prevention of Alzheimer’s Dementia (EPAD), [27], Brain Health Registry). Trials need protections as well. An important need is to address distress when biomarker-based clinical trials are terminated [28]. Protections for research in participants with diminished capacity for decision making also include identifying a study partner or decision tool to identify difficulty with fully informed consent, e.g., [29, 30].
Diversity
Issues of justice are critically important for this workgroup since social determinants of health and disparities are related to biomarkers [31]. Studies that include diverse cohorts are critical for understanding how best to use DRE (e.g., [32]). Whereas initially this group focused on education and mentorship of trainees and young investigators, it became clear that an urgent weakness was training research staff in sensitive outreach to diverse cohorts. This group is developing a publicly available lecture series designed to better train coordinators about issues of cultural sensitivity and diversity, such that we may better enroll and retain underrepresented or underserved communities in clinical research.
NEXT STEPS TOWARD IMPLEMENTATION
Discussing best practices and supporting two way communication
The science in this field is moving quickly and education around a broad framework of general principles of appropriate use and effective care will support more rapid understanding and communication. Education must stress that clinically valid conclusions from DRE typically must be interpreted in a larger context of a comprehensive evaluation, information often absent in research projects or direct to consumer products. Treatable contributors to cognitive dysfunction must not be dismissed by attributing all dysfunction to AD dementia on the basis of amyloid brain positivity or other DRE. It is unclear how to explain these interpretive limitations of DRE to the public to foster understanding and a sense of feeling respected. Groups with inclusive membership can support effective two-way communication to shape messages and that reach larger communities.
Immediate and sustained impact
A positive impact would mean that stakeholders and all who care for people with or at risk for dementia would feel comfortable with decisions and methods of communicating and using DRE. The impact of the group on the field will depend in part on what it produces in collaborative papers, products (e.g., webinars, decision tools) and the degree to which members give credit and acknowledge its contributions to their accomplishments. One urgent need is increasing involvement of historically underrepresented groups in research and policy. At present we informally estimate only about 5% of the AGREEDementia members to be from underrepresented groups and we are working to increase this representation. Because the group is relatively new and completely volunteer based, decisions around leadership fall on whoever is willing to work (e.g., set agendas, lead meetings, organize speakers) and the burden is minimal relative to the opportunities made possible for collaborations. Aside from a small contribution from the Stanford Alzheimer’s Disease Center to support the external website, there are minimal fiscal needs. Collaborations with the NIA publicize valuable resources. If there were a decision to seek financial support to grow efforts, there are various alternatives. One model of financial support could be through grants, another model of support could be through requesting paid membership, and an alternative approach is through professional organizations that provide infrastructure through paid membership such as Alzheimer’s Association International Society to Advance Alzheimer’s Research and Treatment (ISTAART).
Outreach to promote understanding
Broad outreach beyond research centers supports generalizability to clinics and this issue is vital. Most of what is known about people’s reactions to learning about DRE comes from research in carefully selected cohorts and much needs to be learned about how best to integrate with diverse patients in clinics. For example, patients may have comorbid mental health issues that may exacerbate their distress. Whereas more extensively discussed elsewhere in this issue [9], interpreting and using biofluid based biomarker data, particularly with comorbid conditions, is often not straightforward [33]. Many studies have few non-whites and there are differences across groups in biomarker levels and genetics (e.g., [34–36]). Some economically disadvantaged populations are also at disproportionately elevated vascular risk [37, 38] likely in part due to social determinants of health [31]. These issues have implications for how DRE is used and explained.
Understanding limitations of DRE
A careful understanding of measures and relationships between measures is needed. For example, Schindler suggests that brain amyloid levels in cognitively normal individuals are correlated with the age at dementia onset, but because amyloid accumulation plateaus around the time of symptom onset, amyloid levels are not well correlated with dementia severity [34]. Blood biomarkers offer tremendous opportunities but issues related to implementation are critical achieving that potential [32].
