INTRODUCTION
MS is a significant cause of neurological and cognitive disability in young people. Pathologically it is characterized by inflammatory demyelination and, in chronic lesions, axonal loss1. The cause of reduced cortical metabolism described in MS remains uncertain2, 3. While MS is typically regarded as a disease primarily affecting WM, cGM is increasingly complicit in physical and cognitive disease progression. However, the relationships between WM and cGM disease progression remain controversial. Although some studies suggest a relationship between normal appearing WM (NAWM) atrophy and cGM damage4, 5, others suggest that cGM disease progression is either independent from or only partly related to WM abnormalities6, 7. Louapre et al, utilizing DTI at 7T, found a lack of spatial specificity between NAWM tracts and the overlying cGM8. Steenwijk et al9 reported a stronger relationship between cGM atrophy and WM tract pathology in RRMS compared to SPMS patients, concluding that the association between NAWM and cGM becomes increasingly independent with disease progression. The assertion that cGM and WM progression is either dependent or partly independent is supported by histopathological and radiological series demonstrating the role of meningeal mediated processes in both cortical and leucocortical lesion but not WM T2h-l development10, 11.
Although few studies4, 5, 9 have examined the regional relationship between cortical structure and WM disease, the association between regional WM volume and perfusion and cortical volume and perfusion is not previously studied. CBF and CBV reduction are previously shown either in the absence of, or adjusting for, inter-group structural differences suggesting that cortical perfusion could serve as a surrogate of disease severity and tissue integrity under specific conditions12–14. Aviv et al12 demonstrated focal cGM CBV reduction in cognitively impaired compared to preserved SPMS patients after adjusting for global WM T2h-l volumes. Hojjat et al15 demonstrated significant CBF reduction in the absence of structural differences in impaired compared to cognitively-preserved RRMS patients. Lastly, Debernard et al14 found CBF reduction in the absence of cGM volume differences in unimpaired RRMS patients compared to healthy controls (HC) using pCASL perfusion. While prior studies reported regional variation in CBF and CBV, none examined the regional associations between lobar WM (normal appearing and lesional) and cGM volume and perfusion reduction. Consistent with growing evidence for partly independent mechanisms of disease progression in WM and cGM, we hypothesized that an independent association would be found between lobar WM disease and cGM volume and perfusion.
METHODS
Study participants
38 MS (19 each SPMS and RRMS) patients from two tertiary referral MS clinics and 19 healthy, age-matched controls were prospectively recruited over a one-year period. Exclusion criteria included history of drug/alcohol abuse, relapse or steroid use < 6 months, pre-MS psychiatric history, head injuries involving loss of consciousness, cardiac disease, and MRI contraindications. Demographic data was obtained for each subject. This study was approved by local ethics committee and written consent was obtained from each participant prior to study enrollment.
Cognitive testing
All patients and HC underwent MR imaging, neurological examination, and EDSS assessment within one week. Patients were tested using the Minimal Assessment of Cognitive Function in Multiple Sclerosis battery comprising 7 tests covering 5 cognitive domains, including: processing speed, memory, executive function, visuospatial perception, and verbal fluency. Only cognitively-preserved patients were enrolled in the study given the greater potential for confounding pathophysiological factors with greater disease progression characterized by cognitive impairment and the previously published association between cognitive impairment, disease progression and hypoperfusion9, 15, 16.
Image acquisition
All MRI data were acquired on a 3T MRI system (Achieva, Philips Healthcare, The Netherlands) with an 8-channel phased array coil. The MRI sequences included: axial proton density/T2 (TR/TE/flip angle= 2500ms/10.7ms/90°; FOV= 230×230 mm2; acquisition matrix= 256×263; slice thickness= 3 mm); axial T1-weighted TSE (TR/TE/flip angle= 9.5ms/2.3ms/12°; FOV= 240×240 mm2; acquisition matrix= 256×219; slice thickness= 1.2 mm); axial phase-sensitive inversion recovery (TR/TE= 3374ms/15ms; FOV= 230×230 mm2; acquisition matrix= 400×255; slice thickness= 3 mm); axial field-echo, echo-planar dynamic susceptibility contrast (DSC) perfusion (TR/TE/flip angle= 1633ms/30ms/60°; FOV= 220×220 mm2; acquisition matrix= 96×93; slice thickness= 4 mm; no gap; signal bandwidth= 1260 Hz/pixel; sections= 24). During the perfusion scan, ten mL of 1mmol/mL concentrated Gadobutrol (Gadovist, Bayer, Toronto, Canada) was administered by a power injector at a rate of 5 mL/s, followed by 25 mL bolus of saline at 5 mL/s, and a total of 60 images were acquired with the injection occurring at the 5th volume. A segmented inversion recovery look-locker EPI sequence was performed immediately before and after the axial DSC sequence (TR/TE/flip angle= 29ms/14ms/20°; inversion time= 15.8ms; FOV= 220×220 mm2; acquisition matrix= 128×126; 15 k-space lines per acquisition; slice thickness= 4 mm; 60 time points). A 3 second delay occurred following the last imaging time point to facilitate longitudinal magnetization recovery.
