Abstract
Background
The impacts of patient age and gender on immune response (IR) and clinical outcome after cancer vaccines are not known. We hypothesized younger and female patients would have higher IR rates and better survival.
Methods
Patients with resected stage IIB-IV melanoma in three clinical trials (Mel43, Mel44, Mel48) were vaccinated with 12 melanoma-associated peptides restricted by Class I MHC. The cumulative incidence rate of CD8+ T cell responses (direct Interferon-gamma ELIspot assay) by week 7 was compared by age and gender. Overall survival (OS) and disease-free survival (DFS) landmark analyses were compared by Kaplan-Meier estimates and in multivariate analyses.
Results
T cell responses were evaluated in 327 patients, and detected in 50% of males and 48% of females, with no difference in IR by gender or menopausal status. Males had trends toward longer DFS (p=0.12) and OS (p=0.09). Cumulative incidence of IR was higher in patients < 64 years of age versus older patients (p=0.03). OS and DFS were similar by age group (p> 0.50). In multivariate modeling, younger age was associated with better IR (OR 0.40, p-value 0.003), without an impact of age or gender on clinical outcomes.
Conclusion
These data support the hypothesis that older patients are less likely to develop T cell responses to a cancer vaccine. Nonetheless, significant proportions of older patients mount immune responses with comparable survival outcomes. Thus, these data support including older patients in cancer vaccine trials, but suggest value in stratifying patients by age < / > 64 years.
Keywords: Age, Gender, Peptide vaccines, Melanoma, Clinical trials, Outcomes
Introduction
Vaccination as a strategy for eradication of disease has been validated since Jenner's cowpox vaccines in 1796. In recent decades, comparable approaches have been explored for eradication of cancer leading up to the US Food and Drug Administration (FDA) approval of a vaccine for prostate cancer [1-2]. In addition, a peptide vaccine has improved progression-free survival of melanoma patients treated with high-dose interleukin-2 [3]. Many other cancer vaccines have been evaluated, without clear success, but with provocative and encouraging findings for several vaccine approaches [4-7]. Recent advances in checkpoint blockade therapies also offer promise to improve the efficacy of cancer vaccines by blocking cancer-associated immune dysfunction and have been shown to be safe when administered in combination with cancer vaccines [8-9].
The first goal of most cancer vaccines is to induce robust T cell responses against MHC-associated peptides derived from cancer-associated proteins. However, immune responses to currently available cancer vaccines are commonly of low magnitude and may be transient [10-11]. In addition, patient factors may interfere with the immune response (IR) to vaccines. Potential limitations may include patient age and gender; however, their impact on cancer vaccine efficacy is poorly understood. Both age and gender have significant effects on immune function in a wide variety of other clinical settings. It is generally accepted that with increasing age, the T cell repertoire declines, and there is defective induction of T cell memory; however, memory T cell responses induced during youth can persist even in old age [12-13]. Some of these changes, interestingly, have been associated with chronic cytomegalovirus infection [14-15]. Other potential contributing factors may include thymic involution and age-related changes in bone marrow function [12]. Some clinical trials of cancer vaccines have excluded older patients because of concern about immune senescence, but the impact of age on T cell responses to a defined-antigen vaccine has not been well studied.
There is less consensus on the effect of gender on immune function. However, numerous studies highlight the potential impact. Preclinical data implicate sex-related differences in proportions of regulatory T cells, and in T cell trafficking [15]. T cells have also been implicated in the induction and progression of atherosclerotic disease, with recent data showing strong protection against aortic aneurysms in women, apparently mediated in part by differences in immune responses related to estrogens or androgen receptors [16-17]. Also, females tend to mount a more robust and protective immune response to infection, critical illness, and vaccinations [18-21]. Similarly, females also tend to develop immune-mediated diseases more frequently and have overall worse outcomes.
We have performed a series of clinical trials of melanoma vaccines using a mixture of 12 Class I MHC restricted peptides (12MP). In the first study with these peptides, T cell responses were induced to one or more peptides in 100% of 25 patients enrolled, as assessed by ELIspot assay after one in vitro stimulation [22]. In several larger subsequent studies, a more stringent measure of T cell response was employed: immune responses were measured by a direct ex vivo ELIspot assay. The same assay was performed in over 300 patients on 3 separate clinical trials; providing a significant dataset with comparable immunologic assessment. Melanoma patients range widely in age and have large proportions of both male and female patients, making it feasible to assess differences in immune response as a function of both age and gender. Thus, the objectives of the present study were to study these questions in hope that the findings may aid the design and interpretation of future clinical trials of cancer vaccines and combination immunotherapies. Our hypothesis was that younger age and female gender would be associated with higher rates of immune response to a multipeptide cancer vaccine.
