Abstract
Background
Postoperative nausea and vomiting (PONV) are common complications following surgery and anaesthesia. Antiemetic drugs are only partially effective in preventing PONV. An alternative approach is to stimulate the PC6 acupoint on the wrist. This is an update of a Cochrane review first published in 2004, updated in 2009 and now in 2015.
Objectives
To determine the effectiveness and safety of PC6 acupoint stimulation with or without antiemetic drug versus sham or antiemetic drug for the prevention of PONV in people undergoing surgery.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library, Issue 12, 2014), MEDLINE (January 2008 to December 2014), EMBASE (January 2008 to December 2014), ISI Web of Science (January 2008 to December 2014), World Health Organization Clinical Trials Registry, ClinicalTrials.gov, and reference lists of articles to identify additional studies. We applied no language restrictions.
Selection criteria
All randomized trials of techniques that stimulated the PC6 acupoint compared with sham treatment or drug therapy, or combined PC6 acupoint and drug therapy compared to drug therapy, for the prevention of PONV. Interventions used in these trials included acupuncture, electro-acupuncture, transcutaneous electrical acupoint stimulation, transcutaneous nerve stimulation, laser stimulation, capsicum plaster, acu-stimulation device, and acupressure in people undergoing surgery. Primary outcomes were the incidences of nausea and vomiting after surgery. Secondary outcomes were the need for rescue antiemetic therapy and adverse effects.
Data collection and analysis
Two review authors independently extracted the data and assessed the risk of bias domains for each trial. We used a random-effects model and reported risk ratio (RR) with associated 95% confidence interval (95% CI). We used trial sequential analyses to help provide information on when we had reached firm evidence in cumulative meta-analyses of the primary outcomes, based on a 30% risk ratio reduction in PONV.
Main results
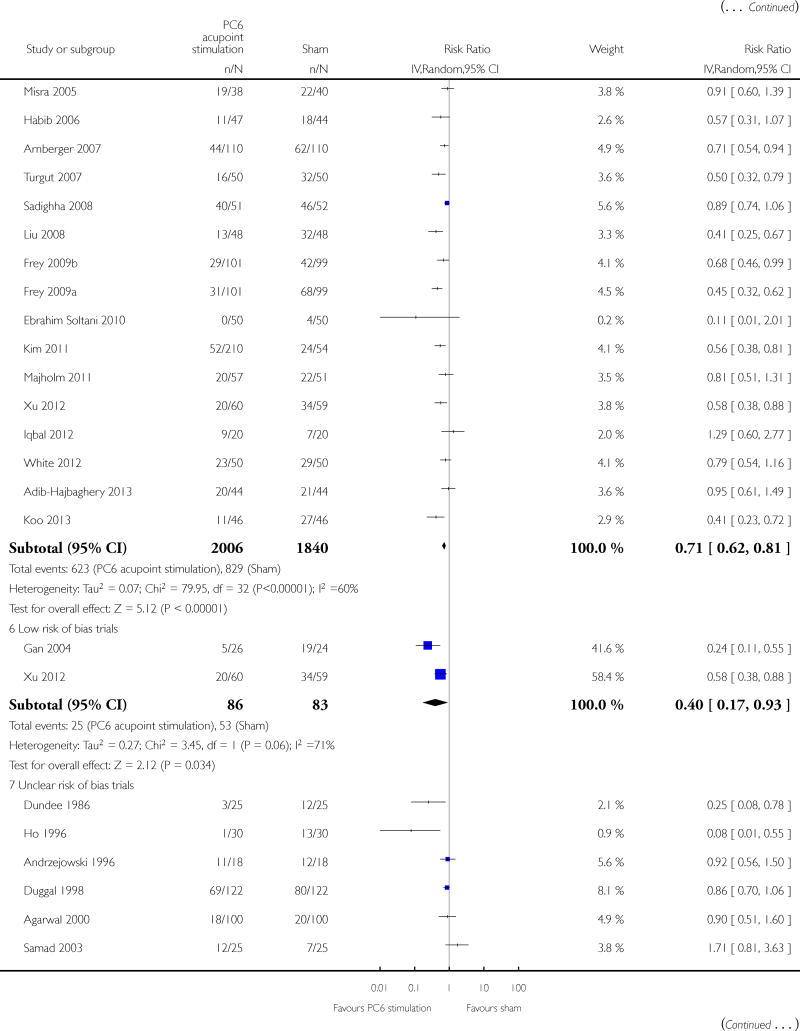
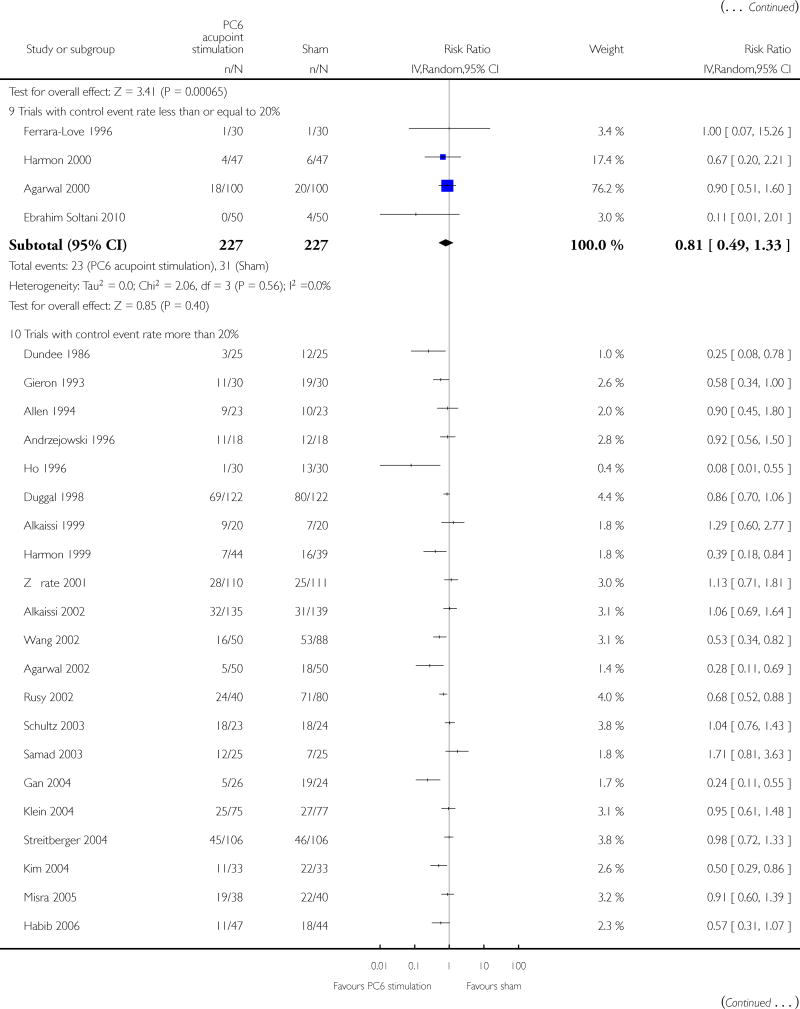
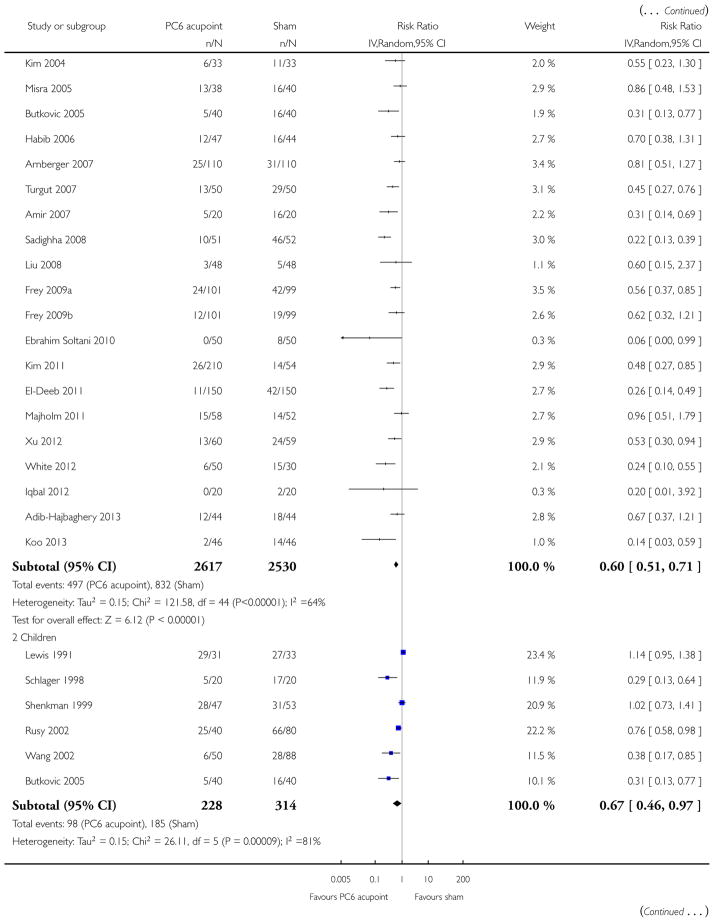
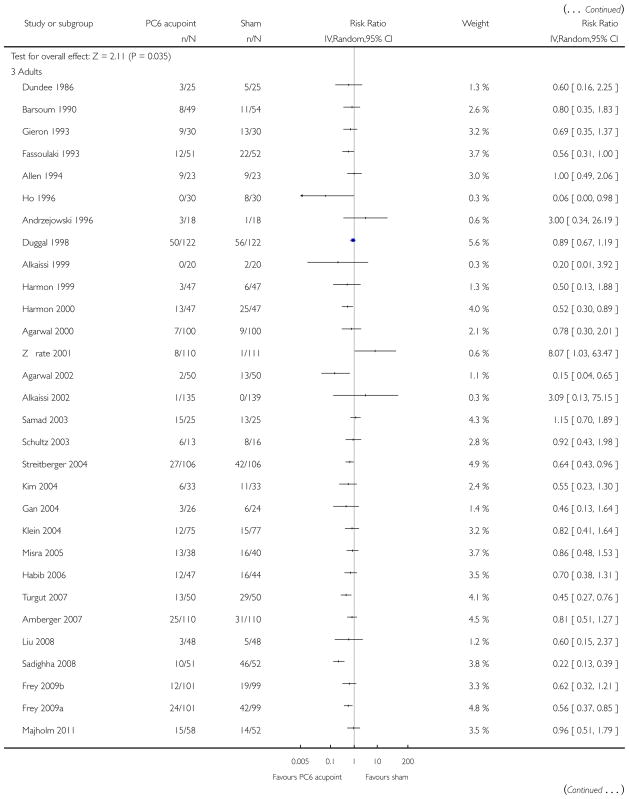
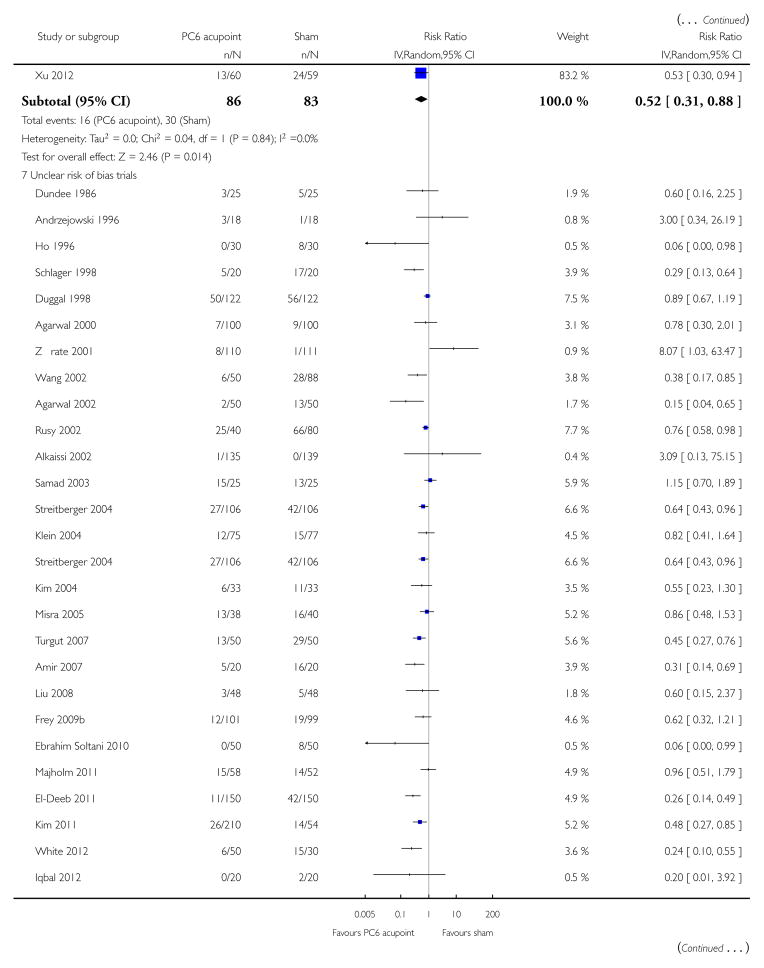
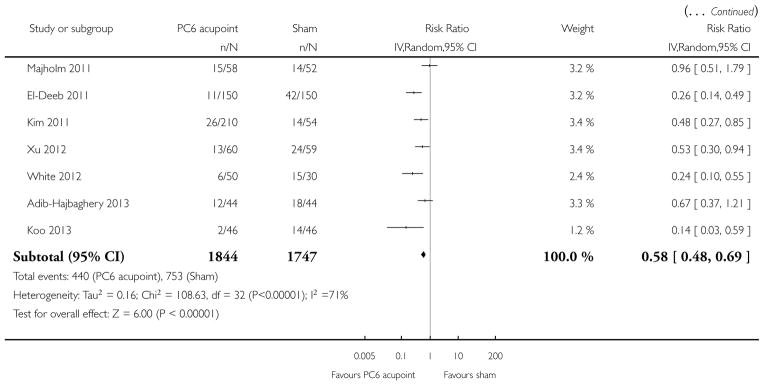
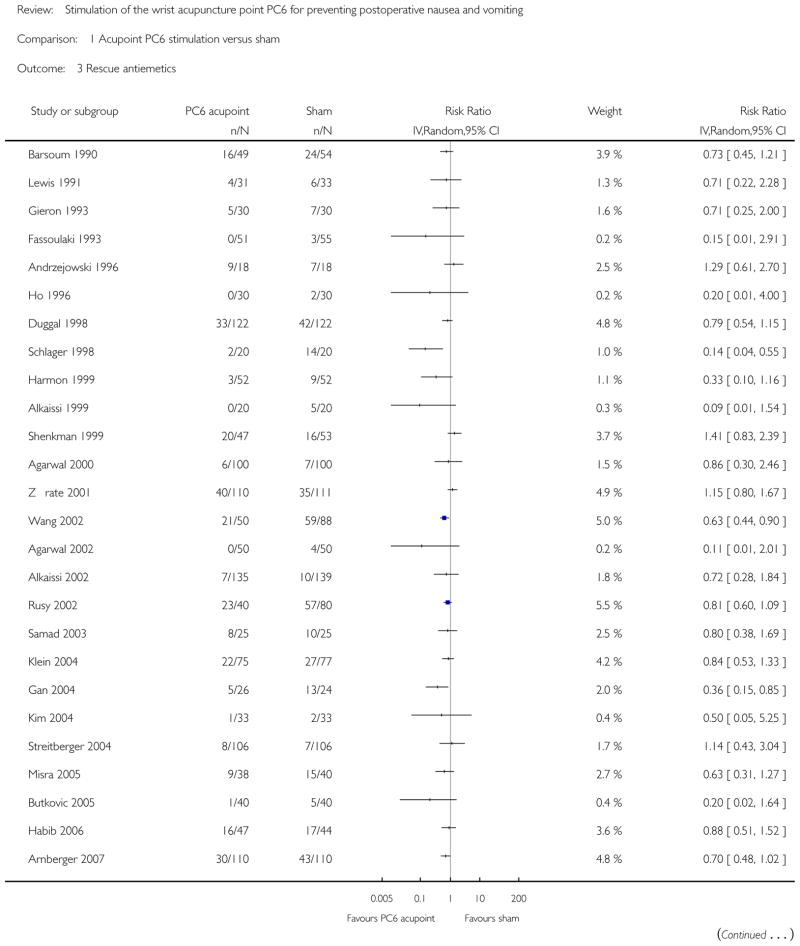
We included 59 trials involving 7667 participants. We rated two trials at low risk of bias in all domains (selection, attrition, reporting, blinding and other). We rated 25 trials at high risk in one or more risk-of-bias domains. Compared with sham treatment, PC6 acupoint stimulation significantly reduced the incidence of nausea (RR 0.68, 95% CI 0.60 to 0.77; 40 trials, 4742 participants), vomiting (RR 0.60, 95% CI 0.51 to 0.71; 45 trials, 5147 participants) and the need for rescue antiemetics (RR 0.64, 95% CI 0.55 to 0.73; 39 trials, 4622 participants). As heterogeneity among trials was substantial and there were study limitations, we rated the quality of evidence as low. Using trial sequential analysis, the required information size and boundary for benefit were reached for both primary outcomes.
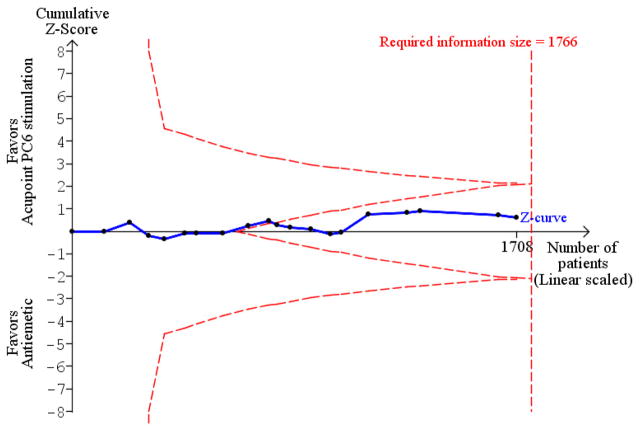
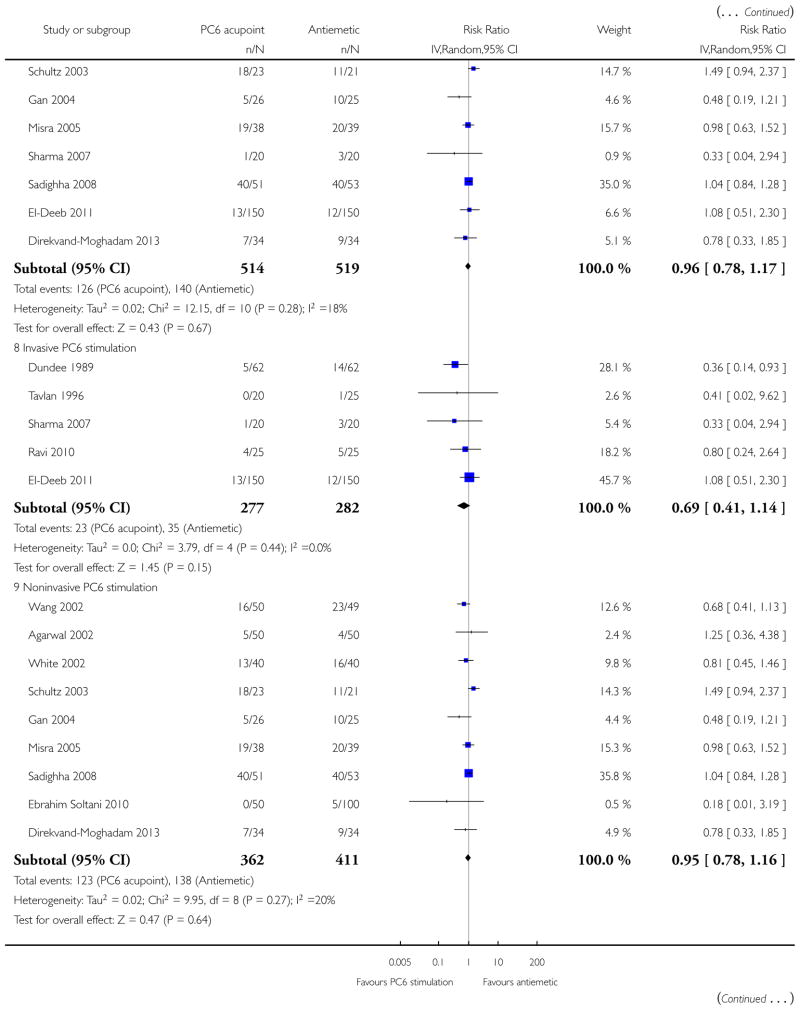
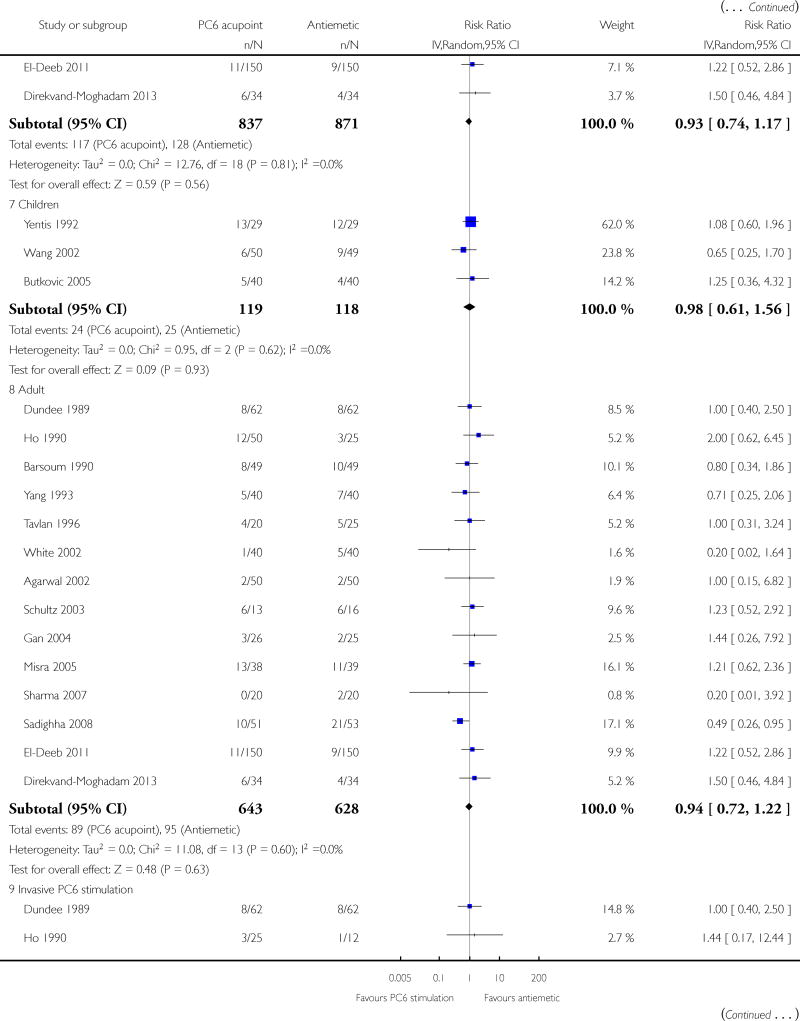
PC6 acupoint stimulation was compared with six different types of antiemetic drugs (metoclopramide, cyclizine, prochlorperazine, droperidol. ondansetron and dexamethasone). There was no difference between PC6 acupoint stimulation and antiemetic drugs in the incidence of nausea (RR 0.91, 95% CI 0.75 to 1.10; 14 trials, 1332 participants), vomiting (RR 0.93, 95% CI 0.74 to 1.17; 19 trials, 1708 participants), or the need for rescue antiemetics (RR 0.87, 95% CI 0.65 to 1.16; 9 trials, 895 participants). We rated the quality of evidence as moderate, due to the study limitations. Using trial sequential analyses, the futility boundary was crossed before the required information size was surpassed for both primary outcomes.
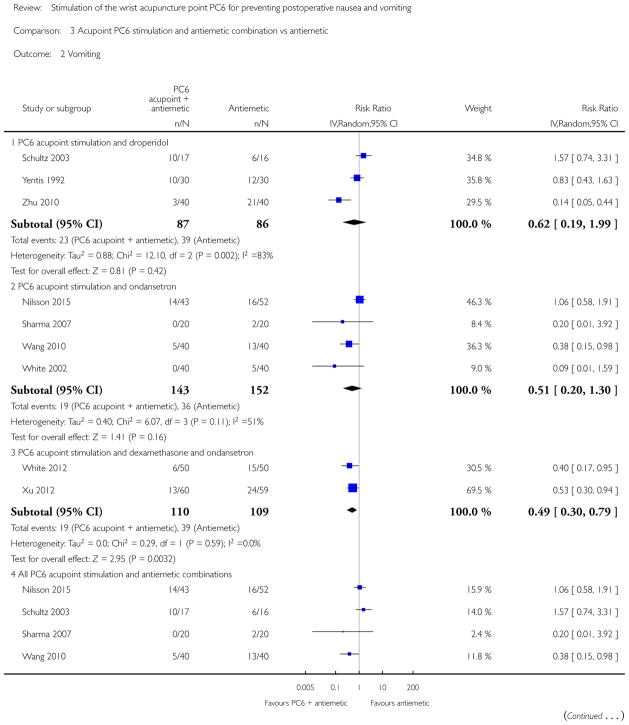
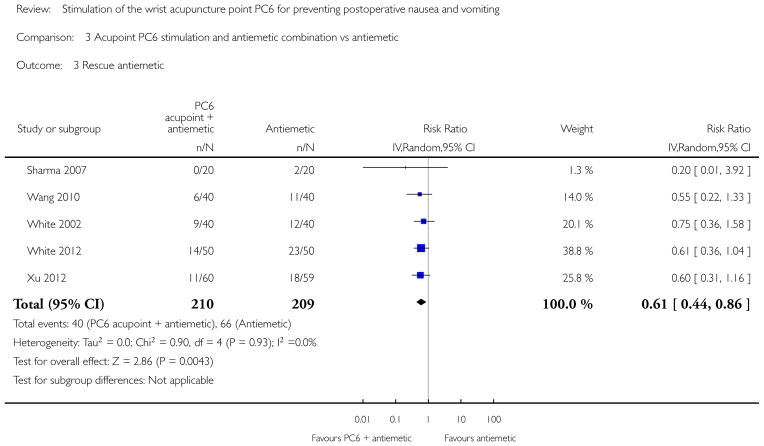
Compared to antiemetic drugs, the combination of PC6 acupoint stimulation and antiemetic therapy reduced the incidence of vomiting (RR 0.56, 95% CI 0.35 to 0.91; 9 trials, 687 participants) but not nausea (RR 0.79, 95% CI 0.55 to 1.13; 8 trials, 642 participants). We rated the quality of evidence as very low, due to substantial heterogeneity among trials, study limitations and imprecision. Using trial sequential analysis, none of the boundaries for benefit, harm or futility were crossed for PONV. The need for rescue antiemetic was lower in the combination PC6 acupoint stimulation and antiemetic group than the antiemetic group (RR 0.61, 95% CI 0.44 to 0.86; 5 trials, 419 participants).
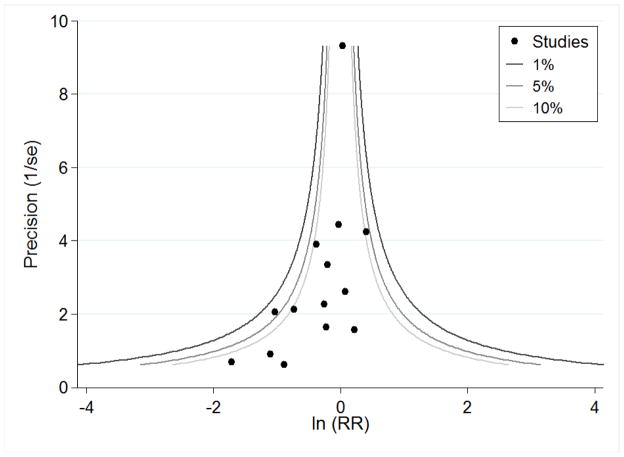
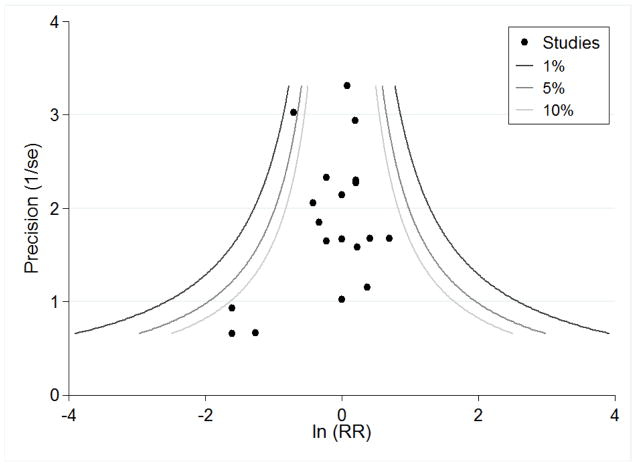
The side effects associated with PC6 acupoint stimulation were minor, transient and self-limiting (e.g. skin irritation, blistering, redness and pain) in 14 trials. Publication bias was not apparent in the contour-enhanced funnel plots.
Authors’ conclusions
There is low-quality evidence supporting the use of PC6 acupoint stimulation over sham. Compared to the last update in 2009, no further sham comparison trials are needed. We found that there is moderate-quality evidence showing no difference between PC6 acupoint stimulation and antiemetic drugs to prevent PONV. Further PC6 acupoint stimulation versus antiemetic trials are futile in showing a significant difference, which is a new finding in this update. There is inconclusive evidence supporting the use of a combined strategy of PC6 acupoint stimulation and antiemetic drug over drug prophylaxis, and further high-quality trials are needed.
INDEX TERMS: Medical Subject Headings (MeSH), *Acupuncture Points, *Wrist, Antiemetics [therapeutic use], Postoperative Nausea and Vomiting [*prevention & control], Randomized Controlled Trials as Topic
MeSH check words: Humans
PLAIN LANGUAGE SUMMARY
Wrist PC6 acupuncture point stimulation to prevent nausea and vomiting after surgery
Review question
Does a review of the evidence support the use of wrist PC6 acupuncture point stimulation (PC6 acupoint) as effective in reducing nausea and vomiting after surgery (PONV), compared to sham (dummy acupoint stimulation) or antiemetics (drugs that relieve nausea and vomiting) in people undergoing surgery? This review updates the evidence published in 2009, and is current to December 2014.
Background
Nausea and vomiting are two of the most common complications (up to 80%) after anaesthesia and surgery. Antiemetics are only partially effective and may cause adverse effects, like sedation and headache. Stimulating a PC6 acupoint, an alternative method, has been reported to reduce PONV with few serious side effects.
Study characteristics
We found 59 relevant studies, conducted between 1986 and 2015, involving 7667 participants undergoing elective surgery. Seven of the trials were conducted in 727 children. The PC6 acupoint stimulation varied from invasive techniques, such as traditional acupuncture needles, to noninvasive techniques, such as acupressure wristbands. PC6 acupoint stimulation was compared with six different types of antiemetic drugs (metoclopramide, cyclizine, prochlorperazine, droperidol. ondansetron and dexamethasone).
Key findings and quality of evidence
Effects of PC6 acupoint stimulation versus sham on PONV
We found a moderate-size effect in children and adults, although there were concerns about study limitations and unexplained variation in the effects. Further studies with sham comparisons are not necessary to confirm this beneficial effect.
Effects of PC6 acupoint stimulation versus antiemetic on PONV
We found no difference in the incidence of PONV. We rated the quality of this evidence as moderate, due to study limitations. Further studies are unlikely to show a difference.
Effects of combining PC6 acupoint stimulation and antiemetic versus antiemetic on PONV
We found a moderate-size effect on postoperative vomiting but not on postoperative nausea. However, there were concerns about study limitations, unexplained variation in effects between studies, and an insufficient number of studies. Further high-quality research on combinations of PC6 acupoint stimulation and antiemetics are needed to reduce uncertainties about this effect on PONV.
