Abstract
Hypothesis:
Prosthetic electrical stimulation can evoke compensatory eye and head movement despite vestibular implant electrode insertion occurring years after prior labyrinthectomy.
Background:
Vestibular implants sense head rotation and directly stimulate the vestibular nerve, bypassing damaged end organs. Animal research and current clinical trials have demonstrated the efficacy of this approach. However, candidacy criteria for vestibular implants currently require presence of a patent labyrinth in the candidate ear and at least aidable hearing in the opposite ear, thus excluding patients who have undergone prior labyrinthectomy for unilateral Meniere’s disease that later progressed to bilateral vestibular hypofunction.
Methods:
Eight years after right unilateral labyrinthectomy, we implanted stimulating electrodes in the previously exenterated right ear ampullae of a rhesus macaque monkey. The left labyrinth had long-standing hypofunction due to intratympanic gentamicin injection and surgical disruption. We used 3-dimensional video-oculography to measure eye movement responses to prosthetic electrical stimulation. We also measured head-movement responses to prosthetic stimulation with the head unrestrained.
Results:
Bilateral vestibular hypofunction was confirmed by absence of vestibulo-ocular reflex responses to whole-body rotation without prosthetic stimulation. For a subset of the implanted electrodes, prosthetic vestibular stimulation evoked robust compensatory eye and head movements. One electrode reliably elicited responses aligned with the implanted ear’s anterior canal nerve regardless of the return electrode used. Similarly, a second electrode also elicited responses consistent with excitation of the horizontal canal nerve. Responses grew quasilinearly with stimulation rate and current amplitude.
Conclusion:
Prosthetic electrical stimulation targeting the vestibular nerve can be effective years after labyrinthectomy, if at least some parts of the vestibular nerve’s ampullary branches remain despite destruction or removal of the membranous labyrinth.
Introduction
The vestibular system senses head movement using sensors in the inner ear. This information is crucial for gaze and posture stabilization via reflex pathways, stable perception of self-motion, and accurate sense of direction. As such, patients with bilateral damage to the vestibular sensors lack these essential functions and suffer from debilitating dizziness, gaze and postural instability, and also impaired sense of direction. One emerging restorative treatment for these patients is vestibular implantation, which substitutes for the damaged periphery by sensing head rotation with a gyroscope and converting the detected motion into electrical stimulation of the ampullary nerves.1 Work in animal models1–3 and ongoing clinical trials4–8 have demonstrated partial restoration of vestibular function (also reviewed in9).
Candidacy criteria for vestibular implantation currently require a patent labyrinth and radiographically normal internal auditory canal anatomy by CT and MRI on the side planned for implantation, as well as hearing in the contralateral ear sufficient to support communication.10 The anatomic criterion is intended to ensure that the vestibular nerve’s semicircular canal branches are present and spatially distinct enough to be selectively excited by electrodes placed in each ampulla. The contralateral hearing criterion reflects the fact that vestibular implantation carries a risk of iatrogenic sensorineural hearing loss in the implanted ear. Those criteria currently preclude implantation of patients with bilateral vestibular hypofunction after having undergone unilateral labyrinthectomy, a scenario that typically arises when a patient thought to have unilateral Meniere’s disease undergoes labyrinthectomy or sacculotomy and later develops vestibular loss in the opposite ear. However, if vestibular implantation is effective in the ear that had undergone labyrinthectomy, then these patients could benefit from vestibular implantation without potentially compromising hearing in the unimplanted ear.
Thus to assess the feasibility and efficacy of vestibular implantation after labyrinthectomy, we implanted a macaque monkey with vestibular prosthesis electrodes targeting the remnants of ampullae in an ear that underwent intratympanic gentamicin treatment and then transmastoid labyrinthectomy 8 years earlier.1 We quantified prosthesis performance by recording the evoked compensatory eye and head movements mediated via the vestibulo-ocular reflex (VOR) and the vestibulo-collic reflex (VCR), respectively (Fig. 1A). Prosthetic stimulation evoked robust eye and head movements in two main directions aligned with the implant labyrinth’s anterior and horizontal canal axes. Responses grew quasilinearly with increasing stimulation rate and current amplitude, reaching response velocities similar to published responses to prosthetic vestibular nerve stimulation of anatomically intact ears. Our findings demonstrate that vestibular implantation can be feasible and efficacious even several years after labyrinthectomy.
Fig1:
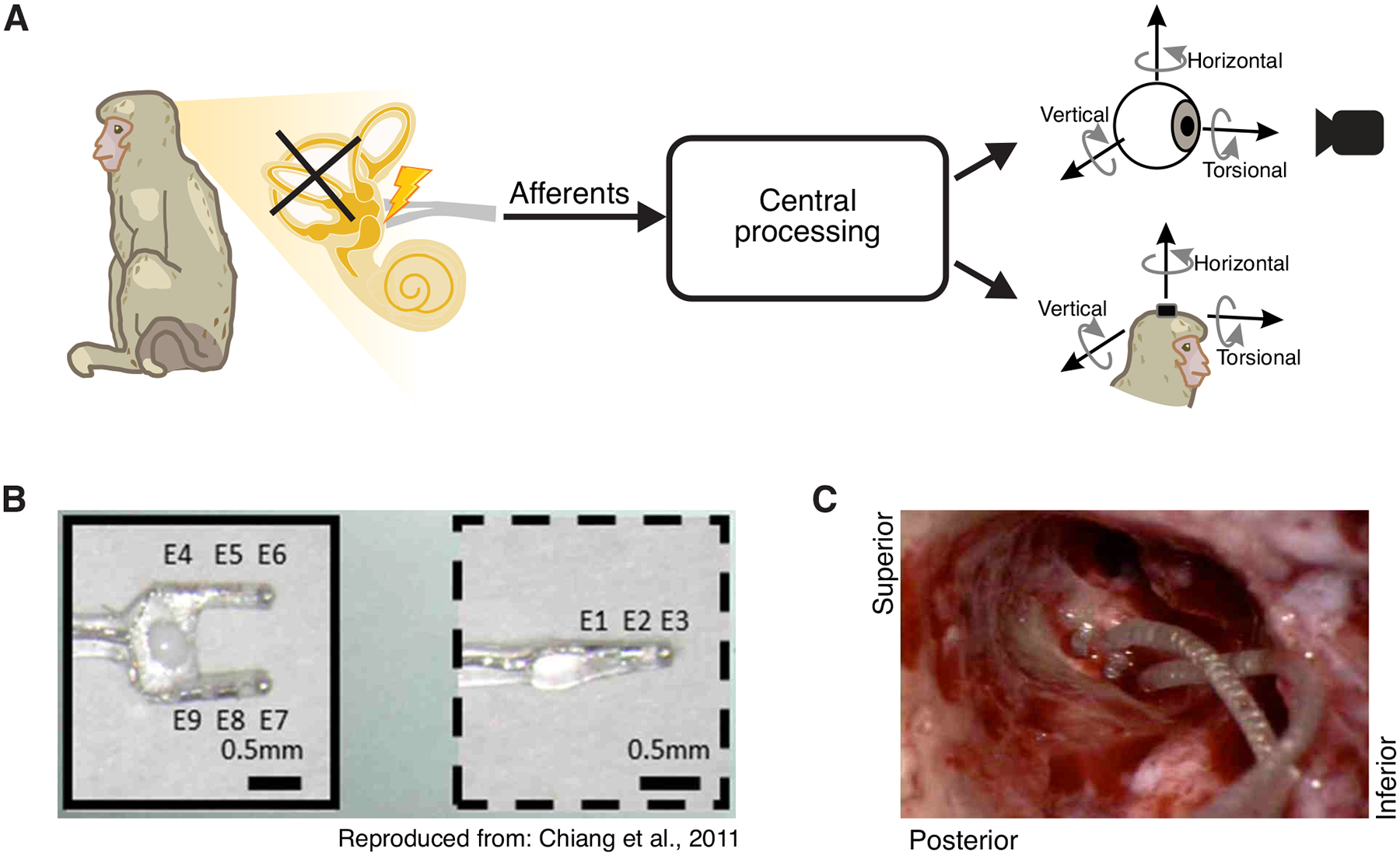
