Abstract
Physical activity intervention studies that focus on improving cognitive function in older adults have increasingly used magnetic imaging resonance (MRI) measures in addition to neurocognitive measures to assess effects on the brain. The purpose of this systematic review was to identify the effects of endurance-focused physical activity randomized clinical trial (RCT) interventions on the brain as measured by MRI in community-dwelling middle-aged or older adults without cognitive impairment. Five electronic databases were searched. The final sample included six studies. None of the studies reported racial or ethnic characteristics of the participants. All studies included neurocognitive measures in addition to MRI. Five of the six interventions included laboratory-based treadmill or bike supervised exercise sessions, while one included community-based physical activity. Physical activity measures were limited to assessment of cardiorespiratory fitness, and pedometer in one study. Due to the lack of adequate data reported, effect sizes were calculated for only one study for MRI measures and two studies for neurocognitive measures. Effect sizes ranged from d = .2-.3 for MRI measures and .2–32 for neurocognitive measures. Findings of the individual studies suggest that MRI measures may be more sensitive to the effects of physical activity than neurocognitive measures. Future studies are needed that include diverse, community-based participants, direct measures of physical activity, and complete reporting of MRI and neurocognitive findings.
Keywords: physical activity, exercise, cognition, brain, MRI, fMRI
Mild cognitive impairment, defined as decline in episodic memory, attention, and cognitive function beyond what is expected due to normal aging (Petersen et al., 2010), occurs in 22% of adults 71 years or older (Petersen et al., 2010; Plassman et al., 2008). Cognitive impairment due to dementia, a distressing chronic disease of cognitive dysfunction that impacts quality of life, independence, and family relations, occurs in 14% of older adults over 71 years (Petersen et al., 2010; Plassman et al., 2007). Cognitive impairment (with or without dementia) is an increasing public health concern due to the rapidly growing older adult population. It is essential that we target modifiable health behaviors that can help offset or delay the onset of cognitive impairment.
There has been increased attention on the effect of physical activity, particularly endurance-focused physical activity (activity that raises heart rate and respiration; American College of Sports Medicine et al., 2009) on cognitive function in middle-aged and older adults (Behrman & Ebmeier, 2014). Most support for a beneficial effect of physical activity on cognition comes from clinical epidemiologic studies that suggest a small but protective effect of physical activity and aerobic fitness on risk of cognitive impairment and decline in healthy cognitive aging (Pignatti, Rozzini, & Trabucchi, 2002; Richards, Hardy, & Wadsworth, 2003; Weuve et al., 2004). In these studies, neurocognitive testing was used to examine specific cognitive functions and abilities, including memory or executive function.
Neurocognitive tests are widely used in physical activity and cognition research because they are relatively inexpensive to implement, have been validated in multiple age groups and races, and can be implemented in clinical or community-based settings (McCarten, 2013; Zygouris & Tsolaki, 2015). Unfortunately, sensitivity of neurocognitive measures in physical activity and cognition studies is questionable (Smith, Potter, McLaren, & Blumenthal, 2013). Not all neurocognitive tests are well-validated in every ethnic or socioeconomic subgroup, and such tests may not be sensitive enough to detect early cognitive impairment (Holtzer et al., 2008). This has led to investigation of more precise and sensitive measures of brain health.
Recently, magnetic resonance imaging (MRI) methods have been used to assess brain health because they allow more sensitive measurement of brain regions and neurophysiological functions (Smith et al., 2013). MRI methods may detect changes in brain structure or neurophysiological functions that are indicative of mild cognitive impairment or dementia disease processes prior to detection by neurocognitive tests. MRI used to assess the link between physical activity and brain structure (e.g., brain volume). Most MRI studies use a physical activity correlational approach. More specifically, anatomical MRI studies have shown that physical activity is associated with reduced loss of brain tissue in the frontal, parietal, and temporal cortices (Kramer, Erickson, & Colcombe, 2006), greater preservation of hippocampal volume (Szabo et al., 2011), and lower volume of white matter lesions (Burzynska et al., 2014).
Functional MRI (fMRI) is used to assess brain activation. Functional MRI research has shown that, compared to less fit older adults, highly fit older adults performing a task with distracting stimuli had greater activation in prefrontal and parietal cortical regions involved in selective attention and inhibitory functioning, and lower activation in an anterior cingulate region that monitors conflict in the central executive system (Colcombe et al., 2004). Furthermore, higher fitness levels in older adults have been associated with enhanced attentional function through increased recruitment of prefrontal cortical regions (Prakash et al., 2011).
There are eight literature reviews recently published that have examined physical activity interventions and cognitive function (Cumming, Tyedin, Churilov, Morris, & Bernhardt, 2012; Farina, Rusted, & Tabet, 2014; Gary & Brunn, 2014; Gates, Fiatarone Singh, Sachdev, & Valenzuela, 2013; Lautenschlager, Cox, & Kurz, 2010; Law, Barnett, Yau, & Gray, 2014; Littbrand, Stenvall, & Rosendahl, 2011; Smith et al., 2013; Snowden et al., 2011). However, most (n = 6) focused on participants with specific disease processes. One other review examined combined cognitive and exercise interventions and did not examine the impact of physical activity separately (Law et al., 2014). All of these reviews focused on the use of neurocognitive measures for cognitive function. Of the two reviews of physical activity interventions with healthy middle-aged and older adults, only Smith and colleagues’ (2013) narrative review addressed the use of MRI measures in two identified studies; the relations between MRI measures and neurocognitive measures were not addressed.
