Abstract
Challenging environments have guided nature in the development of ultrastable protein complexes. Specialized bacteria produce discrete multi-component protein networks called cellulosomes to effectively digest lignocellulosic biomass. While network assembly is enabled by protein interactions with commonplace affinities, we show that certain cellulosomal ligand-receptor interactions exhibit extreme resistance to applied force. Here, we characterize the ligand-receptor complex responsible for substrate anchoring in the Ruminococcus flavefaciens cellulosome using single-molecule force spectroscopy and steered molecular dynamics simulations. The complex withstands forces of 600-750 pN, situating it among the strongest bimolecular interactions reported, equivalent to half the mechanical strength of a covalent bond. Our findings demonstrate force activation and inter-domain stabilization of the complex, and suggest that certain network components serve as mechanical effectors for maintaining network integrity. This detailed understanding of cellulosomal network components can help in the development of biocatalysts for production of fuels and chemicals from renewable plant-derived biomass.
Introduction
Cellulosomes are protein networks designed by nature to degrade lignocellulosic biomass.1 These networks comprise intricate assemblies of conserved subunits including catalytic domains, scaffold proteins, carbohydrate binding modules (CBMs), cohesins (Cohs), dockerins (Docs), and X-modules (XMods) of unknown function. Coh:Doc pairs form complexes with high affinity and specificity,2 and provide connectivity to a myriad of cellulosomal networks with varying Coh:Doc network topology.3–5 The most intricate cellulosome known to date is produced by Ruminococcus flavefaciens (R.f.)6,7 and contains several primary and secondary scaffolds along with over 220 dockerin-bearing protein subunits.8
The importance of cellulolytic enzymes for the production of renewable fuels and chemicals from biomass has highlighted an urgent need for improved fundamental understanding of how cellulosomal networks achieve their impressive catalytic activity.9 Two of the mechanisms known to increase the catalytic activity of cellulosomes are proximity and targeting effects.10 Proximity refers to the high local concentration of enzymes afforded by incorporation into nanoscale networks, while targeting refers to specific binding of cellulosomes to substrates. Protein scaffolds and CBM domains are both critical in this context as they mediate interactions between comparatively large bacterial cells and cellulose particles. Since many cellulosomal habitats (e.g., cow rumen) exhibit strong flow gradients, shear forces will accordingly stress bridging scaffold components mechanically in vivo. Protein modules located at stressed positions within these networks should therefore be preselected for high mechanostability. However, thus far very few studies on the mechanics of carbohydrate-active proteins or cellulosomal network components have been reported.11
In the present study we sought to identify cellulosomal network junctions with maximal mechanical stability. We chose an XMod-Doc:Coh complex responsible for maintaining bacterial adhesion to cellulose in the rumen. The complex links the R. flavefaciens cell wall to the cellulose substrate via two CBM domains located at the N-terminus of the CttA scaffold, as shown in Fig. 1a. The crystal structure of the complex solved by X-ray crystallography12 is shown in Fig. 1b. XMod-Doc tandem dyads such as this one are a common feature in cellulosomal networks. Bulk biochemical assays on XMod-Docs have demonstrated that XMods improve Doc solubility and increase biochemical affinity of Doc:Coh complex formation.13 Crystallographic studies conducted on XMod-Doc:Coh complexes have revealed direct contacts between XMods and their adjacent dockerins.12,14 Additionally, many XMods (e.g., PDB 2B59, 1EHX, 3PDD) have high β-strand content and fold with N- and C-termini at opposite ends of the molecule, suggestive of robust mechanical clamp motifs at work.15,16 These observations are all suggestive of a mechanical role for XMods. Here we perform AFM single-molecule force spectroscopy experiments and steered molecular dynamics simulations in order to understand mechanostability of the XMod-Doc:Coh cellulosomal ligand-receptor complex. We conclude that the high mechanostability we observe originates from molecular mechanisms including stabilization of Doc by the adjacent XMod domain, and catch bond behavior that causes the complex to increase in contact area upon application of force.
Figure 1.
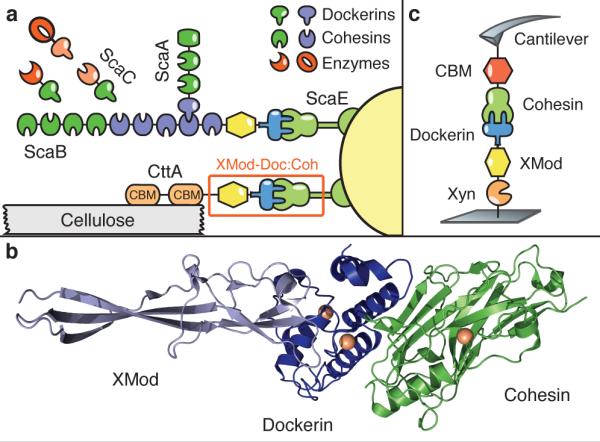
System overview. (a) Schematic of selected components of the R. flavefaciens cellulosome. The investigated XMod-Doc:Coh complex responsible for maintaining bacterial adhesion to cellulose is highlighted in orange. (b) Crystal structure of the XMod-Doc:Coh complex. Ca2+ ions are shown as orange spheres. (c) Depiction of experimental pulling configuration I, with CBM-Coh attached to the cantilever tip and Xyn-XMod-Doc attached to the glass surface.
Results and Discussion
Single-Molecule Experiments
We performed single-molecule force spectroscopy (SMFS) experiments with an atomic force miscroscope (AFM) to probe the mechanical dissociation of XMod-Doc:Coh. Xylanase (Xyn) and CBM fusion domains on the XMod-Doc and Coh modules, respectively, provided identifiable unfolding patterns permitting screening of large datasets of force-distance curves.17–19 Engineered cysteines and/or peptide tags on the CBM and Xyn marker domains were used to covalently immobilize the binding partners in a site-specific manner to an AFM cantilever or cover glass via poly(ethylene glycol) (PEG) linkers. The pulling configuration with Coh-CBM immobilized on the cantilever is referred to as configuration I, as shown in Fig 1c. The reverse configuration with Coh-CBM on the cover glass is referred to as configuration II. In a typical experimental run we collected about 50,000 force extension traces from a single cantilever. We note that the molecules immobilized on the cantilever and glass surfaces were stable over thousands of pulling cycles.
