Summary
The association of early onset wheezing with common viral and bacterial infections has raised significant interest in the role of infections in childhood asthma inception. This article serves to review these relationships among infections, host factors, and asthma inception in childhood.
Keywords: inception, asthma, infection, wheezing, virus, childhood, genetics
Introduction
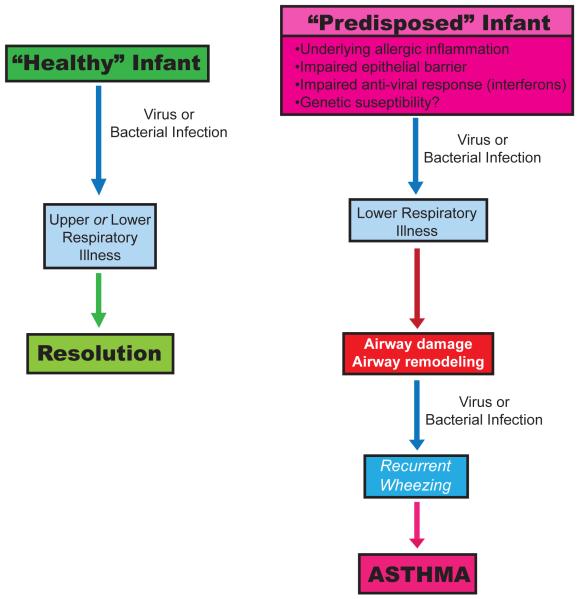
Asthma is a common chronic respiratory disease, responsible for a significant amount of morbidity and mortality in the Western world. Understanding the origins of asthma inception and risk factors for severity of disease can potentially help elucidate modes of early intervention that could alter the disease course, with an aim at primary prevention. The majority of children with a history of asthma and impaired lung function at school age have a history of recurrent wheezing illnesses in early life(1-3). Additionally, throughout childhood and beyond, viral illnesses are the most common triggers of asthma exacerbations.(4) The timing of early onset wheezing with common viral infections has raised significant interest in the role of infections in childhood asthma inception. Furthermore, studies using molecular diagnostics suggest the airway microbiome may be involved in complex infectious relationships that may be altering the disease course of both chronic asthma and associated exacerbations. Host factors such as allergic sensitization, antiviral immune responses, and environmental exposures are additionally thought to modify these relationships(3, 5-8). In this article, we review these relationships among infections, host factors, and asthma inception in childhood (Figure 1).
Figure 1. Pathogenesis of Asthma Inception.
Adapted from Jackson et al, 2010 (73) This figure highlights relationships among infections, environmental and host factors in the inception of asthma.
Epidemiology of Wheezing and Asthma
Asthma, the most common chronic disease of childhood, often presents during the preschool years with wheezing viral respiratory infections. Viral wheezing illnesses during childhood are pervasive, with up to 50% of children having at least one episode of wheezing prior to school age.(1) Viral pathogens have been demonstrated in up to 90% of acute wheezing episodes within the first 3 years of life.(2) Improvements in molecular diagnostic techniques, through the advent of PCR, have enhanced our ability to recognize novel species and types of viruses previously undocumented or underestimated in the wheezing patient (Table 1). While respiratory syncytial virus (RSV) had long been recognized as a cause of lower respiratory tract infections in infants, particularly during the winter season, human rhinovirus (HRV) has been detected in more than 60% of asthma exacerbations, making it a common culprit throughout the year.(9) The previously difficult to culture HRV-C species has now been identified, with data indicating this particular virus may be intrinsically more virulent and likely to incite wheezing.(10) Parainfluenza, coronavirus, influenza, adenovirus, bocavirus, and human metapneumovirus (hMPV), have also been identified in wheezing children, along with bacteria including nontypeable haemophilus influenza, streptococcus pneumoniae, moraxella catarrhalis, mycoplasma pneumoniae and chlamydophila pneumoniae. (11-15)
Table 1.
