Abstract
Factor VIII (FVIII) is an important cofactor in the blood coagulation cascade. A deficiency or dysfunction of FVIII causes hemophilia A, a life-threatening bleeding disorder. FVIII circulates in plasma as a heterodimer comprising 6 domains (heavy chain, A1-A2-B and light chain, A3-C1-C2). Replacement therapy using FVIII is the leading therapy in the management of hemophilia A. However, ∼15% to 30% of patients develop inhibitory antibodies that neutralize the activity of the protein. Neutralizing antibodies to epitopes in the lipid binding region of FVIII are commonly identified in patients’ plasma. In this report, we investigated the effect of O-phospho-L-serine (OPLS), which binds to the lipid binding region, on the immunogenicity of B domain deleted recombinant factor VIII (BDDrFVIII). Sandwich enzyme-linked immunosorbent assay (ELISA) studies showed that OPLS specifically bind to the lipid binding region, localized in the C2 domain of the coagulation factor. Size exclusion chromatography and fluorescence anisotropy studies showed that OPLS interfered with the aggregation of BDDrFVIII. Immunogenicity of free-vs BDDrFVIII-OPLS complex was evaluated in a murine model of hemophilia A. Animals administered subcutaneous (sc) injections of BDDrFVIII-OPLS had lower neutralizing titers compared with animals treated with BDDrFVIII alone. Based on these studies, we hypothesize that specific molecular interactions between OPLS and BDDrFVIII may improve the stability and reduce the immunogenicity of BDDrFVIII formulations.
Keywords: B domain deleted recombinant factor VIII, O-phospho-L-serine, protein formulation, excipient, physical stability, immunogenicity, inhibitor development
Introduction
Factor VIII (FVIII) is a multi-domain heterodimeric glycoprotein composed of a heavy chain (domains A1, A2, and B) and a light chain (domains A3, C1, and C2).1 FVIII is subjected to posttranslational modifications within the endoplasmic reticulum and the Golgi apparatus. Heavy chain polypeptides with molecular weights spanning between 90 and 210 kd are generated because of the limited processing of several proteolytic sites within the B domain.1,2 The light chain of FVIII is homogeneous with a molecular weight of ∼80 kd as this chain is not processed.1,2 Besides the limited proteolysis, rFVIII is subjected to glycosylation, mainly at the level of the B domain.1 Thus, the FVIII heterodimer is a highly heterogeneous glycoprotein with molecular weight in the range of 170 to 290 kd.
FVIII is an important blood coagulation factor that participates in the formation and the proper anchoring of the factor IXa:factor X complex on the surface of the platelet membranes.3 Dysfunction or deficiency of FVIII results in a bleeding disorder known as hemophilia A.4 Administration of recombinant human FVIII (rFVIII) is a therapy employed to control bleeding episodes.5
Treatment with rFVIII is hampered by inhibitor development by induction of neutralizing antibodies in ∼15% to 30% of the patients.5-7 The immune response to therapeutic proteins has major consequences on the pharmacology and efficacy of protein drugs. Antibodies developed against the C2 domain of FVIII8 have been hypothesized to interfere with the protein binding to phosphatidylserine (PS) head group and to perturb the proper assembly of the tenase complex.9,10
The interaction of the PS head group, O-phospho-L-serine (OPLS), with the C2 domain of rFVIII has been shown to reduce the immunogenicity of full-length FVIII.11 Here, we extended the studies to investigate the effect of OPLS, on a truncated form of FVIII, B domain deleted factor VIII (BDDrFVIII).
It has been shown that the B domain does not interact with either factor IXa, factor X, or with the platelet membranes.12 Thus, the deletion of the B domain does not alter the biological activity of the protein.12 Furthermore, the deletion of the B domain decreased considerably the molecular weight of the functional protein, the degree of heterogeneity, and the degree of glycosylation of the heterodimer (Figure 1).13 In addition, BDDrFVIII maintains the same universal dominant epitopes such as C2 domain as the full-length protein, and thus the specific interactions between C2 domain and OPLS may lead to the reduction in the immunogenicity.
Figure 1.

Deletion of B domain decreases the heterogeneity of the heavy chain of FVIII. Sodium dodecyl sulfate - polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions for BDDrFVIII and rFVIII. From left to right: B domain deleted recombinant factor VIII (BDDrFVIII), molecular weight marker (MWM), and recombinant human factor VIII (rFVIII). The position of heavy chain (HC), light chain (LC), and nascent single chain (SC) precursors are indicated.
The data in this study indicated that binding of OPLS to the truncated form of the FVIII improved the stability of the recombinant protein and modulated its immunological properties.
Materials and Methods
Materials
BDDrFVIII (Refacto, Wyeth) was obtained as a gift from Zale P. Bernstein, Dr (Hemophilia Center of Western New York, Buffalo, NY). Normal human plasma and FVIII-deficient human plasma were purchased from Trinity Biotech (Co Wicklow, Ireland). Brain phosphatidylserine (BPS) and dimyristoylphosphatidylcholine (DMPC) were obtained from Avanti Polar Lipids (Alabaster, AL). pyrogen-free water was purchased from Henry Schein Inc (Melville, NY). The monoclonal ESH4 antibody and BDDrFVIII was purchased from American Diagnostica Inc (Greenwich, CT), goat anti-mouse IgG alkaline phosphatase conjugate was purchased from Southern Biotechnology Associates Inc (Birmingham, AL). OPLS and IgG-free bovine serum albumin (BSA) were purchased from Sigma (Rockford, IL). Tris-HCl (4%-15%) precast acrylamide gels were obtained from BioRad (Hercules, CA). All other buffer salts used in the study were purchased from Fisher Scientific (Fairlawn, NJ).
Sodium Dodecyl Sulfate - Polyacrylamide Gel Electrophoresis Under Reducing Conditions
Five micrograms of either BDDrFVIII or control rFVIII were mixed with an equal volume of Laemmli buffer (Biorad, Hercules, CA) and heated for 5 minutes at 90°C. Samples loaded onto the gels were subjected to electrophoresis under a constant voltage of 120 V for 65 minutes. The gel was stained with coomassie blue for visualization of the light and heavy chains.
