Abstract
Human papillomaviruses (HPV) establish persistent infections because of evolved immune evasion mechanisms, particularly HPV-mediated suppression of the immune functions of Langerhans cells (LC), the antigen presenting cells of the epithelium. Polyinosinic-polycytidilic acid (Poly-I:C) is broadly immunostimulatory with the ability to enhance APC expression of costimulatory molecules and inflammatory cytokines resulting in T cell activation. Here we investigated the activation of primary human LC derived from peripheral blood monocytes after exposure to HPV16 virus like particles followed by treatment with stabilized Poly-I:C compounds (s-Poly-I:C), and their subsequent induction of HPV16-specific T cells. Our results indicate that HPV16 particles alone were incapable of inducing LC activation as demonstrated by the lack of costimulatory molecules, inflammatory cytokines, chemokine-directed migration, and HPV16-specific CD8+ T cells in vitro. Conversely, s-Poly-I:C caused significant upregulation of costimulatory molecules and induction of chemokine-directed migration of LC that were pre-exposed to HPV16. In HLA-A*0201-positive donors, s-Poly-I:C treatment was able to induce CD8+ T cell immune responses against HPV16-derived peptides. Thus, s-Poly-I:C compounds are attractive for translation into therapeutics in which they could potentially mediate clearance of persistent HPV infection.
Keywords: papillomavirus, HPV16, Langerhans cells, immune escape
1. Introduction
High-risk human papillomavirus (hrHPV)1 infection leads to the development of several human cancers including cervical, vaginal, vulvar, anal, and head and neck cancers that cause significant morbidity and mortality worldwide [1]. In particular, high risk types HPV16 and HPV18 account for almost 70% of cervical cancer development and the risk of cervical lesion progression is disproportionately high for HPV16 [2, 3]. More than 15% of women that have hrHPV infections cannot initiate an effective immune response against HPV, and among those that do, viral clearance is very slow [4, 5]. This suggests that HPV is escaping immune detection and warrants the investigation of therapeutic treatments that stimulate the immune system to clear HPV infections, especially in women with consecutive positive hrHPV DNA tests.
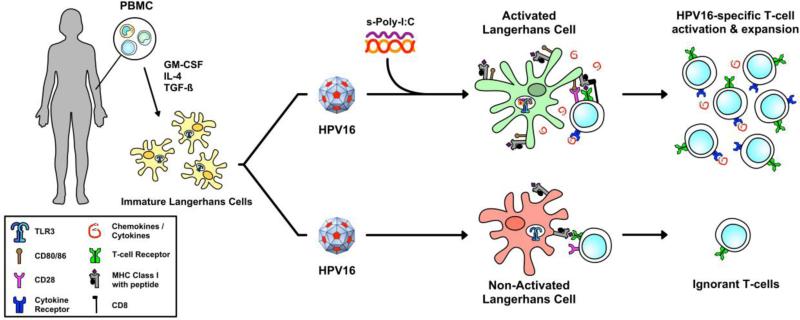
We have previously implicated HPV-mediated suppression of Langerhans cell (LC) immune function as a key mechanism in which HPV evades immune surveillance [6-8]. LC are the local antigen presenting cell (APC) of the epithelial and mucosal layers, making them responsible for initiating immune responses against epithelium invading viruses [9]. Upon proper pathogenic stimulation, LC undergo phenotypic and functional changes including the activation of signaling cascades, the up-regulation of co-stimulatory molecules, and the release of pro-inflammatory cytokines. Activated LC then travel to lymph nodes via chemokine-directed migration where they interact with antigen specific T cells and initiate an adaptive T cell response (reviewed in [10]). However, LC exposed to HPV16 do not become functionally mature APC, exhibit dysregulated cellular signaling suggestive of HPV-induced suppression of LC function, and are therefore unable to initiate HPV16-specific cytotoxic T cell responses [6, 7]. Elegant studies of LC depletion in murine epidermis have challenged the notion that LC are required for initiating immune responses to epicutaneous antigens, instead favoring a model in which dermal dendritic cells (DC) are immunostimulatory and LC are naturally immunoregulatory under homeostatic conditions [11-15]. Despite these intriguing findings, most human LC studies do not demonstrate similar immunosuppressive effects. In contrast, human LC, freshly isolated ex vivo or generated by in vitro differentiation, have a very high capacity for cross-presentation of antigen and inducing both CD8+ and CD4+ T cell responses [16-18].
Toll-like receptors (TLRs) are expressed by APC and recognize pathogen associated molecular patterns (PAMPs). We have previously demonstrated that treatment with a TLR8 agonist can activate LC, whereas a TLR7 agonist does not [19], suggesting that the specific TLR molecule engaged on LC has a profound effect on the resulting immune response. TLR3 is responsible for the detection of viral dsRNA, and TLR3 signaling pathways initiate antiviral and inflammatory responses [20]. TLR3 is found primarily in the endosomes of APC including LC as well as on the surface of epithelial cells [21, 22]. Both natural and synthetic dsRNAs provide warning signals through TLR3, inducing the production of type I IFNs and other cytokines. The synthetic dsRNA viral analog polyinosinic-polycytidylic acid (Poly-I:C) has long been known as the strongest type I IFN inducer recognized by TLR3 [23], and furthermore, can induce LC maturation via TLR3 stimulation [24]. Poly-I:C is a broad inducer of innate immunity and has been investigated clinically for its adjuvant and anti-viral activity [25]. Despite its long-known potential, Poly-I:C is rapidly inactivated by enzymes in blood, and is therefore not ideal for clinical applications [26]. However, Poly-I:C can be stabilized with polypeptides (s-Poly-I:C) thereby promoting its use in the clinic [27]; Poly-I:C stabilized with poly-arginine is known as Poly-ICR whereas Poly-I:C stabilized with poly-lysine and carboxymethylcellulose is known as Poly-ICLC. The primary objective of this study was to investigate whether s-Poly-I:C can overcome HPV-induced immune suppression by functionally activating LC exposed to HPV16, and inducing activation of HPV16-specific T cells in vitro.
2. Materials and Methods
2. 1 Healthy donor material
Ten healthy donors (male and female) between the ages of 24-40 years (average 29 ± 5.7 yrs) were recruited from the USC Health Sciences Campus, and were enrolled into the study after a brief physical and completing a short medical history questionnaire. Eligibility criteria included ability to give informed consent, immune competence for leukapheresis collection, nonpregnant status, and no history of clinical HPV disease. Written informed consent for blood sampling was obtained from all individuals under an approved Institutional Review Board protocol. Low resolution HLA-A2 determination was performed for all participants using standard endpoint PCR and confirmed by flow cytometry using an anti-HLA-A2 antibody on isolated leukocytes. For HLA-A2 positive donors, high resolution HLA-A2 genotyping was performed using the A*02 SSP UniTray Kit (Life Technologies, Carlsbad, CA) to provide allele level typing at the HLA-A2 locus. Blood samples were obtained by leukapheresis to enrich PBMC. Leukocytes were purified immediately after collection using Lymphocyte Separation Media (Corning, Manassas, VA) by density gradient centrifugation, and cryopreserved in liquid nitrogen.
