Abstract
The balance between tumor-promoting and tumor-suppressing immune responses and the difference between them ultimately determine whether a cancer escapes immune recognition mechanisms. Defining the complex relationships between the tumor itself, the tumor environment, and the immune system has been critical in facilitating the development of successful immunotherapies. This review explores the role of oncogenes in inducing cancer-associated inflammation, the local and systemic factors that lead to immune suppression, and immunotherapy approaches to overcome immune privilege.
INTRODUCTION
GI cancers, including colorectal cancer (CRC), gastric cancer, pancreatic cancer, and cancers of the liver and bile duct, are all consistently in the top ten malignancies diagnosed annually in the United States.1 For early-stage cancer, surgical resection remains the mainstay of curative-intent treatment. Current management strategies and treatments are limited primarily by lack of specificity to the cancer cells and by general treatment toxicities that limit full delivery of anticancer agents.2,3 For these reasons, novel therapeutic strategies are urgently needed. One of the more recent and exciting new fields in anticancer therapeutics is immune therapy.
ROLE OF INFECTION IN GI CANCERS AND THE MICROBIOME
Increasing evidence suggests that as many as one third of cancers worldwide are associated with microbial infections. For GI cancers, common examples include Helicobacter pylori associated with gastric cancer, Clonorchis sinensis and Opisthorchis viverrini associated with bile duct cancer, and enterotoxigenic Bacteroides fragilis associated with colon cancer.4 Under normal conditions, an acute inflammatory response is self-limiting. However, under conditions associated with chronic inflammation, the production of reactive oxidative species and inflammatory cytokines can induce DNA damage in proliferating cells, thus leading to the generation of gene mutations or to epigenetic changes. Alternatively, de novo mutation of oncogenes such as K-ras and p53 can directly initiate the cascade of events associated with chronic inflammation.
Despite this new understanding regarding the role of infection in the development of some GI cancers, the clinical observation is that most cancers, including GI cancers and especially pancreatic cancers, are considered poorly immunogenic. In contrast to infectious disease–generated neoantigens, the programmed progression of somatic gene mutations that transforms normal cells into malignant cells generates cancer proteins that are usually altered self-proteins. These proteins are masked from the immune system as a result of immune regulatory mechanisms.
ROLE OF ONCOGENES IN INDUCING CANCER-ASSOCIATED INFLAMMATION
Mutated K-ras is the prototype oncogene known to initiate chronic inflammatory changes within a cancer. As an example, mutated K-ras is the key driver gene that initiates the pancreatic cancer–associated inflammation program. For this reason, cancer-mediated inflammation is thought to be an additional pillar characteristic that defines a cancer.5 The net effect is often a downregulation of any potential immune activity from effector cells capable of recognizing and lysing the malignant cells at this critical location and timing. The balance and difference between tumor-promoting and tumor-suppressing immune response ultimately determines whether a cancer escapes immune recognition mechanisms.
LOCAL AND SYSTEMIC FACTORS THAT LEAD TO OVERALL IMMUNE SUPPRESSION: THE KEY PLAYERS
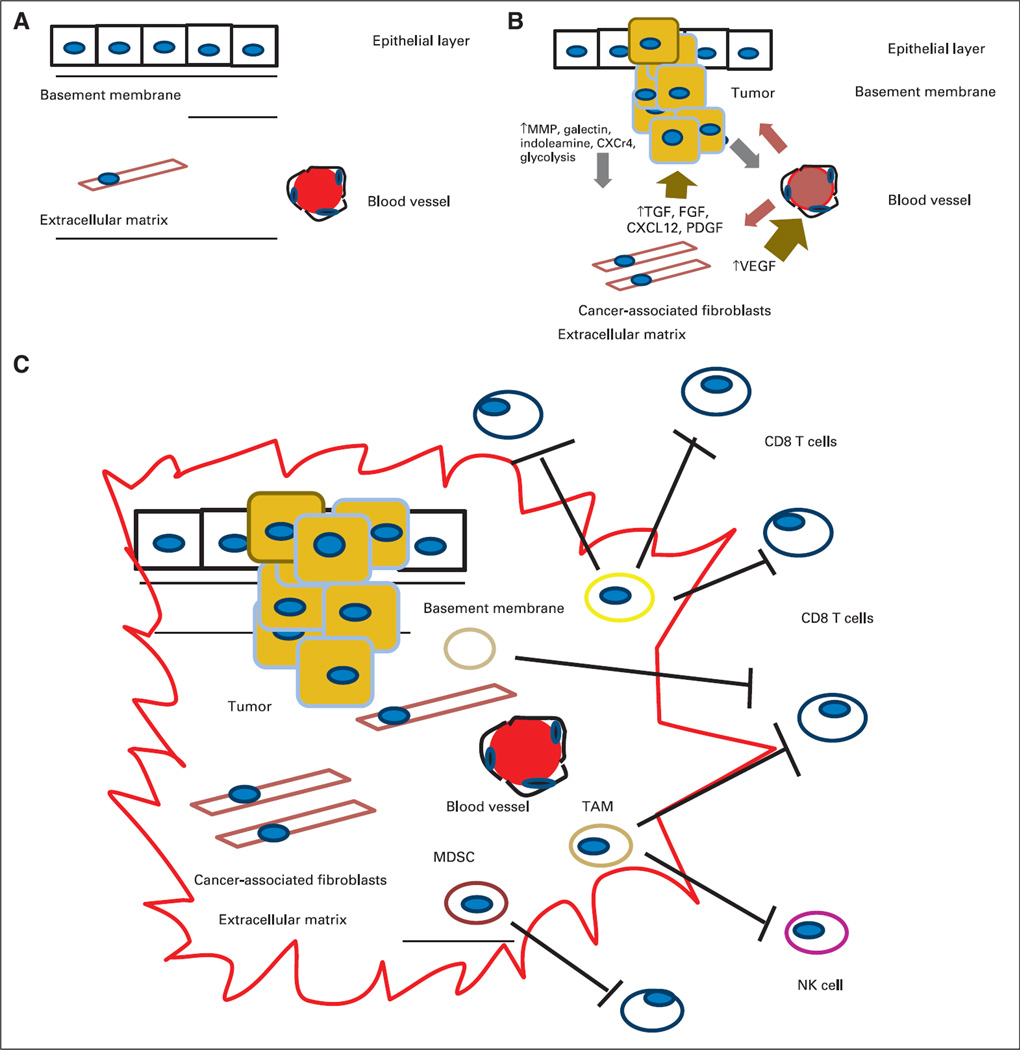
Defining the complex relationships between the tumor, the tumor environment, and the immune system has been critical in facilitating the development of successful immunotherapies. This is particularly true for pancreatic cancer because the generation of genetically engineered mouse models such as KPC mice (LSL-K-rasG12D;LSL-p53R172H/+;Pdx1-Cre) closely reproduces the gradual progression from premalignant to malignant human pancreatic cancer and has greatly accelerated our understanding of the contributions of the tumor, the tumor’s stroma, and the immune response to both (Fig 1).6,7
Fig 1.
