Abstract
Plus strand RNA viruses that replicate in the cytoplasm face challenges in supporting the numerous biosynthetic functions required for replication and propagation. Most of these viruses are genetically simple and rely heavily on co-opting cellular proteins, particularly cellular RNA-binding proteins, into new roles for support of virus infection at the level of virus-specific translation, and building RNA replication complexes. In the course of infectious cycles many nuclear-cytoplasmic shuttling proteins of mostly nuclear distribution are detained in the cytoplasm by viruses and re-purposed for their own gain. Many mammalian viruses hijack a common group of the same factors. This review summarizes recent gains in our knowledge of how cytoplasmic RNA viruses use these co-opted host nuclear factors in new functional roles supporting virus translation and virus RNA replication and common themes employed between different virus groups.
Keywords: RNA virus, enterovirus, poliovirus, flavivirus, norovirus, Hepatitis C virus, coronavirus, PTB, PCBP2, La protein, UNR, SRp20, hnRNP C, hnRNP A1, hnRNP K, hnRNP M, RNA helicase A, NSAP, Tia1/TIAR, G3BP1
Introduction
Viral spread and ultimately pathogenesis requires efficient replication in key host cells that aid spread of the virus within hosts and throughout host populations. RNA viruses are typically small, encoding as little at three genes, and thus must rely on many host factors interacting with viral RNAs to assist with essential replication functions, and control many interaction points within host cells to promote replication. This often results in redirecting host metabolism on several levels to support the infection and at the same time suppress innate host defense systems that are triggered. Comparing plus and minus stranded RNA viruses, there are stark differences at the time of uncoating of genomic viral RNA in the cytoplasm. The plus strand RNA virus genome that is released is naked, however the minus strand RNA virus genome is completely enclosed in a functional nucleocapsid with RNA replicase poised ready to produce transcript mRNAs. Thus, the plus strand virus RNA can, and does, interact with many host RNA binding proteins (RBPs), whereas there is little opportunity for minus strand virus genomic RNA to interact directly with host RBPs. Most RNA-binding proteins are nuclear shuttling proteins and many more nuclear RBPs have been reported to play roles in replication of plus strand RNA viruses than minus strand RNA viruses. Accordingly, this review focuses heavily on plus stranded RNA viruses, particularly mammalian viruses.
RNA viruses interact with a multitude of host factors during the course of infection. Several screening approaches have been employed to identify which of the 15—20,000 proteins that may be expressed in a given cell, are host factors required for RNA virus replication. These include genetic screens in yeast that implicated 130 proteins that could affect plant virus replication (tomato bushy stunt virus) (Y. Jiang et al., 2006) and about 100 genes that affect brome mosaic virus (Kushner et al., 2003; Panavas et al., 2005). RNAi knockdown studies in mammalian cells with Hepatitis C virus (HCV), Dengue virus (DENV) and West Nile virus (WNV) have identified several hundred other genes that affect virus replication. However, many or most of these may function quite indirectly, affecting pathways that produce metabolites or products the virus needs, movement or trafficking of constituents that are directly required, factors that control divalent cation fluxes and ATPase pumps, the stress or innate immune activation levels that counteract general cellular biosynthetic potential, or include general off-target effects from the silencing step. It is likely that the spectrum of factors that directly interact in meaningful ways with virus RNA and proteins will be larger than that known today, but also smaller than the first lists that have emerged from such screenings. Recently the novel approach of thiouracil cross-linking mass spectroscopy (TUXMS) was used to more precisely identify host proteins bound to poliovirus RNA during replication. This procedure identified all proteins known to interact with enterovirus RNA, plus 66 additional factors previously unidentified (Lenarcic et al., 2013). Eight of the new proteins were chosen and validated as playing roles in replication, indicating this new method is powerful and should be applied to other virus systems. However, standard molecular biology and biochemical approaches will still be required to tease out the functions and impact of each of these factors on virus replication. Proteins that interact with viral RNA do not present interesting targets for antiviral development unless it is determined that they play critical roles in virus replication.
Plus strand RNA viruses must translate incoming viral genomic RNA as the first biosynthetic step in replication cycles, thus, control of translation becomes the first battleground with the host that involves co-opted nuclear factors. It makes sense for the virus to utilize the host factors it commonly encounters at sites of replication. Thus, translation regulation involves virus co-opting of cellular translation factors. These are mostly cytoplasmic resident proteins since translation is a cytoplasmic process. However, translation does not occur on transcripts that are naked and devoid of RNA-binding proteins, rather, cellular transcripts are continually bound to a host of RNA binding proteins from the instant they emerge from RNA polymerase during their synthesis. In mammalian cells, RNA binding proteins control most aspects of RNA biology and the RNA cycle; from splicing, transport out of the nucleus, cellular function, transcript-specific translation control, and cytoplasmic localization and mRNA half-life. Mammalian cells encode hundreds of RBPs (~860), most with several splice variants (Castelló et al., 2012). The cytoplasmic milieu encountered by plus strand RNA virus genomes as they are released from capsids is poised to greet the interloper as any other mRNA, with a ready store of RNA binding proteins ready to interact and impart functions. No wonder viruses have evolved to interact with RBP in diverse ways to promote replication. Recent research indicates that interactions of viruses with RBP can both benefit and inhibit virus replication, depending on stages of the replicative cycle. RBP interaction with complexes may afford molecular switching events that promote RNA replication over translation as one example.
The complete virus “interactome” will contain proteins that provide a diverse range of functions during virus replication; including protein and RNA chaperone functions, transport functions, helicases, factors and enzymes that regulate vesicular traffic, membrane functions and lipid metabolism. Notably, many of these are cytoplasmic proteins, thus beyond the purview of this review. Most of the nuclear RBPs are constantly shuttling between the nucleus and cytoplasm, thus, the definition of nuclear factor in this context refers solely to shuttling proteins that are more concentrated in the nucleus under normal physiological conditions.
Nuclear pore disruption: How cytoplasmic viruses get their stuff
An infecting virus may encounter sufficient nuclear shuttling proteins to initiate translation or the first rounds of RNA replication. However, rapidly expanding RNA virus replication requires a growing storehouse of supplies and will quickly expend the limited cytoplasmic supply of many nuclear factors. It was noticed early on during studies of poliovirus-infected cells that many nuclear resident proteins showed improper cytoplasmic localization, including PTB, La autoantigen, Sam68, nucleolin and others (Back et al., 2002; McBride et al., 1996; Meerovitch et al., 1993; Waggoner and Sarnow, 1998). In fact, many viruses disrupt trafficking of proteins and mRNPs at the nuclear pore as a mechanism to restock the cytoplasmic storehouse with needed supplies. This dysregulation is usually required for efficient viral replication and can involve increased export of outbound cargo from the nucleus and/or blockage of inbound cargo from the cytoplasm. Detailed descriptions of the mechanisms involved are beyond the scope of this article, but recent reviews cover advances in virus disruption of nuclear pores (Le Sage and Mouland, 2013; Yarbrough et al., 2014). The range of viruses known to disrupt nuclear-cytoplasmic traffic is diverse and includes viruses that replicate in the cytoplasm (enterovirus, cardiovirus, coronavirus, rhabdovirus) and viruses that replicate in the nucleus (influenza, HIV-1, Herpes simplex virus 1, Adenovirus). The mechanisms that disrupt nuclear transport are also diverse. Just two examples are enteroviruses that employ viral 2A proteinase to cleave at least three nuclear pore proteins (Nup 62, Nup 98, and Nup 153) (N. Park et al., 2008; 2010; Watters and Palmenberg, 2011) and cardiovirus that employs L protein to induce a phosphorylation cascade that results in hyperphosphorylation of Nup 98, Nup62, Nup153 and Nup 214 as well as dysregulation of the pore complex (Bacot-Davis and Palmenberg, 2013; Basta et al., 2014; Porter and Palmenberg, 2009; Ricour et al., 2009; Watters and Palmenberg, 2011). In addition, L protein directly binds and inhibits the active cellular transport protein RAN GTPase (Bacot-Davis and Palmenberg, 2013). Notably some of the Nup targets are the same for both picornavirus genera, an example of convergent evolution providing two different approaches to accomplish similar functional goals.
Hijacked proteins regulate virus translation in IRESomes
ITAFs function in IRESomes
Picornaviruses such as poliovirus (PV) and encephalomyocarditis virus (EMCV) do not contain m7GTP cap structures on genomic RNA to recruit ribosomes, instead they use an internal cap-independent mode of translation that requires a large folded RNA structure to recruit ribosomes called an internal ribosome entry site (IRES). IRES elements have complex RNA folds and are only active when bound with specific proteins called IRES trans-activating factors (ITAFs) that are thought to provide RNA chaperone functions. ITAFs are not canonical translation initiation factors that function in cap-dependent translation but are proteins known to play primary roles in other aspects of RNA biology in the cell. ITAFs are all of cellular, not viral origin, which makes sense for plus strand viruses because the IRES must function before any viral proteins can be synthesized. The IRES plus the required ITAFs and canonical translation factors that make up a functional unit are referred to as IRESomes because they function together as a complex. The functional relationships between ITAFs, canonical translation factors and ribosome recruitment are still unclear but significant strides have been made in understanding them in three virus systems. Although the first ITAFs were discovered in the context of viral IRES translation, many of the same ITAFs are thought to play similar roles promoting cap-independent translation for cellular IRES elements. For example, PTB is an ITAF for BIP, BAG1, Apaf-1, UNR, p53 IRESs; hnRNP A1 is an ITAF for Cyclin D1 and c-myc IRESs; and hnRNP C1/C2 is an ITAF for XIAP and c-myc IRESs (reviewed in (King et al., 2010)).
Types of viral IRESs
There are distinct classes of virus IRESs that are referred to by a somewhat inconsistent and evolving nomenclature. Picornavirus IRES elements can be classified into at least five types based on structure, limited sequence homology and phylogeny. Type 1 IRESs are large ~450 nucleotide segments that are encoded by poliovirus and other enteroviruses; Type 2 IRESs are similarly large and occur in cardioviruses (EMCV), aphthoviruses (FMDV) and parechoviruses; and Type 3 IRES occurs only in Hepatitis A virus (HAV). Type 4 IRESs (sometimes called Class 2 or Type 3) are smaller ~ 330 nucleotide segments and occur in non-picornaviruses (hepatitis C virus and pestiviruses) but are also found among the newer picornavirus families Teschovirus, Sapelovirus, Senecavirus, Tremovirus. Type 5 IRESs are encoded in the newly defined Picornavirus genera Kobuvirus, Salivirus and Paraturdivirus (Sweeney et al., 2012).
ITAFs of Type 1 and Type 2 IRESs
One of the first host proteins ever shown to interact with picornavirus RNA by UV-crosslinking was polypyrimidine tract binding protein 1 (PTB1, shortened to PTB) (Hellen et al., 1993). PTB is a shuttling, but mostly nuclear resident protein that associates with pre-mRNAs and plays roles in premRNA processing, alternative splicing, mRNA metabolism and transport (Kafasla et al., 2010; Sawicka et al., 2008). PTB binds polypyrimidine tracts in pre-mRNA introns to repress exon inclusion but can actually bind quite varied RNA structures. Thus, PTB can also stabilize certain mRNAs against degradation by binding to the 3′ untranslated regions. In virus replication schemes PTB plays roles that support and stimulate cap-independent translation driven by picornavirus and HCV RNA. PTB exists as one of three alternatively spliced isoforms (PTB1, PTB2, PTB4) and contains four-RNA binding domains (RBDs) of the RNP1/RNP2 class (Kafasla et al., 2010; Sawicka et al., 2008).
Though PTB was the first, a wide spectrum of factors, largely nuclear RBPs, have also been proposed as poliovirus ITAFs, including Lupus autoantigen (La) (Meerovitch et al., 1993; 1989), poly(rC)-binding proteins (PCBPs) (Blyn et al., 1996; Gamarnik and Andino, 1997), upstream of N-ras (UNR) (Anderson et al., 2007; Boussadia et al., 2003; Hunt et al., 1999), SRp20 (Bedard et al., 2007) and glycyl-tRNA synthetase (GARS) (Andreev et al., 2012). In order to make sense of the relative impact of this range of factors on basic translation, Pestova and Hellen have used in vitro translation initiation experiments reconstituted with a complete set of purified factors to define the minimal set of factors absolutely required for IRES translation. In these reconstituted translation systems, the HCV class of IRES minimally requires no ITAFs and only translation factor eIF3 to bind ribosomes (Hellen, 2009; Pestova et al., 1998). For both picornaviruses EMCV and PV, several canonical translation factors and only one ITAF is minimally required. EMCV requires only PTB as an ITAF and poliovirus requires only PCBP2 as an ITAF to initiate translation (Pestova et al., 1996; Sweeney et al., 2014). However, minimal requirements in vitro likely do not reflect the conditions of severe competition among mRNAs for ribosomes in cells, suggesting the other factors proposed as ITAFs may play less fundamental, but still critical roles during infection and can contribute to pathogenesis. Additionally, certain ITAFs play crucial roles in regulating the conversion of translation-competent genomic RNAs into replication-competent RNAs.
PTB regulation of Type1 and Type 2 IRESomes
PTB was known as a nuclear splicing factor when its role as an ITAF of poliovirus and EMCV was discovered; a classic hijacked nuclear protein pressed into a new role required for the virus. However, PTB is now known to also support cap-independent translation of cellular IRES-containing mRNAs that were discovered after the viral IRESs. These include Apaf-1, Bag-1 and mutant forms of c-myc transcripts associated with more aggressive tumor growth (Cobbold et al., 2010; Sawicka et al., 2008). Thus PTB can interact with a wide range of RNAs to promote cap-independent translation and may be considered a pro-translation, general ITAF.
Continuous investigations of PTB have revealed biochemical details about how it supports IRESome function in virus translation. The RNA binding function of PTB is modular and split between four RNA-binding domains (RBDs) that are distributed along an overall extended and flexible structure. This extended flexible nature of PTB is important for IRES function. The PTB RBDs recognize short pyrimidine-rich sequences but have distinct RNA structural preferences. The two N-terminal RBDs (RBD1 and RBD2) recognize short pyrimidine tracts contained in loops, while the RBDs3-4 preferentially bind to larger flexible RNA sequences. These features give PTB the ability to bind to a variety of RNA structures and enables PTB to function as a versatile adaptor protein that facilitates formation of many RNA-protein regulatory complexes (Clerte and Hall, 2009).
Recently, EMCV and poliovirus IRES structures have been used most extensively as models to study the ITAF functions of PTB. Mapping studies indicated two PTBs moieties bind the EMCV IRES, one upstream in a non-essential region of the IRES and another in the IRES core. In the core, the orientation of a single PTB binding to IRES was determined by binding of PTB mutants and hydroxyl radical probing. RBD1 and RBD2 bind near the 3′ end of the core IRES sequence, and RBD3 binds near the 5′ end. Binding of PTB to multiple regions on the IRES simultaneously enables stabilization and constraint of the IRES tertiary structure and is consistent with multiple RBD-RNA interactions proposed in the splicing functions of PTB (Oberstrass et al., 2005) (Kafasla et al., 2009). This illuminates how an ITAF mechanistically provides an RNA-chaperone function. PTB is thought to promote RNA looping also, partly because RBDs 3 and 4 bind RNA in anti-parallel directions (Oberstrass et al., 2005) (Lamichhane et al., 2010).
Further work established which of the multiple RBD-IRES interactions are critical for ITAF function. Using PTB with point mutations in various RBDs, it was shown that both poliovirus and EMCV IRES RNAs required simultaneous interaction with three of the four PTB RBDs, however the list of most critical RBDs differed between the viruses (Kafasla et al., 2011). This indicates the modular and elongated nature of PTB can adapt to disparate RNA structures using different sets of RBDs to provide similar chaperone function.
Besides providing an RNA chaperone function, PTB also provides a second critical function as it helps recruit or position the canonical translation initiation factor eIF4GI on the IRES (Fig. 1A). In the case of PV, both PTB and eIF4G bind the same RNA stem loop structure (stem loop V) comprising much of the IRES core. The orientation of PTB binding is such that RBDs 3-4 bind the base of stem loop V, whereas RBDs 1 and 2 bind the stem loop itself in the same region proposed to bind the central domain of eIF4GI. The two proteins do not compete for binding, rather PTB is thought to stimulate IRES activity by ensuring eIF4GI binds in the correct orientation and position to function properly for ribosome entry at a nearby AUG (Kafasla et al., 2010).
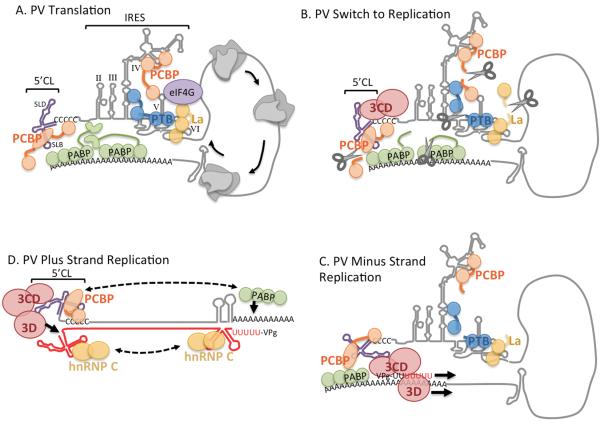
Figure 1.
