Abstract
Glycosylation is recognized as one of the most common modifications on proteins. Recent studies have shown that aberrant expression of α (1,6) fucosyltransferase (FUT8), which catalyzes the transfer of fucose from GDP-fucose to core-GlcNAc of the N-linked glycoproteins, modulates cellular behavior that could lead to the development of aggressive prostate cancer. While the relationship between the abnormal expression of FUT8 and glycoprotein fucosylation in different prostate cancer cells have been demonstrated, there are no evidence if dysregulated fucosylation might be involved in prostate cancer progression from androgen dependent to castration resistant prostate cancer. In this study, using a proteomics approach, we analyzed androgen dependent and androgen resistant LAPC4 cells and identified FUT8 to be significantly overexpressed in the androgen resistant LAPC4 cells. These findings were independently confirmed in LAPC4 cells that were treated with non-steroidal anti-androgen (Bicalutamide) and in the in vivo castrated tumor xenograft models. Similarly, we also demonstrated that overexpression of FUT8 might be responsible for the decreased PSA expression in prostate cancer specimens. To our knowledge this is the first study reporting the functional role of fucosylated enzyme in the development of castration resistant prostate cancer.
Keywords: Androgen resistance; LC-MS/MS; α (1, 6) Fucosyltransferase; Bicalutamide; Prostate cancer
Introduction
Prostate cancer (PCa) is the most common and the second leading cause of cancer death in men in the United States 1. Clinically, organ confined prostate cancer is managed through surgery or localized radiation therapy, however for some patients who recur systemically following treatment or in advance high-risk prostate cancer patients or metastatic disease, the mainstay of treatment is androgen-deprivation therapy (ADT) 2. However, long term ADT leads to the emergence of resistance mechanisms and ultimately the disease progresses to a castration-resistant phenotype which is fatal 3. This transformation from a clinically localized hormone-dependent state to the androgen-resistant phenotype may involve changes in Androgen receptor (AR) and associated pathways 4,5.
Fucosylation of glycoproteins have been shown to play pivotal roles in many aspects of biological processes such as lymphocyte homing, immune responses, fertilization, and development 6. Moreover, aberrant fucosylation, which results from the deficiency or overexpression of fucosyltransferases (FUTs), is associated with variety of human diseases, including cancers 7,8. Unlike other FUTs that are functionally redundant, the α (1,6) fucosyltransferase (FUT8) is the only enzyme responsible for the α 1,6-linked (core) fucosylation that adds fucose to the inner most GlcNAc of an N-linked glycan 9. A growing body of evidence indicates that core fucosylation is important for regulating protein functions 10,11. Transgenic and knockout animal models for core fucosylation have been generated to study the physiological role of FUT8 12,13. Ectopic expression of FUT8 resulted in the steatosis like phenotype in transgenic mice 14, while on the other hand, knocking out FUT8 in mice was reported to dramatically decrease the postnatal survival and inhibition of chemical-induced hepatocellular carcinoma and tumorigenesis 1, 2. Core fucosylation is also crucial for the ligand binding affinity of TGF-β 1 receptor, EGF receptor 15, and integrin α3β1 16. Lacking of the core fucose on these receptors leads to a marked reduction in ligand binding ability and downstream signaling. Furthermore, the increase in core fucosylation on E-cadherin has been shown to strengthen cell-cell adhesion 17.
Overexpression of FUT8 has been observed in several malignant tumors which is linked to the severity of these cancers 18,19. In papillary thyroid carcinoma, higher expression of FUT8 is linked to larger tumor volumes and lymph nodes metastases 20. Similarly, in prostate cancer we have previously observed and reported higher FUT8 expression in aggressive tumors (Gleason 8 and above) compared to its non-aggressive Gleason 6 and lower 21. In addition we have also reported that overexpression of FUT8 in prostate cancer cells was correlated with increased fucosylation of glycoproteins in aggressive prostate cancer cells 22. Here we report that FUT8 overexpression was induced in castration resistant cells and was responsible for the lower PSA production and cell survival in prostate cancer.
Materials and Methods
Cell Lines and reagents
The prostate cancer LAPC4 cell line that harbor the wildtype Androgen receptor that was regularly validated by DNA typing was obtained from Dr. Johns Isaacs in 2016 (Johns Hopkins School of Medicine) which were cultured in Iscove’s Modified Dulbecco’s Medium (IMDM) containing 10% charcoal-stripped fetal calf serum (FBS) (GIBCO, Carlsbad, CA), 100 U of penicillin and 100ug/ml of streptomycin in the presence of 5nM synthetic androgen R1881. PC3 cells lines were previously obtained from American Type Culture Collection (Manassas, VA, USA) and were maintained in T-75 flasks in RPMI-1640. The LNCaP-95 cell line, an androgen independent prostate cancer cell line, was provided by Dr. Alan K. Meeker (Johns Hopkins University) and has been previously described 23,24. LNCaP-95 cells were maintained in Phenol red free and charcoal stripped 10% cFBS media. The LAPC4-AI cell line was developed from the LAPC4 wild type cells in our lab by continuous culturing in charcoal-stripped FBS for a prolong period of six months. All cell lines were routinely tested for mycoplasma contamination using the American Type Tissue Culture Universal Mycoplasma Detection Kit. Primary mouse monoclonal antibodies for AR (AR 441, dilution 1:1000) and polyclonal AR (N-20, dilution 1:1000) were from Santa Cruz Biotech, (Santa Cruz, CA), the polyclonal FUT8 antibody was from R&D systems (Minneapolis, MN), the polyclonal PSA antibody (1:1000) was from DAKO (Carpinteria, CA) Similarly, mAb-β Actin (1:25 000), the Anti-mouse and the Anti-rabbit IgG HRP-conjugated (1:20,000) were from Sigma Aldrich (St. Louis, MO) while the Anti-Sheep IgG HRP-conjugated (1:20,000) was from Thermo Fisher Scientific (Grand Island, NY). Majority of all other chemical reagents and compounds were ordered from Sigma Aldrich, unless otherwise specified. Aminolink resin, spin columns (snap cap), Zeba spin desalting columns were purchased from Life Technologies (Grand Island, NY); Alltech Extract-Clean Carbograph columns were from Fisher Sci. (Waltham, MA). Peptide-N-glycosidase F (PNGase F), denaturing buffer (10×), and reaction buffer (G7; 10×) were from New England Biolabs (Ipswich, MA). Trypsin gold was from Promega (Madison, WI). Orbitrap Velos LC-MS (Thermo) was used for quantitative proteomics analysis.
