Abstract
Idiopathic pulmonary fibrosis (IPF) is a common, progressive and invariably lethal interstitial lung disease with no effective therapy. The key cell driving the development of fibrosis is the myofibroblast. Lipoxin (LX)A4 (LXA4) is an anti-inflammatory lipid, important in the resolution of inflammation and it has potential anti-fibrotic activity. However, the effects of LXA4 on primary human lung myofibroblasts (HLMF) have not previously been investigated. Therefore, the aim of this study was to examine the effects of LXA4 on TGFβ1-dependent responses in IPF- and non-fibrotic control (NFC)-derived HLMFs. HLMFs were isolated from IPF and NFC patients and grown in vitro. The effects of LXA4 on HLMF proliferation, collagen secretion, αSMA expression and Smad2/3 activation were examined constitutively and following TGFβ1 stimulation. The LXA4 receptor (ALXR) was expressed in both NFC- and IPF-derived HLMFs. LXA4 (10−10 M and 10−8 M) reduced constitutive αSMA expression, actin stress fiber formation, contraction and nuclear Smad2/3, indicating regression from a myofibroblast to fibroblast phenotype. LXA4 also significantly inhibited FBS-dependent proliferation, and TGFβ1-dependent collagen secretion, αSMA expression, and Smad2/3 nuclear translocation in IPF-derived HLMFs. LXA4 did not inhibit Smad2/3 phosphorylation. In summary, LXA4 attenuated pro-fibrotic HLMF activity and promoted HLMF regression to a quiescent fibroblast phenotype. LXA4 or its stable analogues delivered by aerosol may offer a novel approach to the treatment of IPF.
Keywords: Idiopathic Pulmonary Fibrosis (IPF), fibrosis, lung, myofibroblasts, Lipoxin A4, differentiation, Smad 2, Smad 3
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive disease with a median survival of only 3 years(1, 2). Patients present with breathlessness and disabling cough, progressing to respiratory failure and a distressful death. The cause is not known, but alveolar cell injury coupled with fibroblast/myofibroblast proliferation and activation are critical in the pathophysiology(3, 4). There is little effective treatment, and there is therefore an urgent unmet clinical need for novel modulators of lung fibrosis and tissue remodelling.
The key cell driving the development of fibrosis is the myofibroblast(5, 6). Myofibroblasts are intermediate in phenotype between fibroblasts and smooth muscle cells, expressing α-smooth muscle actin (αSMA) and exhibiting contractile activity, but they are also the principle cell responsible for the synthesis and deposition of the fibrotic matrix in IPF(7). The increased numbers of myofibroblasts found within IPF lungs occurs in part through the differentiation of resident fibroblasts(8); this involves reorganization of the actin cytoskeleton, increased expression of α-smooth muscle actin (αSMA) and incorporation of actin stress fibers(9), a process regulated by the TGFβ1/Smad pathway(10, 11). Furthermore, myofibroblasts derived from IPF lungs demonstrate enhanced proliferation(12), migration(13), collagen production(14), α-smooth muscle actin (αSMA) expression(15) and actin stress fibre formation(15). The myofibroblast is therefore a highly attractive target for the treatment of IPF. As myofibroblasts rarely persist in healthy lungs, their differentiation is considered a key event in the pathogenesis of IPF, and it is likely that a therapy capable of reversing this phenotypic change would be efficacious.
A family of lipid mediators known as lipoxins, resolvins, protectins and maresins (collectively called resolving mediators for convenience)(16-18), are important for the resolution of inflammation, and are generated soon after a tissue insult. Lipoxins are generated from membrane arachidonic acid through biochemical synthesis involving the enzymes 5- and 15-lipoxygenase (5-LOX, 15-LOX)(18). Several molecules are generated through transcellular synthesis with 15-LOX active in one cell such as an epithelial cell, and 5-LOX active in a second cell such as an inflammatory leukocyte. In addition, aspirin-triggered (AT) forms of these molecules exist (epi-lipoxins, AT-resolvins), where acetylated COX-2 generates the initial metabolite which is then modified further by 5-LOX. Several molecules can also be formed endogenously in the absence of aspirin, possibly via a cytochrome P450-dependent pathway(17), and 5-LOX-independent pathways for the production of protectins and maresins also exist with unicellular synthesis evident(18).
A key feature of these molecules is that they promote resolution of inflammation at low nM concentrations, but are not innately immunosuppressive as they also activate anti-bacterial mechanisms(17, 19). Lipoxin (LX)A4 also has anti-fibrotic activity in a number of model systems. For example, it inhibits PDGF-dependent TGFβ1 production and pro-fibrotic gene expression by renal mesangial cells(20), inhibits mesangial cell proliferation(21), and experimental renal fibrosis(22). LXA4 also inhibitions EMT in renal epithelial cells(23), while knockout of the 12/15-LOX pathway prevents experimental dermal fibrosis(24). With respect to lung fibrosis, LXA4 inhibited connective tissue growth factor-dependent proliferation of a human lung fibroblast cell line(25), while a stable epi-LXA4 analogue reduced bleomycin-induced pulmonary fibrosis in mice(26).
The effects of LXA4 on the function of healthy and IPF-derived primary human lung myofibroblast (HLMF) function are unknown. We hypothesized that LXA4 inhibits constitutive HLMF pro-fibrotic responses and TGFβ1-driven pro-fibrotic activity through the Smad signalling pathway. We therefore investigated the effects of LXA4 on constitutive and TGFβ1-dependent HLMF Smad 2/3 activity, gene transcription, and pro-fibrotic HLMF processes such as contraction, collagen secretion, proliferation and differentiation.
MATERIALS AND METHODS
Human lung myofibroblast isolation, characterisation and culture
Non-fibrotic control (NFC) HLMFs were derived from healthy areas of lung from patients undergoing lung resection for carcinoma at Glenfield Hospital, Leicester, UK. No morphological evidence of disease was found in the tissue samples used for HLMF isolation. IPF HLMFs were derived from patients undergoing lung transplant at the University of Pittsburgh Medical Center, USA, and were shown to have usual interstitial pneumonia (UIP) on histological examination. Myofibroblasts were grown, cultured and characterised as previously described(27). All NFC patients gave informed written consent and the study was approved by the National Research Ethics Service (references 07/MRE08/42 and 10/H0402/12). Written informed consent was also obtained from all IPF subjects, with the protocol approved by the University of Pittsburgh Institutional Review Board.
All cultures demonstrated the typical elongated spindle-shaped fibroblast morphology. All cultures underwent further extensive characterisation via flow cytometry and immunofluorescence. They were found to be predominantly a myofibroblast rich population (99% expressing αSMA) as described previously by us and others (15, 27-30). They expressed αSMA, CD90, fibroblast surface protein (FSP1), and collagen type 1. No contaminating cells were found. Immunostaining for macrophages (CD68), T-cells (CD3), and progenitor cells (CD34) was negative. There was no evidence of cobblestone shaped epithelial cells amongst the cultures. Results of this detailed characterisation have been shown previously(27, 30).
LXA4
LXA4 (5(S),6(R)-Lipoxin A4) (Cayman Chemicals, Michigan, USA) was used at concentrations of 10−10 M and 10−8 M.
Flow cytometry
HLMFs were grown in T25 flasks and serum-starved for 24 h prior to experiments. The myofibroblasts were incubated for 24 h and either left unstimulated or stimulated with TGFβ1 (10 ng/ml)(R&D Systems, Oxford, UK).