Implantation of vestibular prosthesis stimulating electrodes after prior labyrinthectomy. A) Diagram of the vestibular pathways and the behavioral outputs. Top: 3D videooculography of the evoked eye movement (mediated via the vestibulo-ocular reflex). Bottom: 3D gyroscope measurement of the evoked head movement (mediated via the vestibulo-collic reflex). B) Electrode array image. Reproduced from Chiang et al., 2011. C) Photo of the inner ear during electrode implant surgery. The two-pronged array is on the left while the single-pronged array is on the right.
Methods
Animal history and surgical procedures
One macaque monkey (RhF60738G, an 8-kg adult female) was studied. All housing, surgical, and experimental protocols were approved by the Johns Hopkins Animal Care and Use Committee. Animals were housed on a 12-hr light/dark cycle with daily enrichment. Throughout the study, animals were monitored in consultation with the clinical veterinarian staff to ensure physical and emotional wellbeing. The animal was initially fitted with a post for head fixation (detailed methods in1,11). A stainless-steel post was affixed to the skull with stainless steel screws and dental acrylic. To create a model of vestibular implantation after bilateral vestibular hypofunction, the animal was treated bilaterally with intratympanic gentamicin (ITG) injection and then underwent left ear electrode implantation in 2010. Details of the surgical technique for that implantation were described in1. To eliminate the effect of residual hair cell function after ITG, a right labyrinthectomy was performed in 2013. Briefly, a mastoidectomy was performed to access the labyrinth. Each of the three canals and their ampullae were drilled open, the vestibule was opened by joining the ampullotomies, all five vestibular neuroepithelia were removed via curettage, the vestibule was filled with free grafts of muscle and fascia, areas of dural exposure over the paraflocculus (which in rhesus monkeys occupies the roughly spherical volume encircled by the semicircular canals12) were covered with bone pate and fascia, then the wound was sutured closed in layers.
Eight years later, the left ear became unresponsive to prosthetic stimulation following inadvertent administration of direct current. Therefore, the right ear was implanted in 2021 with an electrode array similar to that used on the left side (Fig. 1B).13 Due to the distortion of anatomical landmarks, ampulla locations could only be estimated; however, residual edges of the horizontal and anterior canal ampulla were visible. A two-pronged, four-electrode array was inserted in that location, with electrodes E4/E5 targeting the horizontal canal’s ampullary nerve and electrodes E8/E9 targeting the anterior canal ampullary nerve (Fig. 1C, left array). Scar and ossification in the expected location of the posterior canal ampulla precluded ready identification of the posterior canal’s ampullary nerve. Because the goal of the surgery was to determine if any responses can be elicited at all and because other experiments in the lab focused mainly on horizontal head rotation, a decision was made intraoperatively to place the third electrode array (a single-pronged array with electrodes E1/E2/E3, normally meant to target posterior canal) near the horizontal canal ampulla remnant (Fig. 1C, right array) to increase the likelihood of successfully stimulating the horizontal canal ampullary nerve. Pieces of fascia and bone were packed around each electrode to stabilize its location. A reference electrode (E11) was inserted in extracranial musculature. The animal recovered for two weeks before any experiments were performed.
Supplementary Digital Content 1 shows 3-dimensional reconstructions of a temporal bone CT scan illustrating electrode placement in the left ear (electrodes implanted in an ear that had not previously undergone labyrinthectomy, A) and the right ear (electrodes implanted in a post-labyrinthectomy ear, B). These images reveal that the single-tined electrode array that would normally have been inserted in the right posterior canal ampulla was instead subjacent to the horizontal canal ampulla.
Stimulation paradigms
Direction:
We measured eye- and head-movement response directions for all possible combinations of stimulating and return electrodes. Stimuli consisted of 100-ms pulse trains at 600 pps. Each stimulation pulse was cathodal-first biphasic with the stimulation current of 250 uA or 80% of the threshold current at which twitching of facial musculature was evident during video-oculographic observation. At least 30 pulse trains were delivered for each combination.
Stimulation frequency and current amplitude:
For the best electrode pair for anterior canal (E1E8) and horizontal canal (E5E3) stimulation, pulse trains were delivered as described above but with wider ranges of pulse rate (50, 100, 200, 300, 400, 500, 600, 800, 1000 pps) and current amplitude (125, 188, 250 uA). At lease 30 pulse trains were delivered for each pulse rate and current amplitude. We note that we did not complete a comparable analysis for movements in the posterior canal plane since no combination of electrodes evoked robust response.
Behavioral recordings
The monkey was seated in a primate chair during the stimulation sessions. Eye and head movements were recorded in separate sessions. Treats and water were given intermittently during recording sessions to maintain alertness.