iTRAQ labeling of global tryptic peptides from cell lines
Each iTRAQ (isobaric tags for relative and absolute quantitation) 4-plex reagent was dissolved in 70 μl of ethanol. One mg of each tryptic peptide sample was added into 250 μl of iTRAQ dissolution buffer, then mixed with iTRAQ 4-plex reagent and incubated for one hour at room temperature. iTRAQ channel 114 and 115 was used to label LAPC4 WT samples, iTRAQ 116 and 117 were used for labelling the LAPC4-AI cells. The 4 sets of tagged peptides were combined and purified by SCX column. Then, 10% of the labeled peptides were dried and re-suspended into 0.4% acetic acid solution prior to fractionation for mass spectrometry analysis.
Peptide Fractionation using Basic RPLC
The high-pH RPLC (reverse-phase liquid chromatography) separation was performed on the 1220 Infinity LC system with a Zorbax Extended-C18 analytical column containing 1.8 μM particles (Agilent Technologies, Inc. CA); flow rate was 0.2 mL/min. The mobile-phase A consisted of 10 mM ammonium formate (pH 10) and B consisted of 10 mM ammonium formate and 90% ACN (pH 10). 50 μg peptides were fractioned using the following linear gradient: from 0 to 2% B in 10 min, from 2 to 8% B in 5 min, from 8 to 35% B in 85 min, from 35 to 95% B in 5 min and then held at 95% B for an additional 15 min. Peptides were detected at 215 nm and ninety-six fractions were collected along with the LC separation in a time-based mode from 16 to 112 min. The separated peptides in ninety-six wells were concatenated into 24 fractions by combining four wells to one sample, such as 1, 25, 49, and 73 as fraction one; 2, 26, 50, and 74 as fraction two; and so on. The peptides were then dried in a Speed-Vacuum and stored at −80°C until LC-MS/MS analysis.
LC-MSMS
Peptides were resuspended in 0.1% formic acid in water and subjected to LCMSMS analysis. Peptides from each fraction were separated on a Dionex Ultimate 3000 RSLCnano system (Thermo Scientific) with a 75 μm × 15 cm Acclaim PepMap100 separating column (Thermo Scientific) and with a 2 cm guarding column (Thermo Scientific). The flow rate was 300 nL/min with 0.1% formic acid in water (A) and 0.1% formic acid 95% acetonitrile (B). The gradient profile was: 5–40% B for 90 min. MS analysis was performed using an Orbitrap Velos Pro mass spectrometer (Thermo Scientific). The spray voltage was set at 2.2 kV. Orbitrap spectra (AGC 1×106) were collected with resolution of 30,000 followed by ten data-dependent HCD MS/MS (at a resolution of 7,500, collision energy 35%, activation time 0.1 ms). A dynamic exclusion time was set at 25 sec with a repeat count of 2.
Protein Expression Data Analysis
MS/MS Data and Differential Expression Analysis: We searched our tandem mass spectrometry derived raw data against the RefSeq protein database using the SEQUEST search engine in Proteome Discoverer v1.4. We specified, carbarmidomethylation of cysteine, lysine (K) iTRAQ modifications and N-terminal iTRAQ modification as fixed residue modifications. We specified deamidation at Asparagine and oxidation of methionine as dynamic modifications. Peptide identification false discovery rate (FDR) was specified as 0.01. Parsimonious protein grouping was specified to allow at least one peptide per protein. High confidence PSMs (i.e. peptide spectrum matches better than pre-specified false discovery rate cut-off) were used for protein grouping. Peptide and protein quantifications were based of ratios of iTRAQ reporter ions: 114, 115, 116 and 117. Our specified reporter ion quantification LAPC4-wt/LAPC4-wt was 115/114, The LAPC4-AI/LAPC4-WT ratio done in duplicate were (116/114 and 117/114). Proteome Discoverer reports coefficient of variations (CVs) of unique peptide spectrum matches’ reporter ion ratios. As a second level quality control measure, we set an acceptable limit at less than or equal to 30%. We filtered-out peptide spectrum matches and associated protein with reporter ion ratio CVs greater than 30%.
