Abstract
Background
To study the association between subclinical vascular disease and subsequent development of erectile dysfunction (ED).
Hypothesis
Among a number of subclinical atherosclerosis and vascular dysfunction measurements, the coronary artery calcium (CAC) score is the best predictor of erectile dysfunction.
Methods
After excluding participants taking ED medications at baseline, we studied 1,862 men aged 45-84 years free of known CVD from the prospective Multi-Ethnic Study of Atherosclerosis (MESA) (2000-2002) with comprehensive baseline subclinical vascular disease phenotyping and ED status assessed at MESA visit 5 (9.4±0.5 years after baseline) using a standardized question on ED symptoms. Multivariable logistic regression was used to assess the associations between baseline measures of vascular disease (atherosclerosis domain: coronary artery calcium [CAC], carotid intima-media thickness [cIMT], carotid plaque, ankle-brachial index [ABI]; vascular stiffness/function domain: aortic stiffness, carotid stiffness, brachial flow-mediated dilation [FMD]), and ED symptoms at follow-up.
Results
Mean baseline age was 59.5±9 years and 839 participants (45%) reported ED symptoms at follow-up. Compared to symptom-free individuals, participants with ED had higher baseline prevalence of CAC>100 (36.4% vs 17.2%), cIMT Z-score >75th percentile (35.3% vs 16.6%), carotid plaque score ≥2 (39% vs 21.1%), carotid distensibility <25th percentile (34.6% vs 17.1%), aortic distensibility <25th percentile (34.2% vs 18.7%) and FMD <25th percentile (28.4% vs 21.3%); all p<0.01. Specifically, only CAC>100 (OR 1.43, 95% CI 1.09 – 1.88) and carotid plaque score ≥2 (OR 1.33, 95% CI 1.02 – 1.73) were statistically associated with ED.
Conclusions
Subclinical vascular disease is common in men who later self-report ED. Early detection of subclinical atherosclerosis, particularly advanced CAC and carotid plaque, may provide opportunities for predicting the onset of subsequent vascular ED.
Keywords: Erectile dysfunction, cardiovascular disease, subclinical vascular disease, atherosclerosis, coronary artery calcium
Introduction
Vascular erectile dysfunction (ED) and cardiovascular disease (CVD) share common risk factors [1,2]. Given the high prevalence of CVD in the adult population, it is not surprising that ED is also common, currently affecting 5-20% of adult males and up to 70% of men with overt CVD [3-5]. As a result of the shared upstream pathways, clinical detection of suspected vascular ED has been suggested as a justification to screen for concomitant CVD, and vice versa [5,6].
It is commonly described that ED precedes the onset of clinical CVD. Indeed, studies have shown ED to be an independent predictor of CVD events [7-9]. On the other hand, the temporal relationship between subclinical CVD, which may precede overt clinical CVD by over a decade, and the onset of ED, remains poorly described. Evidence linking ED with subclinical CVD has come mostly from cross-sectional studies [10-13], limiting our understanding of their temporal association. This is an important area of study, as the detection of CVD at the subclinical stage may provide an opportunity for predicting ED.
The Multi-Ethnic Study of Atherosclerosis (MESA) allows some ability to study this question, as healthy adults free of CVD were enrolled at baseline, and ED was self-reported during follow-up. In this study, we sought to explore the association of baseline subclinical vascular disease with subsequent self-reported ED among male participants from MESA using a number of baseline (Visit 1) measurements of subclinical vascular disease. Our secondary goal was to assess which domain of subclinical vascular disease – atherosclerosis or vascular stiffness and dysfunction – was most associated with subsequent ED.
Methods
Study Design
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study aimed at identifying the determinants of CVD, with a special focus on the prognostic implications of subclinical vascular disease, and an emphasis on ethnic diversity. Details of the MESA have been published before [14]. In brief, between July 2000 and August 2002 MESA enrolled a total of 6,814 men and women aged 45–84 years who were free of clinically apparent CVD from six communities geographically dispersed throughout the US. All study participants provided written informed consent. The study protocol was approved by the institutional review board of each study site.
All MESA participants received lab testing and baseline non-contrast cardiac computed tomography (CT) scans, and 6,725 had a baseline carotid ultrasound. In addition, a subset of MESA participants underwent measurement of baseline flow-mediated dilation (FMD) (n=3,026) and cardiac magnetic resonance imaging (MRI) with measurement of aortic distensibility (n=3,530). FMD assessment was sampled using a nested case-cohort strategy that included all MESA participants who had adjudicated CVD events prior to October 10, 2005 (cases, n=182), as well as a random sample of participants without cardiovascular events through the same date (n=2,844). ED status was not formally assessed at baseline, although use of ED medications was captured.