We sorted the data by first searching for contour length increments that matched our specific xylanase and CBM fingerprint domains. After identifying these specific traces (Fig. 2a), we measured the loading rate dependency of the final Doc:Coh ruptures based on bond history. To assign protein subdomains to the observed unfolding patterns, we transformed the data into contour length space using a freely rotating chain model with quantum mechanical corrections for peptide backbone stretching (QM-FRC, Supplementary Note 1, Supplementary Fig. 1).20,21 The fit parameter-free QM-FRC model describes protein stretching at forces greater than 200 pN more accurately than the commonly used worm-like chain (WLC) model.20,22 The resulting contour length histogram is shown in Fig. 2b. Peak-to-peak distances in the histogram represent contour length increments of unfolded protein domains. Assuming a length per stretched amino acid of 0.365 nm and accounting for the folded length of each subdomain, we compared the observed increments to the polypeptide lengths of individual subdomains of the Xyn-XMod-Doc and Coh-CBM fusion proteins. Details on contour length estimates and domain assignments are shown in Supplementary Table 1.
Figure 2.
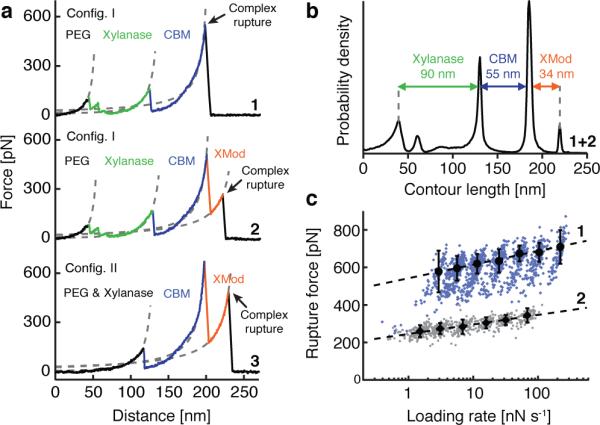
Experimental SMFS unfolding traces. (a) Unfolding fingerprints from pulling configuration I (curves 1 & 2) and configuration II (curve 3). The QM-FRC model (dashed lines) was used to estimate the contour lengths of the unfolded modules. (b) Contour length histogram obtained from 127 force extension traces (Config. I). The peak-to-peak increments correspond to Xyn, CBM and XMod amino acid sequence lengths. (c) Dynamic force spectra for the final Doc:Coh complex rupture peaks obtained from 2122 force-extension traces. The blue points show Doc:Coh ruptures that occurred with an intact XMod, while gray points show ruptures immediately following XMod unfolding. Black circles and diamonds represent the most probable rupture force/loading rate obtained by Gaussian fitting at each pulling speed. Error bars are ±1 standard deviation. Dashed lines are least squares fits to the Bell-Evans model.
Unfolding patterns in configuration I showed PEG stretching followed by a three-peaked Xyn fingerprint (Fig. 1a, top trace, green), which added 90 nm of contour length to the system. Xyn unfolding was followed by CBM unfolding at ~150 pN with 55 nm of contour length added. Finally, the XMod-Doc:Coh complex dissociated at an ultra-high rupture force of ~600 pN. The loading rate dependence of the final rupture event for curves of subtype 1 is plotted in Fig. 2c (blue). The measured complex rupture force distributions are shown in Supplementary Fig. 2.
Less frequently (35-40% of traces) we observed a two-step dissociation process wherein the XMod unfolded prior to Doc:Coh rupture as shown in Fig. 2a, (middle trace, orange). In these cases, the final dissociation exhibited a much lower rupture force (~300 pN) than the preceding XMod unfolding peak, indicating the strengthening effect of XMod was lost, and XMod was no longer able to protect the complex from dissociation at high force. The loading rate dependency of Doc:Coh rupture occurring immediately following XMod unfolding is shown in Fig. 2c (gray).
In configuration II (Fig. 2a, bottom trace), with the Xyn-XMod-Doc attached to the cantilever, the xylanase fingerprint was lost after the first few force extension traces acquired in the dataset. This indicated the Xyn domain did not refold within the timescale of the experiment once unfolded, consistent with prior work.17,18 CBM and XMod unfolding events were observed repeatedly throughout the series of acquired force traces in both configurations I and II, indicating these domains were able to refold while attached to the cantilever over the course of the experiment.
We employed the Bell-Evans model23 (Supplementary Note 2) to analyze the final rupture of the complex through the effective distance to the transition state (Δx) and the natural off-rate (koff). The fits to the model yielded values of Δx = 0.13 nm and koff = 7.3 * 10−7 s−1 for an intact XMod, and Δx = 0.19 nm and koff = 4.7 * 10−4 s−1 for the “shielded” rupture following XMod unfolding (Fig. 2c). These values indicate that the distance to the transition state is increased following XMod unfolding, reflecting an overall softening of the binding interface. Distances to the transition state observed for other ligand-receptor pairs are typically on the order of ~0.7 nm.17 The extremely short Δx of 0.13 nm observed here suggests that mechanical unbinding for this complex is highly coordinated. We further analyzed the unfolding of XMod in the Bell-Evans picture and found values of Δx = 0.15 nm and koff = 2.6 * 10−6 s−1. The loading rate dependence for this unfolding event is shown in Supplementary Fig. 3.