Overall, the side effects related to PC6 acupoint stimulation were minor, transient and self-limiting (e.g. skin irritation, blistering, redness and pain) in 14 studies.
Conclusion
To prevent PONV, the effect of PC6 acupoint stimulation is comparable to antiemetics.
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON [Explanation]
| Acupoint PC6 stimulation versus sham for preventing postoperative nausea and vomiting | ||||||
|---|---|---|---|---|---|---|
| Patient or population: People at risk of postoperative nausea and vomiting Settings: Surgery Intervention: Acupoint PC6 stimulation Comparison: Sham | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sham | Acupoint PC6 stimulation | |||||
| Nausea - All trials | Low | RR 0.68 (0.60 to 0.77) | 4742 (40 studies) | ⊕⊕○○ low1,2 |
||
| 200 per 1000 | 136 per 1000 (120 to 154) | |||||
| Moderate | ||||||
| 400 per 1000 | 272 per 1000 (240 to 308) | |||||
| High | ||||||
| 600 per 1000 | 408 per 1000 (360 to 462) | |||||
| Vomiting - All trials | Low | RR 0.60 (0.51 to 0.71) | 5147 (45 studies) | ⊕⊕○○ low2,3 |
||
| 200 per 1000 | 120 per 1000 (102 to 142) | |||||
| Moderate | ||||||
| 400 per 1000 | 240 per 1000 (204 to 284) | |||||
| High | ||||||
| 600 per 1000 | 360 per 1000 (306 to 426) | |||||
| Rescue antiemetics | 329 per 1000 | 210 per 1000 (181 to 240) | RR 0.64 (0.55 to 0.73) | 4622 (39 studies) | ⊕⊕○○ low4,5 |
|
| Adverse effects | Not estimable | Not estimable | Not estimable | 35 studies6 | Not applicable | See footnote6 |
The basis for the assumed risks for nausea and vomiting is from a consensus panel (Gan 2014) using Apfel’s simplified risk score (Apfel 1999). The assumed risk for rescue antiemetic is the median sham group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio;
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. |
Of the 40 trials, 13 had one or more high risk of bias domains (downgrade 1 point due to study limitations).
Substantial amount of heterogeneity (downgrade 1 point due to inconsistency).
Of the 45 trials, 16 had one or more high risk of bias domains (downgrade 1 point due to study limitations).
Moderate amount of heterogeneity (downgrade 1 point due to inconsistency).
Of the 39 trials, 13 had one or more high risk of bias domains (downgrade 1 point due to study limitations).
Twenty-two trials reported no adverse side effects. Minor, self-limiting and transient adverse effects reported in 13 studies (haematoma, redness, irritation and pain at acupuncture site; redness, swelling, discomfort, blistering at acupoint site when wearing acupressure wristband).
BACKGROUND
Description of the condition
Postoperative nausea and vomiting (PONV) are common complaints after general, regional, or local anaesthesia (Watcha 1992), with incidences up to 80% (Sadhasivam 1999). PONV may lead to delayed recovery from anaesthesia and surgery, unanticipated readmission to hospital and increased overall healthcare costs (Gan 2014).
Drug therapy is only partially effective in preventing or treating PONV (Gin 1994). A systematic review of antiemetic drugs for PONV (Carlisle 2006) showed that eight drugs effectively prevented PONV when compared to placebo: droperidol, metoclopramide, ondansetron, tropisetron, dolasetron, dexamethasone, cyclizine, and granisetron. The risk ratios (RRs) varied between 0.60 and 0.80, depending on the drug and the outcome (Carlisle 2006). Evidence for side effects was sparse: droperidol was sedative (RR 1.32, 95% confidence interval (CI) 1.16 to 1.51) and headache was more common after ondansetron (RR 1.16, 95% CI 1.03 to 1.30) (Carlisle 2006). More recently, a multidisciplinary panel of experts produced guidelines for the prevention or minimization of PONV using prophylactic or rescue therapy, either separately or in combination with non-pharmacological approaches (Gan 2014).
Description of the intervention
As anaesthetists continue to search for more cost-effective approaches to improving patient outcomes, attention has focused on simple, inexpensive, and non-invasive methods to prevent PONV. Concern about the cost and side effects of drugs has led to interest in the use of alternative approaches to preventing emesis.
Various non-pharmacological techniques have been examined in trials as alternatives to antiemetic drugs. These include acupuncture, electro-acupuncture, laser acupuncture, transcutaneous electrical nerve stimulation (TENS), electro-acupoint stimulation, acupressure, and capsicum plaster. Most non-pharmacological studies have focused on stimulation of the wrist at the ‘Pericardium (PC6) acupuncture point’ to reduce nausea and vomiting. The PC6 acupoint lies between the tendons of the palmaris longus and flexor carpi radialis muscles, 4 cm proximal to the wrist crease (Yang 1993).
How the intervention might work
The mechanism by which PC6 acupoint stimulation prevents PONV has not been established in ‘Western’ evidence-based methodology. However, according to Traditional Chinese Medicine theory, surgery interrupts the balanced state of the human body by disturbing the movement of both qi (energy flow) and blood, leading to stomach qi going upward to cause nausea and vomiting (Lv 2013). By regulating the function of the stomach to reduce the adverse flow of qi, PC6 acupoint stimulation may prevent nausea and vomiting (Lv 2013). Other acupoints believed to prevent PONV include Shenmen (H7) (Ming 2002) and Shang Wen (CV13) (Somri 2001).
Why it is important to do this review
Despite supportive literature for the use of PC6 acupoint stimulation in recent consensus guidelines for the management of PONV (Gan 2014), there is currently a lack of widespread uptake of the technique. This may be due to a lack of evidence on the optimal timing, duration and method of PC6 acupoint stimulation (Streitberger 2011), and preference of anaesthesiologists for an immediate pharmacokinetic effect of an antiemetic over a slower onset of PC6 acupoint stimulation effect.
One of the earliest systematic review (Vickers 1996), using a ‘vote counting’ approach, suggested that acupuncture may not be effective in the prevention of PONV. However, the vote-counting approach is not considered an acceptable method of summarizing the results of a systematic review (Petitti 1994).
Our previous systematic review of trials (Lee 1999), including trials published up to 1997, showed no difference between PC6 acupoint stimulation and commonly-used antiemetic drugs in preventing PONV after surgery. This review also indicated that the technique was more effective than placebo (sham treatment or no treatment) in preventing PONV in adults but not in children. However, these results in children were questionable, as they were based largely on trials in which PC6 acupoint stimulation occurred while the central nervous system was depressed by general anaesthesia (White 1999). Another major limitation of our earlier review was that we included both no-treatment and sham-treatment groups. Therefore, we may have overestimated the treatment effect of PC6 acupoint stimulation.
In the last Cochrane review update (Lee 2009) of 40 trials (n = 4858), we showed that there were significant reductions in the incidences of nausea (RR 0.71, 95% CI 0.61 to 0.83), vomiting (RR 0.70, 95% CI 0.59 to 0.83), and the need for rescue antiemetics (RR 0.69, 95% CI 0.57 to 0.83) in the PC6 acupoint stimulation group compared with the sham treatment group. Compared to antiemetic drugs, the incidence of nausea (RR 0.82, 95% CI 0.60 to 1.13), vomiting (RR 1.01, 95% CI 0.77 to 1.31) or the need for rescue antiemetics (RR 0.82, 95% CI 0.59 to 1.13) were similar in the PC6 acupoint stimulation group. Publication bias may have affected the risk ratio estimated for postoperative nausea but not for vomiting (Lee 2006) in the first version of the review published in 2004 (Lee 2004). However, in the next version (Lee 2009), publication bias was not apparent from the contour-enhanced funnel plots.
The rationale for conducting this Cochrane review update was to establish if there is firm evidence for the effect of PC6 acupoint stimulation in reducing the incidence of PONV using trial sequential analysis methodology. We were concerned that repeated updates (Lee 2004; Lee 2009) may introduce spuriously significant results (type 1 error) due to repeated significance testing.
OBJECTIVES
To determine the effectiveness and safety of PC6 acupoint stimulation with or without antiemetic drug versus sham or antiemetic drug for the prevention of PONV in people undergoing surgery.
METHODS
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) of techniques intended to stimulate the PC6 acupoint, compared with either sham treatment or antiemetic drugs, for the prevention of PONV. We defined ‘sham treatment’ as a device applied in a non-PC6 location, or any attempt to imitate (give the illusion of) PC6 acupoint stimulation. Therefore, for trials that assessed acupressure wristbands, we considered wristbands without studs placed at the PC6 acupoint as adequate sham treatment, and we included these trials in the review.
We excluded studies that only reported the severity of postoperative nausea or vomiting or both, and had not reported the incidence of postoperative nausea and vomiting or the need for rescue antiemetic.
Types of participants
We included all surgical patients without age limitation in the review. The age limits for children were defined by each study. We considered all types of surgery.
Types of interventions
Techniques intended to stimulate the PC6 acupoint: acupuncture, electro-acupuncture, laser acupuncture, transcutaneous electrical stimulation, conventional peripheral nerve stimulation, acu-stimulation device, acupressure, and capsicum plaster; versus sham treatment or drug therapy for the prevention of PONV. We grouped these diverse techniques as one entity in the main analysis, consistent with the concept that stimulating the correct acupuncture point is more important than the nature of the stimulus (Mann 1987). There was no restriction on the duration of PC6 acupoint stimulation or when it was applied.
Types of outcome measures
We performed separate meta-analyses for each of the following primary and secondary outcomes. Trials could report more than one primary or secondary outcome:
Primary outcomes
Incidence of postoperative nausea.
Incidence of postoperative vomiting, defined as either retching or vomiting, or both.
We did not combine postoperative nausea and vomiting as we could not be certain that participants who vomited were also nauseated. If the authors reported several incidences of the outcome measure (for example 0 to six hours, six to 24 hours, 0 to 24 hours), we used the longest cumulative follow-up data from the end of surgery (in this case, 0 to 24 hours).
Secondary outcomes
Need for rescue antiemetic drug when prophylaxis failed.
Adverse effects from PC6 acupoint stimulation or antiemetic drug, or both.
Search methods for identification of studies
Electronic searches
We searched the following for relevant trials on 31st December 2014:
The Cochrane Central Register of Controlled Trials (CENTRAL; Issue 12, 2014), in Appendix 1.
Electronic databases: OVID MEDLINE (January 2008 to December 2014), in Appendix 2; OVID EMBASE (January 2008 to December 2014), in Appendix 3; ISI Web of Science (January 2008 to December 2014), in Appendix 4)
World Health Organization Clinical Trials Registry and ClinicalTrial.gov
Reference lists of relevant articles, reviews, and trials.
We combined the following MeSH and text words with the filters for identifying randomized controlled trials: ‘postoperative complications’, ‘nausea and vomiting’, ‘acupuncture’, ‘acupuncture therapy’, ‘acupuncture points’, ‘acupressure’, ‘transcutaneous electric nerve stimulator’, and ‘electro-acupuncture’. There was no language restriction. We excluded studies of PC6 acupoint stimulation to treat established PONV, or to prevent intraoperative nausea or vomiting.
Searching other resources
We did not search for conference proceedings or seek unpublished trials. Grey literature has not been peer-reviewed and there is some evidence that it is of lower quality than published studies (McAuley 2000). Searching unpublished trials may not be worthwhile, as many unpublished trials are of poor or unclear methodological quality (Van Driel 2009).
Data collection and analysis
Selection of studies
We screened titles and abstracts of publications identified from the search, and selected trials that fulfilled our inclusion criteria. There was one disagreement between review authors for inclusion into this systematic review. The third review author adjudicated and decided that the study (Zhu 2010) met the inclusion criteria. We examined all selected trials for duplicate data; where we found duplication, we used the results of the main trial report.
Data extraction and management
We extracted data independently, using a standardized data collection form, and resolved any discrepancies in data extraction by discussion. We collected data on the type, duration, and timing of PC6 acupoint stimulation, as well as the type and dose of prophylactic antiemetic drug. We recorded general details of the participant population and type of surgery. We collected outcome measures as described above for each study group. We did not consider factors such as the severity of PONV or the number of episodes of vomiting. In studies with more than two groups, we avoided double-counting of participants by following the guidelines for analysis in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in included studies
We assessed the quality of the included trials independently, under open conditions. We graded the risk of bias for each study in the domains of sequence generation, allocation concealment, blinding of participants, healthcare providers, and outcome assessors, incomplete outcome data, selective outcome reporting, and comparison of baseline characteristics for each group in a ‘Risk of bias’ table (Higgins 2011). We graded each domain as low risk of bias, unclear (uncertain risk of bias) or high risk of bias, according to the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For summary assessment of the risk of bias within and across studies, we followed the approach outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and rated it as low, unclear or high risk of bias.
We used the GRADE approach to describe the overall quality of the outcome, rating it as high, moderate, low or very low (Guyatt 2011). To make this assessment, we examined the study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates and potential publication bias (Guyatt 2011). We downgraded the quality of evidence from high if there were deficiencies in these domains.
We included the following outcomes in Summary of findings for the main comparison, Summary of findings 2 and Summary of findings 3: incidence of postoperative nausea, incidence of postoperative vomiting, need for rescue antiemetic, and adverse effects.
Measures of treatment effect
For dichotomous data, we reported the risk ratio (RR) and the associated 95% confidence interval (95% CI).
Unit of analysis issues
None.
Dealing with missing data
We analysed data according to the intention-to-treat principle.
Assessment of heterogeneity
We measured heterogeneity using the I2 statistic, a measure of the proportion of total variation in the estimates of treatment effect that is due to heterogeneity between studies rather than due to chance. We described the level of heterogeneity as not important (I2 statistic from 0% to 40%), moderate (I2 statistic from 30% to 60%), substantial (I2 statistic from 50% to 90%) and considerable (I2 statistic from 75% to 100%) (Higgins 2011).
Assessment of reporting biases
We used the contour-enhanced funnel plot to differentiate asymmetry due to publication bias from that due to other factors (Peters 2008), using STATA statistical software (Stata Corporation, College Station, Texas, version 14). Contour-enhanced funnel plots display the area of statistical significance on a funnel plot to improve the correct identification of the presence or absence of publication bias. We used this in conjunction with the ‘trim and fill’ method (Duval 2000) to inform the likely location of missing studies, using STATA statistical software, as suggested by Peters 2008. Publication bias would be expected when the usual funnel plot is asymmetrical but assessment of the contour-enhanced funnel plot indicates that missing studies are located where non-significant studies would be plotted (Peters 2008).
Data synthesis
We used Review Manager 5 to perform the DerSimonian and Laird random-effects model meta-analyses of risk ratios, as we expected that the treatments and conditions in these trials would be heterogeneous. This model incorporates both between-study (different treatment effects) and within-study (sampling error) variability (Mosteller 1996).
We estimated the number needed to treat for an additional beneficial outcome (NNTB) for different baseline risks for nausea and vomiting, using the RR (Smeeth 1999) to assess whether PC6 acupoint stimulation is worthwhile for individuals. We estimated the 95% CI around the NNTB using the method outlined by Altman 1998.
We undertook trial sequential analysis (TSA) to estimate the required information size in meta-analysis, that is, the number of participants needed to provide a reliable and conclusive estimate (Afshari 2015). The required information size was based on a risk ratio reduction of 30% (Apfel 2007), an overall type 1 error of 5%, power at 80%, incidence in the control arm and a model-based heterogeneity correction, using Trial Sequential Analysis software (Thorlund 2011).
Subgroup analysis and investigation of heterogeneity
We undertook exploratory a priori subgroup analyses, which included trials in adults versus children, and trials according to type of PC6 acupoint stimulation (invasive versus noninvasive). To test whether the subgroups were different from one another, we tested the interaction using the technique outlined by Altman 2003.
Sensitivity analysis
We conducted sensitivity analyses to estimate the robustness of results according to the risk of bias (low, unclear, high) and to the control event rate (≤ 20%, > 20%).
RESULTS
Description of studies
Results of the search
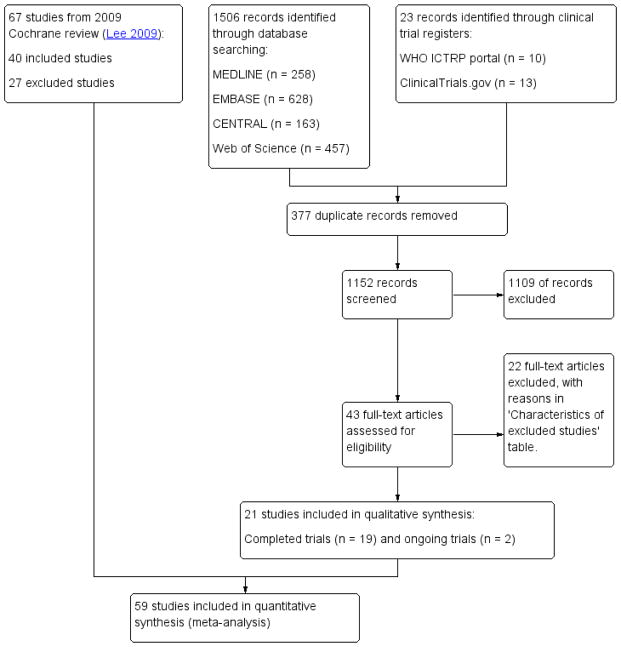
The search identified 43 studies for full-text review. Sixty-seven trials (40 included and 27 excluded) from our previous Cochrane review (Lee 2009) were brought forward for this systematic review. The flow chart (Figure 1) shows the results of the literature search (the number of hits) and the culling process to reduce the total to 59 included studies for meta-analysis. Ongoing trials are described in Characteristics of ongoing studies.
Figure 1.
Study flow diagram.
Table 1.
Characteristics of ongoing studies [ordered by study ID]
| Cooke 2014 | |
| Trial name or title | PC6 acupoint stimulation for prevention of postoperative nausea and vomiting in patients undergoing cardiac surgery |
| Methods | 2-centred, double-blinded, 2-arm, parallel-group RCT |
| Participants | People undergoing primary cardiac surgery |
| Interventions | Group 1: Seaband wristband applied bilaterally to PC6 acupoint on arrival to ICU, covered by light opaque bandage for 36 hours Group 2: Sham Seaband wristband without stud applied to both wrists on arrival to ICU, covered by light opaque bandage for 36 hours |
| Outcomes | Nausea (0 – 6 h, 0 – 12 h, 0 – 24 h, 0 – 36 h), vomiting (0 – 6 h, 0 – 12 h, 0 – 24 h, 0 – 36 h), rescue antiemetic (0 – 36 h) |
| Starting date | February 2015 |
| Contact information | m.cooke@griffith.edu.au |
| Notes | Rescue antiemetic includes metoclopramide, ondansetron, dexamethasone, droperidol. The trial is registered with the Australian and New Zealand Clinical Trials Registry (ACTRN12614000589684) |
| Lv 2013 | |
| Trial name or title | PC6 acupoint stimulation for prevention of postoperative nausea and vomiting in patients undergoing craniotomy |
| Methods | Single-centred, double-blinded, 5-arm, parallel-group RCT |
| Participants | People undergoing craniotomy |
| Interventions | Group 1: Ondansetron 8 mg IV before skin closure and PC6 acupuncture bilaterally for 30 min after regaining consciousness from general anaesthesia with stimulation every 10 min to keep de qi sensation Group 2: Ondansetron 8 mg IV before skin closure and sham PC6 acupuncture bilaterally for 30 min after regaining consciousness from general anaesthesia with no stimulation Group 3: Ondansetron 8 mg IV before skin closure and PC6 stimulation via active TENS electrodes bilaterally for 30 min after regaining consciousness from general anaesthesia with stimulation intensity and frequency set to when de qi sensation is felt Group 4: Ondansetron 8 mg IV before skin closure and PC6 stimulation via inactive TENS electrodes bilaterally for 30 min after regaining consciousness from general anaesthesia with no stimulation intensity and frequency Group 5: Ondansetron 8 mg IV before skin closure. |
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), rescue antiemetic (metoclopramide) |
| Starting date | January 2013 |
| Contact information | zhenjiuhuaxi@163.com. |
| Notes | Protocol published in Trials 2013 May 28;14:153. doi: 10.1186/1745-6215-14-153. This study is registered with the Chinese Clinical Trial Registry: ChiCTR-TRC-13003026 |
h = hours
ICU = Intensive care unit
IV = intravenous
PC6 = pericardium acupoint
RCT = randomized controlled trial
TENS = transcutaneous electrical nerve stimulation
Included studies
We include 59 trials conducted between 1986 and 2015, involving 7667 participants (see Characteristics of included studies). The median sample size of trials was 104 (interquartile range: 75 to 156). All trials but three (Gieron 1993; Kim 2004; Zhu 2010) were published in English. Most trials recruited healthy adults undergoing elective surgery. Seven trials recruited children (Butkovic 2005; Lewis 1991; Rusy 2002; Schlager 1998; Shenkman 1999; Wang 2002; Yentis 1992). Three trials recruited both children and adults (Amir 2007; Ebrahim Soltani 2010; Ravi 2010). Most participants had general anaesthesia. Women having elective Caesarean delivery received spinal anaesthesia in six studies (Direkvand-Moghadam 2013; Duggal 1998; El-Deeb 2011; Habib 2006; Harmon 2000; Ho 1996).
Table 2.
Characteristics of included studies [ordered by study ID]
| Adib-Hajbaghery 2013 | ||
| Methods | Parallel-group, blinded randomized trial, conducted in Iran. Study dates not reported | |
| Participants | 88 people aged 15 – 70 years undergoing appendectomy under general anaesthesia. Exclusion: past history of nausea and vomiting in the past 24 h, prior use of acupressure or acupuncture, history of gastrointestinal or ear disorders, neurological impairment, fever, unanticipated perioperative complications |
|
| Interventions | Acupressure wristband placed at P6 points on both wrists, applied in the recovery room when participant was awake and removed after 7 hours following surgery (n = 44) Sham group was acupressure wristbands without bead on P6 points applied to both wrists, applied in the recovery room when participant was awake and removed after 7 hours following surgery (n = 44) |
|
| Outcomes | Nausea (0 – 7 h), vomiting (0 – 7 h), risk of rescue antiemetic (metoclopramide 10 mg) | |
| Notes | No power calculation done. Funding sources not declared. Authors declare no conflict of interest in the study | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were “randomly allocated to two groups using a dice (odd numbers to the acupressure group and even numbers to the control group.” |
| Allocation concealment (selection bias) | Unclear risk | Insuffient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Participants blinded to intervention. |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “Staff blinded to grouping”. |
| Blinding of outcome assessor (detection bias) All outcomes |
High risk | Researcher and nurse likely to know allocation group. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | Insufficient information. |
| Selective reporting (reporting bias) | High risk | Participant discomfort with wristbands monitored by researchers every 2 hours but not reported |
| Other bias | Low risk | Baseline characteristics for age, body mass index, duration of anaesthesia and incision length were comparable |
| Agarwal 2000 | ||
| Methods | Parallel-group randomized trial, conducted in India. Study dates not reported | |
| Participants | 200 people undergoing endoscopic urological surgery. Exclusion: patient refusal to participate in study, previous history of PONV and motion sickness, impaired renal function with increased urea and creatinine concentrations, diabetes mellitus, obesity, patients receiving antiemetic medication, histamine H2 receptor antagonist within 72 hours of surgery. No participant withdrew from the study. |
|
| Interventions | Acupressure wristband placed at P6 points on both forearms, applied 30 min before induction of anaesthesia and removed after 6 hours following surgery (n = 100) Sham group was the spherical bead of acupressure wristbands placed on posterior surface, applied 30 min before induction of anaesthesia and removed 6 hours after surgery (n = 100) |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), side effects of acupressure, risk of rescue antiemetic drug | |
| Notes | Rescue antiemetic was ondansetron 4 mg IV. No side effects or complications noted in either group. No details about funding source or any declarations of interest among authors | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were assigned to two different groups according to a computer-generated table of random numbers” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “An anesthesiologist blinded to the therapy registered the incidence of nausea and vomiting at three different times in the first 24 hr postoperatively: on arrival of the patient in PACU, and at six hours (time of removal of acupressure wristband) and 24 hr after operation” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | “No patient was excluded after admission to the study”. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable: “Patients were comparable in both the groups as regards to age, sex, height and weight” |
| Agarwal 2002 | ||
| Methods | Parallel-group randomized trial, conducted in India. Study dates not reported | |
| Participants | 150 adults undergoing laparoscopic cholecystectomy. Exclusion: patient refusal to participate in study, previous history of PONV and motion sickness, impaired renal function with increased urea and creatinine concentrations, diabetes mellitus, obesity, patients receiving antiemetic medication, histamine H2 receptor antagonist within 72 hours of surgery |
|
| Interventions | Acupressure wristband placed at P6 points on both forearms, applied 30 min before induction of anaesthesia and removed after 6 hours following surgery (plus normal saline 1 mL IV just before induction of anaesthesia) (n = 50) Sham group was the spherical bead of acupressure wristbands placed on posterior surface, applied 30 min before induction of anaesthesia and removed 6 hours after surgery (plus normal saline 1 mL IV just before induction of anaesthesia) (n = 50) Antiemetic group was ondansetron 4 mg IV just before induction of anaesthesia (plus sham treatment outlined above) (n = 50) |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), risk of rescue antiemetic drug | |
| Notes | Rescue antiemetic was ondansetron 4 mg IV if participant vomited more than once. No side effects or complications noted in any of the groups. Data for outcome (0 – 24 h) obtained by correspondence with author. No details about funding source or any declarations of interest among authors | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomised into three groups of 50 each using a table of random numbers..” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “The incidence of PONV was evaluated by a blinded observer”. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for 150 patients randomized. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable: “Patients were comparable in both the groups as regards to age, sex, height, weight and duration of surgery” |
| Alkaissi 1999 | ||
| Methods | Parallel-group randomized trial, conducted in Sweden. Study dates not reported | |
| Participants | 60 women undergoing day-case minor gynaecological surgery. Exclusion: patients undergoing local anaesthesia and those given prophylactic antiemetic during anaesthesia (n = 10, replaced by randomizing another 10 participants at the end of the study) |
|
| Interventions | Acupressure wristband placed at P6 point on both forearms. Applied before surgery and left on for 24 hours. Draped with a dressing during the stay in the hospital (n = 20) Sham acupressure applied to dorsal side of forearms. Applied before surgery and left on for 24 hours. Draped with a dressing during the stay in the hospital (n = 20) Reference group were informed and anaesthetized in the same way as the other 2 groups (n = 20) |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), risk of rescue antiemetic drugs | |
| Notes | Rescue antiemetics were metoclopramide 10 mg IV at participant’s request; if not effective, then given droperidol 1.25 mg IV. Reference group received no treatment and were not included in data analysis. No details about funding source or any declarations of interest among authors | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “The nurses who asked the patients about nausea, and administered antiemetics on the postoperative ward were not aware of which treatment the patient received or where the PC6 acupoint is located” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “The nurses who asked the patients about nausea, and administered antiemetics on the postoperative ward were not aware of which treatment the patient received or where the PC6 acupoint is located”. These nurses also noted vomiting episodes |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Reasons were given for 10 dropouts, who were replaced by randomising another 10 participants at the end of the study. “The dropouts were evenly distributed between the groups.” No missing data reported for 60 participants analysed |
| Selective reporting (reporting bias) | High risk | Primary outcome (PONV) reported. Description of side effects not given |
| Other bias | Low risk | Demographic data appeared to be comparable. |
| Alkaissi 2002 | ||
| Methods | Parallel-group randomized trial, conducted in Sweden. Study dates not reported | |
| Participants | 410 women undergoing elective gynaecological surgery. No exclusion criteria specified. 30 participants were withdrawn because they were: given local anaesthesia (n = 12), or an antiemetic was given without the criteria for treatment of PONV being met (n = 14), malignant hyperthermia (n = 1), allergy to latex (n = 2), and could not read Swedish (n = 1). These 30 participants were replaced by another 30 at the end of the study period |
|
| Interventions | Acupressure wristband placed on P6 point on both forearms just before start of anaesthesia, left on for 24 h (n = 135) Sham group included acupressure wristbands at non-acupoint on both forearms just before start of anaesthesia, left on for 24 h (n = 139) Reference group received no prophylactic treatment and was not blinded (n = 136) |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), side effects of acupressure, risk of rescue antiemetic (type of drug not described) | |
| Notes | Reference group received no treatment and were not included in data analysis. Adverse effects: wristbands felt uncomfortable, produced red indentation, or caused itching, headache and dizziness, or wrists hurt and tightness of wristband caused swelling or deep marks or blistering at site of stud Financial support was provided by the County Council of Östergötland (Project F98–305) Sweden. No details about any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | “The wrists were wrapped for blinding”. Participants reported outcomes |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “The wrists were wrapped for blinding”. Participants reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Reasons were given for 30 dropouts, who were replaced by randomising another 30 participants at the end of the study. “Withdrawals were evenly distributed between the groups.” No missing data reported for 410 participants analysed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Demographic data appeared to be comparable in Table 2. |
| Allen 1994 | ||
| Methods | Parallel-group randomized trial, conducted in England. Study dates not reported | |
| Participants | 46 women undergoing gynaecological surgery. Exclusions: previous exposure to elasticized wristbands for the prevention of motion sickness |
|
| Interventions | Acupressure wristband placed on P6 point of dominant arm before premedication (90 min before surgery) (n = 23). Duration of treatment not given Sham acupressure wristband placed on dorsum of dominant wrist before premedication (n = 23). Duration of treatment not given |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h). | |
| Notes | Rescue antiemetic was prochlorperazine 12.5 mg IM 4-hourly when necessary. More than 1 dose of prochlorperazine data given (not included in data analysis). No details about funding source or any declarations of interest among authors | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar in participants with no previous experience with this form of acupressure |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | “No patient refused to participate in the study, nor were there any withdrawals” |
| Selective reporting (reporting bias) | High risk | Risk of rescue antiemetic drug (1 or more doses) was not given in the results. Description of side effects not reported |
| Other bias | Low risk | Baseline characteristics were comparable. “The ages and weights of the patients in the two groups were comparable..” |
| Amir 2007 | ||
| Methods | Parallel-group randomized trial, conducted in India. Study dates not reported | |
| Participants | 40 children and adults undergoing middle ear surgery. Exclusion: People with cardiovascular disease, central nervous system problems, previous history of PONV and/or motion sickness, and smokers. No details about withdrawals or loss to follow-up |
|
| Interventions | Group 1: electro-acupuncture at frequency of 4 Hz and current intensity increased to a degree just less than what caused discomfort, given 20 min before induction for duration of surgery (n = 20) Group 2: sham electro-acupuncture. No details given except that participants experienced needle pricks (n = 20) |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), risk of rescue antiemetic drug (0 – 24 h), risk of adverse effects | |
| Notes | Rescue antiemetic was ondansetron 4 mg IV after first episode of PONV and repeated when necessary at 6-hourly intervals. No side effects in sham electro-acupuncture group. Erythema occurred in 3 participants in the electro-acupuncture group No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Informed consent was taken from the selected patients and they were divided into two groups of twenty each using a computer-generated table of random numbers” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “A blinded observed collected postoperative data of PONV”. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for the 20 participants randomized to each group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “Differences in mean age, weight, sex and duration of surgery were statistically insignificant” |
| Andrzejowski 1996 | ||
| Methods | Parallel-group randomized trial, conducted in United Kingdom. Study dates not reported | |
| Participants | 36 women undergoing total abdominal hysterectomy. Exclusions: metal or elastoplast allergy, anticoagulant therapy, local skin disease at P6 acupoint or sham point, or chronic treatment with antiemetics |
|
| Interventions | Semipermanent acupuncture needle inserted at P6 acupoint on both wrists 20 min before induction, left in place until second postoperative day (n = 18) Sham semipermanent acupuncture needle inserted in sham point 20 min before induction, left in place until second postoperative day (n = 18) |
|
| Outcomes | Nausea (0 – 8 h), vomiting (0 – 8 h), risk of antiemetic rescue drug, side effects | |
| Notes | Antiemetic rescue was prochlorperazine 12.5 mg IM when necessary. No side effects reported with interventions. No details about funding source or any declarations of interest among authors | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. “Patients were allocated randomly into one of two groups” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. “This was achieved by concealing the assignment schedule in sealed envelopes which were opened by the investigator just before inserting the needles”. Comment: not sure if envelopes were sequentially numbered and opaque |
| Blinding of patients (performance bias) All outcomes |
Low risk | Assessments were made by the participants, who were blinded to their treatment |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | Assessments were made by the participants, who were blinded to their treatment |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for 36 participants randomized. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “There was no significant difference between the two groups in age, weight, total morphine consumed, or duration of anaesthesia” |
| Arnberger 2007 | ||
| Methods | Parallel-group randomized trial, conducted in Switzerland and Austria. Study dates not reported | |
| Participants | 220 women undergoing elective gynaecological and abdominal laparoscopic surgery of more than 1 hour duration. Exclusion: pregnant and breast-feeding women, and women with eating disorders, obesity (BMI > 35kg/m2), severe renal or liver impairment, central nervous system injury, vertebrobasilar artery insufficiency, vestibular disease, cytostatic therapy, and preoperative vomiting or antiemetic therapy. No participant withdrew from study |
