Abstract
Purpose
To develop a highly accelerated phase contrast cardiac-gated volume flow measurement (4D flow) MR imaging technique based on spiral sampling and dynamic compressed sensing, and to compare with established phase contrast imaging techniques for the quantification of blood flow in abdominal vessels.
Methods
In this prospective IRB approved study, 10 subjects (9 males, mean age 51 y) including 7 patients with liver disease were enrolled. Two 4D flow acquisitions were performed, one using Cartesian sampling with respiratory tracking, the other using spiral sampling and acquired in a breath hold. Cartesian 2D cine phase contrast was also acquired in the portal vein. Two independent observers assessed vessel conspicuity on phase contrast 3D angiogram. Quantitative flow parameters were measured by two independent observers in major abdominal vessels. Inter-technique concordance was quantified using Bland-Altman analysis and Pearson correlation.
Results
There was no significant difference in vessel conspicuity between 4D flow acquisitions (p >0.069, for both observers), while more artifacts were observed with spiral 4D flow (p <0.016). Quantitative measurements in abdominal vessels showed strong correlation between spiral and Cartesian 4D flow techniques (for total flow r = 0.96, p <0.001). For portal venous flow, spiral 4D flow was in better agreement with 2D cine phase contrast (−8.8/9.3 mL/s) than was Cartesian 4D flow (−10.6/14.6 mL/s).
Conclusion
Combining highly efficient spiral sampling with dynamic compressed sensing results in major acceleration for 4D flow MR imaging, which allows comprehensive assessment of abdominal vessel hemodynamics in a breath hold.
INTRODUCTION
Quantitative assessment of abdominal vessel hemodynamics has important clinical applications, including detection of portal hypertension and diagnosis and quantification of arterial stenosis. While Doppler ultrasound is still widely used, the resulting flow parameters are subject to limited reproducibility (1) due to limited spatial window and variable probe orientation. On the other hand, phase contrast MR imaging allows reliable flow measurement (2) with excellent anatomic localization. Due to the slow data acquisition rate in MR imaging, vessel coverage is often limited to a single cross plane, using cardiac-triggered 2D phase contrast (cine 2DPC) MR imaging which may be achieved in a breath hold. Cine 2DPC requires operator expertise to select the vessel of interest, which may decrease precision. Larger volumes can be measured with phase contrast using cardiac-triggered velocity measurement with 3D vessel coverage (4D flow) (3). The 4D flow technique has been recently validated against cine 2DPC as well as Doppler ultrasound (4–9). However for typical applications, slow data acquisition coupled with respiratory motion control for abdominal scans (10) lead to scan times of the order of 10–20 min, which limits clinical acceptance of 4D flow imaging. Various techniques such as parallel imaging (5), radial (11), spiral without acceleration (12) or compressed sensing (13) have been proposed, resulting in acquisition time in the order of 5–15 min.
The objective of our study is to drastically speed up 4D flow, using a combination of efficient accelerated spiral sampling and compressed sensing reconstruction of sub-Nyquist data. Here we validate this novel acquisition method against established phase contrast imaging techniques for flow assessment in major upper abdominal vessels.
METHODS AND MATERIALS
Subjects
This was a HIPAA compliant single-center prospective study, approved by the institutional review board at −. 10 subjects (9 males, 1 female, mean age 51 y, range 30–70 y) were enrolled. Informed signed consent was obtained from all participants. Three healthy volunteers were recruited internally. None of the volunteers had a known history of liver disease or significant alcohol consumption. Seven subjects had chronic liver disease [secondary to HCV (n=3), HBV (n=3), NASH (n=1)]. Among these 7 subjects, 3 had liver cirrhosis diagnosed by histopathology. All subjects were asked to fast for 6 hours before the exam in order to avoid hyper-dynamic flow effects due to caloric intake.