(A) Normal relationship between the epithelial layer, basement membrane, and extracellular matrix (ECM). (B) Interaction between the tumor initiation process and its relationship to the stroma. A pathologic hallmark of pancreatic cancer is the development of an abundant inflammatory response. The inflammatory environment consists of activated stellate cells, ECM proteins, and fibroblasts. These cancer-associated fibroblasts are thought to secrete factors such as transforming growth factor beta (TGF-β). This subsequently leads to the production of ECM components, including collagens, fibroblast growth factor, C-X-C chemokine ligand 12 (CXCL12), proteoglycans, and hyaluronic acid. These in turn secrete tumor-promoting factors that contribute to the tumor’s invasion through the basement membrane via proteolytic enzymes, including matrix metalloproteinases. There are also increases in angiogenic factors such as vascular endothelial growth factor (VEGF) that lead to the development of new blood vessels and changes in vascular permeability that lead to the release of additional ECM-modulating events. (C) Tumor initiation-stroma interaction. Mutated K-ras is thought to be the driver of a cancer-associated inflammation program that leads to predominance and infiltration of immunosuppressive immune cells into the tumor stroma at the expense of effector T cells. Tumors harbor the ability to increase the number regulatory T cells, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) and to upregulate molecules such as C-X-C chemokine receptor type 12. Ultimately, they produce a local immunosuppressive environment ideal for tumor growth. CXCR4, C-X-C chemokine receptor type 4; NK, natural killer (cell); PDGF, platelet-derived growth factor; PGF, placental growth factor.
Tumor Cells
Tumor cells have developed several mechanisms to modulate the immune system and avoid detection by effector immune cells. Examples include cell surface expression of immune system checkpoint ligands such as programmed death-ligand 1 (PD-L1)8,9; secretion of soluble immunosuppressive factors, including transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), interleukin-10 (IL-10), galectin-1, and indoleamine 2,3 dehydrogenase10–12; downregulation of major histocompatibility complex (MHC) class I expression; overexpression of receptors such as C-X-C chemokine receptor type 4 (CXCR4) via upregulation of hypoxia inducible factor 1 alpha (HIF1-α), basic fibroblast growth factor, and epidermal growth factor, which when bound to C-X-C chemokine ligand 12 (CXCL12) can lead to tumor growth, angiogenesis, metastasis, and chemotherapeutic resistance.13,14
Stroma
Both preclinical and clinical studies are now providing strong evidence that genetic alterations alone are not sufficient for tumor development. The tumor-stroma interactions in GI cancers are best illustrated in studies conducted in both human and mouse pancreatic cancer models. In fact, the pathologic hallmark of pancreatic cancer is the development of an abundant inflammatory response (desmoplasia).15,16 This inflammatory environment consists of regulatory immune cell populations, activated stellate cells, extracellular matrix (ECM) proteins, and fibroblasts. These cancer-associated fibroblasts (CAFs) represent the most abundant cell type in the tumor stroma. TGF-β and its isoforms are thought to be early mediators secreted by tumor cells that lead to the activation of CAFs.17 This subsequently leads to the production of ECM components, including collagens, secreted protein acidic and rich in cysteine, osteopontin, osteonectin, elastin, tenascin-C, fibronectin, thrombospondin, proteoglycans, hyaluronic acid, and STAT3. These components in turn secrete tumor-promoting factors that contribute to tumor invasion through the basement membrane via proteolytic enzymes including matrix metalloproteinases 1, 2, and 9; increase in angiogenic factors such as VEGF that lead to new blood vessel development; and changes in vascular permeability leading to the release of additional ECM-modulating events.18,19 Interestingly, at least in the pancreatic cancer genetic mouse models, the increased rigidity of the new stroma has been shown to compress the local vasculature and alter perfusion.20 In addition, other mediators are secreted, including hepatocyte growth factor, platelet-derived growth factor, insulin-like growth factor, and nerve growth factors. Finally, CAFs are also known to secrete chemokines such as stromal cell derived factor 1 (also known as CXCL12). Ultimately, the stroma becomes transformed and is able to support invasion, migration, and tumor growth and is protected by an immune suppressive shield that is devoid of activated killer T cells.21,22
Local and Systemic Immune System
Tumor cells express multiple immune mediators that directly or indirectly block the activity of effector CD4+ and CD8+ T cells and dampen local tumor-infiltrating immune responses.23–25 Galectin-1 expression, for example, is induced by the hypoxic tumor microenvironment. Galectin-1, in turn, induces increased IL-10 production, which results in decreased interferon gamma production by activated T cells.10 Tumors also produce increased amounts of indoleamine 2,3 dehydrogenase, which in turn depletes tryptophan from the tumor microenvironment and decreases T-cell function.11,12,17
