Abstract
Larynx may alternatively serve as a target or organ-at-risk (OAR) in head and neck cancer (HNC) image-guided radiotherapy (IGRT). The objective of this study was to estimate IGRT parameters required for larynx positional error independent of isocentric alignment and suggest population–based compensatory margins. Ten HNC patients receiving radiotherapy (RT) with daily CT-on-rails imaging were assessed. Seven landmark points were placed on each daily scan. Taking the most superior anterior point of the C5 vertebra as a reference isocenter for each scan, residual displacement vectors to the other 6 points were calculated post-isocentric alignment. Subsequently, using the first scan as a reference, the magnitude of vector differences for all 6 points for all scans over the course of treatment were calculated. Residual systematic and random error, and the necessary compensatory CTV-to-PTV and OAR-to-PRV margins were calculated, using both observational cohort data and a bootstrap-resampled population estimator. The grand mean displacements for all anatomical points was 5.07mm, with mean systematic error of 1.1mm and mean random setup error of 2.63mm, while bootstrapped POIs grand mean displacement was 5.09mm, with mean systematic error of 1.23mm and mean random setup error of 2.61mm. Required margin for CTV-PTV expansion was 4.6mm for all cohort points, while the bootstrap estimator of the equivalent margin was 4.9mm. The calculated OAR-to-PRV expansion for the observed residual set-up error was 2.7mm, and bootstrap estimated expansion of 2.9mm. We conclude that the interfractional larynx setup error is a significant source of RT set-up/delivery error in HNC both when the larynx is considered as a CTV or OAR. We estimate the need for a uniform expansion of 5mm to compensate for set up error if the larynx is a target or 3mm if the larynx is an OAR when using a non-laryngeal bony isocenter.
Keywords: Image-guided radiotherapy, head and neck radiotherapy, interfractional larynx setup error, Quality-assurance, Target volume and organ-at-risk margins
Introduction
While intensity modulated radiotherapy (IMRT) has led to the ability to deliver highly conformal radiotherapy (RT) doses, a major limitation in sparing normal tissues while delivering tumoricidal doses to target volumes, after target delineation, is setup error(1, 2). Conceptually, in ICRU62(3) and ICRU 83(4), the planning target volume (PTV) and planning organ at risk volume (PRV) account for this setup error and ensure that precision target delineation does not result in either a geometric miss nor inadvertent normal tissue overdose(5, 6). However, a significant limitation of most current image guided radiation therapy (IGRT) systems is their reliance on a single point reference for corrective setup translations. For example, use of a single isocenter for portal imaging or an index slice or contour for cone-beam CT data(7) has consequences in the head and neck, where target structures or organs-at-risk (OARs) are not necessarily fixed to bony landmarks and experience translational motion during delivery of radiation(8–10). Consequently, despite excellent setup, TV/OAR displacement from the isocenter may occur in a directionally distinct manner(11). Recently, there has been increased interest in efforts to spare the carotid arteries from significant dose for early-stage laryngeal cancer(12–14). To this end, IMRT is therapeutically justified owing to the fact that these cancers are, by and large, highly curable(15) and long-term toxicity therefore becomes a significant consideration in patients with potential decades of survival. Additionally, our group and others have adopted strategies to minimize laryngeal doses for non-larynx head and neck cancers when a low neck match cannot be utilized practically(16). Such approaches are beneficial for organs like larynx where a defined planning organ at risk volume (PRV) margin might be of possible value for plan optimization as it is well documented that laryngeal overdose results in quantifiable toxicity(17).
While masks can serve to reduce patient external motion during treatment, internal target/organ movement is an unavoidable reality that must be considered as well for treatment accuracy. However, it is imperative that the use of IMRT does not result in inadvertent geometric miss which may in aggregate reduce survival probability. For this reason, we sought to ascertain the relative geometric variation in the motion of the larynx relative to a single isocenter (defined as a bony landmark) in order to ensure that our current radiotherapy margins are within evidence-based limits.
The specific aims of the current study are:
Estimation of the relative inter-fraction setup error of the laryngeal apparatus relative to a fixed isocenter, using both experimentally observed CT-on-rails data and robust estimators of population set-up error using a bootstrap methodology.
