Abstract
The epidemiology of deaths due to vaccine-preventable diseases has been significantly and positively altered through the use of vaccines. Despite this, significant challenges remain in vaccine development and use in the third millennium. Both new (Ebola, Chikungunya, West Nile) and re-emerging diseases (measles, mumps, influenza) require the development of new or next-generation vaccines. The global aging of the population, and accumulating numbers of immunocompromised persons, will require new vaccine and adjuvant development to protect large segments of the population. After vaccine development, significant challenges remain globally in the cost and efficient use and acceptance of vaccines by the public. This article raises issues in these two areas and suggests a way forward that will benefit current and future generations.
Keywords: Vaccines, Immunization, Infectious Diseases, Communicable Diseases, Emerging, Social Problems
Introduction
The prevention of infectious diseases by the widespread use of vaccines has demonstrably changed the types and incidence of morbidity and mortality due to vaccine-preventable diseases. In fact, the Centers for Disease Control and Prevention (CDC) has ranked vaccines and widespread sanitation as being responsible for the significant decrease in vaccine-preventable disease incidence and deaths [1]. This has been accomplished in two major ways: first, by the development of safe and effective vaccines; and second, by the widespread utilization of these vaccines among the populace. Both these conditions face threats and new challenges in the third millennium. The first challenge is the need to develop new technologies that enable faster and directed vaccine development for hyper-variable and complex disease-causing organisms, and the need for safe and effective adjuvants that can be used to direct and amplify protective immune responses. The second major challenge is the necessity for the populace, as a whole, to voluntarily accept these vaccines as important determinants of their wellbeing and health. In this review, we outline these challenges (see Figure 1) and suggest how they can be met (see Figure 2) in the third millennium.
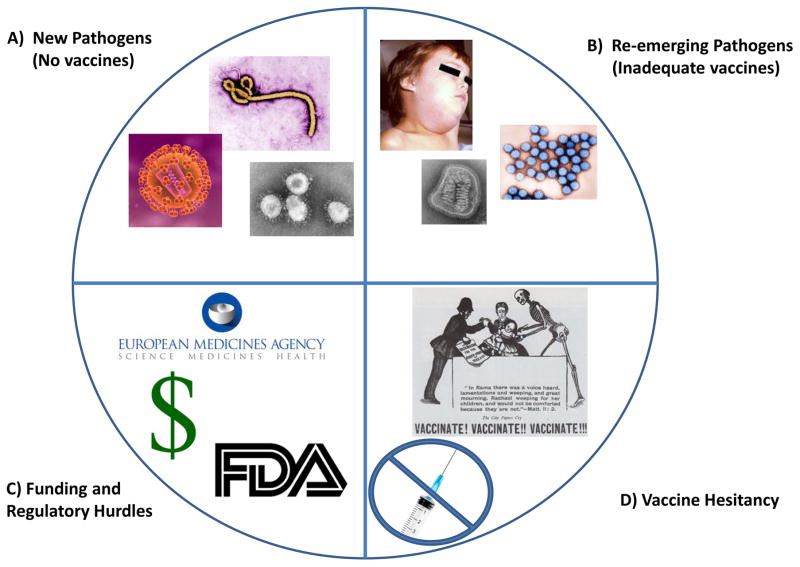
Figure 1. Challenges Facing Vaccines.
A) Pathogens for which we currently lack vaccines (HIV, M. tuberculosis, Ebola, SARS, MERS…). These are completely new pathogens that have not been studied, or are more complex pathogens with immunomodulatory traits or hypervariable genomes for which conventional vaccine development approaches have failed. B) Re-emerging pathogens that current vaccines: have been unable to control or eradicate, elicit marginally protective immunity, have unwanted side effects (rotavirus), require multiple booster immunizations (hepatitis B), or require yearly vaccine reformulation (influenza). C) funding and regulatory issues can be formidable obstacles to the successful research, development, clinical testing, and licensure of new vaccines. These factors may also act as disincentives to even consider vaccine development. D) Anti-vaccination groups are increasingly vocal in their opposition to vaccination. Current communication technologies allow them to rapidly and widely spread their messages against vaccines. As a result safety standards are considerably higher now and public opinion towards a new vaccine must now accurately gauged and considered to an extent never seen before. All images are public domain or are owned by their respective agency/foundation.
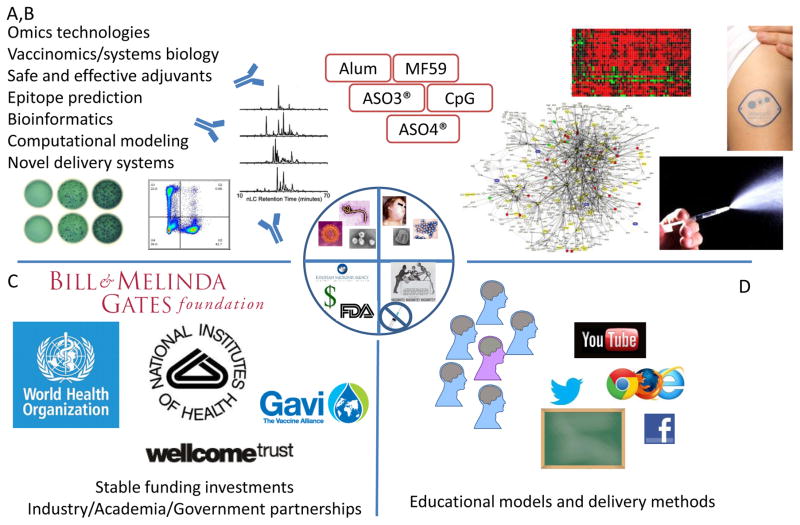
Figure 2. Solutions to the Current Vaccinology Problems.
A,B) Cutting edge technologies allow investigators to study the immune system with unprecedented detail and scope. These technologies, along with novel analytical routines designed to handle the massive datasets, will provide greater insights into immune function and vaccine response. Studies targeting adjuvants and innate immune pathways will also be incorporated into the design of more effective vaccines, perhaps involving novel delivery systems such as the intradermal or intranasal routes pictured. B) International partnerships that bring together scientific leaders from academia, product development expertise from industry, public health officials, and funding/support from private foundations and governmental agencies will be necessary to provide the stable, long-term support and resources necessary to create safe and effective vaccines. C) Coordinated educational efforts that encompass multiple traditional and novel communication platforms will allow widespread delivery of scientific knowledge and data-driven findings. Physicians, healthcare providers, and patients will have open dialogues that acknowledge concerns and provide information tailored to the patient’s preferred learning styles.
Disease challenges: Epidemiology
Despite the immense progress in combating infectious diseases through vaccines, formidable foes remain. HIV, tuberculosis, malaria, and hepatitis C are epidemiologic giants that continue to contribute significantly to global mortality and lack effective vaccines (Table 1). Fortunately, progress has been made on the malaria vaccine front [2].. Some may argue that since we now have highly effective, tolerable treatments for curing hepatitis C, the quest for a hepatitis C vaccine is a moot point; however, the cost and availability of curative therapies is still limited to a select few. HIV and tuberculosis remain the most deadly infectious diseases worldwide. [3].
