Abstract
Background and Aims
Inactivating mutations in MYO5B cause severe neonatal diarrhea in Microvillus Inclusion Disease. Loss of active MYO5B causes the formation of pathognomonic inclusions and aberrations in brush border enzymes.
Methods
We developed three mouse models of germline, constitutively intestinal targeted and inducible intestinal targeted deletion of MYO5B. The mice were evaluated for enterocyte cellular morphology.
Results
Germline MYO5B KO mice showed early diarrhea and failure to thrive with evident microvillus inclusions and loss of apical transporters in the duodenum. IgG was present within inclusions. Apical transporters were lost and inclusions were present in the duodenum, but were nearly absent in the ileum. VillinCre;MYO5BF/F mice showed similar pathology and morphological changes in duodenal enterocytes. In contrast, when MYO5B KO was induced with tamoxifen treatment at 8 weeks of age, VillinCreERT2;MYO5BF/F mice developed severe diarrhea with loss of duodenal brush border enzymes, but few inclusions were observed in enterocytes. However, if tamoxifen is administered to 2-day-old VillinCreERT2;MYO5BF/F mice, prominent microvillus inclusions were observed.
Conclusions
The microvillus inclusions that develop after MYO5B loss reveal the presence of an unrecognized apical membrane trafficking pathway in neonatal duodenal enterocytes. However, the diarrheal pathology after MYO5B loss is due to deficits in transporter presentation at the apical membrane in duodenal enterocytes.
Keywords: Enterocyte trafficking, brush border, Rab11a, Rab8a, Syntaxin 3, NHE3
Introduction
Microvillus Inclusion Disease (MVID) is caused by inactivating mutations in myosin Vb (MYO5B).1–3 Neonates with MVID display severe life-threatening diarrhea usually beginning in the first week of life.4, 5 Recent studies using in vitro investigations in correlation with human patient pathological tissues have revealed insights into the possible pathophysiology of MVID.6–9 Our investigations have demonstrated that MYO5B interactions with Rab8a are responsible for deficits in microvillar structure and trafficking, while interactions between MYO5B and Rab11a are responsible for microvillus inclusion formation.9 Importantly, we were able to demonstrate that microvillus inclusions developed as a result of apical macropinocytosis.9 While in vitro studies using MYO5B knockdown and rescue in CaCo2 and CaCo2-BBE cells have identified important mechanisms that are putatively involved in MVID pathogenesis,3, 7–9 important questions remain about the significance of microvillus inclusions to the diarrhea phenotype and possible implications for treatment.
A recent publication has reported a gene trap MYO5B knockout mouse that demonstrated duodenal microvillus inclusions, but the pups died within 12 hours of birth, limiting the analysis of pathology.10 Additionally, an inducible intestinal MYO5B knockout mouse was recently reported to develop microvillus inclusions within 4 days of MYO5B loss.11 However, the sole reliance on adult mice to study a neonatal disease may hamper direct interpretation of the neonatal mechanisms involved in MVID. We have now developed germline, intestinally targeted and inducible intestinal MYO5B knockout mice. Both germline MYO5B knockout mice and VillinCre;MYO5BF/F mice demonstrated numerous microvillus inclusions in the proximal intestine, but showed few inclusions in the distal small bowel. Inclusions contained IgG, suggesting their derivation from apical macropinocytosis involved with internalization of IgG from maternal milk. However, while 8 week old VillinCreERT2;MYO5BF/F mice induced with a single dose of tamoxifen developed severe diarrhea within 48 hours, they demonstrated few microvillus inclusions. Nevertheless, if 2-day-old VillinCreERT2;MYO5BF/F mice received tamoxifen, they developed prominent microvillus inclusions within three days. Diarrhea in all three mouse models was well correlated with losses in apical transporters in the proximal small intestine. These results demonstrate that microvillus inclusions in MYO5B knockout mice are not directly pathological, but rather reveal the presence of a specialized apical membrane, processing pathway in duodenal enterocytes during the neonatal period. The predominance of pathology in the proximal small intestine suggests that interventions promoting distal intestinal adaptation may represent an important strategy in the treatment of MVID neonates.
Materials and Methods
All authors had access to the study data and had reviewed and approved the final manuscript.
Construction and validation of MYO5B deletion mice
ES cells harboring a Knock-out First allele for MYO5B (Figure 1A) were obtained from the Knockout Mouse Project (KOMP, Davis, CA). ES cells (CSD78985 clone B10) were injected into C57BL/6 mouse blastocysts by the Vanderbilt Stem Cell Shared Resource. Ten chimeric male mice (50–80% chimeric) were produced and mated with albino C57BL/6 mice to produce chimeric founders. One chimera gave germline transmission to black offspring. The heterozygous mice from this line were mated for one year and produced no viable homozygous offspring. The mice were therefore crossed onto CD1 mice and after three generations crossed heterozygous mice gave birth to viable homozygous knockout offspring.
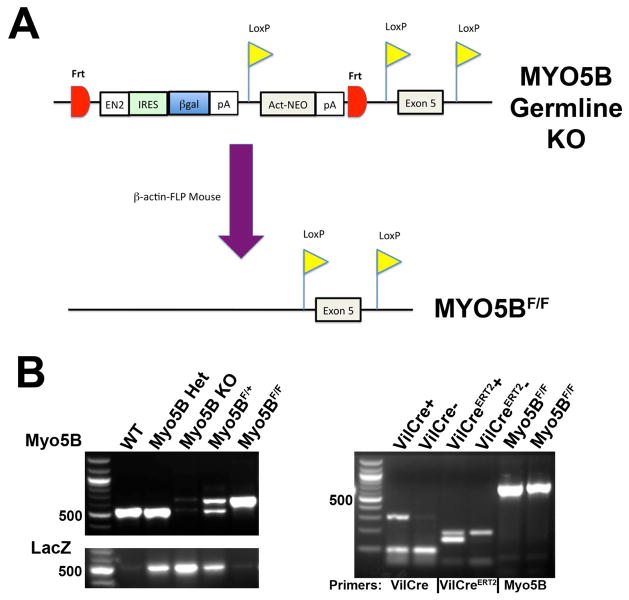
Figure 1. Generation of germline MYO5B KO and MYO5BF/F mice.
(A) Schematic representation of the Knock-out First allele for MYO5B from the Knockout Mouse Project (KOMP) is shown. This construct was utilized to generate germline MYO5B KO mice by early termination of the Myo5B transcription prior to Exon 5. Mice harboring this allele were mated to β-actin-FLP mice to also create MYO5BF/F mice in which LoxP sites flank Exon 5. (B) PCR of genomic DNA shows the different PCR product patterns used to identify the genotype of a mouse. The 100bp DNA Ladder (from NEB) is shown in left lane of gels to denote PCR product size. The 500 bp marker is indicated.
To produce floxed allele mice, the MYO5B+/− mice were bred with Actin-FLP mice to derive MYO5Bflox/+ mice (Figure 1A). The MYO5Bflox/+ heterozygotes were crossed to produce homozygous MYO5Bflox/flox mice (MYO5BF/F). The MYO5BF/F mice were then crossed with both VillinCre and VillinCreERT2 mice to produce VillinCre;MYO5BF/F targeted intestinal MYO5B deletion mice and VillinCreERT2;MYO5BF/F inducible intestinal knockout mice. The care, maintenance, and treatment of animals in these studies followed protocols approved by the Institutional Animal Care and Use Committee of Vanderbilt University.
DNA was isolated from mice tails using DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. All genotyping was confirmed by specific PCR with GoTaq Green Master Mix (Promega, Madison, WI) (Figure 1B) using the following PCR primer pairs: (For Myo5B) MYO5BKO-F2, 5′-CTTGAGTTTGTTTAGTCTCTTGTCCCCTTTG-3′ and MYO5BKO-R3, 5′-CCGCTGACTATGATGGATTGGTTCTTTTC-3′, (For LacZ) LacZ-1, 5′-TGCCGCTCATCCGCCACAT-3′ and LacZ-2, 5′-CACCGATCGCCCTTCCCAACAGT-3′, (For VillinCre) 16775, 5′-GCCTTCTCCTCTAGGCTCGT-3′, 16776, 5′-TATAGGGCAGAGCTGGAGGA-3′, and oIMR9074, 5′-AGGCAAATTTTGGTGTACGG-3′, and (For VillinCreERT2) 5′Cre, 5′-CGCGAACATCTTCAGGTTCT-3′ and 3′Cre, 5′-CAAGCCTGGCTCGACGGCC-3′. The following cycling parameters were used for Myo5B: 95°C(3′), 95°C(30″), 64°C(30″), 72°C(45″), 72°C(5′) with steps 2–4 repeated for 35 cycles. For LacZ and VillinCre PCR, the annealing temperature of 62°C was used while VillinCreERT2 PCR used 58°C.
For validation of Myo5B mRNA loss, total RNA was extracted from frozen duodenum samples using TRIzol (Invitrogen, Carlsbad, CA). Total RNA (1 μg) was treated with RQ1 RNase-free DNase (Promega) and then reverse transcribed using Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. PCR was then performed with Advantage Taq (Clontech, Mountain View, CA) using the following primers: Exon4-sense, 5′-CCTACGAGCAGCTGCCAATCTAC-3′ and Exon5-AS, 5′-GACACCGTCTTGCCTGCTCCAGACTCTC-3′. Cycling was performed at 95°C 15″ and 68C 20″, for 42 cycles.
Western blot analysis
Fresh duodenum from each mouse was placed in 65°C lysis buffer (60 mM Tris pH ~6.8/10 mM EDTA/2% SDS) directly after euthanization. Samples were heated at 65°C for 10 minutes and then spun down for 10 minutes at 3600 rpm at 4°C. Samples were stored at −80°C until time of use. At time of western blot, DTT (final concentration of 0.12 mM) was added to each sample (50 μg of total protein), followed by 10 minutes incubation at 65°C. Samples were resolved on a 4–12 % gradient Novex Bis-Tris gel (Invitrogen) and electrophoretically transferred onto Odyssey nitrocellulose membranes (Li-Cor, Lincoln, NE). Membranes were subsequently dried for 1 hour at room temperature, followed by blocking with Odyssey TBS blocking buffer (Li-Cor) for 1 hour at room temperature. Primary (rabbit anti-MYO5B, 1:500, Sigma Prestige cat# HPA040902 and rabbit anti-VDAC1/porin, 1:1000, Abcam cat# ab15895) and secondary antibodies (IRDye 800CW donkey anti-rabbit IgG, 1:15000, Li-Cor cat# 925-32213) were diluted in the blocking buffer with 0.2% Tween-20. Membranes were visualized using Odyssey Fc Imaging System (Li-Cor).
Determination of mouse weight and stool water content
Mice from all 3 mouse models were weighed at time of euthanasia (at least n=3 for each group). To determine stool water content, stool samples collected from the colon (3 control mice and 3 MYO5B mice) were placed in pre-weighed tubes and then total stool weight was determined. The samples were then desiccated to dryness in a Speedvac and tubes were reweighed. The differences in sample weights pre- and post-desiccation were determined and used to determine percent stool water. Comparisons were performed using a one-tailed Mann-Whitney U test (Prism from GraphPad, La Jolla, CA) with a p-value ≤ 0.05.
Induction of VillinCreERT2;MYO5BF/F with tamoxifen
Cre recombinase was activated in 8–10 week old VillinCreERT2;MYO5BF/F mice (n=9) by one intraperitoneal injection of tamoxifen (2 mg). Mice were sacrificed 3 days after the tamoxifen dose. Tamoxifen-injected MYO5BF/F mice and VillinCreERT2;MYO5BF/+ mice (n=8) were used as controls because no difference was found between induced MYO5BF/F and induced VillinCreERT2;MYO5BF/+ mice. For Cre recombinase activation in neonatal mice, 2 day old VillinCreERT2;MYO5BF/+ (Controls, n=6) and VillinCreERT2;MYO5BF/F (n=4) littermates were given subcutaneous injections of tamoxifen (0.5 mg/g/day) for 2 consecutive days and sacrificed 3 days after the first tamoxifen injection.
Immunofluorescence
For MYO5B KO and VillinCre;MYO5BF/F mice, pups were euthanized between 2–8 days of age. Excised intestines were fixed in 4% paraformaldehyde overnight at 4°C and embedded in paraffin. Sections were deparaffinized and rehydrated before antigen retrieval was performed using Target Retrieval solution (DakoCytomation, Glostrup, Denmark) in a pressure cooker for 15 minutes. Following cool down on ice, sections were blocked with Mouse on Mouse (M.O.M) Basic Kit (Vector Laboratories, Burlingame, CA) for 20 minutes at room temperature (except for mouse IgG staining) and followed by protein block serum-free (Dako) either 1 hour at room temperature or overnight at 4°C. The following primary antibodies were incubated at 4°C overnight: chicken anti-MYO5B (1:200),9, 12 rabbit anti-Ezrin (1:200, Cell Signaling cat.# 3145), goat anti-DPPIV (1:200, R&D Systems cat#AF954), mouse anti-CD10 (1:100, Abcam cat# ab47721), mouse anti-CD10 (1:60, Fitzgerald cat# 10R-CD10aHU) (used for VillinCreERT2;MYO5BF/F neonate studies), rat anti-Lamp2 (1:200, Abcam cat# ab13524), rabbit anti-Rab8a (1:200),9, 13 rabbit anti-Rab11a (1:200, US Biological Life Sciences cat# R0009), rabbit anti-STX3 (1:200, Abcam cat# ab4113), rabbit anti-NHE3 (1:200, a gift from Dr. F. Ghishan, University of Arizona, Phoenix), rabbit anti-pERM (1:200, Cell Signaling cat# 3149), mouse anti-E-cadherin (1:200, BD Biosciences cat# 610181), mouse anti-p120 (1:200, BD Biosciences cat# 610133), mouse anti- Na/K-ATPase (1:50, Millipore cat# 05-369), rabbit anti-lysozyme (1:2000, Abcam cat# ab108508), rabbit anti-Muc2 (1:200, Santa Cruz cat# sc-15334), rabbit anti-Chga (1:100, Abcam cat# ab17064), and rabbit anti-Villin (1:100, Cell Signaling cat# R814). After 3 washes in 1X PBS for 5 minutes each, sections were incubated for 1 hour at room temperature with the appropriate secondary antibodies conjugated for immunofluorescence with Alexa Fluor 488, Alexa Fluor 647, Cy2, Cy3, Cy5, or Cy7 (1:500, Molecular Probes, Eugene, OR or Jackson ImmunoResearch, West Grove, PA). For Ki67 immunofluorescence, sections were incubated with directly conjugated anti-Ki67-Alexa Fluor 647 (1:50, BioLegend cat# 652408) at the same time as the secondary antibodies. Slides were then washed 3 times with 1XPBS and mounted with ProLong Gold Antifade Reagent with DAPI (Invitrogen). For mouse IgG immunofluorescence, sections were incubated with goat anti-mouse IgG (H+L) Alexa Fluor 488 (Molecular Probes cat# A11029) at time of primary antibody incubation. This signal was then amplified by incubation with donkey anti-goat Alexa Fluor 488 (Molecular Probed cat# A11055) at the time of secondary antibody incubation. All sections were analyzed using Zeiss Axiophot microscope equipped with an Axiovision digital imaging system (Zeiss, Jena GmBH, Germany) or an Olympus FV1000 confocal microscope (Tokyo, Japan).