Biomarkers, genetics, therapy, and future clinical practice
Aducanumab demonstrated how therapeutic innovations involving biomarkers and genetics introduce new ethical complexities. Whereas sharing APOE ε4 carrier status with patients has traditionally been discouraged because it only has a slight to moderate effect on dementia risk, APOE ε4 carrier status has additional implications in the content of potential treatment with aducanumab. APOE ε4 carriers have elevated risk for complications from aducanumab, including amyloid-related imaging abnormalities (ARIA) with edema or hemorrhage. Therefore, disclosure of APOE ε4 carrier status to individuals considering treatment with aducanumab may be reasonable because it affects the risks of treatment. Because of these risks, the absence of meaningful clinical improvement, and the expense of the treatment, some clinicians question whether the treatment should be offered [39]. Additionally, although the degree of amyloid positivity may be available in some situations, amyloid status is typically disclosed as either positive or negative. The threshold at which amyloid levels are associated with cognitive impairment may vary depending on factors that may affect resilience or vulnerability to disease, and some groups under-represented in AD research may have significant differences in biomarker levels [40, 41]. Therefore, it is possible that applying a single cut-off for amyloid positivity to all individuals may lead to unjust barriers to therapies [42], although it is not yet clear how to interpret biomarker levels so that they provide appropriate results for all individuals. Using biomarker tests that perform accurately and consistently across diverse groups is critical to healthcare equity [43]. AGREEDementia and other similar open discussion groups can support patients make more informed decisions.
Summary
Effective use of DRE can transform dementia diagnosis and care; however, defining responsible practice and respectful use is complex. Supporting effective collaboration between stakeholders and the community developing and supporting use of DRE requires education around the nuances of interpreting these results while the science progresses rapidly. It requires collaboration among people with diverse expertise such as researchers, ethicists, legal scholars, educators, clinicians, and people living with and at risk for dementia to provide education. AGREEDementia represents an open, welcoming, group that bridges the gap in communication between small, academic, working groups and the general public who struggle to have their voices heard. The group’s hybrid structure in which there is one overall meeting that broadcasts opportunities for synergy and education (All Hands meetings), and smaller, accomplishment-focused, working groups, is effective but difficult to understand without an explanatory, public website. The public website also broadcasts accomplishments and external, educational opportunities while an internal website supports collaboration. Ultimately, ongoing collaboration will be supported through mutual respect for the deeply personal needs of individuals struggling with the risk and effects of dementia.
ACKNOWLEDGMENTS
This research was supported in part by the Stanford Alzheimer’s Disease Research Center (P30AG 066515) and the War Related Illness and Injury Study Center. Multiple other grants from NIA support other authors and their centers including R01AG068206, P30AG066509, P30AG062421, P30AG072931, K0 1AG057796, U24AG041689 and R56AG069130. FFO receives support from The State of São Paulo Research Foundation (FAPESP), grant #2015/10109-5. ACR, JAL and LT receive support from the Office of Research and Development of the Department of Veterans Affairs (VISN 21 MIRECC, RX003152).
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs, the National Institute on Aging (NIA), or any of the organizations of which they are members or from which they receive support. In addition to the authors listed, Nina Silverberg, Grayson Donley, Jessica Harper of NIA contributed intellectually and administratively. Robyn Shapiro has provided legal consultation. Rebecca Edelmayer and other members of the Alzheimer’s Association and Angela Taylor of the Lewy Body Dementia Association supported continued engagement and outreach to new members.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-0458r1).
None of the authors identified relevant COI but most received federal funding, most from the NIA but some from other NIH institutes (NINDS, NIMH) and some from the Office of Research and Development of the Department of Veterans Affairs including the Mental Illness Research Education and Clinical Center (MIRECC). These authors include ACR, DB, NAC, LC, SSD, JG, CEG, JAL, MM, RLN, MWP, ARF, AR, MR, DS, CGS, LT, JT, EMW, SW, LSW, DTZ. In addition, some authors receive funding from the Alzheimer’s Association (JJA, JPC, JDG, NTA). JWA is an unpaid consultant of Memtrax. JPC also receives funding from the Doris Duke Charitable Foundation and serves on a medical advisory board for Humana Healthcare. JLH receives funding from Eisai/Biogen, Lilly, and Biohaven. JDG received support from BrightFocus Foundation, Eli Lilly, Genentech, Biogen, Eisa and also consults with SiteRx. JHL consulted with Genentech and Biogen. FFO received support from FAPESP – The State of São Paulo Research Foundation. SES has analyzed data provided at no cost by C2N Diagnostics to Washington University, but she has not received any personal compensation from any for-profit organizations.
