Abstract
Despite its superior efficacy, clozapine is helpful in only a subset of patients with schizophrenia unresponsive to other antipsychotics. This lack of complete success has prompted the frequent use of various clozapine combination strategies despite a paucity of evidence from randomized controlled trials supporting their efficacy. Pimozide, a diphenylbutylpiperidine, possesses pharmacological and clinical properties distinct from other typical antipsychotics. An open label trial of pimozide adjunctive treatment to clozapine provided promising pilot data in support of a larger controlled trial. Therefore, we conducted a double blind, placebo controlled, parallel designed 12 week trial of pimozide adjunctive treatment added to ongoing optimal clozapine treatment in 53 patients with schizophrenia and schizoaffective disorder partially or completely unresponsive to clozapine monotherapy. An average dose of 6.48 mg/day of pimozide was found to be no better than placebo in combination with clozapine at reducing PANSS total, positive, negative, and general psychopathology scores. There is no suggestion from this rigorously conducted trial to suggest that pimozide is an effective augmenting agent if an optimal clozapine trial is ineffective. However, given the lack of evidence to guide clinicians and patients when clozapine does not work well, more controlled trials of innovative strategies are warranted.
Keywords: clozapine, pimozide, combination, schizophrenia, treatment non-response
INTRODUCTION
The introduction of clozapine provided a more effective treatment option for patients with schizophrenia refractory to first generation (Wahlbeck, et al 1999) and second generation (McEvoy et al 2006) antipsychotic medication. However, only 30%–60% of patients with schizophrenia who are unresponsive to “typical” antipsychotics will respond to clozapine (Lieberman, et al 1994; Kane, et al 1988; Breier, et al 1994; Pickar, et al 1992), prompting the frequent use of various clozapine combination strategies despite a paucity of controlled trials demonstrating the clinical usefulness or safety of such strategies.
Indeed, the majority of reports on clozapine combination strategies are case reports and open label trials. Amongst the few published controlled trials, the most frequently reported are trials with a second antipsychotic drug. To date, clozapine combination strategies with chlorpromazine (Potter, et al 1989), risperidone (Honer, et al 2006; Anil Yağcioğlu, et al 2005; Freudenreich, et al 2007) and aripiprazole (Fleischhacker et, al 2010) have proven ineffective. The only positive results have been reported with sulpiride (Shiloh, et al 1997) and amisulpiride (Genç, et al 2007), however, significant methodological limitations call these results into question.
Pimozide, a diphenylbutylpiperidine, is generally considered a typical antipsychotic with primarily dopamine D2 receptor blocking activity (Pinder, et al 1976). However, unlike other typical antipsychotics, pimozide does not block dopamine autoreceptors (Roth RH 1994; Walters and Roth, 1976), does not produce D2 receptor upregulation (Tecott, et al 1986), lacks affinity for α-adrenergic receptors (Peroutka et al 1977) and is a potent opiate receptor antagonist (Creese, et al 1976). Pimozide has also demonstrated particular efficacy in treating mono-symptomatic delusional disorder (Riding and Munro 1975;Reilly TM 1975; Hamann and Avnstorp 1982; Johnson and Anton 1983;Munro, et al 1985).
On the basis of these data and promising pilot data from an open label trial of pimozide adjunctive treatment to clozapine non-responders (Friedman, et al 1997), we conducted a double blind, placebo controlled 12 week trial of pimozide adjunctive treatment added to ongoing optimal clozapine treatment in patients with schizophrenia and schizoaffective disorder partially or completely unresponsive to clozapine monotherapy. The two main hypotheses tested were: 1) that pimozide adjunctive treatment to clozapine would produce a statistically significant greater reduction in Positive and Negative Syndrome (PANSS) total scores compared to the placebo adjunct and 2) that pimozide adjunctive treatment to clozapine would produce significantly greater reduction in Clinical Global Impression (CGI) score compared to the placebo adjunct.
PATIENTS AND METHODS
Participants
Subjects eligible for this study were those with a Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSMIV) (American Psychiatric Association, 1994) diagnosis of schizophrenia or schizoaffective disorder based on information obtained from the Comprehensive Assessment of Symptoms and History (Andreasen, et al 1992) interview. In addition, potential subjects were treatment unresponsive to an optimal trial of clozapine monotherapy. Treatment non-response was defined as the presence, at 4 consecutive weekly screening phase ratings, of persistent positive psychotic symptoms characterized by PANSS scores of 4 or higher on at least two items from the Positive subscale, a PANSS total score>60 and a CGI≥4; constructs adopted from the U.S. multi-center trial of clozapine (Kane, et al 1988).