Etiology of infection with various outcomes. (Citations in parenthesis)
| Outpatient early life wheezing illnesses |
Illnesses associated with hospitalization |
Illnesses associated with increased asthma risk |
|
|---|---|---|---|
|
Viruses |
|||
|
Bacteria |
|
Viral Infections: The Major Players
HRV and Asthma Inception
HRV is the most frequent cause of the common cold, and has been identified as a pathogen in both the upper and lower airways.(10, 16, 17) HRVs are non-enveloped, single stranded RNA viruses from the Picornaviridae family of viruses. Based upon sequence homology, 3 distinct species of HRV have been identified: HRV-A, HRV-B and HRV-C. HRV-A is composed of >70 types, and HRV-B with >25 types. More recently, a 3rd distinct group of HRV-C has been identified, with a rapidly expanding number of distinct serotypes. This particular species has proven difficult to grow in traditional culture, making their initial detection elusive. HRV-C has been identified frequently from patients hospitalized for fever, respiratory illness, and asthma exacerbations.(10) Children hospitalized with HRV infection tend to be older with a prior history of wheezing, as well as a personal history of atopy.(18) The omnipresent nature of HRV infections throughout the year can be further categorized via species analysis. Data has shown peak incidence of HRV-C types in the fall, timing that correlates with increased asthma exacerbations and hospitalization rates, and with increased HRV-A detection during the spring.(10, 19) There is a relatively low level of HRV-B burden throughout the year.(10) The broad spectrum of illness generated by HRV infections, from asymptomatic to severe respiratory compromise necessitating hospitalization, strongly suggests that host factors modify the impact of infection. As such, allergic sensitization and innate immune response patterns to infection are thought to play a vital role.(5, 6, 20)
Recent evidence has pointed to illnesses caused by HRV infection as a strong predictor of asthma development. In a prospective analysis of outcome in relation to asthma, Kotaniemi and colleagues investigated 100 children hospitalized for wheezing during the first 2 years of life, performing viral RT-PCR from frozen nasal aspirates obtained from the index episode of wheezing.(21) They found that while prior to 6 months of age, RSV was the most commonly isolated viral pathogen, from 6 months onward, HRV was most prevalent in wheezing children. The risk for asthma development by school age was 4x greater in the HRV infected group than for children hospitalized with other respiratory viral illnesses.(21) Kusel et al demonstrated, in a high-risk birth cohort, an association between outpatient wheezing with HRV in infancy and persistent wheezing at 5 years of age, particularly for children with early allergic sensitization.(3) The Childhood Origins of ASThma study (COAST), also a high-risk birth cohort, identified HRV-induced wheezing during infancy as the most significant predictor of persistent wheezing at 3 years of life, and a diagnosis of asthma at age 6 years.(2, 6, 12) These associations were strongest for those with a history of early aeroallergen sensitization(2).
RSV and Asthma
Respiratory Syncytial Virus (RSV), a negative stranded, enveloped, RNA virus of the Paramyxoviridae family, has long been recognized as an important viral pathogen during the winter months, frequently leading to early life wheezing. While RSV is not a predominant cause of asthma exacerbations in the older child or adult, debate has continued on the causal relationship to the subsequent development of asthma.(22-27) Several studies have demonstrated that RSV related severe lower respiratory tract infections (LRTI), particularly those requiring hospitalization, are associated with an increased risk of asthma diagnosis at school age.(28, 29) A prospective cohort study conducted in Sweden by Sigurs and associates (28, 30) used a case-control design to compare 47 hospitalized infants with documented RSV infection in infancy to 97 healthy controls, measuring rates of asthma and allergic sensitization over time. By the 18-year follow up, they found a higher percentage of both current asthma diagnosis (39% vs. 9%, p<0.001) as well as allergic sensitization (41% vs. 14%, p=0.005) in the RSV group than in the control.(30) Using a multivariate analysis approach, RSV infection was found to be the major risk factor in subsequent asthma development (OR 7.2). They concluded that severe early RSV bronchiolitis was associated with increased prevalence and persistence of allergic asthma into early adulthood. The Tucson Children’s Respiratory Study (TCRS) reported persisting respiratory symptoms (wheeze) through early adolescence in subjects who experienced outpatient RSV LRTI during the first 3 years of life. Interestingly, the association to wheeze diminished with age, and lost significance by 13 years of age.(29) The Tennessee Asthma Bronchiolitis Study (TABS), a large unselected population based retrospective study of children identified through a Medicaid database, investigated the relationship between winter virus infection and asthma.(31, 32) They found that the risk of asthma by age 5 increased with the timing of infant birth in relation to the peak of the winter virus season, with subjects born 120 days prior to winter virus peak having the highest risk.(31)
These findings led to speculation that prevention of RSV infection could potentially reduce asthma outcomes. Simoes and colleagues compared a group of premature infants treated with palivizumab, an anti-RSV monoclonal antibody, and compared them to an untreated aged-matched cohort. They found on prospective follow up that the incidence of recurrent wheezing was significantly lower in the palivizumab treated group than for the untreated controls, suggesting that preventing LRTI with RSV could reduce the development of persistent wheezing.(33) A recent randomized controlled trial by Blanken et al showed that otherwise healthy premature infants treated with palivizumab had a relative reduction of 61% (95% CI 56-65) in the number of wheezing days within the first year of life when compared to controls that received a placebo injection during the RSV season. Perhaps even more encouraging was the demonstration of the extended protective benefit from palivizumab, noting a persistent reduction in total wheezing days and respiratory related hospitalizations >2months out from the last palivizumab injection.(34) Whether these findings will translate to asthma prevention is not known and of interest moving forward.