Preparation of the BDDrFVIII - Phospholipids Head Group Complexes
The lyophilized BDDrFVIII was reconstituted, using the buffer supplied by the manufacturer, to a concentration of 1 μg/μL. The protein-phospholipid head group complexes were generated by diluting the 1 μg/μL stock solution of BDDrFVIII with Tris buffer (25 mM Tris, 5 mM CaCl2, 300 mM NaCl, pH 7.0) containing 5, 10, or 20 mM phospholipid head group. The reaction mixtures were incubated at 25°C for 30 minutes. Prior to addition to the protein stock, the buffer was sterilized by filtration through a 0.22-μm Millex-GP filter unit (Millipore Corp, Bedford, MA).
Preparation of the BDDrFVIII-PS Containing Liposomes
Multi-lamellar vesicles were prepared by rehydrating a thin lipid film of DMPC and BPS at 37°C with Tris buffer (25 mM Tris, 5 mM CaCl2, 300 mM NaCl, pH 7.0), as described before.14 The liposomes were extruded 8 times through double-stacked 80-nm polycarbonate membranes using a high-pressure extruder (Lipex Biomembranes Inc, Vancouver, BC, Canada) at ∼200 psi. The size distribution of the particles was monitored with a Nicomp model CW380 size analyzer (Particle Sizing Systems, Santa Barbara, CA). The liposomal-FVIII complex was generated by incubating BDDrFVIII in the presence of small unilamellar vesicles (SUV)s with different lipid concentrations at 37°C for 30 minutes.
Sandwich Enzyme-Linked Immunosorbent Assay
Sandwich enzyme-linked immunosorbent assay (ELISA) studies were performed in Nunc-Maxisorp 96-well plates, coated with ESH4 capture antibody (directed against epitope 2303-2332). Coating was achieved by incubating the plates with 50 μL/well of a 2.5 μg/mL ESH4 antibody solution in carbonate buffer (Na2CO3 200 mM, pH 9.4) for 16 hours at 4°C. The plates were then washed 6 times with 100 μL of phosphate buffer saline (PBS: 10 mM Na2HPO4, 1.8 mM KH2PO4, 14 mM NaCl, 2.7 mM KCl, pH 7.40) containing 0.05% Tween 20 (PBT). The plates were incubated with 200 μL/well of 1% BSA in PBS for 2 hours at room temperature to eliminate nonspecific binding. A washing step using PBT was performed before the incubation with 50 μL of BDDrFVIII-lipidic complexes (150 ng/mL BDDrFVIII) in 1% BSA in PBS for 1 hour at 37°C. After washing with PBT, the plates were incubated in the presence of 100 μL/well of a mixture containing rat anti-human rFVIII polyclonal antibody (1:500 dilution) and goat anti-rat IgG alkaline phosphatase conjugated antibody (1:1000 dilution) for 1 hour at room temperature. The washing step was performed as described above, and the plates were incubated at room temperature with 200 μL of 1mg/mL p-nitrophenyl phosphate solution in diethanolamine buffer (1 M diethanolamine, 0.5 mM CaCl2). Thirty minutes later, the reaction was quenched using 100 μL of 3 N NaOH, and the optical density of the alkaline phosphatase reaction product was determined at 405 nm with a SpectraMax plate reader (Molecular Device Corp, Sunnyvale, CA).
Spectroscopy Studies
Tryptophan intrinsic fluorescence spectra of BDDrFVIII in the presence of OPLS or phosphatidylcholine (PC) head group were acquired on a PTI spectrofluorometer (PTI, Lawrenceville, NJ) equipped with a Xe arc lamp. Stock solutions containing 17 nM BDDrFVIII were prepared in Tris buffer in the absence and presence of 1 mM OPLS. The 2 stock solutions were mixed under different ratios to obtain various protein:phospholipid head group molar ratios. Fluorescence spectra were acquired by exciting the sample at 285 nm and monitoring the emission between 320 and 400 nm. Normalized fluorescence intensity (F330/F0-330, where F330 is the fluorescence intensity at 330 nm at a given phospholipid concentration and F0-330 is the fluorescence intensity at 330 nm of the protein in the absence of the phospholipid) was plotted as a function of the phospholipid head group concentration. The relationship between F330/F0-330, the protein, and the lipid analog concentrations is described by the following set of equations:
| (1) |
| (2) |
| (3) |
where Fmax is the maximal change in F330/F0-330; PL, PT, L, and LT are the protein-OPLS complex, total protein, free OPLS, and total OPLS concentration, respectively; and n is the stoichiometry of the complex. Substituting for L and PL in Equation 1, Equation 4 was derived:
| (4) |
Spectral data were fitted to this equation using nonlinear regression (WinNonLin, Pharsight Corp, Mountain View, CA) to obtain estimates for the phenomenological KD value for the BDDrFVIII-OPLS complex and for Fmax. The fitting was performed for n = 1 (1:1 stoichiometry) and for n > 1. The best fit of the experimental data was observed only for n = 1. Therefore, we assumed a stoichiometry of 1:1 for the OPLS-BDDrFVIII complex. This study assumption is in agreement with a previous study by Purohit et al,11 which employed a 1:1 stoichiometry for the binding of OPLS to recombinant factor VIII.
The total lipid concentration at which the F330/F0-330 approaches Fmax represents the minimal OPLS concentration that provides maximum spectral properties for BDDrFVIII-OPLS complex.
