Abstract
Gene therapy is defined as the treatment of disease by transfer of genetic material into cells. This review will explore methods available for gene transfer as well as current and potential applications for craniofacial regeneration, with emphasis on future development and design. Though non-viral gene delivery methods are limited by low gene transfer efficiency, they benefit from relative safety, low immunogenicity, ease of manufacture, and lack of DNA insert size limitation. In contrast, viral vectors are nature’s gene delivery machines that can be optimized to allow for tissue-specific targeting, site-specific chromosomal integration, and efficient long-term infection of dividing and non-dividing cells. In contrast to traditional replacement gene therapy, craniofacial regeneration seeks to use genetic vectors as supplemental building blocks for tissue growth and repair. Synergistic combination of viral gene therapy with craniofacial tissue engineering will significantly enhance our ability to repair and replace tissues in vivo.
Keywords: gene therapy, gene transfer, vector design, tissue engineering, virus, regeneration
INTRODUCTION
Human gene therapy is defined as the treatment of disorder or disease through transfer of engineered genetic material into human cells, often by viral transduction. Since the introduction of science fiction, the popular press has toyed with the notion of viral gene delivery and its terrifying implications. One of the more recent popular works on the topic is the 2007 remake of Richard Matheson’s classic 1954 novel I Am Legend, which details events following the discovery, release, and mutation of a genetically re-engineered measles virus that was initially hailed as the cure for cancer (Matheson, 1954; Lawrence, 2007). This adapted novel, which has been redone in three instances as a feature film, outlines the seemingly inevitable worldwide destruction that could result from viral gene therapy. With an emotionally stirring history of fictional violence and a debate that provokes both moral and medical issues, it may be surprising that, since 1990, billions of dollars have been spent on hundreds of human viral gene therapy clinical trials. Our society is in the midst of a paradigm shift that began with the discovery of viruses as dangerous infectious agents and will end with the use of viruses to cure disease and regenerate tissues.
On January 19, 1989, the director of the National Institutes of Health (NIH), Dr. James A. Wyngaarden, approved the first clinical protocol to insert a foreign gene into the immune cells of persons with cancer (Roberts, 1989). On September 14, 1990, W. French Anderson and his colleagues at the NIH performed the first approved gene therapy procedure on a four-year-old girl born with severe combined immunodeficiency (SCID) (Anderson, 1990). Despite the viral horror stories written by the popular media, this initial trial was largely a success, and the most recent report on this individual in 2004 noted that she is thriving as an 18-year-old teenager in suburban Cleveland (Springen, 2004). Over the next ten years, 300 clinical gene therapy trials were performed on about 3000 individuals (McKie, 2000). The field was then blackened with the death of an 18-year-old male four days after the introduction of 38 trillion particles of recombinant adenovirus into his liver (Somia and Verma, 2000). Despite this tragedy, we continue to move forward because of the great promise of novel genetic treatments that, when perfected, will likely outshine current methods, such as protein therapy or pharmacotherapeutics, for treatment of many diseases and defects.
We are now nearing the 20-year mark since the first gene therapy trial. Though success has been limited, the future still seems overwhelmingly promising, and we are steadily approaching an acceptable safety record. This review will explore non-viral and viral methods available for transgene introduction as well as their current and potential applications for craniofacial regeneration and therapy, with emphasis on future development and design.
NON-VIRAL GENE DELIVERY
Though this review will focus mostly on viral methods of gene delivery, it is essential to recognize that many advances have been made in the field of non-viral gene therapy. Polymeric gene delivery is desired because of its relative safety, low immunogenicity and toxicity, ease of administration and manufacture, and lack of DNA insert size limitation (Park et al., 2006). The main disadvantage is insufficient gene transfer efficiency due to the need for post-uptake endosomal escape and nuclear translocation of the DNA complex (Park et al., 2006). In this respect, clinical efficiency and specificity standards have not yet been met.
Synthetic Polymers
The main strategy for most synthetic polymer delivery systems is to generate cationic polymers to interact electrostatically with and neutralize negatively charged DNA (Park et al., 2006). This facilitates properties such as protection from DNAses. If a net positive charge is maintained, the polymer/DNA complex can adhere to the cell surface glycocalyx and be internalized by endocytic mechanisms. Unfortunately, the use of endocytic uptake from the external environment perpetuates the need for endocytic escape into the cytosol. This challenge in the polymeric gene delivery field has been addressed by multiple strategies, including incorporation of fusogenic peptides for endosomal membrane binding and disruption (Cho et al., 2003) and by balancing a hydrophobic cholesterol group with hydrophilic polymers to enhance escape (Mahato et al., 2001).
One of the first polymers recognized for its ability to form nanoparticulate polyelectrolyte complexes with DNA was Poly L-lysine (PLL) (Laemmli, 1975). Unfortunately, this cationic material was found to have high cytotoxicity (Choi et al., 1998) and a tendency to aggregate and precipitate (Liu et al., 2001). The solution to this dilemma was found in the form of the flexible, water-soluble polymer polyethylene glycol (PEG). Covalent coupling of PEG, or ‘PEGylation’, of a target molecule such as PLL limits its cytotoxicity and non-specific protein adsorption (Choi et al., 1998). This strategy has also been used with polyethyleneimine (PEI), a cationic gene carrier with superior transfection efficiency and unique buffering properties (Boussif et al., 1995), similarly to reduce the extent of inter-particular aggregation (Mishra et al., 2004; Quick and Anseth, 2004).
In addition to improving the bio-properties of PLL and PEI, PEGylated polymers can be conjugated to specific targeting moieties, such as sugars, antibodies, peptides, and folate (Lee and Kim, 2005). For example, peptide conjugation of the apoB-100 fragment of low-density lipoprotein can increase transfection efficiency in bovine aorta and smooth-muscle cells 150- to 180-fold (Nah et al., 2002), and RGD peptides can allow for increased selection of endothelial cells (Kim et al., 2005). To summarize, synthetic PEGylated polymers such as PLL and PEI are promising gene delivery molecules. Future study in this field is focused on biodegradable polycations such as poly(β-amino ester), poly(2-aminoethyl propylene phosphate), and degradable PEI to decrease cytotoxicity and increase transfection efficiency (Akinc et al., 2003).
Natural Polymers
The natural polymer family contains materials such as cyclodextrin, chitosan, collagen, gelatin, and alginate. When compared with synthetic materials, natural polymers have the advantage of innate environmental responsiveness and the ability to be degraded and remodeled by cell-secreted enzymes. They are non-toxic at both low and high concentrations, are readily incorporated into oral or bolus matrix delivery systems, and can serve as tissue engineering scaffolds (Dang and Leong, 2006). The simplicity of oral delivery and mucoadhesive properties of materials such as chitosan make it an interesting potential polymer for gene delivery and vaccination (Roy et al., 1999). The transfection efficiency of natural polymers such as cyclodextrin, though significantly less than that of virus, is similar to that of PEI and lipofectamine (Gonzalez et al., 1999). Thus, while natural polymers benefit from degradation and remodeling, they still face significant transfection issues due to the requirement for endosomal escape. However, this strategy has been successfully used to increase bone regeneration with polymer ‘gene activated matrix’ containing DNA encoding parathyroid hormone (Bonadio et al., 1999; Chen et al., 2003)
VIRAL GENE DELIVERY
Viruses have undergone millions of years of evolution and are a species-conserved way of introducing DNA to cells (Dewannieux et al., 2006). Scientists are now attempting to fine-tune these gene delivery vehicles for treatment of human disease and defects. Regardless of method selection, there are three universal requirements for viral gene therapy vectors. First, the delivery system must be safe and immunologically inert. Second, it must protect the genetic material from degradation. Third, the vector must encode an effective therapeutic gene that has sustained expression at a defined target site. For true commercial application, the packaged vector must also be easily produced and processed and have a reasonable shelf-life. As we near the 20-year mark from the first human gene therapy clinical trial, significant advances have been made in satisfying these three requirements. However, new objectives—such as tissue-specific targeting, site-specific chromosomal integration, and controlled infection of both dividing and non-dividing cells—have emerged. Though negative publicity has attached a significant stigma to viral gene therapy, it is indeed the most efficient method of gene transfer, and basic research and clinical trials are rapidly moving to overturn the safety concerns.
Our society is in the midst of a paradigm shift that began with the discovery of viruses as dangerous infectious agents and will end with the use of viruses to cure disease and regenerate tissues. However, safety concerns still limit the universal acceptance of this strategy. These concerns include the accidental generation of replication competent viruses during vector production and the packaging or mobilization of the engineered vector by endogenous retroviruses present in the human genome (Connolly, 2002). Either of these could lead to horizontal dissemination of new viruses from gene therapy patients. Localized concerns include random insertion or mutagenesis of the vector leading to cancer, or germ cell alteration resulting in vertical inheritance of the acquired gene (Connolly, 2002). The need for controlled genome integration hit home when two of the 11 persons treated for X-SCID with retrovirally transduced stem cells developed leukemia due to insertion of the transgene near the oncogenic gene LMO2 (Kaiser, 2003). Site-specific chromosomal integration, conditional expression of the transgene only in target cells, and the use of self-inactivating (SIN) retroviral vectors have been proposed (Yu et al., 1986) and may significantly improve the safety of viral therapy. The following sections will review the use of viral vectors for in vivo therapy, emphasizing the construction and advantages of different viruses.