|
| Interventions | P6 group: during anaesthesia, neuromuscular blockade was monitored by a conventional nerve stimulator at a frequency of 1 Hz over the median nerve (first electrode 1 cm proximal to P6 acupoint and second electrode placed 2 cm distal to the P6 acupoint) on the dominant hand (n = 110) Sham group: during anaesthesia, neuromuscular blockade was monitored by a conventional nerve stimulator at a frequency of 1 Hz over the ulnar nerve (first electrode 1 cm proximal to the point at which the proximal flexion crease of the wrist crosses the radial side of the tendon to the flexor carpi ulnaris muscle at the volar side of the wrist and second electrode placed 3 cm proximal to the distal electrode) on the dominant hand (n = 110) |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), risk of rescue antiemetic drug (0 – 24 h), risk of adverse effects | |
| Notes | Rescue antiemetic was ondansetron 4 mg IV if 2 or more episodes of vomiting or persistent nausea; with repetition after 2 hours. No local irritation, redness, contact dermatitis or muscle ache (side effects) were recorded. Nausea (0 – 6 h), vomiting (0 – 6 h), and incidence of rescue antiemetic (0 – 6 h) also reported Support was provided solely from institutional sources. Authors declared no conflict of interests |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “After induction of anaesthesia, patients were assigned to one of two groups using a set of computer-generated random numbers” |
| Allocation concealment (selection bias) | Low risk | “The assignments were kept in sealed, sequentially numbered envelopes until used, and the envelope numbers with the assignment were recorded” |
| Blinding of patients (performance bias) All outcomes |
Low risk | “Patients and PONV evaluators were not informed of the group assignments” |
| Blinding of healthcare providers (performance bias) All outcomes |
High risk | “The attending anaesthesiologist could not be blinded to the group assignment, but he or she was not involved with the PONV assessment” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “Patients and PONV evaluators were not informed of the group assignments” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | “Two hundred twenty patients were recruited for this study without any dropout over the observation period” |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “Demographic and morphometric characteristics and factors likely to influence PONV were similar in the two groups” |
| Barsoum 1990 | ||
| Methods | Parallel-group randomized trial, conducted in England. Study dates not reported | |
| Participants | 162 people undergoing general surgery. 10 participants withdrew because of language or age difficulty with completing analogue score, premature removal of wristbands, and incomplete follow-up data | |
| Interventions | Acupressure wristbands placed on P6 acupoint of both wrists in the recovery room (n = 49) Sham acupressure wristbands (no studs) were applied to both wrists in the recovery room and antiemetics given only if clinically required (n = 54) Antiemetic group was given prochlorperazine 12.5 mg IM with each postoperative opiate injection and when clinically required, and wore an acupressure band without stud on both wrists in the recovery room (n = 49) |
|
| Outcomes | Vomiting (0 – 24 h), risk of rescue antiemetic (prochlorperazine) | |
| Notes | Nausea scores were reported for those participants who could not eat. Number of participants who were free of nausea was not given. Vomiting on postoperative day 2 and 3 also reported. 4 participants reported some local tightness and discomfort (1 of these experienced carpal tunnel-like symptoms) No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar and all participants were told that they were wearing wristbands to try to prevent PONV |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Reasons for withdrawals were given. No missing data reported for the 152 participants analysed |
| Selective reporting (reporting bias) | High risk | Severity of nausea was reported but risk of nausea was not. |
| Other bias | Low risk | Baseline characteristics appeared to be comparable. “It can be seen that the groups were comparable with regard to the range of operation and anaesthetic agents used” |
| Butkovic 2005 | ||
| Methods | Parallel-group randomized trial, conducted in Croatia. Study dates not reported | |
| Participants | 120 children (5 – 14 years) undergoing hernia repair, circumcision, or orchidopexy. Exclusion: children predisposed to nausea and vomiting secondary to gastroesophageal reflux, motion sickness, and inner ear or central nervious system disorders |
|
| Interventions | Group 1: laser acupuncture on P6 acupoint bilaterally for 1 min, 15 min before induction of anaesthesia and IV infusion of saline (n = 40) Group 2: metoclopramide 0.15 mg/kg IV and sham laser on P6 acupoint bilaterally for 1 min, 15 min before induction of anaesthesia (n = 40) Group 3: sham laser stimulation on P6 acupoint bilaterally for 1 min, 15 min before induction of anaesthesia and saline infusion (n = 40) |
|
| Outcomes | Vomiting (0 – 2 h), risk of rescue antiemetic drug. | |
| Notes | Rescue antiemetic was ondansetron 0.1 mg/kg IV if vomiting was severe. No details about funding source or any declarations of interest among authors | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make intervention appear similar |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “Researchers were double-blinded” but no specific details about how blinding was achieved. Comment: probably done |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “Researchers were double-blinded” but no specific details about how blinding was achieved. Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for the 120 children analysed. |
| Selective reporting (reporting bias) | High risk | Description of side effects not included. Nausea not reported because it may be difficult to assess in children |
| Other bias | Low risk | Baseline characteristics were comparable. “Demographic data showed no significant difference among groups” |
| Direkvand-Moghadam 2013 | ||
| Methods | Parallel 3-arm randomized trial, conducted in Iran. Study conducted from September 2011 to October 2012 | |
| Participants | 102 healthy women, aged 18 – 35 years, at first to fourth pregnancy, with normal foetal heart rates, undergoing Caesaren delivery with spinal anaesthesia between 29 September 2011 to 23 October 2012 at University Hospital of Ilam, West of Iran. Exclusion: Acute or chronic diseases associated with nausea and vomiting, carpal tunnel syndrome, preoperative opioids, weights < 50 kg or > 100 kg |
|
| Interventions | Group 1: No P6 treatment group (n = 34) Group 2: Metoclopramide IV before spinal anaesthesia induction (n = 34) Group 3: P6 acupressure wristbands applied to both wrists 15 min before spinal anaesthesia induction and removed 6 hours after surgery (n = 34) |
|
| Outcomes | Nausea (0 – 6 h), vomiting (0 – 6 h), risk of rescue antiemetic drugs (0 – 6 h) | |
| Notes | All treatment groups were used in the analysis. Details of exact type of rescue antiemetic were not given. Power calculation done. Funding from Ilam University of Medical Sciences. No financial or other competing interests declared by authors | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomly assigned to one of the three groups by a trained midwife, with 34 cases in each group, at the obstetrical triage unit, by using a random number chart” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Unclear risk | Insufficent information. |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “The researcher was not aware of grouping of participants”. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “The data collection was carried out by a trained midwife who was not also aware of each medication and who had no idea about the plan of the study. ” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | “None of the 102 enrolled parturients were withdrawn for any reason” |
| Selective reporting (reporting bias) | High risk | Description of side-effects of acupressure or metoclopramide were not reported |
| Other bias | Low risk | Baseline characteristics (age, weight, height, gestational age, duration of surgery” were comparable |
| Duggal 1998 | ||
| Methods | Parallel-group randomized trial, conducted in Canada. Study dates not reported | |
| Participants | 263 women undergoing spinal anaesthesia for elective Caesarean delivery. Excluded: women with a history of hyperemesis gravidarum or if they had received antiemetic medication during the 48 h before surgery. 8 women excluded for failing to wear wristbands for 10 hours, 3 had received prophylactic antiemetics, and 8 were not given standard combination of intrathecal drugs (total 19 withdrawals) |
|
| Interventions | Acupressure wristbands were applied to both wrists just before induction of spinal anaesthesia and worn for 10 hours (n = 122) Sham acupressure wristbands were applied at P6 acupoint (but stud missing) on both wrists just before induction of spinal anaesthesia and worn for 10 hours (n = 122) |
|
| Outcomes | Nausea (0 – 10 h), vomiting (0 – 10 h), risk of rescue antiemetic (type of drug not given), side effects of acupressure. Patients recorded outcome measures on a questionnaire | |
| Notes | Adverse effects of acupressure wristbands: tightness, swollen hands, problems with infusion, itching wrists. Intraoperative nausea and vomiting reported Funding by a grant from the BC Medical Services Foundation. Wristbands were donated by Sea Band UK Ltd. No details of any declarations of interest among the authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A table of random numbers was used to allocate patients to one of two groups” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | “The nature of the bands was therefore unknown to the patient, anaesthetist and investigators for the duration of the study” |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “The nature of the bands was therefore unknown to the patient, anaesthetist and investigators for the duration of the study” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “The nature of the bands was therefore unknown to the patient, anaesthetist and investigators for the duration of the study” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Reasons for withdrawals were given. No missing data reported for the 244 participants analysed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “Demographic analysis revealed no statistically significant difference between subjects in the two groups” |
| Dundee 1986 | ||
| Methods | Parallel-group randomized trial, conducted in Ireland. Study dates not reported | |
| Participants | 75 women undergoing minor gynaecological surgery. | |
| Interventions | Group 1: acupuncture at P6 acupoint with 5 min manual stimulation (1.2 cm 30 gauge needle) after premedication with nalbuphine 10 mg (n = 25) Group 2: sham acupuncture at a dummy point on lateral elbow crease with 5 min manual stimulation (1.2 cm 30 gauge needle) after premedication with nalbuphine 10 mg (n = 25) Group 3: no further treatment after premedication with nalbuphine 10 mg (n = 25) |
|
| Outcomes | Nausea (0 – 6 h), vomiting (0 – 6 h), side effects of treatment | |
| Notes | No side effects noted in either group. Group 3 data were excluded from data analysis. Presence or absence of needle marks and its location may have been observed by the outcome assessor No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | The authors took adequate steps to make interventions appear similar |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “Their assessments were performed by an observer who was unaware of which patients had undergone acupuncture” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for 75 participants analysed. |
| Selective reporting (reporting bias) | Unclear risk | No details about the use of rescue antiemetic in anaesthetic protocol. The risk of rescue antiemetic drug not reported |
| Other bias | Low risk | “The groups were comparable in average age, weight, and duration of anaesthesia” |
| Dundee 1989 | ||
| Methods | Parallel-group randomized trial, conducted in Ireland. Study dates not reported | |
| Participants | 155 women undergoing minor gynaecological surgery. | |
| Interventions | Acupuncture at P6 acupoint with 5 min manual stimulation after premedication (n = 31) Electroacupuncture at P6 acupoint for 5 min after premedication (n = 31) Antiemetic group 1 had cyclizine 50 mg IM after premedication (n = 31) Antiemetic group 2 had metoclopramide 10 mg IM after premedication (n = 31) Reference group had no treatment (n = 31). |
|
| Outcomes | Nausea (0 – 6 h), vomiting (0 – 6 h), side effects of treatment | |
| Notes | For data analysis purposes, manual acupuncture and electro-acupuncture were combined. Reference group received no treatment and were not included in data analysis. This paper reported both controlled and uncontrolled studies of P6 stimulation. Used original data from secondary papers related to this study (Dundee 1989) (note that metoclopramide group was not included in this trial, but the results of other groups are the same). According to the authors, there were no side effects associated with acupuncture groups but some participants complained of drowsiness following antiemetic drug administration. For data analyses, manual acupuncture group was compared with cyclizine, and electroacupuncture group was compared with metoclopramide No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “Patients were visited at 1 h and 6 h after operation by a person who was unaware of the preoperative treatment” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for 155 participants analysed. |
| Selective reporting (reporting bias) | Unclear risk | No details about the use of rescue antiemetic in anaesthetic protocol. The risk of rescue antiemetic drug not reported |
| Other bias | Unclear risk | Demographic comparisons between groups were not given. |
| Ebrahim Soltani 2010 | ||
| Methods | Parallel 4-group randomized trial, conducted in Iran. Study conducted in Iran during 2007 to 2008 | |
| Participants | 200 participants aged 10 – 60 years old, with ASA physical status I to II, undergoing strabismus surgery. Exclusion criteria: nausea or vomiting within 1 week of surgery, local infection near acupoint, symptomatic comorbidities, travel sickness, length of stay in the recovery room more than 2 hours or those receiving any medical therapy before surgery | |
| Interventions | Group 1: sham acupressure wristbands place inappropriately on the posterior surface of both forearms 30 min before induction of anaesthesia plus saline 1ml IV. Removed wristband 6 hours after surgery (n = 50) Group 2: sham acupressure wristbands place inappropriately on the posterior surface of both forearms 30 min before induction of anaesthesia plus metoclopramide 0.2 mg/kg IV immediately before induction. Removed wristband 6 hours after surgery (n = 50) Group 3: sham acupressure wristbands place inappropriately on the posterior surface of both forearms 30 min before induction of anaesthesia plus ondansetron 0.15 mg/kg IV immediately before induction. Removed wristband 6 hours after surgery (n = 50) Group 4: bilateral wristbands on P6 acupoint 30 min before induction of anaesthesia plus saline 1ml IV. Removed wristband 6 hours after surgery (n = 50) |
|
| Outcomes | Nausea (0 – 2 h), Vomiting (0 – 2 h) in recovery room | |
| Notes | Subgroup analysis for adults and children not done as overall population was mixed in age range (10 – 60 years). No incidence for postoperative nausea or vomiting (0 – 24 h) reported No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details. “Patients were randomised into four groups using random numbers, with 50 cases in each group.” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors attempted to blind antiemetic drugs use with saline placebo and used sham acupressure wristbands on non-acupoint |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Wristbands were not covered with dressing. No details about whether healthcare providers were blinded or not |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | Nursing staff recording the PONV were unaware of group allocations |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | “No patient was excluded after admission to the study.” |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | “There was no statistically significant differences with respect to demographic data between groups in the study.” |
| El-Deeb 2011 | ||
| Methods | Parallel 3-group randomized trial, conducted in Egypt. Study dates not reported | |
| Participants | 450 women undergoing elective Caesaren delivery using spinal anaesthesia. Exclusion criteria: previous acupuncture treatment in the last 6 months, nausea or vomiting during 24 h preoperatively, diabetes, hypertension, cardiovascular disease, and any other major systemic comorbidities |
|
| Interventions | Group 1: sham group (normal saline IV and sham electroacupuncture at dorsal side of forearm for 30 minutes) before spinal anaesthesia (n = 150) Group 2: ondansetron group (4 mg ondansetron IV 30 minutes and sham electroacupuncture at dorsal side of forearm for 30 minutes before spinal anaesthesia (n = 150) Group 3: electroacupuncture group (normal saline IV and electroacupuncture at P6 acupoint on both wrists for 30 minutes before spinal anaesthesia (n = 150) |
|
| Outcomes | Postoperative nausea (0 – 6 h), postoperative vomiting (0 – 6 h), rescue antiemetic (ondansetron 4 mg IV, 0 – 6 h), treatment side effects | |
| Notes | “No local (cutaneous) side effects were reported at the acu-stimulation site by any patient in the treatment groups during the 24h study period. No complications were noted” No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details given. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelop used. Comment: not sure if envelopes were sequentially numbered and opaque |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors applied placebo drug and sham electroacupuncture techniques but blinding of participants not specified |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | Authors applied placebo drug and sham electroacupuncture techniques but blinding of healthcare providers not specified |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | Outcomes assessed by “independent anaesthetist who was blinded to group assignment” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for 450 participants analysed. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | “The three groups were not significantly different with respect to demographic characteristics, intraoperative ephedrine dose, gestational age, and duration of surgery” |
| Ertas 2015 | ||
| Methods | Parallel-group double-blinded randomized trial, conducted in Turkey. Study dates not reported | |
| Participants | 62 adult women undergoing gynaecological laparoscopy under general anaesthesia. Exclusion: women who had nausea and vomiting within 24 h before surgery, use of antiemetics or glucocorticoids within 24 hours before surgery, users of pacemakers, pregnant or nursing women, obese women, diseases associated with nausea and vomiting, those switched from laparoscopic to laparotomy |
|
| Interventions | Group 1: ReliefBand applied 15 – 30 min, at 31 Hz, on dominant hand before the operation and activated for 24 hours after surgery (n = 31) Group 2: Sham ReliefBand (electrodes wrapped in a plastic bandage and inactivated) applied 15 – 30 min, at 31 Hz, on dominant hand before the operation for 24 hours after surgery (n = 31) |
|
| Outcomes | Risk of rescue antiemetic (IV metoclopramide 0.5 mg/kg) drug (0 – 24 h) and adverse effects of device. No incidence of postoperative nausea or vomiting reported in the first 24 h after surgery | |
| Notes | Severity of postoperative nausea and vomiting data not used. Authors stated that no adverse effects related to ReliefBand were observed. Power calculation done. Authors declared no financial or conflict of interest. Details about funding support not given | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Random numbers displayed on a list of codes prepared by a computerized system.” |
| Allocation concealment (selection bias) | Low risk | “These codes were written on paper slips, which were placed in numbered opaque sealed envelopes.” |
| Blinding of patients (performance bias) All outcomes |
Low risk | “The patient and the research worker who held the records of the patient had no idea whether the ReliefBand was an authentic or a sham device.” |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “The patient and the research worker who held the records of the patient had no idea whether the ReliefBand was an authentic or a sham device.” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All 62 women followed up. |
| Selective reporting (reporting bias) | High risk | Incidence of postoperative nausea and vomiting (0 – 24 h) not reported |
| Other bias | Low risk | Baseline characteristics (age, height, body weight, duration of anaesthesia, duration of surgery, Apfel risk scores, smoking history, history of PONV) were comparable between groups |
| Fassoulaki 1993 | ||
| Methods | Parallel-group randomized trial, conducted in Greece. Study dates not reported | |
| Participants | 106 women undergoing abdominal hysterectomy. Exclusions: 3 women in the sham group were excluded because they were given metoclopramide in the postoperative period for persistent vomiting (but these data were included for risk of rescue antiemetic given analysis) |
|
| Interventions | Transcutaneous electrical nerve stimulation on the P6 acupoint was applied 30 – 45 min before induction and continued for 6 hours postoperatively (n = 51) Sham group was treated the same way but with the electrical stimulator turned off (n = 52) |
|
| Outcomes | Vomiting (0 – 2 h) without antiemetic rescue, risk of rescue antiemetic (metoclopramide) | |
| Notes | Potential bias if outcome assessor removed plastic bag covering the stimulator. Reported vomiting 2 – 4 h, 4 – 6 h, 6 – 8 h intervals. No data on vomiting (0 – 8 h) No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | “The stimulator, active or inactive, was covered with dark plastic bags, not allowing distinction between active and inactive stimulators” |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | Vomiting was assessed by “an independent observer who was unaware of the patient randomization and of TENS treatment” |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | “Three patients, originally assigned to the control groups, who received postoperatively metoclopramide because of persistent vomiting were eliminated from further vomiting evaluation and consequently from the study”. Comment: may introduce clinically relevant bias in summary effect measure |
| Selective reporting (reporting bias) | High risk | Nausea and side effects were not reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “The two groups did not differ in age, body weight, duration of anaesthesia, and duration of surgery” |
| Ferrara-Love 1996 | ||
| Methods | Parallel-group randomized trial, conducted in United States. Study dates not reported | |
| Participants | 136 adults undergoing orthopaedic, general, plastic, and ‘other’ surgery. Exclusions: 46 participants excluded after randomisation for failure to meet inclusion criteria |
|
| Interventions | Group 1: acupressure wristbands placed on P6 acupoint during surgery until hospital discharge (n = 30) Group 2: sham acupressure wristbands without studs placed on P6 acupoint during surgery until hospital discharge (n = 30) Group 3: reference group had no acupressure treatment (n = 30) |
|
| Outcomes | Nausea in the operating room after surgery, risk of rescue antiemetic drugs in the operating room if nausea persisted and/or emesis occurred | |
| Notes | No treatment group excluded from data analysis. No cumulative outcome data Study was funded by grants from the American Society of PostAnesthesia Nurses and SeaBand, United Kingdom No details about any declarations of interest among authors. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “Randomization was done by birth date with even numbered months and days assigned to the treatment group, odd months and days assigned to the placebo group and combinations of even/odd months and days assigned to the control group” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | PACU staff were blinded. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “Incidence of postoperative nausea and vomiting was documented by the PACU staff who were blinded as to treatment and placebo group” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for the 90 participants analysed. |
| Selective reporting (reporting bias) | High risk | Risk of vomiting and side effects were not reported in the results |
| Other bias | Low risk | Baseline characteristics were comparable. “There were no differences between groups in demographic and perioperative variables” as tested using appropriate univariate statistical tests |
| Frey 2009a | ||
| Methods | Parallel 4-arm randomized trial, conducted at a single German centre. Study dates not reported | |
| Participants | 214 adult women undergoing vaginal hysterectomy requiring general anaesthesia. Exclusion: women with cardiac pacemaker or implanted defibrillator, at risk of malignant hyperthermia, had allergy to nickel/chrome, or change in surgical technique |
|
| Interventions | Group 1: Acu-stimulation (ReliefBand) before induction of anaesthesia at P6 acupoint on dominant forearm for 24 h after surgery (n = 48) Group 2: Acu-stimulation (ReliefBand) after induction of anaesthesia at P6 acupoint on dominant forearm for 24 h after surgery (n = 53) Group 3: Sham acustimulation (inactivated ReliefBand electrodes with a silicone cover) before induction of anaesthesia at P6 acupoint on dominant forearm for 24 h after surgery (n = 49) Group 4: Sham acustimulation (inactivated ReliefBand electrodes with a silicone cover) after induction of anaesthesia at P6 acupoint on dominant forearm for 24 h after surgery (n = 50) |
|
| Outcomes | Nausea (0 – 6 h), vomiting (0 – 6 h), rescue antiemetic (tropisetron 2 mg) | |
| Notes | Combined Groups 1 and 2 as acustimulation group, and Groups 3 and 4 as sham group for analysis. No cumulative incidence of 0 – 24 h outcomes reported No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details. |
| Allocation concealment (selection bias) | Unclear risk | Participants randomized by “drawing a sealed envelope indicating treatment assignment.” No details about envelopes being opaque |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors made efforts to inactivate electrodes and place a silicone cover over the device which “was invisible for both patients and investigators.” |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | Authors made efforts to inactivate electrodes and place a silicone cover over the device which “was invisible for both patients and investigators.” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “The investigators responsible for collecting data were blind to the treatments administered to the study patients.” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 14 participants excluded after randomization due to change in surgical technique, resulting in final sample size of 200 |
| Selective reporting (reporting bias) | High risk | Side effects of active and sham ReliefBand not assessed or reported |
| Other bias | Low risk | Groups were comparable for participant characteristics, duration of surgery and anaesthesia and risk score for PONV |
| Frey 2009b | ||
| Methods | Parallel 4-arm randomized trial (single centre) conducted in Germany. Study dates not reported | |
| Participants | 229 patients, aged more than 18 years with ASA physical status I to III, undergoing laparoscopic cholecystectomy. Exclusion criteria were patients with cardiac pacemaker or implanted cardioverter/defibrillator, at risk of malignant hyperthermia, with allergy to nickel/chrome or change in surgical technique | |
| Interventions | Group 1: Acustimulation (ReliefBand) before induction of anaesthesia at P6 acupoint on dominant forearm for 24 h after surgery (n = 59) Group 2: Acu-stimulation (ReliefBand) after induction of anaesthesia at P6 acupoint on dominant forearm for 24 h after surgery (n = 53) Group 3: Sham acustimulation (inactivated ReliefBand electrodes with a silicone cover) before induction of anaesthesia at P6 acupoint on dominant forearm for 24 h after surgery (n = 59) Group 4: Sham acustimulation (inactivated ReliefBand electrodes with a silicone cover) after induction of anaesthesia at P6 acupoint on dominant forearm for 24 h after surgery (n = 58) |
|
| Outcomes | Nausea (0 – 2 h), vomiting (0 – 2 h), rescue antiemetic (tropisetron 2 mg), side effects of ReliefBand (skin irritation under electrodes) | |
| Notes | Combined Groups 1 and 2 as acustimulation group, and Groups 3 and 4 as sham group for analysis. No cumulative incidence of 0 – 24 h outcomes reported. No details about funding source or any declarations of interest among authors | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details. |
| Allocation concealment (selection bias) | Unclear risk | Participants randomized by “drawing a sealed envelope indicating treatment assignment.” No details about envelopes being opaque |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors made efforts to inactivate electrodes and place a silicone cover over the device which “was invisible for both patients and investigators.” |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | Authors made efforts to inactivate electrodes and place a silicone cover over the device which “was invisible for both patients and investigators.” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “Patients were evaluated for the occurrence of nausea, retching, vomiting, pain and potential side effects of ReliefBand (skin irritation under the electrodes) by an investigator unaware of the patients’ group assignment.” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 29 did not receive allocated intervention because of change of surgical technique. No missing data reported for the 200 participants analysed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. Authors stated “the requirement for rescue medication did not differ significantly between the treatment groups.” |
| Other bias | Low risk | “The demographic and morphometric characteristics and factors likely to influence PONV were not significantly different in the acu-stimulation and sham groups as were intraoperative variables.” |
| Gan 2004 | ||
| Methods | Parallel-group randomized trial, conducted in United States. Study dates not reported | |
| Participants | 77 women undergoing major breast surgery. Exclusion: pregnancy, using permanent cardiac pacemaker, previous experience of acupuncture therapies, received any antiemetic medication or had nausea, vomiting or retching within 24 h of surgery. 2 women withdrew from study |
|
| Interventions | Group 1: ondansetron 4 mg IV given at induction of anaesthesia and sham electro-acupoint stimulation at P6 acupoints (30 – 60 min before induction and continued to the end of surgery) (n = 25) Group 2: electro-acupoint stimulation at P6 bilaterally (30 – 60 min before induction and continued to the end of surgery) and saline IV given at induction of anaesthesia (n = 26) Group 3: sham electro-acupoint stimulation at P6 bilaterally (30 – 60 min before induction and continued to the end of surgery) and saline IV given at induction of anaesthesia (n = 24) |
|
| Outcomes | Nausea (0 – 2 h), vomiting (0 – 2 h), risk of rescue antiemetic drug, adverse effects | |
| Notes | Rescue antiemetic was dexamethasone 8 mg IV when participant’s nausea score > 5 out of 10 for 15 min or longer, 2 emetic episodes within 15 min, or at participant’s request. No redness residue on acupoint site in any groups No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was achieved using a random number generator..” |
| Allocation concealment (selection bias) | Low risk | “…In a sealed envelope technique”. “Study drugs were prepared by the pharmacists not directly involved in the study..”. Comments: the authors appeared to take steps to minimize inadequate allocation concealment |
| Blinding of patients (performance bias) All outcomes |
Low risk | “All patients were also told that the device produced an electrical current that they may or may not feel. The screen on the unit (measuring 4 x 2 cm) was covered with an opaque tape in all groups so that the clinicians and research personnel were unaware if the unit was on or off” |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “All patients were also told that the device produced an electrical current that they may or may not feel. The screen on the unit (measuring 4 x 2 cm) was covered with an opaque tape in all groups so that the clinicians and research personnel were unaware if the unit was on or off” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “Postoperative data were collected by a separate research nurse not involved in the preoperative or intraoperative management of patients” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Reasons for withdrawals were given. No missing data reported for the 75 participants analysed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “There was no difference in patient demographics among the groups” |
| Gieron 1993 | ||
| Methods | Parallel-group randomized trial, conducted in Germany. Study dates not reported | |
| Participants | 90 women undergoing gynaecological operations (6 – 8 h). | |
| Interventions | Group 1: acupressure was carried out by fastening small metal bullets at the P6 acupoint to each wrist by an elastic bandage on the morning of the operation and left on for 24 h (n = 30) Group 2: sham acupressure carried out by applying elastic bandage to P6 acupoint on the morning of the operation and left on for 24 h (n = 30) Group 3: no treatment (n = 30). |
|
| Outcomes | Nausea (0 – 6 h), vomiting (0 – 6 h), risk of rescue antiemetic (metoclopramide) | |
| Notes | No treatment data were excluded from analysis. Also reported separate incidences of nausea and vomiting (0 – 1 h) and (6 – 24 h). No side effects identified in the trial No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
High risk | The outcome assessor was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for 90 participants analysed. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “The anthropometric data, the duration of surgery and the amount of postoperative analgesia were comparable between the three groups” |
| Habib 2006 | ||
| Methods | Parallel-group randomized trial, conducted in United States. Study dates not reported | |
| Participants | 94 women undergoing Caesarean delivery under spinal anaesthesia. Exclusion: previous experience of acupuncture or acustimulation, had experienced vomiting or retching within 24 h before surgery, had taken on antiemetic or a glucocorticoid within 24 h before surgery, or had an implanted pacemaker or defibrillator device. 3 participants withdrew from study because of protocol violations |
|
| Interventions | Transcutaneous acupoint electrical stimulation device on P6 acupoint of the dominant hand 30 – 60 min before surgery. Participants asked to wear wristband for 24 h after surgery (n = 47) Sham transcutaneous acupoint electrical stimulation device on dorsum of wrist of the dominant hand 30 – 60 min before surgery. Participants asked to wear wristband for 24 h after surgery (n = 44) |
|
| Outcomes | Postoperative nausea (0 – 24 h), postoperative vomiting (0 – 24 h), risk of rescue antiemetic | |
| Notes | Intraoperative nausea and vomiting data reported in the paper. Rescue antiemetic was ondansetron 4 mg IV if nausea score was 6 or more, or at participant’s request Study supported, in part, by departmental funds. No details about any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficent information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar. “For blinding, the ReliefBand was covered with opaque gauze that was taped to the wrist” |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “A separate researcher who was unaware of the patient’s randomisation collected that data…” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Reasons for withdrawals given. No missing data reported for 91 participants analysed |
| Selective reporting (reporting bias) | High risk | Side effects not reported |
| Other bias | Low risk | Baseline characteristics were comparable. “The two groups were similar with respect to demographics, parity, history of PONV or motion sickness, smoking status, duration of surgery, blood loss, intraoperative fluids, intraoperative IV fentanyl, intraoperative IV ephedrine, treatment for pruritus, and consumption of oxycodone/acetaminophen tablets” |
| Harmon 1999 | ||
| Methods | Parallel-group randomized trial, conducted in Ireland. Study dates not reported | |
| Participants | 104 women undergoing laparoscopy and dye investigation. Exclusions: obesity, diabetes mellitus, and previous history of PONV |
|
| Interventions | Acupressure on P6 acupoint of right wrist, applied immediately before induction for 20 min, removed before end of surgery (n = 52) Sham acupressure on non-acupoint site, applied before induction for 20 min and removed before end of surgery (n = 52) |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), risk of rescue antiemetic drugs | |
| Notes | Rescue antiemetic was ondansetron 4 mg IV and prochlorperazine 12.5 mg IM. No side effects in either group noted. Some participants did not have outcome data No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was conducted by computer..”. |
| Allocation concealment (selection bias) | Unclear risk | “…And the code was sealed until arrival of the patient in the operating theatre”. Comment: not sure whether envelopes were sequentially numbered and opaque |
| Blinding of patients (performance bias) All outcomes |