Eye movement recordings:
The monkey’s head was restrained in darkness during stimulation. A chair-mounted camera (Blackfly BFS-U3–13Y3M, FLIR) was positioned in front of the left eye for videooculography (VOG) at 500 frames/s. Synchronization pulses from the camera signifying shutter closure times were recorded using a Blackrock acquisition system, which also recorded the stimulation pulse timing at a 30 kHz sampling rate. Pupil position tracking was used to determine horizontal and pitch components of 3-dimensional (3D) angular eye position, and iris-based torsional tracking was performed offline using custom code modified from that by14. The 3D eye position was resampled at 1 kHz and synchronized with the stimulation pulse using the recorded frame trigger timing.
Head movement recordings:
The monkey’s head was allowed to move freely during measurement of vestibulo-cervical responses to prosthetic stimulation. At the start of the trial, a fixation target was presented so that the monkey’s head was initially centered at the same position. Evoked rotational head velocity was recorded at 1 kHz using a gyroscope board (ICM-42688-P, InvenSense/TDK) mounted to the head post, with acquisition times acquired by the Blackrock acquisition system at 30 kHz. The 3D head rotational velocity was then synchronized with the stimulation pulse using the recorded acquisition timing.
Data analysis
All analyses were performed using MATLAB 2019b (Mathworks). Digitized (1 kHz) eye position and head velocity data were low-passed filtered at 100 Hz (51st degree, Hamming window, filtfilt function in MATLAB). Rotation vectors were first calculated from eye position data. The 3-D angular velocity vectors were then computed from the rotation vectors15 and transformed into canal coordinates (right anterior-left posterior: RALP, left anterior-right posterior: LARP, and left horizontal-right horizontal: LHRH).16 Trials with saccades or head shaking were excluded from the dataset. Each condition has at least 10 trials after the exclusion. Eye and head velocity data were aligned based on the start of the stimulation pulse train. The average velocity during the 50-ms period before the stimulation was subtracted from the data to eliminate any constant drift. For eye data, the first maximum or minimum eye velocity during the stimulation period (before any quick phase eye movement) was calculated as the evoked response. For head data, the first maximum or minimum head velocity during the stimulation period and up to 50 ms after (before any rebound head movement) was calculated as the evoked response.
Results
Vestibular prosthesis implanted after a labyrinthectomy evokes robust eye movements
We first focused on the direction of the evoked VOR by pairing all available electrodes for stimulation while recording the VOR eye movements. Figure 2A shows the vector representation of the evoked eye movement for each electrode combination. Peak response velocities reached 400 deg/s. Robust eye movement were evoked in two main directions: one closely aligned with the right anterior canal axis (right anterior/left posterior-RALP, Fig. 2A, blue) and the other closely aligned with the right horizontal canal axis (Fig. 2A, magenta). More specifically, Electrode 8 (E8) when paired with any other electrodes resulted in eye movement aligned with the anterior canal axis responses, whereas Electrode 5 (E5) when paired with electrodes other than E8 evoked eye movements aligned with the horizontal canal axis. Figures 2B and C show example traces of the evoked eye movement using the combinations best for anterior canal stimulation and horizontal canal stimulation, respectively. Consistent with the intraoperative video and post-operative CT imaging showing that the single-tined electrode array was implanted far from the posterior canal ampullary nerve, no combination of electrodes evoked robust eye movements aligned with the right posterior canal axis.
Fig2:
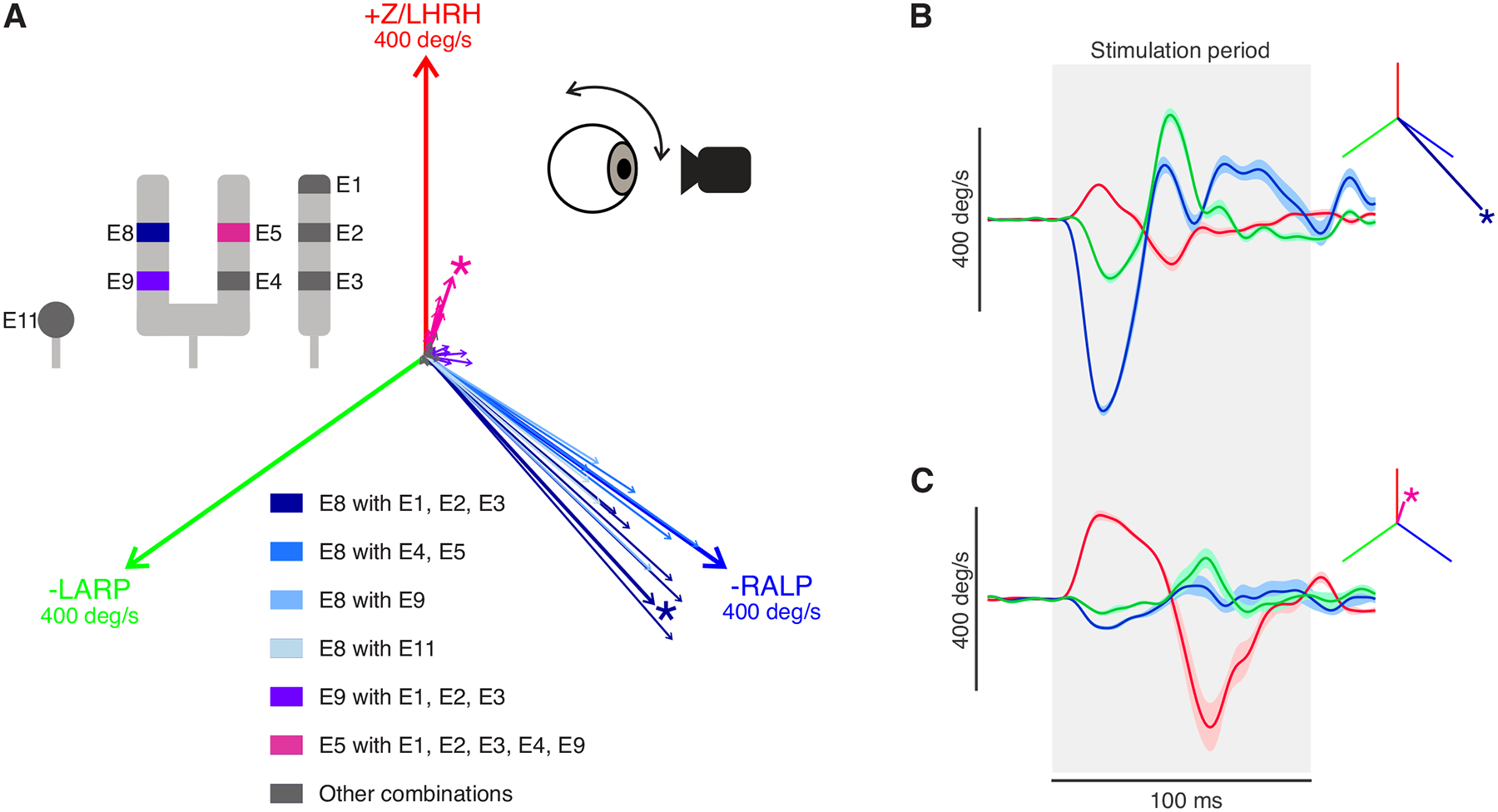
Prosthetic stimulation of vestibular nerves can evoke robust eye movements despite prior labyrinthectomy. A) Vector portrayal of the evoked eye movements using all the possible combinations of electrode sites. Two main evoked directions were found, aligned with the implanted ear’s anterior canal (blue) and horizontal canal (magenta) axes. The magnitude of each canal axis shown is 400 deg/s. The asterisk denotes the best electrode pair for stimulation in each direction. Inset: diagram of the electrode contacts. E8 paired with all other electrodes is shown in blue. E9 paired with E1–3 is in purple. E5 paired with all other electrodes (except E8) is in magenta B) Example eye velocity traces of the evoked VOR in the direction aligned with the anterior canal. Shaded area indicated the SEM. The inset shows the vector representation of the response. C) Example eye velocity traces of the evoked VOR in another direction (aligned with the horizontal canal). Blue, green, and red signify the directions of the -RALP, -LARP, and Z/LHRH, respectively.
