Abstract
Lipids are essential for mammalian cells to maintain many physiological functions. Emerging evidence has shown that cancer cells can develop specific alterations in lipid biosynthesis and metabolism to facilitate their survival and various malignant behaviors. To date, the precise role of cellular lipids and lipid metabolism in viral oncogenesis is still largely unclear with only a handful of literature covering this topic to implicate lipid metabolism in oncogenic virus associated pathogenesis. In this review, we focus on the role of lipid biosynthesis and metabolism in the pathogenesis of the Kaposi’s sarcoma-associated herpesvirus, a common causative factor for cancers arising in the immunocompromised settings.
Keywords: Kaposi’s sarcoma-associated herpesvirus (KSHV), herpesvirus, lipid metabolism
INTRODUCTION
Kaposi’s sarcoma-associated herpesvirus (KSHV, also known as human herpes virus-8, HHV8) is the causative agent for a number of cancers, including Kaposi’s sarcoma (KS), primary effusion lymphoma (PEL) and multicentric Castleman disease (MCD), all of which arise preferentially in immunocompromised patients (Chang et al., 1994; Cesarman et al., 1995; Soulier et al., 1995). Currently, there are four KS isoforms: classic KS affecting elderly men of the Mediterranean; endemic KS, existing in some countries of Central and Eastern Africa; iatrogenic KS, which usually develops in organ transplant recipients with immunosuppression; and epidemic or AIDS-KS, which typically presents with more aggressive features (Mesri et al., 2010). Even though the combined antiretroviral therapy (cART) helps to reduce the total incidence of KS in the western world, KS is still the most common AIDS-associated malignancy and a leading cause of cancer-related morbidity and mortality in AIDS patients (Bonnet et al., 2004). PEL is a B-cell malignancy harboring KSHV which arises preferentially within the pleural or peritoneal cavities of immunocompromised patients (Cesarman et al., 1995). PEL is a rapidly progressing malignancy with a median survival time of approximately 6 months, even after the combinational chemotherapy (Chen et al., 2007). KSHV-associated MCD is a rare lymphoproliferative disorder that frequently arises in HIV+ patients who have a suppressed HIV activity and a relatively preserved CD4 count (Wang et al., 2016). Like other herpesviruses, KSHV establishes a lifelong infection in the host utilizing two major distinct phases: latent infection and lytic replication. During the latent infection–the predominant phase in the majority of infected cells–only a limited number of viral genes are expressed. Provocation by a variety of stimuli induces lytic replication, resulting in new virion assembly and release of infectious viral particles (Schulz, 2006). Previous studies suggest that the oncogenic potential of KSHV is largely dependent upon genes expressed during the viral latency, however, recent data demonstrate that the viral lytic reactivation is critical for infection of naïve cell targets, maintenance of the KSHV reservoir, and tumor development (Aluigi et al., 1996; Lebbe et al., 1997; Grundhoff and Ganem, 2014;).
Lipids form a diverse group of water-insoluble molecules that include triacylglycerides, phosphoglycerides, sterols and sphingolipids. Lipids are essential for mammalian cells to maintain their physiological functions. For instance, fatty acids are the major building blocks for the synthesis of triacylglycerides during the process of energy storage. Phosphoglycerides, together with sterols and sphingolipids are the major structural components of cellular membranes. In addition, lipids as second messengers and hormones also play important roles in many signal transduction pathways. For example, lipids in the cellular membranes have been linked to the functions of several signal transduction pathways, including immunoglobulin E signaling (Sheets et al., 1999), T-cell antigen receptor signaling (Janes et al., 2000), glial-cell-derived neurotrophic factor (GDNF) signaling (Tansey et al., 2000), Ras signaling (Roy et al., 1999) and Hedgehog signaling (Porter et al., 1996). Accumulating evidence has shown that cancer cells develop specific alterations in different aspects of lipid metabolism to facilitate their survival and various malignant behaviors. To date, there are only a handful of studies describing how an oncogenic virus such as KSHV can manipulate host cellular lipid biosynthesis and metabolism to promote viral infection, pathogenesis, and tumorigenesis. In the current review, we summarize recent findings in this new area of KSHV research.