To determine the expression of the LXA4 receptor ALXR, HLMFs were detached using 0.1% trypsin/0.1% EDTA and gated using fibroblast surface antigen, Thy-1 (Merck, Hertforshire, UK). Myofibroblasts were then labelled with APC-conjugated mouse monoclonal anti-FPRL1 (Formyl-peptide receptor-like 1 [FRPL1], also known as ALXR)(R&D Systems) or APC-conjugated isotype control IgG2b antibody (R&D Systems).
To study the inhibitory effects of LXA4 on αSMA expression, HLMFs were incubated in the presence of serum-free medium alone or 0.1% ethanol vehicle control or LXA4 at 10−10 M and 10−8 M. Cells were detached using 0.1% trypsin/0.1% EDTA, washed, then fixed and permeabilized in 4% paraformaldehyde plus 0.1% saponin (Sigma-Aldrich, Poole, Dorset, UK) for 20 minutes on ice. Myofibroblasts were labelled with either: FITC-conjugated mouse monoclonal anti-αSMA (Sigma) or isotype control FITC-conjugated mouse IgG2a; secondary antibodies labelled with FITC were applied if appropriate. Analysis was performed using single colour flow cytometry on a FACScan (BD biosciences, Oxford, UK).
Immunofluorescence
HLMFs were grown on 8-well chamber slides and serum-starved for 24 h prior to the experiment. The cells were left unstimulated and incubated in the presence of 0.1% ethanol vehicle control or LXA4 at 10−10 M and 10−8 M for 48 hours. Cells were then immunostained as described previously(27) using FITC-conjugated mouse monoclonal anti-αSMA (F3777, 10 μg/ml, Sigma) and isotype control FITC-conjugated mouse IgG2a (X0933, 10 μg/ml, Dako, Ely, UK). Cells were mounted with fluorescent mounting medium and cover-slipped. Original images were captured on an epifluorescent microscope (Olympus BX50, Olympus UK Ltd, Southend-on-Sea); grey scale intensity was examined using Cell F imaging software (Olympus UK Ltd). Matched exposures were used for isotype controls.
Actin stress fibres were calculated using a specialised macro on image J designed by Dr Kees Straatman, University of Leicester(15). The macro is capable of providing a quantitative, unbiased score of the number of stress fibres per individual cell by determining the fluctuations of grey scale intensity created by the αSMA staining within the stress fibres.
Collagen Gel Contraction Assay
HLMFs were serum-starved for 24 h and then pre-treated for 24 h with serum-free medium alone, 0.1% ethanol control, LXA4 10−10 M or LXA4 10−8 M. Cells were detached and embedded in collagen gels as described previously (31). TGFβ1 was then added to appropriate wells to a final concentration of 10 ng/ml. Photographs were taken at 0 and 24 h. The surface area was measured at each time point using ImageJ software (http://rsbweb.nih.gov/ij/).
Smad2/3 nuclear localisation
HLMFs were grown on 8-well chamber slides and serum-starved for 24 h prior to the experiment. The cells were either unstimulated or stimulated with TGFβ1 (10 ng/ml) in the presence of serum-free medium alone, 0.1% ethanol control, LXA4 10−10 M or LXA4 10−8 M. After 1 h cells were immunostained using rabbit monoclonal anti-Smad2/3 (0.174 μg/ml, Cell Signalling). Secondary antibody labelled with FITC (F0313, Dako) was applied and the cells counterstained with DAPI (Sigma). Cells were mounted with fluorescent mounting medium and cover-slipped. Images were analysed as above. The intensity of nuclear Smad2/3 staining was quantified by measuring the grey scale intensity of DAPI positive nuclei to whole cell staining.
qRT-PCR
HLMF RNA was isolated using the RNeasy Plus Kit (Qiagen, West Sussex, UK) according to the manufacturer's instructions. Primers were designed for αSMA (ACT2A), forward TTCAATGTCCCAGCCATGTA and reverse GAAGGAATAGCCACGCTCAG, product size 222bp from NCB1 Reference sequence NM_001141945.1; Collagen type 1 (COL1A1), forward TTCTGCAACATGGAGACTGG and reverse CGCCATACTCGAACTGGAATC, Collagen type IV (COL4A1), forward GGACTACCTGGAACAAAAGGG and reverse GCCAAGTATCTCACCTGGATCA, product size 240bp from reference sequence NM_001845.4; β-actin primers were analysed using gene-specific Quantitect Primer Assay primers (Qiagen), HS_ACTB_1_SG. All expression data were normalized to β-actin and corrected using the reference dye ROX. Gene expression was quantified by real-time PCR using the Brilliant SYBR Green QRT-PCR 1-Step Master Mix (Strategene, The Netherlands). PCR products were run on a 1.5% agarose gel to confirm the product amplified was the correct size, and each of the products were sequenced to confirm the specificity of the primers.
Collagen Secretion Assay
HLMFs were cultured in serum-free medium alone or 0.1% ethanol control, and stimulated with TGFβ1 10 ng/ml in the presence of ethanol control, and LXA4 at 10−10 M or 10−8 M for 24 h. Soluble collagen released by HLMFs was quantified using the Sircol collagen assay (Biocolor, County Antrim, UK) according to the manufacturer's instructions (27, 32, 33).
Proliferation Assay
HLMFs were seeded into 6 well plates, and when 50% confluent cells were serum starved for 24 hours in serum-free medium. Cells were then stimulated with serum-free medium plus 0.1% ethanol, 10% FBS medium plus 0.1% ethanol, or 10% FBS plus LXA4 at 10−10 M and 10−8 M. After 48 hours cells were mobilized with 0.1% trypsin/0.1% EDTA and counted using a standard haemocytometer. Cell viability was assessed by Trypan blue exclusion. Results were counted by 2 blinded observers with high agreement (intra-class correlation of 0.969). All conditions were performed in duplicate.
Western blot for Smad proteins
HLMFs were grown in T75 flasks, serum-starved for 24 hours, and stimulated with TGFβ1 (10 ng/ml) in the presence of either serum-free medium alone, 0.1% ethanol control,LXA4 10−10 M or LXA4 10−8 M for 1 hour. HLMFs were detached with 0.1% trypsin/EDTA and washed. Protein was isolated using the RIPA buffer lysis system (Santa Cruz, Germany) and total protein concentration was determined using the DC Bio-Rad protein Assay (Bio-Rad, UK). 30 μg of protein was resolved using 10% Mini-Protean TGX precast gels (Bio-Rad) and then transferred to an immunobilon-P polyvinylidene difluoride membrane, using Trans-blot Turbo transfer packs (Bio-Rad). Membranes were blocked with 5% milk and incubated with rabbit monoclonal anti-phosphorylated-Smad2/3 (0.231 μg/ml, Cell Signalling) or rabbit monoclonal anti-Smad2/3 (0.0087 μg/ml, Cell Signalling). Protein bands were identified by horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence reagent (Amersham, UK). Immunolabelled proteins were visualized using ImageQuant LAS 4000 (GE Healthcare Life Sciences, UK).
Statistical Analysis
Experiments from an individual donor were performed either in duplicate or triplicate and a mean value was derived for each condition. Data distribution across donors was tested for normality using the Kolmogorov-Smirnov test. For parametric data the 1-way ANOVA or repeated measures ANOVA for across-group comparisons was used followed by the appropriate multiple comparison post hoc test; otherwise an unpaired or paired t-test was used. For non-parametric data the Friedman test was used for across group comparisons followed by the appropriate multiple comparison post hoc test, or the Wilcoxon signed rank test was used where there paired groups. GraphPad Prism for windows (version 6, GraphPad Software, San Diego California USA) was used for these analyses. A value of P<0.05 was taken to assume statistical significance and data are represented as mean (± SEM) or median (IQR).