Low risk | “Both patients and nurses were unaware of patient group allocation” |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “Both patients and nurses were unaware of patient group allocation” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “..An anaesthetist blinded to the therapy registered whether nausea, retching or vomiting had occurred” |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | In acupressure group (n = 52), missing nausea and vomiting data in 8 and 5 participants respectively. In sham group (n = 52), missing nausea and vomiting data in 13 and 5 participants respectively |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “The groups were comparable in age, weight and duration of surgical procedure” |
| Harmon 2000 | ||
| Methods | Parallel-group randomized trial, conducted in Ireland. Study dates not reported | |
| Participants | 94 healthy women (18 – 40 years) undergoing elective Caesarean section. Exclusion: previous history of PONV, nausea and vomiting in previous 24 hours, obesity (BMI > 35), diabetes mellitus, or previous experience of acupuncture or acupressure |
|
| Interventions | Acupressure on P6 acupoint on right wrist, applied 5 min before administration of spinal anaesthesia, removed just before assessment 6 hours after discharge to the ward (n = 47) Sham acupressure on non-acupoint site, applied 5 min before administration of spinal anaesthesia, removed just before assessment 6 hours after discharge to the ward (n = 47) |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h). | |
| Notes | Reported separate incidence of intraoperative nausea and vomiting. Rescue antiemetic was ondansetron 4 mg IV during operations, or cyclizine 50 mg IM 8-hourly after operations. Rescue antiemetic use reported as mean dose (no data for risk of rescue cyclizine use). Side effect of acupressure bands was “some localized discomfort in a small number of women” No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “Bands were not visible to the assessing anaesthetist during operations, as patients’ arms were covered with surgical drapes” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “After 6 and 24h, an anaesthetist blinded to the therapy noted whether nausea, retching or vomiting had occurred” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Reasons for withdrawals were given. No missing data reported for 94 participants analysed |
| Selective reporting (reporting bias) | High risk | Risk of rescue cyclizine not reported separately for nausea and vomiting outcomes |
| Other bias | Low risk | Baseline characteristics were comparable. “The groups were comparable with respect to age, weight, height and bupivacaine dose” |
| Ho 1990 | ||
| Methods | Parallel-group randomized trial, conducted in Taiwan. Study dates not reported | |
| Participants | 100 women undergoing laparoscopy. | |
| Interventions | Group 1: electro-acupuncture applied at P6 acupoint on right wrist for 15 min in the recovery room (n = 25) Group 2: transcutaneous electrical nerve stimulation at P6 acupoint on right wrist for 15 min in the recovery room (n = 25) Group 3: antiemetic group was given prochlorperazine 5 mg IV (n = 25) Group 4: no treatment (n = 25). |
|
| Outcomes | Vomiting (0 – 3 h), side effects of treatment groups. | |
| Notes | Reference group received no treatment and was not included in data analysis. Groups 1 and 2 were combined for data analysis, except for subgroup analysis on technique. Side effect of electro-acupuncture were sleepiness and feeling tired No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data were reported for the 100 participants analysed |
| Selective reporting (reporting bias) | High risk | Only vomiting was reported. Authors should have assessed nausea in women and the risk of rescue antiemetic drugs |
| Other bias | Low risk | Baseline characteristics were comparable. “The age, weight, and duration of anaesthesia did not differ significantly among the groups” |
| Ho 1996 | ||
| Methods | Parallel-group randomized trial, conducted in Taiwan. Study dates not reported | |
| Participants | 60 women receiving epidural morphine for post-Caesarean section pain relief. Excluded: previous carpal tunnel syndrome, or those who had experienced nausea or vomiting within 24 h before Caesarean section |
|
| Interventions | Group 1: acupressure wristbands on P6 acupoint of both wrists before administration of spinal anaesthesia. Worn for 48 hours (n = 30) Group 2: sham acupressure wristbands on both wrists but plastic button was blunted in order not to exert pressure on P6 acupoint. Worn for 48 hours (n = 30) |
|
| Outcomes | Nausea (0 – 48 h), vomiting (0 – 48 h), risk of rescue antiemetic drug, side effects of acupressure wristbands | |
| Notes | Rescue antiemetic was metoclopramide. No side effects were noted No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was conducted by computer..”. |
| Allocation concealment (selection bias) | Unclear risk | “… With each code sealed in an envelope to be opened upon the parturient’s arrival in the operating room”. Comment: not sure if envelopes were sequentially numbered and opaque |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “An independent anaesthesiologist blinded to the parturient groups followed up all parturients” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | “All parturients completed the trial and tolerated the bands well” |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “There were no statistically significant difference with respect to age, weight, height, duration of operation, intraoperative blood loss, duration of pain relief, total epidural morphine dosage, percentage of parturients requiring additional analgesics and total time spent wearing bands between the two groups” |
| Iqbal 2012 | ||
| Methods | Parallel-group randomized trial conducted in Pakistan from November 2011 to July 2012 | |
| Participants | 60 participants aged 40 – 60 years, ASA I and II, undergoing laparoscopic surgery. Exclusion: those with a history of severe adverse reactions to NSAIDs, bronchial asthma, kidney or liver dysfunction, bleeding disorders or history of steroids intake within 24 h of surgery |
|
| Interventions | Group 1: acupressure wristband (Seaband) at the P6 acupoint to each wrist and draped with dressing during the stay in hospital. by an elastic bandage (n = 20) Group 2: sham acupressure (Seaband) on dorsal side of both forearms and draped with dressing during the stay in hospital (n = 20) Group 3: no treatment (n = 20). |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), risk of rescue antiemetic drug (metoclopramide 10 mg IV) | |
| Notes | No treatment data were excluded from analysis. No power calculation done No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors made efforts to drape dressing over active and sham wristbands |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “The doctors and nurses giving anesthesia and the nurses on the postoperative ward, although aware that stimulation was being performed were not aware of the location of PC6” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “The doctors and nurses giving anesthesia and the nurses on the postoperative ward, although aware that stimulation was being performed were not aware of the location of PC6” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data were reported for the 60 participants analysed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Demographic data appear similar between acupressure and sham groups |
| Kim 2004 | ||
| Methods | Parallel-group randomized trial conducted in Korea. | |
| Participants | 66 women, ASA physical status I or II, undergoing sevoflurane general anaesthesia for minor breast surgery. Exclusion criteria were women with respiratory, circulatory or neurological disease, liver or kidney dysfunction, nausea or vomiting in the 24 h before surgery, receiving antiemetics, pregnant women and excessively obesity | |
| Interventions | Group 1: Transcutaneous electrical stimulation (ReliefBand) on P6 acupoint 10 min before surgery and left in place for 24 h. Bilateral or unilateral simulation not reported (n = 33) Group 2: Sham transcutaneous electrical stimulation (inactivated ReliefBand) on P6 acupoint 10 min before surgery and left in place for 24 h. Bilateral or unilateral sham simulation not reported (n = 33) |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), risk of rescue antiemetic drug (ondansetron 4 mg IV) | |
| Notes | Descriptive data taken from information in Kim 2012. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Inactivated device that looks similar to the real device. |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient details. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | Unaware of allocated treatment at both baseline and postoperative evaluations |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No reported dropouts or withdrawals. |
| Selective reporting (reporting bias) | Low risk | All expected measured outcomes were reported. |
| Other bias | Low risk | Baseline characteristics were comparable. |
| Kim 2011 | ||
| Methods | Parallel-group randomized trial, conducted in Korea. Study dates not reported | |
| Participants | 264 adult women, with ASA physical status I to II, undergoing laparoscopic hysterectomy. Exclusion criteria were women receiving antiemetics within 24 h of surgery, obesity, neuromuscular, hepatic, or renal diseases, or a history of allergic reactions to the medications used during anaesthesia |
|
| Interventions | Group 1 (group control): 2 surface electrodes placed over ulnar nerve on dominant upper extremity before induction of anaesthesia and removed after anaesthesia in the operating room. Applied 1 Hz single twitch stimulation during anaesthesia maintenance (n = 54) Group 2 (group ST): 2 surface electrodes stimulated the median nerve at P6 acupoint on dominant upper extremity before induction of anaesthesia and removed after anaesthesia in the operating room. Applied 1 Hz single twitch stimulation during anaesthesia maintenance (n = 52) Group 3 (group TOF): 2 surface electrodes stimulated the median nerve at P6 acupoint on dominant upper extremity before induction of anaesthesia and removed after anaesthesia in the operating room. Applied TOF stimulation every 15 seconds during anaesthesia maintenance (n = 53) Group 4 (group DBS): 2 surface electrodes stimulated the median nerve at P6 acupoint on dominant upper extremity before induction of anaesthesia and removed after anaesthesia in the operating room. Applied double-burst stimulation every 20 seconds during anaesthesia maintenance (n = 53) Group 5 (group tetanus): 2 surface electrodes stimulated the median nerve at P6 acupoint on dominant upper extremity before induction of anaesthesia and removed after anaesthesia in the operating room. Applied tetanus stimulation at 50 Hz for 5 seconds every 10 min during anaesthesia maintenance (n = 52) |
|
| Outcomes | Nausea (0 – 6 h), Vomiting (0 – 6 h), rescue antiemetic (ondansetron 4 mg IV), side effects | |
| Notes | Group 1 considered as sham. Groups 2 – 5 combined as 1 acustimulation group. “No side-effects were reported from the electrical stimulation.” Participants in the acustimulation group were more likely to be highly satisfied with PONV management (VAS 7 – 10) at 24 h than sham group (91% versus 75%, P = 0.003) Authors declare no conflicts of interest. No details about funding support for study |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details given. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details given. |
| Blinding of patients (performance bias) All outcomes |
Low risk | “The patients, as well as the anesthesiologist and the nursing staff, were unaware of the patient grouping.” |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “The patients, as well as the anesthesiologist and the nursing staff, were unaware of the patient grouping.” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | Independent outcome assessor “was unaware of the patient randomization and of the neuromuscular monitoring mode.” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No dropouts for 264 participants recruited into study. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | “Baseline characteristics of study participants were similar, as were intraoperative variables.” |
| Klein 2004 | ||
| Methods | Parallel-group randomized trial, conducted in Canada. Study dates not reported | |
| Participants | 152 people undergoing coronary artery bypass graft or valvular surgery. Exclusion: past history of hiatus hernia, heartburn, or previous gastric surgery, morbid obesity, taking antiemetic medications, H2 receptor antagonist, or proton pump inhibitors. No details about withdrawals or loss to follow-up |
|
| Interventions | Acupressure wristbands on P6 acupoint on both wrists before induction of anaesthesia, removed 24 h after extubation (n = 75) Sham acupressure wristbands on P6 acupoint of both wrists before induction of anaesthesia, removed 24 h after extubation. Sham group had band without a bead placed on P6 acupoint (n = 77) |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), risk of rescue antiemetic drug, risk of adverse effects | |
| Notes | Rescue antiemetic was dimenhydrinate 50 mg IV for participants who reported moderate or severe nausea, or who experienced retching or vomiting. No significant adverse effects reported in either group No details about any declarations of interest among authors. Acupressure bands were provided by Sea Band, United Kingdom |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomized by computer-generated random number tables to either acupressure or placebo control groups” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “The anaesthesiologist caring for the patient was not aware of group allocation” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “All patients were assessed for nausea and vomiting by nursing staff in the intensive care unit, who were unaware of treatment allocation” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for the 152 participants analysed. |
| Selective reporting (reporting bias) | Low risk | Reported all expected outcomes. |
| Other bias | Low risk | Baseline characteristics were comparable. “There were no differences between the 2 groups with regard to demographic data and surgical characteristics” |
| Koo 2013 | ||
| Methods | Parallel-group randomized trial that compared capsicum plaster stimulation of P6, K-D2 and sham acupoints. Study conducted in Korea | |
| Participants | 184 adults, aged 21 – 64 years, undergoing thyroid surgery between November 2012 and March 2013. Exclusion: obese, gastro-oesophageal reflux, use of antiemetic, histamine H2-receptor antagonist or tranquillizer within 72 hours before surgery, or respiratory disease |
|
| Interventions | Group 1: Sham P6 and K-D2 inactive tape, similar in appearance to capsicum plaster, applied to both wrists at P6 acupoint and both deltoid 30 min before induction of anaesthesia and left on for 8 h (n = 46) Group 2: Capsicum plaster applied to both wrists at P6 acupoints and inactive tape applied at both deltoids 30 min before induction of anaesthesia and left on for 8 h (n = 46) Group 3: Capsium plaster applied to both K-D2 points on index finger of hand and inactive tape applied at both deltoids 30 min before induction of anaesthesia and left on for 8 h (n = 46) Group 4: Capsium plaster applied to both deltoids and inactive tape applied to both wrists at P6 acupoint 30 min before induction of anaesthesia and left on for 8 h (n = 46) |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), rescue antiemetic (metoclopramide 10 mg IV) | |
| Notes | Groups 3 and 4 were not included in the analysis. Power calculation done. No details about financial support or conflict of interests of authors reported in article | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | “After enrolment, patients were randomized to four groups by sealed envelope.” Comment: no details about use of sequential numbering or opaque envelopes |
| Blinding of patients (performance bias) All outcomes |
Low risk | “The patients and the investigators as well as anesthesiologists and nurses, were unaware of the patient grouping.” |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “The patients and the investigators as well as anesthesiologists and nurses, were unaware of the patient grouping.” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “The patients and the investigators as well as anesthesiologists and nurses, were unaware of the patient grouping.” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | All participants followed-up to 24 h after surgery. “There were no dropouts among the 184 enrolled subjects.” |
| Selective reporting (reporting bias) | High risk | Adverse effects of capsicum plaster not reported. |
| Other bias | Low risk | “The patients’ characteristics, such as sex, age, weight, height, duration of anesthesia, history of PONV, history of motion sickness, nonsmoking status and intraoperative remifentanil use, were comparable between groups.” |
| Lewis 1991 | ||
| Methods | Parallel-group randomized trial, conducted in United States. Study dates not reported | |
| Participants | 66 children undergoing strabismus correction surgery. Excluded: children with anatomical or neurological abnormalities of the upper limbs. 2 children lost to follow-up |
|
| Interventions | Group 1: acupressure wristbands placed on P6 acupoints 1 h before surgery and worn until discharge from hospital (n = 33) Group 2: sham acupressure wristbands without studs placed on P6 acupoints 1 h before surgery and worn until discharge from hospital (n = 33) |
|
| Outcomes | Vomiting (0 – 24 h), risk of rescue antiemetic drug, side effects | |
| Notes | Both types of wristbands were identical unless turned inside out. Rescue antiemetic was droperidol 0.02 mg/kg IV for vomiting. No side effects reported No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | The anaesthetic staff were blinded. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “A second blinded investigator recorded all other perioperative data, including the incidence of postoperative nausea and vomiting in the recovery areas” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Two participants in acupressure group had incomplete data. Comment: unlikely to have a clinically relevant impact on summary estimate |
| Selective reporting (reporting bias) | High risk | Although nausea was an outcome collected in the Methods section it was not reported in the Results because nausea may be difficult to assess in children |
| Other bias | Low risk | Baseline characteristics were comparable. “There were no significant differences between the two groups in their patient characteristics” |
| Liu 2008 | ||
| Methods | Parallel-group randomized trial, conducted in China. Study conducted from June 2006 to July 2007 | |
| Participants | 96 people undergoing laparoscopic cholecystectomy who were aged 18 – 60 years. Exclusions: pregnancy, women experiencing menstrual symptoms, patients with permanent cardiac pace-maker, previous experience with acupuncture therapies before surgery, received antiemetics or experienced nausea, vomiting, or retching within 24 h of surgery. No participants withdrew from study |
|
| Interventions | Group 1: transcutaneous electro-acupoint stimulation using a peripheral nerve stimulator at P6 (2 – 100 Hz, 50 ms, 0.5 – 4 mA) applied 30 to 60 min before induction of anaesthesia, and continued to the end of surgery (n = 48) Group 2: inactive device with similar electrode for transcutaneous electro-acupoint stimulation using a peripheral nerve stimulator at P6 applied 30 – 60 min before induction of anaesthesia, and continued to the end of surgery (n = 48) |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), risk of rescue antiemetic drug (0 – 24 h), adverse effects of transcutaneous electro-acupoint stimulation | |
| Notes | Rescue antiemetic drug was ondansetron 4 mg IV, to participants who had a nausea score of > 5 on a 10-point scale, vomited twice within 15 min, or at the participant’s request. P6 acupoint stimulation was associated with a reduction in the risk of severe nausea (Group 1: 2/48 versus Group 2: 14/48). No redness, swelling, itching, and pain, or other relevant complications at P6 acupoint in the 2 groups No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomized into two groups of 48 in each using a table of random numbers” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “The anesthesiologists and care providers were blinded to the study group” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “Postoperative data were collected by a separate research nurse who was not aware of the preoperative or perioperative management of patients” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | “All 96 patients completed the study”. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “As shown in Tables 1 and 2, the patients’ gender, age, weight, ASA physical status, previous PONV history, duration of surgery or anaesthesia, transfusion amount, operative procedure and doses of opioids in the two groups were not significantly different” |
| Majholm 2011 | ||
| Methods | Parallel-group randomized trial, conducted in Denmark. Study conducted from May 2005 to December 2006 | |
| Participants | 134 healthy non-smoking women undergoing breast surgery given total intravenous anaesthesia. Exclusions: pregnancy, women graded ASA physical status at least III, smoked or had comorbidities that could influence sensitivity in wrists and hands, skin problems at the location of wristband or had experienced nausea or vomiting within 24 h of surgery. Of the 134 participants, 22 withdrew, leaving 112 completing the study |
|
| Interventions | Group 1: acupressure wristbands (Vital-Band) placed on P6 acupoints just before induction and worn until 24 h after surgery, covered with dressing (n = 67) Group 2: sham acupressure wristbands with studs placed on dorsum of the forearm just before induction surgery and worn until 24 h after surgery, covered with dressing (n = 67) |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), risk of rescue antiemetic drug (0 – 24 h), adverse effects associated with wristband | |
| Notes | P6 wristband adverse effects: 19/57 (redness), 7/58 (tenderness), 3/59 (paraesthesia), 8/59 (swelling). Sham wristband adverse effects: 20/53 (redness), 9/53 (tenderness), 1/53 (paraesthesia), 9/52 (swelling). Similar risk of adverse effects between groups for redness (P = 0.59), tenderness (P = 0.59), paraesthesia (P = 0.62) and swelling (P = 0.61) Manufacturer of Vital-Band paid USD 9000 for testing of their device No details about any declarations of interest among authors. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Random allocation sequence was generated by drawing one of these sealed envelopes” and “In order to avoid staff members to figure out the randomization outcome of the last envelopes, we had more sealed randomization envelopes than needed according to the sample size calculation” |
| Allocation concealment (selection bias) | Low risk | “Randomized using opaque sealed envelopes”. |
| Blinding of patients (performance bias) All outcomes |
Low risk | “The wristband was covered with a dressing in such a way that both the patient and the outcome assessors were blinded and unable to discover in which position the acupressure wristband had been applied” |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | No details available. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “The wristband was covered with a dressing in such a way that both the patient and the outcome assessors were blinded and unable to discover in which position the acupressure wristband had been applied” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Reasons for 22 lost to follow-up and discontinued intervention were given |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable for preoperative factors (except history of PONV or motion sickness, or both), intraoperative factors and morphine use in the postoperative period |
| Misra 2005 | ||
| Methods | Parallel-group randomized trial, conducted in India. Study dates not reported | |
| Participants | 123 adults (18 – 52 y) undergoing middle ear surgery. Exclusion: pregnancy, obesity, diabetes mellitus, impaired renal or liver functions; people who had taken H2 antagonists, antiemetics, or psychoactive medication; or had nausea, retching, or vomiting within 48 h before surgery. 3 participants withdrew because: they required administration of dexamethasone (n = 2), and facial nerve injury (n = 1) |
|
| Interventions | Group 1: sham plaster 1 cm × 1 cm patch affixed to P6 acupoint on both forearms 30 min before induction of anaesthesia and normal saline IV at the end of surgery. Plasters removed 6 h after surgery (n = 40) Group 2: capsicum plaster containing capsicum oleoresin 1% w/w 1 cm × 1 cm patch affixed to P6 acupoint on both forearms 30 min before induction of anaesthesia and normal saline IV at the end of surgery. Plasters removed 6 h after surgery (n = 38) Group 3: sham plaster 1 cm × 1 cm patch affixed to P6 acupoint on both forearms 30 min before induction of anaesthesia and ondansetron 4 mg IV at the end of surgery. Plasters removed 6 h after surgery (n = 39) |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), risk of rescue antiemetic drug (0 – 24 h), adverse effects of plaster | |
| Notes | Nausea (0 – 6 h), vomiting (0 – 6 h), incidence of rescue antiemetic (0 – 6 h) also reported. Rescue antiemetic was ondansetron 4 mg IV for participants with persistent nausea for more than 5 min, 2 or more episodes of vomiting/retching, or at participant’s request for PONV treatment. “One patient complained of mild irritation at the site of capsicum plaster application. No other adverse effects attributable to acu-stimulation or ondansetron were observed” No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The subjects were randomly assigned to one of the three groups using a computer-generated random number table” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “Anesthesia was standardized and given by an anesthesiologist blinded to group assignment” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “The incidence of PONV was evaluated within six hours and 24 hr after transfer to the postoperative unit by a blinded observer” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Reasons for withdrawals given. No missing data reported for the 120 participants analysed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “The demographic characteristics of the three groups were similar, as were history of previous PONV and motion sickness” |
| Nilsson 2015 | ||
| Methods | Parallel-group randomized trial, conducted in Sweden. | |
| Participants | 120 adults undergoing elective infratentorial or supratentorial craniotomy from November 2011 to June 2013. Exclusion: mental impairment or communication problems and use of antiemetics within 12 h before surgery | |
| Interventions | Group 1: SeaBand acupressure wristband with plastic button was applied on wrist P6 acupoint (marked by neurosurgical ward nursing staff) on wrist that did not have an intra-arterial catheter at the end of surgery by a nurse anaesthetist (n = 52). Duration of acupressure wristband application was 48 h Group 2: Sham SeaBand acupressure wristband without plastic button was applied on wrist P6 acupoint (marked by neurosurgical ward nursing staff) on wrist that did not have an intra-arterial catheter at the end of surgery by a nurse anaesthetist (n = 60). Duration of sham acupressure wristband application was 48 h Prophylactic IV ondansetron 4 mg was given at the end of surgery to both groups |
|
| Outcomes | Nausea (0 – 48 h), vomiting (0 – 48 h), rescue antiemetic (0 – 48 h), adverse effects related to wristbands | |
| Notes | Authors reported median times, not incidence, that rescue antiemetic (IV ondansetron 1 – 4 mg or droperidol 0.625 – 1.25 mg, or both) were used (0 – 48 h) for each group. No significant difference in proportion of participants requiring antiemetics between groups Power calculation done. The side effects (swelling, bruises, paraesthesia or pain) were equally distributed between P6 acupressure group (n = 7) and sham group (n = 7). Study was supported by the hospitals research foundation. Active and sham wristbands were partly provided by the manufacturer. Authors declared no conflicts of interests | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were “randomly assigned to either the PC6 acupressure group or the sham group using a computer-generated random number table.” |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, prepared by persons not involved in study, contained information about wristband placement and presumably group allocation |
| Blinding of patients (performance bias) All outcomes |
Low risk | “Both the PC6 acupressure bands and the sham bands were covered with a bandage to ensure blinding to the patient and outcome assessor.” |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “Both the PC6 acupressure bands and the sham bands were covered with a bandage to ensure blinding to the patient and outcome assessor.” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 120 randomized but 95 in final analysis (43 in PC6 acupressure group and 52 in sham group). Reasons for withdrawals were described. “There was no difference between the groups in excluded patients (P = 0.406).” |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | “The 2 groups were comparable with respect to medical and demographic characteristics, anesthesia, surgical techniques, risk factors for PONV, and postoperatively administered opioids.” |
| Ravi 2010 | ||
| Methods | Parallel-group randomized trial, conducted in India. Study dates not reported | |
| Participants | 50 people aged 4 – 60 years with ASA physical status I or II undergoing surgery (general, laparoscopic, ENT, paediatric, orthopaedic, obstetric, gynaecological) under general anaesthesia. Exclusion criteria: people with cardiovascular disease, central nervous system problems, previous history of PONV and motion sickness, and smokers | |
| Interventions | Group 1: P6 acupoint injection with 50% 0.2 ml dextrose after induction of anaesthesia (n = 25) Group 2: Ondansetron (50 μg/kg) at end of surgery (n = 25). |
|
| Outcomes | Nausea (0 – 6 h), vomiting (0 – 6 h), rescue antiemetic (ondansetron 4 mg) | |
| Notes | Subgroup analysis for adults and children not done as overall population was mixed in age range (4 – 60 years) No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated random numbers table. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details given. |
| Blinding of patients (performance bias) All outcomes |
Low risk | “Both patients and doctors were unaware of the group allocation.” |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “Both patients and doctors were unaware of the group allocation.” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “An anaesthetist blinded for the study assessed the presence of nausea and vomiting.” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for the 50 participants analysed. |
| Selective reporting (reporting bias) | High risk | Incidence of rescue ondansetron 4 mg for intolerable PONV in recovery room and postoperative ward not reported |
| Other bias | Low risk | Age, sex ratio, weight of participants and duration of surgery were similar between groups |
| Rusy 2002 | ||
| Methods | Parallel-group randomized trial, conducted in United States. Study dates not reported | |
| Participants | 121 children (4 – 18 years) undergoing tonsillectomy with or without adenoidectomy. Exclusions: presence of skin lesions near acupuncture sites, previous and severe PONV, chronic history of nausea and vomiting. 1 child disqualified after enrolment when propofol was administered during the anaesthetic |
|
| Interventions |
|
|
| Outcomes | Vomiting (0 – 24 h), nausea (0 – 24 h), risk of rescue antiemetic drugs | |
| Notes | Rescue antiemetics were ondansetron and droperidol IV. Sham electro-acupuncture and sham reference group data were combined for analysis Funding source was the Jane B Pettit Pain Foundation, Children’s Hospital of Wisconsin. No details about any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A randomized block design procedure was used to assign enrollees to one of three groups..” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “Experienced recovery room nurses, who were blinded to the treatment group, assessed nausea and vomiting” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “Experienced recovery room nurses, who were blinded to the treatment group, assessed nausea and vomiting” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Reason for withdrawal of one participants was given. No missing data reported for 120 participants analysed |
| Selective reporting (reporting bias) | Unclear risk | There was no description about side effects of therapy in the trial, but in the correspondence (Rusy 2002) the authors wrote “There were no noted muscle contractions or patients who complained of paresthesias during the study” |
| Other bias | Low risk | Baseline characteristics were comparable. “The groups were similar for age, sex, weight, analgesics administered, and surgical time (table 1), with no differences found” |
| Sadighha 2008 | ||
| Methods | Parallel-group randomized trial, conducted in Iran. Study dates not reported | |
| Participants | 156 adults undergoing elective laparoscopic cholecystectomy with ASA physical status I to II. Excluded: those with a history of PONV, kidney dysfunction, BMI > 35 kg/m2, use of antiemetics or H2 receptor antagonists within 72 hours of surgery, history of gastrointestinal disease, intra-abdominal pressure > 15 mm Hg, or surgery duration of more than 2 h |
|
| Interventions | Group 1: acupressure wristband at a P6 acupoint before induction until recovery discharge (n = 51) Group 2: metoclopramide 0.2 mg/kg IV at end of surgery and sham acupressure wristband at a non-acupoint before induction until recovery discharge (n = 53) Group 3: no antiemetic and had sham acupressure wristband at a non-acupoint before induction until recovery discharge (n = 52) |
|
| Outcomes | Nausea (recovery), vomiting (no time point specified). | |
| Notes | Power calculation done. Funding source was Shaheed Beheshti University of Medical science. No details about any declarations of interest among authors | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “Patients were randomly assigned to treatment groups according to the last digit of the medical record number.” |
| Allocation concealment (selection bias) | High risk | “Patients were randomly assigned to treatment groups according to the last digit of the medical record number.” |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors used sham acupressure and participants given general anaesthesia would not be aware of any antiemetic drugs given at end of surgery |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient details given. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “Assessors of nausea and vomiting were blinded to the treatment.” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for 156 participants analysed. |
| Selective reporting (reporting bias) | High risk | No treatment side effects or rescue antiemetic use reported. |
| Other bias | Low risk | “Demographic and clinical characteristics of the three groups were similar.” |
| Samad 2003 | ||
| Methods | Parallel-group randomized trial, conducted in Pakistan. Study dates not reported | |
| Participants | 50 people (18 – 60 y) undergoing laparoscopic cholecystectomy. Exclusion: obesity (weight > 80 kg), diabetics, people with history of PONV, people receiving antiemetics and histamine H2 antagonists |
|
| Interventions | Acupressure band on right hand at P6 acupoint ½ h before induction of anaesthesia, and kept on for 6 hours after surgery (n = 25) Sham acupressure band on right hand with plastic bead placed on the dorsum of forearm (n = 25) |
|
| Outcomes | Nausea (0 – 6 h), vomiting (0 – 6 h), risk of rescue antiemetic drug, side effects | |
| Notes | Rescue antiemetic was metoclopramide 10 mg IV for nausea or vomiting. No side effects or complications associated with either intervention No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomly assigned by random table number to either group..” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “A blinded observer in the recovery room (one of the investigator not involved in applying acupressure band) evaluated the patients for presence of nausea and vomiting…” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for 50 participants analysed. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “There was no statistically significant difference with respect to age, sex, weight and duration of surgery between the two groups” |
| Schlager 1998 | ||
| Methods | Parallel-group randomized trial, conducted in Austria. Study dates not reported | |
| Participants | 40 children (3 – 12 years) undergoing strabismus surgery. Excluded: children with gastric or intestinal disease, emesis and vomiting in the previous week, and those who received any medical therapy immediately before surgery. No child withdrew from study |
|
| Interventions | Low-level laser stimulation performed on each P6 acupoint over 30 seconds, 15 min before induction of anaesthesia and 15 min after arriving in the recovery room (n = 20) Sham laser stimulation held on P6 acupoints but laser beam not activated, 15 min before induction of anaesthesia and 15 min after arriving in the recovery room (n = 20) |
|
| Outcomes | Vomiting (0 – 24 h), risk of rescue antiemetic drug. | |
| Notes | Rescue antiemetic was dimenhydrinate suppositories 50 mg. Nurses in the recovery room may not have been blinded to treatment groups. Vomiting (0 – 2 h, 0 – 6 h) also recorded in the paper Funding source was from Helbo-Medizintechnik GmbH and the Ludwig Boltzmann Institute for Acupuncture. No details about any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar. “Neither children nor parents were able to tell if the laser was active” |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for 40 children analysed. |
