Abstract
Exposure to non-inherited maternal antigens (NIMA) in fetal and neonatal life of an F1 backcross (BDF1 female × B6 male) mouse can result in lifelong tolerance to allografts expressing the NIMA (H2d). We have recently shown that the NIMA-specific regulatory T cells were directly correlated with level of maternal microchimerism (MMc) in adult mice, indicating a causative link between the two, and that both Tregs and multi-lineage MMc were dependent on ingestion of milk from a NIMA+ mother during nursing. Yet how maternal cells obtained in fetal and neonatal life are maintained in adult life remains unclear. Since stem cells are deficient in MHC class I & II expression, we hypothesized that maternally derived stem cells that replenish MMc remain throughout life without eliciting immunity, but differentiated maternal cells can either be deleted by alloreactive T and B effector cells or persist, inducing NIMA-specific tolerance. Consistent with this hypothesis, we found maternally-derived lineageneg c-kit+ cells in the bone marrow of most of adult offspring by quantitative PCR; however, only 50% had detectable MMc in lineage+ bone marrow cells. Mesenchymal stem cells (lineageneg and plate-adherent cells) propagated from the bone marrow also contained maternally-derived cells, albeit in 10-fold lower frequency compared with MMc in myeloid lineage (CD11b+ and CD11c+) cells. Maternally-derived cardiac stem cells were also detected in lineageneg c-kit+ cells purified from heart tissue of NIMA-exposed mice, indicating a local pool of stem cells sustaining MMc in a non-lymphoid tissue. Cardiac stem cell MMc correlated with the presence of maternally derived cardiomyocytes. Lastly, liver MMc increased after nursing suggesting a seeding of maternal cells into the liver via breast milk. Whether orally-derived liver MMc also included maternal stem cells, was not determined. Maternal stem cells in bone marrow and tissues of NIMA-exposed mice are likely responsible for sustaining MMc in adult mice, but their presence alone does not guarantee multi-lineage MMc and tolerance.
Keywords: stem cells, regulatory T cells, microchimerism, NIMA, nursing
Introduction
Tolerance to non-inherited maternal antigens (NIMA) was first demonstrated by Owen et al.1 who showed that most Rh− women born of a Rh+ mother failed to produce antibody against Rh antigen when they gave birth to Rh+ children, whereas Rh− women from Rh− mothers did make anti Rh Ab. Similar observations of a privileged status for NIMA were made 34 years later by Claas et al.2 in studies of anti-HLA antibody responses in adults. In clinical studies of HLA haploidentical kidney allografts between siblings, graft survival was significantly better when the mismatched HLAs were NIMAs, than when noninherited paternal antigens (NIPA) were mismatched.3 Similarly, graft-versus-host disease after bone marrow transplantation was significantly less in NIMA-HLA-mismatched siblings than in other haploidentical donor-recipient combinations.4 The benefit of NIMA-HLA-mismatch has recently been found to extend to unrelated umbilical cord blood transplants for hematologic malignancies.5
We have reproduced the beneficial NIMA effect in an F1 backcross breeding (B6 male × BDF1 female) and heterotopic heart Tx model.6 About 57% of the NIMA-H2d-exposed offspring (H2b/b) accepted DBA/2 heart allografts, whereas non-exposed control F1 backcross mice rejected the allograft uniformly within 11 days post transplant. The exposure to NIMAd resulted in CD4+ T regulatory cells (TR) development in some but not all mice.7 This variability in TR induction could account for the variability in allograft tolerance, since only those NIMAd-exposed mice with strong mobilization of CD4+ TR to the graft were able to accept heart allografts long-term.6 Recently, we found that even before transplantation, TR-mediated suppression of DTH and in-vivo lymphoproliferation responses to maternal antigens were directly correlated with level of maternal microchimerism (MMc), suggesting a causative link between the two.8 We also found that adult offspring which had the highest levels of MMc also had the most CD4+ TGFβ+ T regulatory cells. Similarly Mold et al.9 showed that high levels of maternal microchimerism promoted NIMA-specific CD4+ Foxp3+ TR cell proliferation and specific suppression of MLR in human fetal lymph nodes.
Since MMc can be detected in different organs of NIMAd-exposed offspring including heart, lungs and liver, and in various lymphoid and myeloid lineages, including CD4, CD11b and CD11c cells,8 we wondered how these maternal cells are renewed and maintained in adult mice. One possible explanation would be engraftment of maternal progenitor cells that differentiate into the microchimeric cells throughout the life of the offspring.