Conversely, there are several studies that argue against the causality of RSV in asthma inception. Poorisrisak and colleagues looked at 37 pairs of monozygotic twins, differing with respect to hospitalization for RSV-bronchiolitis during infancy, and found no difference with regard to subsequent development of asthma or allergic sensitization by 7 years of age within the twin pairs.(35) While definitive conclusions are limited due to the small sample size of the study, the results argued against the role of severe RSV infection in the development of asthma, as earlier studies had implied. Similarly, a larger twin based study, conducted in Denmark by Thomsen and associates, identified an association between severe RSV illness and asthma, but not a causal relationship.(36)
Other Viruses
Influenza, an RNA virus in the Orthomyxoviridae family, is responsible for a significant amount of respiratory related illness in both children and adults; however, its effect specifically within the asthma population has been debated(37). Studies have demonstrated significant morbidity associated with influenza infection for patients with underlying chronic conditions (such as asthma) when compared to healthy controls.(38) A large prospective study of children 6 months to 59 months of age looked at rates of influenza attributable outpatient visits and hospitalizations for children with asthma, and compared this to otherwise healthy children over a 5 year period of time.(39) They found that the average influenza associated hospitalization rate was 4x greater, with a 2 fold increase in outpatient visits, for the asthma group, than for the nonasthmatic children.(39) However, prospective studies looking to distinguish influenza’s role in the setting of acute asthma exacerbations have failed to show a strong association.(27)
Human Bocavirus (HBoV), a member of the Parvoviridae family, is a relatively newly identified virus, first recognized in 2005 with the aid of molecular sequencing(40). It primarily infects the respiratory tract, and while the virulence in causing symptomatic respiratory symptoms has been debated, data have shown a high degree of co-infection with other viral pathogens, namely HRV, RSV, and adenovirus, particularly in children under age 2 years.(41, 42) These data suggest that its role in early life LRTI may be underappreciated; however, further investigation is needed.
Human metapneumovirus (hMPV), a Paramyxovirus closely related to RSV, has been linked to both asthma inception as well as exacerbations within the pediatric and adult populations.(24, 43-47) A study conducted by Garcia-Garcia and associates investigated the relationship between hMPV bronchiolitis and the subsequent development of wheezing. They compared this outcome, at ages 3 and 5 years, for 55 children who were hospitalized for either hMPV- related bronchiolitis or RSV-bronchiolitis during the first 2 years of life. They found, in a multivariate analysis, that hMPV was the most important risk factor for the development of asthma by the preschool years (OR=15.9). (43) Additionally, data published by Williams et al found a significant association between hMPV and wheezing among children less than 3 years of age; however, the relationship lost significance in children 3 years and older, indicating that hMPV may play an important role during early life, a critical period of lung growth and development.(47)
Bacteria and the Airway Microbiome
Advances in molecular diagnostics have expanded our knowledge of the diversity of the human microbiome and its relation to human diseases. With improved ability to recognize and identify a growing number of bacteria within the airway, the natural progression to investigate their potential role in chronic pulmonary diseases has come to the forefront of focus. Bisgaard et al published data from a large birth cohort study of high risk subjects identified at birth by maternal history of asthma, that noted positive associations between bacterial colonization with streptococcus pneumoniae, haemophilus influenzae, and moraxella catarrhalis (or a combination of these organisms) in the hypopharynx of asymptomatic neonates, and the subsequent development of asthma (prevalence in colonized 33%, not colonized 10%, OR 4.57) within the first 5 years of life. (48) The association was found to be “time specific”, that is, present only for the infants colonized by 1 month but not at 12 months of age. (48) A second study, performed on the same high-risk population, looked at the frequency of bacteria and virus present in airway aspirates during acute wheezing illness.(49) They found that wheezing was significantly associated with bacterial infections (OR 2.9, p<0.001), primarily streptococcus pneumoniae, haemophilus influenzae, and moraxella catarrhalis, and this relationship was similar but distinct from the association with viral infections (OR 2.8, p<0.001)(49). Similar findings were noted by De Shutter et al, who retrospectively analyzed a population of recurrent wheezing children between the ages of 4 and 48 months, who underwent bronchoscopy and BAL after failing to respond to inhaled corticosteroids.(50) 48% of subjects had significant bacterial BAL cultures, with non-typeable haemophilus influenz most common, followed by streptococcus pneumoniae and moraxella catarrhalis, respectively.(50)
Progression from Early Life Wheezing to Asthma
Inflammation in Preschool Children