To our knowledge, no existing literature review has systematically examined physical activity randomized clinical trials (RCTs) for cognition as measured by MRI methods in healthy, community-dwelling middle-aged or older adults. Further, no review has investigated the relations between MRI and neurocognitive measures in such studies. Therefore, the purpose of this review is to identify the effect of endurance-focused physical activity interventions on the brain as measured by MRI in community-dwelling middle-aged or older adults without cognitive impairment. We also aim to determine if there is agreement between MRI and neurocognitive outcome measures in endurance-focused physical activity interventions.
Methods
Design
We retrieved the existing literature on brain MRI outcomes in physical activity RCTs for middle-aged or older adults (Higgins & Green, 2011). We used the Preferred Reporting Items for Reviews and Meta-Analyses (PRISMA) flowsheet and checklist to ensure complete reporting of the evidence-based minimum reporting items (Moher, Liberati, Tetzlaff, Altman, & Group, 2009).
Inclusion Criteria
Inclusion criteria for this review were (a) RCT, (b) implementation of an endurance-focused physical activity intervention (physical activity with an aerobic component that raises heartrate and respirations), (c) brain imaging outcome as measured by MRI, (d) healthy middle-aged or older adult sample without cognitive impairment at baseline, and (e) written in English.
Search Methods
In May 2015, a search was conducted of five databases: PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL), SciVerse Scopus, Health Source: Nursing/Academic Edition, and PsychInfo databases. Next, the Cochrane Database of Systematic Reviews (CDRS) was searched to obtain systematic reviews of physical activity interventions that aim to improve or maintain cognition. Finally, the reference lists of existing literature reviews previously identified by the authors were reviewed.
For the search strategy, we identified search terms according to three main categories: (a) physical activity, (b) cognition, and (c) MRI outcome measure. The search terms of the first category were physical activity (text word), walking (MeSH), exercise (MeSH), leisure activities (MeSH), and lifestyle physical activity (text word). For the second category, the search terms were cognition, cognition disorder, memory, short-term memory, mental recall, recognition (psychology), retention (psychology), memory disorders, attention, and dementia (all MeSH). For the third category, the search terms were magnetic resonance imaging (MeSH), functional magnetic resonance imaging (MeSH), MRI (text word), and fMRI (text word). The qualifier of randomized controlled trial (RCT) was applied on the search to narrow results to studies with RCT designs. The age qualifiers of middle-aged and older adult were also applied to narrow results that included middle-aged and older adults. For example, the full electronic search strategy utilized for CINAHL was the following: (“Physical Activity” OR exercise OR “Leisure activities” OR “Walking” OR “lifestyle physical activity”) AND (cognition OR cognition disorder OR “Auditory perceptual disorders” OR “Memory” OR “Memory, short term” OR “Mental recall” OR “Recognition” OR “Retention” OR “Memory disorders” OR “Attention” OR “Dementia”) AND (MRI OR “magnetic imaging resonance” OR “fMRI” OR “functional magnetic imaging resonance”), with the limits of RCT for design, and middle-aged and older adult for age group. To ensure a complete literature retrieval of papers, we did not limit retrieved papers by publication date. The search strategy was verified by a medical librarian.
Search Outcome
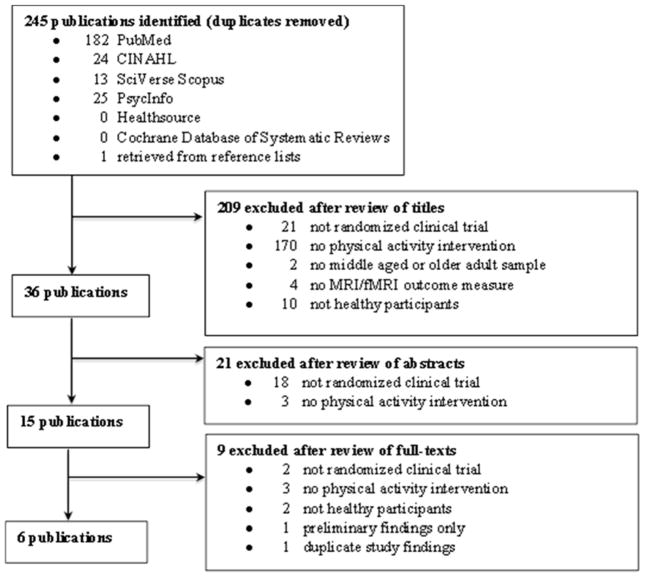
The initial search resulted in 245 unique titles (Figure 1). To evaluate titles for inclusion in the review, two of the authors (JW and SH) independently reviewed the retrieved titles, followed by abstracts, and then full-text articles. Based on title review, 209 papers were excluded. The majority of papers (n = 170) were excluded because there was no physical activity intervention. Next, the abstracts of the 36 remaining papers were evaluated; of these, 21 papers were excluded, with most (n = 18) not having an RCT design. Full-text review was then completed for the remaining 15 papers. The sample resulting from this search strategy was eight papers representing six independent studies (three papers for one study). Since there were three papers representing the same study, two of the three papers that presented preliminary data or a subsample were excluded from this review. JW and SH agreed on 95% of all decisions and reached a mutual consensus for decisions that were incongruent. The final sample included six papers representing six studies.