We found maternally derived lin− c-kit+ stem cells in the bone marrow and the heart of NIMA-exposed F1 backcross offspring. Mesenchymal stem cells derived from the bone marrow also contained rare maternal cells. We also found that exposure to a H-2d heterozygous mother by nursing alone could induce MMc in the liver, suggesting that besides transplacental migration of maternal stem cells during gestation mother’s milk may also contain stem cells capable of traversing the gut barrier of the newborn and colonizing the liver.
Results and Discussion
Bone marrow of NIMAd-exposed offspring contains maternally derived stem cells
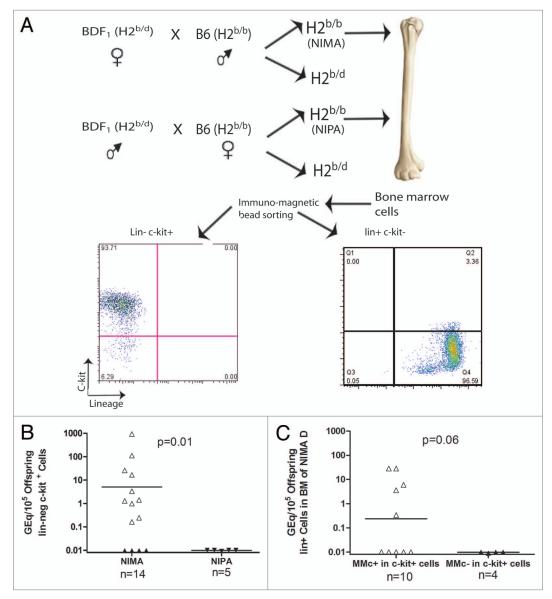
We previously detected maternally derived CD4, CD11b and CD11c cells in the adult offspring.8 These maternal lymphoid cells can be obtained during fetal and neonatal life of the offspring. However, it is not clear how the maternal cells obtained in fetal and neonatal lives can persist in adult offspring since lymphoid cells generally have a relatively short life span. We hypothesized that the maternal stem cells can cross the placenta, occupy a niche within the host, and give rise to differentiated maternal cells throughout the life of the offspring. We wanted to investigate whether maternally derived stem cells are present in the bone marrow (BM) of offspring since BM contains progenitors of both lymphoid and myeloid lineages. Figure 1A shows the purity of the c-kit+ cells isolated from BM cells, which was typically greater than 90%. We found that 10/14 NIMAd-exposed offspring had maternally derived lin− c-kit+ stem cells in their BM, as detected by a sensitive quantitative PCR technique (Fig. 1B). In contrast, none of the BM cells of NIPAd control mice had any detectable H2Dd Mc in the sorted lin−c-kit+ population. When we checked for MMc in lin+ BM cells, we found that only half (5/10) of NIMAd-exposed offspring that had MMc in the c-kit+ cells also had MMc in the lin+ cells (Fig. 1C). As expected, NIMAd-exposed offspring that lacked MMc in BM c-kit+ cells also lacked detectable MMc in the lin+ cells. This data indicates that: (1) MMc in maternally derived hematopoetic stem cells are required for MMc in leukocytes (lin+) and (2) maternal c-kit+ cells present in the offspring BM give rise to viable lin+ cells in some but not all the offspring.
Figure 1.
High incidence of MMc in bone marrow stem cells compared with lineage+ cells in the bone marrow of NIMAd-exposed adult offspring. (A) The figure shows the breeding scheme of NIMAd-exposed and NIPAd control offspring. Lineage−c-kit+ and lineage+ c-kit− cells from the bone marrow of NIMAd-exposed and NIPAd control offspring were sorted using a MACS sorter and the sorted cells were checked for purity using a flow cytometer. (B) DNA was extracted from the sorted cells and checked for maternal H2Dd microchimerism by qPCR. MMc was expressed in GEq/105 offspring cells. 1 GEq/105 offspring cells indicates one maternal cell in 105 offspring cells. (C) Lin+ cells in the BM of NIMAd-exposed offspring mice (either MMc+ or − in c-kit+ bone marrow cells) were checked for MMc.