Quantitative MR perfusion
Quantitative CBF (ml/100g/min), quantitative CBV (ml/100g), and MTT (seconds) were obtained using Bookend MRI perfusion as previously published17. The technique uses pre- and post-gadolinium ‘bookend’ scans to calculate WM quantitative CBV without need for an arterial input function while accounting for the effects of intravascular-to-extravascular water exchange. Tissue concentration-time curve is calculated through arterial input function sampling, allowing relative CBV and relative CBF determination. The central volume principle is used to calculate MTT.
Image processing
Structural T1-and proton density/T2-weighted images were co-registered using linear registration (SPM8; Wellcome Department of Imaging Neuroscience, London, UK). T2h-l and deep GM structures were segmented by a board-certified neuroradiologist (>10 years’ experience) using the trace function in Analyze 8.0 (Mayo Clinic, Rochester, MN, USA). T1-weighted structural images were first segmented into GM and WM masks using the unified segmentation model in SPM8 and checked for accuracy before creating subject-specific NAWM masks by subtracting T2h from the automated WM segmentation. For cortical volumetric analysis, the International Consortium for Brain Mapping lobar (Laboratory of Neuroimaging, Keck School of Medicine, Los Angeles, CA, USA) and MRIcro Brodmann templates (Neuropsychology Laboratory, Columbia, SC, USA) were registered to MNI-152 space using the normalize function in SPM. Structural T1-weighted images, and associated lesional ROIs, and lobar templates were co-registered to the EPI DSC pre-gadolinium images using linear registration (FSL-FLIRT: FMRIB Software Library v5.0) and non-linear intensity modulation and multi-resolution, non-linear registration with four subsampling levels (FSL-FNIRT: FMRIB Software Library v5.0). Global and lobar cGM and WM volumetric and perfusion metrics were then quantified separately for bilateral frontal, parietal, temporal and occipital lobes, as previously described18.
Statistical analysis
Demographic, clinical, volumetric and perfusion data were summarized for HC, RRMS, and SPMS patients using mean and standard deviation for continuous variables, and proportions for categorical variables. To compare RRMS vs. HC, SPMS vs. HC, and SPMS vs. RRMS for demographic variables (i.e., age, gender, educational years, disease duration and EDSS), univariate logistic regression model was conducted. Significant confounding factors were determined and used for perfusion data analysis. Bonferroni corrected p-value < 0.017 (0.05/3) was considered statistically significant for controlling for multiple comparisons among the 3 groups. To compare HC, RRMS, and SPMS cohort differences for the imaging parameter covariates (i.e., CBF, CBV, MTT, lobar GM and WM volume), generalized linear model with logit link function was used after adjusting for confounding factors. GENMOD procedure in Statistical Analysis Software (SAS version 9.4 for Windows, SAS Institute Inc, Cary, NC, USA) was performed to fit the model with Bonferroni adjusted p-value < 0.017 considered statistically significant. Confounding factors of age, disease duration, and EDSS were assessed for multicollinearity by examining tolerance and variance inflation factor (VIF = 1/tolerance) in a regression model using SPSS (IBM Corp., Armonk, NY, USA). A tolerance value <0.1 and VIF >10 were regarded as indicating multicollinearity. Normality was determined using the Shapiro-Wilk test, and anomalous dependent variables were log transformed to fit the data to a normal distribution. Natural log-transformation was applied as appropriate for normalizing the distributions. A general linear regression was implemented to assess the association between GM and WM regional perfusion data, between GM and T2h-l regional perfusion data, between WM and T2h-l regional perfusion data, and between lobar GM and lobar WM volume data while considering confounding factors and expressed as R2.
RESULTS
Clinical characteristics, global volumes and perfusion
MS subgroups did not significantly differ in sex, disease duration and years of education, although the SPMS cohort was older (p=0.0041) and had higher EDSS (p=0.0006) scores than RRMS (Table 1). SPMS patients demonstrated a longer disease duration but this did not reach statistical significance (p=0.02). The SPMS cohort had greater global atrophy in cGM and WM compared to RRMS and HC subjects (p=0.002, p=0.0026 and p=0.0049 and p=0.0011 respectively, Table 2). RRMS exhibited lower global WM (p=0.0115) but not GM volume compared to HC. GM and WM CBF and CBV were reduced and MTT prolonged in SPMS compared to RRMS subjects. GM CBF reduction and MTT prolongation were present in RRMS and SPMS compared to HC. No significant WM CBF or CBV difference was observed for any RRMS/SPMS comparison with HC WM MTT was significantly prolonged for SPMS versus HC. No significant T2h-l volume, CBF or MTT differences were seen between RRMS and SPMS patients, although SPMS patients had higher T2h-l CBV than RRMS patients.
Table 1.
Comparison of demographic and clinical data for HC, RRMS, and SPMS subjects
| Parameter | HC n=19 |
RRMS n=19 |
SPMS n=20 |
RRMS vs HC p-value |
SPMS vs HC p-value |
SPMS vs RRMS p-value |
|---|---|---|---|---|---|---|
| Age (yrs) | 49.0 ± 7.1 | 46.4 ± 7.2 | 55.2 ± 6.5 | 0.27 | 0.0168 | 0.0041 |
| Gender (F/M) | 14/5 | 15/4 | 11/9 | 0.70 | 0.23 | 0.12 |
| Education (yrs) | 16.9 ± 2.9 | 16.1 ± 1.3 | 15.1 ± 2.6 | 0.22 | 0.051 | 0.15 |
| Disease Duration (yrs) | 0.00 | 11.8 ± 5.4 | 16.7 ± 6.5 | NA | NA | 0.0234 |
| EDSS; Median (IQR) | 0.00 | 1.5 (1, 2) | 6 (6, 6.5) | NA | NA | 0.0006 |
All values are mean (SD) except where indicated. EDSS: Expanded Disability Status Scale. Bonferroni corrected p<0.017 is considered statistically significant.
