Abstract
The huKS-IL2 immunocytokine (IC) consists of IL2 fused to a mAb against EpCAM, while the hu14.18-IL2 IC recognizes the GD2 disialoganglioside. They are under evaluation for treatment of EpCAM+ (ovarian) and GD2+ (neuroblastoma and melanoma) malignancies because of their proven ability to enhance tumor cell killing by antibody-dependent cell-mediated cytotoxicity (ADCC) and by antitumor cytotoxic T cells. Here, we demonstrate that huKS-IL2 and hu14.18-IL2 bind to tumor cells via their antibody components and increase adhesion and activating immune synapse (AIS) formation with NK cells by engaging the immune cells’ IL-2 receptors (IL2R). The NK leukemia cell line, NKL (which expresses high affinity IL2Rs), shows fivefold increase in binding to tumor targets when treated with IC compared to matching controls. This increase in binding is effectively inhibited by blocking antibodies against CD25, the α-chain of the IL2R. NK cells isolated from the peritoneal environment of ovarian cancer patients, known to be impaired in mediating ADCC, bind to huKS-IL2 via CD25. The increased binding between tumor and effector cells via ICs is due to the formation of AIS that are characterized by the simultaneous polarization of LFA-1, CD2 and F-actin at the cellular interface. AIS formation of peritoneal NK and NKL cells is inhibited by anti-CD25 blocking antibody and is 50–200% higher with IC versus the parent antibody. These findings demonstrate that the IL-2 component of the IC allows IL2Rs to function not only as receptors for this cytokine but also as facilitators of peritoneal NK cell binding to IC-coated tumor cells.
Keywords: Immunocytokines, Natural killer, Cancer, Immunotherapy, Immune synapse
Introduction
Interleukin-2 (IL-2) is a lymphocytotrophic cytokine that augments helper and cytotoxic T cell as well as natural killer (NK) cell-mediated antitumor immune responses [1]. High levels of IL-2 in the tumor mass are indicative of increased immune cell infiltration which in turn is associated with positive clinical outcomes in cancer patients [2, 3]. IL-2 was therefore proposed as an immunotherapeutic agent for the treatment of various types of tumors [4, 5]. However, systemic administration of high amounts of IL-2, to induce immune-mediated activity against the tumor causes significant toxicity [6, 7]. The immunocytokine approach was developed to enable tumor-reactive mAbs to initiate antitumor immune responses via ADCC while also delivering IL-2 directly into the tumor microenvironment [8, 9].
Immunocytokines (ICs) are recombinant fusion proteins consisting of cytokine molecules, like IL-2, linked in most but not all cases, to the heavy chains of tumor-reactive mAbs. ICs that can bind to antigens expressed on the surface of cancer cells are being investigated for their antitumor effects. Prominent antitumor ICs that have been developed and studied in vitro and in vivo (mouse models and humans) include huKS-IL2 (anti-EpCAM), hu14.18-IL2, L19-IL2, T84.66-IL2 and scFv23-TNF [10–20]. huKS-IL2 and hu14.18-IL2 are constructed using humanized anti-EpCAM (huKS) and humanized anti-disialoganglioside GD2 (14.18) antibodies, respectively [19, 20]. For huKS-IL2 and hu14.18-IL2, a human IL-2 molecule is genetically linked to the carboxy terminus of each IgG heavy chain making the intact molecule bivalent with respect to IL-2. huKS-IL2 and hu14.18-IL2 are being tested in clinical trials in EpCAM positive cancers and in neuroblastoma and melanoma patients, respectively [12, 21]. Antitumor activity has been seen in children with neuroblastoma receiving hu14.18-IL2 in phase II clinical testing [22], and clinical efficacy has been demonstrated for an anti-GD2 antibody combined with IL2 and GM-CSF, when used for neuroblastoma patients in remission [23].
Treatment with ICs results in activation of T cell-mediated antitumor cytotoxicity [24] and in NK cell-mediated control of tumor growth [25, 26]. The antibody component of the IC, upon binding to antigens on the tumor cells, activates NK cells via CD16-Fc interaction [27]. Consequently, tumor cell lysis occurs via ADCC [28]. However, deglycosylated or Fc-epitope lacking ADCC-incompetent ICs are also able to mediate increased tumor cell lysis. Previous studies have demonstrated that ICs may mediate tumor cell lysis, presumably via ADCC-independent mechanisms [18, 29, 30]. In a recently published study [31], we have demonstrated that NK cell lines lacking CD16 but expressing high levels of the high affinity IL2R receptor are able to form an increased number of conjugates with GD2pos target cells in the presence of the hu14.18-IL2 IC, whereas only a basal level of such heterotypic cell–cell interactions were observed upon treatment with the hu14.18 antibody or with simultaneous addition of hu14.18 antibody and IL2. Furthermore, we have also demonstrated that the increased IC-mediated NK-tumor cell conjugations were highly productive as they resulted in a higher level of lysis of the target cells [31].
The ability of the IC to engage the IL2R in conjugating cytotoxic immune cells with tumor cells and thereby increase cytolysis of the targets is likely to be physiologically relevant. NK cells isolated from ovarian cancer patients are deficient in the Fc receptor, CD16, but exhibit increased tumor cell killing in the presence of huKS-IL2 in ex vivo experiments [10, 32]. The huKS-IL2-IL2R interactions with IL2R on these patients’ NK cells, similar to those observed in experiments with the CD16neg NK cell lines [31], may allow the ovarian tumor patients’ CD16-defecient NK cells to more effectively lyse the tumor targets when coated with IC.
Recognition of target cells by NK cells via natural cytotoxicity mechanisms requires the formation of an immune synapse at the site of contact between the effector and tumor cells [33]. NK targeting of tumor cells via ADCC also occurs through the formation of an immune synapse [34]. We therefore hypothesized that after the IC binds to tumor cells via the antibody, it next binds to effector cells via an IL-2/IL-2 receptor (IL2R) interaction. The IL2R then induces activating immune synapse (AIS) formation leading to lysis of the cancer cells via an IL-2 receptor-dependent mechanism.
Here, we demonstrate that tumor cells and NK cells can conjugate through the IL2 receptor when treated with IC. In addition, treatment with IC increases activating immune synapses (AIS) between tumor cells and NK cells. These AIS are formed through the interaction of the IL-2 component of the IC and the IL2R on the NK cell. IC and IL2R are then polarized at the interface between immune cells and targets, indicating that the IL2R can play a role in cell adhesion and polarized cell activation when it encounters the IL2 component of the IC bound to the surface of the target cell. These novel findings elucidating the adhesive and polarizing properties of the IL2R and its ability to contribute to cell–cell conjugate formation may have important implications in how ICs can be used in cancer treatment.
Materials and methods
Cell culture and immunocytokines
OVCAR-3 cells were obtained from ATCC and M21 and NKL cells were kind gifts from Drs. Ralph Reisfeld and Jenny Gumperz, respectively. huKS mAb, huKS-IL2 IC, hu14.18 mAb and hu14.18-IL2 IC were provided by The EMD-Lexigen Research Center (now EMD-Serono) in Billerica MA and were used at 0.25 μg/ml in all experiments. IL-2 (Proleukin, Chiron) was used at 750 U/ml and anti-CD25 blocking antibody (clone GL439) used in all experiments was 10 μg/ml. Unless noted otherwise, statistical analysis of all data was conducted using Mann–Whitney tests and graphs were plotted using the GraphPad Prism software.