To ensure optimal trials of clozapine for all prospective subjects we required a minimum plasma clozapine concentration of 378 ng/ml for a minimum treatment period of 8 weeks prior to study entry. This cutoff was based on the average of minimum effective plasma concentration values suggested by available studies, which ranges from 350 to 420 ng/ml (Perry, et al 1991; Potkin, et al 1994; Kronig, et al 1995; Hasegawa, et al 1993; Miller, et al 1994). Moreover, whie the precise treatment duration required to obtain an optimal response to clozapine remains debated, we based our choice of 8 weeks on the observations of Conley and colleagues (1997).
Subjects were recruited from inpatient and outpatient treatment settings at Mount Sinai Hospital, Pilgrim Psychiatric Center and Manhattan Psychiatric Center in New York following institutional review board approval at these facilities and after informed consent was provided. Following enrollment, the first four weeks of the study were used to assess treatment non-response and stability of symptoms by performing weekly PANSS ratings. Symptom stability was defined as PANSS total score changes < 20% from the first assessment. After demonstrating four weeks of symptom stability, baseline assessments were performed.
Potential subjects were excluded from participation in the study if they were receiving other psychotropic medications in addition to clozapine, with the exception of benzodiazepines, benztropine and valproic acid. Furthermore, once participants entered the symptom stability screening phase of the study no changes to the clozapine dose were permitted, nor was the addition of other psychotropic medication permitted (with the exception of benztropine) for the duration of the study. Participants were also excluded if it was determined that they were abusing alcohol or drugs within the 6 month period preceding study entry as ascertained by history and urine toxicology screen.
Assessments
Severity of positive and negative schizophrenic symptoms was assessed with the Positive and Negative Syndrome Scale (PANSS; Kay 1991). The PANSS contains 30 items, with 7 items rating positive symptoms, 7 rating negative symptoms, and 16 other items assessing “general psychopathology”. Ongoing reliability assessments ensured inter-rater reliability was maintained for all raters above .80 (ICC). Severity of extrapyramidal symptoms was rated with the Extrapyramidal Symptom Rating Scale (ESRS; Chouinard, et al 1979). The EPS domains of abnormal involuntary movements, parkinsonism, dystonias and akathisia are rated for individual body parts on a two dimensional continuum of severity and frequency. Given that the increased risk of extrapyramidal symptoms associated with pimozide threatened the integrity of the blind, assignment of assessments ensured that the rating of the ESRS was carried out by personnel different from those performing the PANSS and functional competence ratings. Functional competence was assessed with the Specific Level of Function scale (SLOF; Schneider, et al 1983). The SLOF consists of 43 items rated on a 5-point likert type scale grouped into 5 functional and behavioral areas. These areas include: social skills, problem behaviors, self care deficits, community living skills and vocational functioning. In addition, blood pressure, pulse, side effects, and laboratory studies were monitored during the study. In accordance with published reports on the use of pimozide, ECGs were monitored at regular intervals during the course of the study (Shapiro, et al 1983). Manual interpretations of every ECG was done to assess rhythm, waveforms and intervals such as QRS, PR, RR and QT intervals. Because the QT interval shortens with increasing heart rate, the QT interval, corrected for heart rate (QTc) was accomplished by the following commonly used formula [QT/Sqrt(RR)] (Heger, et al 1987).
Treatment Design
Following baseline assessments subjects were randomized to double blind treatment with pimozide or matching placebo in a 1:1 fashion. Following randomization, a four week titration period began during which either pimozide or placebo was added to clozapine treatment at a rate of 2 mg per week by a blinded physician to a desired clinical effect or to a maximum dose of 8 mg per day. The maximum dose of pimozide was based on the maximum dose used in our open label trial with pimozide (Friedman, et al 1997). When, in the opinion of the study physician, an optimal dose of study medication was achieved at the end of the four week titration phase, treatment continued with this optimal dose for an additional eight weeks. Compliance of outpatient subjects with study medication was assessed by weekly pill counts.
Data Analysis
Data analysis was carried out by employing the GEE (Generalized Estimation Equation) method to analyze the efficacy data using independent working correlation. Models: Yij= a + b1Groupi+ b2weekj+ b3Groupi×weekj+eij, where Yij= efficacy measurement (ie: PANSS total scores) of ith patients in jth week, Groupi=0 if the ith patient receives treatment A, = 1 otherwise, weekj= j, j=0, 1, 2,…, 12, eij= error, a = intercept, b1= group effect, b2= time effect, b3= Group×time effect. The model also adjusted for the following confounding factors; 1) baseline PANSS scores, 2) clozapine plasma level, 3) clozapine treatment duration, 4) age, 5) age of onset of illness, 6) treatment setting (inpatient vs outpatient), 7) race.