| Selective reporting (reporting bias) | Unclear risk | Risk of nausea was not recorded because it may be difficult to assess in children. Authors stated that “stimulation of PC6 with a low-level laser has no known side effects” |
| Other bias | Low risk | Baseline characteristics were comparable. “There were no significant differences between the groups in age, sex distribution, ASA status, weight, height, duration of anaesthesia, duration of surgery or number of repaired muscles (table 1)” |
| Schultz 2003 | ||
| Methods | Parallel-group randomized trial, conducted in United States. Study conducted from July 1999 to August 2000 | |
| Participants | 103 women undergoing gynaecological surgery. Exclusions: pregnancy, surgery for cancer within the previous 5 years, chemotherapy or radiation therapy within 5 years, an antiemetic within 24 h before surgery, previous use of acupressure bands, or peripheral neuropathy. 40 women withdrew before completion of trial due to non-administration of study drug and change in postoperative plans due to earlier hospital discharge |
|
| Interventions | Group 1: droperidol 1.25 mg IV at induction and acupressure wristband at P6 acupoint on both wrists before surgery (worn up to 48 h after surgery) (n = 30) Group 2: droperidol 1.25 mg IV at induction and sham acupressure wristband at P6 acupoint on both wrists before surgery (worn up to 48 h after surgery). Sham acupressure wristband had flat button which did not exert pressure on P6 acupoint (n = 24) Group 3: normal saline IV at induction and acupressure wristband at P6 acupoint on both wrists before surgery (worn up to 48 h after surgery) (n = 24) Group 4: normal saline IV at induction and sham acupressure wristband at P6 acupoint on both wrists before surgery (worn up to 48 h after surgery) (n = 25) |
|
| Outcomes | Nausea (0 - duration of hospital stay), vomiting (0 - hospital stay) | |
| Notes | Authors replied to our request for unpublished data for incidence of nausea and vomiting during hospital stay. Sea Bands were provided by manufacturer. No details about any declarations of interest among authors | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random-number table. |
| Allocation concealment (selection bias) | Low risk | “Study envelopes with the appropriate acupressure band and drug preparation were prepared by the principal investigator and the study pharmacist…. The packets were kept in a secure area of the surgical admitting department. The envelope, containing the study group designation, was opened by the admitting nurse…” |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes |
High risk | Although 40 women withdrew from the study, reasons were given. “There was no statistically significant difference in the age of the 103 women who continued in the study as compared with 40 women who did not complete the study”. Of the 103 women recruited, 95 and 62 women had complete data for nausea and vomiting during hospital stay respectively. Comment: missing data likely to bias the summary effect measure |
| Selective reporting (reporting bias) | High risk | Risk of side effects and use of rescue antiemetic drugs were not described in the paper |
| Other bias | Low risk | Baseline characteristics appeared to be comparable. There was no difference among the groups for age, type of surgery, duration of surgery, duration of acupressure wristband use |
| Sharma 2007 | ||
| Methods | Parallel-group randomized trial, conducted in India. Study dates not reported | |
| Participants | 60 women undergoing laparoscopic cholecystectomies under general anaesthesia. Exclusion: obesity, previous history of PONV and motion sickness |
|
| Interventions | Group 1: ondansetron 4 mg IV given 10 min after induction of anaesthesia (n = 20) Group 2: bilateral P6 acupuncture 5 min before induction of anaesthesia. Intermittent stimulation was given at P6 acupoints by rotating needle clockwise and anticlockwise up to 30 min (n = 20) Group 3: combination of group 1 and group 2 interventions (n = 20) |
|
| Outcomes | Nausea (0 – 7 h), vomiting (0 – 7 h), risk of rescue antiemetic drug (0 – 7 h), risk of adverse effects | |
| Notes | Rescue antiemetic was metoclopramide 10 mg IV. No pain, bleeding, vasovagal attack, or broken acupuncture needles noted in any of the groups No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
High risk | “Blinding of any form was not possible because acupuncture needles had to be kept in situ in the operating room” |
| Blinding of healthcare providers (performance bias) All outcomes |
High risk | “Blinding of any form was not possible because acupuncture needles had to be kept in situ in the operating room” |
| Blinding of outcome assessor (detection bias) All outcomes |
High risk | “Blinding of any form was not possible because acupuncture needles had to be kept in situ in the operating room” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for 60 women analysed. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “There was no significant difference among the patients in both the groups regarding weight, age, height, gender, hours of preoperative fasting and duration of anesthesia and surgery…” |
| Shenkman 1999 | ||
| Methods | Parallel-group randomized trial, conducted in United States. Study dates not reported | |
| Participants | 100 children (2 – 12 y) undergoing tonsillectomy. Exclusion: congenital heart disease or significant pulmonary disease, predisposition for emesis or actual emesis in the 24 h before surgery, use of medications with antiemetic effects within the 24 h before surgery, infection over an acupuncture point, need for postoperative intubation for more than 1 h, and severe obstructive sleep apnoea |
|
| Interventions | Group 1: acupressure wristband on P6 acupoints of both wrists applied before premedication. Immediately after induction of anaesthesia, wristbands were removed and acupuncture needles were inserted at P6 acupoint on both wrists, left in place until next day. Needles were secured with a strip of tape (n = 47) Group 2: acupressure wristbands applied to sham point on both arms before premedication. Immediately after induction of anaesthesia, wristbands were removed and acupuncture needles were applied to sham point on both arms, left in place until next day. Needles were secured with a strip of tape (n = 53) |
|
| Outcomes | Vomiting (0 – 24 h), risk of rescue antiemetic drug, side effects of acupressure/acupuncture | |
| Notes | Rescue antiemetic was ondansetron IV if 2 or more emetic episodes occurred. Combination of acupressure and acupuncture treatment effect was not analysed in subgroup analysis (invasive versus noninvasive). Proportion of acupuncture site redness and irritation was similar in both groups Funding source from National Institutes of Health General Clinical Research Centre (grant number MRR 02172). Acubands provided by Lifestyle Enterprises, New Jersey. Intradermal needles supplied by OMS Medical Supplies, Massachusetts. No details about any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar. P6 acupoints and sham points on all patients were covered with opaque adhesive tape |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “Postanesthesia care unit and ward nurses who assessed and charted postoperative emesis and medication administration were blinded to the group assignment of each patient” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “Postanesthesia care unit and ward nurses who assessed and charted postoperative emesis and medication administration were blinded to the group assignment of each patient” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for 100 participants analysed. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “There were no differences between the groups with regard to demographics or previous retching, vomiting, or either (table 2)” |
| Streitberger 2004 | ||
| Methods | Parallel-group randomized trial, conducted in Germany. Study was conducted between January and August 2002 | |
| Participants | 212 women undergoing gynaecological or breast surgery under general anaesthesia. Exclusion: acupuncture treatment during the last 6 months, pregnancy, nausea or vomiting during the past 24 h, lymphoedema of the upper limbs, eczematous skin changes at the P6 acupoint, and coagulopathy. 1 woman in the acupuncture group withdrew consent and was treated as a failure in the analysis |
|
| Interventions | Acupuncture group: 52 participants had acupuncture to P6 acupoint on both wrists, 20 min before induction of anaesthesia; another 54 participants had acupuncture to P6 acupoint on both wrists immediately after induction of anaesthesia Sham acupuncture: 51 participants had placebo acupuncture to P6 acupoint on both wrists, 20 min before induction of anaesthesia; another 55 participants had placebo acupuncture to P6 acupoint on both wrists immediately after induction of anaesthesia |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), risk of rescue antiemetic drugs, adverse events related to acupuncture | |
| Notes | Dimenhydinate and dolasetron rescue antiemetics used. Haematomas reported by 1 participant in the acupuncture group and by 2 participants in the placebo acupuncture group. Allergy to sticky plaster reported by 5 participants in each group. No severe adverse reaction reported Funding source from University of Heidelberg (grant number F.203583). No details about any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The patients were randomly distributed by type of surgery (gynaecological or breast) to ensure balance between groups“. Comment: no further details provided in the paper. |
| Allocation concealment (selection bias) | Low risk | “The acupuncturist obtained randomisation allocation by phone from a member of the Coordination Centre for Clinical Trials, University of Heidelberg, who had no contact with study patients. An adequate concealment was thereby assured” |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar. To assess blinding, patients were asked what kind of needle they believe they had received” |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “The patients, the observer of the endpoints, the nurses, the anaesthetists and all other staff members were not informed about the allocation” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “The patients, the observer of the endpoints, the nurses, the anaesthetists and all other staff members were not informed about the allocation” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Reasons for withdrawals given. Intention-to-treat analysis used |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “Baseline characteristics revealed no relevant differences between the two groups (table 1)” |
| Tavlan 1996 | ||
| Methods | Parallel-group randomized trial, conducted in Turkey. Study dates not reported. This study was reported as an abstract | |
| Participants | 65 women (18 – 45 y) undergoing gynaecological laparoscopy. | |
| Interventions | Group 1: ondansetron 8 mg IV before induction (n = 25). Group 2: 0.2 ml 50% dextrose on the P6 acupoint before induction (n = 20) Group 3: 20 ml IV saline before induction. |
|
| Outcomes | Nausea (0 – 1 h), vomiting (0 – 1 h). | |
| Notes | Group 3 (n = 20) not used in the acupoint P6 stimulation versus sham analyses No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data reported for 65 participants analysed. |
| Selective reporting (reporting bias) | Unclear risk | Risk of side effects and rescue antiemetic drugs not given because the article was an abstract |
| Other bias | Low risk | Baseline characteristics were comparable. “No significant differences were observed between the groups in terms of demography” |
| Turgut 2007 | ||
| Methods | Parallel-group randomized trial, conducted in Turkey. Study dates not reported | |
| Participants | 102 women aged 40 – 65 years, with no previous experience of acupressure bands, undergoing elective gynaecological surgery (total abdominal hysterectomy and bilateral salpingo-oophorectomy). 1 participant in acupressure group and 1 in sham group withdrew because of swelling and erythema in treated hand and protocol violation respectively. Exclusion criteria: obesity (BMI > 30), diabetes, history of motion sickness, PONV, or smoking |
|
| Interventions | Acupressure group: wristband with plastic bead positioned at P6 point on both wrists, 30 min before induction of general anaesthesia. Wristbands left on for 24 h (n = 51) Sham group: wristband with plastic bead positioned at non-acupoint site on the dorsal surface of both forearms, 30 min before induction of general anaesthesia. Wristbands left on for 24 h (n = 51) Both groups were educated on the use of participant-controlled analgesia before surgery. Participants received participant-controlled analgesia containing morphine in the postanaesthetic care room, and continued for 24 h |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), rescue antiemetic drug use, adverse effects of wristbands | |
| Notes | Risks of nausea and vomiting on arrival in recovery room reported. No adverse effects or complications were observed due to acupressure wristbands, except for 1 participant in the acupressure group who withdrew due to swelling and erythema of the treated hand. Rescue antiemetic was metoclopramide 10 mg IV No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “The anaesthesiologists caring for the patients were not aware of group assignment” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “The study was observer-blinded”. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Reasons for withdrawal given. No missing data reported for 100 participants analysed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | “Patients of both groups were comparable with regard to age, weight, height, ASA physical status and duration of surgery” |
| Wang 2002 | ||
| Methods | Parallel-group randomized trial, conducted in United States. Study dates not reported | |
| Participants | 190 children (7 – 16 y) undergoing general anaesthesia and outpatient surgical procedures. Exclusions: ASA physical status higher than II and people with a history of developmental delay or prematurity. 3 children were excluded from study because of major study protocol violations |
|
| Interventions | Group 1: after induction, intravenous saline was given. Acupuncture at P6 acupoints on both arms was performed before end of surgery. Injection of 0.2 mL of 50% dextrose using a 1 mL tuberculin syringe with a 25-gauge needle at a depth of 5 to 7 mm from skin (n = 50) Group 2: after induction, droperidol 10 ug/kg IV was given. Superficial skin prick at the P6 acupoint was performed before end of surgery (n = 49) Group 3: after induction, intravenous saline was given. Sham point acupuncture at the dorsum of arms was performed before end of surgery. Injection of 0.2 mL of 50% dextrose using a B-D 1 mL tuberculin syringe with a 25-gauge needle at a depth of 5 to 7 mm from skin (n = 43) Group 4: after induction, intravenous saline was given. Superficial skin prick at the P6 acupoint was performed before end of surgery (n = 45) |
|
| Outcomes | Nausea (0 - recovery room), vomiting (0 - recovery room), risk of rescue antiemetic drug | |
| Notes | Rescue antiemetic was ondansetron IV 0.1 – 4 mg/kg. Groups 3 and 4 were combined and considered as a sham group. No puncture site redness or irritation noted in any of the groups. Late outcomes (discharge to first day after surgery) also reported. No data on outcomes (0 – 24 h) according to author Funding source from Foundation of Anesthesia Education and Research, Society of Pediatric Anesthesia and National Institutes of Health (NICHD R01HD37007-01). No details about any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Yoking randomization (based on computer-generated list) was used for equal distribution of variables that are known to affect the outcome |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar. “Children, parents, surgeons, anesthesiologists, PACU nursing staff, and the research assistant, were all blinded to group assignment” |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “Children, parents, surgeons, anesthesiologists, PACU nursing staff, and the research assistant, were all blinded to group assignment” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “Children, parents, surgeons, anesthesiologists, PACU nursing staff, and the research assistant, were all blinded to group assignment” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Details about withdrawals were given. No missing data reported for 187 children analysed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “There were no differences among the various study groups in regard to baseline demographic characteristics such as age and history of PONV” |
| Wang 2010 | ||
| Methods | Parallel-group randomized trial, conducted in China. Study dates not reported | |
| Participants | 80 people, aged 20 – 60 years, undergoing supratentorial craniotomy. Excluded people were obese (BMI > 30), diabetic, had a history of motion sickness or recent PONV or smoked |
|
| Interventions | Group 1: transcutaneous electrical acupoint stimulation at right wrist P6 acupoints 30 min before induction of anaesthesia, left on for 6 hours after surgery (n = 40) Group 2: sham transcutaneous electrical acupoint stimulation at non-acupoint on dorsal side of the forearm 30 min before induction of anaesthesia, left on for 6 hours after surgery (n = 40) Ondansetron 4 mg IV given as routine antiemetic treatment for each participant before skin closure in both groups |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), rescue antiemetic (metoclopramide 10 mg IV), side effects | |
| Notes | Authors reported that no adverse effects or complications occurred associated with treatment groups No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated random number table. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details given. |
| Blinding of patients (performance bias) All outcomes |
Low risk | “None of the patients had experience with acupuncture electrodes.” Patients were also “unaware whether the sensation was coming from an acupoint or a non-acupoint.” |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | Attending anaesthetist was blinded to treatment allocation. “The screen on the unit was covered with an opaque tape in both groups, so that clinicians and observers were unaware whether the unit was at an acupoint or not.” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | Trained nurse staff did the PONV and were blind to the position of the electrode |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 80 participants were randomized and all completed the study. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Demographic and perioperative characteristics in Table 1 were comparable between groups |
| White 2002 | ||
| Methods | Parallel-group randomized trial, conducted in United States. Study dates not reported | |
| Participants | 120 adults undergoing elective plastic surgery. Excluded: antiemetic medication within 24 h before surgery, pregnancy, using permanent cardiac pacemaker, previous experience with acustimulation treatment, experiencing vomiting or retching within 24 h before surgery. No participants withdrew before discharge from hospital, 5 participants withdrew from study at 72 hours follow-up |
|
| Interventions | Group 1: ondansetron 4 mg and inactive acustimulation device (ReliefBand) at P6 acupoint on arrival in the recovery room. Device worn for 72 hours after surgery (n = 40) Group 2: saline 2 mL and active acustimulation device (ReliefBand) at P6 acupoint on arrival in the recovery room. Device worn for 72 hours after surgery (n = 40) Group 3: ondansetron 4 mg and active acu-stimulation device (ReliefBand) at P6 acupoint on arrival in the recovery room. Device worn for 72 hours after surgery (n = 40) |
|
| Outcomes | Nausea (0 - hospital discharge), vomiting (0 - hospital discharge), risk of rescue antiemetic drug, side effects | |
| Notes | Rescue antiemetic was metoclopramide 10 mg IV if persistent nausea or vomiting, or retching lasting more than 10 min. No swelling at wrist or erythema reported. No outcome measures (0 – 72 h) given in the paper Funding source from department. First author received past funding from both Woodside Biomedical systems and GlaxoSmith-Kline |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomly assigned to one of three treatment groups using a computer-generated random number table…” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | All participants were told that the Relief-Band acu-stimulation device produces a sensation which they may or may not feel to minimize bias. Participants recorded outcome measures in a participant diary |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | All participants were told that the Relief-Band acustimulation device produces a sensation which they may or may not feel to minimize bias. Participants recorded outcome measures in a participant diary |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 1 participant lost to follow-up in ondansetron group, 1 lost to follow-up in acu-stimulation group, and 3 lost to follow-up in combination group. Author used intention to treat analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “The three treatment groups were comparable with respect to demographic characteristics, pre-existing risk factors for development of PONV, and preoperative nausea scores” |
| White 2012 | ||
| Methods | Parallel-group randomized trial, conducted in United States. Study dates not reported | |
| Participants | 100 adult outpatients, with ASA physical status I – II, undergoing major laparoscopic surgery. Exclusion criteria were people receiving antiemetic drugs within 24 hour before surgery, previous experience using acustimulation device for management of pain or emetic symptoms, history of alcohol or drug abuse within last 3 months, or a skin lesion or irritation at P6 acupoints |
|
| Interventions | Group 1: Bilaterial acupressure (Pressure Right) strips on P6 acupoints 30 – 60 min before entering operating room and left in place for 72 h after surgery. Dexamethasone 4 mg IV given before start of surgery, ondansetron 4 mg IV given at end of surgery (n = 50) Group 2: Sham acupressure (no plastic button) strips on P6 acupoints 30 – 60 min before entering operating room and left in place for 72 hours after surgery. Dexamethasone 4 mg IV given before start of surgery, ondansetron 4 mg IV given at end of surgery (n = 50) |
|
| Outcomes | Nausea (0 – 72 h), vomiting (0 – 72 h), rescue antiemetic (metoclopramide 10 mg IV and prochlorperazine 25 mg suppository), side effects of acupressure | |
| Notes | Outcomes 0 – 24 h also reported. Participants in the acupressure group were more likely to be highly satisfied with PONV management at 72 h than sham group (mean difference 18%, 95% CI 1% to 34%). No difference in 48 h or 72 h quality of recovery score between groups. “Incidence of side-effects did not differ between the two study groups (Table 4).” Power calculation done Active and sham Pressure Right acupressure devices were provided by manufacturer. Authors declare no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated randomisation scheme. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details given. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Strips were identical. |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | Placement of acupressure or sham acupressure strips by co-investigator not involved in outcome assessment |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | Blinded observer questioned each participant before discharge and via telephone interviews about outcomes |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 100 participants completed the study and all participants completed the follow-up evaluations |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Demographic characteristics and history of PONV or motion sickness were not significantly different in the 2 antiemetic study groups |
| Xu 2012 | ||
| Methods | Parallel-group randomized trial, conducted in China. Study dates not reported | |
| Participants | 130 adults, ASA physical status I – III, undergoing infratentorial craniotomy. Excluded: those with previous experiences with acupuncture, nausea or vomiting within 24 h before surgery, preoperative use of antiemetics (except dexamethasone), cardiac pacemaker, cardioverter, or defibrillator, pregnant or breastfeeding at time of surgery, obese (BMI > 35), mental retardation or psychiatric illness |
|
| Interventions | Group 1: transcutaneous electrical acupoint stimulation at dominant wrist P6 acupoints 30 min before induction of anaesthesia, left on for 24 hours after surgery (n = 65) Group 2: sham transcutaneous electrical acupoint stimulation at dominant wrist P6 acupoints 30 min before induction of anaesthesia but no electrical stimulation activated, left on for 24 h after surgery (n = 65) Ondansetron 4 mg IV and dexamethasone 10 mg given as routine antiemetic treatment for each participant during surgery in both groups |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), rescue antiemetic (metoclopramide 10 mg IM) | |
| Notes | Power calculation done. Authors reported that no adverse effects (cutaneous irritation, bleeding, nerve injury) occurred associated with treatment groups. Authors have no conflicts of interest. Study supported by grants from Major State Basic Research Development Program of China (973 Program No. 2007CB12502) and National Natural Science Foundation of China (No. 81171235/H0914) | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated random number table. |
| Allocation concealment (selection bias) | Low risk | Sequentially-numbered, opaque sealed envelopes. |
| Blinding of patients (performance bias) All outcomes |
Low risk | “Display screens of the units were concealed from view for patients and other investigators… All patients were told that a tingling or numbing sensation might or might not be felt, regardless of the the group assignment.” |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “Display screens of the units were concealed from view for patients and other investigators.” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “Trained nursing staff, who were blinded to the group assignments, assessed PONV…” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 11 (8%) participants withdrew, probably due to those who could not be extubated within 2 h after surgery or had impaired consciousness in the neurological intensive care unit |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Other bias | Low risk | “No differences in patient demographics, risk factors for PONV, duration of anaesthesia, intraoperative opioids and postoperative analgesic consumption between the two groups.” |
| Yang 1993 | ||
| Methods | Parallel-group randomized trial, conducted in Taiwan. Study dates not reported | |
| Participants | 120 women undergoing gynaecological laparoscopy. | |
| Interventions | Group 1: acupuncture group included participants given an injection of 0.2 mL 50% glucose in water into P6 acupoint before extubation (n = 40) Group 2: antiemetic group was droperidol 20 ug/kg IV on induction of anaesthesia (n = 40) Group 3: no treatment (n = 40). |
|
| Outcomes | Vomiting (0 – 3 h), side effects of acupuncture. | |
| Notes | Reference group received no treatment and was not included in data analysis. Pain at acupoint site noted No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of healthcare providers (performance bias) All outcomes |
Unclear risk | Insufficient information. |
| Blinding of outcome assessor (detection bias) All outcomes |
Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data recorded for 120 participants analysed. |
| Selective reporting (reporting bias) | High risk | Nausea was not reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “There was no statistically significant differences in age, weight, duration of anesthesia or amount of fluid given among the three groups of patients” |
| Yentis 1992 | ||
| Methods | Parallel-group randomized trial, conducted in Canada. Study dates not reported | |
| Participants | 90 children (1 – 16 y) undergoing strabismus surgery. 1 child in each of the 3 groups could not be contacted after surgery | |
| Interventions | Group 1: acupuncture at P6 acupoint on right wrist with 5 min of manual stimulation after induction of anaesthesia (n = 30) Group 2: antiemetic group had 0.075 mg/kg droperidol IV after induction of anaesthesia (n = 30) Group 3: acupuncture (as in Group 1) and droperidol (as in Group 2) treatment (n = 30) |
|
| Outcomes | Vomiting (0 – 48 h), risk of rescue antiemetic drug, side effects of treatment | |
| Notes | Rescue antiemetic was dimenhydrinate IM. Restlessness more frequent in droperidol group than acupuncture group. Risk of vomiting before discharge from hospital also reported in paper No details about funding source or any declarations of interest among authors |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | “Whether or not patients received droperidol, both treatments or acupuncture alone, was unknown to the staff, the patients and their parents” |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “Whether or not patients received droperidol, both treatments or acupuncture alone, was unknown to the staff, the patients and their parents” |
| Blinding of outcome assessor (detection bias) All outcomes |
Unclear risk | Insufficient information. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | One participant in each group lost to follow-up. Comment: unlikely to bias summary estimate. |
| Selective reporting (reporting bias) | High risk | Need for rescue antiemetic not reported in Results. Nausea was not reported because it may have been difficult to assess in younger children |
| Other bias | Low risk | Baseline characteristics were comparable. “Age, weight, number of muscles repaired and duration of anaesthesia did not differ among the groups” |
| Zhu 2010 | ||
| Methods | Parallel-group randomized trial, conducted in China. Study dates not reported | |
| Participants | 120 women undergoing gynaecological laparoscopic surgery, ASA I – II, for general anaesthesia | |
| Interventions | Group 1: dilute droperidol injected into bilateral P6 acupoints using an acupuncture needle at 20 min before surgery. Twisted needle at depth of 2.5 cm to 3 cm to get needling sensation, and retained for 10 min (n = 40) Group 2: 2.5 mg droperidol IV 20 minutes before surgery (n = 40) Group 3: no treatment. |
|
| Outcomes | Nausea (0 – 24 h), vomiting (0 – 24 h), side effects of droperidol | |
| Notes | No treatment data were excluded from analysis. No drowsiness, anxiety or extrapyramidal reactions observed in any groups No details about any declarations of interest among authors. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized into 3 groups using a random-numbers table |
| Allocation concealment (selection bias) | Unclear risk | Sequential but no other details given. |
| Blinding of patients (performance bias) All outcomes |
High risk | Participants unlikely to be blinded as they were conscious in order to feel needling sensation of acupuncture |
| Blinding of healthcare providers (performance bias) All outcomes |
High risk | No attempt to mask sham acupuncture in droperidol group. |
| Blinding of outcome assessor (detection bias) All outcomes |
Unclear risk | Insufficient details given. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No missing data recorded for 120 participants analysed. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Age and type of surgery among groups were comparable. |
| Zárate 2001 | ||
| Methods | Parallel-group randomized trial, conducted at 4 university centres in United States. Study dates not reported | |
| Participants | 250 adults undergoing laparoscopic cholecystectomy. Excluded: people who had taken antiemetic, glucocorticosteroids, or psychoactive medication within 24 hours before the operation; were pregnant; had an implanted cardiac pacemaker or defibrillator device; or had experienced vomiting or retching within 24 h before surgery. 29 adults were excluded because of protocol violations |
|
| Interventions | Group 1: ReliefBand (watch-like acustimulation device) positioned at P6 acupoint before the end of surgery. The device was set to deliver a 25 mA stimulus at 31 Hz. Participants wore the device for 9 hours after surgery (n = 110) Group 2: ReliefBand with no acustimulation positioned at P6 acupoint before end of surgery, worn up to 9 hours after surgery (n = 56) Group 3: ReliefBand with no acustimulation positioned at the dorsal aspect of the wrist before end of surgery, worn up to 9 hours after surgery (n = 55) |
|
| Outcomes | Nausea (0 - arrival in recovery room), vomiting (0 - arrival in recovery room), risk of rescue antiemetic (0 – 2 h), side effects of wristband. Rescue antiemetics were droperidol 0.625 mg IV and ondansetron 4 mg IV | |
| Notes | Group 2 and Group 3 were considered as the sham control group for data analysis. Although the ReliefBand devices were identical in appearance, their placement on the dorsal side of the wrist would have suggested that the participants were in Group 3. Outcomes also evaluated at 45, 90, 120, 240, 360 and 540 min after surgery. No cumulative data recorded (requested data from authors but no reply). Side effects of wristbands were mild cutaneous irritation with erythema Study supported by grants from Woodside Biomedical Inc and the White Mountain Institute. The second and third authors are paid consultants for Woodside Biomedical Inc |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Outpatients who had been fasted overnight were randomly assigned to one of three treatment groups (groups T, S, and P) with a computer-generated random number table” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of patients (performance bias) All outcomes |
Low risk | Authors took adequate steps to make interventions appear similar. “To minimize bias resulting from the presence or absence of the electrical stimulation, all patients were told before the operation that the ReliefBand produces a sensation which ’they might or might not feel” |
| Blinding of healthcare providers (performance bias) All outcomes |
Low risk | “The recovery room nursing staff were unaware of the acu-stimulation treatment group to which the patient had been assigned” |
| Blinding of outcome assessor (detection bias) All outcomes |
Low risk | “The recovery room nursing staff were unaware of the acu-stimulation treatment group to which the patient had been assigned” |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Reasons for withdrawals were given. No missing data recorded for 221 participants analysed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Baseline characteristics were comparable. “The three treatment groups were comparable demographically and with respect to their histories of PONV and motion sickness, baseline nausea score, duration of surgery, and the time the acu-stimulation device was applied before the end of surgery” |
ASA = American Society of Anesthesiologists
BMI = body mass index
cm = centimetres
DBS = double burst stimulation
ENT = Ear, nose, throat
h = hour
Hz = Hertz
H2 = selective histamine type 2 receptor
IM = intramuscular
IV = intravenous
K-D2 = Korean hand acupuncture K-D2 point
Kg = kilograms
mA = milliamperes
mg = milligrams
ml = millilitres
mm = millimetres
n = number of participants
NSAIDs = nonsteroidal anti-inflammatory drugs
P6 = pericardium acupoint
PACU = postoperative anaesthesia care unit
PC = pericardium acupoint
PONV = postoperative nausea and vomiting
ST = single twitch
TENS = transcutaneous electrical nerve stimulation
TOF = train-of-four
VAS = visual analogue scale
y = years
There were 10 types of PC6 acupoint stimulation: needle acupuncture (Dundee 1986; Dundee 1989; Sharma 2007; Streitberger 2004; Yentis 1992); infiltration of dextrose (Ravi 2010; Tavlan 1996; Wang 2002; Yang 1993) or with droperidol (Zhu 2010); semipermanent needles (Andrzejowski 1996); electrical stimulation of needles (Amir 2007; Dundee 1989; El-Deeb 2011; Gan 2004; Ho 1990; Rusy 2002); transcutaneous electrical nerve stimulation (Fassoulaki 1993; Ho 1990), transcutaneous electrical acupoint stimulation (Habib 2006; Wang 2010; Xu 2012), laser stimulation (Butkovic 2005; Schlager 1998); acu-stimulation device (Ertas 2015; Frey 2009a; Frey 2009b; Kim 2004; White 2002; Zárate 2001); and acupressure (Adib-Hajbaghery 2013; Agarwal 2000; Agarwal 2002; Alkaissi 1999; Alkaissi 2002; Allen 1994; Barsoum 1990; Direkvand-Moghadam 2013; Duggal 1998; Ebrahim Soltani 2010; Ferrara-Love 1996; Gieron 1993; Harmon 1999; Harmon 2000; Ho 1996; Iqbal 2012; Klein 2004; Lewis 1991; Majholm 2011; Nilsson 2015; Sadighha 2008; Samad 2003; Schultz 2003; Turgut 2007; White 2012). Three studies used conventional peripheral nerve stimulation (Arnberger 2007; Kim 2011; Liu 2008). One trial used both acupressure and acupuncture (Shenkman 1999). Capsicum plaster at PC6 acupoint was used in two studies (Koo 2013; Misra 2005). The type of surgery; type, timing, and duration of stimulation of the PC6 acupoint; and the follow-up time for assessing PONV varied greatly.
PC6 stimulation was compared with six antiemetic drugs: metoclopramide (Butkovic 2005; Direkvand-Moghadam 2013; Dundee 1989; Ebrahim Soltani 2010; Sadighha 2008); cyclizine (Dundee 1989); prochlorperazine (Barsoum 1990; Ho 1990); droperidol (Schultz 2003; Wang 2002; Yang 1993; Yentis 1992; Zhu 2010); ondansetron (Agarwal 2002; Ebrahim Soltani 2010; El-Deeb 2011; Gan 2004; Misra 2005; Ravi 2010; Sharma 2007; Tavlan 1996; White 2002), dexamethasone plus ondansetron (White 2012).
A combination of PC6 stimulation and antiemetic drug was used as a multimodal therapy in several trials (Schultz 2003; Sharma 2007; Wang 2010; White 2002; White 2012; Xu 2012; Yentis 1992; Zhu 2010).
Excluded studies
We excluded 49 trials. Please see ‘Characteristics of excluded studies’ for more information.