ROLE OF LIPIDS IN THE PRIMARY AND LATENT KSHV INFECTION
In cell culture, KSHV is able to infect various types of human cells, such as B cells, endothelial cells, epithelial cells, and fibroblasts (Dai et al., 2012; Fontana et al., 2014; Kang and Myoung, 2017). Several membrane proteins including heparin sulfate proteoglycan (HSPG), DC-SIGN, integrin α3β1/αvβ3, EphA2 and xCT can act as cellular receptors for KSHV infection in a cell type-dependent manner (Birkmann et al., 2001; Akula et al., 2002; Kaleeba and Berger, 2006; Rappocciolo et al., 2006; Garrigues et al., 2008; Hahn et al., 2012). After binding with these receptors, KSHV can induce the phosphorylation of focal adhesion kinase (FAK) which subsequently leads to the activation of Src, phosphatidylinositol 3-kinase (PI3-K), protein kinase C-ζ (PKC-ζ), Rho-GTPases, mitogen-activated protein kinase kinase (MEK), and extracellular signal regulated kinase 1/2 (ERK1/2) (Naranatt et al., 2003; Sharma-Walia et al., 2004; 2005). Activation of these signaling cascades can facilitate virus entry, its movement in the cytoplasm, and the nuclear delivery of viral DNA. Many of these KSHV induced signaling molecules are associated with lipid rafts micro-domains in the membrane. Previously, Raghu et al. reported that lipid rafts of endothelial cells play critical roles in KSHV infection and gene expression (Raghu et al., 2007). They found that disruption of lipid rafts by methyl β-cyclo dextrin (MβCD) or nystatin significantly inhibited the expression of viral latent gene, Lana (Latency-associated nuclear antigen), and the lytic gene, Rta (Replication and transcription activator). Lana is the only viral protein consistently expressed in all KS-associated malignancies (Dupin et al., 1999) and its major function is to maintain the viral episome in the latently-infected cells (Ballestas et al., 1999; Avey et al., 2015). Rta is a key viral protein initially controlling virus “latent to lytic” switch (Sun et al., 1998). The inhibition of Lana and Rta expression was mainly achieved by suppressing the KSHV-induced PI3-K and RhoA-GTPases activation and reducing the co-localizations of PI3-K and RhoA-GTPases with lipid rafts (Raghu et al., 2007). Since disruption of lipid rafts did not affect KSHV binding and viral DNA internalization, the authors concluded that lipid rafts are mainly required for KSHV-induced microtubule dynamics, virus movement in the cytoplasm, nuclear delivery of viral DNA, and viral gene expression (Raghu et al., 2007). A later study from the same group indicates that at a very early timepoint during infection (~1 min post-infection), an adaptor protein, c-Cbl, can induce the selective translocation of KSHV into the lipid rafts along with the α3β1, αVβ3, and x-CT receptors, leading to a productive infection (Chakraborty et al., 2011). Knock-down of c-Cbl was found to inhibit KSHV infection by preventing micro-pinocytosis and selective virus-receptor translocation, with KSHV being diverted toward a clathrin-lysosomal noninfectious pathway.
One recent study has shown that KSHV infection can activate several components of the lipoxygenase pathway, including 5-lipoxygenase (5LO), leukotriene (LT) A4 hy-lase (LTA4H), and leukotriene B4 (LTB4), a chemo-tactic lipid mediator of the 5LO pathway (Sharma-Walia et al., 2014). Interestingly, blocking the 5LO/LTB4 cascade can inhibit the expression of KSHV-encoded latent protein Lana, the immunomodulatory protein K5, the viral macrophage inflammatory protein 1 (MIP-1), and MIP-2 expression. Taken together, these results clearly indicate that cellular lipids, lipid metabolism and related signaling pathways are involved in KSHV primary infection and subsequent latency establishment. Given its critical role in KSHV infection cycle, the lipid pathway may represent a promising “drug target” to manage KSHV infection.
ROLE OF LIPIDS IN KSHV REACTIVATION AND LYTIC REPLICATION
Like latency, viral reactivation and lytic replication also play important roles in KSHV oncogenesis. A recent study has shown that reactivation can be induced by some short-chain fatty acids (SCFAs) such as phenylbutyrate through inhibiting histone deacetylase (HDAC) activities (Gorres et al., 2014). Consistently, Yu et al. have found that several SCFAs produced by periodontal pathogens such as Porphyromonas gingivalis and Fusobacterium nucleatum can also induce the KSHV lytic reactivation by suppressing HDACs as well as two histone N-lysine methyltransferases (HLMTs): enhancer of zeste homo-log2 (EZH2) and suppressor of variegation 3-9 homo-log1 (SUV39H1) (Yu et al., 2014). These findings indicate that periodontal pathogens may create a unique microenvironment in the oral cavity, which in turns favors KSHV replication and KS development. Indeed, oral cavity involvement represents the initial manifestation of KS in 20%-60% of HIV-associated cases (Flaitz et al., 1997; Lager et al., 2003; Reichart 2003).
