Abstract
Imprinting ensures that the infant forms the caregiver attachment necessary for altricial species survival. In our mammalian model of imprinting, neonatal rats rapidly learn the odor-based maternal attachment. This rapid learning requires reward-evoked locus ceruleus (LC) release of copious amounts of norepinephrine (NE) into the olfactory bulb. This imprinting ends at postnatal day 10 (P10) and is associated with a dramatic reduction in reward-evoked LC NE release. Here we assess whether the functional emergence of LC α2 inhibitory autoreceptors and the downregulation of LC α1 excitatory autoreceptors underlie the dramatic reduction in NE release associated with termination of the sensitive period. Postsensitive period pups (P12) were implanted with either LC or olfactory bulb cannulas, classically conditioned with intracranial drug infusions (P14), and tested for an odor preference (P15). During conditioning, a novel odor was paired with either olfactory bulb infusion of a β-receptor agonist (isoproterenol) to assess the target effects of NE or direct LC cholinergic stimulation combined with α2 antagonists and α1 agonists in a mixture to reinstate neonatal levels of LC autoreceptor activity to assess the source of NE. Pups learned an odor preference when the odor was paired with either olfactory bulb isoproterenol infusion or reinstatement of neonatal LC receptor activity. These results suggest that LC autoreceptor functional changes rather than olfactory bulb changes underlie sensitive period termination.
Keywords: olfactory bulb, locus ceruleus, norepinephrine, acetylcholine, phenylephrine (α1 agonist), idazoxan (α2 antagonist), memory, learning, infant rat, sensitive period, imprinting, development, mother-infant attachment
Introduction
The strong influences of infant experiences on adult life will remain elusive until we understand the unique functions of the neonatal brain that lay the foundation for future brain processing. Childhood experiences, especially within the context of attachment, have a strong impact on emergence of adult mental health and character traits (Bowlby, 1969; Glaser, 2000; Schore, 2001; Teicher et al., 2003). Our mammalian imprinting model explores this issue and has shown that the neonatal rat uses a unique, simplistic learning circuit to acquire the life-sustaining odor attachment to the caregiver. Specifically, without brain areas normally involved in adult learning (nonfunctional hippocampus, amygdala, frontal cortex), the infant must rely on a unique learning circuit involving the olfactory bulb and locus ceruleus (LC) (Rudy and Morledge, 1994; Wilson and Sullivan, 1994; Vermer et al., 1996; Stanton, 2000; Sullivan et al., 2000b; Sullivan, 2003).
In our rat mammalian imprinting model using odor–reward pairings, neonatal rats can rapidly learn an odor-based attachment to their mother. This sensitive period learning is dependent on a simplistic learning circuit involving the olfactory bulb and LC and results in a rapid strong attachment to the mother corresponding with olfactory bulb metabolic and anatomical changes (Wilson et al., 1987; Woo et al., 1987; Wilson and Leon, 1988; Wilson and Sullivan, 1990; McLean et al., 1999; Sullivan, 2001). These learning-induced behavioral and neural changes require norepinephrine (NE) (LC lesion or blocking olfactory bulb NE prevents learning), and an odor preference is learned from odor–NE pairings (LC stimulation or olfactory bulb NE infusions) (Sullivan et al., 1992, 1994, 2000b; Yuan et al., 2003). Although the behavioral and neural changes are dependent on acquisition during the sensitive period, the odor is later important for the mate choice, sex, and maternal behavior of the adult (Pager, 1974; Coopersmith and Leon, 1986; Fillion and Blass, 1986; Woo and Leon, 1987; Moore et al., 1996; Fleming et al., 1999; Shah et al., 2002).
At postnatal day 10 (P10), as the sensitive period ends, pups develop the motor abilities to leave the nest (Bolles and Woods, 1965), and their learning abilities become more adult-like. First, the infant’s learning abilities expand to permit passive avoidance, active avoidance, and inhibitory conditioning (Collier et al., 1979; Blozovski and Cudennec, 1980; Camp and Rudy, 1988; Myslivecek, 1997; Sullivan et al., 2000a). The developmental emergence of amygdala functioning seems to underlie these new learning abilities (Wilson and Sullivan, 1993; Sullivan et al., 2000a). Second, learning diminishes in concert with the loss of NE release to odor and reward (Rangel and Leon, 1995). Because the LC is the sole source of NE for the olfactory bulb, we assess here whether developmental changes in the LC may underlie pups’ loss of rapid, robust odor-preference learning. The LC contains recurrent collaterals, along with corresponding LC NE au-toreceptors to regulate LC function (Berridge and Waterhouse, 2003). Although the LC α2 autoreceptors are present in the neonate (receptor autoradiography and mRNA) (Winzer-Serhan et al., 1999), they do not appear to function until approximately P10, at least in whole animals receiving sensory stimulation during extracellular single-cell LC recordings (Kimura and Nakamura, 1987; Nakamura et al., 1987; Nakamura and Sakaguchi, 1990). Specifically, extracellular recording of single LC neurons in response to sensory stimuli (1 sec air puff or electric shock) elicits prolonged excitation (20 –30 sec) in neonates, whereas the same stimulation produces only a response duration measured in milliseconds in older pups. This developmental response difference appears to be attributable to the functional emergence of α2 inhibitory noradrenergic autoreceptors, resulting in the LC auto-inhibition within milliseconds of activation and the functional attenuation of LC α1 excitatory noradrenergic autoreceptors, which prolong LC neonatal responses (Nakamura et al., 1987; Pieribone et al., 1994; Scheinin et al., 1994). On the basis of these data, we hypothesized that developmental LC autoreceptor changes may be responsible for the failure of older pups to learn the rapid, robust odor preference simply because the LC releases insufficient amounts of NE to support learning. To test this hypothesis, we attempted to reinstate neonatal LC autoreceptor activity in postsensitive period pups through infusions of an auto-receptor agonist–antagonist mixture and assessed whether imprinting-like odor-preference learning could be reinstated. Moreover, because the olfactory bulb is the site of the learning-induced changes, we assessed the role of NE within the olfactory bulb by intrabulbar infusions of an NE β-agonist. Our results suggest that the developmental change in LC autoreceptors rather than the olfactory bulb may underlie, at least in part, sensitive period termination.
Materials and Methods
Subjects
The subjects were male and female Long–Evans rat pups born and bred in our colony at the University of Oklahoma (originally from Harlan Farms). Animals were housed in polypropylene cages (34 × 29 × 17 cm) lined with wood chips and were kept in a temperature (23°C)- and light (7:00 A.M. to 7:00 P.M.)-controlled room. Food and water were available ad libitum. The day of birth was considered P0, and litters were culled to 10 on P1. No more than one male and one female from a litter were used in each experimental condition. All procedures followed University of Oklahoma and National Institutes of Health standards for animal treatment.