RESULTS
Human lung myofibroblasts express LXA4 receptors
LXA4 acts via activation of the ALXR G-protein-coupled receptor (also known as FPR2, FPRL1, FPRH1, RFP, HM63), in low nM concentrations. The functions of this receptor however are cell-specific; in neutrophils LXA4-ALXR interactions inhibit migration but in monocytes these can stimulate chemotaxis(34).
NFC- and IPF-derived HLMFs expressed ALXR (FRPL1) with a whole population shift in comparison to the control antibody, P=0.0056, Mann-Whitney test (Figure 1A). There was a trend for a higher expression in the IPF-derived myofibroblasts, although this was not statistically significant and expression of ALXR did not change following 24 hours of TGFβ1 stimulation (Figure 1B). Thus HLMFs express LXA4 receptors.
Figure 1. HLMFs express ALXR.
(A) Representative histogram for ALXR staining assessed by flow cytometry in HLMFs obtained from an IPF donor. (B) Both NFC (n=3) and IPF-derived (n=3) HLMFs expressed ALXR constitutively, P=0.0056 (pooled data). ALXR expression was not increased following 24 hours of treatment with TGFβ1.
Constitutive αSMA expression and actin stress fibre formation is inhibited by LXA4
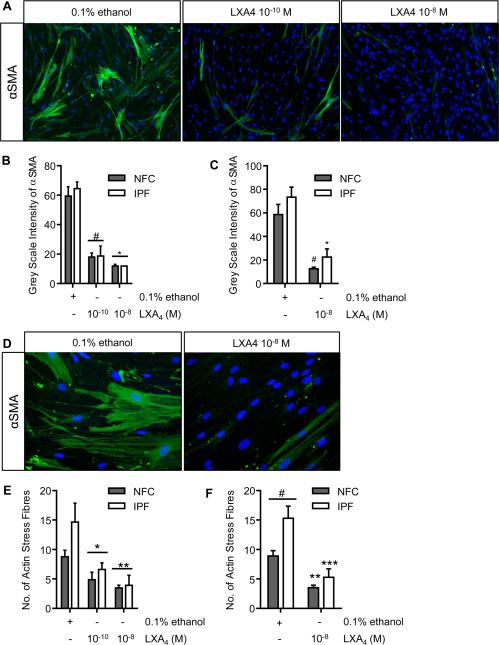
We and others have shown that fibroblasts obtained from human lung parenchyma express high quantities of αSMA and have the typical myofibroblast contractile phenotype(27, 29, 35, 36). Furthermore, these HLMFs derived from IPF donors express higher numbers of actin stress fibres constitutively than cells from NFC donors(15). After 48 hours of treatment with LXA4 (10−10 M and 10−8 M), the amount of constitutive αSMA staining was dose-dependently reduced (Figure 2A-C). Following treatment with LXA4 cells were less stellate and became more spindle like, a similar morphology to inactivated fibroblasts (Figure 2D). IPF-derived HLMFs again expressed more αSMA stress fibres at baseline in comparison to NFC-derived cells, P=0.0177. LXA4 reduced the number of actin stress fibres in both IPF- and NFC-derived cells and to a similar extent (Figure 2E and F).
Figure 2. Constitutive αSMA and stress fibre formation is attenuated by LXA4.
(A) Representative immunofluorescent images from a NFC donor showing the decrease in αSMA expression in HLMFs following incubation with LXA4 (10−10 M and 10−8 M). (B) αSMA expression was assessed by measuring grey scale intensity. LXA4 decreased αSMA expression at 10−10 M and 10−8 M in NFC-derived and IPF-derived HLMFs (n=6 and n=3 respectively, data pooled) (P<0.0001, repeated measures ANOVA, *p=0.0001 and **P<0.0001, corrected by Sidaks multiple comparison test). For each donor a minimum of 10 cells per field were measured and the mean taken. (C) In both NFC- (n=6) and IPF (n=6)-derived HLMFs αSMA was attenuated by LXA4 10−8 M (2 way ANOVA corrected by Sidaks multiple comparison test, NFC #P=0.0004 and IPF *P=0.0002). (D) Cells were noticeably more spindle like following treatment with LXA4; a representative image from an IPF donor is shown. (E) An Image J macro was used to quantify the number of actin filaments and a minimum of 10 cells from each donor was assessed and averaged. LXA4 decreased the number of actin filaments at both 10−10 M and 10−8 M in both NFC- and IPF-derived cells (n=6 and n=4 respectively, data pooled) (P=0.0003, repeated measures ANOVA, *P=0.0135 and **P=0.0005, corrected by Sidaks multiple comparison test). (F) IPF-derived HLMFs expressed increased numbers of smooth muscle actin filaments (stress fibres) in comparison to NFC-derived cells, #P=0.0177. Actin filaments in both NFC- (n=6) and IPF (n=6)-derived HLMFs were attenuated by LXA4 10−8 M (2 way ANOVA corrected by Sidaks multiple comparison test, NFC **P=0.0005 and IPF ***P<0.0001).
Constitutive HLMF contraction is inhibited by LXA4
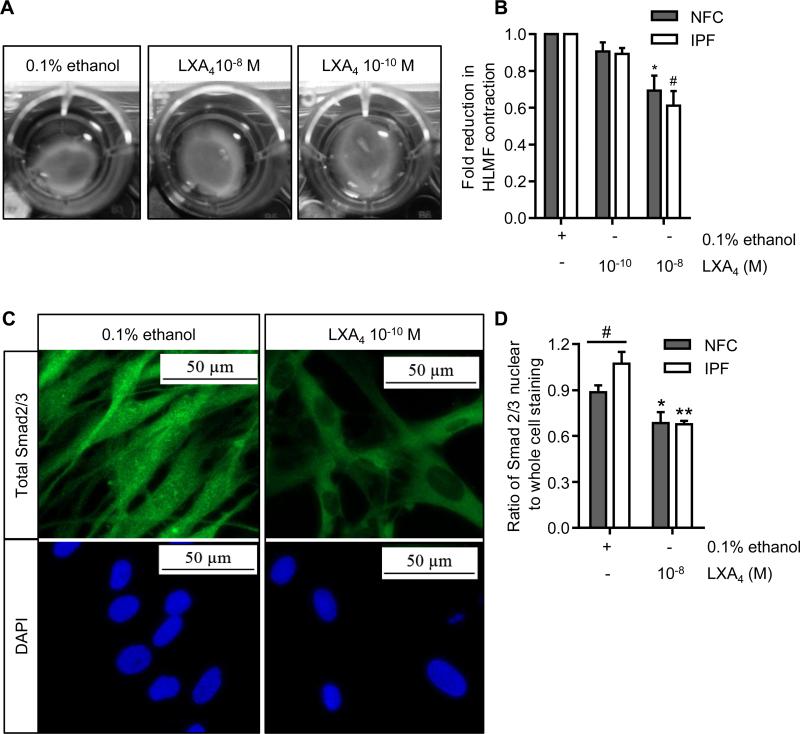
One of the main features of a myofibroblast is its ability to drive tissue repair and wound closure by reorganising the ECM via contraction(37). We therefore investigated the effects of LXA4 on constitutive HLMF contraction. HLMF contraction was examined over 24 h in the presence of LXA4 at 10−10 M and 10−8 M using collagen gels (Figure 3A). With both NFC- and IPF-derived HLMFs, basal contraction was dose-dependently reduced by LXA4 (P=0.0009 and P=0.0007 respectively, for LXA4 at 10−8 M) (Figure 3B).