We recently reported that targeting sphingolipid metabolism by either sphingosine kinase inhibitors or exogenous \ can dramatically induce viral lytic genes expression in KSHV-infected primary endothelial cells or PEL cells (Qin et al., 2014; Dai et al., 2014, 2015). Such induction is at least in part mediated by the suppression of pro-latency viral microRNAs (e.g., miR-K12-1 and miR-K12-11) as well as related signaling pathways (e.g., NF-κB) (Dai et al., 2014).
ROLE OF LIPIDS IN THE SURVIVAL OF KSHV-INFECTED CELLS
Recent studies have shown that cellular lipids and lipid metabolism can regulate the survival of KSHV-infected primary and tumor cells. Having analyzed the metabolic profiles of primary B cells and KSHV+ PEL cells, Bhatt et al. found that KSHV+ PEL cells exhibit greater aerobic glycolysis and fatty acid synthesis than primary B cells (Bhatt et al., 2012). Meanwhile, the major lipid components of eukaryotic cell walls (e.g., phosphatidylcholine and phosphatidylethanolamine) are also more abundant in PEL cells. The fatty acid synthase (FASN), a multienzyme complex involved in the cellular lipids synthesis (Kuhajda et al., 2000), is overexpressed in PEL cells. Moreover, treatment of KSHV+ PEL cells with the FASN inhibitor, C75, can reduce cell viability in a dose-dependent manner (Bhatt et al., 2012).
Delgado et al. have utilized a metabolomic approach to investigate the KSHV mediated global metabolic alterations in latently infected cells (Delgado et al., 2012). They found that ~60 analyzed metabolites were altered after latent infection. Among them, many long chain fatty acids were affected due to the alteration of fatty acid synthesis pathways. Previous studies have shown that fatty acid synthesis is also required for the survival of latently infected endothelial cells and inhibition of key enzymes (e.g., acetyl-CoA carboxylase (Wang et al., 2009) or FASN (Kuhajda et al., 2000)) in this pathway led to apoptosis of infected cells. In contrast, addition of palmitic acid (the fundamental fatty acid precursor) can protect latently infected cells from the acetyl-CoA carboxylase inhibitor, 5-(Tetradecyloxy)-2-Furoic Acid (TOFA)-induced cell death. The same group later reported that the KSHV latent infection also increases peroxisome biogenesis. Interestingly, the proteins involved in peroxisomal lipid metabolism of very long chain fatty acids, such as ABCD3 (a peroxisome-specific lipid transporter) and ACOX1 (Acyl-CoA Oxidase 1, a peroxisomal enzyme), are required for the survival of latently infected cells (Sychev et al., 2017).
Sphingolipid biosynthesis involves hydrolytic conversion of ceramide to sphingosine. Subsequently, sphingosine is phosphorylated by one of two sphingosine kinase isoforms (SphK1 or SphK2) to generate bioactive sphingosine-1-phosphate (S1P) (Ogretmen and Hannun, 2004) (Figure 1). The relative levels of ceramide and S1P ultimately determine the fate of tumor cells, with accumulation of ceramides favoring apoptosis, and accumulation of S1P favoring proliferation (Cuvillier et al., 1996; Ogretmen and Hannun, 2004). SphK can be activated by a variety of tumor-promoting cytokines and growth factors. SphK activation is responsible for a rapid accumulation of intracellular S1P and depletion of ceramide species (Maceyka et al., 2002). S1P can subsequently bind to one of five G protein-coupled S1P receptors (S1PR1-5) and then activate diverse downstream signaling pathways (Strub et al., 2010). Because of their pleiotropic roles, bioactive sphingolipids have evolved as promising therapeutic targets for cancer treatment over the past two decades (Saddoughi et al., 2013). We have recently reported that induction of intracellular ceramide using a novel SphK2 inhibitor (ABC294640) or exogenous ceramide/dihydro(dh)-ceramide species (e.g., C6-Cer or dhC16-Cer) can effectively kill KSHV+primary endothelial cells or PEL tumor cells, but have little effect on KSHV non-infected cell controls (e.g., naïve endothelial cells or B cells) (Qin et al., 2014; Dai et al., 2014, 2015). Further, these compounds can also repress KSHV+PEL tumor progression in vivo and it is likely that this is mediated through interfering with several cell survival/proliferation-associated signaling pathways (e.g., MAPK/ERK, Akt and NF-κB) and up-regulating viral lytic genes and cellular tumor suppressor genes expression (Qin et al., 2014; Dai et al., 2015; Cao et al., 2017). In addition, the KSHV mediated up-regulation of SphK2 (Dai et al., 2014) may also help sensitize KSHV+ cells to sphingolipid targeted therapy.