Cannula implantation
On P12, pups were anesthetized by inhalation (with metofane until tailpinch reflex was eliminated) and placed in an adult stereotaxic apparatus adapted for use with infants. Stainless-steel cannulas (30 gauge tubing) were implanted bilaterally in the olfactory bulb or LC through holes drilled in the overlying skull. Stereotaxic coordinates derived from the atlas of Paxinos et al. (1991) were used as a reference and adapted through pilot work (Sullivan et al., 2000b) for implanting cannulas into the LC (caudal, 1.40 mm; lateral, ±0.60 mm from lambda). The cannulas were lowered 5.5 mm from the surface of the skull, which placed the tip near the LC. The cannula assembly was fixed to the skull with dental cement, and anchor wires (one for the olfactory bulb, two for the LC) were placed ~3 mm from the cannula holes (Gilbert and Cain, 1980). To ensure patency of the cannulas, guide wires were placed in the lumen of the tubing until training. Olfactory bulb cannulas were implanted under visual guidance. After recovery from surgery (generally within 30 min), pups were returned to the litter and dam until training 2 d later.
Drug infusion protocol and conditioning
On P14, pups were placed in training chambers and bilateral cannulas were connected via polyethylene 10 tubing to a syringe pump (Harvard Apparatus, South Natick, MA) driving two microliter syringes (Hamilton, Reno, NV). The cannulas were filled (6 sec for olfactory bulb cannulas and 12 sec for LC cannulas at 0.5 μl/min) with either drug (described below) or vehicle. During both the 10 min behavioral adaptation period and the 10 min training period, drug or vehicle was infused at 0.1 μl/min for a total infusion volume of 2.0 μl as described previously (Sullivan et al., 1992, 2000b). Pups were given a 10 min adaptation period to permit acclimation to the training chamber and to allow drug distribution within the targeted brain area. During the subsequent 10 min, brain infusions continued, and pups were presented with citral odor (0.25 μl; Sigma, St. Louis, MO), which served as the conditioned stimulus (CS). After training, pups were disconnected from the syringe pump and returned to the nest until testing the next day. Throughout adaptation and training, pups were observed continuously to ensure that all pups were healthy and responding normally to experimental stimuli. Pups exhibiting abnormally high (i.e., aversive) or low (i.e., lethargic) behavioral responsiveness were eliminated from the experiment during adaptation or conditioning.
Olfactory bulb infusions
Pups with bilateral cannulas in the olfactory bulbs received either isoproterenol (50 μM; Sigma), an NE β-receptor agonist, or vehicle (saline) (Sullivan et al., 1989).
LC infusions
Pups with cannulas implanted into the LC received one or more of the following compounds: acetylcholine (2 mM acetylcholine chloride; Sigma), which activates the LC (Adams and Foote, 1988); phenylephrine (1 mM L-phenylephrine; Sigma), which is an α1 agonist that stimulates the excitatory LC autoreceptors (Mouly et al., 1995); or idazoxan (2 μM; Sigma), which is an α2 antagonist that blocks the inhibitory LC autoreceptors (Devauges and Sara, 1990; Ivanov and Aston-Jones, 1995; Sullivan et al., 2000b).
Systemic injections
To confirm the noradrenergic basis of LC manipulation effects on odor learning, a separate group of LC-infused pups was injected systemically with the β-receptor antagonist propranolol (20 mg/kg, i.p.) 30 min before training (Sullivan et al., 1989).
Behavior tests
On P15, pups were given one of two tests, depending on the experiments. Both tests, which have been widely used and dependably assess infant learning, are described below. No drugs were infused during testing, and drugs present during training had left the system the previous day (Goodman and Gilman, 1985).
Two-odor choice test
This test measures the amount of time pups spent over a familiar odor (clean pine nest shavings) versus the CS citral odor used during conditioning. The test apparatus consisted of a Plexiglas arena (24 cm long × 14 cm wide) with a wire mesh floor that enabled pups to smell the odor beneath them. The floor was divided into two parts, separated by a 2 cm midline. One side contained the odor CS (0.20 μl of citral placed on a 5 × 5 cm Kimwipe), and the other side contained a familiar pine odor (160 ml of wood chips). Pups were placed on the midline between the two odors (direction was counterbalanced), and the amount of time each pup spent over each odor was monitored for three 60 sec trials, with 10 sec between each trial. Between each trial, the floor was cleaned with distilled water and then dried (Cornwell-Jones, 1981; Sullivan et al., 1989).
Y-maze
This test requires pups to choose between two arms of a Plexiglas Y-maze (start box, 8.5 cm width, 10 cm length, 8 cm height; choice arms, 8.5 × 24 × 8 cm), one containing the citral odor CS and the other containing the familiar odor of pine shavings. Pups were placed in the start box (direction was counterbalanced), and after 5 sec, the door to each alley was opened, at which time pups were given 60 sec to choose an arm. A response was considered a choice when a pup’s entire body was past the entrance to the alley. Occasionally (three pups, only one trial each), pups did not make a choice within 60 sec, and in these cases, the pups were removed and returned to the maze to repeat the trial. Pups received five trials, with 30 sec between trials (Sullivan and Wilson, 1991).
Drug diffusion
To characterize the extent of drug diffusion within and outside of the LC, additional pups were used. On P12, pups were anesthetized by urethane and placed in a stereotaxic apparatus. Holes were drilled through the skull at 1.4 mm posterior to lambda and ±0.60 mm from the midline. A 10 μl Hamilton syringe was lowered 5.5 mm from the surface of the skull, which placed the tip near the LC. The pups were infused with 2 μl of a saline solution of [3H]NE (56.9 Ci · mmol−1 · μm−1, 0.37 Ci · mmol−1 · μl;−1; DuPont NEN, Boston, MA), and 20 min after infusion, the brains were quickly removed and frozen in methyl butane at −45°C. Brains were sliced in 20 μm coronal sections. The slides were apposed to a tritium storage phosphor screen (Amersham Biosciences, Arlington Heights, IL). After 14 d of exposure, the screen was scanned at a pixel density of 50 μm (5000 dots per cm2) with a Storm 820 PhosphorImager (Molecular Dynamics, Sunnyvale, CA). Phosphorimaging of the slides results in a tagged image file format (Tucker et al., 2002).
Histology
After behavioral testing, pups were overdosed with urethane and perfused with saline and 4% formalin. Brains were removed and postfixed in 4% formalin and 30% sucrose. Sections (40 μm) were cut, and cresyl violet staining was used to verify LC cannula placements.
Results
Behavioral observations of pups during training
Behavior was observed during training to ensure that the experimental manipulations did not adversely affect pups. Two pups were excluded from the study for exhibiting hyperactivity during training, and four other pups were excluded for weight loss between surgery and training. Pups gained weight over the course of the experiment, with initial weights of 28.67 gm (SE, ±1.62) and 27.96 gm (SE, ±0.94) for olfactory bulb and LC cannula pups, respectively. After testing, olfactory bulb and LC cannula pups weighed 33.71 gm (SE, ±1.18) and 32.45 gm (SE, ±1.02), respectively.
There were no statistical weight differences at either training or testing between groups for each cannula placement. Pup treatment weight gain did not change over the course of the experiment, although pups that did not gain weight after surgery were discontinued from the experiment (saline vs drug treatment at surgery, training, and testing (ANOVA; F(3,76) = 0.198; NS).