Study population
For the present analysis, all male MESA participants who attended both Visit 1 and Visit 5 were considered for inclusion (N=2,202). Among these, we excluded 52 participants taking ED medications at baseline, 67 in whom ED was not assessed and 221 who answered ‘Don't know’ to the question in regards to their erectile function. This yielded a study population of 1,862 men not taking ED medications at baseline and with self-reported absence or presence of ED symptoms at follow-up (Supplementary Figures 1 and 2).
Clinical and laboratory measurements, and assessment of atherosclerosis, vascular stiffness and vascular function
Full details of the measurements performed in MESA have been reported elsewhere [15-20] and are available in the online Supplementary Materials section.
ED Assessment
Formal assessment of ED symptoms was conducted during follow-up at MESA visit 5 (approximately 9 years after baseline) using a standardized, single direct question on ED symptoms, and mirrors the ED assessment used in the National Health and Nutrition Examination Survey (NHANES) [3]. ED was considered present if the participant reported being “sometimes able” or “never able” to achieve a satisfactory erection during intercourse [21].
Statistical analysis
Baseline demographics, cardiovascular risk factors and subclinical vascular disease measurements of the study population were calculated both overall and by ED status. Differences across ED groups were tested using chi-square tests for categorical variables, and t-tests or Kruskal-Wallis tests for continuous variables.
To determine the independent association between each subclinical vascular disease measure and ED, we used multivariable logistic regression of ED (+) vs. ED (-) on each of the measures (categorized as normal/abnormal). For each measure, three multivariable models were used. Model 1 adjusted for age, race/ethnicity, education, and MESA site. Model 2 further adjusted for smoking status, diabetes, family history, systolic blood pressure, LDL cholesterol, HDL cholesterol, lipid-lowering medication use, anti-hypertensive medication use, and waist circumference. Fully-adjusted models (Model 3) further adjusted for beta-blocker use, Center for Epidemiologic Studies-Depression (CES-D) score (continuous), non-tricyclic anti-depressant medication use, tricyclic anti-depressant medication use, and anti-psychotic medication use.
For these analyses, only missing values of carotid plaque score were imputed (leveraging the original carotid ultrasound data that was performed in essentially all participants), while for FMD and aortic distensibility, a complete case strategy was followed including only those participants in whom the test modality had been performed.
To determine which domain of subclinical vascular disease (atherosclerosis or vascular stiffness/dysfunction) was most associated with ED, we created binary terms for expressing the presence of at least one baseline abnormality in each of the two domains. Thus, when any of the following were present the subclinical atherosclerosis domain was considered abnormal: coronary artery calcium (CAC) >100, ankle-brachial index (ABI) <0.9 or >1.4, carotid intima-media thickness (cIMT) Z-score >75th percentile and/or carotid plaque score ≥2. Similarly, when any of the following were present, the vascular stiffness/dysfunction domain was considered abnormal: aortic distensibility <25th percentile, carotid distensibility <25th percentile and/or flow-mediated dilation <25th percentile. In order to make the domains comparable, for these analyses missing values of FMD and aortic distensibility were also imputed, using multiple imputation and the variables listed in model 3 as predictors. The association between each domain and ED was then tested following the same multivariable logistic regression strategy described above.
A two-tailed p-value <0.05 was considered statistically significant. All calculations were performed using STATA version 13.0 (Stata Corp, College Station, Texas).
Results
Baseline characteristics of the study population are shown in Table 1. Mean age was 59.5 and 42.8% were Caucasian, 23.5% African American, 23.0% Hispanic and 10.6% Chinese American. At MESA visit 5 (9.4 ± 0.5 years after baseline) a total of 839 individuals (45%) reported having ED symptoms.
Table 1. Baseline characteristics of the study population.
| Overall (N=1,862) | ED (-) (N=1,023) | ED (+) (N=839) | P Value | |
|---|---|---|---|---|
| Age, years | 59.5 ± 9 | 55.9 ± 8 | 63.9 ± 9 | <0.001 |
| Race | 0.37 | |||
| Non-Hispanic White | 797 (42.8) | 424 (41.5) | 373 (44.5) | 0.096 |
| African American | 438 (23.5) | 251 (24.5) | 187 (22.3) | 0.13 |
| Chinese American | 198 (10.6) | 116 (11.3) | 82 (9.8) | 0.14 |
| Hispanic | 429 (23.0) | 232 (22.7) | 197 (23.5) | 0.34 |
| ≥ High school degree | 1,631 (87.8) | 917 (89.9) | 714 (85.2) | 0.001 |
| Current smoking | 238 (12.8) | 142 (13.9) | 96 (11.5) | 0.058 |
| Diabetes | 207 (11.1) | 64 (6.3) | 143 (17.1) | <0.001 |
| Family history of CHD | 697 (39.6) | 359 (36.9) | 338 (42.9) | 0.005 |
| Systolic blood pressure, mmHg | 123.9 ± 18 | 120.6 ± 17 | 127.9 ± 19 | <0.001 |
| Anti-hypertensive treatment | 592 (31.8) | 242 (23.7) | 350 (41.7) | <0.001 |
| LDL cholesterol, mg/dL | 117.0 ± 31 | 119.3 ± 30 | 114.2 ± 31 | <0.001 |
| HDL cholesterol, mg/dL | 44.6 ± 11 | 44.7 ± 11 | 44.5 ± 11 | 0.93 |
| Lipid-lowering treatment | 312 (16.8) | 148 (14.5) | 164 (19.6) | 0.002 |
| Body mass index, kg/m2 | 28.1 ± 4 | 27.8 ± 4 | 28.4 ± 5 | 0.003 |
| Waist circumference, cm | 99.5 ± 12 | 97.9 ± 11 | 101.5 ± 12 | <0.001 |
| CES-D, score | 6.1 ± 6 | 5.7 ± 6 | 6.5 ± 7 | 0.034 |
| Non-TCA treatment | 66 (3.5) | 33 (3.2) | 33 (3.9) | 0.20 |
| TCA treatment | 9 (0.5) | 4 (0.4) | 5 (0.6) | 0.26 |
| Anti-psychotic treatment | 2 (0.1) | 1 (0.1) | 1 (0.1) | 0.44 |
| Beta-blocker treatment | 135 (7.3) | 63 (6.2) | 72 (8.6) | 0.022 |
Data presented as number (%), mean ± SD or median (IQR).