Figure 1.
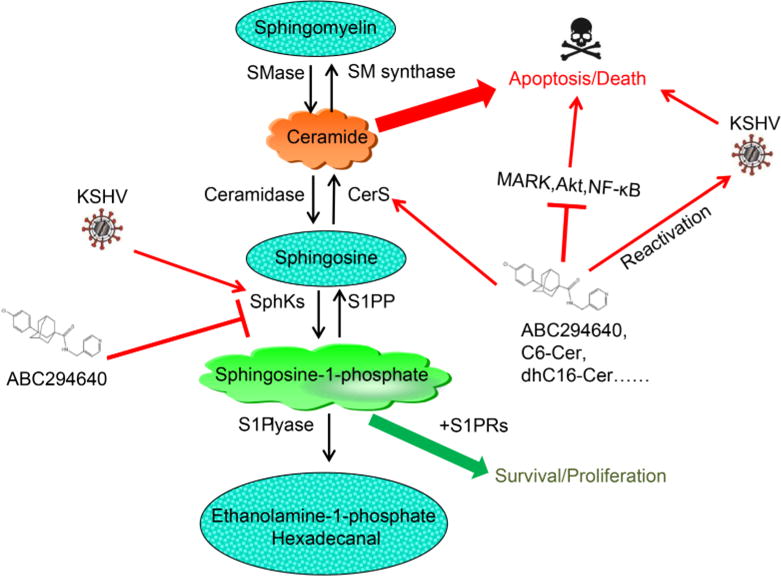
Targeting sphingolipid metabolism in KSHV-infected host cells. CerS, ceramide synthase; S1PP, S1P phosphatase; SphKs, sphingosine kinases; S1PRs, S1P receptors. ABC294640: A novel selective SPHK2 inhibitor; C6-Cer, dhC16-Cer etc: exogenous short- or long-chain ceramides.
LIPIDS AND KSHV-INDUCED ANGIOGENESIS/TRANSFORMATION
One recent study revealed that neutral lipid (NL) content is increased in KSHV-infected human umbilical vein endothelial cells (HUVEC) (Angius et al., 2015). In particular, triglyceride synthesis is boosted in the lytic phase, whereas the cholesteryl ester synthesis rises in the latent phase. Moreover, inhibition of cholesterol esterification significantly reduces neo-tubule formation mainly in latently infected cells, indicating that a reprogramming of cholesteryl ester metabolism is involved in KSHV-mediated neo-angiogenesis and that it may also contribute to the high metastatic potential of the derived-tumors.
It is believed that KSHV-encoded G protein-coupled receptor (vGPCR) is a key molecule in the pathogenesis of KS and that it plays a central role in promoting vascular endothelial growth factor-driven angiogenesis and spindle cell proliferation (Montaner et al., 2003; Grisotto et al., 2006; Wei et al., 2016). Several studies have shown that 1 Alpha, 25-dihydroxyvitamin D3 [1 alpha, 25(OH)(2)D(3)] and its TX527 analog inhibit the growth of vGPCR transformed endothelial cells in vitro and in vivo. The inhibition effects are achieved through a complex of mechanisms including an interaction with vitamin D receptor, down-regulation of the NF-κB pathway and up-regulation of the pro-apoptotic protein, Bim (Gonzalez-Pardo et al., 2010; 2012; 2013; Suares et al., 2015). Taken together, these data indicate the importance of vitamin D as a steroid signaling molecule in vGPCR-transformed endothelial cell proliferation.
CONCLUSION
Our group and others have recently shown that cellular lipids and lipid metabolism play important roles in KSHV-infected cell survival, pathogenesis, and tumorigenesis (summarized in Figure 2). Lipid research has become an exciting direction in the KSHV field. To date, it is still largely unclear how this oncogenic virus manipulates lipid biosynthesis and metabolism during de novo infection and KSHV mediated tumor development. Clinically, there are few data resulting from clinical trials testing the effectiveness of lipids-targeted therapeutics for KSHV-related malignancies. To the best of our knowledge, there’s only one ongoing early phase trial for the evaluation of ABC294640 in patients with refractory/relapsed Diffuse Large B-cell Lymphoma (DLBCL) or Kaposi Sarcoma (KS) directed by Dr. Suki Subbiah (NCT0222 9981). How to accelerate the “bench to bedside” transition in this field is a key question needs to be addressed soon.