Postsensitive period LC activation does not support odor learning
In contrast to neonatal rat pups during the sensitive period (Sullivan et al., 2000b), preweanling (P14) rat pups cannot learn an odor preference by simple activation of the LC during an odor presentation. As shown in Figure 1, only pups that received isoproterenol infused into the olfactory bulb exhibited a preference for the citral odor CS. ACh infusion into the LC, which produces an odor preference in younger neonatal pups, was insufficient to produce an odor preference in these preweanling pups (ANOVA; F(3,26) = 6.834; p < 0.005). Post hoc Fisher tests revealed that olfactory bulb isoproterenol pups spent significantly more time over the CS odor than did the other groups (p < 0.05). It should be noted that there was a difference between the ACh LC group and the vehicle olfactory bulb groups (p < 0.05), but neither of these two groups differed from the vehicle LC group. Preweanling rat pups learn an NE-induced odor preference by reinstating the neonatal LC. As shown in Figure 2, only those pups that received activation of the LC by ACh concurrently with the blockade of LC inhibitory autoreceptors (α2 antagonist, idazoxan) and activation of the LC excitatory autoreceptors (α1 agonist, phenylephrine) during a citral odor presentation exhibited a subsequent preference for the citral odor (F(5,24) = 14.332; p < 0.0001). Pups that received any combination of less than three of these drugs or vehicle did not exhibit learning as determined by post hoc Fisher tests (each group spent significantly less time over the CS; p < 0.001). As illustrated in Figure 3, a systemic blockade of NE receptors during the training prevented the reinstatement of the NE-dependent odor learning seen during the neonatal sensitive period (ANOVA; conditioning group × odor; F(2,17) = 18.338; p < 0.0001; Fisher post hoc tests indicate that the pups injected with propranolol and infused concurrently with acetylcholine, idazoxan, and phenylephrine are significantly different from each of the other groups; p < 0.03).
Figure 1.
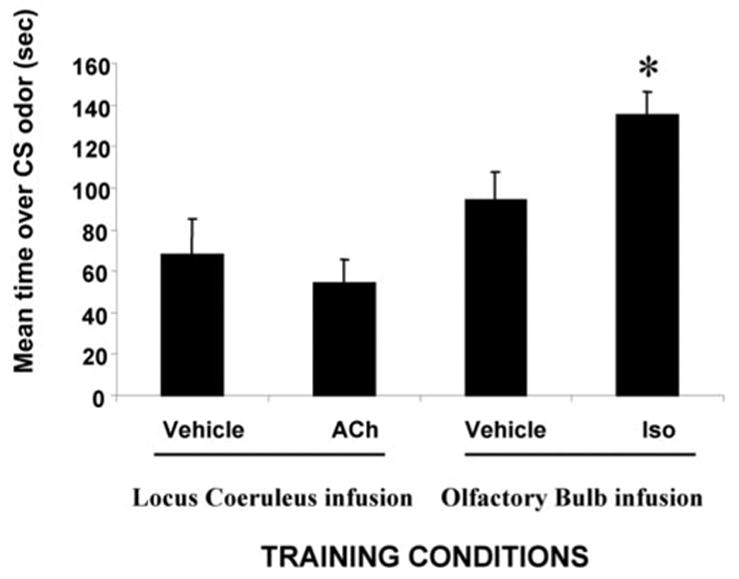
Mean ±SEM time spent over CS odor during the two-odor choice test. Training infusion into the olfactory bulb of the NE receptor agonist isoproterenol (Iso) significantly increased the learned relative odor preference compared with vehicle group. Training infusion into the locus ceruleus of ACh, an LC stimulant, did not produce a significant change in odor preference compared with vehicle groups (n=7–8 per group). The asterisk represents significant differences from all other groups (p <0.05).
Figure 2.
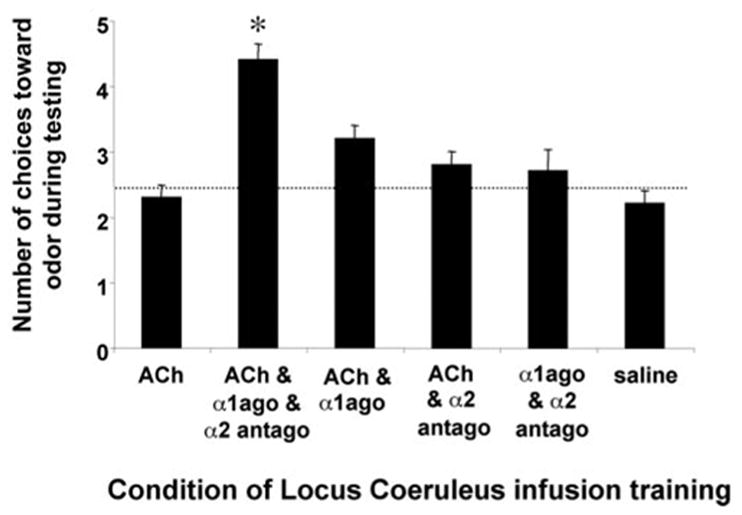
Mean ± SEM number of choices toward the CS odor during the Y-maze test. Training infusion of ACh and the α1 agonist phenylephrine (α1ago) and the α2 antagonist idazoxan (α2 antago) increased the learned relative odor preference compared with the other groups (n = 3–7 per group). The asterisk represents significant differences from all other groups (p < 0.001). The dotted horizontal line represents a chance line.
Figure 3.
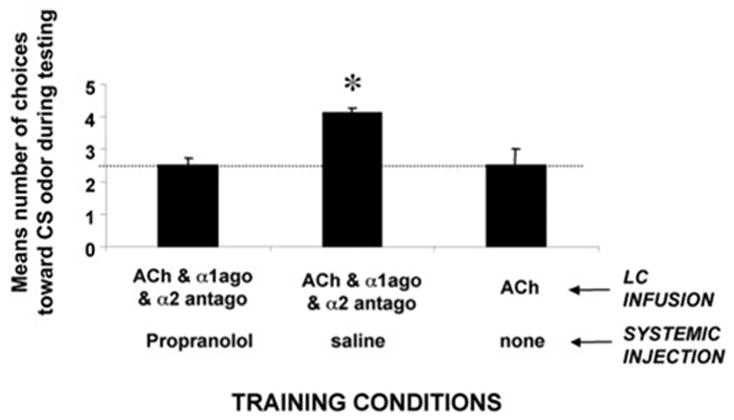
Mean ± SEM number of choices toward the CS odor during the Y-maze test. A pretraining injection of propranolol (20 mg/kg) significantly reduced the learned relative odor preference expressed by pups receiving infusion of ACh and the α1 agonist phenylephrine (α1ago) and the α2 antagonist idazoxan (α2 antago) compared with the control groups. The asterisk represents significant differences from all other groups (p < 0.03). The dotted horizontal line represents a chance line.
Drug diffusion
As demonstrated in Figure 4, the volume of drugs infused into the LC for data illustrated in Figure 2 diffused <1 mm from the LC.