MR imaging acquisition
MR imaging was performed using a 1.5T system (Magnetom Aera, Siemens Healthcare) equipped with a 30-channel body and spine coil array. Phase contrast was acquired without gadolinium contrast agent injection, and consisted of cine 2DPC, Cartesian 4D flow and spiral 4D flow imaging performed in chronological order (acquisition parameters in supplementary Table S1). While Cartesian 4D flow was selected to allow direct comparison with spiral 4D flow, cine 2DPC standard technique was selected because of its wide use for single vessel measurement. Both prototype 4D flow sequences used 4-point velocity encoding (14) of Venc=60 cm/s and were acquired in the same coronal oblique orientation, using a 60-mm slab covering hepatic vessels as well as aortic branches and inferior vena cava (IVC), with approximately matched spatial and temporal resolutions (spatial: 2.5×3.9×5 and 2.5×2.5×5 mm2; temporal: 68.4 and 66.2 ms for Cartesian and spiral respectively). Subjects were asked to keep their arms up to avoid aliasing artifacts. Cartesian 4D flow used a crossed-pair navigator placed on the dome of the spleen to track breathing motion. Cine 2DPC was acquired in the portal vein using Venc=60 cm/s, spatial resolution of 1.6×3.3×7 mm2, temporal resolution 41.8 ms. A plane was selected perpendicular to the main extrahepatic portal vein, using coronal, axial and sagittal HASTE (half-Fourier acquisition single-shot turbo spin-echo) to localize the vessel. Parallel imaging (GRAPPA, generalized autocalibrated partially parallel acquisitions (15)) was used to accelerate both cine 2DPC and Cartesian 4D flow with a reduction factor of R=2.
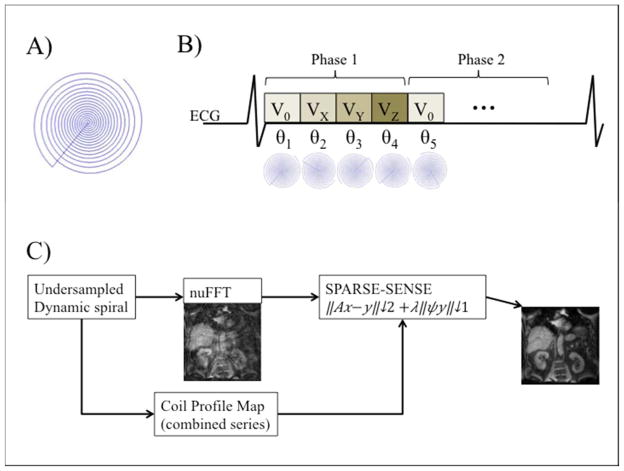
Spiral 4D flow acquisition and reconstruction methods are depicted on Fig. 1 and detailed in supplemental material. Briefly, a 3D stack-of-spirals was acquired. Sub-Nyquist sampling was performed using variable density spirals, with acceleration factor of 6, resulting in 2 acquired shots per 3D phase encoding step. Therefore, the whole volume (12 slices) was acquired in only 24 heartbeats. A dynamic compressed sensing framework was used for reconstruction, which has previously been validated for Cartesian imaging with acceleration factor up to 8 (16–18). Reconstruction routines were implemented using Matlab (Matlab, R2012b; Mathworks, Natick, Mass) and took about 3h per subject on a desktop computer (12-core Intel Xeon, 64 GB RAM, no algorithm parallelization). After compressed sensing reconstruction, phase difference images (Fig. 2) were computed and residual phase offsets were eliminated by fitting a 3rd order polynomial to static tissue (19) for each of the 3 velocity-encoding directions, using Matlab fit function. An example of spiral datasets before and after reconstruction is given in supplementary video S2.
Figure 1.
Sampling and reconstruction strategy for accelerated spiral 4D flow phase contrast imaging. A) Undersampled variable density spiral trajectory. B) Cardiac triggered acquisition. Random rotations were applied to the trajectory for each dynamic frame (velocity encodings and cardiac phases). C) Compressed sensing reconstruction pipeline. The coil sensitivity map was derived from the acquired data itself and inserted in the SPARSE-SENSE encoding operator.
Figure 2.
Example of magnitude, phase difference images (VX, VY and VZ correspond to velocity measured with motion encoding gradient in the R-L, A-P and F-H directions respectively) and 3D angiogram for Cartesian and spiral acquisitions (Venc=60cm/s). The dark lines proximal to the spleen on Cartesian series (arrowhead) show the cross-beam navigator used for respiratory gating. Phase aliasing present in the aorta and vena cava were corrected for during post-processing. The 3D angiogram shows a segmented view of the portal, splenic and superior mesenteric veins with comparable quality and conspicuity.
Image analysis
All analysis of 4D flow data was performed with prototype software 4D Flow 2.4 (Siemens Healthcare) which allows visualization and segmentation of 4D angiographic data as well as measurement of individual vessel. The following abdominal vessels were assessed.
Arteries: abdominal aorta (above celiac axis and below renal arteries), celiac axis, hepatic, splenic, superior mesenteric, and main renal arteries.
Veins: right, middle and left hepatic veins, IVC (below and above renal veins), portal, splenic, superior mesenteric and renal veins.