Cartoon illustrating roles of RBPs in PV replication. A. PV Translation. Host factor PCBP binds both cloverleaf stem-loop B (SLB) and IRES SLIV, PTB binds SLV and together with La helps eIF4G and other canonical translation factors (not shown) bind properly to recruit ribosomes. ITAFs provide RNA chaperone functions and modular binding motifs link and fold different stem loops. Interactions of PABP with PCBP and PABP with initiation factors (eIF4G, eIF4B interactions not depicted) help close the RNA 5′-3′ loop and facilitate ribosomes recycling from stop codon to start codon. Other ITAFs for PV mentioned in the text are not depicted. B. Switch to Replication. Viral polymerase precursor 3CD binds 5′CL SLD, together with cleavage of PCBP, PTB and La by 3Cpro (scissors), converts the template to translation-initiation-incompetent state, plus blocks ribosome recycling and thus ribosomes runoff and clear the template. C. PV Minus Strand Replication. Replicase complex consisting of interacting 3CD-3Dpol complexes initiates replication. The complex is oriented properly via PCBP binding PABP. D. PV Plus Strand Replication. The double-stranded RF replication intermediate (nascent negative strand shown in red) breathes allowing cloverleaf and anti-cloverleaf to form, stabilized by binding PCBP and hnRNP C respectively. Polymerase replicase complex also builds on 5′ CL and initiates replication on negative strand template that is properly positioned for precise-end initiation. hnRNP C can also interact with SL on the 5′ end of the negative strand that also requires breathing to form. In this scenario PABP may be able to rebind to poly(A) tail and hnRNP oligomerization and PABP interaction with PCBP may facilitate genome circularization in double-stranded RF intermediate.
Other picornaviruses also utilize PTB as an ITAF. Coxsackievirus B3 is another enterovirus closely related to poliovirus with a Type 1 IRES, thus there is no surprise that PTB interacts with CVB3 RNA and promotes translation in a similar fashion as an ITAF. However, PTB also interacts with the CVB3 3′ UTR and this interaction may stimulate IRES translation through long-range looping or genome circularization bridged by PTB (Verma et al., 2010). Foot-and-mouth disease virus IRES is a Type 2 IRES structure distantly related to EMCV, sharing little sequence homology but some secondary structural similarity. However, FMDV requires both PTB and ITAF 45 (also known as Ebp1) to minimally support translation, whereas EMCV requires only PTB. The FMDV IRES also binds three of four PTB RBDs, where RBDs 3 and 4 bind distally located regions of the IRES and can function as a minimal IRES when supplied as a truncated form of PTB (Song et al., 2005). Changes in IRES structure were analyzed to compare effects of ITAF binding on RNA conformation shifts in EMCV and FMDV IRESs. Despite the different ITAF requirements, in both cases when ITAFs interacted with their cognate IRESs, similar conformation changes occurred where two domains were brought into closer compacted proximity (Yu et al., 2011a). Thus, PTB provides the same structural role building the functional IRESome, whether alone or in conjunction with other ITAFs.
PCBP regulation of Type1 IRESomes
Poly(rC)-binding protein (PCBP) is the only obligate ITAF For enteroviruses using TYPE 1 IRESs (Sweeney et al., 2014). Unlike PTB, that binds widely separated sites on the EMCV IRES, PCBP binds to a restricted area of stem loop IV of the PV IRES (Fig. 1a). This binding region of SLIV is distant from the IRES core in the two dimensional secondary structure (Blyn et al., 1996). But PCBP also binds stem loop B of the cloverleaf structure (CL) at the 5′ terminus of enterovirus RNA (Fig. 1) (Gamarnik and Andino, 2000; 1997). There are four PCBP isoforms, however PCBP1 and PCBP2 are expressed in more abundance and both bind PV IRES. PCBP1/2 isoforms can form dimers or heterodimers and each can interact with PV IRES RNA, but PCBP2 plays a more dominant role. PCBP binds RNA through three KH domains and interacts 6-fold more strongly with the IRES than cloverleaf RNAs in isolation (Gamarnik and Andino, 2000). PCBP2 is the isoform required for both translation initiation and also for RNA replication. KH1 is the primary domain that interacts with PV IRES domain IV (Silvera et al., 1999; Walter et al., 2002). For closely related CVB3, individual KH1 and KH3 domains of PCBP bind to IRES stem loop IV, KH1 interacts with subdomain IV/C RNA, whereas KH3 interacts with subdomain IV/B (Zell et al., 2008a).
Another host factor, SRp20, interacts with PCBP2 through its KH3 domain and was found to be critical for ITAF function of PCBP2 in cells. In vitro translation in HeLa cell extracts depleted of SRp20 are deficient in supporting poliovirus translation initiation and SRp20 siRNA knockdown in HeLa cells restricted poliovirus translation. (Bedard et al., 2007). Like other ITAFs, SRp20 is strongly relocalized from the nucleus to cytoplasm during poliovirus infection. SRp20 may bind PCBP2 rather than viral RNA directly since deletion of its RRM RNA-interaction motif does not alter its localization during infection, however, this truncated form strongly repressed virus translation, likely via a dominant negative process (Fitzgerald and Semler, 2011).
Roles of other ITAFs for Type 1 IRESs
Many other factors have been shown to promote virus translation and have been considered ITAFs or ITAF ancillary factors, but compared to PCBP and PTB much less is known about their function at the biochemical level in promoting IRES translation. The binding sites of some of these other ITAFs are poorly characterized. La enhances and corrects aberrant translation of PV in reticulocyte lysates that lack other ITAFs (Meerovitch et al., 1993) and 40S ribosome subunit binding to PV IRES is inhibited by a dominant negative La protein (Costa-Mattioli et al., 2004). Nucleolin interacts with poliovirus IRES and enhances IRES-dependent translation (Izumi et al., 2001). UNR has also been reported to interact with IRES elements (PV and rhinovirus) acting as an RNA chaperone and also binds PABP that is bound to poly(A) tails in cellular mRNAs (Anderson et al., 2007; T.-C. Chang et al., 2004; Hunt et al., 1999). Presumably this interaction may also occur with viral polysomes and can contribute to genome looping or circularization that makes continuous translation more efficient. That UNR is important in vivo was demonstrated by gene knockout of both UNR alleles in mouse cells that reduced translation of PV and human rhinovirus (HRV) IRES’s by 90% and could be rescued by introduction of UNR expression plasmid (Boussadia et al., 2003). RNA affinity pulldown of cellular proteins using EV71 5′ UTR bait revealed PTB, unr, PCBP1/2, hnRNP A1 and 10 other proteins. hnRNP A1 interacts with stem-loops II and VI of the EV71 5′UTR, and is proposed to be another enterovirus ITAF. The roles of this interaction in replication are unclear since knockdown of hnRNP A1 had no effect on viral replication. In contrast, knockdown of both hnRNPs A1 and A2 reduced viral RNA synthesis and virus output, suggesting that hnRNP A2 can substitute for hnRNP A1 (Lin et al., 2009), however the relative importance of hnRNP A1 versus PCBP2 or PTB in EV71 replication was not evaluated. GARS is perhaps the most unusual proposed ITAF; a tRNA synthetase housekeeping enzyme that binds the IRES core in the apical portion of domain V that mimics the anticodon-stem-loop of tRNAGly (Andreev et al., 2012).
So what are we to make of all the proposed ITAFs for PV and other closely related enteroviruses? Recently, in vitro reconstitution of translation with purified factors has been accomplished on the PV IRES and other Type 1 IRESs and those experiments indicate that PCBP2 is the only mandatory ITAF required to accomplish 48S ribosome assembly and initiation. PTB showed minor stimulation of translation, and other proposed ITAFs (La, UNR, SRp20, GARS) produced no measureable effect on 43S or 48S translation complex formation in vitro. PCBP2 may also promote recruitment of 43S complexes by direct interaction with eIF3 (Sweeney et al., 2014). The weak stimulation of PCBP2-dependent 48S complex formation by PTB in vitro is likely due to its imposed reorientation of eIF4G binding to the IRES (Kafasla et al., 2010). But many ITAFs promote translation in cells by providing functions that promote efficiency, beyond the minimal requirements for IRES translation initiation. Intracellular conditions encountered by viruses include intense competition among mRNAs for ribosomes in cells, forcing viruses to utilize multiple mechanisms to seize translation control. Thus, the other ITAFs that aid IRES translation efficiency in vivo play less fundamental, but still critical roles during infection and contribute to pathogenesis. Future work will discern new molecular details how this happens.
ITAFs of Type 3 IRESomes
Comparatively little is known about factor requirements for the Type 3 IRES of HAV. Unlike most of its picornavirus cousins, this virus replicates very slowly, does not cause host translation shutoff and is relatively non-cytopathic in tissue culture cells. The IRES is unique in that it requires intact eIF4G1, whereas the PV IRES functions better with only the cleaved central domain of eIF4GI (Borman and Kean, 1997; Hambidge and Sarnow, 1992). Both PTB and PCBP have been implicated as ITAFs for HAV (Yi et al., 2000; B. Zhang et al., 2007). HAV 3Cpro inhibits HAV IRES-dependent translation and cleaves PTB. This finding suggests PTB cleavage regulates the switch from viral translation to RNA replication and strengthens a role of PTB as an ITAF for this IRES (Kanda et al., 2010).
ITAFs of Type 4 IRESomes
The smaller type 4 IRES elements (~300 nt) Hepatitis C virus (HCV) and pestiviruses such as bovine viral diarrheal virus (BVDV) and classical swine fever virus (CSFV) have been extensively studied. The HCV IRES has simpler factor requirements than Type 1 or 2 IRESs. It requires only one canonical initiation factor, eIF2, and no ITAFs to form 48S translation initiation complexes in vitro with reconstituted systems, interacts directly with ribosomes and ribosomal 18S RNA (Malygin et al., 2013; Pestova et al., 1998) and can recruit translation factor eIF3 (Ji et al., 2004; Siridechadilok et al., 2005). Despite its simpler minimal requirements in vitro, like poliovirus, several factors have been described that enhance HCV translation in lysate systems and cells, including La protein. PTB also weakly interacts with the IRES, but appears to attenuate translation instead of stimulate it and its putative role as a functional ITAF involved in ribosome recruitment has been challenged (Brocard et al., 2007; Domitrovich et al., 2005; Ito and Lai, 1999),
Several lines of evidence indicate that La is an ITAF. La enhances translation in the context of the competitive translation environment during infection in cells with replicons or virus. siRNA depletion of La inhibits HCV translation and a dominant negative form of La inhibits translation (Costa-Mattioli et al., 2004) in vivo. Further, the fact that La protein can be exploited as a therapeutic target for HCV suggests a significant role in HCV replication. A cell permeable peptide corresponding to the N-terminal 18 amino acids of La inhibits HCV IRES-mediated translation and also inhibits HCV replication in cells (Fontanes et al., 2009). La-peptide-mediated inhibition of HCV IRES-translation blocked interaction of La and other ITAFs (PTB and PCBP2) with the IRES, but could be rescued with exogenous PTB and PCBP2. This implied that La peptide sequesters PTB and PCBP2 as its mode of action (Fontanes et al., 2009).
Other knockdown experiments implicated several host factors in HCV translation or HCV virus replication in cells, including La, PTB, PCBP2 and proteasome alpha-subunit 7 (PSMA7) (Kruger et al., 2001; Shirasaki et al., 2010). All four factors are induced in Huh-7.5 cells during HCV replication, suggesting the virus has co-opted factors that are naturally up-regulated during infection. shRNA knockdown of La repressed production of HCV core protein 70%, further supporting a role as an ITAF in vivo. La has been reported to be a telomerase component, thus telomerase activity and expression of other telomerase subunits increased coordinately with La induction during chronic HCV infection (Shirasaki et al., 2010). The linkage of HCV-induced La expression to increased telomerase activity in chronically-infected liver may be important in development of hepatocellular carcinoma.
Evidence from in vitro translation experiments in somewhat artificial rabbit reticulocyte lysates suggest that PTB is not a true HCV ITAF that facilitates 40S ribosome subunit binding to the HCV IRES. Rather, PTB may stimulate translation indirectly through bringing other factors bound to the X region of the 3′ UTR into proximity with the IRES (Brocard et al., 2007). PTB is proposed to loop HCV RNA by binding the 3′ UTR X region and 5′ UTR IRES region simultaneously. Another factor, NSAP1 (also called hnRNP Q) stimulates HCV IRES translation by binding an A-rich region downstream of the initiator AUG and facilitating formation of 48S ribosome initiation complex by also binding the 40S ribosome proteins. This is the first reported interaction of an ITAF directly with a ribosome, thus NSAP1 provides more of a translation-factor role than other canonical ITAFs that play “chaperone-like” roles (J. H. Kim et al., 2004; S. M. Park et al., 2011).
ITAFs of Type 5 IRESs
PTB also supports translation initiation of Aichivirus (AV) that contains a novel type of picornavirus IRES that differs structurally from Type 1 and 2 IRESs. In reconstitution experiments this IRES requires interaction with both eIF4G and PTB. Unlike other IRESs, the AV IRES has a strong requirement for a novel nuclear factor DHX29, that is also a ribosome-associated helicase. DHX29 releases the AV IRES initiation codon from a strong stable hairpin to aid anticodon base pairing by the ribosome (Yu et al., 2011b).
ITAFs are determinants of tissue and host tropism
Various ITAFs are either essential for virus translation or play less critical roles that enhance efficiency of translation. High translation efficiency is critical for most viruses to produce sufficient proteases or other factors to control activation of innate immune pathways that would block further replication. Therefore, ITAFs have long been considered host range factors that determine tissue and host tropism and pathogenesis. The attenuated Sabin vaccine strains of poliovirus lost neurovirulence partly through nucleotide changes in the IRES that alter PTB binding (Wimmer et al., 1993). Restricted IRES function will result from a lack of the proper ITAF in certain cells. For instance, a neuronal variant of PTB (nPTB) is required for the neurovirulence of the GDVII strain of TMEV (Pilipenko et al., 2001). PTB1 expression patterns have a powerful tropic effect on a chimeric poliovirus containing the HRV2 IRES element (called PV1(RIPO)). This virus has a growth defect in mouse cells because it cannot interact with endogenous murine PTB (Jahan et al., 2013; 2011). This is proposed to interfere with the thermodynamics of folding of the IRESome into a functional structure. The virus also has a severe neuro-attenuation defect in humans but retains highly specific virulence against glioblastoma cells and HeLa cells that express high levels of PTB (Gromeier et al., 2000).
Nuclear factors that antagonize cap-independent viral translation
Modern proteomic approaches are now being applied to identify the full complement of host factors that interact with viral RNAs, and have identified over 30 proteins that interact with FMDV IRES directly or indirectly (Pacheco et al., 2008). Of these Gemin5 was found to bind to the IRES at a domain 5 hairpin flanked by A/U/C-rich sequences via its C-terminal domain. Gemin5 is the RNA-binding component of the survival of motor neuron (SMN) complex that assembles Sm proteins onto spliceosomal snRNAs. Gemin5 binding did not enhance FMDV translation, but rather inhibited it. RNA structure analysis using 2′hydroxyl acylation analyzed by primer extension (SHAPE) revealed Gemin5 induced conformation changes that out-competed SHAPE changes induced by PTB. Thus Gemin5 may competitively inhibit PTB ITAF binding (Piñeiro et al., 2013). Gemin5 also interacts with the HCV IRES and may provide similar functions (Piñeiro et al., 2013). Gemin5 is cleaved in FMDV infection by L protease (Piñeiro et al., 2012) similar to Gemin3 cleavage by poliovirus that blocks assembly of the SMN complex (Almstead and Sarnow, 2007). Gemin5 cleavage alleviates translational repression of the IRES. The similarity of Gemin 5 and Gemin 3 cleavages suggests that inactivation of the SMN complex may be required by a wide range of picornaviruses.
RNA decay regulator AUF1 (which has 4 isoforms) is cleaved by poliovirus and rhinovirus 3CD proteinase. AUF1 is a factor that binds AU-rich elements in mRNA 3′ UTRs and promotes rapid mRNA decay and turnover. AUF1 follows the familiar pattern of strong relocalization from nucleus to cytoplasm during poliovirus infection (Rozovics et al., 2012). Surprisingly though, AUF1 does not bind PV 3′ UTR but rather binds the IRES at stem loop IV and negatively regulates rhinovirus and poliovirus infections via translation inhibition. However, discordant findings with closely related CVB3 indicated that AUF1 bound to the 3′ UTR (Wong et al., 2013). 3Cpro cleavage inactivates RNA binding function of AUF1 and relieves translation restriction (Cathcart et al., 2013). Thus, both AUF1 and Gemin5 act as a “hijacked” restriction factor in translation and must be remedied by inactivation by a viral proteinase. However, AUF1 may not be a restriction factor for all picornaviruses. EMCV infection induces strong cytoplasmic relocalization of AUF1, but was not cleaved by EMCV 3Cpro, even at late times after infection (Cathcart and Semler, 2014).