Table 2.
Comparison of perfusion and volumetric data for cGM, NAWM and T2h-l between HC, RRMS, and SPMS subjects after adjusting for confounding factors
| Parameter | HC n=19 |
RRMS n=19 |
SPMS n=20 |
RRMS vs HC |
SPMS vs HC |
SPMS vs RRMS |
|
|---|---|---|---|---|---|---|---|
| cGM |
Median (Interquartiles) |
Median (Interquartiles) |
Median (Interquartiles) |
p-value | p-value | p-value | |
| Global volume | 890 (869, 914) | 864 (827, 895) | 673 (646, 697) | 0.10 | 0.0026 | 0.0020 | |
| CBF | 43.20 (33.91, 54.49) | 41.07 (29.85, 55.68) | 34.05 (27.19, 43.68) | <0.0001 | <0.0001 | 0.0011 | |
| CBV | 2.64 (2.02, 3.29) | 2.53 (1.94, 3.37) | 2.40 (1.92, 3.07) | 0.078 | 0.046 | 0.0062 | |
| MTT | 3.77 (3.20, 4.31) | 3.90 (3.32, 4.41) | 4.30 (3.66, 4.99) | 0.0001 | <0.0001 | <0.0001 | |
| NAWM | Global volume | 878 (812, 894) | 818 (738, 850) | 697 (669, 729) | 0.0115 | 0.0011 | 0.0049 |
| CBF | 23.49 (18.69, 27.78) | 21.55 (16.05, 29.50) | 19.54 (16.74, 24.92) | 0.68 | 0.58 | 0.0080 | |
| CBV | 1.59 (1.28, 2.00) | 1.57 (1.17, 2.15) | 1.51 (1.30, 1.98) | 0.89 | 0.16 | 0.0103 | |
| MTT | 4.51 (4.09, 5.10) | 4.62 (4.08, 5.20) | 4.86 (4.28, 5.41) | 0.25 | 0.0005 | 0.0094 | |
| T2h-l | Global volume | 0.00 | 5.4 (1.2, 9.3) | 6.4 (1.0, 10.9) | NA | NA | 0.82 |
| CBF | 0.00 | 12.77 (8.23, 17.24) | 11.15 (8.95, 15.71) | NA | NA | 0.049 | |
| CBV | 0.00 | 0.99 (0, 1.32) | 1.08 (0.83, 1.47) | NA | NA | 0.0026 | |
| MTT | 0.00 | 4.91 (4.00, 5.63) | 5.64 (4.82, 6.30) | NA | NA | 0.43 |
T2h-l: T2-hyperintense lesions. Bonferroni corrected p<0.017 is considered statistically significant. Age was considered as confounding factor for comparing RRMS vs. HC, and SPMS vs. HC; age, EDSS, and disease duration were considered as confounding factors for comparing SPMS vs. RRMS. All volumes are in cm3. CBF ml/100g/m, CBVml/100g, MTT seconds
Lobar volumetric group comparisons
SPMS patients had reduced cGM volumes in the temporal and occipital lobes, and reduced WM volumes in the occipital lobe compared to RRMS patients (Table 3). SPMS patients also demonstrated reduced occipital lobe WM and temporal and occipital cGM compared to HC. Frontal and parietal lobe WM volume reduction was observed for all comparisons and RRMS also demonstrated a reduced temporal WM volume compared to HC. Overall, a weak association was present between lobar cGM and lobar NAWM volume (Table 4, Supplementary Figure 1) in both RRMS (R2 0.14–0.48) and SPMS (R2 0.16–0.48) patients with no statistical significance achieved for any lobar region. Association was stronger in HC (R2 0.53–0.79)
Table 3.