Phase aliasing present in high velocity vessels was corrected for velocity values between Venc and 2*Venc.
4D flow image quality was evaluated by two independent observers (-- and --, radiologists with 3 and 10 years-experience) using the time-averaged 3D angiogram. The following scoring system was adopted: vessel conspicuity and sharpness (0: vessel not seen, 1: severely to moderately blurred, 2: mildly blurred, 3: well delineated) and degree of background artifacts (1: severe, 2: moderate, 3: minimal/none). Observers were not blinded to the type of acquisition, but analyzed all Cartesian data, then all spiral data consecutively to avoid bias resulting from direct comparison.
Quantitative flow analysis was performed by two independent observers (--, a postdoc and --, a medical student with 4 and 1 year experience in image analysis) who measured time-averaged flow and through-plane velocity, vessel area, as well as peak through-plane velocity for each vessel. Previous to analysis, a radiologist identified all vessels of interest. For 4D flow datasets, segmentation was performed using a centerline detection algorithm (20) by selecting at least 2 seeds in the vessel lumen. A planar region of interest was automatically placed perpendicular to the segmented vessel in order to quantify the cross-sectional blood flow. 4D flow processing took 10 to 20 min per case, depending on the number of vessels analyzed. Cine 2DPC was analyzed using software locally developed on Matlab (R2012b). The magnitude image was used to segment the bright-intensity portal vein from darker background tissue, and flow parameters were extracted from the segmented phase image.
Statistical analysis
For qualitative analysis, differences in scores between Cartesian and spiral 4D flow sequences were assessed using paired Wilcoxon test, and inter-reader agreement was evaluated using the weighted Kappa test. For quantitative analysis, comparisons between Cartesian 4D flow, spiral 4D flow and cine 2DPC, as well as inter-observer variability, were given in terms of the Bland-Altman limits of agreement. Pearson correlation and paired T-test were used to compare parameters derived from both 4D flow techniques. All statistical analysis was performed using Matlab R2012b statistical toolbox.
RESULTS
Acquisition time
All 10 exams were successfully completed. The respiratory-triggered 4D flow Cartesian acquisition showed variable acquisition time (250 cardiac cycles, acquired in 6:21 to 20:37 min, depending on navigator efficiency and breathing pattern) with a mean time of 11:21 min. Cine 2DPC and spiral 4D flow were acquired in 17 and 24 heart beats respectively, during breath holding at end expiration.
Image quality
For both observers, there was no significant difference in vessel conspicuity/sharpness between spiral and Cartesian 4D flow in arteries (mean score 2.3 ± 1.0 vs. 2.2 ± 1.1 for observer 1, 2.5 ± 1.0 vs. 2.4 ± 1.0 for observer 2, p >0.297) or veins (score 1.8 ± 1.2 vs. 1.9 ± 1.3 for observer 1, 1.8 ± 0.9 vs. 2.0 ± 1.0 for observer 2, p >0.069) (Fig. 2). However, background artifacts were significantly worse on spiral vs. Cartesian 4D flow (mean score 1.9 ± 0.7 vs. 2.8 ± 0.4 for observer 1, 2.1 ± 0.3 vs. 2.9 ± 0.3 for observer 2, p <0.016 for both). Substantial inter-observer agreement was observed (κ=0.65).
Examples of flow visualization in the portal, superior mesenteric and splenic veins are shown in supplementary videos S3 and S4.
