Abstract
BACKGROUND
Bisphosphonates are the primary therapy for postmenopausal and glucocorticoid-induced osteoporosis. Case series suggest a potential link between prolonged use of bisphosphonates and low-energy fracture of subtrochanteric or diaphyseal femur, as a consequence of over-suppression of bone resorption.
METHODS
Using health care utilization data, we conducted a propensity score-matched cohort study to examine the incidence rates (IRs) and the risk of subtrochanteric or diaphyseal femur fractures among oral bisphosphonate users compared with raloxifene or calcitonin users. A Cox proportional hazards model evaluated the risk of these fractures associated with duration of osteoporosis treatment.
RESULTS
A total of 104 subtrochanteric or diaphyseal femur fractures were observed among 33,815 patients. The estimated IR of subtrochanteric or diaphyseal femur fractures per 1,000 person-years was 1.46 (95% CI 1.11–1.88) among the bisphosphonate users and 1.43 (95% CI 1.06–1.89) among raloxifene/calcitonin users. No significant association between bisphosphonate use and subtrochanteric or diaphyseal femur fractures was found (hazard ratio (HR) 1.03, 95% CI 0.70–1.52), compared with raloxifene/calcitonin. Even with this large study size, we had little precision in estimating the risk of subtrochanteric or diaphyseal femur fractures in patients treated with bisphosphonates for longer than five years (HR 2.02, 95% CI 0.41–10.00).
CONCLUSIONS
The occurrence of subtrochanteric or diaphyseal femur fracture was rare. There was no evidence of an increased risk of subtrochanteric or diaphyseal femur fractures in bisphosphonate users, compared to raloxifene/calcitonin users. However, this study cannot exclude the possibility that long-term bisphosphonate use may increase a risk of these fractures.
Keywords: bisphosphonates, calcitonin, raloxifene, femoral fractures, osteoporosis, side effects
INTRODUCTION
Bisphosphonates decrease bone turnover and increase bone mineral density, by inhibiting osteoclast-mediated bone resorption (1). Because of their clinical efficacy in reducing the risk of fractures in patients with osteopenia or osteoporosis, bisphosphonates have been widely used for the prevention and treatment of osteoporosis (1,2). Over the past few years, a number of case series suggested a potential association between low-energy atypical fracture of the femur and bisphosphonates use (3–11). It is thought that long-term treatment with bisphosphonates might result in adynamic brittle bone, leading to atypical fractures, usually defined as subtrochanteric or diaphyseal femur fractures after minimal or no trauma (8,12). Characteristic radiographic patterns of these fractures include bilateral cortical thickening, a transverse or oblique (≤ 30 degree) fracture with a beaking of the cortex (6,13).
A case series from Sweden estimated that the crude incidence of stress fractures of the femoral shaft in bisphosphonate users was 1 per 1,000 person-years, 50 times higher than that for the untreated women (11). However, these estimates were based on a small number of patients, a short follow-up duration, and uncertain denominators.
A Danish cohort study of 15,561 patients with baseline fracture reported that the hazard ratio (HR) was 1.46 (95% confidence interval, CI, 0.91–2.35) for subtrochanteric or diaphyseal femur fracture and 1.45 (95% CI, 1.21– 1.74) for classical osteoporotic hip fracture in alendronate users compared to no osteoporosis treatment (14). They suggested that subtrochanteric or diaphyseal femur fractures were more likely related to osteoporosis rather than alendronate use (14). The results of the study should be interpreted with caution as the patients were not randomly allocated to the two groups, alendronate or no treatment. In the observational setting, there is always a reason why some patients received a prescription and some did not (15–17). Therefore, the outcomes of the patient groups would not be comparable and the validity of any inferences drawn about the relative effects of treatment would be subject to unmeasured confounding (i.e., confounding by indication) (15,17,18). Data from recent secondary analyses using three large placebo-controlled randomized clinical trials (RCTs) of bisphosphonates showed the occurrence of atypical subtrochanteric or diaphyseal femoral fracture was rare among 14,195 women (0.23 per 1,000 person-years) (19). Of those, 3,673 were treated with alendronate and 3,875 with intravenous zoledronic acid. The HRs for bisphosphonate use compared with placebo ranged from 1.03 to 1.50 with wide 95% CIs including the null value of 1, due to the small number of outcomes. Furthermore, the generalizability of the results from clinical trials may be limited (19). In March 2010, the U.S. Food and Drug Administration (FDA) issued a safety announcement that there was no clear connection between bisphosphonate use and a risk of atypical femur fractures in their ongoing review (20).
Given the limitations in the currently available data, we conducted a large population-based cohort study 1) to estimate the incidence rates (IRs) and HRs of subtrochanteric and diaphyseal femoral fractures in elderly patients treated with oral bisphosphonates compared to those treated with either raloxifene or calcitonin nasal spray, and 2) to examine the potential risk of these fractures associated with treatment duration. To control confounding by indication to a large extent, we used the propensity score-matching method embedded in a new user cohort design comparing two active treatments. A propensity score is the estimated probability of starting treatment A versus starting treatment B, based on preexisting patient characteristics (17,21). Propensity score-matching has been increasingly used as an effective way to adjust a large number of confounders simultaneously even if the outcome is rare (17,18).