Materials and Methods
This study included patients with resected stage IIB to IV melanoma who were clinically free of disease. Patients received a vaccine containing 12MP—a multivalent vaccine derived from melanocytic differentiation antigens and cancer testis antigens—in one of three prospective phase II clinical trials. All patients received 6-10 vaccines, with vaccines 1-6 completed in the first 7 weeks. T cell responses were detected using a direct Interferon-gamma ELIspot assay and measured weekly for the first seven weeks of the study period. Clinical trial design and assay protocols have been reported in detail in prior publications [23-24]. In brief, all three trials assessed the CD8+ T lymphocyte response to 12MP administered in Montanide ISA-51 adjuvant (a form of incomplete Freund's adjuvant, purchased from Seppic, Inc; Paris, France). For each trial, patients were randomized to treatment with that vaccine with or without other immunologic agents:
For the Mel43 trial (NCT00089193), the vaccines included 12MP plus one tetanus toxoid helper peptide (MELITAC 12.1), and patients were randomized to receive granulocyte-macrophage colony-stimulating factor (GM-CSF, arms B, D) or not (arms A, C) as part of the vaccine adjuvant, and to be vaccinated in 1 (arms A, B) or 2 (arms C, D) vaccine sites [25].
For the Mel44 trial (NCT00118274), the four treatment arms included 12MP, but differed in the helper peptides included in the vaccines (tetanus peptide, MELITAC 12.1, Arms A,B; or 6 melanoma helper peptides (6MHP), MELITAC 12.6, Arms C,D), and also differed in whether the patients were pretreated once with one low dose of cyclophosphamide (arms B, D) [23].
For the Mel48 trial (NCT00705640), all patients received the same vaccine of 12MP plus tetanus helper peptide (MELITAC 12.1), but differed in whether the vaccine peptides were administered at one or two sites [26-27].
For all trials, T cell response was assessed on peripheral blood mononuclear cell samples using direct ex vivo ELIspot assay. Requirements for a positive response included at least a doubling of CD8+ T cell response over background and over any pre-existing response as well as an increase of at least 20 per 100,000 CD8+ T cells. More specifically, the following definitions were used: Nvax = number of T cells responding to vaccine peptide; Nneg = number of T cells responding to maximum negative control; and Rvax = Nvax/Nneg. An IMMUNE RESPONSE to the 12MP vaccine was present if all of the following conditions were met: (1) Nvax–Nneg > or equal to 20 cells/100,000 CD8+T cells, (2) Rvax > or equal to 2, (3) (Nvax–1SD) > or equal to (Nneg + 1 SD), and (4) Rvax after vaccination > or equal to 2 × Rvax before vaccination.
After the vaccination period, patients underwent scheduled clinical follow up over a maximum of 10 years based on previously described protocols [23-24]. Disease progression during or after therapy was recorded. This was categorized by gender and age. The primary endpoint was the cumulative incidence of immune response, which was calculated as the proportion of participants mounting an immune response by ELIspot criteria over the first eight weeks. Secondary endpoints included overall survival (OS) and disease-free survival (DFS).
Statistical Analysis
Clinical data were collected from the clinical trials database and medical record, including stage (IIB-IV), clinical trial and trial arm, CD8+ T cell immune response, age, and gender. A histogram was generated to assess for normality of data distributions. At ages higher than 64 years, the decreasing sample size of older patients limited the power to adequately detect differences based on age. Thus, on the basis of our histogram, we defined groups based on a cutoff of 64 years or older in the analyses.
Immune response was assessed weekly and graphed over the first 7 weeks of the study period to yield cumulative incidence plots. Time 0 refers to the administration of the first vaccine. The 7-week cumulative incidence rates of immune response were used to compare groups based on age and gender. The two age groups were defined using the 64 years of age cutoff. A subgroup analysis of female participants was performed by separating younger and older females with a cutoff at 50 years of age or older to approximate the impact of menopause on immune response. Differences in the cumulative incidence of immune response among the groups were assessed using Gray's test [28].
Kaplan-Meier survival estimates and the log-rank test were used to perform time-to-event analyses of OS and DFS combining all study participants. Study arms were divided into 5 groups: Mel43 + GM-CSF, Mel 43 without GM-CSF, Mel44 + tetanus, Mel44 + 6MHP, and Mel48. A subgroup analysis was also performed of the study arms previously found to have the highest proportions of patients with immune response (Mel 43 arms without GM-CSF [25] and Mel 44 arms with tetanus helper peptide [23]). OS was assessed based on a landmark analysis, calculated as the time elapsed from the 8-week post registration date to either death or loss to follow-up, while DFS was calculated as the time elapsed to death, recurrence/progression of disease, or loss to follow-up. Patients experiencing the outcome of interest (IR, OS or DFS) before the 8-week window were excluded from landmark analysis.
A multivariate logistic regression model was developed to study the association of immune response with factors of interest. The factors assessed in the immune response model included gender (female/male), stage (II, III, IV), age (</≥ 64 years of age), and cohort group (high- versus low-responding study arms). The inclusion of cohort group in this analysis was to account for this known confounder, and it should be noted that the aforementioned correlation between immune response and cohort is known to be independent of the factors: age, gender and stage.
Cox proportional hazard modeling (PROC PHREG in SAS 9.4; SAS Institute, Cary, NC) was used to perform landmark time-to-event analyses of OS and DFS. Landmark analysis included predictors – gender, stage, and age as well as immune response captured in the first 8 weeks of the study period. Significance was determined using a Wald test. The model was performed using all study participants, and then on a subset focusing on the participants corresponding to the high responder arms.