Slide preparation and analysis for confocal microscopy
Target cells (7.5 × 104 cells) were mixed with effector cells at a 1:1 ratio in 400 μl phosphate buffered saline containing 1% bovine serum albumin (PBS-BSA) along with the IC, antibodies, IL-2, or CD25 blocking antibody. The cells were centrifuged for 5 min at 100×g and incubated at 37°C at 5% CO2 for 25 min. After incubation, the cells were fixed with 3% paraformaldehyde on poly-L-lysine coated coverslips, washed twice with 150 mM glycine, permeabilized with 0.1% Triton-X for 4 min and blocked overnight or for 30 min with PBS containing 5% goat serum. Some coverslips were stained (on separate coverslips) with murine antibodies, LFA-1 (1:200), CD2 (1:200) and antihuman CD25 non-blocking antibody (clone HB8784, 1 μg) in 5% goat serum or with a cocktail of goat anti-mouse FITC (1:300, Jackson ImmunoResearch), and rhodamine conjugated phalloidin (1:1,000, Invitrogen) for 30 min. Coverslips were washed, stained with DAPI, mounted in mounting media (Invitrogen), dried overnight and visualized using the Bio-Rad Radiance 2,100 MP Rainbow confocal microscope. Fifty conjugates between effector and target cells were counted on each coverslip. Each conjugate was scored for polarization at the contact interface for molecules LFA-1 and F-actin, CD2 and F-actin, CD25, or 14.18-IL2-FITC. Percent AIS formation was determined as: [(number of conjugates showing polarization of molecules/total conjugates) ×100].
Plate adhesion assays
NKL (50,000 cells/well) cells labeled with Calcein AM were added to confluent cultures of OVCAR-3 or M21 tumor targets under the different control and test conditions. After 30 min incubation, the wells were washed twice with PBS-BSA and fluorescence of target cell-bound NKL were quantified using the Victor V-3 plate reader (Perkin Elmer).
Flow cytometry conjugate formation assays
OVCAR-3 and NKL cells were labeled with 0.5 M Cell-Tracker Red (Invitrogen) and 1.25 pM CellTracker Green (Invitrogen), respectively. In other experiments involving M21 and NKL, the cells were labeled with BODIPY 630/650, and CFSE, respectively. The tumor targets and NKL cells were mixed at 1:1 ratio in the presence of IC or other specified test and control conditions. After 30 min incubation, tumor cell–NKL cell conjugation was measured by flow cytometry as described in our previous study [35].
NK cells from patients and healthy donors
Peripheral blood mononuclear cells from health donors and from ovarian cancer patients, and tumor associated NK cells from the peritoneal fluid of patients with ovarian cancer were obtained and purified for in vitro analyses as reported previously [32, 36]. All peripheral blood and peritoneal fluid samples were obtained with approval of the UW Health Sciences Human Subjects committee.
IC binding to patient NK cells
Previously frozen peritoneal fluid mononuclear cells were incubated with Goat IgG for 15 min and then with or without huKS-IL2 in PBS-BSA for 10 min. FITC-conjugated anti-CD25 antibody (clone 2A3, BD Biosciences, 20 μl/test) was added to some tubes and incubated for 10 min. After washing, the cells were labeled with fluorophore conjugated anti-NKp46, CD16, CD56 and CD3 to distinguish NK cells and samples were analyzed for anti-CD25 binding. Four patient samples were used in this analysis. A matched paired t test was conducted for statistical analysis using the statistical program JMP.
Results
Immunocytokines increase NK AIS formation
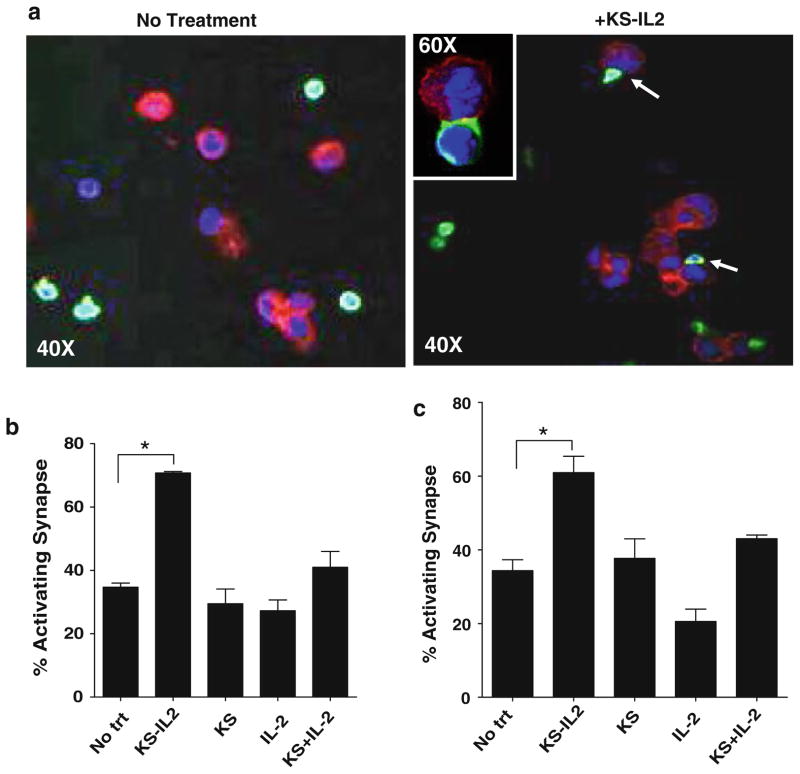
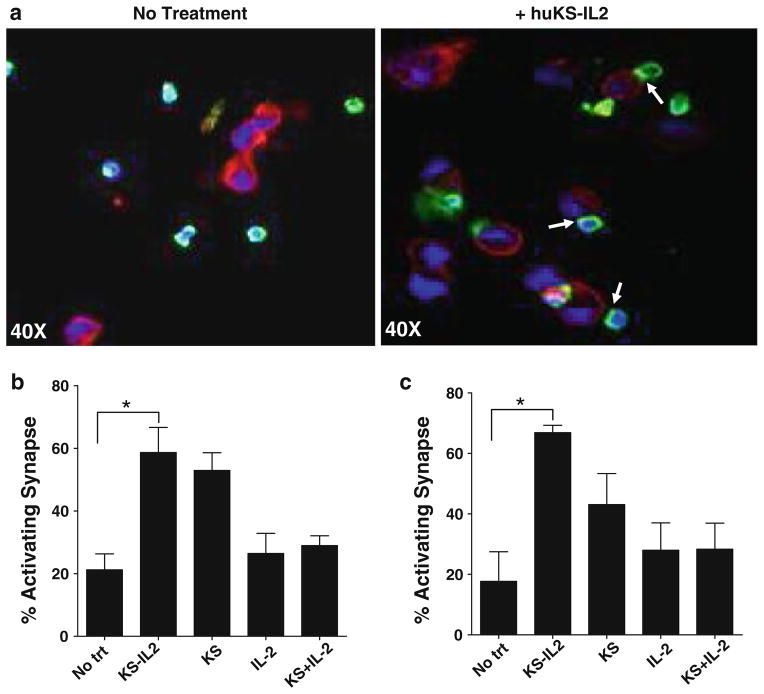
Naive NK cells isolated from the peripheral blood of healthy donors (Fig. 1) and ovarian cancer patients (Fig. 2) formed twofold higher numbers of AIS with the ovarian tumor cell line, OVCAR-3, in the presence of the IC huKS-IL2 as compared to the no treatment controls (Figs. 1a and 2a). Experiments conducted in the presence of huKS-IL2, huKS antibody, IL2, or huKS + IL2 as separate molecules and stained for activating synapse markers LFA-1 and CD2 are shown in Figs. 1b and 2b (LFA-1), and Figs. 1c and 2c (CD2). Staining with F-actin is not shown. Cell conjugates in the presence of huKS-IL2 were polarized more extensively at the IC-mediated synapses as compared to the no treatment controls (Figs. 1b, c and 2b, c). Polarization of LFA-1, CD-2 and F-actin are features that have been well documented with NK synapses formed via the engagement of activating receptors and their corresponding ligands, and confirms these are AIS [37]. No significant increase in AIS over the no treatment controls was observed when experiments with NK cells from healthy donors and ovarian cancer patients were conducted in the presence of the huKS antibody, IL-2, and huKS + IL-2 (Figs. 1b, c and 2b, c).