Flow parameters
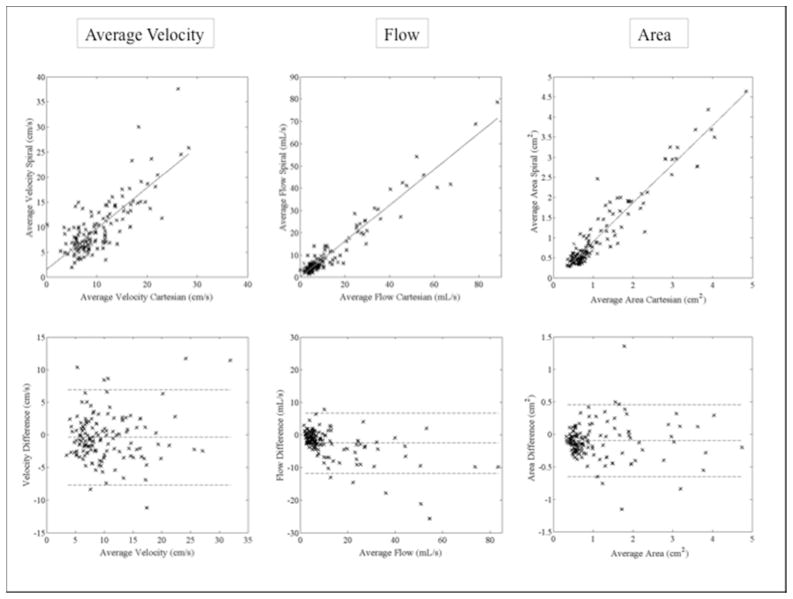
A total of 136 abdominal vessels were measured in all 10 subjects during 4D flow processing. Strong correlation was found between spiral and Cartesian 4D flow parameters for all vessels (r=0.96, 0.95, 0.77 and 0.83 for average flow, average vessel area, average through-plane velocity and peak velocity respectively, p <0.001 for all). Per vessel parameters of 4D flow and cine 2DPC acquisitions are given in Table 1. Bland Altman limits of agreement are given in Table 2. Compared to Cartesian 4D flow (assessed on all upper abdominal vessels), spiral 4D flow resulted in significantly lower vessel area and flow (p <0.001 for both), while no significant difference was noted for average and peak velocity (p=0.19 and 0.17 respectively). For portal venous flow measurement, spiral 4D flow was in better agreement with cine 2DPC than was Cartesian 4D flow, for which the computed average flow was higher. Correlation and Bland-Altman plots are shown in Fig. 3 for all vessels, and in Fig. 4 for portal vein only. Inter-observer agreement was similar for Cartesian 4D flow, spiral 4D flow and cine 2DPC (Table 2).
Table 1.
Flow parameters for Cartesian 4D flow, spiral 4D flow and cine 2D phase contrast, for each major abdominal vessel measured (total number of measured vessels is given in parenthesis). Flow parameters are given as mean ± standard deviation.
| Cartesian 4D flow | Spiral 4D flow | |||||||
|---|---|---|---|---|---|---|---|---|
| Flow | Area | Vavg | Vpeak | Flow | Area | Vavg | Vpeak | |
| Hepatic artery (n=6) | 3.5 ±1.4 | 0.53 ±0.10 | 6.7 ±2.8 | 20.1 ±8.3 | 4.1 ±1.7 | 0.48 ±0.10 | 8.3 ±3.2 | 29.4 ±14.4 |
| Celiac axis (n=8) | 7.2 ±3.0 | 0.56 ±0.10 | 12.9 ±5.2 | 48.6 ±25.8 | 5.8 ±3.8 | 0.46 ±0.11 | 13.5 ±8.4 | 51.2 ±33.2 |
| Splenic artery (n=10) | 5.1 ±4.4 | 0.57 ±0.13 | 8.5 ±6.4 | 29.2 ±27.5 | 4.7 ±3.1 | 0.38 ±0.07 | 12.0 ±5.9 | 34.7 ±15.6 |
| SMA (n=9) | 6.5 ±4.4 | 0.63 ±0.15 | 9.8 ±4.3 | 56.6 ±28.7 | 4.9 ±2.2 | 0.45 ±0.08 | 11.1 ±3.7 | 53.2 ±24.2 |
| Right renal artery (n=7) | 7.1 ±2.2 | 0.56 ±0.12 | 12.8 ±3.8 | 40.6 ±13.5 | 5.0 ±1.8 | 0.46 ±0.07 | 10.7 ±2.5 | 30.6 ±5.9 |