Quantification of fused villi, inclusions, proliferation, and Paneth cells
For quantification of fused villi and inclusions, slides immunostained with the appropriate antibodies were scanned using the Ariol SL-50 automated slide scanner (Genetix, San Jose, CA). Three randomly selected sections of well-oriented duodenum and ileum were selected for quantification. A total approximate intestinal length of 1.5 mm (for MYO5B KO), 2 mm (for VillinCre;MYO5BF/F), or 2.5–3.5 mm (for VillinCreERT2;MYO5BF/F) were quantified per mouse (n=3) from both the duodenum and the ileum for fused villi and inclusions. For fused villi quantification, a villus structure was considered fused if the apical membrane of a villus was observed upon exiting of the crypt (as marked by Ezrin immunostaining), but was later lost in conjunction with a neighboring villus. For example, if two separate villi with marked apical membranes emerged from crypts but later converged and lost sections of apical membrane accompanied by obvious interconnected cells (as marked on the basolateral membrane by p120 immunostaining), two fused villi were counted. For comparisons, the total number of fused villi was then normalized by the linear length of intestine quantified.
The same regions of tissue used for fused villi quantification were used for quantification of inclusions. Inclusions were identified as being round internal Ezrin-positive structures (obvious deep invaginations from the apical membrane not normally found in controls were also included as inclusions). The total number of inclusions were quantified as the total counted number of Ezrin-positive inclusions normalized by the total linear length of tissue examined. For co-labeled inclusions, these same regions were examined for Ezrin and CD10 or Ezrin and DPPIV co-labeled inclusions. All Ezrin-positive inclusions identified for the total inclusion quantification were examined for co-labeling with either CD10 or DPPIV. The numbers of dual-labeled inclusions were presented as the percentage of the total Ezrin-positive inclusions that co-labeled with CD10 or DPPIV.
For quantification of proliferation and presence of Paneth cells, sections immunostained for either Ki67 (proliferation) or lysozyme (Paneth cells) were analyzed. Two regions of randomly selected, well-oriented crypts from the duodenum of each mouse (n=3 for each group) were examined for the total number of Ki67-positive cells. At least 5 crypts from each mouse were quantified. The total number of Ki67-positive cells was normalized by the number of crypts counted to obtain the average number of proliferating cells per crypt. The early maturation of Paneth cells in neonatal mice was quantified by lysozyme immunostaining. Again, two regions of randomly selected, well-oriented crypts (for 5–10 crypts total) from the duodenum of each mouse (n=3) were quantified for the number of lysozyme-positive cells. The total number of lysozyme-positive cells was normalized by the number of crypts counted. All comparisons were performed using a one-tailed Mann-Whitney U test (Prism from GraphPad) with a significant p-value ≤ 0.05.
Electron microscopy
For TEM and SEM preparation, freshly excised duodenum tissue was quickly washed in 0.1M cacodylate buffer. Samples were then fixed in 2.5% glutaraldehyde (in 0.1M sodium phosphate buffer pH 7.4, 0.1M cacodylate buffer) for 30 minutes at room temperature followed by overnight fixation at 4°C. After washing, samples were treated with 1% osmium tetroxide for 1 hour and dehydrated through serial ethanol dilutions (30%, 50%, 70%, 95%, and 100%). For SEM, after ethanol dehydration, samples were incubated with hexamethyldisilazane (HMDS), mounted on stubs, and coated with gold in a sputter coater. Images were obtained using an FEI Quanta 250 scanning electron microscope. For TEM, tissue was incubated with propylene oxide followed by removal of ethanol from the samples prior to infiltration with and embedding in EPON 812 resin (Electron Microscopy Sciences, Hatfield, PA). Ultrathin sections (70 to 80 nm thick) were cut and collected on 300-mesh copper grids. Sections were stained with 2% uranyl acetate and then Reynold’s lead citrate. Images were obtained using a Philips/FEI T-12 Tecnai T12 electron microscope. For quantification of microvilli length and width, TEM images were evaluated for at least 60 microvilli from each mouse using an AMT Image Capture Engine to analyze images obtained with a DVC camera. Statistical differences were determined using a one-tailed Mann-Whitney U test (Prism from GraphPad) with a significant p-value ≤ 0.05.
Results
MYO5B germline KO mice display failure to thrive, aberrant villi structure, and microvillus inclusions
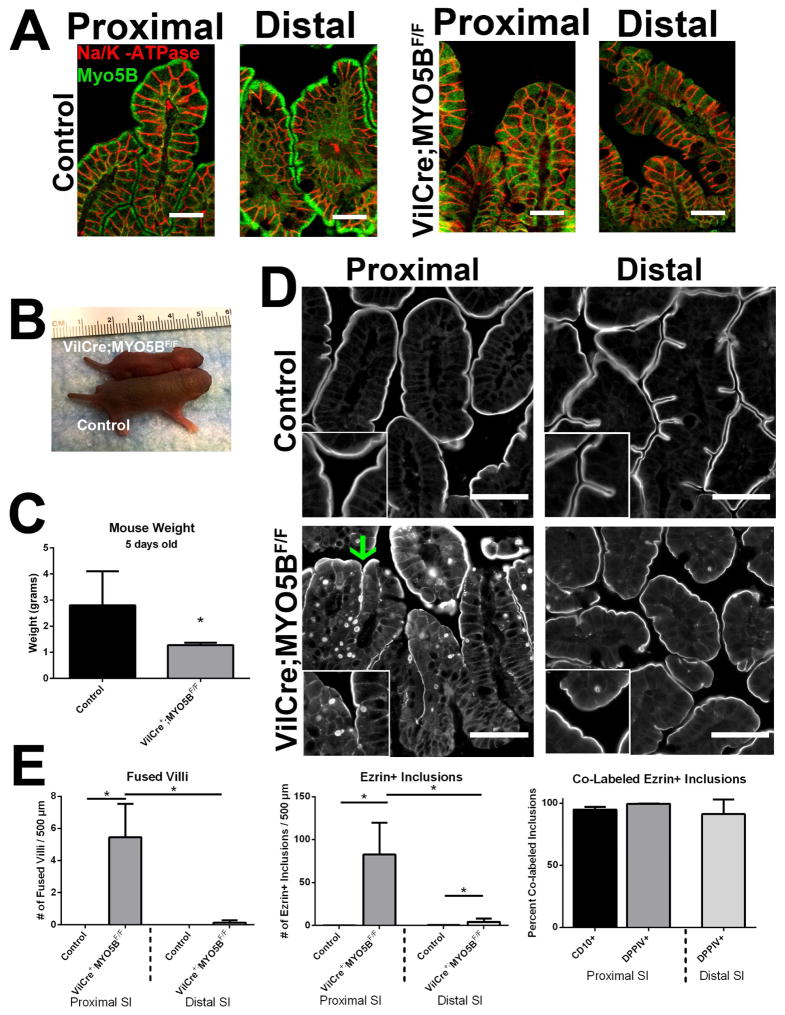
Breeding of C57BL/6 MYO5B heterozygous mice did not yield any viable MYO5B KO pups after one year of breeding, thus C57BL/6 MYO5B heterozygous mice were crossed onto the outbred CD1 background. After three generations, viable C57BL/6;CD1 germline MYO5B KO pups were obtained. Analysis of MYO5B by PCR and western blot confirmed the loss of MYO5B mRNA and protein (Figure 2A and B). Furthermore, immunofluorescence for MYO5B showed loss of MYO5B from the intestine of MYO5B KO mice (Figure 2C). While MYO5B KO mice were indistinguishable from both wildtype and heterozygous littermates at birth, by 3 days old, MYO5B KO mice had a significantly higher percent of water in their stool (Figure 2D). By 5 days old, MYO5B KO mice were visibly smaller and weighed significantly less than both wildtype and heterozygous littermates (Figure 2E and F). No differences in weight or pathology were seen between wildtype and heterozygous MYO5B mice, so both were used as controls in further studies. Of note, colon and liver histology in MYO5B KO mice appeared predominately normal, so further studies focused on the small intestine.
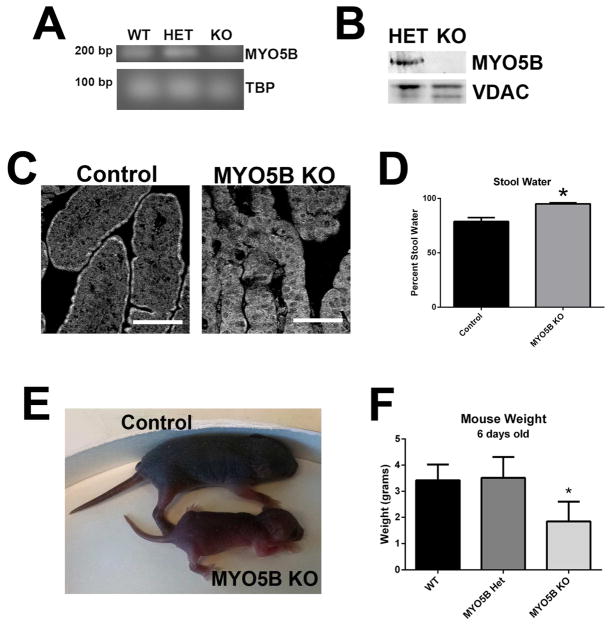
Figure 2. Germline MYO5B KO mice fail to thrive.
(A) PCR of cDNA from WT, MYO5B Het, and MYO5B KO mouse duodenum confirm Myo5B mRNA loss. Tata-box binding protein (TBP) was used as a loading control. (B) Western blot of duodenum confirms MYO5B protein loss in a MYO5B germline KO mouse as compared to a MYO5B heterozygote mouse. VDAC used as a loading control. (C) Immunofluorescence for MYO5B in control duodenum displays subapical staining. This subapical staining was lost in MYO5B KO duodenum. Scale bar = 50 μm (D) The percentage of stool constituted by water was calculated in control and MYO5B KO mice (n=3) MYO5B KO had significantly higher water content in stool collected from the colon. *p-value = 0.05. (E) Picture of control and MYO5B KO littermates at 5 days old shows the reduced size of MYO5B KO mice. (F) Mice weighed at 6 days old display no difference between WT and MYO5B Het mice. MYO5B KO mice were significantly smaller than control littermates (both WT and Het). (n=3) *p-value = 0.05.
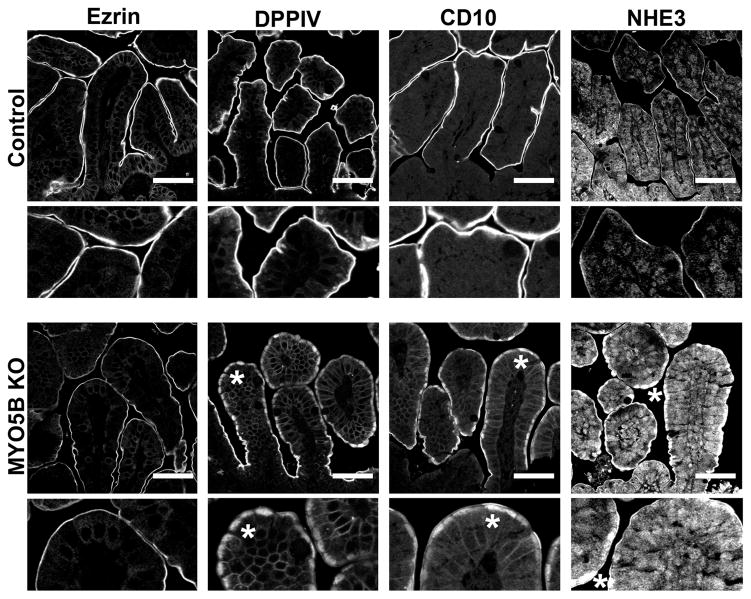
In control duodenum, Ezrin immunostained the microvilli on the apical surface of enterocytes along the villi (Figure 3A). In MYO5B KO duodenum, Ezrin immunostaining revealed fused villi (Figure 3A) with approximately 8 fused villi per 500 μm length of duodenum (Figure 4C). Previous studies have noted villus fusion in MVID patients14 as well as in ezrin KO mice.15 Additionally, we observed intracellular Ezrin-positive inclusions (Figure 3A) at a prevalence of 140 inclusions per 500 μm length of duodenum (Figure 4D). The Ezrin-positive inclusions were typically circular with a diameter of 2–4 μm. The Ezrin-positive inclusions appeared along the entire length of the villi beginning in cells just emerging from the crypts. To examine the effects of MYO5B loss on other enterocyte components previously shown as disrupted in MVID patients,9, 16 MYO5B KO duodenum was immunostained for dipeptidyl peptidase-4 (DPPIV) and CD10. In control proximal small intestine, DPPIV predominately labeled the apical surface with only a small amount of subapical DPPIV observed, and CD10 localized to the apical surface of enterocytes. In contrast, in MYO5B KO duodenum, a significant subapical localization of DPPIV was observed as well as localization in small punctate vesicles and Ezrin-positive inclusions (Figure 3B and C). Similarly, CD10 was markedly decreased along the apical surface and also mislocalized to a subapical compartment and in Ezrin-positive inclusions in MYO5B KO duodenum (Figure 3B and C). Greater than 98% of Ezrin-positive inclusions co-labeled with CD10 and DPPIV (Figure 3C and 4E).
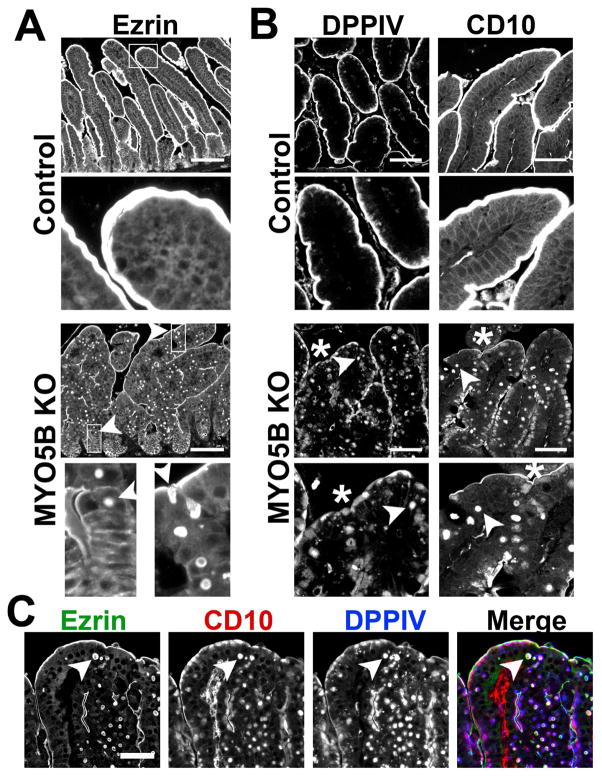
Figure 3. Immunofluorescence of MYO5B KO duodenum.