Fig. 1.
huKS-IL2 increases AIS between primary NK cells and ovarian tumor cells. a Naïve NK cells from peripheral blood of healthy donors were incubated with OVCAR-3 cells in the presence of IC (+huKS-IL2) or absence (no treatment). Cells were fixed onto coverslips and stained for AIS markers LFA-1, CD2 and F-actin. Red = F-actin, Blue = nucleus, Green = LFA-1. OVCAR-3 cells are seen stained mainly as cells with red cytoplasm, while the smaller NK cells are identified by their LFA-1 (green) expression. When huKS-IL2 is present (Right photomicrographs in a “+huKS-IL2”) several NK cells are seen forming synapses with the OVCAR-3 cells. Increased density of LFA-1 is seen at sites of AIS formation (white arrows). Inset in a demonstrates a higher power (60× + zoom) view of an AIS. Pictures were taken using a confocal microscope at 40×. Pictures are representative of four separate donors. AIS formation was quantified by counting NK-OVCAR-3 conjugates showing polarization of LFA-1 (b) or CD2 (c). AIS formation was determined when NK cells and OVCAR-3 cells were incubated in media (no treatment) or media containing huKS-IL2, huKS, IL-2, or huKS + IL2. Percentage of activating synapses was determined as described in Methods. Quantification of coverslips by fluorescent microscopy, shown in the bar graphs, indicates increased AIS when huKS-IL2 is added. Bar graphs are mean of data obtained using NK cells from four individual healthy donors. *P <0.05
Fig. 2.
Enhanced IC-mediated AIS formation between targets and naïve NK cells from peripheral blood of ovarian cancer patients. Naïve NK cells were isolated from peripheral blood of four ovarian cancer patients at the time of diagnosis of the disease. All experiments were conducted similar to those with NK cells from healthy donors (Fig. 1). Photomicrographs (a) show increased AIS formation in the presence of huKS-IL2. Polarization of LFA-1 (b) and CD2 (c) were used as indicators of AIS formation. All other details are same as those provided in the legend for Fig. 1. *P <0.05
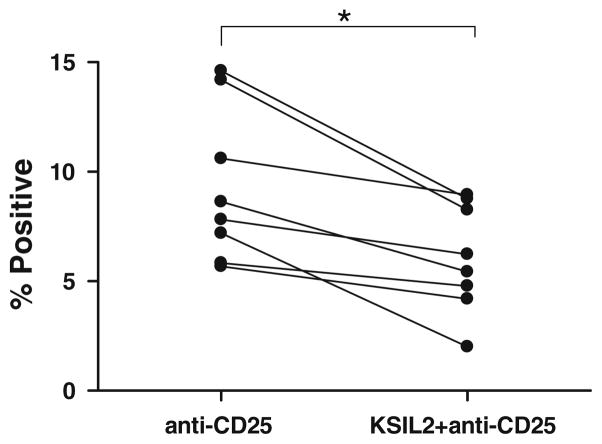
IC binds to peritoneal NK cells of ovarian cancer patients via CD25
The demonstration that huKS antibody (rather than the huKS-IL2 IC) facilitated only a basal level of AIS formation between healthy donor or ovarian cancer patient NK cells and tumor targets (bar graphs for LFA-1 and CD2 in Figs. 1b and 2b and for CD2 in Figs. 1c and 2c) suggested that the Fc receptors (CD16) on those NK cells were not actively using the Fc of the huKS mAb to bind to the tumor cells and create AIS. Furthermore, this also suggested that only a small component of the augmented AIS formation induced by huKS-IL2 in those same experiments occurred via the engagement of CD16 and the Fc portion of the huKS-IL2. Data obtained in our recent study [31] suggest that the increased AIS formation mediated by huKS-IL2 is likely through CD25, the α-chain of the IL2R. We therefore investigated whether the IC could bind to NK cells isolated from the peritoneal fluid of ovarian cancer patients, via interaction with CD25 as these cells are known to be deficient in CD16 (56). In a flow cytometry binding assay, anti-CD25 antibody (clone 2A3, a blocking antibody that inhibits the binding of IL2 to CD25) was excluded from binding to peritoneal NK cells when co-incubated with huKS-IL2 (Fig. 3). These results suggested a direct interaction between the IL-2 component of huKS-IL2 and CD25 expressed on the CD16low peritoneal NK cells from ovarian cancer patients (Fig. 3).
Fig. 3.
NK cells from the peritoneal fluid of ovarian cancer patients can bind to huKS-IL2 via CD25. Pretreatment with huKS-IL2 decreased the amount of binding of anti-CD25 mAb to the cell surface, indicating that huKS-IL2 was binding to patient peritoneal fluid NK cells via CD25. NK cells were gated using CD16, CD56 and NKp46. Four patient samples in duplicate (total of eight points per treatment group) are shown in this figure. Statistical analysis was conducted using the matched pair t test. *P <0.05
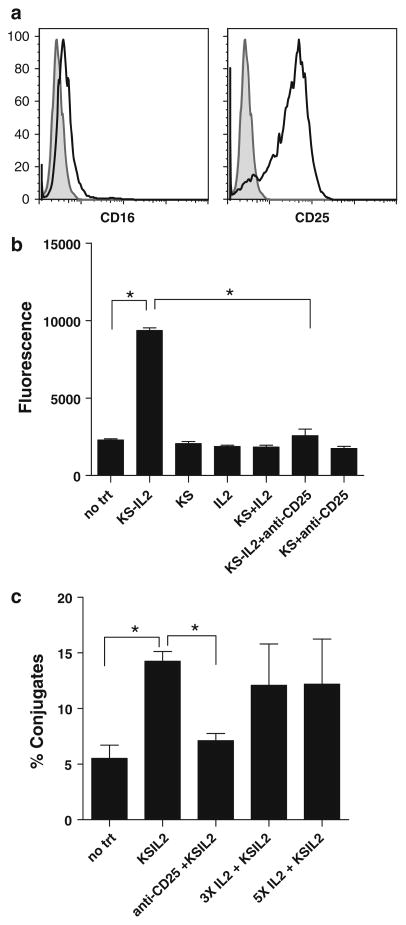
IC-CD25 interaction facilitates AIS NK-tumor cell conjugation
The NK cell leukemia cell line NKL expresses very low levels of CD16 (mean fluorescence intensity (MFI) = 1.2 ± 0.4, determined from four independent experiments) but are highly positive for CD25 (Fig. 4a) [38]. The NKL cells were therefore utilized as a model to study IC–CD25 interactions and their potential role in AIS formation as these cells mimic the CD16-deficient effector cells found in cancer patients [32]. Microtiter plate-based cell adhesion experiments with NKL cells and OVCAR-3 cells in the presence of huKS-IL2 showed a 4–5-fold increase in cell binding as compared to test samples that were treated with huKS mAb, or huKS mAb in combination with IL-2 (Fig. 4b). This cell binding that was facilitated by huKS-IL2 was inhibited to near-baseline levels by the addition of anti-CD25 mAb (Fig. 4b), demonstrating the role of the IL2R in this cell binding. Separate flow cytometry conjugation assays were also performed and demonstrate comparable results with huKS-IL2 (Fig. 4c). The addition of huKS-IL2 caused a threefold increase in detection of hetero-cell conjugates. Once again, IC-facilitated conjugation between NKL and OVCAR-3 cells was nearly abrogated by the anti-CD25 blocking antibody (Fig. 4c).