REFERENCES
- [1].Robillard JM, Feng TL (2017) When patient engagement and research ethics collide: Lessons from a dementia forum. J Alzheimers Dis 59, 1–10. [DOI] [PubMed] [Google Scholar]
- [2].Oslin DW, Chapman S, Duvall SL, Gelernter J, Ingram EP, Kranzler HR, Lehmann LS, Lynch JA, Lynch KG, Pyne JM (2021) Study design and implementation of the PRecision Medicine In MEntal health Care (PRIME Care) Trial. Contemp Clin Trials 101, 106247. [DOI] [PubMed] [Google Scholar]
- [3].Alcalay RN, Kehoe C, Shorr E, Battista R, Hall A, Simuni T, Marder K, Wills AM, Naito A, Beck JC, Schwarzschild MA, Nance M (2020) Genetic testing for Parkinson disease: Current practice,knowledge, and attitudes among US and Canadian movement disordersspecialists. Genet Med 22, 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tyrone JT, Sabbagh MN (2019) Fighting for My Life: How to Thrive in the Shadow of Alzheimer’s, Thomas Nelson. [Google Scholar]
- [5].Roberts JS, Ferber R, Blacker D, Rumbaugh M, Grill JD; Advisory Group on Risk Evidence Education for Dementia (AGREED) (2021) Disclosure of individual research results at federally funded Alzheimer’s Disease Research Centers. Alzheimers Dement (N Y) 7, e12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].AGREEDementia PET Amyloid Decision Tool. https://cf0a52d991.clvaw-cdnwnd.com/9e9d33a0baaf7e6f666a2136d82e2828/200000096-0976509768/MCI-and-Brain-Amyloid-Imaging-Decision-Aid-5.pdf?ph=cf0a52d991
- [7].Walter S, Wheaton B, Huling Hummel C, Tyrone J, Ziolkowski J, Shaffer E, Aggarwal N (2021) Can virtual scientific conferences facilitate two-way learning between dementia researchers and participants? J Prev Alzheimers Dis 8, 387–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lingler JH, Butters MA, Gentry AL, Hu L, Hunsaker AE, Klunk WE, Mattos MK, Parker LA, Roberts JS, Schulz R (2016) Development of a standardized approach to disclosing amyloid imaging research results in mild cognitive impairment. J Alzheimers Dis 52, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Galasko DR, Grill JD, Lingler JH, Heidebrink JL, and for the Symptomatic Subcommittee of the Advisory Group on Risk Evidence Education for Dementia (AGREEDementia) (2022) A blood test for Alzheimer’s disease: It’s about time or not ready for prime time? J Alzheimers Dis 90, 963–966. [DOI] [PubMed] [Google Scholar]
- [10].NIA, Alzheimer’s & Dementia Outreach, Recruitment & Engagement Resources (ADORE), https://www.nia.nih.gov/research/alzheimers-dementia-outreach-recruitment-engagement-resources
- [11].Arias JJ, Tyler AM, Oster BJ, Karlawish J (2021) The proactive patient: Long-term care insurance discrimination risks of Alzheimer’s disease biomarkers. J Law Med Ethics 46, 485–498. [DOI] [PubMed] [Google Scholar]
- [12].Rybak YE, Lai KSP, Ramasubbu R, Vila-Rodriguez F, Blumberger DM, Chan P, Delva N, Giacobbe P, Gosselin C, Kennedy SH, Iskandar H, McInerney S, Ravitz P, Sharma V, Zaretsky A, Burhan AM (2021) Treatment-resistant major depressive disorder: Canadian expert consensus on definition and assessment. Depress Anxiety 38, 456–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stites SD, Vogt NM, Blacker D, Rumbaugh M, Parker MW, for the Advisory Group on Risk Evidence Education for Dementia (AGREED) (2022) Patients asking about APOE gene test results? Here’s what to tell them. J Fam Pract 71, E1–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mozersky J, Roberts JS, Rumbaugh M, Chhatwal J, Wijsman EM, Galasko D, Blacker D; AGREED (2022) Spillover: The approval of new medications for Alzheimer’s disease dementia will impact biomarker disclosure among asymptomatic individuals. J Alzheimers Dis 90, 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rabinovici GD, Karlawish J, Knopman D, Snyder HM, Sperling R, Carrillo MC (2016) Testing and disclosures related to amyloid imaging and Alzheimer’s disease: Common questions and fact sheet summary. Alzheimers Dement 12, 510–515. [DOI] [PubMed] [Google Scholar]