In summary, the following outcomes with corresponding baseline predictors were used:
| 1) Outcome: IR | Predictors: stage, age, gender, cohort group (high- versus low-responder arms) |
| 2a) Outcomes: OS, DFS | Predictors: stage, age, gender, immune response (all participants) |
| 2b) Outcomes: OS, DFS | Predictors: stage, age, gender, immune response (only participants from high responder arms (Mel43 A & C) and (Mel44 A & B) |
Assumptions of the Cox proportional hazard models were assessed using graphical methods. Differences were considered significant for p-values (two-sided) ≤ 0.05.
Results
A total of 332 participants were enrolled in the trials. 327 patients met the inclusion criteria for the study and were evaluated for immune response measurable by Week 8. Six subjects were excluded: two did not have ELIspot assay data and four were omitted due to HLA type mismatch. One individual was a participant in both the Mel43 and Mel48 trials. However, this individual's ELIspot data were complete and included in this analysis only for Mel48.
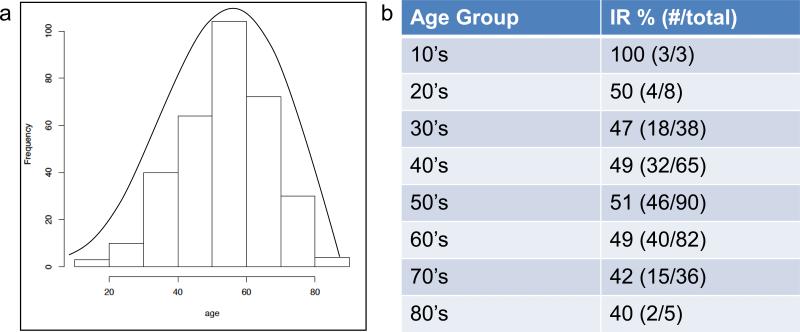
The median age of the study participants was 56 years (range 15 to 87). Histogram representation shows a slightly left-skewed distribution of ages (Figure 1). The number of participants decreases significantly prior to age 40 and after 70 years of age. 15% (49 of 327) were less than or equal to 39 years of age; 10% (34/327) were 71 years of age or older. Based on our defined age cutoff of 64 years of age, 249 of the 327 evaluable participants were in the younger age group while 78 participants were in the older age group. 31% (103 of 327) of patients were women, a roughly 2:1 ratio of males to females. Stage of disease at enrollment included 44 patients with stage IIB/IIC, 45 with stage IIIA, 176 with stage IIIB/IIIC, and 62 with stage IV. Median follow-up was 3.0 years, with a maximum of 5.5 years. Overall, immune responses were detected in 49% of study participants by previously specified ELISpot criteria. Two cohort groups had immune response rates exceeding 70%: Mel 43 arms without GM-CSF [25] and Mel 44 arms with tetanus helper peptide [23]. Patients in these two cohorts were combined to form the high-responder group in the subset analysis (Table 1).
Fig. 1.
Representation of age distribution of study participants. a. Histogram of age range with superimposed line graph b. Immune response by decade and total number of study participants per age group
TABLE 1.
Class I MHC-restricted vaccine (12MP) trial protocols for patients with high-risk melanoma
| Trial | Peptide | Adjuvant | N | Gender, M | Age, <64y | IR+ (%) |
|---|---|---|---|---|---|---|
| Mel43a | 12MP;tetanus | IFA + GM-CSF | 58 | 37 | 47 | 20 (34%) |
| IFA | 60 | 45 | 46 | 44 (73%) | ||
| Mel44a | 12MP;tetanus | IFA+/− cyclophos | 82 | 53 | 57 | 58 (71%) |
| 12MP:6MHP | IFA+/− cyclophos | 85 | 59 | 64 | 18 (21%) | |
| Mel48a,b | 12MP;tetanus | IFA | 42 | 30 | 35 | 20 (48%) |
| Total | 327c | M 224 | <64y 249 | + (49%) | ||
| F 103 | ≥64y 78 | − (51%) |
MHC major histocompatibility complex, 12MP 12 class I MHC-restricted melanoma peptides, IFA incomplete Freund's adjuvant, GM-CSF granulocyte-macrophage colony-stimulating factor, 6MHP 6 class II MHC-restricted melanoma helper peptides, IR Immune response