Lobar GM and WM volume differences in cm3 between HC, RRMS, and SPMS subjects after adjusting for confounding factors
| Parameter | HC (n=19) Median (Interquartiles) |
RRMS (n=19) Median (Interquartiles) |
SPMS (n=20) Median (Interquartiles) |
RRMS vs HC p-value |
SPMS vs HC p-value |
SPMS vs RRMS p-value |
|---|---|---|---|---|---|---|
| Gray Matter | ||||||
| Frontal Lobe | 200.040 (190.352, 167.944) | 199.248 (192.480, 207.896) | 200.352 (193.500, 207.540) | 0.69 | 0.60 | 0.22 |
| Parietal Lobe | 90.296 (83.992, 94.512) | 89.824 (85.048, 95.176) | 93.368 (88.956, 96.352) | 0.72 | 0.03 | 0.095 |
| Temporal Lobe | 122.740 (110.640, 130.560) | 121.336 (114.672, 128.544) | 106.892 (99.432, 112.928) | 0.86 | 0.0044 | 0.0073 |
| Occipital Lobe | 101.088 (98.536, 106.272) | 97.160 (95.728, 102.960) | 72.792 (69.868, 75.196) | 0.10 | 0.0030 | 0.0033 |
| White Matter | ||||||
| Frontal Lobe | 190.568 (170.368, 201.528) | 170.316 (159.520, 189.112) | 146.260 (134.908, 157.136) | <0.0001 | 0.0006 | <0.0001 |
| Parietal Lobe | 101.200 (94.536, 109.152) | 93.272 (84.944, 97.208) | 81.952 (77.736, 87.532) | 0.0001 | 0.0018 | <0.0001 |
| Temporal Lobe | 70.132 (56.648, 79.352) | 68.512 (64.116, 74.016) | 61.208 (51.576, 71.720) | 0.026 | 0.98 | 0.0026 |
| Occipital Lobe | 32.024 (30.248, 35.092) | 29.092 (24.136, 33.768) | 26.864 (23.552, 31.832) | 0.65 | 0.0098 | 0.0003 |
Bonferroni corrected p<0.017 is considered statistically significant. Age was considered as confounding factor for comparing RRMS vs. HC, and SPMS vs. HC; age, EDSS and disease duration were considered as confounding factors for comparing SPMS vs. RRMS. All volumes are in cm3
Table 4.
Relationship between cGM and NAWM regional perfusion data, between GM and T2h-l regional perfusion data, and between Lobar cGM and Lobar NAWM volume data for HC, RRMS, and SPMS subjects after adjusting for confounding factors
| Relationship between | Relationship between | Relationship between | Relationship between Lobar cGM and NAWM Regional volume |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||||
| cGM and NAWM Regional Perfusion | cGM and T2h-l Regional Perfusion | NAWM and T2h-l Regional Perfusion | |||||||||||||||||||
|
| |||||||||||||||||||||
| CBE | CBV | MII | CBE | CBV | MII | CBE | CBV | MII | Volume | ||||||||||||
|
| |||||||||||||||||||||
| Group | Region | R2 | p-value | R2 | p-value | R2 | p-value | R2 | p-value | R2 | p-value | R2 | p-value | R2 | p-value | R2 | p-value | R2 | p-value | R2 | p-value |
|
| |||||||||||||||||||||
| HC | Global | 0.957 | <.0001 | 0.962 | <.0001 | 0.967 | <.0001 | 0.015 | NA | 0.005 | NA | 0.006 | NA | 0.001 | NA | 0 | NA | 0.003 | NA | 0.728 | 0.0002 |
| Frontal - Right | 0.912 | <.0001 | 0.946 | <.0001 | 0.949 | <.0001 | 0.017 | NA | 0.015 | NA | 0 | NA | 0.006 | NA | 0.007 | NA | 0 | NA | 0.525 | 0.0028 | |
| Frontal - Left | 0.898 | <.0001 | 0.959 | <.0001 | 0.91 | <.0001 | 0.034 | NA | 0.024 | NA | 0.001 | NA | 0.007 | NA | 0.005 | NA | 0 | NA | 0.612 | 0.0009 | |
| Parietal - Right | 0.903 | <.0001 | 0.933 | <.0001 | 0.933 | <.0001 | 0.007 | NA | 0.002 | NA | 0.004 | NA | 0 | NA | 0 | NA | 0 | NA | 0.643 | 0.0008 | |
| Parietal - Left | 0.935 | <.0001 | 0.945 | <.0001 | 0.928 | <.0001 | 0.013 | NA | 0.01 | NA | 0.001 | NA | 0 | NA | 0 | NA | 0 | NA | 0.785 | <.0001 | |
| Temporal - Right | 0.918 | <.0001 | 0.905 | <.0001 | 0.96 | <.0001 | 0.001 | NA | 0 | NA | 0.003 | NA | 0.001 | NA | 0.004 | NA | 0.007 | NA | 0.673 | 0.0005 | |
| Temporal - Left | 0.769 | <.0001 | 0.877 | <.0001 | 0.95 | <.0001 | 0.011 | NA | 0.005 | NA | 0.006 | NA | 0.001 | NA | 0 | NA | 0.003 | NA | 0.643 | 0.001 | |
| Occipital - Right | 0.908 | <.0001 | 0.93 | <.0001 | 0.976 | <.0001 | 0.032 | NA | 0.004 | NA | 0.026 | NA | 0.001 | NA | 0.004 | NA | 0.017 | NA | 0.787 | <.0001 | |