Caliciviruses such as feline calicivirus and human norovirus share a non-structural protein coding region that is distantly related to picornaviruses, yet translate by an unusual cap-independent mechanism that does not involve an IRES. Rather, translation of norovirus involves interaction of initiation factor eIF3 with the viral VPg protein covalently linked to the 5′ end (Daughenbaugh et al., 2003; 2006). PTB interacts with both 5′ and 3′ ends of feline calicivirus genomic RNA, and also subgenomic RNA. FCV infection induces nuclear to cytoplasmic relocalization of PTB that is coincident with the switch from early translation to late RNA replication. PTB inhibited FCV translation in vitro, thus PTB is proposed to be a negative regulator that may aid the switch from translation to RNA replication (Karakasiliotis et al., 2010).
Nuclear factors may modulate flavivirus translation
Flavivirus RNA is unique in containing a 5′ cap structure, but no poly(A) tail to facilitate typical PABP binding that promotes 5′-3′ looping. Thus, flaviviruses use cap-dependent translation machinery but it is unknown how viral translation is promoted over cellular translation. Dengue virus promotes both cap-dependent translation and a form of non-canonical translation that is not IRES dependent and does not require a functional m7G cap structure (Edgil et al., 2006). Surprisingly, DENV can bind PABP in an A-rich region upstream of the 3′ SL, resulting in promotion of translation (Polacek et al., 2009) likely by binding cap-binding translation factor eIF4G and promoting 5′-3′ interactions similar to host mRNA (Fig. 2). DENV RNA is also known to bind several host factors that could play roles in translation or RNA replication, including PTB, La, Y box-binding protein1 (YB-1), translation elongation factor eEF-1a and p100/Tudor-SN (De Nova-Ocampo et al., 2002; García-Montalvo et al., 2004; Lei et al., 2011; Paranjape and E. Harris, 2007; Polacek et al., 2009; M. Yocupicio-Monroy et al., 2007; R. M. E. Yocupicio-Monroy et al., 2003). YB-1 binds the 3′ SL of DENV and represses translation, possibly in a role that regulates the switch from translation to RNA replication discussed below (Paranjape and E. Harris, 2007), and binding of eEF-1a to the 3′ SL has no effect on translation (Davis et al., 2007) (Fig. 2). La binds to both 5′ and 3′ UTRs (García-Montalvo et al., 2004) and could play a role stabilizing looped RNA structures via protein bridging and indirectly support translation through ribosome recycling. PTB has been proposed to play positive roles in virus replication but the mechanism(s) remains elusive. PTB relocalizes to the cytoplasm with variable completeness in infections in different cell types. PTB cytoplasmic localization is weak in Huh7 cells, however PTB binds the 3′ stem-loop region of DENV RNA in these infections (Fig.2) and could play roles in RNA looping to promote translation and RNA replication. Nuclear to cytoplasmic relocalization of PTB was associated with increased DENV translation and replication and siRNA knockdown of PTB inhibited replication and overexpression increased replication (Agis-Juárez et al., 2009). However, in Huh7 cells knockdown studies indicated PTB did not have an effect on DENV RNA translation, but promoted negative strand RNA synthesis and interacted with viral protein NS4A (L. Jiang et al., 2009). Thus, additional work is required to pin down the specific mechanisms of PTB-specific stimulation of DENV replication.
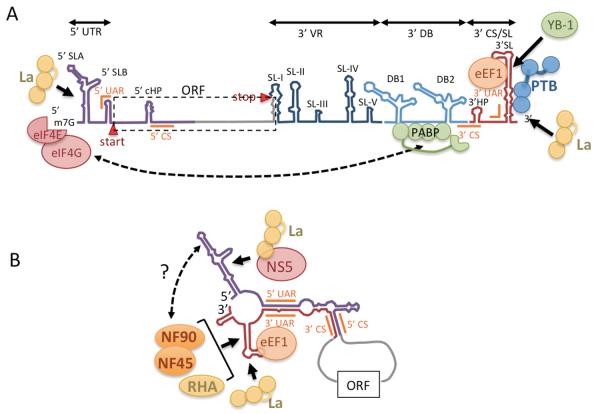
Figure 2.
A. Structural elements of flavivirus 5′ and 3′ UTRs based on Dengue virus sequences. Start and stop codons defining the open reading frame are shown. The 5′ UTR is ~95 nt and includes 5′ SLA, 5′ SLB. The flavivirus 3′ UTR is <380 nt and contains regions with conserved structural elements: VR (dark blue) with SL-I through IV, the 3′ DB region containing duplicate dumbbell structures (DB1 and DB2) (light blue) and the 3′ CS/SL region (red) containing the conserved 3′ SL. All structures from SL-1 through DB-2 participate in pseudoknots (not depicted). To facilitate translation poly(A)-binding protein binds A-rich sequences in the DB region allowing protein-bridge looping to translation factor eIF4G associated with the 5′ cap structure. YB-1 binds the 3′ SL and represses translation and replication. PTB and La bind 3′ UTR may also facilitate translation. B. Long range interaction between complimentary sequences in 5′ and 3′ regions (5′ CS, 3′ CS; 5′ UAR, 3′ UAR) facilitate genomic looping associated with RNA replication. This looping remodels RNA into new conformations that may promote binding of additional host RBPs not associated with translating DENV RNA such as eEF1, and the dsRNA binding proteins NF90, NF45 and RHA that bind the 3′ SL. In HCV, NF90 binds both 5′ and 3′ UTR of HCV RNA and RHA is involved in bridging 5′ and 3′ UTRs (not depicted). A similar arrangement may exist in flaviviruses where NF90 and RHA may also bind 5′ UTR stem loops and stabilize the looped structure to promote negative strand RNA replication. The NS5 RdRp binds to the 5′ SL1 which due to looping helps position the polymerase near the 3′ terminus (Bidet and Garcia-Blanco, 2014). NS5 may also be recruited by La, which binds the 5′ UTR.
Hijacked nuclear proteins regulate viral negative strand RNA replication
In theory, nuclear factors may not be necessary for support of viral RNA synthesis. Each virus synthesizes its own complete RNA-dependent RNA replicase, an enzyme function that is uniquely not present in host cells. Further, in the cytoplasm where virus replication occurs, viruses duplicate functions such as mRNA capping and polyadenylation that are carried out in the nucleus for host transcripts. Thus, one may not expect many dependent interactions between virus RNA replicases and host factors to have evolved to support viral RNA replication. For negative strand viruses that contain RNA covered in nucleocapsid proteins, this may be more expected. However, plus strand RNA viruses make extensive use of host RBPs for important roles in the replicative process, not in RNA polymerase enzymatic activity per se, but in promotion of its temporal and spatial regulation. A key feature for host factors is helping to organize complex 5′-3′ genome interactions in alternate configurations that sequentially promote translation, then RNA synthesis.
Regulated switch from viral translation to RNA replication
All plus strand RNA viruses are faced with the problem that the same genomic template is used for both 5′ to 3′ transit by ribosomes and 3′ to 5′ transit by the viral RNA polymerase. Since translation and RNA replication machinery proceed in opposite directions there must be a regulated switch from translation to RNA replication that clears the template of all elongating ribosomes before negative strand RNA synthesis can take place. For plus strand RNA viruses the details of this regulation are emerging. A common theme is that sufficient translation/production of key virus proteins is required before modifications are triggered in host factor structure/protein interactions that shift host factor roles from promoting translation to promoting RNA synthesis. Accumulating evidence indicates that both translation and negative strand RNA replication occur on templates circularized via complex RNA-RNA, protein-RNA and protein-protein interactions. Further, the switch in template use involves complex shifts in these interactions allowing host factors to change roles simultaneously.
PCBP2 helps mediate a switch from poliovirus translation to RNA replication due to changes in its RNA binding properties. PCBP binds both the IRES and 5′ cloverleaf (CL) stem b, and its binding the cloverleaf stimulates translation early during infection (Gamarnik and Andino, 1998). The PCBP complexed on the 5′ cloverleaf promotes translation in conjunction with the 3′ poly(A) tail in a circularization model based on protein-protein interactions. The C-terminal KH3 domain of PCBP is required to stimulate translation and can be tethered directly to PV RNA and still promote translation. In the current model the 5′CL-PCBP complex interacts with the 3′ poly(A)-PABP complex to form a 5′-3′ circular structure that enhances translation by facilitating ribosome reloading as ribosomes recycle from the stop codon (Fig. 1B) (Ogram et al., 2010). This is consistent with the finding that PCBP2 and 5′ cloverleaf function during de novo assembly of polysomes (Kempf and Barton, 2008).
Thus, PCBP strongly promotes translation by interaction with the 5′ CL in the phase before viral proteins accumulate.
But once virus proteins accumulate from sustained translation, PCBP bound to the 5′ CL also promotes binding of viral polymerase precursor 3CD to the adjacent cloverleaf stem loop D (SLD). The recruited 3CD promotes translation inhibition (Gamarnik and Andino, 1998). Further, cleavage of PCBP1 and 2 mediated by 3Cpro occurs during this phase of the replication cycle (Fig. 1B). Cleavage occurs between the KH2 and KH3 domains, resulting in truncated PCBP2 lacking the KH3 domain that cannot support translation but binds the CL and supports RNA replication (Perera et al., 2007).
In fact, several ITAFs for PV are cleaved by 3Cpro in midphase of the replication cycle. In addition to PCBP, PTB is cleaved by 3Cpro in a reaction that was shown to inhibit PV translation (Back et al., 2002) and La is similarly cleaved (Shiroki et al., 1999). Even though cleavage of PCBP2 and PTB inhibits poliovirus translation and contributes to a switch in RNA template usage, for related rhinoviruses things may be different. PCBP2 and PTB are differentially cleaved during infection with three HRV serotypes in Hela cells but are not cleaved in human lung epithelial cells that sustain productive infections. This suggests some ITAF cleavages may not be required by HRV to mediate a switch in template usage (Chase and Semler, 2014).
Like picornaviruses, HCV must clear genomic RNA templates of ribosomes to allow use by RNA replicase complexes. Several insights have emerged about how HCV regulates the switch and mechanisms involve both host factors and accumulation of nascent HCV proteins. Early studies showed HCV core protein binds to the IRES and linked translation production of HCV core protein to translation repression in a direct feedback loop (J. Zhang et al., 2002). Expression of HCV NS3 protease also has the effect of blocking IRES translation and increasing replicon replication. NS3 protease and La protein compete for binding the IRES in the same SLIV region, thus NS3 was proposed to directly inhibit ITAF binding to the IRES, thereby reducing translation activity (Ray and Das, 2011). Additional work indicated La protein binds to a GCAC sequence near the initiator AUG in SLIV of the IRES, but this enhances RNA replication, not translation, by promoting linkage between 5′ and 3′ UTRs through La (Kumar et al., 2013). Since both La and NS3 have similar dissociation constants for binding HCV IRES RNA, NS3 may not actually evict all the IRES-bound La, but competitively reach a binding equilibrium that promotes replication by recruiting NS5B to a replication complex involving La, NS3 and both ends of the viral RNA (Kumar et al., 2013). Another class of nuclear factors called NF/NFAR proteins NF90/NFAR (nuclear factor associated with dsRNA, also called ILF3) bind to both ends of the genome of HCV and the pestivirus BVDV(Isken et al., 2007; 2003; 2004). NF90/NFAR is part of group of interacting nuclear factors containing dsRNA-binding motifs and multiple isoforms that include NF45 and RNA helicase A (RHA). NF90/NFAR is associated with HCV translation inhibition and increased RNA replication (Isken et al., 2007; 2004; 2003).
Another group used siRNA approaches to show that efficient HCV translation was dependent upon several members of the cellular 5′-3′ deadenylation-dependent mRNA decay pathway, Rck/p54, LSm1, and PatL1. The requirement of these factors for efficient translation was linked to the 5′ and 3′-UTRs. The 3′ UTR also interacted with LSm1-7 complexes. All of these factors are core components of P-bodies where non-translating silenced mRNAs are stored before undergoing decay. This raises the possibility that the decay factors could play roles in the switch to replication, but the proposed role from experiments for HCV interaction with reconstituted LSm1-7 complexes was support of translation (Scheller et al., 2009). It is possible that co-opting P-body components downregulates functions of P-bodies that repress HCV translation. HCV infection results in significant reductions in the number of P-bodies in cells both in vitro and also in hepatocytes from patients infected with HCV (Pérez-Vilaró et al., 2014; 2012).
Host factors in genome circularization
RNA synthesis of viral negative strands initiates replication of genomic templates from the 3′-terminus. Thus, it was a surprise when RNA polymerase complexes for poliovirus was found to bind near the 5′ terminus of the plus strand genome template on the cloverleaf (Andino et al., 1993). This suggested that the template is circularized rather than linear to allow close proximity of the replicase complex and 3′ end of the template where the polymerase must initiate. Cellular mRNAs and viral genomes are thought to translate optimally on RNAs circularized by interactions between translation factors eIF4G, eIF4B and PABP that promote ribosome recycling (Kuyumcu-Martinez et al., 2004). Thus, RNA replication may first begin on templates transitioning from a circular configuration. Indeed, this paradigm seems to be conserved among many plus strand RNA viruses. In viruses, the long-range interactions that circularize templates are mediated by either protein-protein interactions or RNA-RNA interactions or both.
For PV, circularization is also promoted by a complex of PCBP bound to the cloverleaf that interacts with PABP bound to poly(A) tail forming a protein-protein bridge (Herold and Andino, 2001). An analogous arrangement occurs in coronavirus mouse hepatitis virus (MHV) where interaction between 5′ and 3′ ends is promoted by another protein bridge involving PTB bound to 5′ UTR and hnRNP A1 bound to the 3′ UTR (Huang and Lai, 2001). For Dengue virus cyclization occurs via long range RNA-RNA interactions of complementary sequences in the corresponding 5′ and 3′ UTRs ( Filomatori et al., 2011; Lodeiro et al., 2009; Polacek et al., 2009; Sztuba-Solinska et al., 2013; Villordo et al., 2010) (Fig.2). In HCV an RNA sequence in the NS5b gene region interacts with the 3′ UTR via a kissing-loop to facilitate placement of the polymerase at the exact 3′ end of the genome (Friebe et al., 2005).
The latter two examples above emphasize RNA-RNA interactions without factors, but it is possible that host factors could promote or stabilize these long-range interactions. For instance, HCV interactions may be aided by hnRNP A1, which binds ns5B and both ends of the genome (C. S. Kim et al., 2007). Finally, the host RBP NF90/NFAR circularize the HCV genome by binding both ends of the genome. These interactions may occur sequentially after kissing loop and hnRNP A1 interactions form, as NF90 binding blocks translation and stimulates RNA replication (Isken et al., 2007; 2004; 2003). RHA can also play a role in the complex with NF90/NFAR-1 as a bridging factor between the 5′ and 3 UTRs of HCV that promotes genome circularization (Isken et al., 2007). Several of these factors also bind to DENV2 3′ UTR (NF90, NF45, RHA) and NF90 is critical for replication (Gomila et al., 2011). Together, these findings suggest that conformations of circularized genomic RNA can differ between translation and replication. Accordingly, switching between translation and replication can be facilitated by virus recruitment of host factors that provide conformational remodeling of RNA structures.
For the coronavirus mouse hepatitis virus (MHV), crosstalk between the viral UTRs relies on interactions between PTB and hnRNP A1, which bind the 5′ UTR and 3′ UTR, respectively. Mutations that block interaction of these factors with MHV RNA reduce replication and transcription (Huang and Lai, 2001). MHV replication was not restricted in cells that do not express hnRNP A1, since the viral RNA can also bind several other hnRNP proteins in replacement roles. These were identified by RNA affinity with negative strand UTR to be hnRNP A2/B1, hnRNP A/B and hnRNP A3, all which are closely related to hnRNP A1 (Shi et al., 2003).
Nuclear factors regulate picornavirus negative strand RNA synthesis
For enteroviruses there is a clear dependence on co-opted PCBP2 to promote negative strand RNA synthesis built on the platform of the extended 5′ cloverleaf. Only intact PCBP functions in translation but either intact or cleaved (by 3Cpro) PCBP2 can bind the 5′ cloverleaf (CL) on stem-loop B(SLB) (Perera et al., 2007) and promote genome circularization and recruitment of 3CD to the cloverleaf on the adjacent loop, positioning near the 3′ poly (A) template due to the protein-bridge circularization (Herold and Andino, 2001). Initial reports indicated the PCBP binding region was CL SLB, however this was later shown to be insufficient, that an additional C-rich spacer region located just downstream of the CL is also required for PCBP binding. Mutation of six C residues in this region to A abolished replication in HeLa cells but had no effect on translation. It was found that PCBP did not bind to 88 nt CL alone and but did bind 142 nt extended CL including this region (Toyoda et al., 2007). Point mutations in this region strongly affect PV neurovirulent phenotypes (De Jesus et al., 2005). Similarly, PCBP2 was shown to bind the analogous C-rich spacer region in CVB3 RNA (Fig. 1C). PCBP2 did not bind CVB3 CL alone but 3Cpro binding to SLD allowed recruitment of PCBP2 to the minimal CL (Zell et al., 2008b). Also, PCBP KH domains 1 and 3 interact with the extended cloverleaf RNA of CVB3 (Zell et al., 2008a). Taken together these reports make it clear that PV co-opts PCBP to provide multiple functions for virus replication, including: promoting genome circularization, as an ITAF, switching from translation to RNA synthesis, and properly assembling the replicase on the 5′ CL. The CL is a required partner in several of these steps and can be seen as a general promoter for RNA synthesis that provides a platform for binding host factors and polymerase (Vogt and Andino, 2010).