- [16].Rabinovici GD, Gatsonis C, Apgar C, Chaudhary K, Gareen I, Hanna L, Hendrix J, Hillner BE, Olson C, Lesman-Segev OH, Romanoff J, Siegel BA, Whitmer RA, Carrillo MC (2019) Association of amyloid positron emission tomography with subsequent change in clinical management among medicare beneficiaries with mild cognitive impairment or dementia. JAMA 321, 1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Thijssen EH, La Joie R, Wolf A, Strom A, Wang P, Iaccarino L, Bourakova V, Cobigo Y, Heuer H, Spina S, VandeVrede L, Chai X, Proctor NK, Airey DC, Shcherbinin S, Duggan Evans C, Sims JR, Zetterberg H, Blennow K, Karydas AM, Teunissen CE, Kramer JH, Grinberg LT, Seeley WW, Rosen H, Boeve BF, Miller BL, Rabinovici GD, Dage JL, Rojas JC, Boxer AL; Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) investigators (2020) Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med 26, 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].van der Schaar J, Visser LNC, Bouwman FH, Ket JCF, Scheltens P, Bredenoord AL, van der Flier WM (2022) Considerations regarding a diagnosis of Alzheimer’s disease before dementia: A systematic review. Alzheimers Res Ther 14, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Largent EA, Harkins K, van Dyck CH, Hachey S, Sankar P, Karlawish J (2020) Cognitively unimpaired adults’ reactions to disclosure of amyloid PET scan results. PLoS One 15, e0229137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stites SD, Rubright JD, Karlawish J (2018) What features of stigma do the public most commonly attribute to Alzheimer’s disease dementia? Results of a survey of the U.S. general public. Alzheimers Dement 14, 925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Goldman JS, Hahn SE, Catania JW, LaRusse-Eckert S, Butson MB, Rumbaugh M, Strecker MN, Roberts JS, Burke W, Mayeux R, Bird T; American College of Medical Genetics and the National Society of Genetic Counselors (2011) Genetic counseling and testing for Alzheimer disease: Joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet Med 13, 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zallen DT (2018) “Well, good luck with that”: Reactions to learning of increased genetic risk for Alzheimer disease. Genet Med 20, 1462–1467. [DOI] [PubMed] [Google Scholar]
- [23].Schwarz CG, Kremers WK, Therneau TM, Sharp RR, Gunter JL, Vemuri P, Arani A, Spychalla AJ, Kantarci K, Knopman DS, Petersen RC, Jack CR (2019) Identification of anonymous MRI research participants with face-recognition software. N Eng J Med 381, 1684–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hull LE, Chanfreau-Coffinier C, Tuteja S, Berlowitz D, Lehmann LS, Oslin DW, Pyne JM, DuVall SL, Lynch JA (2019) Early adoption of pharmacogenetic testing for veterans prescribed psychotropic medications. Pharmacogenomics 20, 781–789. [DOI] [PubMed] [Google Scholar]
- [25].de Oliveira FF, Chen ES, Smith MC, Bertolucci PHF (2018) Pharmacogenetics of angiotensin-converting enzyme inhibitors in patients with Alzheimer’s disease dementia. Curr Alzheimer Res 15, 386–398. [DOI] [PubMed] [Google Scholar]
- [26].Milne R, Bunnik E, Diaz A, Richard E, Badger S, Gove D, Georges J, Fauria K, Molinuevo JL, Wells K (2018) Perspectives on communicating biomarker-based assessments of Alzheimer’s disease to cognitively healthy individuals. J Alzheimers Dis 62, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Weiner MW, Nosheny R, Camacho M, Truran-Sacrey D, Mackin RS, Flenniken D, Ulbricht A, Insel P, Finley S, Fockler J (2018) The Brain Health Registry: An internet-based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimers Dement 14, 1063–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Largent EA, Karlawish J (2020) Rescuing research participants after Alzheimer trials stop early: Sending out an SOS. JAMA Neurol 77, 413–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Seaman JB, Tenhorst L, Gentry A, Hunsaker A, Lingler JH (2015) Psychometric properties of a decisional capacity screening tool for individuals contemplating participation in Alzheimer’s disease research. J Alzheimers Dis 46, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Largent EA, Bhardwaj T, Clapp JT, Sykes OS, Harkins K, Grill JD (2022) You’ve got a friend in me: How cognitively unimpaired older adults select a study partner to participate with them in Alzheimer’s disease research. J Alzheimers Dis 90, 1023–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Daly T, Mastroleo I, Migliaccio R (2022) Avoiding over-reliance on multi-domain interventions for dementia prevention. J Alzheimers Dis 90, 989–993. [DOI] [PubMed] [Google Scholar]