We next investigated the effects of stimulation frequency and current amplitude by varying these parameters. Stimulation was delivered using the best electrode pair for the anterior canal. As shown in Figs. 3A–C, the evoked eye movement velocity increased as a function of stimulation rate. This relationship was approximately linear up to ~600 Hz, after which the eye velocity started to plateau. A similarly linear relationship was also observed when varying the stimulation current amplitude, though no obvious saturation is observed at the maximum current limit (Figs. 3D–E). Stimulation using the best electrode pair for the horizontal canal likewise showed similar trends (See Supplementary Digital Content 2, which shows responses evoked by the best electrode pair for the horizontal canal).
Fig3:
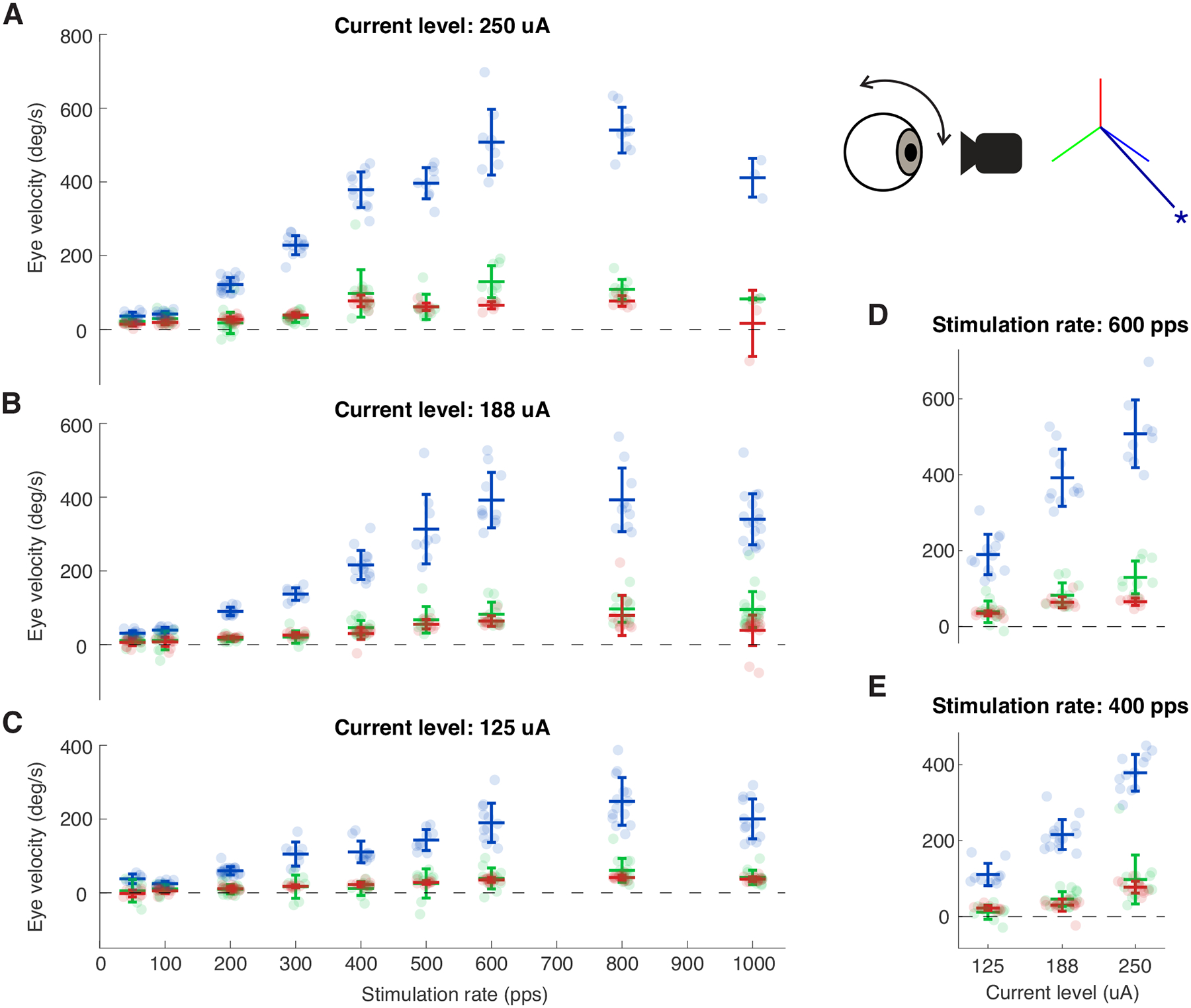
Evoked VOR eye movements show quasilinear relationship as the stimulation rate and the current amplitude increase. A-C) Evoked VOR eye velocity as a function of stimulation rate using 250, 188, and 125 uA respectively. D-E) Evoked eye velocity as a function of current amplitude using 600 and 400 pps, respectively. Inset shows the vector representation of the response of the electrode pair used. Error bars show one standard deviation. Blue, green, and red signify the directions of the -RALP, -LARP, and Z/LHRH, respectively.
