Abstract
Purpose
To evaluate if the formation of protein corona around ferumoxytol nanoparticles can facilitate stem cell labeling for in vivo tracking with MR imaging.
Methods
Ferumoxytol was incubated in media containing human serum (group 1), fetal bovine serum (group 2), StemPro® media (group 3), protamine (group 4) and protamine+heparin (group 5). Formation of a protein corona was characterized by dynamic light scattering (DLS), zeta potential, and liquid chromatography-mass spectrometry (LC-MS). Iron uptake was evaluated by DAB-Prussian blue, Lysosomal staining, and inductively coupled plasma spectrometry (ICP). To evaluate the effect of protein corona on stem cell labeling, human mesenchymal stem cells (hMSC) were labeled with above formulations, implanted into pig knee specimen and investigated with T2-weighted Fast Spin Echo (FSE) and Multi Echo Spin Echo (MESE) sequences on a 3T MR scanner. Data of different groups were compared with a Kruskal-Wallis test.
Results
Compared to bare nanoparticles, all experimental groups showed significantly increased negative zeta values (from −37 to less than −10; p=0.008). Nanoparticles in group 1–3 showed an increased size due to the formation of a protein corona. hMSCs labeled with group 1–5 media revealed significantly shortened T2-relaxation times compared to unlabeled controls (p=0.0012). hMSCs labeled with group 3 and 5 had the highest iron uptake after group 1. After implantation into pig knees, hMSCs labeled with group 1 media revealed significantly shorter T2-relaxation times compared to hMSC labeled with group 2–5 (p=0.0022).
Conclusion
The protein Corona around ferumoxytol nanoparticles can facilitate stem cell labeling for clinical cell tracking with MR imaging.
INTRODUCTION
Imaging techniques are important for monitoring novel cell therapies for tissue regeneration. Therapeutic cells migrate, proliferate, differentiate, and respond to their environment (1). Iron oxide nanoparticles can be used to track transplanted cells in vivo with magnetic resonance (MR) imaging (2). Persistence or disappearance of the iron oxide label at the transplant site, as visualized on MRI, can provide information about successful or unsuccessful engraftment outcomes (3, 4).
Previous studies found that relatively large superparamagnetic iron oxide nanoparticles (SPIO) with diameters of more than 50 nm are phagocytosed by stem cells (5). This leads to more efficient cellular uptake compared to “ultrasmall” superparamagnetic nanoparticles (USPIO) with diameters of less than 50 nm, which are mainly subject to endocytotic cellular uptake (6–8). Therefore, initial approaches for MR-based cell tracking have been almost exclusively performed with SPIO (6, 9, 10). Unfortunately, SPIO has been taken off the market in the US and Europe and have been replaced by USPIO as second-generation nanoparticles, which offer a wider spectrum of clinical applications. Ferumoxytol (Feraheme™) is a Food and Drug Administration (FDA)-approved iron supplement (11), which is composed of USPIO and can be applied “off label” for cell tracking in patients. Since ferumoxytol uptake by stem cells is relatively inefficient (12), previous cell labeling protocols utilized transfection agents such as protamine sulfate, with or without addition of heparin, to shuttle ferumoxytol into the cell (12, 13). However, transfection agent-mediated cell labeling has three disadvantages: First, it requires incubation of positively charged transfection agents with negatively charged nanoparticles. This causes precipitation of some nanoparticles due to loss of surface charges and related safety concerns for clinical applications (14). Second, transfection agent-mediated cell labeling can lead to surface adsorption of nanoparticles instead of internalization, which can impair in vivo cellcell interactions (14, 15). Third, since most transfection agents are not approved for clinical use, adding non-clinically approved transfection agents to clinical protocols can hinder clinical translation (16, 17). Simple incubation protocols would be preferable as these would be easier to apply and not require Investigational New Drug (IND) approval for the transfection agent. It has been recently described that proteins in human serum or serum containing media form a corona around nanoparticles (18). The protein corona increases the nanoparticles’ surface charge and hydrodynamic size (19, 20), which can increase their cellular uptake through endocytosis or phagocytosis (21–23). The purpose of our study was to evaluate if the formation of a protein corona around ferumoxytol nanoparticles can facilitate stem cell labeling for in vivo tracking with MR imaging.
MATERIALS AND METHODS
Hard corona formation
Ferumoxytol (Feraheme™) is composed of ultra-small superparamagnetic particles of iron oxides (USPIO) with an iron oxide core and a carboxymethyldextran coat. The agent has a mean hydrodynamic diameter of 30 nm and an r2 relaxivity of 83 L mmol-1 s-1 at 20 MHz (24). We incubated 100μl of ferumoxytol (concentration 1 mg Fe/mL) with labeling media at 37°C for one hour as follows: Group 1: 900μl of DMEM media supplemented with 10% human serum, Group 2: 900μl of DMEM media with 10% fetal bovine serum, Group 3: 900μl of StemPro® serum free media (StemPro® is a xenogen-free, serum-free, and cGMP compliant media, which contains well characterized proteins specifically formulated for expansion of human mesenchymal stem cells (hMSC) for clinical use), Group 4: 10μg/ml protamine sulfate in 1ml media and Group 5: 60μg/ml protamine sulfate and 2 IU/ml heparin in 1ml media. Groups 4–5 were used as a standard of reference for clinically translatable transfection agent-mediated cell labeling methods. Excess/unbound proteins were removed by centrifugation and washing with phosphate buffered saline (PBS). The pellet was resuspended in 500μl of PBS and used for further experiments. Bare nanoparticles served as control (group 6).