| Occipital - Left | 0.934 | <.0001 | 0.938 | <.0001 | 0.959 | <.0001 | 0.02 | NA | 0.002 | NA | 0.016 | NA | 0.002 | NA | 0.008 | NA | 0.006 | NA | 0.791 | <.0001 | |
|
| |||||||||||||||||||||
| RRMS | Global | 0.957 | <.0001 | 0.934 | <.0001 | 0.941 | <.0001 | 0.609 | 0.0006 | 0.337 | 0.0276 | 0.171 | 0.2003 | 0.687 | 0.0001 | 0.461 | 0.0064 | 0.224 | 0.0751 | 0.243 | 0.1816 |
| Frontal - Right | 0.928 | <.0001 | 0.898 | <.0001 | 0.942 | <.0001 | 0.532 | 0.0018 | 0.687 | 0.0001 | 0.334 | 0.033 | 0.537 | 0.0016 | 0.643 | 0.0003 | 0.386 | 0.013 | 0.334 | 0.3466 | |
| Frontal - Left | 0.94 | <.0001 | 0.925 | <.0001 | 0.951 | <.0001 | 0.094 | 0.5388 | 0.105 | 0.7885 | 0.078 | 0.4339 | 0.091 | 0.3898 | 0.078 | 0.5776 | 0.13 | 0.2115 | 0.284 | 0.3139 | |
| Parietal - Right | 0.935 | <.0001 | 0.881 | <.0001 | 0.87 | <.0001 | 0.464 | 0.0053 | 0.537 | 0.0019 | 0.112 | 0.426 | 0.524 | 0.0027 | 0.633 | 0.0006 | 0.193 | 0.1057 | 0.135 | 0.3923 | |
| Parietal - Left | 0.947 | <.0001 | 0.952 | <.0001 | 0.86 | <.0001 | 0.068 | 0.8027 | 0.083 | 0.9232 | 0.076 | 0.756 | 0.09 | 0.7498 | 0.111 | 0.7182 | 0.118 | 0.2304 | 0.234 | 0.1379 | |
| Temporal - Right | 0.944 | <.0001 | 0.897 | <.0001 | 0.915 | <.0001 | 0.078 | 0.3904 | 0.072 | 0.3912 | 0.093 | 0.7074 | 0.153 | 0.2411 | 0.161 | 0.199 | 0.017 | 0.8927 | 0.389 | 0.0337 | |
| Temporal - Left | 0.982 | <.0001 | 0.978 | <.0001 | 0.876 | <.0001 | 0.073 | 0.6998 | 0.064 | 0.9999 | 0.061 | 0.8038 | 0.063 | 0.6522 | 0.057 | 0.9999 | 0.088 | 0.3333 | 0.482 | 0.0248 | |
| Occipital - Right | 0.844 | <.0001 | 0.807 | <.0001 | 0.966 | <.0001 | 0.172 | 0.2855 | 0.116 | 0.3641 | 0.076 | 0.6229 | 0.135 | 0.4273 | 0.21 | 0.1222 | 0.077 | 0.3821 | 0.462 | 0.0106 | |
| Occipital - Left | 0.892 | <.0001 | 0.905 | <.0001 | 0.969 | <.0001 | 0.134 | 0.4168 | 0.098 | 0.4261 | 0.454 | 0.0074 | 0.133 | 0.3579 | 0.163 | 0.2519 | 0.522 | 0.0018 | 0.654 | 0.0006 | |
|
| |||||||||||||||||||||
| SPMS | Global | 0.961 | <.0001 | 0.956 | <.0001 | 0.977 | <.0001 | 0.694 | 0.0001 | 0.423 | 0.0125 | 0.699 | 0.0003 | 0.752 | <.0001 | 0.516 | 0.0041 | 0.701 | 0.0002 | 0.394 | 0.2388 |
| Frontal - Right | 0.944 | <.0001 | 0.929 | <.0001 | 0.923 | <.0001 | 0.489 | 0.0059 | 0.186 | 0.1723 | 0.294 | 0.22 | 0.67 | 0.0003 | 0.297 | 0.0593 | 0.289 | 0.1336 | 0.179 | 0.6434 | |
| Frontal - Left | 0.94 | <.0001 | 0.931 | <.0001 | 0.941 | <.0001 | 0.634 | 0.0004 | 0.293 | 0.0392 | 0.616 | 0.0018 | 0.703 | 0.0001 | 0.32 | 0.0442 | 0.671 | 0.0006 | 0.159 | 0.6728 | |
| Parietal - Right | 0.951 | <.0001 | 0.949 | <.0001 | 0.925 | <.0001 | 0.414 | 0.0142 | 0.513 | 0.0024 | 0.593 | 0.0022 | 0.401 | 0.0211 | 0.534 | 0.0023 | 0.472 | 0.0078 | 0.218 | 0.2349 | |
| Parietal - Left | 0.925 | <.0001 | 0.908 | <.0001 | 0.951 | <.0001 | 0.539 | 0.0045 | 0.209 | 0.1705 | 0.472 | 0.0287 | 0.555 | 0.003 | 0.26 | 0.0928 | 0.582 | 0.0047 | 0.237 | 0.1695 | |
| Temporal - Right | 0.937 | <.0001 | 0.887 | <.0001 | 0.917 | <.0001 | 0.603 | 0.0086 | 0.524 | 0.0194 | 0.898 | <.0001 | 0.689 | 0.0015 | 0.725 | 0.0015 | 0.872 | <.0001 | 0.304 | 0.3312 | |
| Temporal - Left | 0.849 | <.0001 | 0.903 | <.0001 | 0.958 | <.0001 | 0.678 | 0.0023 | 0.427 | 0.0871 | 0.826 | 0.0003 | 0.842 | <.0001 | 0.547 | 0.0229 | 0.872 | <.0001 | 0.477 | 0.5079 | |
| Occipital - Right | 0.915 | <.0001 | 0.9 | <.0001 | 0.984 | <.0001 | 0.765 | 0.0051 | 0.739 | 0.0135 | 0.884 | 0.0008 | 0.845 | 0.0024 | 0.859 | 0.0035 | 0.878 | 0.0008 | 0.622 | 0.3684 | |
| Occipital - Left | 0.906 | <.0001 | 0.936 | <.0001 | 0.959 | <.0001 | 0.306 | 0.1756 | 0.437 | 0.1732 | 0.879 | 0.0006 | 0.346 | 0.26 | 0.585 | 0.0521 | 0.875 | 0.0006 | 0.458 | 0.3448 | |
NA: the perfusion data was zero for T2h-l in HC subjects, therefore, p-value of T2h-l was not available in the model. The R2 was obtained in the model of cGM regional perfusion data with confounding factors only.