Fig. 4.
NKL cells are a suitable model to study the role of CD25 in IC binding and exhibit increased binding to targets via CD25 in flow cytometry and plate adhesion assays. a CD25 (IL2 receptor alpha chain), and CD16 expression were analyzed by flow cytometry. Shaded area is isotype control. b Fluorescently labeled NKL cells added to confluent, plated OVCAR-3 cells in the presence of the indicated reagents, and then washed off. The residual fluorescence detected is a measure of adherent NKL cells. Increased binding was seen only with huKS-IL2, and this was inhibited with anti-CD25 blocking antibody. c OVCAR-3 and NKL cells were stained with two different cell tracker dyes, and incubated together in the presence of huKS-IL2, anti-CD25 with huKS-IL2, or excess IL2 with huKS-IL2. Cell conjugates were measured using flow cytometry. *P <0.05
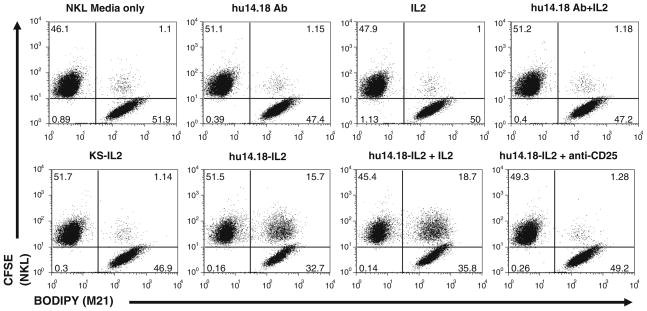
Cell conjugation via IC-CD25 interaction was also observed with the hu14.18-IL2 IC that targets the GD2 epitope. A flow cytometry cell conjugation assay demonstrated increased cell conjugation between NKL cells and M21 melanoma cells in the presence of hu14.18-IL2 (Fig. 5). Anti-CD25 blocking antibody inhibited cell conjugation to the level that was observed with hu14.18 alone (Fig. 5). Specificity was further demonstrated by the result that huKS-IL2 was unable to mediate conjugate formation between the M21 melanoma cells (negative for EpCAM expression) and NKL cells (Fig. 5). Separate studies (not shown) also demonstrated that the hu14.18-IL2 immuno-cytokine facilitated much better NKL cell binding to GD2+ tumor cells in the plate-based assay (as in Fig. 4b) than did IL2 or the hu14.18 mAb.
Fig. 5.
14.18IL2 IC increases conjugate formation between M21 and NKL cells and can be blocked with an anti-CD25 mAb in flow cytometry conjugate assays. M21 and NKL cells were dyed with BODIPY and CFSE, respectively, and incubated together with the depicted treatments and analyzed for conjugate formation. huKS-IL2 was added to demonstrate specificity of the hu14.18-IL2 IC. Pretreatment with blocking anti-CD25 mAb almost completely abrogates conjugate formation. Numbers in dot plots indicate percentage of total counted events in each quadrant, with the upper right quadrant indicating 2-color conjugate events. The results are representative of three independent experiments
CD25 is polarized at the IC-mediated AIS
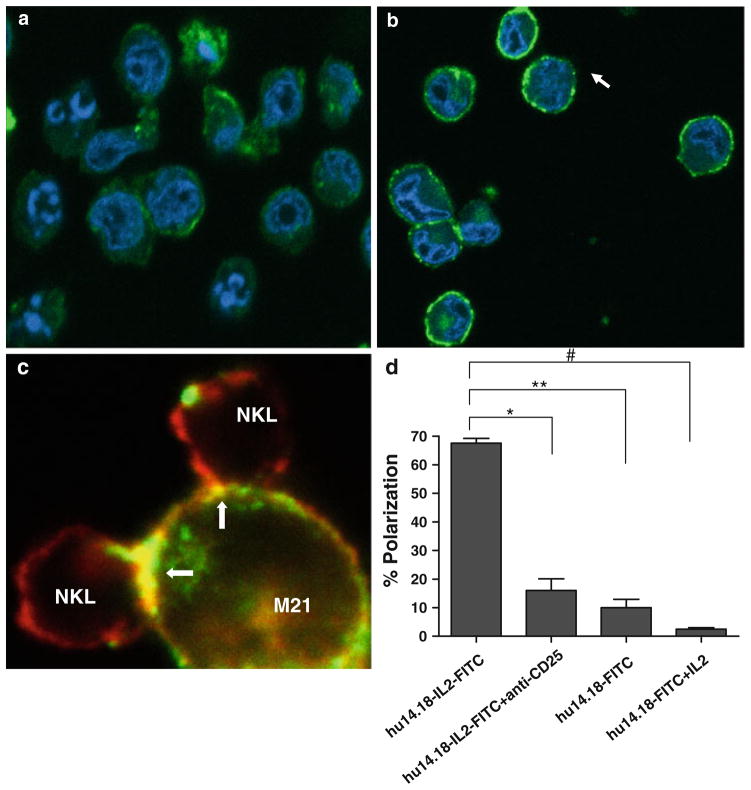
We investigated the involvement of CD25 in the formation of the IC-mediated immune synapse. NKL cells and/or M21 cells were incubated with hu14.18-IL2 that had been directly conjugated with FITC (14.18-IL2-FITC). After 25 min at 37°C, the cells were fixed and examined by confocal microscopy. The monocultures of NKL cells (Fig. 6a) show weak ring fluorescence of the hu14.18-IL2-FITC, indicating that the IL2 receptors on the NKL cells that are binding the IC are distributed evenly on the NKL surface. In Fig. 6a, we also note some low level internalization of the 14.18-IL2-FITC in the NKL cells, consistent with our recent report of this same observation made by 2-color flow cytometry studies [31]. Monocultures of M21 cells cultured with 14.18-IL2-FITC show “ring fluorescence” (Fig. 6b) indicating that the hu14.18-IL2-FITC is binding to GD2 distributed over the entire cell surface on the cell surface. In contrast, the NKL-M21 conjugates formed after treatment with hu14.18-IL2-FITC show significant polarization of the IC at the immune synapse (Fig. 6c). The quantitation of the 14.18-IL2-FITC polarization for M21 + NKL co-cultures, with and without anti-CD25 mAb is shown in Fig. 6d. Note that polarization of 14.18-IL2-FITC was strongly inhibited by the anti-CD25 blocking antibody (Fig. 6d). In parallel, comparable analyses were done with FITC-conjugated hu14.18 mAb (14.18-FITC). Minimal polarization of the 14.18-FITC was observed at the site of contact between NKL and M21 cells, even when soluble IL2 was added (Fig. 6d).