Protein Corona characterization
Dynamic light scattering (DLS) and zeta potential of samples from group 1–6 were measured with Malvern PCS-4700 and Malvern Zetasizer 3000HSa instruments. The protein corona composition in group 1–3 was analyzed with LC-MS (liquid chromatography-mass spectrometry). Protease Max (Promega, Madison, WI, USA), an acid labile surfactant, was added to the nanoparticles, followed by vortexing and sonication. Next, the samples were reduced, alkylated and digested overnight. The digestion was quenched by addition of formic acid, followed by peptide concentration and purification. The peptide pools were dried, reconstituted and injected into an in-house packed C18 reversed phase analytical column. The mass spectrometer was an Orbitrap Fusion set to acquire data in a dependent fashion. Fragmentation was performed on the most intense multiply charged precursor ions. All LC/MS data were analyzed for peptide composition using Preview and Byonic v2.0 software (ProteinMetrics, San Carlos, CA, USA). Data were validated using standard reverse-decoy techniques. Peptide spectral matches and other supporting data were transferred for further analysis using custom tools developed in MATLAB (MathWorks, Natick, MA, USA) to provide visualization and statistical characterization.
A semi-quantitative assessment of the protein amount was conducted through application of a Spectral Counting (SpC) method. The Normalized percentage of Spectral Counts (NpSpC) of each protein, identified in the LC-MS spectra, were calculated by applying the following equation: (25)
Where NpSpCk is the normalized percentage of spectral count (i.e., raw counts of ions) for protein k, SpC is the spectral count identified, and Mw is the molecular weight (in kDa) of the protein k.
Stem cell labeling
Triplicate samples of 1×106 human mesenchymal stromal cells (hMSCs) (Lonza, Basel, Switzerland) were labeled with group 1–3 compositions of ferumoxytol (concentration 100μg/ml) for 5 days and with group 4–5 compositions for 4 hours in serum-free media and 20 hours in 10% fetal bovine serum-containing media (12). Following the labeling procedures, the cells were washed in Dulbecco’s Modified Eagle Medium (DMEM) media, counted and referred to Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES), microscopy and imaging studies.
The cellular iron uptake was measured by ICP-OES and then divided by the cell concentration to provide the iron content per cell.
In addition, cell samples from each group were stained with the “Accustain” Prussian blue kit (Sigma-Aldrich, St Louis, MO, USA) with post-DAB (3,3′-diaminobenzidine) enhancement, and LysoTracker® Red DND 99 (1nM; Invitrogen, Molecular Probes, Eugene, OR, USA).
To further localize ferumoxytol nanoparticles in hMSC, 1×106 cells per group were carefully washed three times with PBS, fixed in formalin, embedded in gelatin, cut in 1mm3 blocks, fixed in 1% Osmium tetroxide in 0.1 M Sodium Cacodylate (EMS, Hatfield, PA, USA), stained with 2% uranyl acetate, dehydrated, embedded in Embed-812 resin (EMS, Hatfield, PA, USA), cut into 100 nm thick sections and placed on 300 mesh formvar-coated nickel grids (Ted Pella Inc., Redding, CA, USA). Electron microscopy was performed on an aberration corrected Titan (FEI, Hillsboro, OR, USA) operated at 300kV equipped with a OneView camera (Gatan, Pleasanton, CA, USA) and a Quantum 966 electron energy loss (EEL) spectrometer (Gatan, Pleasanton, CA, USA). EEL spectra were obtained to confirm the presence of iron nanoparticle with a dispersion of 0.25 eV/channel in microprobe mode. Spectra were background subtracted in Gatan Digital Micrograph software and smoothed via a 5-point average in OriginPro 9.1 prior to plotting.
MR imaging
Triplicate samples of 2×106 hMSCs from each group were mixed with 50μl ficol and placed into 3mm NMR tubes. Additional samples of 2×106 hMSC seeded in agarose scaffold were implanted into 5 mm cartilage defects in the femoral condyle of five pig knee joint specimens. All samples and specimen underwent MR imaging on a clinical 3T MR system (Signa HD 16.0, GE Medical Systems, Milwaukee, WI, USA), using a Mayo Clinic BC-10 MRI Coil, a T2 fat-saturated fast spin echo (FSE) sequence (TR3500, TE30, BT31.25, FOV 10×10 cm, Matrix 192×192, NEX 2, ST1.6mm, EL6), and a multi-echo spin echo (MESE) sequence (TR3500, TE 15, 30, 45, 60, BW31.25, FOV 10×10 cm, Matrix 192×192, NEX 1, ST1.6mm, EL1). T2 relaxation times of all samples were calculated using CineTool® software (GE Medical Systems, Milwaukee, WI, USA).
Statistical analyses
We used a Kruskal-Wallis test to test whether group one was significantly better than the other groups, followed by three post-hoc exact one-sided Wilcoxon tests comparing (i) groups 1–5 vs unlabeled control, (ii) groups 2–5 vs group 1, and (ii) group 1 vs the best two groups of groups 2–5 (Protamine+Heparin and StemPro®). A Bonferroni-adjusted significance level of 0.05/4 = 0.0125 was used. All statistical analyses were done using Stata Release 14.2 (StataCorp LP, College Station, TX).
RESULTS
Ferumoxytol protein corona characterization
Ferumoxytol nanoparticles formed a protein corona (Figure 1) in media containing human serum, fetal bovine serum, cGMP compliant proteins (StemPro®). DLS measurements showed that the corona-covered nanoparticles were more dispersed compared to bare nanoparticles in water as control group (figure 2a). The average size of nanoparticles in group 1, 2, 3, and control was 35.76±2.25 nm, 13.98±0.10 nm, 22.19±1.37 nm, and 16.53±0.94 nm respectively. The average size of nanoparticles in group 1–3 was significantly larger compared to the bare nanoparticles in water (p=0.025). The size of nanoparticles in group 1 was significantly different compared to group 2 and group 3 (p=0.0046).
