Abstract
Background
Epidemiologic studies worldwide have provided substantial evidence of the contributions of environmental exposures to the development of childhood cancer, yet this knowledge has not been integrated into the routine practice of clinicians who care for children with this disease. To identify the basis of this deficit, we sought to assess the environmental history-taking behavior and perceptions of environmental health among pediatric hematologists and oncologists.
Procedure
A web-based survey was sent from June to October 2012 to 427 pediatric oncologists, fellows, and nurse practitioners from 20 U.S. institutions, with an overall response rate of 45%.
Results
Survey responses indicated that environmental exposures are of concern to clinicians. The vast majority of respondents (88%) reported receiving questions from families about the relationship between certain environmental exposures and the cancers they regularly treat. However, a lack of comfort with these topics appears to have limited their discussions with families about the role of environmental exposures in childhood cancer pathogenesis. Although 77% of respondents suspected that some of the cases they saw had an environmental origin, their methods of taking environmental histories varied widely. Over 90% of respondents believed that more knowledge of the associations between environmental exposures and childhood cancer would be helpful in addressing these issues with patients.
Conclusions
Though limited in size and representativeness of participating institutions, the results of this survey indicate a need for increased training for hematology/oncology clinicians about environmental health exposures related to cancer and prompt translation of emerging research findings in biomedical journals that clinicians read.
Keywords: Pediatric hematology/oncology, Epidemiology, Translational research, Medical education, Pediatric environmental health
Introduction
Mortality from childhood malignancies has declined significantly over the past 40 years, largely due to advances in pediatric cancer treatments. [1] Though childhood cancers remain one of the leading causes of death for children 1–14 years old in the U.S. [2], their origin is only partly understood. [3] However, a growing body of literature has implicated environmental hazards in the etiology of certain childhood cancers. The President’s Cancer Panel states in their 2008–2009 Annual Report that, “the true burden of environmentally induced cancer has been grossly underestimated.” [4]
Exposure to ionizing radiation from nuclear accidents, x-rays, or radiation therapy is associated with an increased risk of childhood leukemia [5–7] and solid tumors. [8–10] Exposures to solvents and ambient air pollutants, including benzene, may also contribute to an increased risk of childhood leukemia. [11–14] Evidence suggests a link between parental, prenatal, and childhood exposures to pesticides and childhood leukemia in both residential and occupational settings. [15–21] In utero exposure to household insecticides and indoor pesticides is linked to increased risk of childhood leukemia. [22] Finally, numerous studies from around the world have consistently identified associations between pesticide exposures and risk of lymphomas, brain tumors, and other solid tumors. [19, 23]
The U.S. Surgeon General and the State of California have reported prenatal and postnatal exposures to environmental tobacco smoke to have a suggestive association with childhood leukemia, lymphomas, and brain tumors. [24, 25] The International Agency for Research on Cancer (IARC) has classified tobacco smoke as carcinogenic to smokers’ children with sufficient evidence for hepatoblastoma and limited evidence for childhood leukemia. [26] Paternal smoking, in particular, before conception has also been linked to an increased risk of childhood acute lymphoblastic leukemia (ALL). [27]
Many of the above studies find odds ratios greater than 2 (including meta-analyses) for overall risk or specific exposure strata. The evidence from case-control studies (and meta-analyses based on them) is strengthened by additional studies finding polychlorinated biphenyls and polybrominated diphenyl ethers, fire retardant chemicals in house dust that are associated with elevated risk of childhood leukemia. These studies provide an objective measure of chemical exposure and eliminate recall bias. [28, 29] Due to the rarity of childhood cancer, the ability to form prospective studies is limited. Research collaborations such as the Childhood Leukemia International Consortium (CLIC) and the International Childhood Cancer Cohort Consortium (I4C) provide hope for the future, as pooled data and biospecimens from large-scale studies will help identify more robust findings regarding childhood cancer causation. [30, 31]
Greaves has proposed that a delay in a child’s exposure to common childhood infections may result in an improperly modulated immune system and a subsequent risk of aberrantly high levels of lymphoblastic cell proliferation following the barrage of infections when the child enters day care or preschool. [32] This delayed infection hypothesis has been supported by subsequent studies and meta-analyses showing that children exposed to common infections early in life via social contact (such as day care attendance), are at reduced risk of ALL. [33, 34]
Despite the growing insight into potentially modifiable risk factors for childhood cancer, there is little evidence that this knowledge is being translated to clinical practice. Surveys conducted among general pediatricians show that, while these physicians attach considerable importance to the impact of environmental exposures on children’s health, they spend little time discussing this information with families. [35, 36] A literature review found consistent gaps in knowledge of environmental hazards and confidence in addressing these issues among pediatric healthcare providers in a variety of geographic regions. [36] Despite Institute of Medicine recommendations in 1988 that called for the integration of environmental health concepts into all levels of nursing and medical education, relatively little progress has been made. [37, 38] Few medical schools, pediatric residencies, or nurse practitioner training programs devote substantial training time to environmental contributors to disease. [39–41] Nevertheless, initiatives have begun in both the nursing and medical communities to bridge this gap. [42, 43]
We conducted a survey to learn whether practicing pediatric hematologists and oncologists encounter barriers to integrating environmental research findings into practice similar to those reported by general pediatricians. Specifically, we sought to assess their level of knowledge and attitudes related to potential environmental contributions to childhood cancers and their history-taking practices.