Table 3.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agarwal 2005 | PC6 acupoint stimulation not used. Authors used Korean hand acupressure point K-D2 in the study |
| Al-Sadi 1997 | No sham treatment group used. Control was defined as no intraoperative acupuncture needle at PC6 acupoint |
| Alkaissi 2005 | Participants did not undergo surgery. |
| Cekmen 2007 | PC6 acupoint stimulation not used. Authors used transcutaneous electrical nerve stimulation on neck and mastoid area |
| Chen 2005 | No sham treatment group used. |
| Coloma 2002 | Treatment of established postoperative nausea and vomiting. |
| Dundee 1988 | Incidences of nausea and vomiting were not reported separately |
| Dundee 1991 | 2 different forms of PC6 stimulation (acupuncture + saline, acupuncture + 1% lidocaine). No sham treatment group used |
| El-Bandrawy 2013 | Severity of nausea and vomiting symptoms assessed, not incidence |
| El-Rakshy 2009 | Multiple acupoints used. |
| Fan 1997 | Incidences of nausea and vomiting were not reported separately |
| Fry 1986 | No sham treatment group used. Control was defined as no acupressure treatment. Participants did not know that they were in the trial |
| Grube 2009 | Multiple acupoints used (Hegu, Quchi, Neiguan, Zusanli, Neiting, Sanyinjiao, Daichong) |
| Hirs 2013 | Ambiguous details about group allocation following results of unpublished pilot study |
| Ho 2006 | Prevention of intraoperative nausea and vomiting, not postoperative outcomes |
| Jin 2013 | No sham PC6 acupoint stimulation group. |
| Kabalak 2005 | Both Pericardium and Shangwan acupoints used. No treatment was given to the control group |
| Khan 2004 | Incidences of nausea and vomiting were not reported separately |
| Kim 2002 | Control was defined as an inactive capsicum plaster tape fixed at the Korean hand acupuncture point K-D2 point of both hands |
| Kim 2010 | Control group appears to be no-treatment. Unable to get full text |
| Klaiman 2008 | Incidences of nausea and vomiting were not reported separately |
| Korinenko 2009 | Multiple acupoints used. |
| Larson 2010 | Severity of nausea and vomiting symptoms assessed, not incidence |
| Lee 2008 | No sham treatment group used. |
| Lee 2013 | No sham treatment group used. |
| Liodden 2011 | Control group was standard care without acupuncture/acupressure |
| Lu 2013 | No sham treatment group used. |
| McConaghy 1996 | Treatment of established postoperative nausea and vomiting. |
| McMillan 1994 | All transcutaneous electrical stimulation at PC6 acupoint groups received antiemetics. Incidences of nausea and vomiting were not reported separately for placebo transcutaneous electrical stimulation and transcutaneous electrical stimulation groups |
| Ming 2002 | Multiple acupoints used. |
| Ng 2011 | Incidences of nausea and vomiting were not reported separately |
| Norheim 2010 | Control group was standard care without acupuncture/acupressure |
| Noroozinia 2013 | Prevention of intraoperative nausea and vomiting, as well as postoperative nausea and vomiting. Unclear how long acupressure wristband was applied on for. Metoclopramide given for intraoperative nausea and vomiting will affect subsequent PONV incidence comparisons |
| Ouyang 2009 | No sham treatment group used. Control was defined as no acupuncture at PC6 acupoint before and during anaesthesia |
| Phillips 1994 | No sham treatment group used. No specific details of the type of antiemetic drug used as control |
| Schwager 1996 | Both PC6 and Li4 acupoints stimulated. |
| Shyr 1990 | Control was defined as no acupuncture at PC6 acupoint. |
| Sinha 2011 | Nausea and vomiting during labour and delivery, not postoperative outcomes |
| Somri 2001 | Multiple acupoints used. |
| Stein 1997 | Prevention of intraoperative nausea and vomiting. |
| Tang 2013 | No sham treatment group used. |
| Wang 2014 | Multiple acupoints used. |
| Weightman 1987 | No sham treatment group used. Control was defined as no acupuncture at PC6 acupoint after induction of anaesthesia |
| White 2005 | This study compared 3 prophylactic acu-stimulation treatments: preoperative, postoperative, and both preoperative and postoperative. No sham treatment group used for both preoperative and postoperative acu-stimulation |
| Windle 2001 | Retrospective chart review was used to estimate the incidence of vomiting. Incidences of nausea and vomiting were not considered separately, and results were not presented in the paper |
| Yeh 2010 | Multiple acupoints used for pain and PONV. |
| Yentis 1991 | No sham treatment group used. Control was no acupuncture treatment at PC6 acupoint |
| Yentis 1998 | This study compared acupuncture given before induction, after induction and in the recovery room. No sham treatment or antiemetic drug group for comparison |
| Zheng 2008 | Multiple acupoints used. |
IV - intravenous
K-D2 = Korean hand acupuncture K-D2 point
PC = pericardium acupoint
PONV = postoperative nausea and vomiting
Studies awaiting classification
There are no studies awaiting classification
Ongoing studies
There are two ongoing studies (Cooke 2014; Lv 2013). Please see Characteristics of ongoing studies for more information.
Risk of bias in included studies
A ‘Risk of bias’ graph captures the review authors’ judgements about each ‘Risk of bias’ item, presented as percentages across all included trials (Figure 2). A ‘Risk of bias’ summary captures the review authors’ judgements about each risk of bias item for each included trial (Figure 3). There were two studies with an overall low risk of bias (Gan 2004; Xu 2012), as we rated all key domains ‘low risk’. Of the 25 studies with a high risk of bias (one or more key domains were rated ‘high risk’), 20 of these were due to selective reporting.
Figure 2.
Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
Figure 3.

Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Allocation
Allocation sequence was provided using a computer-generated random numbers table (Agarwal 2000; Amir 2007; Arnberger 2007; Ertas 2015; Gan 2004; Harmon 1999; Ho 1996; Klein 2004; Misra 2005; Ravi 2010; Wang 2010; White 2002; White 2012; Xu 2012; Zárate 2001), a table of random numbers (Agarwal 2002; Direkvand-Moghadam 2013; Duggal 1998; Liu 2008; Samad 2003; Schultz 2003), a block-design procedure (Rusy 2002), a yoking randomization based on a computer-generated list (Wang 2002) and the toss of a dice (Adib-Hajbaghery 2013). Two trials had high risk of selection bias from inadequate sequence generation (Ferrara-Love 1996; Sadighha 2008). Eight of the 59 trials reported adequate allocation concealment (Arnberger 2007; Ertas 2015; Gan 2004; Majholm 2011; Nilsson 2015; Schultz 2003; Streitberger 2004; Xu 2012). In 49 trials the allocation concealment was unclear, and in two trials (Ferrara-Love 1996; Sadighha 2008) it was inadequate.
Blinding
Participants were not blinded in one study (Sharma 2007) because acupuncture needles inserted before induction of anaesthesia had to be kept in situ in the operating room in two of the three intervention groups. There was no blinding of healthcare providers in two studies (Arnberger 2007; Sharma 2007). As an outcome assessor was not blinded in three studies (Adib-Hajbaghery 2013; Gieron 1993; Sharma 2007), detection bias was likely to have occurred.
Incomplete outcome data
Three trials were at high risk of attrition bias (Fassoulaki 1993; Harmon 1999; Schultz 2003).
Selective reporting
Twenty trials did not report all four outcomes: postoperative nausea, postoperative vomiting, rescue antiemetic drugs, and adverse events in their studies (Adib-Hajbaghery 2013; Alkaissi 1999; Allen 1994; Barsoum 1990; Butkovic 2005; Direkvand-Moghadam 2013; Ertas 2015; Fassoulaki 1993; Ferrara-Love 1996; Frey 2009a; Habib 2006; Harmon 2000; Ho 1990; Koo 2013; Lewis 1991; Ravi 2010; Sadighha 2008; Schultz 2003; Yang 1993; Yentis 1992).
Other potential sources of bias
All studies except one (Dundee 1989) reported the between-group comparisons of baseline characteristics.
Effects of interventions
See: Summary of findings for the main comparison Acupoint PC6 stimulation versus sham for preventing postoperative nausea and vomiting; Summary of findings 2 Acupoint PC6 stimulation versus antiemetic drug for preventing postoperative nausea and vomiting; Summary of findings 3 Acupoint PC6 stimulation and antiemetic combination compared to antiemetic for preventing postoperative nausea and vomiting
PC6 acupoint stimulation versus sham treatment
In the few studies (Frey 2009a; Frey 2009b; Streitberger 2004) that directly compared the timing of PC6 acupoint stimulation (pre-versus post-induction), the risk reduction of PONV was similar irrespective of when the acupoint stimulation occurred. Compared to sham acu-stimulation, the odds of nausea within 24 hours after hysterectomy for pre-induction acu-stimulation and post-induction acu-stimulation were 0.31 (95% CI 0.14 to 0.68) and 0.33 (95% CI 0.15 to 0.73) respectively (Frey 2009a). Similarly, compared to sham acu-stimulation, the odds of vomiting within 24 hours after hysterectomy for pre-induction acu-stimulation and post-induction acu-stimulation were 0.37 (95% CI 0.18 to 0.79) and 0.26 (95% CI 0.13 to 0.56) respectively (Frey 2009a). There was no significant difference in the incidence of PONV at two hours after laparoscopic cholecystectomy in the group of acu-stimulation pre-induction compared to post-induction of anaesthesia (Frey 2009b). There was no significant difference in the incidence of PONV at 24 hours when acupuncture was given before induction (RR 0.88, 95% CI 0.60 to 1.28) or after induction (RR 0.82, 95% CI 0.53 to 1.27) (Streitberger 2004).
Primary outcomes
1. Incidence of postoperative nausea
(see Analysis 1.1)
Forty trials examined PC6 acupoint stimulation for the prevention of nausea, in a total of 4742 participants (Analysis 1.1). PC6 acupoint stimulation reduced the incidence of nausea (RR 0.68, 95% CI 0.60 to 0.77) but there was substantial heterogeneity (I2 statistic = 67%). The ‘trim and fill’ method did not trim or add any more studies to the contour-enhanced funnel plot (Figure 4). The estimated NNTB for different baseline risks of nausea is shown in Table 4.
Figure 4.
Contour-enhanced funnel plot of comparison: PC6 acupoint stimulation versus sham for nausea. Contour lines are at 1%, 5% and 10% levels of statistical significance.
There was no interaction effect between the subgroup analyses that were prespecified: children versus adults (Analyses 1.1.2, 1.1.3: Chi2 statistic 0.48, df = 1, P = 0.49); invasive versus noninvasive PC6 acupoint stimulation (Analyses 1.1.4, 1.1.5: Chi2 statistic 1.52, df = 1, P = 0.22). There was also no interaction between trials at low, unclear and high risk of bias (Analyses 1.1.6, 1.1.7, 1.1.8; Chi2 statistic 1.46, df = 2, P = 0.48) or for control event rates (up to 20% or more than 20%) (Analyses 1.1.9, 1.1.10: Chi2 statistic 0.44, df = 1, P = 0.51).
As the heterogeneity among trials was substantial and there were study limitations, we downgraded the evidence from high to low quality. Using trial sequential analysis, the required information size and boundary for benefit were reached for nausea (Figure 5).
Figure 5.
Trial sequential analysis of 40 trials comparing PC6 acupoint stimulation versus sham (despite risk of bias) for postoperative nausea, with control event proportion of 46.8%, diversity of 71%, α of 5%, power of 80%, and relative risk reduction of 30% (Apfel 2007).
2. Incidence of postoperative vomiting, defined as either retching or vomiting, or both
(see Analysis 1.2)
Forty-five trials examined PC6 acupoint stimulation for the prevention of vomiting, in 5147 participants. PC6 acupoint stimulation reduced the incidence of vomiting (RR 0.60, 95% CI 0.51 to 0.71) but there was substantial heterogeneity (I2 statistic = 64%). The ‘trim and fill’ method did not trim or add any more studies to the contour-enhanced funnel plot (Figure 6). The estimated NNTB for different baseline risks of vomiting is shown in Table 4.
Figure 6.
Contour-enhanced funnel plot of comparison: PC6 acupoint stimulation versus sham for vomiting. Contour lines are at 1%, 5% and 10% levels of statistical significance.
Table 4.
Estimated NNTB for preventing PONV (PC6 acupoint stimulation versus sham)
| Control event rate | Nausea | 95% CI | Vomiting | 95% CI |
|---|---|---|---|---|
| 10% | 31 | 25 to 43 | 25 | 20 to 34 |
| 20% | 16 | 13 to 22 | 13 | 10 to 17 |
| 30% | 10 | 8 to 14 | 8 | 7 to 11 |
| 40% | 8 | 6 to 11 | 6 | 5 to 9 |
| 50% | 6 | 5 to 9 | 5 | 4 to 7 |
| 60% | 5 | 4 to 7 | 4 | 3 to 6 |
| 70% | 4 | 4 to 6 | 4 | 3 to 5 |
| 80% | 4 | 3 to 5 | 3 | 3 to 4 |
| 90% | 3 | 3 to 5 | 3 | 2 to 4 |
CI = confidence interval
NNTB = number needed to treat for an additional beneficial outcome
PC6 = pericardium acupoint
PONV = postoperative nausea and vomiting
There was no interaction effect between subgroup analyses that were prespecified: children versus adults (Analyses 1.2.2, 1.2.3: Chi2 statistic 0.33, df = 1, P = 0.63); invasive versus noninvasive PC6 acupoint stimulation (Analyses 1.2.4, 1.2.5: Chi2 statistic 0.56, df = 1, P = 0.45). There was also no interaction between trials at low, unclear and high risk of bias (Analyses 1.2.6, 1.2.7, 1.2.8; Chi2 statistic 0.30, df = 2, P = 0.86) or for control event rates (up to 20% or more than 20%) (Analyses 1.2.9, 1.2.10: Chi2 statistic 1.39, df = 1, P = 0.24).
As the heterogeneity among trials was substantial and there were study limitations, we downgraded the evidence from high to low quality. Using trial sequential analysis, the required information size and boundary for benefit were reached for vomiting (Figure 7).
Figure 7.
Trial sequential analysis of 45 trials comparing PC6 acupoint versus sham (despite risk of bias) for postoperative vomiting, with control event proportion of 32.9%, diversity of 74%, α of 5%, power of 80%, and relative risk reduction of 30% (Apfel 2007).
Secondary outcomes
1. Need for rescue antiemetic drug when prophylaxis failed
The need for a rescue antiemetic was less after PC6 stimulation compared to sham treatment in 39 trials involving 4622 participants (RR 0.64, 95% CI 0.55 to 0.73). There was moderate heterogeneity (I2 statistic = 44%). Three trials did not specify the type of rescue antiemetic drug used (Alkaissi 2002; Duggal 1998; Ferrara-Love 1996). We included the data excluded by one trial for persistent vomiting (Fassoulaki 1993). We downgraded the evidence from high to low quality because of inconsistency between trials and study limitations.
2. Adverse effects from PC6 acupoint stimulation and/or antiemetic drug
Overall, the side effects associated with PC6 acupoint stimulation were minor and self limiting. There were no side effects for participants receiving acupuncture (Dundee 1986; Dundee 1989; Sharma 2007; Wang 2002; Zhu 2010); electroacupuncture (El-Deeb 2011); acupressure (Agarwal 2000; Agarwal 2002; Gieron 1993; Harmon 1999; Ho 1996; Klein 2004; Lewis 1991; Samad 2003); or transcutaneous electro-acupoint stimulation (Arnberger 2007; Gan 2004; Kim 2011; Liu 2008; Wang 2010; Xu 2012).
Haematomas occurred in one participant in the acupuncture group and in two participants in the placebo acupuncture group (Streitberger 2004). Pain was reported at the acupuncture site in one trial (Yang 1993). There was no significant difference in the incidence of redness and irritation at the puncture site between PC6 acupoint stimulation and sham treatment groups (Shenkman 1999). Participants complained of feeling tired and sleepy during electro-acupuncture stimulation (Ho 1990) or had erythema (Amir 2007).
Although no side effects were reported with acu-stimulation (Ertas 2015; White 2002), another trial reported mild cutaneous irritation (Zárate 2001). Three trials (Alkaissi 2002; Barsoum 1990; Duggal 1998) reported that acupressure bands felt uncomfortable, produced red indentation or itching, headache and dizziness, swollen wrists, and blistering at the site of the button. One participant in the acupressure group withdrew from a trial due to swelling and erythema of the wrist (Turgut 2007). The incidence of redness, tenderness, paraesthesia and swelling was similar between active and sham acupressure wristband groups (Majholm 2011; Nilsson 2015). One participant complained of mild irritation at the site of capsicum plaster application (Misra 2005).
PC6 acupoint stimulation versus antiemetic drug
Primary outcomes
1. Incidence of postoperative nausea
Compared to antiemetic drugs, there was no difference in the incidence of postoperative nausea associated with PC6 acupoint stimulation (Analysis 2.1.5: RR 0.91, 95% CI 0.75 to 1.10) in 14 trials involving 1332 participants. There was minor heterogeneity between the trials (I2 statistic = 16%). The ‘trim and fill’ method did not trim or add any more studies to the contour-enhanced funnel plot (Figure 8). We found no interaction effect between the different types of antiemetic drugs (ondansetron, metoclopramide, cyclizine, droperidol) for comparison with PC6 acupoint stimulation (Analyses 2.1.1 to 2.1.4: Chi2 statistic 1.10, df = 3, P = 0.78).
Figure 8.
Contour-enhanced funnel plot of comparison: PC6 acupoint stimulation versus antiemetic for nausea. Contour lines are at 1%, 5% and 10% levels of statistical significance.
There was no interaction effect between subgroup analyses that were prespecified: children versus adults (Analyses 2.1.6, 2.1.7: Chi2 statistic 1.49, df = 1, P = 0.22); invasive versus noninvasive PC6 acupoint stimulation (Analyses 2.1.8, 2.1.9: Chi2 statistic 1.38, df = 1, P = 0.24). There was weak evidence for an interaction effect between trials at low, unclear and high risk of bias (Analyses 2.1.10, 2.1.11, 2.1.12; Chi2 statistic 5.36, df = 2, P = 0.07). There was no interaction effect between control event rate groups (up to 20% or more than 20%) (Analyses 2.1.13, 2.1.14: Chi2 statistic 0, df = 1, P = 0.97).
As there were study limitations, we downgraded the evidence from high to moderate quality. Using trial sequential analysis, the boundary for futility was reached for nausea (Figure 9).
Figure 9.
Trial sequential analysis of 14 trials of PC6 acupoint stimulation versus antiemetic (despite risk of bias) for postoperative nausea, with control event proportion of 25.0%, diversity of 40%, α of 5%, power of 80%, and relative risk reduction of 30% (Apfel 2007).
2. Incidence of postoperative vomiting, defined as either retching or vomiting, or both
Compared to antiemetic drugs, there was no difference in the incidence of postoperative vomiting associated with PC6 acupoint stimulation (Analysis 2.2.6: RR 0.93, 95% CI 0.74 to 1.17) in 19 trials involving 1708 participants. Trial results were homogeneous (I2 = 0%). The ‘trim and fill’ method did not trim or add any more studies to the contour-enhanced funnel plot (Figure 10). There was no interaction effect between the different types of antiemetic drugs used for comparisons with PC6 acupoint stimulation (Chi2 statistic 0.71, df = 4, P = 0.95).
Figure 10.
Contour-enhanced funnel plot of comparison: PC6 acupoint stimulation versus antiemetic for vomiting. Contour lines are at 1%, 5% and 10% levels of statistical significance.
There was no interaction effect between subgroup analyses that were prespecified: children versus adults (Analyses 2.2.7, 2.2.8: Chi2 statistic 0.03, df = 1, P = 0.87); invasive versus noninvasive PC6 acupoint stimulation (Analyses 2.2.9, 2.2.10: Chi2 statistic 0.19, df = 1, P = 0.66). There was no interaction effect between trials at low, unclear and high risk of bias (Analyses 2.2.11, 2.2.12, 2.2.13; Chi2 statistic 0.32, df = 2, P = 0.85). There was no interaction effect between control event rate groups (up to 20% or more than 20%) (Analyses 2.2.14, 2.2.15: Chi2 statistic 0.01, df = 1, P = 0.94).
As there were study limitations, we downgraded the evidence from high to moderate quality. Using trial sequential analysis, the boundary for futility was reached for vomiting (Figure 11).
Figure 11.
Trial sequential analysis of 19 trials comparing PC6 acupoint stimulation versus antiemetic (despite risk of bias) for postoperative vomiting, with control event proportion of 14.7%, diversity of 0%, α of 5%, power of 80%, and relative risk reduction of 30% (Apfel 2007).
Secondary outcomes
1. Need for rescue antiemetic drug when prophylaxis failed
There was no difference in the incidence of requiring rescue antiemetics for PC6 acupoint stimulation compared to pooled antiemetic drugs (RR 0.87, 95% CI 0.65 to 1.16) in nine trials involving 895 participants. Trial results were homogeneous (I2 statistic = 0%). The evidence was of moderate quality due to study limitations.
2. Adverse effects from PC6 acupoint stimulation or antiemetic drug, or both
Restlessness was less frequent in the acupuncture group than after droperidol (RR 0.47, 95% CI 0.26 to 0.87) (Yentis 1992). While there was no puncture site redness, irritation or vasovagal effects (Sharma 2007; Wang 2002), another study reported pain associated with acupuncture (Yang 1993). There was no drowsiness, anxiety or extrapyramidal reactions found in participants given acupuncture or droperidol (Zhu 2010). No complications associated with electro-acupuncture, electro-acupuncture stimulation, acu-stimulation, acupressure, ondansetron were noted in several trials (Agarwal 2002; El-Deeb 2011; Gan 2004; Misra 2005; White 2002). Of the 49 participants in the acupressure wristband group, four reported some local tightness and discomfort (Barsoum 1990).
PC6 acupoint stimulation and antiemetic combination versus sham
One trial examined this comparison of wristband and droperidol versus sham wristband and placebo drug (Schultz 2003). There was no difference between groups for the incidence of nausea (RR 1.19, 95% CI 0.91 to 1.55) and vomiting (RR 1.18, 95% CI 0.63 to 2.21).
PC6 acupoint stimulation and antiemetic combination versus antiemetic
Primary outcomes
1. Incidence of postoperative nausea
Analysis (Analysis 3.1)
The eight trials (n = 642) evaluating the combination of PC6 acupoint stimulation and antiemetic versus antiemetic for preventing postoperative nausea were all conducted in adults. There was no difference in the incidence of postoperative nausea between groups (Analysis 3.1.4: RR 0.79, 95% CI 0.55 to 1.13). There was substantial heterogeneity between the trials (I2 statistic = 72%), which may be explained by the level of invasiveness of the PC6 acupoint stimulation (Analysis 3.1.5 and 3.1.6: subgroup interaction effect was significant, P = 0.03). We found no interaction effect between the different types of PC6 acupoint stimulation antiemetic drug combinations (Chi2 statistic 0.23, df = 2, P = 0.89); level of risk of bias of trials (Chi2 statistic 2.14, df = 2, P = 0.34) or control event rate groups (Chi2 statistic 1.35, df = 1, P = 0.25).
We downgraded the evidence from high to very low quality due to substantial heterogeneity among the trials, study limitations and imprecision of the summary estimate. Using trial sequential analysis, none of the boundaries for benefit, harm or futility were crossed and the required information size of 1743 was far from being reached (Figure 12).
Figure 12.
Trial sequential analysis of 8 trials comparing PC6 acupoint and antiemetic versus antiemetic (despite risk of bias) for postoperative nausea, with control event proportion of 47.5%, diversity of 79%, α of 5%, power of 80%, and relative risk reduction of 30% (Apfel 2007).
2. Incidence of postoperative vomiting, defined as either retching or vomiting, or both
Analysis (Analysis 3.2)
Compared to the antiemetic control groups, the combination of PC6 acupoint stimulation and antiemetic reduced the incidence of vomiting in nine trials involving 687 participants (Analysis 3.2.4: RR 0.56, 95% CI 0.35 to 0.91). There was substantial heterogeneity between the trials (I2 = 61%). There was no interaction effect between different age groups (Analysis 3.2.5 and 3.2.6: Chi2 statistic 1.17, df = 1, P = 0.28), level of invasiveness of the PC6 acupoint stimulation (Analysis 3.2.7 and 3.2.8: Chi2 statistic 0.73, df = 1, P = 0.39), level of risk of bias of trials (Chi2 statistic 0.01, df = 2, P = 1.00) or control event rate (Chi2 statistic 1.97, df = 1, P = 0.16).
As there was substantial heterogeneity among trials, study limitations and imprecision of the summary estimate, we downgraded the evidence from high to very low quality. Using trial sequential analysis, none of the boundaries for benefit, harm or futility were crossed and the required information size of 2058 was far from being reached (Figure 13).
Figure 13.
Trial sequential analysis of 9 trials comparing PC6 acupoint stimulation and antiemetic versus antiemetic (despite risk of bias) for postoperative vomiting, with control event proportion of 32.9%, diversity of 68%, α of 5%, power of 80%, and relative risk reduction of 30% (Apfel 2007).
Secondary outcomes
1. Need for rescue antiemetic drug when prophylaxis failed
Analysis (Analysis 3.3)
The most common type of rescue antiemetic used for PC6 acupoint stimulation and antiemetic combination was metoclopramide (Sharma 2007; Wang 2010; White 2002; Xu 2012). One trial used both metoclopramide and prochlorperazine as rescue antiemetics (White 2012). Overall, participants in the PC6 acupoint stimulation and antiemetic combination group were less likely to require rescue antiemetic than the antiemetic-only comparison group (RR 0.61, 95% CI: 0.44 to 0.86) with no heterogeneity (I2 statistic = 0%) in five trials involving 419 participants. As there were study limitations and imprecision, we rated the overall quality of evidence as low.
2. Adverse effects from PC6 acupoint stimulation and/or antiemetic drug
No major adverse effects were reported in several trials (Sharma 2007; Wang 2010; White 2002; Xu 2012; Zhu 2010). The incidence of headache, fatigue, drowsiness, dizziness, constipation and local discomfort were similar between groups (White 2012).
ADDITIONAL SUMMARY OF FINDINGS [Explanation]
| Acupoint PC6 stimulation versus antiemetic drug for preventing postoperative nausea and vomiting | ||||||
|---|---|---|---|---|---|---|
| Patient or population: People at risk of postoperative nausea and vomiting Settings: Surgery Intervention: Acupoint PC6 stimulation Comparison: Antiemetic drug | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Antiemetic | Acupoint PC6 stimulation | |||||
| Nausea - All antiemetics combined | Low | RR 0.91 (0.75 to 1.10) | 1332 (14 studies) | ⊕⊕⊕○ moderate1 |
||
| 200 per 1000 | 182 per 1000 (150 to 220) | |||||
| Moderate | ||||||
| 400 per 1000 | 364 per 1000 (300 to 440) | |||||
| High | ||||||
| 600 per 1000 | 546 per 1000 (450 to 660) | |||||
| Vomiting - All antiemetics combined | Low | RR 0.93 (0.74 to 1.17) | 1708 (19 studies) | ⊕⊕⊕○ moderate2 |
||
| 200 per 1000 | 186 per 1000 (148 to 234) | |||||
| Moderate | ||||||
| 400 per 1000 | 372 per 1000 (296 to 468) | |||||
| High | ||||||
| 600 per 1000 | 558 per 1000 (444 to 702) | |||||
| Rescue antiemetic | 150 per 1000 | 130 per 1000 (97 to 174) | RR 0.87 (0.65 to 1.16) | 895 (9 studies) | ⊕⊕⊕○ moderate3 |
|
| Adverse effects | See comment | See comment | Not estimable | 11 studies4 | Not applicable | See footnote4 |
The basis for the assumed risks for nausea and vomiting is from a consensus panel (Gan 2014) using Apfel’s simplified risk score (Apfel 1999). The assumed risk for rescue antiemetic is the median antiemetic group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio;
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. |
Of the 14 trials, 5 had one or more high risk of bias domains (downgrade 1 point due to study limitations).
Of the 19 trials, 10 had one or more high risk of bias domains (downgrade 1 point due to study limitations).
Of the 9 trials, 3 had one or more high risk of bias domains (downgrade 1 point due to study limitations).
Eight trials reported no side effects. Three trials reported adverse effects (e.g.. restlessness and pain with acupuncture; local tightness and discomfort with acupressure wristbands).
| Acupoint PC6 stimulation and antiemetic combination compared to antiemetic for preventing postoperative nausea and vomiting | ||||||
|---|---|---|---|---|---|---|
| Patient or population: People at risk of postoperative nausea and vomiting Settings: Surgery Intervention: Acupoint PC6 stimulation and antiemetic combination Comparison: Antiemetic drug | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Antiemetic | Acupoint PC6 stimulation and antiemetic combination | |||||
| Nausea | Low | RR 0.79 (0.55 to 1.13) | 642 (8 studies) | ⊕○○○ very low1,2,3 |
||
| 200 per 1000 | 158 per 1000 (110 to 226) | |||||
| Moderate | ||||||
| 400 per 1000 | 316 per 1000 (220 to 452) | |||||
| High | ||||||
| 600 per 1000 | 474 per 1000 (330 to 678) | |||||
| Vomiting | Low | RR 0.56 (0.35 to 0.91) | 687 (9 studies) | ⊕○○○ very low3,4,5 |
||
| 200 per 1000 | 112 per 1000 (70 to 182) | |||||
| Moderate | ||||||
| 400 per 1000 | 224 per 1000 (140 to 364) | |||||
| High | ||||||
| 600 per 1000 | 336 per 1000 (210 to 546) | |||||
| Rescue antiemetic | 316 per 1000 | 193 per 1000 (139 to 272) | RR 0.61 (0.44 to 0.86) | 419 (5 studies) | ⊕⊕○○ low3,6 |
|
| Adverse effects | See comment | See comment | Not estimable | 6 studies7 | Not applicable | See footnote7 |
The basis for the assumed risks for nausea and vomiting is from a consensus panel (Gan 2014) using Apfel’s simplified risk score (Apfel 1999). The assumed risk for rescue antiemetic is the median antiemetic group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio;
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. |
Of the 8 trials, 2 had one or more high risk of bias domains (downgrade 1 point due to study limitations).
Substantial heterogeneity present (downgrade 1 point due to inconsistency).
Optimal information size is far from reached and/or total number of events is less than 300 (downgrade 1 point due to imprecision).
Of the 9 trials, 3 had one or more high risk of bias domains (downgrade 1 point due to study limitations).
Moderate amount of heterogeneity (downgrade 1 point due to inconsistency).
Of the 5 trials, 1 had one or more high risk of bias domains (downgrade 1 point due to study limitations).
No major adverse effects reported in the trials.
DISCUSSION
Summary of main results
We have shown that PC6 acupoint stimulation reduced the incidence of postoperative nausea and vomiting (PONV) compared to sham treatment. PC6 acupoint stimulation prevented postoperative nausea, vomiting, and need for antiemetic rescue by similar amounts that can be considered clinically significant (see Summary of findings for the main comparison). However, the reasons for substantial heterogeneity are unclear and do not appear to be related to age, invasiveness level of the PC6 acupoint stimulation, risk of bias levels or control event rate, since there were no significant subgroup interactions effects. Nevertheless, compared to sham treatment, the reduction in the incidences of nausea, vomiting, and need for rescue antiemetics with PC6 acupoint stimulation may reduce costs (such as antiemetic drug cost, length of stay in hospital) as well as improve quality of patient care. However, the costs and quality of patient care were not outcomes examined in this systematic review.
Our results suggest that the PC6 acupoint stimulation was as effective as an antiemetic prophylaxis therapy for reducing the incidences of PONV (see Summary of findings 2). However, there was inconclusive evidence for combining PC6 acupoint stimulation and antiemetic as a multimodal approach for preventing PONV (see Summary of findings 3). Many trials either reported no adverse side effects or minor, transient side effects associated with PC6 acupoint stimulation.
New highlights of this review include the results of the trial sequential analyses: (1) no further PC6 acupoint stimulation versus sham trials are needed, and (2) further PC6 acupoint stimulation versus antiemetic trials are futile in showing a significant difference.
Overall completeness and applicability of evidence
The participants included this systematic review are representative of people with varying underlying risk factors for PONV undergoing a range of surgical procedures with various prophylactic antiemetic regimens. The trials were conducted in middle- and high-income countries. Therefore, the results of this systematic review are directly applicable to clinical practice.
A lack of evidence on the optimal timing, duration and method of PC6 acupoint stimulation (Streitberger 2011) may explain the low uptake of PC6 acupoint stimulation in current clinical practice. In the few studies (Frey 2009a; Frey 2009b; Streitberger 2004) that have directly compared the timing of PC6 acupoint stimulation (pre- versus post-induction), the risk reduction of PONV was similar irrespective of when the PC6 acupoint stimulation occurred. No trials in this systematic review compared different durations of PC6 acupoint stimulation. The noninvasive techniques may be more acceptable to anaesthesiologists and patients, as little training is needed to accurately locate the site of PC6 acupoint and administer the stimulation via appropriate devices. Generally, we found no subgroup interaction effects between invasive and noninvasive PC6 acupoint stimulation techniques in all the comparisons examined in this systematic review.
Although outside the scope of this systematic review, the cost effectiveness of PC6 acupoint stimulation has not been examined and would require the collection of direct and indirect healthcare costs related to PC6 acupoint stimulation and antiemetic prophylaxis, and data on the length of stay in hospital, and time to resume normal diet, sleep pattern and normal activities.