METHODS
Data Source and Study Patients
A large cohort study was conducted using health care utilization databases from two U.S. states: 1) Medicare beneficiaries enrolled in the Pharmaceutical Assistance Contract for the Elderly in Pennsylvania from January 1996 through December 2006, and 2) Medicare beneficiaries enrolled in the Pharmaceutical Assistance to the Aged and Disabled in New Jersey from January 1996 through December 2006. Both drug benefits programs provided comprehensive pharmacy coverage with a small or no copayment for the low-income elderly.
We identified subjects who had at least one prescription filled for osteoporosis treatment (oral bisphosphonates, raloxifene, or calcitonin nasal spray) and at least one medical claim during each of three consecutive 6-month periods before the first use of osteoporosis treatment. These criteria ensured their continuous eligibility for at least one year prior to the study entry, to permit us to identify new users of osteoporosis drugs, and to assess their comorbidities and other medications. Propensity score-matching methods were then used to select a subset of oral bisphosphonate users and a combined group of either raloxifene or calcitonin nasal spray users who were compatible with regard to the potential confounders described below (see Table 1 for the variables included in the propensity score calculation) (22).
Table 1.
Characteristics of propensity score-matched study population in 12 months prior to filling their first osteoporosis drug prescription
| Bisphosphonates | Raloxifene/calcitonin | |
|---|---|---|
| N | 17,028 | 16,787 |
|
Demographic factors | ||
| Age, years, | ||
| mean (SD) | 79.9 (6.5) | 80.0 (6.9) |
| Race, White | 16,180 (95) | 15,987 (95.2) |
| Sex, female | 16,474 (96.8) | 16,244 (96.8) |
|
Health care utilization | ||
| No. of visit, | ||
| mean (SD) | 10.6 (6) | 10.5 (6.1) |
| ER visit | 4,505 (26.5) | 4,482 (26.7) |
| No. of all prescription drugs, mean (SD) | 10.4 (6) | 10.5 (6.1) |
| Hospitalization | 6,089 (35.8) | 6,146 (36.6) |
| Nursing home resident | 1,882 (11.1) | 1,991 (11.9) |
|
Comorbidities | ||
| Prior fall | 2,094 (12.3) | 2,119 (12.6) |
| Prior hip fracture | 612 (3.6) | 601 (3.6) |
| Prior vertebral fracture | 1,858 (10.9) | 1,890 (11.3) |
| BMD test | 4,085 (24) | 4,180 (24.9) |
| Hypertension | 11,303 (66.4) | 11,233 (66.9) |
| Chronic kidney disease | 492 (2.9) | 481 (2.9) |
| Chronic liver disease | 207 (1.2) | 191 (1.1) |
| Parkinson’s disease | 586 (3.4) | 598 (3.6) |
| Dementia | 1,039 (6.1) | 1,092 (6.5) |
| Diabetes mellitus | 4,354 (25.6) | 4,312 (25.7) |
| Congestive heart failure | 3,664 (21.5) | 3,728 (22.2) |
| COPD | 4,782 (28.1) | 4,763 (28.4) |
| Inflammatory arthritis | 1,267 (7.4) | 1,258 (7.5) |
| Inflammatory bowel disease | 241 (1.4) | 226 (1.4) |
| Alcoholism | 311 (1.8) | 301 (1.8) |
| Comorbidity Index, mean (SD) | 1.9 (1.9) | 2 (1.9) |
|
Other medications | ||
| Opioids | 6,817 (40) | 6,826 (40.7) |
| Anti-epileptics | 892 (5.2) | 877 (5.2) |
| Proton pump inhibitors | 4,361 (25.6) | 4,441 (26.5) |
| Benzodiazepines | 4,636 (27.2) | 4,614 (27.5) |
| SSRI | 2,654 (15.6) | 2,683 (16) |
| Warfarin | 1,814 (10.7) | 1,786 (10.6) |
| Inhaled steroid | 1,389 (8.2) | 1,391 (8.3) |
| Oral steroid | 2,420 (14.2) | 2,387 (14.2) |
New Jersey and Pennsylvania combined, 2nd drug dispensing and a 90-day lag period are required.
Data are presented in number (%), unless specified.
SD: standard deviation, ER: emergency room, BMD: bone mineral density, COPD: chronic obstructive pulmonary disease, SSRI: selective serotonin reuptake inhibitor
Drug Exposures
We compared new users of oral bisphosphonate with new users of either raloxifene or calcitonin nasal spray. Oral bisphosphonates included in the study were alendronate, risedronate, and etidronate. Ibandronate was not available during the study period. Switchers between different oral bisphosphonate agents were considered as continuous users unless there was a gap between two bisphosphonate drug prescriptions for longer than 90 days.