Figure 1.
Flow chart of search & retrieval process & results.
Measures and Analytic Strategy
Two of the authors (SH and JW) reviewed the six papers, which were then abstracted into narrative tables. The results were coded, checked for inconsistencies, and discussed for final consensus when necessary. The six studies were also assessed for potential risk of bias across studies, as well as within the individual studies.
The six studies were first evaluated in regards to the country where the study took place, design and assessment points, setting, sample, physical activity intervention and control conditions, intervention duration, and intervention adherence (session attendance; Table 1). Next, outcome measures (physical activity, MRI and neurocognitive) and outcome results (group effects and effect sizes) were presented (Table 2). Outcome results for MRI and neurocognitive measures were determined by examining effect sizes (standardized mean differences from baseline to post intervention) based on available published results. We used calculated effect sizes according to standardized formulas with Cohen’s d, depending on the design of the study; and we categorized effect sizes by assigning them, respectively, Cohen’s d of .2, .5, and .8 for small, medium, and large effect (Cohen, 1988). We also examined the significant MRI and neurocognitive findings by comparing the MRI brain regions to the neurocognitive tests the authors assessed and reported in each paper (Table 3).
Table 1.
Sample & Intervention Characteristics of Six Physical Activity Studies for Brain Health with Middle-Aged & Older Adult Samples
| # | Author (Year) | Design | Sample | Intervention | ||||
|---|---|---|---|---|---|---|---|---|
| Inclusion Criteria | Sample Characteristics | Setting | Intervention & Control | Intervention Duration | Attendance, Adherence | |||
| 1 |
Chapman et al. (2013) USA |
RCT T1: baseline T2: 6 weeks T3: 12 weeks |
|
N = 37 (18 intervention, 19 control), Age 57–75 years (M = 64.0 ± 3.9), 73% female | Lab | Intervention
|
12 weeks | Participants attended over 90% of sessions |
| 2 |
Colcombe, et al. (2004) USA |
RCT T1: baseline T2: 25 weeks |
|
N = 29 (intervention & control n not reported), Age 58–77 years (M = 65.6 ± 5.7), 62.1% female, education M = 15.1 years | Not reported | Intervention
|
24 weeks | Not reported |
| 3 |
Erickson et al. (2011) USA |
RCT T1: within 4 weeks of intervention T2: 24 weeks T3: 1 year |
|
N = 120 (60 intervention, 60 control), Age 60–79 years (M = 66.6 ± 5.6), 67% female | Not reported | Intervention
|
1 year | Participants attended 85% of sessions |
| 4 |
Holzschneider et al. (2012) Germany |
RCT T1: baseline T2: 24 weeks |
|
N = 33 (16 intervention [8 spatial, 8 perceptual], 17 control [9 spatial, 8 perceptual]), Age 40–55 years (M = 48.9 ± 3.8), 52% female | Not reported | Intervention
|
24 weeks | Not reported |
| 5 |
Mortimer et al. (2012) China |
RCT T1: baseline T2: 20 weeks T3: 40 weeks |
|
N = 120 (90 intervention [30 Tai Chi, 30 walking, 30 social interaction], 30 control), Age 60–79 years (M = 67.9 ± 5.8), 67% female, education M = 11.7 years | Local park, gym, community center | Intervention (3 groups):
|
40 weeks | Not reported |
| 6 |
Voelcker-Rehage et al. (2011) Germany |
RCT T1: baseline T2: 24 weeks T3: 1 year |
|
N = 44 (10 cardiovascular, 10 coordination, 10 control), Age 63–79 years (M = 69.6 ± 3.8), 64% female, education M = 12.4 years | Not reported | Intervention (2 groups):
|
1 year | Not reported |
Table 2.