Nucleolin is a shuttling RNA helicase that is largely concentrated in the nucleolus and binds pre-rRNAs. Nucleolin was initially reported to bind poliovirus 3′ UTR and enhance replication and may play roles in negative strand RNA replication (Waggoner and Sarnow, 1998). Subsequently another report indicated nucleolin interacts with poliovirus IRES and enhances IRES-dependent translation (Izumi et al., 2001). Whether interactions at two sites on opposite ends of the viral genome requires one nucleolin moiety or looped circularized RNA has not been determined and more precise functional roles of nucleolin have not been identified.
FMDV interacts with RNA helicase A (RHA) and alters its distribution in the cell from mostly nuclear to cytoplasmic. RHA is involved in diverse cellular functions as it enhances gene expression by interacting with CBP/p300 and RNA polymerase II and responds to IFN alpha signaling to increase its activity (Aratani et al., 2001; Fuchsová et al., 2002). During FMDV infection RHA can bind the S domain of the virus 5′ UTR and co-precipitates with FMDV 2C and 3A proteins that function in the replicase complex. Though the precise function of RHA remains undetermined, knockdown of RHA reduces virus titers, indicating RHA plays roles in supporting replication (Lawrence and Rieder, 2009). FMDV-induced movement of RHA out of the nucleus is associated with demethylation or arginine residues at the C terminus and did not require activity of FMDV leader protein. Rather nuclear egress involves demethylation activity provided by Jumonji C-domain containing protein 6 (JmjC) that is stimulated by FMDV (Lawrence et al., 2014).
Nuclear factors promote flavivirus negative strand RNA synthesis
Flavivirus 3′ UTRs are complex structures that contain cis-acting elements required for translation, cyclization and replication. Accordingly, this regulatory RNA region binds several host factors that may play roles in RNA synthesis, including La, PTB, YB-1, eEF1a, NF90, RHA, and NF45 (Fig 2). Flavivirus RNA replication is dependent on many of these co-opted host factors, however not all are thought of as nuclear factors. Translation elongation factor 1A (eEF1A), which is abundant in both the cytoplasm and nucleus, binds to three sites within the 3′ UTR of West Nile virus and other flaviviruses. This interaction supports negative strand RNA synthesis but not translation (Blackwell and Brinton, 1997; Davis et al., 2007). eEF1A immunoprecipitates with the NS5 replicase complex proteins of WNV and colocalizes with replication complexes, further supporting a role for this factor in minus strand RNA synthesis (Davis et al., 2007). Flavivirus RNA shifts to an alternate conformation based on complex long-range binding interactions that form a new panhandle structure. This panhandle structure positions 5′ and 3′ termini close together and promotes RNA replication (Fig 2). Binding of a complex of double-strand binding factors NF90, RHA and NF45 to the 3′ SL likely occurs in concert with the formation of the panhandle and promotes RNA replication (Gomila et al., 2011). The possibility exists that these factors may also bind the 5′ UTR and stabilize the long-range looped structure similar to their role with HCV RNA (Isken et al., 2007). Further, La binds to both 5′ and 3′ UTRs and could play a role stabilizing this structure as well as recruiting NS5 and NS3 (García-Montalvo et al., 2004). The NS5 RdRp binds to the 5′ SL1 which due to looping helps position the polymerase near the 3′ terminus (Bidet and Garcia-Blanco, 2014).
Similar to picornaviruses and HCV, PTB also binds the 5′ UTR of Dengue virus (DENV) and Japanese Encephalitis virus (JEV) (Anwar et al., 2009; S. M. Kim and Jeong, 2006). Although the 5′ UTR binding site suggested a possible role of PTB in flavivirus translation, PTB knockdown inhibits DENV negative strand RNA replication and does not affect virus translation or viral entry. PTB also interacts with NS4A protein, a component of the replicase, further suggesting that PTB plays a role in viral RNA replication (Anwar et al., 2009; L. Jiang et al., 2009). Surprisingly, PTB knockdown did not inhibit JEV replication so its requirement is not conserved. PTB also binds 3′ UTRs of both viruses. Though the 5′ UTR of both viruses are conserved, the differential requirement for PTB may stem from the fact that the 3′ UTRs are divergent. Additionally, PTB translocates to the cytoplasm in DENV-infected cells and is found colocalized with calnexin endoplasmic reticulum marker and NS1 and NS3 (Agis-Juárez et al., 2009). Further work will be required to elucidate precise functional roles of PTB in flavivirus replication.
Nuclear factors regulate HCV negative strand RNA synthesis
For HCV, as discussed above, the switch from translation to RNA replication is stimulated by binding of core and NS3 to the IRES. However, some translation may still occur and be coupled to RNA replication at the same subcellular membrane compartment termed a replicasome. Pulse chase and other analyses in infected cells supported this conclusion since restriction of RNA synthesis with an NS5B polymerase inhibitor reduced translation even when levels of HCV RNA were unaltered (Liu et al., 2012). As with any RNA virus, the overlapping, ongoing replication and translation processes make separation of the roles of host factors very difficult to tease apart.
Even though PTB may not be a bona fide ITAF for HCV, it interacts with the poly(U/C) tract in the HCV 3′ UTR and may promote replication. During infection in Huh7 cells a phosphorylated form of PTB not found in uninfected cells associates with the membrane-bound HCV replication complex (K.-S. Chang and Luo, 2006).
Synaptotagmin-binding cytoplasmic RNA-interacting protein (SYNCRIP)(NSAP1)(hnRNP Q) is another member of the heterogenous nuclear ribonucleoprotein (hnRNP) family that plays roles in mRNA maturation and also binds HCV RNA and enhances IRES-dependent translation. SYNCRIP/hnRNP Q also plays a role in RNA replication as it associates with the replication complex in membrane fractions and colocalizes with nascent virus RNA. Immunodepletion or siRNA knockdown of NSAP1 decreases RNA replication, indicating dual functions of NSAP1 in the HCV replicative cycle (Liu et al., 2009).
Finally, the RNA chaperone nucleolin has also been proposed to play a role in HCV replication, as it binds the virus NS5B RNA polymerase via its glycine-arginine-rich domain. Transient expression of NS5B recruits or sequesters nucleolin from the nucleus to cytoplasm and may modulate the oligomerization of NS5B that is required for RNRP activity (Hirano et al., 2003; Kusakawa et al., 2007).
Nuclear factors regulate alphavirus RNA synthesis
After Sindbis virus (SINV) infection numerous nuclear proteins shift to the cytoplasm including hnRNP K, hnRNP A1, and HuR. hnRNP A1 interacts with the viral 5′ UTR, and with the genomic (G) and subgenomic (SG) RNA promoters. Knockdown of hnRNP A1 resulted in markedly decreased synthesis of G and SG RNA both in infected cells and in vitro (Gui et al., 2010; Lin et al., 2009). A recent report also indicates SINV infection results in cytoplasmic relocation of PTB and TIA1, but no specific function of these factors in replication is known. (Sanz et al., 2014). Several reports indicate SINV nonstructural proteins interact with stress granule nucleating proteins G3BP1 and G3BP2, including nsP4 polymerase (Cristea et al., 2010), nsP3 and nsP2 (Cristea et al., 2006) (Gorchakov et al., 2008). The interaction of G3BP1 with nsP3 has been shown to have the proviral effect of inhibiting stress granule formation (Panas et al., 2012). TIA1 and PTB also enter the cytoplasm during SINV infection but not during ectopic expression of nsP proteins, indicating replicative processes may be required (Sanz et al., 2014). Recently the combined approach of isotope labeling of purified Semliki Forest virus replicase/modified lysosome complexes with proteomics analysis identified 78 host proteins associated with the functional replicase. Several familiar proteins colocalized with replicase including PCBP1, hnRNP M, hnRNP C, and hnRNP K. Silencing experiments indicated that hnRNP M and C are antiviral for SFV, Chikungunya and SINV. Differential silencing results with hnRNP K indicated opposite roles of this RBP in CHIKV and SINV versus SFV. This suggests interactions of host factors with replicase complexes are not always proviral and that the roles of hnRNPs may be interchangeable or more nuanced in various cells/virus combinations (Varjak et al., 2013).
Nuclear factors modulate astrovirus and norovirus RNA replication
PTB also binds the 3′ UTR of astrovirus. Seven host proteins from CaCo2 cell lysates could be cross-linked to 3′-UTR RNA probes in vitro and mobility shift assays indicated that two complexes of host factors form on 3′ UTR probes and that PTB is part of one of the complexes. siRNA knockdown of PTB reduced virus replication, suggesting PTB is required for replication (Espinosa-Hernández et al., 2014). It is unclear if negative strand or plus strand RNA replication is affected by PTB.
Human norovirus was shown to bind La and PTB to the 3′ UTR that contains a small stem-loop structure (Gutiérrez-Escolano et al., 2003), however, since human norovirus cannot be cultivated in vitro, most recent work has focused on murine norovirus that replicates in macrophage cell lines. Murine norovirus RNA contains three stable stem loop structures and a single stranded polypyrimidine (pY) tract within the 3′ terminal stem loop. Both PTB and PCBP bind the pY tract, but this is not essential since viruses lacking this region are viable in cells and in mice. However, pY-deleted virus suffer a fitness cost and are less virulent in mice (Bailey et al., 2010). RNA affinity chromatography-mass spectroscopy identified over 50 host factors that bound to discreet structures in murine norovirus RNA. Many were common to other RNA viruses discussed above, including PTB, La, DDX3, nucleolin, hnRNPK, PCBP1/2, eEF1a and hnRNP A species. siRNA knockdown of PTB, La and DDX3 in RAW264.7 cells resulted in deficient virus replication (Vashist et al., 2012) suggesting these play roles in replication, but much more work will be required to elucidate how these factors function in norovirus infections. A long range 5′-3′ RNA-RNA looping interaction was described in murine norovirus RNA that is stabilized by binding PCBP2 and hnRNP A1. Mutations in the RNA-RNA complimentary binding motif reduced binding of both host factors to RNA and also inhibited RNA replication; and PCBP2 and hnRNP A1 colocalized to virus replication complexes in cells. All these results indicate the looping interaction stabilized by the host factors plays an important roles in replication(López-Manríquez et al., 2013).
Feline calicivirus also binds nucleolin on the 3′ UTR together in a complex with viral protein NS6/7 and infection of feline kidney cells results in nuclear-cytoplasmic relocalization of nucleolin to sites enriched for NS6/7 (Cancio-Lonches et al., 2011). Finally, PTB plays a negative role in calicivirus translation and therefore may promote the switch from translation to replication (Karakasiliotis et al., 2010).
Nuclear factors in coronavirus RNA replication
Several host proteins interact with the 3′ end of the transmissible gastroenteritis coronavirus (TGEV) genome. Genomic RNA ends were used as bait in RNA affinity pulldowns to identify host factors. The only protein to bind the 5′ end was PTB, whereas nine other proteins bound the 3′ terminus RNA, including several hnRNPs, PABP and two amino-acyl tRNA synthetases. Knockdown of PABP, hnRNP Q and glutamyl-prolyl-tRNA synthetase inhibited replication of a replicon, indicating they may play functional roles in replication (Galán et al., 2009). Since hnRNPs oligomerize, they may participate in RNA-protein complexes by bringing transcription-regulating sequences (TRS) TRS-L and TRS-B into close proximity to facilitate coronavirus discontinuous transcription (Sola et al., 2011b).
Further investigation of the role of PTB in TGEV replication indicated PTB affects virus RNA accumulation and relocalizes viral RNAs to novel cytoplasmic domains different from replication-transcription sites. Interestingly, siRNA knockdown of PTB in two cell lines increased virus mRNA levels and virus titer, suggesting PTB interaction inhibits virus replication, the opposite of most other RNA viruses. PTB relocalized from the nucleus to discreet structures that contained TIA1 and TIAR and may be reminiscent of stress granules. These foci also contained viral genomic and subgenomic RNA and PTB could play a role in sequestration of some virus RNA into novel foci involved in posttranscriptional regulation of virus gene expression (Sola et al., 2011a). There is not substantial evidence that the TIA1-foci are bonafide stress granules but may represent replication complexes.
Regulation of plus strand RNA synthesis and RNA processing
Nuclear factors regulate enterovirus plus strand RNA synthesis
Compared to minus strand synthesis, less is known about the synthesis of plus strands from the minus strand anti-genomic template in RNA virus systems such as poliovirus. The template for plus strand synthesis is different, being mostly double-stranded RNA instead of single stranded RNA, and this may have profound influence on the host factors that interact with virus RNA. This double stranded structure is called replicative form (RF) in picornaviruses. hnRNP C supports virus positive strand replication by binding to the anti-cloverleaf that forms on the 3′ end of the minus strand of RF (Fig. 1D). Since the RF template is completely double stranded, the anti-cloverleaf can only form by dsRNA breathing allowing strand separation at the terminus. The strand separation is aided progressively by host factors that recognize the cognate plus strand stem-loop B and anti-stem-loop B, which are PCBP2 and hnRNP C1/C2, respectively and prevent renewed base-pairing that would zip the RF back to completely double-stranded structure. In this situation there is opportunity for the factors PCBP and hnRNP C to interact to support positive strand RNA synthesis, yet this has not been reported. hnRNP C also is relocalized from the nucleus to the cytoplasm during infection and like PCBP2, can interact with PV 3CD polymerase precursor. A current model proposes that hnRNP C maintains the 3′ end of the template in a single strand form via its chaperone function and then recruits the polymerase (Brunner et al., 2005). Using the HeLa in vitro replication system, depletion of hnRNP C inhibited production of plus strand RNA product, and replenishment with full length but not truncated hnRNP C restored RNA synthesis. (Brunner et al., 2005). hnRNP C also interacts with the 5′ end of the negative strand RNA on a stem loop structure that can only form through breathing of the full length double strand RF replication intermediate (Ertel et al., 2010). Although RF is typically depicted in cartoons as a linear structure, this finding raises the possibility that oligomerization of hnRNP C could maintain a loop with both ends of the double-stranded template in close proximity to promote RNA strand synthesis (Fig. 1D). hnRNP C functions in pre-mRNA processing as a tetramer that forms via a coil-coil interactions involving its oligomerization domain (Whitson et al., 2005).
An engineered tandem cloverleaf approach was used to separate functions required for PV minus strand RNA synthesis from plus strand RNA synthesis. This approach revealed several requirements for plus strand synthesis that were surprising. First, the short stem A is essential for plus strand synthesis but not minus strand synthesis, suggesting the stem-loop structure is functional on the anti-cloverleaf. Second, the complete plus strand version of the CL is required, including binding sites for PCBP and 3CD polymerase precursor. A trans-initiation model was proposed where replicase builds on the plus strand CL to prime plus strand synthesis the same way it does for negative strand RNA synthesis (Vogt and Andino, 2010). However, hnRNP C binding to the anti-CL will also help position the 3′ end of the negative strand to allow precise RNA replication initiation by the replicase.
Nuclear factors function in viral RNA processing
After synthesis of PV plus strand transcripts an RNA processing step removes VPg at the 5′ end of a portion of transcripts. All PV RNA associated with polysomes lacks VPg, having been removed by a host unlinkase enzyme, whereas all encapsidated RNA retains VPg. Cellular 5′ tyrosyl-DNA phosphodiesterase-2 (TDP2) is a hijacked cellular DNA repair enzyme that performs the VPg unlinkase function for enteroviruses. PV infection relocalizes the bulk of TDP2 from the nucleus to cytoplasm. A hypothesis predicts that VPg is a capsid packaging signal that must be removed so that a pool of nascent transcripts can continue to engage ribosomes and produce additional virus proteins (Virgen-Slane et al., 2012). The effect of preventing VPg unlinking by using click chemistry to form an uncleavable bond for TDP2 was tested and found to not affect translation or replication efficiency, indicating VPg does not inhibit initial steps in either process (Langereis et al., 2013).
Nuclear factors regulate flavivirus plus strand RNA synthesis
In flaviviruses, plus strand RNA synthesis is initiated by a promoter-protein complex that builds on a conserved stem-loop structure on the 3′ terminal 96 nt of minus strand RNA. This structure is specifically bound by four host proteins, one of which is La autoantigen (R. M. E. Yocupicio-Monroy et al., 2003) and another is TIAR. TIAR and its closely related paralog TIA1 are nuclear proteins that shuttle into the cytoplasm during environmental stress and help nucleate formation of RNA stress granules. TIA1 also binds WNV (-) RNA and both proteins bind WNV RNA through their conserved RRM2 motifs (W. Li et al., 2002). WNV replication was repressed in TIAR knockout cells and mutations of the UAAUU TIAR recognition motif in the 3′ SL affected replication but not translation. Several mutant viral RNAs that only weakly bound TIAR rapidly reverted to wild type phenotypes in vivo, suggested that TIAR interaction was not required for low level minus strand replication but allowed efficient high level plus strand RNA synthesis from the minus strand template (Emara et al., 2008).