Retrovirus
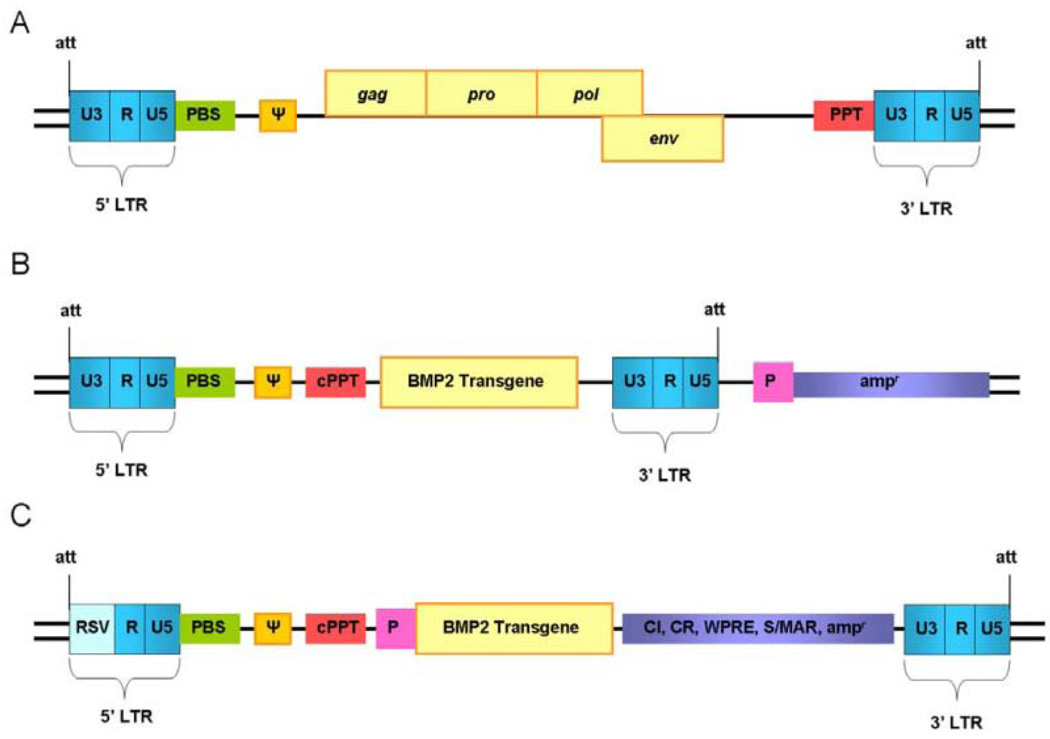
Before we can successfully manipulate retroviral vectors, the composition of their genome must be thoroughly understood. Since the discovery of retroviruses in 1910, when Peyton Rous induced malignancy in chickens by the injection of cell-free filtrates from muscle tumor (VanEpps, 2005), we have gained much insight into their mechanism of action. Three main classes of recombinant retroviruses are used as tools in gene delivery: γ-retroviruses, lentiviruses, and spumaviruses (Chang and Sadelain, 2007). Despite their negative press, exogenous retroviruses have been used in many biological studies and facilitated the discovery of proto-oncogenes (Martin, 2004), the manipulation and investigation of intracellular pathways, and successful ex vivo treatment of persons with hemophilia and SCID (Sumimoto and Kawakami, 2007; Chu et al., 2008; Scheller et al., 2008). Retroviruses are 80- to 100-nm enveloped viruses that contain linear, non-segmented, single-stranded RNA. Retroviruses are naturally self-replicating for viral assembly and re-infection (Kurian et al., 2000). Reverse transcription allows for the generation of double-stranded DNA from the transduced 7- to 12-kBp RNA and subsequent insertion into the genome. Exogenous retroviruses can be subdivided into simple and complex categories based on the composition of their RNA vector. Simple vectors contain three basic genes—gag, pol, and env—which are necessary for viral replication and must be removed prior to gene therapy (Buchschacher, 2001) (Fig. 1A). Identical long terminal repeats (LTRs) are present at each end of the retroviral genome. The LTRs contain promoter, enhancer, and integration sequences which facilitate interaction with attachment sites via integrase (Engelman, 1999). Complex retroviruses contain up to 15 additional accessory genes, such as tat, the transcriptional transactivator for HIV-1, vif, rev, nef, etc. (Frankel and Young, 1998).
Figure 1.
Retroviral vector development for increased efficiency and targeting. (A) Structure of a simple retroviral genome containing coding sequences for gag, pro, pol, and env for replication. (B) Structure of a simple γ-retroviral gene therapy vector with replication coding sequences removed and transgene inserted. Note retention of the dual long-terminal repeats (LTRs), primer binding site (PBS), signal Ψ, attachment sites (att), and polypurine tract (PPT). The U3 component of the 5′LTR is used as a promoter to drive transgene expression. A second heterologous or tissue-specific promoter (P) has been inserted to drive an ampicillin gene to facilitate ex vivo selection of transduced cells. (C) Structure of an enhanced self-inactivating retroviral gene therapy vector (note substitution of the 5′LTR U3 component). The internal promoter (P) is tissue-specific to limit transgene expression. Additional genetic elements—such as chromatin insulators (CI), chromatin structure regulators (CR), a woodchuck hepatitis virus post-transcriptional regulatory element (WPRE), scaffold or matrix attachment regions (S/MAR), or resistance genes—are incorporated to enhance site specificity and integration efficiency while limiting gene silencing.
The use of retrovirus as a gene delivery system necessitates re-engineering of the viral genome to block autonomous replication while maintaining integration efficiency. This is accomplished via the maintenance of cis-acting and removal of trans-acting factors. Five essential components for successful viral gene expression by any LTR-driven γ-retroviral vector include dual-LTRs, att site, primer binding site, signal psi, and the polypurine tract (Zhang and Godbey, 2006) (Fig. 1B). The polypurine tract aids in transport of the pre-integration complex to the nucleus and allows for internal initiation of second-strand DNA synthesis (Zennou et al., 2000). Most γ-retroviral vectors rely on their LTRs to drive robust and ubiquitous transgene expression. Replacement of these with site-specific constitutive promoters is currently limited by expression strength and promoter silencing, but remains an active area of research that could allow for customization of transgene expression level and location. Additional vector design strategies to enhance gene expression and reduce silencing include: deletion of silencing elements (Zufferey et al., 1998), incorporation of robust promoters such as U3 or PGK (Chang and Sadelain, 2007), incorporation of woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) to enhance mRNA transcript stability (Loeb et al., 2002), incorporation of scaffold or matrix attachment regions for anchorage of chromatin with stabilization of chromosomal loops (Agarwal et al., 1998), and use of insulators such as the chicken β-globin locus control region to limit position effect variegation (West et al., 2002) (Fig. 1C). Expression in bacterial plasmid form can be used for easy amplification and incorporation of drug resistance or response genes to facilitate selection ex vivo or expression in vivo (Delviks and Pathak, 1999; Jaalouk et al., 2000). Addition of the gene of interest or resistance gene under the control of a tissue-specific promoter results in a dual-promoter vector designed to enhance selection and integration. However, simultaneous use of two promoters can result in significantly reduced expression of both due to promoter interference (Apperley et al., 1991). SIN vectors which develop a defective promoter in the viral LTR have been used to circumvent this effect (Buchschacher, 2001) (Fig. 1C). Understanding and engineering of these vectors is rapidly advancing. For example, it has been shown that ex vivo transduction of hematopoietic stem cells can be improved through control of cell-cycle stage during virus delivery (Korin and Zack, 1998) and the use of proteosome inhibitors (Goff, 2004).
Retroviruses require genome integration of their vector to function, and most, excluding lentiviruses such as HIV-1, are able to infect only dividing cells (Lewis and Emerman, 1994). Stable integration of retroviral vectors is known to occur near expressed genes and appears to be non-random (Mitchell et al., 2004). This allows for long-term expression of the transgene and makes retroviruses the vector of choice for ex vivo and in vivo transduction of highly replicative populations such as tumor cells and hematopoietic cells for treatment of chronic disease and genetic deficiency (Somia and Verma, 2000). Indeed, LTR-driven γ-retroviral vectors have been used in over 45 ex vivo clinical trials to treat diseases such as hemophilia, SCID, and leukemia (Kohn et al., 2003; NIH, 2008b). To advance gene therapy with retroviral vectors, increased transduction efficiency and gene expression, site-specific chromosomal integration, and cell-specific targeting are necessary. Ex vivo or in vivo γ-retroviral transduction of rapidly dividing cells at sites of wound healing and new bone synthesis may be used in the future to enhance craniofacial tissue regeneration. Expansion of this field to include lentiviral vectors targeting non-dividing cells could allow for the long-term restoration of non-functional salivary gland tissue or repair of quiescent periodontal defects.
Adenovirus
Adenoviridae were initially isolated by Wallace Rowe in 1953 from adenoid explants as the “virus of the common cold” (Rowe et al., 1957; Ginsberg, 1999). They have since become attractive tools for gene therapy, given that their infection is generally self-limiting and non-fatal (Zhang and Godbey, 2006). Adenoviruses are the largest non-enveloped virus and contain linear, double-stranded DNA. Over 50 serotypes have been identified, but the most common in nature and in adenoviral gene therapy are group C human serotypes 2 and 5 (Barnett et al., 2002). The icosahedral adenoviral capsid is made up of hexon and penton proteins, knobbed fibers, and stabilizing minor cement proteins. These surround the core proteins and large 36-kBp adenoviral genome (Verma and Somia, 1997). The ends of the genome have inverted terminal repeats which flank a coding region capable of encoding more than 30 viral genes (Zhang and Godbey, 2006). These genes are termed ‘early’ or ‘late’, depending on their temporal expression. Early genes function as regulatory proteins for viral replication, while late genes encode structural proteins for new virus assembly (Zhang and Godbey, 2006). Entry of adenovirus into the cell occurs when penton base proteins bind integrins for clathrin-mediated endocytosis. Subsequent disruption of the endosome and capsid allows for viral core entry into the nucleus (Russell, 2000). Initiation of the ‘immediate early’ infection phase activates transcription of the E1A gene, a trans-acting transcriptional regulatory factor that is required for early gene activation (E1B, E2A, E2B, E3, E4, viron proteins) (Russell, 2000). The final ‘late’ infection phase activates genes L1 to L5 through complex splicing; viral particles which accumulate in the nucleus are then released via cell lysis (Zhang and Godbey, 2006).
During the engineering of adenoviral vectors for gene delivery, up to 30 kbp of the 36-kbp genome can be replaced with foreign DNA (Smith, 1995). Multiple strategies have been used to produce replication-defective, transforming adenoviral vectors. In first-generation adenoviral vectors, E1 and E3 genes are deleted to allow for a 6.5-kbp insertion. However, cell-line endogenous expression of E1 can lead to E2 expression and viral replication at low levels (Russell, 2000). Other first-generation vectors have used deletion of E2 and E4 regions to allow for an insert of greater than 6.5 kbp (Lusky et al., 1998). These vectors are impaired by limited expression and a robust inflammatory response (Khan et al., 2003). Second-generation ‘gutless’ vectors appear to be the most promising. Gutless vectors retain only the inverted terminal repeats and packaging sequence around the transgene (Russell, 2000). This results in prolonged transgene expression, increased insert size allowance, and reduced immune response (Fleury et al., 2004). Studies have shown that adenoviral gene expression occurs via episome formation, and only 1 in 1000 infectious units can integrate into the genome (Tenenbaum et al., 2003). Though this decreases the risk of insertional mutagenesis, it also limits adenoviral application to high-level transient transgene expression, because the gene is often lost 5 to 20 days post-transduction (Dai et al., 1995). Adenoviruses have the significant advantage of being able to infect both dividing and non-dividing cells (Verma and Somia, 1997). This makes them specially suited for applications involving brain, eye, lung, pancreas, hepatocytes, neurons, and monocytes (Blomer et al., 1997; Kafri et al., 1997). PEGylation and expression of targeting ligands on the viral capsid are being investigated to decrease the immune response and enhance targeting of adenoviral vectors (Eto et al., 2008). A clinical trial with adenovirus to repair salivary gland tissue post-radiation therapy is ongoing (Baum et al., 2006; NIH, 2008b).