Figure 3. Basal HLMF contraction and Smad2/3 nuclear translocation are inhibited by LXA4.
(A) Representative images from a NFC donor showing the spontaneous contraction of HLMFs within a collagen gel and its suppression by LXA4. (B) Constitutive HLMF contraction was significantly attenuated by LXA4 (10−8 M) in both NFC (n=6) and IPF-derived (n=5) cells (2 way ANOVA corrected by Sidaks multiple comparison test, NFC *P=0.0009 and IPF #P=0.0001). (C) Representative images from an IPF donor displaying total Smad2/3 expression within HLMFs. At baseline the HLMFs have staining both in the nucleus and within the cytoplasm, which is reduced upon incubation with LXA4 (10−8 M) for 1 hour. (D) The ratio of total Smad2/3 nuclear to whole cell staining was assessed by measuring grey scale intensity. IPF-derived (n=6) HLMFs demonstrated increased expression of total Smad2/3 within the nucleus in comparison to NFC donors (n=6), (#P=0.043, Mann Whitney U test). LXA4 (10−8 M) significantly attenuated the amount of Smad2/3 localised to the nucleus in both NFC- and IPF-derived cells (2 way ANOVA corrected by Sidaks multiple comparison test, NFC *P=0.0254 and IPF **P=0.0006).
Constitutive HLMF Smad2/3 nuclear translocation is inhibited by LXA4
It has previously been reported that αSMA expression and stress fibre formation in myofibroblasts is regulated in part by the TGFβ1/Smad signalling pathway(10, 11). As LXA4 inhibited both constitutive αSMA expression and HLMF contraction, we examined whether LXA4 disrupts constitutive Smad2/3 nuclear translocation using immunofluorescent staining. HLMFs had detectable Smad2/3 within the nucleus at baseline, and the IPF-derived HLMFs displayed a higher proportion of nuclear staining in comparison to the NFC-derived cells (P=0.043), in keeping with previous work(15). This nuclear Smad2/3 immunostaining was reduced and to a similar extent in both IPF- and NFC-derived HLMFs in the presence of LXA4 (10−8 M) for 1 h (Figure 3C and D).
TGFβ1-induced αSMA expression and contraction are inhibited by LXA4 in HLMFs
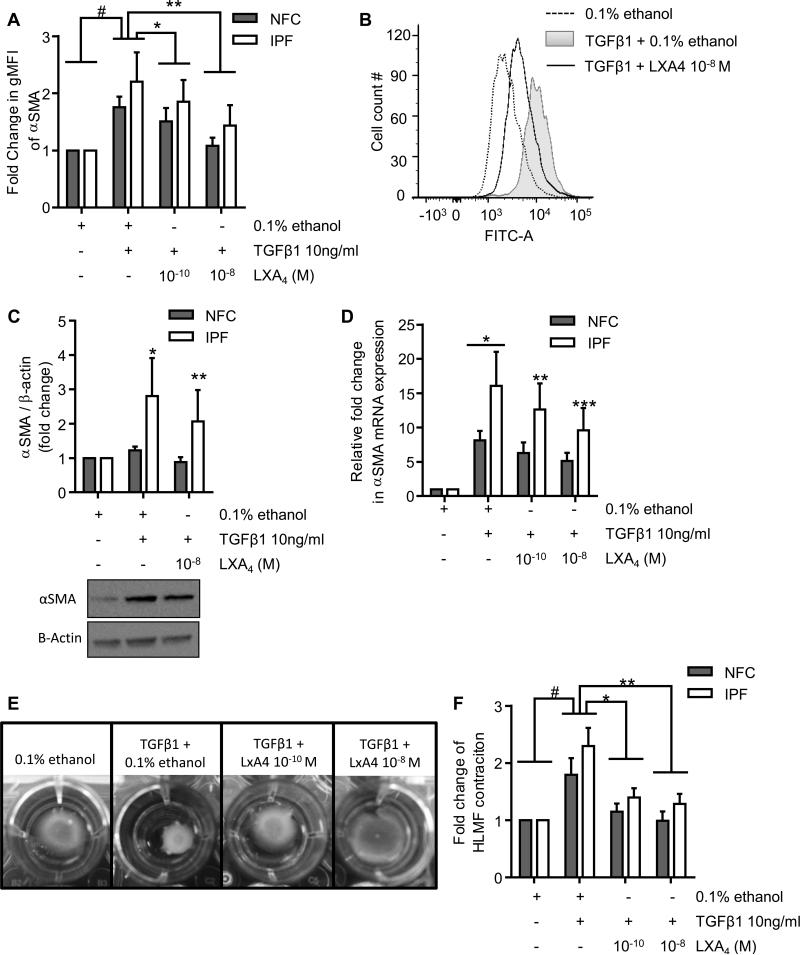
Next we investigated whether LXA4 inhibits TGFβ1-induced pro-fibrotic activity in HLMFs. Cells were treated with TGFβ1 in the presence of 0.1% ethanol control, or LXA4 at 10−10 M and 10−8 M, and flow cytometry was performed. In comparison to vehicle control, TGFβ1 stimulation significantly increased αSMA protein expression in both NFC- and IPF-derived HLMFs, P=0.0070 (Figure 4A). The TGFβ1-dependent increase in αSMA expression was dose-dependently inhibited in both NFC- and IPF-derived HLMFs by LXA4 at 10−10 M (P=0.0106) and 10−8 M (P=0.0076) (Figure 4A and B). This was also confirmed by Western blot analysis, where TGFβ1 increased αSMA expression in IPF-derived HLMFs but not NFC-derived cells, and which was significantly attenuated by LXA4 at 10−8 M (P=0.0274)(Figure 4C).
Figure 4. TGFβ1-dependent increases in αSMA mRNA and protein expression and HLMF contraction are attenuated by LXA4.
(A) Flow cytometry was used to assess the changes in αSMA expression following TGFβ1 (10 ng/ml) stimulation in the presence of LXA4, in both NFC- (n=4) and IPF-derived (n=3) HLMFs. αSMA expression in HLMFs was significantly increased following TGFβ1 stimulation, #P=0.0070 (paired t test). LXA4 significantly attenuated TGFβ1-dependent increases in αSMA expression at 10−10 M and 10−8 M (*P=0.011 and **P=0.008, respectively, Repeated measures ANOVA corrected by Sidaks multiple comparison test, statistics were performed on pooled data). (B) Representative fluorescent histogram showing αSMA expression in HLMFs under the above conditions.(C) Densitometry results of western blot analysis indicating the increased αSMA expression following TGFβ1 (10ng/ml) stimulation for 24 hours in IPF donors *P=0.0344 (paired t test). This increase was significantly attenuated by LXA4 10−8 M, **P=0.0274 (paired t test). No significant difference was found between NFC and IPF-derived HLMFS in terms of TGFβ1-stimulated αSMA expression (P=0.25).(D) qPCR results displaying the relative expression of αSMA in HLMFs following 24 hours of stimulation with TGFβ1 (10 ng/ml) in the presence of 0.1% ethanol or LXA4 at 10−10 M and 10−8 M. TGFβ1 significantly upregulated αSMA mRNA expression in both NFC- (n=6) and IPF-derived (n=5) HLMFs, #P=0.018 (one sample t test). IPF-derived HLMFs demonstrated a significantly greater increase in mRNA expression in comparison to NFC-derived cells, *P=0.0261. TGFβ1-dependent upregulation of αSMA mRNA expression was significantly inhibited in IPF-derived cells in the presence of LXA4 10−10 M and 10−8 M (**P=0.0479 and ***P=0.0033 respectively, 2 way ANOVA, corrected by Sidak's multiple comparison test). (E) Representative images from an IPF donor showing the TGFβ1-stimulated contraction of HLMFs within a collagen gel and its suppression by LXA4. (F) HLMFs from both NFC (n=6) and IPF (n=5) -derived donors displayed increased contraction following TGFβ1(10ng/ml) stimulation (#P=0.0026, One sample t test, pooled data). LXA4 significantly attenuated HLMF contraction in both NFC (n=6) and IPF (n=5) -derived myofibroblasts at 10−10 M and 10−8 M (P=0.0005, repeated measures ANOVA, *P=0.0057 and **P=0.0006, corrected by Dunn's multiple comparison test).