Ten vaccinations were administered for Mel43 and Mel44 and 6 for Mel48
Patients with nonmeasurable disease or equivocal findings may be enrolled in Mel48
One participant belonged to both trials 43 and 48
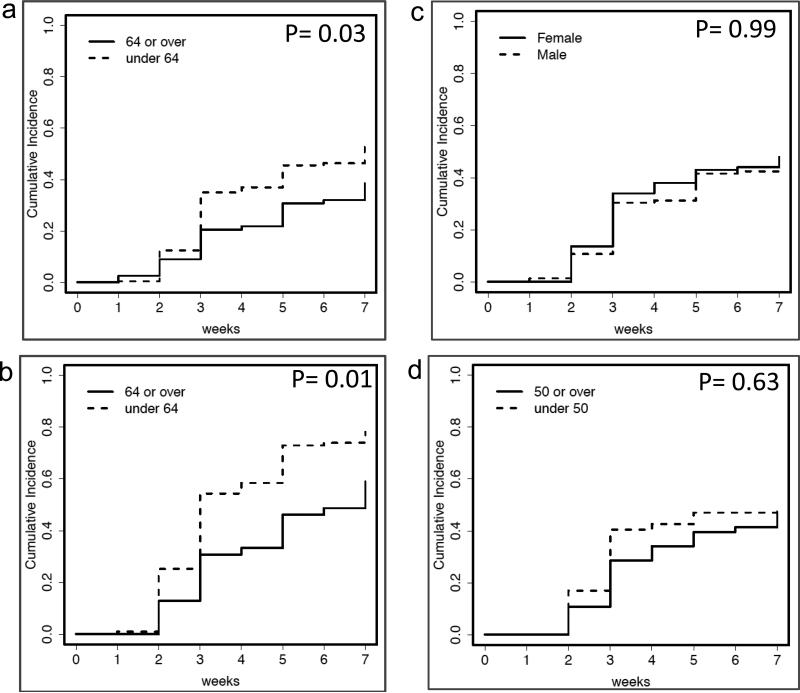
The 7-week cumulative incidence rate for immune response was 53% (130/249) in patients less than 64 years of age but only 38% (30/78) in older patients (p=0.03; Figure 2a). Subset analysis of the high-responder group showed an even more pronounced difference between age groups, p=0.01 (Figure 2b) which suggests a consistent pattern between younger patients and higher cumulative incidence of immune response regardless of clinical trial or clinical arm. The 7-week cumulative incidence rate for immune response was 50% (111/224) in males and 48% (49/103) in females, with no difference by gender (p=0.99; Figure 2c). Age greater than or equal to 50 years, in female patients, was used as a proxy for menopausal status. While younger females responded earlier, the difference equilibrated over time and was not associated with a difference in the cumulative incidence for immune response (p=0.63; Figure 2d).
Fig. 2.
Cumulative incidence of immune response, or the measure of immune response over time, over first 8 weeks of the trial period. a Includes all study participants by age grouping participants into either younger or older than 64 years b. Uses only participants within the high responder arms (Mel 43 without GM-CSF and Mel 44 with 6MHP) stratifying by age c. All study participants by gender d. Includes only female patients and further stratifies them into younger or older than 50 years of age to approximate for menopause
In a multivariate logistic regression model, younger age was again associated with a higher proportion of study participants with immune response to vaccination (OR 0.40, p-value 0.003) while gender and stage did not predict immune response (Table 2). Cohort group referred to high versus low responder groups. Previously published data from our group showed a significant correlation between immune response and adjuvants and helper peptides administered with the 12MP peptides.
Table 2.
Multivariate logistic regression modeling as a function of immune response
| Parameter | Odds Ratio | Confidence Intervals | P- value |
|---|---|---|---|
| Stagea | 0.14b | ||
| III | 1.1 | 0.54-2.2 | 0.8 |
| IV | 0.56 | 0.24-1.3 | 0.2 |
| III vs. IV | 0.52 | 0.27-0.99 | 0.047 |
| Gender | 1.2 | 0.7-2.0 | 0.47 |
| Age | 0.4 | 0.22-0.73 | 0.003 |
| Cohort | 6.6 | 4.0-11.1 | <0.001 |
The reference group is patients with Stage II melanoma unless otherwise stated.
Cumulative P-value for all stages.
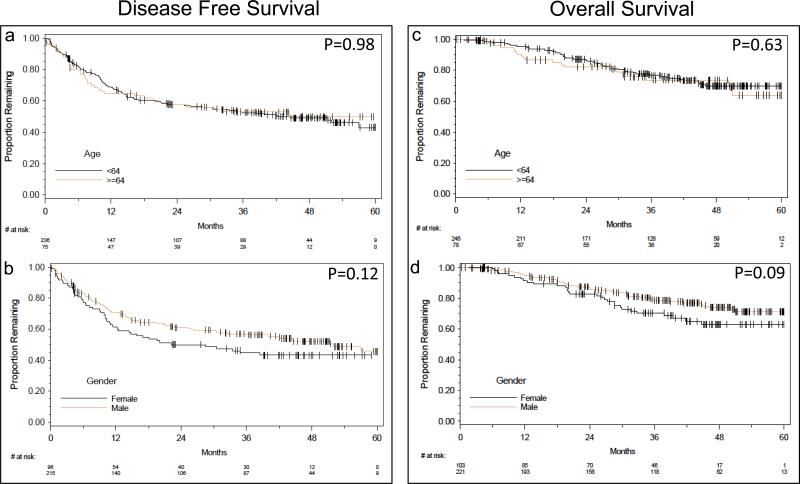
Associations of age and gender with OS and DFS were assessed with Kaplan-Meier estimates. No difference was detected in OS or DFS by age group (log rank p=0.63 and 0.98, respectively). There was a non-significant trend toward improved DFS (p=0.12,) and OS (p=0.09) for males (Figure 3a-d). A subgroup analysis assessed low and high immune response arms for age and gender differences in OS and DFS (Supplementary Figure 1-2). No association was shown among the subgroups. However, the individual clinical trials included in this study were not initially powered with the intention of demonstrating a relationship between these variables if present.