| Left renal artery (n=9) | 6.0 ±1.6 | 0.55 ±0.10 | 11.0 ±2.8 | 38.5 ±14.6 | 5.7 ±2.2 | 0.47 ±0.08 | 12.1 ±3.3 | 31.1 ±9.6 |
| Aorta above celiac (n=8) | 55.4 ±20.6 | 2.80 ±0.85 | 19.7 ±5.6 | 74.4 ±21.1 | 47.5 ±19.3 | 3.00 ±0.83 | 16.4 ±5.9 | 68.3 ±23.8 |
| Aorta below renal (n=9) | 21.9 ±9.8 | 1.71 ±0.46 | 12.7 ±5.5 | 67.1 ±21.3 | 18.5 ±8.1 | 1.71 ±0.28 | 11.4 ±5.1 | 65.4 ±21.7 |
| Portal vein (n=10) | 10.6 ±6.1 | 1.28 ±0.54 | 8.1 ±2.7 | 15.5 ±4.1 | 8.8 ±4.1 | 1.13 ±0.45 | 7.8 ±1.8 | 15.4 ±3.0 |
| SMV (n=6) | 5.0 ±2.4 | 0.83 ±0.17 | 5.9 ±1.8 | 11.6 ±2.8 | 4.1 ±2.5 | 0.80 ±0.36 | 5.1 ±1.0 | 12.0 ±1.9 |
| Splenic vein (n=9) | 4.5 ±2.4 | 0.70 ±0.24 | 6.4 ±2.1 | 13.5 ±4.6 | 4.0 ±1.6 | 0.50 ±0.16 | 7.1 ±2.2 | 14.5 ±4.3 |
| IVC above renal (n=9) | 37.5 ±14.1 | 2.71 ±1.30 | 15.8 ±6.4 | 39.7 ±20.4 | 28.4 ±10.1 | 2.47 ±1.22 | 16.8 ±10.1 | 36.4 ±14.6 |
| IVC below renal (n=9) | 18.6 ±7.4 | 1.91 ±0.91 | 10.4 ±3.4 | 24.3 ±6.3 | 13.5 ±7.7 | 1.66 ±0.79 | 8.6 ±4.3 | 21.3 ±6.7 |
| Right HV (n=6) | 7.2 ±3.8 | 0.94 ±0.35 | 7.2 ±1.6 | 21.4 ±11.3 | 4.2 ±1.9 | 0.68 ±0.20 | 6.2 ±2.7 | 17.3 ±6.2 |
| Middle HV (n=4) | 7.0 ±2.7 | 0.76 ±0.09 | 9.2 ±3.4 | 27.9 ±16.0 | 3.2 ±1.1 | 0.55 ±9.33 | 5.9 ±1.9 | 21.7 ±10.8 |
| Left HV (n=4) | 4.7 ±1.4 | 0.68 ±0.13 | 7.2 ±3.0 | 28.8 ±10.2 | 3.4 ±1.4 | 0.58 ±0.10 | 6.2 ±2.8 | 24.9 ±12.9 |
| Left renal vein (n=7) | 7.7 ±1.7 | 0.94 ±0.19 | 8.3 ±1.8 | 18.3 ±5.7 | 6.6 ±3.3 | 1.00 ±0.60 | 7.0 ±2.3 | 18.1 ±5.6 |
| Right renal vein (n=6) | 6.0 ±1.3 | 0.82 ±0.18 | 7.5 ±2.1 | 15.3 ±5.0 | 4.1 ±1.0 | 0.71 ±0.18 | 6.2 ±2.6 | 14.4 ±5.8 |
| Cine 2D phase contrast | ||||||||
| Portal Vein (n=10) | 8.6 ±2.7 | 1.87 ±0.45 | 4.7 ±1.4 | 7.3 ±1.8 | ||||
Area: time-averaged vessel area in cm2; Flow: time-averaged total flow in mL/s; HV: hepatic vein; IVC: inferior vena cava; SMA: superior mesenteric artery; SMV: superior mesenteric vein; Vavg: time-averaged through-plane velocity in cm/s; Vpeak: peak through-plane velocity in cm/s.
Table 2.
Bland Altman limits of agreement (LA) for comparison of spiral 4D flow, Cartesian 4D flow (both assessed in upper abdominal vessels) and cine 2D phase contrast (assessed in the portal vein). Limits of agreement for inter-observer reproducibility are also given.