Similarly, TGFβ1-dependent stimulation increased αSMA mRNA expression in HLMFs, which was significantly greater in IPF-derived HLMFs compared to NFC-derived cells (P=0.0261). This TGFβ1-dependent αSMA mRNA expression was significantly reduced in IPF-derived HLMFs following treatment with LXA4 at 10−10 and 10−8 M (P=0.0479 and P=0.0033 respectively, 2 way ANOVA)(Figure 4D), but not NFC-derived cells.
To study the effects of LXA4 on TGFβ1-dependent HLMF contraction, HLMFs were cultured within collagen gels and their contraction monitored over 24 hours. Pictures were taken at 0 and 24 hours and the contraction measured using Image J software; representative images are displayed in Figure 4E. TGFβ1 stimulation increased HLMF contraction in comparison to vehicle control (0.1% ethanol)(P=0.0026), with no differences in response between NFC- and IPF-derived cells (Figure 4F). LXA4 significantly reduced TGFβ1-dependent HLMF contraction at both 10−10 M (P=0.0057) and 10−8 M (P=0.0006). Thus LXA4 not only inhibits constitutive HLMF αSMA expression and contraction, but also the increased αSMA expression and contraction induced by the potent pro-fibrotic mediator TGFβ1(38,39).
TGFβ1-induced collagen mRNA expression and collagen secretion is inhibited by LXA4
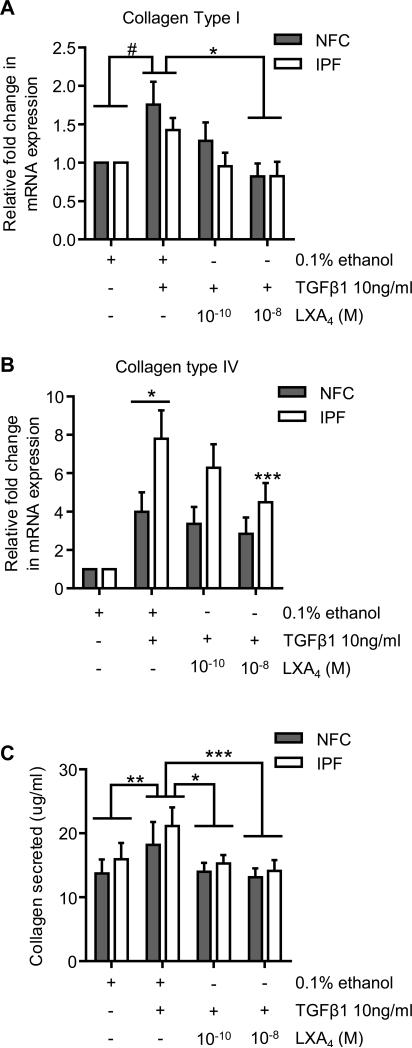
To further elucidate the inhibitory effects of LXA4 we investigated mRNA expression of collagen type I and collagen type IV, which are two of the most abundant collagens found within the IPF lungs (39, 40). Collagen type 1 mRNA expression was significantly increased by TGFβ1 in both NFC- and IPF-derived HLMFs compared to control, P=0.0064 (One sample t test, pooled data)(Figure 5A). This increase was significantly reduced by LXA4 at 10−8 M in both NFC- and IPF-derived cells (P=0.0057, 1 Way ANOVA corrected by Dunns multiple comparison test)(Figure 5A). Similarly, collagen type IV mRNA expression was significantly increased in both NFC- and IPF-derived HLMFs (P=0.0031 and P<0.0001, respectively, 2 way ANOVA corrected by Dunnetts multiple comparison test). IPF-derived HLMFs expressed significantly more collagen type IV mRNA following TGFβ1 stimulation in comparison to NFC-derived cells (P=0.0480, unpaired t test)(Figure 5B). LXA4 dose-dependently inhibited TGFβ1-induced collagen type IV mRNA expression in IPF-derived cells at 10−8 M (P=0.0039, 2 Way ANOVA corrected by Sidaks multiple comparison test).
Figure 5. LXA4 significantly decreases TGFβ1-dependent increases in collagen mRNA expression and collagen secretion.
(A) Collagen type I mRNA expression was significantly upregulated following 24 hours of TGFβ1 stimulation in NFC- and IPF-derived HLMFs (n=6 and n=5 respectively)(#P=0.0064, one sample t test on pooled data). LXA4 significantly reduced TGFβ1-dependent up regulation of collagen type I mRNA at 10−8 M (P=0.0214, 1 way ANOVA, *P=0.0057, corrected by Sidaks multiple comparison test). (B) Collagen type IV mRNA expression was significantly upregulated following 24 hours of TGFβ1 stimulation in NFC and IPF-derived HLMFs (n=7 and n=5 respectively)(P=0.0031 and P<0.0001, respectively, 2 Way ANOVA corrected by Sidaks multiple comparison test). The TGFβ1-dependent increase in collagen type IV mRNA expression was significantly greater in IPF-derived HLMFs in comparison to NFC-derived cells (*P=0.0480, unpaired t-test). The TGFβ1-dependent increase in collagen type IV mRNA expression in IPF HLMFs was significantly reduced by LXA4 at 10−8 M **P=0.0039, 2 Way ANOVA, corrected by Sidaks multiple test). (C) The amount of collagen secreted by both NFC- and IPF-derived HLMFs increased significantly following TGFβ1 (10ng/ml) for 24 hours, with no difference between NFC and IPF (**P=0.0022 on pooled data, NFC n=4 and IPF n=4). LXA4 significantly inhibited collagen secretion at both 10−10 and 10−8 M (*P=0.0015 and ***P=0.0002, 2-way ANOVA, pooled data).
The ability of LXA4 to downregulate TGFβ1-increased collagen mRNA was paralleled by a reduction in total collagen secretion measured using the Sircol Assay. TGFβ1 significantly increased the amount of total collagen secreted by NFC- and IPF-derived cells (P=0.0022), which was dose-dependently inhibited by LXA4 (10−10 M, P=0.0015; 10−8 M, P=0.0002)(Figure 5C).
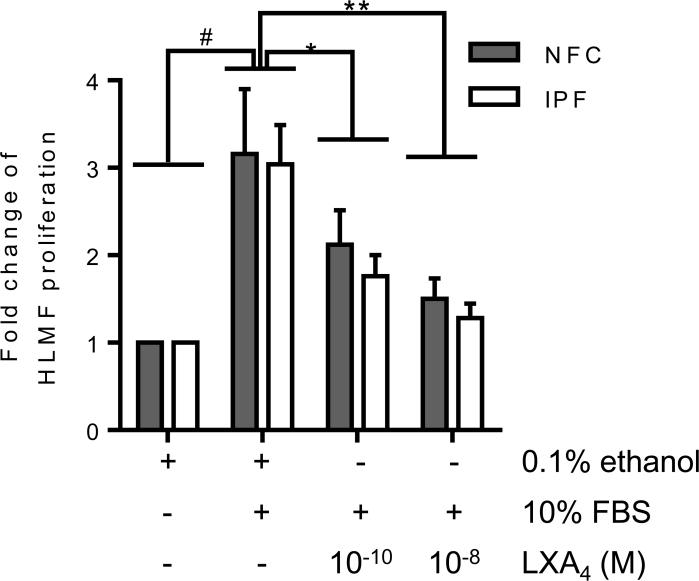
FBS-induced HLMF proliferation is attenuated by LXA4
HLMF proliferation was assessed after 48 hours of stimulation with 10% FBS which significantly increased proliferation (P=0.0006)(Figure 6). In both NFC- and IPF-derived HLMFs, FBS-dependent proliferation was significantly decreased by LXA4 at 10−10 M (P=0.0001) and 10−8 M (P=0.001), (Figure 6). In summary, FBS-induced HLMF proliferation is attenuated by treatment with LXA4.