The exceptionally high rupture forces measured experimentally (Fig. 2) are hugely disproportionate to the XMod-Doc:Coh biochemical affinity, which at KD ~20 nM12 is comparable to typical antibody-antigen interactions. Antibody-antigen interactions, however, will rupture at only ~60 pN at similar loading rates,24 while bimolecular complexes found in muscle exposed to mechanical loading in vivo will rupture at ~140 pN.25 Trimeric titin-telethonin complexes also found in muscle exhibit unfolding forces around 700 pN26 while Ig domains from cardiac titin will unfold at ~200 pN.27 The XMod-Doc:Coh ruptures reported here fell in a range from 600-750 pN at loading rates ranging from 10-100 nN s−1. At around half the rupture force of a covalent gold-thiol bond,28 these bimolecular protein rupture forces are, to the best of our knowledge, among the highest of their kind ever reported. The covalent bonds in this system are primarily peptide bonds in the proteins and C-C and C-O bonds in the PEG linkers. These are significantly more mechanically stable than the quoted gold-thiol bond rupture force (~ 1.2 nN)29 and fall in a rupture force range > 2.5 nN at similar loading rates. Therefore, breakage of covalent linkages under our experimental conditions is highly unlikely. We note that the high mechanostability observed here is not the result of fusing the proteins to the CBM or Xyl domains. The covalent linkages and pulling geometry are consistent with the wild type complex and its dissociation pathway. In vivo, the Coh is anchored to the peptidoglycan cell wall through its C-terminal sortase motif. The XMod-Doc is attached to the cellulose substrate through two N-terminal CBM domains. By pulling the XMod-Doc from through an N-terminal Xyl fusion domain, and the Coh through a C-terminal CBM, we established an experimental pulling geometry that matches loading of the complex in vivo. This pulling geometry was also used in all simulations. The discontinuity between its commonplace biochemical affinity and remarkable resistance to applied force illustrates how this complex is primed for mechanical stability, and highlights differences in the unbinding pathway between dissociation at equilibrium and dissociation induced mechanically along a defined pulling coordinate.
Steered Molecular Dynamics
To elucidate the molecular mechanisms at play that enable this extreme mechanostability, we carried out all-atom steered molecular dynamics (SMD) simulations. The Xyn and CBM domains were not modeled in order to keep the simulated system small and reduce the usage of computational resources. This approximation was reasonable since we have no indication that these domains significantly affect the XMod-Doc:Coh binding strength.30 After equilibrating the crystal structure,12 the N-terminus of XMod-Doc was harmonically restrained while the C-terminus of Coh was pulled away at constant speed. The force applied to the harmonic pulling spring was stored at each time-step. We tested pulling speeds of 0.25, 0.625 and 1.25 Å ns−1, and note that the slowest simulated pulling speed was ~4,000 times faster than our fastest experimental pulling speed of 6.4 μm s−1. This difference is considered not to affect the force profile, but it is known to account for the scale difference in force measured by SMD and AFM.31,32
SMD results showed the force increased with distance until the complex ruptured for all simulations. At the slowest pulling speed of 0.25 Å ns−1 the rupture occurred at a peak force of ~900 pN, as shown in Supplementary Fig. 4 and Supplementary Movie 1. We analyzed the progression and prevalence of hydrogen bonded contacts between the XMod-Doc and Coh domains to identify key residues in contact throughout the entire rupture process and particularly immediately prior to rupture. These residues are presented in Fig. 3a, c, d and Supplementary Fig. 5 and 6. The simulation results clearly reproduced key hydrogen bonding contacts previously identified12 as important for Doc:Coh recognition (Supplementary Fig. 5).
Figure 3.
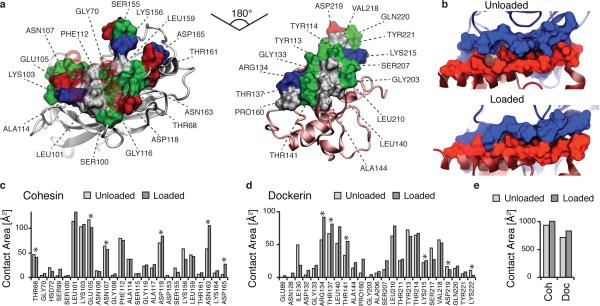
Analysis of binding interface and catch bond mechanism from SMD. (a) Surface plots for the main interacting residues of Coh (left) and Doc (right). Hydrophobic residues are shown in gray, polar residues in green, and negative and positive residues in red and blue, respectively. Both Coh and Doc exhibit a hydrophobic patch in the center of the binding surface that is surrounded by polar and charged residues. (b) Rearrangement of binding residues of Coh (blue) and Doc (red) under force. Following mechanical loading, an interdigitated complex is formed that resembles teeth of a zipper. (c,d) Surface contact area of interacting residues of Coh (c) and Doc (d) in the absence and presence of force. Residues forming prevalent hydrogen bonds are indicated with stars. (e) Total contact surface area of Coh and Doc in unloaded and loaded conformations.
The main interacting residues are shown in Fig. 3a and b. Both Coh and Doc exhibit a binding interface consisting of a hydrophobic center (gray) surrounded by a ring of polar (green) and charged residues (blue, positive; red, negative). This residue pattern suggests the hydrophilic side chains protect the interior hydrophobic core from attack by water molecules, compensating for the flat binding interface that lacks a deep pocket. The geometry suggests a penalty to unbinding that stabilizes the bound state. Further, we analyzed the contact surface areas of interacting residues (Fig. 3b-e). The total contact area was found to increase due to rearrangement of the interacting residues when the complex is mechanically stressed, as shown in Fig. 3e and Supplementary Movie 2. Doc residues in the simulated binding interface clamped down on Coh residues upon mechanical loading, resulting in increased stability and decreased accessibility of water into the hydrophobic core of the bound complex (Fig. 3b). These results suggest that a catch bond mechanism is responsible for the remarkable stability33 under force and provide a molecular mechanism which the XMod-Doc:Coh complex uses to summon mechanical strength when needed, while still allowing relatively fast assembly and disassembly of the complex at equilibrium. The residues that increase most in contact area (Fig. 3c, d) present promising candidates for future mutagenesis studies.