2. 2 Antibodies, reagents and HPV16 viral particles
HLA-ABC FITC, HLA-DP, DQ, DR-FITC, CD40 PE, CD80 FITC, CD86 FITC, CD83 PE, CD1a PE, purified anti-human CCR7, CD14 PE, Langerin PE, TLR3 PE were purchased from BD Biosciences (San Jose, CA). CD40 PE, purified rat IgG2a, goat anti-rat IgG PE, mouse IgG1 FITC, mouse IgG1 PE were purchased from Biolegend (San Diego, CA). Recombinant human (rhu)-CCL21 was purchased from R&D Systems (Minneapolis, MN). Rhu-GM-CSF was manufactured by Berlex (Seattle, WA) while rhu-TGFβ1 and rhu-IL-4 were purchased from Biosource (Carlsbad, CA). Poly-ICR was provided by Nventa Biopharmaceuticals/Akela Pharma (Austin, TX). Poly-ICLC was provided by Oncovir, Inc. (Washington, D.C.) HPV16L1L2 virus-like particles (VLP) and chimeric HPV16L1L2-E7 VLP (HPV16 cVLP) were produced in insect cells and purified as previously described [28]. Endotoxin levels in VLP preparations were found to be below 0.06 EU using an E-toxate kit (Sigma-Aldrich).
2.3 Langerhans cell generation and characterization
LC were generated from human PBMC as previously described [19]. Briefly, PBMC and incubated in complete RPMI media with the addition of 1000 U/mL GM-CSF, 1000 U/mL IL-4, and 10 ng/ml TGFβ for 7 days. LC phenotype was confirmed by flow cytometry as CD1a+Langerin+CD14−. Intracellular TLR3 expression was assessed by fixation and permeabilization (BD Cytofix/Cytoperm solution kit, BD Biosciences) of LC prior to intracellular staining with a TLR3 antibody, following by flow cytometry.
2.4 LC activation assay and flow cytometry
LC were treated with HPV16 VLP prior to stimulation with TLR agonists, and surface markers were detected by flow cytometry as previously described [29]. Briefly, 106 LC were seeded in a 6-well plate and either left untreated, treated with 10 μg HPV16 VLP, or 10 μg HPV16 VLP for 4 h prior to treatment with s-Poly-I:C, or with s-Poly-I:C alone. After 48 h, the cells were harvested, washed, stained for surface MHC I, MHC II, CD80, CD83, CD86, CD40, CCR7 or isotype controls, and analyzed on an FC500 flow cytometer using CXP software (Beckman Coulter). LC were gated on size (large cells) based on forward and side scatter. Geometric mean fluorescence intensities (MFI) were used for data analysis based on logarithmically acquired data.
2.5 In Vitro Migration of LC
Chemokine directed migration towards CCL21 of LC was carried out using transwell plates (Costar, Cambridge, MA) as previously described [30]. Briefly, 2 × 105 LC, untreated or treated with HPV16 VLP, s-Poly-I:C alone, or the combination of HPV16 VLP plus s-Poly-I:C, as indicated above, were added to the upper chamber in triplicate wells and incubated for 4 h at 37°C. After 4 h, cells that migrated to the lower chamber containing CCL21 or media alone were counted using a Z1 Beckman Coulter particle counter. Where indicated, a migration index was calculated as the number of cells migrating to CCL21 over spontaneous migration for each treatment group.
2.6 Cytokine and chemokine analysis
Supernatants from 72 h cultures were assayed in triplicate using the Bio-Plex Suspension Array System (Bio-Rad, Hercules, CA) [29]. Cytokines and chemokines analyzed included IFNα, IL-1β, IL-6, IL-12p70, IP-10, TNFα, MCP-1, MIP-1α, MIP-1β, and RANTES using a custom MilliPlex MAP Human Cytokine/Chemokine Panel per manufacturer's instructions (Millipore, Billerica, MA).
2.7 Mixed lymphocyte reaction (MLR) assay
The MLR assay was performed as previously described [6, 31, 32]. HLA-A*0201 LC were left untreated or treated with HPV16 VLP and s-Poly-I:C and co-cultured with untreated, allogeneic, HLA-mismatched CD4+ and CD8+ T cells purified from different donor PBMC by negative magnetic separation (Miltenyi, San Diego, CA). Responder (R) T cells and irradiated stimulator (S) LC were cultured at R:S ratio of 20:1 in a 96-well round bottom plate in replicates of six per treatment for 5 days. T cells and LC, each cultured alone, and T cells cultured with autologous PBMC were used as negative controls, while T cells cultured with phytohaemagglutinin (PHA, Sigma-Aldrich) serve as a positive control. 3H-thymidine was added after 5 days to measure T cell proliferation. After an additional 18 h, radioactive 3H-thymidine-pulsed cells were harvested and radioactivity counted on a TopCount microplate liquid scintillation counter (Perkin Elmer, Waltham, MA). Proliferation indices were calculated as (mean radioactive cpm experimental/mean cpm of T cells alone).
2.8 In vitro immunization with HPV16 E7
Autologous CD8+ T cells and LC from HLA-A*0201+ donors were co-cultured in vitro over several weeks to elicit primary CD8+ T cell responses against HPV16 E7 using a previously described protocol [6, 30]. LC were generated as described above, and untouched CD8+ T cells were purified from PBMC using a negative selection naïve CD8 T cell isolation kit (Miltenyi Biotec, San Diego, CA). LC were left untreated or exposed to HPV16-L1/L2-E7 cVLP, then left untreated or treated with s-Poly-I:C. As a positive control, other LC treated with s-Poly-I:C were exogenously loaded with HLA-A*0201 binding peptides (E711-20, E782-90 and E786-93). CD8+ T cells and LC were co-cultured with irradiated LC at a 20:1 (R:S) ratio for 7 days at 37 °C. Cultures were restimulated with untreated or treated LC at days 7, 14 and 21. After 28 days T cells were harvested and tested for peptide-specific IFN-γ production by ELISPOT.
2.9 IFN-γ ELISPOT assay
The ELISPOT assay was performed according to an established laboratory protocol. 96-well multiscreen-HTS plates (Millipore) were coated with 10μg/mL anti-human IFNγ (clone 1-D1K, Mabtech, Mariemont, OH) in PBS overnight at 4°C, washed with PBS, and blocked for 2 h with complete media at 37°C. T cells collected after in vitro immunization assays were plated at 2 × 105 cells/well in six replicate wells in the presence or absence of the HLA-A*0201 restricted peptides for 18 h at 37°C, 5% CO2 [30]. Wells were washed with PBS/0.5% Tween-20 and incubated with 1μg/mL biotinylated anti-human IFNγ antibody (clone 7-B6-1, Mabtech), for 2 h followed by incubation for 1 h with streptavidin-HRP (Sigma, St. Louis, MO). Spot development was carried out with 3-amino-9-ethyl-carbazole substrate (Sigma). Spots were counted using the video-imaging KS ELISPOT analysis system (Carl Zeiss, Thornwood, NY). The number of spots in the medium control wells were subtracted from spots detected in antigen-specific stimulation wells and was subsequently calculated as number of HPV peptide-specific T cells per million PBMC.
2.10 Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 (San Diego, CA). Statistical significance of the different assays (activation assay, cytokine and chemokine analysis, migration assay, MLR assay, and in vitro immunization assay) was determined by a one-way ANOVA for overall significance followed by a Tukey's multiple comparisons test comparing all columns for pooled experiments from individual healthy donors. A non-parametric Kruskal-Wallis statistical test following by Dunn's multiple comparisons test was performed to verify the results of the statistical analysis for each assay. Non-parametric Mann-Whitney U tests were performed for representative experiments of technical replicates of single donors performed in triplicate wells. Results with P-values <0.05 were considered significant.