Figure 6. FBS-dependent HLMF proliferation is inhibited by LXA4.
HLMF proliferation was increased following 48 hours of stimulation with FBS and to a similar extent in both NFC- and IPF-derived HLMFs (#P=0.0006, one-sample t test, pooled date, NFC n=5 and IPF n=5). This increase in HLMF proliferation was significantly reduced with LXA4 at both 10−10 M and 10−8 M (P=0.0001, repeated measures ANOVA, *P=0.0001 and **P=0.001 respectively, corrected by Dunns multiple comparison test).
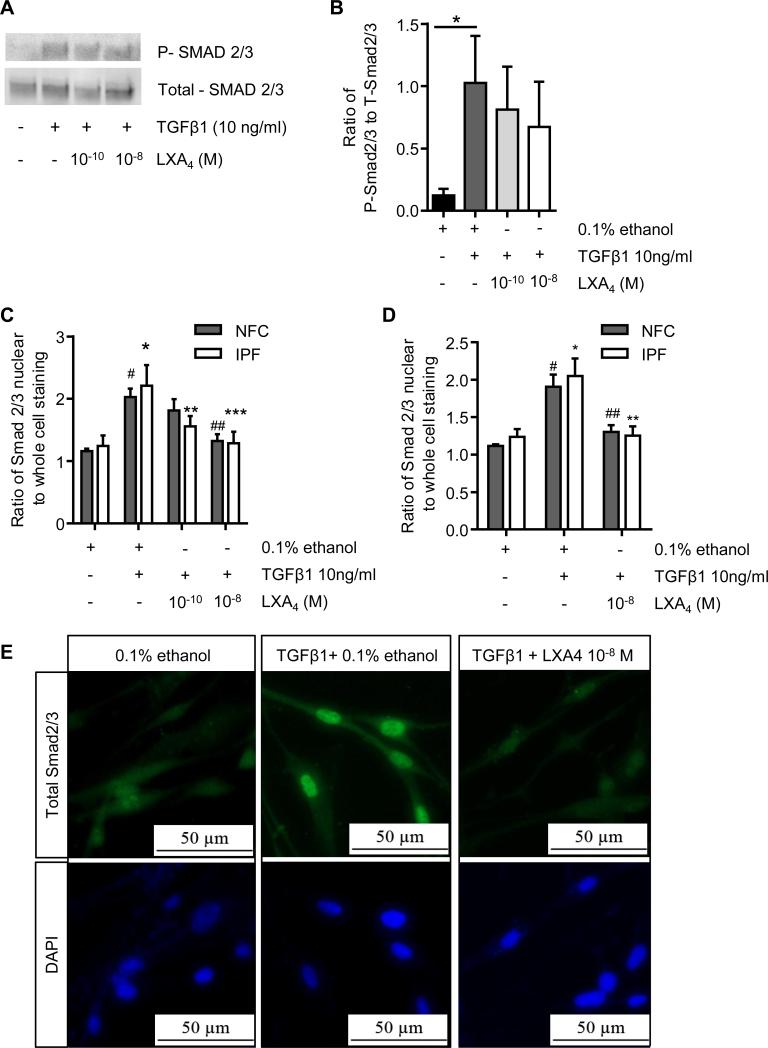
TGFβ1-dependent Smad2/3 nuclear translocation is disrupted by LXA4
The ability of LXA4 to attenuate both constitutive Smad2/3 nuclear localisation and several TGFβ1-dependent cell processes in HLMFs suggested that LXA4 may also attenuate TGFβ1-dependent Smad2/3 nuclear translocation. Phosphorylation is a key initial event in the activation of these Smad proteins. Using Western blot analysis we therefore investigated the effect of LXA4 on TGFβ1-induced phosphorylation of Smad2/3 and the expression of total Smad2/3 in HLMFs. As previously reported in many studies, TGFβ1 significantly increased Smad2/3 phosphorylation in HLMFs (P=0.0110). However, LXA4 at 10−10 M and 10−8 M had no significant effect on the TGFβ1–dependent increase in Smad2/3 phosphorylation in either NFC- or IPF-derived HLMFs (Figure 7A and B).
Figure 7. LXA4 does alter Smad2/3 phosphorylation but exerts it effect through inhibition of Smad2/3 translocation to the nucleus.
(A and B) Using Western blot analysis we investigated the effect of LXA4 on TGFβ1-induced phosphorylation of Smad2/3; a representative image from a NFC donor is shown. TGFβ1 significantly increased Smad2/3 phosphorylation in HLMFs (P=0.0110, NFC n=2 and IPF n=3). However, LXA4 at 10−10 M and 10−8 M had no significant effect on the TGFβ1–dependent increase in Smad2/3 phosphorylation in either NFC- or IPF-derived HLMFs. (C) Immunofluorescent analysis of total Smad2/3 nuclear translocation showed a significant increase in both NFC(n=5) and IPF(n=4) HLMFs (#P=0.0007 and *P=0.0007 respectively). This TGFβ1-dependent increase in total nuclear translocation was significantly and dose-dependently attenuated by LXA4 (2 way ANOVA corrected by Sidaks multiple comparison test, NFC 10−8 M ##P=0.0051 and IPF 10−10 M **P=0.0208 and 10−8 M ***P=0.0011). (D) In both NFC- (n=6) and IPF- (n=6) derived HLMFs TGFβ1-dependent total Smad2/3 nuclear translocation was attenuated by LXA4 10−8 M (2 way ANOVA corrected by Sidaks multiple comparison test, NFC ##P=0.00037 and IPF **P=0.0003. (E) Representative immunofluorescent images from an NFC donor demonstrating the increased nuclear translocation of total Smad2/3 following TGFβ1 stimulation and the inhibition of this by LXA4 (10−8 M).
Once TGFβ1 has induced phosphorylation of Smad2/3, they interact with Smad4, and this complex translocates to the nucleus to initiate gene transcription. Although Smad2/3 phosphorylation was not inhibited, TGFβ1-dependent Smad2/3 nuclear translocation was significantly attenuated by LXA4 (Figure 7C, D and E). Thus LXA4 appears to significantly disrupt TGFβ1-dependent nuclear translocation of Smad2/3, but not Smad2/3 phosphorylation.
DISCUSSION
The myofibroblast is implicated as the key cell driving the progression of IPF through the synthesis of excess fibrotic extracellular matrix and tissue contraction. The myofibroblast is therefore an attractive target for novel anti-fibrotic therapies. Here we have demonstrated that HLMFs express LXA4 receptors, and that LXA4 attenuates i) constitutive HLMF αSMA expression, stress fibre formation, contraction and Smad2/3 nuclear localisation, ii) TGFβ1-dependent increases in HLMF αSMA expression, contraction, collagen mRNA expression, collagen secretion, and Smad2/3 nuclear localisation, and iii) serum-dependent HLMF proliferation.