Fig. 3.
Kaplan-Meier survival graphs of age (top) and gender (bottom) versus disease free survival (DFS) and overall survival (OS) starting at 8 weeks to maximum of 60 months follow up period. DFS is shown on the left column and OS on the right column a. Association between age and DFS b. Effect of gender on DFS c. Age versus overall survival d. Gender versus overall survival
Cox proportional hazard modeling of factors associated with DFS or OS indicated that there was no association between age and disease-free survival (HR 1.03, p=0.90) or overall survival (HR 1.26, p=0.39). There was a trend toward significance between male gender and improved OS (HR 0.66, p=0.07). However, ultimately no difference was shown in DFS (HR 0.76, p = 0.12). In this dataset, immune response to the multi-peptide melanoma vaccine was not associated with DFS or OS (Table 3).
Table 3.
Multivariate analysis of key parameters and clinical outcomes
| Parameter | Hazard Ratio (DFS) | P- value (DFS) | Hazard Ratio (OS) | P- value (OS) |
|---|---|---|---|---|
| Stagea | 0.58b | 0.21b | ||
| III | 1.3 | 0.32 | 1.4 | 0.3 |
| IV | 1.3 | 0.35 | 0.87 | 0.75 |
| III vs IV | 1.0 | 0.91 | 0.6 | 0.12 |
| Gender | 0.76 | 0.12 | 0.66 | 0.07 |
| Age | 1.03 | 0.90 | 1.26 | 0.39 |
| Immune Response | 0.93 | 0.69 | 1.05 | 0.83 |
The reference group is patients with Stage II melanoma unless otherwise stated.
Cumulative P-value for all stages.
Discussion
As immune therapies are providing durable benefit for some cancer patients, there is an increasing need for new immune therapy combinations that may increase the proportion of patients who benefit from therapy. Further, there is a need for criteria to select the best therapeutic options for each patient. Cancer vaccines may expand the proportion of patients who benefit from checkpoint blockade therapy. Checkpoint blockade therapy typically works for patients who already have antigen-specific T cell responses; new cancer vaccines targeting relevant differentiation antigens or neoantigens may induce or expand T cell responses in patients who otherwise would not benefit from checkpoint blockade alone.
Elderly patients are sometimes excluded from vaccine trials because of concern that they may be unable to mount an immune response; however, systematic analysis of CD8 T cell response to cancer vaccines as a function of age has been lacking. Our data support the hypothesis that a decreased proportion of older patients are able to elicit immune responses to cancer vaccines; however, it is noteworthy that immune responses were still detected in a significant subset of older patients (38%, compared to 52% for younger patients). Also, for those patients enrolled in study arms that had higher overall immunogenicity, the fraction of immune response rates seen in older patients approached 59%, compared to 77% for younger patients as shown in Figure 2b.
Interestingly, after adjusting for common confounders, older patients did not have poorer clinical outcomes in this study. Despite overall improvements in outcome measurements in the last two decades, the elderly population has not benefited from technological advancements at the same rate as their younger counterparts. This discrepancy has been replicated in nearly all of the most common cancers [29-30]. There are several explanations for this well-accepted notion and our study findings. Most studies have been based largely on administrative databases offering little clinical context and ability for risk adjustment. This significantly limits the analysis as the elderly have higher risk-adjusted morbidity and mortality. AlHilli et al argues the effect of age is inconsequential once co-morbidities and other known risk factors are adjusted for in patients with endometrial cancer [31]. Furthermore, the authors allude to varying cancer treatment regimens among younger and elderly patients; a practice that is not restricted to endometrial cancer alone [32-33]. Studies that directly measure the effects of aggressive cancer therapies on clinical outcomes in the elderly are limited. This, combined with common hesitation to risk higher rates of complications underscores the need to include this patient group in clinical trials to improve patient selection and to form better predictive models. For the present study, enrollment on the clinical trials required meeting inclusion and exclusion criteria that ensured performance status of 0-1, absence of severe heart disease, and good organ function. Thus, elderly patients with declining functional status were not included. Thus, the conclusions of this report are relevant to elderly patients with good functional status, but may not apply to debilitated older patients.
Our results suggest that it may be reasonable to stratify patients on vaccine trials based on an age cutoff of about 64 years. However, even among older patients, there is a significant probability of CD8+ T cell response to a multipeptide vaccine. Thus, there does not appear to be evidence at this time to limit eligibility of study participants based on age alone. Further studies are necessary to determine the factors that predict an immune response in the elderly to guide patient selection and improve rates of response. The cutoff of 64 years in our study, between younger and older patients, also does not address whether there may be an older age beyond which immunogenicity falls off precipitously. However, there were immune responses in patients of all decades of life evaluated from teenage years into the 80s. We were not able to identify an age beyond 64 years for which immune responses were not detected, but acknowledge that if there had been larger numbers of patients over age 70, the immune response rate estimate may have been precise enough to detect a decrement in immunogenicity for the more elderly group.