Krawiec and colleagues performed bronchoscopy and bronchoalveolar lavage on 20 wheezing children (median age 15 months) with recurrent/persistent wheezing. When compared to normal controls, they found that the wheezing children had 3 times the total BAL cells. The cellular increase was nonspecific, unlike the eosinophilic predominance seen in many older children and adults with asthma(51, 52). They additionally found increased levels of inflammatory mediators PGE2, LTE4 and LTB4, suggesting that ongoing inflammation is present in the airways of young wheezers.(52) Saglani and colleagues performed endobronchial biopsies on both wheezing subjects (aged 3 months to 5 years) and aged matched controls(53). Unlike the pathogenic hallmarks of asthma established for older children and adults, they found no evidence of eosinophillic inflammation nor reticular basement membrane thickening in the infant wheezers (median age 12 months), even in the presence of atopy(54, 55). However, these pathologic markers were noted in slightly older wheezing children (median age 29 months), suggesting that the time between 1 and 3 years of age is a critical period for asthmatic airway remodeling, and that changes are not a congenital phenomenon, but more likely a consequence of environmental exposures and disease development over time (53).
Symptoms & Lung Function Changes in Early Life
Guilbert and colleagues followed a cohort of preschool aged children at high risk of asthma, and found that for children who did not outgrow their wheezing, their symptom burden progressively increased through their preschool years.(56) Those early wheezers with persistent disease have additionally been shown to have diminished lung function by school age. Within the TCRS cohort, children with persistent wheezing beginning prior to age 3, had reductions in lung function at age 6 years that persisted over time.(57) Similarly, the COAST study demonstrated an association between recurrent severe exacerbations in early life that required OCS and reduced lung function at school age.(58) The persistent and progressive nature of disease course noted in these studies suggests that early life remodeling of the airway may be occurring.
Host Factors and Asthma Development
Immunologic Factors
Whether infection leads to asthma versus the counter argument of an underlying predisposition to asthma and early life wheezing illnesses has long been debated, with the search for explanatory mechanisms a goal for many research groups. Multiple cohort studies have identified impaired IFN-γ responses at birth or infancy with recurrent wheezing in early life, but these relationships have not generally persisted to childhood asthma risk (5, 7, 59). Sensitization to aeroallergens is a clear risk factor for asthma development, with recent data suggesting that children who develop sensitization to multiple allergens in early life are at particularly high-risk of asthma inception and severe exacerbations. (60, 61), In fact, a sequential developmental relationship with allergic sensitization leading to HRV-induced wheezing, and subsequent asthma, has recently been identified.(6) This leads one to question: what mechanisms link allergic sensitization to HRV-induced wheezing and asthma?
One potential mechanism involves impairment of antiviral immunity by allergic sensitization and exposure. Children and adults with allergic asthma have been reported to have reductions in peripheral blood mononuclear cell production of types I and III interferons in response to influenza and HRV infection. (62, 63) These abnormalities are associated with expression of the high-affinity IgE receptor (FcεRI) on plasmacytoid dendritic cells (pDCs) and are accentuated by FcεRI crosslinking. Additionally, epithelial cell antiviral responses have been reported to be abnormal in patients with allergic asthma (8, 64), although not all groups have been able to replicate these findings(65)
Other potential pathways that may enhance HRV susceptibility in allergic individuals involve the effects of allergic inflammation on the airway leading to enhanced airway responsiveness, impaired barrier function, and increased mucous secretion (66). It has also been recently proposed that allergic asthmatics may have an impaired ability to “shut-off” HRV-induced inflammation. (67) Finally, HRV can directly lead to a number of airway changes critical to asthma development. Leigh and colleagues looked at the response of airway epithelial cells following infection with HRV, and noted an overall up-regulation of several growth factors important in airway remodeling including amphiregulin, activin A, and VEGF (68). Zhu and associates found HRV infection triggered mucin production via toll like receptor 3, and also caused up-regulation of epidermal growth factor receptor, providing mechanisms for mucus plugging and epithelial remodeling.(69)
Genetic Susceptibility to HRV Infections
Genetic mutations in a variety of single nucleotide polymorphisms (SNPs) related to innate signaling responses have been linked to asthma, leading to further interest in the complex relationship between a gene-by-environment interaction in asthma inception. Variation at the 17q21 locus is the most replicated asthma susceptibility region of the genome, but is not associated with the development of allergic sensitization.(70-72) Caliskan and colleagues recently reported that the increased risk of asthma in the at-risk “TT” genotype at SNP rs7216389 was seen only in children who wheezed with HRV infections in early life.(70) The mechanisms underlying these observations are unknown and of clear interest for further investigation.