Fig. 6.
hu14.18-IL2 is polarized between NKL and M21 cells and polarization is blocked with anti-CD25 blocking antibody. a NKL, b M21 cells, or c co-cultures of NKL and M21 cells were incubated with hu14.18-IL2FITC (as well as other treatments not pictured) for 25 min and then fixed onto a glass coverslip and visualized using confocal microscopy. Images were taken at 40×. Representative images of five separate experiments are shown in a–c. Data in c is a merged image showing 14.18-IL2-FITC (green) and actin (red) staining. Polarization of 14.18-IL2-FITC and actin is observed (yellow stain and yellow arrow) at the AIS between NKL and M21 cells. d Percent polarization was calculated by dividing the number of conjugates that displayed either hu14.18-IL2-FITC or hu14.18-FITC polarized at the synapse between two conjugates divided by the total number of conjugates. IC on the graph denotes hu14.18-IL2- FITC, while Ab denotes hu14.18-FITC. *P <0.03
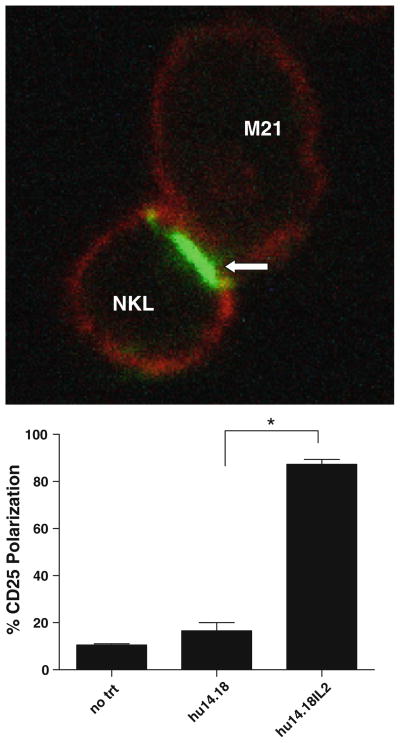
This polarization of 14.18-IL2-FITC (Fig. 6d) suggested that the activating synapse between the NKL cells and the M21 cells involves a localization of the IL2 receptors present on the NKL cell surface. This was confirmed directly by staining NKL-M21 conjugates for the CD25 molecules (Fig. 7). NKL cells were co-incubated with M21 cells and either hu14.18-IL2 or hu14.18 mAb. They were then fixed and counterstained for actin and for CD25 (visualized by using non-blocking anti-CD25 antibodies). When the cells were incubated with hu14.18 mAb, both NKL and M21 cells showed cytoskeletal red-actin staining, and the nonconjugated NKL cells showed “rim-fluorescence” of their CD25 while the M21 cells were CD25 negative. In contrast, when the cells were incubated with hu14.18-IL2, virtually all of the CD25 seen on the NKL cells was localized to the IC-mediated NKL-M21 synapse (Fig. 7a). The level of polarization was quantified by visual enumeration, and only marginal CD25 polarization was observed when NKL and M21 were treated with 14.18 antibody compared to the polarization induced by hu14.18-IL2 (Fig. 7b).
Fig. 7.
CD25 polarizes at the immune synapse. a M21 (melanoma) cells and NKL cells were incubated together with either no treatment, hu14.18, or hu14.18-IL2 for 25 min. Cells were fixed onto a glass coverslip, stained for CD25 (green) using a non-blocking antibody. Cells were visualized using confocal microscopy. Arrow indicates polarization of CD25 between NKL and M21 cells. Images were taken at 40× and digitally zoomed. b Polarization of CD25 was quantified by dividing the number of conjugates between NKLs and M21 s where CD25 was polarized at the synapse by the total number of conjugates. *P < 0.02
Discussion
The data presented in this study conclusively demonstrate a role for CD25 in mediating attachment and formation of AIS by NK cells when these NK cells encounter tumor cells that display cell surface-bound IL2 that was delivered by the tumor-reactive IC. As such, the IC functions as a cross-linking agent. ICs have already been shown to mediate stable conjugate formation between T cells and tumor targets via IL2R [30]. We have also studied the kinetics of IC-IL2R binding and demonstrated that the IC-IL2R complex is rapidly internalized by the NKL cells [31]. Our studies further confirm that ICs presented on the target cells serve as ligands of IL2R and also indicate that IC-facilitated conjugation of NK cells with cancer cells may serve as an important mechanism in the therapeutic control of tumor growth.
NK cells play an important role in the elimination of tumor cells by ADCC as well as natural cytotoxicity (via NKG2D, DNAM-1, and other activating receptors) mechanisms [39–41]. NK cell-mediated killing of tumor targets via both ADCC and natural cytotoxicity mechanisms is enhanced when the effector cells are activated by IL-2 treatment. IL-2 treated NK cells have classically been referred to as lymphokine activated killer (LAK) cells due to their high potential in lysing tumor targets [42]. In the extensive literature available on IL-2 stimulation of NK cells, the mechanism for activation involves the binding of IL-2 to its receptor on the NK cells thereby triggering an activating signaling cascade.
CD25 is the alpha chain of the IL-2 receptor and by itself does not have any signaling capacity [43]. However, on the immune cell surface, CD25 is associated with the beta (CD122) and gamma chains of the IL-2 receptor that have the signaling motifs [44, 45]. Furthermore, CD25 has a very low affinity for IL-2 [46–48]. However, the trimeric IL-2 receptor (CD25 along with the beta and gamma chains) is a high affinity IL-2 receptor. Treatment with IC leads to stimulation of the NK cells via IL-2 thereby enhancing their antitumor responses [49]. Our experiments with NK cells indicate that IC-facilitated binding of effector cells to tumor targets is significantly inhibited by anti-CD25 antibodies that block the recognition of IL-2 by this receptor. Comparable results were also obtained with NKL cells, which are virtually lacking Fc receptors. Therefore, in the presence of tumor cells that have been coated with IC, these NK and NKL cells are coming in contact with a cell surface that is now expressing a lattice of surface-bound IL2. In this setting, the IL2Rs of the NK and NKL cells bind to the tumor cell-bound IL2 and act initially as adhesion molecules, facilitating contact between NK and tumor cells via the IC.
The divalent presentation of IL2 on each IC molecule, and the presentation of cell-bound IL2 on the IC-labeled tumor cells may allow the CD25 molecules on the NK and NKL cells to bind to the IL2 fixed to the tumor cell with significantly greater affinity than that for soluble IL-2 [50]. This theoretical higher affinity of cell-bound IC (versus soluble IL2) to CD25 is consistent with our cell adhesion blocking experiments, where an equimolar amount of excess soluble IL-2 was unable to inhibit IC-mediated binding between NKL cells and M21 (hu14.18-IL2 + IL2 in lower panel of Fig. 5). Similarly, a 3–5-fold excess of soluble IL2 does not inhibit conjugation of NKL cells to huKS-IL2 labeled OVCAR-3 cells (Fig. 4c). In contrast, almost complete inhibition of IC-facilitated NKL-tumor cell binding was observed when the anti-CD25 antibody was used as the blocking agent (Figs. 4b, c and 5), indicating that soluble IL-2 is not a strong competitor of the CD25 interaction between the IL2Rs on NK and NKL cells, and the cell-bound IL2 that IC has delivered to the tumor cell surface. This observation may help explain why in vivo administration of IC is so much more effective in eradicating tumor than is comparable amounts of mono-clonal antibody + soluble IL2 [49]. The IL2 component of the tumor-bound IC may attract effector cells via conjugate formation using CD25 as an adhesion molecule (as seen in vitro in Figs. 4 and 5). Furthermore, recruitment of immune cells to the tumor bed following IC therapy may lead to an increase in IL-2 synthesis and release at the tumor site. However, the secreted soluble IL-2, because of its lower affinity for the IL-2 receptor, is not likely to effectively compete with the tumor cell-bound IC, thereby enabling the IC to continue to recruit effectors to the tumor site [31].