Figure 2.
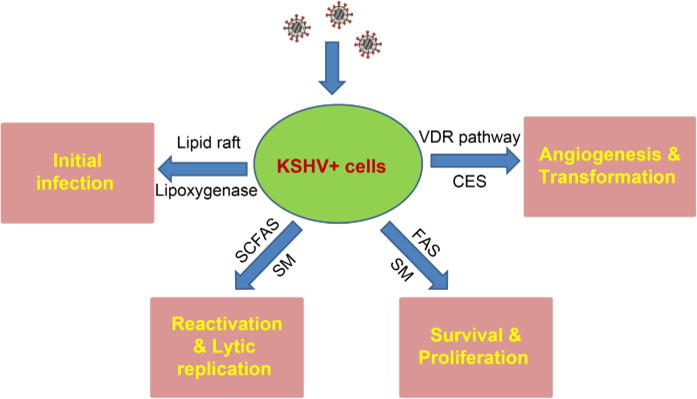
Schematic of recent findings about the contributions of cellular lipids and lipid metabolism to KSHV infection and pathogenesis. SCFAs: short-chain fatty acids; SM: sphingolipid metabolism; FAS: fatty acid synthesis; VDR: Vitamin D receptor; CES: cholesteryl ester synthesis.
Acknowledgments
This work was partially supported by grants from a DOD Career Development Award (CA140437), the Louisiana Clinical and Translational Science Center Pilot grants (U54GM104940 from NIH), a LSU LIFT2 funding, a NIH P20-GM121288-01 subproject, NIH RO1s (AI091526, AI128864, AI101046, and AI106676) as well as awards from the National Natural Science Foundation of China (81472547, 81400164, 81672924 and 81772930). Funding sources had no role in the study design, data collection/analysis, decision to publish, and/or manuscript preparation.
Footnotes
ORCID: 0000-0002-9905-1275
ORCID: 0000-0002-6818-8535
COMPLIANCE WITH ETHICS GUIDELINES
The authors declare that they have no conflicts of interest. This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Akula SM, Pramod NP, Wang FZ, Chandran B. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell. 2002;108:407–419. doi: 10.1016/s0092-8674(02)00628-1. [DOI] [PubMed] [Google Scholar]
- Aluigi MG, Albini A, Carlone S, Repetto L, De Marchi R, Icardi A, Moro M, Noonan D, Benelli R. KSHV sequences in biopsies and cultured spindle cells of epidemic, iatrogenic and Mediterranean forms of Kaposi’s sarcoma. Res Virol. 1996;147:267–275. doi: 10.1016/0923-2516(96)82285-0. [DOI] [PubMed] [Google Scholar]
- Angius F, Uda S, Piras E, Spolitu S, Ingianni A, Batetta B, Pompei R. Neutral lipid alterations in human herpesvirus 8-infected HUVEC cells and their possible involvement in neo-angiogenesis. BMC Microbiol. 2015;15:74. doi: 10.1186/s12866-015-0415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avey D, Brewers B, Zhu F. Recent advances in the study of Kaposi’s sarcoma-associated herpesvirus replication and pathogenesis. Virol Sin. 2015;30:130–145. doi: 10.1007/s12250-015-3595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballestas ME, Chatis PA, Kaye KM. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science. 1999;284:641–644. doi: 10.1126/science.284.5414.641. [DOI] [PubMed] [Google Scholar]
- Bhatt AP, Jacobs SR, Freemerman AJ, Makowski L, Rathmell JC, Dittmer DP, Damania B. Dysregulation of fatty acid synthesis and glycolysis in non-Hodgkin lymphoma. Proc Natl Acad Sci U S A. 2012;109:11818–11823. doi: 10.1073/pnas.1205995109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkmann A, Mahr K, Ensser A, Yaguboglu S, Titgemeyer F, Fleckenstein B, Neipel F. Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8. 1. J Virol. 2001;75:11583–11593. doi: 10.1128/JVI.75.23.11583-11593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet F, Lewden C, May T, Heripret L, Jougla E, Bevilacqua S, Costagliola D, Salmon D, Chene G, Morlat P. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101:317–324. doi: 10.1002/cncr.20354. [DOI] [PubMed] [Google Scholar]
- Cao Y, Qiao J, Lin Z, Zabaleta J, Dai L, Qin Z. Up-regulation of tumor suppressor genes by exogenous dhC16-Cer contributes to its anti-cancer activity in primary effusion lymphoma. Oncotarget. 2017;8:15220–15229. doi: 10.18632/oncotarget.14838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, ValiyaVeettil M, Sadagopan S, Paudel N, Chandran B. c-Cbl-mediated selective virus-receptor translocations into lipid rafts regulate productive Kaposi’s sarcoma-associated herpesvirus infection in endothelial cells. J Virol. 2011;85:12410–12430. doi: 10.1128/JVI.05953-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569–576. doi: 10.1634/theoncologist.12-5-569. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Dai L, Plaisance-Bonstaff K, Voelkel-Johnson C, Smith CD, Ogretmen B, Qin Z, Parsons C. Sphingosine kinase-2 maintains viral latency and survival for KSHV-infected endothelial cells. PLoS One. 2014;9:e102314. doi: 10.1371/journal.pone.0102314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Qin Z, Defee M, Toole BP, Kirkwood KL, Parsons C. Kaposi sarcoma-associated herpesvirus (KSHV) induces a functional tumor-associated phenotype for oral fibroblasts. Cancer Lett. 2012;318:214–220. doi: 10.1016/j.canlet.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Trillo-Tinoco J, Bai A, Chen Y, Bielawski J, Del Valle L, Smith CD, Ochoa AC, Qin Z, Parsons C. Ceramides promote apoptosis for virus-infected lymphoma cells through induction of ceramide synthases and viral lytic gene expression. Oncotarget. 2015;6:24246–24260. doi: 10.18632/oncotarget.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado T, Sanchez EL, Camarda R, Lagunoff M. Global metabolic profiling of infection by an oncogenic virus: KSHV induces and requires lipogenesis for survival of latent infection. PLoS Pathog. 2012;8:e1002866. doi: 10.1371/journal.ppat.1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, van Marck E, Salmon D, Gorin I, Escande JP, Weiss RA, Alitalo K, Boshoff C. Distribution of human herpesvirus-8 latently infected cells in Kaposi’s sarcoma, multicentric Castleman’s disease, and primary effusion lymphoma. Proc Natl Acad Sci U S A. 1999;96:4546–4551. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaitz CM, Jin YT, Hicks MJ, Nichols CM, Wang YW, Su IJ. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences (KSHV/HHV-8) in oral AIDS-Kaposi’s sarcoma: a PCR and clinicopathologic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:259–264. doi: 10.1016/s1079-2104(97)90014-7. [DOI] [PubMed] [Google Scholar]
- Fontana JM, Mygatt JG, Conant KL, Parsons CH, Kaleeba JA. Kaposi’s Sarcoma-Associated Herpesvirus Subversion of the Anti-Inflammatory Response in Human Skin Cells Reveals Correlates of Latency and Disease Pathogenesis. J Skin Cancer. 2014;2014:246076. doi: 10.1155/2014/246076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigues HJ, Rubinchikova YE, Dipersio CM, Rose TM. Integrin alphaVbeta3 Binds to the RGD motif of glycoprotein B of Kaposi’s sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor. J Virol. 2008;82:1570–1580. doi: 10.1128/JVI.01673-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Pardo V, D’Elia N, Verstuyf A, Boland R, Russo de Boland A. NFkappaB pathway is down-regulated by 1alpha, 25(OH)(2)-vitamin D(3) in endothelial cells transformed by Kaposi sarcoma-associated herpes virus G protein coupled receptor. Steroids. 2012;77:1025–1032. doi: 10.1016/j.steroids.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Pardo V, Martin D, Gutkind JS, Verstuyf A, Bouillon R, de Boland AR, Boland RL. 1 Alpha, 25-dihydroxyvitamin D3 and its TX527 analog inhibit the growth of endothelial cells transformed by Kaposi sarcoma-associated herpes virus G protein-coupled receptor in vitro and in vivo. Endocrinology. 2010;151:23–31. doi: 10.1210/en.2009-0650. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Pardo V, Verstuyf A, Boland R, Russo de Boland A. Vitamin D analogue TX 527 down-regulates the NF-kappaB pathway and controls the proliferation of endothelial cells transformed by Kaposi sarcoma herpesvirus. Br J Pharmacol. 2013;169:1635–1645. doi: 10.1111/bph.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorres KL, Daigle D, Mohanram S, Miller G. Activation and repression of Epstein-Barr Virus and Kaposi’s sarcoma-associated herpesvirus lytic cycles by short- and medium-chain fatty acids. J Virol. 2014;88:8028–8044. doi: 10.1128/JVI.00722-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisotto MG, Garin A, Martin AP, Jensen KK, Chan P, Sealfon SC, Lira SA. The human herpesvirus 8 chemokine receptor vGPCR triggers autonomous proliferation of endothelial cells. J Clin Invest. 2006;116:1264–1273. doi: 10.1172/JCI26666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundhoff A, Ganem D. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J Clin Invest. 2004;113:124–136. doi: 10.1172/JCI200417803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn AS, Kaufmann JK, Wies E, Naschberger E, Panteleev-Ivlev J, Schmidt K, Holzer A, Schmidt M, Chen J, Konig S, Ensser A, Myoung J, Brockmeyer NH, Sturzl M, Fleckenstein B, Neipel F. The ephrin receptor tyrosine kinase A2 is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus. Nat Med. 2012;18:961–966. doi: 10.1038/nm.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes PW, Ley SC, Magee AI, Kabouridis PS. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin Immunol. 2000;12:23–34. doi: 10.1006/smim.2000.0204. [DOI] [PubMed] [Google Scholar]
- Kaleeba JA, Berger EA. Kaposi’s sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science. 2006;311:1921–1924. doi: 10.1126/science.1120878. [DOI] [PubMed] [Google Scholar]
- Kang S, Myoung J. Primary lymphocyte infection models for KSHV and its putative tumorigenesis mechanisms in B cell lymphomas. J Microbiol. 2017;55:319–329. doi: 10.1007/s12275-017-7075-2. [DOI] [PubMed] [Google Scholar]
- Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci U S A. 2000;97:3450–3454. doi: 10.1073/pnas.050582897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lager I, Altini M, Coleman H, Ali H. Oral Kaposi’s sarcoma: a clinicopathologic study from South Africa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:701–710. doi: 10.1016/s1079-2104(03)00370-6. [DOI] [PubMed] [Google Scholar]
- Lebbe C, de Cremoux P, Millot G, Podgorniak MP, Verola O, Berger R, Morel P, Calvo F. Characterization of in vitro culture of HIV-negative Kaposi’s sarcoma-derived cells. In vitro responses to alfa interferon. Arch Dermatol Res. 1997;289:421–428. doi: 10.1007/s004030050215. [DOI] [PubMed] [Google Scholar]
- Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201. doi: 10.1016/s1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Molinolo A, Bugge TH, Sawai ET, He Y, Li Y, Ray PE, Gutkind JS. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi’s sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell. 2003;3:23–36. doi: 10.1016/s1535-6108(02)00237-4. [DOI] [PubMed] [Google Scholar]
- Naranatt PP, Akula SM, Zien CA, Krishnan HH, Chandran B. Kaposi’s sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-zeta-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J Virol. 2003;77:1524–1539. doi: 10.1128/JVI.77.2.1524-1539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- Qin Z, Dai L, Trillo-Tinoco J, Senkal C, Wang W, Reske T, Bonstaff K, Del Valle L, Rodriguez P, Flemington E, Voelkel-Johnson C, Smith CD, Ogretmen B, Parsons C. Targeting sphingosine kinase induces apoptosis and tumor regression for KSHV-associated primary effusion lymphoma. Mol Cancer Ther. 2014;13:154–164. doi: 10.1158/1535-7163.MCT-13-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Caballero A, Sivakumar R, Varga L, Bottero V, Chandran B. Lipid rafts of primary endothelial cells are essential for Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8-induced phosphatidylinositol 3-kinase and RhoA-GTPases critical for microtubule dynamics and nuclear delivery of viral DNA but dispensable for binding and entry. J Virol. 2007;81:7941–7959. doi: 10.1128/JVI.02848-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappocciolo G, Jenkins FJ, Hensler HR, Piazza P, Jais M, Borowski L, Watkins SC, Rinaldo CR., Jr DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J Immunol. 2006;176:1741–1749. doi: 10.4049/jimmunol.176.3.1741. [DOI] [PubMed] [Google Scholar]
- Reichart PA. Oral manifestations in HIV infection: fungal and bacterial infections, Kaposi’s sarcoma. Med Microbiol Immunol. 2003;192:165–169. doi: 10.1007/s00430-002-0175-5. [DOI] [PubMed] [Google Scholar]
- Roy S, Luetterforst R, Harding A, Apolloni A, Etheridge M, Stang E, Rolls B, Hancock JF, Parton RG. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat Cell Biol. 1999;1:98–105. doi: 10.1038/10067. [DOI] [PubMed] [Google Scholar]