Results
Development of Androgen Resistant Cell Line and Mass Spectrometry Based Proteomic Analysis
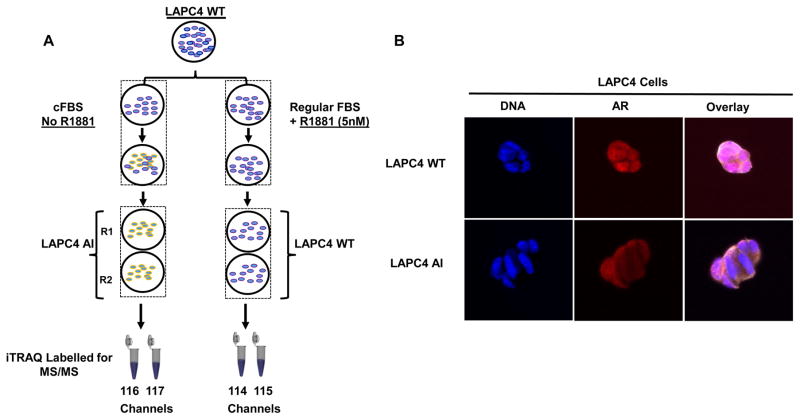
The wild type LAPC4 prostate cancer cell line was continuously cultured in charcoal dextran stripped fetal bovine serum (cFBS) containing medium for more than six months to obtain androgen resistant LAPC4-AI cells as shown by schematics (Figure 1A). We evaluated the androgen receptor (AR) status of these cells by I.F (Immunofluorescence) microscopy and compared the AR localization between the LAPC4-AI grown in charcoal stripped FBS containing medium and the wildtype LAPC4 cells that were grown in normal FBS containing media supplemented with 1nM of synthetic androgens (R1881). As shown in the Figure 1B, cells that were resistant to androgen (LAPC4-AI) had a predominant cytoplasmic AR localization compared to the wildtype LAPC4 cells, where majority of the AR was found inside the nucleus. To evaluate whether changes in protein expression might be playing a role in the development of castration resistant phenotype, we performed a global proteomic analysis using LC-MS/MS Mass Spectrometry on these cells. Briefly, cell lysate from the wildtype LAPC4 and androgen resistant (LAPC4-AI) were subjected to the 4-plex iTRAQ labelling before combining them together Supplemental Figure 1. 50ug of protein samples labelled with iTRAQ were subjected to basic reverse phase liquid chromatography (bRPLC). A total of 96 fractions were collected and concentrated into 24 fractions. Each bRPLC fraction was analyzed by LC-MS/MS to obtain both qualitative and quantitative information on the proteome of LAPC4 and LAPC4-AI cells. At 1% spectral FDR, a total of 3,365 protein groups were identified with a minimum of two peptides per protein (Supplemental Table S1).
Figure 1.
Schematic showing the workflow for generating LAPC4 AI cell line and iTRAQ 4 plex (114, 115, 116 & 117) labelling of the samples for Mass Spectrometer analysis (A). The cytoplasmic and nuclear localization of AR in LAPC4 WT and LAPC4 AI cells (B).
FUT8 Overexpression in Androgen Resistant LAPC4 Cells
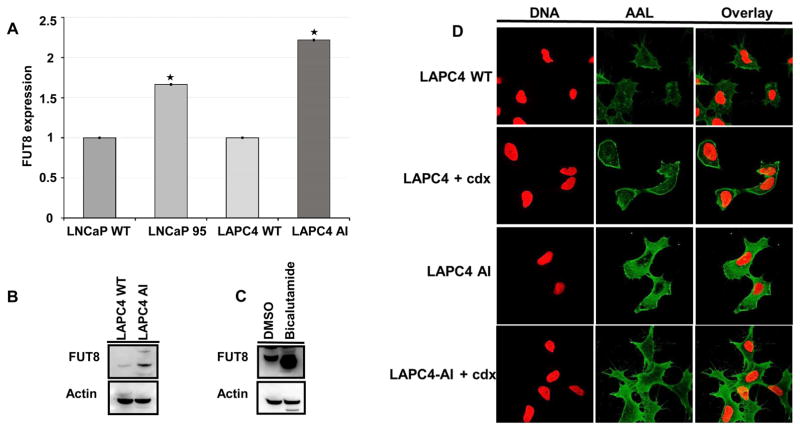
To evaluate whether glycosylation was related to androgen resistance, we searched for the detected N-linked glycosylated related enzymes in the globally identified proteins and were able to detect a total of 20 N-linked glycosylated related enzymes (Table. 1). To determine which N-Linked glycosylated related enzymes were differentially expressed between the LAPC4-AI androgen resistant and wild type prostate cancer cells, we compared the iTRAQ ratios between the wildtype (114, 115) to that of LAPC4-AI (116, 117). As shown in the Table 1, the α-1, 6-fucosyltransferase (FUT8) which is responsible for the core fucosylation of many cellular glycoproteins was significantly overexpressed (more than 3 fold) in LAPC4-AI cells compared to that of the androgen sensitive wildtype LAPC4 cells. To further investigate whether overexpression of FUT8 in androgen resistant prostate cancer cells was due to the upregulation of FUT8 mRNA, we analyzed the LAPC4-AI and LAPC4 wildtype cells along with the LNCaP and LNCaP-95 prostate cancer cell lines using qRT-PCR analysis. The relative quantitative mRNA analysis from wild types (LNCaP and LAPC4) and the androgen resistant prostate cancer cells (LNCaP-95 and LAPC4-AI) revealed overexpression of FUT8 in both androgen resistant prostate cancer cell lines (Figure 2A). In order to determine whether FUT8 protein expression was present in androgen resistant LAPC4 AI cells, we performed western blot analysis using the wildtype LAPC4 and the androgen resistant LAPC4-AI cells. As shown in the Figure 2B, FUT8 protein was overexpressed in LAPC4-AI cells compared to that of the wildtype cells. We further examined six different prostate cancer cell lines including the LNCaP-AI (androgen independent) and PC3 (androgen resistant) prostate cancer cells and found higher expression of FUT8 in LNCaP-AI and PC3 cells compared to the wildtype LNCaP cells (Supplemental Figure 2)
Table 1.