Inflammatory immune cells such as dendritic cells and macrophages, when activated through engagement with antigen, display a metabolic profile similar to that of a glycolytic tumor cell. This involves a shift in metabolism away from oxidative phosphorylation under normal oxygen conditions toward aerobic glycolysis, a phenomenon known as the Warburg effect. This change in macrophages rapidly provides energy and metabolic intermediates for the biosynthesis of additional immune and inflammatory proteins. The generation of lactate as an additional byproduct of aerobic glycolysis further stimulates a proinflammatory storm by generating the hypoxic factor HIF1-α.26
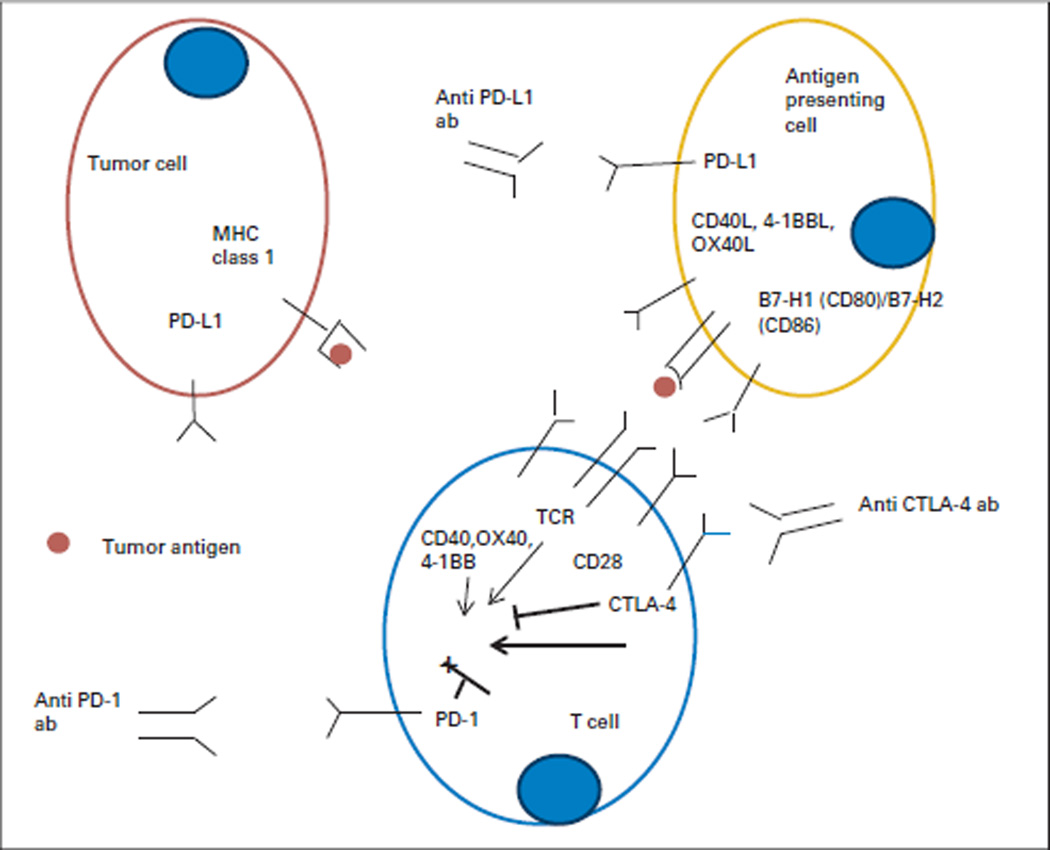
Immune checkpoint modulation is another well-described mechanism by which tumor cells control the local immune response. In the normal host setting, immune checkpoint molecules modulate the T-cell response to antigens by either upregulating costimulatory pathways or downregulating coinhibitory pathways of immune signaling. Programmed cell death protein 1 (PD-1) is a coinhibitory receptor that downregulates T-cell activity in peripheral tissues during inflammation, thus preventing increased collateral tissue damage during an immune response and preventing the development of autoimmunity. PD-1 is activated by its ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC), which are both upregulated during an inflammatory response. Tumor cells of various malignancies have been shown to upregulate PD-L1 as a mechanism that dampens the local T-cell response by decreasing cytokine production and T-cell proliferation. In GI malignancies, PD-L1 upregulation occurs in pancreatic cancer, CRC, and gastric cancer (Fig 2).27–29
Fig 2.
Immune costimulatory and coinhibitory ligands and receptors involved in T-cell activation and inhibition. Tumor cells are poor antigen-presenting cells because they often do not possess or downregulate class I antigens. The activation of the adaptive immune response begins when activated macrophages and specialized antigen-presenting cells and/or dendritic cells process antigens and present antigens onto appropriate major histocompatibility complex (MHC) class I and II molecules where they can be recognized by a T cell with the appropriate T-cell receptor that recognizes the specific bacterial and/or viral antigen pep-tide. In the context of a second costimulatory signal consisting of the B7 family of receptors on the antigen-presenting cell and CD28 on the T cell, the combination of signal 1 and signal 2 leads to maximal immune activation. ab, antibody; CTLA-4, cytotoxic T-lymphocyte antigen-4; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TCR, T-cell receptor.
Tumors naturally attract and activate several immune cell populations with regulatory functions that normally infiltrate inflamed normal tissue to prevent autoimmune activity such as CD4+CD25+FOXP3+ regulatory T cells (Tregs). Increased numbers of Tregs are identified in the tumor microenvironment in most GI cancers and have been shown to have a presence even in premalignant lesions in pancreatic cancers. These cells in turn suppress the proliferation of tumor-specific CD4+ and CD8+ effector T cells as well as natural killer cells. Patients with pancreatic cancer have increased numbers of Tregs at the tumor site and in the circulation.30–32 It has been reported that a low percentage of Tregs in the circulation 1 year after resection correlates with improved survival.33
In addition, increased numbers of tumor-infiltrating myeloid-derived suppressor cells (MDSCs) further suppress T-cell proliferation and increase T-cell apoptosis.11,17 MDSCs are immature myeloid cells that suppress both innate and adaptive immunity. MDSCs inhibit the function of effector T cells and natural killer cells and promote the development of Tregs. Increased numbers of circulating MDSCs is an independent poor prognostic factor in patients with pancreatic cancer.34 In addition, as a direct result of substances produced by the tumor microenvironment such as IL-10 and TGF-β, tumor-associated macrophages (TAMs) switch their differentiation from M1 (proinflammatory macrophages) to M2 (anti-inflammatory macrophages), which in turn have protumor properties. The identification and localization of MDSCs and TAMs in the surrounding preinvasive pancreatic cancer lesions known as pancreatic intraductal neoplasia is supporting evidence that these suppressor immune cells closely follow the histologic progression of pancreatic cancer.35
IMMUNOTHERAPY APPROACHES
Tumor Vaccines Targeting GI Cancers in the Clinic
Several proteins such as carcinoembryonic antigen, mutated K-ras, BRAF, PI3K, the mucin family of proteins (MUC1 and MUC5), telomerase, human epidermal growth factor receptor 2 (HER2), and gastrin are overexpressed in several GI cancers (Table 1) 47,48 Vaccines and antibodies designed to target these antigens have been tested in clinical trials either alone or by using viral vectors or dendritic cells.36,37,49
Table 1.