Adeno-associated Virus
Adeno-associated virus (AAV) is a parvovirus of the Dependovirus genus that was discovered in 1965 as a co-infecting agent of adenovirus preparations (Carter, 2005). The first infectious clone of AAV serotype 2 to be used for human gene therapy was generated in 1982 (Samulski et al., 1982). Since that time, AAV serotypes 1–12 and over 100 AAV variants have been isolated (Wu et al., 2006). AAV is a non-enveloped DNA virus with a 22-nm icosahedral capsid containing a 4.7-kBp linear single-stranded DNA genome. Coding capacity is limited to 4.5 kBp, but may be extended by splitting the sequence between 2 viruses that can later concatamerize after transduction (Nakai et al., 2000). The genome contains 2 unique open reading frames (ORFs) which encode 4 replication proteins and 3 capsid proteins, respectively (Ding et al., 2005). Inverted GC-rich self-complementary terminal repeats flank the ORFs and are the only cis-acting factors required for genome replication and packaging (Ding et al., 2005). Two ORF-encoded trans-acting proteins required for viral replication are rep, which controls viral replication and integration, and cap, which encodes structural components of the capsid. Though site-specific integration on chromosome 19 occurs if rep is maintained (Kotin et al., 1990), it is generally removed from rAAV-engineered vectors. Advantages of AAVs include low immunogenicity, lack of pathogenicity, a wide range of infectivity with potential cell-/tissue-specific targeting, and the ability to establish long-term latent transgene expression in both dividing and non-dividing cells.
AAVs are naturally replication-deficient and require a helper virus for replication and dissemination (Zhang and Godbey, 2006). Self-limiting infection, coupled with their ability to stably infect dividing and non-dividing cells, makes them an excellent target for in vivo gene therapy and craniofacial applications. It is generally accepted that AAV vectors persist as non-integrated circular episomal concatemers, and research shows an integration frequency of less than 1 in 30 million particles in studies of AAV delivery to muscle in rabbits (Schnepp et al., 2003; Schultz and Chamberlain, 2008). Infection occurs through binding of viral proteins to charged heparin sulfate proteoglycans (Summerford and Samulski, 1998) and is potentially enhanced by interactions with alpha-V-beta-5 integrins (Summerford et al., 1999) and human fibroblast growth factor receptor-1 (Qing et al., 1999). After clathrin-mediated endocytosis, endosomal escape, and nuclear translocation, AAV can produce latent long-term infection via episome formation in which the transgene reaches maximum expression levels after an incubation period of 4 to 8 wks and remains stable for up to 2–3 yrs in animal models (Thomas et al., 2004; Manno et al., 2006). Though it is becoming less of an issue, one of the challenges facing viral engineers is the large-scale amplification of AAVs. Baculovirus expression systems for rAAV2 vector production in SF9 cells show promise for large-scale production (Urabe et al., 2002). However, current clinical trials are limited by their reliance on transient production systems and still require complete elimination of helper virus during production (Kay et al., 2000).
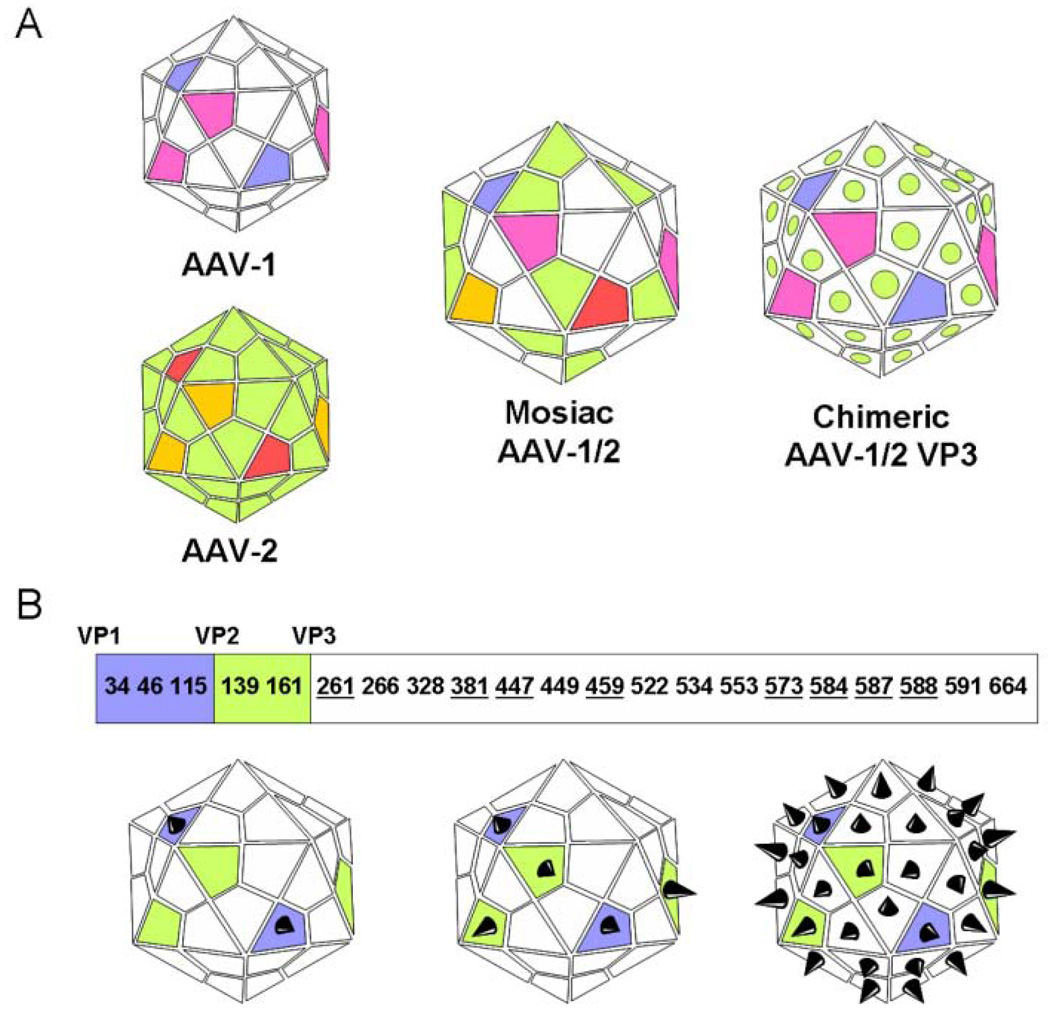
The primary goal for rAAV engineering is to improve transduction efficiency to decrease vector loading while increasing target specificity. An initial successful effort to improve transduction was a switch from single-strand to self-complementary recombinant AAV vectors, to bypass the rate-limiting second-strand DNA synthesis step (McCarty et al., 2001). Because of high variation among capsids, AAV vectors have inherent tissue-targeting abilities that can be enhanced with capsid re-engineering. For example, AAV6 demonstrates increased transduction efficiency in skeletal muscle (Gao et al., 2002), and AAV4 shows preference for the CNS (Davidson et al., 2000). DNA shuffling and cloning technologies are currently being used to generate extensive libraries of recombinant AAVs that display diverse tissue specificity and potential to evade host-neutralizing antibodies (Perabo et al., 2006; Li et al., 2008). The crystal structure of the AAV2 capsid was solved in 2002 (Xie et al., 2002). AAV virons have icosahedral capsids made of 60 copies of VP1, VP2, and VP3 proteins encoded by the second genomic ORF in a variable predicted ratio of 1:1:18 (Muzyczka and Warrington, 2005). VP1 and VP2 are variable between AAV serotypes (Wu et al., 2006). Mosaic vectors (capsid structure derived from subunits of different serotypes) or chimeric vectors (capsid proteins modified by domain or amino acid swapping between serotypes) have been generated through trans-capsidation or marker-rescue/domain-swapping (Wu et al., 2006) (Fig. 2A) to enable the infection of tissues refractory to transduction by naturally occurring AAV vectors or to limit AAV infection to specific tissues (Wu et al., 2006). Insertion of peptide ligands, conjugate-based targeting, and presentation of large protein ligands on the AAV capsid are additional strategies that have been used to enhance targeting and transduction of rAAVs (Muzyczka and Warrington, 2005) (Fig. 2B). Insertion sites for peptide-encoding DNA sequences are limited to maintain infectivity of the viron. A 14-residue core RGD peptide motif insertion is possible in VP3 at residues 261, 381, 447, 459, 573, 584, 587, and 588 (Girod et al., 1999; Shi and Bartlett, 2003) (Fig. 2B). Integrin-RGD interactions could be exploited by craniofacial tissue engineers to enhance infection of endothelial cells and localization of rAAV to matrix-laden sites such as bone and tooth.
Figure 2.
AAV capsid engineering for enhanced transduction and tissue-specific targeting. (A) Mosaic vectors (capsid structure derived from subunits of different serotypes) or chimeric vectors (capsid proteins modified by domain or amino acid swapping between serotypes) have been generated through trans-capsidation or marker-rescue/domain-swapping (Wu et al., 2006). Seemingly limitless engineered combinations of the 12 identified AAV serotypes and over 100 AAV variants can be generated to enhance tissue targeting and transduction. (B) Insertion of peptide ligands and their presentation on the AAV capsid is a strategy that has been used to enhance targeting and transduction by rAAVs (Muzyczka and Warrington, 2005). The AAV capsid is made up of 3 subunits—VP1 (white), VP2 (green), and VP3 (blue)—in a variable ratio of 1:1:18. Each of these subunits shares a conserved VP3 sequence, with VP2 building upon VP3, and VP1 building upon VP2, as shown. These similarities can be exploited to regulate surface expression of an incorporated peptide. For example, if the peptide sequence is incorporated into the coding section unique to VP1, the peptide will be expressed only by that capsid protein. There is a limited number of sites that can support peptide insertion while maintaining viron infectivity. A 14-residue core RGD peptide motif insertion is possible in VP3 at residues 261, 381, 447, 459, 573, 584, 587, and 588 (Girod et al., 1999; Shi and Bartlett, 2003). Integrin-RGD interactions could be exploited by craniofacial tissue engineers to enhance infection of endothelial cells and localization of rAAV to matrix-laden sites such as bones and teeth. Panel B adapted from Muzyczka and Warrington, 2005.
CLINICAL GENE THERAPY
State of the Field
There are three main strategies for gene delivery: in vivo, in vitro, and ex vivo. Though the most direct method is in vivo injection, this approach lacks the improved patient safety of in vitro and ex vivo methods. Systemic delivery is desirable if the target tissue is not directly accessible. However, this method often results in low specificity of gene expression, risks of toxicity due to the high vector concentration required, and potential damage to the function of healthy tissues (Zhang and Godbey, 2006). Alternatively, matrix-based delivery allows for tissue-specific gene delivery, higher localized loading of DNA or virus, and increased control over the structural microenvironment (Dang and Leong, 2006). Thus far, human in vivo clinical trials have introduced adenovirus, AAV, retrovirus, and herpes simplex virus by intravenous (IV) injection, intra-tissue injection, or lung aerosol (Kemeny et al., 2006). In contrast, ex vivo trials have focused on stable retroviral transduction of rapidly dividing populations such as CD8+ T-cells, hematopoietic stem cells, hepatocytes, and fibroblasts, followed by IV or local re-introduction. At the time of this publication, a search of the NIH Genetic Modification Clinical Research Information System (GeMCRIS) revealed 908 total gene therapy clinical trial entries in the database (NIH, 2008a). At clinicaltrials.gov, a search for interventions with “gene transfer” OR “gene therapy” returned 174 studies, of which 145 are viral-based, with 84 active, 48 completed, and 7 terminated. This cross-section of results translates to 1605 persons who have participated in this subset of completed gene therapy trials and nearly 5000 total active or anticipated participants, based on each study’s documented enrollment since 1990. The following sections will briefly review the progress of gene therapy since 1990.