The ability of WNV and DENV to hijack TIAR and TIA1 from the nucleus and place it into new roles in RNA synthesis provides another advantage; hijacking also sequesters these proteins from their role in forming stress granules (SG) (Emara and Brinton, 2007). Many viruses induce SGs by interrupting cellular pathways and homeostasis, particularly protein synthesis, and by triggering activation of PKR. These virus-derived perturbations drive SG formation through phosphorylation of eIF2 or other mechanisms (Kedersha et al., 2013; 1999; Reineke and Lloyd, 2013). Accumulating evidence suggests SGs may provide platforms to activate innate immune functions and they are seen as antiviral (Lloyd, 2013). Indeed, many viruses have evolved mechanisms to suppress SG formation, and co-opting key SG nucleating factors such as TIAR and G3BP1 is one mechanism employed by flaviviruses and HCV (Ariumi et al., 2011; Emara and Brinton, 2007; Ruggieri et al., 2012). Further experiments with chimeras of WNV that had different RNA replication efficiencies and different abilities to induce or control SGs indicated that early viral RNA synthesis cannot exceed the ability to protect product dsRNA with virus-induced membranes. Virus-induced membranes block access of PKR to the dsRNA intermediates. Viruses that replicate too quickly overwhelm the limited virus-induced membranes that protect dsRNA, allowing PKR activation and SGs formation that inhibits replication (Courtney et al., 2012).
Regulation of subgenomic RNA synthesis by host RPBs
Several plus strand viruses have multiple open reading frames and use subgenomic plus strand transcripts (sgRNA) to express structural proteins. The synthesis of subgenomic plus strand RNA is regulated differently than genomic RNA, however is still controlled by transcription elements that function in cis or trans. This provides the opportunity for subgenomic transcription elements to assemble alternate replicase complexes containing different host factors than those modulating genomic RNA replication.
Coronaviruses promote sgRNA transcription using interactions between 5′ terminal leader sequences and intergenic (IG) sequences just upstream of each ORF. One host factor, hnRNP A1 binds to both leader and IG sequences in MHV negative strand template RNA ((H. P. Li et al., 1997). Overexpression of hnRNP A1 promotes MHV RNA replication while a dominant negative hnRNP A1 reduces replication (Shi et al., 2000) and the interaction of hnRNP A1 with the leader and IG sequences is critical for in vitro sgRNA transcription (X. Zhang et al., 1999). hnRNP A1 could function by recruiting virus N nucleocapsid protein (Wang and X. Zhang, 1999). PTB is another prominent nuclear factor that may regulate sgRNA transcription as it binds the plus strand leader sequence and also the 5′ UTR in minus strand RNA in reactions that promote sgRNA replication specifically (Huang and Lai, 1999; H. P. Li et al., 1999).
The alphavirus Sindbis virus induces a shift of hnRNP K from the nucleus to cytoplasm where it colocalizes with viral nonstructural protein 2 and co-immunoprecipitates with subgenomic but not genomic RNA, nsP1 and nsP2. siRNA knockdown of hnRNP K reduced gene expression from a subgenomic promoter. These findings indicate that hnRNP K has a role, details yet to be determined, in sgRNA synthesis (Burnham et al., 2007).
Host RBPs regulate stability of viral RNA
Viruses must maintain the integrity of their viral RNAs and suppress RNA decay pathways. Alphavirus and coronavirus genomes are capped and poly-adenylated, retaining two modifications that stabilize mRNAs against RNA decay. However, binding of certain host factors to virus RNA can also protect against RNA decay machinery. HuR is associated with prolonged mRNA stability in cells and binds to U-rich elements (UREs) in alphavirus 3′ UTRs (Garneau et al., 2008; Sokoloski et al., 2010). As with many other factors, HuR is selectively relocalized to the cytoplasm during Sindbis virus infection. Two other alphaviruses, Ross River virus and Chikungunya virus, lack the conserved 3′ UREs but still tightly bind HuR via alternative binding elements (Dickson et al., 2012). Knockdown of HuR increased decay of Sindbis virus RNAs and reduced virus replication yields in both human and mosquito cells, indicating that HuR binding to SINV RNA blocks components of the cellular RNA decay pathway (Sokoloski et al., 2010).
Other viruses lack either 5′ m7GTP caps or poly(A) tails and may specifically co-opt or target host factors to protect against decay. Many of these are resident cytoplasmic factors but some are nuclear shuttling proteins. The PV 5′ CL binds PCBP to support RNA replication as discussed above, but this also stabilizes PV plus strand RNA in HeLa lysates. A mutant PCBP protein that cannot bind the CL was associated with increased decay of PV RNA in the HeLa in vitro replication system and it was proposed that PCBP binding blocks Xrn1 exonuclease activity. This was supported by showing capped PV RNAs bypassed the requirement for PCBP2 in stability assays (Murray et al., 2001). Poliovirus also achieves greater RNA stability through cleavage and proteolytic degradation of RNA decay factors Xrn1, Dcp1a and Pan3 by poliovirus proteinases and virus-activated proteasomal decay (Dougherty et al., 2011). Further, 3Cpro cleavage of the RNA destabilizing factor AUF1 likely contributes to poliovirus RNA stability (Rozovics et al., 2012; Wong et al., 2013).
Flaviviruses generate short non-coding RNAs (sfRNA) from the 3′ UTR due to the ability of this structure to stall and sequester Xrn1. DENV2 sfRNA accumulates during infection and acts a sponge and binds host factors G3BP1, G3BP2 and Caprin1. Sequestration of G3BP was linked to dysregulation of translation of several mRNAs of specific interferon stimulated genes, thus interfering in innate immunity (Bidet et al., 2014). Importantly sfRNA also sequesters Xrn1, and results in dysregulation of host mRNA stability and accumulation of uncapped mRNA decay intermediates in cells (Moon et al., 2012).
The stem-loops and U-rich tract contained in the 3′ UTR of HCV bind La protein and inhibit decay of HCV RNA in HeLa extracts (Spångberg et al., 2001). Similar to alphaviruses, the HCV 3′ URE can bind HuR and knockdown of HuR expression in cells resulted in reduced HCV translation and RNA replication using replicon models (Korf et al., 2005; Spångberg et al., 2000). The stimulatory effects of HuR on IRES translation were confirmed using dicistronic vectors in rabbit reticulocyte lysates, Xenopus laevis oocytes and HeLa cell extracts but may not involve direct interaction of HuR with the IRES (Rivas-Aravena et al., 2009). Though HuR is associated with RNA stability in many systems, its importance for HCV RNA remains unclear. Proteomics analysis indicates more than 70 host proteins are enriched from cells with HCV 3′ UTR probes and several of these are associated with regulation of RNA stability in cells, including YB-1, NF90, hnRNP C and hnRNP D (D. Harris et al., 2006).
Conclusions
The discussion herein mostly focused on nuclear shuttling RBPs and their roles in regulating mammalian plus strand RNA virus translation and RNA replication. However, cytoplasmic viruses exploit many other types of cytoplasmic factors involved in translation regulation, membrane formation and remodeling, and virus assembly and egress that were not discussed. As research on RNA virus replication has continued, several common themes have emerged. First, the array of host proteins that play significant roles in replication will continue to grow as the newly expanded list of interacting factors identified from proteomics approaches undergo analysis. Second, although many host proteins are hijacked by viruses, several ubiquitous host proteins, such as PTB, PCBP and hnRNP A1 are used by several classes of viruses, often in different ways. Third, the production of key virus proteins shifts the function of some host factors from support of translation to RNA replication. Fourth, the RNA chaperone functions described for host factors in supporting IRESome activity often provide similar or analogous chaperone functions supporting RNA structures and long range interactions in RNA synthesis. Fifth, the new concept of host factors being sequestered or sponged away from normal host roles may be common, and may be a variant form of hijacking. Sixth, some interactions of host factors with virus RNA inhibit replication or translation and must be opposed by viruses.
Many nuclear host factors discussed are highly conserved, which can enable cross-species infection in the case of alphavirus and flaviviruses, but also restrict virus tissue tropism and control pathogenesis in mammals where gene expression patterns are so variable. Also, the complete lists of factors used by any one virus are unique, evidence that viruses evolve many different ways to take over cell functions. However, many of the diverse host factors that are hijacked perform related functions for the virus in replication.
Our understanding of co-opted factors, though expanding rapidly, is far from complete. We certainly do not have a comprehensive list of factors utilized by any single virus. Future expansion of proteomics research and new approaches such as TUX-MS will fill in the catalog, but substantial traditional biochemical wet bench science will be required to tease apart functions and impact of newly discovered factors, many of which may be novel to science as a whole. Adaptation of in vitro replication systems used for poliovirus to study other virus families will help dissect the roles of factors in viral replication complexes, particularly where the same factor may play roles in sequential replicative processes. Finally, the increased use of super-resolution fluorescence microscopy and cryo-electron microscopy of whole cells will also be important to finely localize factors and virus-induced structures in cells.
Nuclear shuttling host proteins are commonly hijacked by RNA viruses to support replication.
A limited group of ubiquitous RNA binding proteins are commonly hijacked by a broad range of viruses.
Key virus proteins alters roles of RNA binding proteins in different stages of virus replication.
Acknowledgements
Research in the author’s laboratory is supported by NIH Public Health Service Grant AI50237, by NCI, National Institutes of Health Cancer Center Support Grant P30CA1251230; and by National Institutes of Health Grants HD007495, DK56338, and CA125123 (to the Integrated Microscopy Core at Baylor College of Medicine).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agis-Juárez RA, Galván I, Medina F, Daikoku T, Padmanabhan R, Ludert JE, del Angel RM. Polypyrimidine tract-binding protein is relocated to the cytoplasm and is required during dengue virus infection in Vero cells. J. Gen.Virol. 2009;90:2893–2901. doi: 10.1099/vir.0.013433-0. doi:10.1099/vir.0.013433-0. [DOI] [PubMed] [Google Scholar]
- Almstead LL, Sarnow P. Inhibition of U snRNP assembly by a virus-encoded proteinase. Genes Dev. 2007;21:1086–1097. doi: 10.1101/gad.1535607. doi:10.1101/gad.1535607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EC, Hunt SL, Jackson RJ. Internal initiation of translation from the human rhinovirus-2 internal ribosome entry site requires the binding of Unr to two distinct sites on the 5′ untranslated region. J Gen Virol. 2007;88:3043–3052. doi: 10.1099/vir.0.82463-0. doi:10.1099/vir.0.82463-0. [DOI] [PubMed] [Google Scholar]
- Andino R, Rieckhof GE, Achacoso PL, Baltimore D. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5′-end of viral RNA. EMBO J. 1993;12:3587–3598. doi: 10.1002/j.1460-2075.1993.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev DE, Hirnet J, Terenin IM, Dmitriev SE, Niepmann M, Shatsky IN. Glycyl-tRNA synthetase specifically binds to the poliovirus IRES to activate translation initiation. Nucl. Acids Res. 2012;40:5602–5614. doi: 10.1093/nar/gks182. doi:10.1093/nar/gks182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar A, Leong KM, Ng ML, Chu JJH, Garcia-Blanco MA. The polypyrimidine tract-binding protein is required for efficient dengue virus propagation and associates with the viral replication machinery. J. Biol. Chem. 2009;284:17021–17029. doi: 10.1074/jbc.M109.006239. doi:10.1074/jbc.M109.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aratani S, Fujii R, Oishi T, Fujita H, Amano T, Ohshima T, Hagiwara M, Fukamizu A, Nakajima T. Dual roles of RNA helicase A in CREB-dependent transcription. Mol. Cell. Biol. 2001;21:4460–4469. doi: 10.1128/MCB.21.14.4460-4469.2001. doi:10.1128/MCB.21.14.4460-4469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariumi Y, Kuroki M, Kushima Y, Osugi K, Hijikata M, Maki M, Ikeda M, Kato N. Hepatitis C Virus Hijacks P-Body and Stress Granule Components around Lipid Droplets. J. Virol. 2011;85:6882–6892. doi: 10.1128/JVI.02418-10. doi:10.1128/JVI.02418-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SH, Kim YK, Kim WJ, Cho S, Oh HR, Kim J-E, Jang SK. Translation of polioviral mRNA is inhibited by cleavage of polypyrimidine tract-binding proteins executed by polioviral 3C(pro) J. Virol. 2002;76:2529–2542. doi: 10.1128/jvi.76.5.2529-2542.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacot-Davis VR, Palmenberg AC. Encephalomyocarditis virus Leader protein hinge domain is responsible for interactions with Ran GTPase. Virology. 2013;443:177–185. doi: 10.1016/j.virol.2013.05.002. doi:10.1016/j.virol.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D, Karakasiliotis I, Vashist S, Chung LMW, Rees J, Reese J, McFadden N, Benson A, Yarovinsky F, Simmonds P, Goodfellow I. Functional analysis of RNA structures present at the 3′ extremity of the murine norovirus genome: the variable polypyrimidine tract plays a role in viral virulence. J. Virol. 2010;84:2859–2870. doi: 10.1128/JVI.02053-09. doi:10.1128/JVI.02053-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basta HA, Bacot-Davis VR, Ciomperlik JJ, Palmenberg AC. Encephalomyocarditis virus leader is phosphorylated by CK2 and syk as a requirement for subsequent phosphorylation of cellular nucleoporins. J. Virol. 2014;88:2219–2226. doi: 10.1128/JVI.03150-13. doi:10.1128/JVI.03150-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard KM, Daijogo S, Semler BL. A nucleo-cytoplasmic SR protein functions in viral IRES-mediated translation initiation. EMBO J. 2007;26:459–467. doi: 10.1038/sj.emboj.7601494. doi:10.1038/sj.emboj.7601494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidet K, Dadlani D, Garcia-Blanco MA. G3BP1, G3BP2 and CAPRIN1 are required for translation of interferon stimulated mRNAs and are targeted by a dengue virus non-coding RNA. PLoS Path. 2014;10:e1004242. doi: 10.1371/journal.ppat.1004242. doi:10.1371/journal.ppat.1004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidet K, Garcia-Blanco MA. Flaviviral RNAs: weapons and targets in the war between virus and host. Biochem. J. 2014;462:215–230. doi: 10.1042/BJ20140456. doi:10.1042/BJ20140456. [DOI] [PubMed] [Google Scholar]
- Blackwell JL, Brinton MA. Translation elongation factor-1 alpha interacts with the 3′ stem-loop region of West Nile virus genomic RNA. J. Virol. 1997;71:6433–6444. doi: 10.1128/jvi.71.9.6433-6444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyn LB, Swiderek KM, Richards O, Stahl DC, Semler BL, Ehrenfeld E. Poly(rC) binding protein 2 binds to stem-loop IV of the poliovirus RNA 5′ noncoding region: identification by automated liquid chromatography-tandem mass spectrometry. Proc. Natl. Acad. Sci. USA. 1996;93:11115–11120. doi: 10.1073/pnas.93.20.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman AM, Kean KM. Intact eukaryotic initiation factor 4G is required for hepatitis A virus internal initiation of translation. Virology. 1997;237:129–136. doi: 10.1006/viro.1997.8761. doi:10.1006/viro.1997.8761. [DOI] [PubMed] [Google Scholar]
- Boussadia O, Niepmann M, Créancier L, Prats AC, Dautry F, Jacquemin-Sablon H. Unr Is Required In Vivo for Efficient Initiation of Translation from the Internal Ribosome Entry Sites of both Rhinovirus and Poliovirus. J. Virol. 2003;77:3353–3359. doi: 10.1128/JVI.77.6.3353-3359.2003. doi:10.1128/JVI.77.6.3353-3359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard M, Paulous S, Komarova AV, Deveaux V, Kean KM. Evidence that PTB does not stimulate HCV IRES-driven translation. Virus Genes. 2007;35:5–15. doi: 10.1007/s11262-006-0038-z. doi:10.1007/s11262-006-0038-z. [DOI] [PubMed] [Google Scholar]