Figure 4.
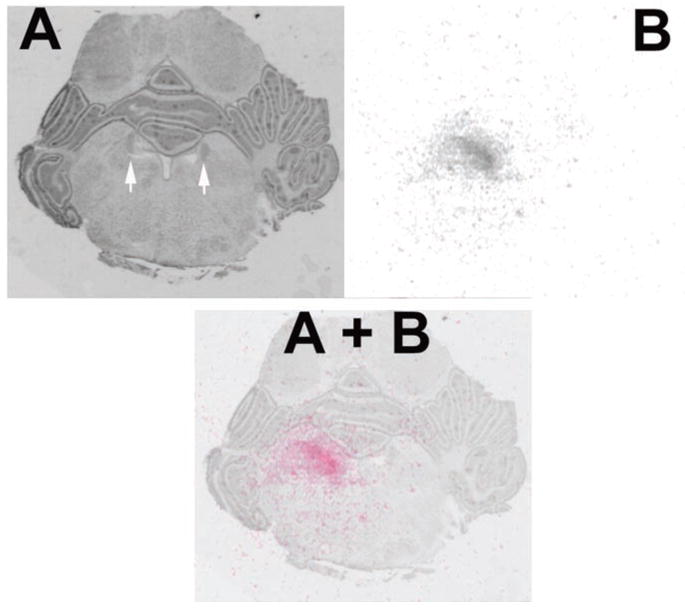
A, Section from a P14 pup counterstained with cresyl violet. The white arrows mark the locus ceruleus bilaterally. Actual cannula tip placement is outside the plane of this section. B, The same section as in A at the same magnification and orientation, characterizing the extent of [ 3H]NE drug diffusion within the LC. A+B, Color overlay of [ 3H]NE diffusion on the histological section showing drug diffusion over the region of the locus ceruleus.
Histology
Cannula tip placements for cannulas directed at the LC are shown in Figure 5. All tip placements were <1 mm from the LC except for one pup, which was excluded from the experiment. This pup, which received an infusion of ACh concurrently with phenylephrine and idazoxan, did not exhibit a subsequent preference for the citral odor, unlike pups receiving the same infusion into the LC.
Figure 5.
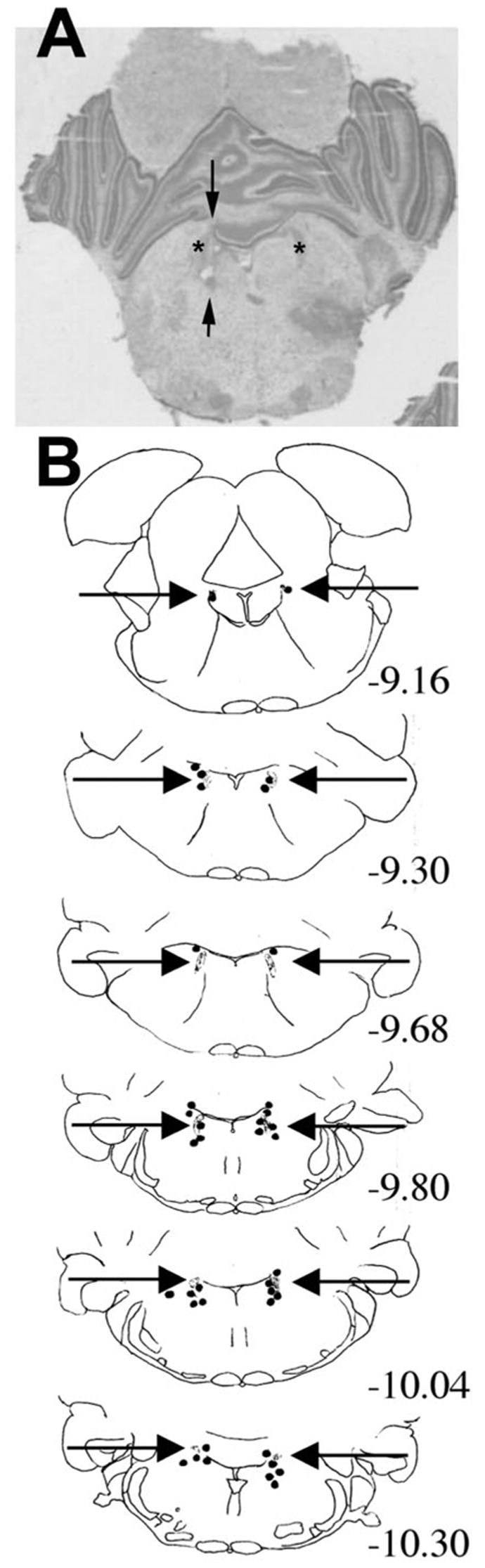
A, Representative location of cannula tip near the locus ceruleus. The cannula tip placement is marked by the arrows. The location of the locus ceruleus is illustrated by an asterisk. B, Locations of cannula tips (dots) in rats used for the experiment shown in Figure 2. The locus ceruleus is illustrated in gray and marked by the horizontal arrows. Corresponding sections from the adult stereotaxic atlas of Paxinos et al. (1991) were determined, and the relative distance from bregma for each coronal section on the basis of the adult atlas is noted on the right.
Discussion
These results suggest that the developmental emergence of auto-inhibition and attenuation of autoexcitation in the LC may be responsible, at least in part, for the termination of the infant rat’s sensitive period for learning. Our results have shown that preweanling (P14) pharmacological LC manipulations intended to reinstate neonatal sensitive period LC function (attenuating LC autoinhibition and amplifying LC autoexcitation) are sufficient to reinstate the neonatal NE-dependent odor learning. Specifically, in postsensitive period P14 pups, we reversed the LC characteristics that emerge at P10 by blocking the LC α2 inhibitory autoreceptors and activating the LC α1 excitatory autoreceptors. The pharmacologically neotenized LC, combined with LC cholinergic stimulation paired with a novel odor, was sufficient to produce an odor preference that was blocked by systemic blockade of NE β receptors.
Our results also suggest that the olfactory bulb remains plastic even after the sensitive period has terminated, because simply increasing olfactory bulb NE is sufficient to produce the rapid NE-dependent odor preference. Indeed, increasing NE via LC stimulation or direct olfactory bulb infusions showed that the olfactory bulb maintains its ability to produce rapid acquisition of an odor preference. These results complement previous results on the important role of NE in developmental plasticity other than learning, such as that which occurs in the developing visual system (Kasamatsu and Pettigrew, 1979; Kasamatsu et al., 1979; Bear and Singer, 1986; Shirokawa et al., 1989).
These results are in sharp contrast to the effects of NE on learning in adults, in which NE appears to have a modulatory effect on acquisition and on attention consolidation (Gold and Van Buskirk, 1975; Liang et al., 1990; Selden et al., 1990; McGaugh et al., 1996; Roozendaal et al., 1999; Feenstra et al., 2001). Specifically, similarly to neonates, neural correlates of learning could be achieved by large NE infusions directly into the olfactory bulb, hippocampus, and auditory cortex of the adult rat, suggesting that the mature brain retains at least some potential for NE-dependent plasticity, provided that sufficient levels of NE are available (Gray et al., 1986; Harley et al., 1996; Chaulk and Harley, 1998; Harley, 1998; Edeline, 1999).