N-Glycosylated related enzymes identified in global iTRAQ data
| Gene | Protein | Description | Coverage | # Peptides | # PSMs | Gene IDs | LAPC4WT (115)/LAPC4 T(114) | LAPC4 AI (116)/LAPC4 WT(114) | LAPC4 AI (117)/LAPC4 WT(114) |
|---|---|---|---|---|---|---|---|---|---|
| DAD1 | Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit DAD1 | defender against cell death 1, isoform CRA_a | 26.55 | 3 | 32 | 1603 | 1.310 | 0.986 | 1.030 |
| B4GALT1 | Beta-1,4-galactosyltransferase 1 | unnamed protein product | 8.14 | 2 | 6 | 2683 | 0.871 | 0.595 | 0.582 |
| MAN2A1 | Alpha-mannosidase 2 | PREDICTED: alpha-mannosidase 2 isoform X1 | 7.49 | 7 | 18 | 4124 | 1.231 | 0.687 | 0.691 |
| PRKCSH | Glucosidase 2 subunit beta | PRKCSH protein, partial | 13.44 | 7 | 47 | 5589 | 1.092 | 0.768 | 0.743 |
| RPN1 | Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 | unnamed protein product | 53.05 | 27 | 322 | 6184 | 1.226 | 0.910 | 0.901 |
| RPN2 | Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 2 | RPN2 protein, partial | 39.23 | 13 | 107 | 6185 | 1.138 | 0.916 | 0.992 |
| MOGS | Mannosyl-oligosaccharide glucosidase | mannosyl-oligosaccharide glucosidase isoform 2 | 15.18 | 10 | 37 | 7841 | 1.047 | 0.990 | 0.986 |
| B4GALT3 | Beta-1,4-galactosyltransferase 3 | unnamed protein product | 12.78 | 3 | 5 | 8703 | 0.794 | 1.262 | 1.131 |
| DPM1 | Dolichol-phosphate mannosyltransferase subunit 1 | Dolichyl-phosphate mannosyltransferase polypeptide 1, catalytic subunit | 24.23 | 5 | 23 | 8813 | 1.227 | 1.031 | 1.161 |
| ALG3 | Dol-P-Man:Man(5)GlcNAc(2)-PP-Dol alpha-1,3-mannosyltransferase | ALG3 protein, partial | 5.08 | 2 | 5 | 10195 | 1.261 | 0.811 | 0.808 |
| GANAB | GANABGANABNeutral alpha-glucosidase AB | glucosidase, alpha; neutral AB, isoform CRA_d | 28.3 | 20 | 157 | 23193 | 1.035 | 1.113 | 1.074 |
| GANAB | GANABGANABNeutral alpha-glucosidase AB | glucosidase, alpha; neutral AB, isoform CRA_a | 25.73 | 21 | 164 | 23193 | 1.047 | 1.102 | 1.078 |
| ST8SIA5 | Alpha-2,8-sialyltransferase 8E | asparagine-linked glycosylation 6 homolog isoform CRA_d | 6.32 | 3 | 5 | 29929 | 1.118 | 0.776 | 0.793 |
| DPM3 | Dolichol-phosphate mannosyltransferase subunit 3 | dolichyl-phosphate mannosyltransferase polypeptide 3, isoform CRA_a | 23.91 | 2 | 17 | 54344 | 1.121 | 0.920 | 0.881 |
| UGGT1 | UDP-glucose:glycoprotein glucosyltransferase 1 | UDP-glucose ceramide glucosyltransferase-like 1 | 10.58 | 17 | 37 | 56886 | 1.063 | 1.085 | 1.053 |
| ALG8 | Probable dolichyl pyrophosphate Glc1Man9GlcNAc2 alpha-1,3-glucosyltransferase | glucosyltransferase, partial | 4.32 | 2 | 4 | 79053 | 1.056 | 0.599 | 0.629 |
| ALG2 | Alpha-1,3/1,6-mannosyltransferase ALG2 | unnamed protein product | 8.06 | 2 | 5 | 85365 | 1.065 | 2.104 | 2.111 |
| RFT1 | Protein RFT1 homolog | PREDICTED: protein RFT1 homolog isoform X4 | 13.02 | 6 | 10 | 91869 | 0.994 | 0.696 | 0.745 |
| STT3B | Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit STT3B | STT3, subunit of the oligosaccharyltransferase complex, homolog B | 18.04 | 15 | 108 | 2E+05 | 1.163 | 1.031 | 1.029 |
| FUT8 | Alpha-(1,6)-fucosyltransferase | Chain X, Crystal Structure Of Human Alpha 1,6-fucosyltransferase, Fut8 | 14.26 | 8 | 18 | 2530 | 0.949 | 3.041 | 3.047 |
Figure 2.
Relative quantitative qRT PCR analysis for mRNA expression of FUT8 in wildtype (LNCaP and LAPC4) and in androgen resistant LNCaP-95 and LAPC4 AI prostate cancer cells, Error bars represent mean ±SE and * indicates P ≤ 0.05 (A). Western blot analysis of FUT8 in LAPC4 WT and LAPC4 AI prostate cancer cells (B). Western Blot analysis of FUT8 in LAPC4 WT cells treated with or without bicalutamide (10uM) for 72hrs (C). Confocal microscopy for the AAL lectin in LAPC4 WT or bicalutamide (cdx) treated or LAPC4 AI and LAPC4 AI treated with 10uM of bicalutamide (D).
To evaluate if pharmacological inhibition of AR signaling with the non-steroidal competitive AR antagonist bicalutamide exerts similar changes in FUT8 expression, we treated wildtype LAPC4 cells with 1uM of bicalutamide or mock control (DMSO) in normal growth medium. Cells harvested after 72hr of treatment were subjected to western blot analysis for FUT8 expression. Beta actin was included to ensure equal amount of loading across the lanes. As shown in the Figure 2C, there was a significant increase in the FUT8 expression compared to the mock treated controls. We next sought to determine whether the increase levels of FUT8 expression in castration resistant LAPC4 AI cells was functional. We evaluated the fucosylated status of the wildtype and androgen resistant LAPC4 cells by performing the Aleuria aurantia (AAL) lectin analysis using confocal microscopy and by AAL lectin blot analysis. AAL has been previously shown to specifically bind to the α −1,6 linked core fucose of the N-acetylglucosamine of glycopeptides. As shown in the Figure 2D a higher staining of AAL was observed in bicalutamide-treated (casadox, cdx) or androgen resistant LAPC4 AI cells compared to the wildtype suggesting a functional overexpression of FUT8 in castration resistant cells. (Figure 2D). Similar results were obtained by AAL lectin blot in LAPC4 AI cells when compared to the LAPC4 wildtype cells. (Supplemental Figure 3A).