| Flow | Area | Vavg | Vpeak | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| ALL VESSELS | Inter-technique | Spiral 4D vs. Cartesian 4D | Mean | −2.6 | −0.10 | −0.4 | −1.5 |
| LA | −11.8/6.6 | −0.66/0.45 | −7.7/6.9 | −27.2/24.3 | |||
|
| |||||||
| Inter-observer | Cartesian 4D | Mean | −0.7 | −0.02 | −0.4 | −1.4 | |
| LA | −8.7/7.3 | −0.44/0.40 | −6.2/5.4 | −36.1/33.2 | |||
|
| |||||||
| Spiral 4D | Mean | −0.9 | −0.02 | −0.8 | −1.2 | ||
| LA | −7.2/5.4 | −0.60/0.57 | −5.7/4.1 | −23.8/21.3 | |||
|
| |||||||
| PORTAL VEIN | Inter-technique | Spiral 4D vs. Cartesian 4D | Mean | −1.8 | −0.15 | −0.3 | −0.1 |
| LA | −11.8/8.2 | −0.94/0.65 | −6.1/5.5 | −8.9/8.8 | |||
|
| |||||||
| Cartesian 4D vs. cine 2D | Mean | 2.1 | −0.59 | 3.4 | 8.2 | ||
| LA | −10.6/14.6 | −1.55/0.38 | −3.3/10.0 | −0.4/16.8 | |||
|
| |||||||
| Spiral 4D vs. cine 2D | Mean | 0.2 | −0.73 | 3.1 | 8.1 | ||
| LA | −8.8/9.3 | −1.51/0.04 | −0.6/6.8 | 2.3/14.0 | |||
|
| |||||||
| Inter-observer | Cartesian 4D | Mean | 0.2 | −0.03 | 0.2 | −0.1 | |
| LA | −3.5/3.9 | −0.34/0.29 | −3.3/3.6 | −4.7/4.6 | |||
|
| |||||||
| Spiral 4D | Mean | −1.1 | −0.12 | −0.2 | 0.2 | ||
| LA | −5.1/2.8 | −0.79/0.56 | −1.8/1.3 | −1.6/2.1 | |||
|
| |||||||
| Cine 2D | Mean | −0.6 | 0.11 | −0.6 | −0.3 | ||
| LA | −5.2/4.1 | −0.26/0.49 | −3.4/2.2 | −2.1/1.4 | |||
Area: time-averaged vessel area in cm2; Flow: time-averaged total flow in mL/s; HV: hepatic vein; IVC: inferior vena cava; LA: limits of agreement; SMA: superior mesenteric artery; SMV: superior mesenteric vein; Vavg: time-averaged through-plane velocity in cm/s; Vpeak: peak through-plane velocity in cm/s
Figure 3.
Correlation (top row) and Bland-Altman plots (bottom row) comparing flow parameters in 136 abdominal vessels measured in 10 subjects using spiral and Cartesian 4D flow. For each subject, all abdominal vessels that were successfully segmented were included in the analysis. Bland Altman limits of agreement are given in Table 2.
Figure 4.
Bland-Altman plots showing the comparison of spiral vs. Cartesian 4D flow, Cartesian vs. cine 2D and spiral vs. cine 2D in the portal vein measured in 10 subjects. Bland Altman limits of agreement are given in Table 2. The velocity and area bias observed for cine 2D phase contrast vs. 4D flow techniques may be due to imperfect plane alignment (which does not affect total flow). Average portal venous flow was 10.6, 8.8 and 8.6 mL/s using Cartesian 4D flow, spiral 4D flow and cine 2D phase contrast respectively.
DISCUSSION
We have demonstrated that combining efficient spiral sampling with dynamic compressed sensing reconstruction allows for substantial acceleration of 4D flow acquisition (from an average of approximately 11 min down to a breath hold) while providing similar performance for flow parameter estimation when compared to standard Cartesian 4D flow and cine 2D phase contrast (2DPC) measurements in vivo.
The primary goal of phase contrast imaging is to quantify blood flow in pathologies such as portal hypertension or for diagnosing vascular obstruction/stenosis. Cartesian 4D flow and cine 2DPC have been previously validated against Doppler ultrasound and were thus selected to assess the performance of the novel spiral acquisition technique. Comparing spiral and Cartesian 4D flow quantification in abdominal vessels, we found high correlation between the two techniques. Furthermore, in our small series spiral 4D flow was in better agreement with cine 2DPC than was Cartesian 4D flow for portal venous flow measurement, which needs to be verified in a larger series. This could be due to the fact that both cine 2DPC and spiral 4D flow were acquired in a breath hold, thereby limiting motion-related blurring, while Cartesian 4D flow required respiratory triggering. Increased blurring may also explain the significantly higher flow and vessel area measured with Cartesian 4D flow, compared with spiral 4D flow. Of note, cine 2DPC resulted in lower velocity and higher area when compared to both 4D flow techniques in the portal vein, which may be due to imperfect alignment of the measurement plane at scan time. However such an error negligibly affects total flow rate measurement, for which cine 2DPC had comparable values to 4D flow.
Robust analysis is an important condition for reliable quantitative flow measurement, which should yield low inter-observer variability. For all abdominal vessels, inter-observer agreement of spiral 4D flow was similar or better than that of Cartesian 4D flow. For portal venous flow measurement, similar inter-observer agreement was found for spiral 4D flow, Cartesian 4D flow and cine 2DPC, indicating equivalent performance of the novel spiral acquisition compared to standard methods.
Acquisition times for both 4D flow techniques were significantly different. The required number of cardiac cycles was more than 10 times lower for spiral (24 heartbeats) than for Cartesian (250 heartbeats). Furthermore, dual triggering in Cartesian 4D flow lead to poor efficiency (about 35%) and longer scan time. On the other hand, spiral acquisition collected all necessary data within a breath hold without need for respiratory navigator. Such a fast acquisition could be exploited to increase spatio-temporal resolution or to acquire multiple velocity encoding to assess arterial and venous flow in separate acquisitions.