Formation of the AIS is essential for NK cells to mediate lysis via their activating receptors [33, 51]. Lysis of target cells by ADCC is also associated with immune synapse formation [34, 52]. The AIS is associated with polarization of LFA-1 and CD2 along with the activating receptors [37]. Our studies with fresh human NK cells show that the huKS-IL2 IC induces significantly greater AIS than does the corresponding huKS mAb, even when additional soluble IL2 is added (Fig. 1b, c). These conjugates formed by the IC show the typical phenotype of AIS, with LFA-1 and CD2 polarized to the synapse. This result suggests that the IL2 component of the IC, when fixed to the tumor surface, functions as an additional adhesive target that also induces effector polarization. Using NKL cells as a model, we demonstrate that CD25 not only facilitates binding of the effector cells to tumor targets but is also polarized to the site of the immune synapse (Fig. 7). A schematic diagram is included as Supplemental File 1, to demonstrate the relationships of GD2 distribution on M21 tumor cells, IL2R on NKL cells and the localization of FITC-conjugated hu14.18-IL2 to these M21 and NKL cells, when cultured alone, or when synapses are formed via the IC, based on the actual photomicrographs shown in Figs. 6 and 7. Separate analyses have recently demonstrated that these same NKL cells mediate greater GD2-specific killing of M21 cells when co-cultured with hu14.18-IL2 than when cultured with hu14.18 mAb + IL2 [31], indicating that the AIS facilitated between NKL and M21 cells by hu14.18-IL2 also leads to lysis. Future studies will evaluate the potential association between CD25 polarization (by cell-bound IC) and the actin cytoskeleton and WIP and WASp proteins that are required for the transport of the signaling molecules and perforin granules to the NK cell AIS [53, 54].
Studies in ovarian cancer patients have shown that NK cells isolated from the tumor microenvironment express reduced levels of the Fc receptor CD16 [32, 55]. This down-regulation of CD16 is likely brought about by the interaction of cancer antigens with tumor infiltrating NK cells [56]. NK cells from the peritoneal environment of ovarian cancer patients therefore exhibit reduced ability to mediate ADCC as compared to circulating NK cells of the same patient; however, these cells demonstrate enhanced cytotoxicity in the presence of IC (10). The data we have generated with the FcR-deficient NKL cells suggests that NK-mediated antitumor activity with IC molecules may proceed even without activation of FcRs, as the IL2 that the IC brings to the tumor cell surface can induce adhesion and activation of AISs on NK cells. Our results are also consistent with in vivo antitumor effects seen with other ICs in settings where FcR-mediated ADCC was impossible [20, 25, 29].
Our in vitro data presented here are also consistent with a recent clinical observation from our phase II study of hu14.18-IL2 in children with relapsed/refractory neuroblastoma [22]. An analysis of KIR/KIR-ligand genotype showed that antitumor-response (or clinical improvement) was associated with KIR/KIR-ligand mismatch [57]. This suggests that the clinical effect of IC in these patients is mediated by NK cells, as shown in vitro in this report. That same study [57] also evaluated for possible associations between FcγR genotypes and response. In contrast to other studies showing an association between high affinity genotypes for the FcγRIIIa receptor and clinical responses to other therapeutic mAbs [58, 59], our study of hu14.18-IL2 showed no trend toward any association of high affinity FcγRIIIa alleles and response [57]. While this lack of any trend toward association may reflect the small cohort of patients in this study, the data presented in this in vitro report provide another hypothesis. Namely, it may be that the activation of NK-tumor cell synapses via the IC may utilize either IL2Rs or FcRs (or both), thereby circumventing the potential need for (or benefit of) high affinity FcγRIIIa alleles. Further clinical confirmatory studies and in vitro analyses are needed to test this hypothesis.
Supplementary Material
Acknowledgments
Funding for this research was provided by grants from the Department of Defense (#W81XWH-04-1-0102), Ovarian Cancer Research Fund (UW/UWM.05), start-up funds from the Department of Obstetrics and Gynecology to MSP, a charitable donation from Jean McKenzie to JC and MSP, a T32 training grant to JAAG, and by U10CA98543 COG Group Chair Grant, and R01-CA-32685-25, P30-CA14520, CA87025, CA81403, RR03186, and grants from the Midwest Athletes for Childhood Cancer Fund, The Crawdaddy Foundation, The St. Baldrick’s Foundation, The Evan Dunbar Foundation and Abbie’s Fund to PMS. We are deeply grateful to Kathy Schell for her advice and help and acknowledge the support provided by the University of Wisconsin Comprehensive Cancer Centers Flow Cytometry facility which is supported by a core grant (CA14520) from the National Institutes of Health, to Lance Rodenkirk for help with confocal microscopy and image analysis, and to Lori Scardino for help with the schematic figure.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00262-011-1072-9) contains supplementary material, which is available to authorized users.
Contributor Information
Jennifer A. A. Gubbels, Department of Obstetrics and Gynecology, University of Wisconsin-Madison, Madison, WI 53711, USA
Brian Gadbaw, Department of Pediatrics, University of Wisconsin-Madison, Madison, WI 53711, USA.
Ilia N. Buhtoiarov, Department of Human Oncology, University of Wisconsin-Madison, Madison, WI 53711, USA
Sachi Horibata, Department of Obstetrics and Gynecology, University of Wisconsin-Madison, Madison, WI 53711, USA.
Arvinder K. Kapur, Department of Obstetrics and Gynecology, University of Wisconsin-Madison, Madison, WI 53711, USA
Dhara Patel, Department of Obstetrics and Gynecology, University of Wisconsin-Madison, Madison, WI 53711, USA.
Jacquelyn A. Hank, Department of Human Oncology, University of Wisconsin-Madison, Madison, WI 53711, USA
Stephen D. Gillies, Provenance Biopharmaceuticals Corp., Waltham, MA, USA
Paul M. Sondel, Email: pmsondel@humonc.wisc.edu, Department of Pediatrics, University of Wisconsin-Madison, Madison, WI 53711, USA. Department of Human Oncology, University of Wisconsin-Madison, Madison, WI 53711, USA. 4159 Wisconsin Institutes for Medical Research, 1111 Highland Ave, Madison, WI 53705, USA
Manish S. Patankar, Email: patankar@wisc.edu, Department of Obstetrics and Gynecology, University of Wisconsin-Madison, Madison, WI 53711, USA. H4/657 CSC, 600 Highland Avenue, Madison, WI 53792-6188, USA
Joseph Connor, Email: jpconnor@wisc.edu, Department of Obstetrics and Gynecology, University of Wisconsin-Madison, Madison, WI 53711, USA. Clinical Science Center-H4/650, 600 Highland Ave, Box 6188, Madison, WI 53792, USA.