Effect of surgical castration on the FUT8 expression in LAPC4 xenograft animal model
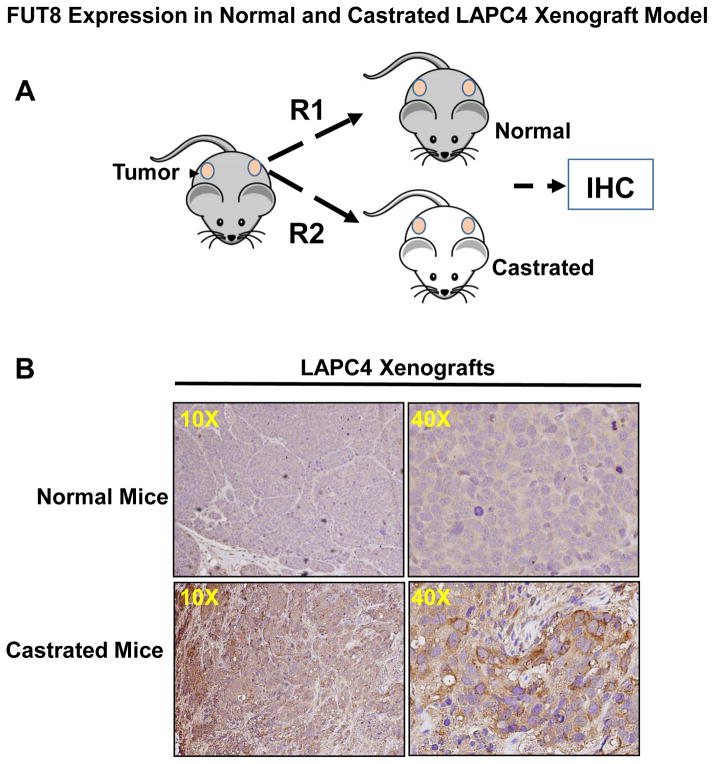
Prostate cancer xenograft mimics many features of human prostate tumors, which make it a valuable model to study the mechanisms of progression to castration resistant prostate cancer. To further determine whether androgen ablation was indeed responsible for the overexpression of FUT8 in prostate cancer, we moved to a more biological relevant mice model. LAPC4 wildtype cells at a density of 1× 106 in PBS were mixed in 1:3 ratio with 1× Matrigel (BD biosciences) and implanted into the dorsal flanks of athymic nude mice. Once tumors were established and reached to tumor volume of ~1cm3, animals were divided into two groups. Castration was performed in one group of animals by bilateral orchiectomy. Mice in control group were shame-operated under anesthesia to localize sex organs but without orchiectomy. At the end of the experiment (5 weeks after castration) all animals were sacrificed and tumor were removed for immunohistochemical studies. As shown in the Figure 3 animals that were castrated have a significantly higher FUT8 staining in the tumor xenograft compared to that of the normal uncastrated animals. These data independently confirm FUT8 overexpression in the in vivo castration resistant LAPC4 prostate cancer xenograft model.
Figure 3.
Schematic showing LAPC4 cells implanted as xenografts on the lower back of athymic male nude mice, after the tumor volume reached around 1cm, mice were either castrated or mocked operated (A). Tumors were harvested by the end of experiments and subjected to IHC for FUT8 expression (B).
Overexpression of FUT8 Reduces the Production of PSA in Prostate Cancer Cells
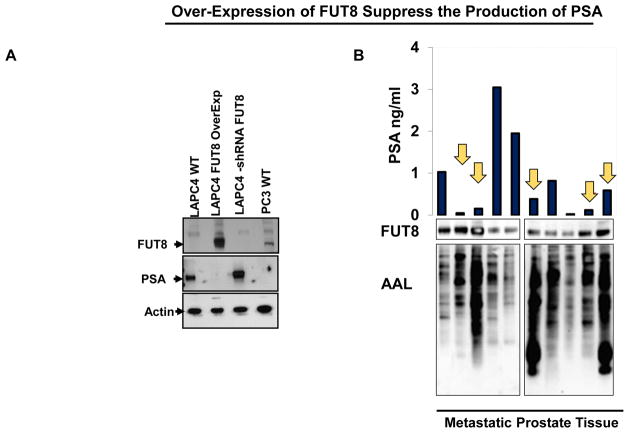
FUT8 expression was induced by androgen ablation in LAPC4 cells, we next inquired whether exogenous expression of FUT8 in the LAPC4 cells have any biological activity on the PSA expression in these cells. LAPC4 cells that were stably selected to expressed FUT8 (pCMV6-FUT8-Myc-DDK) or shRNA against FUT8 (pLKO-shRNA FUT8) or empty vectors (pCMV6 were subjected to western blot analysis for PSA and FUT8 expression. Protein lysates from PC3 cells an androgen resistant prostate cancer cell line that had previously shown to express higher levels of FUT8 was included as a positive control for FUT8 expression. As shown in the Figure 4A, LAPC4 cells that were transfected with FUT8 overexpressing plasmid had a significant reduction in the PSA expression compared to the mock transfected cells. Similarly, knocking down FUT8 in the LAPC4 cells causes an increase in the total PSA suggesting an inhibitory role of FUT8 on the PSA signaling. To further confirm these findings we evaluated ten different metastatic prostate cancer tissues for PSA and FUT8 expression using western blot analysis. The AAL lectin blot analysis was also performed using the same lysates to confirm the functional FUT8 overexpression in these specimens. As shown in the Figure 4B, tissue samples that has higher expression of FUT8 except Lane 8, had a significantly lower PSA production and vice versa which was in accordance with our in vitro western blot analysis Figure 4A suggesting the inhibitory role of FUT8 on the PSA expression in metastatic prostate cancer tissues.
Figure 4.
Western Blot analysis for PSA and FUT8 in LAPC4 cells that were transfected with plasmid carrying wildtype FUT8 or shRNA against FUT8 or vector control. PC3 cells were included as a positive control for FUT8 and negative control for PSA expression. Beta actin was included to ensure equal amount of loading across the lanes (A). Inverse association between FUT8 and PSA expression in ten metastatic prostate tumor samples (R = − 0.321). AAL lectin blot was included to confirm the functional increase of FUT8 in all ten samples (B).