3. Results
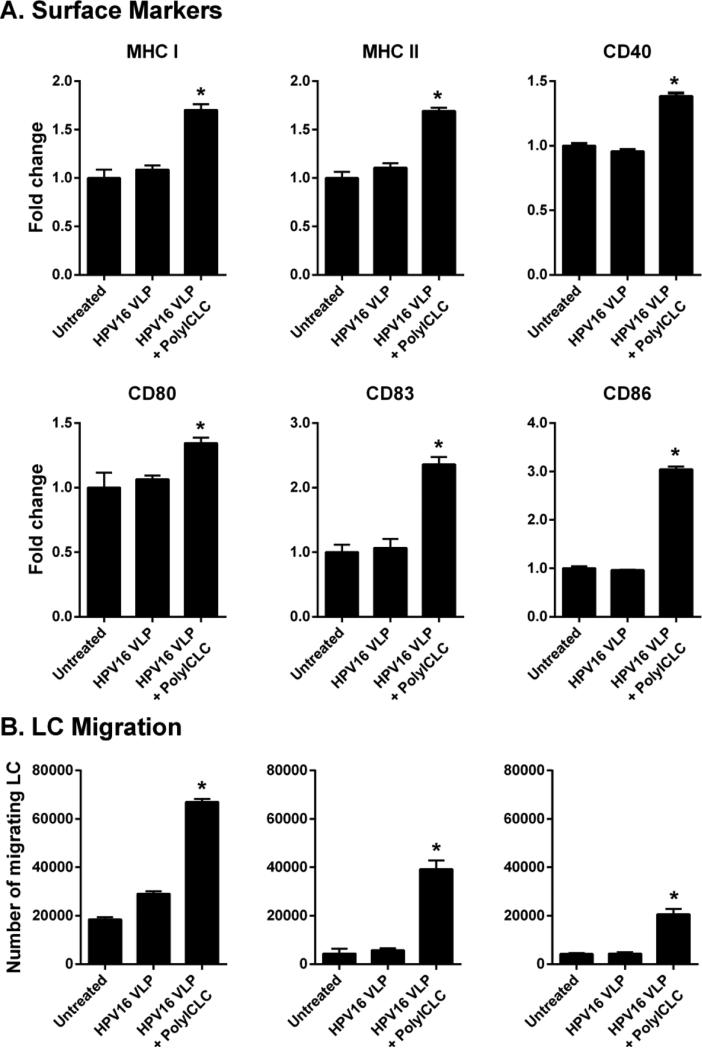
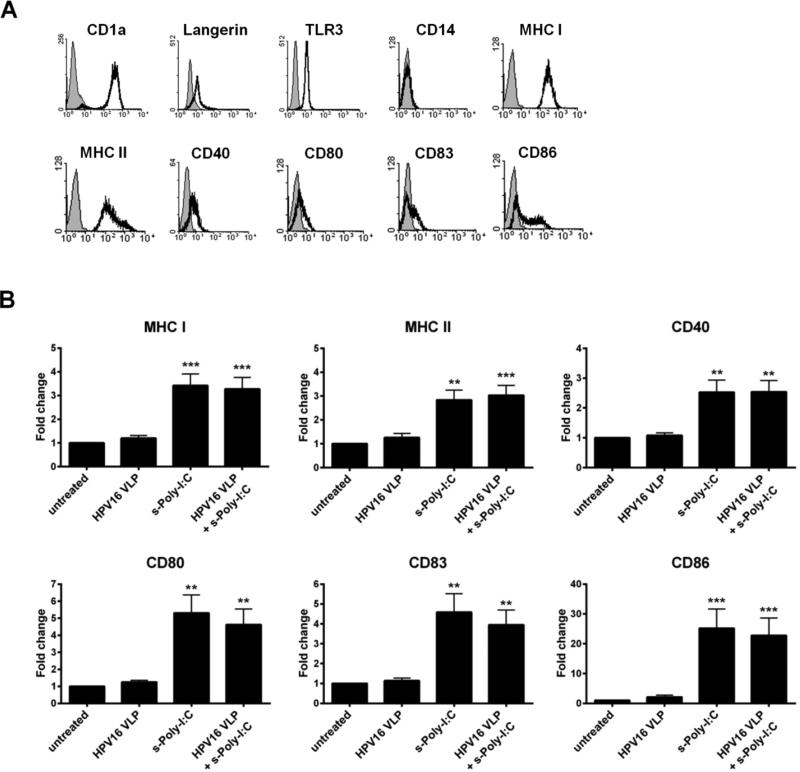
3.1 s-Poly-I:C (Poly-ICR) induces upregulation of MHC and costimulatory molecules on HPV16-exposed LC
The phenotype of immature LC generated from PBMC was defined as high expression of CD1a, Langerin, MHC class I and class II (Fig. 1a), similar to what has been shown for LC isolated from skin ex vivo [21]. LC were positive for intracellular staining of TLR3, negative for the monocytic marker CD14, and expressed low levels of CD40, CD80, CD86 and the maturation marker CD83. These immature LC were exposed to HPV16, followed by treatment with s-Poly-I:C (Poly-ICR) and were then analyzed for the expression of MHC and T cell co-stimulatory molecules. Exposure of LC to HPV16 VLP did not change expression of MHC and activation markers compared to unexposed LC (Fig. 1b), similar to what has been previously described [6]. In contrast, quantification of activation-associated markers from all donors indicated that treatment of LCs with s-Poly-I:C after pre-exposure to HPV16 caused a significant upregulation of all surface markers analyzed (Fig. 1b), as measured by higher levels of fluorescent staining (mean fluorescence intensity) in all LC, not just a proportion of cells (data not shown). Treatment of LC with s-Poly-I:C alone expectedly resulted in upregulation of LC activation markers, in some cases (CD80, CD83, CD86) resulting in a non-significant but higher level of stimulation than with the combination, consistent with the idea that the presence of HPV16 VLP are somewhat suppressive to LC activation (Fig. 1b). These results indicate s-Poly-I:C is able to induce phenotypic activation of LC despite the presence of HPV16, suggesting a potential reversal of the immune suppression caused by HPV16 that we have previously demonstrated to be a dysregulation of the PI3K/AKT pathway [7].
Fig. 1.
Poly-ICR induces upregulation of MHC and costimulatory molecules on LC. (A) Immature LC were stained for CD1a, langerin, intracellular TLR3, CD14, MHC I, MHC II, CD40, CD80, CD83, and CD86 and assessed via flow cytometry; isotype control in gray. (B) Immature LC were left untreated or exposed to HPV16 VLP. Subsequently, cells were treated with s-Poly-I:C (5 μg/mL Poly-ICR) for 48h. Controls were left untreated, exposed to HPV16 VLP alone, or treated with s-Poly-I:C alone. Average fold increase in LC surface marker expression. Data represent mean fold increase in surface marker expression (± SEM) relative to untreated LC based on mean fluorescence intensity (MFI) (N=10 healthy donors). **p<0.01, ***p<0.001 compared to untreated LC and LC exposed to HPV16 (one-way ANOVA, Tukey's post-test).