Maternally derived mesenchymal stem cells (MSCs) NIMAd-exposed offspring
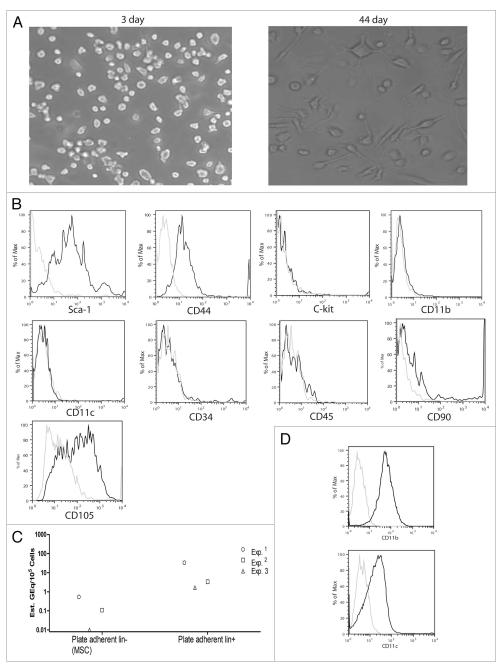
After we detected maternal c-kit+ stem cells in the bone marrow, we wanted to know whether the bone marrow also contains maternally derived MSCs. For this, we cultured bone marrow cells in MSC media. Figure 2A shows representative images of the culture at day 3 and day 44. At day 3, most of the plate adherent cells are round indicating that they are macrophages/dendritic cells. By day 44, MSCs grow and become dominant in the culture. After 6 weeks, we sorted lin− cells from the plate adherent cells using an autoMACS sorter. We used different cell surface markers of mouse MSCs to determine whether the lin− plate adherent cells contained MSCs. The cells expressed stem cell antigen 1 (Sca-1) and CD44 (Fig. 2B), which are considered as important markers for mouse MSCs.10 Unlike hematopoietic stem cells mouse MSCs do not express c-kit.11 The cells did not contain CD11b+ (macrophages) and CD11c+ (dendritic cells) cells (Fig. 2B), which are major contaminants in MSC culture.11 We checked for maternal H2Dd signal in the DNA extracted from MSCs and plate adherent lin+ cells using the qPCR technique. We had very weak H2Dd signals in the MSCs and about ten-fold stronger signals in the plate adherent lin+ cells (Fig. 2C). We found that the plate adherent lin+ cells were mostly CD11b+/CD11clow (Fig. 2D), which confirms our previously published data that maternal cells were mostly found in the CD11b+ and CD11c+ populations of the bone marrow.8 We did not detect any H2Dd microchimerism in any of the cell populations in NIPAd control group (data not shown).
Figure 2.
Cultured Bone marrow cells of NIMAd-exposed offspring contain maternally-derived mature myeloid lineages (CD11b+) and mesenchymal stem cells. (A) Bone marrow cells from NIMAd-exposed offspring were cultured in MSC media for one and half months. (B) Plate adherent lin− cells were sorted using a MACS sorter and checked for markers that define mouse mesenchymal stem cells. The dark and light lines represent staining for different cell surface antigens and corresponding isotypes, respectively. (C) Plate adherent lin− and lin+ cells from four different NIMAd-exposed offspring were pooled, DNA was extracted and MMc was checked by qPCR. Each dot represents a group of four NIMAd-exposed offspring. (D) Plate adherent lin+ cells in the MSC culture was checked for their CD11b and CD11c expressions. The dark and light lines represent staining for cell surface antigens (CD11b and CD11c) and corresponding isotypes, respectively.
Maternally derived cardiac stem cells in NIMAd-exposed offspring
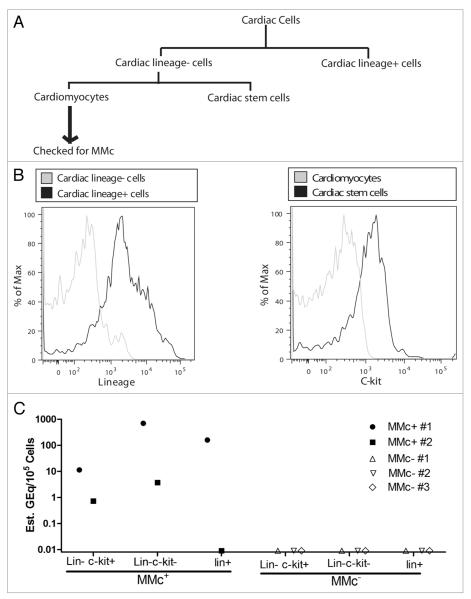
We previously found that the heart is one of the organs exhibiting a high frequency of MMc and that it contained maternal cells in both lineage− and lineage+ fractions.8 We wanted to know whether heart tissue contained maternally derived stem cells. We sorted different cell fractions from the heart: lin−c-kit+ (cardiac stem cells), lin−c-kit− (mainly cardiomyocytes) and lin+ (hematopoietic cells) using a MACS sorter (Fig. 3A and B). Different cell populations were further purified using a FACS sorter to obtain >99% purity of the cell populations. DNA was extracted from lin−c-kit− cells and checked for MMc using a qPCR technique (Fig. 3A). The different cell fractions from offspring having detectable MMc in their cardiomyocytes (lin−c-kit− cells) were pooled and represented as MMc+ in Figure 3C, whereas the cell fractions from offspring without detectable MMc in the lin−c-kit− cells were pooled and represented as MMc−. The pooled samples were then checked for MMc in both lin+ and lin− fractions (Fig. 3C), We found that offspring with a maternal DNA signal in their cardiomyocytes also had maternally derived cardiac stem cells (Fig. 3C). Pooled samples from hearts that were MMc− in their cardiomyocytes also did not have detectable maternal cells in cardiac stem cell or lineage+ populations (Fig. 3C). This data suggests that the maternally derived cardiac stem cells replenish the supply of maternally derived cardiomyocytes in the offspring.