Figure 1.
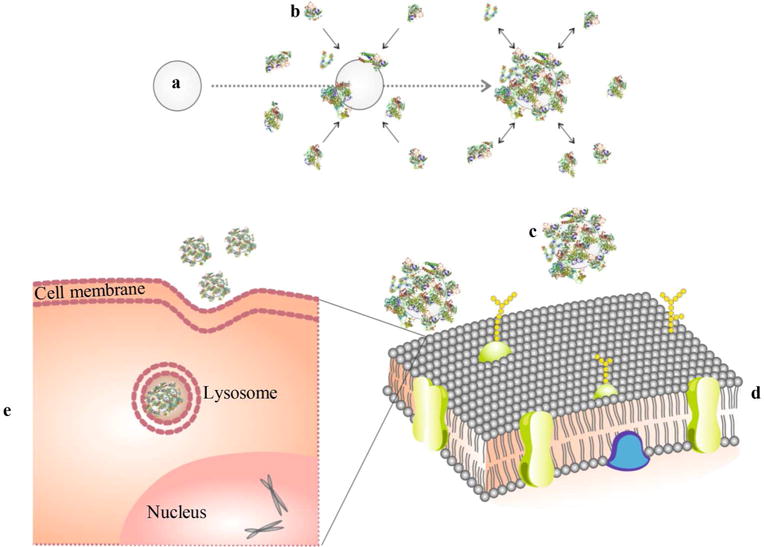
Schematic design of protein corona reaction with ferumoxytol, and its cellular uptake. (a) Iron oxide nanoparticle, (b) proteins in culture media, (c) nanoparticle covered with protein corona (d) approaches the cell membrane, and (e) protein-covered nanoparticle in a lysosome/endosome in a cell.
Figure 2.
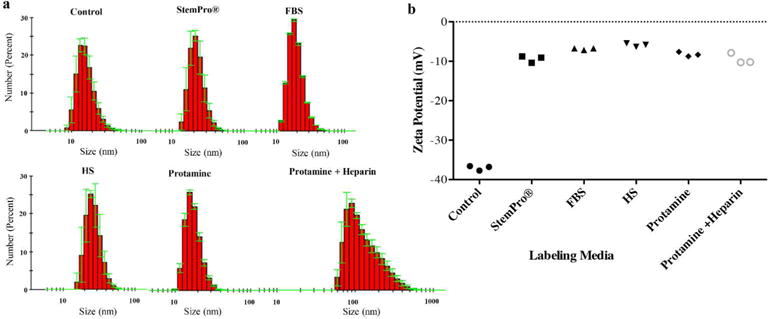
Protein Corona characterization. (a) DLS analysis of ferumoxytol covered by protein corona in different culture media. (b) Zeta potential measurement of ferumoxytol covered by protein corona in different culture media.
The zeta potential of nanoparticles in group 1–5 (minus 5.87±0.45 mV; minus 6.87±0.24 mV, minus 9.42±0.85 mV, minus 8.24±0.34 mV, and minus 9.46±0.79 mV respectively) was significantly different compared to the average zeta potential of bare nanoparticles in water (minus 37.03±.59 mV, p=0.0012). In addition, the zeta potential of nanoparticles in group 1 was significantly smaller compared to group 2–5 (p=0.0022; figure 2b).
To further evaluate the composition of the protein corona around ferumoxytol nanoparticles in group 1–3, we evaluated the type and size of corona proteins. Following incubation with human or fetal serum containing media, the corona was composed of proteins with a molecular weight of less than 30 kDa. The most frequent proteins (NpSpC>5) in human serum, fetal bovine serum, and cGMP compliant protein-containing media (StemPro®) included apolipoprotein A-I, hemoglobin alpha, and serum albumin, respectively (Tables 1–3 and Supplemental Table 1–3).
Table 1.
Representative hard corona proteins associated with ferumoxytol after incubation in media with 10% FBS, as identified by LC-MS/MS.
| Uniprot accession number | Protein name | NpSpC |
|---|---|---|
| P01966 | Hemoglobin alpha | 16.02±2.68 |
| B0JYN6 | Alpha-2-HS-glycoprotein | 10.59± 1.38 |
| P02768 | Serum albumin | 7.45±0.49 |
| P81644 | Apolipoprotein A-II | 7.37±0.87 |
| P02081 | Hemoglobin beta | 6.94±0.38 |
| P34955 | Alpha-1-antiproteinase | 5.04±0.47 |
| P15497 | Apolipoprotein A-I | 4.89±0.72 |
| Q9TRP4 | Fetuin | 2.93±1.76 |
| Q03247 | Apolipoprotein E | 1.06±0.13 |
| P19035 | Apolipoprotein C-III | 0.99±0.34 |
Data are displayed as means and standard deviations of three individual tests.
Table 3.
Representative hard corona proteins associated with ferumoxytol after incubation in StemPro® media, as identified by LC-MS/MS.
| Uniprot accession number | Protein name | NpSpC |
|---|---|---|
| P02768 | Serum albumin | 70.93±1.46 |
| Q2TBU0 | Haptoglobin | 2.39±0.27 |
| P19035 | Apolipoprotein C-III | 2.34±1.89 |
| Q3ZBQ9 | APOM protein | 1.95±0.11 |
| P18902 | Retinol-binding protein 4 | 1.89±0.86 |
| P60712 | Actin, cytoplasmic 1 | 1.433±0.25 |
| Q03247 | Apolipoprotein E | 1.27±0.35 |
| Q3ZCF0 | Dynactin subunit 2 | 1.20±0.15 |
Data are displayed as means and standard deviations of three individual tests.