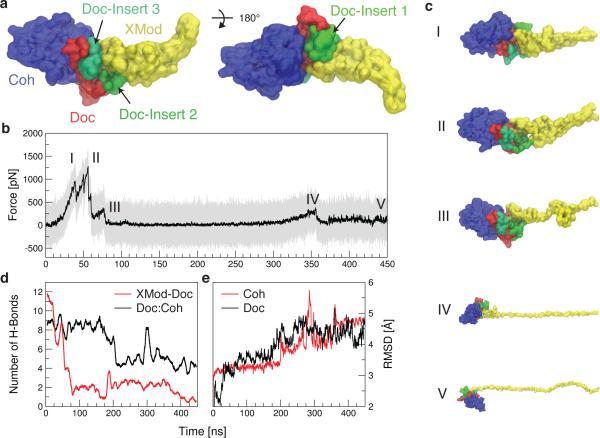
Among the 223 Doc sequences from R. flavefaciens, 6 subfamilies have been explicitly identified using bioinformatics approaches.8 The XMod-Doc investigated here belongs to the 40-member Doc family 4a. A conserved feature of these Doc modules is the presence of 3 sequence inserts that interrupt the conserved duplicated F-hand motif Doc structure. In our system, these Doc sequence inserts make direct contacts with XMod in the crystallized complex (Fig. 1), and suggest an interaction between XMod and Doc that could potentially propagate to the Doc:Coh binding interface. To test this, an independent simulation was performed to unfold XMod (Fig. 4). The harmonic restraint was moved to the C-terminus of XMod so that force was applied from the N- to C-terminus of XMod only, while leaving Doc and Coh unrestrained. The results (Fig. 4b) showed XMod unfolded at forces slightly higher than but similar to the XMod-Doc:Coh complex rupture force determined from the standard simulation at the same pulling speed. This suggested XMod unfolding prior to Doc:Coh rupture was not probable, but could be observed on occasion due to the stochastic nature of domain unfolding. This was consistent with experiments where XMod unfolding was observed in ~35-40% of traces. Furthermore, analysis of the H-bonding between Doc and XMod (Fig. 4d, red) indicated loss of contact as XMod unfolded, dominated by contact loss between the 3 Doc insert sequences and XMod. Interestingly, XMod unfolding clearly led to a decrease in H-bonding between Doc and Coh at a later stage (~200 ns) well after XMod had lost most of its contact with Doc, even though no force was being applied across the Doc:Coh binding interface. This provided evidence for direct stabilization of the Doc:Coh binding interface by XMod. As shown in Fig. 4e, the root mean squared deviation (RMSD) of Doc increased throughout the simulation as XMod unfolded. Coh RMSD remained stable until it started to lose H-bonds with Doc. Taken together this suggests that as XMod unfolded, Coh and Doc became more mobile and lost interaction strength, potentially explaining the increase in Δx from 0.13 to 0.19 nm upon unfolding of XMod in the experimental datasets. Apparently the XMod is able to directly stabilize the Doc:Coh interface, presumably through contact with Doc insert sequences that then propagate this stabilizing effect to the Doc:Coh binding interface.
Figure 4.
SMD shows unfolding of XMod destabilizes Doc:Coh binding interface. XMod was unfolded by moving the harmonic restraint to the C terminus of XMod while the N terminus was moved at 0.625 Å ns−1. (a) Surface representation of XMod-Doc:Coh complex with Doc insert sequences. Coh is shown in blue, Doc in red and green (inserts), and XMod in yellow. (b) Force time trace of XMod unfolding. The domain starts to unfold in several substeps starting at ~ 400 pN. Snapshots at different time steps are labelled I-V and are shown in (c). (d) Average number of hydrogen bonds between Doc:Coh (black) and XMod-Doc (red). XMod-Doc contact is dominated by the insert sequences 1–3. (e) Root mean squared deviation (RMSD) of Doc (black) and Coh (red).
In summary, we investigated an ultrastable XMod-Doc:Coh complex involved in bacterial adhesion to cellulose. While previously the role of XMod functioning in tandem XMod-Doc dyads was unclear,12,14 we show that XMod serves as a mechanical stabilizer and force-shielding effector subdomain in the ultrastable ligand-receptor complex. The Doc:Coh complex presented here exhibits one of the most mechanically robust protein-protein interactions reported thus far, and points towards new mechanically-stable artificial multi-component biocatalysts for industrial applications, including production of second-generation biofuels.
Methods
Site Directed Mutagenesis
Site-Directed Mutagenesis of R. flavefaciens strain FD1 Chimeric Cellulosomal Proteins. A pET28a vector containing the previously cloned R. flavefaciens CohE from ScaE fused to cellulose-binding module 3a (CBM3a) from C. thermocellum, and a pET28a vector containing the previously cloned R. flavefaciens XMod-Doc from the CttA scaffoldin fused to the XynT6 xylanase from Geobacillus stearothermophilus12 were subjected to QuikChange mutagenesis34 to install the following mutations: A2C in the CBM and T129C in the xylanase, respectively.
For the construction of the native configuration of the CohE-CBM A2C fusion protein Gibson Assembly35 was used. For further analysis CohE-CBM A2C was modified with a QuikChange PCR36 to replace the two cysteins (C2 and C63) in the protein with alanine and serine (C2A and C63S). All mutagenesis products were confirmed by DNA sequencing analysis.
The XynT6-XDoc T129C was constructed using the following primers:
5’-acaaggaaggtaagccaatggttaatgaatgcgatccagtgaaacgtgaac-3’
5’-gttcacgtttcactggatcgcattcattaaccattggcttaccttccttgt-3’
The CBM-CohE A2C was constructed using the following primers:
5’-ttaactttaagaaggagatataccatgtgcaatacaccggtatcaggcaatttgaag-3’
5’-cttcaaattgcctgataccggtgtattgcacatggtatatctccttcttaaagttaa-3’
The CohE-CBM C2A C63S was constructed using the following phosphorylated primers:
5’-ccgaatgccatggccaatacaccgg-3’
5’-cagaccttctggagtgaccatgctgc-3’
Expression and Purification of Xyn-XMod-Doc
The T129C Xyn-XMod-Doc protein was expressed in E. coli BL21 cells in kanamycin-containing media that also contained 2 mM calcium chloride, overnight at 16° C. After harvesting, cells were lysed using sonication. The lysate was then pelleted, and the supernatant fluids were applied to a Ni-NTA column and washed with tris-buffered saline (TBS) buffer containing 20 mM imidazole and 2 mM calcium chloride. The bound protein was eluted using TBS buffer containing 250 mM imidazole and 2 mM calcium chloride. The solution was dialyzed with TBS to remove the imidazole, and then concentrated using an Amicon centrifugal filter device and stored in 50% (v/v) glycerol at ~ 20° C. The concentrations of the protein stock solutions were determined to be ~ 5 mg mL−1 by absorption spectrophotometry.