Treatment of Disease
Gene therapy is specially suited for long-term delivery of a transgene to persons with a single genetic deficiency that is not amenable to protein or pharmacokinetic therapy. This was the premise of the first successful gene therapy clinical trials that inserted genes ex vivo into CD34+ cells to treat persons with SCID (Anderson, 1990; Blaese et al., 1993). Amazingly, persistence of the adenosine deaminase (ADA) transgene was noted in peripheral blood leukocytes 12 yrs post-therapy without adverse events (Muul et al., 2003). Since 1990, clinical treatment of genetic diseases—including cystic fibrosis, hemophilia, Leber congenital amaurosis, muscular dystrophy, ornithine transcarbamylase deficiency, Pompe disease, and Gaucher’s disease—has been attempted, with promising documented success (Aiuti et al., 2007; Alexander et al., 2007). Following the SCID trials, treatment of cystic fibrosis by re-introduction of the cystic fibrosis transmembrane regulator (CFTR) chloride ion channel to lung epithelial cells was highly targeted and was the first use of rAAV in humans (Flotte et al., 2003). However, like many other in vivo and ex vivo clinical trials, transduction efficiency was generally insufficient to improve clinical parameters significantly. Apart from SCID, the most promising documented results for genetic deficiency correction have been the replacement of factor IX (F-IX) in hemophilia. Studies by Avigen Inc. have examined rAAV2-mediated F-IX delivery to the liver. In dogs, therapeutic levels of F-IX were achieved for multiple years following vector treatment (Manno et al., 2006). In humans, delivery of rAAV2.F-IX through the hepatic artery achieved therapeutic levels of F-IX expression for approximately 8 wks (Aiuti et al., 2007). It appears that cell-mediated immunity to the rAAV2 capsid limits expression in humans. Thus, immunomodulation and capsid engineering may make F-IX therapy a near-future reality (Krebsbach et al., 2003; Manno et al., 2006). Gene therapy is also highly desired for the treatment of neurologic and other chronic disease. Clinical trials have been implemented and/or completed for the treatment of HIV/AIDS, arthritis, angina pectoris, solid tumors, Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, Batten disease, Canavan disease, and familial hypercholesterolemia (Aiuti et al., 2007; Alexander et al., 2007; NIH, 2008a,b). Despite the many hurdles, most clinical trials are progressing steadily, with treatments for angina pectoris (Henry et al., 2007), prostate cancer (Freytag et al., 2007), non-small-cell lung cancer, and head and neck cancer now entering phase III clinical trials (NIH, 2008b).
GENE THERAPY FOR CRANIOFACIAL REGENERATION
More than 85% of the United States population requires repair or replacement of a craniofacial structure, including bone, tooth, temporomandibular joint, salivary gland, and mucosa. Regeneration of oral and craniofacial tissues presents a formidable challenge that requires synthesis of basic science, clinical science, and engineering technology. Identification of appropriate scaffolds, cell sources, and spatial and temporal signals are necessary to optimize development of a single tissue, hybrid organs consisting of multiple tissues, or tissue interface. In contrast to traditional replacement gene therapy, craniofacial regeneration via gene therapy seeks to use genetic vectors as supplemental building blocks for tissue growth and repair. Synergistic combination of viral gene therapy with craniofacial tissue engineering will significantly enhance our ability to repair and regenerate tissues in vivo.
Head and Neck Squamous Cell Carcinoma (HNSCC)
Though the treatment of HNSCC does not directly fall in the category of craniofacial regeneration, it is the most well-developed use of gene therapy in the craniofacial region. There are three main strategies to target any solid tumor with gene therapy. First, immunomodulatory therapy seeks to increase the visibility of the tumor cells to the immune system in vivo or to modify the effector cells ex vivo to increase targeting of the tumor via the introduction of specific gene expression. In 2007, the dendric cell vaccine ‘Provenge’ was deemed safe and preliminarily approved by the FDA advisory panel in a 13 to 4 vote for the treatment of prostate cancer. However, it was later denied final approval and is currently being re-evaluated (Moyad, 2007). Second, oncolytic viruses have been developed that can selectively target, multiply in, and destroy cancer cells (Dambach et al., 2006). A phase II clinical trial of OncoVex (GM-CSF), with combined chemoradiotherapy in locally advanced head and neck cancers, is ongoing (Aiuti et al., 2007). In addition, the ‘H101’ oncolytic adenovirus has undergone phase I–III clinical trials for treating head and neck cancer and is now approved for use in China (Yu and Fang, 2007). Third, suicide genes such as herpes simplex thymidine kinase can be introduced to cancer cells to increase their susceptibility to anti-viral drugs such as acyclovir (Niculescu-Duvaz and Springer, 2005). As mentioned above, application of these methods for treatment of various cancers comprises the majority of the current phase III clinical gene therapy trials (NIH, 2008b). Additional strategies of interest for specific targeting of HNSCC include local viral introduction of genes encoding p53 (Clayman et al., 1999; Yoo et al., 2004), endostatin (Lin et al., 2007), and non-viral IL-2/IL-12 (O’Malley et al., 2005).
Mineralized Tissues
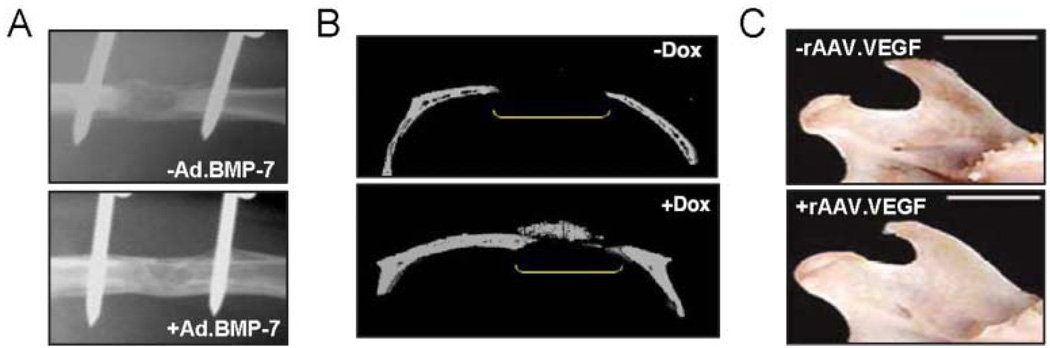
Animal-model-based gene therapy and engineering of individual craniofacial structures such as bone and cartilage have firmly established a productive relationship, and novel approaches to regeneration of complex mineralized tissues such as tooth (Nakashima et al., 2006) and TMJ (Rabie et al., 2007) are just beginning to emerge. Clinical protein delivery of PDGF-B or bone morphogenetic proteins (BMPs) at periodontal defect sites is well-known to enhance repair and healing of bone and gingiva (Kaigler et al., 2006). Gene delivery can allow for localized sustained protein expression at therapeutic levels and can overcome recombinant protein delivery issues such as cost, half-life, supra-physiologic dosing, and poor retention. In support of this, studies have shown that use of adenovirus expressing PDGF-B for treatment of periodontal defects demonstrates better results than continuous protein therapy (Jin et al., 2004; Franceschi, 2005) (Table). Adenoviral-, retroviral-, and AAV-mediated delivery of osteogenic genes has been demonstrated to enhance fracture repair and intramembranous or endochondral bone formation in vivo in animal models (Table). To meet clinical needs, gene delivery must be safe, simple, and cost-effective. Thus, focus on in vivo strategies which avoid primary cell isolation and long-term culture is ideal. An “expedited ex vivo” bone regeneration strategy has recently been proposed in which explants of adipose tissue or muscle directly transduced with Ad.BMP-2 without culture can be re-implanted at defect sites to enhance regeneration of critical-sized rat femoral defects (Betz et al., 2008) (Fig. 3A). In addition, studies to release virus directly from biomaterials have been effective for bone regeneration in animal models (Hu et al., 2007)
Table.
Virally Transduced Genes for Regeneration of Craniofacial Tissues
| Gene | Application | Virus | References |
|---|---|---|---|
| BMP-4 | Bone repair | Retrovirus, Adenovirus | Shen 2004, Wright 2002, Lin 2006 |
| BMP-6 | Bone repair | Adenovirus | Jane 2002 |
| BMP-9 | Bone repair | Adenovirus | Alden 2000 |
| Shh | Bone repair | Retrovirus | Edwards 2005 |
| caAlk2 | Bone repair | AAV | Ulrich-Vinther 2007 |
| VEGF | Bone repair, Condylar growth | Retrovirus, Adenovirus, AAV | Tarkka 2003, Rabie 2007, Jiang 2008 |
| BMP-2 | Bone repair, Chondrogenesis | Retrovirus, Adenovirus | Lee 2002, Chang 2003, Hu 2007, Smith 2000 |
| BMP-7 | Bone repair, Chondrogenesis | Retrovirus, Adenovirus | Jin 2003, Krebsbach 2000, Hidaka 2001 |
| TGF-B1 | Chondrogenesis | Retrovirus, Adenovirus, AAV | Lee 2001, Smith 2000, Zhao 2002, Pagnotto 2007 |
| SOX9 | Chondrogenesis | Retrovirus, Adenovirus, AAV | Li 2004, Cucchiarini 2007 |
| IGF-1 | Chondrogenesis | Adenovirus, AAV | Smith 2000, Izal 2008 |
| GDF5 | Chondrogenesis | Adenovirus | Feng 2008 |
| AQP-1 | Salivary gland repair | Adenovirus, AAV | Baum 2006 |
| PDGF-B | Wound healing, Bone repair | Retrovirus, Adenovirus | Breitbart 2001, Jin 2004 |
BMP, bone morphogenetic protein; Shh, sonic hedgehog; caAlk2, constitutively active activin-like kinase 2; TGF-β1, transforming growth factor beta 1; IGF, insulin-like growth factor; GDF, growth and differentiation factor; AQP, aquaporin; PDGF, platelet-derived growth factor.