Prosthesis-evoked head movements show similar trend to the eye movements
VCR head movements evoked by all available electrode combinations could be grouped into two main directions: one aligned with the right anterior canal axis and the other aligned with the right horizontal canal axis (Fig. 4A). E8 when paired with any other electrode primarily evoked anterior canal-aligned head movement, while E5 when paired with the electrodes other than E8 evoke head movements aligned with horizontal canal stimulation. Figs. 4 B andC show example traces of head movement responses elicited using the best combinations for the anterior and horizontal canals, respectively. As was the case for eye movements, no combination of electrodes evoked robust head movements aligned with the right posterior canal axis.
Fig4:
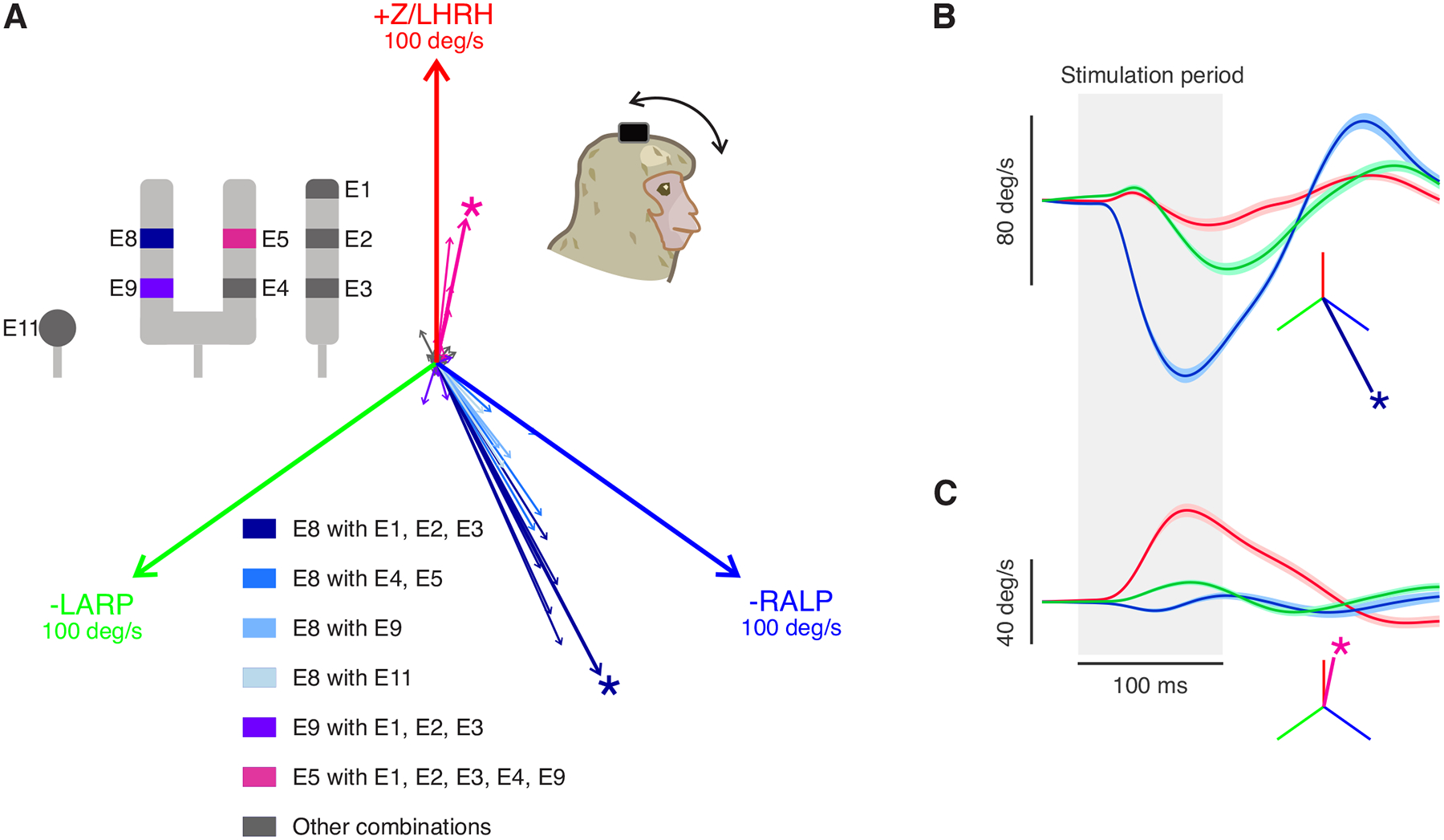
Prosthetic stimulation of vestibular nerves can evoke robust head movements despite prior labyrinthectomy. A) Similar to Fig. 2 but shows vector portrayal of the evoked head movements using all the possible combinations of electrode sites. Again, two main evoked directions were found, aligned with the implanted ear’s anterior canal (blue) and horizontal canal (magenta) axes. The magnitude of each canal axis shown is 100 deg/s. The asterisk denotes the best electrode pair for stimulation in each direction. Inset: diagram of the electrode contacts. E8 paired with all other electrodes is shown in blue. E9 paired with E1–3 is in purple. E5 paired with all other electrodes (except E8) is in magenta B) Example head velocity traces of the evoked VOR in the direction aligned with the anterior canal. Shaded area indicated the SEM. The inset shows the vector representation of the response. C) Example eye velocity traces of the evoked VOR in another direction (aligned with the horizontal canal). Blue, green, and red signify the directions of the -RALP, -LARP, and Z/LHRH, respectively.
Varying stimulation frequency of the best electrode combination for anterior canal stimulation yielded a linear relationship between head velocity and stimulation up to ~400 Hz (Figs. 5A–C). When varying the stimulation current amplitude, the evoked head velocity also showed a quasilinear relationship up to the maximum current used (Figs. 5D–E). Stimulation using the best electrode pair for the horizontal canal showed comparable trends (See Supplementary Digital Content 3, which shows responses evoked by the best electrode pair for the horizontal canal).