Expression and Purification of Coh-CBM
The Coh-CBM C2A, C63S fusion protein was expressed in E. coli BL21(DE3) RIPL in kanamycin and chloramphenicole containing ZYM-5052 media37 overnight at 22° C. After harvesting, cells were lysed using sonication. The lysate was then pelleted, and the supernatant fluids were applied to a Ni-NTA column and washed with TBS buffer. The bound protein was eluted using TBS buffer containing 200 mM imidazole. Imidazole was removed with a polyacrylamide gravity flow column. The protein solution was concentrated with an Amicon centrifugal filter device and stored in 50% (v/v) glycerol at −80° C. The concentrations of the protein stock solutions were determined to be ~ 5 mg mL−1 by absorption spectrophotometry.
Sample Preparation
In sample preparation and single-molecule measurements calcium supplemented TBS buffer (Ca-TBS) was used (25mM TRIS, 72mM NaCl, 1mM CaCl2, pH 7.2). Cantilevers and cover glasses were functionalized according to previously published protocols18,38. Briefly, cantilevers and cover glasses were cleaned by UV-ozone treatment and piranha solution, respectively. Levers and glasses were silanized using (3-aminopropyl)-dimethyl-ethoxysilane (APDMES) to introduce surface amine groups. Amine groups on the cantilevers and cover glasses were subsequently conjugated to a 5 kDa NHS-PEG-Mal linker in sodium borate buffer. Disulfide-linked dimers of the Xyl-XMod-Doc proteins were reduced for 2 hours at room temperature using a TCEP disulfide reducing bead slurry. The protein/bead mixture was rinsed with Ca-TBS measurement buffer, centrifuged at 850 rcf for 3 minutes, and the supernatant was collected with a micropipette. Reduced proteins were diluted with measurement buffer (1:3 (v/v) for cantilevers, and 1:1 (v/v) for cover glasses), and applied to PEGylated cantilevers and cover glasses for 1 h. Both cantilevers and cover glasses were then rinsed with Ca-TBS to remove unbound proteins, and stored under Ca-TBS prior to force spectroscopy measurements. Site specific immobilization of the Coh-CBM-ybbR fusion proteins to previously PEGylated cantilevers or coverglasses was carried out according to previously published protocols39. Briefly, PEGylated cantilevers or coverglasses were incubated with Coenzyme A (CoA) (20 mM) stored in coupling buffer for 1h at room temperature. Levers or surfaces were then rinsed with Ca-TBS to remove unbound CoA. Coh-CBM-ybbR fusion proteins were then covalently linked to the CoA surfaces or levers by incubating with Sfp phosphopantetheinyl transferase for 2 hours at room 37° . Finally, surfaces or levers were subjected to a final rinse with Ca-TBS and stored under Ca-TBS prior to measurement.
Single-Molecule Force Spectroscopy Measurements
SMFS measurements were performed on a custom built AFM40 controlled by an MFP-3D controller from Asylum Research running custom written Igor Pro (Wavemetrics) software. Cantilever spring constants were calibrated using the thermal noise / equipartition method41. The cantilever was brought into contact with the surface and withdrawn at constant speed ranging from 0.2–6.4 μm s−1. An x-y stage was actuated after each force-extension trace to expose the molecules on the cantilever to a new molecule at a different surface location with each trace. Typically 20,000–50,000 force-extension curves were obtained with a single cantilever in an experimental run of 18-24 hours. A low molecular density on the surface was used to avoid formation of multiple bonds. While the raw datasets contained a majority of unusable curves due to lack of interactions or nonspecific adhesion of molecules to the cantilever tip, select curves showed single-molecule interactions. We filtered the data using a combination of automated data processing and manual classification by searching for contour length increments that matched the lengths of our specific protein fingerprint domains: Xyl (~89 nm) and CBM (~ 56 nm). After identifying these specific traces, we measured the loading rate dependency of the final Doc:Coh ruptures based on bond history.
Data Analysis
Data were analyzed using previously published protocols17,18,22. Force extension traces were transformed into contour length space using the QM-FRC model with bonds of length b = 0.11 nm connected by a fixed angle γ = 41° and and assembled into barrier position histograms using cross-correlation. Detailed description of the contour length transformation can be found in Supplementary Note 1 and Supplementary Fig. 1.
For the loading rate analysis, the loading rate at the point of rupture was extracted by applying a line fit to the force vs. time trace in the immediate vicinity prior to the rupture peak. The loading rate was determined from the slope of the fit. The most probable rupture forces and loading rates were determined by applying Gaussian fits to histograms of rupture forces and loading rates at each pulling speed.
Molecular Dynamics Simulations
The structure of the XMod-Doc:Coh complex had been solved by means of X-ray crystallography at 1.97Å resolution and is available at the protein data bank (PDB:4IU3). A protonation analysis performed in VMD42 did not suggest any extra protonation and all the amino acid residues were simulated with standard protonation states. The system was then solvated, keeping also the water molecules present in the crystal structure, and the net charge of the protein and the calcium ions was neutralized using sodium atoms as counter-ions, which were randomly arranged in the solvent. Two other systems, based on the aforementioned one, were created using a similar salt concentration to the one used in the experiments (75 mM of NaCl). This additional salt caused little or no change in SMD results. The overall number of atoms included in MD simulations varied from 300,000 in the majority of the simulations to 580,000 for the unfolding of the X-Mod.