- Brunner JE, Nguyen JHC, Roehl HH, Ho TV, Swiderek KM, Semler BL. Functional interaction of heterogeneous nuclear ribonucleoprotein C with poliovirus RNA synthesis initiation complexes. J. Virol. 2005;79:3254–3266. doi: 10.1128/JVI.79.6.3254-3266.2005. doi:10.1128/JVI.79.6.3254-3266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham AJ, Gong L, Hardy RW. Heterogeneous nuclear ribonuclear protein K interacts with Sindbis virus nonstructural proteins and viral subgenomic mRNA. Virology. 2007;367:212–221. doi: 10.1016/j.virol.2007.05.008. doi:10.1016/j.virol.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Cancio-Lonches C, Yocupicio-Monroy M, Sandoval-Jaime C, Galvan-Mendoza I, Urena L, Vashist S, Goodfellow I, Salas-Benito J, Gutiérrez-Escolano AL. Nucleolin interacts with the feline calicivirus 3′ untranslated region and the protease-polymerase NS6 and NS7 proteins, playing a role in virus replication. J. Virol. 2011;85:8056–8068. doi: 10.1128/JVI.01878-10. doi:10.1128/JVI.01878-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelló A, Fischer B, Eichelbaum K, Horos R, Beckmann BM, Strein C, Davey NE, Humphreys DT, Preiss T, Steinmetz LM, Krijgsveld J, Hentze MW. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149:1393–1406. doi: 10.1016/j.cell.2012.04.031. doi:10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- Cathcart AL, Rozovics JM, Semler BL. Cellular mRNA decay protein AUF1 negatively regulates enterovirus and human rhinovirus infections. J. Virol. 2013;87:10423–10434. doi: 10.1128/JVI.01049-13. doi:10.1128/JVI.01049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart AL, Semler BL. Differential restriction patterns of mRNA decay factor AUF1 during picornavirus infections. J. Gen.Virol. 2014;95:1488–1492. doi: 10.1099/vir.0.064501-0. doi:10.1099/vir.0.064501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K-S, Luo G. The polypyrimidine tract-binding protein (PTB) is required for efficient replication of hepatitis C virus (HCV) RNA. Virus Res. 2006;115:1–8. doi: 10.1016/j.virusres.2005.06.012. doi:10.1016/j.virusres.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Chang T-C, Yamashita A, Chen C-YA, Yamashita Y, Zhu W, Durdan S, Kahvejian A, Sonenberg N, Shyu A-B. UNR, a new partner of poly(A)-binding protein, plays a key role in translationally coupled mRNA turnover mediated by the c-fos major coding-region determinant. Genes Dev. 2004;18:2010–2023. doi: 10.1101/gad.1219104. doi:10.1101/gad.1219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase AJ, Semler BL. Differential cleavage of IRES trans-acting factors (ITAFs) in cells infected by human rhinovirus. Virology. 2014;449:35–44. doi: 10.1016/j.virol.2013.10.030. doi:10.1016/j.virol.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerte C, Hall KB. The domains of polypyrimidine tract binding protein have distinct RNA structural preferences. Biochemistry. 2009;48:2063–2074. doi: 10.1021/bi8016872. doi:10.1021/bi8016872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold LC, Wilson LA, Sawicka K, King HA, Kondrashov AV, Spriggs KA, Bushell M, Willis AE. Upregulated c-myc expression in multiple myeloma by internal ribosome entry results from increased interactions with and expression of PTB-1 and YB-1. Oncogene. 2010 doi: 10.1038/onc.2010.31. doi:10.1038/onc.2010.31. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Svitkin Y, Sonenberg N. La autoantigen is necessary for optimal function of the poliovirus and hepatitis C virus internal ribosome entry site in vivo and in vitro. Mol. Cell. Biol. 2004;24:6861–6870. doi: 10.1128/MCB.24.15.6861-6870.2004. doi:10.1128/MCB.24.15.6861-6870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney SC, Scherbik SV, Stockman BM, Brinton MA. West nile virus infections suppress early viral RNA synthesis and avoid inducing the cell stress granule response. J. Virol. 2012;86:3647–3657. doi: 10.1128/JVI.06549-11. doi:10.1128/JVI.06549-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristea IM, Carroll J-WN, Rout MP, Rice CM, Chait BT, MacDonald MR. Tracking and elucidating alphavirus-host protein interactions. J. Biol. Chem. 2006;281:30269–30278. doi: 10.1074/jbc.M603980200. doi:10.1074/jbc.M603980200. [DOI] [PubMed] [Google Scholar]
- Cristea IM, Rozjabek H, Molloy KR, Karki S, White LL, Rice CM, Rout MP, Chait BT, MacDonald MR. Host factors associated with the Sindbis virus RNA-dependent RNA polymerase: role for G3BP1 and G3BP2 in virus replication. J. Virol. 2010;84:6720–6732. doi: 10.1128/JVI.01983-09. doi:10.1128/JVI.01983-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughenbaugh KF, Fraser CS, Hershey JWB, Hardy ME. The genome-linked protein VPg of the Norwalk virus binds eIF3, suggesting its role in translation initiation complex recruitment. 2003;22:2852–2859. doi: 10.1093/emboj/cdg251. doi:10.1093/emboj/cdg251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughenbaugh KF, Wobus CE, Hardy ME. VPg of murine norovirus binds translation initiation factors in infected cells. Virol J. 2006;3:33. doi: 10.1186/1743-422X-3-33. doi:10.1186/1743-422X-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WG, Blackwell JL, Shi P-Y, Brinton MA. Interaction between the cellular protein eEF1A and the 3′-terminal stem-loop of West Nile virus genomic RNA facilitates viral minus-strand RNA synthesis. J. Virol. 2007;81:10172–10187. doi: 10.1128/JVI.00531-07. doi:10.1128/JVI.00531-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesus N, Franco D, Paul A, Wimmer E, Cello J. Mutation of a single conserved nucleotide between the cloverleaf and internal ribosome entry site attenuates poliovirus neurovirulence. J. Virol. 2005;79:14235–14243. doi: 10.1128/JVI.79.22.14235-14243.2005. doi:10.1128/JVI.79.22.14235-14243.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nova-Ocampo M, Villegas-Sepúlveda N, del Angel RM. Translation elongation factor-1alpha, La, and PTB interact with the 3′ untranslated region of dengue 4 virus RNA. Virology. 2002;295:337–347. doi: 10.1006/viro.2002.1407. doi:10.1006/viro.2002.1407. [DOI] [PubMed] [Google Scholar]
- Dickson AM, Anderson JR, Barnhart MD, Sokoloski KJ, Oko L, Opyrchal M, Galanis E, Wilusz CJ, Morrison TE, Wilusz J. Dephosphorylation of HuR protein during alphavirus infection is associated with HuR relocalization to the cytoplasm. J. Biol. Chem. 2012;287:36229–36238. doi: 10.1074/jbc.M112.371203. doi:10.1074/jbc.M112.371203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domitrovich AM, Diebel KW, Ali N, Sarker S, Siddiqui A. Role of La autoantigen and polypyrimidine tract-binding protein in HCV replication. Virology. 2005;335:72–86. doi: 10.1016/j.virol.2005.02.009. doi:10.1016/j.virol.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Dougherty JD, White JP, Lloyd RE. Poliovirus-mediated disruption of cytoplasmic processing bodies. J. Virol. 2011;85:64–75. doi: 10.1128/JVI.01657-10. doi:10.1128/JVI.01657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgil D, Polacek C, Harris E. Dengue virus utilizes a novel strategy for translation initiation when cap-dependent translation is inhibited. J. Virol. 2006;80:2976–2986. doi: 10.1128/JVI.80.6.2976-2986.2006. doi:10.1128/JVI.80.6.2976-2986.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emara MM, Brinton MA. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc. Natl. Acad. Sci. USA. 2007;104:9041–9046. doi: 10.1073/pnas.0703348104. doi:10.1073/pnas.0703348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emara MM, Liu H, Davis WG, Brinton MA. Mutation of mapped TIA-1/TIAR binding sites in the 3′ terminal stem-loop of West Nile virus minus-strand RNA in an infectious clone negatively affects genomic RNA amplification. J. Virol. 2008;82:10657–10670. doi: 10.1128/JVI.00991-08. doi:10.1128/JVI.00991-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel KJ, Brunner JE, Semler BL. Mechanistic consequences of hnRNP C binding to both RNA termini of poliovirus negative-strand RNA intermediates. J. Virol. 2010;84:4229–4242. doi: 10.1128/JVI.02198-09. doi:10.1128/JVI.02198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Hernández W, Velez-Uriza D, Valdés J, Vélez-Del Valle C, Salas-Benito J, Martínez-Contreras R, García-Espítia M, Salas-Benito M, Vega-Almeida T, De Nova-Ocampo M. PTB Binds to the 3′ Untranslated Region of the Human Astrovirus Type 8: A Possible Role in Viral Replication. PLoS ONE. 2014;9:e113113. doi: 10.1371/journal.pone.0113113. doi:10.1371/journal.pone.0113113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filomatori CV, Iglesias NG, Villordo SM, Alvarez DE, Gamarnik AV. RNA sequences and structures required for the recruitment and activity of the dengue virus polymerase. J. Biol Chem. 2011;286:6929–6939. doi: 10.1074/jbc.M110.162289. doi:10.1074/jbc.M110.162289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KD, Semler BL. Re-localization of Cellular Protein SRp20 during Poliovirus Infection: Bridging a Viral IRES to the Host Cell Translation Apparatus. PLoS Path. 2011;7:e1002127. doi: 10.1371/journal.ppat.1002127. doi:10.1371/journal.ppat.1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanes V, Raychaudhuri S, Dasgupta A. A cell-permeable peptide inhibits hepatitis C virus replication by sequestering IRES transacting factors. Virology. 2009;394:82–90. doi: 10.1016/j.virol.2009.08.012. doi:10.1016/j.virol.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe P, Boudet J, Simorre J-P, Bartenschlager R. Kissing-loop interaction in the 3′ end of the hepatitis C virus genome essential for RNA replication. J. Virol. 2005;79:380–392. doi: 10.1128/JVI.79.1.380-392.2005. doi:10.1128/JVI.79.1.380-392.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchsová B, Novák P, Kafková J, Hozák P. Nuclear DNA helicase II is recruited to IFN-alpha-activated transcription sites at PML nuclear bodies. J. Cell. Biol. 2002;158:463–473. doi: 10.1083/jcb.200202035. doi:10.1083/jcb.200202035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán C, Sola I, Nogales A, Thomas B, Akoulitchev A, Enjuanes L, Almazán F. Host cell proteins interacting with the 3′ end of TGEV coronavirus genome influence virus replication. Virology. 2009;391:304–314. doi: 10.1016/j.virol.2009.06.006. doi:10.1016/j.virol.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamarnik AV, Andino R. Two functional complexes formed by KH domain containing proteins with the 5′ noncoding region of poliovirus RNA. RNA. 1997;3:882–892. [PMC free article] [PubMed] [Google Scholar]
- Gamarnik AV, Andino R. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev. 1998;12:2293–2304. doi: 10.1101/gad.12.15.2293. doi:10.1101/gad.12.15.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamarnik AV, Andino R. Interactions of viral protein 3CD and poly(rC) binding protein with the 5′ untranslated region of the poliovirus genome. J. Virol. 2000;74:2219–2226. doi: 10.1128/jvi.74.5.2219-2226.2000. doi:10.1128/JVI.74.5.2219-2226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Montalvo BM, Medina F, del Angel RM. La protein binds to NS5 and NS3 and to the 5“ and 3” ends of Dengue 4 virus RNA. Virus Res. 2004;102:141–150. doi: 10.1016/j.virusres.2004.01.024. doi:10.1016/j.virusres.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Garneau NL, Sokoloski KJ, Opyrchal M, Neff CP, Wilusz CJ, Wilusz J. The 3′ untranslated region of sindbis virus represses deadenylation of viral transcripts in mosquito and Mammalian cells. J. Virol. 2008;82:880–892. doi: 10.1128/JVI.01205-07. doi:10.1128/JVI.01205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomila RC, Martin GW, Gehrke L. NF90 binds the dengue virus RNA 3′ terminus and is a positive regulator of dengue virus replication. PLoS ONE. 2011;6:e16687. doi: 10.1371/journal.pone.0016687. doi:10.1371/journal.pone.0016687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorchakov R, Garmashova N, Frolova E, Frolov I. Different types of nsP3-containing protein complexes in Sindbis virus-infected cells. J. Virol. 2008;82:10088–10101. doi: 10.1128/JVI.01011-08. doi:10.1128/JVI.01011-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc. Natl. Acad. Sci. USA. 2000;97:6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui H, Lu C-W, Adams S, Stollar V, Li M-L. hnRNP A1 interacts with the genomic and subgenomic RNA promoters of Sindbis virus and is required for the synthesis of G and SG RNA. J. Biomed. Sci. 2010;17:59. doi: 10.1186/1423-0127-17-59. doi:10.1186/1423-0127-17-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Escolano AL, Vázquez-Ochoa M, Escobar-Herrera J, Hernández-Acosta J. La, PTB, and PAB proteins bind to the 3(′) untranslated region of Norwalk virus genomic RNA. Biochem Biophys Res Commun. 2003;311:759–766. doi: 10.1016/j.bbrc.2003.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambidge SJ, Sarnow P. Translational enhancement of the poliovirus 5′ noncoding region mediated by virus-encoded polypeptide 2A. Proc. Natl. Acad. Sci. USA. 1992;89:10272–10276. doi: 10.1073/pnas.89.21.10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D, Zhang Z, Chaubey B, Pandey VN. Identification of cellular factors associated with the 3′-nontranslated region of the hepatitis C virus genome. Mol. Cell. Proteomics. 2006;5:1006–1018. doi: 10.1074/mcp.M500429-MCP200. doi:10.1074/mcp.M500429-MCP200. [DOI] [PubMed] [Google Scholar]
- Hellen CU, Witherell GW, Schmid M, Shin SH, Pestova TV, Gil A, Wimmer E. A cytoplasmic 57-kDa protein that is required for translation of picornavirus RNA by internal ribosomal entry is identical to the nuclear pyrimidine tract-binding protein. Proc. Natl. Acad. Sci. USA. 1993;90:7642–7646. doi: 10.1073/pnas.90.16.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellen CUT. IRES-induced conformational changes in the ribosome and the mechanism of translation initiation by internal ribosomal entry. Biochim. Biophys. Acta. 2009;1789:558–570. doi: 10.1016/j.bbagrm.2009.06.001. doi:10.1016/j.bbagrm.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold J, Andino R. Poliovirus RNA replication requires genome circularization through a protein-protein bridge. Mol. Cell. 2001;7:581–591. doi: 10.1016/S1097-2765(01)00205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M, Kaneko S, Yamashita T, Luo H, Qin W, Shirota Y, Nomura T, Kobayashi K, Murakami S. Direct interaction between nucleolin and hepatitis C virus NS5B. J. Biol. Chem. 2003;278:5109–5115. doi: 10.1074/jbc.M207629200. doi:10.1074/jbc.M207629200. [DOI] [PubMed] [Google Scholar]
- Huang P, Lai MM. Polypyrimidine tract-binding protein binds to the complementary strand of the mouse hepatitis virus 3′ untranslated region, thereby altering RNA conformation. J. Virol. 1999;73:9110–9116. doi: 10.1128/jvi.73.11.9110-9116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Lai MM. Heterogeneous nuclear ribonucleoprotein a1 binds to the 3′-untranslated region and mediates potential 5′-3′-end cross talks of mouse hepatitis virus RNA. J. Virol. 2001;75:5009–5017. doi: 10.1128/JVI.75.11.5009-5017.2001. doi:10.1128/JVI.75.11.5009-5017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SL, Hsuan JJ, Totty N, Jackson RJ. unr, a cellular cytoplasmic RNA-binding protein with five cold-shock domains, is required for internal initiation of translation of human rhinovirus RNA. Genes Dev. 1999;13:437–448. doi: 10.1101/gad.13.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isken O, Baroth M, Grassmann CW, Weinlich S, Ostareck DH, Ostareck-Lederer A, Behrens S-E. Nuclear factors are involved in hepatitis C virus RNA replication. RNA. 2007;13:1675–1692. doi: 10.1261/rna.594207. doi:10.1261/rna.594207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isken O, Grassmann CW, Sarisky RT, Kann M, Zhang S, Grosse F, Kao PN, Behrens S-E. Members of the NF90/NFAR protein group are involved in the life cycle of a positive-strand RNA virus. EMBO J. 2003;22:5655–5665. doi: 10.1093/emboj/cdg562. doi:10.1093/emboj/cdg562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isken O, Grassmann CW, Yu H, Behrens S-E. Complex signals in the genomic 3′ nontranslated region of bovine viral diarrhea virus coordinate translation and replication of the viral RNA. RNA. 2004;10:1637–1652. doi: 10.1261/rna.7290904. doi:10.1261/rna.7290904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Lai MM. An internal polypyrimidine-tract-binding protein-binding site in the hepatitis C virus RNA attenuates translation, which is relieved by the 3′-untranslated sequence. Virology. 1999;254:288–296. doi: 10.1006/viro.1998.9541. doi:10.1006/viro.1998.9541. [DOI] [PubMed] [Google Scholar]
- Izumi RE, Valdez B, Banerjee R, Srivastava M, Dasgupta A. Nucleolin stimulates viral internal ribosome entry site-mediated translation. Virus Res. 2001;76:17–29. doi: 10.1016/s0168-1702(01)00240-4. doi:10.1016/S0168-1702(01)00240-4. [DOI] [PubMed] [Google Scholar]