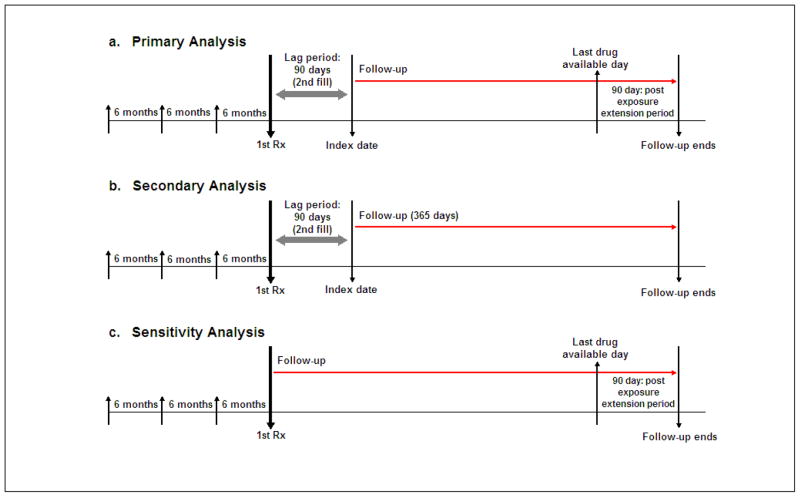
For both the primary (“as treated”) and secondary (“first exposure carried forward”) analyses, in which a lag period of 90 days was required, follow-up began 91 days after filling the first prescription of either exposures of interest. The second prescription fill for the same exposure group was required during the 90-day lag period. (Figure 1)
Figure 1. Study Design.
Subjects were required to have at least one claim each during the prior three 6-month intervals. For both the primary (“as treated”) and secondary (“first exposure carried forward”) analyses, follow-up began at the 91st day after filling the first prescription of either exposures of interest. The second prescription fill for the same exposure drug group was required during the 90-day lag period. For the primary analysis (a), we continued the follow-up until the 90 days after the last drug available date. Last drug available date was calculated with a number of days of supply after the last prescription fill date. For the secondary analysis (b), the follow-up continued until 365 days after the index date. Patients were considered “always exposed” for the first exposure drug group during the follow-up period. In a sensitivity analysis (c), follow-up began at the first prescription fill and ended 90 days after the last drug available date.
For the primary analysis, subjects were followed up until the 90 days after the last drug available date, assuming that bisphosphonates have a long-duration of action. Last drug available date was calculated with a number of days of supply after the last prescription fill date. For the secondary analysis, mimicking an intention-to-treat analysis used in clinical trials, subjects were followed up for 365 days and considered “always exposed” on the basis of the first exposure, regardless of drug discontinuation or switching, drug during the follow-up period.
We also performed a sensitivity analysis (“as treated with no lag period”), in which the follow-up started at the date of the first prescription fill and continued until the 90 days after the last drug available date. (Figure 1).
Outcomes
We used definitions of subtrochanteric (ICD-9 820.22) or diaphyseal (ICD-9 821.0x) femur fracture based on primary hospital discharge diagnosis codes. In a recent validation study, administrative claims-based algorithms using the primary hospital discharge diagnosis codes to identify cases of subtrochanteric or diaphyseal femur fracture yielded high positive predictive values between 0.75 and 0.86 (23). The primary outcome of interest was a combined endpoint of subtrochanteric and diaphyseal femur fractures. When a patient has both subtrochanteric and diaphyseal femur fractures, it was counted as a single fracture. We also evaluated whether the outcomes were related to major trauma based on various diagnoses codes (see Appendix 2).
Patients were censored at the earliest time of the following events during the follow-up period: 1) occurrence of the first outcome, 2) occurrence of typical hip fracture, defined with ICD 9 820.0–820.1 and 820.8–820.9, 3) admission to nursing home, 4) end of study period, or 5) death. Typical hip fractures were considered censoring events as most patients with hip fractures would get surgically repaired (24) and therefore have different risks for subsequent fractures of the femur. Due to incomplete prescription data among nursing home residents in the study database, subjects were censored at the time of nursing home admission. Subjects who did not have any dispensing during the lag period and who had censoring events during the lag period were excluded from the analyses.
Covariates
Patient characteristics potentially related to a future femur fracture were assessed using the data from the 12 months prior to the first prescription fill date. These characteristics included demographic factors (age, sex, race and state), calendar year, nursing home resident, health care utilization factors (acute care hospitalizations, emergency room visits, and number of physician visits and different medications), other recorded comorbidities (prior falls, prior hip or vertebral fractures, bone mineral density test, alcoholism, Parkinson’s disease, dementia, chronic kidney or liver disease, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, heart failure, inflammatory arthritis, and inflammatory bowel disease), and use of other medications likely associated with bone metabolism or fall risks (oral or inhaled glucocorticoids, anticonvulsants, benzodiazepines, selective serotonin reuptake inhibitors (SSRIs), beta blockers, warfarin, proton pump inhibitors, and opioids).
To quantify patients’ comorbidities, we additionally calculated the Deyo-adapted Charlson Comorbidity Index based on ICD-9-CM from the 12 months prior to the first prescription fill date(25,26). The Comorbidity Index is a summary score, based on 19 major medical conditions including myocardial infarction, pulmonary, renal, hepatic disease, diabetes, cancer, human immunodeficiency virus infection, etc. A score of 0 represents absence of comorbidity and a higher score indicates a greater number of comorbid conditions. Duration of treatment with either oral bisphosphonates or raloxifene/calcitonin was assessed for subgroup analysis.
Statistical Analyses
Logistic regression models were developed to calculate the propensity score of individual patients in each state, Pennsylvania or New Jersey. The propensity score is the probability of initiating oral bisphosphonates versus either raloxifene or calcitonin nasal spray as a function of all the potential confounders listed in Table 1 and calendar year. Propensity scores were calculated at their first prescription fill date. Patients in each group (oral bisphosphonates vs. raloxifene/calcitonin) were then matched 1:1 to the second decimal place of the estimated propensity scores. After the propensity score-matching, subjects from two states were pooled for all the analyses. The characteristics of patients in each group were compared before and after the propensity score-matching.