The MD simulations in the present study were performed employing the NAMD molecular dynamics package43,44. The CHARMM36 force field45,46 along with the TIP3 water model47 was used to describe all systems. The simulations were done assuming periodic boundary conditions in the NpT ensemble with temperature maintained at 300 K using Langevin dynamics for pressure, kept at 1 bar, and temperature coupling. A distance cut-off of 11.0 Å was applied to short-range, non-bonded interactions, whereas long-range electrostatic interactions were treated using the particlemesh Ewald (PME)48 method. The equations of motion were integrated using the r-RESPA multiple time step scheme44 to update the van der Waals interactions every two steps and electrostatic interactions every four steps. The time step of integration was chosen to be 2 fs for all simulations performed. Prior to the MD simulations all the systems were submitted to an energy minimization protocol for 1000 steps. The first two nanoseconds of the simulations served to equilibrate systems before the production runs that varied from 40 to 450 ns in the ten different simulations that were carried out. The equilibration step consisted of 500 ps of simulation where the protein backbone was restrained and 1.5 ns where the system was completely free and no restriction or force was applied. During the equilibration the initial temperature was set to zero and was constantly increased by 1 K every 100 MD steps until the desired temperature (300 K) was reached.
To characterize the coupling between dockerin and cohesin we performed SMD simulations49 of constant velocity stretching (SMD-CV protocol) employing 3 different pulling speeds: 1.25 Å ns−1, 0.625 Å ns−1, and 0.25 Å ns−1. In all simulations, SMD was employed by restraining the position of one end of the XMod-Doc domain harmonically (center of mass of ASN5), and moving a second restraint point, at the end of the Coh domain (center of mass of GLY210), with constant velocity in the desired direction. The procedure is equivalent to attaching one end of a harmonic spring to the end of a domain and pulling on the other end of the spring. The force applied to the harmonic spring is then monitored during the time of the molecular dynamics simulation. The pulling point was moved with constant velocity along the z axis and due to the single anchoring point and the single pulling point the system is quickly aligned along the z axis. Due to the flexibility of the linkers this approach reproduces the experimental setup. All analyses of MD trajectories were carried out employing VMD42 and its plugins. Secondary structures were assigned using the Timeline plugin, which employs STRIDE criteria50. Hydrogen bonds were assigned based on two geometric criteria for every trajectory frame saved: first, distances between acceptor and hydrogen should be less than 3.5 Å; second, the angle between hydrogen-donor-acceptor should be smaller than 30°. Surface contact areas of interacting residues were calculated employing Volarea51 implemented in VMD. The area is calculated using a probe radius defined as an in silico rolling spherical probe that is screened around the area of the dockerin exposed to the cohesin and the also the cohesin area exposed to the dockerin.
Supplementary Material
Acknowledgement
The authors gratefully acknowledge funding from an advanced grant of the European Research Council. This work was supported by grants from the National Institutes of Health (NIH, 9P41GM104601 to KS) and the National Science Foundation (NSF, MCB-1157615 to KS). Simulations made use of the Texas Advanced Computing Center (TACC) as part of the Extreme Science and Engineering Discovery Environment (XSEDE, MCA93S028 to KS) and the NCSA Blue Waters sustained-petascale supercomputer as part of the general allocations (Simulations of Cellulosomal Subunits: Components of a Molecular Machinery for Depolymerization of Feedstock for Production of Second Generation Biofuels, to KS). A grant to EAB, HEG and MAN from GIF, the German-Israeli Foundation for Scientific Research and Development is also noted. Additional support was obtained from grants (No. 1349) to EAB from the Israel Science Foundation (ISF) and the United States-Israel Binational Science Foundation (BSF), Jerusalem, Israel. EAB. is the incumbent of The Maynard I. and Elaine Wishner Chair of Bio-organic Chemistry. MAN acknowledges funding from Society in Science - The Branco Weiss Fellowship program administered by ETH Zürich, Switzerland.
Footnotes
Author contributions statement.
Performed and analyzed SMFS experiments: C.S., K.H.M., L.F.M., M.A.J., E.D., M.A.N.; Performed and analyzed MD simulations: R.C.B., K.S.; Provided proteins and DNA cloning vectors: W.O., D.B.F., E.A.B. Wrote and edited the manuscript: C.S., K.H.M., R.C.B., E.A.B., K.S., H.E.G., M.A.N.; Supervised research: E.A.B., K.S., H.E.G., and M.A.N.
Competing financial interests statement.
The authors declare no competing financial interests.
Accession codes
Plasmids used in this study are available through Addgene (https://www.addgene.org) under the following accession codes: Xylanase-Xmodule-Dockerin: 60865; Cohesin-CBM: 60866.