(A) Ezrin immunostaining in control mice show fully formed brush border along the length of single villi. The inset shows no internal Ezrin staining. In MYO5B KO duodenum, Ezrin labeled internal inclusions in addition to the apical brush border. The inset at left shows inclusions formed in cells just exiting the crypt (arrowhead). Inclusions were also found forming along the apical membrane (arrowhead, right inset). Ezrin immunostaining also revealed fused villi in MYO5B KO duodenum. Scale bar = 100 μm. (B) DPPIV and CD10 both labeled the apical brush border in control mice with no internal structures labeled (inset). In MYO5B KO duodenum, DPPIV and CD10 was collapsed from the apical surface into a diffuse subapical localization (asterisk) as well as localized to internal inclusions (arrowheads). DPPIV is also found localized to small punctate vesicles (DPPIV inset). Scale bar = 50 μm. (C) MYO5B duodenum immunostained for Ezrin (green), CD10 (red), and DPPIV (blue) show Ezrin-positive inclusions co-labeled with CD10 and DPPIV (arrowhead). Scale bar = 50 μm.
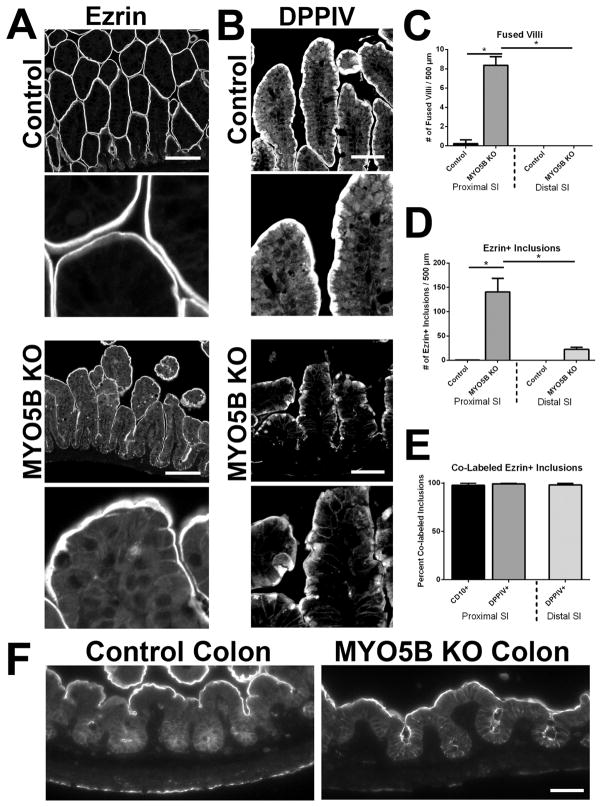
Figure 4. Comparison of distal small intestine and colon.
(A) Distal small intestine from control and MYO5B KO mice was immunolabeled for Ezrin. In control distal small intestine, Ezrin labeled the apical brush border with no intracellular immunoreactivity, as found in the proximal intestine (Inset). Distal MYO5B KO small intestine displayed only occasional fused villi and few Ezrin-positive inclusions along with the apical membrane labeling. Scale bar = 100 μm. (B) In control distal small intestine, DPPIV labeled the apical membrane with a small subapical population. DPPIV localization in MYO5B KO distal small intestine appeared similar to control tissue with a compact subapical localization in addition to the apical membrane. Additionally, fewer DPPIV-positive intracellular inclusions were found. Scale bar = 50 μm. (C) The number of fused villi was counted in both the proximal and distal intestine in control and MYO5B KO mice. MYO5B KO proximal intestine had approximately 8 fused villi per 500 μm length of intestine, which was significantly increased from the controls. A decreasing gradient in the number of fused villi was observed in MYO5B KO intestine with only rare fused villi found in the distal small intestine. *p-value = 0.05. (D) MYO5B KO duodenum had approximately 140 Ezrin-positive inclusions per 500 μm of intestine. Again, the number of inclusions was significantly decreased in MYO5B KO distal small intestine as compared to MYO5B KO duodenum. *p-value = 0.05. (E) The percent of Ezrin-positive inclusions that co-labeled with CD10 or DPPIV was quantified. Over 98% of all Ezrin-positive inclusions were dual-labeled with Ezrin and CD10 or Ezrin and DPPIV. No difference in co-labeled inclusions was found between the proximal and distal small intestine. (F) Colon from control and MYO5B KO mice was immunolabeled for Ezrin. No microvillus inclusions were observed in MYO5B KO colon. Scale bar = 50 μm.
The proximal small intestine was compared to the distal small intestine in MYO5B KO mice to examine differences along the length of the small intestine. Ezrin labeled the apical surfaces along the villi of the control distal small intestine, outlining individual villi as seen in the control duodenum. While MYO5B KO duodenum had numerous fused villi, Ezrin immunostaining of the MYO5B KO distal small intestine revealed single villi similar to the controls (Figure 4A and C). Likewise, significantly fewer Ezrin-positive inclusions were observed in the distal small intestine than in the proximal intestine of MYO5B KO mice (Figure 4A and D). No inclusions were noted in the colonic mucosa of MYO5B KO mice (Figure 4F).
We next utilized DPPIV immunostaining to examine expression along the intestinal tract. CD10 expression was not used for distal small intestine analysis because we noted CD10 expression significantly decreases in normal murine distal small intestine (data not shown). In control distal small intestine, DPPIV labeled the apical surface as well as a small subapical compartment (Figure 4B). DPPIV immunostaining of MYO5B KO mouse distal small intestine demonstrated few intracellular inclusions, similar to the Ezrin immunostaining (Figure 4B). However, when Ezrin-positive inclusions were observed in the distal intestine, they usually also stained for DPPIV (Figure 4E). Additionally, in MYO5B KO intestine, the localization of DPPIV to small punctate vesicles in the duodenum was not observed in the distal small intestine (Figure 4B). These findings indicate that MYO5B loss induces a gradient of pathology along the length of the small intestine with the predominate pathology located in the proximal intestine.
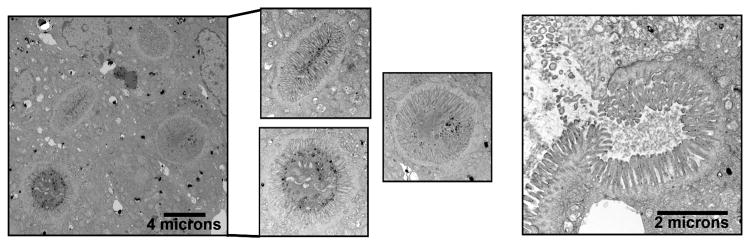
To examine the ultrastructure of the inclusions found predominately in the duodenum, we performed transmission electron microscopy (TEM) on duodenum from 7-day-old MYO5B KO mice. By TEM analysis, inclusions with microvilli were observed inside enterocytes (Figure 5). In addition, omega-shaped invaginations containing apical microvilli were observed at the apical surface. Thus, MYO5B germline KO mice develop microvillus inclusions as seen in MVID patients.
Figure 5. Transmission electron microscopy identification of microvillus inclusions.
TEM analysis of MYO5B KO duodenum revealed inclusions with fully formed microvilli, confirming the formation of microvillus inclusions. Several microvillus inclusions were visualized within the enterocytes of the duodenum (left panels). Scale bar = 4 μm. Additionally, microvillus inclusion was visualized at the apical membrane with characteristic omega shaped invaginations of the brush border microvilli (right panel). Scale bar = 2 μm.
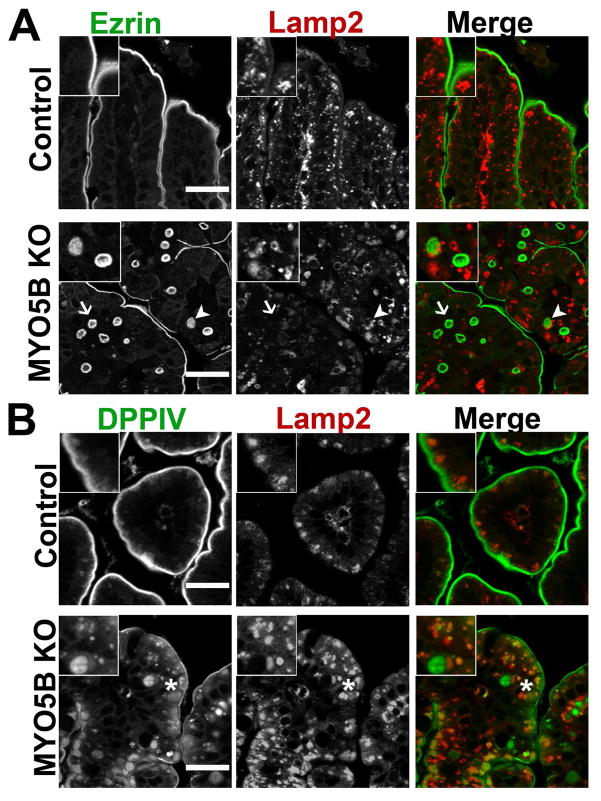
In MYO5B knockdown Caco2-BBE cells, internalized DPPIV was trafficked to lysosomes.9 To examine if the internal DPPIV punctate vesicles or the Ezrin-positive microvillus inclusions were localized with lysosomes, we co-immunostained for DPPIV or Ezrin with Lamp2. In control duodenum, Lamp2-positive lysosomes were located subapically just under the Ezrin- and DPPIV-positive brush border (Figure 6A and B). In MYO5B KO duodenum, a subset of Ezrin-positive inclusions were not localized near Lamp2-positive lysosomes, while other Ezrin-positive inclusions appeared to be closely surrounded by but not co-localized with Lamp2-positive lysosomes (Figure 6A). Similarly, internal DPPIV found on microvillus inclusions did not co-localize with Lamp2 (Figure 6B). However, the DPPIV-positive punctate vesicles in KO duodenum co-labeled with Lamp2, suggesting that a portion of the internalized DPPIV not found in Ezrin-positive microvillus inclusions was diverted to lysosomes (Figure 6B).
Figure 6. Trafficking of Ezrin-positive inclusions and DPPIV.
(A) Duodenum from control and MYO5B KO mice were co-stained for Ezrin (green) and Lamp2 (red). In control enterocytes, Lamp2-positive lysosomes concentrated just under the apical surface. Upon MYO5B loss, a subset of Ezrin-positive inclusions were closely surrounded or ‘cupped’ by Lamp2-positive lysosomes (arrowhead). However, other Ezrin-positive inclusions were not located near Lamp2 (arrow). Scale bar = 25 μm. (B) In controls, the small DPPIV population occasionally found in the subapical region co-localized with Lamp2. In MYO5B KO duodenum, the subset of DPPIV that mistrafficked to small punctate vesicles was found to co-label with Lamp2 (asterisk). Scale bar = 25 μm.
Germline MYO5B loss disrupts apical trafficking but not microvilli formation or basolateral protein localization
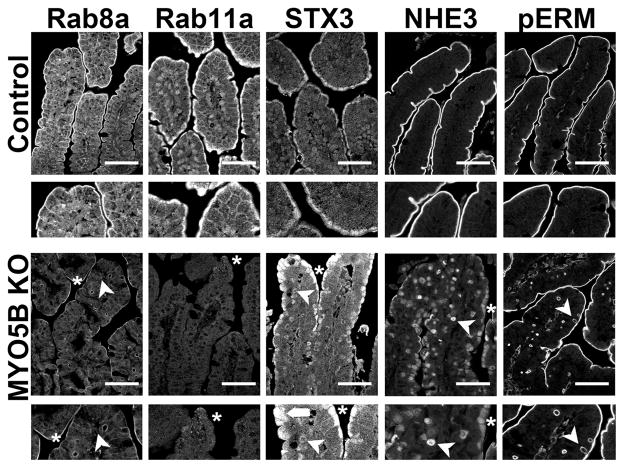
To determine the effects of MYO5B loss on apical trafficking and polarity, we examined immunostaining for apical trafficking proteins (Rab8a, Rab11a, and STX3), the apical sodium-hydrogen exchanger 3 protein (NHE3), and phosphorylated-Ezrin-Radixin-Moesin (pERM), as a reflection of phosphorylated ezrin in microvilli. Recent studies have shown that loss of MYO5B results in dispersion of MYO5B binding proteins, Rab8a and Rab11a.9, 11 In control duodenum, Rab8a and Rab11a were predominately concentrated in the subapical compartment (Figure 7). In MYO5B KO duodenum, Rab8a was significantly decreased from its normal subapical location and was instead localized to inclusions (Figure 7). Additionally, normal Rab11a localization was lost and only occasional inclusions were labeled with Rab11a in MYO5B KO duodenum (Figure 7). Loss of Rab11a from enterocytes has been shown to alter the localization of the apical SNARE protein, STX3.17 STX3 was limited to the subapical compartment in normal neonate duodenum. In MYO5B KO duodenum, STX3 was more dispersed into the cytoplasm as well as localized to some microvillus inclusions (Figure 7). Similar to the disruption of the apical trafficking proteins, the apical NHE3 was also mislocalized. While NHE3 was found strictly on the apical membrane in normal duodenum, in MYO5B KO mice, NHE3 was collapsed into a diffuse subapical localization as well as localized in inclusions (Figure 7). To examine the effects of MYO5B loss on a structural component of the apical microvilli, we immunostained MYO5B KO intestine for pERM. While the apical pERM localization was retained in MYO5B KO intestine, pERM also clearly labeled inclusions (Figure 7), further validating the presence of mature microvilli within the inclusions and suggesting the retention of apical microvilli on enterocytes along the villi.
Figure 7. Disruption of apical trafficking in MYO5B KO duodenum.
Control (top row) and MYO5B KO (bottom row) duodenal sections were immunostained for apical proteins (Rab8a, Rab11a, STX3, NHE3, and pERM). Apical trafficking proteins, Rab8a, Rab11a, and STX3 were subapically concentrated in control tissue with Rab8a also labeling along lateral membranes. Rab8a and Rab11a were lost from the apical membrane (asterisk) and occasionally localized to inclusions (arrowhead) in MYO5B KO duodenum. STX3 was collapsed into a broad and diffuse subapical region (asterisk and arrow) as well as localized to inclusions (arrowhead). The apical exchanger NHE3 was normally located on the apical membrane of enterocytes in the small intestine. Upon MYO5B loss, NHE3 was mislocalized to a diffuse subapical compartment (asterisk) and labels inclusions (arrowhead). Additionally, a microvilli structural protein, pERM, was examined to further investigate the status of microvilli in MYO5B KO tissue. pERM in control duodenum labeled only on the apical surface of the villus enterocytes. As with Ezrin, in MYO5B KO duodenum, pERM immunostaining identified microvillus inclusions inside enterocytes as well as the formation of microvillus inclusions at the apical surface of enterocytes (arrowhead). Scale bar = 50 μm.