Table 1.
Major Global Infectious Disease Challenges That Lack Effective Vaccines
| Pathogen/Disease | Global Morbidity and Mortality | Vaccine Challenge |
|---|---|---|
| HIV | 37 million people worldwide living with HIV end of 2014; 1.2 million deaths due to HIV in 2014[100] | Highly variable virus; unclear immune correlates of protection |
| Tuberculosis | 9.6 million new cases of tuberculosis active infection and 1.5 million deaths in 2014[101] | Unclear immune correlates of protection; 1/3 of world’s population infected with latent tuberculosis |
| Malaria | 214 million cases of malaria and 438 000 deaths in 2015[102] | Antigenic variation during stages of infection; complex host-parasite interaction; unclear immune correlates of protection |
| Hepatitis C (HCV) | 130–150 million people are infected; 500,000 deaths occur each year due to HCV-related liver disease[103] | Genetic diversity among viral strains; hypervariable virus; unclear immune correlates of protection |
| Dengue | 390 million infections may occur each year, of which 96 million manifest clinically[104] | Four virus serotypes; lack of adequate animal disease model; incomplete understanding of immune correlates of protection |
The recent Ebola outbreaks have resulted in 28,639 reported cases with 11,316 deaths as of January 2016[4] and have raised the spotlight on the need for readily available and effective vaccines against lethal emerging pathogens. Over the past year, significant progress has been rapidly made on vaccines for prevention of Ebola virus disease [5–8]. The design of clinical vaccine trials in the midst of ongoing outbreaks using novel cluster randomized controlled trial designs with ring vaccination is a notable early third-millennium achievement [9]. However, as important as this study design is, would it not have been better if an effective vaccine had already been developed and available?
As the authors of a recent New England Journal of Medicine perspective article point out, prior to the Ebola outbreaks of 2013–2015, at least seven Ebola vaccine candidates had been tested in primates with promising results; however, only one had been tested in healthy humans, and no vaccine had reached later phase clinical trials that would lead to licensure. The authors propose that vaccine development is currently facing a crisis due to several factors: 1) the pathogens for which we lack vaccines are complex and require significant investment; 2) there are fewer vaccine manufacturers that are able to contribute the required resources for development of new vaccines; 3) and the current business model prioritizes the development of vaccines with large market potential [10]. Vaccine development for other emerging infections (e.g., Middle East respiratory syndrome, or MERS; severe acute respiratory syndrome, or SARS; Chikungunya; West Nile; Dengue; and Lyme) face these same roadblocks (Table 2).
Table 2.
Emerging and Reemerging Viral Pathogens
| Virus/Disease | Case Frequency | Main Geographic Distribution |
|---|---|---|
| Ebola | 2013–2015 outbreaks: 28, 639 cases and 11316 deaths[105] | Central and West Africa |
| Marburg | Sporadic outbreaks; largest was in Angola in 2004 with 252 cases[106] | Central Africa |
| Lassa Fever | 100000–300000 cases/year[107] | West Africa |
| SARS | 8098 cases during 2003 outbreak[108] | Southeast Asia |
| MERS-CoV | 1638 cases since 2012 [109] | Arabian peninsula |
| Chikungunya | > 1.3 million cases as of April 2015 in the Americas[110] | Africa, Southeast Asia, Americas |
| Zika | Not available[111] | South/Central America since May, 2015 (previously Africa, Asia) |
SARS = severe acute respiratory syndrome
MERS-CoV = Middle East respiratory syndrome coronavirus
These challenging pathogens for which we lack promising vaccines, along with the newly emerging pathogens that pose pandemic potential, are not the only reasons we need more vaccine research and development. The pathogens for which we currently have licensed vaccines remain adversaries that continue to challenge vaccine development.
Current Vaccine Deficiencies
Although remarkably effective, currently licensed vaccines have some limitations. In fact, vaccine failure (i.e., the failure for the recipient to either develop or maintain protective immunity) takes place despite the receipt of a single dose, and/or several doses of vaccines, such as measles-mumps-rubella (MMR), hepatitis A and B [11], influenza [12], varicella [13], Haemophilus influenzae type b (Hib) containing acellular pertussis (DTaP-Hib) [14], and other vaccines. Antigenic changes in influenza viruses require us to reformulate and administer the vaccines every year. We and others have demonstrated that host genetics (SNPs in HLA and other immune response genes) and other host factors may play a significant role in variations in adaptive immune response to vaccination, including vaccine failure [15–19].
However, there are some limitations with vaccines containing live attenuated (MMR, varicella, smallpox, zoster, yellow fever) or killed whole/inactivated (polio, influenza, Hepatitis A) microbial organisms, antigenic subunits of these pathogens (pertussis, pneumococcus, anthrax), or genetically engineered (hepatitis B, cholera, HPV) vaccines. The main deficits in these vaccines are the requirement for a cold chain, the induction of low seroconversion rates in some subpopulations, interference from pre-existing maternal antibodies, the requirement for multiple doses, and, for vaccines containing live organisms, the incapability to use the vaccine in immunocompromised individuals. Recently, there has also been a resurgence in pertussis (whooping cough) incidence in many countries around the world with many cases occurring in previously vaccinated children and adolescents [20,21]
New viral vaccine candidates have recently been introduced for varicella-zoster, influenza, dengue and other viruses; however, much remains to be done. For example, the short-term efficacy of the live virus herpes zoster vaccine was demonstrated to be 69.8% in a randomized study of over 22,500 vaccine recipients [22]. Zoster vaccine efficacy was shown to decrease with increasing age at vaccination [23]. An AS01-adjuvanted varicella-zoster virus glycoprotein E (gE) subunit vaccine has undergone clinical trials where it demonstrated acceptable safety and enhanced immunogenicity; the vaccine is under review by the FDA with likely approval in 2017 [24,25]. The world’s first tetravalent dengue vaccine has been recently approved for the prevention of dengue infection that is caused by all four dengue virus serotypes in children and adults 9 to 45 years of age [26]. Two seasonal influenza vaccines, the MF59-adjuvanted influenza vaccine and the high-dose influenza vaccine, are now licensed for use in older adults (≥ 65 years of age). It has been shown that the MF59 adjuvant increases humoral immune response to various influenza strains, including cross-reactive strains [27,28]. A large influenza vaccine study in 7,082 individuals demonstrated significantly higher immunogenicity of MF59-adjuvanted vaccine compared to a conventional non-adjuvanted influenza vaccine for both homologous and heterologous influenza A strains [29]. The immunogenicity and efficacy of high-dose influenza vaccine (60 ug hemagglutinin per strain), compared to standard-dose vaccine (15 ug hemagglutinin per strain), was assessed in a large vaccine study in 31,989 older individuals, which demonstrated the higher immunogenicity of high-dose influenza vaccine compared to conventional vaccine and the improved protection against influenza [30]. However, there is some evidence that AS03 adjuvants used in pandemic influenza vaccines may be associated with the development of autoimmune and neurological disorders, like narcolepsy [31]. Thus, we need a better understanding of determinants of vaccine immunogenicity – both markers that correlate with protection (innate and adaptive antibody and T-cell-mediated cellular) and those that control vaccine failure [32].