Determination of the PTV expansions required for laryngeal-targeting radiotherapy for larynx cancers
Estimation of PRV expansions required for laryngeal sparing-radiotherapy for non-laryngeal head and neck cancers
Materials/Methods
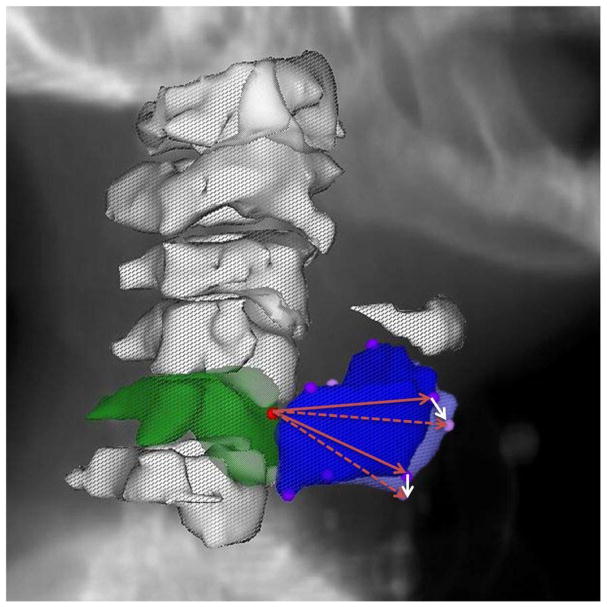
Daily DICOM-RT from a series of ten patients previously enrolled on an adaptive RT study(18, 19) were de-archived after IRB approval. Daily non-contrast CT-on-rails (350 mm FOV, 1×1×2.5 mm voxel dimensions)(20, 21) scans were acquired as detailed previously(18, 19), and imported into a treatment planning system (Pinnacle, Philips Healthcare, Andover, MA). For each daily CT-on-rails DICOM 3-D image, the C5 vertebra and thyroid cartilage were identified and 7 reference points were manually placed as a point of interest (POI) at the superior-most voxel of the anterior aspect of the C5 vertebrae, the superior-most and inferior-most voxels of the anterior aspect of thyroid cartilage, as well as the superior-most and inferior-most voxels of the most lateral aspect of the right and left thyroid cartilage cornua see (Fig. 1). The selection of larynx six POIs was based on the fact that thyroid cartilage is the largest laryngeal cartilage that forms the external framework of the larynx and houses its structural components with strong attachments. The selected landmark points shape a 3-dimensional framework of the upper and lower most boundaries of the thyroid cartilage in the midline and bilaterally to best represent laryngeal motion.
Fig. 1.
Visual depiction of the selected points of interest (POIs) with the red circle at the most superior aspect of the anterior C5 vertebra (3-D reconstructed contour in green) representing the fixed isocenteric reference and the violet circles represent selected POIs of the thyroid cartilage on Day 1 (3-D reconstructed contour in blue) while the pink circles represent the same POIs on Day 2 (not all POIs are visible because of the overlap) but in different spatial location caused by laryngeal inter-fraction motion. White arrows show example of vector displacements of two POIs relative to their original position in relation to the fixed isocenter of Day 1 (solid red arrow) in Day two (dashed red arrow)
The most superior, anterior point of the C5 vertebra was defined a priori as a fixed origin for each daily scan. On each daily CT-on-rails scan, vector displacements were obtained to the other 6 reference POIs relative to this origin. All such vectors were brought to the same origin, and as such the vectors represent the motion of the larynx relative to this origin. Conversely, the larynx represented by the 6-point structure, represents a 3-dimensional registered object moving relative to a fixed point. This isolates laryngeal motion changes relative to a point in the bony anatomy independent of errors in patient setup: the geometry is the same in every CT and any setup changes would result in only translational or rotational shifts which would be negated by the use of vectors. Assuming each patient’s initial verification (Day 1) scan was treated as a “gold standard” reference, vector differences between the initial verification scan and each daily CT-on-rails scan was calculated serially. The magnitudes of these vector differences were collected representing the daily shifts of the entire larynx relative to a fixed point from a planned setup, and as a measure of the intrinsic movement of the larynx (i.e. the motion of the larynx despite immobilization and changes in day-to-day controlled setup) see (Fig. 1).
The mean magnitude of vector displacement over all days was calculated for each patient at each POI. From these mean values, a grand means was calculated at each point to characterize the cumulative displacement for each POI. The systematic error for the population was defined as the standard deviation of the grand mean. The random error for each individual was determined to be the standard deviation about an individual’s mean value for each vector, while the population random error is given by the root mean square of individual random errors (see supplementary figure 1). The results from these calculations, the observational cohort systematic error (Σcohort) and the random error (σcohort), were used to calculate the necessary CTV-to-PTV correction sufficient for 90% of patients to receive 95% of the nominal dose for each POI, using Van Herk’s formula (22).
Additionally, using this data, the necessary PRV is calculated based on work by McKenzie et al. (23) and is calculated as:
To calculate robust non-parametric estimates for inference, bootstrap resampling was applied using Efron’s bootstrap methodology. Using an iterative resampling-replacement method, a cumulative 1,000 random distributions for each POI, was drawn from each individual patient’s distribution of daily shifts using the original 1854 individual experimentally-derived daily POI displacement measures. The resultant 6×105 resampled distributions (i.e. 6 POIs × 10 original patient distributions × 10,000 replacement/resampling iterations) were then used to generate a robust systematic error (Σbootstrap) and random error (σbootstrap) for robust probabilistic estimation of the population-level magnitude of larynx inter-fractional motion at each POI. Likewise, a 95% tolerance interval (i.e., a 95% confidence interval of a range encompassing 95% of all displacements) was derived as an estimator of the internal target volume for both cohort and bootstrap distributions (95% TIcohort and 95% TIbootstrap).