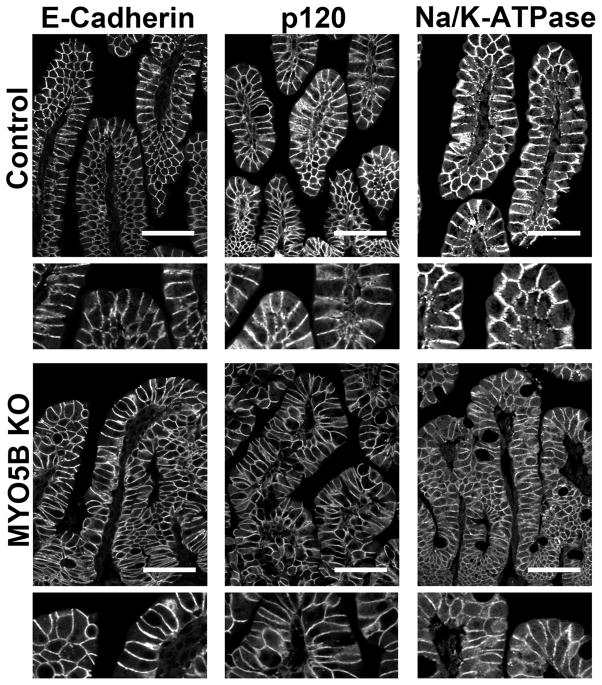
To study the effects of MYO5B loss on basolateral trafficking, we immunostained for E-cadherin, p120, and Na/K-ATPase. All 3 proteins were found on the basolateral membrane in control duodenum. This basolateral localization was maintained in MYO5B KO duodenum (Figure 8). Additionally, immunostaining of these 3 basolateral proteins highlighted the fusion of villi and the potential disorganization of enterocytes in these fused villi. This disorganization caused by villi fusion may confound the observation of subtle changes in basolateral trafficking. No changes were found for immunostaining of tight junction proteins (data not shown).
Figure 8. Immunofluorescence staining for basolateral trafficking.
Duodenum sections were immunostained for junctional proteins E-cadherin and p120 and the basolateral Na/K-ATPase. All three proteins were localized to the basolateral membrane in control duodenum (top row). Similar basolateral localization was observed in MYO5B KO duodenum (bottom row), thus showing no disruption in basolateral trafficking upon MYO5B loss. Scale bar = 50 μm.
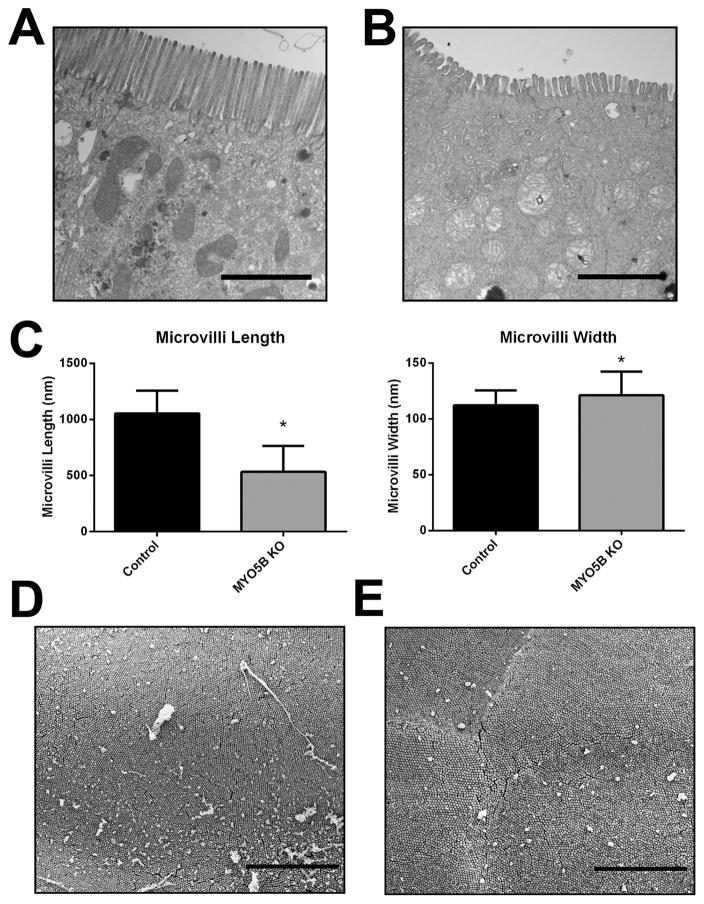
While germline deletion of MYO5B led to a disruption and mislocalization of a number of apical trafficking proteins, Ezrin and pERM staining suggests the presence of structurally intact apical microvilli in MYO5B KO mice. Thus, we performed TEM and scanning electron microscopy (SEM) to assess the presence of apical microvilli in the enterocytes. TEM of control duodenum showed long, straight microvilli along the apical surface (Figure 9A). Apical microvilli were retained in MYO5B KO duodenum (Figure 9B). However, similar to the previously reported Rab11a KO mouse,17, 18 MYO5B KO duodenum displayed significantly shortened and wider apical microvilli (Figure 9B and C). Although microvilli were shorter in comparison to control duodenum (Figure 9D), normal packing of microvilli was found in MYO5B KO duodenum (Figure 9E), suggesting that microvilli formation was not significantly altered upon loss of MYO5B in neonates.
Figure 9. TEM and SEM analysis of apical microvilli.
(A) Control duodenum was imaged by TEM. Images show long and straight microvilli on the apical surface of enterocytes. Scale bar = 2 μm. (B) TEM imaging of MYO5B KO duodenum revealed shortened apical microvilli. Scale bar = 2 μm. (C) The length and width of apical microvilli from control and MYO5B KO duodenum was measured. In MYO5B KO duodenum, microvilli were half the height of control microvilli. Additionally, microvilli were slightly wider in MYO5B KO duodenal enterocytes. *p-value < 0.05. (D) Control duodenum examined by SEM demonstrated tightly packed microvilli. Scale bar = 5 μm. (E) By SEM, tightly packed microvilli were visualized in MYO5B KO duodenum showing that packing remained largely unchanged even with shortened microvilli. Scale bar = 5 μm.
Disruption of apical trafficking, but not microvillus inclusion formation, is present in embryonic MYO5B KO duodenum
We examined embryonic (E18.5) duodenum from control and MYO5B KO littermates to determine the onset of the apical trafficking disruption and the formation of microvillus inclusions. Embryonic duodenum was immunostained for Ezrin, DPPIV, CD10, and NHE3. Embryonic control duodenum displayed strict apical localization of each marker (Figure 10), similar to that seen in neonatal control duodenum. While neonatal MYO5B KO duodenum displayed numerous Ezrin-positive inclusions (beginning at 1 day old), embryonic E18.5 MYO5B KO duodenum did not possess Ezrin-positive inclusions (Figure 10). However, mislocalization of the apical markers DPPIV, CD10, and NHE3 into a subapical compartment was observed in the embryonic KO duodenum (Figure 10).
Figure 10. Immunofluorescence staining of embryonic duodenum.
Embryonic (E18.5) duodenum from control and MYO5B KO littermates was immunostained for apical markers to examine mistrafficking and microvillus inclusion formation. As in neonates, all markers (Ezrin, DPPIV, CD10, and NHE3) labeled the apical membrane in control embryonic duodenum (top row). In contrast to neonates, Ezrin-positive inclusions were not observed in MYO5B KO duodenum suggesting that microvillus inclusions were not formed until after birth. However, DPPIV, CD10, and NHE3 staining was collapsed to a subapical localization (asterisk) not found in the control littermates. These findings show apical mistrafficking is present before birth, but microvillus inclusions likely do not form until after birth. Scale bar = 50 μm.
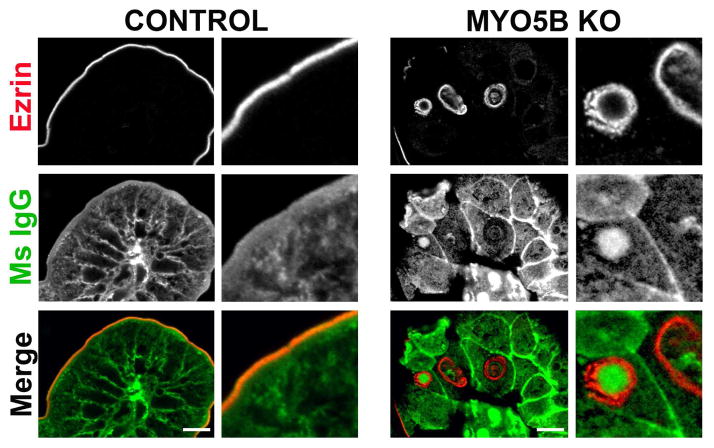
As previous studies have suggested that the duodenum is primed for maternal IgG uptake in neonatal rodents,19–21 we dual immunostained for murine IgG and Ezrin to assess the presence of maternal IgG inside the inclusions found in MYO5B KO neonates. Neonatal MYO5B KO duodenum sections were immunostained with an Alexa 488-conjugated goat anti-mouse IgG antibody to label native mouse IgG from maternal milk. No significant internal regions of concentrated IgG were found in control neonatal duodenum (Figure 11). In MYO5B KO duodenum, while not all inclusions immunostained for IgG, a prominent subset of Ezrin-positive inclusions did co-label with murine IgG (Figure 11). These findings support the origin of microvillus inclusions through internalization of apical membrane.
Figure 11. IgG internalization in Ezrin-positive inclusions.
Mouse IgG internalization was visualized by immunofluorescence staining for mouse IgG (green) and Ezrin (red). To detect endogenous mouse IgG, the Mouse on Mouse block kit could not be used, thus resulting in high background signal from interstitial cells. Nonetheless, no significant internal mouse IgG was observed in control duodenum (left panels). In contrast, in MYO5B KO duodenum, mouse IgG was found inside Ezrin-positive inclusions (right panels). Scale bar = 10 μm.
Germline MYO5B loss leads to crypt hyperproliferation and early maturation of Paneth cells
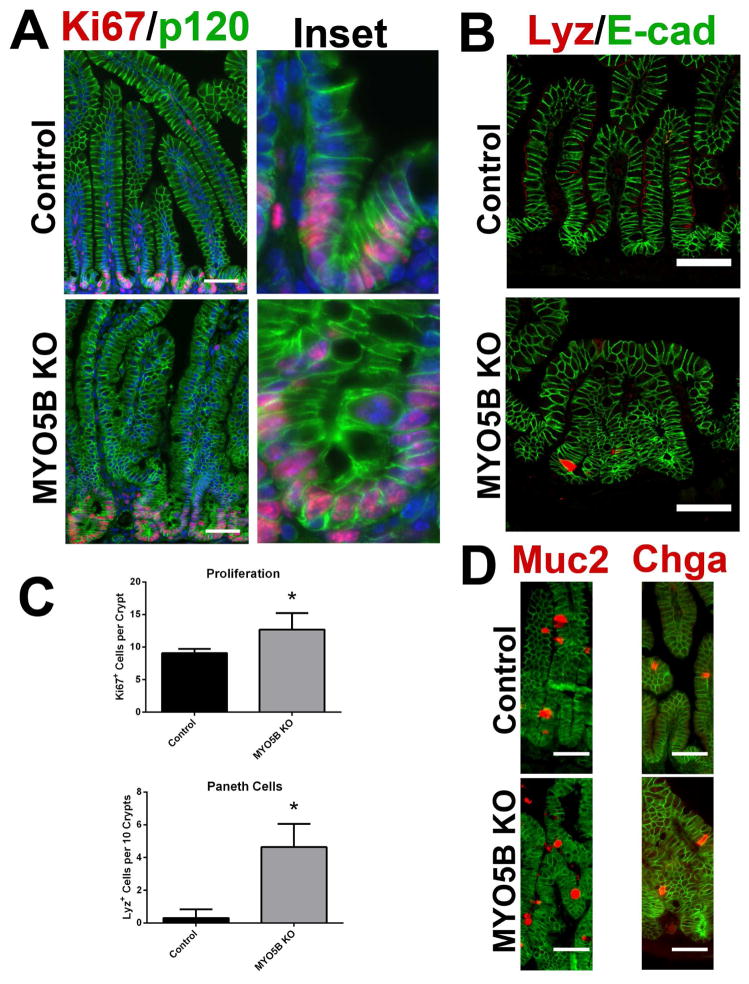
Because MYO5B loss results in aberrant villi structure, we examined the effects of MYO5B loss on proliferation and the cell lineages in the duodenum. Ki67 immunostaining demonstrated a significant increase in proliferating cells within MYO5B KO crypts as compared to control crypts (12 cells per crypt versus 9 cells per crypt, respectively) (Figure 12A and C). No proliferating cells were observed outside of crypts in MYO5B KO duodenum. Lysozyme immunostaining was used to assess the presence of Paneth cells in the neonatal mice. Paneth cells do not normally mature in neonatal mice until 14 days of age.22 Accordingly, in control duodenum, only rare Lysozyme-positive Paneth cells were observed (Figure 12B and C). In MYO5B KO duodenum, the early maturation of Paneth cells within the crypts was seen (Figure 12B and C). In contrast to the crypt, no significant differences were observed in the differentiation of secretory cell lineages along the villi including enteroendocrine cells (labeled by chromogranin A) and goblet cells (labeled by Muc2) (Figure 12D).
Figure 12. Proliferation and cell lineages in MYO5B KO duodenum.
(A) Ki67 was utilized as a marker of proliferation. Sections were stained for Ki67 (red) and p120 (green). Ki67-positive proliferating cells were confined to the short crypts in control mice. In MYO5B KO mice, an increase in proliferation was observed as well as an expansion of the crypt. DAPI (blue). Scale bar = 50 μm. (B) To determine the status of Paneth cells, sections were immunostained for Lysozyme (red) and E-cad (green). A premature maturation of Paneth cells was observed in MYO5B KO duodenum. Scale bar = 50 μm (C) The number of Ki67-positive proliferating cells per crypt was quantified for both control and MYO5B KO duodenum. A small but significant increase in proliferation was found in MYO5B KO duodenum. *p-value = 0.05. As seen in panel B, Lysozyme-positive Paneth cells appear earlier in MYO5B KO than in control littermates. While less than 1 Paneth cell per 10 crypts was found in control duodenum, more than 4 Paneth cells per 10 crypts were counted in MYO5B KO duodenum *p-value = 0.05. (D) Tissue sections were co-stained for lineage markers Muc2 or Chga (red) and p120 (green). Goblet cells (Muc2-positive) and enteroendocrine cells (Chga-positive) were present in both control and MYO5B KO tissue. Scale bar = 50 μm.
Intestine-targeted MYO5B deletion in mice recapitulates the pathology in germline MYO5B KO mice
To examine the role of MYO5B specifically in the intestine, we generated intestine-targeted MYO5B deletion mice by crossing VillinCre mice to MYO5BF/F to produce VillinCre;MYO5BF/F mice. In contrast to the germline MYO5B KO mice, viable VillinCre;MYO5BF/F pups were obtained on a C57BL/6 background. MYO5B immunostaining in intestinal sections confirmed loss of MYO5B along the length of the small intestine (Figure 13A) and colon (data not shown). Nevertheless, VillinCre;MYO5BF/F failed to thrive and did not survive past 6 days of age. While indistinguishable from control littermates at birth, by 5 days of age, VillinCre;MYO5BF/F mice displayed evidence of dehydration and were visibly smaller (Figure 13B and C). No differences in weight or pathology were observed between MYO5BF/F and VillinCre;MYO5BF/+ mice, and thus both were used as controls in further studies.
Figure 13. Characterization of VillinCre;MYO5BF/F intestine.