As an example, rates of primary (i.e., lack of antibody following vaccination) and secondary (i.e., waning or inadequate antibody following vaccination) measles vaccine failure are projected to be 2–10% and <0.25%, respectively [33,34]. Similarly, after three or more doses of hepatitis B vaccine, 5%–10% of persons fail to mount protective titers (≥10 mIU/ml) of hepatitis B surface antigen antibodies [35,36]. Furthermore, the current inactivated influenza vaccine is deficient in its ability to mount an effective immune response in many aging and older individuals. In fact, it protects, on average, only 30–40% of adults ≥ 65 years old versus 70–90% of younger adults from contracting influenza viral infection [37].
Although vaccines are one of the most effective public health means for preventing disease and death, they are not perfect and can cause side effects, unforeseen reactions, or vaccine adverse events (AEs). Fortunately, most reactions associated with vaccines are mild, such as redness, soreness, swelling, rash or fever, and transient. Serious AEs after vaccination are uncommon, but they may involve Guillain-Barré syndrome (after swine flu vaccine, under one case per 100,000 vaccinated) [38–40], narcolepsy (following a AS03-adjuvanted influenza vaccine, one case per ~16,000 vaccinated) [41,42], severe rash and allergic reaction (after influenza, MMR and yellow fever vaccines, one case per ~100,000) [43–45], seizures and acute encephalopathy (after whole-cell pertussis vaccine, the estimated risk is 6 to 9 cases per 100,000 vaccinated) [46,47], and paralytic polio (after live attenuated oral polio vaccine, 1 case per 2–3 million vaccinated) [48]. Clinical and laboratory research is critical for determining the causal mechanisms of such AEs. Rapid advances in the field of “adversomics,” which our group defined as being “the application of immunogenomics and systems biology to understand the genetic and non-genetic drivers of vaccine AEs at the molecular level,” are informing the development of new vaccines [49,50].
The ultimate goal of vaccine research is to develop better vaccines and new adjuvants against major infectious diseases, including safe and effective vaccines for tuberculosis, rotavirus, malaria, HIV and other emerging and reemerging pathogens. Advancements in healthcare have contributed to the aging of the global population and the growing number of persons with immunocompromising medical conditions. Along the same lines, the population of many developed nations is aging rapidly and so the impact of immunosenescence, or the age-related decline in immune function, must be considered. This is especially important for diseases, such as influenza, with a disproportionate public health impact on older individuals. Additional vaccine strategies are needed to overcome the immunosenescence and immunodeficiencies of these populations. Further efforts are also needed to better understand age- and gender-related changes and defects in the regulation and function of innate and adaptive immune responses to vaccines. This will be essential to the development of vaccines or treatments that overcome immunosenescence [51–53].
Acceptance of Vaccines
The continued, widespread acceptance of vaccines remains a critical component to the overall effectiveness of those vaccines in maintaining individual and herd immunity, and therefore public health strategy. While a large percentage of the population still readily accepts vaccines as safe and reliable, a growing subset of the population believes that vaccines are unsafe or potentially even deadly. Generally speaking, this subset believes that vaccines have a higher potential to be dangerous than actually contracting the disease, leading them to make choices against vaccination. Therefore, it is critical that those in the healthcare profession are able to help individuals choose to accept vaccines. However, numerous issues and determinants play out to create hesitancy or refusal to accept vaccines.
In an article by Poland and Bronson [54], the authors discussed the importance of examining the layers of closely linked factors (proximate determinants) and external factors (non-proximate determinants) affecting vaccine decision making, to address vaccine acceptance or hesitancy. This critical, and often overlooked, point was summarized by saying:
“The reality of childhood vaccination uptake…is much more complex. Proximate determinants don’t exist in vacuums. They can and do interact with each other and they can vary from person to person, from group to group and over time in complex, non-intuitive ways. Additionally, non-proximate determinants can also affect how vaccine uptake plays out for both individuals and groups. Psychological, ethical, social, cultural, political, economic, ecological and historical factors, not to mention interpersonal, institutional and state power structures can also influence whether or not children receive vaccines [54].”
In the third millennium, people are now more “connected” than ever before due to ease in global travel, the increase in technology availability and internet access, and the globalization of our society; this in turn complicates the issue of vaccine hesitancy or acceptance. As humans don’t exist in a vacuum, neither do the decisions they make. In this millennium, it is critical to address these issues by understanding the various lenses through which individuals make decisions: their personal beliefs, their individual preferred cognitive styles, and the proximate and non-proximate determinants that impact their lives.
In a recent editorial, two of us (CMP, GAP) [55] outlined six preferred cognitive styles an individual may employ in decision making regarding one’s health. A cognitive style is simply a preferred way of thinking that helps an individual understand and process the world. When healthcare workers (HCWs) seek to understand an individual’s preferred cognitive style and communicate with them based on that style, they can help address vaccine hesitancy in a way that the patient best understands. As a result, the patient may choose to engage in healthy behaviors and choices. The task of processing vaccine hesitancy or rejection with a patient is extremely important, and to do so in such a way that the patient has the best chance of making a wise decision. The Preferred Cognitive Styles and Decision-Making model emphasizes that medicine is increasingly moving to a more individualized style of care, and using a patient’s preferred cognitive style (thus employing psychology and mental health within the medical office) allows patients to engage with an even more personalized style of care, while increasing the probability that through health education patients make better informed vaccine decisions.[54–56]
New Vaccine Development
As described in the previous sections, new vaccine products have multiple obstacles to overcome: 1) the current set of pathogens for which we desperately need new vaccines are considerably more complex than previous vaccine targets; 2) empirical approaches to vaccine development (Isolate, Inactivate, and Inject) do not work for current pathogens; 3) regulatory hurdles are greater now than they have been in the past; and 4) an increasingly risk-averse culture demands significantly higher safety standards for vaccines. These challengesare counterbalanced by the tremendous advances that have been made in the fields of immunology and vaccinologythat provide exciting tools to overcome these challenges.
Despite the phenomenal success of early vaccines, we still do not fully understand, at a mechanistic level, the rules governing immunogenicity and the development of protective immunity. A collective endeavor to address this knowledge gap, entitled the “Human Vaccine Project,” [57] is bringing together researchers, academic institutions, public health agencies, industry, philanthropic foundations, and non-governmental agencies to define the underlying rules of immunogenicity in order to develop safer and more effective vaccines [58,59].