Sub-Nyquist sampling as performed in our study with an acceleration factor R=6 requires advanced reconstruction techniques, otherwise undesirable artifacts may result. Although good performance was reached with respect to flow quantification, image quality was found to be worse for spiral 4D flow. There are multiple ways to improve the image quality. First, spiral reconstruction may benefit from correction techniques (21, 22). Second, compressed sensing reconstruction may use extra regularization terms promoting angiogram (23, 24) or phase characteristic (25) properties. This could be evaluated in future studies.
There are limitations to this study. First, the spiral technique was validated in vivo against phase contrast-based methods, but not against Doppler ultrasound. Second, the small numbers in our validation cohort did not enable us to compare between healthy subjects and patients with liver disease. Finally, the reconstruction algorithm efficiency should be improved in order to provide images at scan time. This could be addressed by exploiting recent advances in parallel computing for MR imaging (26).
In conclusion, we have demonstrated that 4D flow data collection can be reduced down to a breath hold, by implementing efficient accelerated spiral sampling coupled with dynamic compressed sensing reconstruction. In abdominal vessels, good vascular conspicuity was observed, although with decreased image quality, and quantitative parameters derived from the novel technique were in strong agreement with established phase contrast techniques such as cine 2D phase contrast and Cartesian 4D flow.
Supplementary Material
Advances in knowledge
Efficient data acquisition for 4D flow MR imaging in the abdomen can be achieved in a single breath-hold using a combination of accelerated spiral sampling and dynamic compressed sensing reconstruction.
Flow parameters measured in major abdominal vessels were in strong agreement with Cartesian 4D flow and cine 2D phase contrast MR imaging techniques, while reducing the acquisition time from 10 min down to a breath hold.
Implications for patient care
The proposed technique allows integration of a fast 4D flow protocol in a clinical setup, thereby providing a comprehensive hemodynamic assessment of multiple abdominal vessels with minimal impact on scan time.
Summary statement
Combining highly efficient spiral sampling with dynamic compressed sensing results in major acceleration for 4D flow MR imaging, and allows comprehensive assessment of abdominal vessel hemodynamics in a breath hold.
Acknowledgments
Grant support: NIDDK Grant 1R01DK087877
References
- 1.Paulson EK, Kliewer MA, Frederick MG, Keogan MT, DeLong DM, Nelson RC. Doppler US measurement of portal venous flow: variability in healthy fasting volunteers. Radiology. 1997;202(3):721–4. doi: 10.1148/radiology.202.3.9051024. [DOI] [PubMed] [Google Scholar]
- 2.Hara AK, Burkart DJ, Johnson CD, et al. Variability of consecutive in vivo MR flow measurements in the main portal vein. AJR Am J Roentgenol. 1996;166(6):1311–5. doi: 10.2214/ajr.166.6.8633438. [DOI] [PubMed] [Google Scholar]
- 3.Markl M, Chan FP, Alley MT, et al. Time-resolved three-dimensional phase-contrast MRI. J Magn Reson Imaging. 2003;17(4):499–506. doi: 10.1002/jmri.10272. [DOI] [PubMed] [Google Scholar]
- 4.Stankovic Z, Csatari Z, Deibert P, et al. Normal and altered three-dimensional portal venous hemodynamics in patients with liver cirrhosis. Radiology. 2012;262(3):862–73. doi: 10.1148/radiol.11110127. [DOI] [PubMed] [Google Scholar]
- 5.Stankovic Z, Frydrychowicz A, Csatari Z, et al. MR-based visualization and quantification of three-dimensional flow characteristics in the portal venous system. J Magn Reson Imaging. 2010;32(2):466–75. doi: 10.1002/jmri.22248. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson J, Carlhall CJ, Dyverfeldt P, Engvall J, Bolger AF, Ebbers T. Semi-automatic quantification of 4D left ventricular blood flow. J Cardiovasc Magn Reson. 2010;12:9. doi: 10.1186/1532-429X-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frydrychowicz A, Wieben O, Niespodzany E, Reeder SB, Johnson KM, Francois CJ. Quantification of thoracic blood flow using volumetric magnetic resonance imaging with radial velocity encoding: in vivo validation. Invest Radiol. 2013;48(12):819–25. doi: 10.1097/RLI.0b013e31829a4f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nordmeyer S, Riesenkampff E, Crelier G, et al. Flow-sensitive four-dimensional cine magnetic resonance imaging for offline blood flow quantification in multiple vessels: a validation study. J Magn Reson Imaging. 2010;32(3):677–83. doi: 10.1002/jmri.22280. [DOI] [PubMed] [Google Scholar]