In Vitro Activity of BDDrFVIII and BDDrFVIII-OPLS Complex
The in vitro specific activity of BDDrFVIII in the presence and absence of OPLS was measured by the activated partial thromboplastin time (aPTT) assay.15 In brief, samples containing free BDDrFVIII or BDDrFVIII-OPLS complexes were mixed with FVIII-deficient human plasma. Following addition of platelin L (phospholipid) reagent and CaCl2, the clotting time of each sample was measured with a Coag-A-Mate XM coagulation analyzer (Organon Teknika Corp, Durham, NC). The activity of BDDrFVIII-OPLS complex was extrapolated from a standard curve generated using BDDrFVIII standards. Data were presented as relative specific activity, computed as the specific activity of the BDDrFVIII-OPLS complex normalized against the specific activity of BDDrFVIII standards.
High Performance Size Exclusion Chromatography Studies
Size exclusion chromatography (SEC) experiments were performed using a Biosep-SEC-S4000 4.6 × 300 mm column (Phenomenex, Torrance, CA) maintained at a constant temperature using a Shimadzu CTO-10A column oven (Shimadzu Corp, Kyoto, Japan).16 The chromatography system consisted of a Waters 510 high-performance liquid chromatography (HPLC) pump (Waters Corp, Milford, MA), a Shimadzu SIL-10A auto-injector, equipped with a sample cooler unit, and 2 HPLC detectors connected in series (Shimadzu RF-10A XL fluorescence detector and Shimadzu SPD-10A UV detector). The chromatograms were recorded and data analyzed using a Shimadzu CR-8A integrator. All components except for the HPLC pump were connected to a Shimadzu SCL-10A controller. The experiments were performed under isocratic conditions at 0.4 mL/min using Tris buffer (25 mM Tris, 0.3M NaCl, 5mM CaCl2, pH 7.0). The time delay between the 2 detectors is ∼0.08 minutes.
Free BDDrFVIII and BDDrFVIII-OPLS complexes containing different OPLS concentrations were subjected to thermal stress for 90 minutes at 50°C. Samples generated by this method were stored at 4°C on the sample cooler rack prior to aggregate content analysis. Excitation and emission for the fluorescence detector were set to 285 nm and 330 nm, respectively. The UV detector was set to monitor absorbance at 215 nm.
Steady-state Fluorescence Anisotropy
Temperature-dependent steady-state emission anisotropy (r) studies were performed using a PTI instrument equipped with large-aperture motorized Glan-Thompson polarizing prisms and a Peltier unit, as described earlier.11 In brief, data points were acquired from 20°C to 80°C at a heating rate of 1°C/min. The protein and the phospholipid-head group concentration were maintained constant at 17 nM and 10 mM, respectively. The instrument-specific G-factor (G) was determined at room temperature as the ratio between I∥ and I, whereI∥ and I represent the emission intensity with the excitation and the emission polarizer oriented parallel and at 90°, respectively. G was held fixed over the entire range of study. Excitation and emission were set at 285 and 330 nm, respectively. Emission slit widths were set to 4 nm.
Anisotropy (r) was calculated according to Equation 517:
| (5) |
Data were fitted to a sigmoidal curve using the WinNonLin software package (Pharsight).
In Vivo Studies
Immunogenicity studies for free BDDrFVIII and BDDrFVIII-OPLS complex were performed in hemophilic mice 8 to 12 weeks old, bearing a targeted deletion in exon 16 of the FVIII gene, as described before.18 In brief, the immunization procedure consisted of 4 sc injections of BDDrFVIII or BDDrFVIII-OPLS (containing 1 μg protein in 100 μL buffer and/or 10 mM phospholipid head group) at weekly intervals. Blood samples were obtained at the beginning of the sixth week by cardiac puncture. Samples were added at a 10:1 (vol/vol) ratio to acid citrate dextrose (ACD) (85 mM sodium citrate, 110 mM D-glucose and 71 mM citric acid). Plasma was separated by centrifugation and stored at -80°C until analysis. All studies were performed in accordance with the guidelines of Institutional Animal Care and Use Committee (IACUC) at the University at Buffalo, The State University of New York.
Measurement of Inhibitory Anti-rFVIII Antibodies Titers
Neutralizing anti-rFVIII antibodies were detected using the Nijmegen modification of the Bethesda assay.19 Dilutions of plasma samples (1:8 to 1:16 000) were mixed with normal human plasma. Residual FVIII activity was measured in duplicates, using the 1-stage aPTT assay. By definition, 1 Bethesda Unit (BU) is the neutralizing activity that produces 50% inhibition of the FVIII activity. The point of 50% inhibition was determined by linear regression of those data points falling within the range of approximately 20% to 80% inhibition.
Statistical Analysis
Statistical Analysis was performed by 1-way analysis of variance (ANOVA), followed by Dunnett post hoc analysis, using the Minitab 14 software application (Minitab Inc, State College, PA).
Results and Discussion
Interaction of BDDrFVIII With OPLS
It has been shown that interaction of FVIII with PS-containing membranes is critical for the proper function of the tenase complex.3,20 Gilbert and Drinkwater reported that PS lipid binds with high affinity to rFVIII.21 Based on X-ray diffraction studies, the interaction is mediated via lipid binding region within the C2 domain of the molecule.9 Additional studies have indicated that the lipid binding region, 2303-2332, containing 4 hydrophobic loops interacts with PS-rich membranes.22
To investigate whether phosphatidylserine analogs bind to the lipid binding region of BDDrFVIII, we performed Sandwich ELISA studies. The mouse monoclonal antibody ESH4, which binds to the lipid binding region 2303-2332,23 was used as a capture antibody; whereas a rat polyclonal antibody was used as probe antibody. If the OPLS or other phospholipids analogs could bind to the lipid binding region of the protein, they would compete with ESH4, and a reduction in binding of BDDrFVIII to the capture antibody should be observed.