Female gender is commonly thought to be immunoprotective [19-20,34]. However, our study found that gender did not significantly impact immune response and was not associated with improved clinical outcome. The trend toward improved survival in males is an unexpected finding, as males with melanoma generally have significantly worse outcomes than females, stage-for-stage [35-36]. Gender differences in melanoma histology have been reported, with males having higher rates of ulceration and a more frequent absence of tumor-infiltrating lymphocytes [36]. Those findings suggest a more aggressive tumor biology in males [37]. There is also evidence of gender differences in T lymphocyte infiltration in other human tissues, primarily in the cardiovascular literature involving hypertension, vascular/endothelial injury, oxidative stress and shock [38]. Thus, there may be complex and interacting gender-related differences in immune function and tumor biology that explain the trend towards improved overall survival without a concurrent difference in immune response rate after vaccination. Ongoing investigations are pursuing differences in sex hormones and the effect on the cytokine profile and microenvironment [16,21] However, the summary findings of this study are that there were not significant differences in immune response or clinical outcome between males and females receiving a multipeptide vaccine. Thus, these data support evaluation of immune responses to cancer vaccines independent of patient gender.
The study has both strengths and limitations. Data were derived in a prospective manner despite being reviewed retrospectively. To our knowledge, the combined data from the participants of three clinical trials leads to the largest patient volume in humans assessing gender and age differences to a multi-peptide melanoma vaccine. All patients in these 3 studies were vaccinated with 12MP, and the immune responses were all evaluated in the same manner by the same laboratory. However, the vaccine adjuvants and helper peptides used varied among the trials and study arms. They included GM-CSF, cyclophosphamide, and 6MHP vs. tetanus peptide. Subsequent analysis revealed 6MHP and GM-CSF, but not cyclophosphamide, to have independent effects on CD8+ T cell responses to the 12MP vaccine. It is possible that these variables may have diluted our results; however, the higher fraction of immune response seen in younger patients were observed consistently across all studies and study arm subsets, and there was no detectable trend to an impact of gender on CD8+ T cell response in study subsets. Additional host factors could have also contributed to results, such as comorbidities, homeopathic medicine use, and nutritional status; however, the entry criteria excluded patients with major co-morbidities.
Conclusions
In summary, a multi-peptide vaccine can be administered to patients regardless of gender or age, with expectation of immunogenicity in all patient subsets. We do recommend considering stratification of patients by age when immune response is the primary endpoint. The ability to mount a response was observed at age 64 suggesting this to be a reasonable age cutoff. As cancer vaccines are optimized and combined effectively with other immune therapies, these findings support study of such combination immunotherapies, even in older patients, whose tumors commonly lack BRAF mutations, and thus lack good targeted therapy options.
Supplementary Material
Acknowledgement
This research was funded in part by the National Institutes of Health [NIH]/National Cancer Institute [NCI] grants NIH R01 CA057653 and CA 118386 and NIH R21 CA103528 awarded to Craig Slingluff, T32 CA163177 awarded to Yinin Hu/Craig Slingluff, and K25 CA181638 awarded to Nolan Wages, and Cancer Center Support Grant P30 CA044579.
List of Abbreviations
- 6MHP
6 melanoma helper peptides
- 12MP
12 Class I MHC restricted peptides
- DFS
Disease-free survival
- FDA
Food and Drug Administration
- IR
Immune response
Footnotes
Data included in this journal article submission was previously presented in two separate portions at the Society of Immunotherapy of Cancer 2014 Annual Meeting as a poster presentation and the 2015 Academic Surgical Congress as a plenary oral presentation
Conflict of Interest
Craig Slingluff is an inventor of several peptides included in the vaccine that was administered during the clinical trials studied within this paper. The University of Virginia Licensing and Ventures Group holds the patents for those peptides, which have been licensed through the Ludwig Institute for Cancer Research to Glaxo Smith Kline. The remaining authors have nothing to disclose or competing interests in association with this study.
Contributor Information
Adriana G Ramirez, Department of Surgery, University of Virginia, Charlottesville, United States of America, PO Box 800709, Charlottesville, VA 22908.
Nolan A Wages, Department of Public Health Sciences, University of Virginia, Charlottesville, United States of America, Charlottesville, VA 22908.
Yinin Hu, Department of Surgery, University of Virginia, Charlottesville, United States of America, Charlottesville, VA 22908.
Mark E Smolkin, Department of Public Health Sciences, University of Virginia, Charlottesville, United States of America, Charlottesville, VA 22908.
Craig L Slingluff, Jr, Division of Surgical Oncology, University of Virginia, Charlottesville, United States of America, Charlottesville, VA 22908.