The IRs of subtrochanteric or diaphyseal fractures were calculated among propensity score matched-patients in each treatment group. Cox proportional hazard analyses were used to estimate the HRs and the 95% CIs of the risk of subtrochanteric or diaphyseal femur fractures among oral bisphosphonate users compared to raloxifene/calcitonin users. Since we matched the groups on propensity scores containing potential confounders, the Cox regression models only contained a variable for the exposures of interest, with raloxifene/calcitonin as the reference exposure. We tested the proportional hazards assumption for each exposure of interest with respect to each of the fracture outcomes via the Kolmogorov supremum test (27). We also constructed adjusted Kaplan-Meier fracture-free survival curves and inspected two-way log-rank tests. A Cox model stratified by treatment duration (less than 1 year, 1–2 years, 3–4 years, and 4 years or more) was used to assess the association between the risk of fractures and duration of treatment. All analyses were conducted using SAS Statistical Software version 9.2 (SAS Institute Inc., Cary, NC).
This work was approved by Brigham and Women’s Hospital’s Institutional Review Board. Data use agreements were in place with Medicare and the state pharmacy benefit programs that supplied information for the study database. All potentially traceable personal identifiers were removed from the data before analyses to protect patients’ privacy.
RESULTS
Cohort Selection
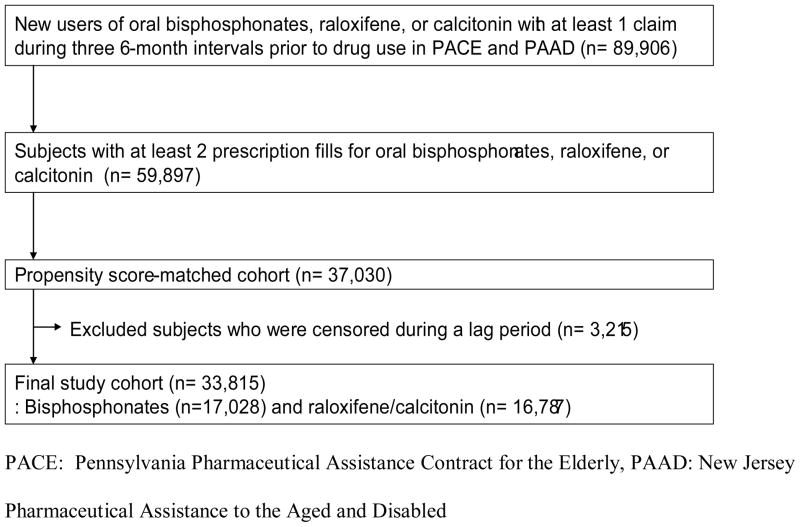
Figure 2 illustrates our cohort selection process for the primary and secondary analyses. Of 89,906 new users of oral bisphosphonates, raloxifene, or calcitonin nasal spray with at least one claim each during the prior three 6-month intervals, 59,897 subjects had at least two consecutive prescription fills. After 1:1 propensity score-matching, a total of 37,030 subjects with at least two prescription fills for osteoporosis drugs were identified. We excluded 3,215 patients who had a censoring event during the 90-day lag period. Our final cohort consisted of 17,028 oral bisphosphonate users and 16,787 raloxifene/calcitonin users.
Figure 2.
Flow diagram of cohort selection
For the sensitivity analysis, a total of 59,642 subjects with at least one prescription fill for osteoporosis drugs were identified after 1:1 propensity score-matching.
Patient Characteristics
The baseline characteristics of propensity score-matched patients with at least two prescription fills are listed in Table 1. The mean age was 79.9 years (standard deviation (SD) 6.5) in bisphosphonate users and 80.0 years (SD 6.9) in the raloxifene/calcitonin users. 97% were women and 95% were Caucasians in both groups. A mean duration of follow-up was 2.13 years (SD 2.21); however, more than 4,000 patients had a follow-up longer than 5 years. Approximately 84 % of the subjects in the bisphosphonate group were treated with alendronate, 14% with risedronate, and 2% with etidronate. 72% of the subjects in the raloxifene/calcitonin group were treated with calcitonin. The propensity score-matched cohorts had more similar health care utilization patterns, comorbidities, and use of other medications than the unmatched cohorts (see Appendix 2).
Subtrochanteric or Diaphyseal Fractures
A total of 104 subtrochanteric or diaphyseal femur fractures were observed for our primary analysis. Only two major trauma-related fractures in the propensity score-matched cohorts were noted and excluded from the analysis. Incidence rates for subtrochanteric or diaphyseal femur fracture were calculated in the propensity score-matched cohorts (Table 2). The primary analysis estimated that there were 1.46 subtrochanteric or diaphyseal femur fractures (0.92 subtrochanteric and 0.61 diaphyseal) per 1,000 person-years among the bisphosphonate users. The IRs were similar among raloxifene/calcitonin users. Similar IRs across both groups were noted in both the secondary and sensitivity analyses (Table 2).
Table 2.