Abbreviations: ED: Erectile dysfunction; CHD: Coronary heart disease; LDL: Low-density lipoprotein; HDL: High-density lipoprotein; CES-D: Center for epidemiologic studies depression scale; TCA: Tricylcic Antidepressant.
Table 2 shows the prevalence of abnormal subclinical vascular disease measures by ED group. Individuals with ED symptoms at visit 5 had a higher baseline prevalence of every abnormal atherosclerosis measurement (p<0.001 all, except for abnormal ABI, p=0.09) compared to ED symptom-free individuals. Similarly, ED (+) individuals also had a higher baseline prevalence of every abnormal vascular stiffness/dysfunction measurement (p<0.01 all). The proportion of participants with ED symptoms at follow-up across each baseline measure of subclinical vascular disease is displayed in Figure 1. ED was more common with increasing severity of measures of subclinical atherosclerosis, as well as with decreasing vascular distensibility and FMD. The highest proportion of ED (+) participants was observed among those with baseline CAC>100.
Table 2. Prevalence of baseline abnormal values for each subclinical vascular disease measure, by ED status.
| Subclinical vascular disease measurements | Overall (N=1,862) | ED (-) (N=1,023) | ED (+) (N=839) | P Value |
|---|---|---|---|---|
| Atherosclerosis | ||||
| Cardiac CT | ||||
| CAC score >100 | 481 (25.8) | 176 (17.2) | 305 (36.4) | <0.001 |
| Carotid Ultrasound | ||||
| cIMT Z-score >75th Perc. | 461 (25.0) | 168 (16.6) | 293 (35.3) | <0.001 |
| Carotid Plaque ≥2 | 543 (29.2) | 216 (21.1) | 327 (39.0) | <0.001 |
| Ankle-Brachial Index | ||||
| ABI <0.9 or >1.4 | 57 (3.1) | 25 (2.5) | 32 (3.8) | 0.09 |
| Vascular Stiffness & Dysfunction | ||||
| Carotid Ultrasound | ||||
| Distensibility Coefficient <25th Perc | 450 (25.0) | 169 (17.1) | 281 (34.6) | <0.001 |
| Aortic MRI | ||||
| Aortic Distensibility <25th Perc. (N=999) | 249 (24.9) | 111 (18.7) | 138 (34.2) | <0.001 |
| Flow-Mediated Dilation | ||||
| FMD <25th Perc. (N=993) | 243 (24.5) | 117 (21.3) | 126 (28.4) | 0.009 |
Data presented as number (%).
CAC, cIMT, ABI and carotid distensibility measurements were available in all study participants. Carotid plaque scores were available in 74.2% participants and were imputed in the remaining 25.8%. Aortic distensibility and FMD measurements were available in 999 and 993 study participants, respectively, and a complete case strategy was followed.
Abbreviations: CT: Computed Tomography; CAC: Coronary artery calcium; cIMT: Carotid intima-media thickness; ABI: Ankle-brachial index; MRI: Magnetic Resonance Imaging; FMD: Flow-mediated dilation.
Figure 1.