(Alden et al., 2000; Baum et al., 2006; Breitbart et al., 2001; Chang et al., 2003; Cucchiarini et al., 2007; Edwards et al., 2005; Feng et al., 2008; Hidaka et al., 2001; Hu et al., 2007; Izal et al., 2008; Jane et al., 2002; Jiang et al., 2008; Jin et al., 2004; Jin et al., 2003; Krebsbach et al., 2000; Lee et al., 2002; Lee et al., 2001; Li et al., 2004; Lin et al., 2006; Pagnotto et al., 2007; Rabie et al., 2007; Shen et al., 2004; Smith et al., 2000; Tarkka et al., 2003; Ulrich-Vinther, 2007; Wright et al., 2002; Zhao et al., 2002)
Figure 3.
Gene therapy for bone regeneration. (A) An “expedited ex vivo” bone regeneration strategy has recently been proposed in which explants of adipose tissue or muscle can be directly transduced with Ad.BMP-2 without culture. This has shown promising results in the regeneration of critical-sized rat femoral defects (Betz et al., 2008). (B) A vector for rAAV-based BMP-2 gene delivery regulated by the tetracycline-sensitive promoter (TetON) has been generated (Gafni et al., 2004). Calvarial defect bone formation was noted in mice only after the administration of Doxycycline via the drinking water to induce BMP-2 expression. This represents a novel strategy for localized inducible gene expression. (C) Local injection of rAAV-VEGF to the mandibular condyle of rats results in increased condylar growth after 60 days, as demonstrated by increased condyle width and length (Rabie et al., 2007).
Inducible vector systems, use of rAAV, and transduction of novel osteogenic factors have outstanding potential for mineralized tissue regeneration. In addition to vector design and capsid engineering for cell-specific transduction, we must now consider the use of systemic drug-inducible vector components. For example, an early study using a retroviral vector demonstrated dexamethasone-inducible GFP expression from transduced BMSCs in vitro (Jaalouk et al., 2000). Researchers have gone on to explore doxycycline-inducible ‘tetON’ promoter systems. In these systems, selective induction of BMP-2 or BMP-4 expression achieved by administration of oral doxycycline can allow for localized induction of bone formation only at vector-containing sites in vivo (Gafni et al., 2004; Peng et al., 2004) (Fig. 3B). The field of rAAV-mediated bone repair is rapidly advancing and promises superior safety, tissue targeting, and high in vivo transduction efficiency of non-dividing cells. In the past 5 years, proof-of-principle studies have been completed and have shown positive results for AAV transduction of bone-forming cells and enhanced healing of osseous defects from in vivo application of rAAV expressing constitutively active activin receptor-like kinase-2 (caAlk2), VEGF/RANKL, and ex vivo BMP-7 (Kang et al., 2007; Ulrich-Vinther, 2007) (Table). The use of caAlk2, a receptor that mediates BMP signaling, is emerging as an interesting gene therapy target, because of its low required therapeutic expression level and inability to be blocked by native BMP antagonist noggin and chordin (Zhang et al., 2003; Koefoed et al., 2005; Ulrich-Vinther, 2007).
Successful engineering of teeth and the TMJ is challenging and requires the generation of functional interfaces. The introduction of BMPs in vivo to exposed pulp tissue has been proposed as a novel strategy for odontoblast transduction to enhance dentin regeneration and repair (Nakashima et al., 2006). However, gene therapy has not yet been applied to the field of total tooth engineering (Young et al., 2005; Hu et al., 2006). Engineering of the TMJ requires the creation of functional bone and cartilage with an appropriate transition zone. Investigators have generated such osteochondral grafts by seeding differentiated pig chondrocytes and Ad.BMP7-transduced human gingival fibroblasts onto biphasic PLLA/hydroxyapatite composite scaffolds and implanting them subcutaneously into N: Nih-bg-nu-xid immunocompromised mice (Schek et al., 2004, 2005). Marrow-containing vascularized bone, mature cartilage, and a defined mineralized interface can be generated within 4 wks of implantation (Schek et al., 2004, 2005). A second approach to TMJ repair is the in vivo introduction of therapeutic genes to the mandibular condyle. Recent work has demonstrated successful rAAV2-mediated transduction of VEGF to condylar tissue in vivo that subsequently enhanced mandibular condylar growth (Rabie et al., 2007) (Fig. 3C). These pioneering studies provide proof-of-principle evidence for the fabrication of a physiologic osteochondral graft and direct TMJ transduction that may be developed to treat persons with TMJ disorders or developmental deformities.
Salivary Gland
Loss of salivary gland function can result as a pharmacologic side-effect, from radiation therapy, or as a consequence of autoimmune diseases such as Sjögren’s syndrome. In addition to direct repair of non-functional glandular tissue, researchers are working to develop an engineered salivary gland substitute that could be implanted in place of the parotid gland (Aframian and Palmon, 2008). Unlike acinar cells, ductal epithelial cells are incapable of fluid secretion. Because researchers have been unable to isolate and expand acinar cells in vitro, identification and localization of membrane proteins required for ionic gradient formation and fluid flow in acinar cells have informed efforts to modify ductal cell populations by gene transfer. Acinar cells require 4 membrane proteins to generate an osmotic gradient for unidirectional fluid movement: (1) the N+K+-ATPase, used to maintain membrane potential; (2) a Ca2+-activated K+ channel; (3) the secretory isoform of the Na+/K+/2Cl− co-transporter; and (4) the apical membrane-bound Ca2+-activated Cl− channel (Melvin et al., 2005; Aframian and Palmon, 2008). Salivation occurs in response to agonists that generate an increase in intracellular Ca2+ concentration and is facilitated by osmotic gradient-directed fluid movement through water channels in the apical membrane, known as aquaporins (AQP) (Melvin et al., 2005). It is now recognized that isolated ductal epithelial cells lack expression of AQP and, as such, cannot mediate fluid movement (Tran et al., 2006). Re-introduction of transient AQP expression by adenoviral transduction has been successful in rhesus monkey parotid duct cells in vitro (Tran et al., 2005) and rat and mini-pig salivary gland tissue in vivo (Baum et al., 2006). Indeed, attempts to restore salivary flow by in vivo transduction of adenovirus encoding AQP1 into remaining glandular tissue of persons treated with radiation for head and neck cancer is the first human craniofacial repair gene therapy clinical trial and is currently ongoing (Baum et al., 2006; NIH, 2008b).
Wound Healing/Mucosa
Engineering of skin and mucosal equivalents is essential for the esthetic reconstruction of individuals disfigured by trauma, resective surgery, or severe burns. Skin is composed of layered dermis and epidermis in a configuration that must be preserved for optimum regeneration. The first attempts to repair damaged skin and mucosa with an engineered graft did not occur until the 1980s (Madden et al., 1986). Skin with both dermal and epidermal components, such as Dermagraft™ (Purdue et al., 1997) and Apligraf™, used for coverage of burns and acute wounds (Eaglstein et al., 1995), was the first FDA-approved tissue-engineered construct that has been put into clinical practice. Clinically, a product known as gene-activated matrix (GAM) has been developed as an enhanced skin graft substitute. GAM for wound-specific delivery of adenovirus vector encoding PDGF-B to improve healing of diabetic ulcers is currently in Phase II clinical trials (Gu et al., 2004; NIH, 2008b). It is reasonably expected that these developments could be expanded to enhance wound healing and tissue repair in the craniofacial region (Jin et al., 2004).
CONCLUSION: PROSPECTS AND CHALLENGES
Since the beginning of human gene therapy in 1990, nearly 1000 clinical trials have been initiated. Patient follow-up for as much as 18 yrs post-gene transfer has been generally positive, with isolated tragedy (Muul et al., 2003). It is encouraging that many gene therapy trials for single-gene and complex disorders are now complete, vector selection and design strategies have significantly improved, and a safety profile is nearly established, as evidenced by the many current phase III clinical trials. Though strategies such as ex vivo transduction of cells with integrating retrovirus are promising, and early success led to high hopes, it is essential to keep our expectations of gene therapy realistic, because future development will require slow, stepwise progress. As we near the 20-year mark for gene therapy and begin its integration with craniofacial engineering, our focus must evolve to include expansion of placebo-controlled clinical trials, development of targeted vectors to increase transduction efficiency and to overcome the immune response, and consideration of the concept of ‘genotoxicity’ testing as a fundamental feature of gene therapy research.
ACKNOWLEDGMENTS
Parts of the authors’ research discussed in this manuscript were supported by NIH/NIDCR Tissue Engineering and Regeneration, T32 DE07057 (PHK/ELS) and R01 DE 13835 (PHK).