Incidence rates (IRs) for subtrochanteric or diaphyseal femur fracture per 1,000 person-years in the propensity score-matched population
| Bisphosphonates | Raloxifene/calcitonin | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | N patients | N events | Person- years | IR * (95% CI) | N patients | N events | Person- years | IR * (95% CI) |
|
Primary (as treated) analysis | ||||||||
| Subtrochanteric or diaphyseal femur fracture | 17,028 | 57 | 39,095 | 1.46 (1.11–1.88) | 16,787 | 47 | 32,836 | 1.43 (1.06–.89) |
| Subtrochanteric femur fracture | 17,028 | 36 | 39,098 | 0.92 (0.66–1.3) | 16,787 | 34 | 32,836 | 1.04 (0.73–1.43) |
| Diaphyseal femur fracture | 17,028 | 24 | 39,095 | 0.61 (0.40–0.90) | 16,787 | 13 | 32,836 | 0.40 (0.22–0.66) |
|
Secondary (first exposure carried forward) analysis | ||||||||
| Subtrochanteric or diaphyseal femur fracture | 17,028 | 22 | 15,817 | 1.39 (0.89–2.07) | 16,787 | 21 | 15,333 | 1.37 |
| Subtrochanteric femur fracture | 17,028 | 15 | 15,817 | 0.95 (0.55–1.53) | 16,787 | 16 | 15,333 | 1.04 (0.62–1.66) |
| Diaphyseal femur fracture | 17,028 | 8 | 15,817 | 0.51 (0.24–0.96) | 16,787 | 5 | 15,333 | 0.33 (0.12–0.72) |
|
Sensitivity(as treated with no lag period) analysis | ||||||||
| Subtrochanteric or diaphyseal femur fracture | 29,780 | 81 | 58,344 | 1.39(1.11–1.72) | 29,743 | 72 | 46,959 | 1.53 (1.21–1.92) |
| Subtrochanteric femur fracture | 29,780 | 41 | 58,347 | 0.70 (0.51–0.94) | 29,743 | 45 | 46,936 | 0.96 (0.71–1.27) |
| Diaphyseal femur fracture | 29,780 | 46 | 58,344 | 0.79 (0.58–1.04) | 29,743 | 27 | 46,960 | 0.57 (0.39–0.83) |
in 1,000 person-years, CI: confidence interval
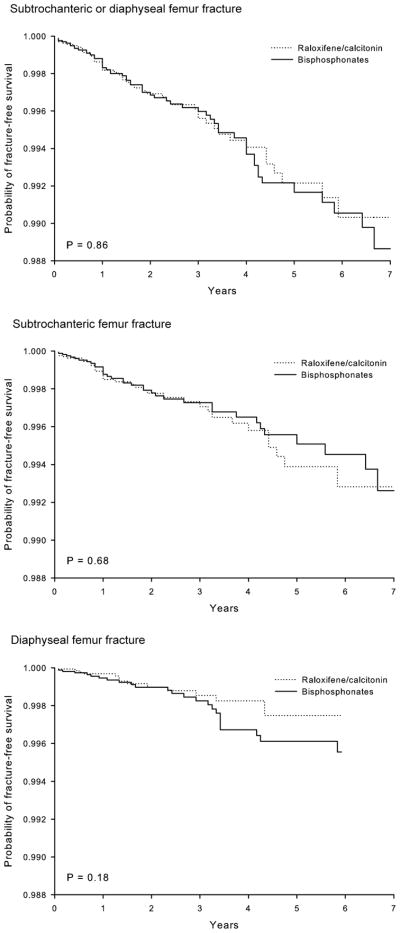
Hazard ratios for each fracture events were estimated with Cox regression models in the propensity score-matched cohorts (see Table 3). In the primary analysis, oral bisphosphonates were not associated with a significantly increased risk of subtrochanteric or diaphyseal (HR 1.03, 95% CI 0.70 – 1.52), subtrochanteric (HR 0.90, 95% CI 0.56 – 1.44), or diaphyseal femur fractures (HR 1.57, 95% CI 0.80–3.09), compared with raloxifene/calcitonin. However, due to the wide confidence interval with a relatively small number of events, we cannot exclude the increased risk for diaphyseal fractures of the femur associated with use of oral bisphosphonates. Similar results were observed in both the secondary and sensitivity analyses. For every model, the result of the Kolmogorov-type supremum test was not significant (all p-values > 0.50). Therefore, the “proportional hazards assumption” was not violated in our models. Figure 3 displays the Kaplan-Meier fracture-free survival curves over the follow-up period for the primary analysis. The rates of subtrochanteric or diaphyseal femur fractures did not meaningfully differ among two groups.
Table 3.
Hazard ratios (95% confidence intervals) for subtrochanteric or diaphyseal femur fracture in the propensity score-matched population
| Fractures | Bisphosphonates | Raloxifene/calcitonin |
|---|---|---|
|
Primary (as treated) analysis | ||
| Subtrochanteric or diaphyseal femur fracture | 1.03 (0.70–1.52) | 1.00 |
| Subtrochanteric femur fracture | 0.90 (0.56–1.44) | 1.00 |
| Diaphyseal femur fracture | 1.57 (0.80–3.09) | 1.00 |
|
Secondary (first exposure carried forward) analysis | ||
| Subtrochanteric or diaphyseal femur fracture | 1.02 (0.56–1.85) | 1.00 |
| Subtrochanteric femur fracture | 0.91 (0.45–1.84) | 1.00 |
| Diaphyseal fracture | 1.55 (0.51–4.75) | 1.00 |
|
Sensitivity(as treated with no lag period) analysis | ||
| Subtrochanteric or diaphyseal femur fracture | 0.91 (0.66–1.26) | 1.00 |
| Subtrochanteric femur fracture | 0.74 (0.48–1.12) | 1.00 |
| Diaphyseal femur fracture | 1.41 (0.87–2.27) | 1.00 |
in 1,000 person-years
Figure 3.