Description of Outcome Measures & Results of Six Physical Activity Studies for Brain Health with Middle-Aged & Older Adult Samples
| # | Author (Year) | Measures | Outcome results | |||
|---|---|---|---|---|---|---|
| PA | MRI | Neurocognitive (NC) | Group Effects | Outcomes (Effect Sizes) | ||
| 1 | Chapman et al. (2013) | Maximal oxygen consumption (VO2max) Borg rating of perceived exertion |
fMRI: cerebral blood flow |
|
MRI: Intervention group had a greater increase in resting cerebral blood flow in the anterior cingulate region compared to the control (p < .05) NC: Intervention group had a significant improvement in immediate (1.6 vs −2.3, p = .003) & delayed memory (2.0 vs. −0.3, p = .03) when compared to the control from T1 to T3 |
Unable to calculate |
| 2 | Colcombe, et al. (2004) | Maximal oxygen uptake (VO2max) Resting heartrate Rockport 1-mile walk test |
fMRI: modified Eriksen flanker paradigm to assess cortical plasticity |
|
MRI: Intervention group had significantly greater level of task-related activity in attentional control areas, but lower activity in task-related activity in the anterior cingulate region (greater overall cortical plasticity) NC: Intervention group had 11% reduction in reaction time [(t(15)2.49, p < 0.04] |
Unable to calculate |
| 3 | Erickson et al. (2011) | Maximal oxygen uptake (VO2max): treadmill protocol | MRI: brain volume |
|
MRI: Intervention group had a significant increase in volume of the left & right hippocampus from T1 to T3 compared to the control (2.12% & 1.97% vs. −1.40% & −1.43%) NC: There were no significant group differences from T1 to T3 in the spatial memory task. However, higher aerobic fitness levels at baseline (r = 0.31; p < 0.001) & after intervention (r = 0.28; p < 0.004) were associated with better memory performance on the spatial memory task |
MRI: L hippocampus: .21 R hippocampus: .20 L anterior hippocampus: .29 R anterior hippocampus: .30 L posterior hippocampus: .12 R posterior hippocampus: .19 L caudate nucleus: .09 R caudate nucleus: .09 Thalamus: .01 BDNF: .11 NC: Unable to calculate |
| 4 | Holzschneider et al. (2012) | Maximal oxygen uptake (VO2peak): 3-min treadmill or cycle ergometer protocol | fMRI: virtual maze task with retrieval assessment to assess spatial learning capacity |
|
MRI: The cycling/spatial intervention group had change in brain activation from T1 to T2, which correlated positively with the change in VO2peak in the medial frontal gyrus (r = .85; t = 6.03) & the cuneus (r = .81; t = 5.14) NC: No significant differences between physical training groups. |
Unable to calculate |
| 5 | Mortimer et al. (2012) | Number of steps per week as measured by pedometer | MRI: brain volume | Neuropsychological battery: Digit Span, Bell Cancellation Test, Rey-Osterrieth Complex Figure (copying & recall), Stroop Test, Chinese Auditory Verbal Learning Test, Category Verbal Fluency Test, WAIS-R Similarities Test, Trail-Making Test, Clock-Drawing Test, Boston Naming Test, & Mattis Dementia Rating Scale |
MRI: Tai Chi & Social groups had significant increases in brain volume (p < 0.05) in both the Tai Chi (t = 2.28) & Social Intervention (t = 2.03) groups when compared with control. No significant findings for the walking group. NC: Walking group had significant improvement in Rey Figure copying (t = −.063, p = .05), Tai Chi group had significant improvements in Mattis Dementia Rating Scale total score (t = 2.98, p = 0.004), Trailmaking Test (p = 0.002); delayed recognition on the Auditory Verbal Learning Test (t = 2.66, p = 0.009), verbal fluency for animals (t = 2.60, p = 0.01) |
MRI: Unable to calculate NC: (for those with complete data available) Verbal Learning Test - delayed: .23 Stroop Color Word Test: −.32* |
| 6 | Voelcker-Rehage et al. (2011) | Maximal oxygen uptake (VO2peak): submaximal graded exercise modified Porszasz treadmill protocol Motor fitness assessment: feet tapping, stick-fall test, one-leg-stand |
fMRI: Flanker Task to assess brain activation patterns |
|
MRI: Both intervention groups had decreased activation in the prefrontal areas compared to the control. The coordination group had increased activation in the inferior frontal gyrus, thalamus & caudate, & superior parietal lobule. NC: Both cardiovascular intervention [F(2, 39) = 4.87, p = 0.013, η2 = 0.20] & coordination intervention groups [F(2, 39) = 4.10, p = 0.024, η2 = 0.17] had improved performance accuracy in the Flanker Task. The coordination group had improved performance accuracy [F(2, 39) = 5.51, p = 0.008, η2 = 0.22] & speed [F(2, 39) = 11.82, p < 0.001, η2 = 0.38] in the Visual Search Task |
MRI: Unable to calculate NC: Flanker test % correct: −.07 Flanker test reaction time: −.10* Visual search task % correct: .20 Visual search task reaction time: −.24* |
Table 3.
MRI Brain Region & Neurocognitive Measures Findings & Significance
| Study | MRI Brain Region | Sig. | Neurocognitive Measures | Sig. |
|---|---|---|---|---|
| Chapman et al. (2013) | Anterior cingulate region | + | Executive function: Trails A & B | NS |
| Memory – California Verbal Learning Test II, Wechsler Memory Scale IV | ||||
| Colcombe, et al. (2004) | Anterior cingulate region | − | Behavioral conflict: reaction time | − |
| Middle, superior frontal gyrus | + | |||
| Superior parietal lobule | + | |||
| Erickson et al. (2011) | L/R anterior/posterior hippocampus | + | Spatial memory task: computer test | NS |
| L/R caudate nucleus | NS | |||
| Holzschneider et al. (2012) | Medial front gyrus | + | Spatial cognition: viewpoint, path integration task | NS |
| Cuneus | + | Spatial cognition: viewpoint, path integration task | NS | |
| Hippocampus, retrosplenial cortex, parahippocampal gyrus, frontal region, temporal region, cingulate region | NS | Spatial cognition: viewpoint, path integration task | NS | |
| Occipital region | NS | Perceptual cognition: visual discrimination task | NS | |
| Mortimer et al. (2012) | Whole brain volume | NS | ||
| Rey Figure (copying) | − | |||
| WAIS digit span, Bell cancellation test, Stroop Test, Auditory Verbal Learning Test, Category Verbal Fluency, WAIS Similarities, Trails A & B Time, Clock drawing test, Boston Naming Test, Mattis Dementia Rating Scale | NS | |||
| Voelcker-Rehage et al. (2011) | R medial frontal gyrus | NS | Executive control: modified Flanker Task | + |
| Perceptual speed: Visual Search Task | NS | |||
| L anterior cingulate | NS | Executive control: modified Flanker Task | NS | |
| R posterior cingulate | NS | Executive control: modified Flanker Task | NS | |
| R parahippocampal gyrus | NS | Executive control: modified Flanker Task | NS | |
| Perceptual speed: Visual Search Task | NS | |||
| R superior temporal gyrus | NS | |||
| R lentiform nucleus | NS | |||
| L parahippocampal gyrus | − | |||
| R superior, middle temporal gyrus | − | |||
| L anterior cingulate | − | Executive control: modified Flanker Task | NS | |
| IQ: neuropsychological battery (Digit-Symbol Substitution & Identical picture task, Figural Analogies & Letter Series, Paired Associate Test, Verbal Fluency, Vocabulary Test) | NS |
Note. NS = not significant, + = significant positive change, − = significant negative change. Blank cells indicate that there was no corresponding MRI brain region or neurocognitive test.