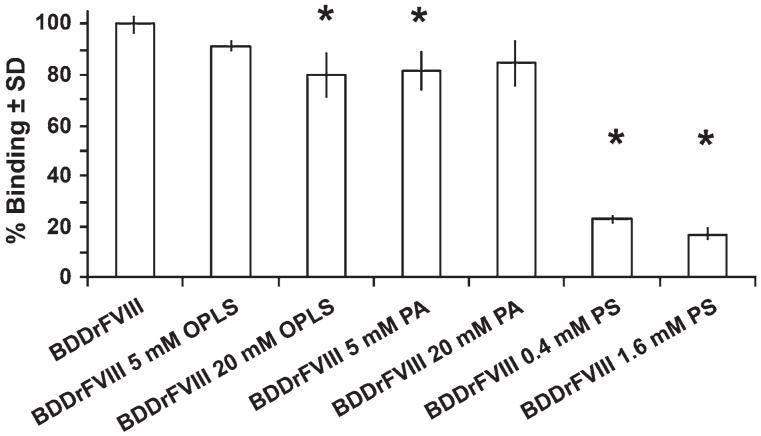
As shown in Figure 2, a concentration-dependent decrease in ESH4 binding was observed for the BDDrFVIII-OPLS complexes, suggesting that OPLS interacts with the lipid binding region of BDDrFVIII (Figure 2). In contrast, the relative binding of ESH4 to BDDrFVIII in the presence of other negatively charged phospholipid head group (phosphatidic acid [PA]) was independent of the concentration of the lipid analog, indicating that the interaction between FVIII with PS is specific. To investigate further the specificity of BDDrFVIII with PS-containing particulates, experiments were performed in the presence of PS-containing liposomes. The binding of the ESH4 antibody to PS liposomes was also dependent on the lipid concentration (Figure 2), in agreement with previously published studies.22 The mechanism of monoclonal antibody binding to FVIII associated with PS-containing liposomes is different from that observed for FVIII in complex with OPLS or PA, head group of respective phospholipids. This observation further substantiated that the affinity and specificity of the protein toward lipids can be increased several fold by both electrostatic (between PS head group and BDDrFVIII) and hydrophobic (between the PS acyl chains and the hydrophobic loops of BDDrFVIII lipid binding region) interactions for the proper anchoring of BDDrFVIII on the surface of lipidic membranes.
Figure 2.
Sandwich enzyme-linked immunosorbent assay (ELISA) for free and phospholipid analog complexes of BDDrFVIII. Binding of monoclonal antibody ESH4 (2302-2332) to BDDrFVIII in the presence of lipid head group or liposomes (DMPC:BPS [70:30]). In all preparations, BDDrFVIII concentration was kept constant (150 ng/mL) and lipid concentrations are indicated. The error bars represent standard deviation (± SD; n = 4). * depicts statistical significance (P < .05, 1-way analysis of variance (ANOVA); Dunnet post hoc analysis). BDDrFVIII indicates B domain deleted recombinant factor VIII; OPLS, O-phospho-L-serine; PA, phosphatidic acid; PS, phosphatidylserine; DMPC, dimyristoylphosphatidylcholine; and BPS, brain phosphatidylserine.
Effect of OPLS on the Conformation of BDDrFVIII and the Affinity Constant of the BDDrFVIII-OPLS Complex
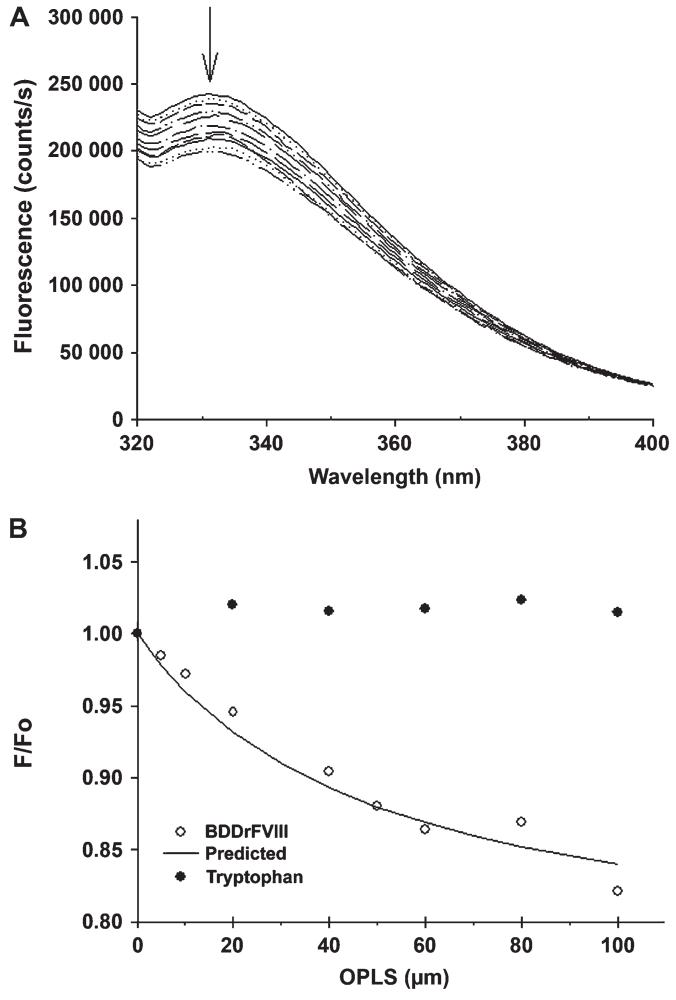
Intrinsic fluorescence spectroscopy is sensitive to changes in the tertiary structure of the proteins. To investigate the effect of OPLS on the tertiary structure of BDDrFVIII, fluorescence spectra were acquired in the presence of various OPLS concentrations. As shown in Figure 3A, the fluorescence intensity of FVIII was altered as the concentration of OPLS was increased. The intensity decreased as the OPLS concentration was increased from 5 to 100 μM. However, no change in emission wavelength maximum was observed. Addition of OPLS to a 400-nM L-tryptophan (Trp) solution did not affect the intensity of the fluorophore(Figure 3B), ruling out any quenching effects of OPLS on the Trp intrinsic fluorescence. In line with previous observations for FVa and FVIII and their interactions with short chain phospholipids and phospholipid analogs,11,24 we interpreted that the spectral differences reflected small conformational changes in the protein structure, as a result of interaction with OPLS. Fitting the data to Equation 4 (see Methods section and Figure 3B) allowed the determinations of an apparent KD value of ∼70.2 μM, suggesting that the affinity of BDDrFVIII toward OPLS was weaker compared with the affinity to PS containing lipid bilayer or platelets membrane (KD ∼5.1 nM25).