Results
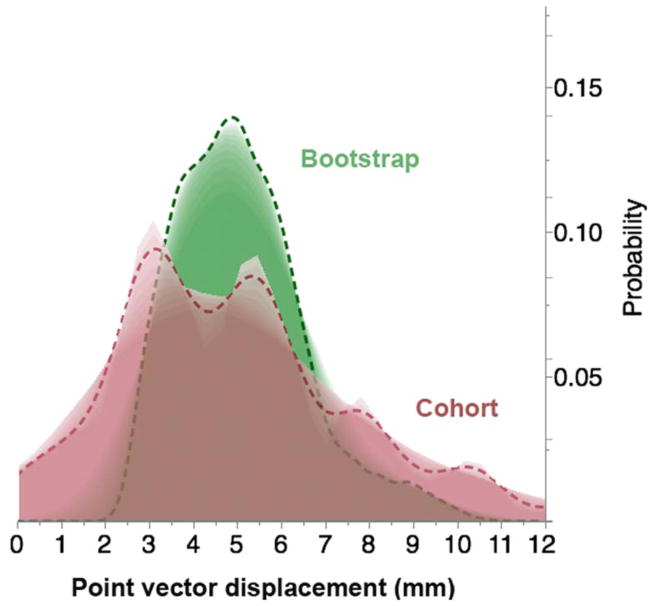
A total of 309 daily CT-on-rails DICOM images were utilized for all ten patients, an average of 31 scans per patient, with a minimum of 25 scans and a maximum of 35 daily scans. Mean observational cohort (n=10 patients) displacement for each anatomic POI ranged from 4.77 to 5.30 mm with a grand mean of 5.07 mm over-and-above the correction for bony landmark set-up error among all points. Calculated systematic error Σcohort for all POIs ranged between 1.01–1.38 mm, with a mean Σcohort of 1.1 mm across all points, and random error σcohort of 2.48 – 2.87 mm with a mean σcohort of 2.63 mm across all points. For all sites the bootstrapped POI mean displacement ranged from 4.85–5.35 mm, with a grand bootstrapped POI mean displacement of 5.09 mm. Fig. 2 illustrates the difference in POI vector displacement distribution probability between the studied cohort and its bootstrap resampling.
Fig. 2.
Shadowgram showing the difference in distribution probability of points of interest vector displacement over treatment time between the studied cohort and its bootstrap resampling.
Calculated Σbootstrap for all POIs ranged between 1.06–1.46 mm, with a mean Σbootstrap of 1.23 mm across all resampled points, and σbootstrap of 2.45–2.85 mm with a mean σbootstrap of 2.61 mm (Table 1).
Table 1.
Statistical data corresponding to individual POIs for the studied cohort and the bootstrap validation.
| Point | Mean, Cohort vector displacement (mm) | Mean, Bootstrapped vector displacement (mm) | Σ, Cohort Systematic Error (mm) | σ, Cohort Random Error (mm) | Σ, Bootstrapped Systematic Error (mm) | σ, Bootstrapped Random Error (mm) |
|---|---|---|---|---|---|---|
| Most Superior Aspect of the Anterior Thyroid Cartilage | 4.77 | 4.85 | 1.17 | 2.59 | 1.24 | 2.57 |
| Most Inferior Aspect of Anterior Thyroid Cartilage | 4.97 | 5.01 | 1.01 | 2.65 | 1.06 | 2.62 |
| Most Superior Aspect of Left Superior Cornu of Left Thyroid Cartilage | 5.16 | 5.10 | 1.1 | 2.48 | 1.20 | 2.46 |
| Most Inferior Aspect of Left Inferior Cornu of Thyroid Cartilage | 5.09 | 5.10 | 1.23 | 2.48 | 1.30 | 2.45 |
| Most Superior Aspect of Right Superior Cornu of Thyroid Cartilage | 5.3 | 5.35 | 1.38 | 2.87 | 1.46 | 2.85 |
| Most Inferior Aspect of Right Inferior Cornu of Thyroid Cartilage | 5.08 | 5.13 | 1.07 | 2.73 | 1.13 | 2.69 |
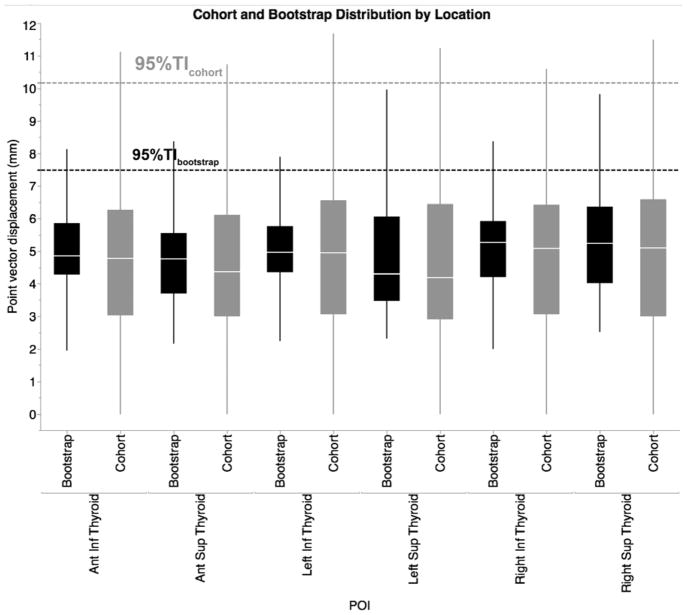
For the observed patient cohort, the one-sided upper limit ensuring 95% coverage of all residual displacements (95% TIcohort) was 10.18mm, while the equivalent bootstrap-estimated population limit (95% TIbootstrap) dropped to 7.52mm (Fig. 3).