Results
The six papers were published between 2004 and 2013 (Chapman et al., 2013; Colcombe et al., 2004; Erickson et al., 2011; Holzschneider, Wolbers, Röder, & Hötting, 2012; Mortimer et al., 2012; Voelcker-Rehage, Godde, & Staudinger, 2011). Of the six studies, three were conducted in the United States (Chapman et al., 2013; Colcombe et al., 2004; Erickson et al., 2011), two in Europe (both in Germany; Holzschneider et al., 2012; Voelcker-Rehage et al., 2011), and one in Asia (China; Mortimer et al., 2012). There did not appear to be a risk of bias within the individual studies or across studies. We excluded two papers from the same study prior to data extraction and analysis, preventing that particular risk of bias.
Sample Characteristics and Setting
Inclusion and exclusion criteria
All six studies included sedentary community-dwelling middle-aged (n = 1; Holzschneider et al., 2012) and/or older adults (Chapman et al., 2013; Colcombe et al., 2004; Erickson et al., 2011; Mortimer et al., 2012; Voelcker-Rehage et al., 2011). However, only three studies provided specific age parameters. Some studies included criteria to exclude those with neurological conditions, specifically those with cognitive impairment or dementia (n = 5; Chapman et al., 2013; Colcombe et al., 2004; Erickson et al., 2011; Mortimer et al., 2013; Voelcker-Rehage et al., 2011) or stroke (n = 2; Mortimer et al., 2012; Voelcker-Rehage et al., 2011). Most of the studies (5/6) also specified criteria regarding psychiatric status, including no history of psychiatric conditions (n = 1; Colcombe et al., 2004), no evidence of depressive symptoms (n = 2; Erickson et al., 2011; Mortimer et al., 2012), and no history of psychiatric conditions or depressive symptoms (n = 2; Chapman et al., 2013; Holzschneider et al., 2012). Other specific health-related exclusion criteria were no vascular disease related to cardiovascular disease or diabetes (Mortimer et al., 2012). As expected, all of the studies had standard exclusion criteria related to MRI procedures. Additional inclusion criteria were adequate visual acuity and right-handedness (Erickson et al., 2011).
Sample characteristics
The sample sizes reported in the six studies ranged from 37 to 120. The mean age for participants was 65.5 ± 5.1, ranging from 48.9 to 69.6 years. All six studies reported findings on men and women, and they all had more women than men (range = 55% to 72% women). Interestingly, none of the studies reported racial or ethnic characteristics of the participants. Four studies reported years of education; participants completed on average 13.8 years. No study reported income level.
Setting
Of the two studies that reported the setting, interventions took place in a research laboratory (Chapman et al., 2013) and a variety of community sites, including a community center, park, and gym (Mortimer et al., 2012). All of the physical activity interventions appear to be have been supervised or monitored.
Intervention Characteristics
The interventions for all six studies involved physical activity training, during which participants engaged in aerobic activity two or three times per week ranging from 40 to 60 minutes per session (Table 1). The aerobic activity included walking (n = 4; Colcombe et al., 2004; Erickson et al., 2011; Mortimer et al., 2012; Voelcker-Rehage et al., 2010), cycling (Holzschneider et al., 2012), or a choice between walking and cycling (Chapman et al., 2013). One study had a second intervention condition involving coordination training designed to improve fine- and gross-motor coordination such as balance (Voelcker-Rehage et al., 2010). Another study provided two additional intervention conditions including Tai Chi and a social interaction group that self-selected discussion topics (Mortimer et al., 2012).
The attention control group most commonly received non-endurance stretching, flexibility, or toning training (n = 4; Colcombe et al., 2004; Erickson et al., 2011; Holzschneider et al., 2012; Voelcker-Rehage et al., 2011). A fifth study provided an attention control group with periodic phone calls to keep them in the study and another employed a wait-list control (Chapman et al., 2013). One study randomly assigned participants from both study conditions (aerobic endurance group and non-endurance control) to one of two different cognitive training sessions (spatial or perceptual) during the last month of the intervention (Holzschneider et al., 2012).
Intervention duration ranged from 12 weeks to 1 year. Attendance was defined as percentage of intervention sessions attended by participants. Only three of six studies reported attendance. For a 12-week program, participants attended 90% of the sessions (Chapman et al., 2013), and for the second study participants attended 85% (Erickson et al., 2011) at 1 year.