Selected Completed Immunotherapy Trials
| Reference | Disease | Phase | No. of Patients | Line of Therapy | Antigen/Strategy | Clinical End Point |
|---|---|---|---|---|---|---|
| Marshall et al36 | CEA-expressing cancers | I | 58* | 36 of 58 received more than two lines |
CEA | Increased survival trend for patients receiving rF CEA + TRICOM + GM-CSF; rV CEA-TRICOM + rF CEA; TRICOM + GM-CSF |
| Morse et al37 | Metastatic CRC | II† | 74 | Minimum of 2 months perioperative chemotherapy |
CEA-MUC1; DC- poxvirus PANVAC v PANVAC + GM-CSF |
Recurrence-free survival at 2 years was similar (47% and 55%); hepatic or lung metastases completely resected |
| Beatty et al42 | First-line metastatic pancreatic cancer |
I | 22 | First line | CD40 agonist | Median PFS, 5.2 months |
| Royal et al43 | Locally advanced metastatic pancreatic cancer |
II | 27 | Chemotherapy refractory |
Ipilimumab | No responses |
| Brahmer et al44 | Multiple tumors | I | 207 (18 CRC, 14 pancreatic cancer, 7 gastric cancer) |
Chemotherapy refractory |
Anti-PD-1, BMS- 936559 |
Durable responders in melanoma, non–small-cell lung, renal, and ovarian cancer |
| Muro et al45 | Gastric cancer | Ib | 39 (PD-L1– positive |
Chemotherapy refractory |
Pembrolizumab | Response rate, approximately 32% |
| Le et al39 | Mesothelin-expressing cancers |
I | 22 | Refractory | Mesothelin- Listeria |
Median OS, 8.4 months; 37% of patient population survived more than 15 months |
| Le et al38 | Metastatic pancreatic cancer |
II† | 30 | Refractory | Ipilimumab v GVAX- ipilimumab |
OS, 3.6 v 5.7 months; 1-year survival, 7% v 27% |
| Le et al40 | Metastatic pancreatic cancer |
II† | 90 | Refractory; 51% had more than two regimens |
2:1 GVAX-CRS- 207 v GVAX |
OS, 6.1 v 3.9 months (GVAX- ipilimumab v GVAX for patients who received at least three doses [two GVAX and one CRS- 207 or three GVAX]); OS, 9.7 v 4.6 months (GVAX-ipilumumab v GVAX) |
| Hardacre et al41 | Resected pancreatic cancer | II | 70 | Adjuvant | Alpha Gal | 1-year DFS, 62% |
| Tran et al46 | Metastatic bile duct | Single Patient |
1 | Refractory | erbb2IP-TIL adoptive cell transfer |
Maximum reduction of 30% target liver and lung lesions at 7 months; stable for 13 months |
Abbreviations: alpha Gal, alpha(1,3)Galactosyl epitope; CEA, carcinoembryonic antigen; CRC, colorectal cancer; CRS-207, live-attenuated Listeria-expressing mesothelin; DC, dendritic cell; DFS, disease-free survival; erbb2IP, erbb2 interacting protein; GM-CSF, granulocyte-macrophage colony-stimulating factor; GVAX, whole-cell pancreatic tumor vaccine; MUC1, mucin 1; OS, overall survival; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PFS, progression-free survival; rF, recombinant fowlpox; rV, recombinant vaccinia; TIL, tumor-infiltrating lymphocyte; TRICOM, triad of costimulatory molecules B&-1, ICAM-1, LFA-3.
Cohorts: rF CEA + TRICOM ± GM-CSF, rV CEA-TRICOM + rF CEA, and TRICOM ± GM-CSF.
Randomized.