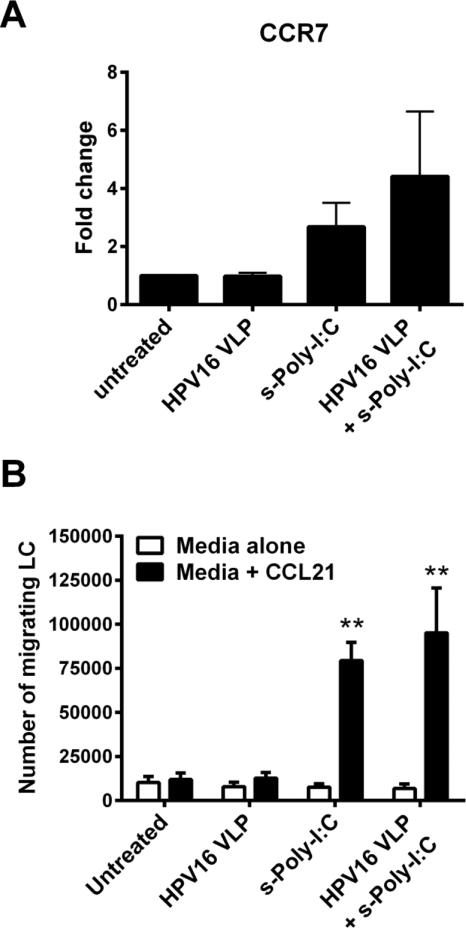
3.2 Poly-ICR induces HPV16-exposed LC to migrate to CCL21
LC chemokine-directed migration to regional lymph nodes after receiving maturation signals in the periphery is required for successful interaction with naïve T cells [33]. As an in vitro correlate of LC migration in vivo, a transwell chemotaxis assay to CCL21 was used to assess the migratory capacity of LC after exposure to HPV16 followed by treatment with s-Poly-I:C (Poly-ICR). CCL21 is a chemokine that is expressed in lymphoid organs and signals through the maturation-induced CCR7 receptor on LC during migration to lymph nodes [33]. Treatment of LC with either s-Poly-I:C alone, or with s-Poly-I:C post HPV16 exposure resulted in an increased trend in CCR7 expression (Fig. 2a) and a significant increase in migration capacity towards CCL21 compared to untreated LC or LC exposed to HPV16 alone (Fig. 2b). Although CCR7 expression was highly variable between individual donors, all six individual donors tested for LC migration responded with similar robustness, indicating that even low levels of induced CCR7 expression are sufficient to trigger chemokine-directed migration.
Fig. 2. Poly-ICR induces HPV16-exposed LC to upregulate CCR7 and migrate to CCL21 in vitro.
LC were exposed to HPV16 prior to s-Poly-I:C (Poly-ICR) treatment as described. (A) CCR7 expression was analyzed by flow cytometry. Data represent mean fold increase in CCR7 expression (± SEM) relative to untreated LC based on MFI (N=10 individual donors). (B) In vitro migration assay. LC were analyzed for migration through a transwell insert to CCL21 or medium alone. Shown is the mean number of LC migrating to CCL21 (black bars) compared to spontaneous migration (± SEM) relative to untreated LC (N=6 individual donors). **p<0.01 compared to untreated LC and LC exposed to HPV16 (one-way ANOVA, Tukey's post-test).
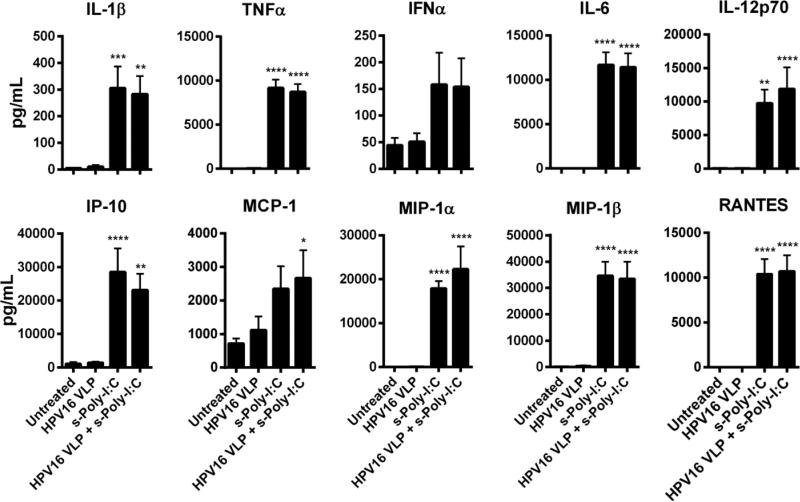
3.3 Poly-ICR induces HPV16-exposed LC to produce high levels of inflammatory cytokines and chemokines
Induction of T cell responses against virus-infected cells requires APC to produce Th1 inducing cytokines and chemokines to prime CD8+ T cells against viral antigens and recruit innate immune cells to participate in eradication of virus-infected cells. Poly-I:C is well known for inducing a type I interferon response through activation of transcription factors that leads to the production of additional inflammatory cytokines and chemokines [20, 23]. Therefore, LC were tested for the ability to secrete a wide variety of cytokines and chemokines after exposure to HPV16 followed by treatment with s-Poly-I:C (Poly-ICR). Only treatment of s-Poly-I:C alone or HPV16-exposed LC plus s-Poly-I:C resulted in a significant increase in the magnitude of cytokines and chemokines produced, most notably TNFα IFNα, IL-1β, IL-6, IL-12p70, IFN-γ-inducible protein 10 (IP-10), MCP-1, MIP-1α, MIP-1β, and RANTES (Fig. 3). These results demonstrate that HPV16-exposed LC are able to secrete inflammatory cytokines and chemokines that can activate and attract T cells to the site of antigen priming after treatment with s-Poly-I:C.
Fig. 3. Poly-ICR induces HPV16-exposed LC to produce high levels of inflammatory cytokines and chemokines.
LC were exposed to HPV16 prior to treatment with s-Poly-I:C (Poly-ICR) (N=7 individual donors). Cell supernatants were analyzed for a panel of ten cytokines and chemokines using a Bio-Plex Suspension Array System. Data represent the mean (± SEM) analyte concentration. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to untreated LC and LC exposed to HPV16 (one-way ANOVA, Tukey's post-test).
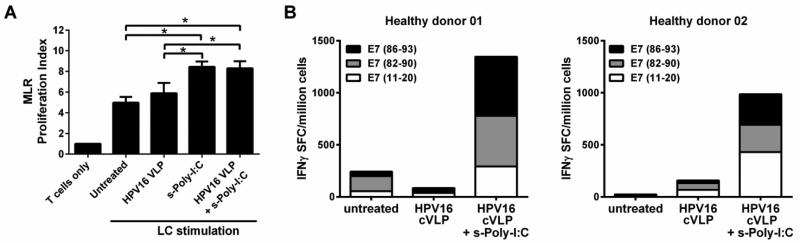
3.4 HPV16-exposed LC treated with Poly-ICR induce HPV16-specific CD8+ T cell responses
Activated LC are potent stimulators of T cell proliferation [34]. To determine whether s-Poly-I:C (Poly-ICR) treated HPV16-exposed LC demonstrate an increased ability to stimulate naïve T cell proliferation, an in vitro MLR assay was performed. Untreated, HPV16-exposed, or HPV16-exposed and then s-Poly-I:C treated LC were co-cultured with allogeneic T cells. LC treated with s-Poly-I:C after HPV16 exposure or s-Poly-I:C alone demonstrated a significant enhancement of T cell stimulatory capacity compared to both untreated LC alone and LC exposed to HPV16 VLP (Fig. 4a). To analyze the ability of LC to induce antigen-specific T cells, we next tested whether LC pre-incubated with HPV16 and then treated with Poly-ICR induced an HPV16-specific CD8+ T cell response in five of the HLA-A2+ donors after an in vitro immunization assay followed by an IFNγ ELISPOT assay. In these experiments, HPV16 L1L2-E7 cVLP containing the E7 protein were used [28]. cVLP contain a fusion protein of L2-E7, which encapsidates the E7 protein inside the VLP and is delivered to LC as a viral antigen. Defined HLA-A*0201 binding E7 peptides [35] were used to detect the breadth and magnitude of HPV16 E7-specific CD8+ T cell reactivity induced. LC exposed to HPV16 cVLP and subsequently treated with s-Poly-I:C were able to induce IFNγ secreting E7 peptide-specific CD8+ T cells specific for all three epitopes, E711-20, E782-90, and E786-93, when compared to untreated LC or LC exposed to HPV16 cVLP alone (Fig. 4b,). T cell responses tested against pooled E7 peptides were found to be additive of individual peptide responses, indicating that diverse T cells recognized each peptide individually (data not shown). Collectively, these results demonstrate that LC exposed to HPV16 particles and subsequently to s-Poly-I:C were able to become functionally active and capable of inducing T cell proliferation and an HPV16-specific CD8+ T cell response.