Fig. 3.
Distributional boxplot of geometric vector displacement of cohort POIs and its bootstrap validation (Pale line within the box indicates median value, while the box limits indicating the 25th and 75th percentiles. The lines represent the 10th and 90th percentiles, and the horizontal dotted lines represent the 95% TI)
Using van Herk’s formula(22), for the observed cohort, the margin required for CTV-to-PTV expansion to ensure 90% population coverage with 95% of prescribed dose (PTVcohort) was 4.6 mm over-and-above the correction for bony landmark set-up error, while the calculated bootstrap estimator of the equivalent requisite coverage margin (PTVbootstrap) was 4.9 mm. Moreover, using McKenzie formula(24), the calculated OAR-to-PRV expansion for the observed residual set-up error (PRVcohort) was 2.7 mm, closely approximating the bootstrap estimated expansion over all POIs (PRVbootstrap) of 2.9 mm.
Discussion
The goal of in-room image-guided radiotherapy (IGRT) is to improve treatment delivery via a reduction of PTV volumes by imaging patients before or during treatment. Consequently, high frequency head and neck IGRT may be used as a feedback mechanism, ensuring accuracy of patient setup, and providing an opportunity to adjust the PTV(25, 26) or PRV(27) to account for institutionally dependent set-up error(28, 29).
In HNC, the accuracy of RT is of extreme importance owing to the close proximity of many OARs to the target volumes; the larynx, as either a target (PTV) or avoidance (PRV) structure is no exception. Our efforts herein define evidence-based, institutional margins for cases when the laryngeal apparatus is a target (i.e. larynx cancer)(12–14, 30) and define reasonable pre-planning PRV margins to avoid beam-path toxicities when laryngeal sparing is desired(31, 32) (i.e. oropharynx/oral cavity cancers receiving elective neck radiation when a low-neck match approach is not feasible)(16, 33).
In HNC treatment, immobilization masks are used to minimize interfractional variation in patient set-up and motion during radiation delivery. The amount of error in daily setup in an immobilized patient has been studied previously (34–36) and CTV-PTV corrections necessary have been suggested previously using a variety of IGRT devices (7, 37). Interestingly, while studies have been performed to determine the effect that setup error and the movement of a patient as a whole can have on RT accuracy in HNCs, there has been a lack of analysis of the effects of independent TV/OAR/ROI motion, although this data is beginning to emerge(9, 21, 27, 28, 38).
In head and neck cancers, the larynx provides an ideal model for non-isocentric treatment planning and patient set-up as it potentially has large translational displacements relative to bony landmarks due to its flexible attachments. Additionally, the cartilaginous structures of the larynx are capable, on some level, of deformation throughout treatment, thus necessitating the use of multiple points in the assessment of this study. As an illustration, when serial daily CT scans are concatenated in movie format (see Supplement) the concomitant effect of bony and laryngeal set-up error is better appreciated. With traditional patient set-up and immobilization the large, dynamic changes in the laryngeal apparatus, as seen in the supplemental material, are not appreciated or accounted for with daily isocentric alignment to rigid structures. Furthermore, as highly-conformal approaches become the standard of practice, IGRT implementation can further improve outcomes through the direct assessment of interfractional laryngeal variability and laryngeal motion during radiation delivery. For example, at our institution, the entire larynx is routinely treated in cases with locally-advanced disease; evaluation of kilovoltage portal radiography allows alignment of laryngeal structures directly rather than solely relying on bony landmarks – a feat that would have proven difficult in the megavoltage portal imaging era. Additionally, volumetric approaches including CT-on-rails or cone-beam CT may be utilized with similar purpose. When using the larynx in toto for positional alignment, the proposed margins may be utilized directly for CTV-PTV expansion, whereas with a non-larynx based reference (e.g. bony landmark) the PTV margins require superimposition for isocentric alignment of the reference structure. Our data show that utilization of IMRT for early stage larynx cancers(14) and emerging interest in even more aggressive and technical IGRT strategies necessitate the need to mitigate intra-fractional setup error(39–41) and thus require excellent geometric accuracy throughout the set-up and delivery of treatment. For instance, CT-on-rails has impressive isocentric alignment performance characteristics (reported by Shiu et al.(42) with <0.5mm directional error and <1mm cumulative isocentric error when aligning to spinal structures).