Materials and Methods
An online survey was sent from June to October 2012 to 20 clinical sites: 18 pediatric cancer treatment centers in California, plus the Dana-Farber Cancer Institute (Boston, MA) and the University of Utah Huntsman Cancer Institute (Salt Lake City, UT). Physicians who received the survey were identified through their participation as a clinical collaborator with the California Childhood Leukemia Study (CCLS) or their affiliations with one of the above institutions. [27] A single physician at each site was asked to distribute the online survey to all attending physicians, fellows, and nurse practitioners who were members of hematology/oncology or stem cell transplant services. All responses were collected anonymously with no respondent identifying information. Responses were collected using SurveyMonkey, Inc. of Palo Alto, CA. [44] A reminder email was sent to participants who had not completed the survey after approximately 4 weeks.
The survey consisted of 11 questions pertaining to demographic information, perceptions regarding the causes of childhood cancer, history-taking behaviors and training, patient experiences, and home practices; with multiple and open-ended responses allowed [see Supplemental Appendix I for the full-text survey]. A Likert scale of 1–5 (“strongly disagree” to “strongly agree”) was used to assess attitudes, and two questions allowed for open-ended responses. A pilot survey was conducted at Lucile Packard Children’s Hospital at Stanford University (n = 22), and slight modifications were made to the instrument based on these results. However, these pilot responses were not included in the final analyses. Descriptive analyses (frequencies, percentages, standard deviations) were performed overall and by respondents’ characteristics (type of position and years in practice), using SAS 9.2 (SAS Institute, Cary, NC). The survey was approved by the Institutional Review Boards at Stanford University, Dana-Farber Cancer Institute, and the University of Utah.
Results
The survey was distributed to 427 physicians and nurse practitioners, with 191 responding (overall response rate of 45%). The majority of respondents were attending physicians, most of whom had over 10 years of practice in pediatric hematology/oncology (Table I).
Table I.
Characteristics of respondents
| Position | Number (%) |
|---|---|
| Attending physician | 117 (61) |
| Fellow | 40 (21) |
| Nurse practitioner | 34 (18) |
| Years in Practice | |
| 0–5 | 65 (34) |
| 5–10 | 40 (21) |
| 10+ | 86 (45) |
Participants were asked about their beliefs regarding the likely causes of leukemia in children. Their responses included genetics (92%), health status (e.g., stress, prenatal care and nutrition; 25%), environmental exposures (e.g., chemicals, contamination, second hand smoke, infections, and radiation; 78%), and none of the above (7%) (Table II). Open-ended responses varied, but commonly included: “all of the above,” previous exposures to chemotherapy agents, infertility treatments, and simply “bad luck.” A majority of respondents (61%) agreed that environmental exposures were important contributors to childhood cancer (mean Likert score 3.65).
Table II.
Percentage of respondents to selected survey questions
| Which of the following do you think are likely causes of leukemia in children? a | |
| Genetics | 92% |
| Health status | 25% |
| Environmental exposures | 78% |
| None of the above | 7% |
| Other | 13% |
|
| |
| In your opinion, are environmental exposures important contributors to childhood cancer? b | |
| Strongly agree | 11% |
| Agree | 50% |
| Neutral | 25% |
| Disagree | 13% |
| Strongly disagree | 2% |
|
| |
| How often, if ever, have you had a case that you suspected was related to something in the patient’s environment? b | |
| Frequently | 1% |
| Occasionally | 26% |
| Rarely | 50% |
| Never | 23% |
|
| |
| How often, if ever, have you received questions from parents or family members about potential workplace or environmental exposures as possible causes of disease? b | |
| Frequently | 48% |
| Occasionally | 40% |
| Rarely | 11% |
| Never | 1% |
|
| |
| What is your current level of comfort with discussing potential environmental sources of exposure in relation to disease with your patients and their family? b | |
| Very comfortable | 7% |
| Somewhat comfortable | 49% |
| Somewhat uncomfortable | 35% |
| Not at all comfortable | 9% |
Multiple responses were allowed,
Percentages may not be add up to exactly one hundred due to rounding.
When asked about their routine history-taking practices, the participants most frequently obtained information about parental occupations, household tobacco smoke, and radiation exposures (Figure 1). Nearly 25% reported not asking about any of the factors mentioned in the survey. Only 7% had reported ever receiving training in taking an environmental history.
Figure 1.