Fig. 4. HPV16-exposed LC treated with Poly-ICR induce T cell proliferation and an HPV16-specific CD8+ T cell response.
(A) Mixed lymphocyte reaction. Proliferation of allogeneic T cells by HPV16-exposed LC treated with Poly-ICR. LC were left untreated or exposed to HPV16 VLP for 6 h (N=4 individual donors). Subsequently, the cells were treated with s-Poly I:C (5 μg/mL Poly-ICR) for 48 hr. LC were co-cultured with purified MHC-mismatched T cells from a healthy donor for 6 days in triplicate wells. 3H-thymidine was added during the last 18 hr of culture. Shown is the mean cpm thymidine uptake (± SEM) *p<0.05 compared to untreated LC (one-way ANOVA, Tukey's post-test). (B) In vitro immunization. Purified CD8+ T cells were co-cultured with s-Poly-I:C (5 μg/mL Poly-ICR) treated or untreated autologous LC for 4 weeks with weekly restimulations in an in vitro immunization assay. LC were loaded with HPV16 L1L2-E7 cVLP, then treated with s-Poly-I:C or left untreated. T cells were then tested for IFN-γ secretion in response to HLA-A*0201 binding peptides by ELISPOT analysis. The number of spots representing IFNγ secreting cells were averaged over eight replicate wells. Data show two representative HLA-A*0201+ donors following s-Poly-I:C treatment of LC out of five donors tested.
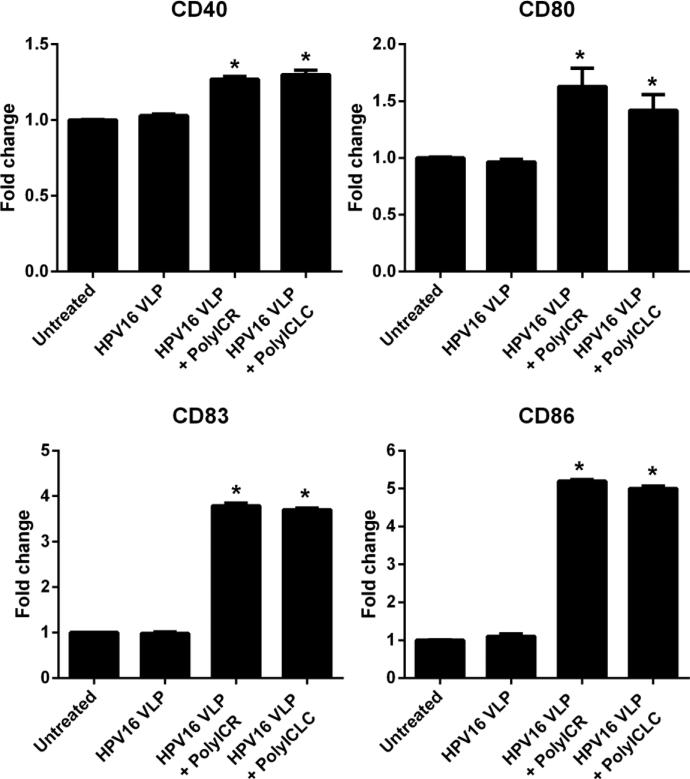
3.5 Poly-ICR and Poly-ICLC induce similar activated phenotypes in HPV16-exposed LC
The above results show that s-Poly-I:C stabilized with poly-arginine (Poly-ICR) induces phenotypically and functionally activated LC that were pre-exposed to HPV16. Poly-I:C stabilized with poly-lysine (poly-ICLC, Hiltonol), is an investigational dsRNA compound that has been used in several past and present clinical trials as a vaccine adjuvant [36-38]. Since Poly-ICLC can be more rapidly translated to clinical studies in HPV+ women, we wanted to determine its activity on HPV16-exposed LC and we hypothesized that LC treatment with Poly-ICLC would similarly activate LC compared to Poly-ICR. To examine this, LC were either left untreated or exposed to HPV16 prior to treatment with Poly-ICR or Poly-ICLC and then analyzed for cell-surface expression of costimulatory molecules. The results demonstrate that LC treated with either Poly-ICR or Poly-ICLC after HPV16 exposure had significantly increased expression of the costimulatory molecules CD40, CD80, CD83, and CD86 compared to untreated and HPV16 only groups (Fig. 5). Notably, the level of expression induced by Poly-ICR and Poly-ICLC was similar for all markers tested when using the optimal concentration of each s-Poly-I:C compound as determined by a dose titration (5 μg/mL Poly-ICR and 50 μg/mL Poly-ICLC; Fig. S1). These results demonstrate that both s-Poly-I:C compounds induce a similar activated phenotype in HPV16-exposed LC.
Fig. 5. Poly-ICLC induces upregulation of costimulatory molecules on LC similar to Poly-ICR.
Immature LC were left untreated or exposed to HPV16 VLP prior to treatment with s-Poly-I:C (Poly-ICR or Poly-ICLC). Control cells were left untreated or were exposed to HPV16. Expression of CD40, CD80, CD83, and CD86 and was then analyzed by flow cytometry. The average fold increase in LC surface marker expression from one representative donor out of four tested is presented. Data shown is the mean fold increase in surface marker expression (± SD) of triplicate values relative to untreated LC based on MFI. *p=0.05 (Mann-Whitney U test).
3.6 Poly-ICLC induces LC activation in HPV16-exposed LC
Since Poly-ICR and Poly-ICLC induced similar increases in the expression of costimulatory molecules, we next sought to determine if Poly-ICLC could induce functionally activated LC in a comparable manner to what was observed for Poly-ICR. Therefore, LC were treated with Poly-ICLC after pre-exposure to HPV16, and LC activation was evaluated as the upregulation of MHC and costimulatory molecules, in vitro chemokine-directed migration, and secretion of inflammatory cytokines and chemokines.
Poly-ICLC was able to activate LC that had been pre-exposed to HPV16 such that expression of the MHC I, MHC II, CD40, CD80, CD83, and CD86 were significantly increased (Fig. 6a). Treatment of HPV16-exposed LC with Poly-ICLC resulted in a significant increase in migration capacity towards CCL21 compared to untreated LC or LC exposed to HPV16 alone (Fig. 6b). Similar to Poly-ICR, treatment of HPV16-exposed LC with Poly-ICLC resulted in a significant increase in the amount of cytokines and chemokines secreted (Table S1). Similar to what we observed for Poly-ICR (Fig 1-3), LC treatment with Poly-ICLC alone caused similar levels of phenotypic and functional activation to that observed with LC treatment of Poly-ICLC after exposure to HPV16 (data not shown). Taken together these results indicate that Poly-ICLC can induce the functional activation of HPV16-exposed LC, providing a strong rationale for testing Poly-ICLC in future clinical applications.
Fig. 6. Poly-ICLC induces LC activation in HPV16-exposed LC.
(A) Poly-ICLC induces upregulation of MHC and costimulatory molecules on LC. LC were left untreated or exposed to HPV16 prior to treatment with s-Poly-I:C (50 μg/mL Poly-ICLC), then analyzed by flow cytometry for indicated surface markers. Control cells were left untreated or exposed to HPV16 alone. Data shown is the mean fold increase in surface marker expression (± SD) of replicate values relative to untreated LC based on MFI of one representative donor out of four tested. *p=0.05, (Mann-Whitney U test). (B) Poly-ICLC induces HPV16-exposed LC to migrate to chemokine CCL21 in vitro. LC were exposed to HPV16 prior to s-Poly-I:C (50 μg/mL Poly-ICLC). LC were analyzed for migration to medium or medium supplemented with CCL21. Shown is the mean number (± SD) of LC migrating to CCL21 (black bars) compared to spontaneous migration (white bars) of three separate donors performed in triplicate relative to untreated LC. *p=0.05 compared to HPV16 only LC (Mann-Whitney U test).