In non-laryngeal HNC, our data is equally important because OARs require dosimetric boundaries as well to ensure that over-dosage does not occur. This is conceptually represented as a PRV(43). At our institution, a low-neck match is used whenever feasible(16, 33, 44); however, alignment of the isocenter for the IMRT field must still account for potential laryngeal set-up error during treatment in order to ensure that unanticipated dose overlap does not occur. Several authors have demonstrated that extraneous, but modifiable, laryngeal dose is associated with significant acute and long-term toxicities(45, 46). Institutions using a full neck IMRT strategy would be prudent to consider PRV margination with magnitudes comparable to those listed in order to ensure attempts at organ-sparing are effectively realized.
Our data suggest that a population-based CTV-PTV margin of 5 mm reasonably accounts for larynx motion if the larynx is a target structure or 3 mm if it is planned as a PRV before dose calculation (i.e. pre-optimization), using established margination recipes(22, 24). These adjustments sufficiently account for geometric error associated of the laryngeal apparatus during set-up and treatment execution, ensuring precise delivery of the prescribed dose for all measured values. Our study used a post-isocentric alignment, suggesting that if a bony reference isocenter is used, an additional margin is required. However, if the laryngeal anatomy itself is used as alignment reference the use of a bony (C5) isocenter may be obviated. Institutionally we now align directly to laryngeal structures.
There are several caveats inherent in our data; first, this series represents a limited number of patients from a single institution with serial imaging for predominately oropharyngeal cancers. Furthermore, our exclusive use of daily CT-on-rails does not allow for evaluation of intrafractional respiratory or swallowing motion associated with the larynx(41, 47, 48) which may require additional margination, and which we and other groups are currently investigating. The use of bootstrap resampling serves as a corrective for our limited sample size, as the large number of iterative daily measurements (>25 for any patient, 1854 total measurements) and the large resampling run (n=10,000 per patient, per POI) allows robust inferential estimation, at least for similar populations. The bootstrap distribution (and resultant systematic, random error, and confidence intervals) are designed to represent a large-scale population from which the sampled experimental set is potentially drawn. Consequently, as expected some difference exist (as the resampled population central parameters will be of more utility, as compared to experimental cohort data), but on the whole, the magnitude of difference between bootstrap and experimental cohort for systematic and random error was for all measures <0.2mm (exceedingly small). This, in fact, suggests our presented experimental data largely would reflect any given head and neck cancer patient larynx motion distribution that might be seen in a significant patient cohort.
This is, however, to our knowledge, the first study to utilize diagnostic quality imaging via daily CT-on-rails to evaluate interfractional motion of the larynx. It serves as a model study to evaluate interfractional organ motion for delivery of IGRT as well as a benchmark for institutional IGRT margination recipes.
Conclusions
In conclusion, interfractional larynx setup error is a source of significant potential geometric error, even after pristine isocentric alignment, in HNC treated with radiation therapy both when the larynx is treated as a target (e.g. larynx primaries) or as a normal tissue avoidance structure (e.g. oropharyngeal cancer). We estimate the need for a uniform CTV-to-PTV expansion of approximately 5mm to compensate for daily isocenter-independent set-up error if the larynx is a target or an OAR-to-PRV margin of 3 mm if the larynx is an OAR when using a non-laryngeal isocenter with comparable immobilization platforms.
Supplementary Material
Acknowledgments
Dr. Abdallah Mohamed acknowledges the support by a UICC American Cancer Society Beginning Investigators Fellowship funded by the American Cancer Society.
Footnotes
PACS numbers: 87.55.D-, 87.55.Qr
References
- 1.van Herk M, Remeijer P, Lebesque JV. Inclusion of geometric uncertainties in treatment plan evaluation. International journal of radiation oncology, biology, physics. 2002;52(5):1407–22. doi: 10.1016/s0360-3016(01)02805-x. Epub 2002/04/17. [DOI] [PubMed] [Google Scholar]
- 2.Miszczyk L, Tarnawski R, Skladowski K. The impact of delivered dose errors on local control of irradiated advanced laryngeal cancer. Neoplasma. 2000;47(2):133–6. Epub 2000/09/14. [PubMed] [Google Scholar]
- 3.Prescribing, recording, and reporting photon beam therapy (supplement to ICRU Report 50). 1999 1999. Report No.