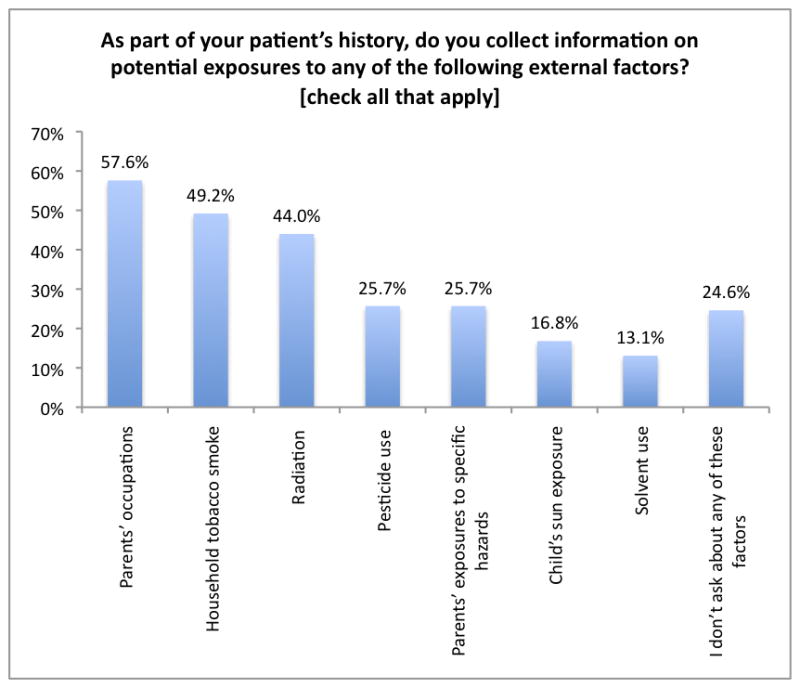
As part of your patient’s history, do you collect information on potential exposures to any of the following external factors?
Although half of the respondents reported rarely suspecting that a case was related to some factor in the patient’s environment, a large majority (88%) either “frequently” or “occasionally” received questions from parents or family members about potential workplace or environmental exposures contributing to their child’s disease. Forty four percent of respondents felt either “somewhat uncomfortable” or “not at all comfortable” discussing the disease implications of environmental exposures with patients and their families (Table II). An overwhelming majority (92%) stated they would find it helpful to have more information regarding the association between childhood cancers and environmental exposures in order to answer questions from parents, patients, or family members.
Respondents with 0–5 years in oncology practice were more likely to focus on genetics as a likely cause of childhood leukemia than practitioners with 5–10 and 10+ years of experience (98%, 85%, and 91%, respectively; p = 0.04). Clinicians with 10+ years in practice were more likely to ask about exposure to pesticides, solvents, and paternal exposures to specific environmental hazards (p = 0.01) (Table III). Responses also varied across types of position (Table IV). Eighty-five percent of nurse practitioners agreed or strongly agreed that environmental exposures were important contributors to cancer induction, with only one nurse practitioner disagreeing with this statement (mean Likert score 4.0). By contrast, only 58% of fellows and 55% of attending physicians agreed or strongly agreed (p = 0.03). However, nurse practitioners were also more likely to be uncomfortable with discussing potential sources of exposure in relation to disease with patients and family, compared to other positions (p < 0.001). Attending physicians were significantly more likely to ask about pesticide use, solvents, and parental occupation than other provider types while taking a patient’s history (p = 0.01, p = 0.01, and p = 0.001 respectively) (Table IV). Close to 25% of all respondents did not ask patients or their families about any of the queried factors associated with childhood cancers.
Table III.
Responses to selected survey questions stratified by years in practice
| Question | 0–5 years % (SD)a |
5–10 years % (SD) |
10+ years % (SD) |
P-Value |
|---|---|---|---|---|
|
| ||||
| Which of the following do you think are likely causes of leukemia in children? b | ||||
| □ Genetics | 98 (3) | 85 (11) | 91 (2) | 0.04 |
| □ Health status | 29 (11) | 18 (12) | 24 (9) | 0.40 |
| □ Environmental exposures | 77 (10) | 85 (11) | 76 (9) | 0.48 |
| □ Other | 6 (6) | 13 (10) | 17 (8) | 0.12 |
| □ None of the above | 3 (4) | 10 (9) | 8 (6) | 0.32 |
|
| ||||
| As part of your patient’s history, information is collected on potential exposures to which of the following external factors? b | ||||
| □ Household tobacco smoke | 46 (12) | 48 (15) | 52 (11) | 0.73 |
| □ Pesticide use | 15 (9) | 20 (12) | 36 (10) | 0.01 |
| □ Radiation | 35 (12) | 40 (15) | 52 (11) | 0.10 |
| □ Solvent use | 8 (6) | 5 (7) | 21 (9) | 0.01 |
| □ Child’s sun exposure | 14 (8) | 15 (11) | 20 (8) | 0.59 |
| □ Parents’ occupations | 48 (12) | 58 (15) | 65 (10) | 0.10 |
| □ Parents’ exposures to specific hazards | 17 (9) | 18 (12) | 36 (10) | 0.01 |
| □ I don’t ask about any of these factors | 25 (11) | 33 (14) | 21 (9) | 0.37 |
SD = standard deviation,
Multiple responses were allowed
Note: We conducted stratified analyses for all questions by years in practice, and found no statistically significant differences other than those reported above.
Table IV.