Lobar perfusion group comparisons
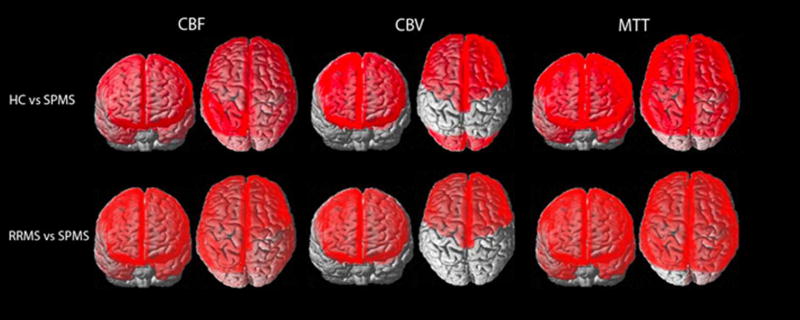
The distribution of significant lobar cortical perfusion differences between group comparisons is demonstrated in the Figure. Lobar cGM CBF reduction and MTT increase was present in all lobes in SPMS patients compared to the other groups, with the exception of MTT increase within the left occipital lobe for the SPMS versus RRMS comparison, which did not reach statistical significance. Lobar cGM CBV reduction was present in the bilateral frontal lobes of SPMS patients compared to the other groups, and in the bilateral occipital lobes of SPMS versus HC. No significant lobar cGM CBF, CBV reduction or MTT prolongation was found between RRMS and HCs. No significant lobar perfusion differences were observed between any group comparison for NAWM. Strong association was shown between cGM and NAWM global and lobar perfusion for all group comparisons and lobes (R2 0.77–0.98, p<0.0001; Table 4, Supplementary Figure 2). Overall, SPMS patients demonstrated stronger associations between lobar T2h-l and cGM and NAWM perfusion compared to RRMS patients (Table 4, Supplementary Figure 3 and 4; cGM CBF R2 0.31–0.77 and NAWM CBF R2 0.35–0.85 versus cGM CBF R2 0.07–0.61 and NAWM CBF R2 0.06–0.69 respectively).
Figure.
Whole brain depiction of perfusion differences in cortical Gray matter between HC, RRMS and SPMS patients. Units for CBF (ml/100g/m), CBV (ml/100g), MTT (seconds)
Associations between perfusion and volumetric data
No significant associations were found between perfusion and volumetric data in any regression analysis (i.e., for cGM, T2h-l or NAWM).
DISCUSSION
We demonstrated a weak association between lobar volumes of cGM and NAWM in MS patients despite cGM and NAWM volume reduction with increasing disease severity. Similarly, although lobar NAWM and cGM perfusion were highly correlated, the distribution of lobar cGM perfusion reduction was distinct from underlying lobar NAWM perfusion which showed no significant between-group differences. These results do not conflict with the notion that the pathophysiology of WM and cGM disease may occur independently and that the strength of association varies relating to the disease severity. The strong association between cGM and NAWM perfusion and lack of association with volumetric measures suggests a potential role for perfusion as an independent surrogate of disease activity.
Weak associations between NAWM volume and GM volume and perfusion in the present study argues against a mechanism of secondary cGM anterograde or retrograde axonal degeneration and suggests independent pathophysiological processes acting on WM and GM. This assertion is supported by various pathological and imaging studies8, 22–24 which have shown that cGM lesions may develop prior to the appearance of WM plaques22, arise independently of, and are poorly correlated with T2h-l formation23,24. A number of pathological and imaging papers have increasingly implicated an independent etiology for cGM lesion formation attributed either to the direct presence of meningeal-derived neurotoxic substances or secondary microglial activation mediated through meningeal/supbial inflammation and manifest as a gradient of demyelination centered upon the subpial cortex24, 27.