Because few tumor antigens have been identified, the whole tumor cell has been the best source of immunogens. The entire tumor cell provides an unbiased method for allowing the immune system to determine which tumor antigens are the best for activating an immune response against. An allogeneic granulocyte-macrophage colony-stimulating factor (GM-CSF) –secreting whole-cell pancreatic tumor vaccine (GVAX) approach was tested initially in sequence with adjuvant chemoradiotherapy in patients who had resected pancreatic adenocarcinoma. In particular, the molecule GM-CSF is secreted by the irradiated tumor cell and deposited locally (ie, site of the vaccinating tumor cells). This local secretion of GM-CSF then recruits dendritic cells to the site of the vaccine to take up the tumor proteins and prime T-cell responses. Several phase I and II studies have been reported in both adjuvant and chemotherapy refractory metastatic pancreatic cancer.38,50–52 Mesothelin-specific CD8+ T-cell responses have also correlated with improved survival following whole-cell vaccination.53 Mesothelin is a cell surface tumor-associated antigen that is overexpressed in the majority of pancreatic adenocarcinomas and is postulated to be involved in cell adhesion and metastases.54 Other vaccines that have shown encouraging results in pancreatic cancer include the Listeria-based vaccine CRS-207 (live-attenuated Listeria-expressing mesothelin).39 CRS-207 has been studied in combination with GVAX on the basis of findings from a phase I study in which three patients with metastatic poorly differentiated adenocarcinoma (PDA) who had received prior GVAX had survival greater than 15 months.38 This prime/boost study (in which the first vaccine was given as a means of jumpstarting the immune system [prime] and the antigen was readministered to build on the overall immune response [boost]) demonstrated a prolonged survival in a heavily pretreated group of patients who received low-dose cyclophosphamide-GVAX with CRS-207 compared with those who received cyclophosphamide-GVAX alone.40 It is important to note however, that in the LSL-K-rasG12D; LSL-p53R172H/+; Pdx1-Cre genetically engineered pancreatic cancer mouse model, tumor-derived GM-CSF was essential for suppressing antigen-specific T cells in the stroma.55,56 This was explained by the fact that unopposed GM-CSF secreted locally in the tumor microenvironment without a counteracting signal contributed to recruitment of suppressive monocytes and subsequent immune suppression. However, in the case of vaccination at multiple intradermal sites, as is performed with GVAX, GM-CSF levels peak at 48 hours after vaccination and diminish to zero by 96 hours. This allows recruitment and activation of antigen-presenting cells at a natural immunizing site, which facilitates antitumor adaptive immunity, especially in the setting of simultaneous immune checkpoint blockade.50
The most compelling data for the role of vaccines in initiating antitumor immune responses comes from a recent neoadjuvant study that assessed the effects of GVAX given with a low-dose of cyclophosphamide to target suppressive Tregs 2 weeks before surgical resection of pancreatic tumors. That study identified for the first time vaccine-induced intratumoral tertiary lymphoid aggregates in the majority of resected surgical specimens. These tertiary lymphoid structures were shown to be regulatory, that is, they induced antigen-specific T cells that could still be downregulated by immune checkpoint signals within the tumor, including PD-L1. That study provided the first example of immune-based therapy converting a nonimmunogenic neoplasm into an immunogenic neoplasm by inducing infiltration of T cells and development of tertiary lymphoid structures in the tumor microenvironment.57 Studies are already underway that use combinations of GVAX, CRS-207, and anti-PD-1 monoclonal antibodies (mAbs) as immune strategies in several clinical settings, including neoadjuvant therapy, adjuvant therapy, and metastatic disease (NCT02243371; GVAX Pancreas Vaccine [With CY] and CRS-207 With or Without Nivolumab).
Another whole-cell vaccine platform is algenpantucel-L (NewLink Genetics, Ames, IA). This vaccine is derived from two human PDA cell lines (HAPa-1 and HAPa-2) that have been genetically modified to express alpha(1,3)Galactosyl epitopes. The rationale is to induce complement and antibody-dependent cell-mediated hyperacute rejection of the vaccine through anti-alpha(1,3)Galactosyl antibodies that are already present in most patients because of the presence of bacterial flora in the intestinal tract. A phase II clinical trial investigating the addition of algenpantucel-L immunotherapy to adjuvant gemcitabine chemotherapy and chemoradiotherapy in 70 patients with resected PDA was recently completed. Of interest, patients who received a higher dose of vaccine in the study (300 million v 100 million cells per dose) had an increase in 12-month disease-free survival and 12-month overall survival.41 A larger follow-up adjuvant study using algenpantucel-L at 300 million cells per dose has recently been completed; results will be forthcoming.
IMMUNE ANTIBODIES
Antibodies That Target Tumor Antigens
To date, mAbs have been the most successful form of immunotherapy clinically. mAbs mediate antitumor activity via antibody-dependent cell-mediated cytotoxicity, phagocytosis, and complement-dependent cytotoxicity. Advantages of mAbs include specific targeting of tumor cells while sparing normal tissue, relative ease of administration, and low toxicity profile. Major disadvantages include the absence of direct T-cell activation, which therefore precludes T-cell–mediated cytotoxic killing and the generation of memory immune responses. In addition, a potential limiting factor in the use of mAbs involves tumor heterogeneity. Specific examples are antibodies that target the HER2 protein (trastuzumab) and VEGF receptor 2 (VEGFR2; ramucirumab) approved in gastric cancer and antibodies that target VEGF (bevacizumab) and epidermal growth factor receptor (cetuximab, panitumumab) approved in CRC. There are additional clinical trials (still accruing patients) that target other GI cancer proteins such as MUC5 (NCT01040000; Phase 2 Study of NPC-1C Chimeric Monoclonal Antibody to Treat Pancreatic and Colorectal Cancer). As an alternative approach, immunoconjugates can combine the specificity of mAbs with the potency of cytotoxic moieties. 90-Yttrium and 177-lutetium–labeled somatostatin have been examined in hepatocellular cancer and in neuroendocrine cancers. Dual affinity re-targeting molecules are multispecific antibodies capable of targeting two or more antigens simultaneously. At least one study that uses a colon cancer antigen (gpA33) together with a CD3 T-cell receptor for patients with chemotherapy refractory CRC will soon be tested in an early-phase clinical trial (NCT02248805; Phase 1 Study of MGD007 in Relapsed/Refractory Metastatic Colorectal Carcinoma) as will dual affinity re-targeting molecules that target epidermal growth factor receptor and CD3 (NCT01420874; Anti-CD3 x Anti-Erbitux® Armed Activated T Cells [Phase Ib] for Gastrointestinal [GI] Cancer).
Antibodies That Target Costimulatory Molecules
Although vaccines can induce T-cell responses against tumor antigens, significant clinical responses have not yet been observed. Emerging data together with recent clinical findings, such as the induction of regulatory lymphoid infiltrates following GVAX, strongly support the need to combine antibodies that either enhance costimulatory signals or downregulate inhibitory signals with vaccines that induce an adaptive response to achieve the most potent antitumor immune responses.
The CD40 pathway is one example that demonstrates the potential of targeting a stimulatory signal within the pancreatic tumor microenvironment. CD40 engagement of macrophages and/or dendritic cells within the pancreatic tumor stroma upregulates surface expression of MHC and additional costimulatory molecules and augments T-cell activation. On the basis of strong preclinical data, this strategy was tested in a first-in-human clinical trial in patients with solid tumors (including two patients with cholangiocarcinoma) by using the humanized CD40 agonist CP-870,893.58 A subsequent study tested CP-870,893 administered after gemcitabine in 22 previously untreated patients with advanced pancreatic cancer.42 A follow-up study is planned that will use the CD40 agonist RO7009789 (previously known as CP-870,893) in patients with resectable pancreatic cancer; one dose of RO7009789 will be administered as a single agent before surgery followed by four cycles of adjuvant therapy with gemcitabine plus nab-paclitaxel plus RO7009789.