Figure 3.
Effect of OPLS on tertiary structure of BDDrFVIII. (A) Intrinsic fluorescence spectra of BDDrFVIII in the presence of various concentrations of OPLS (from top to bottom: 0, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100 μM). The peak maximum is indicated by the arrow. (B) Changes in F/F0 as a function of OPLS concentration. The protein and the L-tryptophan (L-Trp) concentration were 17 nM and 400 nM, respectively. Excitation wavelength was set at 285 nm and emission was monitored at 330 nm for protein-containing samples and at 348 nm for the L-Trp solution. BDDrFVIII indicates B domain deleted recombinant factor VIII and OPLS, O-phospho-L-serine.
Effect of OPLS on the BDDrFVIII Biological Activity
In order to investigate if OPLS could have an effect on the biological activity of BDDrFVIII, the relative specific activity in the presence of different concentrations of OPLS was determined. As can be seen in Table 1, at concentration of up to 20 mM OPLS, no significant changes in specific activity were observed, indicating that OPLS does not interfere with the biological activity of the protein.
Table 1.
Effect of OPLS on the In Vitro Activity of BDDrFVIII*
| OPLS | Relative Specific Activity of BDDrFVIII (% mean ± SD) |
|---|---|
| 0 mM | 99.4 ± 14.1 |
| 5 mM | 101 ± 3.2 |
| 20 mM | 95.4 ± 3.2 |
OPLS indicates O-phospho-L-serine; BDDrFVIII, B domain deleted recombinant factor VIII; and SD, standard deviation.
Effect of OPLS on the Physical Stability of BDDrFVIII
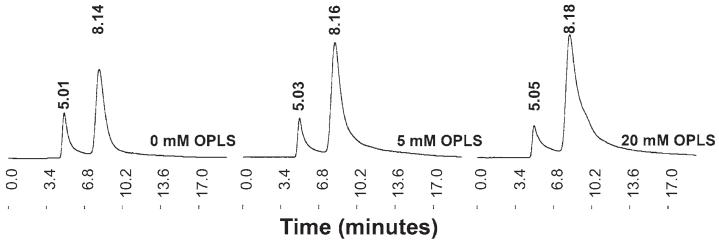
It has been reported that both rFVIII and BDDrFVIII have a tendency to aggregate because of small conformational changes in the lipid binding region.26 In order to test the hypothesis that the interaction of OPLS with the lipid binding region could reduce the propensity of the protein to aggregate, BDDrFVIII was subjected to thermal stress in the presence of different concentrations of OPLS. The aggregate content of the samples was determined using SEC, as described in the Methods section. The native BDDrFVIII was characterized by a single peak that eluted at ∼8.15 minutes (Figure 4). The exposure to elevated temperatures resulted in the formation of aggregated species that eluted in the void volume of the column (∼5.0 minutes). The ratio of the area under the curve of the aggregated peak to the native peak (ie, peak area ratio [PAR]) is a measure of the relative population of the aggregated and native species. In the absence of OPLS, the PAR parameter was estimated to be 0.365. As the concentration of OPLS is increased from 0 to 20 mM, the area of the peak corresponding to native protein is increasing at the expense of the aggregated one, suggesting that OPLS interfered with the aggregation process and exerted a stabilizing effect (Table 2). The decrease in PAR from 0.365 to 0.165 (0 and 20 mM OPLS, respectively) indicated a decrease in the aggregated species from ∼27% to 14% in the presence of OPLS.
Figure 4.
Effect of O-phospho-L-serine (OPLS) on the aggregation behavior of B domain deleted recombinant factor VIII (BDDrFVIII). Size exclusion chromatography profiles of BDDrFVIII subjected to thermal stress in the presence of different concentrations of OPLS. Samples were incubated at 50°C for 90 minutes and cooled to room temperature before analysis. Elution time was shown in minutes. The peak at ∼8.1 minutes corresponded to the native protein and the peak at ∼5.0 minutes represented the aggregated species. The Y-axis is shown in arbitrary units.
Table 2.
Size Exclusion Chromatography—Peak Area Ratio and Relative Total Peak Area of the Aggregated Peak to the Native Peak as a Function of OPLS Concentration*
| OPLS | Peak Area Ratio | Relative Total Peak Area |
|---|---|---|
| 0 mM | 0.365 | 100% |
| 5 mM | 0.218 | 171% |
| 20 mM | 0.165 | 210% |
OPLS indicates O-phospho-L-serine.
Furthermore, the total peak area is a measure of the total concentration of soluble species in a sample that can be resolved by gel filtration. As can be seen from the data in Table 2, the total peak area increased with the concentration of OPLS, suggesting that, in the presence of the lipid analogs, less insoluble species were formed under thermal stress. This observation further substantiated that OPLS interfered with the aggregation process of BDDrFVIII.