- [32].Karikari T (2022) Blood tests for Alzheimer’s disease: Increasing efforts to expand and diversify research participation is critical for widespread validation and acceptance. J Alzheimers Dis 90, 967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Syrjanen JA, Campbell MR, Algeciras-Schimnich A, Vemuri P, Graff-Radford J, Machulda MM, Bu G, Knopman DS, Jack CR Jr, Petersen RC, Mielke MM (2022) Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement 18, 1128–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schindler SE, Cruchaga C, Joseph A, McCue L, Farias FHG, Wilkins CH, Deming Y, Henson RL, Mikesell RJ, Piccio L, Llibre-Guerra JJ, Moulder KL, Fagan AM, Ances BM, Benzinger TLS, Xiong C, Holtzman DM, Morris JC (2021) African Americans have differences in CSF soluble TREM2 and associated genetic variants. Neurol Genet 7, e571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Blue EE, Horimoto A, Mukherjee S, Wijsman EM, Thornton TA (2019) Local ancestry at APOE modifies Alzheimer’s disease risk in Caribbean Hispanics. Alzheimers Dement 15, 1524–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Brickman AM, Manly JJ, Honig LS, Sanchez D, Reyes-Dumeyer D, Lantigua RA, Lao PJ, Stern Y, Vonsattel JP, Teich AF, Airey DC, Proctor NK, Dage JL, Mayeux R (2021) Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement 17, 1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schneider BC, Gross AL, Bangen KJ, Skinner JC, Benitez A, Glymour MM, Sachs BC, Shih RA, Sisco S, Manly JJ (2015) Association of vascular risk factors with cognition in a multiethnic sample. J Gerontol B Psychol Sci Soc Sci 70, 532–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tosto G, Bird TD, Bennett DA, Boeve BF, Brickman AM, Cruchaga C, Faber K, Foroud TM, Farlow M, Goate AM, Graff-Radford NR, Lantigua R, Manly J, Ottman R, Rosenberg R, Schaid DJ, Schupf N, Stern Y, Sweet RA, Mayeux R (2016) The role of cardiovascular risk factors and stroke in familial Alzheimer disease. JAMA Neurol 73, 1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Moghavem N, Henderson VW, Greicius MD (2021) Medicare should not cover aducanumab as a treatment for Alzheimer’s disease. Ann Neurol 90, 331–333. [DOI] [PubMed] [Google Scholar]
- [40].Deters KD, Napolioni V, Sperling RA, Greicius MD, Mayeux R, Hohman T, Mormino EC (2021) Amyloid PET imaging in self-identified non-Hispanic Black participants of the Anti-Amyloid in Asymptomatic Alzheimer’s disease (A4) study. Neurology 96, e1491–e1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Morris JC, Schindler SE, McCue LM, Moulder KL, Benzinger TL, Cruchaga C, Fagan AM, Grant E, Gordon BA, Holtzman DM (2019) Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol 76, 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Vyas DA, Eisenstein LG, Jones DS (2020) Hidden in plain sight - reconsidering the use of race correction in clinical algorithms. N Engl J Med 383, 874–882. [DOI] [PubMed] [Google Scholar]
- [43].Schindler SE, Karikari TK, Ashton NJ, Henson RL, Yarasheski KE, West T, Meyer MR, Kirmess KM, Li Y, Saef B, Moulder KL, Bradford D, Fagan AM, Gordon BA, Benzinger TLS, Balls-Berry J, Bateman RJ, Xiong C, Zetterberg H, Blennow K, Morris JC (2022) Effect of race on prediction of brain amyloidosis by plasma Aβ42/Aβ40, phosphorylated tau, and neurofilament light. Neurology 99, e245–e257. [DOI] [PMC free article] [PubMed] [Google Scholar]