Figure 3.
MMc in cardiac stem cells is associated with MMc in cardiomyocytes. (A) Lin+ cells were sorted from cells extracted from the hearts of NIMAd-exposed offspring using a MACS sorter. C-kit+ cells (cardiac stem cells) were sorted from the lin− fraction. The lin−c-kit− cells are mostly cardiomyocytes. The cells were further sorted using a FACS sorter to obtain 100% purity. Cardiomyocytes were checked for MMc. Different cell fractions isolated from the hearts of the offspring having detectable or undetectable MMc in the cardiomyocte fractions were pooled and represented as MMc+ and MMc−, respectively. (B) Different cardiac cell populations after MACS sorting. (C) DNA was extracted from the different cardiac cell populations from MMc+ and MMc− offspring. They were checked for MMc. Each dot represents MMc level in a pooled cell fraction of 4–5 NIMAd-exposed offspring.
Oral exposure to NIMA increases MMc in the liver
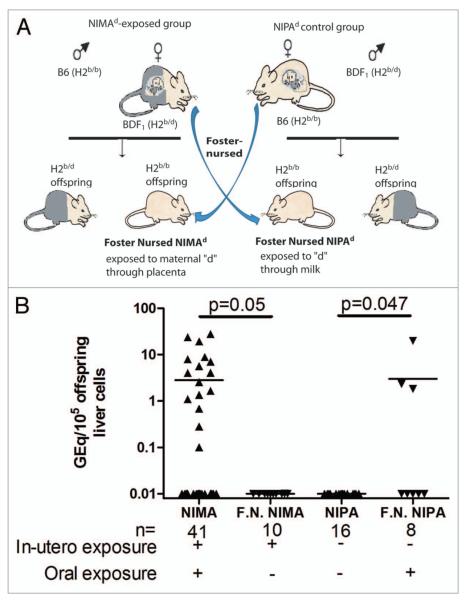
We previously showed that in absence of oral exposure to maternal antigens offspring lost not only regulation to maternal antigens but also MMc, indicating a critical role of oral exposure to maternal antigens in maintaining MMc.8 We wanted to investigate whether oral exposure to maternal antigens alone could induce maternal microchimerism. For this, we switched the mothers of NIMAd-exposed and NIPAd control offspring just after their birth so that they were foster nursed by B6 female and BDF1 female mice, respectively (Fig. 4A). We used NIMAd-exposed and NIPAd control offspring nursed by their biological mothers as controls for this experiment. When the NIPAd control offspring were foster nursed by BDF1 female mice, some (3/8) of them had H2Dd microchimerism in the liver, which was significantly different (p = 0.047) from that in NIPAd control offspring (n = 16) nursed by their own mothers, none of which had any detectable level of H2Dd microchimerism (Fig. 4B). This data indicates that oral exposure to maternal antigens alone can induce MMc in the liver. We also found a significant (p = 0.05) loss of H2Dd microchimerism in the livers of NIMAd-exposed offspring in the absence of oral exposure to H2d antigens (Fig. 4B). About 40% (16/41) of the NIMAd-exposed offspring nursed by their biological mothers had detectable levels of H2Dd microchimerism in the livers, whereas none of the NIMAd-exposed offspring foster nursed by B6 female mice had detectable levels of H2Dd microchimerism in the livers, which confirms our previously published data that oral exposure to maternal antigens is necessary to maintain MMc in offspring.