- Saddoughi SA, Ogretmen B. Diverse functions of ceramide in cancer cell death and proliferation. Adv Cancer Res. 2013;117:37–58. doi: 10.1016/B978-0-12-394274-6.00002-9. [DOI] [PubMed] [Google Scholar]
- Schulz TF. The pleiotropic effects of Kaposi’s sarcoma herpesvirus. J Pathol. 2006;208:187–198. doi: 10.1002/path.1904. [DOI] [PubMed] [Google Scholar]
- Sharma-Walia N, Chandran K, Patel K, Veettil MV, Marginean A. The Kaposi’s sarcoma-associated herpesvirus (KSHV)-in-duced 5-lipoxygenase-leukotriene B4 cascade plays key roles in KSHV latency, monocyte recruitment, and lipogenesis. J Virol. 2014;88:2131–2156. doi: 10.1128/JVI.02786-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Walia N, Krishnan HH, Naranatt PP, Zeng L, Smith MS, Chandran B. ERK1/2 and MEK1/2 induced by Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J Virol. 2005;79:10308–10329. doi: 10.1128/JVI.79.16.10308-10329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Walia N, Naranatt PP, Krishnan HH, Zeng L, Chandran B. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-de-pendent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J Virol. 2004;78:4207–4223. doi: 10.1128/JVI.78.8.4207-4223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets ED, Holowka D, Baird B. Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FcepsilonRI and their association with detergent-resistant membranes. J Cell Biol. 1999;145:877–887. doi: 10.1083/jcb.145.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay MF, Clauvel JP, Raphael M, Degos L, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv Exp Med Biol. 2010;688:141–155. doi: 10.1007/978-1-4419-6741-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suares A, Russo de Boland A, Verstuyf A, Boland R, Gonzalez-Pardo V. The proapoptotic protein Bim is up regulated by 1alpha, 25-dihydroxyvitamin D3 and its receptor agonist in endothelial cells and transformed by viral GPCR associated to Kaposi sarcoma. Steroids. 2015;102:85–91. doi: 10.1016/j.steroids.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Sun R, Lin SF, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi’s sarcoma-associated herpesvirus. Proc Natl Acad Sci U S A. 1998;95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sychev ZE, Hu A, DiMaio TA, Gitter A, Camp ND, Noble WS, Wolf-Yadlin A, Lagunoff M. Integrated systems biology analysis of KSHV latent infection reveals viral induction and reliance on peroxisome mediated lipid metabolism. PLoS Pathog. 2017;13:e1006256. doi: 10.1371/journal.ppat.1006256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey MG, Baloh RH, Milbrandt J, Johnson EM., Jr GFRalpha-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron. 2000;25:611–623. doi: 10.1016/s0896-6273(00)81064-8. [DOI] [PubMed] [Google Scholar]
- Wang C, Xu C, Sun M, Luo D, Liao DF, Cao D. Acetyl-CoA carboxylase-alpha inhibitor TOFA induces human cancer cell apoptosis. Biochem Biophys Res Commun. 2009;385:302–306. doi: 10.1016/j.bbrc.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HW, Pittaluga S, Jaffe ES. Multicentric Castleman disease: Where are we now? Semin Diagn Pathol. 2016;33:294–306. doi: 10.1053/j.semdp.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Zhu Q, Ding L, Liang Q, Cai Q. Manipulation of the host cell membrane by human γ-herpesviruses EBV and KSHV for pathogenesis. Virol Sin. 2016;31:395–405. doi: 10.1007/s12250-016-3817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Shahir AM, Sha J, Feng Z, Eapen B, Nithianantham S, Das B, Karn J, Weinberg A, Bissada NF, Ye F. Short-Chain Fatty Acids from Periodontal Pathogens Suppress Histone Deacetylases, EZH2, and SUV39H1 To Promote Kaposi’s Sarcoma-Associated Herpesvirus Replication. J Virol. 2014;88:44664479. doi: 10.1128/JVI.03326-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