REFERENCES
- Aframian DJ, Palmon A. Current status of the development of an artificial salivary gland. Tissue Eng Part B Rev. 2008;14:187–198. doi: 10.1089/ten.teb.2008.0044. [DOI] [PubMed] [Google Scholar]
- Agarwal M, Austin TW, Morel F, Chen J, Bohnlein E, Plavec I. Scaffold attachment region-mediated enhancement of retroviral vector expression in primary T cells. J Virol. 1998;72:3720–3728. doi: 10.1128/jvi.72.5.3720-3728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiuti A, Bachoud-Levi AC, Blesch A, Brenner MK, Cattaneo F, Chiocca EA, et al. Progress and prospects: gene therapy clinical trials (part 2) Gene Ther. 2007;14:1555–1563. doi: 10.1038/sj.gt.3303033. [DOI] [PubMed] [Google Scholar]
- Akinc A, Anderson DG, Lynn DM, Langer R. Synthesis of poly(beta-amino ester)s optimized for highly effective gene delivery. Bioconjug Chem. 2003;14:979–988. doi: 10.1021/bc034067y. [DOI] [PubMed] [Google Scholar]
- Alden TD, Beres EJ, Laurent JS, Engh JA, Das S, London SD, et al. The use of bone morphogenetic protein gene therapy in craniofacial bone repair. J Craniofac Surg. 2000;11:24–30. doi: 10.1097/00001665-200011010-00005. [DOI] [PubMed] [Google Scholar]
- Alexander BL, Ali RR, Alton EW, Bainbridge JW, Braun S, Cheng SH, et al. Progress and prospects: gene therapy clinical trials (part 1) Gene Ther. 2007;14:1439–1447. doi: 10.1038/sj.gt.3303001. [DOI] [PubMed] [Google Scholar]
- Anderson WF. September 14, 1990: the beginning. Hum Gene Ther. 1990;1:371–372. doi: 10.1089/hum.1990.1.4-371. [DOI] [PubMed] [Google Scholar]
- Apperley JF, Luskey BD, Williams DA. Retroviral gene transfer of human adenosine deaminase in murine hematopoietic cells: effect of selectable marker sequences on long-term expression. Blood. 1991;78:310–317. [PubMed] [Google Scholar]
- Barnett BG, Crews CJ, Douglas JT. Targeted adenoviral vectors. Biochim Biophys Acta. 2002;1575:1–14. doi: 10.1016/s0167-4781(02)00249-x. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Zheng C, Cotrim AP, Goldsmith CM, Atkinson JC, Brahim JS, et al. Transfer of the AQP1 cDNA for the correction of radiation-induced salivary hypofunction. Biochim Biophys Acta. 2006;1758:1071–1077. doi: 10.1016/j.bbamem.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Betz VM, Betz OB, Harris MB, Vrahas MS, Evans CH. Bone tissue engineering and repair by gene therapy. Front Biosci. 2008;13:833–841. doi: 10.2741/2724. [DOI] [PubMed] [Google Scholar]
- Blaese RM, Culver KW, Chang L, Anderson WF, Mullen C, Nienhuis A, et al. Treatment of severe combined immunodeficiency disease (SCID) due to adenosine deaminase deficiency with CD34+ selected autologous peripheral blood cells transduced with a human ADA gene. Amendment to clinical research project, Project 90-C-195, January 10, 1992. Hum Gene Ther. 1993;4:521–527. doi: 10.1089/hum.1993.4.4-521. [DOI] [PubMed] [Google Scholar]
- Blomer U, Naldini L, Kafri T, Trono D, Verma IM, Gage FH. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–759. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart AS, Grande DA, Laser J, Barcia M, Porti D, Malhotra S, et al. Treatment of ischemic wounds using cultured dermal fibroblasts transduced retrovirally with PDGF-B and VEGF121 genes. Ann Plast Surg. 2001;46:555–561. doi: 10.1097/00000637-200105000-00016. [DOI] [PubMed] [Google Scholar]
- Buchschacher GL., Jr Introduction to retroviruses and retroviral vectors. Somat Cell Mol Genet. 2001;26(1–6):1–11. doi: 10.1023/a:1021014728217. [DOI] [PubMed] [Google Scholar]
- Carter BJ. Adeno-associated virus vectors in clinical trials. Hum Gene Ther. 2005;16:541–550. doi: 10.1089/hum.2005.16.541. [DOI] [PubMed] [Google Scholar]
- Chang AH, Sadelain M. The genetic engineering of hematopoietic stem cells: the rise of lentiviral vectors, the conundrum of the LTR, and the promise of lineage-restricted vectors. Mol Ther. 2007;15:445–456. doi: 10.1038/sj.mt.6300060. [DOI] [PubMed] [Google Scholar]
- Chang SC, Chuang HL, Chen YR, Chen JK, Chung HY, Lu YL, et al. Ex vivo gene therapy in autologous bone marrow stromal stem cells for tissue-engineered maxillofacial bone regeneration. Gene Ther. 2003;10:2013–2019. doi: 10.1038/sj.gt.3302106. [DOI] [PubMed] [Google Scholar]
- Chen H, Frankenburg EP, Goldstein SA, McCauley LK. Combination of local and systemic parathyroid hormone enhances bone regeneration. Clin Orthop Relat Res. 2003;416:291–302. doi: 10.1097/01.blo.0000079443.64912.18. [DOI] [PubMed] [Google Scholar]
- Cho YW, Kim JD, Park K. Polycation gene delivery systems: escape from endosomes to cytosol. J Pharm Pharmacol. 2003;55:721–734. doi: 10.1211/002235703765951311. [DOI] [PubMed] [Google Scholar]
- Choi YH, Liu F, Kim JS, Choi YK, Park JS, Kim SW. Polyethylene glycol-grafted poly-L-lysine as polymeric gene carrier. J Control\Release. 1998;54:39–48. doi: 10.1016/s0168-3659(97)00174-0. [DOI] [PubMed] [Google Scholar]
- Chu K, Cornetta KG, Econs MJ. Efficient and stable gene expression into human osteoclasts using an HIV-1-based lentiviral vector. DNA Cell Biol. 2008;27:315–320. doi: 10.1089/dna.2007.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayman GL, Frank DK, Bruso PA, Goepfert H. Adenovirus-mediated wild-type p53 gene transfer as a surgical adjuvant in advanced head and neck cancers. Clin Cancer Res. 1999;5:1715–1722. [PubMed] [Google Scholar]
- Connolly JB. Lentiviruses in gene therapy clinical research. Gene Ther. 2002;9:1730–1734. doi: 10.1038/sj.gt.3301893. [DOI] [PubMed] [Google Scholar]
- Cucchiarini M, Thurn T, Weimer A, Kohn D, Terwilliger EF, Madry H. Restoration of the extracellular matrix in human osteoarthritic articular cartilage by overexpression of the transcription factor SOX9. Arthritis Rheum. 2007;56:158–167. doi: 10.1002/art.22299. [DOI] [PubMed] [Google Scholar]
- Dai Y, Schwarz EM, Gu D, Zhang WW, Sarvetnick N, Verma IM. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambach MJ, Trecki J, Martin N, Markovitz NS. Oncolytic viruses derived from the gamma34.5-deleted herpes simplex virus recombinant R3616 encode a truncated UL3 protein. Mol Ther. 2006;13:891–898. doi: 10.1016/j.ymthe.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Dang JM, Leong KW. Natural polymers for gene delivery and tissue engineering. Adv Drug Deliv Rev. 2006;58:487–499. doi: 10.1016/j.addr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci USA. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delviks KA, Pathak VK. Development of murine leukemia virus-based self-activating vectors that efficiently delete the selectable drug resistance gene during reverse transcription. J Virol. 1999;73:8837–8842. doi: 10.1128/jvi.73.10.8837-8842.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewannieux M, Harper F, Richaud A, Letzelter C, Ribet D, Pierron G, et al. Identification of an infectious progenitor for the multiple-copy HERV-K human endogenous retroelements. Genome Res. 2006;16:1548–1556. doi: 10.1101/gr.5565706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Zhang L, Yan Z, Engelhardt JF. Intracellular trafficking of adeno-associated viral vectors. Gene Ther. 2005;12:873–880. doi: 10.1038/sj.gt.3302527. [DOI] [PubMed] [Google Scholar]
- Eaglstein WH, Iriondo M, Laszlo K. A composite skin substitute (graftskin) for surgical wounds. A clinical experience. Dermatol Surg. 1995;21:839–843. doi: 10.1111/j.1524-4725.1995.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Edwards PC, Ruggiero S, Fantasia J, Burakoff R, Moorji SM, Paric E, et al. Sonic hedgehog gene-enhanced tissue engineering for bone regeneration. Gene Ther. 2005;12:75–86. doi: 10.1038/sj.gt.3302386. [DOI] [PubMed] [Google Scholar]
- Engelman A. In vivo analysis of retroviral integrase structure and function. Adv Virus Res. 1999;52:411–426. doi: 10.1016/s0065-3527(08)60309-7. [DOI] [PubMed] [Google Scholar]
- Eto Y, Yoshioka Y, Mukai Y, Okada N, Nakagawa S. Development of PEGylated adenovirus vector with targeting ligand. Int J Pharm. 2008;354:3–8. doi: 10.1016/j.ijpharm.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Feng G, Wan Y, Balian G, Laurencin CT, Li X. Adenovirus-mediated expression of growth and differentiation factor-5 promotes chondrogenesis of adipose stem cells. Growth Factors. 2008;26:132–142. doi: 10.1080/08977190802105917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury S, Driscoll R, Simeoni E, Dudler J, von Segesser LK, Kappenberger L, et al. Helper-dependent adenovirus vectors devoid of all viral genes cause less myocardial inflammation compared with first-generation adenovirus vectors. Basic Res Cardiol. 2004;99:247–256. doi: 10.1007/s00395-004-0471-x. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Zeitlin PL, Reynolds TC, Heald AE, Pedersen P, Beck S, et al. Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)-CFTR vector in adult cystic fibrosis patients: a two-part clinical study. Hum Gene Ther. 2003;14:1079–1088. doi: 10.1089/104303403322124792. [DOI] [PubMed] [Google Scholar]
- Franceschi RT. Biological approaches to bone regeneration by gene therapy. J Dent Res. 2005;84:1093–1103. doi: 10.1177/154405910508401204. [DOI] [PubMed] [Google Scholar]
- Frankel AD, Young JA. HIV-1: fifteen proteins and an RNA. Annu Rev Biochem. 1998;67:1–25. doi: 10.1146/annurev.biochem.67.1.1. [DOI] [PubMed] [Google Scholar]
- Freytag SO, Stricker H, Peabody J, Pegg J, Paielli D, Movsas B, et al. Five-year follow-up of trial of replication-competent adenovirus-mediated suicide gene therapy for treatment of prostate cancer. Mol Ther. 2007;15:636–642. doi: 10.1038/sj.mt.6300068. [DOI] [PubMed] [Google Scholar]
- Gafni Y, Pelled G, Zilberman Y, Turgeman G, Apparailly F, Yotvat H, et al. Gene therapy platform for bone regeneration using an exogenously regulated, AAV-2-based gene expression system. Mol Ther. 2004;9:587–595. doi: 10.1016/j.ymthe.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HS. The life and times of adenoviruses. Adv Virus Res. 1999;54:1–13. doi: 10.1016/s0065-3527(08)60363-2. [DOI] [PubMed] [Google Scholar]
- Girod A, Ried M, Wobus C, Lahm H, Leike K, Kleinschmidt J, et al. Genetic capsid modifications allow efficient re-targeting of adeno-associated virus type 2. Nat Med. 1999;5:1438. doi: 10.1038/71021. [DOI] [PubMed] [Google Scholar]
- Goff SP. Retrovirus restriction factors. Mol Cell. 2004;16:849–859. doi: 10.1016/j.molcel.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Gonzalez H, Hwang SJ, Davis ME. New class of polymers for the delivery of macromolecular therapeutics. Bioconjug Chem. 1999;10:1068–1074. doi: 10.1021/bc990072j. [DOI] [PubMed] [Google Scholar]