Figure 4.
Impact of nursing on liver microchimerism in adult offspring. (A) BDF1 female mice were crossed with B6 male mice to obtain NIMAd-exposed offspring that were exposed to maternal H2d antigens in-utero. The breeding pair was switched to obtain NIPAd control offspring that were not exposed to H2d antigens in-utero. Just after their birth, the mothers of the both kinds of offspring were switched so that NIMAd-exposed NIPAd control offspring were nursed by B6 and BDF1 females, respectively. This resulted in only in-utero and oral exposure to H2d antigens in foster nursed NIMAd and NIPAd offspring, respectively. (B) DNA was extracted from the livers of the foster nursed offspring. NIMAd-exposed and NIPAd control mice nursed by their biological mothers were used as controls.
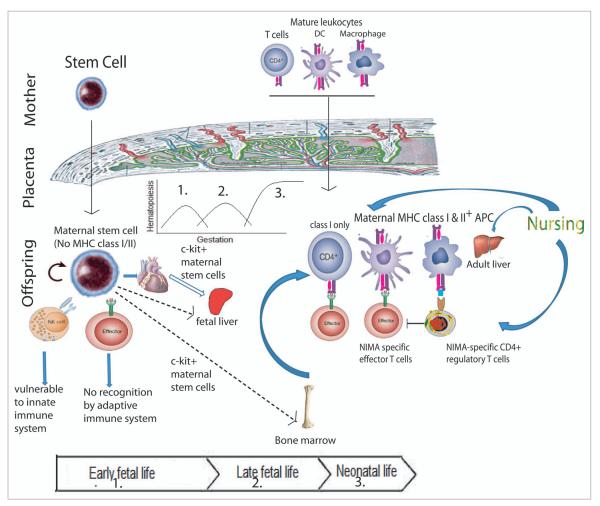
Figure 5 shows a hypothetical model of maternal-fetal cell trafficking that could account for the data presented here and previous papers by our lab and others. Transfer of fetal stem cells across the placenta into the mother has been suggested by different researchers.12-14 By simultaneous immunohistochemistry and in-situ hybridization of X and Y chromosomes, Stevens et al.15 found maternally-derived cardiomyocytes, hepatocytes, renal tubular cells and beta cells in pancreas of male infants. It is unlikely that offspring would obtain parenchymal cells from their mother in fetal life since these cells are not present in maternal blood, suggesting a pluripotent maternal stem cell engraftment that is supported by the murine data presented here.
Figure 5.
Proposed model of microchimerism-induced tolerance to alloantigens. During fetal life, maternal stem cells can cross placenta and engraft into different organs of offspring. They are immune-privileged and lack MHC expression. Thus they can avoid immune mediated rejection. However, the lineage+ cells differentiated from the stem cells can express MHC class I/II and can be detected and killed by NIMA-specific T cells of the offspring. These effector T cells can be blocked by NIMA-specific regulatory T cells generated via oral exposure to NIMA and liver MMc engraftment during nursing.
Our data shows that maternally-derived c-kit+ stem cells are present in the bone marrow and heart of adult offspring (Fig. 5, left). Although not depicted in Figure 5, stem cell and mature leukocyte traffic across the placental barrier is bi-directional. For example, Khosrotehrani et al.16 crossed Rag-deficient (lack T and B cells) female mice with wild type male mice and recovered functional T and B cells of fetal origin from the spleen of the female mice during pregnancy. Bianchi et al.12 detected CD34+ male progenitor cells in the peripheral blood of one woman out of eight women who were pregnant with male offspring at least twenty seven years before the blood sampling. Our finding of rare mesenchymal stem cells of maternal origin in the bone marrow of NIMA-exposed mice (Fig. 2C) is paralleled by the demonstrated presence of MSCs of fetal origin has been in the bone marrow of women decades after pregnancy with male fetuses.17 However, since the frequency of maternally derived MSCs is very low and the isolated MSCs are not 100% pure, we cannot rule out the possibility of lin+ cell contamination in MSCs. Similarly, other stem cells sorted using a MACS sorter are presumed stem cells since they are not 100% pure. As shown in the model, mature T, B and myeloid cells may also cross placental barrier; however, these cells would be expected to be relatively short lived (Fig. 5, right).