Kaplan-Meier curves for fracture-free survival in oral bisphosphonates vs. raloxifene/calcitonin nasal spray
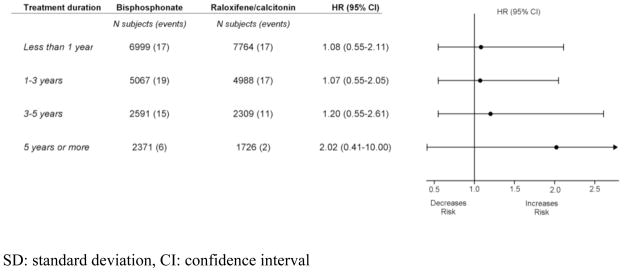
Overall no significant differences were noted between the two groups for the risk of subtrochanteric or diaphyseal fracture of the femur stratified by the treatment duration (see Figure 4), although the HR was 2.02 with a wide confidence interval (95% CI 0.41–10.00) among those treated for longer than five years (2,371 bisphosphonate users compared to 1,726 raloxifene/calcitonin users).
Figure 4.
Hazard ratios (HR) for subtrochanteric or diaphyseal femur fractures, according to osteoporosis treatment duration
COMMENTS
Bisphosphonates are widely used for the prevention and treatment of osteopenia and osteoporosis. Common side effects such as heartburn, esophageal irritation, and musculoskeletal pain, are well-known, while little data, particularly from prospective studies with a long-term follow up, exist on the questionable association between atypical femur fractures and use of bisphosphonates (13,28). In this large propensity score-matched cohort study using health care utilization data, there was no difference in the risk of subtrochanteric or diaphyseal femur fractures in bisphosphonate users compared with raloxifene/calcitonin users. Occurrence of these fractures among both bisphosphonate and raloxifene/calcitonin users was rare in this study.
The estimated IR of subtrochanteric or diaphyseal femur fractures was 1.46 per 1,000 person-years among the bisphosphonate users and 1.43 per 1,000 person-years among the raloxifene/calcitonin users. The results of the primary analysis (HR 1.03, 95% CI 0.70–1.52) indicate that an increase in the rate of subtrochanteric or diaphyseal femur fracture associated with oral bisphosphonate uses by more than 0.74 per 1,000 person-years can be excluded with a confidence level of 95% (29).
Our study has several important implications. We compared the risk of subtrochanteric or diaphyseal femur fracture between two active osteoporosis treatment groups with the propensity score-matching method to minimize confounding by indication. In addition, we utilized multiple approaches in the study design and analysis and obtained consistent results. Our results on the IRs of subtrochanteric or diaphyseal femur fractures are similar to those from the Danish cohort study (14), but somewhat higher than the results (0.25 per 1,000 person-years) from two recent studies (19,30). Although the IRs in our study might have been overestimated as we could not assess whether all the subtrochanteric or diaphyseal femur fractures in our study had characteristic radiographic findings of atypical fracture, such as a simple transverse fracture with cortical thickening (6), morphologic evaluation of fractures with radiographs were not done in two other previous studies (14,30) and only available in a subset of subjects in the secondary analyses of three RCTs (19). Therefore, the difference in these rates are probably related to the characteristics of our study population (i.e., study size, mostly female (> 95%), users of osteoporosis drugs, low socioeconomic status, and a greater number of medical comorbidities and prescription drugs).
Several case series suggested a risk of atypical femur fracture particularly with long-term use of bisphosphonates (3,6,7,9). In our subgroup analysis of 4,097 patients, the relative hazard associated with long-term use of bisphosphonates (over five years) compared to raloxifene/calcitonin use was 2.02 (95% CI 0.41–10.00). Similar results were noted in two earlier studies, although both studies were based on a much smaller number of the long-term users and the outcomes. There were only five subtrochanteric or diaphyseal femur fractures among 178 patients with alendronate use for longer than six years in the Danish cohort study (HR 1.37, 95% CI 0.22–8.62) (14) and two atypical subtrochanteric or diaphyseal femur fractures among 662 patients with alendronate use for longer than five years in the Fracture Intervention Trial (FIT) Long-Term Extension trial (HR 1.33, 95% CI 0.12–14.67) (19). Studies that did not observe statistically significant changes merit special attention to their statistical power to detect a clinically meaningful change (31). Even though we conducted a large-scale cohort study, it is still possible that we did not have sufficient power to detect the excess risk for such a rare outcome.
In a study by Black et al (19), the number needed to treat with a bisphosphonate to observe one excess atypical femur fracture was 725, based on a hypothetical relative risk of 3.0 compared to placebo. We estimated that 450 patients would need to be treated with a bisphosphonate for more than five years to observe one excess subtrochanteric or diaphyseal femur fracture, assuming a hypothetical relative risk of 3.0 compared to those treated with raloxifene or calcitonin. Given the results from the FIT which showed treating 81 postmenopausal women with osteoporosis with alendronate for over four years would prevent one hip fracture (32), the benefit clearly outweighs even with such a high hypothetical risk.