The differences in the role of NE in neonatal and adult learning appear to be attributable to developmental differences in the response of the LC to sensory stimuli. The infant LC and adult LC are both activated by sensory stimuli, but the LC of the adult is less likely than the LC of the infant to respond to non-noxious stimuli (Foote et al., 1980; Kimura and Nakamura, 1985; Nakamura and Sakaguchi, 1990; Selden et al., 1990; Harris and Fitzgerald, 1991; Harley and Sara, 1992; Aston-Jones et al., 1994; Sara et al., 1995; Vankov et al., 1995; Mansour et al., 2003). Additionally, the LC of the adult habituates after repeated (or even single) stimulus presentations, whereas the LC of the infant fails to habituate (Nakamura et al., 1987; Nakamura and Sakaguchi, 1990; Vankov et al., 1995). Even more dramatic is the response duration of the neonatal LC compared with the preweanling–adult LC, with a 1 sec presentation of tactile stimulation causing a response of a few milliseconds in the LC of the adult but a 20 –30 sec response in the LC of the infant (Nakamura et al., 1987). These developmental differences in LC activity are reflected in dramatic age differences in stimulus-evoked release of NE in the olfactory bulb, with neonatal pups (sensitive-period age pups) releasing significantly more NE than slightly older pups (postsensitive period pups) (Rangel and Leon, 1995). This developmental decrease in the sensory-evoked release of NE in the olfactory bulb occurs despite the extensive increase in NE centrifugal fibers innervating the bulb at this developmental stage (McLean and Shipley, 1991). Together with the present results, these findings suggest that developmental changes in the LC may be responsible for the increasingly subtle role of NE in learning and memory as rats mature.
Throughout the lifespan, attachments continue to be formed within the context of mate selection, mating, and care of the young. Although the neural circuitry learning for these behaviors is more complex than that seen in the neonate, the importance of NE in learning re-emerges (Levy et al., 1990; Brennan and Keverne, 1997; Insel and Young, 2001). There is some evidence to suggest that the LC changes to accommodate the rapid learning required in these reproduction-related learning situations. Specifically, similarly to the LC of the neonatal rat pup, spontaneous activity is greatly diminished during pregnancy, although the amount of NE released is greatly enhanced (Nakamura et al., 1988).
The large release of NE during neonatal rat learning appears to underlie the neural changes uniquely associated with learning during the sensitive period. During acquisition, the NE from the LC maintains the responsiveness of mitral cells (primary output neurons of the olfactory bulb), whereas the mitral cells of control animals (backward, odor only) quickly habituate (Wilson et al., 1987). During consolidation, a cascade of molecular events is implicated in odor-preference learning in neonatal rats. An important interaction between serotonin and NE permits increasing mitral cell cAMP and cAMP response element-binding protein phosphorylation (Yuan et al., 2003; Zhang et al., 2003). This causes modifications in the olfactory bulb that last into adulthood, including enhanced odor-evoked glomerular layer 2-DG uptake and Fos-like immunoreactivity (Coopersmith and Leon, 1984; Sullivan and Leon, 1986; Johnson et al., 1995), modified odor response patterns of mitral–tufted cells (Wilson et al., 1987), and morphological changes in the glomerular layer (Woo et al., 1987). The acquisition of these olfactory bulb learning-induced changes is limited to the sensitive period (Woo et al., 1987; Sullivan and Wilson, 1991).
As described above, as the pup’s sensitive period ends, rapid odor-preference conditioning ends. However, other types of learning emerge, such as fear conditioning, inhibitory conditioning, and passive avoidance (Collier et al., 1979; Blozovski and Cudennec, 1980; Camp and Rudy, 1988; Myslivecek, 1997; Sullivan et al., 2000a). The functional emergence of the amygdala seems to be responsible for the emergence of the learned fear system (odor, 0.5 mA shock) (Sullivan et al., 2000a). Moreover, the functional emergence of the pup’s ability to release corticosterone in response to shock and other stressors may be related to the ability of the amygdala to function in the fear conditioning situation (odor, 0.5 mA shock) (Moriceau and Sullivan, 2003, 2004). Thus, developmental changes in pups’ learning abilities likely reflect the myriad of developmental brain changes occurring during early development. We hypothesized that the changing learning system reflects the changes in developmental demands placed on pups as new behaviors such as walking emerge. Other developmental changes in learning have been documented to coincide with weaning and with the emergence of hippocampal-dependent spatial learning and potentiated startle (Hunt and Campbell, 1999; Richardson et al., 2000; Stanton, 2000).
Although some prenatal olfactory experience may contribute to the pup’s olfactory attachment to the mother, postnatal experience in the form of classical conditioning appears necessary for attachment (Thoman et al., 1968; Galef and Sherry, 1973; Johanson and Hall, 1982; Pedersen et al., 1982; Alberts and May, 1984; Sullivan et al., 1986a,b). A similar role for somatosensory learning, particularly the facial whiskers, may also be important in early attachment (Polan and Hofer, 1998; Landers and Sullivan, 1999b). Both somatosensory and olfactory learning are NE dependent (Landers and Sullivan, 1999a). Because of the late maturation of the auditory and visual systems in the rat, dependence on odor and somatosensory learning may be critical for survival (Moye and Rudy, 1985, 1987; Hunt and Campbell, 1999; Richardson et al., 2000). Learning in the olfactory system seems to be particularly important in several behaviors critical for survival (i.e., nipple attachment, huddling, orientation to the dam), and damage to the olfactory system or the source of the odor stimuli greatly reduces survival (Singh and Tobach, 1975; Hofer, 1976). Together, these results suggest that a unique learning circuitry in the neonate has evolved to ensure that infants form a rapid, robust attachment to the mother (Sullivan et al., 2000b; Hofer and Sullivan, 2001). This unique neonatal learning circuit may be particularly important in the infant rat, in which structures important to adult learning have not yet developed [i.e., the amygdala (Sullivan et al., 2000a), the hippocampus (Rudy and Morledge, 1994), and the cerebellum (Freeman and Nicholson, 2000)]. The present results are a further indication that the LC is a fundamental component of this unique learning circuit, and that the special balance of NE autoreceptors within the developing LC helps define a sensitive period that is critical for infant learning and survival.
Footnotes
This work was supported by National Institute of Child Health and Human Development Grant HD33402 and National Science Foundation Grant IBN0117234 and by the Oklahoma Center for the Advancement of Science and Technology and University of Oklahoma research funds to R.M.S. We thank Donald Wilson for assistance in this project.