Effect of OPLS in the Thermal Unfolding of BDDrVIII
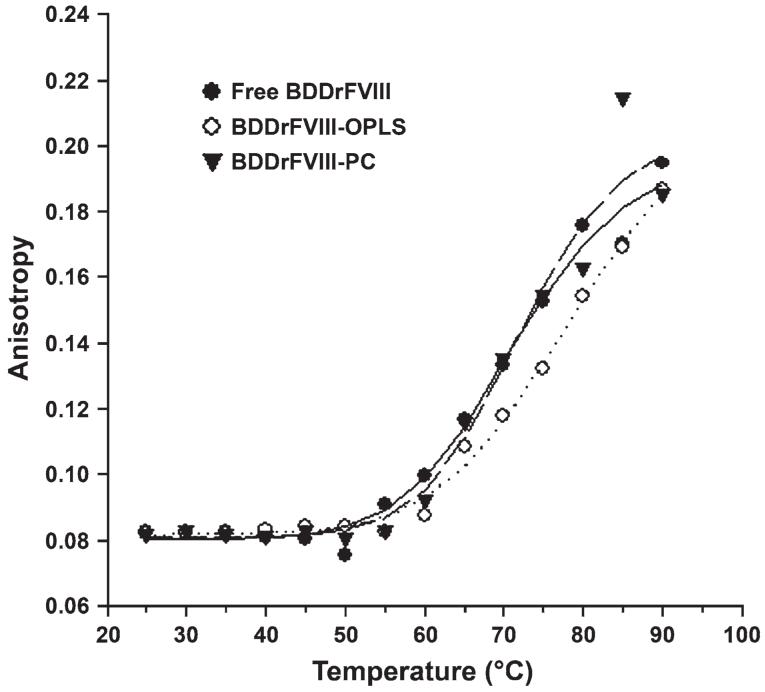
To investigate the effect of OPLS on the thermal unfolding of BDDrFVIII, fluorescence anisotropy measurements were acquired in the absence and presence of OPLS. Anisotropy is a parameter that is dependent on the molecular volume of the species. The anisotropy values should increase with increase in hydrodynamic radius following aggregation of the protein.17 As can be seen in Figure 5, there was no significant change in anisotropy values over the temperature range of 20°C to 45°C in the case of free BDDrFVIII, suggesting that the protein does not undergo self-association under those conditions. However, at temperatures greater than 50°C, the anisotropy increased sharply, suggesting the formation of aggregates. The formation of aggregated protein species was further supported by the isothermal SEC studies (Figure 4). In the presence of OPLS, the melting profile shifted to higher temperatures. The increase in anisotropy values in the presence of OPLS was observed only at higher temperatures (above 60°C) compared with the free protein (55°C), indicating that the aggregation was delayed by OPLS. To investigate whether this stabilizing effect is due to the specific interaction between protein and OPLS, a control experiment was performed in the presence of PC as there is no specific interaction between PC and BDDrFVIII.27 As can be seen in Figure 5, only OPLS, but not PC, can delay the onset of the aggregation process, emphasizing that (1) the molecular interaction between protein and OPLS is required for the stabilizing effect and (2) conformational changes in the lipid binding domain initiate the aggregation of FVIII.
Figure 5.
Effect of O-phospho-L-serine (OPLS) on the thermal unfolding of B domain deleted recombinant factor VIII (BDDrFVIII). Steady-state fluorescence anisotropy measurements in the absence and presence of phospholipids analog (10 mM). Changes in anisotropy were monitored over the temperature range of 20°C to 80°C using a heating rate of 1°C/min. Excitation and emission were set at 285 nm and 333 nm, respectively. PC indicates phosphatidylcholine.
The aggregation and stability of factor VIII have been studied extensively (Grillo et al,28 Derrick et al,29 Fatouros et al,30 Wang and Kelner,31 and Ramani et al16,26).Factor VIII is susceptible to aggregation, and it leads to loss of activity. Grillo et al28 have shown that the aggregation is the result of minor conformational changes in the tertiary structure of the protein. Based on biophysical and biochemical studies, Ramani et al26 have shown that the conformational changes in the lipid binding domain may be at least partly responsible for the initiation of the aggregation. Since OPLS binds to this region, it is possible that this ligand binding may inhibit the aggregation process. At this point, the molecular events that contribute to the stabilization of the protein are not clear. In the case of the recombinant human interleukin-1 receptor antagonist and other proteins, the addition of the excipients into the formulation caused a small increase in the free energy between the native and the intermediate/denatured states that turned out to be sufficient in suppressing the aggregation process and in increasing the shelf-life of the products.32-34 Similarly, it is possible that the weak binding of OPLS to BDDrFVIII (KD = 70.2 μM) might cause sufficient stabilization and might be ample to prevent the aggregation of the protein. In addition, we speculate that OPLS binds to the lipid binding region, inducing small conformational changes that, in turn, could inhibit the aggregation process. Further, it is likely that OPLS-mediated stabilization may also involve partially unfolded states, thus interfering with the aggregation kinetics. Despite the fact that OPLS specifically binds to factor VIII, it is possible that OPLS mediated stabilization of proteins can be extended to other proteins.
Immunological Properties of BDDrFVIII-OPLS Complex
Based on the presence of immunodominant epitopes within C2 domain8 and its interaction with lipid membranes,9,21,22,35 we hypothesize that the interaction of OPLS with the lipid binding region of the C2 domain would have an effect on the immunological properties of BDDrFVIII. We tested the immune properties of BDDrFVIII-OPLS complex and of the free protein in a murine model of hemophilia A. Subcutaneous route of administration was chosen to magnify immune response to enable statistical analysis.11
The neutralizing antibody titers were determined using the Nijmegen modification of the Bethesda assay.7 Following 4 doses of 1 μg BDDrFVIII, the inhibitory antibody titers for animals treated with BDDrFVIII-OPLS complex were 177 ± 36 BU/mL (± SEM, n = 9). These titers were significantly lower than that observed for animals given free-BDDrFVIII (461 ± 85 BU/mL [±SEM, n = 12])(Figure 6). The magnitude of observed response is higher in this animal model than that observed in hemophilia A patients, possibly because of the administration of a human protein in a non-tolerized, non-human model via the sc route at a dose that was 6-fold higher than usually used in clinical situations. Nevertheless, the type and mechanism of antibody development are similar between murine and humans.36
Figure 6.
Effect of O-phospho-L-serine (OPLS) on the immunological properties of B domain deleted recombinant factor VIII (BDDrFVIII). Inhibitory titers from individual animals administered free-BDDrFVIII or BDDrFVIII-OPLS complexes are shown as open symbols. The horizontal bar depicts the mean inhibitory antibody titers. * depicts statistical significant differences (P < .05). On the Y-axis, BU indicates Bethesda Unit.