Physical Activity, MRI, and Neurocognitive Measures
We examined study measures related to physical activity, MRI, and neurocognition (Table 2). Attendance was the only reported measure of dose of the physical activity intervention. None of the studies assessed the quantity or duration of the physical activity intervention through use of self-report questionnaires or accelerometers. Mortimer and colleagues (2012), however, used a pedometer to determine number of weekly steps. Five of the studies included a measure of cardiorespiratory fitness, which is related to physical activity; four assessed VO2max or VO2peak, with a graded treadmill test (Erickson et al., 2011; Voelcker-Rehage et al., 2011), Rockport 1-mile walk test (Colcombe et al., 2004), or choice of treadmill or cycle ergometer (Holzschneider et al., 2012). One study did not report the method for obtaining VO2max (Chapman et al., 2013). Cardiorespiratory fitness was most commonly used to determine intensity range for each participant during the physical activity intervention. Chapman and colleagues (2013) also assessed perceived exertion, and Colcombe and colleagues (Colcombe et al., 2004) assessed resting heartrate, both of which are related to cardiorespiratory fitness. One study also assessed motor fitness (e.g., feet tapping, one-leg-stand; Voelcker-Rehage et al., 2011).
For MRI measures, two studies reported use of MRI to measure brain volume (Erickson et al., 2011; Mortimer et al., 2012). Of the others, one each measured cerebral blood flow (Chapman et al., 2013), cortical plasticity (Colcombe et al., 2004), spatial learning capacity (Holzschneider et al., 2012), and brain activation patterns (Voelcker-Rehage et al., 2011).
Neurocognitive tests were reported in all studies. Executive function (n = 3; Chapman et al., 2013; Mortimer et al., 2012; Voelcker-Rehage et al., 2011) was most frequently assessed, followed by spatial cognition or spatial memory (n = 3; Erickson et al., 2011; Holzschneider et al., 2012; Mortimer et al., 2012), verbal memory (n = 3; Chapman et al., 2013; Mortimer et al., 2012) and perceptual speed (n = 2; Holzschneider et al., 2012; Voelcker-Rehage et al., 2011).
Outcome Results
All but one study reported significant improvements in MRI measures for the endurance-focused physical activity intervention groups. For the two studies that reported brain volume, one reported relative increases in brain volume for the endurance-focused physical activity intervention group compared to the control (Erickson et al., 2011). The other study did not have significant effects of the walking intervention (Mortimer et al., 2012). Four of the other studies found significant differences between the endurance-focused physical activity intervention group and either the wait-list control group (Chapman et al., 2013) or the non-endurance toning/stretching group (Colcombe et al., 2004; Holzschneider et al., 2012; Voelcker-Rehage et al., 2011). The significant changes in the endurance-focused physical activity intervention were: (a) higher resting cerebral blood flow in the anterior cingulate region (Chapman et al., 2013); (b) greater level of task-related activities in attentional control areas of brain (middle frontal gyrus, superior front gyrus, superior parietal lobes) and reduced level of activity in the anterior cingulate cortex (Colcombe et al., 2004); (c) change in brain activation in the medial frontal gyrus and cuneus positively related to change in VO2peak (Holzschneider et al., 2012); and (d) reduced activity in the anterior cingulate cortex, but lower task-related activity in the attentional control areas (Voelcker-Rehage et al., 2011).
We were able to calculate effect sizes of the physical activity intervention on MRI outcomes for only one study (Erickson et al., 2011). This study had positive but small effect sizes for four regions: left hippocampus (d = .21), right hippocampus (d = .20), left anterior hippocampus (d = .29), and right anterior hippocampus (d = .3). Effect sizes to measure the impact of endurance-focused physical activity interventions on neurocognitive measures were calculated for two studies (Mortimer et al., 2012; Voelcker-Rehage et al., 2011). Both studies had small positive effects on neurocognitive measures, specifically in executive function (d = .32; Mortimer et al., 2012), perceptual speed (d = .20–24; Voelcker-Rehage et al., 2011), and delayed verbal memory (d = .23; Mortimer et al., 2012).
Agreement between MRI and Neurocognitive Outcome Measures
We compared the significant findings of the MRI brain regions examined in each study with the corresponding neurocognitive tests assessed (Table 3). There was one concordant finding for significant effects of physical activity on the neurocognitive test of behavioral conflict (executive function) and on the anterior cingulate region (Colcombe et al., 2004). Most inconsistent findings were in the direction of physical activity having significant effects on MRI findings and non-significant effects on the neurocognitive tests. Most findings on the effects of physical activity were non-significant for both MRI and neurocognitive tests.
Discussion
This review of six studies examined the effect of endurance-focused physical activity RCTs on the brain, as measured by MRI, in community-dwelling middle-aged or older adults without cognitive impairment or dementia. We also analyzed the effects of these endurance-focused physical activity interventions on MRI and neurocognitive measures. The six studies reviewed demonstrated modest effects of the physical activity intervention on MRI and neurocognitive measures (i.e., for those that could be calculated).