Conclusions and Future Directions
Both viral and bacterial infections in early life cause a significant amount of morbidity, with potentially life-long implications. Their identification during wheezing illnesses and their subsequent presence during exacerbations once disease has been established suggest an import role in airway inflammatory responses, which may lead to the development of various asthma phenotypes. The implications for proving a causal relationship could open new doors for asthma treatment and prevention through the use of vaccination and direct antimicrobials, or indirect means of enhancing the immune response in high-risk individuals. Studies focusing on early prevention, such as the case with palivizumab and RSV, are encouraging in demonstrating that early intervention may modify subsequent risk and morbidity from recurrent wheezing illnesses. These findings foreshadow the potential therapeutic and preventative role for strategies against other respiratory pathogens, notably HRV. The relationships among infections, host responses, and asthma inception remain complex and an important area for future study.
Acknowledgments
Supported by NIH grants: P01 HL70831, 5U10HL098090 and by the Clinical and Translational Science Award program through the National Institutes of Health National Center for Advancing Translational Sciences grant UL1TR000427
Citations
- 1.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. N.Engl.J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 2.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, Dasilva DF, Tisler CJ, Gern JE, Lemanske RF., Jr Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, Sly PD. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston SL, Pattemore PK, Sanderson G, Smith S, Campbell MJ, Josephs LK, Cunningham A, Robinson SB, Myint SH, Ward ME, Tyrrell DAJ, Holgate ST. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996;154:654–660. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 5.Gern JE, Brooks GD, Meyer P, Chang A, Shen K, Evans MD, Tisler C, Dasilva D, Roberg KA, Mikus LD, Rosenthal LA, Kirk CJ, Shult PA, Bhattacharya A, Li Z, Gangnon R, Lemanske RF., Jr Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol. 2006;117:72–78. doi: 10.1016/j.jaci.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, Lee WM, Gern JE, Lemanske RF., Jr Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;185:281–285. doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007;120:835–841. doi: 10.1016/j.jaci.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 8.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J.Exp.Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O’Toole S, Myint SH, Tyrrell DA. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller EK, Edwards KM, Weinberg GA, Iwane MK, Griffin MR, Hall CB, Zhu Y, Szilagyi PG, Morin LL, Heil LH, Lu X, Williams JV. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123:98–104. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston SL, Martin RJ. Chlamydophila pneumoniae and Mycoplasma pneumoniae: a role in asthma pathogenesis? Am J Respir Crit Care Med. 2005;172:1078–1089. doi: 10.1164/rccm.200412-1743PP. [DOI] [PubMed] [Google Scholar]
- 12.Lemanske RF, Jr., Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, Kirk CJ, Reisdorf E, Roberg KA, Anderson EL, Carlson-Dakes KT, Adler KJ, Gilbertson-White S, Pappas TE, Dasilva DF, Tisler CJ, Gern JE. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman D, Printz S, Ben Yaakov M, Lazarovich Z, Ohana B, Friedman MG, Dvoskin B, Leinonen M, Boldur I. Atypical pathogen infection in adults with acute exacerbation of bronchial asthma. Am J Respir Crit Care Med. 2003;167:406–410. doi: 10.1164/rccm.200209-996OC. [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal LA, Avila PC, Heymann PW, Martin RJ, Miller EK, Papadopoulos NG, Peebles RS, Jr., Gern JE. Viral respiratory tract infections and asthma: the course ahead. J Allergy Clin Immunol. 2010;125:1212–1217. doi: 10.1016/j.jaci.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bezerra PG, Britto MC, Correia JB, Duarte Mdo C, Fonceca AM, Rose K, Hopkins MJ, Cuevas LE, McNamara PS. Viral and atypical bacterial detection in acute respiratory infection in children under five years. PloS one. 2011;6:e18928. doi: 10.1371/journal.pone.0018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, Fraenkel DJ, Meyer J, Lackie PM, Sanderson G, Holgate ST, Johnston SL. Rhinoviruses infect the lower airways. Journal of Infectious Diseases. 