Responses to selected survey questions stratified by position
| Question | Attending Physician % (SD)a |
Fellow % (SD) |
Nurse Practitioner % (SD) |
P-Value |
|---|---|---|---|---|
|
| ||||
| In your opinion, are environmental exposures important contributors to childhood cancer? b | ||||
| □ Strongly Agree OR Agree | 55 (9) | 58 (15) | 85 (12) | 0.03 |
| □ Neutral | 29 (8) | 25 (13) | 12 (11) | |
| □ Strongly Disagree OR Disagree | 16 (7) | 18 (12) | 3 (6) | |
|
| ||||
| What is your current level of comfort with discussing potential environmental sources of exposure in relation to disease with your patients and their family? b | ||||
| □ Very comfortable | 11 (6) | 0 | 3 (6) | < 0.001 |
| □ Somewhat comfortable | 61 (9) | 40 (15) | 18 (13) | |
| □ Somewhat uncomfortable | 24 (8) | 43 (15) | 62 (16) | |
| □ Not at all comfortable | 4 (4) | 18 (12) | 18 (13) | |
|
| ||||
| As part of your patient’s history, information is collected on potential exposures to which of the following external factors? c | ||||
| □ Household tobacco smoke | 50 (9) | 48 (15) | 47 (4) | 0.91 |
| □ Pesticide use | 33 (9) | 13 (10) | 15 (12) | 0.01 |
| □ Radiation | 50 (9) | 30 (14) | 38 (16) | 0.06 |
| □ Solvent use | 19 (7) | 5 (7) | 3 (6) | 0.01 |
| □ Child’s sun exposure | 15 (6) | 13 (10) | 29 (15) | 0.09 |
| □ Parents’ occupations | 68 (8) | 35 (15) | 50 (17) | 0.001 |
| □ Parents’ exposures to specific hazards | 31 (8) | 13 (10) | 24 (14) | 0.07 |
| □ I don’t ask about any of these factors | 22 (8) | 25 (13) | 32 (16) | 0.48 |
|
| ||||
| Do you and your family do anything at home to avoid exposures to potential environmental hazards (e.g. pesticides, cleaning products, organic foods, plastics, etc.)? (yes vs. no) | ||||
| □ Yes | 70 (8) | 40 (15) | 85 (12) | < 0.001 |
SD = standard deviation,
Percentages may not be add up to exactly one hundred due to rounding,
Multiple responses were allowed.
Note: We conducted a stratified analysis for all questions by position, and found no statistically significant differences other than those reported above and in the text.
Sixty seven percent of all respondents engaged in at least one practice to protect themselves and their families from potentially hazardous environmental exposures (e.g., pesticides, cleaning products, organic foods, plastics, etc.). Fellows were significantly less likely to report participating in behaviors in their own home that might avoid exposures to chemicals associated with health risks than were nurse practitioners or attending physicians (p < 0.001) (Table IV). Those who attempted to avoid exposures at home were more likely to agree or strongly agree that environmental exposures are important contributors to the development of childhood leukemia (69%, SD: 8%, p = 0.001). Among those providers who attempted to avoid home exposures and also agreed or strongly agreed that environmental factors are important contributors to childhood cancers, only 53% were somewhat or very comfortable discussing these issues with their patients.
Discussion
This survey found that a majority of clinicians agreed that environmental exposures were important contributors to childhood cancers, but remained inconsistent in their history-taking for these events. Although most practitioners routinely received questions about the relationship between environmental exposures and disease, few were entirely comfortable addressing these issues. Over 90% of respondents believed they would benefit from more information on this topic.
Physician-patient communications
Previous surveys of the general public in the U.S. indicate widespread beliefs that the environment plays an important role in various health problems, and that parents would like more information from their pediatricians regarding environmental health topics. [45, 46] The findings of our survey support these previous results. Many respondents (48%) reported being frequently asked about environmental exposures to potential carcinogens by patients or their families. Greater familiarity with the emerging research on environmental contributions to childhood cancer would allow clinicians to be more responsive to these questions.
Providers with 5 or fewer years of experience were more likely to highlight genetics as a cause of childhood leukemia compared to providers with more experience, perhaps reflecting differences in curriculum and training that highlight more recent genetic studies. This group was also less likely to incorporate environmental health questions into their routine patient histories. Generational differences might account for this difference, as the more experienced clinicians were educated and trained during the height of the environmental movement, which could lead to greater awareness of environmental impacts on health. [47, 48]
Barriers to integration into practice
Our survey results indicate that clinical hematologists and oncologists engage in a variety of environmental history-taking practices. Many participants reported frequently asking about parental occupation, but not about any specific environmental hazards associated with that occupation. Anecdotal evidence and survey results suggest that clinicians have reservations about the appropriateness of asking the patients’ families questions related to environmental exposures and other carcinogens. One factor contributing to this perception may be the notion that clinicians do not have a major role in assessing etiology, and that such questions could raise the parents’ anxiety and guilt with little benefit to treatment outcomes. Though anticipation of negative parental reaction has been similarly cited by pediatricians as a common barrier to intervening with parents who smoke, the vast majority of smoking parents show strong support for addressing smoking at office visits. [49, 50]
Twenty-five percent of clinicians did not ask about any of the environmental factors mentioned in our survey, while 75% routinely asked at least some questions related to assessing environmental exposures. For both groups, a better grounding in the literature could ensure that responses to questions from patients and families regarding environmental hazards are addressed promptly and accurately. This assumption is supported by our result that providers uniformly believed that more information on environmental health research relevant to childhood cancer would be helpful.