References
- 1.Doi RH, Kosugi A. Cellulosomes: plant-cell-wall-degrading enzyme complexes. Nat. Rev. Microbiol. 2004;2:541–551. doi: 10.1038/nrmicro925. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho A, Dias F, Prates JAM, Nagy T, Gilbert HJ, Davies GJ, Ferreira L, Romão M, Fontes C. Cellulosome assembly revealed by the crystal structure of the cohesin– dockerin complex. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13809–13814. doi: 10.1073/pnas.1936124100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith SP, Bayer EA. Insights into cellulosome assembly and dynamics: from dissection to reconstruction of the supramolecular enzyme complex. Curr. Opin. Struct. Biol. 2013;23:686–694. doi: 10.1016/j.sbi.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Bayer EA, Lamed R, White BA, Flint HJ. From cellulosomes to cellulosomics. Chem. Rec. 2008;8:364–377. doi: 10.1002/tcr.20160. [DOI] [PubMed] [Google Scholar]
- 5.Demain AL, Newcomb M, Wu JHD. Cellulase, clostridia, and ethanol. Microbiol. Mol. Biol. Rev. 2005;69:124–154. doi: 10.1128/MMBR.69.1.124-154.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jindou S, Brulc JM, Levy-Assaraf M, Rincon MT, Flint HJ, Berg ME, Wilson MK, White BA, Bayer EA, Lamed R, Borovok I. Cellulosome gene cluster analysis for gauging the diversity of the ruminal cellulolytic bacterium Ruminococcus flavefaciens. FEMS Micro-biol. Lett. 2008;285:188–194. doi: 10.1111/j.1574-6968.2008.01234.x. [DOI] [PubMed] [Google Scholar]
- 7.Ding SY, Rincon MT, Lamed R, Martin JC, McCrae SI, Aurilia V, Shoham Y, Bayer EA, Flint HJ. Cellulosomal Scaffoldin-Like Proteins from Ruminococcus flavefaciens. J. Bacteriol. 2001;183:1945–1953. doi: 10.1128/JB.183.6.1945-1953.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rincon MT, Dassa B, Flint HJ, Travis AJ, Jindou S, Borovok I, Lamed R, Bayer EA, Henrissat B, Coutinho PM, Antonopoulos DA, Miller MEB, White BA. Abundance and Diversity of Dockerin-Containing Proteins in the Fiber-Degrading Rumen Bacterium, Ruminococcus flavefaciens FD-1. PLoS One. 2010;5:e12476. doi: 10.1371/journal.pone.0012476. doi:10.1371/journal.pone.0012476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Himmel ME, Ding S-Y, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science. 2007;315:804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- 10.Fierobe H-P, Bayer EA, Tardif C, Czjzek M, Mechaly A, Bélaïch A, Lamed R, Shoham Y, Bélaïch J-P. Degradation of cellulose substrates by cellulosome chimeras Substrate targeting versus proximity of enzyme components. J. Biol. Chem. 2002;277:49621–49630. doi: 10.1074/jbc.M207672200. [DOI] [PubMed] [Google Scholar]
- 11.Valbuena A, Oroz J, Hervás R, Vera AM, Rodríguez D, Menéndez M, Sulkowska JI, Cieplak M, Carrión-Vázquez M. On the remarkable mechanostability of scaffoldins and the mechanical clamp motif. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13791–13796. doi: 10.1073/pnas.0813093106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salama-Alber O, Jobby MK, Chitayat S, Smith SP, White BA, Shimon LJW, Lamed R, Frolow F, Bayer EA. Atypical cohesin-dockerin complex responsible for cell-surface attachment of cellulosomal components: binding fidelity, promiscuity, and structural buttresses. J. Biol. Chem. 2013;288:16827–16838. doi: 10.1074/jbc.M113.466672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams JJ, Webb BA, Spencer HL, Smith SP. Structural Characterization of Type II Dockerin Module from the Cellulosome of Clostridium thermocellum: Calcium-Induced Effects on Conformation and Target Recognition âĂă. Biochemistry. 2005;44:2173–2182. doi: 10.1021/bi048039u. [DOI] [PubMed] [Google Scholar]
- 14.Adams JJ, Pal G, Jia Z, Smith SP. Mechanism of bacterial cell-surface attachment revealed by the structure of cellulosomal type II cohesin-dockerin complex. Proc. Natl. Acad. Sci. U. S. A. 2006;103:305–310. doi: 10.1073/pnas.0507109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sikora M, Cieplak M. Mechanical stability of multidomain proteins and novel mechanical clamps. Proteins Struct. Funct. Bioinf. 2011;79:1786–1799. doi: 10.1002/prot.23001. [DOI] [PubMed] [Google Scholar]
- 16.Brunecky R, Alahuhta M, Bomble YJ, Xu Q, Baker JO, Ding S-Y, Himmel ME, Lunin VV. Structure and function of the Clostridium thermocellum cellobiohydrolase A X1-module repeat: enhancement through stabilization of the CbhA complex. Acta Crystallogr. 2012;68:292–299. doi: 10.1107/S0907444912001680. [DOI] [PubMed] [Google Scholar]
- 17.Stahl SW, Nash MA, Fried DB, Slutzki M, Barak Y, Bayer EA, Gaub HE. Single-molecule dissection of the high-affinity cohesin-dockerin complex. Proc. Natl. Acad. Sci. U. S. A. 2012;109:20431–20436. doi: 10.1073/pnas.1211929109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jobst MA, Schoeler C, Malinowska K, Nash MA. Investigating Receptor-ligand Systems of the Cellulosome with AFM-based Single-molecule Force Spectroscopy. J. Vis. Exp. 2013;82:e50950. doi: 10.3791/50950. doi:10.3791/50950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otten M, Ott W, Jobst MA, Milles LF, Verdorfer T, Pippig DA, Nash MA, Gaub HE. From genes to protein mechanics on a chip. Nat. Methods. 2014 doi: 10.1038/nmeth.3099. doi:10.1038/nmeth.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livadaru L, Netz RR, Kreuzer HJ. Stretching Response of Discrete Semiflexible Polymers. Macromolecules. 2003;36:3732–3744. [Google Scholar]
- 21.Hugel T, Rief M, Seitz M, Gaub H, Netz R. Highly Stretched Single Polymers: Atomic-Force-Microscope Experiments Versus Ab-Initio Theory. Phys. Rev. Lett. 2005;94:048301. doi: 10.1103/PhysRevLett.94.048301. doi:10.1103/PhysRevLett.94.048301. [DOI] [PubMed] [Google Scholar]
- 22.Puchner EM, Franzen G, Gautel M, Gaub HE. Comparing Proteins by Their Unfolding Pattern. Biophys. J. 2008;95:426–434. doi: 10.1529/biophysj.108.129999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merkel R, Nassoy P, Leung A, Ritchie K, Evans E. Energy landscapes of receptor–ligand bonds explored with dynamic force spectroscopy. Nature. 1999;397:50–53. doi: 10.1038/16219. [DOI] [PubMed] [Google Scholar]