RESULTS
76 subjects were consented, 16 subjects were excluded during the four week symptom stability screening period prior to randomization due to withdrawal of consent or a failure to meet inclusion and exclusion criteria. Seven additional subjects were randomized to a haloperidol adjunctive pilot study. 53 subjects were randomized to study drug; 28 to placebo and 25 to pimozide (see Figure 1). 23 of the 28 subjects randomized to placebo, and 22 of the 25 subjects randomized to pimozide completed all 12 weeks of the study. One subject randomized to pimozide terminated early due to severe EPS, while two terminated early because of adverse events deemed unrelated to study medication (delirium due to a UTI, exacerbation of hemorrhoidal bleeding). Five of the 28 subjects randomized to placebo terminated early; two subjects due to persistently severe psychotic symptoms necessitating treatment changes by the treating physician not allowable by the protocol, one due to an episode of bigeminy, one due to hypotension, and one withdrew consent to continue participation (Figure 1).
Figure 1.
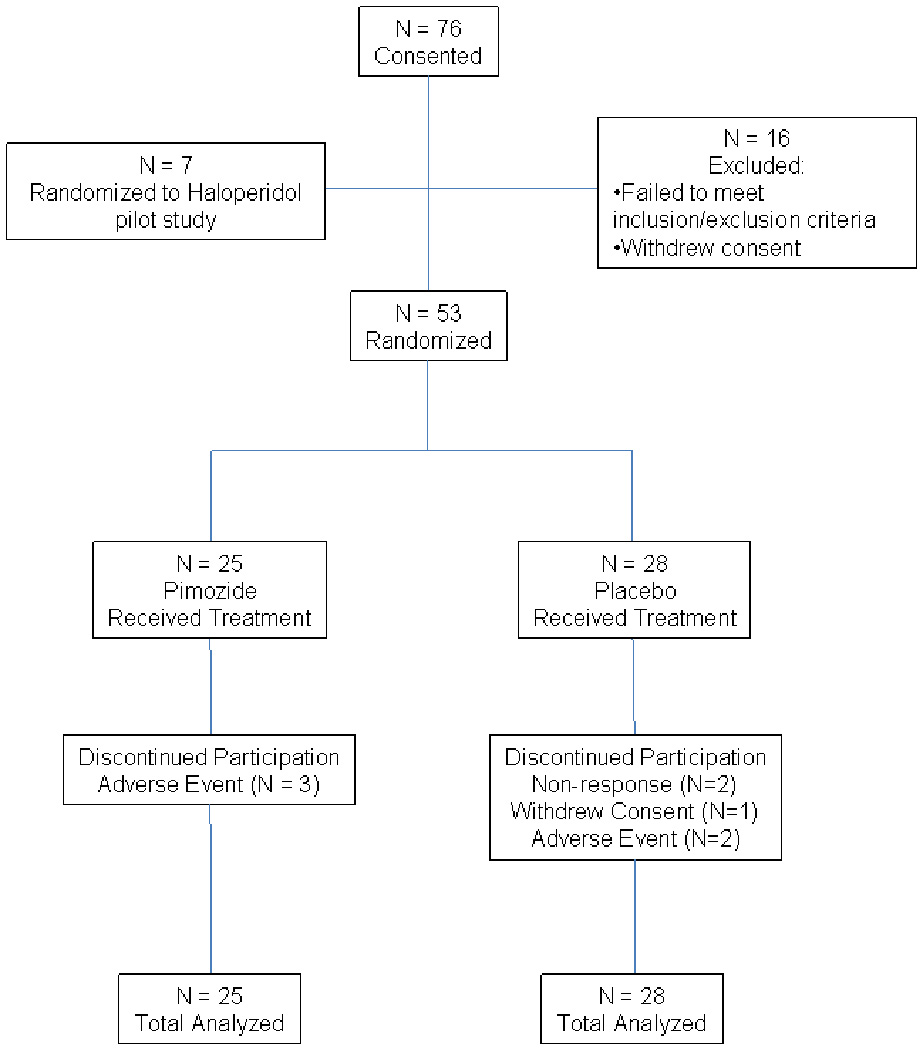
CONSORT diagram showing the flow of participants through each stage of the Pimozide study.
Table 1 shows baseline clinical and demographic data for the pimozide and placebo treatment groups. Both groups had similar baseline plasma clozapine levels above the therapeutic threshold. The group randomized to pimozide showed a trend towards lower baseline PANSS total scores. The group randomized to placebo had a greater proportion of Caucasian subjects while the group randomized to pimozide had a greater proportion of African American subjects (p = .04). No other differences were noted.
Table 1.