References
- 1.Richards JM. Therapeutic uses of interleukin-2 and lymphokine-activated killer (LAK) cells. Blood Rev. 1989;3:110–119. doi: 10.1016/0268-960x(89)90006-4. [DOI] [PubMed] [Google Scholar]
- 2.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 3.Nelson BH. The impact of T-cell immunity on ovarian cancer outcomes. Immunol Rev. 2008;222:101–116. doi: 10.1111/j.1600-065X.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 4.Bubenik J, Indrova M, Perlmann P, Berzins K, Mach O, Kraml J, Toulcova A. Tumour inhibitory effects of TCGF/IL-2/-containing preparations. Cancer Immunol Immunother. 1985;19:57–61. doi: 10.1007/BF00199313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bubenik J, Perlmann P, Indrova M, Simova J, Jandlova T, Neuwirt J. Growth inhibition of an MC-induced mouse sarcoma by TCGF (IL 2)-containing preparations. Preliminary report. Cancer Immunol Immunother. 1983;14:205–206. doi: 10.1007/BF00205362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316:889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 7.Lotze MT, Matory YL, Rayner AA, Ettinghausen SE, Vetto JT, Seipp CA, Rosenberg SA. Clinical effects and toxicity of interleukin-2 in patients with cancer. Cancer. 1986;58:2764–2772. doi: 10.1002/1097-0142(19861215)58:12<2764::aid-cncr2820581235>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.Lode HN, Xiang R, Becker JC, Gillies SD, Reisfeld RA. Immunocytokines: a promising approach to cancer immunotherapy. Pharmacol Ther. 1998;80:277–292. doi: 10.1016/s0163-7258(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 9.Reisfeld RA, Becker JC, Gillies SD. Immunocytokines: a new approach to immunotherapy of melanoma. Melanoma Res. 1997;7(Suppl 2):S99–S106. [PubMed] [Google Scholar]
- 10.Connor JP, Felder M, Hank J, Harter J, Gan J, Gillies SD, Sondel P. Ex vivo evaluation of anti-EpCAM immunocytokine huKS-IL2 in ovarian cancer. J Immunother. 2004;27:211–219. doi: 10.1097/00002371-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Neal ZC, Yang JC, Rakhmilevich AL, Buhtoiarov IN, Lum HE, Imboden M, Hank JA, Lode HN, Reisfeld RA, Gillies SD, Sondel PM. Enhanced activity of hu14.18-IL2 immunocytokine against murine NXS2 neuroblastoma when combined with interleukin 2 therapy. Clin Cancer Res. 2004;10:4839–4847. doi: 10.1158/1078-0432.CCR-03-0799. [DOI] [PubMed] [Google Scholar]
- 12.Osenga KL, Hank JA, Albertini MR, Gan J, Sternberg AG, Eickhoff J, Seeger RC, Matthay KK, Reynolds CP, Twist C, Krailo M, Adamson PC, Reisfeld RA, Gillies SD, Sondel PM. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children’s Oncology Group. Clin Cancer Res. 2006;12:1750–1759. doi: 10.1158/1078-0432.CCR-05-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson EE, Lum HD, Rakhmilevich AL, Schmidt BE, Furlong M, Buhtoiarov IN, Hank JA, Raubitschek A, Colcher D, Reisfeld RA, Gillies SD, Sondel PM. Intratumoral immunocytokine treatment results in enhanced antitumor effects. Cancer Immunol Immunother. 2008;57:1891–1902. doi: 10.1007/s00262-008-0519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner K, Schulz P, Scholz A, Wiedenmann B, Menrad A. The targeted immunocytokine L19-IL2 efficiently inhibits the growth of orthotopic pancreatic cancer. Clin Cancer Res. 2008;14:4951–4960. doi: 10.1158/1078-0432.CCR-08-0157. [DOI] [PubMed] [Google Scholar]
- 15.Schliemann C, Palumbo A, Zuberbuhler K, Villa A, Kaspar M, Trachsel E, Klapper W, Menssen HD, Neri D. Complete eradication of human B-cell lymphoma xenografts using rituximab in combination with the immunocytokine L19-IL2. Blood. 2009;113:2275–2283. doi: 10.1182/blood-2008-05-160747. [DOI] [PubMed] [Google Scholar]
- 16.Wong JY, Chu DZ, Williams LE, Liu A, Zhan J, Yamauchi DM, Wilczynski S, Wu AM, Yazaki PJ, Shively JE, Leong L, Raubitschek AA. A phase I trial of (90)Y-DOTA-anti-CEA chimeric T84.66 (cT84.66) radioimmunotherapy in patients with metastatic CEA-producing malignancies. Cancer Biother Radiopharm. 2006;21:88–100. doi: 10.1089/cbr.2006.21.88. [DOI] [PubMed] [Google Scholar]
- 17.Wong JYC, Chu DZ, Yamauchi DM, Williams LE, Liu A, Wilczynski S, Wu AM, Shively JE, Doroshow JH, Raubitschek AA. A phase I radioimmunotherapy trial evaluating 90yttrium-labeled anti-carcinoembryonic antigen (CEA) chimeric T84.66 in patients with metastatic CEA-producing malignancies. Clin Cancer Res. 2000;6:3855–3863. [PubMed] [Google Scholar]
- 18.Lyu MA, Kurzrock R, Rosenblum MG. The immunocytokine scFv23/TNF targeting HER-2/neu induces synergistic cytotoxic effects with 5-fluorouracil in TNF-resistant pancreatic cancer cell lines. Biochem Pharmacol. 2008;75:836–846. doi: 10.1016/j.bcp.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Gillies SD, Reilly EB, Lo KM, Reisfeld RA. Antibody-targeted interleukin 2 stimulates T-cell killing of autologous tumor cells. Proc Natl Acad Sci USA. 1992;89:1428–1432. doi: 10.1073/pnas.89.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiang R, Lode HN, Dolman CS, Dreier T, Varki NM, Qian X, Lo KM, Lan Y, Super M, Gillies SD, Reisfeld RA. Elimination of established murine colon carcinoma metastases by antibody-interleukin 2 fusion protein therapy. Cancer Res. 1997;57:4948–4955. [PubMed] [Google Scholar]
- 21.Ko YJ, Bubley GJ, Weber R, Redfern C, Gold DP, Finke L, Kovar A, Dahl T, Gillies SD. Safety, pharmacokinetics, and biological pharmacodynamics of the immunocytokine EMD 273066 (huKS-IL2): results of a phase I trial in patients with prostate cancer. J Immunother. 2004;27:232–239. doi: 10.1097/00002371-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Shusterman S, London W, Gillies SD, Hank JA, Voss S, Seeger RC, Reynolds CP, Kimball J, Albertini MA, Wagner B, Gan J, Eickhoff J, DeSantes KD, Cohn SL, Hecht T, Gadbaw B, Reisfeld RA, Maris JM, Sondel PM. Anti-tumor activity of hu14.18-IL2 in relapsed/refractory neuroblastoma patients: a Children’s Oncology Group (COG) phase II study. J Clin Oncol. 2010;28:4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu AL, Gilman A, Ozkaynak MF, London WB, Kreissman S, Chen H, Smith M, Anderson B, Villablanca J, Matthay KK, Shimada H, Grupp SA, Seeger R, Reynolds CP, Buxton A, Reisfeld RA, Gillies SD, Cohn SL, Maris JM, Sondel PM. Chimeric Anti-GD2 antibody with GM-CSF, IL2 and 13-cis retinoic acid for high-risk neuroblastoma: a children’s oncology group (COG) phase 3 study. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker JC, Pancook JD, Gillies SD, Furukawa K, Reisfeld RA. T cell-mediated eradication of murine metastatic melanoma induced by targeted interleukin 2 therapy. J Exp Med. 1996;183:2361–2366. doi: 10.1084/jem.183.5.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pancook JD, Becker JC, Gillies SD, Reisfeld RA. Eradication of established hepatic human neuroblastoma metastases in mice with severe combined immunodeficiency by antibody-targeted interleukin-2. Cancer Immunol Immunother. 1996;42:88–92. doi: 10.1007/s002620050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker JC, Pancook JD, Gillies SD, Mendelsohn J, Reisfeld RA. Eradication of human hepatic and pulmonary melanoma metastases in SCID mice by antibody-interleukin 2 fusion proteins. Proc Natl Acad Sci USA. 1996;93:2702–2707. doi: 10.1073/pnas.93.7.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillies SD, Lan Y, Lo KM, Super M, Wesolowski J. Improving the efficacy of antibody-interleukin 2 fusion proteins by reducing their interaction with Fc receptors. Cancer Res. 1999;59:2159–2166. [PubMed] [Google Scholar]