References
- 1.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 2.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 3.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisma J, et al. Gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunsvig PF, Kyte JA, Kersten C, Sundstrum S, Muller M, Nyakas M, Hansen GL, Gaudemack G, Aamdal S. Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin Cancer Res. 2011;17:6847–6857. doi: 10.1158/1078-0432.CCR-11-1385. doi: 10.1158/1078-0432.CCR-11-1385. [DOI] [PubMed] [Google Scholar]
- 5.Okada H, Butterfield LH, Hamilton RL, Hoji A, Sakaki M, Ahn BJ, et al. Induction of robust type-I CD8+ T-cell responses in WHO grade 2 low-grade glioma patients receiving peptide-based vaccines in combination with poly-ICLC. Clin Cancer Res. 2015;21:286–294. doi: 10.1158/1078-0432.CCR-14-1790. doi: 10.1158/1078-0432.CCR-14-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiriveedhi V, Tucker N, Herndon J, Li L, Sturmoski M, Ellis M, et al. Safety and preliminary evidence of biologic efficacy of a mammaglobin-a DNA vaccine in patients with stable metastatic breast cancer. Clin Cancer Res. 2014;20:5964–5975. doi: 10.1158/1078-0432.CCR-14-0059. doi: 10.1158/1078-0432.CCR-14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weihrauch MR, Ansen S, Jurkiewicz E, Geisen C, Xia Z, Anderson KS, et al. Phase I/II combined chemoimmunotherapy with carcinoembryonic antigen-derived HLA-A2-restricted CAP-1 peptide and irinotecan, 5-fluorouracil, and leucovorin in patients with primary metastatic colorectal cancer. Clin Cancer Res. 2005;11:5993–6001. doi: 10.1158/1078-0432.CCR-05-0018. doi: 10.1158/1078-0432.CCR-05-0018. [DOI] [PubMed] [Google Scholar]
- 8.Agarwalla P, Barnard Z, Fecci P, Dranoff G, Curry W. Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. J Immunother. 2012;35:385–389. doi: 10.1097/CJI.0b013e3182562d59. doi: 10.1097/CJI.0b013e3182562d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorensen MR, Holst PJ, Steffensen MA, Christensen JP, Thomsen AR. Adenoviral vaccination combined with CD40 stimulation and CTLA-4 blockage can lead to complete tumor regression in a murine melanoma model. Vaccine. 2010;28:6757–6764. doi: 10.1016/j.vaccine.2010.07.066. doi: 10.1016/j.vaccine.2010.07.066. [DOI] [PubMed] [Google Scholar]
- 10.Toungouz M, Libin M, Bulte F, Faid L, Lehman F, Duriau D, et al. Transient expansion of peptide-specific lymphocytes producing IFN-gamma after vaccination with dendritic cells pulsed with MAGE peptides in patients with mage-A1/A3-positive tumors. J Leukoc Biol. 2001;69:937–943. 2001. [PubMed] [Google Scholar]
- 11.Slingluff CL, Petroni G, Yamshchikov GV, Hibbitts S, Grosh WW, Chianese-Bullock KA, et al. Immunologic and clinical outcomes of vaccination with a multiepitope melanoma peptide vaccine plus low-dose interleukin-2 administered either concurrently or on a delayed schedule. JCO. 2004;22:4474–4485. doi: 10.1200/JCO.2004.10.212. doi: 10.1200/JCO.2004.10.212. [DOI] [PubMed] [Google Scholar]
- 12.Nikolich-Žugich J. Aging of the T cell compartment in mice and humans: from no naive expectations to foggy memories. J Immunol. 2014;193:2622–2629. doi: 10.4049/jimmunol.1401174. doi: 10.4049/jimmunol.1401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawelec G, Goldeck D, Derhovanessian E. Inflammation, ageing and chronic disease. Current Opinion in Immunology. 2014;29:23–28. doi: 10.1016/j.coi.2014.03.007. doi: 10.1016/j.coi.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Derhovanessian E, Maier AB, Hahnel K, McElhaney JE, Slagboom EP, Pawelec G. Latent infection with cytomegalovirus is associated with poor memory CD4 responses to influenza A core proteins in the elderly. J Immunol. 2014;193:3624–3631. doi: 10.4049/jimmunol.1303361. doi: 10.4049/jimmunol.1303361. [DOI] [PubMed] [Google Scholar]
- 15.Sandberg K, Ji H, Hay M. Sex-specific immune modulation of primary hypertension. Cell Immunol. 2014;294:95–101. doi: 10.1016/j.cellimm.2014.12.001. doi: 10.1016/j.cellimm.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laser A, Ghosh A, Roelofsdd K, Sadig O, McEvoy B, DiMustro P, Eliason J, Upchurch GR. Increased estrogen receptor alpha in experimental aortic aneurysms in females compared with males. J Surg Res. 2014;186:467–474. doi: 10.1016/j.jss.2013.07.050. doi: 10.1016/j.jss.2013.07.050d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang C, Lee SO, Wang R, Yeh S, Chang T. Androgen receptor (AR) physiological roles in male and female reproductive systems: lessons learned from AR-knockout mice lacking AR in selective cells. Biol Reprod. 2013;89:21–21. doi: 10.1095/biolreprod.113.109132. (2013). doi: 10.1095/biolreprod.113.109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. The Lancet infectious diseases. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neuroscience & Biobehavioral Reviews. 2000;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. (2000). doi: 10.1016/S0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 20.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 21.Knöferl MW, Angele MK, Diodato MD, Schwacha MG, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Annals of Surgery. 2002;235:105–112. doi: 10.1097/00000658-200201000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slingluff CL, Petroni GR, Chianese-Bullock KA Smolkin ME, Hibbitts S, Murphy C, et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res. 2007;13:6386–6395. doi: 10.1158/1078-0432.CCR-07-0486. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]
- 23.Slingluff CL, Petroni GR, Chianese-Bullock KA, Smolkin M, Ross MI, Haas NB, et al. Randomized multicenter trial of the effects of melanoma- associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. JCO. 2011;29:2924–2932. doi: 10.1200/JCO.2010.33.8053. doi: 10.1200/JCO.2010.33.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slingluff CL. A multipeptide vaccine in melanoma patients with evaluation of the injection site microenvironment. [23 January 2015];ClinicalTrials.gov. 2014 https://clinicaltrials.gov/ct2/show/NCT00705640.