We have learned an incredible amount about the immune system in the last decade, including the characterization of new T helper subsets, regulatory T cells, dendritic cells, and macrophages; [60–64] the recognition of the role of the microbiome and innate immunity in the development and maintenance of robust adaptive immunity; [65] the development and use of novel adjuvants;;[66,67] and a growing body of research aimed at developing a comprehensive understanding the immune response as an interconnected, networked, complex system. [68–70].
Advances in the tools, technologies, and research reagents available for the study of biological systems have also seen recent dramatic changes (Table 3). Next-generation sequencing technologies now allow investigators to rapidly sequence pathogen genomes, conduct global gene expression studies with increased sensitivity, identify rare mRNA transcripts, perform alternate splicing, conduct genome-wide DNA methylation analysis, perform ChIP-Seq, and numerous other applications [71]. These technologies are now being adapted to the study of single cells [72]. Advances in multiparameter flow cytometry and the integration of mass spectrometry (CyTOF) enable researchers to simultaneously study 40+ parameters of cellular phenotype and function at an individual cell basis [73–75]. Cutting-edge bioinformatics algorithms and routines capable of handling “Big Data” are providing sophisticated methodologies to integrate, visualize, and interpret these datasets [76–80].
Table 3.
New Technologies for Vaccine Research.
| Technology or Tool | Description |
|---|---|
| Next Generation Sequencing | Massively parallel sequencing platforms that generate millions of bases of sequence reads in a relatively short timeframe. These platforms have a wide variety of applications including, but not limited to: whole genome sequencing, transcriptome profiling, or identification of genome-wide patterns of miRNA patterns or DNA methylation. |
| Proteomics | Mass spectrometry techniques for the unbiased, semi-quantitative characterization of the entire protein content of a sample. |
| CyTOF | Mass cytometry. Single cell analysis platform combining mass spectrometry with flow cytometry. Antibodies are tagged with heavy metal ions and used to stain cell populations of interest. Spectral overlap limits flow cytometry to ~20 markers. CyTOF has increased signal resolution and potentially offers 10X as many parameters. |
| Vaccinomics / Systems Biology | An approach to understanding variations in immune response to vaccines that utilizes high-dimensional technologies to fully characterize, at an omics level, the perturbations elicited by vaccination. This rich dataset is then analyzed using bioinformatic tools and computational modeling to create a predictive model of immune responses that can be tested and improved through iterative experimental cycles. |
| Single Cell Sequencing | A set of methods for capturing the transcriptome of individual cells. Methods include: STRT, SMART-Seq, Quartz-Seq, and CEL-Seq |
| Epitope Prediction Algorithms | A set of computational tools that use binding motif databases, artificial neural networks, hidden Markov models, support vector machines or other methods to predict MHC-binding peptides in protein sequences. Examples include: SYFPEITHI, BIMAS, PREDEP, RANKPEP |
We have coined the term “Vaccinomics” for systems biology approaches that include immunogenetics and immunogenomics to studying immune responses to vaccines, and applying this knowledge to directed vaccine development. Similar approaches such as “Systems Vaccinology” or “Computational Immunology” [32,68,70,81–91] have also been coined. Vaccinomics is a collaborative approach that brings together biology, computer science, statistics, and bioinformatics. Together, these multidisciplinary teams perturb the immune system in carefully controlled ways and collect high-dimensional datasets characterizing the immune response at multiple levels (the transcriptome, the epigenome, the proteome, the metabolome, the lipidome—including individual molecules, cell populations and surface/functional phenotypes, and tissues/organs). The data is then organized, integrated, and mined using sophisticated computer algorithms and bioinformatics approaches in order to develop predictive and/or mechanistic models of immune function [76]. These models are then tested in a new round of experimentation that, in turn, yields additional insights and raises more biological questions.
We believe that this work will be crucial toward understanding the complex interplay between human beings and the pathogens of high public health interest [92]. These types of studies will allow a rationale, logical, and directed approach to developing novel vaccines and improving current ones.
More effective vaccines are also needed for certain population groups. Successful vaccination of older individuals is often complicated by pre-existing medical conditions and medicine usage.. Similarly, infants and young children have immune systems that have not yet fully developed and respond to infection/vaccination differently than adults [93,94]. The development of more effective vaccines and treatment options for the very young and for older individuals will require a more comprehensive understanding of how the various components of the immune response collectively respond to immunization or infection [95–99]. Vaccinomics approaches integrating high-dimensional datasets and predictive modeling will likely play a central role in these efforts.
The Way Forward
The above brief review highlights the challenges facing the field of vaccinology in the third millennium. There are a few “foundational pillars” around which teams of scientists must collaborate: issues of emerging diseases that threaten health of humans and animals; the need for novel vaccine adjuvants; the challenges of vaccine research and development financing; and the science that is developing around the critical need to communicate, educate—and convince—the populace to endorse and accept vaccines.
We also call attention to the growing need for bioinformatics in 21st century vaccine development. Currently, however, few bioinformaticians are involved in vaccine research; given the early growth of the field, the tools and analytic routines needed for assisting new vaccine development are at an early stage of development. Such tools are needed if we are to take advantage of the economies of “big data”[76].
To the above mix, it is apparent that the costs associated with new vaccine development continue to increase, often requiring $1 billion to get to licensure. This, in turn, requires stable and sustained investment, and a more seamless integration of vaccine financing from basic research, to preclinical development, to clinical development, to population utilization. Currently, this route is expensive, fragmented, discordant, disconnected, and uncertain. This state of affairs leads to more expense, delays in bringing new vaccine candidates into clinical trials, and results in continuing unmet public health needs. A recent editorial reviewed some of these issues and suggested the development of a global vaccine development fund [10]. We find this novel concept to be compelling, as it is highly likely to galvanize the necessary teams of scientists and financial and regulatory infrastructure needed in order to make achievable progress and meet the needs of a diverse and changing global public health need.
Highlights.
Vaccines have eradicated smallpox, nearly eradicated polio, and resulted in > 99.9% reduction in incidence rates for many diseases
There are significant challenges facing vaccine development for current and emerging diseases (Ebola, HIV, Zika, Malaria).
New vaccine and adjuvant development is necessary to improve immunogenicity and safety profiles of vaccines.