(A) Proximal and distal small intestine from control and VillinCre;MYO5BF/F mice was immunostained for MYO5B (green) and Na/K-ATPase (red). A clear subapical concentration of MYO5B was observed in control tissue, however this immunoreactivity was lost in VillinCre;MYO5BF/F intestine (proximal and distal). Of note, no significant changes were observed in Na/K-ATPase expression and localization. Scale bar = 25 μm. (B) An image of a VillinCre;MYO5BF/F mouse and a control littermate mouse show an obvious size difference. (C) Mice weighed at 5 days old showed that VillinCre;MYO5BF/F mice were approximately half the weight of control littermates. *p-value = 0.05 (D) Ezrin immunofluorescence revealed fused villi (green arrow) and the presence of Ezrin-positive inclusions in VillinCre;MYO5BF/F proximal small intestine. However, only occasional inclusions were observed in the VillinCre;MYO5BF/F distal small intestine and rarely fused villi (lower right panel). Scale bar = 50 μm. (E) Similar to germline MYO5B KO mice, VillinCre;MYO5BF/F mice showed a gradient of phenotype from proximal to distal small intestine. Approximately 5 fused villi per 500 μm length of proximal intestine was measured, which decreased to only rare fused villi in the distal small intestine. Likewise, the VillinCre;MYO5BF/F small intestine displayed a significantly fewer inclusions in the distal small intestine compared to the proximal. Again, over 98% of all Ezrin-positive inclusions also labeled with CD10 or DPPIV with no significant differences found between the proximal and distal small intestine. *p-value = 0.05.
Ezrin immunostaining in VillinCre;MYO5BF/F duodenum showed fused villi at a rate of 5.4 fused villi per 500 μm length of intestine (Figure 13D and E). In addition, Ezrin-positive inclusions were found within the enterocytes along the villi in the proximal small intestine (averaging 82 Ezrin-positive inclusions per 500 μm length of intestine) (Figure 13D and E). We then examined the number of fused villi and Ezrin-positive inclusions in the distal small intestine of VillinCre;MYO5BF/F mice. Similar to MYO5B KO mice, a decreasing gradient was observed between the proximal and distal small intestine. Only rare fused villi were observed in the VillinCre;MYO5BF/F mouse distal small intestine and the presence of Ezrin-positive inclusions was significantly less frequent (less than 5 Ezrin-positive inclusions per 500 μm length of intestine) (Figure 13D and E). However, regardless of the location of the Ezrin-positive inclusions (proximal versus distal), >97% of all Ezrin-positive inclusions co-labeled with CD10 and DPPIV (Figure 13E).
VillinCre;MYO5BF/F mice display disruption of apical trafficking, but not basolateral protein localization or microvilli formation
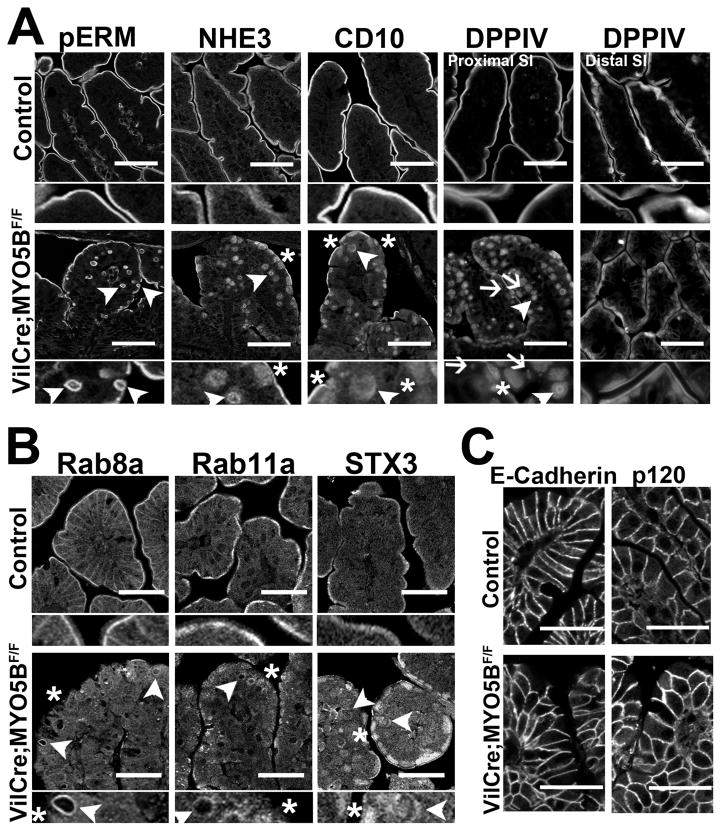
While normal localization of pERM to the apical brush border was retained in VillinCre;MYO5BF/F mouse duodenum, pERM-positive inclusions were also observed in the cytoplasm of enterocytes along the villi (Figure 14A). Similar to the apical trafficking disruption found in germline MYO5B KO mice, strict apical localization of NHE3, CD10, and DPPIV was lost in VillinCre;MYO5BF/F mouse enterocytes (Figure 14A). Localization of these proteins expanded into a more diffuse subapical region as well as localizing to the internal inclusions found in VillinCre;MYO5BF/F mouse duodenum. DPPIV was also dispersed into punctate vesicles within the enterocyte cytoplasm (Figure 14A). Examination of DPPIV localization in VillinCre;MYO5BF/F mouse distal small bowel demonstrated near absence of the internal DPPIV-positive punctate vesicles found in the proximal small intestine of VillinCre;MYO5BF/F mice (Figure 14A). While a more diffuse subapical DPPIV localization was noted in the distal small intestine of VillinCre;MYO5BF/F mice than in controls, the clear presence of apical brush border DPPIV localization, in addition to the lack of DPPIV-positive inclusions and punctate vesicles, further demonstrates the presence of a gradient of pathological changes upon loss of MYO5B along the small intestine.
Figure 14. Disruption of apical trafficking in VillinCre;MYO5BF/F duodenum.
(A) Control (top row) and VillinCre;MYO5BF/F (bottom row) duodenal sections were immunostained for apical proteins (pERM, NHE3, CD10 and DPPIV). pERM staining in control duodenum demonstrated microvilli located only on the apical surface of the villi enterocytes. In VillinCre;MYO5BF/F duodenum, pERM immunostaining identified microvillus inclusions inside enterocytes as well as the formation of microvillus inclusions at the apical surface of enterocytes (arrowhead). The apical exchanger NHE3 was normally located on the apical membrane of enterocytes in the small intestine. Upon MYO5B loss, NHE3 was mislocalized to a diffuse subapical compartment (asterisk) and labeled in inclusions (arrowhead). CD10 and DPPIV both labeled the apical brush border in control mice. In VillinCre;MYO5BF/F duodenum, CD10 and DPPIV were relocated into a diffuse subapical localization (asterisk) as well as into internal inclusions (arrowheads). DPPIV was also found localized to small punctate vesicles (arrows). In VillinCre;MYO5BF/F distal small intestine (last column), while some DPPIV subapical accumulation was observed, no apparent overall difference was noted in comparison to control. Scale bar = 50 μm. (B) Apical trafficking proteins, Rab8a, Rab11a and STX3 were subapically concentrated in control tissue with Rab8a also labeling along lateral membranes. Rab8a and Rab11a were lost from the apical membrane with a small accumulation in the subapical regions (asterisk) and occasionally localized to inclusions (arrowheads) in VillinCre;MYO5BF/F duodenum. STX3 was collapsed into a diffuse subapical region (asterisk) as well as localized to inclusions (arrowheads). Scale bar = 50 μm. (C) Control and VillinCre;MYO5BF/F duodenum were immunostained for E-cadherin and p120. In both controls and VillinCre;MYO5BF/F duodenum, E-cadherin and p120 labeled the basolateral membranes with no obvious differences between them. Scale bar = 50 μm.
To examine the effect of MYO5B loss on apical trafficking and polarity, VillinCre;MYO5BF/F mouse duodenum was immunostained for the apical trafficking proteins Rab8a, Rab11a, and STX3. In control duodenum, Rab8a, Rab11a, and STX3 were concentrated to the subapical region in enterocytes, while Rab8a also had distribution along the lateral membrane (Figure 14B). In VillinCre;MYO5BF/F mouse duodenum, Rab8a and Rab11a occasionally localized to inclusions, but overall were predominately dispersed throughout the cytoplasm or lost in enterocytes (Figure 14B). Immunostaining for STX3 showed relocation into a more diffuse subapical distribution, as well as localization in the inclusions (Figure 14B).
The effects of MYO5B loss on basolateral trafficking were examined by immunostaining for Na/K-ATPase, E-cadherin, and p120. Loss of MYO5B from VillinCre;MYO5BF/F intestine did not significantly affect Na/K-ATPase localization to the basolateral membrane (Figure 13A). Similarly, normal localization of E-cadherin and p120 to the lateral membranes was observed in VillinCre;MYO5BF/F mouse duodenum (Figure 14C). While, as previously noted, disruption of villi structure and resulting disorganization of enterocytes may obscure subtle changes in localization of these basolateral proteins, no significant changes were found in the localization of basolateral proteins.
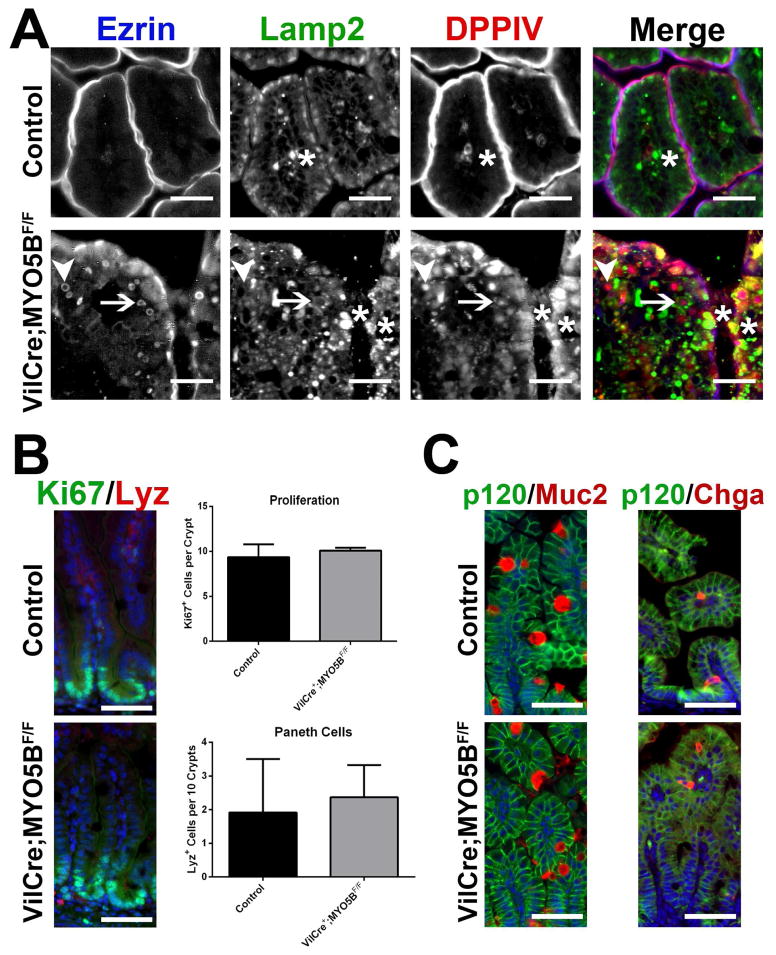
We next examined if internal Ezrin-positive inclusions or the internalized DPPIV were co-localized with Lamp2-positive lysosomes. While some Ezrin-positive inclusions did not localize near Lamp2 expression, other Ezrin-positive inclusions were surrounded or ‘cupped’ by Lamp2-positive lysosomes, but did not directly co-localize with Lamp2 (Figure 15A). The two populations of internalized DPPIV not associated with Ezrin-positive inclusions (both diffuse subapical DPPIV and DPPIV in small punctate vesicles) both co-labeled with Lamp2 (Figure 15A). These data show that at least a portion of the internalized apical proteins are likely diverted to lysosomes.
Figure 15. Localization of lysosomes and characterization of cellular make-up in villi.
(A) Duodenum from control and VillinCre;MYO5BF/F duodenum was triple-stained for Ezrin (blue), Lamp2 (green), and DPPIV (red). In control enterocytes, Lamp2-positive lysosomes concentrated just under the Ezrin and DPPIV-positive apical surface. In VillinCre;MYO5BF/F duodenum, a subset of Ezrin-positive inclusions were closely surrounded or ‘cupped’ by Lamp2-positive lysosomes (arrow). However, other Ezrin-positive inclusions were not located near Lamp2 (arrowhead). DPPIV internalized into small vesicles co-localized with Lamp2 (asterisks). Scale bar = 25 μm. (B). Control and VillinCre;MYO5BF/F duodenum was analyzed for proliferating cells (Ki67 in green) and the early maturation of Paneth cells (Lysozyme in red). No significant differences were found between the two mouse strains. DAPI (blue) Scale bar = 50 μm. (C) Sections were immunostained for Muc2 (red, left panel) and Chga (red, right panel) with p120 (green). Similar to the germline MYO5B KO, no significant difference was observed between the control littermates and VillinCre;MYO5BF/F duodenums. DAPI (blue). Scale bar = 50 μm.
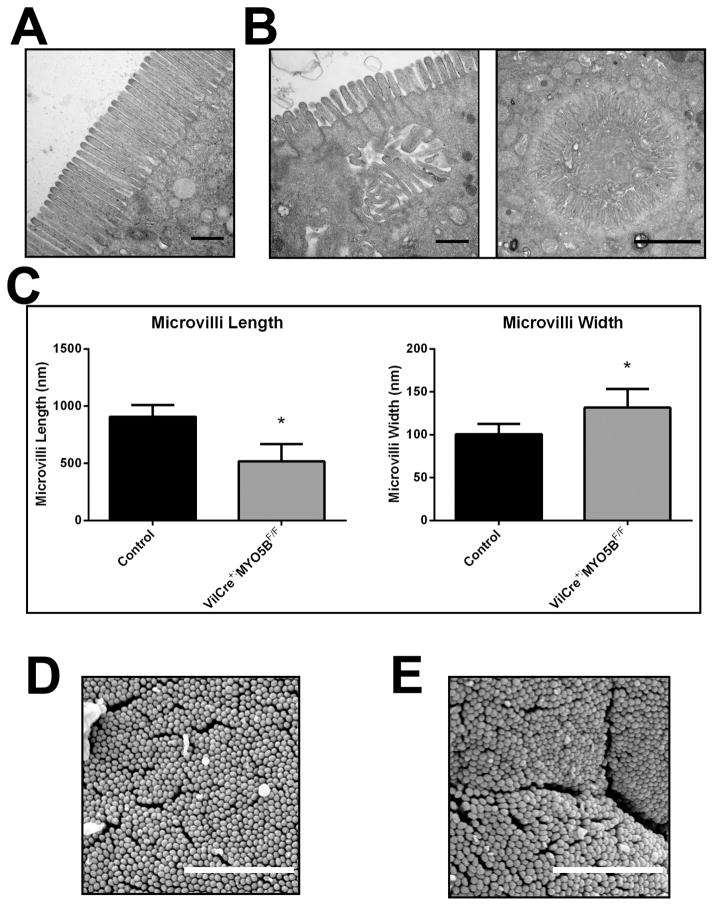
The ultrastructure of VillinCre;MYO5BF/F mouse duodenal enterocytes was analyzed by SEM and TEM. While VillinCre;MYO5BF/F mouse enterocytes maintained apical microvilli, these apical microvilli were significantly shorter and wider than microvilli found in control enterocytes (Figure 16A, B, and C). Additionally, inclusions with mature microvilli were frequently observed in VillinCre;MYO5BF/F enterocytes by TEM (Figure 16B). Although apical microvilli in VillinCre;MYO5BF/F enterocytes were significantly shorter, SEM showed that this shortened length did not significantly affect packing or organization (Figure 16D and E).