We found that 70% (10/14) of the NIMAd-exposed adult offspring had maternally derived lin− c-kit+ stem cells in their bone marrow, which were probably obtained during fetal life since 100% of mice had MMc in at least one of their organs when tested just after the birth.8 Only 50% (5/10) had MMc in the lin+ cells. This finding indicates that maternally derived c-kit+ cells engraft in most of the offspring; however, in only 50% of the offspring can they successfully colonize the host with lin+ cell progeny. Stem cells are immune privileged (Fig. 5, left): for example embryonic stem cells have been shown to express low levels of MHC class I and no MHC class II and thus, can avoid adaptive immune-mediated rejection,18 while still subject to innate (NK-cell)19 immune surveillance. Hematopoietic stem cells expressing minor hitocompatibility antigens, e.g., H60 can also be targeted by NK cells.20 Some stem cells, e.g., MSCs evade destruction by host immune cells by inhibiting Th1 cytokines, e.g., IFNγ and producing immunosuppressive cytokines, e.g., TGFβ and IL-10 favoring regulatory T cell induction.21,22 However, when self-renewing stem cells differentiate into mature cell lineages, they express MHC class I and in some lineages, class II molecules and thereby can easily be detected by offspring effector T cells (Fig. 5). However, deletion of differentiated maternal cells depends upon NIMA-specific effector and regulatory T cell balance (Fig. 5, right). The finding that in 50% of the offspring maternally derived c-kit+ cells can give rise to lin+ cells is consistent with our previous finding that about 50% of the NIMAd-exposed offspring had NIMA-specific regulatory T cells.8
In the current paper, we also demonstrated that nursing alone could generate MMc in the offspring liver without any transfer of maternal cells during fetal life, which was consistent with the finding by Zhou et al.23 Therefore, we proposed two ways of obtaining maternal cells by offspring (Fig. 5): (1) In fetal life, offspring obtain maternal stem cells, which can replenish MMc throughout the life of the offspring. (2) In neonatal life, offspring may get maternal cells through nursing. However, oral exposure to the maternal antigens alone was not enough to induce NIMA-specific tolerance (data not shown). Offspring that were exposed to maternal antigens through placenta during fetal life, but were not nursed by their biological mothers (so no oral exposure to maternal antigens) exhibited neither MMc nor NIMA-specific tolerance.8 Exposure to the maternal antigens both through placenta during fetal life and through nursing during neonatal life were required to induce NIMA-specific tolerance. From these findings, we conclude that maternally derived stem cells obtained during pregnancy engraft into different organs of offspring, which give rise to differentiated maternal cells. Deletion of maternal cells by NIMA-specific effector T cells is blocked by NIMA-specific regulatory T cells that are generated by oral exposure to NIMA (Fig. 5).8,24 The liver of the newborn mouse may be analogous to the human fetal lymph node9 as the anatomic site for preferential induction of NIMA-specific Treg cells.
The engraftment of maternally derived stem cells may be critically important for HSC and solid organ allograft outcome.3,5 However, maternally derived stem cells alone do not induce NIMA-specific tolerance. They need to differentiate into certain lineages to induce tolerance. However, at this point we don’t know which lineages are the important ones. We anticipate that MHC class II+, including CD11b+ and CD11c+ maternal macrophages and dendritic cells may be important since they may be involved in presentation of maternal antigens to NIMA-specific Tregs that suppress T effector cells (Fig. 5) and protect a NIMA-containing DBA/2 heart allograft from rejection.6
Materials and Methods
Source of mice, breeding and typing
C57 BL/6 (B6;H-2b/b), DBA/2 (H-2d/d) and B6D2F1 (BDF1;H-2b/d) were purchased from Harlan Sprague Dawley (Indianapolis, IN). F1 backcross breeding was performed to obtain offspring exposed or non-exposed to non-inherited maternal antigens as described previously.6 Briefly, BDF1 female mice were crossed with B6 male mice (Fig. 1A). About 50% of the offspring were homozygous for H2b/b and would thus be exposed to maternal H2d antigens in-utero during fetal life and orally in neonatal life. We call them NIMAd-exposed offspring. We switched the breeding pair to obtain NIPAd control offspring; 50% of these control mice were also H2b/b. They have the same genetic background as NIMAd-exposed mice; however, they were not exposed to the H2d antigens since the mother (B6 female) does not have H2d antigens. All offspring were weaned after 21 days of birth and typed by flow cytometry using anti-H2Kd antibody (BD Biosciences, San Jose, CA).