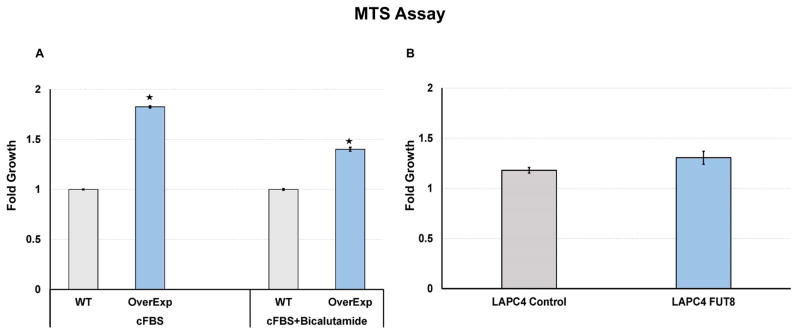
Overexpression of FUT8 support cell proliferation in castrated condition
To further demonstrate whether FUT8 overexpression in prostate cancer cells promotes the proliferation of cells in normal or androgen ablated conditions, we preformed the MTS assay on the LAPC4 cells that were stably selected to overexpress FUT8 (LAPC4-FUT8) or control vectors (LAPC4 Ctr). Briefly, LAPC4-FUT8 or control cells plated at the density of 2× 104 cells in quadruplets were grown in the charcoal stripped FBS (cFBS) containing IMDM media or cFBS containing media with 10 uM bicalutamide or DMSO for three days or in normal FBS containing media. By the end of three days cells were subjected to the MTS assay to examine cell growth. Data from the MTS assay were normalized to the 0hr reading from the replica plates. Fold growth were calculated by normalizing MTS readings to the wildtype control cells. As shown in the Figure 5A, cells that were selected to overexpress FUT8 (LAPC4-FUT8) had a significant higher readings compared to the wildtype control. Similarly, in the presence of bicalutamide (10uM), more of LAPC4-FUT8 cells were detected by the MTS assay compared to the wildtype control cells. We also evaluated the proliferation of LAPC4-FUT8 and control cells in normal FBS containing IMDM media supplemented with 1nM of R1881 and found no growth differences between LAPC4-FUT8 and control cells Figure 5B. In addition to the LAPC4 cell line model we also confirmed these observations in LNCaP model that were stably selected to express FUT8 (LNCaP-FUT8) (Supplemental Figure 3B). Together these data suggesting a positive role for FUT8 in the castration resistance biology of prostate cancer. Further studies are underway in our laboratory to explicitly identify mechanisms underlying these phenomena.
Figure 5.
MTS assay was performed to evaluate the proliferation of LAPC4 WT and LAPC4 cells that were stably selected to overexpress FUT8 in charcoal stripped FBS (cFBS) containing media or in the presence of bicalutamide (10uM) (A). LAPC4 control or LAPC4-FUT8 cells grown in regular growth media containing 10% FBS and 5nM R1881 (B). Error bars indicate ± SEM and * indicates P ≤ 0.05.
Discussion
One of the underlying cause of glycosylation changes in cancer is the dysregulated expression of glycosyltransferases and associated proteins within the cancer cell 30. Fucosylated glycans have been shown to play essential roles in many aspects of mammalian processes6; while its dysregulation was responsible for a variety of human diseases including cancers 7,8. Unlike other fucosyltrasferase, FUT8 is the only enzyme responsible for the α 1,6-linked (core) fucosylation of proteins. Our initial studies with FUT8 in prostate cancer specimens demonstrated a strong correlated between FUT8 expression and aggressive prostate cancer. In this study we discovered a novel role for FUT8 overexpression which might be responsible for driving castration resistance in prostate cancer. Using a global proteomic approach we were able to identify FUT8 as one of the significantly overexpressed glycosyltransferases proteins in the LAPC4-AI cells which were further verified by other androgen resistant models (Figures. 2, 3).
Androgen Receptor (AR) signaling is a major player in all aspects of prostate biology 31. AR activation is responsible for the development of the prostate and is required in the control of prostatic cell growth, differentiation, function, and survival 5. During prostate cancer, it is the aberrant AR signaling that results in the uncontrolled proliferation and survival of prostate cancer cells especially in advanced hormone refractory disease where AR became active in the absence of ligand. Blocking the AR pathway in prostate cancer patient can be achieved by several mechanism including surgical orchiectomy or chemically with luteinizing hormone-releasing hormone (LHRH) agonists, LHRH antagonists, or anti-androgens 32,33. Although these therapies do seem to work but the effects on prostate cancer cells are very transient 34. To demonstrate whether glycosyltransferase were involved in the development of this phenotype, we treated the LAPC4 cells with bicalutamide, a non-steroidal anti-androgens and identified FUT8 to be overexpressed in these cells suggesting a role of glycosylation in the biology of androgen ablation (Figure 1C). Interestingly, we have previously shown negligible levels of FUT8 expression in LNCaP cells compared to the more aggressive PC3 prostate cancer cells 21, however when LNCaP cells were grown in charcoal stripped media for several passages (LNCaP-95) there was an increase in the FUT8 expression (Figure 1A) indicating androgen ablation as a requisite for the FUT8 overexpression in the AR positive prostate cancer cells. Similarly, we found the increase in FUT8 levels in these cells was a functional increase as the ALL lectin microscopy (Figure 2D) and Lectin blot (Supplemental Figure 3A) showed higher levels of staining in the LAPC4 AI or LAPC4 AI cells treated with bicalutamide.