To understand the biochemical function of specific proteins in the corona, a bioanalytical approach (18) was used to classify the quantity of proteins that mediate complement activation, coagulation, acute phase response, apolipoproteins, and tissue leakage (Figure 3b). In the FBS and HS groups, 19.4% and 16.4% of all proteins in the corona were apolipoproteins. In the StemPro® group, 20% of all proteins in the corona were acute phase response proteins (9.7%) and apolipoproteins (10.3%).
Figure 3.
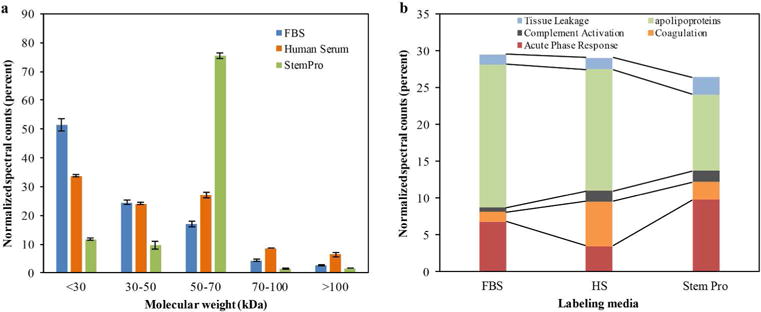
Classification of proteins in the hard corona of ferumoxytol nanoparticles, after incubation with protein-containing media: (a) Normalized spectral counts (NpSpC) of proteins of different molecular weight ranges in the hard corona of ferumoxytol nanoparticles. (b) NpSpC of proteins with different physiological functions in the hard corona of ferumoxytol nanoparticles, after incubation with human serum (HS)- or fetal bovine serum (FBS)-containing media or StemPro® media. Data are displayed as means and standard errors of three samples per group.
Cellular iron uptake
3,3′ Diaminobenzidine (DAB) enhanced Prussian blue stains demonstrated marked iron uptake for hMSC labeled with ferumoxytol in all groups (Figure S1a). The quantitative iron uptake, as determined by ICP-OES, was significantly higher (p≤0.001) for hMSCs labeled with ferumoxytol in group 1 (4.01±0.02 pg/cell) compared to other labeling methods (group 2: 1.88±0.27 pg/cell, group 3: 2.55±0.01 pg/cell), including the standard transfection protocols, group 4: 1.18±0.28 pg/cell, and group 5: 2.98±0.01 pg/cell; Figure S1b).
All labeling protocols lead to an increased quantity of lysosomes/endosomes in labeled compared to unlabeled hMSC, as shown on immunofluorescent stains (lysoTracker® Red DND-99). This suggests a similar mode of lysosomal nanoparticle uptake for all labeling protocols (Figure S2). The presence of iron oxide nanoparticles in lysosomes/endosomes was confirmed by transmission electron microscopy and electron energy loss spectroscopy (EELS) (Figure 4). No nanoparticles were observed in any other compartment of the cells. Imaging and EELS of an unlabeled control revealed no presence of nanoparticles in the cells.
Figure 4.
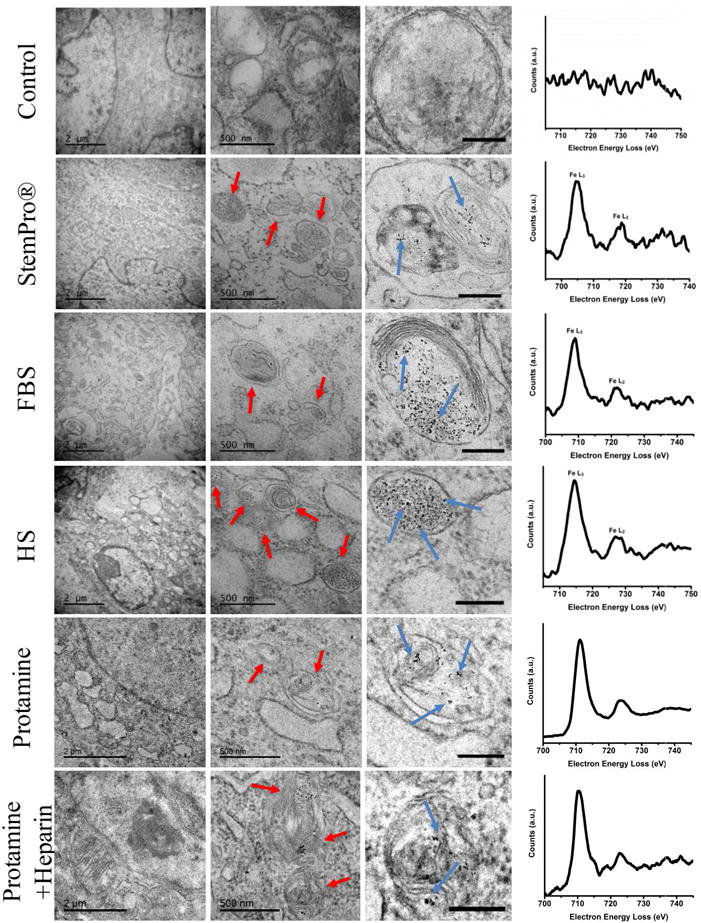
Ferumoxytol compartmentalization in hMSC after exposure to different labeling media: Electron microscopy of similarly treated cells shows nanoparticle-containing lysosomes/endosomes (red arrows) and the iron nanoparticles (blue arrows) in all samples that have been exposed to ferumoxytol (scale bar on left column, middle column, and right column are 2μm, 500nm, and 200nm respectively). Electron energy loss spectra (right) confirm the presence of iron in lysosomes/endosomes of labeled cells and the absence of iron in the unlabeled control.