- Jahan N, Wimmer E, Mueller S. A Host-Specific, Temperature-Sensitive Translation Defect Determines the Attenuation Phenotype of a Human Rhinovirus/Poliovirus Chimera, PV1(RIPO) J. Virol. 2011;85:7225–7235. doi: 10.1128/JVI.01804-09. doi:10.1128/JVI.01804-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan N, Wimmer E, Mueller S. Polypyrimidine tract binding protein-1 (PTB1) is a determinant of the tissue and host tropism of a human rhinovirus/poliovirus chimera PV1(RIPO) PLoS ONE. 2013;8:e60791. doi: 10.1371/journal.pone.0060791. doi:10.1371/journal.pone.0060791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Fraser CS, Yu Y, Leary J, Doudna JA. Coordinated assembly of human translation initiation complexes by the hepatitis C virus internal ribosome entry site RNA. Proc. Natl. Acad. Sci. USA. 2004;101:16990–16995. doi: 10.1073/pnas.0407402101. doi:10.1073/pnas.0407402101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Yao H, Duan X, Lu X, Liu Y. Polypyrimidine tract-binding protein influences negative strand RNA synthesis of dengue virus. Biochem. Biophys. Res. Commun. 2009;385:187–192. doi: 10.1016/j.bbrc.2009.05.036. doi:10.1016/j.bbrc.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Serviene E, Gal J, Panavas T, Nagy PD. Identification of essential host factors affecting tombusvirus RNA replication based on the yeast Tet promoters Hughes Collection. J. Virol. 2006;80:7394–7404. doi: 10.1128/JVI.02686-05. doi:10.1128/JVI.02686-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafasla P, Lin H, Curry S, Jackson RJ. Activation of picornaviral IRESs by PTB shows differential dependence on each PTB RNA-binding domain. RNA. 2011;17:1120–1131. doi: 10.1261/rna.2549411. doi:10.1261/rna.2549411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafasla P, Morgner N, Pöyry TAA, Curry S, Robinson CV, Jackson RJ. Polypyrimidine tract binding protein stabilizes the encephalomyocarditis virus IRES structure via binding multiple sites in a unique orientation. Mol. Cell. 2009;34:556–568. doi: 10.1016/j.molcel.2009.04.015. doi:10.1016/j.molcel.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Kafasla P, Morgner N, Robinson CV, Jackson RJ. Polypyrimidine tract-binding protein stimulates the poliovirus IRES by modulating eIF4G binding. EMBO J. 2010;29:3710–3722. doi: 10.1038/emboj.2010.231. doi:10.1038/emboj.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T, Gauss-Müller V, Cordes S, Tamura R, Okitsu K, Shuang W, Nakamoto S, Fujiwara K, Imazeki F, Yokosuka O. Hepatitis A virus (HAV) proteinase 3C inhibits HAV IRES-dependent translation and cleaves the polypyrimidine tract-binding protein. J. Viral Hepat. 2010;17:618–623. doi: 10.1111/j.1365-2893.2009.01221.x. doi:10.1111/j.1365-2893.2009.01221.x. [DOI] [PubMed] [Google Scholar]
- Karakasiliotis I, Vashist S, Bailey D, Abente EJ, Green KY, Roberts LO, Sosnovtsev SV, Goodfellow IG. Polypyrimidine tract binding protein functions as a negative regulator of feline calicivirus translation. PLoS ONE. 2010;5:e9562. doi: 10.1371/journal.pone.0009562. doi:10.1371/journal.pone.0009562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Ivanov P, Anderson P. Stress granules and cell signaling: more than just a passing phase? Trends Biochem. Sci. 2013;38:494–506. doi: 10.1016/j.tibs.2013.07.004. doi:10.1016/j.tibs.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell. Biol. 1999;147:1431–1442. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf BJ, Barton DJ. Poly(rC) binding proteins and the 5′ cloverleaf of uncapped poliovirus mRNA function during de novo assembly of polysomes. J. Virol. 2008;82:5835–5846. doi: 10.1128/JVI.01513-07. doi:10.1128/JVI.01513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Seol SK, Song O-K, Park JH, Jang SK. An RNA-binding protein, hnRNP A1, and a scaffold protein, septin 6, facilitate hepatitis C virus replication. J. Virol. 2007;81:3852–3865. doi: 10.1128/JVI.01311-06. doi:10.1128/JVI.01311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Paek KY, Ha SH, Cho S, Choi K, Kim CS, Ryu SH, Jang SK. A cellular RNA-binding protein enhances internal ribosomal entry site-dependent translation through an interaction downstream of the hepatitis C virus polyprotein initiation codon. Mol. Cell. Biol. 2004;24:7878–7890. doi: 10.1128/MCB.24.18.7878-7890.2004. doi:10.1128/MCB.24.18.7878-7890.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Jeong YS. Polypyrimidine tract-binding protein interacts with the 3′ stem-loop region of Japanese encephalitis virus negative-strand RNA. Virus Res. 2006;115:131–140. doi: 10.1016/j.virusres.2005.07.013. doi:10.1016/j.virusres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- King HA, Cobbold LC, Willis AE. The role of IRES trans-acting factors in regulating translation initiation. Biochem. Soc. Trans. 2010;38:1581–1586. doi: 10.1042/BST0381581. doi:10.1042/BST0381581. [DOI] [PubMed] [Google Scholar]
- Korf M, Jarczak D, Beger C, Manns MP, Krüger M. Inhibition of hepatitis C virus translation and subgenomic replication by siRNAs directed against highly conserved HCV sequence and cellular HCV cofactors. J Hepatol. 2005;43:225–234. doi: 10.1016/j.jhep.2005.02.046. doi:10.1016/j.jhep.2005.02.046. [DOI] [PubMed] [Google Scholar]
- Kruger M, Beger C, Welch PJ, Barber JR, Manns MP, Wong-Staal F. Involvement of proteasome alpha-subunit PSMA7 in hepatitis C virus internal ribosome entry site-mediated translation. Mol. Cell. Biol. 2001;21:8357–8364. doi: 10.1128/MCB.21.24.8357-8364.2001. doi:10.1128/MCB.21.24.8357-8364.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Ray U, Das S. Human La protein interaction with GCAC near the initiator AUG enhances hepatitis C Virus RNA replication by promoting linkage between 5“ and 3” untranslated regions. J. Virol. 2013;87:6713–6726. doi: 10.1128/JVI.00525-13. doi:10.1128/JVI.00525-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakawa T, Shimakami T, Kaneko S, Yoshioka K, Murakami S. Functional interaction of hepatitis C Virus NS5B with Nucleolin GAR domain. Journal of Biochemistry. 2007;141:917–927. doi: 10.1093/jb/mvm102. doi:10.1093/jb/mvm102. [DOI] [PubMed] [Google Scholar]
- Kushner DB, Lindenbach BD, Grdzelishvili VZ, Noueiry AO, Paul SM, Ahlquist P. Systematic, genome-wide identification of host genes affecting replication of a positive-strand RNA virus. Proc. Natl. Acad. Sci. USA. 2003;100:15764–15769. doi: 10.1073/pnas.2536857100. doi:10.1073/pnas.2536857100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyumcu-Martinez NM, Van Eden ME, Younan P, Lloyd RE. Cleavage of poly(A)-binding protein by poliovirus 3C protease inhibits host cell translation: a novel mechanism for host translation shutoff. Mol. Cell. Biol. 2004;24:1779–1790. doi: 10.1128/MCB.24.4.1779-1790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane R, Daubner GM, Thomas-Crusells J, Auweter SD, Manatschal C, Austin KS, Valniuk O, Allain FH-T, Rueda D. RNA looping by PTB: Evidence using FRET and NMR spectroscopy for a role in splicing repression. Proc. Natl. Acad. Sci. USA. 2010;107:4105–4110. doi: 10.1073/pnas.0907072107. doi:10.1073/pnas.0907072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langereis MA, Feng Q, Nelissen FHT, Virgen-Slane R, van der Heden van Noort GJ, Maciejewski S, Filippov DV, Semler BL, van Delft FL, van Kuppeveld FJM. Modification of picornavirus genomic RNA using “click” chemistry shows that unlinking of the VPg peptide is dispensable for translation and replication of the incoming viral RNA. Nucl. Acids Res. 2013 doi: 10.1093/nar/gkt1162. doi:10.1093/nar/gkt1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P, Conderino JS, Rieder E. Redistribution of demethylated RNA helicase A during foot-and-mouth disease virus infection: role of Jumonji C-domain containing protein 6 in RHA demethylation. Virology. 2014;452-453:1–11. doi: 10.1016/j.virol.2013.12.040. doi:10.1016/j.virol.2013.12.040. [DOI] [PubMed] [Google Scholar]
- Lawrence P, Rieder E. Identification of RNA helicase A as a new host factor in the replication cycle of foot-and-mouth disease virus. J. Virol. 2009;83:11356–11366. doi: 10.1128/JVI.02677-08. doi:10.1128/JVI.02677-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Huang Y, Zhang H, Yu L, Zhang M, Dayton A. Functional interaction between cellular p100 and the dengue virus 3′ UTR. J. Gen. Virol. 2011;92:796–806. doi: 10.1099/vir.0.028597-0. doi:10.1099/vir.0.028597-0. [DOI] [PubMed] [Google Scholar]
- Le Sage V, Mouland AJ. Viral subversion of the nuclear pore complex. Viruses. 2013;5:2019–2042. doi: 10.3390/v5082019. doi:10.3390/v5082019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarcic EM, Landry DM, Greco TM, Cristea IM, Thompson SR. Thiouracil cross-linking mass spectrometry: a cell-based method to identify host factors involved in viral amplification. J. Virol. 2013;87:8697–8712. doi: 10.1128/JVI.00950-13. doi:10.1128/JVI.00950-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HP, Huang P, Park S, Lai MM. Polypyrimidine tract-binding protein binds to the leader RNA of mouse hepatitis virus and serves as a regulator of viral transcription. J. Virol. 1999;73:772–777. doi: 10.1128/jvi.73.1.772-777.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HP, Zhang X, Duncan R, Comai L, Lai MM. Heterogeneous nuclear ribonucleoprotein A1 binds to the transcription-regulatory region of mouse hepatitis virus RNA. Proc. Natl. Acad. Sci. USA. 1997;94:9544–9549. doi: 10.1073/pnas.94.18.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li Y, Kedersha N, Anderson P, Emara M, Swiderek KM, Moreno GT, Brinton MA. Cell proteins TIA-1 and TIAR interact with the 3′ stem-loop of the West Nile virus complementary minus-strand RNA and facilitate virus replication. J. Virol. 2002;76:11989–12000. doi: 10.1128/JVI.76.23.11989-12000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J-Y, Shih S-R, Pan M, Li C, Lue C-F, Stollar V, Li M-L. hnRNP A1 interacts with the 5′ untranslated regions of enterovirus 71 and Sindbis virus RNA and is required for viral replication. J. Virol. 2009;83:6106–6114. doi: 10.1128/JVI.02476-08. doi:10.1128/JVI.02476-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HM, Aizaki H, Choi KS, Machida K, Ou JJH, Lai MMC. SYNCRIP (synaptotagmin-binding, cytoplasmic RNA-interacting protein) is a host factor involved in hepatitis C virus RNA replication. Virology. 2009;386:249–256. doi: 10.1016/j.virol.2009.01.018. doi:10.1016/j.virol.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HM, Aizaki H, Machida K, Ou JHJ, Lai MMC. Hepatitis C virus translation preferentially depends on active RNA replication. PLoS ONE. 2012;7:e43600. doi: 10.1371/journal.pone.0043600. doi:10.1371/journal.pone.0043600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd RE. Regulation of stress granules and P-bodies during RNA virus infection. WIREs RNA. 2013;4:317–331. doi: 10.1002/wrna.1162. doi:10.1002/wrna.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodeiro MF, Filomatori CV, Gamarnik AV. Structural and functional studies of the promoter element for dengue virus RNA replication. J. Virol. 2009;83:993–1008. doi: 10.1128/JVI.01647-08. doi:10.1128/JVI.01647-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Manríquez E, Vashist S, Urena L, Goodfellow I, Chavez P, Mora-Heredia JE, Cancio-Lonches C, Garrido E, Gutiérrez-Escolano AL. Norovirus genome circularization and efficient replication are facilitated by binding of PCBP2 and hnRNP A1. J. Virol. 2013;87:11371–11387. doi: 10.1128/JVI.03433-12. doi:10.1128/JVI.03433-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malygin AA, Kossinova OA, Shatsky IN, Karpova GG. HCV IRES interacts with the 18S rRNA to activate the 40S ribosome for subsequent steps of translation initiation. Nucl. Acids Res. 2013 doi: 10.1093/nar/gkt632. doi:10.1093/nar/gkt632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AE, Schlegel A, Kirkegaard K. Human protein Sam68 relocalization and interaction with poliovirus RNA polymerase in infected cells. Proc. Natl. Acad. Sci. USA. 1996;93:2296–2301. doi: 10.1073/pnas.93.6.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerovitch K, Pelletier J, Sonenberg N. A cellular protein that binds to the 5′-noncoding region of poliovirus RNA: implications for internal translation initiation. Genes Dev. 1989;3:1026–1034. doi: 10.1101/gad.3.7.1026. [DOI] [PubMed] [Google Scholar]
- Meerovitch K, Svitkin YV, Lee HS, Lejbkowicz F, Kenan DJ, Chan EK, Agol VI, Keene JD, Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J. Virol. 1993;67:3798–3807. doi: 10.1128/jvi.67.7.3798-3807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SL, Anderson JR, Kumagai Y, Wilusz CJ, Akira S, Khromykh AA, Wilusz J. A noncoding RNA produced by arthropod-borne flaviviruses inhibits the cellular exoribonuclease XRN1 and alters host mRNA stability. RNA. 2012;18:2029–2040. doi: 10.1261/rna.034330.112. doi:10.1261/rna.034330.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KE, Roberts AW, Barton DJ. Poly(rC) binding proteins mediate poliovirus mRNA stability. RNA. 2001;7:1126–1141. doi: 10.1017/s1355838201010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstrass FC, Auweter SD, Erat M, Hargous Y, Henning A, Wenter P, Reymond L, Amir-Ahmady B, Pitsch S, Black DL, Allain FH-T. Structure of PTB bound to RNA: specific binding and implications for splicing regulation. Science. 2005;309:2054–2057. doi: 10.1126/science.1114066. doi:10.1126/science.1114066. [DOI] [PubMed] [Google Scholar]
- Ogram SA, Spear A, Sharma N, Flanegan JB. The 5“CL-PCBP RNP complex, 3” poly(A) tail and 2A(pro) are required for optimal translation of poliovirus RNA. Virology. 2010;397:14–22. doi: 10.1016/j.virol.2009.11.006. doi:10.1016/j.virol.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco A, Reigadas S, Martínez-Salas E. Riboproteomic analysis of polypeptides interacting with the internal ribosome-entry site element of foot-and-mouth disease viral RNA. Proteomics. 2008;8:4782–4790. doi: 10.1002/pmic.200800338. doi:10.1002/pmic.200800338. [DOI] [PubMed] [Google Scholar]
- Panas MD, Varjak M, Lulla A, Eng KE, Merits A, Karlsson Hedestam GB, McInerney GM. Sequestration of G3BP coupled with efficient translation inhibits stress granules in Semliki Forest virus infection. Mol. Biol. Cell. 2012;23:4701–4712. doi: 10.1091/mbc.E12-08-0619. doi:10.1091/mbc.E12-08-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panavas T, Serviene E, Brasher J, Nagy PD. Yeast genome-wide screen reveals dissimilar sets of host genes affecting replication of RNA viruses. Proc. Natl. Acad. Sci. USA. 2005;102:7326–7331. doi: 10.1073/pnas.0502604102. doi:10.1073/pnas.0502604102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjape SM, Harris E. Y box-binding protein-1 binds to the dengue virus 3′-untranslated region and mediates antiviral effects. J. Biol. Chem. 2007;282:30497–30508. doi: 10.1074/jbc.M705755200. doi:10.1074/jbc.M705755200. [DOI] [PubMed] [Google Scholar]
- Park N, Katikaneni P, Skern T, Gustin KE. Differential targeting of nuclear pore complex proteins in poliovirus-infected cells. J. Virol. 2008;82:1647–1655. doi: 10.1128/JVI.01670-07. doi:10.1128/JVI.01670-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park N, Skern T, Gustin KE. Specific cleavage of the nuclear pore complex protein Nup62 by a viral protease. J. Biol. Chem. 2010;285:28796–28805. doi: 10.1074/jbc.M110.143404. doi:10.1074/jbc.M110.143404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Paek KY, Hong KY, Jang CJ, Cho S, Park JH, Kim JH, Jan E, Jang SK. Translation-competent 48S complex formation on HCV IRES requires the RNA-binding protein NSAP1. Nucl. Acids Res. 2011 doi: 10.1093/nar/gkr509. doi:10.1093/nar/gkr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera R, Daijogo S, Walter BL, Nguyen JHC, Semler BL. Cellular protein modification by poliovirus: the two faces of poly(rC)-binding protein. J. Virol. 2007;81:8919–8932. doi: 10.1128/JVI.01013-07. doi:10.1128/JVI.01013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova TV, Hellen CU, Shatsky IN. Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Mol. Cell. Biol. 1996;16:6859–6869. doi: 10.1128/mcb.16.12.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova TV, Shatsky IN, Fletcher SP, Jackson RJ, Hellen CU. A prokaryotic-like mode of cytoplasmic eukaryotic ribosome binding to the initiation codon during internal translation initiation of hepatitis C and classical swine fever virus RNAs. Genes Dev. 1998;12:67–83. doi: 10.1101/gad.12.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Vilaró G, Fernández-Carrillo C, Mensa L, Miquel R, Sanjuan X, Forns X, Pérez-del-Pulgar S, Díez J. Hepatitis C virus infection inhibits P-body granule formation in human livers. J. Hepatol. 2014 doi: 10.1016/j.jhep.2014.11.018. doi:10.1016/j.jhep.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Pérez-Vilaró G, Scheller N, Saludes V, Díez J. Hepatitis C virus infection alters p-body composition but is independent of p-body granules. J. Virol. 2012;86:8740–8749. doi: 10.1128/JVI.07167-11. doi:10.1128/JVI.07167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipenko EV, Viktorova EG, Guest ST, Agol VI, Roos RP. Cell-specific proteins regulate viral RNA translation and virus-induced disease. EMBO J. 2001;20:6899–6908. doi: 10.1093/emboj/20.23.6899. doi:10.1093/emboj/20.23.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeiro D, Fernandez N, Ramajo J, Martínez-Salas E. Gemin5 promotes IRES interaction and translation control through its C-terminal region. Nucl. Acids Res. 2013;41:1017–1028. doi: 10.1093/nar/gks1212. doi:10.1093/nar/gks1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeiro D, Ramajo J, Bradrick SS, Martínez-Salas E. Gemin5 proteolysis reveals a novel motif to identify L protease targets. Nucl. Acids Res. 2012;40:4942–4953. doi: 10.1093/nar/gks172. doi:10.1093/nar/gks172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacek C, Foley JE, Harris E. Conformational changes in the solution structure of the dengue virus 5“ end in the presence and absence of the 3” untranslated region. J. Virol. 2009;83:1161–1166. doi: 10.1128/JVI.01362-08. doi:10.1128/JVI.01362-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacek C, Friebe P, Harris E. Poly(A)-binding protein binds to the non-polyadenylated 3′ untranslated region of dengue virus and modulates translation efficiency. J Gen Virol. 2009;90:687–692. doi: 10.1099/vir.0.007021-0. doi:10.1099/vir.0.007021-0. [DOI] [PubMed] [Google Scholar]