Figure 16. TEM and SEM analysis of apical microvilli in VillinCre;MYO5BF/F mice.
(A) Control duodenum was imaged by TEM. Images showed long and straight microvilli on the apical surface of enterocytes. Scale bar = 500 nm. (B) TEM imaging of VillinCre;MYO5BF/F duodenum revealed shortened apical microvilli and the presence of internal inclusions with obvious microvilli. Scale bar = 500 nm (left panel). TEM analysis also revealed internal microvillus inclusions with fully formed microvilli. Scale bar = 2 μm (right panel) (C) The length and width of apical microvilli from control and VillinCre;MYO5BF/F duodenum was measured. In VillinCre;MYO5BF/F duodenum, microvilli were significantly shorter than control microvilli. Additionally, microvilli were also wider in VillinCre;MYO5BF/F duodenal enterocytes. *p-value < 0.05. (D) Control duodenum examined by SEM demonstrated tightly packed microvilli. Scale bar = 2 μm. (E) By SEM, tightly packed microvilli were visualized in VillinCre;MYO5BF/F duodenum showing that packing remained largely unchanged even with shortened microvilli. Scale bar = 2 μm.
VillinCre;MYO5BF/F mice do not display changes in proliferation or cell lineage differentiation
Proliferation in VillinCre;MYO5BF/F duodenal sections was examined by immunostaining for Ki67. As in control duodenum, proliferating cells were confined within crypts (Figure 15B). No significant difference was found in the number of proliferating crypt cells between control and VillinCre;MYO5BF/F intestines (Figure 15B). In contrast to germline MYO5B KO intestine, only rare Lysozyme-positive Paneth cells were observed in VillinCre;MYO5BF/F duodenum (Figure 15C). Similarly, no significant differences in the presence of goblet cells or enteroendocrine cells in VillinCre;MYO5BF/F duodenum were observed (Figure 15C).
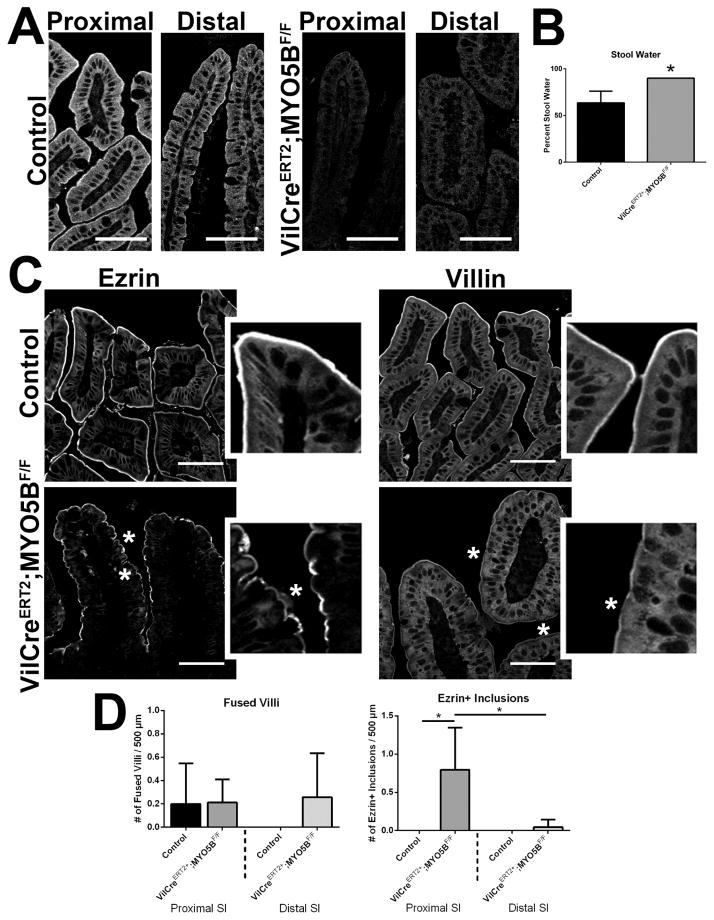
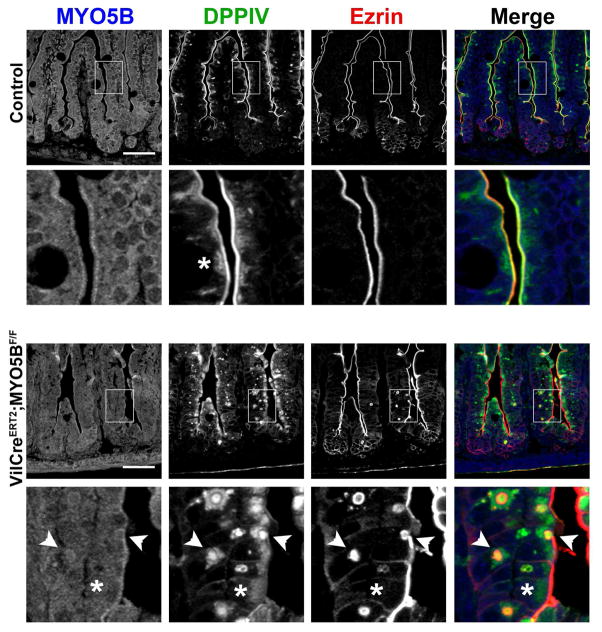
Prominent mistrafficking and apical microvilli loss, but not microvillus inclusions, are the predominate pathologies of inducible intestinal targeted MYO5B deletion in adult mice
Inducible VillinCreERT2;MYO5BF/F C57BL/6 mice were obtained by crossing MYO5BF/F mice to VillinCreERT2 mice. Induction of MYO5B loss in VillinCreERT2;MYO5BF/F mice was achieved by administration of one intraperitoneal injection of 2 mg of Tamoxifen to 8–10 week old mice. Mice displayed signs of distress and were sacrificed 3 days after the Tamoxifen injection. Tamoxifen-injected MYO5BF/F mice were used as controls. MYO5B loss in both proximal and distal small intestine was confirmed by MYO5B immunostaining (Figure 17A). While no weight differences were observed between VillinCreERT2;MYO5BF/F and control mice, VillinCreERT2;MYO5BF/F mice did develop diarrhea as confirmed by a significantly higher percentage of water present in the stool (Figure 17B). Based on our findings from the neonatal loss of MYO5B, we first examined VillinCreERT2;MYO5BF/F mouse small intestines by Ezrin immunostaining. In In the tamoxifen-induced VillinCreERT2;MYO5BF/F mouse duodenum, Ezrin was also found on the apical microvilli, although it appeared as a fainter thinner band along the apical surface or even was completely lost in some cells (Figure 17C). Notably, only occasional Ezrin-positive inclusions were seen. Indeed, less than one Ezrin-positive inclusion was found per 500 μm length of duodenum (and only rarely in the distal small intestine) (Figure 17D). Additionally, no evidence for fused villi was found in either proximal or distal VillinCreERT2;MYO5BF/F small intestine (Figure 17D).
Figure 17. Characterization of VillinCreERT2;MYO5BF/F mouse small intestine in 8-week-old mice induced with tamoxifen.
8 week old VillinCreERT2;MYO5BF/F mice were treated with a single 2 mg i.p. dose of tamoxifen to induce intestine KO of MYO5B. (A) Proximal and distal small intestine from control and VillinCreERT2;MYO5BF/F mice were immunostained for MYO5B. A clear subapical concentration of MYO5B was observed in control tissue, however MYO5B immunofluorescence was lost in VillinCreERT2;MYO5BF/F mice in both proximal and distal intestine. Scale bar = 50 μm. (B) The percentage of stool constituted by water was determined in control and VillinCreERT2;MYO5BF/F mice. VillinCreERT2;MYO5BF/F mice had significantly higher water content in stool collected from the colon (90% stool water). *p-value = 0.05. (C) Ezrin immunostaining in control mice showed fully formed brush border along the length of the villi. The inset shows a strong sharp band of Ezrin-positive brush border. In VillinCreERT2;MYO5BF/F duodenum, Ezrin labeled a thin band along the brush border suggesting a shortening or loss of apical microvilli (asterisk). Of note, only occasional Ezrin-positive inclusions were observed in VillinCreERT2;MYO5BF/F mouse intestines. Similarly, Villin labeled a bright and wide brush border in control duodenums. In VillinCreERT2;MYO5BF/F duodenums, the apical Villin stain was much thinner and even absent in some locations (asterisk) with only rare inclusions observed. Scale bar = 50 μm. (D) Fused villi and the total number of Ezrin-positive inclusions were quantified. No difference was observed in the number of fused villi in control and VillinCreERT2;MYO5BF/F proximal and distal small intestine. There was a significant increase in the number of Ezrin-positive inclusions as compared to control in the proximal intestine. However, the total number of inclusions was only 0.75 Ezrin-positive inclusions per 500 μm of tissue in VillinCreERT2;MYO5BF/F duodenums. *p-value = 0.05.
Because Ezrin immunostaining suggested a shortened or even lost brush border with only a few intracellular inclusions in VillinCreERT2;MYO5BF/F mouse duodenum, we then immunostained for another microvilli brush border structural protein, Villin. In control duodenum, Villin lined the wide brush border along the apical surface of enterocytes (Figure 17C). While Villin was still localized to the apical surface in VillinCreERT2;MYO5BF/F duodenum, it labeled a thinner band along the enterocyte apical surface and was even absent in some regions (Figure 17C). Again, only occasional Villin-positive inclusions were found within the enterocytes. These findings suggest that the apical brush border was disrupted upon MYO5B loss in adult mice, but microvillus inclusion formation (as observed by Ezrin or Villin immunoreactivity) could not account for the diarrhea and dehydration observed in VillinCreERT2;MYO5BF/F mice.
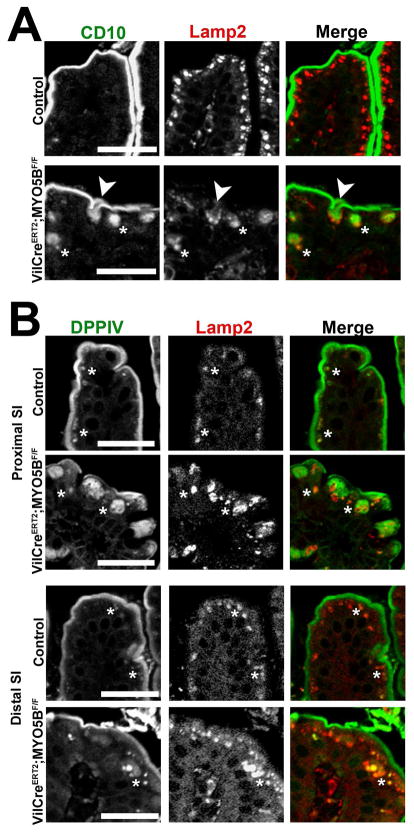
To examine the localization of nonstructural apical components, duodenum sections from tamoxifen-induced adult VillinCreERT2;MYO5BF/F mice were immunostained for CD10 and DPPIV. Loss of MYO5B in VillinCreERT2;MYO5BF/F mouse duodenum resulted in the mislocalization of both CD10 and DPPIV. CD10 was collapsed into a broad and diffuse subapical localization as well as diverted to vesicular structures (Figure 18A). DPPIV was also mislocalized to a subapical region, in addition to localization in punctate vesicles inside enterocytes (Figure 18B). As in neonates, Lamp2-postive lysosomes were concentrated to a narrow subapical region just under the CD10- and DPPIV-labeled brush border in control adult duodenum. However, in VillinCreERT2;MYO5BF/F mouse duodenum, a subset of internalized CD10 co-localized with Lamp2 (Figure 18A). Similarly, a portion of internalized DPPIV also co-localized with Lamp2 (Figure 18B). Thus, upon MYO5B loss, in the duodenum internalized apical proteins, CD10 and DPPIV, were relocalized to Lamp2-postive lysosomes. Nevertheless, little internalized DPPIV was observed in the enterocytes of the distal small intestine (Figure 18B).
Figure 18. Internalization and trafficking of CD10 and DPPIV to lysosomes.
(A) Duodenum from control and VillinCreERT2;MYO5BF/F mice were co-stained for CD10 (green) and Lamp2 (red). In control enterocytes, Lamp2-positive lysosomes were located just under the CD10-labeled apical surface. Upon induced MYO5B loss, a subset of CD10 was internalized (asterisks and arrow). Lamp2 was found surrounding CD10 as it was internalized from the apical surface (arrow), and in addition Lamp2 co-localized with internal CD10 populations (asterisks). Scale bar = 25 μm. (B) Proximal and distal small intestine from controls and VillinCreERT2;MYO5BF/F mice were immunolabeled for DPPIV (green) and Lamp2 (red). In both proximal and distal small intestine controls, DPPIV predominately resided at the brush border, but a small DPPIV population was found in the subapical region co-localized with Lamp2 (asterisks). In VillinCreERT2;MYO5BF/F duodenum, DPPIV was collapsed into a diffuse subapical region as well as to small punctate vesicles (asterisks). The DPPIV-labeled vesicles, along with a portion of the diffuse DPPIV staining, co-labeled with Lamp2 (asterisks). In VillinCreERT2;MYO5BF/F distal small intestine, most DPPIV was retained on the apical membrane with a small subset of internalized DPPIV found in the small vesicles (asterisk). As in the duodenum, this internalized DPPIV population co-labeled with Lamp2 (asterisk). Scale bar = 25 μm.