Numerous papers have examined the spatial relationship between lobar T2h-l and cGM integrity using quantitative and functional parameters other than perfusion. A recent correlative study of quantitative cortical T2* at 7T and 3T-derived surface and tract based analysis found a correlation between WM tract DTI and cGM integrity although this was not spatially specific, reflecting a common sensitivity to MS pathological changes8. Steenwijk et al9 used DTI at 3T to investigate the association between regional GM atrophy and pathology in anatomically connected WM tracts in RRMS, SPMS and primary-progressive MS patients demonstrating a relationship between NAWM tract FA and deep GM and cGM. The model of variance associated with cGM thickness was greatest in RRMS patients but declined in SPMS and primary-progressive patients. Strong association between NAWM integrity and cGM thickness was found only in the mildly impaired group when patients were dichotomized by EDSS category of 4. The authors concluded that NAWM integrity contributes to cGM atrophy only in early MS. Bodini et al4 used Tract-Based Spatial Statistics to explore the relationship between cGM atrophy and FA in connected NAWM tracts in primary-progressive MS patients, and found that only 4/11 regions studied showed a quantitative association between reduced NAWM FA and GM atrophy. Jehna et al5 found spatial interdependence between focal cortical volumes, lesion location and probabilistic fiber pathways, suggesting that WM tracts and cGM volume are regionally dependent and injured due to similar disease processes suggesting that lesional axonal transection20 leads to Wallerian degeneration and retrograde GM atrophy. Their study was performed in “low disabled” individuals with significantly lower age (29.5yrs) and disease duration (7.3yrs) compared to the present cohort. In contradistinction, we did not demonstrate a stronger association between NAWM/ T2h-l and cGM volume or perfusion with earlier disease, likely explained by longer disease duration and older age in our RRMS group compared to Jehna’s cohort and the different functional techniques used. The near universally stronger cGM and NAWM perfusion association and deteriorating perfusion metrics with disease progression also confirmed in prior studies12,14,16, 26, 27 suggests that perfusion is sensitive to a common pathophysiological mechanism reflecting concomitant but not necessarily co-dependent cGM and WM pathology in MS. Findings are supported by a recently reported DTI study8 suggesting that perfusion could serve as a useful surrogate of disease activity in addition to routine structural imaging.
Limitations of the study are the lobar rather than functional domain approach adopted to examine associations between NAWM, T2h-l and cGM. This could result in functionally unrelated regions being included within the lobar cGM assessed. However, a lobar approach is previously used in a recent publication showing that the presence of juxtacortical T2h-l may affect the degree of lobar cortical thinning28. Alternative approaches assessing association between large-scale functional brain networks and cGM integrity may provide greater insight into the volumetric and functional spatial relationship and the effect on cognition25. Greater insight into the association between NAWM, T2h-l and cGM may be illustrated by a longitudinal rather than a cross sectional study design, therefore representing a limitation of the present study. Lastly, the small sample size is relatively modest limiting generalizability to a broader MS patient population. Despite these sample size limitations we were able to demonstrate important differences in association between volumetric and perfusion variables.
In conclusion, the weak spatial association between WM disease and cGM atrophy does not conflict with the notion of an independent pathophysiology of WM and cGM disease. Perfusion reduction with disease severity particularly in cGM suggests that perfusion is sensitive to the pathophysiological mechanism of MS disease severity and may be a useful surrogate of cortical disease progression.
Supplementary Material
Acknowledgments
Grant funding:
This study was supported by Canadian institute of Health Research operating grant #130366, National Institute of Health (NIH) /EB017928, American Heart Association grants 20380798 and 14PRE20380810 as well as a Biogen fellowship funding award.
Abbreviations
- cGM
cortical GM
- HC
healthy control
- NAWM
normal-appearing white matter
- RRMS
relapsing-remitting multiple sclerosis
- SPMS
secondary progressive multiple sclerosis
- T2h-l
T2-hyperintense lesions
- EDSS
expanded disability status scale
References
- 1.Rashid W, Parkes LM, Ingle GT, et al. Abnormalities of cerebral perfusion in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2004;75:1288–93. doi: 10.1136/jnnp.2003.026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Keyser J, Steen C, Mostert JP, et al. Hypoperfusion of the cerebral white matter in multiple sclerosis: possible mechanisms and pathophysiological significance. J Cereb Blood Flow Metab. 2008;28:1645–51. doi: 10.1038/jcbfm.2008.72. [DOI] [PubMed] [Google Scholar]
- 3.Sun X, Tanaka M, Kondo S, Okamoto K, Hirai S. Clinical significance of reduced cerebral metabolism in multiple sclerosis: A combined pet and mri study. Ann Nucl Med. 1998;12:89–94. doi: 10.1007/BF03164835. [DOI] [PubMed] [Google Scholar]
- 4.Bodini B, Khaleeli Z, Cercignani M, Miller DH, Thompson AJ, Ciccarelli O. Exploring the relationship between white matter and gray matter damage in early primary progressive multiple sclerosis: An in vivo study with tbss and vbm. Human brain mapping. 2009;30:2852–2861. doi: 10.1002/hbm.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jehna M, Langkammer C, Khalil M, Fuchs S, Reishofer G, Fazekas F, et al. An exploratory study on the spatial relationship between regional cortical volume changes and white matter integrity in multiple sclerosis. Brain connectivity. 2013;3:255–264. doi: 10.1089/brain.2012.0108. [DOI] [PubMed] [Google Scholar]