Discrete Statistics, Mean (SD) or count (%) for the comparison of baseline clinical and demographic data for placebo and pimozide treated groups.
| Variable | Treatment |
p-value | |
|---|---|---|---|
| Placebo (N = 28) Mean (SD) | Pimozide (N = 25) Mean (SD) | ||
| Age (Years) | 44.4 (8.7) | 45.5 (10.2) | .66 |
| Education (Years) | 11.8 (2.1) | 11.2 (2.3) | .38 |
| Age of Illness Onset (Years) | 18.5 (4.6) | 19.2 (4.2) | .58 |
| Clozapine Dose (mg/day) | 478.1 (150.2) | 518.8 (117.3) | .30 |
| Plasma Clozapine Concentration (ng/ml) | 555.5 (147.7) | 558.1 (152.5) | .95 |
| Duration of Clozapine Treatment (Months) | 20.8 (25.2) | 16.8 (14.7) | .53 |
| PANSS Total | 90.7 (16.0) | 82.9 (15.1) | .08 |
| PANSS Positive | 22.3 (4.9) | 21.0 (4.9) | .36 |
| PANSS Negative | 24.6 (6.4) | 21.9 (4.4) | .08 |
| PANSS General | 44.0 (8.1) | 40.0 (9.0) | .11 |
| SLOF Physical Functioning | 24.77 (.81) | 24.36 (.99) | .11 |
| SLOF Personal Care | 30.81 (2.56) | 30.28 (4.67) | .61 |
| SLOF Interpersonal Relationships | 22 (5.62) | 24.56 (4.19) | .07 |
| SLOF Social Acceptability | 32.85 (2.49) | 32.48 (3.14) | .64 |
| SLOF Activities | 39 (12) | 38.92 (10.48) | .97 |
| SLOF Work Skills | 17.08 (6.35) | 17.36 (5.05) | .86 |
| Variable | Count (%) | Count (%) | p-value |
| Gender | |||
| Female | 8 (29%) | 4 (16%) | .34 |
| Male | 20 (71%) | 21 (84%) | |
| Ethnicity | |||
| Asian | 1 (4%) | 0 (0%) | .04 |
| Black | 4 (14%) | 13 (52%) | |
| Hispanic | 6 (12%) | 5 (20%) | |
| White | 17 (61%) | 7 (28%) | |
| Treatment Setting | |||
| Inpatient | 19(68%) | 15(60%) | .47 |
| Outpatient | 9(32%) | 10(40%) | |
| Valproic Acid Treatment | |||
| No | 18 (64%) | 17 (68%) | .96 |
| Yes | 10 (36%) | 8 (32%) | |
The average daily dose of pimozide utilized during the treatment phase of the study was 6.48 mg/day (SD=2.18). GEE modeling demonstrated no significant treatment by time interaction in favor of pimozide on PANSS total score change over the 12 week study period (p = .53), PANSS Positive score change (p = .55), PANSS Negative score change (p = .15), PANSS General Psycvhopathology score change (p = .52), nor CGI score change (p=.15) (Figure 2, Table 2). Exploratory analyses showed no significant difference in SLOF subscale change scores between the two treatment groups (Table 2.). Analysis of safety data (Table 2) showed no significant changes in plasma clozapine levels, blood glucose, cholesterol or triglycerides. There was a modest increase in QTc interval associated with pimozide treatment (mean = 9 mSec) compared with placebo (mean = −1.5 mSec), the difference was not statistically significant (p=.19). Although there were no significant differences in between placebo and pimozide on the change in scores for the parkinsonism, dystonia, dyskinesia subscales of the ESRS, there was a trend towards a greater frequency of hypersalivation in the pimozide group compared with the placebo group (32% versus 11%) (p=.09).
Figure 2.
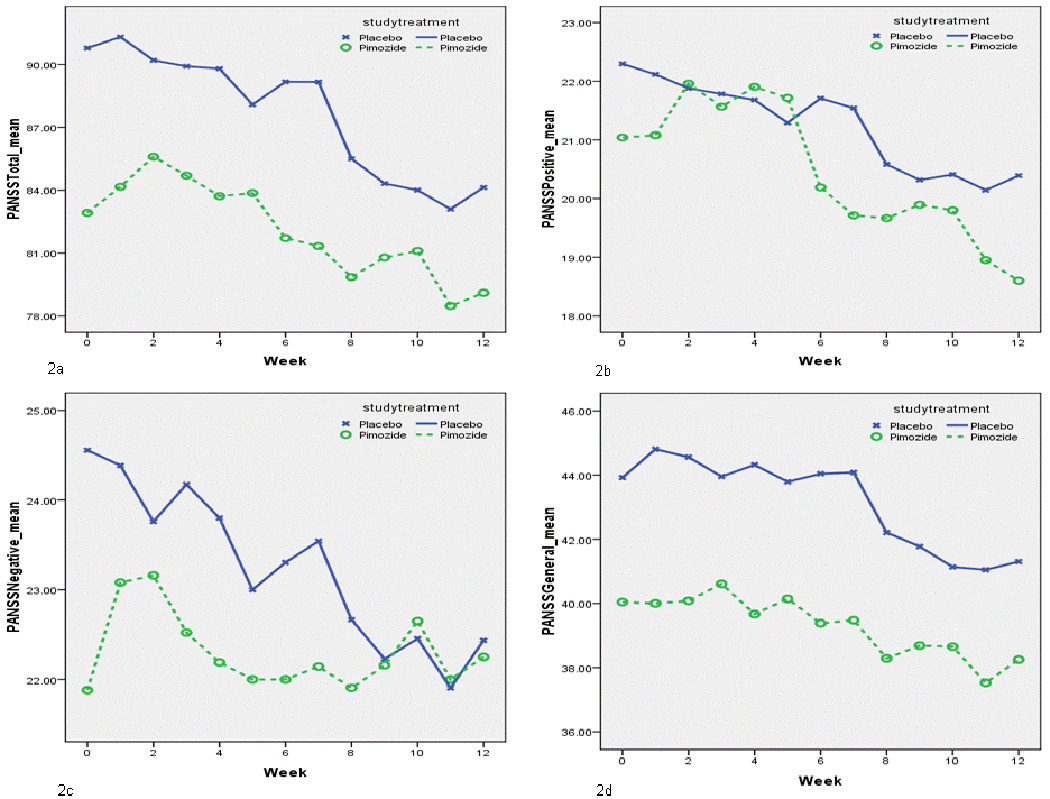
Line and symbol plot of weekly means of; 2a) PANSS Total scores, 2b) PANSS Positive scores, 2c) PANSS Negative socres, 2d) PANSS General Psychopathology scores for pimozide and placebo treated groups.