Fig5:
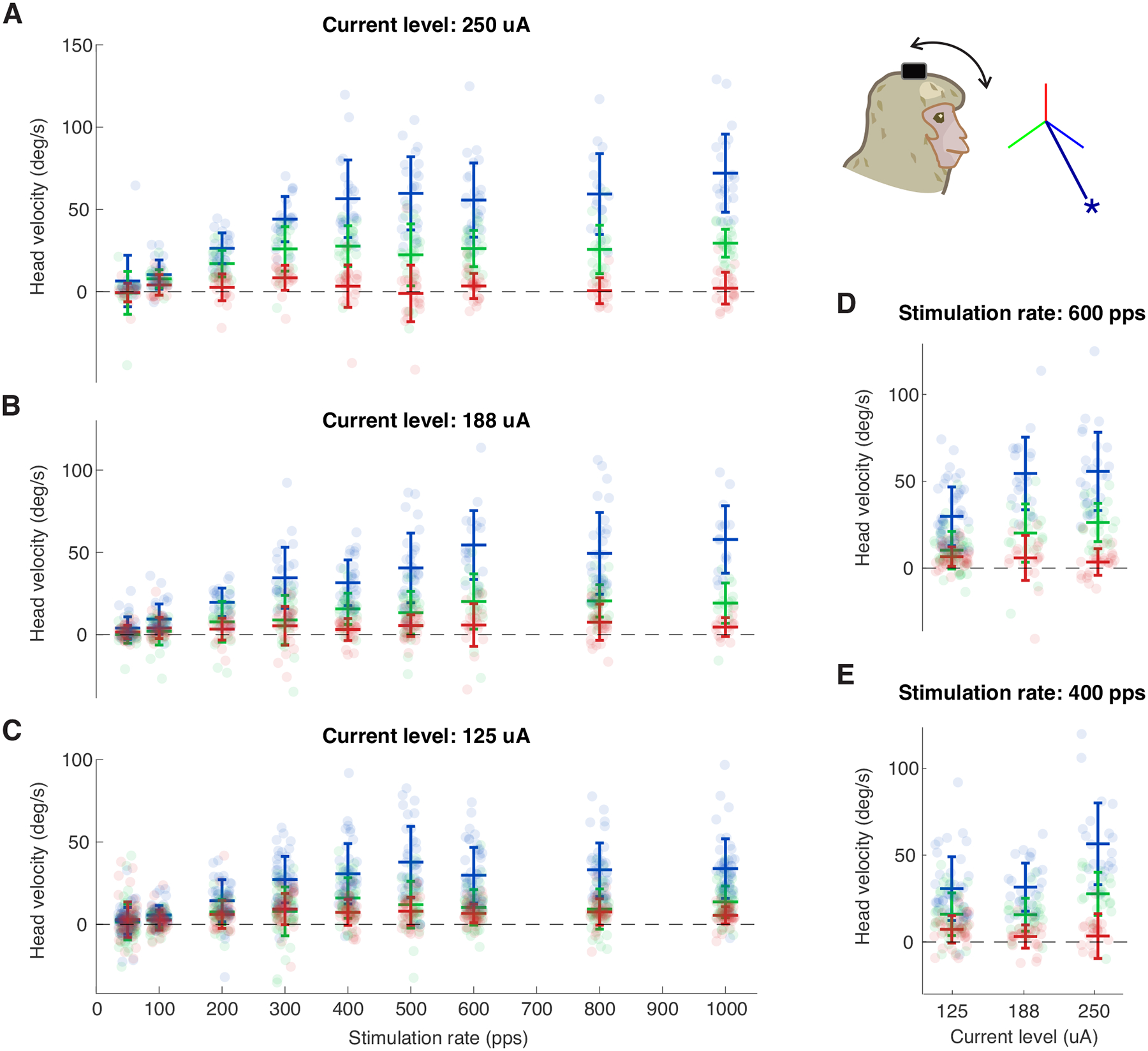
Evoked VCR head movements show quasilinear relationship as the stimulation rate and the current amplitude increase. A-C) Similar to Fig. 3 but shows the evoked VCR head velocity as a function of stimulation rate using 250, 188, and 125 uA, respectively. D-E) Evoked head velocity as a function of current amplitude using 600 and 400 pps, respectively. Inset shows the vector representation of the response of the electrode pair used. Error bars show one standard deviation. Blue, green, and red signify the directions of the -RALP, -LARP, and Z/LHRH, respectively.
Discussion
Here we investigated the feasibility of implanting a vestibular prosthesis in an ear that has previously undergone labyrinthectomy. Our results show that, even after eight years after labyrinthectomy, a vestibular prosthesis implanted in a Macaque monkey can evoke robust eye and head movements in two distinct directions, aligned in this case with the axes of the anterior and horizontal canals. Responses increased quasilinearly with stimulation rate and current amplitude. These trends are comparable to those reported in previous studies of implants where surgeries were performed on previously healthy or gentamicin-treated ears.1,3,17 Taken together our results provide evidence that vestibular function can be partially restored by prosthetic stimulation even after labyrinthectomy. This favorable finding is likely only possible when Scarpa’s ganglion remains intact, and directionally specific prosthetic stimulation would be difficult to achieve if the prior surgery destroyed all ampullary nerve branches distal to the ganglion. Indeed, our demonstration that post-labyrinthectomy vestibular implantation can yield robust, directionally-specific responses in animals indicates that prior labyrinthectomy should not necessarily be an absolute contraindication to vestibular implantation in humans.
Study limitations
During the implantation surgery, partially preserved medial walls of the horizontal and anterior ampullae provided helpful landmarks and likely signified preservation of at least part of the post-ganglion segment of the vestibular nerve’s ampullary branches to those canals. In contrast, neither the posterior canal ampulla remnant nor the singular canal was readily apparent. Post-labyrinthectomy, pre-implantation CT imaging was not available for this case. It could have provided helpful surgical guidance (e.g., showing whether remnants of the medial ampulla walls are still present) and information the extent of tissue removal during labyrinthectomy.18 Although preoperative imaging is not normally needed to identify ampullae in an ear with normal anatomy, pre-surgical imaging to aid in surgical planning would likely increase the success rate of post-labyrinthectomy electrode implantation.
As described above, the posterior canal ampulla remnant was not readily apparent intraoperatively, and we decided to place the single-tined array near the horizontal canal ampulla remnant (instead of the posterior canal ampulla) to maximize the chance of getting at least one electrode near excitable remnants of the horizontal ampullary nerve in the post-labyrinthectomy ear. Supplementary Digital Content 1B shows the temporal bone CT of the right ear, confirming that all the electrode arrays are near the horizontal and anterior canal ampullae. That post-operative CT reconstruction does show what appears to be part of the posterior canal remnant, which may have been implantable had we persisted in drilling to reach it. This hindsight serves to emphasize the potential value of pre-operative imaging of post-labyrinthectomy ears to facilitate surgical planning and better target remnants of the ampullae.