Quality of the evidence
The quality of evidence was variable, depending on the PC6 acupoint stimulation intervention and comparison group examined. The degree of risk of biases across trials also varied, with few trials (Gan 2004; Xu 2012) rated at low risk of bias. Selective reporting of outcomes was the most common risk of bias. The need for rescue antiemetic and side effects associated with PC6 acupoint stimulation and antiemetics were outcomes not always collected and reported. Thus, the impact of selective reporting bias on the summary effect estimates is unknown. When there was substantial heterogeneity, the reasons were often unknown. There may be subtle differences between inactive ReliefBand and SeaBands with studs removed, when placed over the PC6 acupoint. Despite possible differences in sham efficacy and intrinsic bias, we analysed these sham treatments as one group. The evidence base is likely to remain low when PC6 acupoint stimulation is compared to sham, since the threshold for a statistically significant treatment effect has been reached. The evidence base for PC6 acupoint stimulation as an alternative to antiemetic is likely to remain moderate, as the threshold for futility has been reached.
Potential biases in the review process
Publication bias may be common for RCTs of Traditional Chinese Medicine (Tang 1999). The contour-enhanced funnel plots for nausea and vomiting showed no evidence of publication bias. The addition of another 19 studies examining PC6 acupoint stimulation for PONV since the previous version of this review (Lee 2009) did not change the relative risk estimates much. Thus, we are confident that publication bias is minimal in this review.
Agreements and disagreements with other studies or reviews
The results of this updated Cochrane review cannot be directly compared with those reported by Cheong 2013, as the methodology was different. For example, there were differences in the selection of controls, inclusion of other acupoints with PC6, timing of PONV and types of PC6 acupoint stimulation technique subgroup analyses chosen. Nevertheless, the results of PC6 electro-acupoint stimulation versus sham for postoperative nausea (RR 0.49, 95% CI 0.38 to 0.63) and postoperative vomiting (RR 0.50, 95% CI 0.36 to 0.70) in the first 24 hours (Cheong 2013) are in agreement with our review.
AUTHORS’ CONCLUSIONS
Implications for practice
Given that adverse effects associated with PC6 acupoint stimulation are minor and transient, the number needed to treat for an additional beneficial (NNTB) outcome (Table 4) suggests that P6 acupoint stimulation is worthwhile when the baseline risk of PONV is high (i.e. above 60% as defined by Gan 2014). For example, the NNTB (95% CI) is 5 (4 to 7) for nausea and 4 (3 to 6) for vomiting at baseline risk of 60%. PC6 acupoint stimulation may be considered as an alternative to antiemetics in people in whom exposure is undesirable, for example, pregnant or breast-feeding women, and those with contraindications to antiemetics (Streitberger 2011). We do not have sufficient evidence to determine the effects of multimodal PC6 acupoint stimulation and antiemetic on the prevention of PONV.
Implications for research
The results of the trial sequential analyses suggest that no further PC6 acupoint stimulation versus sham trials are needed, and that further PC6 acupoint stimulation versus antiemetic trials would be futile in showing a significant difference. There is a need for high-quality trials to examine whether combinations of PC acupoint stimulation and antiemetic interventions (that is, multimodal prophylaxis) works better than each component alone and whether they interact. An ongoing trial (Lv 2013) may provide more insight into the comparative effectiveness of combining ondansetron and acupuncture against ondansetron and transcutaneous electrical nerve stimulation of PC6 acupoint. More importantly, future trials should include more clinically relevant outcomes, such as quality of recovery, to draw meaningful conclusions.
Acknowledgments
We would like to thank Jane Cracknell (Managing Editor), Andy Smith (content editor), Vibeke E Horstmann (statistical editor), Leopold HJ Eberhart, Ashraf S Habib (peer reviewers), and Rosanna Fennessy (consumer referee) for their help and editorial advice during the preparation of this systematic review. Ms Patricia Tong helped with editing the plain language summary.
We would like to acknowledge Mary Done’s contribution to the original published review (Lee 2004). We thank Drs Fang Zhu and Jinglan Mu for their help in translating papers (Zhu 2010; Tang 2013) into English.
SOURCES OF SUPPORT
Internal sources
Department of Anaesthesia and Intensive Care, The Chinese University of Hong Kong, Hong Kong.
External sources
2008 and 2014 Cochrane Complementary Medicine Field Bursary, USA.
This work was partially funded by Grant Number R24 AT001293 from the National Center for Complementary and Alternative Medicine (NCCAM). The contents of this systematic review are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM or the National Institutes of Health.
APPENDICES
Appendix 1. Search strategy for CENTRAL, The Cochrane Library
-
#1
MeSH descriptor postoperative complications explode all trees
-
#2
MeSH descriptor Postoperative Nausea and Vomiting explode all trees
-
#3
MeSH descriptor nausea explode all trees
-
#4
MeSH descriptor vomiting explode all trees
-
#5
(nausea in All Text or vomiting in All Text)
-
#6
(#1 or #2 or #3 or #4 or #5)
-
#7
MeSH descriptor acupuncture explode all trees
-
#8
MeSH descriptor acupuncture therapy explode all trees
-
#9
MeSH descriptor acupuncture points explode all trees
-
#10
MeSH descriptor acupressure explode all trees
-
#11
MeSH descriptor Transcutaneous Electric Nerve Stimulation explode all trees
-
#12
MeSH descriptor electroacupuncture explode all trees
-
#13
(electroacupuncture in All Text or electro-acupuncture in All Text)
-
#14
acupressure in All Text
-
#15
acupunct* in All Text
-
#16
(nerve in All Text near/6 stimulat* in All Text)
-
#17
(#7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16)
-
#18
(#6 and #17)
Appendix 2. Search strategy for SilverPlatter MEDLINE (Ovid SP)
exp Postoperative Complications/
exp Postoperative Nausea/
exp nausea/
exp vomiting/
(nausea or vomiting or emesis).mp.
or/1–5
exp acupuncture/
exp acupuncture therapy/
exp acupuncture points/
exp acupressure/
exp Transcutaneous Electric Nerve Stimulation/
exp electroacupuncture/
electro?acupunct*.mp.
acupressure.mp.
acupunct*.mp.
(electro* adj6 (nerv* and stimulat*)).mp.
or/7–16
6 and 17
(CLINICAL-TRIAL.pt. or randomized.ab. or placebo.ab. or clinical trials.sh. or randomly.ab. or trial.ti.) and humans.sh.
18 and 19
limit 20 to yr=“2008 -Current”
Appendix 3. Search strategy for SilvePlatter EMBASE (Ovid SP)
exp postoperative complication/
exp postoperative nausea/
exp postoperative vomiting/
exp nausea/
exp vomiting/
(nausea or vomiting or emesis).mp.
or/1–6
exp acupuncture/
exp acupuncture analgesia/
exp electroacupuncture/
exp acupressure/
exp transcutaneous nerve stimulation/
(acupressure or acupunct* or electro?acupunct*).mp.
(electro* adj6 (nerv* and stimulat*)).mp.
or/8–14
7 and 15
RANDOMIZATION/
RANDOMIZED-CONTROLLED-TRIAL/
CONTROLLED-STUDY/
MULTICENTER-STUDY/
(RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER*).ti,ab.
((SINGL* or DOUBL* or TREBL* or TRIPL*) adj6 (BLIND* or MASK*)).ti,ab.
or/17–22
23 and 16
limit 24 to yr=“2008 -Current”
Appendix 4. Search strategy for ISI Web of Science
-
#1
TS=pos$toperative complication*
-
#2
TS=nausea OR TS=vomiting OR TS=emesis
-
#3
#2 OR #1
-
#4
TS=acupunct* OR TS=electro$acupunct* or TS=acupressure
-
#5
TS=(electro* OR transcutaneous) AND TS=(nerv* AND stimulat*)
-
#6
#5 OR #4
-
#7
TS=(random* or clinical or control* or multi$cent* SAME trial* or stud*)
-
#8
TS=(singl* or doubl* or trebl* or tripl* SAME blind* or mask* or method*)
-
#9
TS=(random* or allocat* or compar* or factorial* or follow$up or placebo* or prospective)
-
#10
#9 OR #8 OR #7
-
#11
#10 AND #6 AND #3
DATA AND ANALYSES
Comparison 1. Acupoint PC6 stimulation versus sham
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea | 40 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All trials | 40 | 4742 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.60, 0.77] |
| 1.2 Children | 2 | 258 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.51, 0.80] |
| 1.3 Adults | 36 | 4344 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.61, 0.79] |
| 1.4 Invasive PC6 stimulation | 7 | 896 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.39, 0.80] |
| 1.5 Noninvasive PC6 stimulation | 33 | 3846 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.62, 0.81] |
| 1.6 Low risk of bias trials | 2 | 169 | Risk Ratio (IV, Random, 95% CI) | 0.40 [0.17, 0.93] |
| 1.7 Unclear risk of bias trials | 20 | 2496 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.56, 0.82] |
| 1.8 High risk of bias trials | 11 | 1090 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.55, 0.85] |
| 1.9 Trials with control event rate less than or equal to 20% | 4 | 454 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.49, 1.33] |
| 1.10 Trials with control event rate more than 20% | 36 | 4288 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.59, 0.77] |
| 2 Vomiting | 45 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 All trials | 45 | 5147 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.51, 0.71] |
| 2.2 Children | 6 | 542 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.46, 0.97] |
| 2.3 Adults | 37 | 4465 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.51, 0.72] |
| 2.4 Invasive PC6 stimulation | 7 | 896 | Risk Ratio (IV, Random, 95% CI) | 0.51 [0.34, 0.76] |
| 2.5 Noninvasive PC6 stimulation | 37 | 4151 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.50, 0.73] |
| 2.6 Low risk of bias trials | 2 | 169 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.31, 0.88] |
| 2.7 Unclear risk of bias trials | 26 | 3583 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.48, 0.72] |
| 2.8 High risk of bias trials | 14 | 1373 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.45, 0.83] |
| 2.9 Trials with control event rate less than or equal to 20% | 12 | 1556 | Risk Ratio (IV, Random, 95% CI) | 0.75 [0.51, 1.11] |
| 2.10 Trials with control event rate more than 20% | 33 | 3591 | Risk Ratio (IV, Random, 95% CI) | 0.58 [0.48, 0.69] |
| 3 Rescue antiemetics | 39 | 4622 | Risk Ratio (IV, Random, 95% CI) | 0.64 [0.55, 0.73] |
Comparison 2. Acupoint PC6 stimulation versus antiemetic drug
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea | 14 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Ondansetron | 9 | 843 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.65, 1.13] |
| 1.2 Metoclopramide | 4 | 334 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.38, 1.36] |
| 1.3 Cyclizine | 1 | 62 | Risk Ratio (IV, Random, 95% CI) | 0.5 [0.14, 1.82] |
| 1.4 Droperidol | 2 | 143 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.47, 2.19] |
| 1.5 All antiemetics combined | 14 | 1332 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.75, 1.10] |
| 1.6 Children | 1 | 99 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.41, 1.13] |
| 1.7 Adult | 11 | 1033 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.78, 1.17] |
| 1.8 Invasive PC6 stimulation | 5 | 559 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.41, 1.14] |
| 1.9 Noninvasive PC6 stimulation | 9 | 773 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.78, 1.16] |
| 1.10 Low risk of bias trials | 1 | 51 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.19, 1.21] |
| 1.11 Unclear risk of bias trials | 8 | 975 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.63, 1.04] |
| 1.12 High risk of bias trials | 5 | 306 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.89, 1.29] |
| 1.13 Trials with control event rate less than or equal to 20% | 6 | 685 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.52, 1.52] |
| 1.14 Trials with control event rate more than 20% | 8 | 647 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.69, 1.13] |
| 2 Vomiting | 19 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Ondansetron | 9 | 843 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.68, 1.54] |
| 2.2 Metoclopramide | 5 | 414 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.43, 1.74] |
| 2.3 Prochlorperazine | 2 | 173 | Risk Ratio (IV, Random, 95% CI) | 1.15 [0.48, 2.77] |
| 2.4 Cyclizine | 1 | 62 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.21, 2.13] |
| 2.5 Droperidol | 4 | 266 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.64, 1.43] |
| 2.6 All antiemetics combined | 19 | 1708 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.74, 1.17] |
| 2.7 Children | 3 | 237 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.61, 1.56] |
| 2.8 Adult | 14 | 1271 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 2.9 Invasive PC6 stimulation | 8 | 734 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.70, 1.41] |
| 2.10 Noninvasive PC6 stimulation | 12 | 974 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.67, 1.21] |
| 2.11 Low risk of bias trials | 1 | 51 | Risk Ratio (IV, Random, 95% CI) | 1.44 [0.26, 7.92] |
| 2.12 Unclear risk of bias trials | 8 | 975 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.66, 1.40] |
| 2.13 High risk of bias trials | 10 | 682 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.68, 1.21] |
| 2.14 Trials with control event rate less than or equal to 20% | 15 | 1440 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.70, 1.29] |
| 2.15 Trials with control event rate more than 20% | 4 | 268 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.60, 1.43] |
| 3 Rescue antiemetic | 9 | 895 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.65, 1.16] |
Comparison 3. Acupoint PC6 stimulation and antiemetic combination vs antiemetic
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea | 8 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 PC6 acupoint stimulation and droperidol | 2 | 128 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.15, 4.10] |
| 1.2 PC6 acupoint stimulation and ondansetron | 4 | 295 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.47, 1.33] |
| 1.3 PC6 acupoint stimulation, dexamethasone and ondansetron | 2 | 219 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.50, 0.93] |
| 1.4 All PC6 acupoint stimulation and antiemetic combinations | 8 | 642 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.55, 1.13] |
| 1.5 Invasive PC6 stimulation | 2 | 120 | Risk Ratio (IV, Random, 95% CI) | 0.28 [0.11, 0.75] |
| 1.6 Noninvasive PC6 stimulation | 6 | 522 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.62, 1.25] |
| 1.7 Low risk of bias trials | 1 | 119 | Risk Ratio (IV, Random, 95% CI) | 0.58 [0.38, 0.88] |
| 1.8 Unclear risk of bias trials | 4 | 355 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.61, 1.20] |
| 1.9 High risk of bias trials | 3 | 168 | Risk Ratio (IV, Random, 95% CI) | 0.58 [0.12, 2.66] |
| 1.10 Trials with control event rate less than or equal to 20% | 1 | 40 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.01, 2.60] |
| 1.11 Trials with control event rate more than 20% | 7 | 602 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.56, 1.16] |
| 2 Vomiting | 9 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 PC6 acupoint stimulation and droperidol | 3 | 173 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.19, 1.99] |
| 2.2 PC6 acupoint stimulation and ondansetron | 4 | 295 | Risk Ratio (IV, Random, 95% CI) | 0.51 [0.20, 1.30] |
| 2.3 PC6 acupoint stimulation and dexamethasone and ondansetron | 2 | 219 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.30, 0.79] |
| 2.4 All PC6 acupoint stimulation and antiemetic combinations | 9 | 687 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.35, 0.91] |
| 2.5 Children | 1 | 60 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.43, 1.63] |
| 2.6 Adult | 8 | 627 | Risk Ratio (IV, Random, 95% CI) | 0.51 [0.29, 0.91] |
| 2.7 Invasive PC6 stimulation | 3 | 180 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.08, 1.40] |
| 2.8 Noninvasive PC6 stimulation | 6 | 507 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.38, 1.11] |
| 2.9 Low risk of bias trials | 1 | 119 | Risk Ratio (IV, Random, 95% CI) | 0.53 [0.30, 0.94] |
| 2.10 Unclear risk of bias trials | 4 | 355 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.25, 1.09] |
| 2.11 High risk of bias trials | 4 | 213 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.19, 1.64] |
| 2.12 Trials with control event rate less than or equal to 20% | 2 | 120 | Risk Ratio (IV, Random, 95% CI) | 0.13 [0.02, 1.04] |
| 2.13 Trials with control event rate more than 20% | 7 | 567 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.37, 1.00] |
| 3 Rescue antiemetic | 5 | 419 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.44, 0.86] |
Analysis 1.1.
Comparison 1 Acupoint PC6 stimulation versus sham, Outcome 1 Nausea.
Analysis 1.2.
Comparison 1 Acupoint PC6 stimulation versus sham, Outcome 2 Vomiting.
Analysis 1.3.
Comparison 1 Acupoint PC6 stimulation versus sham, Outcome 3 Rescue antiemetics.
Analysis 2.1.
Comparison 2 Acupoint PC6 stimulation versus antiemetic drug, Outcome 1 Nausea.
Analysis 2.2.
Comparison 2 Acupoint PC6 stimulation versus antiemetic drug, Outcome 2 Vomiting.
Analysis 2.3.
Comparison 2 Acupoint PC6 stimulation versus antiemetic drug, Outcome 3 Rescue antiemetic.
Analysis 3.1.
Comparison 3 Acupoint PC6 stimulation and antiemetic combination vs antiemetic, Outcome 1 Nausea.
Analysis 3.2.
Comparison 3 Acupoint PC6 stimulation and antiemetic combination vs antiemetic, Outcome 2 Vomiting.
Analysis 3.3.
Comparison 3 Acupoint PC6 stimulation and antiemetic combination vs antiemetic, Outcome 3 Rescue antiemetic.
Footnotes
CONTRIBUTIONS OF AUTHORS
Anna Lee (AL) initiated and designed the review, extracted the data, conducted statistical analyses, wrote the first draft of this updated review, and incorporated comments from Cochrane peer reviewers into the final version. Simon Chan and Lawrence Fan provided comments on data extraction forms, extracted the data, and commented on all drafts of this updated review.
DECLARATIONS OF INTEREST
Anna Lee has no conflicts relating to this review.
Simon KC Chan has no conflicts relating to this review.
Lawrence TY Fan has no conflicts relating to this review.
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
This updated review includes a comparison between a multimodal PC6 acupoint stimulation and an antiemetic drug prophylactic therapy versus antiemetic drug, and trial sequential analyses for PONV.
References
* Indicates the major publication for the study
References to studies included in this review
- Adib-Hajbaghery 2013 {published data only}.Adib-Hajbaghery M, Etri M, Hosseainian M, Mousavi MS. Pressure to the p6 acupoint and post-appendectomy pain, nausea, and vomiting: a randomized clinical trial. Journal of Caring Science. 2013;2(2):115–22. doi: 10.5681/jcs.2013.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal 2000 {published data only}.Agarwal A, Pathak A, Gaur A. Acupressure wristbands do not prevent postoperative nausea and vomiting after urological endoscopic surgery. Canadian Journal of Anaesthesia. 2000;47(4):319–24. doi: 10.1007/BF03020945. [DOI] [PubMed] [Google Scholar]
- Agarwal 2002 {published and unpublished data}.Agarwal A, Bose N, Gaur A, Singh U, Gupta MK, Singh D. Acupressure and ondansetron for postoperative nausea and vomiting after laparoscopic cholecystectomy. Canadian Journal of Anesthesia. 2002;49(6):554–60. doi: 10.1007/BF03017380. [DOI] [PubMed] [Google Scholar]
- Alkaissi 1999 {published data only}.Alkaissi A, Stålnert M, Kalman S. Effect and placebo effect of acupressure (P6) on nausea and vomiting after outpatient gynaecological surgery. Acta Anaesthesiologica Scandinavica. 1999;43(3):270–4. doi: 10.1034/j.1399-6576.1999.430306.x. [DOI] [PubMed] [Google Scholar]
- Alkaissi 2002 {published data only}.Alkaissi A, Evertsson K, Johnsson V, Ofenbartl L, Kalman S. P6 acupressure may relieve nausea and vomiting after gynecological surgery: an effectiveness study in 410 women. Canadian Journal of Anesthesia. 2002;49:1034–9. doi: 10.1007/BF03017897. [DOI] [PubMed] [Google Scholar]
- Allen 1994 {published data only}.Allen DL, Kitching AJ, Nagle C. P6 acupressure and nausea and vomiting after gynaecological surgery. Anaesthesia and Intensive Care. 1994;22(6):691–3. doi: 10.1177/0310057X9402200608. [DOI] [PubMed] [Google Scholar]
- Amir 2007 {published data only}.Amir SH, Bano S, Khan RM, Ahmed M, Zia F, Nasreen F. Electro-stimulation at P6 for prevention of PONV. Journal of Anaesthesiology Clinical Pharmacology. 2007;23:383–86. [Google Scholar]
- Andrzejowski 1996 {published data only}.Andrzejowski J, Woodward D. Semi-permanent acupuncture needles in the prevention of postoperative nausea and vomiting. Acupuncture in Medicine. 1996;14:68–70. [Google Scholar]
- Arnberger 2007 {published data only}.Arnberger M, Stadelmann K, Alischer P, Ponert R, Melber A, Greif R, et al. Monitoring of neuromuscular blockade at the P6 acupuncture point reduces the incidence of postoperative nausea and vomiting. Anesthesiology. 2007;107(6):903–8. doi: 10.1097/01.anes.0000290617.98058.d9. [DOI] [PubMed] [Google Scholar]
- Barsoum 1990 {published data only}.Barsoum G, Perry EP, Fraser IA. Postoperative nausea is relieved by acupressure. Journal of the Royal Society of Medicine. 1990;83(2):86–9. doi: 10.1177/014107689008300209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butkovic 2005 {published data only}.Butkovic D, Toljan S, Matolic M, Kralik S, Radesić L. Comparison of laser acupuncture and metoclopramide in PONV prevention in children. Pediatric Anesthesia. 2005;15(1):37–40. doi: 10.1111/j.1460-9592.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Direkvand-Moghadam 2013 {published data only}.Direkvand-Moghadam A, Khosravi A. Effect of acupressure on post-operative nausea and vomiting in cesarean section: a randomised controlled trial. Journal of Clinical and Diagnostic Research. 2013;7(10):2247–9. doi: 10.7860/JCDR/2013/5702.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal 1998 {published data only}.Duggal KN, Douglas MJ, Peter EA, Merrick PM. Acupressure for intrathecal narcotic-induced nausea and vomiting after caesarean section. International Journal of Obstetric Anesthesia. 1998;7:231–6. doi: 10.1016/s0959-289x(98)80044-7. [DOI] [PubMed] [Google Scholar]
- Dundee 1986 {published data only}.Dundee JW, Chestnutt WN, Ghaly RG, Lynas AG. A traditional Chinese acupuncture: a potentially useful antiemetic? British Medical Journal (Clinincal Research edition) 1986;293(6547):583–4. doi: 10.1136/bmj.293.6547.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundee 1989 {published data only}.Dundee JW, Fitzpatrick KTJ, Ghaly RG. Is there a role for acupuncture in the treatment of postoperative nausea and vomiting? Anesthesiology. 1987;67(3A):A165. [Google Scholar]; * Dundee JW, Ghaly RG, Bill KM, Chestnutt WN, Fitzpatrick KTJ, Lynas AGA. Effect of stimulation of the P6 antiemetic point on postoperative nausea and vomiting. British Journal of Anaesthesia. 1989;63(5):612–8. doi: 10.1093/bja/63.5.612. [DOI] [PubMed] [Google Scholar]; Ghaly RG, Fitzpatrick KT, Dundee JW. Antiemetic studies with traditional Chinese acupuncture. A comparison of manual needling with electrical stimulation and commonly used antiemetics. Anaesthesia. 1987;42(10):1108–10. doi: 10.1111/j.1365-2044.1987.tb05180.x. [DOI] [PubMed] [Google Scholar]
- Ebrahim Soltani 2010 {published data only}.Ebrahim Soltani A, Mohammadinasab H, Goudarzi M, Arbabi S, Mohtaram R, Afkham K, et al. Acupressure using ondansetron versus metoclopramide on reduction of postoperative nausea and vomiting after strabismus surgery. Archives of Iranian Medicine. 2010;13(4):288–93. [PubMed] [Google Scholar]; * Ebrahim Soltani AR, Mohammadinasab H, Goudarzi M, Arbabi S, Mohammadinasab A, Mohammadinasab F, et al. Comparing the efficacy of prophylactic p6 acupressure, ondansetron, metoclopramide and placebo in the prevention of vomiting and nausea after strabismus surgery. Acta Medica Iranica. 2011;49(4):208–12. [PubMed] [Google Scholar]
- El-Deeb 2011 {published data only}.El-Deeb AM, Ahmady MS. Effect of acupuncture on nausea and/or vomiting during and after cesarean section in comparison with ondansetron. Journal of Anesthesia. 2011;25(5):698–703. doi: 10.1007/s00540-011-1198-0. [DOI] [PubMed] [Google Scholar]
- Ertas 2015 {published data only}.Ertas G, Bengi Sener E, Kaya C, Ozkan F, Ustun YB, Koksal E. Effects of P6 acustimulation with the ReliefBand on postoperative nausea and vomiting in patients undergoing gynecological laparoscopy. Holistic Nursing Practice. 2015;29(1):6–12. doi: 10.1097/HNP.0000000000000061. [DOI] [PubMed] [Google Scholar]
- Fassoulaki 1993 {published data only}.Fassoulaki A, Papilas K, Sarantopoulos C, Zotou M. Transcutaneous electrical nerve stimulation reduces the incidence of vomiting after hysterectomy. Anesthesia and Analgesia. 1993;76(5):1012–4. doi: 10.1213/00000539-199305000-00017. [DOI] [PubMed] [Google Scholar]
- Ferrara-Love 1996 {published data only}.Ferrara-Love R, Sekeres L, Bircher NG. Nonpharmacological treatment of postoperative nausea. Journal of Perianesthesia Nursing. 1996;11(6):378–83. doi: 10.1016/s1089-9472(96)90048-9. [DOI] [PubMed] [Google Scholar]
- Frey 2009a {published data only}.Frey UH, Scharmann P, Löhlein C, Peters J. P6 acustimulation effectively decreases postoperative nausea and vomiting in high-risk patients. British Journal of Anaesthesia. 2009;102(5):620–5. doi: 10.1093/bja/aep014. [DOI] [PubMed] [Google Scholar]
- Frey 2009b {published data only}.Frey UH, Funk M, Löhlein C, Peters J. Effect of P6 acustimulation on postoperative nausea and vomiting in patients undergoing a laparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica. 2009;53(10):1341–7. doi: 10.1111/j.1399-6576.2009.02081.x. [DOI] [PubMed] [Google Scholar]
- Gan 2004 {published data only}.Gan TJ, Jiao KR, Zenn M, Georgiade G. A randomized controlled comparison of electro-acupoint stimulation or ondansetron versus placebo for the prevention of postoperative nausea and vomiting. Anesthesia and Analgesia. 2004;99(4):1070–5. doi: 10.1213/01.ANE.0000130355.91214.9E. [DOI] [PubMed] [Google Scholar]
- Gieron 1993 {published data only}.Gieron C, Wieland B, Von der Laage D, Tolksdorf W. Acupressure in the prophylaxis of postoperative nausea and vomiting. Der Anaesthesist. 1993;42(4):221–6. [PubMed] [Google Scholar]
- Habib 2006 {published data only}.Habib AS, Itchon-Ramos N, Phillips-Bute BG, Gan TJ Duke Women’s Anesthesia (DWA) Research Group. Transcutaneous acupoint electrical stimulation with the ReliefBand for the prevention of nausea and vomiting during and after Cesarean delivery under spinal anesthesia. Anesthesia and Analgesia. 2006;102(2):581–4. doi: 10.1213/01.ane.0000189217.19600.5c. [DOI] [PubMed] [Google Scholar]
- Harmon 1999 {published data only}.Harmon D, Gardiner J, Harrison R, Kelly A. Acupressure and the prevention of nausea and vomiting after laparoscopy. British Journal of Anaesthesia. 1999;82(3):387–90. doi: 10.1093/bja/82.3.387. [DOI] [PubMed] [Google Scholar]
- Harmon 2000 {published data only}.Harmon D, Ryan M, Kelly A, Bowen M. Acupressure and prevention of nausea and vomiting during and after spinal anaesthesia for Caesarean section. British Journal of Anaesthesia. 2000;84(4):463–7. doi: 10.1093/oxfordjournals.bja.a013471. [DOI] [PubMed] [Google Scholar]
- Ho 1990 {published data only}.Ho RT, Jawan B, Fung ST, Cheung HK, Lee JH. Electro-acupuncture and postoperative emesis. Anaesthesia. 1990;45(4):327–9. doi: 10.1111/j.1365-2044.1990.tb14744.x. [DOI] [PubMed] [Google Scholar]
- Ho CM, Hseu SS, Tsai SK, Lee TY. Effect of P6 acupressure on prevention of nausea and vomiting after epidural morphine for post-Cesarean section pain relief. Acta Anaesthesiologica Scandinavica. 1996;40(3):372–5. doi: 10.1111/j.1399-6576.1996.tb04448.x. [DOI] [PubMed] [Google Scholar]
- Iqbal 2012 {published data only}.Iqbal U, Khan A, Sheikh F. Whether does acupressure (P6) prevent nausea and vomiting in patients undergoing laparoscopic surgery. Pakistan Journal of Medical and Health Sciences. 2012;6(4):973–5. [Google Scholar]
- Kim 2004 {published data only}.Kim SI, Yoo IS, Park HN, Ok SY, Kim SC. Transcutaneous electrical stimulation of the P6 acupoint reduces postoperative nausea after minor breast surgery. Korean Journal of Anaesthesiology. 2004;47:834–9. Identified from Kim KH et al. PLoS ONE 2012 review article. [Google Scholar]
- Kim 2011 {published data only}.Kim YH, Kim KS, Lee HJ, Shim JC, Yoon SW. The efficacy of several neuromuscular monitoring modes at the P6 acupuncture point in preventing postoperative nausea and vomiting. Anesthesia and Analgesia. 2011;112(4):819–23. doi: 10.1213/ANE.0b013e31820f819e. [DOI] [PubMed] [Google Scholar]
- Klein 2004 {published data only}.Klein AA, Djaiani G, Karski J, Carroll J, Karkouti K, McCluskey S, et al. Acupressure wristbands for the prevention of postoperative nausea and vomiting in adults undergoing cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia. 2004;18(1):68–71. doi: 10.1053/j.jvca.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Koo 2013 {published data only}.Koo MS, Kim KS, Lee HJ, Jeong JS, Lee JW. Antiemetic efficacy of capsicum plaster on acupuncture points in patients undergoing thyroid operation. Korean J Anesthesiol. 2013;65(6):539–43. doi: 10.4097/kjae.2013.65.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis 1991 {published data only}.Lewis IH, Pryn SJ, Reynolds PI, Pandit UA, Wilton NCT. Effect of P6 acupressure on postoperative vomiting in children undergoing outpatient strabismus correction. British Journal of Anaesthesia. 1991;67(1):73–8. doi: 10.1093/bja/67.1.73. [DOI] [PubMed] [Google Scholar]