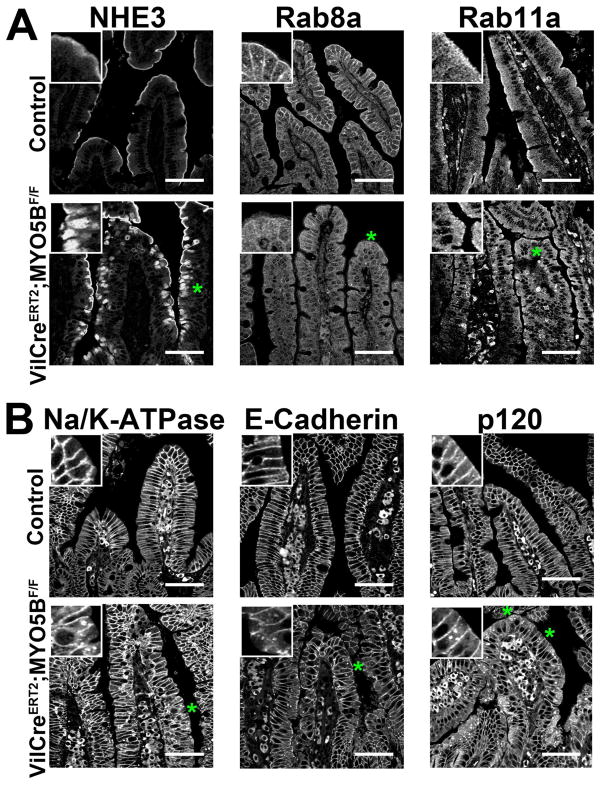
As apical proteins were mislocalized in tamoxifen-induced VillinCreERT2;MYO5BF/F mouse duodenum, disruption of apical trafficking and polarity was investigated. In VillinCreERT2;MYO5BF/F mouse duodenum, NHE3 was mislocalized to a diffuse and broad subapical compartment (Figure 19A). To further investigate apical trafficking, Rab8a and Rab11a expression and distribution were assessed. The predominant subapical concentration of Rab8a was lost in VillinCreERT2;MYO5BF/F enterocytes. Instead, Rab8a was more dispersed in a cytoplasmic vesicular distribution, although some Rab8a was still found subapically and along the lateral membrane (Figure 19A). Similarly, Rab11a was also redistributed from the subapical region to diffuse cytoplasmic vesicles (Figure 19A).
Figure 19. Disruption of apical and basolateral trafficking in VillinCreERT2;MYO5BF/F mice.
(A) Control and VillinCreERT2;MYO5BF/F duodenum was stained for an apical exchanger protein (NHE3) and for apical trafficking proteins (Rab8a and Rab11a). The normal apical localization of NHE3 (top left panel) found in control duodenum was collapsed to a diffuse pattern in the cytoplasm in VillinCreERT2;MYO5BF/F duodenum (green asterisk). Correspondingly, the normal concentrated subapical localization of Rab8a and Rab11a was disrupted in VillinCreERT2;MYO5BF/F duodenum (green asterisks). In VillinCreERT2;MYO5BF/F duodenum, Rab8a and Rab11a was prominently lost from subapical region and diverted to a very diffuse cytoplasmic localization. Scale bar = 50 μm. (B) Basolateral trafficking was analyzed by immunostains for Na/K-ATPase, E-cadherin, and p120 in control and VillinCreERT2;MYO5BF/F duodenum. In control duodenum, all three proteins were tightly localized to the basolateral membrane of enterocytes. While some normal basolateral localization was retained in VillinCreERT2;MYO5BF/F duodenum, aberrant small internal punctate vesicles were also observe for Na/K-ATPase, E-cadherin, and p120 (green asterisks and insets) suggesting that loss of MYO5B disrupts basolateral trafficking. Scale bar = 50 μm.
While our studies here have shown that MYO5B loss in neonates did not cause significant changes in basolateral protein localization, previous studies have reported disruption of basolateral trafficking upon MYO5B loss.2, 3, 8, 9, 11 Again, Na/K-ATPase, E-Cadherin, and p120 immunostaining was assessed as an indicator of disruption of basolateral trafficking. In VillinCreERT2;MYO5BF/F mouse enterocytes, Na/K-ATPase was still localized to the basolateral membrane, however, it was also observed in cytoplasmic vesicles in some cells (Figure 19B). Similarly, E-cadherin was also mislocalized to cytoplasmic vesicles in addition to the normal basolateral localization (Figure 19B). The most prominent effect of MYO5B loss on basolateral proteins was the mislocalization of p120. Loss of MYO5B in adult duodenum resulted in p120 mislocalization to cytoplasmic vesicles similar to that found with Na/K-ATPase and E-Cadherin (Figure 19B). Additionally, p120 was also dispersed into a diffuse cytoplasmic distribution in some enterocytes (Figure 19B). These findings suggest that MYO5B loss in adult duodenum can disrupt basolateral trafficking dynamics.
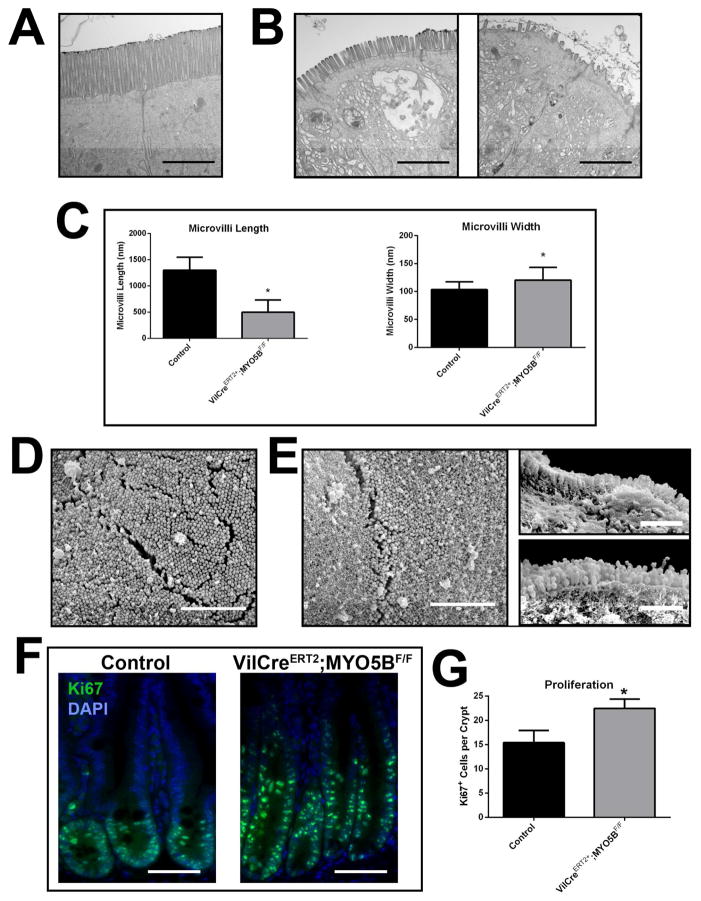
Based on our evidence of diminished Ezrin and Villin immunostaining of the brush border in adult VillinCreERT2;MYO5BF/F mouse enterocytes, we performed TEM and SEM analysis to assess the status of apical microvilli. By TEM analysis, we observed a heterogeneous pattern for microvilli upon MYO5B loss in adult duodenal enterocytes. Some areas of enterocytes had shorter and wider microvilli in tamoxifen-induced VillinCreERT2;MYO5BF/F enterocytes (Figure 20A, B, and C), similar to the pattern found in the neonatal MYO5B loss models. In contrast to the neonates, some regions of enterocytes displayed only few and sparse microvilli or even complete loss of microvilli (Figure 20B). In areas of short microvilli, the microvilli remained normally packed as assessed by SEM (Figure 20E). Cross-sections of other regions revealed malformed microvilli that appeared to be pinched into segments (Figure 20E). This analysis of ultrastructure confirmed that MYO5B loss in adult enterocytes resulted in aberrant microvilli structure or even microvilli loss in some enterocytes. This disturbance of the brush border, in addition to the apical and basolateral mistrafficking, may contribute to the resulting diarrhea seen after induction of MYO5B loss in adult VillinCreERT2;MYO5BF/F mice.
Figure 20. Proliferation and EM analysis of VillinCreERT2;MYO5BF/F duodenum.
(A) TEM images of control duodenum showed long and straight microvilli on the apical surface of enterocytes. Scale bar = 2 μm. (B) By TEM analysis, VillinCreERT2;MYO5BF/F duodenum revealed varying aberrations in the apical microvilli. Frequent areas of shortened apical microvilli (left panel) were observed as well as patches of apical microvilli denudation (right panel). Scale bar = 2 μm. (C) The length and width of apical microvilli from control and VillinCreERT2;MYO5BF/F duodenal enterocytes was quantified. In VillinCreERT2;MYO5BF/F duodenal enterocytes, microvilli that were observed were significantly shorter than control microvilli. Additionally, apical microvilli were also wider in VillinCreERT2;MYO5BF/F duodenal enterocytes. *p-value < 0.05. (D) Control duodenum examined by SEM demonstrated tightly packed microvilli. Scale bar = 2 μm. (E) By SEM, areas of tightly packed microvilli were visualized in VillinCreERT2;MYO5BF/F duodenum as were areas of shortened microvilli. Scale bar = 2 μm (left panel) Additionally, SEM analysis of some cross-sectioned enterocytes further confirmed areas of short microvilli (top right panel, Scale bar = 1 μm) as well as revealed aberrant microvilli morphology such as a ‘linked’ appearance (bottom right panel, Scale bar = 1 μm). (F) Proliferation in control and VillinCreERT2;MYO5BF/F duodenum was examined via Ki67 (green) and DAPI (blue) immunofluorescence. Proliferation (noted by Ki67 immunoreactivity) was restricted to the crypts in both control and VillinCreERT2;MYO5BF/F duodenum. Scale bar = 50 μm. (G) The number of Ki67-positive cells per crypt was quantified for comparison. A significant increase in proliferation was seen in VillinCreERT2;MYO5BF/F duodenal crypts compared to control duodenal crypts. *p-value = 0.05.
VillinCreERT2;MYO5BF/F mice exhibit crypt hyperproliferation
VillinCreERT2;MYO5BF/F duodenum sections were immunostained for Ki67 to examine the effect of MYO5B loss on crypt cells. A significant increase in proliferating cells within VillinCreERT2;MYO5BF/F crypts was observed as compared to control crypts (22 cells per crypt to 15 cells per crypt, respectively) (Figure 20F and G). These findings resembled the hyperproliferation observed in germline MYO5B KO mice. No proliferating cells were observed outside of crypts in VillinCreERT2;MYO5BF/F duodenum.
Induced MYO5B loss in VillinCreERT2;MYO5BF/F neonates causes microvillus inclusion formation
Together, our studies of both neonate and adult MYO5B loss suggest that the neonatal duodenum is primed for microvillus inclusion formation, consistent with the relative absence of inclusions after induction of MYO5B loss in adults. To test this hypothesis, we induced MYO5B loss in 2 day old VillinCreERT2;MYO5BF/F and sacrificed the neonates 3 days later (the same timeline used in adult VillinCreERT2;MYO5BF/F mouse studies). MYO5B loss was achieved in the bottom portion of most villi (Figure 21). As seen in the other neonatal MYO5B loss models as well as in VillinCreERT2;MYO5BF/F adult mice, DPPIV was mislocalized to a diffuse subapical region upon MYO5B loss (Figure 21). Ezrin immunostaining also revealed the development of numerous Ezrin-positive inclusions in the same enterocytes with DPPIV mislocalization. The number of Ezrin-positive inclusions more closely resembled the results found in the germline or constitutive VillinCre directed neonatal MYO5B KO mice.
Figure 21. Neonatal induction of MYO5B loss in VillinCreERT2;MYO5BF/F mice.
Tamoxifen induced MYO5B loss in 2 day old littermates of controls (top row and second inset row) and VillinCreERT2;MYO5BF/F mice (third row and bottom inset row) was examined to analyze the formation of microvillus inclusions in neonates as compared to adult VillinCreERT2;MYO5BF/F mice. Sections of duodenum were triple-stained for MYO5B (blue), DPPIV (green), and Ezrin (red). In controls, MYO5B localized to the subapical region just below the DPPIV and Ezrin-positive co-labeled brush border. Some diffuse internal DPPIV is observed in controls (asterisk). In VillinCreERT2;MYO5BF/F neonatal duodenum, the prominent MYO5B subapical localization was lost in the bottom portion of villi. Of note, MYO5B was not completely lost from the upper half of villi. DPPIV was lost from the apical membrane and collapsed into a prominent, diffuse subapical region (asterisk). Furthermore, in contrast to adult VillinCreERT2;MYO5BF/F mice, VillinCreERT2;MYO5BF/F neonates formed numerous internal Ezrin-positive inclusions in the duodenum (arrowheads) that co-labeled with DPPIV. These inclusions resemble the microvillus inclusions found in in MYO5B KO and VillinCre;MYO5BF/F neonatal mice. Scale bar = 50 μm.
Discussion
The present results suggest that the microvillus inclusions associated with loss of functional MYO5B are not central to the pathological symptoms of Microvillus Inclusion Disease. Thus, induction of MYO5B loss in adult mice led to rapid development of diarrhea, but did not induce the formation of significant numbers of microvillus inclusions. Nevertheless, germline and constitutive targeted knockout of MYO5B did demonstrate clear microvillus inclusion formation in the duodenum. In addition, in conditional KO mice induced at 2 days of age, we also observed formation a prominent numbers of inclusions in the duodenum. It therefore appears likely that the deletion of MYO5B in the neonatal period has exposed a critical apical membrane internalization paradigm that is specifically active in the neonatal period. The most likely pathway revealed would be an apical macropinocytotic route, as we previously demonstrated in CaCo2-BBE cells.9 Internalization and transcytosis of maternal IgG’s in the neonatal period is a critical process for immune protection of newborns.23 Previous investigations in rats have noted that this pathway is substantially down-regulated after weaning.24 The relative absence of microvillus inclusions in conditional allele mice induced at eight weeks of age suggests that this apical macropinocytotic pathway is abolished after the neonatal period. Importantly, we have demonstrated here that microvillus inclusions in MYO5B germline knockout mice contained IgG. This apical macropinocytotic route may complement during the neonatal period the previously described internalization pathways that may provide for ongoing IgG internalization in the distal intestine and after weaning.25 These findings support the origin of microvillus inclusions as macropinocytotic events and electron micrographs demonstrate the formation of the inclusions from invaginations of the apical membrane. Our previous studies in CaCo2-BBE cell MYO5B knockdown and restitution studies have demonstrated the origin of microvillus inclusions from internalization of apical membrane.9 Interestingly, previous studies have demonstrated that FcRN internalization of IgG in CaCo2 cells occurs through an apical internalization pathway most consistent with macropinocytosis.26 All of these results suggest that the presence of microvillus inclusions reflects internalization of apical membranes in neonatal duodenal enterocytes. The manifestation of the inclusions therefore would represent a failure of enterocytes to recycle these membranes through a MYO5B and Rab11a-dependent process, as previous observed in CaCo2 cells.9 Indeed, while this manuscript was under review, Huber and colleagues have provided further detail of the deficits in Rab11a and Rab8a-mediated apical recycling in CaCo2 cells with gene editing mediated loss of MYO5B.27
While microvillus inclusions may not account for the pathology of diarrheal disease, it appears more likely that symptoms accrue from deficits in apical delivery or maintenance of critical transporters and enzymes in the microvillar brush border. As we have previously described in both Navajo MVID patients and MYO5B CaCo2-BBE knockdown lines,9 DPPIV was prominently observed in Lamp2-positive lysosomes in the MYO5B knockout mouse models. This phenotype was present in both germline mice and in adult mice with induced intestinal knockout. Also as in previous studies,11, 17 we found dispersal of the normal subapical localization of both Rab8a and Rab11a. These results appear compatible with a failure to execute appropriate recycling of apical membrane constituents and their eventual shunting to the lysosomes for degradation. An absence of apical transporters such as NHE3 (sodium hydrogen exchanger 3) or SGLT1 (sodium glucose cotransporter 1) could account for the severe diarrhea.