In rhesus monkeys and other nonhuman primates, the cerebellum’s paraflocculus extends into the roughly spherical volume enclosed by the three semicircular canals, somewhat limiting access to the posterior canal’s ampullary nerve in the singular nerve canal via the transmastoid approach we used for implantation.12 In retrospect, we could have removed the posterior ear canal wall, closed off the ear canal, and approached the posterior canal ampulla remnant or singular nerve canal from anterior of the facial nerve. In humans, the paraflocculus does not occupy the space enclosed by the semicircular canals, so the posterior canal ampulla and singular canal are easier to reach via a transmastoid approach without risking dural injury.
This study utilized only acute stimulation. Future studies might investigate how continuous stimulation affects eye and head movement responses after central adaptation (i.e., directional plasticity,19 plasticity in the VOR and VCR pathways11,20) to long-term, continuously motion-modulated prosthetic stimulation.
Clinical implications
Implications of this nonhuman primate case generalize to care of human patients in several ways. First, the case suggests that a history of prior labyrinthectomy (specifically destruction of the membranous labyrinth without complete removal of identifiable remnants of the anteromedial walls of the ampullae) need not be considered an absolute contraindication to vestibular implantation, because it is possible to achieve effective and semi-selective vestibular implant stimulation in that setting. Based on the knowledge gained from this sentinel nonhuman primate case, vestibular implant surgeons might reconsider the current consensus that “patients without a patent labyrinth are not yet considered for intralabyrinthine electrode insertion.”10 A change in that candidacy criterion would be especially important for patients who become severely disabled by bilateral vestibular hypofunction after losing vestibular sensation in the “only-hearing, only-balancing” ear after contralateral labyrinthectomy – a history shared by multiple applicants referred for participation in the vestibular implant trial currently underway at our institution.
Second, this case led to the realization that, when performing a labyrinthectomy for treatment of Meniere’s disease, surgeons should consider the possibility that the patient might eventually undergo vestibular implantation in that ear. With this knowledge, the surgeon might take greater care to preserve the medial and anteromedial walls of the ampullae (despite removing the neuroepithelia), leave enough of each canal’s thin segment intact to facilitate finding the ampullae during a subsequent vestibular implantation surgery, and put fat grafts in the spaces left after opening and exenterating the ampullae and vestibule, with the goal of filling those spaces with tissue that should be readily identifiable (on preoperative imaging and intraoperative exam) and easily removed at the time of vestibular implantation.
Third, this case indicates that when reviewing computed tomography imaging studies for a patient who has undergone labyrinthectomy, clinicians should note the status of the medial and anteromedial walls of the ampullae and the bone channels that connect the ampullae to the internal auditory canal. Presence of those structures after labyrinthectomy, as our post-implantation CT imaging and physiologic results show was true in this case, suggests anatomic preservation of vestibular nerve branches lateral to Scarpa’s ganglion that are spatially distinct enough to allow effective and semi-selective prosthetic stimulation.
Supplementary Material
Supplementary Digital Content 1: Temporal bone computed tomography (CT) 3-dimensional reconstructions contrasting electrode locations in the animal’s left (anatomically normal pre-implantation) and right (post-labyrinthectomy) ears. A) Electrode placement in the left ear. The two-pronged array occupies and targets the horizontal and anterior canal ampullae, while the single-tined array occupies and targets the posterior canal ampulla. B) Electrode placement in the right ear, which is newly implanted post-labyrinthectomy and is the focus of the current study. All three arrays are located near the horizontal and anterior canal ampullae. Segmentation keys: blue = labyrinth, yellow = internal auditory canal, red = paraflocculus, purple = two-pronged array, green = single-tined array.
Supplementary Digital Content 2: Evoked VOR eye movements show quasilinear relationship as the stimulation rate and the current amplitude increase. Similar to Fig. 3 but using a different electrode pair (for horizontal canal axis). A-C) Evoked VOR eye velocity as a function of stimulation rate using 250, 188, and 125 uA respectively. D-E) Evoked eye velocity as a function of current amplitude using 600 and 400 pps, respectively. Inset shows the vector representation of the response of the electrode pair used. Error bars show one standard deviation. Blue, green, and red signify the directions of the -RALP, -LARP, and Z/LHRH, respectively.
Supplementary Digital Content 3: Evoked VCR head movements show quasilinear relationship as the stimulation rate and the current amplitude increase. Similar to Fig. 5 but using a different electrode pair (for horizontal canal axis). A-C) Evoked head velocity as a function of stimulation rate using 250, 188, and 125 uA, respectively. D-E) Evoked head velocity as a function of current amplitude using 600 and 400 pps, respectively. Inset shows the vector representation of the response of the electrode pair used. Error bars show one standard deviation. Blue, green, and red signify the directions of the -RALP, -LARP, and Z/LHRH, respectively.
Funding:
This work was supported by National Institutes of Health grants R01DC002390 (KEC, CCDS), R01DC018061 (KEC), and R01DC009255 (CCDS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
- Supplementary Digital Content 1. Figure that shows temporal bone computed tomography (CT) 3-dimensional reconstructions contrasting electrode locations in the animal’s ears. pdf
- Supplementary Digital Content 2. Figure that shows eye movements evoked by the best electrode pair for the horizontal canal. pdf
- Supplementary Digital Content 3. Figure that shows head movements evoked by the best electrode pair for the horizontal canal. pdf
Competing interests:
I have read the journal’s policy and the authors of this manuscript have the following competing interests:
Dr. Della Santina is an inventor of and holds a royalty interest in pending and awarded patents related to technologies discussed in this report. He holds an equity interest in and is the founder of Labyrinth Devices, LLC, which has a partnership with MED-EL GmbH. Johns Hopkins University holds royalty interests in the patents described above and an equity interest in Labyrinth Devices, LLC. The terms of these arrangements are managed by the JHU Office of Policy Coordination in accordance with the university’s policies on interaction with industry and conflict of interest.