- Liu 2008 {published data only}.Liu YY, Duan SE, Cai MX, Zou P, LY, Li YL. Evaluation of transcutaneous electroacupoint stimulation with the train-of-four mode for preventing nausea and vomiting after laparoscopic cholecystectomy. Chinese Journal of Integrative Medicine. 2008;14(2):94–7. doi: 10.1007/s11655-008-0094-4. [DOI] [PubMed] [Google Scholar]
- Majholm 2011 {published data only}.Majholm B, Møller AM. Acupressure at acupoint P6 for prevention of postoperative nausea and vomiting: a randomised clinical trial. European Journal of Anaesthesiology. 2011;28(6):412–9. doi: 10.1097/EJA.0b013e32833f6f42. [DOI] [PubMed] [Google Scholar]
- Misra 2005 {published data only}.Misra MN, Pullani AJ, Mohamed ZU. Prevention of PONV by acustimulation with capsicum plaster is comparable to ondansetron after middle ear surgery. Canadian Journal of Anesthesia. 2005;52(5):485–9. doi: 10.1007/BF03016527. [DOI] [PubMed] [Google Scholar]
- Nilsson 2015 {published data only}.Nilsson I, Karlsson A, Lindgren L, Bergenheim T, Koskinen LO, Nilsson U. The efficacy of P6 acupressure with Sea-Band in reducing postoperative nausea and vomiting in patients undergoing craniotomy: a randomized, double-blinded, placebo-controlled study. Journal of Neurosurgical Anesthesiology. 2015;27(1):42–50. doi: 10.1097/ANA.0000000000000089. [DOI] [PubMed] [Google Scholar]
- Ravi 2010 {published data only}.Ravi M, Babu G, Somasekharam N, Dinesh M, Asha N, Hamsa J. Comparative efficacy of acupuncture at p6 point with 0.2ml 50% dextrose and inj ondansetron 50ug kg-1 iv for preventing postoperative nausea and vomiting. Journal of Anaesthesiology Clinical Pharmacology. 2010;26:237–9. [Google Scholar]
- Rusy 2002 {published data only}.Rusy LM, Hoffman GM, Weisman SJ. Electroacupuncture prophylaxis of postoperative nausea and vomiting following pediatric tonsillectomy with or without adenoidectomy. Anesthesiology. 2002;96(2):300–5. doi: 10.1097/00000542-200202000-00013. [DOI] [PubMed] [Google Scholar]
- Sadighha 2008 {published data only}.Sadighha A, Nural N. Acupressure wristbands versus metoclopramide for the prevention of postoperative nausea and vomiting. Annals of Saudi Medicine. 2008;28(4):287–91. doi: 10.5144/0256-4947.2008.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad 2003 {published data only}.Samad K, Afshan G, Kamal R. Effect of acupressure on postoperative nausea and vomiting in laparoscopic cholecystectomy. Journal of the Pakistan Medical Association. 2003;53(2):68–72. [PubMed] [Google Scholar]
- Schlager 1998 {published data only}.Schlager A, Offer T, Baldissera I. Laser stimulation of acupuncture point P6 reduces postoperative vomiting in children undergoing strabismus surgery. British Journal of Anaesthesia. 1998;81(4):529–32. doi: 10.1093/bja/81.4.529. [DOI] [PubMed] [Google Scholar]
- Schultz 2003 {published data only}.Schultz AA, Andrews AL, Goran SF, Mathew T, Sturdevant N. Comparison of acupressure bands and droperidol for reducing post-operative nausea and vomiting in gynecologic surgery patients. Applied Nursing Research. 2003;16(4):256–65. doi: 10.1016/s0897-1897(03)00057-0. [DOI] [PubMed] [Google Scholar]
- Sharma 2007 {published data only}.Sharma S, Goswami U. Evaluation of acupuncture for antiemetic prophylaxis. Journal of Anaesthesiology Clinical Pharmacology. 2007;23:401–4. [Google Scholar]
- Shenkman 1999 {published data only}.Shenkman Z, Holzman RS, Kim C, Ferrari LR, DiCanzio J, Highfield ES, et al. Acupressure-acupuncture antiemetic prophylaxis in children undergoing tonsillectomy. Anesthesiology. 1999;90(5):1311–6. doi: 10.1097/00000542-199905000-00015. [DOI] [PubMed] [Google Scholar]
- Streitberger 2004 {published data only}.Schneider A, Löwe B, Streitberger K. Perception of bodily sensation as a predictor of treatment response to acupuncture for postoperative nausea and vomiting prophylaxis. Journal of Alternative and Complementary Medicine. 2005;11(1):119–125. doi: 10.1089/acm.2005.11.119. [DOI] [PubMed] [Google Scholar]; * Streitberger K, Diefenbacher M, Bauer A, Conradi R, Bardenheuer H, Martin E, et al. Acupuncture compared to placebo-acupuncture for postoperative nausea and vomiting prophylaxis: a randomised placebo-controlled patient and observer blind trial. Anaesthesia. 2004;59(2):142–9. doi: 10.1111/j.1365-2044.2004.03577.x. [DOI] [PubMed] [Google Scholar]
- Tavlan 1996 {published data only}.Tavlan A, Baltaci B, Alptekin A, Ceyhan A, Unal N. A comparison of the effect of single dose ondansetron and P6 (neiguan) acupuncture point on postoperative nausea and vomiting for gynaecological laparoscopy. European Journal of Anaesthesiology. 1996;13:164. [Google Scholar]
- Turgut 2007 {published data only}.Turgut S, Ozalp G, Dikmen S, Savil S, Tuncel G, Kadiogullari N. Acupressure for postoperative nausea and vomiting in gynaecological patients receiving patient-controlled analgesia. European Journal of Anaesthesiology. 2007;24(1):87–91. doi: 10.1017/S0265021506001190. [DOI] [PubMed] [Google Scholar]
- Wang 2002 {published data only}.Wang SM, Kain ZN. P6 acupoint injections are as effective as droperidol in controlling early postoperative nausea and vomiting in children. Anesthesiology. 2002;97(2):359–66. doi: 10.1097/00000542-200208000-00012. [DOI] [PubMed] [Google Scholar]
- Wang 2010 {published data only}.Wang XQ, Yu JL, Du ZY, Xu R, Jiang CC, Gao X. Electroacupoint stimulation for postoperative nausea and vomiting in patients undergoing supratentorial craniotomy. Journal of Neurosurgical Anesthesiology. 2010;22(2):128–31. doi: 10.1097/ANA.0b013e3181c9fbde. [DOI] [PubMed] [Google Scholar]
- White 2002 {published data only}.White PF, Issioui T, Hu J, Jones SB, Coleman JE, Waddle JP, et al. Comparative efficacy of acustimulation (ReliefBand) versus ondansetron (Zofran) in combination with droperidol for preventing nausea and vomiting. Anesthesiology. 2002;97(5):1075–81. doi: 10.1097/00000542-200211000-00008. [DOI] [PubMed] [Google Scholar]
- White 2012 {published data only}.White PF, Zhao M, Tang J, Wender RH, Yumul R, Sloninsky AV, et al. Use of a disposable acupressure device as part of a multimodal antiemetic strategy for reducing postoperative nausea and vomiting. Anesthesia and Analgesia. 2012;115(1):31–7. doi: 10.1213/ANE.0b013e3182536f27. [DOI] [PubMed] [Google Scholar]
- Xu 2012 {published data only}.Xu M, Zhou SJ, Jiang CC, Wu Y, Shi WL, Gu HH, et al. The effects of P6 electrical acustimulation on postoperative nausea and vomiting in patients after Infratentorial craniotomy. Journal of Neurosurgical Anesthesiology. 2012;24(4):312–6. doi: 10.1097/ANA.0b013e31825eb5ef. [DOI] [PubMed] [Google Scholar]
- Yang 1993 {published data only}.Yang LC, Jawan B, Chen CN, Ho RT, Chang KA, Lee JH. Comparison of P6 acupoint injection with 50% glucose in water and intravenous droperidol for prevention of vomiting after gynecological laparoscopy. Acta Anaesthesiologica Scandinavica. 1993;37(2):192–4. doi: 10.1111/j.1399-6576.1993.tb03699.x. [DOI] [PubMed] [Google Scholar]
- Yentis 1992 {published data only}.Yentis SM, Bissonnette B. Ineffectiveness of acupuncture and droperidol in preventing vomiting following strabismus repair in children. Canadian Journal of Anaesthesia. 1992;39(2):151–4. doi: 10.1007/BF03008646. [DOI] [PubMed] [Google Scholar]
- Zárate 2001 {published data only}.Zárate E, Mingus M, White PF, Chiu JW, Scuderi P, Loskota W, et al. The use of transcutaneous acupoint electrical stimulation for preventing nausea and vomiting after laparoscopic surgery. Anesthesia and Analgesia. 2001;92(3):629–35. doi: 10.1097/00000539-200103000-00014. [DOI] [PubMed] [Google Scholar]
- Zhu 2010 {published data only}.Zhu HX, Xu YJ, Meng SF, Feng H, Liu Y, Su XJ. Preventive effect of acupoint injection at neiguan (PC 6) on postoperative nausea and vomiting after laparoscopic gynecologic surgery. Zhongguo zhen Jiu: Chinese Acupuncture and Moxibustion. 2010;30(1):72–4. [PubMed] [Google Scholar]
References to studies excluded from this review
- Agarwal 2005 {published data only}.Agarwal A, Dhiraaj S, Tandon M, Singh PK, Singh U, Pawar S. Evaluation of capsaicin ointment at the Korean hand acupressure point K-D2 for prevention of postoperative nausea and vomiting. Anaesthesia. 2005;60(12):1185–8. doi: 10.1111/j.1365-2044.2005.04402.x. [DOI] [PubMed] [Google Scholar]
- Alkaissi 2005 {published data only}.Alkaissi A, Ledin T, Odkvist LM, Kalman S. P6 acupressure increases tolerance to nauseogenic motion stimulation in women at high risk for PONV. Canadian Journal of Anesthesia. 2005;52(7):703–9. doi: 10.1007/BF03016557. [DOI] [PubMed] [Google Scholar]
- Al-Sadi 1997 {published data only}.Al-Sadi M, Newman B, Julious SA. Acupuncture in the prevention of postoperative nausea and vomiting. Anaesthesia. 1997;52(7):658–61. doi: 10.1111/j.1365-2044.1997.143-az0147.x. [DOI] [PubMed] [Google Scholar]
- Cekmen 2007 {published data only}.Cekmen N, Salman B, Keles Z, Aslan M, Akcabay M. Transcutaneous electrical nerve stimulation in the prevention of postoperative nausea and vomiting after elective laparoscopic cholecystectomy. Journal of Clinical Anesthesia. 2007;19(1):49–52. doi: 10.1016/j.jclinane.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Chen 2005 {published data only}.Chen HM, Chang FY, Hsu CT. Effect of acupressure on nausea, vomiting, anxiety and pain among post-cesarean section women in Taiwan. Kaohsiung Journal of Medical Sciences. 2005;21(8):341–50. doi: 10.1016/S1607-551X(09)70132-9. [DOI] [PubMed] [Google Scholar]
- Coloma 2002 {published data only}.Coloma M, White PF, Ogunnaike BO, Markowitz SD, Brown PM, Lee AQ, et al. Comparison of acustimulation and ondansetron for the treatment of established postoperative nausea and vomiting. Anesthesiology. 2002;97(6):1387–92. doi: 10.1097/00000542-200212000-00009. [DOI] [PubMed] [Google Scholar]
- Dundee 1988 {published data only}.Dundee JW, Milligan KR, McKay AC. Influence of intraoperative acupuncture and droperidol on postoperative emesis. British Journal of Anaesthesia. 1988;61:116P–7. [Google Scholar]
- Dundee 1991 {published data only}.Dundee JW, Ghaly G. Local anesthesia blocks the antiemetic action of P6 acupuncture. Clinical Pharmacology and Therapeutics. 1991;50(1):78–80. doi: 10.1038/clpt.1991.106. [DOI] [PubMed] [Google Scholar]
- El-Bandrawy 2013 {published data only}.El-Bandrawy AM, Emara HM, Ghareeb HO. Transcutaneous electrical acupoint stimulation versus acupressure on postoperative nausea and vomiting after abdominal hysterectomy. British Journal of Medical Research. 2013;3(4):2247–55. doi: 10.9734/BJMMR/2013/4005. [DOI] [Google Scholar]
- El-Rakshy 2009 {published data only}.El-Rakshy M, Clark SC, Thompson J, Thant M. Effect of intraoperative electroacupuncture on postoperative pain, analgesic requirements, nausea and sedation: a randomised controlled trial. Acupuncture in Medicine. 2009;27(1):9–12. doi: 10.1136/aim.2008.000075. [DOI] [PubMed] [Google Scholar]
- Fan 1997 {published data only}.Fan CF, Tanhui E, Joshi S, Trivedi S, Hong Y, Shevde K. Acupressure treatment for prevention of postoperative nausea and vomiting. Anesthesia and Analgesia. 1997;84(4):821–5. doi: 10.1097/00000539-199704000-00023. [DOI] [PubMed] [Google Scholar]
- Fry 1986 {published data only}.Fry ENS. Acupressure and postoperative vomiting. Anaesthesia. 1986;41(6):661–2. doi: 10.1111/j.1365-2044.1986.tb13083.x. [DOI] [PubMed] [Google Scholar]
- Grube 2009 {published data only}.Grube T, Uhlemann C, Weiss T, Meissner W. Influence of acupuncture on postoperative pain, nausea and vomiting after visceral surgery: a prospective, randomized comparative study of metamizole and standard treatment. Schmerz. 2009;23(4):370–6. doi: 10.1007/s00482-009-0798-1. [DOI] [PubMed] [Google Scholar]
- Hirs 2013 {published data only}.Hirs I, Lukic A, Fumic NN, Kekic M, Kotaran J. Acupressure and metoclopramide comparison in postoperative nausea and vomiting prevention on laparotomy patients. Acupuncture and Related Therapies. 2013;1(4):42–5. [Google Scholar]
- Ho 2006 {published data only}.Ho CM, Tsai HJ, Chan KH, Tsai SK. P6 acupressure does not prevent emesis during spinal anesthesia for Cesarean delivery. Anesthesia and Analgesia. 2006;102(3):900–3. doi: 10.1213/01.ane.0000195553.82409.00. [DOI] [PubMed] [Google Scholar]
- Jin 2013 {published data only}.Jin W, Lu Y, Chen SD, Qin JL, Fang JQ, Wang JL. Efficacy of preventing postoperative nausea and vomiting after thyroid tumor surgery by TAES at neiguan (P1): a clinical observation. Chinese Journal of Integrated Traditional and Western Medicine. 2013;33(9):1199–202. [PubMed] [Google Scholar]
- Kabalak 2005 {published data only}.Kabalak AA, Akcay M, Akcay F, Gogus N. Transcutaneous electrical acupoint stimulation versus ondansetron in the prevention of postoperative vomiting following pediatric tonsillectomy. Journal of Alternative and Complementary Medicine. 2005;11(3):407–13. doi: 10.1089/acm.2005.11.407. [DOI] [PubMed] [Google Scholar]
- Khan 2004 {published data only}.Khan RM, Maroof M, Hakim S, Jain D, Ashraf M. Intraoperative stimulation of the P6 point controls postoperative nausea and vomiting following laparoscopic surgery. Canadian Journal of Anesthesia. 2004;51(7):740–1. doi: 10.1007/BF03018437. [DOI] [PubMed] [Google Scholar]
- Kim 2002 {published data only}.Kim KS, Koo MS, Jeon JW, Park HS, Seung IS. Capsicum plaster at the Korean hand acupuncture point reduces postoperative nausea and vomiting after abdominal hysterectomy. Anesthesia and Analgesia. 2002;95(4):1103–7. doi: 10.1097/00000539-200210000-00059. [DOI] [PubMed] [Google Scholar]
- Kim 2010 {published data only}.Kim NC, Yoo JB, Cho MS, Shin EJ, Hahm TS. Effects of Nei-Guan acupressure on nausea, vomiting and level of satisfaction for gynecological surgery patients who are using a patient-controlled analgesia. Journal of Korean Academy of Nursing. 2010;40:423–32. doi: 10.4040/jkan.2010.40.3.423. [DOI] [PubMed] [Google Scholar]
- Klaiman 2008 {published data only}.Klaiman P, Sternfeld M, Deeb Z, Roth Y, Golan A, Ezri T, et al. Magnestic acupressure for management of postoperative nausea and vomiting: a preliminary study. Minerva Anestesiologica. 2008;74(11):635–42. [PubMed] [Google Scholar]
- Korinenko 2009 {published data only}.Korinenko Y, Vincent A, Cutshall SM, Li Z, Sundt TM., 3rd Efficacy of acupuncture in prevention of postoperative nausea in cardiac surgery patients. Annals of Thoracic Surgery. 2009;88(2):537–42. doi: 10.1016/j.athoracsur.2009.04.106. [DOI] [PubMed] [Google Scholar]
- Larson 2010 {published and unpublished data}.Larson JD, Gutowski KA, Marcus BC, Rao VK, Avery PG, Stacey DH, et al. The effect of electroacustimulation on postoperative nausea, vomiting and pain in outpatient plastic surgery patients: a prospective randomized, blinded, clinical trial. Plastic and Reconstructive Surgery. 2010;125(3):989–94. doi: 10.1097/PRS.0b013e3181ccdc23. [DOI] [PubMed] [Google Scholar]
- Lee 2008 {published data only}.Lee MY, Min HS. Effects of the Nei-Guan acupressure by wrist band on postoperative nausea and vomiting after middle ear surgery. Taehan Kanho Hakhoe Chi. 2008;38(4):503–12. doi: 10.4040/jkan.2008.38.4.503. [DOI] [PubMed] [Google Scholar]
- Lee 2013 {published data only}.Lee S, Lee MS, Choi DH, Lee SK. Electroacupuncture on P6 prevents opioid-induced nausea and vomiting after laparoscopic surgery. Chinese Journal of Integrated Medicine. 2013;19(4):277–81. doi: 10.1007/s11655-013-1425-7. [DOI] [PubMed] [Google Scholar]
- Liodden 2011 {published data only}.Liodden I, Howley M, Grimsgaard AS, Fønnebø VM, Borud EK, Alraek T, et al. Perioperative acupuncture and postoperative acupressure can prevent postoperative vomiting following paediatric tonsillectomy or adenoidectomy: a pragmatic randomised controlled trial. Acupuncture in Medicine. 2011;29(1):9–15. doi: 10.1136/aim.2010.002915. [DOI] [PubMed] [Google Scholar]
- Lu 2013 {published data only}.Lü JQ, Feng RZ, Pan H, Li N. A randomized controlled clinical trial for acupuncture stimulation of Neiguan (PC 6) to prevent postoperative nausea and vomiting. Acupuncture Research. 2013;38(3):245–8. [PubMed] [Google Scholar]
- McConaghy 1996 {published data only}.McConaghy P, Bland D, Swales H. Acupuncture in the management of postoperative nausea and vomiting in patients receiving morphine via a patient-controlled analgesia system. Acupuncture in Medicine. 1996;14(1):2–5. [Google Scholar]
- McMillan 1994 {published data only}.McMillan CM. Transcutaneous electrical stimulation of Neiguan antiemetic acupuncture point in controlling sickness following opioid analgesia in major orthopaedic surgery. Physiotherapy. 1994;80:5–9. [Google Scholar]
- Ming 2002 {published data only}.Ming JL, Kuo BI, Lin JG, Lin LC. The efficacy of acupressure to prevent nausea and vomiting in postoperative patients. Journal of Advanced Nursing. 2002;39(4):343–51. doi: 10.1046/j.1365-2648.2002.02295.x. [DOI] [PubMed] [Google Scholar]
- Ng 2011 {published data only}.Ng MC, Jones AY, Cheng LC. The role of acu-TENS in hemodynamic recovery after open-heart surgery. Evidence-Based Complementary and Alternaternative Medicine. 2011;2011:301974. doi: 10.1093/ecam/neq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norheim 2010 {published data only}.Norheim AJ, Liodden I, Howley M. Implementation of acupuncture and acupressure under surgical procedures in children: a pilot study. Acupuncture in Medicine. 2010;28(2):71–3. doi: 10.1136/aim.2009.002220. [DOI] [PubMed] [Google Scholar]
- Noroozinia 2013 {published data only}.Noroozinia H, Mahoori A, Hasani E, Gerami-Fahim M, Sepehrvand N. The effect of acupressure on nausea and vomiting after cesarean section under spinal anesthesia. Acta Medica Iranica. 2013;51(3):163–7. [PubMed] [Google Scholar]
- Ouyang 2009 {published data only}.Ouyang MW, Qin ZS, Lin CS, Gu MN. Prophylactic effect of acupuncture on nausea and vomiting after laparoscopic operation. Zhongguo zhen Jiu: Chinese Acupuncture and Moxibustion. 2009;29(11):915–8. [PubMed] [Google Scholar]
- Phillips 1994 {published data only}.Phillips K, Gill L. The use of simple acupressure bands reduces post-operative nausea. Complementary Therapies in Medicine. 1994;2:158–60. [Google Scholar]
- Schwager 1996 {published data only}.Schwager KL, Baines DB, Meyer RJ. Acupuncture and postoperative vomiting in day-stay paediatric patients. Anaesthesia and Intensive Care. 1996;24(6):674–7. doi: 10.1177/0310057X9602400607. [DOI] [PubMed] [Google Scholar]
- Shyr 1990 {published data only}.Shyr MH, Hsu JC, Wu YW, Hui YL, Tan PPC. P6 acupoint injection reduced postoperative nausea and vomiting. Ma Tsui Hsueh Tsa Chi Anaesthesiologica Sinica. 1990;28(3):357–60. [PubMed] [Google Scholar]
- Sinha 2011 {published data only}.Sinha A, Paech MJ, Thew ME, Rhodes M, Luscombe K, Nathan E. A randomised, double-blinded, placebo-controlled study of acupressure wristbands for the prevention of nausea and vomiting during labour and delivery. International Journal of Obstetric Anesthesia. 2011;20(2):110–7. doi: 10.1016/j.ijoa.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Somri 2001 {published data only}.Somri M, Vaida SJ, Sabo E, Yassain G, Gankin I, Gaitini LA. Acupuncture versus ondansetron in the prevention of postoperative vomiting. Anaesthesia. 2001;56(10):927–32. doi: 10.1046/j.1365-2044.2001.02209.x. [DOI] [PubMed] [Google Scholar]
- Stein 1997 {published data only}.Stein DJ, Birnbach DJ, Danzer BI, Kuroda MM, Grunebaum A, Thys DM. Acupressure versus intravenous metoclopramide to prevent nausea and vomiting during spinal anesthesia for cesarean section. Anesthesia and Analgesia. 1997;84(2):342–5. doi: 10.1097/00000539-199702000-00018. [DOI] [PubMed] [Google Scholar]
- Tang 2013 {published data only}.Tang W, Ma W, Fu GQ, Yuan L, Shen WD. Impacts of electroacupuncture at different frequencies on the postoperative nausea and vomiting of patients with laparoscopic surgery. Zhonggui zhen Jiu: Chinese Acupuncture and Moxibustion. 2013;33(2):159–62. [PubMed] [Google Scholar]
- Wang 2014 {published data only}.Wang H, Xie Y, Zhang Q, Xu N, Zhong H, Dong H, et al. Transcutaneous electric acupoint stimulation reduces intra-operative remifentanil consumption and alleviates postoperative side-effects in patients undergoing sinusotomy: a prospective, randomized, placebo-controlled trial. British Journal of Anaesthesia. 2014;112(6):1075–82. doi: 10.1093/bja/aeu001. [DOI] [PubMed] [Google Scholar]
- Weightman 1987 {published data only}.Weightman WM, Zacharias M, Herbison P. Traditional Chinese acupuncture as an antiemetic. British Medical Journal (Clinical Research edition) 1987;295(6610):1379–80. doi: 10.1136/bmj.295.6610.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White 2005 {published data only}.White PF, Hamza MA, Recart A, Coleman JE, Macaluso AR, Cox L, Jaffer O, Song D, Rohrich R. Optimal timing of acustimulation for antiemetic prophylaxis as an adjunct to ondansetron in patients undergoing plastic surgery. Anesthesia and Analgesia. 2005;100(2):367–72. doi: 10.1213/01.ANE.0000144425.16116.0A. [DOI] [PubMed] [Google Scholar]
- Windle 2001 {published data only}.Windle PE, Borromeo A, Robles H, Ilacio-Uy V. The effects of acupressure on the incidence of postoperative nausea and vomiting in postsurgical patients. Journal of Perianesthesia Nursing. 2001;16(3):158–62. doi: 10.1053/jpan.2001.24040. [DOI] [PubMed] [Google Scholar]
- Yeh 2010 {published data only}.Yeh ML, Chung YC, Chen KM, Tsou MY, Chen HH. Acupoint electrical stimulation reduces acute postoperative pain in surgical patients with patient-controlled analgesia: a randomized controlled study. Alternative Therapies in Health and Medicine. 2010;16:10–18. [PubMed] [Google Scholar]
- Yentis 1991 {published data only}.Yentis SM, Bissonnette B. P6 acupuncture and postoperative vomiting after tonsillectomy in children. British Journal of Anaesthesia. 1991;67(6):779–80. doi: 10.1093/bja/67.6.779. [DOI] [PubMed] [Google Scholar]
- Yentis 1998 {published data only}.Yentis SM, Vashisht S. The effect of timing of P6 acupuncture on post-operative vomiting following major gynaecological surgery. Acupuncture in Medicine. 1998;16:10–13. [Google Scholar]
- Zheng 2008 {published data only}.Zheng LH, Sun H, Wang GN, Liang J, Wu HX. Effect of transcutaneous electrical acupoint stimulation on nausea and vomiting induced by patient controlled intravenous analgesia with tramadol. Chinese Journal of Integrative Medicine. 2008;14(1):61–4. doi: 10.1007/s11655-007-9006-2. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Cooke 2014 {published data only}.Cooke M, Rickard C, Rapchuk I, Shekar K, Marshall AP, Comans T, et al. P6 acupoint stimulation for the prevention of postcardiac surgery nausea and vomiting: a protocol for a two-group, parallel, superiority randomised clinical trial. BMJ Open. 2014;4(11):e006179. doi: 10.1136/bmjopen-2014-006179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv 2013 {published data only}.Lv JQ, Feng RZ, Li N. P6 acupoint stimulation for prevention of postoperative nausea and vomiting in patients undergoing craniotomy: study protocol for a randomized controlled trial. Trials. 2013;14:153. doi: 10.1186/1745-6215-14-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
- Afshari 2015.Afshari A, Wetterslev J. When may systematic reviews and meta-analyses be considered reliable? European Journal of Anaesthesiology. 2015;32(2):85–7. doi: 10.1097/EJA.0000000000000186. [DOI] [PubMed] [Google Scholar]
- Altman 1998.Altman DG. Confidence intervals for the number needed to treat. British Medical Journal. 1998;317(7168):1309–12. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman 2003.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfel 1999.Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91(3):693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- Apfel 2007.Apfel CC, Kranke P, Piper S, Rüsch D, Kerger H, Steinfath M, et al. Nausea and vomiting in the postoperative phase. Expert- and evidence-based recommendations for prophylaxis and therapy [Übelkeit und erbrechen in der postoperativen phase. Der Anaesthesist. 2007;56(11):1170–80. doi: 10.1007/s00101-007-1210-0. [DOI] [PubMed] [Google Scholar]
- Carlisle 2006.Carlisle JB, Stevenson CA. Drugs for preventing postoperative nausea and vomiting. Cochrane Database of Systematic Reviews. 2006;(3):CD004125. doi: 10.1002/14651858.CD004125.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong 2013.Cheong KB, Zhang JP, Huang Y, Zhang ZJ. The effectiveness of acupuncture in prevention and treatment of postoperative nausea and vomiting--a systematic review and meta-analysis. PLoS One. 2013;8(12):e82474. doi: 10.1371/journal.pone.0082474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval 2000.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Gan 2014.Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesthesia and Analgesia. 2014;118(1):85–113. doi: 10.1213/ANE.0000000000000002. [DOI] [PubMed] [Google Scholar]
- Gin 1994.Gin T. Recent advances in the understanding and management of postoperative nausea and vomiting. Annals of Academic Medicine of Singapore. 1994;23(6 Suppl):114–9. [PubMed] [Google Scholar]
- Guyatt 2011.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology. 2011;64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Higgins 2011.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. updated March 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- Kim 2012.Kim KH, Kong JC, Choi JY, Choi TY, Shin BC, McDonald S, et al. Impact of including Korean randomized controlled trials in Cochrane reviews of acupuncture. PLoS One. 2012;7(10):e47619. doi: 10.1371/journal.pone.0047619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee 2006.Lee A, Copas JB, Henmi M, Gin T, Chung RCK. Publication bias affected the estimate of postoperative nausea in an acupoint stimulation systematic review. Journal of Clinical Epidemiology. 2006;59(9):980–3. doi: 10.1016/j.jclinepi.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Mann 1987.Mann F. Textbook of Acupuncture. London: William Heinemann Medical Books; 1987. [Google Scholar]
- McAuley 2000.McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet. 2000;356(9237):1228–31. doi: 10.1016/S0140-6736(00)02786-0. [DOI] [PubMed] [Google Scholar]
- Mosteller 1996.Mosteller F, Colditz GA. Understanding research synthesis (meta-analysis) Annual Review of Public Health. 1996;17:1–23. doi: 10.1146/annurev.pu.17.050196.000245. [DOI] [PubMed] [Google Scholar]
- Peters 2008.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology. 2008;61(10):991–6. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Petitti 1994.Petitti DB. Meta-analysis, Decision Analysis and Cost-Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine. New York: Oxford University Press; 1994. [Google Scholar]
- Sadhasivam 1999.Sadhasivam S, Saxena A, Kathirvel S, Kannan TR, Trikha A, Mohan V. The safety and efficacy of prophylactic ondansetron in patients undergoing modified radical mastectomy. Anesthesia and Analgesia. 1999;89(6):1340–5. doi: 10.1097/00000539-199912000-00002. [DOI] [PubMed] [Google Scholar]
- Smeeth 1999.Smeeth L, Haines A, Ebrahim S. Numbers needed to treat derived from meta-analyses - sometimes informative, usually misleading. BMJ. 1999;318(7197):1548–51. doi: 10.1136/bmj.318.7197.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streitberger 2011.Streitberger K, Kranke P. Evidence for the efficacy of acupressure for preventing post-operative nausea and vomiting: an ongoing debate. European Journal of Anaesthesiology. 2011;28(6):396–8. doi: 10.1097/EJA.0b013e3283412529. [DOI] [PubMed] [Google Scholar]
- Tang 1999.Tang JL, Zhan SY, Ernst E. Review of randomised controlled trials of traditional Chinese medicine. BMJ. 1999;319(7203):160–2. doi: 10.1136/bmj.319.7203.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorlund 2011.Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis (TSA) Copenhagen, Denmark: Copenhagen Trial Unit, Centre for Clinical Intervention Research; 2011. www.ctu.dk/tsa. [Google Scholar]
- Van Driel 2009.Van Driel ML, De Sutter A, De Maeseneer J, Christiaens T. Searching for unpublished trials in Cochrane reviews may not be worth the effort. Journal of Clinical Epidemiology. 2009;62(8):838–44. doi: 10.1016/j.jclinepi.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Vickers 1996.Vickers AJ. Can acupuncture have specific effects on health? A systematic review of acupuncture antiemesis trials. Journal of the Royal Society of Medicine. 1996;89(6):303–11. doi: 10.1177/014107689608900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watcha 1992.Watcha MF, White PF. Postoperative nausea and vomiting: its etiology, treatment and prevention. Anesthesiology. 1992;77(1):162–84. doi: 10.1097/00000542-199207000-00023. [DOI] [PubMed] [Google Scholar]
- White 1999.White PF, Watcha MF. Has the use of meta-analysis enhanced our understanding of therapies for postoperative nausea and vomiting? Anesthesia and Analgesia. 1999;88(6):1200–2. doi: 10.1097/00000539-199906000-00002. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Lee 1999.Lee A, Done ML. The use of nonpharmacologic techniques to prevent postoperative nausea and vomiting: a meta-analysis. Anesthesia and Analgesia. 1999;88(6):1362–9. doi: 10.1097/00000539-199906000-00031. [DOI] [PubMed] [Google Scholar]
- Lee 2001.Lee A, Done ML. Acupoint P6 stimulaton for preventing postoperative nausea and vomiting. Cochrane Database of Systematic Reviews. 2001;(4) doi: 10.1002/14651858.CD003281. [DOI] [Google Scholar]
- Lee 2004.Lee A, Done ML. Stimulation of the wrist acupuncture point P6 for preventing postoperative nausea and vomiting. Cochrane Database of Systematic Reviews. 2004;(3):CD 003281. doi: 10.1002/14651858.CD003281.pub2. [DOI] [PubMed] [Google Scholar]
- Lee 2009.Lee A, Fan LT. Stimulation of the wrist acupuncture point P6 for preventing postoperative nausea and vomiting. Cochrane Database of Systematic Reviews. 2009;(2) doi: 10.1002/14651858.CD003281.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]