- 28.Neal ZC, Imboden M, Rakhmilevich AL, Kim KM, Hank JA, Surfus J, Dixon JR, Lode HN, Reisfeld RA, Gillies SD, Sondel PM. NXS2 murine neuroblastomas express increased levels of MHC class I antigens upon recurrence following NK-dependent immunotherapy. Cancer Immunol Immunother. 2004;53:41–52. doi: 10.1007/s00262-003-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillies SD, Lan Y, Williams S, Carr F, Forman S, Raubitschek A, Lo KM. An anti-CD20-IL-2 immunocytokine is highly efficacious in a SCID mouse model of established human B lymphoma. Blood. 2005;105:3972–3978. doi: 10.1182/blood-2004-09-3533. [DOI] [PubMed] [Google Scholar]
- 30.Lustgarten J, Marks J, Sherman LA. Redirecting effector T cells through their IL-2 receptors. J Immunol. 1999;162:359–365. [PubMed] [Google Scholar]
- 31.Buhtoiarov IN, Neal Z, Jan J, Buhtoiarova TN, Hank JA, Yamane B, Rakhmilevich AL, Patankar MS, Gubbels JAA, Reisfeld RA, Gillies SD, Sondel PM. Differential internalization of hu14.18-IL2 by human NK cells and human tumor cells: influence on conjugate formation, cytotoxicity and infiltration into tumors. J Leukoc Biol. 2010;89:625–638. doi: 10.1189/jlb.0710422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belisle JA, Gubbels JA, Raphael CA, Migneault M, Rancourt C, Connor JP, Patankar MS. Peritoneal natural killer cells from epithelial ovarian cancer patients show an altered phenotype and bind to the tumour marker MUC16 (CA125) Immunology. 2007;122:418–429. doi: 10.1111/j.1365-2567.2007.02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis DM, Chiu I, Fassett M, Cohen GB, Mandelboim O, Strominger JL. The human natural killer cell immune synapse. Proc Natl Acad Sci USA. 1999;96:15062–15067. doi: 10.1073/pnas.96.26.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joshi T, Ganesan LP, Cheney C, Ostrowski MC, Muthusamy N, Byrd JC, Tridandapani S. The PtdIns 3-kinase/Akt pathway regulates macrophage-mediated ADCC against B cell lymphoma. PLoS ONE. 2009;4:e4208. doi: 10.1371/journal.pone.0004208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gubbels JA, Belisle J, Onda M, Rancourt C, Migneault M, Ho M, Bera TK, Connor J, Sathyanarayana BK, Lee B, Pastan I, Patankar MS. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gubbels JA, Felder M, Horibata S, Belisle JA, Kapur A, Holden H, Petrie S, Migneault M, Rancourt C, Connor JP, Patankar MS. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol Cancer. 2010;9:11. doi: 10.1186/1476-4598-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng X, Wang Y, Wei H, Sun R, Tian Z. LFA-1 and CD2 synergize for the ERK1/2 activation in the NK cell immunological synapse. J Biol Chem. 2009;284:21280–21287. doi: 10.1074/jbc.M807053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson MJ, Cochran KJ, Cameron C, Le JM, Tantravahi R, Ritz J. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp Hematol. 1996;24:406–415. [PubMed] [Google Scholar]
- 39.Lanier LL, Ruitenberg JJ, Phillips JH. Functional and biochemical analysis of CD16 antigen on natural killer cells and granulocytes. J Immunol. 1988;141:3478–3485. [PubMed] [Google Scholar]
- 40.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waldhauer I, Steinle A. NK cells and cancer immuno-surveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 42.Rosenstein M, Yron I, Kaufmann Y, Rosenberg SA. Lymphokine-activated killer cells: lysis of fresh syngeneic natural killer-resistant murine tumor cells by lymphocytes cultured in interleukin 2. Cancer Res. 1984;44:1946–1953. [PubMed] [Google Scholar]
- 43.Hatakeyama M, Minamoto S, Taniguchi T. Intracyto-plasmic phosphorylation sites of Tac antigen (p55) are not essential for the conformation, function, and regulation of the human interleukin 2 receptor. Proc Natl Acad Sci USA. 1986;83:9650–9654. doi: 10.1073/pnas.83.24.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rickert M, Wang X, Boulanger MJ, Goriatcheva N, Garcia KC. The structure of interleukin-2 complexed with its alpha receptor. Science. 2005;308:1477–1480. doi: 10.1126/science.1109745. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Rickert M, Garcia KC. Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science. 2005;310:1159–1163. doi: 10.1126/science.1117893. [DOI] [PubMed] [Google Scholar]
- 46.Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- 47.Greene WC, Leonard WJ. Human interleukin 2 receptor (Tac antigen) Methods Enzymol. 1987;150:682–700. doi: 10.1016/0076-6879(87)50115-x. [DOI] [PubMed] [Google Scholar]
- 48.Greene WC, Leonard WJ. The human interleukin-2 receptor. Annu Rev Immunol. 1986;4:69–95. doi: 10.1146/annurev.iy.04.040186.000441. [DOI] [PubMed] [Google Scholar]
- 49.Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;91:1706–1715. [PubMed] [Google Scholar]
- 50.Gestwicki JE, Cairo CW, Strong LE, Oetjen KA, Kiessling LL. Influencing receptor-ligand binding mechanisms with multivalent ligand architecture. J Am Chem Soc. 2002;124:14922–14933. doi: 10.1021/ja027184x. [DOI] [PubMed] [Google Scholar]
- 51.Orange JS, Harris KE, Andzelm MM, Valter MM, Geha RS, Strominger JL. The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc Natl Acad Sci USA. 2003;100:14151–14156. doi: 10.1073/pnas.1835830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Spriel AB, Leusen JH, van Egmond M, Dijkman HB, Assmann KJ, Mayadas TN, van de Winkel JG. Mac-1 (CD11b/ CD18) is essential for Fc receptor-mediated neutrophil cytotoxicity and immunologic synapse formation. Blood. 2001;97:2478–2486. doi: 10.1182/blood.v97.8.2478. [DOI] [PubMed] [Google Scholar]
- 53.Krzewski K, Chen X, Strominger JL. WIP is essential for lytic granule polarization and NK cell cytotoxicity. Proc Natl Acad Sci USA. 2008;105:2568–2573. doi: 10.1073/pnas.0711593105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krzewski K, Chen X, Orange JS, Strominger JL. Formation of a WIP-, WASp-, actin-, and myosin IIA-containing multiprotein complex in activated NK cells and its alteration by KIR inhibitory signaling. J Cell Biol. 2006;173:121–132. doi: 10.1083/jcb.200509076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai P, Rabinowich H, Crowley-Nowick PA, Bell MC, Mantovani G, Whiteside TL. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin Cancer Res. 1996;2:161–173. [PubMed] [Google Scholar]
- 56.Patankar MS, Yu J, Morrison JC, Belisle JA, Lattanzio FA, Deng Y, Wong NK, Morris HR, Dell A, Clark GF. Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecol Oncol. 2005;99:704–713. doi: 10.1016/j.ygyno.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 57.Delgado DC, Hank J, Kolesar J, Lorentzen D, Gan J, Seo S, Kim KM, Shusterman S, Gillies SD, Reisfeld RA, Yang R, Gadbaw B, DeSantes KD, London WB, Seeger RC, Maris J, Sondel PM. The role for genotypes of killer Ig-like receptors (KIRs), their ligands, and Fcγ receptors on responses of neuroblastoma patients to Hu14.18-IL2: a children’s oncology group report. Cancer Res. 2010;70:9554–9561. doi: 10.1158/0008-5472.CAN-10-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 59.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.