Table 2.
Mean change scores and standard deviations of efficacy and safety variables for placebo and pimozide treated groups. p-values generated by GEE analyses of efficacy and safety data.
| Variable Change from Baseline To Week 12 | Treatment | p-value | |
|---|---|---|---|
| Placebo Mean Change (SD) | Pimozide Mean Change (SD) | ||
| Symptom Severity | |||
| PANSS Total | −3.59 (10.15) | −0.75 (10.60) | .53 |
| PANSS Positive | −1.05 (2.98) | −1.30 (2.56) | .55 |
| PANSS Negative | −1.59 (4.46) | 0.65 (4.65) | .14 |
| PANSS General | −0.95 (5.26) | −0.15 (4.97) | .52 |
| CGI | −0.35 (0.57) | −0.14 (0.56) | .15 |
| Functional Skills | |||
| SLOF Physical Function | 0.18 (0.66) | 0 (1.28) | .55 |
| SLOF Personal Care | 0.50 (3.19) | 0.28 (2.65) | .78 |
| SLOF Interpersonal Relationships | 0.41 (3.19) | 0.52 (5.38) | .93 |
| SLOF Social Acceptability | 0.18 (2.81) | 0.52 (3.16) | .70 |
| SLOF Work Skills | 0.18 (3.03) | 0.52 (4.02) | .75 |
| Extrapyramidal Symptom Severity | |||
| ESRS Parkinsonism | 1.37 (4.27) | 1.59 (4.37) | .86 |
| ESRS Dystonia | 0.50 (1.98) | −0.09 (1.63) | .27 |
| ESRS Dyskinesia | −0.21 (4.01) | −0.05 (1.89) | .86 |
| Other Safety Measures | |||
| Clozapine Plasma Concentration (ng/ml) | −27.3 (138.6) | 2.5 (163.4) | .48 |
| QTc (mSec) | −1.50 (29.59) | 9.04 (24.70) | .19 |
| Heart Rate | 2.16 (7.33) | 1.59 (12.39) | .85 |
| Systolic Blood Preesure (mm Hg) | −1.37 (13.39) | 0.91 (10.59) | .52 |
| Diastolic Blood Pressure (mm Hg) | 1.04 (8.74) | −0.32 (9.39) | ,61 |
| Absolute Neutrophil Count (count/mm3) | −0.40 (1.52) | 0.27 (1.07) | .10 |
| Plasma Glucose level (mg/dL) | −0.10 (14.20) | 4.31 (18.87) | .37 |
| Total Cholesterol Level (mg/dL) | −12.29 (40.01) | −1.56 (25.34) | .37 |
| Triglyceride Level (mg/dL) | −28.35 (60.35) | −5.19 (46.60) | .23 |
DISCUSSION
The results of this randomized controlled trial do not support the efficacy of pimozide adjunctive treatment to clozapine in patients with schizophrenia and schizoaffective disorder unresponsive to clozapine monotherapy. Similar to several other clozapine augmentation trials, the positive results of our initial open label study (Friedman et al 1997) were not confirmed in this double blind, placebo controlled study. Although moderately sized, it is possible that this investigation was under-powered to demonstrate more modest significant results. However, based on the data from this study, the number of subjects required to detect a treatment difference at an alpha<.05, with 80% power, using two tailed tests would be: 1) 424 for PANSS Total, 2) 3,876 for PANSS Positive, 3) 130 for PANSS Negative, 4) 1292 for PANSS General Psychopathology scores. Even with these sample sizes, the differences for PANSS Total, Negative, and General Psychopathology scores would favor placebo.