New educational methods and outreach programs are needed to combat misinformation and vaccine hesitancy
Acknowledgments
The authors were supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under award numbers U01AI089859, N01AI40065, R37AI048793, and R01AI033144.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest Statement
Dr. Poland is the chair of a Safety Evaluation Committee for novel investigational vaccine trials being conducted by Merck Research Laboratories. Dr. Poland offers consultative advice on vaccine development to Merck & Co. Inc., CSL Biotherapies, Avianax, Dynavax, Novartis Vaccines and Therapeutics, Emergent Biosolutions, Adjuvance, and Microdermis. Drs. Poland and Ovsyannikova hold two patents related to vaccinia and measles peptide research. JA Whitaker receives funding for Mayo Clinic research from Pfizer Independent Grants for Learning and Change. These activities have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are conducted in compliance with Mayo Clinic Conflict of Interest policies. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of special note have been highlighted as:
of special interest (*)
of outstanding interest (**)
- 1.Centers for Disease Control and Prevention. MMWR. 1999;48:621–629. [Google Scholar]
- 2.RTSS Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. [Date accessed: February 12, 2016];HIV/AIDS Fact Sheet No. 360. 2015 http://www.who.int/mediacentre/factsheets/fs360/en/
- 4.World Health Organization. [Date accessed: February 12, 2016];Ebola Situation Report. 2015 http://apps.who.int/iris/bitstream/10665/183400/1/Ebolasitrep_26Aug2015_eng.pdf?ua=1.
- 5.Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, Camacho A, Carroll MW, Doumbia M, Draguez B, Duraffour S, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet. 2015;386:857–66. doi: 10.1016/S0140-6736(15)61117-5. [DOI] [PubMed] [Google Scholar]
- 6.Marzi A, Robertson SJ, Haddock E, Feldmann F, Hanley PW, Scott DP, Strong JE, Kobinger G, Best SM, Feldmann H. EBOLA VACCINE. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science. 2015;349:739–742. doi: 10.1126/science.aab3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarwar UN, Costner P, Enama ME, Berkowitz N, Hu Z, Hendel CS, Sitar S, Plummer S, Mulangu S, Bailer RT, et al. Safety and immunogenicity of DNA vaccines encoding Ebolavirus and Marburgvirus wild-type glycoproteins in a phase I clinical trial. J Infect Dis. 2015;211:549–557. doi: 10.1093/infdis/jiu511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kibuuka H, Berkowitz NM, Millard M, Enama ME, Tindikahwa A, Sekiziyivu AB, Costner P, Sitar S, Glover D, Hu Z, et al. Safety and immunogenicity of Ebola virus and Marburg virus glycoprotein DNA vaccines assessed separately and concomitantly in healthy Ugandan adults: a phase 1b, randomised, double-blind, placebo-controlled clinical trial. Lancet. 2015;385:1545–1554. doi: 10.1016/S0140-6736(14)62385-0. [DOI] [PubMed] [Google Scholar]
- 9.Consortium EçSRVT. The ring vaccination trial: a novel cluster randomised controlled trial design to evaluate vaccine efficacy and effectiveness during outbreaks, with special reference to Ebola. BMJ. 2015;351:h3740. doi: 10.1136/bmj.h3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Plotkin SA, Mahmoud AA, Farrar J. Establishing a Global Vaccine-Development Fund. N Engl J Med. 2015;373:297–300. doi: 10.1056/NEJMp1506820. This paper summarizes the challenges inherent in meeting global infectious disease threats with new vaccines, and proposes a novel approach to meeting this challenge. [DOI] [PubMed] [Google Scholar]
- 11.Cardell K, Akerlind B, Sallberg M, Fryden A. Excellent response rate to a double dose of the combined hepatitis A and B vaccine in previous nonresponders to hepatitis B vaccine. J Infect Dis. 2008;198:299–304. doi: 10.1086/589722. [DOI] [PubMed] [Google Scholar]
- 12.Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis. 2008;197:490–502. doi: 10.1086/524146. [DOI] [PubMed] [Google Scholar]
- 13.Michalik DE, Steinberg SP, LaRussa PS, Edwards KM, Wright PF, Arvin AM, Gans HA, Gershon AA. Primary vaccine failure after 1 dose of varicella vaccine in healthy children. J Infect Dis. 2008;197:944–949. doi: 10.1086/529043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McVernon J, Andrews N, Slack MP, Ramsay ME. Risk of vaccine failure after Haemophilus influenzae type b (Hib) combination vaccines with acellular pertussis. Lancet. 2003;361:1521–1523. doi: 10.1016/s0140-6736(03)13171-6. [DOI] [PubMed] [Google Scholar]
- 15**.Poland GA, Ovsyannikova IG, Jacobson RM, Smith DI. Heterogeneity in vaccine immune response: the role of immunogenetics and the emerging field of vaccinomics. Clin Pharmacol Ther. 2007;82:653–664. doi: 10.1038/sj.clpt.6100415. This paper summarizes the new vaccinomics approach for vaccine development. [DOI] [PubMed] [Google Scholar]
- 16.Ovsyannikova IG, Dhiman N, Jacobson RM, Poland GA. Human leukocyte antigen polymorphisms: variable humoral immune responses to viral vaccines. Exp Rev Vaccines. 2006;5:33–43. doi: 10.1586/14760584.5.1.33. [DOI] [PubMed] [Google Scholar]
- 17.Clifford HD, Hayden CM, Khoo SK, Naniche D, Mandomando IM, Zhang G, Richmond P, Le Souef PN. Polymorphisms in key innate immune genes and their effects on measles vaccine responses and vaccine failure in children from Mozambique. Vaccine. 2012;30:6180–6185. doi: 10.1016/j.vaccine.2012.07.063. [DOI] [PubMed] [Google Scholar]
- 18.Gelder CM, Lambkin R, Hart KW, Fleming D, Williams OM, Bunce M, Welsh KI, Marshall SE, Oxford J. Associations between human leukocyte antigens and nonresponsiveness to influenza vaccine. J Infect Dis. 2002;185:114–117. doi: 10.1086/338014. [DOI] [PubMed] [Google Scholar]
- 19.Davila S, Froeling FE, Tan A, Bonnard C, Boland GJ, Snippe H, Hibberd ML, Seielstad M. New genetic associations detected in a host response study to hepatitis B vaccine. Genes Immunity. 2010;11:232–238. doi: 10.1038/gene.2010.1. [DOI] [PubMed] [Google Scholar]
- 20*.Plotkin SA. The pertussis problem. Clin Infect Dis. 2014;58:830–833. doi: 10.1093/cid/cit934. This review discusses the resurgence of pertussis (whooping cough) and possible improvements in pertussis vaccines. [DOI] [PubMed] [Google Scholar]
- 21.Chiappini E, Stival A, Galli L, de Martino M. Pertussis re-emergence in the post-vaccination era. BMC Infect Dis. 2013;13:151. doi: 10.1186/1471-2334-13-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmader KE, Levin MJ, Gnann JW, Jr, McNeil SA, Vesikari T, Betts RF, Keay S, Stek JE, Bundick ND, Su SC, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50–59 years. Clin Infect Dis. 2012;54:922–928. doi: 10.1093/cid/cir970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 24.Chlibek R, Bayas JM, Collins H, de la Pinta ML, Ledent E, Mols JF, Heineman TC. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults >=50 years of age. J Infect Dis. 2013;208:1953–1961. doi: 10.1093/infdis/jit365. [DOI] [PubMed] [Google Scholar]