- 25.Slingluff CL, Petroni GR, Olson WC, Smolkin ME, Ross MI, Haas NB, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15:7036–7044. doi: 10.1158/1078-0432.CCR-09-1544. doi: 10.1158/1078-0432.CCR-09-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris RC, Chianese-Bullock KA, Petroni GR, Schaefer JT, Brill LB, Molhoek KR, Deacon DH, Patterson JW, Slingluff CL. The vaccine-site microenvironment induced by injection of incomplete Freund's adjuvant, with or without melanoma peptides. J Immunotherapy. 2012;35:78–88. doi: 10.1097/CJI.0b013e31823731a4. doi: 10.1097/CJI.0b013e31823731a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salerno EP, Shea SM, Olson WC, Petroni GR, Smolkin ME, McSkimming C, Chianese-Bullock KA, Slingluff CL. Activation, dysfunction and retention of T cells in vaccine sites after injection of incomplete Freund's adjuvant, with or without peptide. Cancer Immunology Immunotherapy. 2013;62:1149–1159. doi: 10.1007/s00262-013-1435-5. doi: 10.1007/s00262-013-1435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. doi: 10.2307/2241622. [Google Scholar]
- 29.Zeng C, Wen W, Morgans AK, Pao W, Shu X, Zheng W. Disparities by Race, Age, and Sex in the Improvement of Survival for Major Cancers: Results From the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol. 2015;1:88–96. doi: 10.1001/jamaoncol.2014.161. doi: 10.1001/jamaoncol.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jafari MD, Jafari F, Halabi WJ, Nguyen VQ, Pigazzi A, Carmichael JC, Mills SD, Stamos MJ. Colorectal Cancer Resections in the Aging US Population: A Trend Toward Decreasing Rates and Improved Outcomes. JAMA Surg. 2014;149:557–564. doi: 10.1001/jamasurg.2013.4930. doi: 10.1001/jamasurg.2013.4930. [DOI] [PubMed] [Google Scholar]
- 31.AlHilli MM, Bakkum-Gamez JN, Mariani A, Weaver AL, McGree ME, Keeney GL, Jatoi A, Dowdy SC, Podratz KC. Risk-adjusted outcomes in elderly endometrial cancer patients: implications of the contrasting impact of age on progression-free and cause-specific survival. Gynecologic Oncolology. 2015;138:133–140. doi: 10.1016/j.ygyno.2015.04.010. doi: 10.1016/j.ygyno.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Nadpara P, Madhavan SS, Tworek C. Guideline-concordant timely lung cancer care and prognosis among elderly patients in the United States: A population-based study. Cancer Epidemiology. 2015 doi: 10.1016/j.canep.2015.06.005. In Press. doi: 10.1016/j.canep.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen RC, Royce TJ, Extermann M, Reeve BB. Impact of age and comorbidity on treatment and outcomes in elderly cancer patients. Semin Radiat Oncol. 2012;22:265–271. doi: 10.1016/j.semradonc.2012.05.002. doi: 10.1016/j.semradonc.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, Tibshirani RJ, Davis MM. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. P Natl Acad Sci USA. 2014;111:869–874. doi: 10.1073/pnas.1321060111. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stidham KR, Johnson JL, Seigler HF. Survival superiority of females with melanoma. A multivariate analysis of 6383 patients exploring the significance of gender in prognostic outcome. AMA Arch Surg. 1994;129:316–324. doi: 10.1001/archsurg.1994.01420270094020. doi:10.1001/archsurg.1994.01420270094020. [DOI] [PubMed] [Google Scholar]
- 36.Morgese F, Berardi R, Sampaolesi C, Torniai M, Marcantognini G, Giacchetti A, et al. Gender differences and outcome of melanoma patients. Journal of Translational Medicine. 2015;13(Suppl 1) 13 doi: 10.1186/1479-5876-13-S1-P13. [Google Scholar]
- 37.Donizy P, Kaczorowshi M, Halon A, Leskiewivz M, Kozyra C, Matkowski R. Paucity of tumor-infiltrating lymphocytes is an unfavorable prognosticator and predicts lymph node metastases in cutaneous melanoma patients. Anticancer Res. 2015;35:351–358. [PubMed] [Google Scholar]
- 38.Pollow DP, Uhrlaub J, Romero-Aleshire MJ, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension. 2014;64:384–390. doi: 10.1161/HYPERTENSIONAHA.114.03581. doi: 10.1161/HYPERTENSIONAHA.114.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.