2000;181:1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 17.Miller EK, Lu X, Erdman DD, Poehling KA, Zhu Y, Griffin MR, Hartert TV, Anderson LJ, Weinberg GA, Hall CB, Iwane MK, Edwards KM. Rhinovirus-associated hospitalizations in young children. The Journal of infectious diseases. 2007;195:773–781. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jartti T, Lehtinen P, Vuorinen T, Ruuskanen O. Bronchiolitis: age and previous wheezing episodes are linked to viral etiology and atopic characteristics. Pediatr Infect Dis J. 2009;28:311–317. doi: 10.1097/INF.0b013e31818ee0c1. [DOI] [PubMed] [Google Scholar]
- 19.Sears MR, Johnston NW. Understanding the September asthma epidemic. J Allergy Clin Immunol. 2007;120:526–529. doi: 10.1016/j.jaci.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heymann PW, Platts-Mills TA, Johnston SL. Role of viral infections, atopy and antiviral immunity in the etiology of wheezing exacerbations among children and young adults. Pediatr Infect Dis J. 2005;24:S217–222. doi: 10.1097/01.inf.0000188164.33856.f9. discussion. [DOI] [PubMed] [Google Scholar]
- 21.Kotaniemi-Syrjanen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy--the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr.Allergy Immunol. 2005;16:386–392. doi: 10.1111/j.1399-3038.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 23.Lacaze-Masmonteil T, Roze JC, Fauroux B. Incidence of respiratory syncytial virus-related hospitalizations in high-risk children: Follow-up of a national cohort of infants treated with Palivizumab as RSV prophylaxis. Pediatric Pulmonology. 2002;34:181–188. doi: 10.1002/ppul.10175. [DOI] [PubMed] [Google Scholar]
- 24.Manoha C, Espinosa S, Aho SL, Huet F, Pothier P. Epidemiological and clinical features of hMPV, RSV and RVs infections in young children. J.Clin.Virol. 2007;38:221–226. doi: 10.1016/j.jcv.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Schauer U, Hoffjan S, Bittscheidt J, Kochling A, Hemmis S, Bongartz S, Stephan V. RSV bronchiolitis and risk of wheeze and allergic sensitisation in the first year of life. Eur Respir J. 2002;20:1277–1283. doi: 10.1183/09031936.02.00019902. [DOI] [PubMed] [Google Scholar]
- 26.Smyth RL, Openshaw PJ. Bronchiolitis. Lancet. 2006;368:312–322. doi: 10.1016/S0140-6736(06)69077-6. [DOI] [PubMed] [Google Scholar]
- 27.Khetsuriani N, Kazerouni NN, Erdman DD, Lu X, Redd SC, Anderson LJ, Teague WG. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119:314–321. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, Kjellman B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 29.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 30.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, Gustafsson PM. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 31.Wu P, Dupont WD, Griffin MR, Carroll KN, Mitchel EF, Gebretsadik T, Hartert TV. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carroll KN, Wu P, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, Hartert TV. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123:1055–1061. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, Kimpen JL. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42. 42. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 34.Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JL, Bont L. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368:1791–1799. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 35.Poorisrisak P, Halkjaer LB, Thomsen SF, Stensballe LG, Kyvik KO, Skytthe A, Schioetz PO, Bisgaard H. Causal direction between respiratory syncytial virus bronchiolitis and asthma studied in monozygotic twins. Chest. 2010;138:338–344. doi: 10.1378/chest.10-0365. [DOI] [PubMed] [Google Scholar]
- 36.Thomsen SF, van der Sluis S, Stensballe LG, Posthuma D, Skytthe A, Kyvik KO, Duffy DL, Backer V, Bisgaard H. Exploring the association between severe respiratory syncytial virus infection and asthma: a registrybased twin study. Am J Respir Crit Care Med. 2009;179:1091–1097. doi: 10.1164/rccm.200809-1471OC. [DOI] [PubMed] [Google Scholar]