- 25.Chlibek R, Smetana J, Pauksens K, Rombo L, Van den Hoek JA, Richardus JH, Plassmann G, Schwarz TF, Ledent E, Heineman TC. Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: a phase II, randomized, controlled study. Vaccine. 2014;32:1745–1753. doi: 10.1016/j.vaccine.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. New Engl J Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 27.Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, Malzone C, Castellino F, Gentile C, McNally T, et al. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci U S A. 2009;106:7962–7967. doi: 10.1073/pnas.0903181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ansaldi F, Bacilieri S, Durando P, Sticchi L, Valle L, Montomoli E, Icardi G, Gasparini R, Crovari P. Cross-protection by MF59-adjuvanted influenza vaccine: neutralizing and haemagglutination-inhibiting antibody activity against A(H3N2) drifted influenza viruses. Vaccine. 2008;26:1525–1529. doi: 10.1016/j.vaccine.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 29.Frey SE, Reyes MR, Reynales H, Bermal NN, Nicolay U, Narasimhan V, Forleo-Neto E, Arora AK. Comparison of the safety and immunogenicity of an MF59(R)-adjuvanted with a non-adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine. 2014;32:5027–5034. doi: 10.1016/j.vaccine.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 30.DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. New Engl J Med. 2014;371:635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 31.Montplaisir J, Petit D, Quinn MJ, Ouakki M, Deceuninck G, Desautels A, Mignot E, De Wals P. Risk of narcolepsy associated with inactivated adjuvanted (AS03) A/H1N1 (2009) pandemic influenza vaccine in Quebec. PLos ONE. 2014;9:e108489. doi: 10.1371/journal.pone.0108489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poland GA, Kennedy RB, McKinney BA, Ovsyannikova IG, Lambert ND, Jacobson RM, Oberg AL. Vaccinomics, adversomics, and the immune response network theory: Individualized vaccinology in the 21st century. Semin Immunol. 2013;25:89–103. doi: 10.1016/j.smim.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anders JF, Jacobson RM, Poland GA, Jacobsen SJ, Wollan PC. Secondary failure rates of measles vaccines: a metaanalysis of published studies. Pediatr Infect Dis J. 1996;15:62–66. doi: 10.1097/00006454-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Poland GA, Jacobson RM, Thampy AM, Colbourne SA, Wollan PC, Lipsky JJ, Jacobson SJ. Measles re-immunization in children seronegative after initial immunization. J Amer Med Assoc. 1997;277:1156–1158. [PubMed] [Google Scholar]
- 35.Cardell K, Fryden A, Normann B. Intradermal hepatitis B vaccination in health care workers. Response rate and experiences from vaccination in clinical practise. Scand J Infect Dis. 1999;31:197–200. doi: 10.1080/003655499750006272. [DOI] [PubMed] [Google Scholar]
- 36.Alper CA, Kruskall MS, Marcus-Bagley D, Craven DE, Katz AJ, Brink SJ, Dienstag JL, Awdeh Z, Yunis EJ. Genetic prediction of nonresponse to hepatitis B vaccine. New Eng J Med. 1989;321:708–712. doi: 10.1056/NEJM198909143211103. [DOI] [PubMed] [Google Scholar]
- 37.Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004;103:133–138. doi: 10.1016/j.virusres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 38.Marks JS, Halpin TJ. Guillain-Barre syndrome in recipients of A/New Jersey influenza vaccine. J Amer Med Assoc. 1980;243:2490–2494. [PubMed] [Google Scholar]
- 39.Haber P, DeStefano F, Angulo FJ, Iskander J, Shadomy SV, Weintraub E, Chen RT. Guillain-Barre syndrome following influenza vaccination. J Amer Med Assoc. 2004;292:2478–2481. doi: 10.1001/jama.292.20.2478. [DOI] [PubMed] [Google Scholar]
- 40.Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, Retailliau HF, Eddins DL, Bryan JA. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976--1977. Am J Epidemiol. 1979;110:105–123. doi: 10.1093/oxfordjournals.aje.a112795. [DOI] [PubMed] [Google Scholar]
- 41.Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, Himanen SL, Hublin C, Julkunen I, Olsen P, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLos ONE. 2012;7:e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Partinen M, Saarenpaa-Heikkila O, Ilveskoski I, Hublin C, Linna M, Olsen P, Nokelainen P, Alen R, Wallden T, Espo M, et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLos ONE. 2012;7:e33723. doi: 10.1371/journal.pone.0033723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santillan Salas CF, Mehra S, Pardo Crespo MR, Juhn YJ. Asthma and severity of 2009 novel H1N1 influenza: a population-based case-control study. J Asthma. 2013;50:1069–1076. doi: 10.3109/02770903.2013.834505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freitas DR, Moura E, Araujo G, Cardoso A, Scheidt P, Ferraz E, Madalosso G, Chen RT, Hatch DL. Investigation of an outbreak of hypersensitivity-type reactions during the 2004 national measles-mumps-rubella vaccination campaign in Brazil. Vaccine. 2013;31:950–954. doi: 10.1016/j.vaccine.2012.11.095. [DOI] [PubMed] [Google Scholar]
- 45.Lawrence GL, Burgess MA, Kass RB. Age-related risk of adverse events following yellow fever vaccination in Australia. Commun Dis Intell Q Rep. 2004;28:244–248. [PubMed] [Google Scholar]
- 46.Patel MK, Patel TK, Tripathi CB. Diphtheria, pertussis (whooping cough), and tetanus vaccine induced recurrent seizures and acute encephalopathy in a pediatric patient: Possibly due to pertussis fraction. J Pharmacol Pharmacother. 2012;3:71–73. doi: 10.4103/0976-500X.92514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barlow WE, Davis R, Glasser JW, Rhodes PH, Thompson RS, Mullooly JP, Black SB, Shinefield HR, Ward JI, Marcy M, et al. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. New Engl J Med. 2001;345:656–661. doi: 10.1056/NEJMoa003077. [DOI] [PubMed] [Google Scholar]
- 48.Higashigawa M, Maegawa K, Honma H, Yoshino A, Onozato K, Nashida Y, Fujiwara T, Inoue M. Vaccine-associated paralytic poliomyelitis in an infant with perianal abscesses. J Infect Chemother. 2010;16:356–359. doi: 10.1007/s10156-010-0065-5. [DOI] [PubMed] [Google Scholar]
- 49.Poland GA, Ovsyannikova IG, Jacobson RM. Adversomics: the emerging field of vaccine adverse event immunogenetics. Pediatr Infect Dis J. 2009;28:431–432. doi: 10.1097/INF.0b013e3181a6a511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Whitaker JA, Ovsyannikova IG, Poland GA. Adversomics: a new paradigm for vaccine safety and design. Exp Rev Vaccines. 2015:1–13. doi: 10.1586/14760584.2015.1038249. This review outlines the current state of adversomics research and known associations and mechanisms of vaccine adverse events and reactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen WH, Kozlovsky BF, Effros RB, Grubeck-Loebenstein B, Edelman R, Sztein MB. Vaccination in the elderly: an immunological perspective. Trends Immunol. 2009;30:351–359. doi: 10.1016/j.it.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Effros RB. Problems and solutions to the development of vaccines in the elderly. Immunol Allergy Clin North Am. 2003;23:41–55. doi: 10.1016/s0889-8561(02)00055-3. [DOI] [PubMed] [Google Scholar]