Antibodies That Target Immune Checkpoints
Immunotherapy has finally become a cancer treatment modality. Antibodies that inhibit immune checkpoint signals within tumors are the game changers. Two have already been approved for the treatment of metastatic melanoma. Antagonist antibodies that target cytotoxic T-lymphocyte antigen-4 (CTLA-4) and PD-1 signals on T cells activate pre-existing melanoma-specific T cells. Both agents have demonstrated efficacy as single agents and in combination for metastatic melanoma, lung cancer, renal cancer, and others. Three mAbs in this group, the anti-CTLA-4 mAb ipilimumab and the anti-PD-1 mAbs pembrolizumab and nivolumab, are approved by the US Food and Drug Administration for the treatment of metastatic melanoma. Additional ongoing studies are testing these agents in many other cancers, including colorectal, gastric, and pancreatic cancers.
A small phase I study with only 11 patients was completed in a chemotherapy refractory population, and no responses were reported for the three patients with CRC.59 A phase II study using ipilimumab was tested in 27 patients with locally advanced and/or metastatic pancreatic cancer. There were no responders by classic RECIST criteria, but one patient experienced a delayed response after initial progressive disease. This patient developed new metastases after two doses of ipilimumab (progressive disease). However, continued administration per protocol led to a significant delayed regression of the primary tumor and multiple liver lesions.43 More recently, the anti-PD-L1 antibody BMS-936559 was tested in 207 patients with solid tumor (18 patients with CRC, 14 patients with pancreatic cancer, and seven patients with gastric cancer). Although there were radiographic responses seen in patients with other cancers (melanoma, renal cell cancer, non–small-cell lung cancer, and ovarian cancer), there were no responses seen in the patients with GI cancer.44 In addition, the anti-PD-1 antibody pembrolizumab was tested in treatment refractory gastric cancer that had PD-L1–positive tumors in the stroma or in ≥ 1% of tumor cells. Overall, treatment was well tolerated with a response rate of 32%.45 A study is planned using pembrolizumab and chemotherapy for CRC, gastroesophageal, and pancreaticobiliary cancers (NCT02268825; Phase I/IIA Study MK-3475 With Chemotherapy in Patients With Advanced GI Cancers [MK-3475 GI]).
There are several reasons why these immune checkpoint agents fail to show responses in GI cancers. Unlike melanoma, renal cancer, and some lung cancers, most GI cancers do not naturally induce effector T-cell responses. In addition, preclinical pancreatic cancer models suggest that the stroma provides a formidable barrier to effector T-cell infiltration. A recent neoadjuvant study demonstrated the ability of GVAX to induce lymphoid structures and effector T cells that can infiltrate pancreatic tumors. However, the infiltration of effector T cells was associated with production of interferon gamma, which in turn upregulates immune checkpoints including the PD-1/PD-L1 signaling pathway. This response to infiltrating interferon gamma–expressing effector T cells has been referred to as adaptive resistance. A small pilot study compared the checkpoint inhibitor ipilimumab alone with GVAX given to induce and activate pancreatic cancer–specific T cells along with ipilimumab given to block the CTLA-4 pathway from turning the T cells off once they have been activated. That study demonstrated tumor responses of 27% 1-year survival in the combination arm versus 7% 1-year survival in the ipilimumab alone arm.38 That study provided support for the need to combine a T-cell–inducing agent such as a vaccine with these immune checkpoint inhibitors in patients with GI cancers in which T cells do not naturally exist. In the genetically engineered mouse model of pancreatic cancer (LSL-K-rasG12D;LSL-p53R172H/+; Pdx1-Cre), the stromal environment inhibits activated T cells from infiltrating into the tumor. However, immune control of pancreatic cancer growth could be achieved by first depleting CXCL12-expressing carcinoma-associated fibroblasts, a major contributor to the stromal barrier.14
Rrenewed enthusiasm in immunotherapy has led many groups to review previous pathology specimens and identify the immune characteristics of tumors along with histology and genetic features. It is now recognized that CRC and other GI cancers such as small bowel, ampullary, and gastric cancers that have microsatellite instability (MSI-high) or CpG island methylator phenotype tumors have been associated with extensive tumor-infiltrating lymphocytes and, in general, a better prognosis when compared with microsatellite stable tumors.60 One possible explanation is that this increase in CD3+ and CD8+ intratumoral lymphocytes is a direct result of increased immunologic recognition of mutated proteins on the cell surface of tumor cells. This likely explains why single-agent immune checkpoint inhibitors are showing greater activity in cancers with high mutation frequency burdens such as MSI-high tumors.
MSI-high tumors are thought to be present in 15% of CRCs and in approximately 20% of gastric cancers in the US population. However, in less common cancers, the presence of MSI-high tumors is more difficult to estimate, and the literature reports ranges of 0% to 22% for ampullary cancer, 0% to 3% for pancreatic cancer, and 5% to 45% for small bowel cancers.61–63 Clinical trials are studying anti-PD-1 mAbs in patients with GI cancer with MSI-high tumors to test the hypothesis that MSI-high tumors respond more effectively to checkpoint mAbs (NCT01876511).
It is important to point out that these immune-modulating agents do have immune-mediated toxicities. These immune-modulating agents are not cancer T cell–specific. Rather, they will enhance the activation status of other T-cell populations in the patient. High rates of autoimmune toxicities, including colitis, nephritis, hypophysitis, pleuritis, and hepatitis, have been reported with ipilimumab (up to 85% with the highest dose of 10 mg/kg). The rate of toxicity with PD-1/PD-L1 blockade is more modest but can still result in severe grade 3 to 4 autoimmunity and occasional death despite attempts to manage the autoimmunity.43,44,59 Future studies will be needed to determine how best to control the non-cancer T cells to minimize these autoimmune events.