References
- Adams LM, Foote SL. Effects of locally infused pharmacological agents on spontaneous and sensory-evoked activity of the locus coeruleus. Brain Res Bull. 1988;21:395–400. doi: 10.1016/0361-9230(88)90151-7. [DOI] [PubMed] [Google Scholar]
- Alberts JR, May B. Nonnutritive, thermotactile induction of filial huddling in rat pups. Dev Psychobiol. 1984;17:161–181. doi: 10.1002/dev.420170207. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiac P, Alexinsky R. Locus ceruleus neurons in the monkey are selectively activated by attended stimuli in a vigilance task. J Neurosci. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Blozovski D, Cudennec A. Passive avoidance learning in the young rat. Dev Psychobiol. 1980;13:513–518. doi: 10.1002/dev.420130510. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Woods PJ. The ontogeny of behavior in the albino rat. Anim Behav. 1965;12:427–441. [Google Scholar]
- Bowlby J. Attachment. Vol. 1. New York: Basic Books; 1969. Attachment and loss. [Google Scholar]
- Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Prog Neurobiol. 1997;51:457–481. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev Psychobiol. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Chaulk PC, Harley CW. Intracerebroventricular norepinephrine potentiation of the perforant path-evoked potential in dentate gyrus of anesthetized and awake rats: a role for both alpha and beta adrenoceptor activation. Brain Res. 1998;787:59 –70. doi: 10.1016/s0006-8993(97)01460-1. [DOI] [PubMed] [Google Scholar]
- Collier AC, Mast J, Meyer DR, Jacobs CE. Approach-avoidance conflict in preweanling rats: a developmental study. Anim Learn Behav. 1979;7:514 –520. [Google Scholar]
- Coopersmith R, Leon M. Enhanced neural response to familiar olfactory cues. Science. 1984;225:849 –851. doi: 10.1126/science.6474157. [DOI] [PubMed] [Google Scholar]
- Coopersmith R, Leon M. Enhanced neural response by adult rats to odors experienced early in life. Brain Res. 1986;371:400 –403. doi: 10.1016/0006-8993(86)90384-7. [DOI] [PubMed] [Google Scholar]
- Cornwell-Jones JA. Conspecific odor preferences of male albino rats are reversed by intracerebral 6-hydroxydopamine. Brain Res. 1981;213:379–385. doi: 10.1016/0006-8993(81)90242-0. [DOI] [PubMed] [Google Scholar]
- Devauges V, Sara SJ. Activation of the noradrenergic system facilitates an attentional shift in the rat. Behav Brain Res. 1990;39:19 –28. doi: 10.1016/0166-4328(90)90118-x. [DOI] [PubMed] [Google Scholar]
- Edeline JM. Learning-induced physiological plasticity in the thalamo-cortical sensory systems: a critical evaluation of receptive field plasticity, map changes and their potential mechanisms. Prog Neurobiol. 1999;57:165–224. doi: 10.1016/s0301-0082(98)00042-2. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Vogel M, Botterblom MH, Joosten RN, De Bruin JP. Dopamine and noradrenaline efflux in the rat prefrontal cortex after classical aversive conditioning to an auditory cue. Eur J Neurosci. 2001;13:1051–1054. doi: 10.1046/j.0953-816x.2001.01471.x. [DOI] [PubMed] [Google Scholar]
- Fillion TJ, Blass EM. Infantile experience with suckling odors determined adult sexual behavior in male rats. Science. 1986;231:729 –731. doi: 10.1126/science.3945807. [DOI] [PubMed] [Google Scholar]
- Fleming AS, O’Day DH, Kraemer GW. Neurobiology of mother-infant interactions: experience and central nervous system plasticity across development and generations. Neurosci Biobehav Rev. 1999;23:673–685. doi: 10.1016/s0149-7634(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci USA. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Nicholson DA. Developmental changes in eye blink conditioning and neuronal activity in the cerebellar interpositus nucleus. J Neurosci. 2000;20:813–819. doi: 10.1523/JNEUROSCI.20-02-00813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galef GG, Sherry DF. Mother’s milk: medium for transmission of cues reflecting the flavor of mother’s diet. J Comp Physiol Psychol. 1973;83:374 –378. doi: 10.1037/h0034665. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Cain DP. Electrode implantation in infant rats for kindling and chronic brain recording. Behav Brain Res. 1980;1:553–555. doi: 10.1016/0166-4328(80)90010-8. [DOI] [PubMed] [Google Scholar]
- Glaser D. Child abuse and neglect and the brain: a review. J Child Psychol Psychiatr. 2000;41:97–116. [PubMed] [Google Scholar]
- Gold PE, Van Buskirk RB. Facilitation of time dependent memory processes with posttrial epinephrine injections. Behav Biol. 1975;13:145–153. doi: 10.1016/s0091-6773(75)91784-8. [DOI] [PubMed] [Google Scholar]
- Goodman A, Gilman LS. In: The pharmacological basis of therapeutics. Gilman AG, Goodman LS, Haff T, Murad F, editors. New York: MacMillan; 1985. pp. 1459–1489. [Google Scholar]
- Gray CM, Freeman WJ, Skinner JE. Chemical dependencies of learning in the rabbit olfactory bulb: acquisition of the transient spatial pattern change depends on norepinephrine. Behav Neurosci. 1986;100:585–596. doi: 10.1037//0735-7044.100.4.585. [DOI] [PubMed] [Google Scholar]
- Harley CW. Noradrenergic long-term potentiation in the dentate gyrus. Adv Pharmacol. 1998;42:952–956. doi: 10.1016/s1054-3589(08)60905-9. [DOI] [PubMed] [Google Scholar]
- Harley CW, Sara SJ. Locus coeruleus burst induced glutamate trigger delayed perforant path spike amplitude potentiation in the dentate gyrus. Exp Brain Res. 1992;89:581–587. doi: 10.1007/BF00229883. [DOI] [PubMed] [Google Scholar]
- Harley CW, Lalies MD, Nutt DJ. Estimating the synaptic concentration of norepinephrine in dentate gyrus which produces beta-receptor mediated long-lasting potentiation in vivo using microdialysis and intracerebroventricular norepinephrine. Brain Res. 1996;710:293–298. doi: 10.1016/0006-8993(95)01443-8. [DOI] [PubMed] [Google Scholar]
- Harris GC, Fitzgerald RD. Locus ceruleus involvement in the learning of classically conditioned bradycardia. J Neurosci. 1991;11:2314 –2320. doi: 10.1523/JNEUROSCI.11-08-02314.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer MA. Olfactory denervation: its biological and behavioral effects in infant rats. J Comp Physiol. 1976;90:829 –838. doi: 10.1037/h0077272. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Sullivan RM. Toward a neurobiology of attachment. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge, MA: MIT; 2001. pp. 599–616. [Google Scholar]
- Hunt PS, Campbell BA. Developmental dissociation of the components of conditioned fear. In: Bouton ME, Fanselow MS, editors. Learning, motivation and cognition. Washington, DC: American Psychological Association; 1999. pp. 53–74. [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nature Rev Neurosci. 2001;2:1–8. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Aston-Jones G. Extranuclear dendrites of locus coeruleus neurons: activation by glutamate and modulation of activity by alpha adrenoceptors. J Neurophysiol. 1995;74:2427–2436. doi: 10.1152/jn.1995.74.6.2427. [DOI] [PubMed] [Google Scholar]