- 9.Valverde I, Nordmeyer S, Uribe S, et al. Systemic-to-pulmonary collateral flow in patients with palliated univentricular heart physiology: measurement using cardiovascular magnetic resonance 4D velocity acquisition. J Cardiovasc Magn Reson. 2012;14:25. doi: 10.1186/1532-429X-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markl M, Harloff A, Bley TA, et al. Time-resolved 3D MR velocity mapping at 3T: improved navigator-gated assessment of vascular anatomy and blood flow. J Magn Reson Imaging. 2007;25(4):824–31. doi: 10.1002/jmri.20871. [DOI] [PubMed] [Google Scholar]
- 11.Johnson KM, Lum DP, Turski PA, Block WF, Mistretta CA, Wieben O. Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Magn Reson Med. 2008;60(6):1329–36. doi: 10.1002/mrm.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigfridsson A, Petersson S, Carlhall CJ, Ebbers T. Four-dimensional flow MRI using spiral acquisition. Magn Reson Med. 2012;68(4):1065–73. doi: 10.1002/mrm.23297. [DOI] [PubMed] [Google Scholar]
- 13.Hsiao A, Lustig M, Alley MT, Murphy MJ, Vasanawala SS. Evaluation of valvular insufficiency and shunts with parallel-imaging compressed-sensing 4D phase-contrast MR imaging with stereoscopic 3D velocity-fusion volume-rendered visualization. Radiology. 2012;265(1):87–95. doi: 10.1148/radiol.12120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelc NJ, Bernstein MA, Shimakawa A, Glover GH. Encoding strategies for three-direction phase-contrast MR imaging of flow. J Magn Reson Imaging. 1991;1(4):405–13. doi: 10.1002/jmri.1880010404. [DOI] [PubMed] [Google Scholar]
- 15.Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47(6):1202–10. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 16.Feng L, Otazo R, Jung H, et al. Accelerated cardiac T2 mapping using breath-hold multiecho fast spin-echo pulse sequence with k-t FOCUSS. Magn Reson Med. 2011;65(6):1661–9. doi: 10.1002/mrm.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim D, Dyvorne HA, Otazo R, Feng L, Sodickson DK, Lee VS. Accelerated phase-contrast cine MRI using k-t SPARSE-SENSE. Magn Reson Med. 2011 doi: 10.1002/mrm.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn Reson Med. 2010;64(3):767–76. doi: 10.1002/mrm.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker PG, Cranney GB, Scheidegger MB, Waseleski G, Pohost GM, Yoganathan AP. Semiautomated method for noise reduction and background phase error correction in MR phase velocity data. J Magn Reson Imaging. 1993;3(3):521–30. doi: 10.1002/jmri.1880030315. [DOI] [PubMed] [Google Scholar]
- 20.Gulsun MATH. Segmentation of carotid arteries by graph-cuts publications using centerline models. SPIE. 2010:7625. [Google Scholar]
- 21.Chen W, Meyer CH. Semiautomatic off-resonance correction in spiral imaging. Magn Reson Med. 2008;59(5):1212–9. doi: 10.1002/mrm.21599. [DOI] [PubMed] [Google Scholar]
- 22.Robison RK, Devaraj A, Pipe JG. Fast, simple gradient delay estimation for spiral MRI. Magn Reson Med. 2010;63(6):1683–90. doi: 10.1002/mrm.22327. [DOI] [PubMed] [Google Scholar]
- 23.Thompson RB, McVeigh ER. Real-time volumetric flow measurements with complex-difference MRI. Magn Reson Med. 2003;50(6):1248–55. doi: 10.1002/mrm.10637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwak Y, Nam S, Akcakaya M, et al. Accelerated aortic flow assessment with compressed sensing with and without use of the sparsity of the complex difference image. Magn Reson Med. 2012 doi: 10.1002/mrm.24514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao F, Noll DC, Nielsen JF, Fessler JA. Separate magnitude and phase regularization via compressed sensing. IEEE Trans Med Imaging. 2012;31(9):1713–23. doi: 10.1109/TMI.2012.2196707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen MS, Sorensen TS. Gadgetron: An open source framework for medical image reconstruction. Magn Reson Med. 2012 doi: 10.1002/mrm.24389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.