Informing research agendas
Historically, alert clinicians have recognized environmental exposure trends in their patient populations, and brought them to the attention of public health authorities. This was seen in cases of mesothelioma and lung cancer in asbestos workers, and vaginal adenocarcinoma in women born to diethylstilbestrol-exposed mothers, among others. [51–53] Investigations of these cancer clusters led to the identification of previously unrecognized human carcinogens. By bringing an awareness of potential environmental etiologies to their oncology practice, clinicians can play an important role in raising issues in the research community and assisting investigators and public health officials in deciding potential areas of study.
Further illustration of this point can be seen in a recent case report, where the authors identified four cases of congenital fibrosarcoma linked to prenatal exposure to petroleum derivatives. [53] Through the use of a routine pediatric environmental health history questionnaire, the authors were able to compare case histories with toxicological databases and identify exposures in each case to compounds associated with the development of fibrosarcoma in animals. [53] Although this case series does not establish a causal association, it may form the hypothesis for a full-scale study.
Health professionals use peer-reviewed journals, consultations with peers, and conference attendance as their primary sources of reliable information for clinical decision-making. [54, 55] We reviewed abstracts from the 2011 and 2012 American Society of Pediatric Hematology/Oncology (ASPHO) meetings to select those that examined causation or environmental risk factors (characterized broadly to include factors like diet and infectious agents). Of 569 abstracts, 8% dealt with questions of causation, and only 1% mentioned any environmental risk factors. During the same 2-year period, a PubMed search showed that 48 papers were published specifically on the topic of childhood cancers and environmental risk factors. However, these papers appeared primarily in nonclinical journals, such as Environmental Health Perspectives, American Journal of Epidemiology, and Cancer Causes & Control. It is likely that nonclinical journals do not have a widespread readership among busy clinicians. [56]
We reviewed federal funding to assess the proportion of resources devoted to studying environmental causes of childhood cancers. During 2010 and 2011, the National Institutes of Health (NIH) awarded 3–7% of its total funding for childhood leukemia research to studies evaluating environmental etiologies1. The majority of funding for this research comes from the National Institute for Environmental Health Sciences (NIEHS). The National Cancer Institute (NCI) contributed around 1% of its funding for all childhood cancer toward environmental risk research. [57, 58] Despite an evolving understanding of environmental exposures associated with leukemia, funding for research that might inform activities aimed at prevention of childhood cancer remains limited.
Limitations
Though this is a relatively small survey, it is the first characterization of pediatric hematology-oncology practitioners’ current perception of the importance of environmental exposures to their patient care responsibilities. Surveys, as a research tool, have disadvantages with respect to the data collected and representativeness of the sample. In our survey design, participants were restricted to specific responses, limiting the potential details collected. Two questions allowed for open-ended responses, which increased the depth of our investigation.
Our survey response rate of 45% may introduce bias in our results, as no information about the non-responders was collected. For example, if those who had a higher concern for environmental associations were more likely to respond to the survey, these perspectives would have been overestimated in our sample. Email surveys of general practitioners have reported response rates similar to ours [59], however a study found response rates among specialty-fellows to be lower than non-specialists. [60] In general, email surveys have significantly lower response rates than mailed questionnaires. [61, 62] We aimed to increase the response rate and ensure more representativeness of the sample by sending a reminder email to participants. The survey was distributed to clinicians at selected institutions and does not comprise a representative sample of all practitioners in this field. This may affect the generalizability of our results if clinicians at these institutions differ substantially in their attitudes and practices from their counterparts across the country.
Conclusion
This survey identifies an opportunity for improved training in and awareness of environmental health research among attending physicians, fellows, and nurse practitioners working in the field of pediatric hematology/oncology. To help bridge this gap, environmental health researchers and epidemiologists should publish their relevant findings in journals that are widely read by pediatric hematologists/oncologists and nurse practitioners.
Educational opportunities should also be made available at national meetings, as our survey revealed significant interest in increasing baseline knowledge of environmental factors contributing to cancer. Training in environmental history-taking, introduction of basic environmental history questions into the electronic medical record, and implementation of self-administered patient questionnaires during medical visits that address environmental exposures may aid in simplifying the collection of relevant information in the medical records of children with cancer. Moreover, the self-administered questionnaire may address the hesitancy of some clinicians to ask these questions during history taking. Ultimately, having gained a better understanding of current research findings, practitioners will utilize this information in ways appropriate to their particular practice setting.
Our results highlight the need for better integration of environmental health awareness in pediatric oncology practice and training. The translation of rigorous environmental health research findings to clinicians would potentially improve provider-patient communications, enhance data collection, and promote the role of alert clinicians in identifying sentinel events. With the convergence of research from CLIC and other groups, the body of evidence supporting environmental associations with childhood cancers is growing. Hazards, such as those mentioned in the Introduction, have already gained a significant body of supporting information. As the environment continues to change and more hazards are identified, there is an ever-present need for pediatric oncologists to keep pace with this emerging research.
Acknowledgments
In memory of Pat Buffler who guided this work and made epidemiology important in our lifetime. She strongly motivated others to get involved and never saw a reason to fail. We would also like to acknowledge Yang Wang, Pagan Morris, and Sandra Luna-Fineman for their contributions.