ADOPTIVE CELL TRANSFER
With the adoptive cell transfer approach, T cells are removed from the tumor tissue (tumor-infiltrating lymphocytes), expanded ex vivo, and reinfused back to the patient at cell doses of approximately 1 × 10−9 cells following a nonmyeloablative lymphocyte-depleting preparative regimen. This allows manipulation of the T cells by priming the cells to tumor antigens or by transfection with recombinant DNA encoding for T-cell receptors specifically directed toward tumor antigens. This approach has been used successfully in a patient with chemotherapy refractory bile duct cancer in which an erbb2-interacting protein expressed by the cancer was targeted.46 The adoptive cell transfer approach is currently being tested in clinical trials for pancreatic cancer that use an anti-mesothelin chimeric antigen receptor (NCT01583686; CAR T Cell Receptor Immunotherapy Targeting Mesothelin for Patients With Metastatic Cancer).
CHIMERIC ANTIGEN RECEPTORS
Chimeric antigen receptors (CARs) are recombinant receptors that combine the specificity of an antigen-specific antibody with the activating functions of T cells. Unlike T-cell antigen receptors, CARs engage their target independent of antigen processing by the target cell and independent of MHC. CARs are grouped into three generations of increasing costimulatory activity. These CARs share the extracellular domain that engages the target via a single-chain variable fragment derived from an antibody. First-generation CARs include only CD3ζ as an intracellular signaling domain, whereas second-generation CARs include a single costimulatory domain derived from either CD28 or 4-1BB; third-generation CARs include two costimulatory domains (CD28 and 4-1BB) and other costimulatory molecules.64
Although the published literature suggests that antigen-specific targeted CARs are safe, the safety profiles are still fairly limited. In one study, a patient with colon cancer treated with HER2/neu CAR T cells died 5 days after the adoptive transfer; this patient died of what appears to have been a cytokine storm and respiratory failure triggered by the recognition of the low levels of antigens on lung epithelial cells.65
FUTURE DIRECTIONS AND CHALLENGES
The limitations of currently available immunotherapy for GI malignancies became clear as we began to appreciate the complex interplay between the tumor, the supporting tumor microenvironment, and the immune system at both the local and systemic level. As illustrated by GM-CSF and TAMs, the context in which different signals are received and at what time, how they are delivered, and the location of delivery can determine whether the signal is ultimately immune suppressive or activating. Preclinical models have already revealed the synergy between immunotherapy and other targeted therapeutics, including using the appropriate costimulatory molecules and integrating what has recently been discovered regarding checkpoint inhibitors. Critical concepts have been learned from the preclinical pancreatic cancer genetically engineered mouse models and from completed clinical trials in pancreatic cancer that use neoadjuvant GVAX. First-line treatment with agents that deplete or inhibit key immune-suppressing stroma molecules and that provide costimulatory support, treatment using vaccines that induce an immune response in nonimmunogenic cancers, or a combination of these agents should be the first step toward recruiting activated T cells into the tumor. Once activated T cells infiltrate the tumor environment, subsequent administration of immune checkpoint inhibitors can achieve maximum immune efficacy.
However, there are still many challenges that must be overcome. Despite the approval of ipilimumab and pembrolizumab for advanced melanoma and the use of these agents for GI cancers in clinical trials, there is still much to be learned. Monitoring immunologic parameters has been an integral component of all completed and ongoing clinical trials discussed in this review. Although several ongoing studies testing anti-PD-1 antibodies are measuring PD-1 or PD-L1 expression on tumors, it remains to be determined whether cell surface expression of PD-1 on T cells or PD-L1 on tumor cells will be validated as a predictive biomarker. Another challenge relates to the traditional evaluation of antitumor response in immunotherapy trials because both conventional and nonconventional (ie, scenarios that include radiographic stable disease or partial response following an initial increase in tumor burden that might have otherwise led to a patient coming off study secondary to disease progression) responses have been reported. GI cancer studies focused on immunotherapy have shown that it can take more than 3 months to observe a radiographic effect in some patients. It has also been demonstrated that these clinical and radiographic effects can be durable once they occur. A similar concern is how to define and grade immune-mediated adverse events.
As we move to the next phase of studies that will combine multimodality immune therapies, we are reminded that there is still much to learn regarding the safety profiles of agents given in combination to patients who may already have baseline GI, liver function, and endocrine abnormalities from their underlying cancer or as complications from prior treatment. We are at a key moment in which we can develop improved methods to deliver potentially multiple key antigens to a tumor environment that can be manipulated to become more receptive to immune infiltration of effector T cells. The possibility of overcoming immune privilege and delivering personalized immunotherapy might one day become a reality.