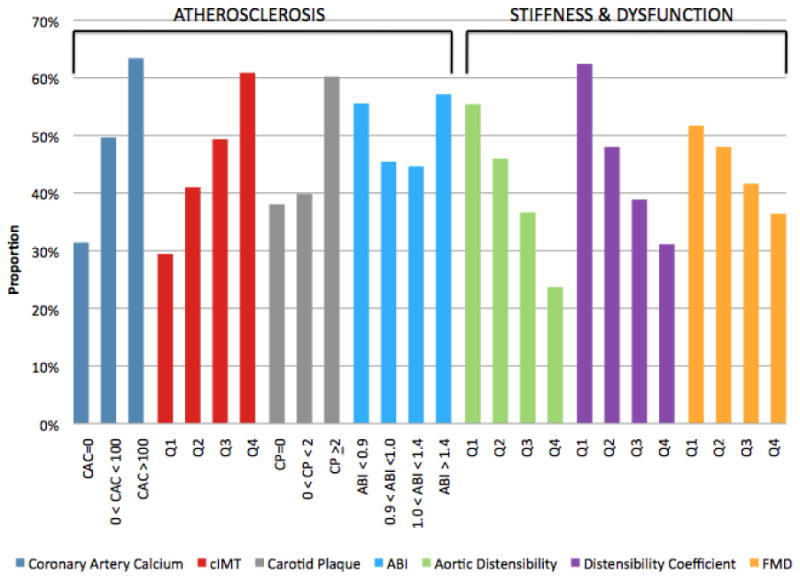
Proportion with ED symptoms at 9-year follow-up by baseline subclinical vascular disease measure. Abbreviations: CAC: Coronary artery calcium; Q: Quartile; cIMT: Carotid intima-media thickness; CP: Carotid plaque; ABI: Ankle-brachial index; FMD: Flow-mediated dilation.
Table 3 shows the multivariable-adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for self-reported ED for each subclinical vascular disease measure. All baseline abnormalities, except for abnormal ABI, were strongly associated with ED in unadjusted models. However, in fully adjusted models only CAC>100 and carotid plaque ≥2 remained statistically associated with subsequent ED.
Table 3. Associations between baseline subclinical vascular disease abnormalities and ED at 9-year follow-up.
| Subclinical vascular disease measurements | Unadjusted | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Atherosclerosis | ||||
| Cardiac CT | ||||
| CAC > 100 | 2.75 (2.22 – 3.41) | 1.56 (1.22 – 2.00) | 1.40 (1.07 – 1.83) | 1.43 (1.09 – 1.88) |
| Carotid Ultrasound | ||||
| cIMT Z-score >75th Percentile | 2.74 (1.87 – 2.88) | 1.48 (1.15 – 1.91) | 1.29 (0.98 – 1.71) | 1.28 (0.97 – 1.70) |
| Carotid Plaque ≥2 | 2.39 (1.95 – 2.93) | 1.41 (1.11 – 1.78) | 1.34 (1.03 – 1.74) | 1.33 (1.02 – 1.73) |
| Ankle Brachial Index (ABI) | ||||
| ABI<0.9 or >1.4 | 1.59 (0.93 – 2.70) | 0.98 (0.53 – 1.81) | 0.90 (0.46 – 1.76) | 0.84 (0.42 – 1.66) |
| Vascular Stiffness & Dysfunction | ||||
| Carotid Ultrasound | ||||
| Distensibility Coefficient <25th Perc. | 2.57 (2.06 – 3.20) | 1.57 (1.22 – 2.04) | 1.29 (0.90 – 1.59) | 1.21 (0.91 – 1.63) |
| Aortic MRI | ||||
| Aortic Distensibility <25th Perc. (N=999) | 2.26 (1.69 – 3.03) | 1.10 (0.78 – 1.56) | 1.05 (0.72 – 1.53) | 1.05 (0.71 – 1.54) |
| Flow-Mediated Dilation | ||||
| FMD <25th Perc. (N=993) | 1.48 (1.11 – 1.96) | 1.23 (0.88 – 1.70) | 1.22 (0.85 – 1.76) | 1.27 (0.88 – 1.84) |
Data presented as odds ratios and 95% confidence intervals.
CAC, cIMT, ABI and carotid distensibility measurements were available in all study participants. Carotid plaque scores were available in 74.2% participants and were imputed in the remaining 25.8%. Aortic distensibility and FMD measurements were available in 999 and 993 study participants, respectively, and a complete case strategy was followed.
Model 1 adjusted for age, race, education, MESA site.
Model 2 adjusted for Model 1 + smoking, diabetes, family history, systolic blood pressure, LDL cholesterol, HDL cholesterol, lipid-lowering medications, anti-hypertensive medications, and waist circumference.
Model 3 adjusted for Model 2 + beta-blockers, depression scale, non-tricyclic antidepressants, tricyclic antidepressants, anti-psychotic medications.
Abbreviations: CT: Computed Tomography; CAC: Coronary artery calcium; cIMT: Carotid intima-media thickness; ABI: Ankle-brachial index; MRI: Magnetic Resonance Imaging; FMD: Flow-mediated dilation.