DNA extraction and quantitative polymerase chain reaction (qPCR) and analysis
DNA was extracted from tissues using QIAamp DNA extraction kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol and quantified using a spectro-photometer (Beckman Coulter, Fullerton, CA). The detailed technique of qPCR and the sequences of primers and probe specific for maternal H2Dd sequence were described in details previously.8 Briefly, DNA was amplified and quantified by qPCR using an iCycler thermal cycler (Bio-Rad Laboratories, Hercules, CA). BDF1 DNA was diluted into B6 DNA in different ratios (1:10–1:106) to obtain a standard curve. Maternal DNA in the samples was quantified using the standard curve. The reaction was performed in 50 μl of total reaction volume with 1 μg of genomic DNA (equivalent to about 106 cells), 20 pm of each primer, 7.5 pm of probe and 25 μl of Taqman Universal PCR Mastermix (Applied Biosystem). The qPCR program used was 50°C for 2 minutes, 95°C for 10 minutes, followed by fifty cycles of 95°C for 15 seconds and 60°C for 1 minute.
Cell sorting
The heart was perfused using cold PBS through the ascending aorta after harvesting to remove blood from coronary vessels. The heart was cut into half longitudinally and rinsed with cold PBS to remove blood from the chambers of the heart. Single cell suspensions of heart tissue were obtained using dispase II and collagenase (Roche, Indianapolis, IN) according to the manufacturer’s direction. Bone marrow cells were collected from femur, tibia and radius of offspring. The cardiac cells and bone marrow cells were stained with magnetic bead-conjugated lineage cocktail antibodies [CD5, CD45R (B220), CD11b, Gr-1 (Ly-6G/C), 7-4 and Ter-119] (Miltenyi Biotech, Auburn, CA). The lin+ cells stained with magnetic bead-conjugated antibodies and unconjugated lin− cells were sorted using a MACS sorter (Miltenyi Biotech, Auburn, CA) according to the manufacturer’s protocol. Lin− cells were stained with magnetic bead-conjugated anti-c-kit antibody and the stained cells were sorted using the MACS sorter.
Culturing of mesenchymal stem cells (MSC)
Sterile single cell suspension was prepared from the bone marrow of NIMAd-exposed and NIPAd control mice in mouse MSC media containing 5% horse serum, 5% fetal bovine serum, 1% non-essential amino acids, 2 mM L-glutamine and 1% penicillin/streptomycin in DMEM. The cells were cultured on plastic petri dishes in 37°C and 5% CO2 for 6 weeks. The media was changed every 2–3 days and cells were checked under microscope.
Flow cytometry
The purity of mouse MSCs was determined by a FACScaliber (BD Biosciences, San Jose, CA). The cells were stained for 30 minutes with antibodies against Sca-1, CD-44, c-kit, CD11b, CD11c, CD34 and CD45 (BD Biosciences, San Jose, CA). The data were analyzed using either a Cellquest or FlowJo (Treestar, Ashland, OR) software.
Acknowledgements
We would like to thank Steve Schumacher for technical assistance in typing mice, and Dr. Entela Lushaj and Dr. Deepika Rajesh for help in culturing MSCs. The research was supported by National Institute of Health Grant R01 AI066229.
Abbreviations
- Mc
microchimerism
- MMc
maternal microchimerism
- NIMA
non-inherited maternal antigen
- NIPA
non-inherited paternal antigen
- TR
T regulatory cells
- TE
T effector cells
- Lin
hematopoietic lineage
References
- 1.Owen RD, Wood HR, Foord AG, Sturgeon P, Baldwin LG. Evidence for actively acquired tolerance to Rh antigens. Proc Natl Acad Sci USA. 1954;40:420–4. doi: 10.1073/pnas.40.6.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claas FH, Gijbels Y, van der Velden-de Munck J, van Rood JJ. Induction of B cell unresponsiveness to noninherited maternal HLA antigens during fetal life. Science. 1988;241:1815–7. doi: 10.1126/science.3051377. [DOI] [PubMed] [Google Scholar]
- 3.Burlingham WJ, Grailer AP, Heisey DM, Claas FH, Norman D, Mohanakumar T, et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. 1998;339:1657–64. doi: 10.1056/NEJM199812033392302. [DOI] [PubMed] [Google Scholar]
- 4.van Rood JJ, Loberiza FRJ, Zhang MJ, Oudshoorn M, Claas F, Cairo MS, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99:1572–7. doi: 10.1182/blood.v99.5.1572. [DOI] [PubMed] [Google Scholar]