- Johanson IB, Hall WG. Appetitive conditioning in neonatal rats: conditioned orientation to a novel odor. Dev Psychobiol. 1982;15:379 –397. doi: 10.1002/dev.420150410. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Duong H, Nguyen V, Leon M. A learned odor evokes an enhanced Fos-like glomerular response in the olfactory bulb of young rats. Brain Res. 1995;699:192–200. doi: 10.1016/0006-8993(95)00896-x. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T, Pettigrew JD. Preservation of binocularity after monocular deprivation in the striate cortex of kittens treated with 6-hydroxydopamine. J Comp Neurol. 1979;185:139 –161. doi: 10.1002/cne.901850109. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T, Pettigrew JD, Ary M. Restoration of visual cortical plasticity by local microperfusion of norepinephrine. J Comp Neurol. 1979;185:163–181. doi: 10.1002/cne.901850110. [DOI] [PubMed] [Google Scholar]
- Kimura F, Nakamura S. Locus coeruleus neurons in the neonatal rat: electrical activity and responses to sensory stimulation. Brain Res. 1985;23:301–305. doi: 10.1016/0165-3806(85)90055-0. [DOI] [PubMed] [Google Scholar]
- Kimura F, Nakamura S. Postnatal development of alpha-adrenoceptor-mediated autoinhibition in the locus coeruleus. Brain Res. 1987;432:21–26. doi: 10.1016/0165-3806(87)90004-6. [DOI] [PubMed] [Google Scholar]
- Landers M, Sullivan RM. Norepinephrine and associative conditioning in the neonatal rat somatosensory system. Brain Res Dev Brain Res. 1999a;114:261–264. doi: 10.1016/s0165-3806(99)00026-7. [DOI] [PubMed] [Google Scholar]
- Landers M, Sullivan RM. Vibrissae evoked behavior and conditioning before functional ontogeny of the somatosensory vibrissae cortex. J Neurosci. 1999b;19:5131–5137. doi: 10.1523/JNEUROSCI.19-12-05131.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy F, Gervais R, Kindermann U, Orgeur P, Piketty V. Importance of β-noradrenergic receptors in the olfactory bulb of sheep for recognition of lambs. Behav Neurosci. 1990;104:464 –469. doi: 10.1037//0735-7044.104.3.464. [DOI] [PubMed] [Google Scholar]
- Liang KC, McGaugh JL, Yao HY. Involvement of amygdala pathways in the influence of post-training intra-amygdala norepinephrine and peripheral epinephrine on memory storage. Brain Res. 1990;508:225–233. doi: 10.1016/0006-8993(90)90400-6. [DOI] [PubMed] [Google Scholar]
- Mansour AA, Babstock DM, Penney JH, Martin GM, McLean JH, Harley CW. Novel objects in a holeboard probe the role of the locus coeruleus in curiosity: support for two modes of attention in the rat. Behav Neurosci. 2003;117:621–631. doi: 10.1037/0735-7044.117.3.621. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: interaction with other brain systems. Proc Natl Acad Sci USA. 1996;93:13508 –13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JH, Shipley MT. Postnatal development of the noradrenergic projection from the locus coeruleus to the olfactory bulb in the rat. J Comp Neurol. 1991;304:467–477. doi: 10.1002/cne.903040310. [DOI] [PubMed] [Google Scholar]
- McLean JH, Harley CW, Darby-King A, Yuan Q. pCREB in the neonate rat olfactory bulb is selectively and transiently increased by odor preference-conditioned training. Learn Mem. 1999;6:608 –618. doi: 10.1101/lm.6.6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CL, Jordan L, Wong L. Early olfactory experience, novelty and choice of sexual partner by male rats. Physiol Behav. 1996;60:1361–1367. doi: 10.1016/s0031-9384(96)00249-1. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Corticosterone influences on mammalian imprinting. International Society for Developmental Psychobiology Abstr; New Orleans. November.2003. [Google Scholar]
- Moriceau S, Sullivan RM. Corticosterone influences on mammalian neonatal sensitive period learning. Behav Neurosci. 2004 doi: 10.1037/0735-7044.118.2.274. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly AM, Eleegouby A, Ravel N. A study of the effects of noradrenaline in the rat olfactory bulb using evoked field potential response. Brain Res. 1995;681:47–57. doi: 10.1016/0006-8993(95)00280-4. [DOI] [PubMed] [Google Scholar]
- Moye TB, Rudy JW. Ontogenesis of learning. VI. Learned and unlearned responses to visual stimulation in the infant hooded rat. Dev Psychobiol. 1985;18:395–409. doi: 10.1002/dev.420180505. [DOI] [PubMed] [Google Scholar]
- Moye TB, Rudy JW. Ontogenesis of trace conditioning in young rats: dissociation of associative and memory processes. Dev Psychobiol. 1987;20:405–414. doi: 10.1002/dev.420200405. [DOI] [PubMed] [Google Scholar]
- Myslivecek J. Inhibitory learning and memory in newborn rats. Prog Neurobiol. 1997;53:399 –430. doi: 10.1016/s0301-0082(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Sakaguchi T. Development and plasticity of the locus coeruleus. A review of recent physiological and pharmacological experimentation. Prog Neurobiol. 1990;34:505–526. doi: 10.1016/0301-0082(90)90018-c. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kimura F, Sakaguchi T. Postnatal development of electrical activity in the locus ceruleus. J Neurophysiol. 1987;58:510 –524. doi: 10.1152/jn.1987.58.3.510. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Sakaguchi T, Shirokawa T, Yamatodani A, Maeyama K, Wada H. Changes in the electrical activity of locus coeruleus neurons in pregnant rats. Neurosci Lett. 1988;85:329 –332. doi: 10.1016/0304-3940(88)90587-3. [DOI] [PubMed] [Google Scholar]
- Pager J. A selective modulation of olfactory bulb electrical activity in relation to the learning of palatability in hungry and satiated rats. Physiol Behav. 1974;12:189 –195. doi: 10.1016/0031-9384(74)90172-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Tork I, Tecott LH, Valentino KL. Atlas of the developing rat brain. San Diego: Academic; 1991. [Google Scholar]
- Pedersen PE, Williams CL, Blass EM. Activation and odor conditioning of suckling behavior in 3-day-old albino rats. J Exp Psychol Anim Behav Process. 1982;8:329 –341. [PubMed] [Google Scholar]
- Pieribone VA, Nicholas AP, Dagerlind A, Hokfelt R. Distribution of α1 adrenoreceptors in rat brain revealed by in situ hybridization experiments utilizing subtype specific probe. J Neurosci. 1994;14:4252–4268. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polan HJ, Hofer MA. Olfactory preference for mother over home nest shavings by newborn rats. Dev Psychobiol. 1998;33:5–20. [PubMed] [Google Scholar]
- Rangel S, Leon M. Early odor preference training increases olfactory bulb norepinephrine. Brain Res Dev Brain Res. 1995;85:187–191. doi: 10.1016/0165-3806(94)00211-h. [DOI] [PubMed] [Google Scholar]
- Richardson R, Paxinos G, Lee J. Ontogeny of conditioned odor potentiation of startle. Behav Neurosci. 2000;114:1167–1173. [PubMed] [Google Scholar]
- Roozendaal B, Nguyen BT, Power AE, McGaugh JL. Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proc Natl Acad Sci USA. 1999;96:11642–11647. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav Neurosci. 1994;108:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Dyon-Laurent C, Herve A. Novelty seeking behavior in the rat is dependent upon the integrity of the noradrenergic system. Brain Res Cogn Brain Res. 1995;2:181–187. doi: 10.1016/0926-6410(95)90007-1. [DOI] [PubMed] [Google Scholar]
- Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau RT., Jr Distribution of α2 adrenergic receptor subtype gene expression in rat brain. Brain Res Mol Brain Res. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Schore AN. The effects of early relational trauma on right brain development, affect regulation, and infant mental health. Inf Ment Health J. 2001;22:201–269. [Google Scholar]
- Selden NR, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1990;42:335–350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- Shah A, Oxley G, Lovic V, Fleming AS. Effects of preweaning exposure to novel maternal odors on maternal responsiveness and selectivity in adulthood. Dev Psychobiol. 2002;41:187–196. doi: 10.1002/dev.10064. [DOI] [PubMed] [Google Scholar]
- Shirokawa T, Kasamatsu T, Kuppermann BD, Ramachandran VS. Noradrenergic control of ocular dominance plasticity in the visual cortex of dark reared cats. Brain Res Dev Brain Res. 1989;47:303–308. doi: 10.1016/0165-3806(89)90187-9. [DOI] [PubMed] [Google Scholar]
- Singh PJ, Tobach E. Olfactory bulbectomy and nursing behavior in rat pups. Dev Psychobiol. 1975;8:151–164. doi: 10.1002/dev.420080207. [DOI] [PubMed] [Google Scholar]
- Stanton ME. Multiple memory systems, development and conditioning. Behav Brain Res. 2000;110:25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- Sullivan RM. Unique characteristics of neonatal classical conditioning: the role of the amygdala and locus coeruleus. Integr Physiol Behav Sci. 2001;36:293–307. doi: 10.1007/bf02688797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM. Developing a sense of safety: the neurobiology of neonatal attachment. Ann NY Acad Sci. 2004 doi: 10.1196/annals.1301.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Brain Res. 1986;27:278 –282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. Neural correlates of conditioned odor avoidance in infant rats. Behav Neurosci. 1991;105:307–312. doi: 10.1037//0735-7044.105.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Brake S, Hofer MA. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Dev Psychobiol. 1986a;19:615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Brake S, Hofer MA, Williams CL. Huddling and independent feeding in neonatal rats is enhanced by conditioned change in behavioral state. Dev Psychobiol. 1986b;19:625–635. doi: 10.1002/dev.420190613. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Leon M. Norepinephrine and learning-induced plasticity in infant rat olfactory system. J Neurosci. 1989;9:3998 –4006. doi: 10.1523/JNEUROSCI.09-11-03998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Zyzak D, Skierkowski P, Wilson DA. The role of olfactory bulb norepinephrine in early olfactory learning. Brain Res Dev Brain Res. 1992;70:279 –282. doi: 10.1016/0165-3806(92)90207-d. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Lemon C, Gerhardt G. Bilateral 6-OHDA lesions of the locus coeruleus impair associative olfactory learning in newborn rats. Brain Res. 1994;643:306 –309. doi: 10.1016/0006-8993(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy: ontogeny of conditioned fear and the amygdala. Nature. 2000a;407:38 –39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Stackenwalt G, Nasr F, Lemon C, Wilson DA. Association of an odor with activation of olfactory bulb noradrenergic β receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behav Neurosci. 2000b;114:957–962. doi: 10.1037/0735-7044.114.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersena SL, Polcarib A, Andersena CM, Navaltae CP, Kima DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Thoman E, Wetzel A, Levine S. Learning in the neonatal rat. Anim Behav. 1968;16:54 –57. doi: 10.1016/0003-3472(68)90108-5. [DOI] [PubMed] [Google Scholar]
- Tucker DL, Tucker N, Conway T. Gene expression profiling of the pH response in Escherichia coli. J Bacteriol. 2002;183:6551–6558. doi: 10.1128/JB.184.23.6551-6558.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankov A, Herve-Minvielle A, Sara SJ. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci. 1995;7:1180 –1187. doi: 10.1111/j.1460-9568.1995.tb01108.x. [DOI] [PubMed] [Google Scholar]
- Vermer RW, Van Vulpen EH, Van Uum JF. Prefrontal development of amygdaloid projections to the prefrontal cortex in the rat studied with retrograde and anterograde tracers. J Comp Neurol. 1996;376:75–96. doi: 10.1002/(SICI)1096-9861(19961202)376:1<75::AID-CNE5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Leon M. Noradrenergic modulation of olfactory bulb excitability in the postnatal rat. Brain Res Dev Brain Res. 1988;42:69 –75. doi: 10.1016/0165-3806(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Olfactory associative conditioning in infant rats with brain stimulation as reward. I. Neurobehavioral consequences. Brain Res Dev Brain Res. 1990;53:215–221. doi: 10.1016/0165-3806(90)90009-n. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Role of the amygdala complex in early olfactory associative learning. Behav Neurosci. 1993;107:254 –263. doi: 10.1037//0735-7044.107.2.254. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Neurobiology of associative learning in the neonate: early olfactory learning. Behav Neural Biol. 1994;61:1–18. doi: 10.1016/s0163-1047(05)80039-1. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM, Leon M. Single unit analysis of postnatal olfactory learning: modified olfactory bulb output response patterns to learned attractive odors. J Neurosci. 1987;7:3154 –3162. doi: 10.1523/JNEUROSCI.07-10-03154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Broide RS, Chen Y, Leslie FM. Highly sensitive radioactive in situ hybridization using full-length hydrolyzed riboprobes to detect alpha 2 adrenoreceptor subtype mRNAs in adult and developing rat brain. Brain Res Brain Res Protoc. 1999;3:229 –241. doi: 10.1016/s1385-299x(98)00043-9. [DOI] [PubMed] [Google Scholar]
- Woo CC, Leon M. Sensitive period for neural and behavioral response development to learned odors. Brain Res. 1987;433:309 –313. doi: 10.1016/0165-3806(87)90038-1. [DOI] [PubMed] [Google Scholar]
- Woo CC, Coopersmith R, Leon M. Localized changes in olfactory bulb morphology associated with early olfactory learning. J Comp Neurol. 1987;263:113–125. doi: 10.1002/cne.902630110. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, McLean JH, Knopfel T. Optical imaging of odor preference memory in the rat olfactory bulb. J Neurophysiol. 2003;87:3156 –3159. doi: 10.1152/jn.00917.2001. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Okutani F, Yagi F, Inoue S, Kaba H. Facilitatory effect of ritanserin is mediated by dopamine D(1) receptors on olfactory learning in young rats. Dev Psychobiol. 2003;37:246 –252. [PubMed] [Google Scholar]