Supplementary Figure 3 shows the mean proportion of participants with ED symptoms among individuals with abnormalities in 0, 1 or 2 subclinical vascular disease domains. The number and proportion of abnormal subclinical vascular disease domains by ED group is displayed in Supplementary Tables 1 and 2. Among men with ED, 75% had at least 1 subclinical vascular disease abnormality at baseline
The multivariable-adjusted ORs and 95% CIs for ED by identity and number of abnormal vascular domains are displayed in Figure 2 and Supplementary Figure 4, respectively. When individual domains were compared, the presence of abnormalities in the atherosclerosis domain but not in the stiffness/dysfunction domain was statistically associated with subsequent ED symptoms in fully adjusted models (OR 1.30, 95% CI 1.01 – 1.68) (Figure 2). When considering the number of abnormal domains, there was a graded increase in the odds of ED with a greater number of abnormalities, with the strongest association seen when abnormalities were present in both domains (OR 1.53, 95% CI 1.05 – 2.24) (Supplementary Figure 4).
Figure 2.
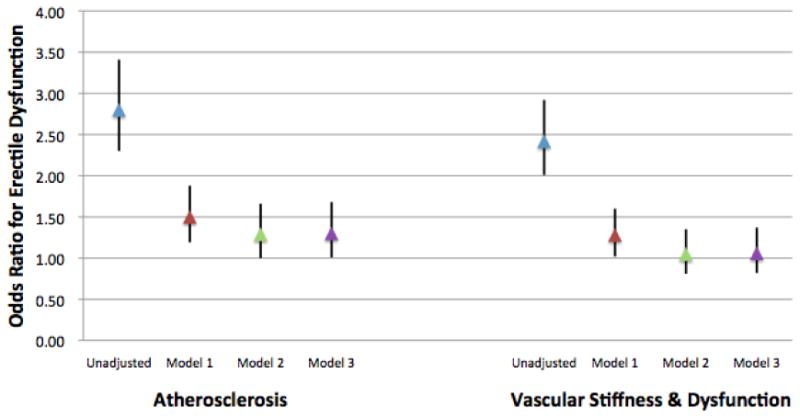
Multivariable adjusted odds ratios for erectile dysfunction for the presence of at least one abnormality in each domain of subclinical vascular disease. Data presented as odds ratios and 95% confidence intervals. Model 1 adjusted for age, race, education, MESA site. Model 2 adjusted for Model 1 + smoking, diabetes, family history, systolic blood pressure, LDL cholesterol, HDL cholesterol, lipid-lowering medications, anti-hypertensive medications, and waist circumference. Model 3 adjusted for Model 2 + beta-blockers, depression scale, non-tricyclic antidepressants, tricyclic antidepressants, anti-psychotic medications. CAC, cIMT, ABI and carotid distensibility measurements were available in all study participants. Carotid plaque scores were available in 74.2% participants, and were imputed in the remaining 25.8%. Aortic distensibility measurements were available in 999 participants and were imputed in the remaining 863. FMD measurements were available in 993 participants and were imputed in the remaining 869.
Discussion
In this ethnically diverse male population from MESA, baseline subclinical vascular disease was common and multiple vascular abnormalities tended to cluster in individuals who subsequently reported ED symptoms. While all vascular disease measurements were associated with ED in unadjusted analyses, only baseline CAC >100 and carotid plaque score ≥2 were statistically associated with subsequent ED after adjusting for traditional risk factors and other potential confounders. Similarly, when measurements were pooled in two main vascular domains, the presence of baseline subclinical atherosclerosis was more closely associated with ED than abnormalities in the stiffness/dysfunction domain. Our findings suggest that the early detection of subclinical vascular disease, particularly coronary calcification and carotid plaque, may provide an opportunity for predicting the onset of subsequent vascular ED.
ED is commonly classified as psychogenic, organic or mixed. Among its organic causes, vascular ED is considered the most prevalent mechanism [22]. The relationship between CVD and vascular ED has been object of great research interest given that these disorders share upstream pathophysiologic pathways and risk factors. Cross-sectional studies have found the coexistence of clinically overt CVD and ED symptoms being a frequent phenomenon [23,24]. Moreover, prospective studies have reported consistent associations between ED and incident CVD events [7-9], with ED symptoms preceding overt CVD by 2-3 years on average [25], highlighting the role of ED as a precursor of CVD events and the value of ED assessment in CVD risk prediction. These findings have resulted in experts advocating for the systematic evaluation of ED symptoms as part of a standard CVD risk assessment in men [5,6].
On the other hand, the temporal relationship between subclinical CVD, which precedes CVD symptoms by over a decade and can be easily measured with tools such as CAC, and ED is less understood. Studies have found strong correlations between subclinical CVD, ED symptoms and/or definite vascular ED diagnosed using penile Doppler [10-13]. Nevertheless, the cross-sectional nature of those studies has precluded the characterization of their temporal relationship. Thus, to date no study had assessed the association between subclinical vascular disease at baseline and subsequent self-reported ED symptoms, nor had tested the specific associations between different atherosclerotic and vascular stiffness/dysfunction measurements and ED.