Research reported in this publication was supported by the National Institutes of Health (NIEHS)/EPA award numbers 1P01ES018172-01 (NIEHS)/RD83451101 (USEPA). M. Miller and C. Zachek were also supported for this publication by the cooperative agreement award number 1 U61TS000238-01 from the Agency for Toxic Substances and Disease Registry (ATSDR). Its contents are the responsibility of the authors and do not necessarily represent the official views of the Agency for Toxic Substances and Disease Registry (ATSDR). Neither EPA nor ATSDR endorse the purchase of any commercial products or services mentioned in PEHSU publications.
Footnotes
A broad definition of environment was used to include factors such as diet and infection, together with more traditional factors. Only projects that focused on cancer as the primary endpoint were considered. Projects exclusively focusing on adult populations were excluded, as well as those that investigated mechanisms that might be broadly implicated in cancer development.
Conflict of Interest Statement
None of the authors has a conflict of interest.
References
- 1.National Cancer Institute. [Accessed February 27, 2013];A snapshot of pediatric cancers. 2012 Available at: http://www.cancer.gov/aboutnci/servingpeople/cancer-statistics/snapshots.
- 2.Howlader N, Noone AM, Karapcho M, et al., editors. National Cancer Institute. [Accessed February 27, 2013];SEER cancer statistics review, 1975–2008. Based on November 2010 SEER data submission. 2011 Available at: http://seer.cancer.gov/csr/1975_2008/
- 3.National Cancer Institute. [Accessed February 27, 2013];Fact sheet: Childhood cancers. 2008 Available at: http://www.cancer.gov/cancertopics/factsheet/Sites-Types/childhood.
- 4.President’s Cancer Panel. Reducing environmental cancer risk: What we can do now. Bethesda, MD: National Cancer Institute, President’s Cancer Panel; 2010. [Accessed February 27, 2013]. p. 240. Published April 2010. Available at: http://deainfo.nci.nih.gov/advisory/pcp/annualReports/pcp08-09rpt/PCP_Report_08-09_508.pdf. [Google Scholar]
- 5.Boice JD, Jr, Miller RW. Childhood and adult cancer after intrauterine exposure to ionizing radiation. Teratology. 1999;59(4):227–233. doi: 10.1002/(SICI)1096-9926(199904)59:4<227::AID-TERA7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 6.Chokkalingam AP, Bartley K, Wiemels JL, et al. Haplotypes of DNA repair and cell cycle control genes, X-ray exposure, and risk of childhood acute lymphoblastic leukemia. Cancer Causes Control. 2011;22(12):1721–1730. doi: 10.1007/s10552-011-9848-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Infante-Rivard C, Mathonnet G, Sinnett D. Risk of childhood leukemia associated with diagnostic irradiation and polymorphisms in DNA repair genes. Environ Health Perspect. 2000;108(6):495–498. doi: 10.1289/ehp.00108495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moysich KB, Menezes RJ, Michalek AM. Chernobyl-related ionising radiation exposure and cancer risk: an epidemiological review. Lancet Oncol. 2002;3(5):269–279. doi: 10.1016/s1470-2045(02)00727-1. [DOI] [PubMed] [Google Scholar]
- 9.Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol. 1997;70:130–139. doi: 10.1259/bjr.70.830.9135438. [DOI] [PubMed] [Google Scholar]
- 10.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds P, Von Behren J, Gunier RB, et al. Childhood cancer incidence rates and hazardous air pollutants in California: An exploratory analysis. Environ Health Perspect. 2003;111(4):663–668. doi: 10.1289/ehp.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scelo G, Metayer C, Zhang L, et al. Household exposure to paint and petroleum solvents, chromosomal translocations, and the risk of childhood leukemia. Environ Health Perspect. 2009;117(1):133–139. doi: 10.1289/ehp.11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steffen C, Auclerc MF, Auvrignon A, et al. Acute childhood leukaemia and environmental exposure to potential sources of benzene and other hydrocarbons; a case-control study. Occup Environ Med. 2004;61:773–778. doi: 10.1136/oem.2003.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinceti M, Rothman KJ, Crespi CM, et al. Leukemia risk in children exposed to benzene and PM10 from vehicular traffic: A case-control study in an Italian population. Eur J Epidemiol. 2012;27(10):781–790. doi: 10.1007/s10654-012-9727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward MH, Colt JS, Metayer C, et al. Residential exposure to polychlorinated biphenyls and organochlorine pesticides and risk of childhood leukemia. Environ Health Perspect. 2009;117(6):1007–1013. doi: 10.1289/ehp.0900583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner MC, Wigle DT, Krewski D. Residential pesticides and childhood leukemia: A systematic review and meta-analysis. Environ Health Perspect. 2010;118(1):33–41. doi: 10.1289/ehp.0900966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Infante-Rivard C, Labuda D, Krajinovic M, et al. Risk of childhood leukemia associated with exposure to pesticides and with gene polymorphisms. Epidemiology. 1999;10(5):481–487. [PubMed] [Google Scholar]
- 18.Infante-Rivard C, Weichenthal S. Pesticides and childhood cancer: An update of Zahm and Ward’s 1998 review. J Toxicol Environ Health B Crit Rev. 2007;10(1–2):81–99. doi: 10.1080/10937400601034589. [DOI] [PubMed] [Google Scholar]