- 4.Prescribing, Recording, and Reporting Photon-Beam Intensity-Modulated Radiation Therapy (IMRT): Contents. Journal of the ICRU. 2010;10(1) doi: 10.1093/jicru/ndq002. NP-NP. [DOI] [Google Scholar]
- 5.Purdy JA. Current ICRU definitions of volumes: limitations and future directions. Seminars in radiation oncology. 2004;14(1):27–40. doi: 10.1053/j.semradonc.2003.12.002. Epub 2004/01/31. [DOI] [PubMed] [Google Scholar]
- 6.van Herk M. Errors and margins in radiotherapy. Seminars in radiation oncology. 2004;14(1):52–64. doi: 10.1053/j.semradonc.2003.10.003. Epub 2004/01/31. [DOI] [PubMed] [Google Scholar]
- 7.Fuller CD, Scarbrough TJ, Sonke JJ, Rasch CR, Choi M, Ting JY, et al. Method comparison of automated matching software-assisted cone-beam CT and stereoscopic kilovoltage x-ray positional verification image-guided radiation therapy for head and neck cancer: a prospective analysis. Physics in medicine and biology. 2009;54(24):7401–15. doi: 10.1088/0031-9155/54/24/010. Epub 2009/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dionisi F, Palazzi MF, Bracco F, Brambilla MG, Carbonini C, Asnaghi DD, et al. Set-up errors and planning target volume margins in head and neck cancer radiotherapy: a clinical study of image guidance with on-line cone-beam computed tomography. International journal of clinical oncology. 2013;18(3):418–27. doi: 10.1007/s10147-012-0395-7. Epub 2012/03/06. [DOI] [PubMed] [Google Scholar]
- 9.van Kranen S, van Beek S, Rasch C, van Herk M, Sonke JJ. Setup uncertainties of anatomical sub-regions in head-and-neck cancer patients after offline CBCT guidance. International journal of radiation oncology, biology, physics. 2009;73(5):1566–73. doi: 10.1016/j.ijrobp.2008.11.035. Epub 2009/03/25. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Garden AS, Lo J, Ang KK, Ahamad A, Morrison WH, et al. Multiple regions-of-interest analysis of setup uncertainties for head-and-neck cancer radiotherapy. International journal of radiation oncology, biology, physics. 2006;64(5):1559–69. doi: 10.1016/j.ijrobp.2005.12.023. Epub 2006/04/04. [DOI] [PubMed] [Google Scholar]
- 11.Ove R, Cavalieri R, Noble D, Russo SM. Variation of neck position with image-guided radiotherapy for head and neck cancer. American journal of clinical oncology. 2012;35(1):1–5. doi: 10.1097/COC.0b013e3181fe46bb. Epub 2011/02/01. [DOI] [PubMed] [Google Scholar]
- 12.Camingue P, Christian R, Ng D, Williams P, Amin M, Roniger DL. Comparison of external beam treatment techniques for T1–2, N0, M0 glottic cancers. Medical dosimetry: official journal of the American Association of Medical Dosimetrists. 2012;37(2):221–4. doi: 10.1016/j.meddos.2011.08.002. Epub 2012/03/01. [DOI] [PubMed] [Google Scholar]
- 13.Chera BS, Amdur RJ, Morris CG, Mendenhall WM. Carotid-sparing intensity-modulated radiotherapy for early-stage squamous cell carcinoma of the true vocal cord. International journal of radiation oncology, biology, physics. 2010;77(5):1380–5. doi: 10.1016/j.ijrobp.2009.07.1687. Epub 2009/11/28. [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal DI, Fuller CD, Barker JL, Jr, Mason B, Garcia JA, Lewin JS, et al. Simple carotid-sparing intensity-modulated radiotherapy technique and preliminary experience for T1–2 glottic cancer. International journal of radiation oncology, biology, physics. 2010;77(2):455–61. doi: 10.1016/j.ijrobp.2009.04.061. Epub 2009/08/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendenhall WM, Werning JW, Hinerman RW, Amdur RJ, Villaret DB. Management of T1–T2 glottic carcinomas. Cancer. 2004;100(9):1786–92. doi: 10.1002/cncr.20181. Epub 2004/04/28. [DOI] [PubMed] [Google Scholar]
- 16.Dabaja B, Salehpour MR, Rosen I, Tung S, Morrison WH, Ang KK, et al. Intensity-modulated radiation therapy (IMRT) of cancers of the head and neck: comparison of split-field and whole-field techniques. International journal of radiation oncology, biology, physics. 2005;63(4):1000–5. doi: 10.1016/j.ijrobp.2005.03.069. Epub 2005/06/28. [DOI] [PubMed] [Google Scholar]
- 17.Caglar HB, Tishler RB, Othus M, Burke E, Li Y, Goguen L, et al. Dose to larynx predicts for swallowing complications after intensity-modulated radiotherapy. International journal of radiation oncology, biology, physics. 2008;72(4):1110–8. doi: 10.1016/j.ijrobp.2008.02.048. Epub 2008/05/13. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz DL, Garden AS, Shah SJ, Chronowski G, Sejpal S, Rosenthal DI, et al. Adaptive radiotherapy for head and neck cancer--dosimetric results from a prospective clinical trial. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2013;106(1):80–4. doi: 10.1016/j.radonc.2012.10.010. Epub 2013/02/02. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz DL, Garden AS, Thomas J, Chen Y, Zhang Y, Lewin J, et al. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. International journal of radiation oncology, biology, physics. 2012;83(3):986–93. doi: 10.1016/j.ijrobp.2011.08.017. Epub 2011/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker JL, Jr, Garden AS, Ang KK, O’Daniel JC, Wang H, Court LE, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. International journal of radiation oncology, biology, physics. 2004;59(4):960–70. doi: 10.1016/j.ijrobp.2003.12.024. Epub 2004/07/06. [DOI] [PubMed] [Google Scholar]