- 37.Glezen WP. Asthma, influenza, and vaccination. J Allergy Clin Immunol. 2006;118:1199–1206. doi: 10.1016/j.jaci.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 38.Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283:499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- 39.Miller EK, Griffin MR, Edwards KM, Weinberg GA, Szilagyi PG, Staat MA, Iwane MK, Zhu Y, Hall CB, Fairbrother G, Seither R, Erdman D, Lu P, Poehling KA. Influenza burden for children with asthma. Pediatrics. 2008;121:1–8. doi: 10.1542/peds.2007-1053. [DOI] [PubMed] [Google Scholar]
- 40.Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006;78:1232–1240. doi: 10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allander T. Human bocavirus. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2008;41:29–33. doi: 10.1016/j.jcv.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 42.Allander T, Jartti T, Gupta S, Niesters HG, Lehtinen P, Osterback R, Vuorinen T, Waris M, Bjerkner A, Tiveljung-Lindell A, van den Hoogen BG, Hyypia T, Ruuskanen O. Human bocavirus and acute wheezing in children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;44:904–910. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Garcia ML, Calvo C, Casas I, Bracamonte T, Rellan A, Gozalo F, Tenorio T, Perez-Brena P. Human metapneumovirus bronchiolitis in infancy is an important risk factor for asthma at age 5. Pediatr.Pulmonol. 2007;42:458–464. doi: 10.1002/ppul.20597. [DOI] [PubMed] [Google Scholar]
- 44.Williams JV. Human Metapneumovirus: An Important Cause of Respiratory Disease in Children and Adults. Current infectious disease reports. 2005;7:204–210. doi: 10.1007/s11908-005-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams JV, Crowe JE, Jr., Enriquez R, Minton P, Peebles RS, Jr., Hamilton RG, Higgins S, Griffin M, Hartert TV. Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. The Journal of infectious diseases. 2005;192:1149–1153. doi: 10.1086/444392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, Wright PF, Crowe JE., Jr Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams JV, Tollefson SJ, Heymann PW, Carper HT, Patrie J, Crowe JE. Human metapneumovirus infection in children hospitalized for wheezing. J Allergy Clin Immunol. 2005;115:1311–1312. doi: 10.1016/j.jaci.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, Brasholt M, Heltberg A, Vissing NH, Thorsen SV, Stage M, Pipper CB. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 49.Bisgaard H, Hermansen MN, Bonnelykke K, Stokholm J, Baty F, Skytt NL, Aniscenko J, Kebadze T, Johnston SL. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Schutter I, Dreesman A, Soetens O, De Waele M, Crokaert F, Verhaegen J, Pierard D, Malfroot A. In young children, persistent wheezing is associated with bronchial bacterial infection: a retrospective analysis. BMC pediatrics. 2012;12:83. doi: 10.1186/1471-2431-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferguson AC, Whitelaw M, Brown H. Correlation of bronchial eosinophil and mast cell activation with bronchial hyperresponsiveness in children with asthma. J Allergy Clin Immunol. 1992;90:609–613. doi: 10.1016/0091-6749(92)90133-m. [DOI] [PubMed] [Google Scholar]
- 52.Krawiec ME, Westcott JY, Chu HW, Balzar S, Trudeau JB, Schwartz LB, Wenzel SE. Persistent wheezing in very young children is associated with lower respiratory inflammation. Am J Respir Crit Care Med. 2001;163:1338–1343. doi: 10.1164/ajrccm.163.6.2005116. [DOI] [PubMed] [Google Scholar]
- 53.Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, Bush A, Jeffery PK. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med. 2007;176:858–864. doi: 10.1164/rccm.200702-212OC. [DOI] [PubMed] [Google Scholar]
- 54.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:S28–38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- 55.Saglani S, Malmstrom K, Pelkonen AS, Malmberg LP, Lindahl H, Kajosaari M, Turpeinen M, Rogers AV, Payne DN, Bush A, Haahtela T, Makela MJ, Jeffery PK. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005;171:722–727. doi: 10.1164/rccm.200410-1404OC. [DOI] [PubMed] [Google Scholar]