The mechanism of the reduction in immunogenicity observed for the treatment with BDDrFVIII-OPLS complex compared with administration of free BDDrFVIII is not completely understood. It is possible that OPLS binding to lipid binding region induces minor conformational changes in this immunodominant epitope that, in turn, could alter the processing of the protein by the immune system. This finding is consistent with this study’s preliminary data, which showed that OPLS suppressed the T-cell proliferation and the secretion of relevant cytokines (data not shown). This observation is further supported by the fact that PS could modulate the antibody response against antigens, by the induction of the synthesis of anti-inflammatory cytokines, such as the transforming growth factor-beta.37 In addition, OPLS offers the additional advantage as an excipient in that its binding to FVIII reduces aggregation of the protein. It has been shown that aggregated protein species are expected to promote an adverse immune response, as it has been shown in the case of the human growth hormone and other therapeutic proteins.38,39 Thus, it is possible that OPLS may act as a multifunctional excipient for FVIII and could be useful for improving stability and reducing the immunogenicity of FVIII preparations.
Conclusion
Overall, the data presented here demonstrate that specific molecular interaction of BDDrFVIII occurs with OPLS resulting in less protein aggregation and less immunogenicity.
ACKNOWLEDGMENTS
The authors thank the Pharmaceutical Sciences Instrumentation Facility, University at Buffalo (UB), for the use of PTI spectrofluorometer. We are grateful to Drs Kazazian and Sarkar of the University of Pennsylvania, Philadelphia, PA, for providing the FVIII knockout mice. We express gratitude to Dr Straubinger (UB) for his suggestions and for the use of the ELISA plate washer. This work was supported by NHLBI, National Institute of Health grant R01 HL-70227 to SVB.
References
- 1.Kaufman RJ, Wasley LC, Dorner AJ. Synthesis, processing, and secretion of recombinant human factor VIII expressed in mammalian cells. J Biol Chem. 1988;263:6352–6362. [PubMed] [Google Scholar]
- 2.Bovenschen N, Rijken DC, Havekes LM, van Vlijmen BJ, Mertens K. The B domain of coagulation factor VIII interacts with the asialoglycoprotein receptor. J Thromb Haemost. 2005;3:1257–1265. doi: 10.1111/j.1538-7836.2005.01389.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman RJ. Biological regulation of factor VIII activity. Annu Rev Med. 1992;43:325–339. doi: 10.1146/annurev.me.43.020192.001545. [DOI] [PubMed] [Google Scholar]
- 4.Larner AJ. The molecular pathology of haemophilia. Q J Med. 1987;63:473–491. [PubMed] [Google Scholar]
- 5.Klinge J, Ananyeva NM, Hauser CA, Saenko EL. Hemophilia A—from basic science to clinical practice. Semin Thromb Hemost. 2002;28:309–322. doi: 10.1055/s-2002-32667. [DOI] [PubMed] [Google Scholar]
- 6.Lollar P. Molecular characterization of the immune response to factor VIII. Vox Sang. 2002;83:403–408. doi: 10.1111/j.1423-0410.2002.tb05342.x. [DOI] [PubMed] [Google Scholar]
- 7.Fijnvandraat K, Bril WS, Voorberg J. Immunobiology of inhibitor development in hemophilia A. Semin Thromb Hemost. 2003;29:61–68. doi: 10.1055/s-2003-37940. [DOI] [PubMed] [Google Scholar]
- 8.Reding MT, Okita DK, Diethelm-Okita BM, Anderson TA, Conti-Fine BM. Human CD4+ T-cell epitope repertoire on the C2 domain of coagulation factor VIII. J Thromb Haemost. 2003;1:1777–1784. doi: 10.1046/j.1538-7836.2003.00251.x. [DOI] [PubMed] [Google Scholar]
- 9.Pratt KP, Shen BW, Takeshima K, Davie EW, Fujikawa K, Stoddard BL. Structure of the C2 domain of human factor VIII at 1.5 A resolution. Nature. 1999;402:439–442. doi: 10.1038/46601. [DOI] [PubMed] [Google Scholar]
- 10.Pratt KP, Qian J, Ellaban E, et al. Immunodominant T-cell epitopes in the factor VIII C2 domain are located within an inhibitory antibody binding site. Thromb Haemost. 2004;92:522–528. doi: 10.1160/TH03-12-0755. [DOI] [PubMed] [Google Scholar]
- 11.Purohit VS, Jr, Ramani K, Jr, Sarkar R, Jr, Kazazian HH, Jr, Balasubramanian SV. Lower inhibitor development in hemophilia A mice following administration of recombinant factor VIII-O-phospho-L-serine complex. J Biol Chem. 2005;280:17593–17600. doi: 10.1074/jbc.M500163200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toole JJ, Pittman DD, Orr EC, Murtha P, Wasley LC, Kaufman RJ. A large region (approximately equal to 95 kDa) of human factor VIII is dispensable for in vitro procoagulant activity. Proc Natl Acad Sci USA. 1986;83:5939–5942. doi: 10.1073/pnas.83.16.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pittman DD, Alderman EM, Tomkinson KN, Wang JH, Giles AR, Kaufman RJ. Biochemical, immunological, and in vivo functional characterization of B-domain-deleted factor VIII. Blood. 1993;81:2925–2935. [PubMed] [Google Scholar]
- 14.Ramani K, Balasubramanian SV. Fluorescence properties of Laurdan in cochleate phases. Biochim Biophys Acta. 2003;1618:67–78. doi: 10.1016/j.bbamem.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Over J. Methodology of the one-stage assay of Factor VIII (VIII:C) Scand J Haematol Suppl. 1984;41:13–24. doi: 10.1111/j.1600-0609.1984.tb02764.x. [DOI] [PubMed] [Google Scholar]
- 16.Ramani K, Purohit V, Middaugh CR, Balasubramanian SV. Aggregation kinetics of recombinant human FVIII (rFVIII) J Pharm Sci. 2005;94:2023–2029. doi: 10.1002/jps.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lakowicz J. Principles of Fluorescence Spectroscopy. Kluwer Academic/Plenum; New York, NY: 1999. [Google Scholar]