The most common shortcoming of the few published controlled trials of clozapine combination treatments is the lack of data demonstrating optimal treatment with clozapine monotherapy prior to entry into combination treatment studies. An optimal trial requires a minimum therapeutic plasma concentration of clozapine and minimum duration of treatment. Often, therapeutic doses of clozapine are assumed by evaluating dose-effect relationships, with no regard for plasma levels. However, several studies have found very large inter-individual variability in plasma concentrations of clozapine at fixed doses (Perry et al 1994;Potkin et al 1994;Olesen et al 1995). Moreover, several studies suggest that a minimum effective plasma concentration from 350 to 420 ng/ml (Perry et al 1994;Potkin et al 1994;Kronig et al 1995;Hasegawa et al 1993;Miller et al 1994) is required to ensure optimal clinical response. Both conditions, a minimum period of stable pre-study treatment and a minimum plasma clozapine concentration at baseline and throughout the study, were fulfilled in our study.
Clinicians need to consider the issue of an optimal clozapine monotherapy trial when interpreting the positive results of unreplicated studies with clozapine augmenting agents like sulpiride (Shiloh, et al 1997), amisulpiride (Genc, et al 2007), and risperidone (Josiassen, et al 2005). Although these studies demonstrated significant improvement in schizophrenia symptoms with these combination treatments compared to clozapine treatment alone (Shiloh, et al 1997; Josiassen, et al 2005) and clozapine combined with quetiapine (Genc, et al 2007), neither baseline nor follow-up clozapine plasma concentrations were reported. Therefore, it is possible that one or both treatment groups did not have, on average, therapeutic clozapine levels at baseline, and/or experienced pharmacokinetic interactions with the study drug elevating clozapine plasma concentrations. These factors may have significantly confounded the results of these studies. Indeed, when baseline clozapine concentrations were demonstrated to be similar and above minimum therapeutic concentration thresholds for both treatment groups, the risperidone plus clozapine combination was shown not to be superior to clozapine alone in a rigorous double blind placebo controlled trial conducted by Honer and colleagues (2006). Moreover, two additional studies failed to replicate the positive results of the first risperidone augmentation trial (Yagcioglu et al 2005;Freudenreich et al 2007).
In addition to the data on lack of efficacy of these combination treatments these trials provide, clinicians should consider the safety data reported by these studies. For example, the risperidone plus clozapine combination demonstrated a tendency towards increasing fasting glucose levels (Honer, et al 2006), and producing greater levels of sedation, akathisia and prolactin elevations (Yagcioglu, et al 2005) when compared to clozapine monotherapy. Potential side effects of these combinations should factor into the clinician’s decision to engage in such treatment strategies, especially when data supporting their efficacy is tenuous or absent.
However, even before clinicians consider any clozapine combination strategy, an optimal clozapine trial should be ensured as was done in this trial. In those cases where side effects limit the clinician’s abilitiy to attain therapeutic plasma concentrations, these should be addressed before initiating a combination strategy. Only after these issues have been addressed should the clinician consider clozapine augmentation strategies as the next step to improve response. However, there is no suggestion from this rigorously conducted trial that pimozide is an effective augmenting agent if an optimal clozapine trial is ineffective. Therefore, given the continued lack of evidence to guide clinicians and patients when clozapine does not work well, more controlled trials of innovative strategies are warranted.
Acknowledgements
This work was supported by grant number MH067806-01A2 awarded by the NIMH to Dr. Joseph I. Friedman.
Footnotes
ClinicalTrials.gov Identifier: NCT00158223
Disclosure/Conflict of Interest
Joseph I Friedman reports reports no financial relationships with commercial interests.
Jean-Pierre Lindenmayer has received grant support from Johnson and Johnson, Eli Lilly and Company, Pfizer, Astra Zeneca, Otsuka and Dainippon-Sumitomo.
Dr. Lindenmayer has served as a consultant to Eli Lilly, Johnson & Johnson, Merck and Shire Pharma.
Frances Alcantara has received grant support from Johnson and Johnson, Eli Lilly and Company, Pfizer, Astra Zeneca, Dainippon-Sumitomo and Otsuka.
Stephanie Bowler reports no financial relationships with commercial interests.
Parak Mohan has received grant support from Johnson and Johnson, Eli Lilly and Company, Pfizer, Astra Zeneca, Dainippon-Sumitomo and Otsuka.
Leonard White reports no financial relationships with commercial interests.
Adel Iskander has received grant support from Johnson and Johnson, Eli Lilly and Company, Pfizer, Astra Zeneca, Dainippon-Sumitomo and Otsuka.
Michael Parrella reports no financial relationships with commercial interests.