The glycosylation on the cell surface proteins including epidermal growth factor receptor (EGFR) 35 has been shown to play role in cancer biology and cellular proliferation36,37. TGF-α which is known to specifically bind the EGFR receptor and activates the intracellular signaling pathways was shown to play role in the prostate cell growth 38. Therefore, there was a plausible hypothesis that overexpression of FUT8 may contribute to the expression profiles of EGFR or TGF-α, however, based on the LC-MS/MS mass spectrometry iTRAQ data, we found downregulated expression of EGFR and TGF-α proteins, thus nullifying the role of EGFR and TGF-α in androgen resistant LAPC4 AI development. To further investigate the phenomena of FUT8-androgen nexus, we recapitulated the androgen ablation condition using surgical orchiectomy LAPC4 mouse xenograft model (Figure 3). Our finding revealed that FUT8 overexpression was in fact driven by androgen ablation in prostate cancer cells. To better understand whether overexpression of FUT8 might be responsible for castration resistant phenotype in prostate cancer cells we ectopically overexpressed FUT8 in LAPC4 cells and found that overexpression of FUT8 in LAPC4 cells suppressed the production of PSA while knocking down the endogenous FUT8 with shRNA in LAPC4 cells significantly enhanced PSA production (Figure 3A). These results were in accordance with the patient samples where overexpression of FUT8 had lower PSA values and vice versa (Figure 3B). Further studies are underway in the laboratory to understand whether FUT8 overexpression is acting as a driver in the development of castration resistant phenotypes which may be used as therapeutic target to overcome prostate cancer resistance to antiandrogen based therapies.
Conclusion
We presented evidence that dysregulated fucoyslation might be playing role in the development of castration resistant prostate cancer, thus extending the notion to include aberrant glycosylation and glycosylated related enzymes to the growing list of mechanistic pathways involved in prostate cancer.
Supplementary Material
Acknowledgments
We are very much thankful to Ms. Ashley Deng for proof reading our manuscript. This work was supported by National Institute of Health, National Cancer Institute, the Early Detection Research Network (EDRN, U01CA152813), the Clinical Proteomics Tumor Analysis Consortium (CPTAC, U24CA160036), National Heart Lung and Blood Institute, Program of Excellence in Glycosciences (PEG, P01HL107153).
List of abbreviations
- AR
Androgen receptor
- FUT8
α (1, 6) fucosyltransferase
- ADT
Androgen Deprivation Therapy
- PSA
Prostate specific antigen
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Aragon-Ching JB, Dahut WL. Novel Androgen Deprivation Therapy (ADT) in the Treatment of Advanced Prostate Cancer. Drug Discov Today Ther Strateg. 2010;7(1–2):31–35. doi: 10.1016/j.ddstr.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aschelter AM, Giacinti S, Caporello P, Marchetti P. Genomic and epigenomic alterations in prostate cancer. Front Endocrinol (Lausanne) 2012;3:128. doi: 10.3389/fendo.2012.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lonergan PE, Tindall DJ. Androgen receptor signaling in prostate cancer development and progression. J Carcinog. 2011;10:20. doi: 10.4103/1477-3163.83937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y, Bolton EC, Jones JO. Androgens and androgen receptor signaling in prostate tumorigenesis. J Mol Endocrinol. 2015;54(1):R15–29. doi: 10.1530/JME-14-0203. [DOI] [PubMed] [Google Scholar]
- 6.Chen CY, Jan YH, Juan YH, Yang CJ, Huang MS, Yu CJ, et al. Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer. Proc Natl Acad Sci U S A. 2013;110(2):630–635. doi: 10.1073/pnas.1220425110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma B, Simala-Grant JL, Taylor DE. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;16(12):158R–184R. doi: 10.1093/glycob/cwl040. [DOI] [PubMed] [Google Scholar]
- 8.Sturla L, Fruscione F, Noda K, Miyoshi E, Taniguchi N, Contini P, et al. Core fucosylation of N-linked glycans in leukocyte adhesion deficiency/congenital disorder of glycosylation IIc fibroblasts. Glycobiology. 2005;15(10):924–934. doi: 10.1093/glycob/cwi081. [DOI] [PubMed] [Google Scholar]
- 9.Kurimoto A, Kitazume S, Kizuka Y, Nakajima K, Oka R, Fujinawa R, et al. The absence of core fucose up-regulates GnT-III and Wnt target genes: a possible mechanism for an adaptive response in terms of glycan function. J Biol Chem. 2014;289(17):11704–11714. doi: 10.1074/jbc.M113.502542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lux A, Yu X, Scanlan CN, Nimmerjahn F. Impact of immune complex size and glycosylation on IgG binding to human FcgammaRs. J Immunol. 2013;190(8):4315–4323. doi: 10.4049/jimmunol.1200501. [DOI] [PubMed] [Google Scholar]
- 11.Raymond C, Robotham A, Spearman M, Butler M, Kelly J, Durocher Y. Production of alpha2,6-sialylated IgG1 in CHO cells. MAbs. 2015;7(3):571–583. doi: 10.1080/19420862.2015.1029215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuda T, Hashimoto H, Okayasu N, Kameyama A, Onogi H, Nakagawasai O, et al. Alpha1,6-fucosyltransferase-deficient mice exhibit multiple behavioral abnormalities associated with a schizophrenia-like phenotype: importance of the balance between the dopamine and serotonin systems. J Biol Chem. 2011;286(21):18434–18443. doi: 10.1074/jbc.M110.172536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Inoue S, Gu J, Miyoshi E, Noda K, Li W, et al. Dysregulation of TGF-beta1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc Natl Acad Sci U S A. 2005;102(44):15791–15796. doi: 10.1073/pnas.0507375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Li W, Ikeda Y, Miyagawa JI, Taniguchi M, Miyoshi E, et al. Ectopic expression of alpha1,6 fucosyltransferase in mice causes steatosis in the liver and kidney accompanied by a modification of lysosomal acid lipase. Glycobiology. 2001;11(2):165–174. doi: 10.1093/glycob/11.2.165. [DOI] [PubMed] [Google Scholar]