MR imaging
In vitro, MR images demonstrated significant MR signal effects of all labeled hMSC when compared to unlabeled hMSCs (figure 5). Compared to unlabeled controls (23.62±2.39 ms), mean T2-relaxation times were significantly (p=0.0012) shorter for hMSC labeled with ferumoxytol in group 1 (10.78±0.73 ms), group 2 (15.91±1.36 ms), group 3 (15.07±0.55 ms), group 4 (19.2±0.17 ms), and group 5 (14.58±0.24 ms). In addition, T2 relaxation times of hMSC labeled with ferumoxytol in group 1 (10.78±0.73 ms) were significantly shorter compared to hMSC labeled in groups 2–5 (p=0.0022).
Figure 5.
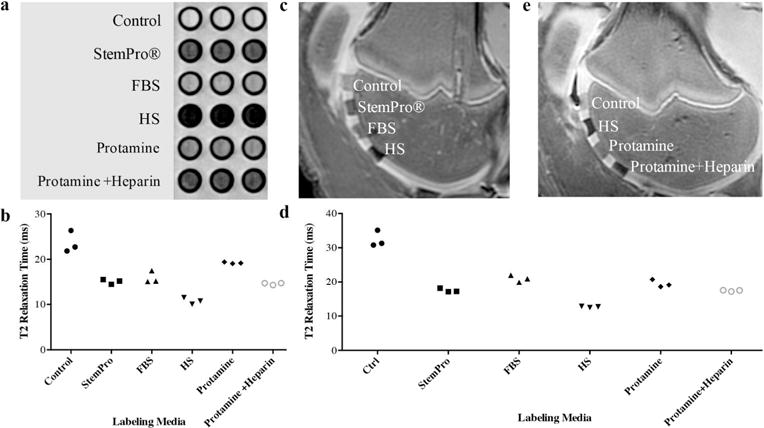
In vitro and ex vivo MR signal of labeled and unlabeled hMSCs at 3 Tesla: (a) Axial T2-weighted MR image of 2×106 hMSC in test tubes with 50μl Ficoll. Unlabeled control cells demonstrate positive (bright) T2-signal, while cells labeled with ferumoxytol in FBS, HS, and StemPro® media demonstrate negative (dark) T2-signal. (b) Corresponding T2 relaxation times show significant T2-shortening of hMSC labeled with ferumoxytol in HS-containing media compared to FBS, StemPro® media as well as protamine and protamine + heparin transfection media. Data are displayed as mean data of three experiments in each group with standard errors. (c) Representative sagittal MR image of unlabeled hMSC and hMSCs with ferumoxytol in FBS, HS, and StemPro® media, seeded in agarose scaffold and implanted in cartilage defects of a pig femur. (d) Representative sagittal MR image of unlabeled hMSC and hMSCs labeled with ferumoxytol in HS, protamine, and protamine + heparin media, seeded in agarose scaffold and implanted in cartilage defects of a pig femur. (e) Corresponding T2 relaxation times of unlabeled, and labeled hMSCs implants.
After implantation of hMSCs into pig knee joints, all labeled hMSC transplants could be clearly delineated from native cartilage and demonstrated significant T2-relaxation time shortening (figure 5c–f) compared to unlabeled hMSC. Group 1 cell labeling resulted in significantly (p=0.0022) shorter T2-relaxation times (12.68±0.11 ms) of labeled cell transplants compared to group 2 (20.94±1.05 ms), group 3 (17.50±0.33 ms), group 4 (19.48±1.13 ms), and group 5 (17.42±0.21 ms) (figure 5c–f).
DISCUSSION
Our data showed that labeling of hMSC with ferumoxytol could be facilitated by generating a protein corona around nanoparticles through incubation in protein containing media. Previous studies have reported the formation of a protein corona around nanoparticles (18, 26, 27). However, to the best of our knowledge, the effect of the protein corona on the labeling efficacy of stem cells has not been investigated.
Ferumoxytol (Feraheme™) is a USPIO and FDA-approved iron supplement (11), which exerts strong signal effects on MR images (13, 28) and can thus be applied “off label” for cell labeling and cell tracking purposes in patients. However, due to their small size, ferumoxytol nanoparticles have a reported low cellular uptake and require transfection-agent assisted protocols for cell labeling (12, 13). Our data showed that the formation of a protein corona could facilitate ferumoxytol uptake by human stem cells. Previous investigators reported the formation of a protein corona around nanoparticles after interaction with protein containing fluids (18, 29, 30). The protein corona increased the nanoparticles’ surface charge and hydrodynamic size (19, 20), which led to increased cellular uptake through endocytosis or phagocytosis (21–23). Accordingly, the larger size and reduced zeta potential of our protein-coated ferumoxytol nanoparticles compared to bare nanoparticles in PBS might explain the observed increased lysosomal uptake of ferumoxytol into hMSC. Furthermore, the protein corona layer could reduce the repulsive interactions between the cell membrane and nanoparticles (as the cell membrane has a net negative charge). Our data showed that the formation of a protein corona increased the zeta potential of ferumoxytol nanoparticles from minus 37.03±0.59 mV to minus 5.87±0.45 mV. Our team previously found that ferumoxides and ferucarbotran can label stem cells by simple incubation through endocytosis or phagocytosis (3, 6). Both of these agents have a larger size compared to ferumoxytol and in addition, ferucarbotran has a negative zeta potential. Thus, although cationic transfection agents can improve cellular uptake, efficient labeling has been previously achieved with negatively charged nanoparticles.