Confounding bias is the major barrier to using large administrative claims databases for pharmacoepidemiologic research. One could avoid the issue of confounding bias by conducting a RCT (15). However, there are a number of important limitations in RCTs to study long-term safety of drugs, such as insufficient sample sizes, inadequate duration of follow-up, generalizability, ethical issues, and substantial cost. We therefore conducted a large population-based cohort study and attempted to minimize this bias by selecting new users of osteoporosis drugs and matching them based on a propensity score that included many potentially important confounders, resulting in the well-balanced cohorts with respect to measured variables in the database. However, it is possible that differences still exist between the groups resulting in residual confounding; due to unmeasured confounders (e.g., calcium and vitamin D intake, bone mineral density, body mass index, and frailty) not included in the propensity score calculation. We included both female and male patients in this study, although the majority of the patients (97%) were female. 540 male patients in the raloxifene/calcitonin group were calcitonin users as raloxifene is only indicated in female patients.
Other important potential limitations include misclassification of exposures and outcomes. While we used pharmacy claims data which is considered as one of the best data sources for the drug exposure to identify the exposure in this study (33), the actual patient adherence to the medication is unknown. The outcomes in the present study were identified by the diagnosis codes from administrative claims. Although the accuracy of the specific codes used in this study has been recently validated in other claims data(23), we could not verify diagnoses of subtrochanteric or diaphyseal femur fracture based on specific radiographic characteristics in the study database. However, the impact of this misclassification bias is most likely non-differential between bisphosphonates and raloxifene/calcitonin users.
In conclusion, we found no significant differences in the risk of either subtrochanteric or diaphyseal fractures of the femur between users of oral bisphosphonates and raloxifene/calcitonin nasal spray. Despite the large study size, however, we still had little precision in estimating the risk of subtrochanteric or diaphyseal femur fractures associated with use of bisphosphonates for more than five years. Thus, we cannot rule out the possibility of an increased risk of these femur fractures associated with long-term use of bisphosphonates.
Supplementary Material
Appendix 1.
Definition of trauma-related injuries
| Type of injury | ICD-9 code |
|---|---|
| Major trauma | E800–848, E881–884, E908–909, E916–928 |
| Open femoral fracture and distal end femoral fracture | 820.1x, 820.3x, 820.9,x 821.1x, 821.2x, 821.3x |
| Crushing injury of lower limb | 928 |
| Crushing injury of multiple or unspecified sites | 929 |
ICD-9: International Classification of Diseases -9
Acknowledgments
Dr. Kim has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. There was no funding or support specific to the study.
Dr. Kim is supported by the National Institutes of Health grant (T32 AR055885 and K23 AR059677).
Dr. Schneeweiss is principal investigator of the Brigham and Women’s Hospital DEcIDE Center on Comparative Effectiveness Research, funded by the Agency for Healthcare Research and Quality, and of the Harvard-Brigham Drug Safety and Risk Management Research Contract, funded by the US Food and Drug Administration. Dr. Schneeweiss is a paid member of scientific advisory boards for HealthCore and ii4sm and has received consulting fees from WHISCON, RTI Health Solutions, the Lewin Group, and HealthCore. Dr. Schneeweiss has received an Investigator-initiated research from Pfizer.
Dr. Katz is supported by the National Institutes of Health grants (K24 AR02123 and P60 AR47782).
Dr. Solomon is supported by the National Institutes of Health grants (K24 AR055989, P60 AR047782, R21 DE018750, and R01 AR056215). Dr. Solomon has received research support from Abbott Immunology, Amgen, and support for an educational course from BMS.
References
- 1.Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032–45. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guyatt G, Cranney A, Griffith L, Walter S, Krolicki N, Favus M, Rosen C. Summary of meta-analyses of therapies for postmenopausal osteoporosis and the relationship between bone density and fractures. Endocrinol Metab Clin North Am. 2002;31 (3):659–79. doi: 10.1016/s0889-8529(02)00024-5. [DOI] [PubMed] [Google Scholar]
- 3.Kwek E, Koh J, Howe T. More on atypical fractures of the femoral diaphysis. N Engl J Med. 2008;359(3):316–7. doi: 10.1056/NEJMc080861. [DOI] [PubMed] [Google Scholar]
- 4.Lee P, Seibel M. More on atypical fractures of the femoral diaphysis. N Engl J Med. 2008;359(3):317. [PubMed] [Google Scholar]
- 5.Edwards M, McCrae F, Young-Min S. Alendronate-related femoral diaphysis fracture--what should be done to predict and prevent subsequent fracture of the contralateral side? Osteoporos Int. 2010;21(4):701–3. doi: 10.1007/s00198-009-0986-y. [DOI] [PubMed] [Google Scholar]
- 6.Lenart B, Lorich D, Lane J. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med. 2008;358(12):1304–6. doi: 10.1056/NEJMc0707493. [DOI] [PubMed] [Google Scholar]
- 7.Capeci C, Tejwani N. Bilateral low-energy simultaneous or sequential femoral fractures in patients on long-term alendronate therapy. J Bone Joint Surg Am. 2009;91 (11):2556–61. doi: 10.2106/JBJS.H.01774. [DOI] [PubMed] [Google Scholar]