In our study, the presence of atherosclerotic abnormalities was more strongly associated with subsequent ED than those from the stiffness/dysfunction domain. One potential explanation could be a preponderance of atherosclerosis as the key mechanism underlying vascular ED. However, atherosclerosis and endothelial dysfunction are strongly correlated processes [22, 26-28] and their differentiation may not be fully clear from the subclinical vascular disease measures in our study. Moreover, cIMT and ABI, which are measurements of atherosclerosis in the carotid arteries and lower limbs, were not independently associated with subsequent ED when evaluated as individual measures. Thus, our findings may also be interpreted as the result of specific features and limitations of each of the tests used for assessing subclinical vascular disease. Indeed, both ABI and cIMT may be strongly affected by variability caused by device and quantification tool used [29,30]. Similarly, measurements of vascular distensibility and FMD must be performed in standardized conditions, are also associated with technical issues [31] and require the use of calculations combining several parameters, which may result in even larger measurement error. This might have impacted the accuracy of the measurements, biasing their associations with ED towards the null.
Such limitations are less likely to occur with more standardized measurements such as CT scanning for CAC scoring and carotid plaque measurements. Indeed, among all subclinical vascular disease measurements assessed, baseline CAC >100 and carotid plaque score ≥2 had the strongest associations with ED symptoms at follow-up. CAC is a robust marker of coronary atherosclerosis [32], and is currently considered the most powerful single CVD prognostic test [33,34]. Studies have shown CAC not only accurately predicting coronary heart disease events [35], but also improving the prediction of cerebrovascular events [36] beyond clinical risk factors. On the other hand, carotid plaque measurements have shown a good performance for predicting cerebrovascular events in population-based studies [20]. Our findings highlight the potential value of CAC and carotid plaque measurements as prognostic tests, with the ability to improve the prediction of events in a number of vascular beds at the same time, including the onset of vascular ED.
Our findings have important clinical implications. The detection of subclinical coronary and/or carotid atherosclerosis in male patients may allow the identification of individuals at an increased risk not only for future CVD events, but also of ED symptoms. This information could be used in the context of a physician-patient discussion to further motivate adherence to lifestyle and pharmacologic recommendations among male patients, as means for improving both their cardiovascular and sexual health. Regular physical activity, cardiovascular-healthy diets and statin therapy have shown to slow the progression of ED, postpone or even prevent the transition from functional to structural ED, and improve ED status [37,38].
Study limitations
Our study has limitations that deserve consideration. First, the MESA assessment of ED status is not formally prospective or longitudinal, as ED symptoms were first assessed at MESA visit 5, and the single question used does not differentiate between existing and new-onset ED symptoms. Therefore, no information was available regarding the prevalence of ED at baseline, or regarding the exact date of ED onset, which precludes time-to-event analyses. Nevertheless, given our exclusion of participants on phosphodiesterase inhibitors at baseline (2.5% of men), together with the MESA protocol requirement to be generally “healthy” at inclusion, it is reasonable to expect that most participants were free of ED at baseline, although this cannot be definitely quantified. Despite the limitations of this “shifted-time” cross-sectional approach, our study assessing for the first time the associations between subclinical vascular disease and ED symptoms after 9 years of follow-up overcomes some of the limitations of previous studies, in which both processes were measured at a single time point.
Second, the single question used for assessing ED did not allow us to identify the underlying mechanisms of ED, and the study protocol did not include further assessment of participants with self-reported ED symptoms. Also, without the objective measure of ED status using penile Doppler, the social stigma regarding ED may have caused participants to conceal their ED symptoms. Nonetheless, the single-question approach for ED self-assessment is considered a reliable tool for assessing ED in population-based studies, and has shown a high correlation (r coefficient ranging 0.71–0.78) with assessments using more detailed questionnaires [21]. Finally, some variables included in Model 3 are associated with other covariates included in the models, which may have resulted in over-adjustment. Nevertheless, previous studies have shown factors such as beta-blocker use to be relevant potential confounders in the association between CVD and ED [25].
Our study also has important strengths. The detailed subclinical vascular disease phenotyping, with assessment of multiple measures of subclinical atherosclerosis and vascular stiffness/dysfunction, allowed assessing the specific associations between each of them and subsequent ED symptoms, both individually and grouped by vascular disease domains. This yielded highly unique data not currently present in the ED and men's health literature. Furthermore, the large sample size and the thorough assessment of clinically-relevant features at baseline allowed for a comprehensive adjustment for potential confounders including CVD risk factors, socio-economic status features, depression, and medication use.
Conclusions
We observed a strong, independent association between the presence of baseline subclinical atherosclerosis, particularly coronary artery calcification and carotid plaque, and self-reported ED approximately 9 years after baseline measurement. Though further studies are needed to better delineate the temporal relationship between both processes, our findings highlight the potential role of integrative prognostic tools such as CAC and carotid plaque assessment, which provide an opportunity for predicting multiple vascular events including the onset of ED symptoms. This information should inform future men's health guidelines, and may further engage men in preventive health care and CVD risk discussions.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The information contained herein was derived in part from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
Funding: This research was supported by contracts N01-HC-95159, N01- HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01- HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01- HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR. This publication was developed under a STAR research assistance agreement, No. RD831697 (MESA Air), awarded by the U.S Environmental Protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication. MC-A was funded by a research grant from the Spanish Society of Cardiology.