In addition to the effects of size and surface charge, we found that the amount and type of proteins in labeling media could alter the composition of the corona and stem cell labeling efficacy. An observed higher uptake of ferumoxytol by hMSCs in human serum containing media compared to FBS containing or StemPro® media could be due to the higher surface charge of human serum (minus 5.87±0.45 mV) compared to both FBS (minus 6.87±0.24 mV) and StemPro® (minus 9.42±0.85 mV). A higher surface charge causes lower repulsive electrostatic interactions between cell membranes and nanoparticles (31), thereby improving lysosomal uptake (31).
A recent report suggested that C3 apolipoproteins in the protein corona significantly decrease and H1 apolipoproteins in the protein corona significantly increase the cellular uptake of nanoparticles (21). We found the opposite: hMSC incubated with HS-containing media demonstrated increased C3 apolipoproteins, decreased H1 apolipoproteins, and increased nanoparticle uptake compared to hMSC incubated with StemPro®. The observed discrepancy could be due to the cell vision effect, i.e. a cellular response to nanoparticles related to detoxification strategies in response to nanoparticles (32) as different cell types have different cell receptors on their surfaces and use different pathways to respond to nanoparticles (33). Another reason could be overriding effects of other proteins and protein conformation in the corona composition, as different nanoparticles were used in these studies.
Other investigators have previously reported a significantly reduced uptake of corona-coated nanoparticles into cells compared to incubation with bare nanoparticles (34). The observed discrepancy could be due to different exposure environments which affect the nanoparticle uptake amount and intracellular location.
For cell transfection, nanoparticles are incubated with transfection agents in serum-free media to avoid interference of proteins with the formation of nanoparticle-transfection agent complexes (12, 13). Labeling of stem cells with nanoparticles in serum free media beyond 4 hours is not possible because serum-deprived cells develop an apoptosis (35). Using classical transfection protocols, the association of the nanoparticles with transfection agents in a first step prevents a corona formation in the second step, when serum is added. To our surprise, a simplification of the labeling protocol towards a one-step incubation of stem cells in protein-containing media without transfection agent lead to increased labeling efficiencies, which will facilitate clinical translations. Ferumoxytol could be added to any standard media for expansion of allogeneic cell products, without any need to modify the cell culture process or wash the cells.
As our ultimate goal is clinical translation, we focused on clinically applicable nanoparticles, which are negatively charged. Positively charged nanoparticles can produce more distinct disruption of the plasma membrane, greater mitochondrial and lysosomal impairment and importantly, are not clinically available to date (36). The main reason is that positively charged nanoparticles are covered by opsonin-based proteins in the blood, which are rapidly removed by the immune system (37).
Our team previously reported another clinically applicable approach for in vivo labeling of autologous bone marrow cells (28). After intravenous injection, ferumoxytol is taken up by hMSC in the bone marrow. hMSC harvested from a bone marrow aspirate can be tracked after transplantation into the same patient. By comparison, the approach described here can be applied to allogeneic cells, “off the shelf” hMSC products and therapeutic cells that are expanded in cell culture for a long time period.
Our results showed better hMSC labeling efficiencies after incubation of hMSC in human serum containing labeling media compared to StemPro® media. For future clinical applications, we suggest using autologous (patient-derived) serum instead of commercially available human serum, to increase the patient’s safety. This labeling approach could be readily used to monitor ongoing phase II and III studies of novel cell therapeutics.
CONCLUSION
We developed a new, transfection-agent free stem cell labeling approach, which takes advantage of the formation of a protein corona around ferumoxytol nanoparticles in protein-containing media. Protein Corona mediated cell labeling represents a new and readily clinically translatable method for labeling “off the shelf” cell products with ferumoxytol and will be applied for stem cell tracking in patients.
Supplementary Material
Figure S1: In vitro evaluation of fenunoxytol-uptake by liMSCs (a) DAB-Prussian blue staining of unlabeled hMSCs (control) and hMSC labeled with fenunoxytol in StemPro®, FBS. HS. protamine, and protamine + heparin containing media, (b) The corresponding average amount of iron per cell, as measured by ICP-OES analysis.
Figure S2: Fenunoxytol compartmentalization in hMSC after exposure to different labeling media: LysoTracker® Red DND-99 immunofluorescent stains for lysosomes/endosomes (red) in hMSC labeled with fenunoxytol in StemPro®. FBS. HS. protamine, and protamine + heparin containing media. All cells exposure to fenunoxytol show increased numbers of lysosomes/ endosomes compared to controls (DAPI represents cell nucleus: Red fluorescent demonstrates lysosomes/endosomes: 100pm scale bar).
Table 2.
Representative hard corona proteins associated with ferumoxytol after incubation in media with 10% HS, as identified by LC-MS/MS.
| Uniprot accession number | Protein name | NpSpC |
|---|---|---|
| P02647 | Apolipoprotein A-I | 6.36±0.38 |
| P02649 | Apolipoprotein E | 4.29±0.12 |
| P02768 | Serum albumin | 3.74±0.57 |
| P0CG05 | Ig lambda-2 chain C regions | 3.61±0.15 |
| P02654 | Apolipoprotein C-I | 2.21±0.17 |
| P55056 | Apolipoprotein C-IV | 1.94±0.26 |
| P02652 | Apolipoprotein A-II | 1.93±0.20 |
| P04114 | Apolipoprotein B-100 | 1.85±0.39 |
| P02656 | Apolipoprotein C-III | 1.66±0.47 |
| P01024 | Complement C3 | 1.66±0.11 |
| P01834 | Ig kappa chain C region | 1.62±0.53 |
| P02655 | Apolipoprotein C-II | 1.46±0.21 |
| P35542 | Serum amyloid A-4 protein | 1.29±0.14 |
| P01876 | Ig alpha-1 chain C region | 1.26±0.05 |
| P00738 | Haptoglobin | 1.13±0.09 |
| P06727 | Apolipoprotein A-IV | 1.04±0.09 |
Data are displayed as means and standard deviations of three individual tests.