4. Discussion
The life cycle of hrHPV is strictly intraepithelial, and consequently viral antigens should be processed and presented by LC, the professional APC that reside in the epithelium [39]. Various studies have found that HPV16 VLP can bind to and stimulate the activation of human dendritic cells (DC) [32, 40], providing evidence that HPV16 can induce the maturation of APC subsets. Immature LC express low levels of phenotypic activation markers and cytokines in the absence of activating stimuli. Therefore, exposure to HPV16 would not be expected to cause a reduction of those levels. However, based on our previous data, we know that the interaction of HPV16 and other hrHPV types with LC results in a suppressive signaling cascade through interference with the PI3-K/Akt signaling pathway, suggestive of active immune suppression [7, 8]. Moreover, we have determined defective LC activation to be dependent on the presence of the HPV16 L2 minor capsid protein binding to a cell surface receptor that causes a decrease in phenotypic activation and stimulatory capacity of LC [41, 42]. In this regard, LC may be uniquely manipulated by HPV compared to other immune cells or DC subsets. Because other HPV genotypes such as high-risk (HPV18, HPV31, and HPV45), low-risk (HPV11), and a cutaneous (HPV5) genotype, also suppress LC activation through a similar mechanism [8], an immune modulating treatment such as s-Poly-I:C shown for HPV16 is likely to have efficacy against other hrHPV types as well.
The field of LC biology is still evolving as new technologies allow unprecedented investigation into the functions of discreet populations of APC subsets [43]. Although mouse studies are pointing towards an immunoregulatory role for LC under homeostatic conditions, it is not presently clear whether the situation in genetically engineered mice is representative of the in vivo function of human LC. One barrier preventing correlation of human studies with mouse in vivo experiments is the disparate expression of various surface receptors used for phenotyping DC subsets and relative functional plasticity of APC depending on the presence of different inflammatory stimuli. Indeed, recent transcriptional profiling of purified mouse and human DC subsets reveals that human LC are most functionally similar to the mouse CD8α+CD103+ dermal DC subpopulation [44]. Moreover, both of these populations are highly adept at cross-priming naïve CD8+ T cells into effector CTLs. It is also very likely that the actual function of LC in situ, whether mouse or human, is determined by stimuli from the environment including danger signals, epithelial cell cross-talk, and presence of other immune cells and soluble mediators [45]. Regardless of the current controversies as to the nature and functions of LC, it is evident that HPV either specifically manipulates LC in a suppressive manner, or takes advantage of the inherent immunoregulatory functions of LC, thereby avoiding immune detection and viral clearance; however, our data demonstrate this situation can be reversed by providing an immunostimulatory TLR3 signal with s-Poly-I:C that allows maturation steps to proceed.
Here we demonstrate that TLR3 is expressed by monocyte-derived LC. Likewise, it has been demonstrated that LC freshly isolated from human skin also express TLR3 and are capable of responding to dsRNA [21]. Highly-purified LC freshly isolated from human epidermis are strongly activated by Poly-I:C as indicated by marked increases in the expression of CD40, CD80, and CD86 [24]. Furthermore, Poly-I:C treatment commits LC to induce CD8+ T cell responses more effectively than dermal DC [46]. Renn et al. demonstrated that LC derived from CD34+ umbilical cord progenitor cells responded better to Poly-I:C than do monocyte derived DC [47]. While these previous in vitro reports indicate that Poly-I:C may be promising in inducing human LC-mediated immune responses, these studies do not consider the rapid degradation of non-stabilized Poly-I:C by serum nucleases present in humans and non-human primates [26], limiting their applicability to clinical research, which is why only stabilized Poly-I:C compounds were chosen for the current investigations.
The results of the current study show that LC are not activated by and do not induce an adaptive immune response to HPV16 alone. Despite this, LC from all donors were able to become functionally active APC, upregulating MHC and costimulatory molecules, undergoing chemokine-directed migration, secreting inflammatory cytokines and chemokines, and inducing CD8+ T cell responses after treatment with s-Poly-I:C (either Poly-ICR or Poly-ICLC) in vitro. However, our study has limitations in that the LC are blood-derived rather than isolated from the cervical mucosa. Despite this, the aforementioned LC studies of others suggest that freshly isolated epidermal LC and LC derived from monocytes or CD34+ progenitors respond similarly to Poly-I:C. Additionally, our culture system does not replicate the complex interactions that take place in vivo between epithelial cells, LC, and lymphocytes along with cytokine cross-talk.
Two studies have demonstrated the utility of using Poly-I:C in the adjuvant setting for HPV-related disease. Intravaginal treatment with Poly-I:C after systemic vaccination has been shown to increase mucosally associated E7-specific CD8+ T cell responses in mice [48]. Increased mucosal trafficking of T cells in that model is likely the result of chemokine upregulation, as it has been shown in this study and by others that Poly-I:C is a strong inducer of chemokine secretion. A dose-dependent increase in the secretion of the chemokine IL-8/CXCL8 was observed when human endocervical, ectocervical, and vaginal epithelial cells were treated with Poly-I:C in vitro, indicating that cells at the cervical transformation zone, which is highly susceptible to hrHPV infection, would be responsive to TLR3 agonists [49]. Poly-ICLC has also been used to enhance the systemic Th1 immune responses against a HPV16 capsomer vaccine in rhesus macaques, supporting the induction of strong anti-HPV16 L1 antibody responses [50]. Taken together, these studies suggest that TLR3 activation may act through multiple mechanisms of action to stimulate both innate and adaptive immunity to promote HPV clearance if applied in the right context. For example, one could envision short-term topical application of Poly-ICLC in a cervical cap or in a dissolvable patch that could be applied to the cervix of women with recently detected hrHPV-positive test results obtained during regular screening intervals in order to stimulate LC activation while virus transcriptional activity is still present.
In the current study, we demonstrated that LC responded strongly to two different poly-peptide stabilized forms of poly-I:C. Poly-ICR induced robust responses in LC, and these results were then confirmed with clinical grade Poly-ICLC. Effective concentrations for LC activation by unstabilized Poly-I:C in in vitro studies has been reported between 10-25 μg/mL [24, 46], which is similar to concentrations used for Poly-ICR and Poly-ICLC in the current study. The effective dose of Poly-ICLC needed to achieve equivalent activation marker upregulation, for example, was five to ten times the effective dose of Poly-ICR (Fig. S1). The differences in the optimal concentrations for Poly-ICR and Poly-ICLC could be due to variations in manufacturing practices, the latter being a cGMP-produced compound, or the use of poly-arginine versus poly-lysine as a stabilization component. In vitro, the mechanism of action of either Poly-I:C or stabilized Poly-I:C (Poly-ICLC) appears to result in similar gene expression and transcriptional profiles after stimulation of PBMC, suggesting that the improved in vivo activity of Poly-ICLC may be related to the stabilization effect rather than differences in TLR3 ligation [51]. While both stabilized compounds tested in this study demonstrate the capability of initiating potent LC-mediated immune responses in the presence of HPV16, Poly-ICLC, which is produced in clinical grade batches by Oncovir under the name Hiltonol, is currently being used in clinical trials including studies to stimulate immunity against solid tumors and in dendritic cells vaccines [36-38].