- 53.Grubeck-Loebenstein B, Della BS, Iorio AM, Michel JP, Pawelec G, Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clin Exp Res. 2009;21:201–209. doi: 10.1007/BF03324904. [DOI] [PubMed] [Google Scholar]
- 54*.Poland CM, Brunson EK. The need for a multi-disciplinary perspective on vaccine hesitancy and acceptance. Vaccine. 2015;33:277–279. doi: 10.1016/j.vaccine.2014.11.022. This paper explores cross-disciplinary approaches to the issues of vaccine hesitancy and rejection. [DOI] [PubMed] [Google Scholar]
- 55.Poland CM, Poland GA. Vaccine education spectrum disorder: the importance of incorporating psychological and cognitive models into vaccine education. Vaccine. 2011;29:6145–6148. doi: 10.1016/j.vaccine.2011.07.131. [DOI] [PubMed] [Google Scholar]
- 56.Poland CM, Poland GA. Vaccination as a public health measure: challenges. In: Cockerham WC, Dingwall R, Quah SR, editors. The Wiley-Blackwell Encyclopedia of Health, Illness, Behavior and Society. Wiley-Blackwell; 2014. [Google Scholar]
- 57**.Koff WC, Gust ID, Plotkin SA. Toward a human vaccines project. Nat Immunol. 2014;15:589–592. doi: 10.1038/ni.2871. This article provides a rational for the development of the “Human Vaccines Project” to accelerate the development of vaccines against major global diseases in humans. [DOI] [PubMed] [Google Scholar]
- 58.Koff WC, Burton DR, Johnson PR, Walker BD, King CR, Nabel GJ, Ahmed R, Bhan MK, Plotkin SA. Accelerating next-generation vaccine development for global disease prevention. Science. 2013;340:1232910. doi: 10.1126/science.1232910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schenkelberg T, Kieny MP, Bianco AE, Koff WC. Building the Human Vaccines Project: strategic management recommendations and summary report of the 15–16 July 2014 business workshop. Exp Rev Vaccines. 2015;14:629–636. doi: 10.1586/14760584.2015.1013466. [DOI] [PubMed] [Google Scholar]
- 60.Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who’s who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. 2013;43:2797–2809. doi: 10.1002/eji.201343751. [DOI] [PubMed] [Google Scholar]
- 61.Geginat J, Paroni M, Maglie S, Alfen JS, Kastirr I, Gruarin P, De Simone M, Pagani M, Abrignani S. Plasticity of human CD4 T cell subsets. Front Immunol. 2014;5:630. doi: 10.3389/fimmu.2014.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Durand M, Segura E. The known unknowns of the human dendritic cell network. Front Immunol. 2015;6:129. doi: 10.3389/fimmu.2015.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Ann Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 65*.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. This is an important review regarding emerging principles of innate control of adaptive immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valdez Y, Brown EM, Finlay BB. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014;35:526–537. doi: 10.1016/j.it.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 68**.Pulendran B. Systems vaccinology: probing humanity’s diverse immune systems with vaccines. Proc Natl Acad Sci U S A. 2014;111:12300–12306. doi: 10.1073/pnas.1400476111. This paper provides a review of a systems vaccinology approach and its application to studying vaccine-induced immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Obermoser G, Presnell S, Domico K, Xu H, Wang Y, Anguiano E, Thompson-Snipes L, Ranganathan R, Zeitner B, Bjork A, et al. Systems scale interactive exploration reveals quantitative and qualitative differences in response to influenza and pneumococcal vaccines. Immunity. 2013;38:831–844. doi: 10.1016/j.immuni.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70**.Oberg AL, Kennedy RB, Li P, Ovsyannikova IG, Poland GA. Systems biology approaches to new vaccine development. Curr Opin Immunol. 2011;23:436–443. doi: 10.1016/j.coi.2011.04.005. This paper outlines in detail the new field of vaccinomics approaches to the design of novel vaccine candidates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reuter JA, Spacek DV, Snyder MP. High-throughput sequencing technologies. Mol Cell. 2015;58:586–597. doi: 10.1016/j.molcel.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Navin NE. Advances and applications of single-cell sequencing technologies. Mol Cell. 2015;58:598–609. doi: 10.1016/j.molcel.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bendall SC, Nolan GP, Roederer M, Chattopadhyay PK. A deep profiler’s guide to cytometry. Trends Immunol. 2012;33:323–332. doi: 10.1016/j.it.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ornatsky O, Bandura D, Baranov V, Nitz M, Winnik MA, Tanner S. Highly multiparametric analysis by mass cytometry. J Immunol Methods. 2010;361:1–20. doi: 10.1016/j.jim.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 75.Bjornson ZB, Nolan GP, Fantl WJ. Single-cell mass cytometry for analysis of immune system functional states. Curr Opin Immunol. 2013;25:484–494. doi: 10.1016/j.coi.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oberg AL, McKinney BA, Schaid DJ, Pankratz VS, Kennedy RB, Poland GA. Lessons learned in the analysis of high-dimensional data in vaccinomics. Vaccine. 2015 doi: 10.1016/j.vaccine.2015.04.088. S0264-410X:00574-00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakaya HI, Pulendran B. Vaccinology in the era of high-throughput biology. Philos Trans R Soc Lond B Biol Sci. 2015:370. doi: 10.1098/rstb.2014.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zak DE, Tam VC, Aderem A. Systems-level analysis of innate immunity. Ann Rev Immunol. 2014;32:547–577. doi: 10.1146/annurev-immunol-032713-120254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79*.Germain RN, Meier-Schellersheim M, Nita-Lazar A, Fraser ID. Systems biology in immunology: a computational modeling perspective. Ann Rev Immunol. 2011;29:527–585. doi: 10.1146/annurev-immunol-030409-101317. This is an important review of the computational modeling methods used to create computational models and to conduct simulations of immune function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Brevern AG, Meyniel JP, Fairhead C, Neuveglise C, Malpertuy A. Trends in IT Innovation to Build a Next Generation Bioinformatics Solution to Manage and Analyse Biological Big Data Produced by NGS Technologies. BioMed Res Int. 2015;2015:904541. doi: 10.1155/2015/904541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poland GA, Kennedy RB, Ovsyannikova IG. Vaccinomics and personalized vaccinology: Is science leading us toward a new path of directed vaccine development and discovery? PLoS Path. 2011;7:e1002344. doi: 10.1371/journal.ppat.