Advances in Knowledge.
We developed a new, transfection-agent free labeling approach, which takes advantage of the formation of a protein corona layer around ferumoxytol nanoparticles in protein-containing media.
Human serum protein corona-mediated cell labeling enabled significantly higher ferumoxytol uptake by human Mesenchymal Stem Cells (hMSC) compared to transfection agent-mediated labeling techniques (p<0.05).
Summary Statement.
Protein Corona mediated cell labeling represents a new and readily clinically translatable method for labeling “off the shelf” cell products with ferumoxytol and will be applied for stem cell tracking in patients.
Acknowledgments
The data presented in the paper was partly acquired on instruments in Stanford School of Medicine Falk cardiovascular research center imaging facility. Part of this work was performed at the Stanford Nano Shared Facilities (SNSF). This work was supported by NIH grants #2R01AR054458 and R21AR066302 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Acknowledgments of research support for the study:
This work was supported by NIH grant 2R01AR054458 and R21AR066302 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Steven Madsen is supported by the Center for Cancer Nanotechnology Excellence and Translational Diagnostics (CCNE-TD) grant funded by NCI-NIH to Stanford University U54CA199075.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures of Conflicts of Interest:
The authors declare no competing financial interest.
References
- 1.Bartel RL. Chapter 8 - Stem Cells and Cell Therapy: Autologous Cell Manufacturing A2 - Atala, Anthony. In: Allickson JG, editor. Translational Regenerative Medicine. Boston: Academic Press; 2015. pp. 107–12. [Google Scholar]
- 2.Long CM, Bulte JW. In vivo tracking of cellular therapeutics using magnetic resonance imaging. Expert opinion on biological therapy. 2009;9(3):293–306. doi: 10.1517/14712590802715723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henning TD, Wendland MF, Golovko D, et al. Relaxation effects of ferucarbotran-labeled mesenchymal stem cells at 1.5T and 3T: discrimination of viable from lysed cells. Magn Reson Med. 2009;62(2):325–32. doi: 10.1002/mrm.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daldrup-Link HE, Nejadnik H. MR Imaging of Stem Cell Transplants in Arthritic Joints. Journal of stem cell research & therapy. 2014;4(2):165. doi: 10.4172/2157-7633.1000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nejadnik H, Henning TD, Castaneda RT, et al. Somatic differentiation and MR imaging of magnetically labeled human embryonic stem cells. Cell transplantation. 2012;21(12):2555–67. doi: 10.3727/096368912X653156. [DOI] [PubMed] [Google Scholar]
- 6.Henning TD, Gawande R, Tavri S, et al. MR Imaging of Ferumoxides labeled Mesenchymal Stem Cells in Cartilage Defects: in vitro and in vivo investigations. Molecular Imaging in press. 2011 [PMC free article] [PubMed] [Google Scholar]
- 7.Castaneda R, Khurana A, Khan R, Daldrup-Link HE. Labeling stem cells with ferumoxytol, an FDA-approved iron oxide nanoparticle. J Vis Exp. 2011 doi: 10.3791/3482. accepted for publication. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daldrup-Link HE, Rudelius M, Oostendorp RA, et al. Targeting of hematopoietic progenitor cells with MR contrast agents. Radiology. 2003;228(3):760–7. doi: 10.1148/radiol.2283020322. [DOI] [PubMed] [Google Scholar]
- 9.Daldrup-Link HE, Rudelius M, Piontek G, et al. Migration of iron oxide-labeled human hematopoietic progenitor cells in a mouse model: in vivo monitoring with 1.5-T MR imaging equipment. Radiology. 2005;234(1):197–205. doi: 10.1148/radiol.2341031236. [DOI] [PubMed] [Google Scholar]
- 10.Sutton EJ, Henning TD, Boddington S, et al. In vivo magnetic resonance imaging and optical imaging comparison of viable and nonviable mesenchymal stem cells with a bifunctional label. Molecular imaging. 2010;9(5):278–90. [PMC free article] [PubMed] [Google Scholar]
- 11.Lu M, Cohen MH, Rieves D, Pazdur R. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J Hematol. 2010;85(5):315–9. doi: 10.1002/ajh.21656. [DOI] [PubMed] [Google Scholar]
- 12.Thu MS, Bryant LH, Coppola T, et al. Self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine for cell tracking by magnetic resonance imaging. Nature medicine. 2012;18(3):463–7. doi: 10.1038/nm.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khurana A, Nejadnik H, Chapelin F, et al. Ferumoxytol: a new, clinically applicable label for stem-cell tracking in arthritic joints with MRI. Nanomedicine (Lond) 2013;8(12):1969–83. doi: 10.2217/nnm.12.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montet-Abou K, Montet X, Weissleder R, Josephson L. Cell internalization of magnetic nanoparticles using transfection agents. Molecular imaging. 2007;6(1):1–9. [PubMed] [Google Scholar]
- 15.Montet-Abou K, Montet X, Weissleder R, Josephson L. Transfection agent induced nanoparticle cell loading. Molecular imaging. 2005;4(3):165–71. doi: 10.1162/15353500200505100. [DOI] [PubMed] [Google Scholar]