Collectively our observations suggest that the TLR3 agonist s-Poly-I:C is a promising therapeutic molecule that can stimulate LC immune function without potential interference or suppression of LC signaling by HPV, and result in LC capable of stimulating anti-HPV CD8+ T cell-mediated immune responses. Looking forward, due to its pharmaceutical grade availability, good safety profile in human clinical studies, and current results reported herein, the use of Poly-ICLC to promote LC activation in vivo in hrHPV positive women should be explored further clinically in order to induce anti-HPV immunity and enhance viral clearance.
Supplementary Material
Acknowledgements
We thank the many healthy donors who enrolled into this study. W.M. Kast holds the Walter A. Richter Cancer Research Chair. This work was supported by NIH grants R01 CA074397 and RC2 CA148298, and the L.K. Whittier Foundation. Support from the Karl H. and Ruth M. Balz Trust, Sammie's Circle, and the Norris Auxiliary Women is gratefully acknowledged. Andrew Woodham was supported by a TL1 Scholar award from the SC CTSI (NIH/NCRR/NCATS) Grant # TL1TR000132. Additional support for Andrew Woodham from the ARCS Foundation John and Edith Leonis Award is greatly appreciated. Multiplex ELISA assays were carried out in the Norris Comprehensive Cancer Center Beckman Center for Immune Monitoring that is supported in part by Cancer Center Support Grant P30CA014089 from the NCI. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the Whittier Foundation, the NCI, or the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: APC, antigen presenting cell; DC, dendritic cell; HPV, human papillomavirus; hrHPV, high-risk HPV; HPV16, HPV type 16; LC, Langerhans cell; MLR, mixed lymphocyte reaction; PAMP, pathogen associated molecular pattern; s-Poly-I:C, stabilized polyinosinic-polycytidilic acid; TLR3, toll-like receptor 3; cVLP, chimeric virus-like particle; VLP, virus-like particle
Disclosures
GMM holds ownership interest in Akela Pharma, Inc. (formerly Nventa). AMS holds ownership interest in Oncovir, Inc.
List of Supplemental Materials
Fig. S1. CD86 expression on LC after titration of s-Poly-I:C (PolyICR or PolyICLC)
Table S1. Poly-ICLC induces HPV16-exposed LC to produce high levels of inflammatory cytokines and chemokines.
References Cited
- 1.Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J, Bray F, Plummer M, Franceschi S. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 2.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 3.Schiffman M, Herrero R, Desalle R, Hildesheim A, Wacholder S, Rodriguez AC, Bratti MC, Sherman ME, Morales J, Guillen D, Alfaro M, Hutchinson M, Wright TC, Solomon D, Chen Z, Schussler J, Castle PE, Burk RD. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337:76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Giuliano AR, Harris R, Sedjo RL, Baldwin S, Roe D, Papenfuss MR, Abrahamsen M, Inserra P, Olvera S, Hatch K. Incidence, prevalence, and clearance of type-specific human papillomavirus infections: The Young Women's Health Study. J Infect Dis. 2002;186:462–469. doi: 10.1086/341782. [DOI] [PubMed] [Google Scholar]
- 5.Rositch AF, Koshiol J, Hudgens MG, Razzaghi H, Backes DM, Pimenta JM, Franco EL, Poole C, Smith JS. Patterns of persistent genital human papillomavirus infection among women worldwide: a literature review and meta-analysis. Int J Cancer. 2013;133:1271–1285. doi: 10.1002/ijc.27828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fausch SC, Da Silva DM, Rudolf MP, Kast WM. Human papillomavirus virus-like particles do not activate Langerhans cells: a possible immune escape mechanism used by human papillomaviruses. J Immunol. 2002;169:3242–3249. doi: 10.4049/jimmunol.169.6.3242. [DOI] [PubMed] [Google Scholar]
- 7.Fausch SC, Fahey LM, Da Silva DM, Kast WM. Human papillomavirus can escape immune recognition through Langerhans cell phosphoinositide 3-kinase activation. J Immunol. 2005;174:7172–7178. doi: 10.4049/jimmunol.174.11.7172. [DOI] [PubMed] [Google Scholar]
- 8.Da Silva D, Movius C, Raff A, Brand H, Skeate J, Wong M, Kast W. Suppression of Langerhans cell activation is conserved amongst human papillomavirus α and β genotypes, but not a μ genotype. Virology. 2014;452-453:279–286. doi: 10.1016/j.virol.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 10.Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol. 2014;14:417–428. doi: 10.1038/nri3683. [DOI] [PubMed] [Google Scholar]
- 11.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Bobr A, Olvera-Gomez I, Igyarto BZ, Haley KM, Hogquist KA, Kaplan DH. Acute ablation of Langerhans cells enhances skin immune responses. J Immunol. 2010;185:4724–4728. doi: 10.4049/jimmunol.1001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shklovskaya E, O'Sullivan BJ, Ng LG, Roediger B, Thomas R, Weninger W, Fazekas de St Groth B. Langerhans cells are precommitted to immune tolerance induction. Proc Natl Acad Sci USA. 2011;108:18049–18054. doi: 10.1073/pnas.1110076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flacher V, Tripp CH, Mairhofer DG, Steinman RM, Stoitzner P, Idoyaga J, Romani N. Murine Langerin+ dermal dendritic cells prime CD8+ T cells while Langerhans cells induce cross-tolerance. EMBO Mol Med. 2014;6:1191–1204. doi: 10.15252/emmm.201303283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, Reiter Y, Banchereau J, Ueno H. Functional specializations of human epidermal langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratzinger G, Baggers J, de Cos MA, Yuan J, Dao T, Reagan JL, Munz C, Heller G, Young JW. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal interstitial or monocyte-derived dendritic cells. J Immunol. 2004;173:2780–2791. doi: 10.4049/jimmunol.173.4.2780. [DOI] [PubMed] [Google Scholar]
- 18.Polak ME, Newell L, Taraban VY, Pickard C, Healy E, Friedmann PS, Al-Shamkhani A, Ardern-Jones MR. CD70-CD27 interaction augments CD8+ T-cell activation by human epidermal Langerhans cells. J Invest Dermatol. 2012;132:1636–1644. doi: 10.1038/jid.2012.26. [DOI] [PubMed] [Google Scholar]
- 19.Fahey LM, Raff AB, Da Silva DM, Kast WM. Reversal of human papillomavirus-specific T cell immune suppression through TLR agonist treatment of Langerhans cells exposed to human papillomavirus type 16. J Immunol. 2009;182:2919–2928. doi: 10.4049/jimmunol.0803645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng YS, Xu F. Anticancer function of polyinosinic-polycytidylic acid. Cancer biology & therapy. 2010;10:1219–1223. doi: 10.4161/cbt.10.12.13450. [DOI] [PubMed] [Google Scholar]
- 21.Flacher V, Bouschbacher M, Verronese E, Massacrier C, Sisirak V, Berthier-Vergnes O, de Saint-Vis B, Caux C, Dezutter-Dambuyant C, Lebecque S, Valladeau J. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol. 2006;177:7959–7967. doi: 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]
- 22.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto M, Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C). Advanced drug delivery reviews. 2008;60:805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Furio L, Billard H, Valladeau J, Peguet-Navarro J, Berthier-Vergnes O. Poly(I:C)-Treated human langerhans cells promote the differentiation of CD4+ T cells producing IFN-gamma and IL-10. The J Invest Derm. 2009;129:1963–1971. doi: 10.1038/jid.2009.21. [DOI] [PubMed] [Google Scholar]