While this manuscript was in preparation, two other groups have reported mouse models for MYO5B deletion. Carton-Garcia, et al. reported a germline gene-trap knockout mouse for MYO5B.10 These mice died in their first day of life. As seen in our studies, they developed microvillus inclusions in the duodenum. This early demise for the mice made further detailed analysis difficult. We were unable to produce viable germline offspring on a pure C57BL/6 background, and this seems to correlate with the findings in these gene-trap knockouts. With breeding onto the outbred CD1 strain, we were able to extend survival to about a week while preserving the MVID phenotype. Schneeberger, et al. have recently reported an inducible intestinally targeted MYO5B deletion mouse based on the VillinCreERT2 driver and a fourth exon floxed allele.11 Many of the findings are similar to those we have reported here including induction of diarrhea, timing of loss of apical transporters and alterations in apical and basolateral trafficking. Nevertheless, the report appears to differ from our findings on prevalence of microvillus inclusions following induction of MYO5B KO in adult mice. It seems likely that the numbers of inclusions in this previous investigation may have been overestimated because of a lack of comparison to either induction in neonatal mice or germline or constitutive KO mice. In addition, the absolute numbers of inclusions may vary in inducible mice based on strain differences and the exact time of induction versus the transition to adolescence after weaning, a time when one would expect to see a down regulation of apical macropinocytosis due to the cessation of breast feeding. The predominance of findings in our studies suggests that formation of microvillus inclusions in duodenal enterocytes is far more pronounced in neonates.
Our results in the MYO5B knockout strains also demonstrate that the manifestations of pathological changes are region dependent. The previous reports in germline gene-trap and adult inducible MYO5B KO did not comment on regional differences within the small intestine.10, 11 As one might expect for a mechanism involved in apical internalization of maternal IgG, the microvillus inclusions in both germline and VillinCre;MYO5BF/F mice were predominant in the duodenum and proximal jejunum and were essentially absent in the ileum and colon. Previous investigations have noted microvillus inclusions are less frequent in the colonocytes of MVID patients.28 In the germline and constitutive intestinal knockout mice, we also observed less prominent changes in apical transporters in the ileum. These findings indicate that the diarrheal phenotype may accrue predominantly from a duodenal deficit in absorption. Previous investigations have documented various differences in the physiology of duodenum, jejunum and ileum.29 Most notably, in studies of massive small bowel resection, the ileum has a higher capacity to hypertrophy.30 This phenomenon is often critical for the recovery of function in babies with short gut syndrome.31 If our findings in mice can be corroborated in children with MVID, then MVID diarrhea may be amenable to treatment with strategies for exclusion of the duodenum and promotion of ileal adaptation.
It is important to note that the MYO5B knockout mice did not recapitulate a number of the phenotypic changes usually associated with MVID.9 First of all, we did not observe complete loss of microvilli in germline knockouts and VillinCre;MYO5BF/F mice. Rather these mice showed an intact brush borders with short microvilli. These findings are in agreement with those in the MYO5B gene-trap KO model.10 This microvillar phenotype is similar to that observed in Rab11a knockout mice.17, 18 Interestingly, in the tamoxifen-induced VillinCreERT2;MYO5BF/F mice, we did observe microvillar denudation in some cells. A similar phenomenon was also reported by Schneeberger, et al. in inducible MYO5B KO in adult mice.11 Some of these differences may be due to age-dependent observations. In the germline KO mouse models, observations were focused on mice in their first week of life, whereas the inducible mice were essentially adults. Similarly, the human MVID children are often not biopsied until their third month of life. In addition, the MVID children have usually spent weeks on total parenteral nutrition without luminal alimentation. A lack of luminal alimentation is usually associated with varying levels of villus atrophy.32–34 Thus, the intestinal phenotype observed in MVID patients in their second or third month of life would reflect a combination of changes from the genetic disease along with the impact of extended bowel rest. We also did not observe the gross changes in junctional proteins such as E-cadherin in the germline and targeted knockout mice, so these more prominent losses in epithelial polarity observed in MVID children may reflect ramifications between disease and the lack of alimentation. Indeed, loss of E-cadherin was observed in a mouse model of total parenteral nutrition.35 Alternatively, it is possible that dominant negative mutations, such as the P660L MYO5B mutation in the Navajo, elicit a stronger phenotype than the knockout. The pathology in true human MYO5B knockouts has not been evaluated in depth. It is also notable that we did not observe any significant impact on the liver in germline knockouts. Similar results have been reported recently in the gene-trap MYO5B knockout mouse.10 Liver disease in MVID patients has previously been noted as quite variable.36 Early in vitro studies had suggested that MYO5B was critical for proper assembly and function of the liver bile canaliculus.37 However, liver disease in MVID patients may reflect an effect of extended parenteral nutrition from birth or a more subtle genetic deficit that is amplified in the presence of parenteral nutrition by MYO5B loss.
In summary, we have examined the effects of MYO5B loss in three separate mouse models reflecting germline deletion, constitutive intestinal deletion and inducible intestinal deletion in both adults and neonates. Our findings demonstrate that microvillus inclusions are an indicator of MYO5B loss and likely reflect inhibition of recycling out of apical macropinosomes in the enterocytes of the proximal small intestine. Thus, this mutation has revealed a previously unrecognized process operating in neonatal duodenal enterocytes. Nevertheless, our findings support the concept that the true pathology of the disease is manifested in the loss of proper trafficking and recycling of transporters to the apical brush border in enterocytes9, 27. Our findings suggest that these apical transporters are prominently diverted to degradation pathways in the lysosome. Thus, treatment of MVID must focus on re-establishment of proper recycling, replacement of diseased enterocytes or facilitation of mechanisms that may still be operable for distal intestine adaptation.
Synopsis.
Three mouse models of MYO5B recapitulate the features of Microvillus Inclusion Disease in neonatal mice, including the formation of inclusions and loss of apical transporters in the duodenum. The studies reveal the presence of a novel apical membrane internalization pathway in neonatal duodenal enterocytes.
Acknowledgments
This work was supported by the NIH RO1 grants DK70856 and DK48370 to J.R.G. V.G.W. was supported by T32 DK007673.
Confocal and structured illumination fluorescence microscopy as well as TEM and SEM imaging was performed through the use of the VUMC Cell Imaging Shared Resource and histological sectioning was performed by Translational Pathology Shared Resource, both supported by National Institute of Health (NIH) Grants CA68485, DK20593, DK58404 and HD15052. Mouse models were developed in the Shared Resources of the Vanderbilt Stem Cell Center supported by NIH Grants CA68485. Fluorescence slide imaging was performed on an Ariol SL-50 digitizing scanner in the VUMC Digital Histology Shared Resource.
Footnotes
The authors have declared that no conflict of interest exists.
Author Contributions:
Weis: Participated in study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript.
Knowles: Participated in study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript.
Choi: Participated in acquisition of data; analysis and interpretation of data; edited manuscript.
Goldstein: Participated in acquisition of data; analysis and interpretation of data.
Williams: Participated in acquisition of data; analysis and interpretation of data; edited manuscript.
Manning: Participated in acquisition of data; analysis and interpretation of data.
Roland: Participated in study concept and design, edited manuscript.
Lapierre: Participated in acquisition of data; analysis and interpretation of data, edited manuscript.
Goldenring: Participated in study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Erickson RP, Larson-Thome K, Valenzuela RK, et al. Navajo microvillous inclusion disease is due to a mutation in MYO5B. Am J Med Genet A. 2008;146A:3117–9. doi: 10.1002/ajmg.a.32605. [DOI] [PubMed] [Google Scholar]
- 2.Muller T, Hess MW, Schiefermeier N, et al. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat Genet. 2008;40:1163–5. doi: 10.1038/ng.225. [DOI] [PubMed] [Google Scholar]
- 3.Ruemmele FM, Muller T, Schiefermeier N, et al. Loss-of-function of MYO5B is the main cause of microvillus inclusion disease: 15 novel mutations and a CaCo-2 RNAi cell model. Hum Mutat. 2010;31:544–51. doi: 10.1002/humu.21224. [DOI] [PubMed] [Google Scholar]
- 4.Davidson GP, Cutz E, Hamilton JR, et al. Familial enteropathy: a syndrome of protracted diarrhea from birth, failure to thrive, and hypoplastic villus atrophy. Gastroenterology. 1978;75:783–90. [PubMed] [Google Scholar]
- 5.Ruemmele FM, Schmitz J, Goulet O. Microvillous inclusion disease (microvillous atrophy) Orphanet J Rare Dis. 2006;1:22. doi: 10.1186/1750-1172-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ameen NA, Salas PJ. Microvillus inclusion disease: a genetic defect affecting apical membrane protein traffic in intestinal epithelium. Traffic. 2000;1:76–83. doi: 10.1034/j.1600-0854.2000.010111.x. [DOI] [PubMed] [Google Scholar]
- 7.Kravtsov D, Mashukova A, Forteza R, et al. Myosin 5b loss of function leads to defects in polarized signalling: Implication for Microvillus Inclusion Disease pathogenesis and treatment. Am J Physiol Gastrointest Liver Physiol. 2014;307:G992–G1001. doi: 10.1152/ajpgi.00180.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thoeni CE, Vogel GF, Tancevski I, et al. Microvillus inclusion disease: loss of myosin Vb disrupts intracellular traffic and cell polarity. Traffic. 2013;15:22–42. doi: 10.1111/tra.12131. [DOI] [PubMed] [Google Scholar]
- 9.Knowles BC, Roland JT, Krishnan M, et al. Myosin Vb uncoupling from RAB8A and RAB11A elicits microvillus inclusion disease. J Clin Invest. 2014;124:2947–62. doi: 10.1172/JCI71651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carton-Garcia F, Overeem AW, Nieto R, et al. Myo5b knockout mice as a model of microvillus inclusion disease. Sci Rep. 2015;5:12312. doi: 10.1038/srep12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneeberger K, Vogel GF, Teunissen H, et al. An inducible mouse model for microvillus inclusion disease reveals a role for myosin Vb in apical and basolateral trafficking. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1516672112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lapierre LA, Avant KM, Caldwell CM, et al. Phosphorylation of Rab11-FIP2 regulates polarity in MDCK cells. Mol Biol Cell. 2012;23:2302–18. doi: 10.1091/mbc.E11-08-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roland JT, Kenworthy AK, Peranen J, et al. Myosin Vb Interacts with Rab8a on a Tubular Network Containing EHD1 and EHD3. Mol Biol Cell. 2007;18:2828–2837. doi: 10.1091/mbc.E07-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhekne HS, Hsiao NH, Roelofs P, et al. Myosin Vb and Rab11a regulate phosphorylation of ezrin in enterocytes. J Cell Sci. 2014;127:1007–17. doi: 10.1242/jcs.137273. [DOI] [PubMed] [Google Scholar]
- 15.Saotome I, Curto M, McClatchey AI. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell. 2004;6:855–64. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Groisman GM, Amar M, Livne E. CD10: a valuable tool for the light microscopic diagnosis of microvillous inclusion disease (familial microvillous atrophy) Am J Surg Pathol. 2002;26:902–7. doi: 10.1097/00000478-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Knowles BC, Weis VG, Yu S, et al. Rab11a regulates Syntaxin 3 localization and microvillus assembly in enterocytes. J Cell Sci. 2015;128:1617–26. doi: 10.1242/jcs.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobajima T, Yoshimura S, Iwano T, et al. Rab11a is required for apical protein localisation in the intestine. Biol Open. 2014;4:86–94. doi: 10.1242/bio.20148532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones EA, Waldmann TA. The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. J Clin Invest. 1972;51:2916–27. doi: 10.1172/JCI107116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Israel EJ, Taylor S, Wu Z, et al. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology. 1997;92:69–74. doi: 10.1046/j.1365-2567.1997.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–7. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 22.Bry L, Falk P, Huttner K, et al. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc Natl Acad Sci U S A. 1994;91:10335–9. doi: 10.1073/pnas.91.22.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah U, Dickinson BL, Blumberg RS, et al. Distribution of the IgG Fc receptor, FcRn, in the human fetal intestine. Pediatr Res. 2003;53:295–301. doi: 10.1203/01.PDR.0000047663.81816.E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodewald R. Intestinal transport of antibodies in the newborn rat. J Cell Biol. 1973;58:189–211. doi: 10.1083/jcb.58.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He W, Ladinsky MS, Huey-Tubman KE, et al. FcRn-mediated antibody transport across epithelial cells revealed by electron tomography. Nature. 2008;455:542–6. doi: 10.1038/nature07255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato K, Nagai J, Mitsui N, et al. Effects of endocytosis inhibitors on internalization of human IgG by Caco-2 human intestinal epithelial cells. Life Sci. 2009;85:800–7. doi: 10.1016/j.lfs.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Vogel GF, Klee KM, Janecke AR, et al. Cargo-selective apical exocytosis in epithelial cells is conducted by Myo5B, Slp4a, Vamp7, and Syntaxin 3. J Cell Biol. 2015;211:587–604. doi: 10.1083/jcb.201506112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips AD, Jenkins P, Raafat F, et al. Congenital microvillous atrophy: specific diagnostic features. Arch Dis Child. 1985;60:135–40. doi: 10.1136/adc.60.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.James PS, Smith MW, Tivey DR. Single-villus analysis of disaccharidase expression by different regions of the mouse intestine. J Physiol. 1988;401:533–45. doi: 10.1113/jphysiol.1988.sp017177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowling RH, Booth CC. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967;32:139–49. [PubMed] [Google Scholar]
- 31.Tappenden KA. Mechanisms of enteral nutrient-enhanced intestinal adaptation. Gastroenterology. 2006;130:S93–9. doi: 10.1053/j.gastro.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 32.Sun X, Spencer AU, Yang H, et al. Impact of caloric intake on parenteral nutrition-associated intestinal morphology and mucosal barrier function. JPEN J Parenter Enteral Nutr. 2006;30:474–9. doi: 10.1177/0148607106030006474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakamoto K, Hirose H, Onizuka A, et al. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J Surg Res. 2000;94:99–106. doi: 10.1006/jsre.2000.5937. [DOI] [PubMed] [Google Scholar]
- 34.Buchman AL, Moukarzel AA, Bhuta S, et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr. 1995;19:453–60. doi: 10.1177/0148607195019006453. [DOI] [PubMed] [Google Scholar]
- 35.Feng Y, Sun X, Yang H, et al. Dissociation of E-cadherin and beta-catenin in a mouse model of total parenteral nutrition: a mechanism for the loss of epithelial cell proliferation and villus atrophy. J Physiol. 2009;587:641–54. doi: 10.1113/jphysiol.2008.162719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girard M, Lacaille F, Verkarre V, et al. MYO5B and bile salt export pump contribute to cholestatic liver disorder in microvillous inclusion disease. Hepatology. 2014;60:301–10. doi: 10.1002/hep.26974. [DOI] [PubMed] [Google Scholar]
- 37.Wakabayashi Y, Dutt P, Lippincott-Schwartz J, et al. Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells. Proc Natl Acad Sci U S A. 2005;102:15087–92. doi: 10.1073/pnas.0503702102. [DOI] [PMC free article] [PubMed] [Google Scholar]