- 6.Calabrese M, Gallo P. Magnetic resonance evidence of cortical onset of multiple sclerosis. Multiple sclerosis. 2009;15:933–941. doi: 10.1177/1352458509106510. [DOI] [PubMed] [Google Scholar]
- 7.Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: A longitudinal study. Annals of neurology. 2008;64:255–265. doi: 10.1002/ana.21436. [DOI] [PubMed] [Google Scholar]
- 8.Louapre C, Govindarajan ST, Gianni C, Cohen-Adad J, Gregory MD, Nielsen AS, et al. Is the relationship between cortical and white matter pathologic changes in multiple sclerosis spatially specific? A multimodal 7-t and 3-t mr imaging study with surface and tract-based analysis. Radiology. 2016;278:524–535. doi: 10.1016/j.nicl.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steenwijk MD, Daams M, Pouwels PJ, L JB, Tewarie PK, Geurts JJ, et al. Unraveling the relationship between regional gray matter atrophy and pathology in connected white matter tracts in long-standing multiple sclerosis. Human brain mapping. 2015;36:1796–1807. doi: 10.1002/hbm.22738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mainero C, Louapre C, Govindarajan ST, Gianni C, Nielsen AS, Cohen-Adad J, et al. A gradient in cortical pathology in multiple sclerosis by in vivo quantitative 7 t imaging. Brain: a journal of neurology. 2015;138:932–945. doi: 10.1093/brain/awv011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klaver R, De Vries HE, Schenk GJ, Geurts JJG. Grey matter damage in multiple sclerosis: A pathology perspective. Prion. 2013;7(1):66–75. doi: 10.4161/pri.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aviv RI, Francis PL, Tenenbein R, et al. Decreased frontal lobe gray matter perfusion in cognitively impaired patients with secondary-progressive multiple sclerosis detected by the bookend technique. AJNR Am J Neuroradiol. 2012;33:1779–85. doi: 10.3174/ajnr.A3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peruzzo D, Castellaro M, Calabrese M, Veronese E, Rinaldi F, Bernardi V, et al. Heterogeneity of cortical lesions in multiple sclerosis: An mri perfusion study. J Cereb Blood Flow Metab. 2013;33:457–463. doi: 10.1038/jcbfm.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debernard L, Melzer TR, Van Stockum S, et al. Reduced grey matter perfusion without volume loss in early relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2014;85:544–51. doi: 10.1136/jnnp-2013-305612. [DOI] [PubMed] [Google Scholar]
- 15.Hojjat SP, Cantrell CG, Vitorino R, Feinstein A, Shirzadi Z, MacIntosh BJ, et al. Regional reduction in cortical blood flow among cognitively impaired adults with relapsing-remitting multiple sclerosis patients. Multiple sclerosis. 2016;11:1421–1428. doi: 10.1177/1352458515622696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hojjat SP, Cantrell CG, Carroll TJ, Vitorino R, Feinstein A, Zhang L, et al. Perfusion reduction in the absence of structural differences in cognitively impaired versus unimpaired RRMS patients. Multiple sclerosis. 2016;13:1685–1694. doi: 10.1177/1352458516628656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vakil P, Lee JJ, Mouannes-Srour JJ, Derdeyn CP, Carroll TJ. Cerebrovascular occlusive disease: Quantitative cerebral blood flow using dynamic susceptibility contrast mr imaging correlates with quantitative h2[15o] pet. Radiology. 2013;266:879–86. doi: 10.1148/radiol.12120756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah MK, Shin W, Parikh VS, Ragin A, Mouannes J, Bernstein RA, et al. Quantitative cerebral mr perfusion imaging: Preliminary results in stroke. J Magn Reson Imaging. 2010;32:796–802. doi: 10.1002/jmri.22302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain : a journal of neurology. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 20.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 21.D'Haeseleer M, Cambron M, Vanopdenbosch L, De Keyser J. Vascular aspects of multiple sclerosis. Lancet Neurol. 2011;10:657–666. doi: 10.1016/S1474-4422(11)70105-3. [DOI] [PubMed] [Google Scholar]
- 22.Gh Popescu BF, Lucchinetti CF. Meningeal and cortical grey matter pathology in multiple sclerosis. BMC Neurology. 2012;12:11. doi: 10.1186/1471-2377-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bo L, Geurts JJ, van der Valk P, Polman C, Barkhof F. Lack of correlation between cortical demyelination and white matter pathologic changes in multiple sclerosis. Arch Neurol. 2007;64:76–80. doi: 10.1001/archneur.64.1.76. [DOI] [PubMed] [Google Scholar]
- 24.Sethi V, Yousry T, Muhlert N, Tozer DJ, Altmann D, Ron M, et al. A longitudinal study of cortical grey matter lesion subtypes in relapse-onset multiple sclerosis. Journal of neurology, neurosurgery, and psychiatry. 2016;87:750–753. doi: 10.1136/jnnp-2015-311102. [DOI] [PubMed] [Google Scholar]
- 25.Glascher J, Rudrauf D, Colom R, Paul LK, Tranel D, Damasio H, et al. Distributed neural system for general intelligence revealed by lesion mapping. Proc Natl Acad Sci U S A. 2010;107:4705–4709. doi: 10.1073/pnas.0910397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis PL, Chia TL, Jakubovic R, et al. Extensive white matter dysfunction in cognitively impaired patients with secondary-progressive multiple sclerosis. American Journal of Neuroradiology. 2014;35(10):1910–5. doi: 10.3174/ajnr.A3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis PL, Jakubovic R, O'Connor P, et al. Robust perfusion deficits in cognitively impaired patients with secondary-progressive multiple sclerosis. American Journal of Neuroradiology. 2013;34:62–67. doi: 10.3174/ajnr.A3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pareto D, Sastre-Garriga J, Auger C, et al. Juxtacortical Lesions and Cortical Thinning in Multiple Sclerosis. American Journal of Neuroradiology. 2015;36(12):2270–2276. doi: 10.3174/ajnr.A4485. doi: https://doi.org/10.3174/ajnr.A4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.