- 8.Odvina C, Zerwekh J, Rao D, Maalouf N, Gottschalk F, Pak C. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90 (3):1294–301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 9.Ing-Lorenzini K, Desmeules J, Plachta O, Suva D, Dayer P, Peter R. Low-energy femoral fractures associated with the long-term use of bisphosphonates: a case series from a Swiss university hospital. Drug Saf. 2009;32(9):775–85. doi: 10.2165/00002018-200932090-00002. [DOI] [PubMed] [Google Scholar]
- 10.Neviaser A, Lane J, Lenart B, Edobor-Osula F, Lorich D. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22(5):346–50. doi: 10.1097/BOT.0b013e318172841c. [DOI] [PubMed] [Google Scholar]
- 11.Schilcher J, Aspenberg P. Incidence of stress fractures of the femoral shaft in women treated with bisphosphonate. Acta Orthopaedica. 2009;80(4):413–5. doi: 10.3109/17453670903139914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armamento-Villareal R, Napoli N, Diemer K, Watkins M, Civitelli R, Teitelbaum S, Novack D. Bone turnover in bone biopsies of patients with low-energy cortical fractures receiving bisphosphonates: a case series. Calcif Tissue Int. 2009;85(1):37–44. doi: 10.1007/s00223-009-9263-5. [DOI] [PubMed] [Google Scholar]
- 13.Watts N, Diab D. Long-Term Use of Bisphosphosphonates in Osteoporosis. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2009-1947. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Abrahamsen B, Eiken P, Eastell R. Subtrochanteric and diaphyseal femur fractures in patients treated with alendronate: a register-based national cohort study. J Bone Miner Res. 2009;24(6):1095–102. doi: 10.1359/jbmr.081247. [DOI] [PubMed] [Google Scholar]
- 15.Csizmadi I, Collet J-P. Bias and confounding in pharmacoepidemiology. In: Strom B, Kimmel S, editors. Textbook of Pharmacoepidemiology. John Wiley & Sons, Ltd; 2006. pp. 167–71. [Google Scholar]
- 16.Schneeweiss S, Patrick A, Stürmer T, Brookhart M, Avorn J, Maclure M, Rothman K, Glynn R. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med Care. 2007;45(10 Supl 2):S131–42. doi: 10.1097/MLR.0b013e318070c08e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneeweiss S. A basic study design for expedited safety signal evaluation based on electronic healthcare data. Pharmacoepidemiol Drug Saf. 2010;19(8):858–68. doi: 10.1002/pds.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Agostino RJ. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 19.Black D, Kelly M, Genant H, Palermo L, Eastell R, Bucci-Rechtweg C, Cauley J, Leung P, Boonen S, Santora A, de Papp A, Bauer D the Fracture Intervention Trial and HORIZON Pivotal Fracture Trial Steering Committees. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med. 2010;362(19):1761–71. doi: 10.1056/NEJMoa1001086. [DOI] [PubMed] [Google Scholar]
- 20.FDA. FDA Drug Safety Communication: Ongoing safety review of oral bisphosphonates and atypical subtrochanteric femur fractures. 3/10/2010 2010. [Google Scholar]
- 21.Ray W. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 22.Rubin D. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 Pt 2):757–63. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 23.Narongroeknawin P, Patkar N, Shakoory B, Jain A, Curtis J, Delzell E, Lander P, Lopez-Ben R, Pitt M, Safford M, Volgas D, Saag K. Identification of subtrochanteric and diaphyseal femoral fractures using administrative claims data. Ann Rheum Dis. 2010;69(Suppl 3 ):646. doi: 10.1016/j.jocd.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyamoto R, Kaplan K, Levine B, Egol K, Zuckerman J. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596–607. doi: 10.5435/00124635-200810000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Deyo R, Cherkin D, Ciol M. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 26.Schneeweiss S, Seeger J, Maclure M, Wang P, Avorn J, Glynn R. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154(9):854–64. doi: 10.1093/aje/154.9.854. [DOI] [PubMed] [Google Scholar]
- 27.Lin D, Wei L, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80(3):557–72. [Google Scholar]
- 28.Solomon D, Rekedal L, Cadarette S. Osteoporosis treatments and adverse events. Curr Opin Rheumatol. 2009;21(4):363–8. doi: 10.1097/BOR.0b013e32832ca433. [DOI] [PubMed] [Google Scholar]
- 29.Goodman S, Berlin J. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med. 1994;121(3):200–6. doi: 10.7326/0003-4819-121-3-199408010-00008. [DOI] [PubMed] [Google Scholar]
- 30.Nieves J, Bilezikian J, Lane J, Einhorn T, Wang Y, Steinbuch M, Cosman F. Fragility fractures of the hip and femur: incidence and patient characteristics. Osteoporos Int. 2009;21(3):399–408. doi: 10.1007/s00198-009-0962-6. [DOI] [PubMed] [Google Scholar]
- 31.Freiman J, Chalmers T, Smith HJ, Kuebler R. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial. Survey of 71 “negative” trials. N Engl J Med. 1978;299:690–4. doi: 10.1056/NEJM197809282991304. [DOI] [PubMed] [Google Scholar]
- 32.Cummings S, Black D, Thompson D, Applegate W, Barrett-Connor E, Musliner T, Palermo L, Prineas R, Rubin S, Scott J, Vogt T, Wallace R, Yates A, LaCroix A. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280(24):2077–82. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 33.Strom B. Overview of automated databases in pharmacoepidemiology. In: Strom B, Kimmel S, editors. Textbook of Pharmacoepidemiology. John Wiley & Sons, Ltd; 2006. pp. 167–71. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.