- Gu DL, Nguyen T, Gonzalez AM, Printz MA, Pierce GF, Sosnowski BA, et al. Adenovirus encoding human platelet-derived growth factor-B delivered in collagen exhibits safety, biodistribution, and immunogenicity profiles favorable for clinical use. Mol Ther. 2004;9:699–711. doi: 10.1016/j.ymthe.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Henry TD, Grines CL, Watkins MW, Dib N, Barbeau G, Moreadith R, et al. Effects of Ad5FGF-4 in patients with angina: an analysis of pooled data from the AGENT-3 and AGENT-4 trials. J Am Coll Cardiol. 2007;50:1038–1046. doi: 10.1016/j.jacc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Hidaka C, Quitoriano M, Warren RF, Crystal RG. Enhanced matrix synthesis and in vitro formation of cartilage-like tissue by genetically modified chondrocytes expressing BMP-7. J Orthop Res. 2001;19:751–758. doi: 10.1016/S0736-0266(01)00019-5. [DOI] [PubMed] [Google Scholar]
- Hu B, Nadiri A, Kuchler-Bopp S, Perrin-Schmitt F, Peters H, Lesot H. Tissue engineering of tooth crown, root, and periodontium. Tissue Eng. 2006;12:2069–2075. doi: 10.1089/ten.2006.12.2069. [DOI] [PubMed] [Google Scholar]
- Hu WW, Wang Z, Hollister SJ, Krebsbach PH. Localized viral vector delivery to enhance in situ regenerative gene therapy. Gene Ther. 2007;14:891–901. doi: 10.1038/sj.gt.3302940. [DOI] [PubMed] [Google Scholar]
- Izal I, Acosta CA, Ripalda P, Zaratiegui M, Ruiz J, Forriol F. IGF-1 gene therapy to protect articular cartilage in a rat model of joint damage. Arch Orthop Trauma Surg. 2008;128:239–247. doi: 10.1007/s00402-007-0407-7. [DOI] [PubMed] [Google Scholar]
- Jaalouk DE, Eliopoulos N, Couture C, Mader S, Galipeau J. Glucocorticoid-inducible retrovector for regulated transgene expression in genetically engineered bone marrow stromal cells. Hum Gene Ther. 2000;11:1837–1849. doi: 10.1089/10430340050129468. [DOI] [PubMed] [Google Scholar]
- Jane JA, Jr, Dunford BA, Kron A, Pittman DD, Sasaki T, Li JZ, et al. Ectopic osteogenesis using adenoviral bone morphogenetic protein (BMP)-4 and BMP-6 gene transfer. Mol Ther. 2002;6:464–470. doi: 10.1006/mthe.2002.0691. [DOI] [PubMed] [Google Scholar]
- Jiang J, Fan CY, Zeng BF. Osteogenic differentiation effects on rat bone marrow-derived mesenchymal stromal cells by lentivirus-mediated co-transfection of human BMP2 gene and VEGF165 gene. Biotechnol Lett. 2008;30:197–203. doi: 10.1007/s10529-007-9532-1. [DOI] [PubMed] [Google Scholar]
- Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004;9:519–526. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin QM, Anusaksathien O, Webb SA, Rutherford RB, Giannobile WV. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J Periodontol. 2003;74:202–213. doi: 10.1902/jop.2003.74.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafri T, Blomer U, Peterson DA, Gage FH, Verma IM. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- Kaigler D, Cirelli JA, Giannobile WV. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin Drug Deliv. 2006;3:647–662. doi: 10.1517/17425247.3.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J. Gene therapy. Seeking the cause of induced leukemias in X-SCID trial. Science. 2003;299:495. doi: 10.1126/science.299.5606.495. [DOI] [PubMed] [Google Scholar]
- Kang Y, Liao WM, Yuan ZH, Sheng PY, Zhang LJ, Yuan XW, et al. In vitro and in vivo induction of bone formation based on adeno-associated virus-mediated BMP-7 gene therapy using human adipose-derived mesenchymal stem cells. Acta Pharmacol Sin. 2007;28:839–849. doi: 10.1111/j.1745-7254.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- Kemeny N, Brown K, Covey A, Kim T, Bhargava A, Brody L, et al. Phase I, open-label, dose-escalating study of a genetically engineered herpes simplex virus, NV1020, in subjects with metastatic colorectal carcinoma to the liver. Hum Gene Ther. 2006;17:1214–1224. doi: 10.1089/hum.2006.17.1214. [DOI] [PubMed] [Google Scholar]
- Khan TA, Sellke FW, Laham RJ. Gene therapy progress and prospects: therapeutic angiogenesis for limb and myocardial ischemia. Gene Ther. 2003;10:285–291. doi: 10.1038/sj.gt.3301969. [DOI] [PubMed] [Google Scholar]
- Kim WJ, Yockman JW, Lee M, Jeong JH, Kim YH, Kim SW. Soluble Flt-1 gene delivery using PEI-g-PEG-RGD conjugate for anti-angiogenesis. J Control Release. 2005;106:224–234. doi: 10.1016/j.jconrel.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Koefoed M, Ito H, Gromov K, Reynolds DG, Awad HA, Rubery PT, et al. Biological effects of rAAV-caAlk2 coating on structural allograft healing. Mol Ther. 2005;12:212–218. doi: 10.1016/j.ymthe.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Kohn DB, Sadelain M, Dunbar C, Bodine D, Kiem HP, Candotti F, et al. American Society of Gene Therapy (ASGT) ad hoc subcommittee on retroviral-mediated gene transfer to hematopoietic stem cells. Mol Ther. 2003;8:180–187. doi: 10.1016/s1525-0016(03)00212-0. [DOI] [PubMed] [Google Scholar]
- Korin YD, Zack JA. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotin RM, Siniscalco M, Samulski RJ, Zhu XD, Hunter L, Laughlin CA, et al. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebsbach PH, Gu K, Franceschi RT, Rutherford RB. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Hum Gene Ther. 2000;11:1201–1210. doi: 10.1089/10430340050015248. [DOI] [PubMed] [Google Scholar]
- Krebsbach PH, Zhang K, Malik AK, Kurachi K. Bone marrow stromal cells as a genetic platform for systemic delivery of therapeutic proteins in vivo: human factor IX model. J Gene Med. 2003;5:11–17. doi: 10.1002/jgm.292. [DOI] [PubMed] [Google Scholar]
- Kurian KM, Watson CJ, Wyllie AH. Retroviral vectors. Mol Pathol. 2000;53:173–176. doi: 10.1136/mp.53.4.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Characterization of DNA condensates induced by poly(ethylene oxide) and polylysine. Proc Natl Acad Sci USA. 1975;72:4288–4292. doi: 10.1073/pnas.72.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence F., director . “I Am Legend.”. Warners Brothers Pictures [movie]; 2007. [Google Scholar]
- Lee JY, Peng H, Usas A, Musgrave D, Cummins J, Pelinkovic D, et al. Enhancement of bone healing based on ex vivo gene therapy using human muscle-derived cells expressing bone morphogenetic protein 2. Hum Gene Ther. 2002;13:1201–1211. doi: 10.1089/104303402320138989. [DOI] [PubMed] [Google Scholar]
- Lee KH, Song SU, Hwang TS, Yi Y, Oh IS, Lee JY, et al. Regeneration of hyaline cartilage by cell-mediated gene therapy using transforming growth factor beta 1-producing fibroblasts. Hum Gene Ther. 2001;12:1805–1813. doi: 10.1089/104303401750476294. [DOI] [PubMed] [Google Scholar]
- Lee M, Kim SW. Polyethylene glycol-conjugated copolymers for plasmid DNA delivery. Pharm Res. 2005;22:1–10. doi: 10.1007/s11095-004-9003-5. [DOI] [PubMed] [Google Scholar]
- Lewis PF, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Asokan A, Wu Z, Van Dyke T, DiPrimio N, Johnson JS, et al. Engineering and selection of shuffled AAV genomes: a new strategy for producing targeted biological nanoparticles. Mol Ther. 2008;16:1252–1260. doi: 10.1038/mt.2008.100. [DOI] [PubMed] [Google Scholar]
- Li Y, Tew SR, Russell AM, Gonzalez KR, Hardingham TE, Hawkins RE. Transduction of passaged human articular chondrocytes with adenoviral, retroviral, and lentiviral vectors and the effects of enhanced expression of SOX9. Tissue Eng. 2004;10:575–584. doi: 10.1089/107632704323061933. [DOI] [PubMed] [Google Scholar]
- Lin L, Fu X, Zhang X, Chen LX, Zhang JY, Yu CL, et al. Rat adipose-derived stromal cells expressing BMP4 induce ectopic bone formation in vitro and in vivo. Acta Pharmacol Sin. 2006;27:1608–1615. doi: 10.1111/j.1745-7254.2006.00449.x. [DOI] [PubMed] [Google Scholar]
- Lin X, Huang H, Li S, Li H, Li Y, Cao Y, et al. A phase I clinical trial of an adenovirus-mediated endostatin gene (E10A) in patients with solid tumors. Cancer Biol Ther. 2007;6:648–653. doi: 10.4161/cbt.6.5.4004. [DOI] [PubMed] [Google Scholar]
- Liu G, Molas M, Grossmann GA, Pasumarthy M, Perales JC, Cooper MJ, et al. Biological properties of poly-L-lysine-DNA complexes generated by cooperative binding of the polycation. J Biol Chem. 2001;276:34379–34387. doi: 10.1074/jbc.M105250200. [DOI] [PubMed] [Google Scholar]
- Loeb JE, Weitzman MD, Hope TJ. Enhancement of green fluorescent protein expression in adeno-associated virus with the woodchuck hepatitis virus post-transcriptional regulatory element. Methods Mol Biol. 2002;183:331–340. doi: 10.1385/1-59259-280-5:331. [DOI] [PubMed] [Google Scholar]
- Lusky M, Christ M, Rittner K, Dieterle A, Dreyer D, Mourot B, et al. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J Virol. 1998;72:2022–2032. doi: 10.1128/jvi.72.3.2022-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden MR, Finkelstein JL, Staiano-Coico L, Goodwin CW, Shires GT, Nolan EE, et al. Grafting of cultured allogeneic epidermis on second- and third-degree burn wounds on 26 patients. J Trauma. 1986;26:955–962. doi: 10.1097/00005373-198611000-00001. [DOI] [PubMed] [Google Scholar]
- Mahato RI, Lee M, Han S, Maheshwari A, Kim SW. Intratumoral delivery of p2CMVmIL-12 using water-soluble lipopolymers. Mol Ther. 2001;4:130–138. doi: 10.1006/mthe.2001.0425. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Martin GS. The road to Src. Oncogene. 2004;23:7910–7917. doi: 10.1038/sj.onc.1208077. [DOI] [PubMed] [Google Scholar]
- Matheson R. I am legend. 1997 ed. New York: Tor Books; 1954. Sept 15, [Google Scholar]
- McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- McKie R. Gene researchers face crisis as man’s saviour turns killer. London: The Observer; 2000. Jan. 2, [Google Scholar]
- Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol. 2004;83:97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- Mitchell RS, Beitzel BF, Schroder AR, Shinn P, Chen H, Berry CC, et al. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2004;2(8):E234. doi: 10.1371/journal.pbio.0020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyad MA. What happened to the Provenge (Sipuleucel-T) vaccine for hormone refractory prostate cancer? Urol Nurs. 2007;27:256–257. [PubMed] [Google Scholar]
- Muul LM, Tuschong LM, Soenen SL, Jagadeesh GJ, Ramsey WJ, Long Z, et al. Persistence and expression of the adenosine deaminase gene for 12 years and immune reaction to gene transfer components: long-term results of the first clinical gene therapy trial. Blood. 2003;101:2563–2569. doi: 10.1182/blood-2002-09-2800. [DOI] [PubMed] [Google Scholar]
- Muzyczka N, Warrington KH., Jr Custom adeno-associated virus capsids: the next generation of recombinant vectors with novel tropism. Hum Gene Ther. 2005;16:408–416. doi: 10.1089/hum.2005.16.408. [DOI] [PubMed] [Google Scholar]