- 21.Neubauer E, Dong L, Followill DS, Garden AS, Court LE, White RA, et al. Assessment of shoulder position variation and its impact on IMRT and VMAT doses for head and neck cancer. Radiation oncology. 2012;7:19. doi: 10.1186/1748-717X-7-19. Epub 2012/02/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Herk M, Remeijer P, Rasch C, Lebesque JV. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. International journal of radiation oncology, biology, physics. 2000;47(4):1121–35. doi: 10.1016/s0360-3016(00)00518-6. Epub 2000/06/23. [DOI] [PubMed] [Google Scholar]
- 23.McKenzie A, van Herk M, Mijnheer B. Margins for geometric uncertainty around organs at risk in radiotherapy. Radiother Oncol. 2002;62(3):299–307. doi: 10.1016/S0167-8140(02)00015-4. Pii S0167-8140(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 24.McKenzie AL, van Herk M, Mijnheer B. The width of margins in radiotherapy treatment plans. Physics in medicine and biology. 2000;45(11):3331–42. doi: 10.1088/0031-9155/45/11/315. Epub 2000/12/01. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki M, Nishimura Y, Nakamatsu K, Okumura M, Hashiba H, Koike R, et al. Analysis of interfractional set-up errors and intrafractional organ motions during IMRT for head and neck tumors to define an appropriate planning target volume (PTV)- and planning organs at risk volume (PRV)-margins. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2006;78(3):283–90. doi: 10.1016/j.radonc.2006.03.006. Epub 2006/03/28. [DOI] [PubMed] [Google Scholar]
- 26.Astreinidou E, Bel A, Raaijmakers CP, Terhaard CH, Lagendijk JJ. Adequate margins for random setup uncertainties in head-and-neck IMRT. International journal of radiation oncology, biology, physics. 2005;61(3):938–44. doi: 10.1016/j.ijrobp.2004.11.016. Epub 2005/02/15. [DOI] [PubMed] [Google Scholar]
- 27.Delana A, Menegotti L, Bolner A, Tomio L, Valentini A, Lohr F, et al. Impact of residual setup error on parotid gland dose in intensity-modulated radiation therapy with or without planning organ-at-risk margin. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] 2009;185(7):453–9. doi: 10.1007/s00066-009-1888-9. Epub 2009/08/29. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Garden AS, Zhang Y, Zhang L, Dong L. Variable planning margin approach to account for locoregional variations in setup uncertainties. Medical physics. 2012;39(8):5136–44. doi: 10.1118/1.4737891. Epub 2012/08/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozano EM, Perez LA, Torres J, Carrascosa C, Sanz M, Mendicote F, et al. Correction of systematic set-up error in breast and head and neck irradiation through a no-action level (NAL) protocol. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2011;13(1):34–42. doi: 10.1007/s12094-011-0614-0. Epub 2011/01/18. [DOI] [PubMed] [Google Scholar]
- 30.Gomez D, Cahlon O, Mechalakos J, Lee N. An investigation of intensity-modulated radiation therapy versus conventional two-dimensional and 3D-conformal radiation therapy for early stage larynx cancer. Radiation oncology. 2010;5:74. doi: 10.1186/1748-717X-5-74. Epub 2010/08/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenthal DI, Chambers MS, Fuller CD, Rebueno NC, Garcia J, Kies MS, et al. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. International journal of radiation oncology, biology, physics. 2008;72(3):747–55. doi: 10.1016/j.ijrobp.2008.01.012. Epub 2008/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz DL, Hutcheson K, Barringer D, Tucker SL, Kies M, Holsinger FC, et al. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. International journal of radiation oncology, biology, physics. 2010;78(5):1356–65. doi: 10.1016/j.ijrobp.2009.10.002. Epub 2010/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amdur RJ, Liu C, Li J, Mendenhall W, Hinerman R. Matching intensity-modulated radiation therapy to an anterior low neck field. International journal of radiation oncology, biology, physics. 2007;69(2 Suppl):S46–8. doi: 10.1016/j.ijrobp.2007.04.091. Epub 2007/10/18. [DOI] [PubMed] [Google Scholar]
- 34.Velec M, Waldron JN, O’Sullivan B, Bayley A, Cummings B, Kim JJ, et al. Cone-beam CT assessment of interfraction and intrafraction setup error of two head-and-neck cancer thermoplastic masks. International journal of radiation oncology, biology, physics. 2010;76(3):949–55. doi: 10.1016/j.ijrobp.2009.07.004. Epub 2010/01/09. [DOI] [PubMed] [Google Scholar]