- 16.Fu Y, Kraitchman DL. Stem cell labeling for noninvasive delivery and tracking in cardiovascular regenerative therapy. Expert review of cardiovascular therapy. 2010;8(8):1149–60. doi: 10.1586/erc.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soenen SJ, De Smedt SC, Braeckmans K. Limitations and caveats of magnetic cell labeling using transfection agent complexed iron oxide nanoparticles. Contrast media & molecular imaging. 2012;7(2):140–52. doi: 10.1002/cmmi.472. [DOI] [PubMed] [Google Scholar]
- 18.Tenzer S, Docter D, Kuharev J, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nature nanotechnology. 2013;8(10):772–81. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 19.Huhn D, Kantner K, Geidel C, et al. Polymer-coated nanoparticles interacting with proteins and cells: focusing on the sign of the net charge. ACS nano. 2013;7(4):3253–63. doi: 10.1021/nn3059295. [DOI] [PubMed] [Google Scholar]
- 20.Rocker C, Potzl M, Zhang F, Parak WJ, Nienhaus GU. A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles. Nature nanotechnology. 2009;4(9):577–80. doi: 10.1038/nnano.2009.195. [DOI] [PubMed] [Google Scholar]
- 21.Ritz S, Schottler S, Kotman N, et al. Protein corona of nanoparticles: distinct proteins regulate the cellular uptake. Biomacromolecules. 2015;16(4):1311–21. doi: 10.1021/acs.biomac.5b00108. [DOI] [PubMed] [Google Scholar]
- 22.Kim JA, Salvati A, Aberg C, Dawson KA. Suppression of nanoparticle cytotoxicity approaching in vivo serum concentrations: limitations of in vitro testing for nanosafety. Nanoscale. 2014;6(23):14180–4. doi: 10.1039/c4nr04970e. [DOI] [PubMed] [Google Scholar]
- 23.Fleischer CC, Payne CK. Nanoparticle-cell interactions: molecular structure of the protein corona and cellular outcomes. Accounts of chemical research. 2014;47(8):2651–9. doi: 10.1021/ar500190q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon GH, von Vopelius-Feldt J, Fu Y, et al. Ultrasmall supraparamagnetic iron oxide-enhanced magnetic resonance imaging of antigen-induced arthritis: a comparative study between SHU 555 C, ferumoxtran-10, and ferumoxytol. Invest Radiol. 2006;41(1):45–51. doi: 10.1097/01.rli.0000191367.61306.83. [DOI] [PubMed] [Google Scholar]
- 25.Monopoli MP, Walczyk D, Campbell A, et al. Physical-chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. Journal of the American Chemical Society. 2011;133(8):2525–34. doi: 10.1021/ja107583h. [DOI] [PubMed] [Google Scholar]
- 26.Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(38):14265–70. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kah JC, Chen J, Zubieta A, Hamad-Schifferli K. Exploiting the protein corona around gold nanorods for loading and triggered release. ACS nano. 2012;6(8):6730–40. doi: 10.1021/nn301389c. [DOI] [PubMed] [Google Scholar]
- 28.Khurana A, Chapelin F, Beck G, et al. Iron administration before stem cell harvest enables MR imaging tracking after transplantation. Radiology. 2013;269(1):186–97. doi: 10.1148/radiol.13130858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walczyk D, Bombelli FB, Monopoli MP, Lynch I, Dawson KA. What the cell “sees” in bionanoscience. Journal of the American Chemical Society. 2010;132(16):5761–8. doi: 10.1021/ja910675v. [DOI] [PubMed] [Google Scholar]
- 30.Cedervall T, Lynch I, Lindman S, et al. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(7):2050–5. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nel AE, Madler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nature materials. 2009;8(7):543–57. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 32.Mahmoudi M, Saeedi-Eslami SN, Shokrgozar MA, et al. Cell “vision”: complementary factor of protein corona in nanotoxicology. Nanoscale. 2012;4(17):5461–8. doi: 10.1039/c2nr31185b. [DOI] [PubMed] [Google Scholar]
- 33.Laurent S, Burtea C, Thirifays C, Hafeli UO, Mahmoudi M. Crucial ignored parameters on nanotoxicology: the importance of toxicity assay modifications and “cell vision”. PloS one. 2012;7(1):e29997. doi: 10.1371/journal.pone.0029997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lesniak A, Fenaroli F, Monopoli MP, Aberg C, Dawson KA, Salvati A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS nano. 2012;6(7):5845–57. doi: 10.1021/nn300223w. [DOI] [PubMed] [Google Scholar]
- 35.Zhu W, Chen J, Cong X, Hu S, Chen X. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem cells. 2006;24(2):416–25. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]
- 36.Panariti A, Miserocchi G, Rivolta I. The effect of nanoparticle uptake on cellular behavior: disrupting or enabling functions? Nanotechnol Sci Appl. 2012;5:87–100. doi: 10.2147/NSA.S25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61(6):428–37. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: In vitro evaluation of fenunoxytol-uptake by liMSCs (a) DAB-Prussian blue staining of unlabeled hMSCs (control) and hMSC labeled with fenunoxytol in StemPro®, FBS. HS. protamine, and protamine + heparin containing media, (b) The corresponding average amount of iron per cell, as measured by ICP-OES analysis.
Figure S2: Fenunoxytol compartmentalization in hMSC after exposure to different labeling media: LysoTracker® Red DND-99 immunofluorescent stains for lysosomes/endosomes (red) in hMSC labeled with fenunoxytol in StemPro®. FBS. HS. protamine, and protamine + heparin containing media. All cells exposure to fenunoxytol show increased numbers of lysosomes/ endosomes compared to controls (DAPI represents cell nucleus: Red fluorescent demonstrates lysosomes/endosomes: 100pm scale bar).