- 25.Vercammen E, Staal J, Beyaert R. Sensing of viral infection and activation of innate immunity by toll-like receptor 3. Clin Microbiol Rev. 2008;21:13–25. doi: 10.1128/CMR.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Clercq E. Degradation of poly(inosinic acid) - poly(cytidylic acid) [(I)n - (C)n] by human plasma. Eur J Biochem / FEBS. 1979;93:165–172. doi: 10.1111/j.1432-1033.1979.tb12807.x. [DOI] [PubMed] [Google Scholar]
- 27.Levy HB, Baer G, Baron S, Buckler CE, Gibbs CJ, Iadarola MJ, London WT, Rice J. A modified polyriboinosinic-polyribocytidylic acid complex that induces interferon in primates. J Infect Dis. 1975;132:434–439. doi: 10.1093/infdis/132.4.434. [DOI] [PubMed] [Google Scholar]
- 28.Greenstone HL, Nieland JD, de Visser KE, De Bruijn ML, Zkirnbauer R, Roden RBS, Lowy DR, Kast WM, Schiller JT. Chimeric papillomavirus virus-like particles elicit antitumor immunity against E7 oncoprotein in an HPV16 tumor model. Proc Natl Acad Sci USA. 1998;95:1800–1805. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodham AW, Raff AB, Da Silva DM, Kast WM. Molecular analysis of human papillomavirus virus-like particle activated langerhans cells in vitro. Methods Mol Biol. 2015;1249:135–149. doi: 10.1007/978-1-4939-2013-6_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan L, Woodham AW, Da Silva DM, Kast WM. Functional Analysis of HPV-Like Particle-Activated Langerhans Cells In Vitro. Methods Mol Biol. 2015;1249:333–350. doi: 10.1007/978-1-4939-2013-6_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muul LM, Silvin C, James SP, Candotti F. Measurement of proliferative responses of cultured lymphocytes. Curr Protocols Immunol / edited by John E Coligan [et al] 2008 doi: 10.1002/0471142735.im0710s82. Chapter 7:Unit 7 10 11-17 10 24. [DOI] [PubMed] [Google Scholar]
- 32.Rudolf MP, Fausch SC, Da Silva DM, Kast WM. Human dendritic cells are activated by chimeric human papillomavirus type-16 virus-like particles and induce epitope-specific human T cell responses in vitro. J Immunol. 2001;166:5917–5924. doi: 10.4049/jimmunol.166.10.5917. [DOI] [PubMed] [Google Scholar]
- 33.Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Ann Rev Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 34.Stingl G, Katz SI, Clement L, Green I, Shevach EM. Immunologic functions of Ia-bearing epidermal Langerhans cells. J Immunol. 1978;121:2005–2013. [PubMed] [Google Scholar]
- 35.Kast WM, Brandt RM, Sidney J, Drijfhout JW, Kubo RT, Grey HM, Melief CJ, Sette A. Role of HLA-A motifs in identification of potential CTL epitopes in human papillomavirus type 16 E6 and E7 proteins. J Immunol. 1994;152:3904–3912. [PubMed] [Google Scholar]
- 36.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, Nelson SF, Liau LM. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Can Res. 2011;17:1603–1615. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, Zeh H, Holtzman MP, Reinhart TA, Whiteside TL, Butterfield LH, Hamilton RL, Potter DM, Pollack IF, Salazar AM, Lieberman FS. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salazar AM, Erlich RB, Mark A, Bhardwaj N, Herberman RB. Therapeutic in situ autovaccination against solid cancers with intratumoral poly-ICLC: case report, hypothesis, and clinical trial. Cancer Immunol Res. 2014;2:720–724. doi: 10.1158/2326-6066.CIR-14-0024. [DOI] [PubMed] [Google Scholar]
- 39.Stanley MA. Epithelial cell responses to infection with human papillomavirus. Clin Microbiol Rev. 2012;25:215–222. doi: 10.1128/CMR.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenz P, Day PM, Pang YY, Frye SA, Jensen PN, Lowy DR, Schiller JT. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol. 2001;166:5346–5355. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- 41.Woodham AW, Raff AB, Raff LM, Da Silva DM, Yan L, Skeate JG, Wong MK, Lin YG, Kast WM. Inhibition of langerhans cell maturation by human papillomavirus type 16: a novel role for the annexin A2 heterotetramer in immune suppression. J Immunol. 2014;192:4748–4757. doi: 10.4049/jimmunol.1303190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fahey LM, Raff AB, Da Silva DM, Kast WM. A major role for the minor capsid protein of human papillomavirus type 16 in immune escape. J Immunol. 2009;183:6151–6156. doi: 10.4049/jimmunol.0902145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crozat K, Guiton R, Guilliams M, Henri S, Baranek T, Schwartz-Cornil I, Malissen B, Dalod M. Comparative genomics as a tool to reveal functional equivalences between human and mouse dendritic cell subsets. Immunol Rev. 2010;234:177–198. doi: 10.1111/j.0105-2896.2009.00868.x. [DOI] [PubMed] [Google Scholar]
- 44.Artyomov MN, Munk A, Gorvel L, Korenfeld D, Cella M, Tung T, Klechevsky E. Modular expression analysis reveals functional conservation between human Langerhans cells and mouse cross-priming dendritic cells. J Exp Med. 2015 doi: 10.1084/jem.20131675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romani N, Brunner PM, Stingl G. Changing views of the role of Langerhans cells. J Invest Dermatol. 2012;132:872–881. doi: 10.1038/jid.2011.437. [DOI] [PubMed] [Google Scholar]
- 46.van der Aar AM, de Groot R, Sanchez-Hernandez M, Taanman EW, van Lier RA, Teunissen MB, de Jong EC, Kapsenberg ML. Cutting edge: virus selectively primes human langerhans cells for CD70 expression promoting CD8+ T cell responses. J Immunol. 2011;187:3488–3492. doi: 10.4049/jimmunol.1101105. [DOI] [PubMed] [Google Scholar]
- 47.Renn CN, Sanchez DJ, Ochoa MT, Legaspi AJ, Oh CK, Liu PT, Krutzik SR, Sieling PA, Cheng G, Modlin RL. TLR activation of Langerhans cell-like dendritic cells triggers an antiviral immune response. J Immunol. 2006;177:298–305. doi: 10.4049/jimmunol.177.1.298. [DOI] [PubMed] [Google Scholar]
- 48.Domingos-Pereira S, Decrausaz L, Derre L, Bobst M, Romero P, Schiller JT, Jichlinski P, Nardelli-Haefliger D. Intravaginal TLR agonists increase local vaccine-specific CD8 T cells and human papillomavirus-associated genital-tumor regression in mice. Mucosal Immunol. 2013;6:393–404. doi: 10.1038/mi.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersen JM, Al-Khairy D, Ingalls RR. Innate immunity at the mucosal surface: role of toll-like receptor 3 and toll-like receptor 9 in cervical epithelial cell responses to microbial pathogens. Biol Reproduction. 2006;74:824–831. doi: 10.1095/biolreprod.105.048629. [DOI] [PubMed] [Google Scholar]
- 50.Stahl-Hennig C, Eisenblatter M, Jasny E, Rzehak T, Tenner-Racz K, Trumpfheller C, Salazar AM, Uberla K, Nieto K, Kleinschmidt J, Schulte R, Gissmann L, Muller M, Sacher A, Racz P, Steinman RM, Uguccioni M, Ignatius R. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathogens. 2009;5:e1000373. doi: 10.1371/journal.ppat.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caskey M, Lefebvre F, Filali-Mouhim A, Cameron MJ, Goulet JP, Haddad EK, Breton G, Trumpfheller C, Pollak S, Shimeliovich I, Duque-Alarcon A, Pan L, Nelkenbaum A, Salazar AM, Schlesinger SJ, Steinman RM, Sekaly RP. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J Exp Med. 2011;208:2357–2366. doi: 10.1084/jem.20111171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.