- 35.Rotondo RL, Sultanem K, Lavoie I, Skelly J, Raymond L. Comparison of repositioning accuracy of two commercially available immobilization systems for treatment of head-and-neck tumors using simulation computed tomography imaging. International journal of radiation oncology, biology, physics. 2008;70(5):1389–96. doi: 10.1016/j.ijrobp.2007.08.035. Epub 2008/01/22. [DOI] [PubMed] [Google Scholar]
- 36.Boda-Heggemann J, Walter C, Rahn A, Wertz H, Loeb I, Lohr F, et al. Repositioning accuracy of two different mask systems-3D revisited: comparison using true 3D/3D matching with cone-beam CT. International journal of radiation oncology, biology, physics. 2006;66(5):1568–75. doi: 10.1016/j.ijrobp.2006.08.054. Epub 2006/11/28. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Zhu XR, Zhang L, Dong L, Tung S, Ahamad A, et al. Comparison of 2D radiographic images and 3D cone beam computed tomography for positioning head-and-neck radiotherapy patients. International journal of radiation oncology, biology, physics. 2008;71(3):916–25. doi: 10.1016/j.ijrobp.2008.01.008. Epub 2008/04/09. [DOI] [PubMed] [Google Scholar]
- 38.Hamming-Vrieze O, van Kranen SR, van Beek S, Heemsbergen W, van Herk M, van den Brekel MW, et al. Evaluation of tumor shape variability in head-and-neck cancer patients over the course of radiation therapy using implanted gold markers. International journal of radiation oncology, biology, physics. 2012;84(2):e201–7. doi: 10.1016/j.ijrobp.2012.03.014. Epub 2012/05/09. [DOI] [PubMed] [Google Scholar]
- 39.Levendag PC, Teguh DN, Keskin-Cambay F, Al-Mamgani A, van Rooij P, Astreinidou E, et al. Single vocal cord irradiation: a competitive treatment strategy in early glottic cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2011;101(3):415–9. doi: 10.1016/j.radonc.2011.05.026. Epub 2011/06/15. [DOI] [PubMed] [Google Scholar]
- 40.Osman SO, Astreinidou E, de Boer HC, Keskin-Cambay F, Breedveld S, Voet P, et al. IMRT for image-guided single vocal cord irradiation. International journal of radiation oncology, biology, physics. 2012;82(2):989–97. doi: 10.1016/j.ijrobp.2010.12.022. Epub 2011/02/09. [DOI] [PubMed] [Google Scholar]
- 41.Osman SO, de Boer HC, Astreinidou E, Gangsaas A, Heijmen BJ, Levendag PC. On-line cone beam CT image guidance for vocal cord tumor targeting. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2009;93(1):8–13. doi: 10.1016/j.radonc.2009.05.015. Epub 2009/06/16. [DOI] [PubMed] [Google Scholar]
- 42.Shiu AS, Chang EL, Ye JS, Lii M, Rhines LD, Mendel E, et al. Near simultaneous computed tomography image-guided stereotactic spinal radiotherapy: an emerging paradigm for achieving true stereotaxy. International journal of radiation oncology, biology, physics. 2003;57(3):605–13. doi: 10.1016/s0360-3016(03)00792-2. [DOI] [PubMed] [Google Scholar]
- 43.Manning MA, Wu Q, Cardinale RM, Mohan R, Lauve AD, Kavanagh BD, et al. The effect of setup uncertainty on normal tissue sparing with IMRT for head-and-neck cancer. International journal of radiation oncology, biology, physics. 2001;51(5):1400–9. doi: 10.1016/s0360-3016(01)01740-0. Epub 2001/12/01. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Shrieve DC, Hitchcock YJ. Comparison of cervical esophagus dose-volumes for three radiotherapy techniques for head and neck cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2008;87(2):274–80. doi: 10.1016/j.radonc.2007.12.014. Epub 2008/01/22. [DOI] [PubMed] [Google Scholar]
- 45.Amin N, Reddy K, Westerly D, Raben D, DeWitt P, Chen C. Sparing the larynx and esophageal inlet expedites feeding tube removal in patients with stage III–IV oropharyngeal squamous cell carcinoma treated with intensity-modulated radiotherapy. The Laryngoscope. 2012;122(12):2736–42. doi: 10.1002/lary.23597. Epub 2012/09/20. [DOI] [PubMed] [Google Scholar]
- 46.Eisbruch A, Kim HM, Feng FY, Lyden TH, Haxer MJ, Feng M, et al. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. International journal of radiation oncology, biology, physics. 2011;81(3):e93–9. doi: 10.1016/j.ijrobp.2010.12.067. Epub 2011/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osman SO, de Boer HC, Heijmen BJ, Levendag PC. Four-dimensional CT analysis of vocal cords mobility for highly focused single vocal cord irradiation. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2008;89(1):19–27. doi: 10.1016/j.radonc.2008.05.016. Epub 2008/06/17. [DOI] [PubMed] [Google Scholar]
- 48.Bradley JA, Paulson ES, Ahunbay E, Schultz C, Li XA, Wang D. Dynamic MRI analysis of tumor and organ motion during rest and deglutition and margin assessment for radiotherapy of head-and-neck cancer. International journal of radiation oncology, biology, physics. 2011;81(5):e803–12. doi: 10.1016/j.ijrobp.2010.12.015. Epub 2011/02/09. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.