Fibroblasts and myofibroblasts demonstrate marked functional and phenotypic heterogeneity between tissues and across species. As an example, there is a marked difference in the phenotype of fibroblasts grown from human airway and lung parenchyma, with those from lung spontaneously displaying a myofibroblast phenotype(29, 35, 36). When considering novel therapeutic targets, it is therefore important to study cells from the species, tissue compartment and disease of interest. Expression of the LXA4 receptor ALXR (FRLP1) has been demonstrated previously in human synovial fibroblasts by RT-PCR(41), and these cells and rat fibroblast cell lines respond to LXA4 implying functional receptor expression (22-41). Here we have demonstrated that ALXR protein is expressed on the surface of the majority of parenchymal HLMFs derived from both NFC and IPF tissue.
In a rat fibroblast cell line LXA4 at 10−9 M attenuated TGFβ1-dependent Smad2 phosphorylation (but not Smad3 phosphorylation) and MAP kinase activation, and inhibited TGFβ1-dependent gene transcription (22). We also found that LXA4 inhibited TGFβ1-dependent gene transcription, but in contrast to Börgeson and colleagues, we could not detect inhibition of Smad2/3 phosphorylation, although there was reduced TGFβ1-dependent nuclear Smad2/3 translocation. The means by which LXA4 would prevent nuclear translocation of activated Smads without affecting phosphorylation is intriguing and will require further work to elucidate the mechanism. Nevertheless, the ability of LXA4 to inhibit Smad nuclear localisation is in keeping with its ability to inhibit TGFβ1-dependent gene transcription, and the downstream production of collagen and αSMA in HLMFs.
Several phenotypic differences have been observed previously between control and IPF-derived HLMFs in culture, suggesting that there may be genetic and/or epigenetic changes that promote a pro-fibrotic HLMF phenotype in patients who develop IPF. Interestingly, we found that LXA4 not only reduced TGFβ1-dependent pro-fibrotic responses, but promoted HLMF differentiation towards a fibroblast phenotype by reducing constitutive αSMA actin expression and actin stress fibre formation. This in turn is likely to explain the reduced constitutive HLMF contraction evident in collagen gels. This occurred in conjunction with a reduction in nuclear Smad2/3 immunostaining which occurred within 1 hour of LXA4 exposure, suggesting there is a component of constitutive Smad2/3 signalling in HLMFs at rest. This ability of LXA4 to promote de-differentiation towards a fibroblast phenotype suggests that if there is a degree of “constitutive” pro-fibrotic HLMF activity in IPF lungs due to genetic/epigenetic factors, LXA4 or a stable analogue might be a particularly useful therapy.
In addition to the inhibition of TGFβ1-dependent stimulation, we found that LXA4 also inhibited HLMF proliferation induced by serum, and this is in keeping with previous work showing that LXA4, inhibited CTGF-dependent proliferation of a human fibroblast cell line(25). Previous studies using LXA4 in cell culture systems suggest it is highly active in the nM range. Our data are consistent with this, with effects readily evident in HLMFs at 10−10 M (0.1 nM), although more consistent at 10−8 M.
In summary we have shown that LXA4 inhibits many TGFβ1-dependent pro-fibrotic responses in healthy and IPF-derived HLMFs, which may result from the inhibition of Smad2/3 nuclear translocation. Furthermore, LXA4 promotes HLMF de-differentiation in the resting state, suggesting it may have the potential to reverse the fibrotic process. In support of this, and of particular relevance to this study, a stable epi-LXA4 analogue markedly inhibited bleomycin-induced pulmonary fibrosis in mice when administered either preventively or curatively, with reversal of fibrosis evident in the latter (26). This was associated with a reduction in the accumulation and differentiation of myofibroblasts in the lung parenchyma. Thus there are consistent in vitro and in vivo data from primary HLMFs and the mouse bleomycin model respectively, indicating that LXA4 or its stable analogues may not only prevent the progression of lung fibrosis, but potentially reverse it. This indicates that clinical trials of LXA4 analogues should be considered in patients with IPF.
Acknowledgments
Funding: This work was supported by Medical Research Council, project grant number MR/K018213/1. The work was also supported in part by the National Institute for Health Research Leicester Respiratory Biomedical Research Unit, and the United States National Institutes of Health NIAMS grant P30 AR061271 and K24 AR060297. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR the Department of Health or the NIH.
Footnotes
Competing interests: None
References
- 1.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 2.Gribbin J, Hubbard RB, Le Jeune I, Smith CJ, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006;61:980–985. doi: 10.1136/thx.2006.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strieter RM. Pathogenesis and natural history of usual interstitial pneumonia: the whole story or the last chapter of a long novel. Chest. 2005;128:526S–532S. doi: 10.1378/chest.128.5_suppl_1.526S. [DOI] [PubMed] [Google Scholar]
- 4.King TE, Jr., Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 5.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 6.Gharaee-Kermani M, Hu B, Phan SH, Gyetko MR. Recent Advances in Molecular Targets and Treatment of Idiopathic Pulmonary Fibrosis: Focus on TGF beta Signaling and the Myofibroblast. Curr. Med. Chem. 2009;16:1400–1417. doi: 10.2174/092986709787846497. [DOI] [PubMed] [Google Scholar]
- 7.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am. J. Pathol. 1994;145:114–125. [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders YY, Kumbla P, Hagood JS. Enhanced myofibroblastic differentiation and survival in Thy-1(-) lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2007;36:226–235. doi: 10.1165/rcmb.2006-0178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J. Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am. J. Respir. Cell. Mol. Biol. 2003;29 doi: 10.1165/rcmb.2003-0063OC. [DOI] [PubMed] [Google Scholar]
- 11.Gu L, Zhu Y, Yang X, Guo Z, Xu W, Tian X. Effect of TGF-beta/Smad signaling pathway on lung myofibroblast differentiation. Acta Pharmacol. Sin. 2007;28:382–391. doi: 10.1111/j.1745-7254.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 12.Jordana M, Befus AD, Newhouse MT, Bienenstock J, Gauldie J. Effect of Histamine on Proliferation of Normal Human Adult Lung Fibroblasts. Thorax. 1988;43:552–558. doi: 10.1136/thx.43.7.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suganuma H, Sato A, Tamura R, Chida K. Enhanced migration of fibroblasts derived from lungs with fibrotic lesions. Thorax. 1995;50:984–989. doi: 10.1136/thx.50.9.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoda H, Yokoyama A, Nishino R, Nakashima T, Ishikawa N, Haruta Y, Hattori N, Naka T, Kohno N. Overproduction of collagen and diminished SOCS1 expression are causally linked in fibroblasts from idiopathic pulmonary fibrosis. Biochem. Biophys. Res. Commun. 2007;353:1004–1010. doi: 10.1016/j.bbrc.2006.12.128. [DOI] [PubMed] [Google Scholar]