- Nah JW, Yu L, Han SO, Ahn CH, Kim SW. Artery wall binding peptide-poly(ethylene glycol)-grafted-poly(L-lysine)-based gene delivery to artery wall cells. J Control Release. 2002;78:273–284. doi: 10.1016/s0168-3659(01)00499-0. [DOI] [PubMed] [Google Scholar]
- Nakai H, Storm TA, Kay MA. Increasing the size of rAAV-mediated expression cassettes in vivo by intermolecular joining of two complementary vectors. Nat Biotechnol. 2000;18:527–532. doi: 10.1038/75390. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Iohara K, Zheng L. Gene therapy for dentin regeneration with bone morphogenetic proteins. Curr Gene Ther. 2006;6:551–560. doi: 10.2174/156652306778520665. [DOI] [PubMed] [Google Scholar]
- Niculescu-Duvaz I, Springer CJ. Introduction to the background, principles, and state of the art in suicide gene therapy. Mol Biotechnol. 2005;30:71–88. doi: 10.1385/MB:30:1:071. [DOI] [PubMed] [Google Scholar]
- NIH. Bethesda, MD: US National Institutes of Health; Genetic Modification Clinical Research Information System (GeMCRIS) 2008a
- NIH. ClinicalTrials.gov: US National Institutes of Health. 2008b http://www.clinicaltrials.gov/
- O’Malley BW, Jr, Li D, McQuone SJ, Ralston R. Combination non-viral interleukin-2 gene immunotherapy for head and neck cancer: from bench top to bedside. Laryngoscope. 2005;115:391–404. doi: 10.1097/00005537-200503000-00002. [DOI] [PubMed] [Google Scholar]
- Pagnotto MR, Wang Z, Karpie JC, Ferretti M, Xiao X, Chu CR. Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007;14:804–813. doi: 10.1038/sj.gt.3302938. [DOI] [PubMed] [Google Scholar]
- Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58:467–486. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Peng H, Usas A, Gearhart B, Young B, Olshanski A, Huard J. Development of a self-inactivating tet-on retroviral vector expressing bone morphogenetic protein 4 to achieve regulated bone formation. Mol Ther. 2004;9:885–894. doi: 10.1016/j.ymthe.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Perabo L, Endell J, King S, Lux K, Goldnau D, Hallek M, et al. Combinatorial engineering of a gene therapy vector: directed evolution of adeno-associated virus. J Gene Med. 2006;8:155–162. doi: 10.1002/jgm.849. [DOI] [PubMed] [Google Scholar]
- Purdue GF, Hunt JL, Still JM, Jr, Law EJ, Herndon DN, Goldfarb IW, et al. A multicenter clinical trial of a biosynthetic skin replacement, Dermagraft-TC, compared with cryopreserved human cadaver skin for temporary coverage of excised burn wounds. J Burn Care Rehabil. 1997;18(1 Pt 1):52–57. doi: 10.1097/00004630-199701000-00009. [DOI] [PubMed] [Google Scholar]
- Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- Quick DJ, Anseth KS. DNA delivery from photocrosslinked PEG hydrogels: encapsulation efficiency, release profiles, and DNA quality. J Control Release. 2004;96:341–351. doi: 10.1016/j.jconrel.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Rabie AB, Dai J, Xu R. Recombinant AAV-mediated VEGF gene therapy induces mandibular condylar growth. Gene Ther. 2007;14:972–980. doi: 10.1038/sj.gt.3302943. [DOI] [PubMed] [Google Scholar]
- Roberts L. Human gene transfer test approved. Science. 1989;243:473. doi: 10.1126/science.2911753. [DOI] [PubMed] [Google Scholar]
- Rowe WP, Huebner RJ, Bell JA. Definition and outline of contemporary information on the adenovirus group. Ann NY Acad Sci. 1957;67:255–261. doi: 10.1111/j.1749-6632.1957.tb46048.x. [DOI] [PubMed] [Google Scholar]
- Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan-DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5:387–391. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- Russell WC. Update on adenovirus and its vectors. J Gen Virol. 2000;81(Pt 11):2573–2604. doi: 10.1099/0022-1317-81-11-2573. [DOI] [PubMed] [Google Scholar]
- Samulski RJ, Berns KI, Tan M, Muzyczka N. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc Natl Acad Sci USA. 1982;79:2077–2081. doi: 10.1073/pnas.79.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schek RM, Taboas JM, Segvich SJ, Hollister SJ, Krebsbach PH. Engineered osteochondral grafts using biphasic composite solid free-form fabricated scaffolds. Tissue Eng. 2004;10:1376–1385. doi: 10.1089/ten.2004.10.1376. [DOI] [PubMed] [Google Scholar]
- Schek RM, Taboas JM, Hollister SJ, Krebsbach PH. Tissue engineering osteochondral implants for temporomandibular joint repair. Orthod Craniofac Res. 2005;8:313–319. doi: 10.1111/j.1601-6343.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- Scheller EL, Chang J, Wang CY. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J Dent Res. 2008;87:126–130. doi: 10.1177/154405910808700206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepp BC, Clark KR, Klemanski DL, Pacak CA, Johnson PR. Genetic fate of recombinant adeno-associated virus vector genomes in muscle. J Virol. 2003;77:3495–3504. doi: 10.1128/JVI.77.6.3495-3504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz BR, Chamberlain JS. Recombinant adeno-associated virus transduction and integration. Mol Ther. 2008;16:1189–1199. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HC, Peng H, Usas A, Gearhart B, Cummins J, Fu FH, et al. Ex vivo gene therapy-induced endochondral bone formation: comparison of muscle-derived stem cells and different subpopulations of primary muscle-derived cells. Bone. 2004;34:982–992. doi: 10.1016/j.bone.2004.01.028. [DOI] [PubMed] [Google Scholar]
- Shi W, Bartlett JS. RGD inclusion in VP3 provides adeno-associated virus type 2 (AAV2)-based vectors with a heparan sulfate-independent cell entry mechanism. Mol Ther. 2003;7:515–525. doi: 10.1016/s1525-0016(03)00042-x. [DOI] [PubMed] [Google Scholar]
- Smith AE. Viral vectors in gene therapy. Annu Rev Microbiol. 1995;49:807–838. doi: 10.1146/annurev.mi.49.100195.004111. [DOI] [PubMed] [Google Scholar]
- Smith P, Shuler FD, Georgescu HI, Ghivizzani SC, Johnstone B, Niyibizi C, et al. Genetic enhancement of matrix synthesis by articular chondrocytes: comparison of different growth factor genes in the presence and absence of interleukin-1. Arthritis Rheum. 2000;43:1156–1164. doi: 10.1002/1529-0131(200005)43:5<1156::AID-ANR26>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Somia N, Verma IM. Gene therapy: trials and tribulations. Nat Rev Genet. 2000;1:91–99. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- Springen K. Using genes as medicine. Newsweek. 2004 Dec 6; [Google Scholar]
- Sumimoto H, Kawakami Y. Lentiviral vector-mediated RNAi and its use for cancer research. Future Oncol. 2007;3:655–664. doi: 10.2217/14796694.3.6.655. [DOI] [PubMed] [Google Scholar]
- Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerford C, Bartlett JS, Samulski RJ. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- Tarkka T, Sipola A, Jamsa T, Soini Y, Yla-Herttuala S, Tuukkanen J, et al. Adenoviral VEGF-A gene transfer induces angiogenesis and promotes bone formation in healing osseous tissues. J Gene Med. 2003;5:560–566. doi: 10.1002/jgm.392. [DOI] [PubMed] [Google Scholar]
- Tenenbaum L, Lehtonen E, Monahan PE. Evaluation of risks related to the use of adeno-associated virus-based vectors. Curr Gene Ther. 2003;3:545–565. doi: 10.2174/1566523034578131. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Storm TA, Huang Z, Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran SD, Wang J, Bandyopadhyay BC, Redman RS, Dutra A, Pak E, et al. Primary culture of polarized human salivary epithelial cells for use in developing an artificial salivary gland. Tissue Eng. 2005;11:172–181. doi: 10.1089/ten.2005.11.172. [DOI] [PubMed] [Google Scholar]
- Tran SD, Sugito T, Dipasquale G, Cotrim AP, Bandyopadhyay BC, Riddle K, et al. Re-engineering primary epithelial cells from rhesus monkey parotid glands for use in developing an artificial salivary gland. Tissue Eng. 2006;12:2939–2948. doi: 10.1089/ten.2006.12.2939. [DOI] [PubMed] [Google Scholar]
- Ulrich-Vinther M. Gene therapy methods in bone and joint disorders. Evaluation of the adeno-associated virus vector in experimental models of articular cartilage disorders, periprosthetic osteolysis and bone healing. Acta Orthop Suppl. 2007;78:1–64. [PubMed] [Google Scholar]
- Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- VanEpps HL. Peyton Rous: father of the tumor virus. J Exp Med. 2005;201:320. doi: 10.1084/jem.2013fta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma IM, Somia N. Gene therapy—promises, problems and prospects. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- West AG, Gaszner M, Felsenfeld G. Insulators: many functions, many mechanisms. Genes Dev. 2002;16:271–288. doi: 10.1101/gad.954702. [DOI] [PubMed] [Google Scholar]
- Wright V, Peng H, Usas A, Young B, Gearhart B, Cummins J, et al. BMP4-expressing muscle-derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice. Mol Ther. 2002;6:169–178. doi: 10.1006/mthe.2002.0654. [DOI] [PubMed] [Google Scholar]
- Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A, et al. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci USA. 2002;99:10405–10410. doi: 10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo GH, Piechocki MP, Oliver J, Lonardo F, Zumstein L, Lin HS, et al. Enhancement of Ad-p53 therapy with docetaxel in head and neck cancer. Laryngoscope. 2004;114:1871–1879. doi: 10.1097/01.mlg.0000147914.51239.ed. [DOI] [PubMed] [Google Scholar]
- Young CS, Abukawa H, Asrican R, Ravens M, Troulis MJ, Kaban LB, et al. Tissue-engineered hybrid tooth and bone. Tissue Eng. 2005;11:1599–1610. doi: 10.1089/ten.2005.11.1599. [DOI] [PubMed] [Google Scholar]
- Yu SF, von Ruden T, Kantoff PW, Garber C, Seiberg M, Ruther U, et al. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc Natl Acad Sci USA. 1986;83:3194–3198. doi: 10.1073/pnas.83.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- Zhang D, Schwarz EM, Rosier RN, Zuscik MJ, Puzas JE, O’Keefe RJ. ALK2 functions as a BMP type I receptor and induces Indian hedgehog in chondrocytes during skeletal development. J Bone Miner Res. 2003;18:1593–1604. doi: 10.1359/jbmr.2003.18.9.1593. [DOI] [PubMed] [Google Scholar]
- Zhang X, Godbey WT. Viral vectors for gene delivery in tissue engineering. Adv Drug Deliv Rev. 2006;58:515–534. doi: 10.1016/j.addr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Zhao J, Jia L, Chen D, Xiao J, Pan X, Jing X. Adenovirus-mediated TGF-beta(1) gene transfer to human degenerative lumbar intervertebral disc cells. Chin Med J (Engl) 2002;115:409–412. [PubMed] [Google Scholar]
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]