Footnotes
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Judy Wang
Stock or Other Ownership: Provectus Biopharmaceuticals (I), OncoSec Medical (I)
Research Funding: Astex Therapeutics (Inst)
Kim Reiss
Employment: Champions Oncology (I)
Stock or Other Ownership: Champions Oncology (I)
Research Funding: Champions Oncology (I)
Rina Khatri
No relationship to disclose
Elizabeth Jaffee
Research Funding: Aduro Biotech
Patents, Royalties, Other Intellectual Property: GVAX (Inst), CRS-207 (Inst)
Dan Laheru
No relationship to disclose
REFERENCES
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Elkord E, Hawkins RE, Stern PL. Immunotherapy for gastrointestinal cancer: Current status and strategies for improving efficacy. Expert Opin Biol Ther. 2008;8:385–395. doi: 10.1517/14712598.8.4.385. [DOI] [PubMed] [Google Scholar]
- 3.Mocellin S. New strategies to improve the efficacy of colorectal cancer vaccines: From bench to bedside. Curr Opin Investig Drugs. 2006;7:1052–1061. [PubMed] [Google Scholar]
- 4.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 7.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 9.Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 10.Martínez-Bosch N, Fernández-Barrena MG, Moreno M, et al. Galectin-1 drives pancreatic carcinogenesis through stroma remodeling and Hedgehog signaling activation. Cancer Res. 2014;74:3512–3524. doi: 10.1158/0008-5472.CAN-13-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi N, Kubota K, Kato S, et al. FOXP3+ regulatory T cells and tumoral indoleamine 2,3-dioxygenase expression predicts the carcinogenesis of intraductal papillary mucinous neoplasms of the pancreas. Pancreatology. 2010;10:631–640. doi: 10.1159/000308966. [DOI] [PubMed] [Google Scholar]
- 12.Brandacher G, Perathoner A, Ladurner R, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: Effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee S, Behnam Azad B, Nimmagadda S, et al. The intricate role of CXCR4 in cancer. Adv Cancer Res. 2014;124:31–82. doi: 10.1016/B978-0-12-411638-2.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghiringhelli F, Ménard C, Terme M, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waghray M, Yalamanchili M, di Magliano MP, et al. Deciphering the role of stroma in pancreatic cancer. Curr Opin Gastroenterol. 2013;29:537–543. doi: 10.1097/MOG.0b013e328363affe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haqq J, Howells LM, Garcea G, et al. Pancreatic stellate cells and pancreas cancer: Current perspectives and future strategies. Eur J Cancer. 2014;50:2570–2582. doi: 10.1016/j.ejca.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Micke P, Ostman A. Exploring the tumour environment: Cancer-associated fibroblasts as targets in cancer therapy. Expert Opin Ther Targets. 2005;9:1217–1233. doi: 10.1517/14728222.9.6.1217. [DOI] [PubMed] [Google Scholar]
- 22.Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 24.Vonderheide RH, Bayne LJ. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol. 2013;25:200–205. doi: 10.1016/j.coi.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sideras K, Braat H, Kwekkeboom J, et al. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat Rev. 2014;40:513–522. doi: 10.1016/j.ctrv.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Quante M, Wang TC. Inflammation and stem cells in gastrointestinal carcinogenesis. Physiology (Bethesda) 2008;23:350–359. doi: 10.1152/physiol.00031.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate antitumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol. 2013;94:41–53. doi: 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: Implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307–314. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vonderheide RH, Bajor DL, Winograd R, et al. CD40 immunotherapy for pancreatic cancer. Cancer Immunol Immunother. 2013;62:949–954. doi: 10.1007/s00262-013-1427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaer DA, Hirschhorn-Cymerman D, Wolchok JD, et al. Targeting tumor-necrosis factor receptor pathways for tumor immunotherapy. J Immunother Cancer. 2014;2:7. doi: 10.1186/2051-1426-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Curr Opin Immunol. 2013;25:230–237. doi: 10.1016/j.coi.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto T, Yanagimoto H, Satoi S, et al. Circulating CD4+CD25+ regulatory T cells in patients with pancreatic cancer. Pancreas. 2012;41:409415. doi: 10.1097/MPA.0b013e3182373a66. [DOI] [PubMed] [Google Scholar]
- 34.Gabitass RF, Annels NE, Stocken DD, et al. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark CE, Hingorani SR, Mick R, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 36.Marshall JL, Gulley JL, Arlen PM, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–731. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 37.Morse MA, Niedzwiecki D, Marshall JL, et al. A randomized phase II study of immunization with dendritic cells modified with poxvectors encoding CEA and MUC1 compared with the same poxvectors plus GM-CSF for resected metastatic colorectal cancer. Ann Surg. 2013;258:879–886. doi: 10.1097/SLA.0b013e318292919e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le DT, Brockstedt DG, Nir-Paz R, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: Phase I studies of safety and immune induction. Clin Cancer Res. 2012;18:858–868. doi: 10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le DT, Wang-Gilliam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardacre JM, Mulcahy M, Small W, et al. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: A phase 2 study. J Gastrointest Surg. 2013;17:94–100. doi: 10.1007/s11605-012-2064-6. [DOI] [PubMed] [Google Scholar]
- 42.Beatty GL, Torigian DA, Chiorean EG, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286–6295. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muro K, Bang Y, Shankaran V, et al. A phase 1b study of pembrolizumab (Pembro; MK-3475) in patients with advanced gastric cancer. Madrid, Spain: European Society for Medical Oncology 2014 Congress; 2014. Sep 26–30, (abstr LBA15) [Google Scholar]
- 46.Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez-Martín C, Lopez-Rios F, Aparicio J, et al. A critical review of HER2-positive gastric cancer evaluation and treatment: From trastuzumab, and beyond. Cancer Lett. 2014;351:30–40. doi: 10.1016/j.canlet.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Bilusic M, Heery CR, Arlen PM, et al. Phase I trial of a recombinant yeast-CEA vaccine (GI-6207) in adults with metastatic CEA-expressing carcinoma. Cancer Immunol Immunother. 2014;63:225–234. doi: 10.1007/s00262-013-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 50.Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: A phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 51.Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma: A phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–335. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: A pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pastan I, Hassan R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res. 2014;74:2907–2912. doi: 10.1158/0008-5472.CAN-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bayne LJ, Beatty GL, Jhala N, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pylayeva-Gupta Y, Lee KE, Hajdu CH, et al. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lutz ER, Wu AA, Bigelow E, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2:616–631. doi: 10.1158/2326-6066.CIR-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vonderheide RH, Flaherty KT, Khalil M, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 59.O’Mahony D, Morris JC, Quinn C, et al. A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin Cancer Res. 2007;13:958–964. doi: 10.1158/1078-0432.CCR-06-1974. [DOI] [PubMed] [Google Scholar]
- 60.Kim WK, Park M, Kim YJ, et al. Identification and selective degradation of neopeptide-containing truncated mutant proteins in the tumors with high microsatellite instability. Clin Cancer Res. 2013;19:3369–3382. doi: 10.1158/1078-0432.CCR-13-0684. [DOI] [PubMed] [Google Scholar]
- 61.Williams AS, Huang WY. The analysis of microsatellite instability in extracolonic gastrointestinal malignancy. Pathology. 2013;45:540–552. doi: 10.1097/PAT.0b013e3283653307. [DOI] [PubMed] [Google Scholar]
- 62.Ogino S, Nosho K, Irahara N, et al. Lympho-cytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15:6412–6420. doi: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colo-rectal cancer, and prognosis: Cohort study and literature review. J Pathol. 2010;222:350–366. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maus MV, Grupp SA, Porter DL, et al. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123:2625–2635. doi: 10.1182/blood-2013-11-492231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]