- 5.van Rood JJ, Stevens CE, Smits J, Carrier C, Carpenter C, Scaradavou A. Reexposure of cord blood to noninherited maternal HLA antigens improves transplant outcome in hematological malignancies. Proc Natl Acad Sci USA. 2009;106:19952–7. doi: 10.1073/pnas.0910310106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrassy J, Kusaka S, Jankowska-Gan E, Torrealba JR, Haynes LD, Marthaler BR, et al. Tolerance to noninherited maternal MHC antigens in mice. J Immunol. 2003;171:5554–61. doi: 10.4049/jimmunol.171.10.5554. [DOI] [PubMed] [Google Scholar]
- 7.Molitor-Dart ML, Andrassy J, Kwun J, Kayaoglu HA, Roenneburg DA, Haynes LD, et al. Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart allograft tolerance. J Immunol. 2007;179:6749–61. doi: 10.4049/jimmunol.179.10.6749. [DOI] [PubMed] [Google Scholar]
- 8.Dutta P, Molitor-Dart M, Bobadilla JL, Roenneburg DA, Yan Z, Torrealba JR, et al. Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice. Blood. 2009;114:3578–87. doi: 10.1182/blood-2009-03-213561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–5. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrepfer S, Deuse T, Lange C, Katzenberg R, Reichenspurner H, Robbins RC, et al. Simplified protocol to isolate, purify and culture expand mesenchymal stem cells. Stem Cells Dev. 2007;16:105–7. doi: 10.1089/scd.2006.0041. [DOI] [PubMed] [Google Scholar]
- 11.Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4:102–6. doi: 10.1038/nprot.2008.221. [DOI] [PubMed] [Google Scholar]
- 12.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93:705–8. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta P, Burlingham WJ. Tolerance to noninherited maternal antigens in mice and humans. Curr Opin Organ Transplant. 2009;14:439–47. doi: 10.1097/MOT.0b013e32832d6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CP, Lee MY, Huang JP, Aplin JD, Wu YH, Hu CS, et al. Trafficking of multipotent mesenchymal stromal cells from maternal circulation through the placenta involves vascular endothelial growth factor receptor-1 and integrins. Stem Cells. 2008;26:550–61. doi: 10.1634/stemcells.2007-0406. [DOI] [PubMed] [Google Scholar]
- 15.Stevens AMHH, Kiefer MM, Rutledge JC, Nelson JL. Chimeric maternal cells with tissue-specific antigen expression and morphology are common in infant tissues. Pediatr Dev Pathol. 2008;21 doi: 10.2350/08-07-0499.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khosrotehrani K, Leduc M, Bachy V, Huu S Nguyen, Oster M, Abbas A, et al. Pregnancy allows the transfer and differentiation of fetal lymphoid progenitors into functional T and B cells in mothers. J Immunol. 2008;180:889–97. doi: 10.4049/jimmunol.180.2.889. [DOI] [PubMed] [Google Scholar]
- 17.O’Donoghue K, Chan J, de la Fuente J, Kennea N, Sandison A, Anderson JR, et al. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364:179–82. doi: 10.1016/S0140-6736(04)16631-2. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Baroja ML, Majumdar A, Chadwick K, Rouleau A, Gallacher L, et al. Human embryonic stem cells possess immune-privileged properties. Stem Cells. 2004;22:448–56. doi: 10.1634/stemcells.22-4-448. [DOI] [PubMed] [Google Scholar]
- 19.Durkin ET, Jones KA, Rajesh D, Shaaban AF. Early chimerism threshold predicts sustained engraftment and NK-cell tolerance in prenatal allogeneic chimeras. Blood. 2008;112:5245–53. doi: 10.1182/blood-2007-12-128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabayoyong WB, Salas JG, Bonde S, Zavazava N. HOXB4-transduced embryonic stem cell-derived Lin-c-kit+ and Lin-Sca-1+ hematopoietic progenitors express H60 and are targeted by NK cells. J Immunol. 2009;183:5449–57. doi: 10.4049/jimmunol.0901807. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 22.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhou L, Yoshimura Y, Huang Y, Suzuki R, Yokoyama M, Okabe M, et al. Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast-feeding after birth. Immunology. 2000;101:570–80. doi: 10.1046/j.1365-2567.2000.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molitor ML, Haynes LD, Jankowska-Gan E, Mulder A, Burlingham WJ. HLA class I noninherited maternal antigens in cord blood and breast milk. Hum Immunol. 2004;65:231–9. doi: 10.1016/j.humimm.2003.12.006. [DOI] [PubMed] [Google Scholar]