- 18.Wu H, Reding M, Qian J, et al. Mechanism of the immune response to human factor VIII in murine hemophilia A. Thromb Haemost. 2001;85:125–133. [PubMed] [Google Scholar]
- 19.Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg M, Mauser-Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII:C inhibitors: improved specificity and reliability. Thromb Haemost. 1995;73:247–251. [PubMed] [Google Scholar]
- 20.Saenko EL, Scandella D, Yakhyaev AV, Greco NJ. Activation of factor VIII by thrombin increases its affinity for binding to synthetic phospholipid membranes and activated platelets. J Biol Chem. 1998;273:27918–27926. doi: 10.1074/jbc.273.43.27918. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert GE, Drinkwater D. Specific membrane binding of factor VIII is mediated by O-phospho-L-serine, a moiety of phosphatidylserine. Biochemistry. 1993;32:9577–9585. doi: 10.1021/bi00088a009. [DOI] [PubMed] [Google Scholar]
- 22.Purohit VS, Ramani K, Kashi RS, Durrani MJ, Kreiger TJ, Balasubramanian SV. Topology of factor VIII bound to phosphatidylserine-containing model membranes. Biochim Biophys Acta. 2003;1617:31–38. doi: 10.1016/j.bbamem.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Griffin BD, Micklem LR, McCann MC, James K, Pepper DS. The production and characterisation of a panel of ten murine monoclonal antibodies to human procoagulant factor VIII. Thromb Haemost. 1986;55:40–46. [PubMed] [Google Scholar]
- 24.Zhai X, Srivastava A, Drummond DC, Daleke D, Lentz BR. Phosphatidylserine binding alters the conformation and specifically enhances the cofactor activity of bovine factor Va. Biochemistry. 2002;41:5675–5684. doi: 10.1021/bi011844d. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Gabriel DA. The physical exchange of factor VIII (FVIII) between von Willebrand factor and activated platelets and the effect of the FVIII B-domain on platelet binding. Biochemistry. 1997;36:10760–10767. doi: 10.1021/bi970052+. [DOI] [PubMed] [Google Scholar]
- 26.Ramani K, Purohit VS, Miclea RD, Middaugh CR, Balasubramanian SV. Lipid binding region (2303-2332) is involved in aggregation of recombinant human FVIII (rFVIII) J Pharm Sci. 2005;94:1288–1299. doi: 10.1002/jps.20340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falls LA, Furie B, Furie BC. Role of phosphatidylethanolamine in assembly and function of the factor IXa-factor VIIIa complex on membrane surfaces. Biochemistry. 2000;39:13216–13222. doi: 10.1021/bi0009789. [DOI] [PubMed] [Google Scholar]
- 28.Grillo AO, Edwards KL, Kashi RS, et al. Conformational origin of the aggregation of recombinant human factor VIII. Biochemistry. 2001;40:586–595. doi: 10.1021/bi001547t. [DOI] [PubMed] [Google Scholar]
- 29.Derrick TS, Kashi RS, Durrani M, Jhingan A, Middaugh CR. Effect of metal cations on the conformation and inactivation of recombinant human factor VIII. J Pharm Sci. 2004;93:2549–2557. doi: 10.1002/jps.20167. [DOI] [PubMed] [Google Scholar]
- 30.Fatouros A, Liden Y, Sjostrom B. Recombinant factor VIII SQ—stability of VIII:C in homogenates from porcine, monkey and human subcutaneous tissue. J Pharm Pharmacol. 2000;52:797–805. doi: 10.1211/0022357001774651. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Kelner DN. Correlation of rFVIII inactivation with aggregation in solution. Pharm Res. 2003;20:693–700. doi: 10.1023/a:1023271405005. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter JF, Kendrick BS, Chang BS, Manning MC, Randolph TW. Inhibition of stress-induced aggregation of protein therapeutics. Methods Enzymol. 1999;309:236–255. doi: 10.1016/s0076-6879(99)09018-7. [DOI] [PubMed] [Google Scholar]
- 33.Kendrick BS, Carpenter JF, Cleland JL, Randolph TW. A transient expansion of the native state precedes aggregation of recombinant human interferon-gamma. Proc Natl Acad Sci USA. 1998;95:14142–14146. doi: 10.1073/pnas.95.24.14142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kendrick BS, Chang BS, Arakawa T, et al. Preferential exclusion of sucrose from recombinant interleukin-1 receptor antagonist: role in restricted conformational mobility and compaction of native state. Proc Natl Acad Sci USA. 1997;94:11917–11922. doi: 10.1073/pnas.94.22.11917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoilova-McPhie S, Villoutreix BO, Mertens K, Kemball-Cook G, Holzenburg A. 3-Dimensional structure of membrane-bound coagulation factor VIII: modeling of the factor VIII heterodimer within a 3-dimensional density map derived by electron crystallography. Blood. 2002;99:1215–1223. doi: 10.1182/blood.v99.4.1215. [DOI] [PubMed] [Google Scholar]
- 36.Qian J, Jr, Borovok M, Jr, Bi L, Jr, Kazazian HH, Jr, Hoyer LW. Inhibitor antibody development and T-cell response to human factor VIII in murine hemophilia A. Thromb Haemost. 1999;81:240–244. [PubMed] [Google Scholar]
- 37.Hoffmann PR, Kench JA, Vondracek A, et al. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. J Immunol. 2005;174:1393–1404. doi: 10.4049/jimmunol.174.3.1393. [DOI] [PubMed] [Google Scholar]
- 38.Moore WV, Leppert P. Role of aggregated human growth hormone (hGH) in development of antibodies to hGH. J Clin Endocrinol Metab. 1980;51:691–697. doi: 10.1210/jcem-51-4-691. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg AS. Effects of protein aggregates: an immunologic perspective. AAPS J. 2006;8:E501–E507. doi: 10.1208/aapsj080359. [DOI] [PMC free article] [PubMed] [Google Scholar]