Despite the potential sensitivity of MRI measures, it is important to note that the non-specific nature of such measures is a limitation. More research is necessary to identify the effects of physical activity interventions on specific brain changes, such as plasticity or neurogenesis (Thomas, Dennis, Bandettini, & Johansen-Berg, 2012). When comparing results from neurocognitive and MRI measures, we found overlap for only one significant finding (significant decreases in reaction time were associated with decreased activity in the anterior cingulated cortex). The absence of overlap in other areas seemed largely due to the general lack of significant findings for both ways of evaluating intervention impact. In addition, there were no corresponding neurocognitive measures for two of the significant MRI findings. These findings may also support suggestions that MRI measures may be more sensitive to the effects of physical activity than neurocognitive measures (Smith et al., 2013); however, further research is needed to explore this.
Several limitations to this review must be considered. First, we limited our review to RCTs only, which is the strongest study design. This excluded studies of physical activity interventions that used other designs. As mentioned earlier, we were only able to calculate effect sizes for three studies. This limitation precludes broad statements regarding the effects of these endurance-focused physical activity interventions on the brain.
This review shows that physical activity interventions that target brain health continue to be a promising area of research. These interventions have the potential to impact brain structure, and possibly cognitive function, in the middle-aged and older adult population; however, additional investigation is needed. Future RCTs should include larger community-based samples that represent diverse populations, and we encourage authors to provide effects sizes or adequate data to calculate effect sizes. In addition to including indirect measures of physical activity, such as cardiorespiratory fitness, studies should include direct measures of lifestyle physical activity, such as accelerometer. To our knowledge, no study that compares endurance-focused physical activity to cognitive training has used MRI measures to assess impact on brain structure and function. Future research should address this gap. Moreover, given the time needed for behavioral changes to impact specific areas of brain function, interventions of greater duration and with longer follow-up may permit identification of consistent effects for specific brain regions and the corresponding neurocognitive tests (Smith et al., 2013).
Acknowledgments
Funding Acknowledgements: National Institute of Nursing Research, National Institutes of Health F31 NR015372
Jonas Nurse Leader Scholar, Jonas Center for Nursing and Veterans Healthcare
Midwest Nursing Research Society Dissertation Grant
Contributor Information
Shannon Halloway, Rush University College of Nursing, 1251 W. Fletcher, Unit H, Chicago, IL 60657.
JoEllen Wilbur, Email: JoEllen_Wilbur@rush.edu, Rush University College of Nursing, Professor and Associate Dean for Research, Endowed, Independence Foundation Chair in Nursing.
Michael E. Schoeny, Email: Michael_Schoeny@rush.edu, Rush University College of Nursing, Associate Professor.
Konstantinos Arfanakis, Email: Konstantinos_Arfanakis@rush.edu, Rush Alzheimer’s Disease Center, Rush University Medical Center, Professor. Director, MRI Research, Department of Biomedical Engineering, Medical Imaging Research Center, Illinois Institute of Technology.
References
- American College of Sports Medicine. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, … Skinner JS. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Medicine and Science in Sports and Exercise. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- Behrman S, Ebmeier KP. Can exercise prevent cognitive decline? The Practitioner. 2014;258(1767):17–21. 2–3. [PubMed] [Google Scholar]
- Burzynska AZ, Chaddock-Heyman L, Voss MW, Wong CN, Gothe NP, Olson EA, … Kramer AF. Physical activity and cardiorespiratory fitness are beneficial for white matter in low-fit older adults. PloS One. 2014;9(9):e107413. doi: 10.1371/journal.pone.0107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Aslan S, Spence JS, DeFina LF, Keebler MW, Didehbani N, Lu H. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Frontiers in Aging Neuroscience. 2013;5 doi: 10.3389/fnagi.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. L. Erlbaum Associates; 1988. [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, … Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming TB, Tyedin K, Churilov L, Morris ME, Bernhardt J. The effect of physical activity on cognitive function after stroke: a systematic review. International Psychogeriatrics. 2012;24(4):557–567. doi: 10.1017/S1041610211001980. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, … Kramer AF. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre C, Chamari K, Mucci P, Massé-Biron J, Préfaut C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. International Journal of Sports Medicine. 2002;23(6):415–421. doi: 10.1055/s-2002-33735. [DOI] [PubMed] [Google Scholar]
- Farina N, Rusted J, Tabet N. The effect of exercise interventions on cognitive outcome in Alzheimer’s disease: a systematic review. International Psychogeriatrics. 2014;26(1):9–18. doi: 10.1017/S1041610213001385. [DOI] [PubMed] [Google Scholar]
- Gary RA, Brunn K. Aerobic exercise as an adjunct therapy for improving cognitive function in heart failure. Cardiology Research and Practice. 2014;2014:157508. doi: 10.1155/2014/157508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates N, Fiatarone Singh MA, Sachdev PS, Valenzuela M. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. The American Journal of Geriatric Psychiatry. 2013;21(11):1086–1097. doi: 10.1016/j.jagp.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. 2011 Retrieved from http://handbook.cochrane.org/