David Adler has received speaker honoraria from Eli Lilly and Company. David Adler is currently employed as a research psychiatrist by Neurobehavioral Research, Inc (Cedarhurst, NY).
Nicholas D Tsopelas reports no financial relationships with commercial interests.
Wei-Yann Tsai reports no financial relationships with commercial interests.
Vladan Novakovick reports no financial relationships with commercial interests.
Philip D. Harvey has served as a consultant for Eli Lilly and Company, Merck and Company, Shire Pharma, Johnson and Johnson, and Dainippon Sumitomo America. He has grant support from AstraZeneca.
Kenneth L. Davis’s wife receives royalty income from Janssen Pharma and Shire Pharma from sale of a patented drug for the treatment of Alzheimer’s disease. The other authors report no financial relationships with commercial interests.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental health disorders. 4th ed. Washington DC: 1994. [Google Scholar]
- Andreasen NC, Flaum M, Arndt S. The comprehensive assessment of symptoms and history (CASH): An instrument for assessing psychopathology and diagnosis. Archives of General Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Anil Yağcioğlu AE, Kivircik Akdede BB, Turgut TI, Tümüklü M, Yazici MK, Alptekin K, Ertuğrul A, Jayathilake K, Göğüş A, Tunca Z, Meltzer HY. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. J Clin Psychiatry. 2005;66:63–72. doi: 10.4088/jcp.v66n0109. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, Carpenter WT., Jr Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151:20–26. doi: 10.1176/ajp.151.1.20. [DOI] [PubMed] [Google Scholar]
- Chouinard G, Ross-Chouinard A. Extrapyramidal symptom rating scale – Manual. Canada: Montreal, Quebec; 1979. [Google Scholar]
- Creese I, Feinberg AP, Snyder SH. Butyrphenone influence on the opiate receptor. European Journal of Pharmacology. 1976;36:231–235. doi: 10.1016/0014-2999(76)90277-6. [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW, Heikkinen ME, Olié JP, Landsberg W, Dewaele P, McQuade RD, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol. 2010:1–11. doi: 10.1017/S1461145710000490. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Freudenreich O, Henderson DC, Walsh JP, Culhane MA, Goff DC. Risperidone augmentation for schizophrenia partially responsive to clozapine: a double-blind, placebo-controlled trial. Schizophr Res. 2007;92:90–94. doi: 10.1016/j.schres.2006.12.030. [DOI] [PubMed] [Google Scholar]
- Friedman J, Ault K, Powchik P. Pimozide augmentation for the treatment of schizophrenic patients who are partial responders to clozapine. Biological Psychiatry. 1997;42:522–523. doi: 10.1016/S0006-3223(97)00227-8. [DOI] [PubMed] [Google Scholar]
- Genç Y, Taner E, Candansayar S. Comparison of clozapine-amisulpride and clozapine-quetiapine combinations for patients with schizophrenia who are partially responsive to clozapine: a single-blind randomized study. Adv Ther. 2007;24:1–13. doi: 10.1007/BF02849987. [DOI] [PubMed] [Google Scholar]
- Hamann K, Avnstorp C. Delusions of infestation treated by pimozide: a double-blind crossover clinical study. Acta Derm Venereol. 1982;62:55–58. [PubMed] [Google Scholar]
- Hasegawa M, Gutierrez-Esteinou R, Way L, Meltzer HY. Relationship between clinical efficacy and clozapine concentrations in plasma in schizophrenia: effect of smoking. J Clin Psychopharmacol. 1993;13:383–390. [PubMed] [Google Scholar]
- Heger JW, Niemann JT, Criley JM. Cardiology for the house officer: second edition. Copyright 1987. Baltimore MD: Williams & Wilkins; 1987. [Google Scholar]
- Honer WG, Thornton AE, Chen EY, Chan RC, Wong JO, Bergmann A, et al. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N Engl J Med. 2006;354:472–482. doi: 10.1056/NEJMoa053222. [DOI] [PubMed] [Google Scholar]
- Johnson GC, Anton RF. Pimozide in delusions of parasitosis. J Clin Psychiatry. 1983;44:233. [PubMed] [Google Scholar]
- Josiassen RC, Joseph A, Kohegyi E, Stokes S, Dadvand M, Paing WW, et al. Clozapine augmented with risperidone in the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2005;162:130–136. doi: 10.1176/appi.ajp.162.1.130. [DOI] [PubMed] [Google Scholar]
- Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- Kay SR. Positive and negative syndromes in schizophrenia. New York: Brunner/Mazel; 1991. [Google Scholar]