Abbreviations and Acronyms
- CAC
Coronary artery calcium
- CES-D
Center for Epidemiologic Studies-Depression
- cIMT
Carotid intima-media thickness
- CT
Computed tomography
- CVD
Cardiovascular disease
- ED
Erectile dysfunction
- FMD
Flow-mediated dilation
- MESA
Multi-Ethnic Study of Atherosclerosis
- MRI
Magnetic resonance imaging
Footnotes
Disclosures: None of the authors report conflicts of interest pertaining to this work.
References
- 1.Fung MM, Bettencourt R, Barrett-Connor E. Heart disease risk factors predict erectile dysfunction 25 years later: the Rancho Bernardo study. J Am Coll Cardiol. 2004;43:1405–11. doi: 10.1016/j.jacc.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 2.Grover SA, Lowensteyn I, Kaouache M, Marchand S, Coupal L, DeCarolis E, Zoccoli J, Defoy I. The prevalence of erectile dysfunction in the primary care setting: importance of risk factors for diabetes and vascular disease. Arch Intern Med. 2006;166:213–219. doi: 10.1001/archinte.166.2.213. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the U. S Am J Med. 2007;120:151–7. doi: 10.1016/j.amjmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 4.NIH Consensus Development Panel on Impotence. NIH Consensus Conference: impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 5.Jackson G, Nehra A, Miner M, Billups KL, Burnett AL, Buvat J, Carson CC, Cunningham G, Goldstein I, Guay AT, Hackett G, Kloner RA, Kostis JB, Montorsi P, Ramsey M, Rosen R, Sadovsky R, Seftel AD, Shabsigh R, Vlachopoulos C, Wu FC. The Assessment of vascular risk in men with erectile dysfunction: the role of the cardiologist and general physician. Int J Clin Pract. 2013;67(11):1163–72. doi: 10.1111/ijcp.12200. [DOI] [PubMed] [Google Scholar]
- 6.Miner M, Nehra A, Jackson G, Bhasin S, Billups KL, Burnett AL, Buvat J, Carson C, Cunningham G, Ganz P, Goldstein I, Guay A, Hackett G, Kloner RA, Kostis JB, LaFlamme KE, Montorsi P, Ramsey M, Rosen R, Sadovsky R, Seftel A, Shabsigh R, Vlachopoulos C, Wu F. All Men with Vasculogenic Erectile Dysfunction Require a Cardiovascular Workup. Am J Med. 2014;127(3):174–82. doi: 10.1016/j.amjmed.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Inman BA, Sauver JL, Jacobson DJ, McGree ME, Nehra A, Lieber MM, Roger VL, Jacobsen SJ. A population-based, longitudinal study of erectile dysfunction and future coronary artery disease. Mayo Clin Proc. 2009;84:108–13. doi: 10.4065/84.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlachopoulos C, Terentes-Printzios D, Ioakeimidis N, Aznaouridis KA, Stefanadis CI. Prediction of cardiovascular events and all-cause mortality with erectile dysfunction: a systemic review and meta-analysis of cohort studies. Circ Cardiovasc Qual Outcomes. 2013;6(1):99–109. doi: 10.1161/CIRCOUTCOMES.112.966903. [DOI] [PubMed] [Google Scholar]
- 9.Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294:2996–3002. doi: 10.1001/jama.294.23.2996. [DOI] [PubMed] [Google Scholar]
- 10.Chirulia E, D'Amico R, Ratti C, Granata AR, Romagnoli R, Modena MG. Subclinical coronary artery atherosclerosis in patients with erectile dysfunction. J Am Coll Cardiol. 2005;46(8):1503–6. doi: 10.1016/j.jacc.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 11.Yaman O, Gulpinar O, Hasan T, Ozdol C, Ertas FS, Ozgenci E. Erectile Dysfunction may predict coronary artery disease: relationship between coronary artery calcium scoring and erectile dysfunction severity. Int Urol Neprhol. 2008;40(1):117–23. doi: 10.1007/s11255-007-9293-8. [DOI] [PubMed] [Google Scholar]
- 12.Jackson G. Erectile dysfunction and asymptomatic coronary artery disease: frequently detected by computed tomography coronary angiography but not by exercise electrocardiography. Int J Clin Pract. 2013;67(11):1159–62. doi: 10.1111/ijcp.12275. [DOI] [PubMed] [Google Scholar]
- 13.El Sakka AI, Morsy AM. Screening for ischemic heart disease in patients with erectile dysfunction: role of penile Doppler ultrasonography. Urology. 2004;64:346–50. doi: 10.1016/j.urology.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Bild D, Bluemke D, Burke G, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: Objective and design. Am J Epidemiol. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 15.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 16.Carr J, Nelson J, Wong N, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 17.Folsom AR, Kronmal RA, Detrano RC, O'Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atheroslcerosis. Arch Intern Med. 2008;168(12):1333–9. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malayeri AA, Natori S, Bahrami H, Bertoni AG, Kronmal R, Lima JA, Bluemke DA. Relation of aortic wall thickness and distensibility to cardiovascular risk factors (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2008;102:491–6. doi: 10.1016/j.amjcard.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediate dilation for incident cardiovascular events in a population-based study: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2009;120:502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, Gottesman RF, Kronmal R, Budoff MJ, Burke GL, Folsom AR, Liu K, Kaufman J, Stein JH. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8(1):pii. doi: 10.1161/CIRCIMAGING.114.002262. e002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derby CA, Araujo AB, Johannes CB, Feldman HA, McKinlay JB. Measurement of erectile dysfunction in population-based studies: the use of a single question self-assessment in the Massachusetts Male Aging Study. Int J Impot Res. 2000;12:197–204. doi: 10.1038/sj.ijir.3900542. [DOI] [PubMed] [Google Scholar]
- 22.Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381(9861):153–65. doi: 10.1016/S0140-6736(12)60520-0. [DOI] [PubMed] [Google Scholar]
- 23.Solomon H, Man JW, Wierzbicki AS, Jackson G. Relation of erectile dysfunction to angiographic coronary artery disease. Am J Cardiol. 2003;91(2):230–1. doi: 10.1016/s0002-9149(02)03113-2. [DOI] [PubMed] [Google Scholar]
- 24.Montorsi F, Briganti A, Salonia A, Rigatti P, Margonato A, Macchi A, Galli S, Ravagnani PM, Montorsi P. Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur Urol. 2003;44:360–5. doi: 10.1016/s0302-2838(03)00305-1. discussion 364–5. [DOI] [PubMed] [Google Scholar]
- 25.Montorsi P, Ravagnani PM, Galli S, Rotatori F, Veglia F, Briganti A, Salonia A, Deho F, Rigatti P, Montorsi F, Fiorentini C. Association between erectile dysfunction and coronary artery disease. Role of coronary clinical presentation and extent of coronary vessels involvement: the COBRA trial Eur Heart J. 2006;27:2632–2639. doi: 10.1093/eurheartj/ehl142. [DOI] [PubMed] [Google Scholar]
- 26.Solomon H, Man JW, Jackson G. Erectile dysfunction and the cardiovascular patient: endothelial dysfunction is the common denominator. Heart. 2003;89:251–3. doi: 10.1136/heart.89.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bivalacqua TJ, Usta MF, Champion HC, Kadowitz PJ, Hellstrom WJ. Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J Androl. 2003;24:S17–37. doi: 10.1002/j.1939-4640.2003.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 28.Ganz P. Erectile dysfunction: pathophysiologic mechanisms pointing to underlying cardiovascular disease. Am J Cardiol. 2005;96(12B):8M–12M. doi: 10.1016/j.amjcard.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Davies JH, Kenkre J, Williams EM. Current utility of the ankle-brachial index (ABI) in general practice: implications for its use in cardiovascular disease screening. BMC Fam Pract. 2014;15:69. doi: 10.1186/1471-2296-15-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Touboul PJ. Intima-media thickness of carotid arteries. Front Neurol Neurosci. 2015;36:31–9. doi: 10.1159/000366234. [DOI] [PubMed] [Google Scholar]
- 31.Ghiadoni L, Salvetti M, Muiesan ML, Taddei S. Evaluation of endothelial function by flow mediated dilation: methodological issues and clinical importance. High Blood Press Cardiovasc Prev. 2015;22(1):17–22. doi: 10.1007/s40292-014-0047-2. [DOI] [PubMed] [Google Scholar]
- 32.Budoff MJ, Achenback S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JA, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 33.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O'Connell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters SAE, den Ruijter HM, Bots ML, Moons KG. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart. 2012;98(3):177–84. doi: 10.1136/heartjnl-2011-300747. [DOI] [PubMed] [Google Scholar]
- 35.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 36.Gibson AO, Blaha MJ, Arnan MK, Sacco RL, Szklo M, Herrington DM, Yeboah J. Coronary artery calcium and incident cerebrovascular events in an asymptomatic cohort: the MESA study. JACC Cardiovasc Imaging. 2014;7(11):1108–15. doi: 10.1016/j.jcmg.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hannan JL, Maio MT, Komolova M, Adams MA. Beneficial impact of exercise and obesity interventions on erectile function and its risk factors. J Sex Med. 2009;6:254–61. doi: 10.1111/j.1743-6109.2008.01143.x. [DOI] [PubMed] [Google Scholar]
- 38.Kostis J, Dobrzynski J. The effect of statins on erectile function; a meta-analysis of randomized trials. J Sex Med. 2014;11(7):1626–35. doi: 10.1111/jsm.12521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