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kennedy RB, Poland GA. The Top Five “Game Changers” in Vaccinology: Toward Rational and Directed Vaccine Development. Omics. 2011;15:533–537. doi: 10.1089/omi.2011.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poland GA, Ovsyannikova IG, Kennedy RB, Haralambieva IH, Jacobson RM. Vaccinomics and a new paradigm for the development of preventive vaccines against viral infections. Omics. 2011;15:625–636. doi: 10.1089/omi.2011.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ovsyannikova IG, Poland GA. Vaccinomics: current findings, challenges and novel approaches for vaccine development. Am Assoc Pharm Sci. 2011;13:438–444. doi: 10.1208/s12248-011-9281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haralambieva IH, Poland GA. Vaccinomics, predictive vaccinology and the future of vaccine development. Future Microbiol. 2010;5:1757–1760. doi: 10.2217/fmb.10.146. [DOI] [PubMed] [Google Scholar]
- 86.Poland GA, Oberg AL. Vaccinomics and bioinformatics: accelerants for the next golden age of vaccinology. Vaccine. 2010;28:3509–3510. doi: 10.1016/j.vaccine.2010.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Poland GA, Jacobson RM, Ovsyannikova IG. Trends affecting the future of vaccine development and delivery: The role of demographics, regulatory science, the anti-vaccine and consumer culture and vaccinomics. Vaccine. 2009;27:3240–3244. doi: 10.1016/j.vaccine.2009.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poland GA, Ovsyannikova IG, Jacobson RM. Personalized vaccines: the emerging field of vaccinomics. Exp Opin Biol Ther. 2008;8:1659–1667. doi: 10.1517/14712598.8.11.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89**.Tsang JS. Utilizing population variation, vaccination, and systems biology to study human immunology. Trends Immunol. 2015;36:479–493. doi: 10.1016/j.it.2015.06.005. This paper discusses recent developments in the field of systmes biology with emphasis on baseline correlates of vaccine responses, sources of immune-state variability, as well as relevant features of study design, data generation, and computational analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pappalardo F, Flower D, Russo G, Pennisi M, Motta S. Computational modelling approaches to vaccinology. Pharmacol Res. 2015;92:40–45. doi: 10.1016/j.phrs.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 91.Mooney M, McWeeney S, Canderan G, Sekaly RP. A systems framework for vaccine design. Curr Opin Immunol. 2013;25:551–555. doi: 10.1016/j.coi.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gottschalk RA, Martins AJ, Sjoelund VH, Angermann BR, Lin B, Germain RN. Recent progress using systems biology approaches to better understand molecular mechanisms of immunity. Semin Immunol. 2013;25:201–208. doi: 10.1016/j.smim.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate Immune Function by Toll-like Receptors: Distinct Responses in Newborns and the Elderly. Immunity. 2012;37:771–783. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Levy O, Goriely S, Kollmann TR. Immune response to vaccine adjuvants during the first year of life. Vaccine. 2013;31:2500–2505. doi: 10.1016/j.vaccine.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boraschi D, Italiani P. Immunosenescence and vaccine failure in the elderly: strategies for improving response. Immunol Letters. 2014;162:346–353. doi: 10.1016/j.imlet.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 96*.Poland GA, Ovsyannikova IG, Kennedy RB, Lambert ND, Kirkland JL. A systems biology approach to the effect of aging, immunosenescence and vaccine response. Curr Opin Immunol. 2014;29C:62–68. doi: 10.1016/j.coi.2014.04.005. This is a recent paper exploring the issues of immunosenescence and vaccine immune responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duraisingham SS, Rouphael N, Cavanagh MM, Nakaya HI, Goronzy JJ, Pulendran B. Systems biology of vaccination in the elderly. Curr Topics Microbiol Immunol. 2013;363:117–142. doi: 10.1007/82_2012_250. [DOI] [PubMed] [Google Scholar]
- 98.Whiting CC, Siebert J, Newman AM, Du HW, Alizadeh AA, Goronzy J, Weyand CM, Krishnan E, Fathman CG, Maecker HT. Large-Scale and Comprehensive Immune Profiling and Functional Analysis of Normal Human Aging. PLos ONE. 2015;10:e0133627. doi: 10.1371/journal.pone.0133627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Banchereau R, Baldwin N, Cepika AM, Athale S, Xue Y, Yu CI, Metang P, Cheruku A, Berthier I, Gayet I, et al. Transcriptional specialization of human dendritic cell subsets in response to microbial vaccines. Nat Commun. 2014;5:5283. doi: 10.1038/ncomms6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.UNAIDS. [Date Accessed: February 12, 2016];Fact sheet 2015. http://www.unaids.org/en/resources/campaigns/HowAIDSchangedeverything/factsheet.
- 101.World Health Organization. [Date Accessed: February 12, 2016];Global tuberculosis report 2015. http://www.who.int/tb/publications/global_report/en/
- 102.World Health Organization. [Date Accessed: February 12, 2016];Malaria fact sheet. 2016 http://www.who.int/mediacentre/factsheets/fs094/en/
- 103.World Health Organization. [Date Accessed: February 12, 2016];Hepatitis C fact sheet no. 164. 2015 http://www.who.int/mediacentre/factsheets/fs164/en/
- 104.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.World Health Organization. [Date Accessed: February 12, 2016];Ebola situation reports. 2016 http://apps.who.int/ebola/ebola-situation-reports.
- 106.Centers for Disease Control and Prevention. [Date Accessed: February 12, 2016];Marburg hemorrhagic fever. 2014 http://www.cdc.gov/vhf/marburg/resources/outbreak-table.html.
- 107.Centers for Disease Control and Prevention. [Date Accessed: February 12, 2016];What you need to know about Lassa Fever. 2015 http://www.cdc.gov/vhf/lassa/pdf/what-you-need-to-know-about-lassa-factsheet.pdf.
- 108.Centers for Disease Control and Prevention. [Date Accessed: February 12, 2016];SARS basics fact sheet. 2012 http://www.cdc.gov/sars/about/fs-sars.html.
- 109.World Health Organization. [Date Accessed: February 12, 2016];Middle East respiratory syndrome coronavirus (MERS-CoV) 2016 http://www.who.int/emergencies/mers-cov/en/
- 110.World Health Organization. [Date Accessed: February 12, 2016];Chikungunya fact sheet no. 327. 2015 http://www.who.int/mediacentre/factsheets/fs327/en/
- 111.World Health Organization. Microcephaly/Zika virus. [Date Accessed: February 12, 2016];Latest Zika situation report. 2016 http://www.who.int/emergencies/zika-virus/en/