- Porter FW, Palmenberg AC. Leader-induced phosphorylation of nucleoporins correlates with nuclear trafficking inhibition by cardioviruses. J. Virol. 2009;83:1941–1951. doi: 10.1128/JVI.01752-08. doi:10.1128/JVI.01752-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray U, Das S. Interplay between NS3 protease and human La protein regulates translation-replication switch of Hepatitis C virus. Sci Rep. 2011;1:1. doi: 10.1038/srep00001. doi:10.1038/srep00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke LC, Lloyd RE. Diversion of stress granules and P-bodies during viral infection. Virology. 2013;436:255–267. doi: 10.1016/j.virol.2012.11.017. doi:10.1016/j.virol.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricour C, Delhaye S, Hato SV, Olenyik TD, Michel B, van Kuppeveld FJM, Gustin KE, Michiels T. Inhibition of mRNA export and dimerization of interferon regulatory factor 3 by Theiler’s virus leader protein. J. Gen. Virol. 2009;90:177–186. doi: 10.1099/vir.0.005678-0. doi:10.1099/vir.0.005678-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-Aravena A, Ramdohr P, Vallejos M, Valiente-Echeverría F, Dormoy-Raclet V, Rodríguez F, Pino K, Holzmann C, Huidobro-Toro JP, Gallouzi I-E, López-Lastra M. The Elav-like protein HuR exerts translational control of viral internal ribosome entry sites. Virology. 2009;392:178–185. doi: 10.1016/j.virol.2009.06.050. doi:10.1016/j.virol.2009.06.050. [DOI] [PubMed] [Google Scholar]
- Rozovics JM, Chase AJ, Cathcart AL, Chou W, Gershon PD, Palusa S, Wilusz J, Semler BL. Picornavirus modification of a host mRNA decay protein. MBio. 2012;3 doi: 10.1128/mBio.00431-12. doi:10.1128/mBio.00431-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri A, Dazert E, Metz P, Hofmann S, Bergeest J-P, Mazur J, Bankhead P, Hiet M-S, Kallis S, Alvisi G, Samuel CE, Lohmann V, Kaderali L, Rohr K, Frese M, Stoecklin G, Bartenschlager R. Dynamic oscillation of translation and stress granule formation mark the cellular response to virus infection. Cell Host Microbe. 2012;12:71–85. doi: 10.1016/j.chom.2012.05.013. doi:10.1016/j.chom.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz MA, Garcia-Moreno M, Carrasco L. Inhibition of Host Protein Synthesis by Sindbis Virus: Correlation with Viral Rna Replication and Release of Nuclear Proteins to the Cytoplasm. Cell Microbiol. 2014 doi: 10.1111/cmi.12381. doi:10.1111/cmi.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicka K, Bushell M, Spriggs KA, Willis AE. Polypyrimidine-tract-binding protein: a multifunctional RNA-binding protein. Biochem. Soc. Trans. 2008;36:641–647. doi: 10.1042/BST0360641. doi:10.1042/BST0360641. [DOI] [PubMed] [Google Scholar]
- Scheller N, Mina LB, Galão RP, Chari A, Giménez-Barcons M, Noueiry A, Fischer U, Meyerhans A, Díez J. Translation and replication of hepatitis C virus genomic RNA depends on ancient cellular proteins that control mRNA fates. Proc. Natl. Acad. Sci. USA. 2009;106:13517–13522. doi: 10.1073/pnas.0906413106. doi:10.1073/pnas.0906413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ST, Huang P, Li HP, Lai MM. Heterogeneous nuclear ribonucleoprotein A1 regulates RNA synthesis of a cytoplasmic virus. EMBO J. 2000;19:4701–4711. doi: 10.1093/emboj/19.17.4701. doi:10.1093/emboj/19.17.4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ST, Yu G-Y, Lai MMC. Multiple type A/B heterogeneous nuclear ribonucleoproteins (hnRNPs) can replace hnRNP A1 in mouse hepatitis virus RNA synthesis. J. Virol. 2003;77:10584–10593. doi: 10.1128/JVI.77.19.10584-10593.2003. doi:10.1128/JVI.77.19.10584-10593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki T, Honda M, Mizuno H, Shimakami T, Okada H, Sakai Y, Murakami S, Wakita T, Kaneko S. La protein required for internal ribosome entry site-directed translation is a potential therapeutic target for hepatitis C virus replication. J. Infect. Dis. 2010;202:75–85. doi: 10.1086/653081. doi:10.1086/653081. [DOI] [PubMed] [Google Scholar]
- Shiroki K, Isoyama T, Kuge S, Ishii T, Ohmi S, Hata S, Suzuki K, Takasaki Y, Nomoto A. Intracellular redistribution of truncated La protein produced by poliovirus 3Cpro-mediated cleavage. J. Virol. 1999;73:2193–2200. doi: 10.1128/jvi.73.3.2193-2200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvera D, Gamarnik AV, Andino R. The N-terminal K homology domain of the poly(rC)-binding protein is a major determinant for binding to the poliovirus 5′-untranslated region and acts as an inhibitor of viral translation. J. Biol. Chem. 1999;274:38163–38170. doi: 10.1074/jbc.274.53.38163. doi:10.1074/jbc.274.53.38163. [DOI] [PubMed] [Google Scholar]
- Siridechadilok B, Fraser CS, Hall RJ, Doudna JA, Nogales E. Structural roles for human translation factor eIF3 in initiation of protein synthesis. Science. 2005;310:1513–1515. doi: 10.1126/science.1118977. doi:10.1126/science.1118977. [DOI] [PubMed] [Google Scholar]
- Sokoloski KJ, Dickson AM, Chaskey EL, Garneau NL, Wilusz CJ, Wilusz J. Sindbis virus usurps the cellular HuR protein to stabilize its transcripts and promote productive infections in mammalian and mosquito cells. Cell Host Microbe. 2010;8:196–207. doi: 10.1016/j.chom.2010.07.003. doi:10.1016/j.chom.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola I, Galán C, Mateos-Gómez PA, Palacio L, Zúñiga S, Cruz JL, Almazán F, Enjuanes L. The polypyrimidine tract-binding protein affects coronavirus RNA accumulation levels and relocalizes viral RNAs to novel cytoplasmic domains different from replication-transcription sites. J. Virol. 2011a;85:5136–5149. doi: 10.1128/JVI.00195-11. doi:10.1128/JVI.00195-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola I, Mateos-Gómez PA, Almazán F, Zúñiga S, Enjuanes L. RNA-RNA and RNA-protein interactions in coronavirus replication and transcription. RNA Biol. 2011b;8:237–248. doi: 10.4161/rna.8.2.14991. doi:10.4161/rna.8.2.14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Tzima E, Ochs K, Bassili G, Trusheim H, Linder M, Preissner KT, Niepmann M. Evidence for an RNA chaperone function of polypyrimidine tract-binding protein in picornavirus translation. RNA. 2005;11:1809–1824. doi: 10.1261/rna.7430405. doi:10.1261/rna.7430405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spångberg K, Wiklund L, Schwartz S. HuR, a protein implicated in oncogene and growth factor mRNA decay, binds to the 3′ ends of hepatitis C virus RNA of both polarities. Virology. 2000;274:378–390. doi: 10.1006/viro.2000.0461. doi:10.1006/viro.2000.0461. [DOI] [PubMed] [Google Scholar]
- Spångberg K, Wiklund L, Schwartz S. Binding of the La autoantigen to the hepatitis C virus 3′ untranslated region protects the RNA from rapid degradation in vitro. J. Gen. Virol. 2001;82:113–120. doi: 10.1099/0022-1317-82-1-113. [DOI] [PubMed] [Google Scholar]
- Sweeney TR, Abaeva IS, Pestova TV, Hellen CUT. The mechanism of translation initiation on Type 1 picornavirus IRESs. EMBO J. 2014;33:76–92. doi: 10.1002/embj.201386124. doi:10.1002/embj.201386124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney TR, Dhote V, Yu Y, Hellen CUT. A distinct class of internal ribosomal entry site in members of the Kobuvirus and proposed Salivirus and Paraturdivirus genera of the Picornaviridae. J. Virol. 2012;86:1468–1486. doi: 10.1128/JVI.05862-11. doi:10.1128/JVI.05862-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztuba-Solinska J, Teramoto T, Rausch JW, Shapiro BA, Padmanabhan R, Le Grice SFJ. Structural complexity of Dengue virus untranslated regions: cis-acting RNA motifs and pseudoknot interactions modulating functionality of the viral genome. Nucl. Acids Res. 2013;41:5075–5089. doi: 10.1093/nar/gkt203. doi:10.1093/nar/gkt203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H, Franco D, Fujita K, Paul AV, Wimmer E. Replication of poliovirus requires binding of the poly(rC) binding protein to the cloverleaf as well as to the adjacent C-rich spacer sequence between the cloverleaf and the internal ribosomal entry site. J. Virol. 2007;81:10017–10028. doi: 10.1128/JVI.00516-07. doi:10.1128/JVI.00516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varjak M, Saul S, Arike L, Lulla A, Peil L, Merits A. Magnetic fractionation and proteomic dissection of cellular organelles occupied by the late replication complexes of Semliki Forest virus. J. Virol. 2013;87:10295–10312. doi: 10.1128/JVI.01105-13. doi:10.1128/JVI.01105-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashist S, Urena L, Chaudhry Y, Goodfellow I. Identification of RNA-protein interaction networks involved in the norovirus life cycle. J. Virol. 2012;86:11977–11990. doi: 10.1128/JVI.00432-12. doi:10.1128/JVI.00432-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma B, Bhattacharyya S, Das S. Polypyrimidine tract-binding protein interacts with coxsackievirus B3 RNA and influences its translation. J. Gen.Virol. 2010;91:1245–1255. doi: 10.1099/vir.0.018507-0. doi:10.1099/vir.0.018507-0. [DOI] [PubMed] [Google Scholar]
- Villordo SM, Alvarez DE, Gamarnik AV. A balance between circular and linear forms of the dengue virus genome is crucial for viral replication. RNA. 2010;16:2325–2335. doi: 10.1261/rna.2120410. doi:10.1261/rna.2120410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgen-Slane R, Rozovics JM, Fitzgerald KD, Ngo T, Chou W, van der Heden van Noort GJ, Filippov DV, Gershon PD, Semler BL. An RNA virus hijacks an incognito function of a DNA repair enzyme. Proc. Natl. Acad. Sci. USA. 2012 doi: 10.1073/pnas.1208096109. doi:10.1073/pnas.1208096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt DA, Andino R. An RNA element at the 5′-end of the poliovirus genome functions as a general promoter for RNA synthesis. PLoS Path. 2010;6:e1000936. doi: 10.1371/journal.ppat.1000936. doi:10.1371/journal.ppat.1000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner S, Sarnow P. Viral ribonucleoprotein complex formation and nucleolar-cytoplasmic relocalization of nucleolin in poliovirus-infected cells. J. Virol. 1998;72:6699–6709. doi: 10.1128/jvi.72.8.6699-6709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter BL, Parsley TB, Ehrenfeld E, Semler BL. Distinct poly(rC) binding protein KH domain determinants for poliovirus translation initiation and viral RNA replication. J. Virol. 2002;76:12008–12022. doi: 10.1128/JVI.76.23.12008-12022.2002. doi:10.1128/JVI.76.23.12008-12022.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang X. The nucleocapsid protein of coronavirus mouse hepatitis virus interacts with the cellular heterogeneous nuclear ribonucleoprotein A1 in vitro and in vivo. Virology. 1999;265:96–109. doi: 10.1006/viro.1999.0025. doi:10.1006/viro.1999.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters K, Palmenberg AC. Differential processing of nuclear pore complex proteins by rhinovirus 2A proteases from different species and serotypes. J. Virol. 2011;85:10874–10883. doi: 10.1128/JVI.00718-11. doi:10.1128/JVI.00718-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson SR, LeStourgeon WM, Krezel AM. Solution structure of the symmetric coiled coil tetramer formed by the oligomerization domain of hnRNP C: implications for biological function. J. Mol. Biol. 2005;350:319–337. doi: 10.1016/j.jmb.2005.05.002. doi:10.1016/j.jmb.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Wimmer E, Hellen CU, Cao X. Genetics of poliovirus. Annu. Rev. Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. doi:10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- Wong J, Si X, Angeles A, Zhang J, Shi J, Fung G, Jagdeo J, Wang T, Zhong Z, Jan E, Luo H. Cytoplasmic redistribution and cleavage of AUF1 during coxsackievirus infection enhance the stability of its viral genome. FASEB J. 2013 doi: 10.1096/fj.12-226498. doi:10.1096/fj.12-226498. [DOI] [PubMed] [Google Scholar]
- Yarbrough ML, Mata MA, Sakthivel R, Fontoura BMA. Viral subversion of nucleocytoplasmic trafficking. Traffic. 2014;15:127–140. doi: 10.1111/tra.12137. doi:10.1111/tra.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Schultz DE, Lemon SM. Functional significance of the interaction of hepatitis A virus RNA with glyceraldehyde 3-phosphate dehydrogenase (GAPDH): opposing effects of GAPDH and polypyrimidine tract binding protein on internal ribosome entry site function. J. Virol. 2000;74:6459–6468. doi: 10.1128/jvi.74.14.6459-6468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocupicio-Monroy M, Padmanabhan R, Medina F, del Angel RM. Mosquito La protein binds to the 3′ untranslated region of the positive and negative polarity dengue virus RNAs and relocates to the cytoplasm of infected cells. Virology. 2007;357:29–40. doi: 10.1016/j.virol.2006.07.042. doi:10.1016/j.virol.2006.07.042. [DOI] [PubMed] [Google Scholar]
- Yocupicio-Monroy RME, Medina F, Reyes-del Valle J, del Angel RM. Cellular proteins from human monocytes bind to dengue 4 virus minus-strand 3′ untranslated region RNA. J. Virol. 2003;77:3067–3076. doi: 10.1128/JVI.77.5.3067-3076.2003. doi:10.1128/JVI.77.5.3067-3076.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Abaeva IS, Marintchev A, Pestova TV, Hellen CUT. Common conformational changes induced in type 2 picornavirus IRESs by cognate trans-acting factors. Nucl. Acids Res. 2011a;39:4851–4865. doi: 10.1093/nar/gkr045. doi:10.1093/nar/gkr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Sweeney TR, Kafasla P, Jackson RJ, Pestova TV, Hellen CU. The mechanism of translation initiation on Aichivirus RNA mediated by a novel type of picornavirus IRES. EMBO J. 2011b;30:4423–4436. doi: 10.1038/emboj.2011.306. doi:10.1038/emboj.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zell R, Ihle Y, Effenberger M, Seitz S, Wutzler P, Görlach M. Interaction of poly(rC)-binding protein 2 domains KH1 and KH3 with coxsackievirus RNA. Biochem Biophys Res Commun. 2008a;377:500–503. doi: 10.1016/j.bbrc.2008.09.156. doi:10.1016/j.bbrc.2008.09.156. [DOI] [PubMed] [Google Scholar]
- Zell R, Ihle Y, Seitz S, Gündel U, Wutzler P, Görlach M. Poly(rC)-binding protein 2 interacts with the oligo(rC) tract of coxsackievirus B3. Biochem. Biophys. Res. Commun. 2008b;366:917–921. doi: 10.1016/j.bbrc.2007.12.038. doi:10.1016/j.bbrc.2007.12.038. [DOI] [PubMed] [Google Scholar]
- Zhang B, Seitz S, Kusov Y, Zell R, Gauss-Müller V. RNA interaction and cleavage of poly(C)-binding protein 2 by hepatitis A virus protease. Biochem. Biophys. Res. Commun. 2007;364:725–730. doi: 10.1016/j.bbrc.2007.09.133. doi:10.1016/j.bbrc.2007.09.133. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yamada O, Yoshida H, Iwai T, Araki H. Autogenous translational inhibition of core protein: implication for switch from translation to RNA replication in hepatitis C virus. Virology. 2002;293:141–150. doi: 10.1006/viro.2001.1270. doi:10.1006/viro.2001.1270. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li HP, Xue W, Lai MM. Formation of a ribonucleoprotein complex of mouse hepatitis virus involving heterogeneous nuclear ribonucleoprotein A1 and transcription-regulatory elements of viral RNA. Virology. 1999;264:115–124. doi: 10.1006/viro.1999.9970. doi:10.1006/viro.1999.9970. [DOI] [PubMed] [Google Scholar]