- Kronig MH, Munne RA, Szymanski S, Safferman AZ, Pollack S, Cooper T, et al. Plasma clozapine levels and clinical response for treatment-refractory schizophrenic patients. Am J Psychiatry. 1995;152:179–182. doi: 10.1176/ajp.152.2.179. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Safferman AZ, Pollack S, Szymanski S, Johns C, Howard A, et al. Clinical effects of clozapine in chronic schizophrenia: response to treatment and predictors of outcome. Am J Psychiatry. 1994;151:1744–1752. doi: 10.1176/ajp.151.12.1744. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA, et al. CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163:600–610. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- Miller DD, Fleming F, Holman TL, Perry PJ. Plasma clozapine concentrations as a predictor of clinical response: a follow-up study. J Clin Psychiatry. 1994;55:117–121. [PubMed] [Google Scholar]
- Munro A, O'Brien JV, Ross D. Two cases of "pure" or "primary" erotomania successfully treated with pimozide. Can J Psychiatry. 1985;30:619–622. doi: 10.1177/070674378503000813. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, U'Prichard DC, Greenberg DA, Snyder SH. Neuroleptic drug interactions with norepinephrine alpha receptor binding sites in rat brain. Neuropharmacology. 1977;16:549–556. doi: 10.1016/0028-3908(77)90023-5. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Miller DD, Arndt SV, Cadoret RJ. Clozapine and norclozapine plasma concentrations and clinical response of treatment-refractory schizophrenic patients. Am J Psychiatry. 1991;148:231–235. doi: 10.1176/ajp.148.2.231. [DOI] [PubMed] [Google Scholar]
- Pickar D, Owen RR, Litman RE, Konicki E, Gutierrez R, Rapaport MH. Clinical and biologic response to clozapine in patients with schizophrenia. Crossover comparison with fluphenazine. Arch Gen Psychiatry. 1992;49:345–353. doi: 10.1001/archpsyc.1992.01820050009001. [DOI] [PubMed] [Google Scholar]
- Pinder RM, Brogden RN, Swayer R, Speight TM, Spencer R, Avery GS. Pimozide: a review of its pharmacological properties and therapeutic uses in psychiatry. Drugs. 1976;12:1–40. doi: 10.2165/00003495-197612010-00001. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Bera R, Gulasekaram B, Costa J, Hayes S, Jin Y, et al. Plasma clozapine concentrations predict clinical response in treatment-resistant schizophrenia. J Clin Psychiatry. 1994;55:133–136. [PubMed] [Google Scholar]
- Potter WZ, Ko GN, Zhang LD, Yan WW. Clozapine in China: a review and preview of US/PRC collaboration. Psychopharmacology (Berl) 1989;99:S87–S91. doi: 10.1007/BF00442568. [DOI] [PubMed] [Google Scholar]
- Reilly TM. Letter: Pimozide in monosymptomatic psychosis. Lancet. 1975;7921:1385–1386. doi: 10.1016/s0140-6736(75)92302-8. [DOI] [PubMed] [Google Scholar]
- Riding BE, Munro A. Letter: Pimozide in monosymptomatic psychosis. Lancet. 1975;7903:400–401. doi: 10.1016/s0140-6736(75)91323-9. [DOI] [PubMed] [Google Scholar]
- Roth RH. Dopamine autoreceptors: pharmacology, function and comparison with post-synaptic dopamine receptors. Communications In Psychopharmacology. 1994;3:429–433. [PubMed] [Google Scholar]
- Schneider LC, Streuening EL. SLOF: A behavioral rating scale for assessing the mentally ill. Social Work Research and Abstracts. 1983;6:9–21. doi: 10.1093/swra/19.3.9. [DOI] [PubMed] [Google Scholar]
- Shapiro AK, Shapiro E, Eisenkraft GJ. Treatment of Gilles de la Tourette syndrome with pimozide. Am J Psychiatry. 1983;140:1183–1186. doi: 10.1176/ajp.140.9.1183. [DOI] [PubMed] [Google Scholar]
- Shiloh R, Zemishlany Z, Aizenberg D, Weizman A. Sulpiride adjunction to clozapine in treatment-resistant schizophrenic patients: a preliminary case series study. Eur Psychiatry. 1997;12:152–155. doi: 10.1016/S0924-9338(97)80205-2. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Kwong LL, Uhr S, Peroutka S. Differential modulation of dopamine D2 receptors by chronic haloperidol, nitrendipine and pimozide. Biological Psychiatry. 1986;21:1114–1122. doi: 10.1016/0006-3223(86)90219-2. [DOI] [PubMed] [Google Scholar]
- Wahlbeck K, Cheine M, Essali A, Adams C. Evidence of clozapine's effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156:990–999. doi: 10.1176/ajp.156.7.990. [DOI] [PubMed] [Google Scholar]
- Walters JR, Roth RH. Dopaminergic neurons: an in vivo system for measuring drug interactions with presynaptic receptors. Naunyn Schmiedebergs Arch Pharmacol. 1976;296:5–14. doi: 10.1007/BF00498834. [DOI] [PubMed] [Google Scholar]