- 15.Venkatachalam MA, Weinberg JM. New wrinkles in old receptors: core fucosylation is yet another target to inhibit TGF-beta signaling. Kidney Int. 2013;84(1):11–14. doi: 10.1038/ki.2013.95. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Itoh S, Wang X, Isaji T, Miyoshi E, Kariya Y, et al. Deletion of core fucosylation on alpha3beta1 integrin down-regulates its functions. J Biol Chem. 2006;281(50):38343–38350. doi: 10.1074/jbc.M608764200. [DOI] [PubMed] [Google Scholar]
- 17.Osumi D, Takahashi M, Miyoshi E, Yokoe S, Lee SH, Noda K, et al. Core fucosylation of E-cadherin enhances cell-cell adhesion in human colon carcinoma WiDr cells. Cancer Sci. 2009;100(5):888–895. doi: 10.1111/j.1349-7006.2009.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito Y, Miyauchi A, Yoshida H, Uruno T, Nakano K, Takamura Y, et al. Expression of alpha1,6-fucosyltransferase (FUT8) in papillary carcinoma of the thyroid: its linkage to biological aggressiveness and anaplastic transformation. Cancer Lett. 2003;200(2):167–172. doi: 10.1016/s0304-3835(03)00383-5. [DOI] [PubMed] [Google Scholar]
- 19.Hutchinson WL, Du MQ, Johnson PJ, Williams R. Fucosyltransferases: differential plasma and tissue alterations in hepatocellular carcinoma and cirrhosis. Hepatology. 1991;13(4):683–688. [PubMed] [Google Scholar]
- 20.Miyoshi E, Ito Y, Miyoshi Y. Involvement of aberrant glycosylation in thyroid cancer. J Oncol. 2010;2010:816595. doi: 10.1155/2010/816595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Chen J, Li QK, Peskoe SB, Zhang B, Choi C, et al. Overexpression of alpha (1,6) fucosyltransferase associated with aggressive prostate cancer. Glycobiology. 2014;24(10):935–944. doi: 10.1093/glycob/cwu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah P, Wang X, Yang W, Toghi Eshghi S, Sun S, Hoti N, et al. Integrated Proteomic and Glycoproteomic Analyses of Prostate Cancer Cells Reveal Glycoprotein Alteration in Protein Abundance and Glycosylation. Mol Cell Proteomics. 2015;14(10):2753–2763. doi: 10.1074/mcp.M115.047928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoti N, Shah P, Hu Y, Yang S, Zhang H. Proteomics analyses of prostate cancer cells reveal cellular pathways associated with androgen resistance. Proteomics. 2017;17(6) doi: 10.1002/pmic.201600228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pflug BR, Reiter RE, Nelson JB. Caveolin expression is decreased following androgen deprivation in human prostate cancer cell lines. Prostate. 1999;40(4):269–273. doi: 10.1002/(sici)1097-0045(19990901)40:4<269::aid-pros9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Yang S, Li Y, Shah P, Zhang H. Glycomic analysis using glycoprotein immobilization for glycan extraction. Anal Chem. 2013;85(11):5555–5561. doi: 10.1021/ac400761e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S, Wang M, Chen L, Yin B, Song G, Turko IV, et al. QUANTITY: An Isobaric Tag for Quantitative Glycomics. Sci Rep. 2015;5:17585. doi: 10.1038/srep17585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang S, Zhang H. Glycomic analysis of glycans released from glycoproteins using chemical immobilization and mass spectrometry. Curr Protoc Chem Biol. 2014;6(3):191–208. doi: 10.1002/9780470559277.ch140085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang SJ, Zhang H. Glycan analysis by reversible reaction to hydrazide beads and mass spectrometry. Anal Chem. 2012;84(5):2232–2238. doi: 10.1021/ac202769k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masiello D, Cheng S, Bubley GJ, Lu ML, Balk SP. Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J Biol Chem. 2002;277(29):26321–26326. doi: 10.1074/jbc.M203310200. [DOI] [PubMed] [Google Scholar]
- 30.Hauselmann I, Borsig L. Altered tumor-cell glycosylation promotes metastasis. Front Oncol. 2014;4:28. doi: 10.3389/fonc.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell SM, Christiaens V, Voulgaraki D, Waxman J, Claessens F, Bevan CL. Mechanisms of androgen receptor signalling via steroid receptor coactivator-1 in prostate. Endocr Relat Cancer. 2004;11(1):117–130. doi: 10.1677/erc.0.0110117. [DOI] [PubMed] [Google Scholar]
- 32.Gomella LG. Effective testosterone suppression for prostate cancer: is there a best castration therapy? Rev Urol. 2009;11(2):52–60. [PMC free article] [PubMed] [Google Scholar]
- 33.Oefelein MG, Resnick MI. Effective testosterone suppression for patients with prostate cancer: is there a best castration? Urology. 2003;62(2):207–213. doi: 10.1016/s0090-4295(03)00331-5. [DOI] [PubMed] [Google Scholar]
- 34.Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32(49):5501–5511. doi: 10.1038/onc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaszuba K, Grzybek M, Orlowski A, Danne R, Rog T, Simons K, et al. N-Glycosylation as determinant of epidermal growth factor receptor conformation in membranes. Proc Natl Acad Sci U S A. 2015;112(14):4334–4339. doi: 10.1073/pnas.1503262112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Contessa JN, Bhojani MS, Freeze HH, Rehemtulla A, Lawrence TS. Inhibition of N-linked glycosylation disrupts receptor tyrosine kinase signaling in tumor cells. Cancer Res. 2008;68(10):3803–3809. doi: 10.1158/0008-5472.CAN-07-6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandes H, Cohen S, Bishayee S. Glycosylation-induced conformational modification positively regulates receptor-receptor association: a study with an aberrant epidermal growth factor receptor (EGFRvIII/DeltaEGFR) expressed in cancer cells. J Biol Chem. 2001;276(7):5375–5383. doi: 10.1074/jbc.M005599200. [DOI] [PubMed] [Google Scholar]
- 38.Onagbesan OM, Peddie MJ. The expression of transforming growth factor alpha receptor protein and its activation in chicken ovarian granulosa cells of maturing follicles. Histochem J. 1998;30(9):647–656. doi: 10.1023/a:1003544926637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.