- 19.Zahm SH, Ward MH. Pesticides and childhood cancer. Environ Health Perspect. 1998;106 (Suppl. 3):893–908. doi: 10.1289/ehp.98106893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rull RP, Gunier R, Von Behren J, et al. Residential proximity to agricultural pesticide applications and childhood acute lymphoblastic leukemia. Environ Res. 2009;109(7):891–899. doi: 10.1016/j.envres.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wigle DT, Turner MC, Krewski D. A systematic review and meta-analysis of childhood leukemia and parental occupational pesticide exposure. Environ Health Perspect. 2009;117:1505–1513. doi: 10.1289/ehp.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma X, Buffler PA, Gunier RB, et al. Critical windows of exposure to household pesticides and risk of childhood leukemia. Environ Health Perspect. 2002;110(9):955–960. doi: 10.1289/ehp.02110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wigle DT, Arbuckle TE, Turner MC, et al. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J Toxicol Environ Health B Crit Rev. 2008;11(5–6):373–517. doi: 10.1080/10937400801921320. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: A report of the surgeon general. Atlanta, GA: Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. Chapter Five: Reproductive and developmental effects from exposure to secondhand smoke; pp. 165–256. [Google Scholar]
- 25.Office of Environmental Health Hazard Assessment. Proposed identification of environmental tobacco smoke as a toxic air contaminant. Part B: Health effects. California Environmental Protection Agency; [Accessed February 27, 2013]. Published June 24, 2005. Available at: http://www.oehha.org/air/environmental_tobacco/pdf/app3partb2005.pdf. [Google Scholar]
- 26.Cogliano VJ, Baan R, Straif K, et al. Preventable exposures associated with human cancers. J Natl Cancer Inst. 2011;103(24):1827–3189. doi: 10.1093/jnci/djr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metayer C, Zhang L, Wiemels JL, et al. Tobacco smoke exposure and the risk of childhood acute lymphoblastic and myeloid leukemias by cytogenetic subtype. Cancer Epidemiol Biomarkers Prev. 2013;22(9):1600–1611. doi: 10.1158/1055-9965.EPI-13-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward MH, Colt JS, Metayer C, et al. Residential exposure to polychlorinated biphenyls and organochlorine pesticides and risk of childhood leukemia. Environ Health Perspect. 2009;117(6):1007–1013. doi: 10.1289/ehp.0900583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward MH, Colt JS, Deziel NC, et al. Residential Levels of Polybrominated Diphenyl Ethers and Risk of Childhood Acute Lymphoblastic Leukemia in California. Environ Health Perspect. 2014;122(10):1110–1116. doi: 10.1289/ehp.1307602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metayer C, Milne E, Clavel J, et al. The Childhood Leukemia International Consortium. Cancer Epidemiol. 2013;37(3):336–347. doi: 10.1016/j.canep.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown RC, Dwyer T, Kasten C, et al. Cohort profile: The International Childhood Cancer Cohort Consortium (I4C) Int J Epdemiol. 2007;36(4):724–730. doi: 10.1093/ije/dyl299. [DOI] [PubMed] [Google Scholar]
- 32.Greaves MF. Infection, immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer. 2006;6:193–203. doi: 10.1038/nrc1816. [DOI] [PubMed] [Google Scholar]
- 33.Urayama KY, Buffler PA, Gallagher ER, et al. A meta-analysis of the association between day-care attendance and childhood acute lymphoblastic leukaemia. Int J Epidemiol. 2010;9(3):718–732. doi: 10.1093/ije/dyp378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma X, Buffler PA, Wiemels JL, et al. Ethnic difference in daycare attendance, early infections, and risk of childhood acute lymphoblastic leukemia. Cancer Epidemiol Biomarkers Prev. 2005;14:1928–1934. doi: 10.1158/1055-9965.EPI-05-0115. [DOI] [PubMed] [Google Scholar]
- 35.Kilpatrick N, Frumkin H, Trowbridge J, et al. The environmental history in pediatric practice: A study of pediatricians’ attitudes, beliefs, and practices. Environ Health Perspect. 2002;110:823–827. doi: 10.1289/ehp.02110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trasande L, Newman N, Long L, et al. Translating knowledge about environmental health to practitioners: Are we doing enough? Mt Sinai J Med. 2010;77:114–123. doi: 10.1002/msj.20158. [DOI] [PubMed] [Google Scholar]
- 37.McCurdy LE, Roberts J, Rogers B, et al. Incorporating environmental health into pediatric medical and nursing education. Environ Health Perspect. 2004;112:1755–1760. doi: 10.1289/ehp.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Institute of Medicine. Role of the primary care physicians in occupational and environmental medicine. Washington, DC: National Academies Press; 1988. [PubMed] [Google Scholar]
- 39.Roberts JR, Gitterman BA. Pediatric environmental health education: A survey of US pediatric residency programs. Ambul Pediatr. 2003;3:57–59. doi: 10.1367/1539-4409(2003)003<0057:peheas>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Schenk M, Popp SM, Neale AV, et al. Environmental medicine content in medical school curricula. Acad Med. 1996;71:499–501. doi: 10.1097/00001888-199605000-00022. [DOI] [PubMed] [Google Scholar]
- 41.Bellack JP, Musham C, Hainer A, et al. Environmental health competencies: A survey of U.S. nurse practitioner programs. J Nurs Educ. 1996;35(2):74–81. doi: 10.3928/0148-4834-19960201-07. [DOI] [PubMed] [Google Scholar]
- 42.Wilborne-Davis P, Kirkland KH, Mulloy KB. A model for physician education and consultation in pediatric environmental health—the Pediatric Environmental Health Specialty Units (PEHSU) program. Pediatr Clin North Am. 2007;54:1–13. doi: 10.1016/j.pcl.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 43.National Environmental Education Foundation (NEEF) [Accessed July 8, 2013];Position statement: Health professionals and environmental health education. Published January 13, 2004. Available at: http://www.neefusa.org/pdf/PositionStatement.pdf.