- Holtzer R, Goldin Y, Zimmerman M, Katz M, Buschke H, Lipton RB. Robust norms for selected neuropsychological tests in older adults. Archives of Clinical Neuropsychology. 2008;23(5):531–541. doi: 10.1016/j.acn.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschneider K, Wolbers T, Röder B, Hötting K. Cardiovascular fitness modulates brain activation associated with spatial learning. NeuroImage. 2012;59(3):3003–3014. doi: 10.1016/j.neuroimage.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. Journal of Applied Physiology. 2006;101(4):1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- Lautenschlager NT, Cox K, Kurz AF. Physical activity and mild cognitive impairment and Alzheimer’s disease. Current Neurology and Neuroscience Reports. 2010;10(5):352–358. doi: 10.1007/s11910-010-0121-7. [DOI] [PubMed] [Google Scholar]
- Law LLF, Barnett F, Yau MK, Gray MA. Effects of combined cognitive and exercise interventions on cognition in older adults with and without cognitive impairment: A systematic review. Ageing Research Reviews. 2014;15:61–75. doi: 10.1016/j.arr.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Legault C, Jennings JM, Katula JA, Dagenbach D, Gaussoin SA, Sink KM … SHARP-P Study Group. Designing clinical trials for assessing the effects of cognitive training and physical activity interventions on cognitive outcomes: The Seniors Health and Activity Research Program Pilot (SHARP-P) study, a randomized controlled trial. BMC Geriatrics. 2011;11:27. doi: 10.1186/1471-2318-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littbrand H, Stenvall M, Rosendahl E. Applicability and effects of physical exercise on physical and cognitive functions and activities of daily living among people with dementia: A systematic review. American Journal of Physical Medicine and Rehabilitation. 2011;90(6):495–518. doi: 10.1097/PHM.0b013e318214de26. [DOI] [PubMed] [Google Scholar]
- McCarten JR. Clinical evaluation of early cognitive symptoms. Clinics in Geriatric Medicine. 2013;29(4):791–807. doi: 10.1016/j.cger.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JA, Ding D, Borenstein AR, DeCarli C, Guo Q, Wu Y, … Chu S. Changes in brain volume and cognition in a randomized trial of exercise and social interaction in a community-based sample of non-demented Chinese elders. Journal of Alzheimer’s Disease. 2012;30(4):757–766. doi: 10.3233/JAD-2012-120079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald WD, Gunzelmann T, Rupprecht R, Hagen B. Differential effects of single versus combined cognitive and physical training with older adults: The SimA study in a 5-year perspective. European Journal of Ageing. 2006;3(4):179–192. doi: 10.1007/s10433-006-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, … Rocca WA. Prevalence of mild cognitive impairment is higher in men: The Mayo Clinic Study of Aging. Neurology. 2010;75(10):889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatti F, Rozzini R, Trabucchi M. Physical activity and cognitive decline in elderly persons. Archives of Internal Medicine. 2002;162(3):361–362. doi: 10.1001/archinte.162.3.361. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, … Wallace RB. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, … Wallace RB. Prevalence of cognitive impairment without dementia in the United States. Annals of Internal Medicine. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash RS, Voss MW, Erickson KI, Lewis JM, Chaddock L, Malkowski E, … Kramer AF. Cardiorespiratory fitness and attentional control in the aging brain. Frontiers in Human Neuroscience. 2011;4:229. doi: 10.3389/fnhum.2010.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahe J, Petrelli A, Kaesberg S, Fink GR, Kessler J, Kalbe E. Effects of cognitive training with additional physical activity compared to pure cognitive training in healthy older adults. Clinical Interventions in Aging. 2015;10:297–310. doi: 10.2147/CIA.S74071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M, Hardy R, Wadsworth MEJ. Does active leisure protect cognition? Evidence from a national birth cohort. Social Science and Medicine (1982) 2003;56(4):785–792. doi: 10.1016/s0277-9536(02)00075-8. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Potter GG, McLaren ME, Blumenthal JA. Impact of aerobic exercise on neurobehavioral outcomes. Mental Health and Physical Activity. 2013;6(3):139–153. doi: 10.1016/j.mhpa.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden M, Steinman L, Mochan K, Grodstein F, Prohaska TR, Thurman DJ, … Anderson LA. Effect of exercise on cognitive performance in community-dwelling older adults: Review of intervention trials and recommendations for public health practice and research. Journal of the American Geriatrics Society. 2011;59(4):704–716. doi: 10.1111/j.1532-5415.2011.03323.x. [DOI] [PubMed] [Google Scholar]
- Szabo AN, McAuley E, Erickson KI, Voss M, Prakash RS, Mailey EL, … Kramer AF. Cardiorespiratory fitness, hippocampal volume, and frequency of forgetting in older adults. Neuropsychology. 2011;25(5):545–553. doi: 10.1037/a0022733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AG, Dennis A, Bandettini PA, Johansen-Berg H. The Effects of Aerobic Activity on Brain Structure. Frontiers in Psychology. 2012;3 doi: 10.3389/fpsyg.2012.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelcker-Rehage C, Godde B, Staudinger UM. Cardiovascular and coordination training differentially improve cognitive performance and neural processing in older adults. Frontiers in Human Neuroscience. 2011;5 doi: 10.3389/fnhum.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Kang JH, Manson JE, Breteler MMB, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. The Journal of the American Medical Association. 2004;292(12):1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- Zygouris S, Tsolaki M. Computerized cognitive testing for older adults: A review. American Journal of Alzheimer’s Disease and Other Dementias. 2015;30(1):13–28. doi: 10.1177/1533317514522852. [DOI] [PMC free article] [PubMed] [Google Scholar]