- 56.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, Bacharier LB, Lemanske RF, Jr., Strunk RC, Allen DB, Bloomberg GR, Heldt G, Krawiec M, Larsen G, Liu AH, Chinchilli VM, Sorkness CA, Taussig LM, Martinez FD. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 57.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, Taussig LM, Wright AL, Martinez FD. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172:1253–1258. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Brian AL, Lemanske RF, Jr., Evans MD, Gangnon RE, Gern JE, Jackson DJ. Recurrent severe exacerbations in early life and reduced lung function at school age. J Allergy Clin Immunol. 2012;129:1162–1164. doi: 10.1016/j.jaci.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Copenhaver CC, Gern JE, Li Z, Shult PA, Rosenthal LA, Mikus LD, Kirk CJ, Roberg KA, Anderson EL, Tisler CJ, Dasilva DF, Hiemke HJ, Gentile K, Gangnon RE, Lemanske RF., Jr Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med. 2004;170:175–180. doi: 10.1164/rccm.200312-1647OC. [DOI] [PubMed] [Google Scholar]
- 60.Simpson A, Tan VY, Winn J, Svensen M, Bishop CM, Heckerman DE, Buchan I, Custovic A. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med. 2010;181:1200–1206. doi: 10.1164/rccm.200907-1101OC. [DOI] [PubMed] [Google Scholar]
- 61.Stoltz DJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Gern JE, Lemanske RF., Jr Specific patterns of allergic sensitization in early childhood and asthma & rhinitis risk. Clin Exp Allergy. 2013;43:233–241. doi: 10.1111/cea.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Durrani SR, Montville DJ, Pratt AS, Sahu S, Devries MK, Rajamanickam V, Gangnon RE, Gill MA, Gern JE, Lemanske RF, Jr., Jackson DJ. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012;130:489–495. doi: 10.1016/j.jaci.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, Gan VN, Gruchalla RS. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J.Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Contoli M, Caramori G, Mallia P, Johnston S, Papi A. Mechanisms of respiratory virus-induced asthma exacerbations. Clin Exp Allergy. 2005;35:137–145. doi: 10.1111/j.1365-2222.2005.02163.x. [DOI] [PubMed] [Google Scholar]
- 65.Bochkov YA, Hanson KM, Keles S, Brockman-Schneider RA, Jarjour NN, Gern JE. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kloepfer KM, Gern JE. Virus/allergen interactions and exacerbations of asthma. Immunol Allergy Clin North Am. 2010;30:553–563. vii. doi: 10.1016/j.iac.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Subrata LS, Bizzintino J, Mamessier E, Bosco A, McKenna KL, Wikstrom ME, Goldblatt J, Sly PD, Hales BJ, Thomas WR, Laing IA, LeSouef PN, Holt PG. Interactions between innate antiviral and atopic immunoinflammatory pathways precipitate and sustain asthma exacerbations in children. J.Immunol. 2009;183:2793–2800. doi: 10.4049/jimmunol.0900695. [DOI] [PubMed] [Google Scholar]
- 68.Leigh R, Oyelusi W, Wiehler S, Koetzler R, Zaheer RS, Newton R, Proud D. Human rhinovirus infection enhances airway epithelial cell production of growth factors involved in airway remodeling. J Allergy Clin Immunol. 2008;121:1238–1245. doi: 10.1016/j.jaci.2008.01.067. [DOI] [PubMed] [Google Scholar]
- 69.Zhu L, Lee PK, Lee WM, Zhao Y, Yu D, Chen Y. Rhinovirus-induced major airway mucin production involves a novel TLR3-EGFR-dependent pathway. Am J Respir Cell Mol Biol. 2009;40:610–619. doi: 10.1165/rcmb.2008-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caliskan M, Bochkov YA, Kreiner-Moller E, Bonnelykke K, Stein MM, Du G, Bisgaard H, Jackson DJ, Gern JE, Lemanske RF, Jr., Nicolae DL, Ober C. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson WO. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Halapi E, Gudbjartsson DF, Jonsdottir GM, Bjornsdottir US, Thorleifsson G, Helgadottir H, Williams C, Koppelman GH, Heinzmann A, Boezen HM, Jonasdottir A, Blondal T, Gudjonsson SA, Jonasdottir A, Thorlacius T, Henry AP, Altmueller J, Krueger M, Shin HD, Uh ST, Cheong HS, Jonsdottir B, Ludviksson BR, Ludviksdottir D, Gislason D, Park CS, Deichmann K, Thompson PJ, Wjst M, Hall IP, Postma DS, Gislason T, Kong A, Jonsdottir I, Thorsteinsdottir U, Stefansson K. A sequence variant on 17q21 is associated with age at onset and severity of asthma. European journal of human genetics : EJHG. 2010;18:902–908. doi: 10.1038/ejhg.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jackson DJ, Lemanske RF., Jr The role of respiratory infections in childhood asthma inception. Immunol Allergy Clin North Am. 2010;30:513–522. doi: 10.1016/j.iac.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]