- 44. [Accessed May 16, 2013];SurveyMonkey. Environmental exposures: Attitudes and practices in Pediatric Oncology survey. Published June 19, 2012. Available at: https://www.surveymonkey.com/s/Pediatric_Oncology_and_Environment_Survey.
- 45.Centers for Disease Control and Prevention. Public opinion about public health – United States, 1999. MMWR Morb Mortal Wkly Rep. 2003;49:258–260. [PubMed] [Google Scholar]
- 46.Stickler GB, Simmons PS. Pediatricians’ preferences for anticipatory guidance topics compared with parental anxieties. Clin Pediatr (Phila) 1995;34:384–387. doi: 10.1177/000992289503400709. [DOI] [PubMed] [Google Scholar]
- 47.Torgler B, García Valiñas MA, Macintyre A. Differences in preferences towards the environment: The impact of a gender, age and parental effect. [Accessed July 8, 2013];Nota di lavoro, Fondazione Eni Enrico Mattei: CCMP, Climate change modeling and policy. 2008 Available at: http://hdl.handle.net/10419/40684.
- 48.Iizuka M Economic Commission for Latin America and the Caribbean (ECLAC) [Accessed July 8, 2013];Role of environmental awareness in achieving sustainable development. Published November 23, 2000. Available at: http://www.eclac.org/publicaciones/xml/4/8824/lcr1961i.pdf.
- 49.Cluss PA, Moss D. Parent attitudes about pediatricians addressing parental smoking. Ambul Pediatr. 2002;2(6):485–488. doi: 10.1367/1539-4409(2002)002<0485:paapap>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 50.Moss D, Cluss PA, Mesiano M, et al. Accessing adult smokers in the pediatric setting: What do parents think? Nicotine Tob Res. 2006;8(1):67–75. doi: 10.1080/14622200500431809. [DOI] [PubMed] [Google Scholar]
- 51.Fowler PBS, Sloper JC, Warner EC. Exposure to asbestos and mesothelioma of the pleura. Br Med J. 1964;2(5403):211–213. doi: 10.1136/bmj.2.5403.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina: Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284(15):878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 53.Ortega-García JA, Soldin OP, López-Hernández FA, et al. Congenital fibrosarcoma and history of prenatal exposure to petroleum derivatives. Pediatrics. 2012;130(4):e1019–e1025. doi: 10.1542/peds.2011-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schein M, Paladugu R, Sutija VG, et al. What American surgeons read: A survey of a thousand Fellows of the American College of Surgeons. Current Surg. 2000;57(3):252–258. doi: 10.1016/s0149-7944(00)00177-x. [DOI] [PubMed] [Google Scholar]
- 55.Stinson ER, Mueller DA. Survey of health professionals’ information habits and needs. Conducted through personal interviews. JAMA. 1980;243(2):140–143. [PubMed] [Google Scholar]
- 56.McKibbon KA, Haynes RB, McKinlay RJ, et al. Which journals do primary care physicians and specialists access from an online service? J Med Libr Assoc. 2007;95(3):246–254. doi: 10.3163/1536-5050.95.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.U.S. Department of Health and Human Services. National Institutes of Health. National Cancer Institute. [Accessed January 28, 2013];NCI Funded Research Portfolio database. Available at: http://fundedresearch.cancer.gov/nciportfolio/
- 58.U.S. Department of Health and Human Services. National Institutes of Health. [Accessed May 18, 2013];Estimates of Funding for Various Research Condition, and Disease Categories (RCDC) Published April 10, 2013. Available at: http://report.nih.gov/categorical_spending.aspx.
- 59.Braithwaite D, Emery J, de Lusignan S, et al. Using the Internet to conduct surveys of health professionals: a valid alternative? Fam Pract. 2003;20:545–551. doi: 10.1093/fampra/cmg509. [DOI] [PubMed] [Google Scholar]
- 60.Cull WL, O’Connor KG, Sharp S, et al. Response rates and response bias for 50 surveys of pediatricians. Health Serv Res. 2005;40(1):213–226. doi: 10.1111/j.1475-6773.2005.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.VanGeest JB, Johnson TP, Welch VL. Methodologies for improving response rates in surveys of physicians: A systematic review. Eval Health Prof. 2007;30:303–321. doi: 10.1177/0163278707307899. [DOI] [PubMed] [Google Scholar]
- 62.McMahon SR, Iwamoto M, Massoudi MS, et al. Comparison of e-mail, fax, and postal surveys of pediatricians. Pediatrics. 2003;111:299–303. doi: 10.1542/peds.111.4.e299. [DOI] [PubMed] [Google Scholar]