- 15.Roach KM, Wulff H, Feghali-Bostwick C, Amrani Y, Bradding P. Increased constitutive αSMA and Smad2/3 expression in idiopathic pulmonary fibrosis myofibroblasts is KCa3.1-dependent. Respiratory Research. 2014;15 doi: 10.1186/s12931-014-0155-5. doi:10.1186/s12931-014-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serhan CN. Systems approach to inflammation resolution: identification of novel anti-inflammatory and pro-resolving mediators. Journal of Thrombosis and Haemostasis. 2009;7:44–48. doi: 10.1111/j.1538-7836.2009.03396.x. [DOI] [PubMed] [Google Scholar]
- 17.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature Reviews Immunology. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids. 2010;1801:1260–1273. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodgers K, McMahon B, Mitchell D, Sadlier D, Godson C. Lipoxin A(4) modifies platelet-derived growth factor-induced profibrotic gene expression in human renal mesangial cells. Am. J. Pathol. 2005;167:683–694. doi: 10.1016/S0002-9440(10)62043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell D, Rodgers K, Hanly J, McMahon B, Brady HR, Martin F, Godson C. Lipoxins inhibit Akt/PKB activation and cell cycle progression in human mesangial cells. Am. J. Pathol. 2004;164:937–946. doi: 10.1016/S0002-9440(10)63181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boergeson E, Docherty NG, Murphy M, Rodgers K, Ryan A, O'Sullivan TP, Guiry PJ, Goldschmeding R, Higgins DF, Godson C. Lipoxin A(4) and benzo lipoxin A(4) attenuate experimental renal fibrosis. Faseb Journal. 2011;25:2967–2979. doi: 10.1096/fj.11-185017. [DOI] [PubMed] [Google Scholar]
- 23.Wu S, Zhang Y, Tao H, Dong L. Lipoxin A(4) Inhibits Transition of Epithelial to Mesenchymal Cells in Proximal Tubules. Am. J. Nephrol. 2010;32:122–136. doi: 10.1159/000315121. [DOI] [PubMed] [Google Scholar]
- 24.Kroenke G, Reich N, Scholtysek C, Akhmetshina A, Uderhardt S, Zerr P, Palumbo K, Lang V, Dees C, Distler O, Schett G, Distler JHW. The 12/15-lipoxygenase pathway counteracts fibroblast activation and experimental fibrosis. Ann. Rheum. Dis. 2012;71:1081–1087. doi: 10.1136/annrheumdis-2011-200745. [DOI] [PubMed] [Google Scholar]
- 25.Wu SH, Wu XH, Lu C, Dong L, Chen ZQ. Lipoxin A(4) inhibits proliferation of human lung fibroblasts induced by connective tissue growth factor. American Journal of Respiratory Cell and Molecular Biology. 2006;34:65–72. doi: 10.1165/rcmb.2005-0184OC. [DOI] [PubMed] [Google Scholar]
- 26.Martins V, Valenca SS, Farias-Filho FA, Molinaro R, Simoes RL, Ferreira TPT, Silva P. M. R. e., Hogaboam CM, Kunkel SL, Fierro IM, Canetti C, Benjamim CF. ATLa, an Aspirin-Triggered Lipoxin A(4) Synthetic Analog, Prevents the Inflammatory and Fibrotic Effects of Bleomycin-Induced Pulmonary Fibrosis. Journal of Immunology. 2009;182:5374–5381. doi: 10.4049/jimmunol.0802259. [DOI] [PubMed] [Google Scholar]
- 27.Roach K, Duffy S, Coward W, Feghali-Bostwick C, Wulff H, Bradding P. The K+ Channel KCa3.1 as a Novel Target for Idiopathic Pulmonary Fibrosis. PLoS ONE. 2013;8:e85244. doi: 10.1371/journal.pone.0085244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roach KM, Feghali-Bostwick C, Wulff H, Amrani Y, Bradding P. Human lung myofibroblast TGFbeta1-dependent Smad2/3 signalling is Ca(2+)-dependent and regulated by KCa3.1 K(+) channels. Fibrogenesis Tissue Repair. 2015;8 doi: 10.1186/s13069-015-0022-0. 5-015-0022-0. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou X, Wu W, Hu H, Milosevic J, Konishi K, Kaminski N, Wenzel SE. Genomic Differences Distinguish the Myofibroblast Phenotype of Distal Lung from Airway Fibroblasts. Am. J. Respir. Cell Mol. Biol. 2011 doi: 10.1165/rcmb.2011-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moiseeva EP, Roach K, Leyland ML, Bradding P. The Adhesion Receptor Cadm1 on Mast Cells Mediates Adhesion to Lung Fibroblasts and Smooth Muscle. Thorax. 2011;66:A18–A18. [Google Scholar]
- 31.Woodman L, Siddiqui S, Cruse G, Sutcliffe A, Saunders R, Kaur D, Bradding P, Brightling C. Mast Cells Promote Airway Smooth Muscle Cell Differentiation via Autocrine Up-Regulation of TGF-β1. The Journal of Immunology. 2008;181:5001–5007. doi: 10.4049/jimmunol.181.7.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang M, Sharma S, Zhu LX, Keane MP, Luo J, Zhang L, Burdick MD, Lin YQ, Dohadwala M, Gardner B, Batra RK, Strieter RM, Dubinett SM. IL-7 inhibits fibroblast TGF-beta production and signaling in pulmonary fibrosis. J. Clin. Invest. 2002;109:931–937. doi: 10.1172/JCI14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Distler JHW, Jüngel A, Huber LC, Schulze-Horsel U, Zwerina J, Gay RE, Michel BA, Hauser T, Schett G, Gay S, Distler O. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis & Rheumatism. 2007;56:311–322. doi: 10.1002/art.22314. [DOI] [PubMed] [Google Scholar]
- 34.Chiang N, Serhan CN, Dahlen S, Drazen JM, Hay DWP, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: Potent ligand-specific and stereoselective actions in vivo. Pharmacol. Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 35.Pechkovsky DV, Hackett TL, An SS, Shaheen F, Murray LA, Knight DA. Human Lung Parenchyma but Not Proximal Bronchi Produces Fibroblasts with Enhanced TGF-{beta} Signaling and {alpha}-SMA Expression. Am. J. Respir. Cell Mol. Biol. 2010;43:641–651. doi: 10.1165/rcmb.2009-0318OC. [DOI] [PubMed] [Google Scholar]
- 36.Kotaru C, Schoonover KJ, Trudeau JB, Huynh ML, Zhou X, Hu H, Wenzel SE. Regional fibroblast heterogeneity in the lung: implications for remodeling. Am. J. Respir. Crit. Care Med. 2006;173:1208–1215. doi: 10.1164/rccm.200508-1218OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomasek J, Gabbiani G, Hinz B, Chaponnier C, Brown R. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature Reviews Molecular Cell Biology. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa T, Nakao A, Sumiyoshi K, Tsuchihashi H, Ogawa H. SB-431542 inhibits TGF-β-induced contraction of collagen gel by normal and keloid fibroblasts. J. Dermatol. Sci. 2005;39:33–38. doi: 10.1016/j.jdermsci.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Raghu G, Masta S, Meyers D, Narayanan AS. Collagen Synthesis by Normal and Fibrotic Human Lung Fibroblasts and the Effect of Transforming Growth Factor-β. American Journal of Respiratory and Critical Care Medicine. 1989;140:95–100. doi: 10.1164/ajrccm/140.1.95. [DOI] [PubMed] [Google Scholar]
- 40.Selman M, Pardo A, Barrera L, Estrada A, Watson SR, Wilson K, Aziz N, Kaminski N, Zlotnik A. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. American Journal of Respiratory and Critical Care Medicine. 2006;173:188–198. doi: 10.1164/rccm.200504-644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sodin-Semrl S, Taddeo B, Tseng D, Varga J, Fiore S. Lipoxin A(4) inhibits IL-1 beta-induced IL-6, IL-8, and matrix metalloproteinase-3 production in human synovial fibroblasts and enhances synthesis of tissue inhibitors of metalloproteinases. Journal of Immunology. 2000;164:2660–2666. doi: 10.4049/jimmunol.164.5.2660. [DOI] [PubMed] [Google Scholar]