- 24.Morfill J, Blank K, Zahnd C, Luginbühl B, Kühner F, Gottschalk K-E, Plückthun A, Gaub HE. Affinity-Matured Recombinant Antibody Fragments Analyzed by Single-Molecule Force Spectroscopy. Biophys. J. 2007;93:3583–3590. doi: 10.1529/biophysj.107.112532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berkemeier F, Bertz M, Xiao S, Pinotsis N, Wilmanns M, Gräter F, Rief M. Fast-folding α-helices as reversible strain absorbers in the muscle protein myomesin. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14139–14144. doi: 10.1073/pnas.1105734108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertz M, Wilmanns M, Rief M. The titin-telethonin complex is a directed, superstable molecular bond in the muscle Z-disk. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13307–13310. doi: 10.1073/pnas.0902312106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marszalek PE, Lu H, Li H, Carrión-Vázquez M, Oberhauser AF, Schulten K, Fernandez JM. Mechanical unfolding intermediates in titin modules. Nature. 1999;402:100–103. doi: 10.1038/47083. [DOI] [PubMed] [Google Scholar]
- 28.Grandbois M, Beyer M, Rief M, Clausen-Schaumann H, Gaub HE. How Strong Is a Covalent Bond? Science. 1999;283:1727–1730. doi: 10.1126/science.283.5408.1727. [DOI] [PubMed] [Google Scholar]
- 29.Xue Y, Li X, Li H, Zhang W. Quantifying thiol-gold interactions towards the efficient strength control. Nat. Commun. 2014;5:4348. doi: 10.1038/ncomms5348. doi:10.1038/ncomms5348. [DOI] [PubMed] [Google Scholar]
- 30.Bomble YJ, Beckham GT, Matthews JF, Nimlos MR, Himmel ME, Crowley MF. Modeling the self-assembly of the cellulosome enzyme complex. J. Biol. Chem. 2011;286:5614–5623. doi: 10.1074/jbc.M110.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sotomayor M, Schulten K. Single-molecule experiments in vitro and in silico. Science. 2007;316:1144–1148. doi: 10.1126/science.1137591. [DOI] [PubMed] [Google Scholar]
- 32.Grubmüller H, Heymann B, Tavan P. Ligand binding: Molecular mechanics calculation of the streptavidin biotin rupture force. Science. 1996;271:997–999. doi: 10.1126/science.271.5251.997. [DOI] [PubMed] [Google Scholar]
- 33.Thomas W, Forero M, Yakovenko O, Nilsson L, Vicini P, Sokurenko E, Vogel V. Catch-bond model derived from allostery explains force-activated bacterial adhesion. Biophys. J. 2006;90:753–764. doi: 10.1529/biophysj.105.066548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W, Malcolm BA. Two-stage PCR protocol allowing introduction of multiple mutations, deletions and insertions using QuikChange site-directed mutagenesis. Biotechniques. 1999;26:680–682. doi: 10.2144/99264st03. [DOI] [PubMed] [Google Scholar]
- 35.Gibson DG, Young L, Chuang R-Y, Venter JC, Hutchison CA, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 36.Sawano A, Miyawaki A. Directed evolution of green fluorescent protein by a new versatile PCR strategy for site-directed and semi-random mutagenesis. Nucleic. Acids Res. 2000;28:e78–e78. doi: 10.1093/nar/28.16.e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Studier FW. Protein production by auto-induction in high-density shaking cultures. Protein Expression Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Zimmermann JL, Nicolaus T, Neuert G, Blank K. Thiol-based, site-specific and covalent immobilization of biomolecules for single-molecule experiments. Nat. Protoc. 2010;5:975–985. doi: 10.1038/nprot.2010.49. [DOI] [PubMed] [Google Scholar]
- 39.Yin J, Lin AJ, Golan DE, Walsh CT. Site-specific protein labeling by Sfp phosphopantetheinyl transferase. Nat. Protoc. 2006;1:280–285. doi: 10.1038/nprot.2006.43. [DOI] [PubMed] [Google Scholar]
- 40.Gumpp H, Stahl SW, Strackharn M, Puchner EM, Gaub HE. Ultrastable combined atomic force and total internal fluorescence microscope. Rev. Sci. Instrum. 2009;80:063704. doi: 10.1063/1.3148224. doi: 10.1063/1.3148224. [DOI] [PubMed] [Google Scholar]
- 41.Hutter JL, Bechhoefer J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993;64:1868. doi: 10.1063/1.1143970. [Google Scholar]
- 42.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J. Mol. Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 43.Kalé L, Skeel R, Bhandarkar M, Brunner R, Gursoy A, Krawetz N, Phillips J, Shinozaki A, Varadarajan K, Schulten K. NAMD2: Greater Scalability for Parallel Molecular Dynamics. J. Comput. Phys. 1999;151:283–312. [Google Scholar]
- 44.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, Schulten K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Best RB, Zhu X, Shim J, Lopes PEM, Mittal J, Feig M, MacKerell AD., Jr. Optimization of the Additive CHARMM All-Atom Protein Force Field Targeting Improved Sampling of the Backbone ϕ, ψ, and Side-Chain χ 1and χ 2Dihedral Angles. J. Chem. Theory Comput. 2012;8:3257–3273. doi: 10.1021/ct300400x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacKerell AD, et al. All-Atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins. J. Phys. Chem. B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 47.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983;79:926–934. [Google Scholar]
- 48.Darden T, York D, Pedersen L. Particle mesh Ewald: An Nlog(N) method for Ewald sums in large systems. J. Chem. Phys. 1993;98:10089–10092. [Google Scholar]
- 49.Izrailev S, Stepaniants S, Balsera M, Oono Y, Schulten K. Molecular dynamics study of unbinding of the avidin-biotin complex. Biophys. J. 1997;72:1568–1581. doi: 10.1016/S0006-3495(97)78804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frishman D, Argos P. Knowledge-based protein secondary structure assignment. Proteins Struct. Funct. Bioinf. 1995;23:566–579. doi: 10.1002/prot.340230412. [DOI] [PubMed] [Google Scholar]
- 51.Ribeiro JV, Tamames JAC, Cerqueira NMFSA, Fernandes PA, Ramos MJ. Volarea - A Bioinformatics Tool to Calculate the Surface Area and the Volume of Molecular Systems. Chem. Biol. Drug Des. 2013;82:743–755. doi: 10.1111/cbdd.12197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.