References
- 1.Dai C, Fridman GY, Davidovics NS, Chiang B, Ahn JH, Della Santina CC. Restoration of 3D vestibular sensation in rhesus monkeys using a multichannel vestibular prosthesis. Hear Res. 2011;281(1–2):74–83. doi: 10.1016/j.heares.2011.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karmali F, Haburcakova C, Gong W, Della Santina CC, Merfeld DM, Lewis RF. An Implanted Vestibular Prosthesis Improves Spatial Orientation in Animals with Severe Vestibular Damage. J Neurosci. 2021;41(17):3879LP–3888. doi: 10.1523/JNEUROSCI.2204-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutros PJ, Valentin NS, Hageman KN, Dai C, Roberts DC, Della Santina CC. Nonhuman primate vestibuloocular reflex responses to prosthetic vestibular stimulation are robust to pulse timing errors caused by temporal discretization. J Neurophysiol. 2019;121(6):2256–2266. doi: 10.1152/jn.00887.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutros PJ, Schoo DP, Rahman MA, et al. Continuous vestibular implant stimulation partially restores eye-stabilizing reflexes. JCI Insight. 2019;4(22). doi: 10.1172/jci.insight.128397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow MR, Ayiotis AI, Schoo DP, et al. Posture, Gait, Quality of Life, and Hearing with a Vestibular Implant. N Engl J Med. 2021;384(6):521–532. doi: 10.1056/nejmoa2020457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips JO, Ling L, Nie K, et al. Vestibular implantation and longitudinal electrical stimulation of the semicircular canal afferents in human subjects. J Neurophysiol. 2015;113(10):3866–3892. doi: 10.1152/jn.00171.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornos AP, Guinand N, Van De Berg R, et al. Artificial balance: Restoration of the vestibulo-ocular reflex in humans with a prototype vestibular neuroprosthesis. Front Neurol. 2014;5 APR:66. doi: 10.3389/fneur.2014.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis RF, Haburcakova C, Gong W, et al. Vestibular prosthesis tested in rhesus monkeys. In: Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS.; 2011:2277–2279. doi: 10.1109/IEMBS.2011.6090573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sluydts M, Curthoys I, Vanspauwen R, et al. Electrical Vestibular Stimulation in Humans: A Narrative Review. Audiol Neurotol. 2020;25(1–2):6–24. doi: 10.1159/000502407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Berg R, Ramos A, van Rompaey V, et al. The vestibular implant: Opinion statement on implantation criteria for research. J Vestib Res. 2020;30:213–223. doi: 10.3233/VES-200701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell DE, Della Santina CC, Cullen KE. Plasticity within non-cerebellar pathways rapidly shapes motor performance in vivo. Nat Commun. 2016;7(May):1–13. doi: 10.1038/ncomms11238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedjoudje A, Hayden R, Dai C, et al. Virtual Rhesus Labyrinth Model Predicts Responses to Electrical Stimulation Delivered by a Vestibular Prosthesis. J Assoc Res Otolaryngol. 2019;20(4):313–339. doi: 10.1007/s10162-019-00725-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang B, Fridman GY, Dai C, Rahman MA, Della Santina CC. Design and performance of a multichannel vestibular prosthesis that restores semicircular canal sensation in rhesus monkey. IEEE Trans Neural Syst Rehabil Eng. 2011;19(5):588–598. doi: 10.1109/TNSRE.2011.2164937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otero-Millan J, Roberts DC, Lasker A, Zee DS, Kheradmand A. Knowing what the brain is seeing in three dimensions: A novel, noninvasive, sensitive, accurate, and low-noise technique for measuring ocular torsion. J Vis. 2015;15(14):11. doi: 10.1167/15.14.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haslwanter T Mathematics of three-dimensional eye rotations. Vision Res. 1995;35(12):1727–1739. doi: 10.1016/0042-6989(94)00257-M [DOI] [PubMed] [Google Scholar]
- 16.Carriot J, Jamali M, Chacron MJ, Cullen KE. The statistics of the vestibular input experienced during natural self-motion differ between rodents and primates. J Physiol. 2017;595(8):2751–2766. doi: 10.1113/JP273734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell DE, Dai C, Rahman MA, Ahn JH, Della Santina CC, Cullen KE. Head Movements Evoked in Alert Rhesus Monkey by Vestibular Prosthesis Stimulation: Implications for Postural and Gaze Stabilization. PLoS One. 2013;8(10):1–12. doi: 10.1371/journal.pone.0078767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedjoudje A, Schoo DP, Ward BK, Carey JP, Della Santina CC, Pearl M. Vestibular Implant Imaging. Am J Neuroradiol. 2021;42(2):370LP–376. doi: 10.3174/ajnr.A6991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai C, Fridman GY, Chiang B, et al. Directional Plasticity Rapidly Improves 3D Vestibulo-Ocular Reflex Alignment in Monkeys Using a Multichannel Vestibular Prosthesis. J Assoc Res Otolaryngol. 2013;14(6):863–877. doi: 10.1007/s10162-013-0413-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell DiE, Della Santina CC, Cullen KE. Plasticity within excitatory and inhibitory pathways of the vestibulo-spinal circuitry guides changes in motor performance. Sci Rep. 2017;7(1):1–15. doi: 10.1038/s41598-017-00956-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Digital Content 1: Temporal bone computed tomography (CT) 3-dimensional reconstructions contrasting electrode locations in the animal’s left (anatomically normal pre-implantation) and right (post-labyrinthectomy) ears. A) Electrode placement in the left ear. The two-pronged array occupies and targets the horizontal and anterior canal ampullae, while the single-tined array occupies and targets the posterior canal ampulla. B) Electrode placement in the right ear, which is newly implanted post-labyrinthectomy and is the focus of the current study. All three arrays are located near the horizontal and anterior canal ampullae. Segmentation keys: blue = labyrinth, yellow = internal auditory canal, red = paraflocculus, purple = two-pronged array, green = single-tined array.
Supplementary Digital Content 2: Evoked VOR eye movements show quasilinear relationship as the stimulation rate and the current amplitude increase. Similar to Fig. 3 but using a different electrode pair (for horizontal canal axis). A-C) Evoked VOR eye velocity as a function of stimulation rate using 250, 188, and 125 uA respectively. D-E) Evoked eye velocity as a function of current amplitude using 600 and 400 pps, respectively. Inset shows the vector representation of the response of the electrode pair used. Error bars show one standard deviation. Blue, green, and red signify the directions of the -RALP, -LARP, and Z/LHRH, respectively.
Supplementary Digital Content 3: Evoked VCR head movements show quasilinear relationship as the stimulation rate and the current amplitude increase. Similar to Fig. 5 but using a different electrode pair (for horizontal canal axis). A-C) Evoked head velocity as a function of stimulation rate using 250, 188, and 125 uA, respectively. D-E) Evoked head velocity as a function of current amplitude using 600 and 400 pps, respectively. Inset shows the vector representation of the response of the electrode pair used. Error bars show one standard deviation. Blue, green, and red signify the directions of the -RALP, -LARP, and Z/LHRH, respectively.


